Abstract
Poor wound healing after diabetes mellitus remains a challenging problem, and its pathophysiological mechanisms have not yet been fully elucidated. Persistent bleeding, disturbed regulation of inflammation, blocked cell proliferation, susceptible infection and impaired tissue remodeling are the main features of diabetic wound healing. Conventional wound dressings, including gauze, films and bandages, have a limited function. They generally act as physical barriers and absorbers of exudates, which fail to meet the requirements of the whol diabetic wound healing process. Wounds in diabetic patients typically heal slowly and are susceptible to infection due to hyperglycemia within the wound bed. Once bacterial cells develop into biofilms, diabetic wounds will exhibit robust drug resistance. Recently, the application of stimuli-responsive hydrogels, also known as “smart hydrogels”, for diabetic wound healing has attracted particular attention. The basic feature of this system is its capacities to change mechanical properties, swelling ability, hydrophilicity, permeability of biologically active molecules, etc., in response to various stimuli, including temperature, potential of hydrogen (pH), protease and other biological factors. Smart hydrogels can improve therapeutic efficacy and limit total toxicity according to the characteristics of diabetic wounds. In this review, we summarized the mechanism and application of stimuli-responsive hydrogels for diabetic wound healing. It is hoped that this work will provide some inspiration and suggestions for research in this field.
Keywords: Diabetic wound, Biomaterials, Skin tissue engineering, Hydrogel, Smart responsiveness
Graphical abstract

1. Introduction
As a complex metabolic disease that impairs the health of people worldwide with increasing prevalence, the number of diabetic patients worldwide was estimated to be 285 million in 2010 and is projected to rise to 439 million by 2030 [1,2]. The hazards of diabetes are associated with many serious complications, including poor wound healing, cardiovascular disease, eye disease, nerve damage, and lower extremity amputation due to foot ulcers. Approximately 20% of diabetic patients worldwide suffer from poor diabetic wound healing [3]. In diabetic wounds, skin regeneration is often disrupted, which prevent wounds from healing normally. More than half of diabetic wounds develop into chronic wounds, with increased risks of amputation and death. Additionally, diabetic wounds are 60–70% more likely to recur, which makes their treatment cost significantly more expensive [[4], [5], [6]]. Consequently, the high morbidity rate due to the complex pathogenesis and susceptibility increases the cost and difficulty of treatment for diabetic wounds, which places a heavy burden both on the health of patients and health care systems worldwide [[7], [8], [9]].
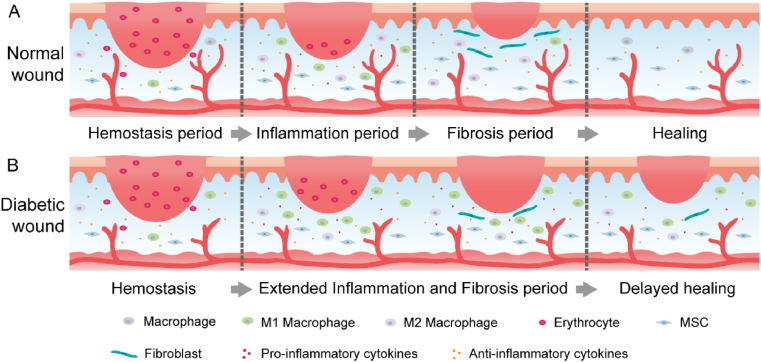
Normal wound healing usually proceeds through specific stages of haemostasis, inflammation, proliferation and remodeling, while in diabetic wounds, this process may be disrupted or delayed due to various deleterious factors. Biochemical disorders in diabetic wounds, including hyperglycaemia, dyslipidaemia, and insulin resistance, can cause tissue damage that hinders the normal wound healing process (Fig. 1) [10]. These harmful factors lead to excessive inflammation in diabetic wounds followed by continuous destruction of the wound tissue and prolong wound healing [11]. The abnormality of immune cells in diabetic wounds makes it difficult to suppress inflammation and greatly increases the susceptibility to infection. Once a chronic wound becomes infected, increased inflammation will persist and keep the wound in a constant cycle of infection, inflammation, and inadequate repair. In addition, studies have shown that another key factor in the nonhealing of diabetic wounds is excessive oxidative stress and decreased antioxidant capacity of tissues, which causes a redox imbalance [12]. The normal vascular system is in a quiescent state due to the balance between proangiogenic factors (e.g., vascular endothelial growth factor A and fibroblast growth factor) and antiangiogenic factors (e.g., angiopoietin 1 and pigment epithelium-derived factors). When the vasculature is at rest, blood vessels can be adequately perfused to deliver adequate nutrients and oxygen to tissues [13]. However, diabetic states can significantly disrupt this balance and cause reduced angiogenesis, which hinders the normal wound healing and restoration of a healthy vascular system [14]. Recent studies have revealed that neuropathy is also associated with an impaired wound healing process [15], due to the oxidative stress caused by diabetes that mediates nerve cell dysfunction and death. Apart from hyperglycaemia, the reduction of several neuropeptides associated with neuropathy impairs the vasodilation and angiogenesis. Decreased vasodilation leads to microcirculatory disturbance and functional wound ischaemia [16]. Therefore, how to treat diabetic wounds more effectively has always been a huge challenge for the medical system.
Fig. 1.
Schematic representation of the main stages of normal wound healing (A) and delayed healing of diabetic wounds (B).
In the past, the treatment of diabetic wounds was not unlike traditional wound treatment, i.e., it mainly uses dry dressings such as bandages and gauze with antibiotic supplements to absorb wound exudates and prevent infection. However, as a passive intervention strategy, the use of dry dressings fails to adapt to the diabetic wound microenvironment and may cause secondary damage due to adhesion between dressings and wounds [[17], [18], [19], [20]]. In addition, the lack of knowledge of the most appropriate treatment and management of diabetic wounds by health care professionals contributes to delayed wound healing. Recently, tissue engineering strategies are seeking advanced bioactive platforms that can artificially mimic or fully restore the function of native skin tissue. The healing of diabetic wounds does not follow the normal phases of wound healing as acute wounds do, and diabetic wound healing may stall at a particular stage of the process, which prolongs wound healing. To address this concern, modern functional wound dressings have attracted great attention in this field.
The significant therapeutic effects of modern functional wound dressings, including nanofibre, hydrophilic colloids, foams, and hydrogels, have been demonstrated in diabetic wounds. These new dressings can provide a moist, antibacterial healing environment and carry bioactive molecules or drugs to accelerate wound healing. The capacity of wet dressings to speed up the healing process has been tested [21]. The method of delivering drugs through nanomaterials to reduce the dose of drugs with increased drug utilization efficiency is considered a promising strategy to promote wound healing [22,23]. At present, most nanomaterials are widely used as drug carriers. However, some nanomaterials have a low loading capacity and have difficulties in avoiding the toxic effects of the carrier itself. They also have a single function in the treatment of wounds and cannot keep the wound moist and isolated from the external environment [24,25]. Hydrogels with three-dimensional network structures are soft materials that can be physically and chemically tailored for various applications in the biomedical field. In addition to their strong water absorption and moisturizing properties, hydrogels have many similar physical and chemical properties to the natural extracellular matrix (ECM), which provide a more ideal management for diabetic wounds [26,27]. Similar to a 3D carrier, hydrogels can be easily loaded with drugs or cytokines that specifically target the main difficulties of diabetic wounds, such as blood sugar control agents, antibacterial agents, and angiogenic factors. Many designed strategies of hydrogels have been proposed and developed for wound healing. However, among these strategies, conventional designed hydrogels as a drug delivery system mainly rely on the diffusion of the drug itself or its own solution to release the drug, which is uncontrollable and may not achieve the expected effect of drug delivery.
Recently, smart hydrogels with stimulus-responsive functions have attracted considerable attention. Briefly, stimulus-responsive hydrogels can respond to internal physiological signals (i.e., temperature [28,29], pH [30,31], redox [32,33], glucose [34,35], or enzymes [36,37]), external stimuli (i.e., temperature [38,39], magnetic field [40,41], pressure [42,43], reactive oxygen species (ROS) [44,45], light [46,47] and electric field [48,49]) or a combination of the above, which can reversibly or irreversibly change the physical properties or chemical structure (Fig. 2). Therapeutic strategies based on smart responsive hydrogels offer a promising platform to address the multistage complexity and difficult regulation of molecular expression during chronic wound healing [50,51]. “Smart” hydrogels can be easily cross-linked using different chemical or physical strategies. These hydrogels have high tensile strength, compressive elasticity, flexibility strong skin adhesion, the ability to attach to complex curved surfaces and dynamic objects, the ability to adapt to complex wounds and other kinematic properties. Smart hydrogels show rapid and autonomous self-recovery after damage caused by external forces and may maintain their structural stability during the wound healing process. With these properties, they can adapt to complex wound changes to ensure a complete fit between wound and dressing to facilitate access and release of the contained medication. Smart hydrogels also have adjustable chemical and physical properties, are biocompatible, biodegradable and create a moist environment similar to an extracellular matrix for wound healing. Smart hydrogels can change their shape/size/volume in response to different stimuli and may have other functional properties to facilitate targeted drug release to specific injury areas [52]. This smart on-demand stimulus-responsive hydrogel can control drug release, maximize therapeutic efficacy and reduce adverse side effects because they can their ability to rapidly detect and respond to the diabetic environment and deliver therapeutic effects without damaging healthy tissues [53,54]. Therefore, designing and developing stimuli-responsive hydrogels based on the complex pathophysiological microenvironment of diabetes provides a promising avenue to better manage diabetic wounds.
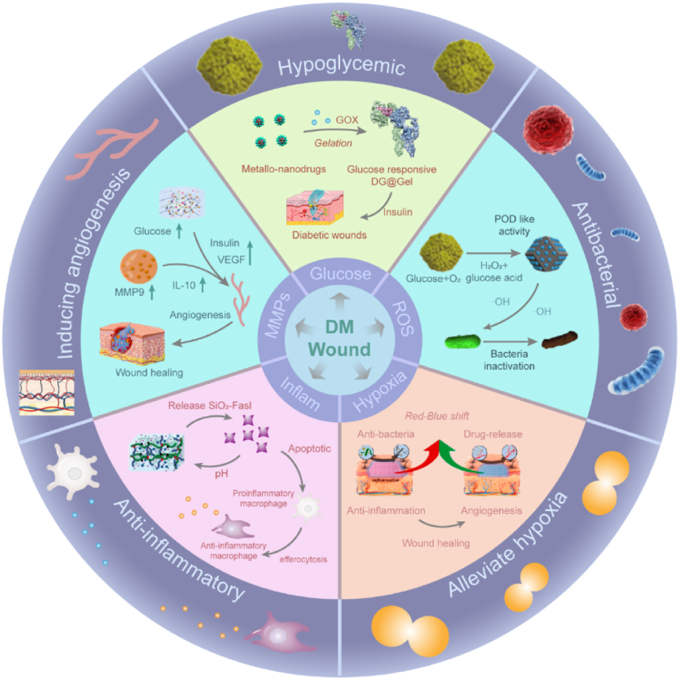
Fig. 2.
Typical alterations in diabetic wounds and a summary of the classification and biological functions of smart hydrogels.
Herein, we review various stimulatory responsive hydrogel structures and their release mechanisms and systematically describe their applications in diabetic wound healing. Furthermore, we highlight the existing evidence for the application of various stimuli-responsive hydrogels in diabetic wounds and summarize the mechanisms and limitations of stimuli-responsive hydrogels. This review will provide scientific guidance for the clinical application of stimuli-responsive hydrogels and promote the development of smart medical materials.
2. Advances in understanding the pathophysiology of diabetic wounds
In healthy individuals, wound healing is generally a dynamic and orderly process of skin repair, which can be divided into four overlapping but distinct phases: hemostasis, inflammation, proliferation and remodeling [55]. However, the wound healing process may be disturbed in some particular circumstances, such as diabetes mellitus [56,57]. Diabetes is able to impair wound healing in various ways and stages. First, diabetes-related hyperglycemia leads to elevated levels of advanced glycation end products (AGEs) in blood. AGEs directly lead to the formation of high concentrations of ROS and reactive nitrogen species (RNS). When the level of ROS/RNS exceeds the antioxidant capacity of tissue, it causes damage to the host tissue and perpetuates inflammation [58,59]. Second, high blood sugar causes the narrowing and occlusion of the capillaries around the wound. So that nutrients and oxygen failed to be transported smoothly. Worse still, changes in hemoglobin glycosylation and erythrocyte membrane structure aggravate the insufficient oxygen and nutrient supply, contributing to dysregulated macrophage polarization. Activation of macrophages can enhance the abnormal apoptosis of fibroblasts and keratinocytes with an excessive release of proinflammatory cytokines. Furthermore, a sustained period of inflammation promotes the recruitment of macrophages into the wound site, which maintains the inflammatory state of the wound via polarization, forming a positive feedback loop [60].
pH plays an important role in the cellular processes of wound healing [61]. As one of the basic parameters of the diabetic wound microenvironment, changes in pH influence these four phases of cellular processes and cell cycle processes. A series of pathophysiological changes accompany the different wound healing stages, and the microenvironmental pH is constantly and dynamically altered [62]. The normal pH of the skin is weakly acidic (5.5–6) [63], but chronic wounds have an elevated pH on their surface due to microvascular leakage [64]. As a result, the pH on the surface of chronic wounds becomes more alkaline, which can cause increased inflammation of the wound, resulting in a prolonged failure to heal. Even worse, this alkaline microenvironment is conducive to bacterial growth, resulting in an increase in the number of bacteria on the surface of the wound, which can lead to wound infection [65]. However, chronic wounds with pus or necrotic tissue show an acidic pH. Also, as chronic wounds heal, the physiological microenvironment begin to naturally restore the acidic environment [64,66,67]. Therefore, it is important to make changes according to the changes in pH during the wound healing process to accelerate the wound healing.
ROS are key regulators of several stages of wound healing. It is well established that an appropriate level of ROS can act as a signal transduction to enhance wound healing through the accumulation of inflammatory cells and enhanced bacteriostatic effects [12,68,69]. However, the redox imbalance caused by excessive oxidative stress and decreased antioxidant capacity of tissues can result in the prolonged healing time of diabetic wounds. In diabetic patients, the activated polyol pathway, hexosamine pathway, AGE pathway, PKC pathway and some other pathways jointly stimulate the overproduction of ROS in mitochondria [[70], [71], [72]]. Studies have found that high levels of ROS not only impair normal collagen synthesis and re-epithelialization in fibroblasts and keratinocytes, but also induce an increase in serine proteases and matrix metalloprotein (MMP) leading to an excess inflammatory response with the degradation of ECM, growth factors and protease inhibitors [[73], [74], [75]]. In addition, hypoxia arising from vascular defects deprives diabetic wounds of proper perfusion, leading to the increased release of ROS and degradation of proteins. These proteolytic lesions result in severe ECM degradation that prevents normal matrix-cell interactions required for wound repair [74,75]. Oxidative stress can also hinder diabetic wound healing through skin damage, neuropathy, ischemic lesions, etc. In conclusion, diabetic wounds have become a difficult problem for researchers due to their complex microenvironment (Fig. 3).
Fig. 3.
Differences between the normal wound microenvironment and the diabetic wound microenvironment.
3. Application of stimuli-responsive hydrogels in diabetic wounds
In standard hydrogel therapeutic systems, the active substance is released through shrinkage, swelling, diffusion and matrix breakdown of the hydrogel. If the properties of a hydrogel can change in response to the surrounding environment, it is called a stimuli-responsive hydrogel, which is also known as a smart hydrogel [76]. Smart on-demand stimuli-responsive hydrogels can rapidly deliver therapeutic effects in disease environments and maintain the physiological health of cells and tissues [77,78]. Based on these concepts, biofunctional materials with intelligent stimulus-responsive therapeutic and regenerative capabilities have been developed, which can respond to external physical stimuli (such as ultrasound, light irradiation, electric and magnetic fields), endogenous disease microenvironments (such as excessive expressed ROS, pH, specific ion concentration, secreted enzymes, specific immune environment) or a combination of these factors through reversible or irreversible changes in physical properties or chemical structure [[79], [80], [81]]. Among these biomaterials, stimuli-responsive hydrogels have achieved initial success in promoting diabetic wound healing. Here we classify and summarize the current application of stimuli-responsive hydrogel dressings for diabetic wound healing according to different stimuli conditions.
3.1. pH-responsive hydrogels
pH can serve as a significant indicator of the wound status because it is associated with many physiological processes, including bacterial infection and angiogenesis [82]. The pH of normal skin or healed wounds is slightly acidic (pH: 4–6) due to the production of fatty acids and amino acids secreted by keratinocytes [83]. However, chronic wounds can persist for months in alkaline conditions (pH: 7–9), which facilitates the colonization and growth of pathogenic microorganisms and makes the wounds more susceptible to bacterial infection [82]. Therefore, monitoring wound pH can clarify the wound status and provide meaningful indicators for early warning of infection risk. When the pH in the surrounding environment changes, the pH-responsive hydrogel will change its volume and release the drug [84,85]. pH-responsive hydrogels consist of polymers with acidic or basic groups that can exchange protons depending on pH. When the pH and ionic strength of the environment change, the degree of dissociation of these groups also changes, which decreases the crosslinking points of the gel network and a change in the swelling degree of the hydrogel [86,87]. For example, polymers with acidic groups such as carboxyl groups form new hydrogen bonds under low pH conditions, which reduces the hydrogel volume. Therefore, the drug release rate of this hydrogel significantly decreases when the pH decreases. In contrast, when the pH increases, the hydrogel swells due to the electrostatic repulsion between the ionized carboxyl groups. As a result, more water is adsorbed into the hydrogel, followed by faster drug release. Similarly, for polymers containing basic groups, acidic conditions will increase the volume of the hydrogel, while basic conditions will make the hydrogel shrink. Hence, the diffusion and release rates of drugs from pH-responsive hydrogels are controlled by regulating the shrinkage or swelling of the hydrogels. Commonly used acid-responsive materials are protonated polymers, such as polymethacrylic acid (PMAA), polyacrylic acid (PAA), and phenylboronic acids (PBAs).
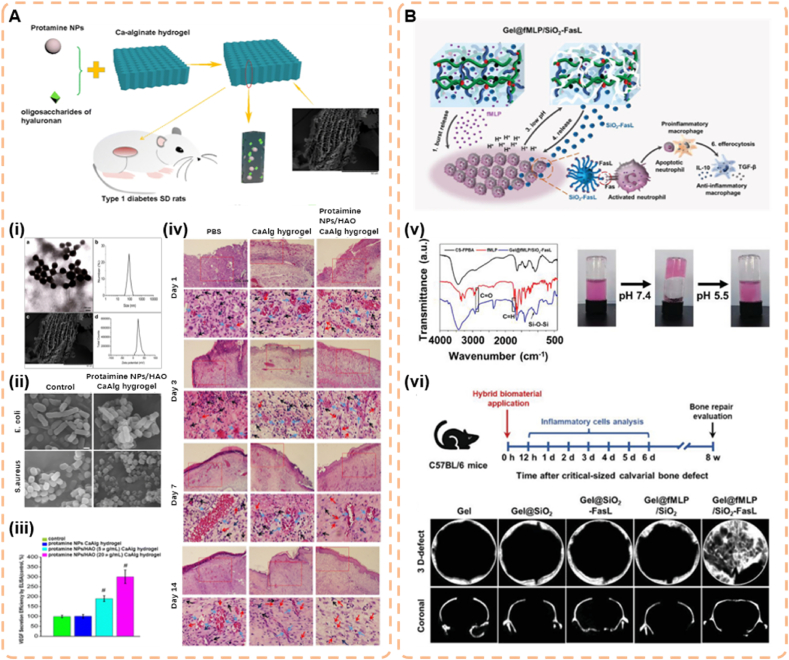
As mentioned above, due to the increased pH in the early stages of diabetic wounds, bacterial colonization and biofilm formation are accelerated, prolonging the inflammatory state and impairing angiogenesis. Therefore, bacterial infection can be better inhibited in the early stages of diabetic wounds [88]. Wang et al. fabricated a novel pH-responsive calcium alginate (CaAlg) hydrogel loaded with protamine nanoparticles (NPs) and hyaluronan oligosaccharides (HAO) (Fig. 4A). As a pH-sensitive dressing material, CaAlg can more quickly and completely trigger drug release. The authors first evaluated the swelling capacity of the hydrogels and found that the protamine NP/HAO CaAlg hydrogels could absorb more water, and the swelling ratio increased with the pH of the medium. In the medium of pH 3.0, most of the carboxylic acid groups in the CaAlg hydrogel exist in the form of –COOH, which causes polymer-polymer bonding and shrinking of the CaAlg hydrogel. When CaAlg hydrogels were soaked in PBS solution at pH 6.0 and 8.0, the carboxylic acid groups in CaAlg hydrogels were ionized and mainly existed in the form of –COO−. The swelling of the hydrogel results from electrostatic repulsion between –COO− groups. The easy formation of hydrogen bonds between -CoO− and H2O significantly enhances this trend, so at pH 8.0, the hydrogels significantly swells in a liquid medium mimicking diabetic wounds. Second, for the drug release process, when the pH value of the simulated acidic microenvironment is 3.0, the gel shrinks due to the protonation of ionized CoO− groups, which reduces the drug release rate. In the diabetic trauma stimulation medium at pH 8.0, the CaAlg hydrogel swells due to the absorption of more water, destroys and loosens the CaAlg gel structure, which can rapidly promote the release of NPs and HAO. The protamine NP/HAO CaAlg hydrogel has a fast cumulative drug release process and releases 98.7% of HAO within 8 h, and the drug release rate at pH 8.0 is approximately 2.2 times that at 3.0. These results demonstrate the increased drug release of the CaAlg hydrogel at pH 8.0 due to its pH dependence. The mechanism of faster drug release may result from the increased absorption of CaAlg hydrogels under weakly alkaline conditions, which promotes the faster release of protamine NPs and HAO from CaAlg hydrogels. As a result, protamine NP/HAO CaAlg hydrogels can specifically and effectively deliver drugs to diabetic wounds [89]. However, the drug release properties of alginate hydrogels have several major drawbacks. First, the alkaline pH environment of alginate hydrogels more rapidly degrades, which may lead to an explosive release of the drug. Second, the degradation of alginate hydrogels is controlled by the diffusion of cations, which is a slow and uncontrollable process. Finally, the weak mechanical properties and lack of cellular interactions of this hydrogel may affect practical applications [90]. During the healing process of diabetic wounds, the wound surface will gradually return to acidic conditions. This restoration of an acidic environment is a natural physiological response that restores various internal cellular processes and improves wound hypoxia, which has positive implications for wound healing. Therefore, Liu et al. combined formyl-methyl-leucine-phenylalanine (fMLP) and FasL-coupled silica NPs (SiO_2-FasL) to synthesize a PBA-based polymer hydrogel (Gel@fMLP/SiO_2-FasL) and loaded them into the hydrogel matrix (Fig. 4B). The hydrogel produced significant pH-induced dissociation when the pH decreased to ensure the rapid release of SiO2-FasL mediated by low pH. In vitro release profiles from the hydrogels indicated that acidic pH significantly promoted the release of SiO2-FasL from Gel@fMLP/SiO2-FasL, which demonstrated the pH responsiveness of this hydrogel [91]. Although PBA-based hydrogels have more stable pH responsiveness, covalently bonded hydrogels are not sufficiently sensitive at the small pH ranges in tissues with local acidosis (diabetic wounds). The responsive sensitivity must be selective, i.e., the hydrogel must adjust the drug release on demand in a timely and rapid manner. Simultaneously, no drug is released at physiological pH to ensure maximum biosafety [92].
Fig. 4.
(A)The primary hypothesis of this study. Protamine NPs and HAO were loaded into the pores of Ca-alginate hydrogel and showed complete and fast release at the alkaline diabetic wound site. Protamine NPs/HAO Ca-alginate hydrogel was placed on the wound in streptozotocin-induced type 1 diabetic rats, and protamine NPs exhibited antibacterial activity by damaging the cell wall, thus reducing the inflammatory response. Moreover, HAO significantly enhanced HUVEC migration and capillary-like tube formation with HAO-induced stimulation of VEGF, leading to rapid diabetic wound healing. (i) Schematic representation of protaimine NPs/HAO CaAlg hydrogel. TEM image of protamine NPs and DLS analysis of the obtained protamine NPs. SEM image of protamine NPs/HAO CaAlg hydrogel and zeta potential distribution of protamine NPs. (ii) SEM images of bacteria treated with protamine NPs/HAO CaAlg hydrogel. (iii) VEGF secretion by HUVECs treated with different kinds of CaAlg hydrogels by ELISA. (iv) H&E staining of tissue sections treated with PBS, CaAlg hydrogel, and protaimine NPs/HAO CaAlg hydrogel on days 1, 3, 7, and 14 (Reproduced with permission of Ref. [89]). (B)Schematic illustration of Gel@fMLP/SiO2-FasL for transiently heightened inflammatory response to initiate refractory wound healing. SiO2-FasL, silicon dioxide nanoparticles conjugated with FasL; Gel@fMLP/SiO2-FasL, pH-responsive hydrogel matrix loaded with fMLP and SiO2-FasL. (v) IR spectrometry of CS-FPBA, fMLP and Gel@fMLP/SiO2-FasL. Photographs of the gelation process of Gel@fMLP/SiO2-FasL with a solution containing RhB (representing fMLP) and SiO2-FasL after incubation at pH 7.4. The decomposition of Gel@fMLP/SiO2-FasL at pH 5.5. (vi) Critical-sized calvarial bone defects were generated in 8-week-old female C57BL/6 mice and treated with hydrogels hybridized with different components. The mice were sacrificed at different time points post operation for further examination. Micro-CT analysis of bone defects (Reproduced with permission of Ref. [91]).
Due to the lack of injectability, mechanical stability, and self-healing, the application of pH-responsive hydrogels consisting of ionic polymers was limited in minimally invasive implants and elastic tissues. In addition, the pH range in local acidotic tissues is too small for some conventional pH-responsive hydrogels [53]. Recently, dynamic covalent chemistry has attracted considerable attention in the preparation of pH-responsive hydrogels. Dynamic bonds can be permanent covalent bonds or reversibly formed bonds. Due to the formation of covalent bonds, these hydrogels are more stable than hydrogels cross-linked through noncovalent interactions [93]. Schiff base formation has become one of the most common methods to synthesize of pH-responsive hydrogels. In mildly acidic environments, Schiff bases or imines with pH sensitivity and reversibility are formed by the condensation reaction of aldehydes and nucleophilic amines. Hence, hydrogels with Schiff base bonds can serve as drug delivery systems to treat local acidosis.
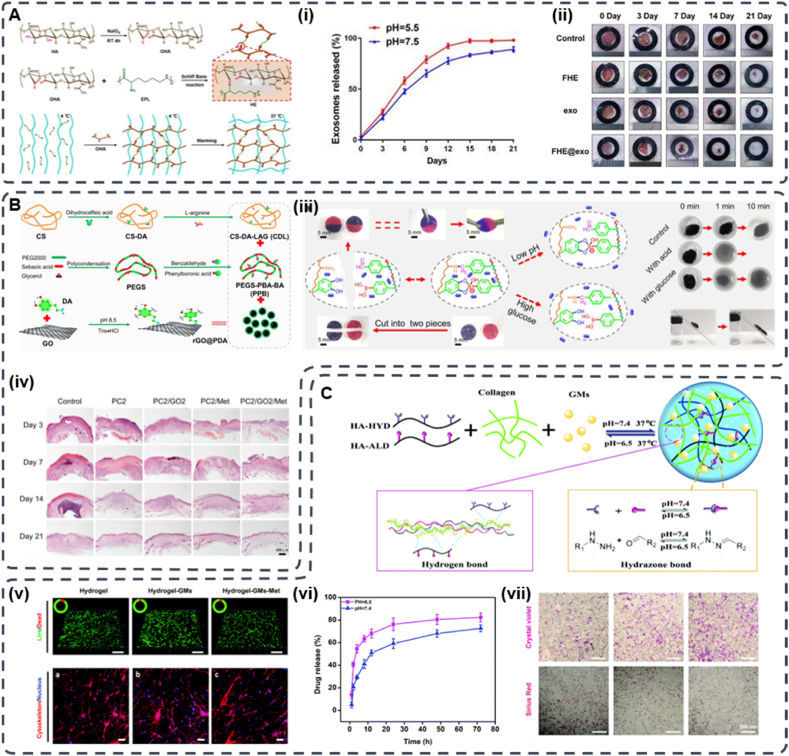
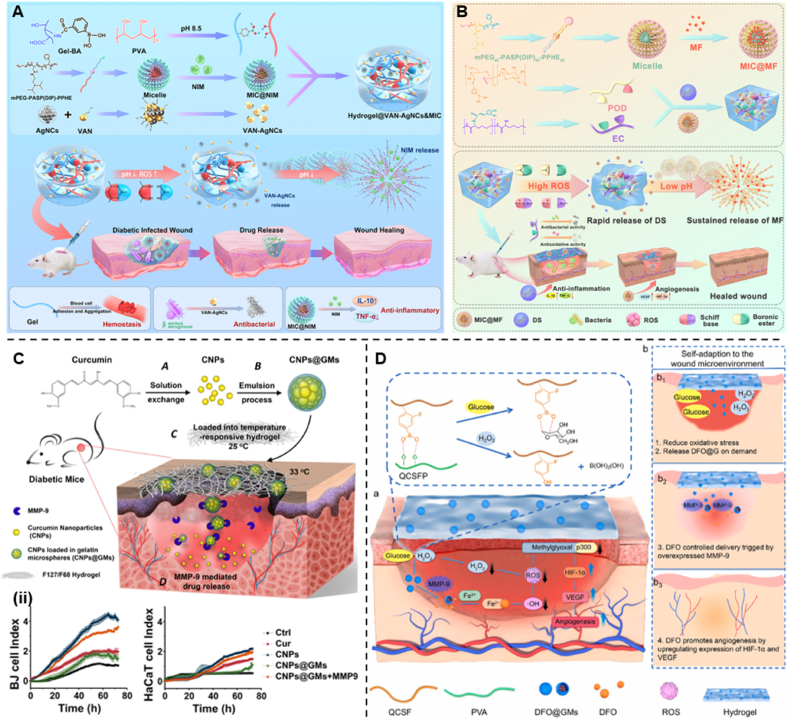
Wang et al. [94] synthesized an injectable FHE@exosome hydrogel composed of Pluronic F127 (F127), oxidized hyaluronic acid (OHA), and Poly-ε-l-lysine (EPL) (Fig. 5A). The hydrogel was fabricated via the reversible Schiff base reaction between OHA and EPL and the thermal response properties of F127. The FHE@exosome hydrogel exhibits self-healing ability, injectability, and excellent biocompatibility and has pH-responsive drug delivery capability. Due to the higher hydrolytic stability of Schiff base bonds at neutral pH, the release rate of FHE hydrogels at pH 7.5 is slower, and fewer exosomes are released than those at pH 5.5. These results suggest that FHE hydrogels are suitable for drug delivery to tissues with acidic microenvironments (diabetic wounds) and do not significantly affect nontargeted healthy tissues. Similarly, Liang et al. introduced a pH-responsive injectable hydrogel with Schiff base bonds. The pH/glucose dual-responsive hydrogel PBA-BA/CS-DALAG (PC) was prepared based on the Schiff base of dihydrocaffeic acid and l-arginine cografted chitosan (CS-DA-LAG) and phenylboronic acid-benzaldehyde bifunctional polyethylene glycol ester (PEGS-PBA-BA). Moreover, polydopamine-coated graphene oxide (rGO@pda) and metformin (Met) were added to improve the ability to promote the recovery of diabetic wounds (Fig. 5B). Under acidic conditions, the pH responsiveness of the drug was tested by using PC/GO/Met hydrogels to simulate diabetic wounds and physiological pH conditions. The gel had a higher crosslink density at pH 7.4 than at pH 5.5. The release of metformin was lower because the Schiff base structure has higher hydrolytic stability under neutral pH conditions. In contrast, when the pH of the PC/GO/Met hydrogel was 5.5, the amount of drug released was 12.7% higher than that at pH 7.4, possibly due to the dissociation of the Schiff base structure under acidic conditions. These results suggest that PC/GO/Met hydrogels are suitable for drug delivery into the acidic microenvironment of diabetic wounds with reduced drug effects on other tissues [95].
Fig. 5.
(A) Synthesis of oxidized hyaluronic acid (HA); Schiff base reaction between oxidized HA and polypeptide (ε-poly-l-lysine, EPL); thermal-responsive sol-gel process of double network hydrogel composed of F127-EPL and oxidized HA. (i) pH-dependent release profile of loaded exosomes in the FHE hydrogel. (ii) Representative images of the healing process in wounds treated with FHE, exosomes, FHE@exosome and control (Reproduced with permission of Ref. [94]). (B) Schematic diagram of the preparation and application of the PC/GO/Met hydrogel. Preparation of dihydrocaffeic acid and l-arginine cografting chitosan (CS-DA-LAG) and phenylboronic acid and benzaldehyde difunctionalized polyethylene glycol-co-poly (glycerol sebacic acid) (PEGS-PBA-BA) and polydopamine coated rGO (rGO@PDA). (iii) Schematic of self-healing, responsive drug release, and removability given by dual dynamic bonds: self-healing mechanism and representative pictures of PC hydrogel and release mechanism of metformin in response to pH and glucose and representative display pictures of removability. (iv) Representative HE staining results of wound tissue on days 3, 7, 14, and 21 (Reproduced with permission of Ref. [95]). (C)The sketch map of “Double-H bonds” in the hydrogel. (v) Live/dead staining of NIH-3T3 cells (scale bar = 200 mm, green – live cells, and red – dead cells) and the cytoskeleton staining of NIH-3T3 cells 3D cultured in hydrogels. (vi) Drug release curves of Met from HA–COL-GMs. (vii) Migrated fibroblast staining and collagen secretion staining (Reproduced with permission of Ref. [98]).
Hydrogels with Schiff base bonds are self-healing and biocompatible and can be beneficial for diabetic wound healing. Due to their excellent biocompatibility and self-healing properties, hydrogels composed of Schiff base bonds can be injected in situ and integrated as a whole and can be position- or shape-independent. Notably, while Schiff base bonds provide self-healing and stimulus responsiveness to hydrogels, they reduce the bulk mechanical strength and long-term stability due to their reversibility. Overall, hydrogels with Schiff base bonds have a promising future in skin tissue engineering due to their simplicity, reversibility, and pH responsiveness [96,97].
Another pH-responsive dynamic build is double hydrogen bonds, which have been reported to treat diabetic wounds. Jia et al. suggested the use of another dynamically constructed pH-responsive hydrogel for diabetic wounds: double hydrogen bonds (hydrogen bonds and hydrazone bonds) (Fig. 5C). In this study, the authors designed an injectable, self-healing, and pH-responsive “double H-bond” (hydrogen and hydrazone bond) cross-linked hyaluronic acid-collagen (HA-Col) hydrogel, followed by methionine gelatine microspheres (GMs) incorporated into HA-Col hydrogels to form pH-responsive hyaluronic acid hydrogels. The stability of hydrogels cross-linked through such dynamic bonds was largely pH dependent. The drug release profiles of Met-loaded hydrogels were studied at pH 6.5 and 7.4; after 24 h, the release of Met at pH 6.5 significantly exceeded that at pH 7.4 because of the instability of the hydrazone bond under acidic conditions [98]. This study has increased the diversity of dynamic crosslinked networks in hydrogels. Therefore, skin tissue engineering still requires the development of next-generation smart hydrogels using various types of dynamic bonds.
pH-responsive hydrogel injectable hydrogels have been intensively explored for practical application to treat diabetic wounds and have made significant progress. When the pH of the pathological tissue differs from that of normal tissue, pH-responsive hydrogels can be selected for drug delivery applications. Despite the significant progress that has been made, some issues remain to be addressed. First, the change in pH in the treated area of the local wound is not significant and therefore does not stimulate the release of the drug from the many pH-responsive hydrogels, which makes it difficult to achieve a therapeutic effect. Second the use of pH-responsive hydrogels may cause adverse local tissue reactions due to the difficult clinical prediction of pH at the site of disease. Finally, it is a great challenge to economically and conveniently synthesize pH-responsive hydrogels with well-defined physical and chemical properties [99,100].
3.2. Temperature-responsive hydrogels
In response to temperature changes in the external environment, temperature-responsive hydrogels change their volume to release drugs [101,102]. Temperature is the most common trigger to modulate the properties of hydrogels. A short response time, simple operation, and adjustable switching temperature contribute to its wide application. Temperature-responsive hydrogels have both hydrophobic and hydrophilic groups, so an increase or decrease in temperature will affect the hydrophobic interactions and hydrogen bonds between polymer chains, which change the structure and volume of the hydrogel [103]. Thermoresponsive polymers are generally divided into two opposite types according to their solubility changes with temperature: polymers with a lower critical solution temperature (LCST) or polymers with an upper critical solution temperature (UCST) [104]. The LCST is the critical temperature below which the hydrogel contracts or shrinks, which decreases its water solubility. This volume change of the hydrogel is called the negative temperature dependence. Negative thermosensitive hydrogels are usually composed of polymer chains with a high proportion of hydrophobic groups. When the temperature is higher than the LCST, the hydrophobic interaction force between hydrophobic units of the polymer chain is stronger than the hydrogen bond interaction force, which results in the volume shrinkage of the hydrogel. However, at temperatures below the LCST, the hydrogel swells due to the increased force of hydrogen bonds between the hydrophilic units of the polymer chain and water. Notably, this trend becomes more pronounced as the temperature increases. In other words, the persistent inflammatory response in chronic wounds can cause an increased local temperature, this may increase the local drug concentration and have the advantage of preventing its dispersion in healthy tissue [105]. The LCST can be altered due to different ratios of hydrophilic and hydrophobic groups in the polymer: a high proportion of hydrophobic or hydrophilic groups can lead to low or high LCST, respectively. Compared with LCST-type hydrogels, most polymers are more soluble in water with increasing temperature in UCST hydrogels, which is positive temperature dependent. Positive thermosensitive hydrogels swell above the UCST but shrink below this temperature [106,107]. Compared with LCST-type hydrogels, UCST hydrogels are less explored, mainly because of the few polymer candidates available. Common thermoresponsive hydrogels are composed of natural polymers [108,109], peptides [110,111], and copolymers based on PCL [[112], [113], [114]], poly (N-isopropyl acrylamide) (PNIPAAm) [115,116], poly (d,l-lactide) (PLA) [117,118], PEG [114,119], and poly (amino ester urethane) (PAEU) [120].
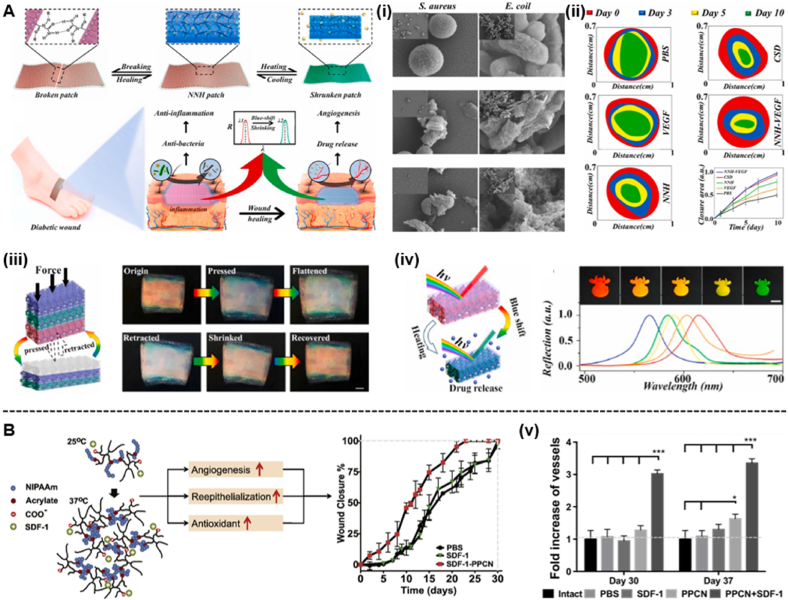
As a temperature-responsive polymer for drug delivery, PNIPAAm can hydrate and dehydrate with changes in temperature. Since the LCST of PNIPAAm is 32–34 °C, which is close to body temperature, PNIPAAm has been used in various biomedical applications [121]. When the temperature is higher than the LCST, the balance of hydrophilic and hydrophobic forces of the PNIPAAm hydrogel changes, which results a transition from coiled to spherical. Therefore, the PNIPAAm hydrogel has a reduced volume at physiological temperature [122]. Chen et al. created responsive and self-healing structurally coloured supramolecular hydrogel patches with N-acryloyl glycinamide (NAGA) and 1-vinyl-1,2,4-triazole (VTZ) hybrid polymers as inverse opal scaffolds and temperature-responsive PNIPAM hydrogels as fillers (Fig. 6A). To verify the properties of the temperature-responsive hydrogel patch, the (NAGA-VTZ) & PNIPAM hydrogel (NNH) patch was first placed in a simulated chronic infection wound environment, and the shrinkage process was observed at 37 °C and suitable pH. The volume of the NNH patch was gradually reduced and its structural colour changed from red to green, which results from the decreasing distance between adjacent nanopore centres in the inverse opal. These results suggest that the NNH patch with colour-sensitivity properties to temperature changes can play a crucial role in wound infection monitoring and has broad prospects in clinical use. To demonstrate the effect of temperature changes on the release of FITC-BSA, the authors performed drug release experiments under body temperature stimulation (37 °C) and room temperature (25 °C). The body temperature stimulation (37 °C) exceeded the volume phase transition temperature (VPTT) of the PNIPAM hydrogel, which made the patch shrink and exhibited more rapid drug release than that at room temperature. Additionally, the dynamic changing ability of different NNH patches to temperature stimuli was investigated. The results showed that the displacement ratio of the plaque gradually decreased with increasing PNIAPM concentration. A higher hydrogel monomer concentration corresponded to greater intermolecular forces and more resistance to temperature effects. Furthermore, the hydrogel patch has temperature-responsive properties due to the presence of PNIPAM hydrogel. The hydrogel patch utilizes an inverse opal structure with temperature changes and colour-sensitive properties to monitor wound infection, effectively manage the wound, and guide clinical treatment [123]. However, PNIPAAm-based hydrogels have several limitations. First, PNIPAAm-based hydrogels exhibit a low compressive modulus and poor elastic recovery after drug loading and insufficient drug release when the temperature changes [[124], [125], [126]]. Most importantly, the hydrogel is a nonbiodegradable hydrogel because the cross-linked bonds in the PNIPAAm hydrogel are not biodegradable. Although the hydrogel has excellent cytocompatibility, it can cause a local inflammatory response that can prevent diabetic wound healing. The nondegradable nature of PNIPAM hydrogels limits their usefulness in many biomedical applications [101]. Therefore, many studies have attempted to introduce a biodegradable and biocompatible linker to the PNIPAM backbone. For example, Zhu et al. reported an injectable hydrogel of a thermosensitive biodegradable polymer loaded with poly (polyethylene glycol citrate-N-isopropylacrylamide) (PPCN) to treat diabetic wounds (Fig. 6B) [127].
Fig. 6.
(A) Schematic representation of the excellent performance of the NNH patches and their application to diabetic wounds. (i) Images of NNH patches against S. aureus and E. coli from scanning electron microscope (SEM). (ii) In vivo diabetic wound healing. The size and quantification of the wound closure area from the PBS group, VEGF group, NNH patch group, CSD group and NNH-VEGF patch group. (iii) Scheme of the compression of NNH bulk. Representative of compression and retraction of NNH bulk. (iv) Scheme of the temperature-responsive feature of the NNH patches. Reflection images and spectra of NNH patches at a temperature of 37 °C (Reproduced with permission of Ref. [123]). (B) Scheme of the antioxidant thermoresponsive hydrogel. (v) Quantification of the functional blood vessel density (Reproduced with permission of Ref. [127]).
Another important feature of thermosensitive hydrogels is that they are liquid or semisolid at room temperature and undergo a sol-to-gel transition when exposed to body temperature. This feature allows the hydrogel to be loaded with therapeutic compounds in a liquid state and easily administered [128]. LCST is the temperature below which hydrogels in liquid or semisolid form are completely soluble/miscible and above which the increase in hydrophobicity and insolubility leads to the formation of gels. Therefore, for injectable biomedical applications, the LCST of the hydrogel solution must be higher than 25 °C and lower than 37 °C. For hydrogels cured at low temperatures, such as gelatine hydrogels, there is a UCST, but above this temperature, the compatibility/solubility of the hydrogel in the gel state significantly increases [129].
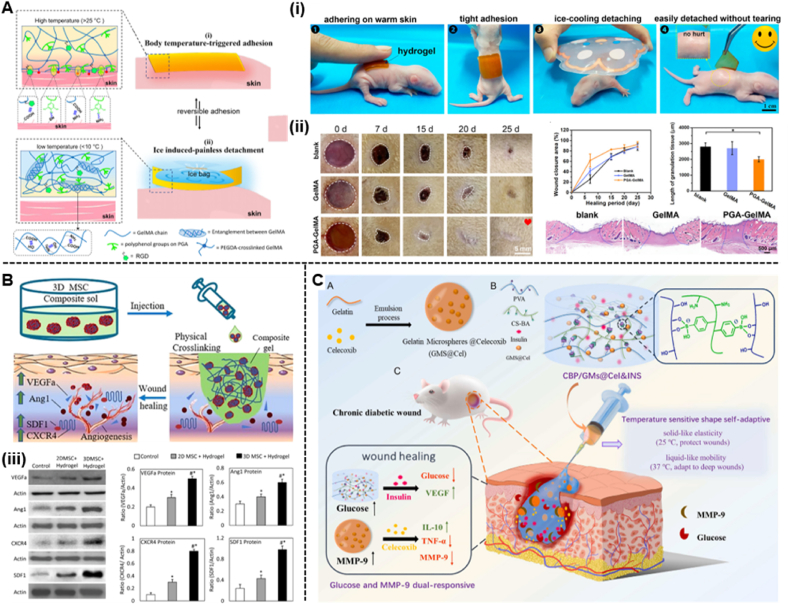
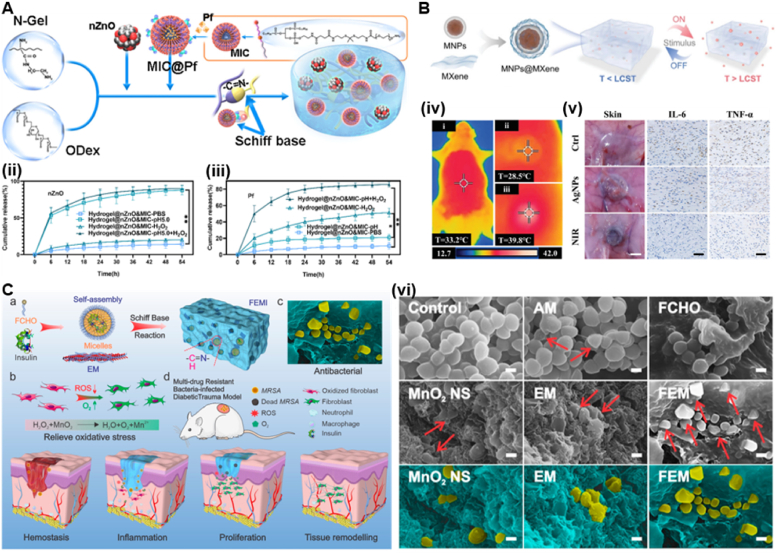
Recently, temperature-sensitive hydrogel systems developed using natural and synthetic polymers have found a wide range of applications in the biomedical field. Thermosensitive hydrogels can form a gel at higher temperatures and revert to a liquid at lower temperatures within a certain temperature range. Thermal gels that can gel in the range of 25–37 °C to undergo a phase transition from room temperature to body temperature are noteworthy in the biomedical field. Due to the phase change at physiological temperatures, thermogels have been used to manage diabetic wounds [130]. For example, Jiang et al. [131] reported a temperature-responsive hydrogel patch composed of polymeric gallic acid (PGA) and gelatine methacryloyl (GelMA) to treat diabetic wounds. Due to the high density of noncovalent bonds, the PGA-GelMA hydrogel has temperature-triggered adhesion and detachment properties (Fig. 7A). Once in contact with the skin surface, the GelMA chains breakdown at body temperature (37 °C), form a soft hydrogel that is easily bonded to the skin surface. Meanwhile, the freely mobile GelMA in the hydrogel chain exposes many reactive motifs, including amino and carboxyl groups, which enable the hydrogel to form multiple interfacial bonds on the skin surface and exhibit strong adhesion. After the ice pack is cooled, the GelMA chains are re-entangled due to the formation of intermolecular hydrogen bonds, restrict the movement of the GelMA chains and break the adhesive bonds. In vivo experiments also demonstrate that the hydrogels are skin-friendly for nonallergic adhesion and nondestructive separation. Similarly, Yang et al. [132] developed a novel therapeutic strategy based on injectable heat-sensitive chitosan/collagen/β-glycerophosphate hydrogels and 3D MSC spheroids to accelerate wound healing in a mouse model of diabetes (Fig. 7B).
Fig. 7.
(A) Schematic illustrations of the body temperature-triggered gentle adhesion and ice-cooling-induced painless detachment of the PGAGelMA hydrogel. (i) Photographs of adhering and triggerable removal of the PGA-GelMA hydrogel on an infant rat skin surface. (ii) Digital images of the wound healing process in all groups and quantification of the wound closure area at different time intervals. Representative H&E staining images of wound samples in different groups on Day 25 and quantification of the length of granulation tissue (Reproduced with permission of Ref. [131]). (B) Schematic illustrations of the thermosensitive Injectable Chitosan/Collagen/β-Glycerophosphate Composite Hydrogels. (iii) Expression of paracrine cytokines in wound tissues (Reproduced with permission of Ref. [132]). (C) The design strategy of glucose and MMP-9 dual-responsive shape self-adaptive hydrogels for treating chronic diabetic wound (Reproduced with permission of Ref. [139]).
Although temperature-sensitive and injectable hydrogels have shown great potential in different biomedical applications, these hydrogels still have some limitations in the preparation and injection process. For example, a long time of low temperature is required in the preparation process to form a uniform sol, and due to the high viscosity of the sol at room temperature, it may be difficult to inject due to clogging of pinholes [133]. More importantly, it is difficult to balance the fluid-like and solid-like properties of hydrogels composed of dynamic covalent bonds (e.g., boronic acid bonds [134,135], Schiff base reactions [136,137]) or noncovalent bonds (e.g., hydrogen bonds [138]). Hydrogels with predominantly fluid properties do not provide sufficient mechanical properties to protect wounds, while hydrogels with predominantly solid properties have difficulty quickly filling irregular deep wounds. To address this issue, Zhou et al. [139] fabricated a hydrogel with temperature-sensitive shape-adaptive behaviour to promote diabetic wound healing (Fig. 7C). Due to the presence of boronate linkages, the hydrogel is liquid at body temperature (37 °C) and solid at room temperature (25 °C). Hence, the thermosensitive hydrogel has more fluid-like properties at skin wound temperature to fill deep wounds and protect wounds through its solid-like properties which proves that the hydrogel exhibits stronger shape adaptability to deep wounds.
Temperature-responsive hydrogels can be rapidly transformed from a flowing solution state to a semisolid gel with nonchemical cross-linking properties by temperature changes. Compared with traditional drug delivery methods, temperature-responsive gels have the advantages of being highly targeted and having fewer toxic side effects, which can effectively reduce the cost of patient treatment and improve the quality of patient compliance survival. However, temperature-responsive hydrogels have some obvious limitations. Since the mechanical properties of the scaffold for cell growth should match those of the host tissue, the poor mechanical properties of temperature-responsive hydrogels simply cannot satisfy the mechanical strength required for cells of different tissues. In addition, the slow response rate of temperature-responsive hydrogels makes them unable to release sufficient drug due to the small difference in temperature between pathological and normal tissues in vivo [140].
3.3. Glucose-responsive hydrogels
Diabetes is a group of metabolic diseases characterized by hyperglycaemia that seriously threatens human health. The treatment of chronic wounds such as diabetic ulcers has always been a huge challenge. Impaired angiogenesis, reduced growth factors, and hyperglycaemia during diabetic wound healing lead to delayed wound healing and cause other complications. Hyperglycaemia also makes the wound more susceptible to microbial infection [141]. In this context, wound dressings that can respond to blood glucose levels have received widespread attention in the management of diabetic wounds. Among many of the proposed systems, glucose-responsive hydrogel-based drug self-regulating delivery systems can release necessary drugs in response to changes in blood glucose concentration. Glucose-responsive hydrogels are mainly divided into three categories: glucose oxidase (GOx) hydrogels, concanavalin A (ConA) hydrogels and PBA hydrogels [142].
3.3.1. Glucose oxidase hydrogels
A common glucose-responsive hydrogel that has been widely used in recent decades is GOx hydrogels. GOx is an enzyme that can oxidize monosaccharides, nitroalkanes, etc. The typical synthesis method of GOx hydrogels uses pH-responsive hydrogels as the backbone and GOx as the glucose-sensitive segment [143]. The pH-responsive hydrogel contains immobilized GOx with glucose-sensing properties. In the presence of glucose and oxygen, GOx can catalyse glucose to gluconic acid, as shown in the following reaction:
| Gicacilucose + H2O + O2 → Gluconic acid + H2O2 |
Due to the pH-responsive backbone, glucose is converted to gluconic acid at high blood glucose levels, which changes the ambient pH. This triggers a volume change in the pH-responsive hydrogel, which changes its pore size, triggers the release of the encapsulated drug from the hydrogel and makes it valuable in regulating (self-regulating) drug delivery. There are two distinct mechanisms in self-regulating drug release. When the pH decreases, the volume of the pH-responsive hydrogel shrinks or collapses to release the drug from the hydrogel network. In contrast, when the pH increases, the volume of the pH-responsive hydrogel swells, and the drug is released [142].
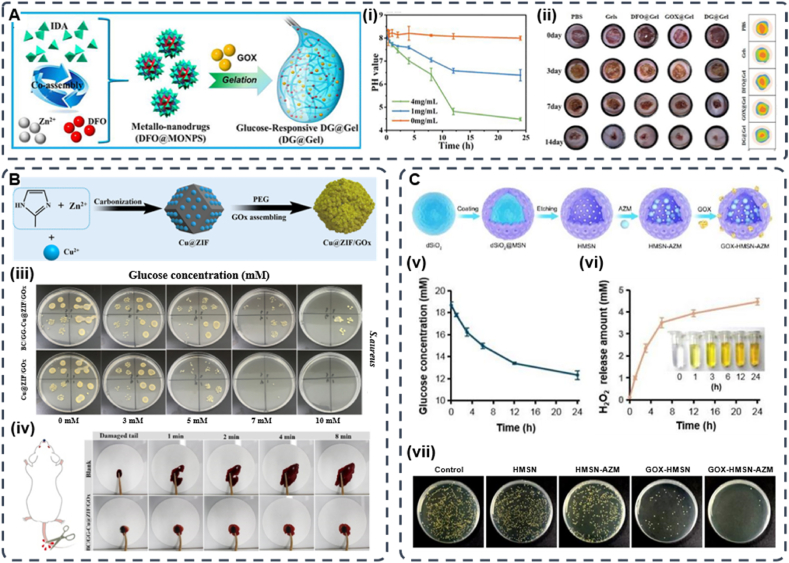
Yang et al. designed a glucose-responsive multifunctional metal-organic drug-loaded hydrogel (DG@Gel) that incorporated GOx to heal diabetic wounds (Fig. 8A). In this investigation, the authors added glucose solutions of different concentrations to the DG@Gel hydrogels and incubated them for 24 h to measure the pH changes. They found that when the glucose concentration increased, the pH of the solution significantly decreased. The pH dropped to approximately 4.3 when approaching the 4 mg/mL glucose solution of the diabetic wound glucose concentration. The significant decrease in pH during this process implied that GOx at diabetic wounds might facilitate the degradation of the hydrogel and the release of loaded deferoxamine mesylate (DFO). In subsequent in vivo applications, DG@Gel significantly promoted local diabetic wound healing without significantly affecting the systemic blood glucose, which indicates that DG@Gel may serve as a feasible candidate for diabetic wound repair [144]. Metal-organic hydrogels loaded with GOx have achieved applications in diabetic wounds, but the ZIF system has more distinct advantages. ZIF-8 readily decomposes in acidic environments (pH 5.0–6.0), and drug delivery and release via pH control is beneficial for diabetic wound healing. Most importantly, ZIF-8 has hollow nanomaterials with adjustable dimensions and unique 3D internal voids that prevent the leakage of GOx and maintain its biological activity. The good hydrothermal and chemical stability of ZIF-8 ensures that the system is not damaged by external stimuli [145]. Similarly, the emergence of metal-organic frameworks (MOFs) with a large specific surface area provides new ideas for solving drug delivery problems [146,147]. Zhang et al. first assembled GOx on a copper ion-doped graphitized imidazole framework (ZIF-8) to construct a MOF-based biomimetic nanoreactor Cu@ZIF/GOx. Then Cu@ZIF/GOx was encapsulated in a biocompatible hybrid hydrogel composed of bacterial cellulose (BC) and guar gum (GG) (Fig. 8B). Due to the abundant glucose around diabetic wounds, glucose can be consumed by GOx to generate H2O2 and gluconic acid, which subsequently trigger the Cu-mediated peroxidase (POD)-mimetic activity of Cu@ZIF to generate oxidative•OH to kill bacteria. In addition, the BC/GG-Cu@ZIF/GOx hydrogel exhibits a high water swelling ratio, excellent biocompatibility, and rapid haemostasis both in vitro and in vivo [148]. For diabetic wounds, bacterial infection and bacterial biofilm formation are more likely to occur. Since the high-glucose environment around the wound creates an excess source of nutrients for bacteria and promotes biofilm formation, the conventional use of antibiotics has a very limited effect. Therefore, researchers expect to reduce the local glucose in diabetic wounds through the catalytic reaction of GOx to improve the therapeutic effect of antibiotics. Shi et al. developed GOx and azithromycin (AZM) bifunctional hollow mesoporous silica NPs (GOx-HMSN- AZM) with antibacterial and local hypoglycaemic effects to promote infected diabetic wound healing (Fig. 8C). This is attributed to the special spatial structure, high specific surface area, and modification sites of HMSN, which can realize GOx functionalization of the material and load AZM into its lumen [149].
Fig. 8.
(A) Schematic of the preparation of DG@Gel. (i) Change in the pH of DG@Gel over 24 h in different glucose concentrations. (ii) Photographs of wound tissues treated with different sample groups on days 0, 3, 7, and 14, and wound healing boundaries in different treatment groups in vivo (Reproduced with permission of Ref. [144]). (B) Schematic illustration of preparing procedure of Cu@ZIF and Cu@ZIF/GOx. (iii) Antibacterial efficacy of Cu@ZIF/GOx treated with different concentration of glucose after 6 h incubation (Reproduced with permission of Ref. [148]). (C) Schematic illustration of the synthetic route of GOx-HMSN-AZM. (v) He glucose concentration variation under the catalyst of 200 μg/mL GOx-HMSN-AZM on different reaction times. (vi) The concentration of generated H2O2 catalyzed by 200 μg/mL GOx-HMSN-AZM on different reaction times. (vii) Photographs of bacterial colonies formed by S. aureus in biofilms which were treated with 500 μg/mL of PBS, HMSN, HMSN-AZM, GOx-HMSN and GOx-HMSN-AZM (Reproduced with permission of Ref. [149]).
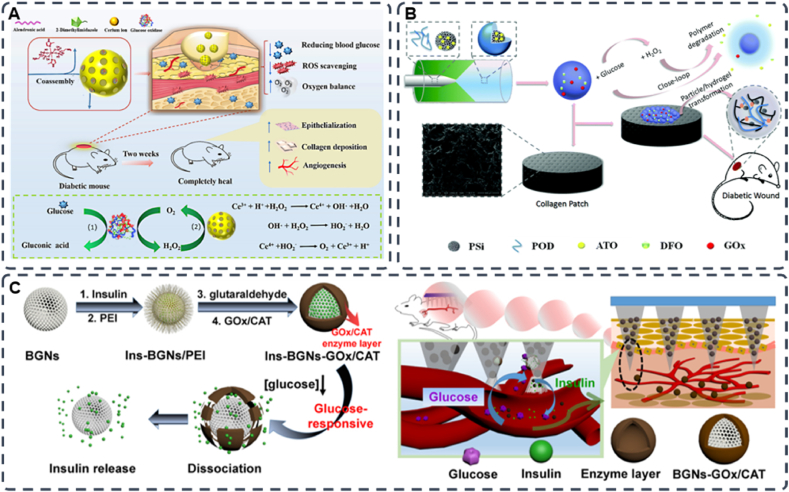
However, hydrogels containing GOx are insensitive to glucose and may leak drugs prematurely because of their low mechanical strength [150,151]. More importantly, the accumulation of H2O2 caused by the rapid removal of glucose may disrupt the redox homeostasis of the local wound microenvironment, generate excess ROS and cause toxic side effects [[152], [153], [154]]. These are the reasons for the limited application of GOx. A viable solution to this problem is to create a carrier that consumes its harmful byproducts and sequentially generate the required downstream actions. Yu et al. developed a glucose/ROS cascade reaction nanometre (CHA@GOx) coassembled by special dual ligands (alendronic acid and 2-methylimidazole) and Gox (Fig. 9A). The hydrogel has superoxide dismutase and catalase (CAT) mimetic activities, which can remove H2O2, the secondary product of GOx, and produce a cascade effect to further catalyse its production of oxygen. This process improves hypoxia while addressing the side effects of H2O2 accumulation. In vitro and in vivo experiments demonstrate that CHA@GOx can remove excess glucose and ROS and facilitate the cell tube formation and cell migration. In a mouse model with diabetic wounds, CHA@GOx also enhanced re-epithelialization and collagen deposition, which can significantly promote diabetic wound healing [155]. Similarly, Liu et al. developed a self-regulating dynamic nanohybrid material that could sensitively respond to a hyperglycaemic microenvironment. The authors synthesized a core/shell structured nanohybrid, where drug-loaded porous silicon (PSi) nanoparticles were encapsulated by a H2O2 responsive polymer, 4-(hydroxymethyl)-phenyl-boronic acid pinacol ester conjugated with oxidized dextran (POD) (Fig. 9B). Then the nanocomposite was embedded in a collagen patch for application in diabetic wounds. Furthermore, the excess aldehydes on the outer surface of POD can immobilize GOx by forming pH-responsive dynamic covalent bonds to simultaneously anchor other amine-containing drugs. When glucose is elevated, GOx can initiate the glucose oxidation and produce gluconic acid and H2O2. Then, POD can consume the produced H2O2 for degradation to further release the encapsulated drug. Interestingly, oxidized dextran (Odex), which is a degradation product of POD, can form a hydrogel in situ with the collagen membrane, which creates a moist environment around the diabetic wound and changes the stiffness of the patch to mimic the elastic properties of natural soft tissue [156].
Fig. 9.
(A) Schematic diagram of Ce-driven coassembly multi-enzymatic activity of nanozyme for diabetic wound healing (Reproduced with permission of Ref. [155]). (B) Schematic illustration of the construction of glucose responsive delivery system and the orchestrated cascade for diabetic wound care. The detrimental side product of H2O2, was utilized and consumed, further aiding the close-loop system (Reproduced with permission of Ref. [156]). (C) Schematic representation of the glucose-sensitive insulin delivery system using glucose-sensitive BGNs; after the treatment with diabetic rats, the glucose-sensitive insulin released from the MNs in vivo (Reproduced with permission of Ref. [158]).
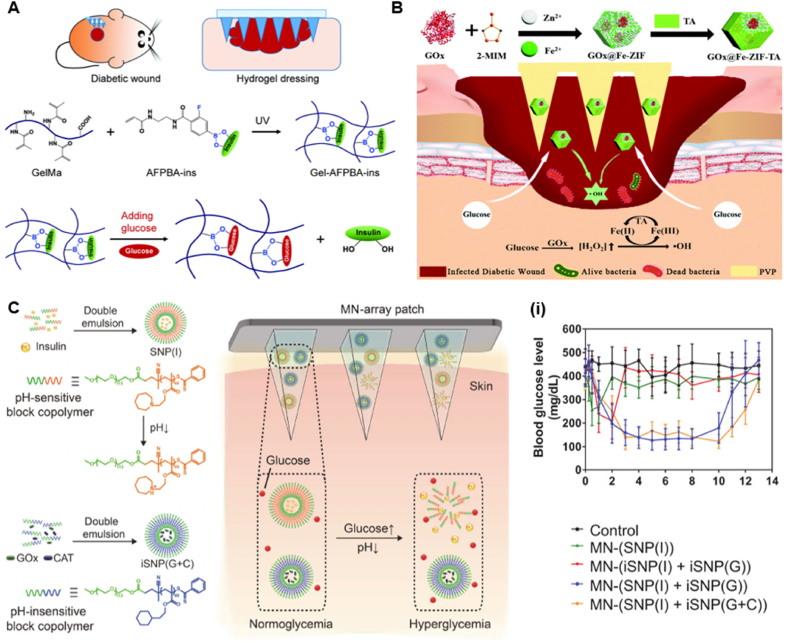
Another transdermal drug delivery method is the microneedle drug delivery system (MDDS), which has attracted attention due to its painless, noninvasive route of administration [157]. MDDS have recently attracted much attention. MNs offer many advantages as an emerging drug delivery modality, such as macromolecular drug delivery, ease of administration, painlessness, targeted drug targeting, and improved drug delivery efficiency. Researchers have developed glucose-responsive microneedles to treat diabetic wounds. Jiang et al. synthesized BGNs with an enzyme layer by electrostatic interaction of polyethyleneimine PEI and enzymes (GOx/CAT) on the surface of bioactive glass nanoparticles (BGNs). The material combines the porous properties of BGNs and glucose sensitivity of the enzyme layer. Then the authors combined polymer microneedles (MNs) with glucose-responsive mesoporous bioactive glass nanoparticles (BGNs) to treat diabetic wounds via transdermal delivery of insulin (Fig. 9C). After the MN has been inserted into the skin, the insulin-loaded BGN can easily spread to the surrounding skin tissue. Glucose in body fluids diffuses and penetrates into the complex enzyme layer (CEL), where it is converted into gluconic acid and H2O2 under the catalysis of GOx/CAT, which decreases the pH of CEL. CAT can also consume the generated H2O2 for degradation to catalyse its production of oxygen to improve hypoxia. Gluconate lowers the pH of the microenvironment and increases the permeability of the enzymatic layer, which structurally disrupt the CEL and releases the preloaded drug (insulin) from the BGN [158].
3.3.2. Phenylboronic acid (PBA)-based hydrogels
As mentioned above, GOx is the most common protein-based glucose response system in the treatment of diabetic wounds. However, the composition of proteins is susceptible to environmental changes due to instability, antigenicity and high cost. Therefore, they are not suitable for storage or long-term use [[159], [160], [161]]. PBA-based materials have been investigated for glucose-responsive drug delivery in recent years. Unlike protein-based systems such as Gox, PBA-based materials have versatile reactivity and high stability, which make them ideal for use in physiological conditions [162]. PBA is can reversibly combine with diols, including glucose, to form a stable five-membered ring complex. Therefore, PBA-modified polymers with diol-containing polymers enable the hydrogels to release the encapsulated drugs subsequently in response to blood glucose level changes. The principle of the PBA-mediated glucose-sensitive drug delivery system is based on the reversible reaction between PBA and cis-diol compounds. In aqueous solutions with higher pH than the pKa of PBA (pKa≈8.6) [[163], [164], [165]], most PBA moieties are negative and relatively hydrophilic. However, when the pH of the solution is lower than the pKa of PBA, the PBA moiety is neutral and hydrophobic. When glucose is added, the charged form of phenyl borate can form a stable complex with glucose through a reversible covalent bond. Thus, the equilibrium shifts in the direction of increasing the hydrophilic (single) forms of phenylborates. The increased hydrophilicity of the PBA-modified material results in its swelling, disassembly, and degradation, which releases the payload [151,166].
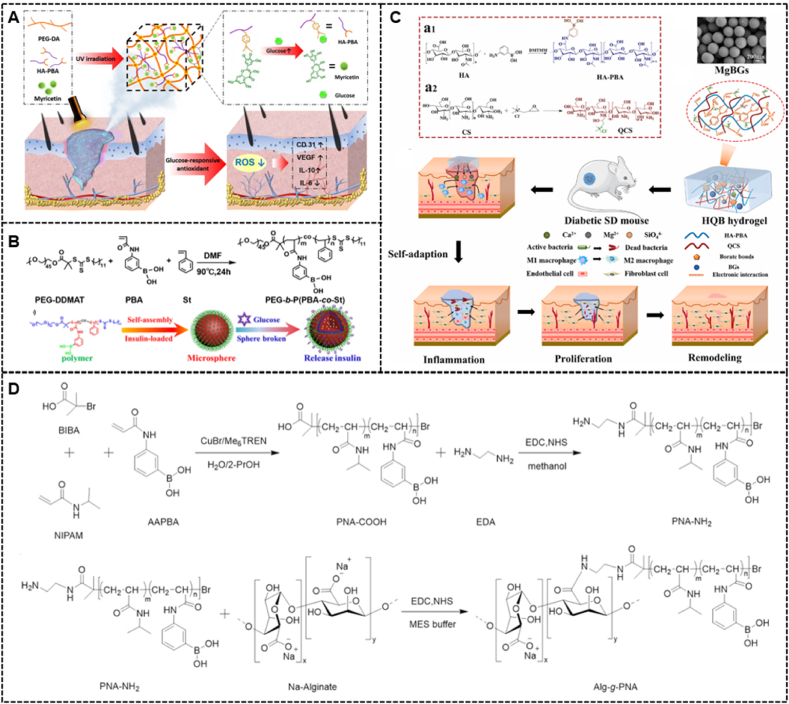
To treat diabetic wounds, the decomposition and degradation of hydrogels have achieved practical application. In the presence of glucose, drug release due to breakdown and degradation relies on breaking the structure of the hydrogel. Xu et al. [167] fabricated a novel glucose-responsive hydrogel for diabetic wound healing. They modified PBA with unique glucose sensitivity to a hyaluronic acid (HA) chain and added it to a polyethylene glycol diacrylate (PEGDA) hydrogel matrix to produce a hybrid hydrogel (PEGDA/HAPBA). Then, through the formation of dynamic boronic acid bonds between the polyphenol group of myricetin and the phenylboronic acid group of HA-PBA, the myricetin (MY) molecule was immobilized into the hybrid hydrogel to obtain the PEG-DA/HA-PBA/MY(PHM) hybrid hydrogel (Fig. 10A). Additionally, the authors measured myricetin release by adding (PHM) hybrid hydrogel to PBS and PBS containing glucose. Their data indicated that elevated glucose levels accelerated the release rate of myricetin (>60.0%) and increased the total release amount (>170%), which implies that the PHH hybrid hydrogel with unique glucose responsiveness served as a promising candidate for diabetic wound therapy. The application of chitosan systems in drug delivery has attracted great interest because their physicochemical properties can be easily and precisely altered [168]. Zhu et al. synthesized polyethylene glycol poly [3-acrylamidophenylboronic acid-copolystyrene] (PEGb-P(PBA-co-St)) for insulin loading to form insulin micelles. Insulin micelles and epidermal growth factor (EGF) were embedded in the composite hydrogel (Fig. 10B). When the glucose concentration increases, the micelle structure can release the loaded insulin due to the binding of phenylboronic acid and glucose, which decreases the blood sugar level. Simultaneously, with the increase in glucose concentration, the release rate of insulin loaded in micelles significantly increases. In vivo experiments revealed that when insulin micelles were combined with glucose and cleaved, the released insulin promoted the reduction in blood glucose concentration and indirectly improve the repair efficiency and directly promoted wound repair [169]. In complex diabetic wounds (with sinus tracts or fistulas), conventional hydrogels do not completely cover the wound, which results in a high rate of wound recurrence due to the inability to internally heal the wound, since conventional hydrogels cannot adapt to various wound shapes and there is limited treatment of diabetic wounds [170]. Therefore, it is necessary to develop a hydrogel with autoadaptation to trea diabetic wounds. Recently, bioactive metal ions and bioactive glasses (BGs) have been increasingly used in soft tissue [[171], [172], [173], [174], [175]]. Zhu et al. synthesized an injectable, adaptive, self-healing, and adhesive hydrogel dressing formed by the phenylborate ester bond within diols and hyaluronic acid modified with phenylboronic acid (HA-PBA) and electrostatic interactions between HA-PBA, quaternized chitosan (QCS), and magnesium-containing bioactive glasses (MgBGs). (Fig. 10C). Interestingly, the introduction of MgBGs enhanced the mechanical properties of the hydrogels and endowed them with bioactivity. In the degradation experiments of the hydrogel dressing under different conditions, the loss of hydrogel mass was more severe when the glucose increased or the pH decreased, which is due to the cleavage of the boronic ester bond under conditions of high glucose concentration and low pH. In vitro experiments demonstrate that the hydrogel is a multifunctional hydrogel with excellent antibacterial properties and self-healing, injectable, and adhesive properties. In vivo experiments also demonstrate the positive effect of this hydrogel on diabetic wound healing [176]. However, injectable hydrogels based on dynamic covalent bonds also have some limitations. For example, the requirements of thick needles and high injection pressure may increase pain [[177], [178], [179], [180]]. Temperature-responsive injectable hydrogels are the most commonly used hydrogels for in situ drug delivery because they can undergo a phase transition through the temperature difference. When glucose-responsive hydrogels have temperature-responsive properties, they can be injected in a sol state and locally transformed into a gel state at body temperature. Moreover, patient comfort in this injection process is improved due to the relatively lower injection pressure and thinner needles. Hu et al. reported a novel injectable hydrogel for the in situ delivery and controlled release of insulin to treat diabetes. They copolymerized 3-acrylamidophenylboronic acid (AAPBA) and N-isopropylacrylamide (NIPAM) and grafted them with alginate to obtain a temperature-sensitive/glucose dual-responsive copolymer alginate-g-P (NIPAM-co-AAPBA) (Alg-g-PNA) (Fig. 10D). When injected into the local site, since the LCST of Alg-g-PNA is lower than body temperature (37 °C, the copolymer solution rapidly becomes a gel locally. When the pH rises higher than the pKa of Alg-g-PNA, due to the presence of glucose, the PBA group in Alg-g-PNA can effectively combine with the cis-diol structure of glucose to form borate. Borate, which is hydrophilic in nature, increases with increasing glucose concentration and makes the LCST of Alg-g-PNA exceed 37 °C. At this point, the gel transforms into a sol state and rapidly releases insulin locally. In contrast, a reduction in borate amount after blood glucose concentrations dropped to normal levels results in an LCST of Alg-g-PNA below 37 °C. The Alg-g-PNA copolymer changes from a sol state to a gel state and stopps releasing insulin. Therefore, the hydrogel can self-regulate the release of insulin at the action site, which may provide a very promising strategy to treat diabetic wounds [181].
Fig. 10.
(A) Diagram illustrating the PHM Hydrogel Platform for Diabetic Wound Healing (Reproduced with permission of Ref. [167]). (B) The synthetic route of PEG-b-P(PBA-co-St), self-assembly of PEG-b-P(PBA-co-St) micelles and insulin release from these micelles in the presence of glucose (Reproduced with permission of Ref. [169]). (C) The fabrication schematic and mechanism of a bioactive HQB hydrogel and its application on chronic wound healing (Reproduced with permission of Ref. [176]). (D) Synthetic Routes of PNA-NH2 and Alg-g-PNA. (b) Copolymers (Reproduced with permission of Ref. [181]).
3.3.3. Glucose-responsive microneedles (MN)
Although PBA-based hydrogels are sensitive to glucose, the limitations of implantation of bulk hydrogels and injection of hydrogels have resulted in limited applications of glucose-sensitive hydrogels for self-regulating drug delivery [182]. In recent years, glucose-responsive hydrogel microneedle (MN) patches, which can detect blood glucose levels and regulate insulin release over time, have become a research hotspot in insulin release [183,184]. A glucose-responsive MN is an efficient blood glucose-responsive system that links blood glucose sensing and insulin release and may reduce the risk of hypoglycaemia [185,186]. Glucose-sensitive MNs typically consist of insulin, a glucose-sensing element, and a cross-linking agent. Glucose-sensing elements are generally composed of GOx, glucose-binding protein, and PBA [187]. When the hydrogel microneedles are inserted into the skin, the skin interstitial fluid can be rapidly absorbed and swelled to a hydrogel state, which results in a slow and continuous release of the loaded drug. By controlling the crosslinking density to adjust the swelling ability of the hydrogel polymer, the transdermal absorption efficiency and drug release behaviour can be improved. Due to the toughness of the cross-linked matrix, the hydrogel MNs can be completely removed from the skin without any polymer residue, which is required for frequent dosing [188,189]. Therefore, the advantages of the MN patch are: precise drug loading, a high drug loading rate, instant transdermal drug delivery, unique swelling ability, no polymer residue, easy removal after treatment, appropriate biosafety, etc. [190].
The most widely used glucose-responsive delivery systems are PBA and its derivatives. The high stability and persistence of PBA in physiological environments make it a reliable glucose sensor in glucose-responsive MNs. Guo et al. designed a glucose-responsive insulin-releasing hydrogel for microneedle dressing. A hydrogel system composed of biocompatible methacrylate gelatin, the glucose-responsive monomer 4-(2-acrylamidoethylcarbamoyl)-3-fluorophenylboronic acid (AFPBA) and glucose insulin (INS) replicated the polydimethylsiloxane mold and prepared a gel-forming-polymethyl methacrylate-insulin hydrogel MN dressing (Fig. 11A). In vitro experiments found that after the Gel-AFPBA-INS hydrogel was soaked in glucose solution, the pore size ratio of the hydrogel was significantly larger than that in PBS solution, and the pore size of the hydrogel gradually increased with increasing concentrations of glucose. The change in pore size of the hydrogel is consistent with the change in its morphological expansion. After soaking in the glucose solution, the glucose molecules diffused into the hydrogel. The anionic boronate species in the hydrogel will preferentially bind to the glycol of glucose molecules to form cyclic boronate esters. The formation of borate anions increases the osmotic pressure of the hydrogel, resulting in swelling of the hydrogel. The authors also assessed the effect of different concentrations of glucose on the kinetics of insulin release from the hydrogels. Insulin from hydrogels was released more rapidly in high glucose environments, and the drug release profile was dependent on glucose concentration. In contrast, the drug release rate was significantly slowed down in low-sugar or sugar-free PBS solution. In vivo experiments, the authors found that Gel-AFPBA-INS hydrogels healed faster in diabetic wounds, and the blood glucose levels in this group of mice were significantly lower than those in other groups, suggesting that controlling blood sugar can significantly promote wound healing. Hydrogel microneedle dressings have sufficient mechanical properties, excellent biocompatibility, glucose-responsive release behaviour, etc., and show great potential in diabetic wound management [191]. However, in the face of the complex diabetic microenvironment, hierarchical therapy has gradually attracted the attention of researchers [192]. Hierarchical therapy not only improves the utilization of drugs, but also allows the use of substances produced upstream to achieve certain biological functions or to eliminate potentially toxic substances. For example, Yang et al. encapsulated GOx into Fe-doped ZIF and constructed a hierarchically porous MOF based on a properly controlled tannic acid (TA) etching process. Since the etching of MOF with TA can form hierarchical channels, the Fe(II)/Fe(III) redox conversion can be realized. Finally, it was wrapped in soluble polyvinylpyrrolidone (PVP) to obtain stimuli-responsive microneedles for infected diabetic wound healing (Fig. 11B). The authors used Escherichia coli as an example to study the glucose-responsive antibacterial activity of GOx@Fe-ZIF-TA. When the glucose concentration increased, colonies could no longer be observed in the GOx@Fe-ZIF-TA group, which indicated that GOx@Fe-ZIF-TA had good antibacterial activity under high glucose concentrations. These results confirmed that GOx@FeZIF-TA can kill pathogens in a glucose-responsive manner for use in treating diabetic-infected wounds. Under the local acidic environment generated by gluconic acid, TA is able to reduce Fe(III) to Fe(II), catalyse the degradation of H2O2 to generate ROS, and achieve an antibacterial effect [193]. Although the advent of microneedling has made drug delivery convenient and painless, it still has some limitations. The main problem of microneedling technology is the risk of skin irritation and redness. In addition, the lack of drug loading of microneedling can significantly limit its development. Moreover, the insufficient mechanical strength and poor control of drug delivery of microneedles also hinder their practical application [194]. Environmental changes in the body are generally subtle and instantaneous due to the buffering effect of the physiological environment. Therefore, the development of microtargets that can respond rapidly to the environment is more relevant for diabetic wound therapy. For the treatment of diabetes, it has always been a huge challenge to accurately monitor blood sugar and give suitable hypoglycemic drugs, leading to serious complications, including hypoglycemia, blindness, and amputation [195,196]. Previous reports confirmed that poly (2-(hexamethyleneimino) ethyl methacrylate) (PHMEMA) possesses ultrasensitive pH responsiveness with a response time less than 5 ms [197]. Luo et al. designed an ultra-pH-responsive and glucose-responsive MN array patch for insulin delivery. The authors first encapsulated insulin in pH-sensitive nanoparticles (SNPs(I)) composed of polyethylene glycol-b-PHMEMA (mPEGb-PHMEMA). iSNPs were subsequently prepared with polyethylene glycol poly (2-cyclohexyl ethyl methacrylate) (mpeg-b-PCHMA). To alleviate the H2O2 generated during glucose oxidation, CAT and GOx were coencapsulated in iSNPs (iSNPs(G + C)). Finally, for convenient and painless administration, both iSNP(G + C) and SNP(I) were incorporated into the MNs (Fig. 11C). In vitro experiments found that the insulin release rate increased significantly with increasing glucose concentration, showing that the amount of insulin released was glucose concentration-dependent. Under hyperglycemic conditions, GOx in iSNP(G + C) catalyzes the oxidation of glucose to gluconic acid, resulting in a local pH decrease and triggering rapid protonation of the tertiary amino group of the PHMEMA fragment. This transition from hydrophobicity to hydrophilicity triggers the structural disassembly of SNP(I), leading to the rapid release of insulin. At the same time, the CAT loaded in iSNP(G + C) is able to consume excessive H2O2, thereby reducing the damage to normal skin. In addition, the MN patch also showed excellent regulation and a rapid response to blood glucose levels (BGLs) [198].
Fig. 11.
(A) Responsive microneedle dressing for diabetic wound healing. Diabetic wounds in mice treated with the hydrogel-based microneedle dressing. Preparation of glucose-responsive insulin-releasing Gel–AFPBA–ins hydrogels and mechanism of glucose-responsive insulin release from the prepared hydrogels (Reproduced with permission of Ref. [191]). (B) Schematic illustration of preparation of hierarchically porous glucose-responsive antibacterial GOx@Fe-ZIF-TA and penetration of MOF-based MNs into infected diabetic wounds to release antibacterial particles for infected diabetic wound treatment (Reproduced with permission of Ref. [193]). (C) Scheme of MN-array patch integrated with pH-sensitive insulin nanoformulations for glucose-responsive insulin delivery. The chemical structure and formation of SNP(I) and iSNP(G + C) and schematic illustration of the MN-array patch loaded with SNP(I) and iSNP(G + C) for in vivo insulin delivery triggered by a hyperglycemic state to release more insulin. (i) Blood glucose levels of type 1 diabetic mice after treatment with blank MNs as control, MNs loaded with SNP(I), MNs loaded with iSNP(I) and iSNP(G), MNs loaded with SNP(I) and iSNP(G), MNs loaded with SNP(I) and iSNP(G + C) (Reproduced with permission of Ref. [198]).
Glucose-responsive hydrogels are usually based on GOx, ConA and PBA, which can respond to glucose. Therefore, they have been used in glucose sensing systems to manage diabetic wound. Despite the great progress that has been made in glucose-responsive hydrogels, many limitations in this field should be addressed before their real application. Despite the advantages of biocompatibility and biodegradability, GOx and ConA are unstable due to environmental changes that can cause denaturation and loss of activity. Although PBA-responsive hydrogels have good stability as synthetic glucose-based materials, PBA-responsive hydrogels have disadvantages such as poor glucose selectivity, poor biodegradability, and a slow response rate. The advent of MNs has provided additional advantages and approaches to treat diabetic wounds, but some limitations remain, such as the stability of the glucose sensing portion, large safety in animals or humans, and possible local production of toxic substances [142].
3.4. ROS-responsive hydrogels
ROS are a class of highly reactive ions and free radicals produced by the human body, including superoxide, hypochlorite ion, and singlet oxygen, which can be transformed from one to the other through a series of reactions [199]. The three main sites of endogenous ROS production are mitochondria, endoplasmic reticulum, and NADPH oxidase, which generate superoxide [200]. Moderate levels of ROS play a crucial role in regulating cellular signalling pathways, inflammation, and cell proliferation [201]. However, when ROS overwhelm the antioxidant defence system, oxidative stress can cause toxic effects, which are associated with a range of pathological conditions, including diabetes [202].
ROS-responsive hydrogels are hydrogels that can sense oxidative stress in the local environment, modulate cellular behaviour, facilitate the release of desired drugs, and eliminate excess ROS production. Therefore, ROS-responsive hydrogels provide a promising practical tool for the presence of high levels of ROS locally in diabetic wounds. To make hydrogels ROS-responsive, the current strategy is to add ROS-responsive moieties to the backbone of the block copolymer to form the hydrogel or use polymers with ROS-responsive side chains. When ROS are elevated in the surrounding environment, the ROS-responsive unit can lead to the controlled release of drugs by degrading bonds in an oxidative environment, which leads to hydrophobic-to-hydrophilic transition or polymer chain scission. Most importantly, several factors affecting the drug loading and drug release should also be considered. These factors affect the structure, morphology, and mechanical properties of hydrogels and regulate the removal time of hydrogels [203].
As stated before, low levels of ROS are of great significance to promote normal wound healing [204,205]. Nevertheless, immune cells produce a large amount of ROS in diabetic wounds, which exceeds their antioxidant capacity. The accumulation of ROS can produce a long-term inflammatory response that makes the wound vulnerable and inhibits tissue regeneration around diabetic wounds [204]. With the progress of the antioxidant theory in recent years, various antioxidant hydrogels for chronic wound healing have been fabricated by antioxidant drug loading or antioxidant group modification [206]. For example, Zhao et al. mixed PVA (alcohol hydroxyl group) and N1-(4-boronobenzyl)-N3-(4-bor- onophenyl)-N1, N1, N3, N3-tetramethylpropane-1, 3-diaminium (TPA) (phenylboronic acid) to obtain a ROS-responsive hydrogel after reaction crosslinking. Then, they evaluated the degradation behaviour of the ROS-responsive hydrogel in PBS containing different concentrations of H2O2. Their results showed that the structure of the hydrogel was gradually destroyed after the incubation with H2O2, while the degradation of the hydrogel was accelerated with increasing H2O2 concentration. Moreover, the effectiveness of the ROS-responsive hydrogel in inhibiting H2O2 production and promoting wound healing has been demonstrated in a mouse infected wound model [207]. Likewise, Wang et al. synthesized a boronate-based ROS-responsive hydrogel. Since boronate esters can be selectively degraded by ROS, the hydrogels help to modulate inflammatory responses and induce drug release through ROS scavenging. The authors synthesized a DPE stock solution using DL-dithiothreitol, polyethylene glycol diacrylate and phenylboronic acid-modified ε-polylysine (EPL). This hydrogel can serve as a physical barrier to fill wound defects for protection by light-induced in situ gelation. The authors found that DPE2 exhibited rapid EPL release by testing the release properties of EPL from hydrogels in an H2O2 PBS solution. Furthermore, the release rate of EPL incubated with H2O2 PBS solution was higher than that incubated with PBS alone. In vivo experiments indicate that DPE2 has an excellent antibacterial effect and significant anti-inflammatory activity and can promote collagen deposition. These results indicated the important role of DPE2 hydrogels in promoting infected diabetic wound healing [208].
Despite obvious developments in ROS responsiveness, a single responsive hydrogel cannot satisfy the growing demand due to the constantly tricky and complex environment of diabetic wounds. Therefore, the development of multiresponsive hydrogels is warranted. In recent years, the formation of reversible covalent chemical bonds, including Schiff base bonds, disulfide bonds, and borate esters, has been considered a potential strategy to design injectable multiresponsive hydrogels. Many of these dynamic covalent bonds can be broken down in response to stimuli from the surrounding environment, such as pH, ROS, temperature, enzymes, etc. [[209], [210], [211]] Wang et al. designed a boronate bond-based hydrogel (hydrogel & vancomycin-conjugated silver nanocluster (VAN-AgNC) & micelle (MIC)) for application in infected diabetic wounds (Fig. 12A). Due to the dynamic phenylboronic acid-diol ester bond, the hydrogel exhibits dual-responsive properties to pH and ROS. In vitro degradation results show that the degradation rate of the hydrogel is slightly slower in PBS than in low pH and H2O2. In the presence of low pH and high ROS, the stable hydrogels rapidly transform into a liquid-like state and rapidly released VAN-AgNCs, which was attributed to the dissociation of the phenylboronic acid-diol ester bonds in the hydrogels. The subsequent sustained release of NIM from the MIC under acidic conditions result from the collapse of the MIC in an acidic environment. These results demonstrate the programmed release of encapsulated VAN-AgNCs and NIMs in an inflammatory environment. This approach gradually treats infected chronic diabetic wounds by providing antimicrobial therapy prior to administrating anti-inflammatory therapy. Additionally, the smart hydrogel has hemostatic and anti-inflammatory functions with excellent cytocompatibility in vivo, which can strongly promote the healing of infected chronic diabetic wounds [212]. Similarly, Xu et al. synthesized a glucose- and ROS-responsive smart hydrogel using boronate linkages to treat diabetic wounds [213]. However, from a clinical perspective, the use of single-factor delivery to treat diabetic wounds is highly inefficient. This is not surprising considering the complexity of the diabetic wound healing process. For the process of wound repair, biological phenomena, including angiogenesis, inflammation and tissue remodeling, are complex, interconnected and controlled in a spatiotemporal manner. Therefore, there is a need to design a material that can control the release of multiple factors so that specific factors have different releases over time. Recently, dual-dynamic covalently constructed smart hydrogels have begun to be applied to diabetic wounds. Wu et al. fabricated a dual-dynamic covalently constructed hydrogel to spatiotemporally control the release of diclofenac sodium (DS) and mangiferin (MF) through an ROS/pH dual response, which inhibited the inflammatory response and promoted the healing of diabetic infected wounds (Fig. 12B). Due to the breakdown of the gel network after the hydrolysis of Schiff bases and boronate linkages, when the DS&MIC@MF hydrogel was added to HCl or H2O2, the hydrogel gradually disintegrated in a short time. Both MF and DS particles achieved controlled release under acidic and oxidative conditions. Although MF could be continuously and slowly released over a week, the amount of DS particles released at 24 h was significantly higher than that of MF. Their data suggested that the sequential collapse of the hydrogel and MIC leads to the spatiotemporal release of DS and MF. This delivery property is beneficial for infected diabetic wound healing because the DS&MIC@MF hydrogel with antibacterial and antioxidant properties is involved in the anti-apoptotic activity and promotion of angiogenesis. More importantly, the spatiotemporal transmission behaviour of DS and MF is highly consistent with the programmed process of infected wound healing [214].
Fig. 12.
(A) Diagrams displaying the formation of NIM-Loaded Micelle, VAN-AgNCs, Hydrogels, and the mechanisms of accelerating wound healing (Reproduced with permission of Ref. [212]). (B) Schematic diagram of the fabrication procedures of the DS&MIC@MF embedded POD/CE hydrogels and illustration of spatiotemporally drugs release behaviour of the hydrogel, and the mechanism of the hydrogel for accelerating wound healing on the infected diabetic cutaneous wound model (Reproduced with permission of Ref. [214]). (C) Schematic representations of CNPs@GMs/hydrogel preparation and the process of drug release at the wound bed in diabetic mice. Preparation of pure CNPs via a solution exchange method. CNPs loaded into GMs by the emulsion process to get CNPs@GMs. CNPs@GMs mixed with the thermos-sensitive hydrogel and covered on the wound in diabetic mice. Under the microenvironment of a nonhealing wound, GMs were degraded by MMPs, and specifically the drug was specifically released. (ii) Cell migration/invasion in real time was monitored by the xCELLigence RTCA for 72 h (Reproduced with permission of Ref. [223]). (D) The fabrication and application of self-adaptive DFO@G-QCSFP for accelerating diabetic wound healing on the full-thickness diabetic wound of a diabetic SD rat. a) The chemical structure of the hydrogel and the mechanism of the hydrogel for accelerating diabetic wound healing. b) The self-adaption of the hydrogel to wound microenvironment. The hydrogel reduced oxidative stress and released DFO@G on demand. Then DFO was released and promoted angiogenesis (Reproduced with permission of Ref. [224]).
ROS-responsive hydrogels have been developed as some of the most promising biomaterials in biomedicine. ROS-responsive hydrogels are antioxidant hydrogels that stimulate drug release through redox reactions and have been extensively studied, specifically in chronic wound healing and cardiovascular diseases. However, the clinical application of ROS-responsive hydrogels remains limited mainly because the difference in ROS between pathological and normal tissues is not obvious. In addition, the adequacy of the controlled redox homeostasis cannot be resolved, which causes a large uncertainty in the release of loaded drugs.
3.5. Other responsive hydrogels
MMPs are a family of zinc- and calcium-dependent endopeptidases that play a role in many normal physiological functions [215,216]. Studies have shown that the overexpression of MMP-9 hinders chronic diabetic wound healing, and the reduction in MMP-9 expression promotes the healing of diabetic wounds [217,218]. Therefore, wound dressings that inhibit the expression of MMP-9 provide a scientific basis for diabetic wound healing. A strategy to generate MMP responsiveness is to incorporate matrix metal oxide matrices into carriers, but most of the available natural matrices are macromolecular proteins, which limits their use in drug delivery [219]. In contrast, synthetic MMP substrates are mostly short linear polypeptides that can be easily incorporated into NPs, which has attracted great attention in the field [220,221]. Due to the small size of the MMP-sensitive polypeptides, the physicochemical properties of the NPs are not significantly affected by loading them into the NPs [222]. However, the presence of MMPs in nanomaterials can affect their structure, conformation, hydrophobicity/hydrophilicity, and charge changes, which are sufficient to affect the size, morphology and stability of these NPs. These changes may disintegrate, aggregate, and rearrange NPs to achieve responsive effects.
Liu et al. reported the encapsulation of an MMP-9 responsive and thermosensitive dual-responsive hydrogel to improve diabetic wound healing. The authors first self-assembled curcumin (Cur) to obtain CuR nanoparticles (CNPs) and subsequently encapsulated them in GMs to obtain CNPS@GMs. Finally, CNPS@GMs were mixed with a temperature-sensitive hydrogel (CNPS@GMs/hydrogel) to cover diabetic skin wounds (Fig. 12C). Since the CNPs were encapsulated in GMs, the hydrogel could respond to MMP-9 in diabetic skin wounds. In the release experiments in vitro, the authors found that with increasing MMP-9 concentration, the Cur released by CNPs@GMs gradually increased. This result suggested that a large amount of Cur could be released from CNPs@GMs to disrupt GMs by treatment with MMP-9. Nevertheless, without MMP-9, the Cur release of CNPs@GMs was much lower than that in the presence of MMP-9, which confirms that CNPs@GMs can specifically react with MMP-9 and release Cur through cleavage. In a diabetic wound model, CNPs@GMs exhibited excellent wound healing promotion and excellent biocompatibility [223]. Similarly, Shao et al. synthesized a microsphere-based MMP-9-responsive hydrogel to manage diabetic wounds. Due to the boronate bond, the hydrogel reacts to high concentrations of glucose and H2O2, which can more effectively treat diabetic wounds. The hydrogel exhibits excellent MMP-9 responsiveness, glucose responsiveness, biocompatibility, and the ability to facilitate diabetic wound healing [224]. Sonamuthu et al. [225] designed a natural protein hydrogel wound dressing based on MMP-9-responsive l-carnosine dipeptide induction for the accelerated treatment of infected diabetic wounds (Fig. 12D). The histidine residue (b-alanine-histidine) of the l-carnosine dipeptide has a strong zinc ion binding ability and inactivates MMP-9 through its strong complexation with zinc ions at the active centre of MMP-9. Therefore, l-carnosine-based biohydrogels can effectively enhance diabetic wound healing by inhibiting MMP-9.
3.6. Dual stimulus-response strategy for diabetic wounds
Due to the multifactorial nature of diabetic wounds, single-responsive hydrogel dressings have limited effects on complete wound healing. As a result, multiresponsive dressings have been developed to better heal wound by satisfying multiple stimuli. With the increasing demand for the precision of drug controlled release, different stimuli can be combined to synergistically regulate drug release and improve the therapeutic effect of drug loading. Due to the synergistic effect of multiple treatment modalities, combination therapy can often achieve better therapeutic effects than single treatment modalities. Therefore, multiresponsive hydrogel dressings are rationally designed with multiple therapeutic modalities and provide a platform for chronic diabetic wounds. For instance, Guo et al. designed a PH and ROS dual-responsive hydrogel with intelligent targeting of refractory wound characteristics (Fig. 13A). They achieved low pH and ROS stimuli-responsive reversal of the harmful microenvironment in wounds by introducing Schiff base bonds and ROS-responsive paeoniside (Pf) micelles to the backbone of the hydrogel. In degradation assays in vitro, the hydrogels decomposed significantly faster over time when exposed to H2O2 and an acidic environment than in PBS solutions. More importantly, we found that zinc oxide nanoparticles (nZnO) and MIC@Pf could be released from the hydrogel due to pH-sensitive Schiff base bonds in the hydrogel, but Pf release from MIC particles was mediated by ROS-induced MIC collapse. Guided. Therefore, this hydrogel can promote infected chronic diabetic wound healing in a step-by-step manner by rapidly releasing nZnO followed by sustained release of Pf-mediated continuous antibiotic and angiogenic activity. In a diabetic wound model, the hydrogel accelerates wound healing by combining hemostatic and antibacterial activities, alleviating inflammatory responses, and promoting angiogenic activities [226]. However, infected diabetic wounds are susceptible to multidrug-resistant bacterial infections due to their long healing time. Due to the strong infectiousness, high recurrence rate, and high lethality of multidrug-resistant bacteria, treating this type of bacterium has been a great clinical challenge. In addition, Wang et al. obtained FeMI hydrogels by a Schiff group reaction of insulin-preloaded Pluronic F127 (FCHO) micelles and ε-polylysine functionalized manganese dioxide nanosheets (EM) (Fig. 13C). The authors investigated the in vitro insulin release behaviour of FEMI hydrogels and found that the release of insulin under acidic and oxidative conditions was significantly greater than that of the control group, showing a unique pH/redox dual-responsive insulin release profile. These results are attributed to the destruction of the cross-linking points of the Schiff bases in the acidic environment and the decomposition of EM nanosheets by H2O2-promoted redox reactions. Animal experiments have demonstrated that FeMI hydrogels can reduce inflammation, promote angiogenesis, and facilitate granulation tissue growth to enhance diabetic wound healing [227].
Fig. 13.
(A) Illustration of microenvironment-Responsive Hydrogel fabrication stragety. (ii) The release tendency of nZnO and Pf (iii) over 54 h (Reproduced with permission of Ref. [226]). (B) Schematic illustration of the preparation of stimuli-responsive MXene-based hydrogel system. The formation and drug release process of the MXene-based hydrogel system. (iv) Thermal images of AgNPs-loaded MXene-based hydrogel applied to the rat subcutaneous before and after near-infrared irradiation. (v) Photos of the subcutaneous tissue of the diabetic rats and immunostaining of IL-6 and TNF-α (Reproduced with permission of Ref. [231]). (C) Schematic diagram illustrating FEMI hydrogel for MDR bacteria-infected diabetic wound healing. (vi) Representative SEM images of MRSA cells after different treatments. The yellow pseudocolor indicates the MRSA bacterial cells and blue pseudocolor indicates the surrounding materials (Reproduced with permission of Ref. [227]).
Although many stimuli including pH, ROS and temperature have been applied in the treatment of chronic wounds, alternating magnetic fields and near-infrared have unique advantages, including tissue penetration and high-sensitivity remote control capabilities [[228], [229], [230]]. A smart responsive hydrogel drug delivery system developed by Yang et al. is composed of MXene-wrapped Fe3O4@SiO2 magnetic colloid and PNIPAM-alginate double network hydrogel (Fig. 13B). As a temperature-responsive polymer, PNIPAM repels water and shrinks near the LCST. In other words, the hydrogel system shrinks with increasing temperature of MNPs@MXene in a near-infrared or alternating magnetic field, thereby precisely controlling the release of silver nanoparticles (AgNPs). Furthermore, in a deep wound diabetic rat model, MNPs@MXene hydrogels not only possessed excellent cytocompatibility and biological utility but also exhibited desirable properties. These characteristics suggest that they may serve as promising candidates for the treatment of chronic wounds [231].
4. Conclusion and future perspectives
Diabetes mellitus is one of the fastest growing diseases globally, and the poor wound healing caused by diabetes remains a significant clinical problem. During chronic wound repair, bacterial infections are inevitable and may even lead to serious complications. Therefore, better control of infection is considered one of the most critical challenges in skin tissue engineering. When a normal wound is infected, although local immune cells can play a bactericidal role, the bacteria can still cause persistent inflammation at the local site causing the healing process of the inflammatory phase to be prolonged indefinitely resulting in nonhealing of the wound. However, in diabetic wounds, many harmful factors prolong the healing process of the inflammatory phase of the wound. First, hyperglycemia increases oxygen consumption leading to cellular hypoxia and promoting the production of ROS. Second, the local hypoxic state is further enhanced by the lack of oxygen delivery due to impaired angiogenesis of the wound microvasculature. The hypoxic state and increased ROS in diabetic wounds significantly increase the inflammatory response of the wound. Finally, the increase in proinflammatory cytokines and AGEs around the local wound further exacerbates the inflammatory response. Hence, the inflammatory period of diabetic wound healing is more permanent than that of normal infected wound healing,increasing the difficulty of treatment [232,233].
Hydrogels have been widely applied in wound therapy owing to their unique properties, including biocompatibility, hydrophilicity and biodegradability. However, the drug release of conventional designed hydrogels is uncontrollable and may not achieve the ideal treatment effect. Recently, responsive hydrogels that can change their properties in response to environmental stimuli have provided a new idea for research in this field. Stimuli-responsive hydrogels are able to sense changes in key environments (e.g., changes in pH, glucose, temperature, and enzymes) and release the optimal dose of drug to the target area at the appropriate time. Glucose-responsive hydrogels, in particular, have the potential to significantly improve compliance and quality of life in diabetic patients. In addition, using physiological factors as triggers, sustained and stable release of therapeutic drugs can be achieved for the long-term treatment of diabetic wounds. As we have discussed in this review, smart responsive hydrogels are very attractive in the management of diabetic wounds. Different responsive strategies have different advantages, so it is essential that we choose the right stimulus response when designing the hydrogel. Second choosing a hydrogel that matches the healing process is another important factor that will not only improve drug utilization but also better promote wound healing. As the reactivity of gel systems evolves, it is clear that the combined application of two or more reactive strategies holds great promise for gel carriers, as this allows for more precise control of gel degradation and drug release [234].
The degradation of nanomaterials, including hydrogels, is a key parameter for scaffold suitability due to the need to accommodate cell proliferation and extracellular matrix production. The appropriate rate of degradation of biomaterials is very important for biomedical applications. For example, determining the optimal degradation rate and water content of a hydrogel can better accelerate wound healing. For wound healing, degradable nanomaterials must have the following properties: first, the nanomaterial must not only be cytocompatible, but the degradation products should be nontoxic and able to be metabolized and cleared from the body. Second, the degradation time should be matched to the healing or regeneration process, otherwise a mechanical barrier would be formed to prevent the normal healing process. Finally the nanomaterial should have mechanical properties suitable for the particular application and the mechanical properties should change with degradation to match the healing or regeneration process [235,236].
The use of smart hydrogels as delivery vehicles is a popular area for future research. Despite these achievements, smart hydrogels still have serious limitations as a drug delivery system, including insufficient responsiveness, poor mechanical properties, lack of biocompatibility, and poor biodegradability. There are also many critical issues that need to be investigated in future clinical applications. First, many of the processes and parameters involved in diabetic wound healing are dynamic, which makes it difficult to find an ideal dressing that can simultaneously meet the requirements of the entire wound healing process [237]. Despite the encouraging results of the studies outlined in this review, further evaluation is needed for the potential application of reactive hydrogels. Second, in targeted drug delivery systems, although two or more reactive strategies have been developed for combined application, it remains a great challenge to integrate the different stimuli and exploit synergistic effects. Finally, many smart hydrogels have achieved desirable rapid results in vitro or in experimental animals [238,239], but their true performance all need to be re-evaluated in humans because the switch in drug release is subject to interference from more complex stimuli. The appropriate concentration of responsive hydrogels is still unknown, so the dose and release rate of hydrogel-loaded drugs should be precisely quantified to avoid adverse effects [240]. Therefore, research on the development of smart hydrogels with good biocompatibility, biodegradability and appropriate controlled release properties is a major goal of future research. As a new clinical drug delivery system, hydrogels may be a promising candidate for the management of local wounds.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 81871788, 82202672, 82072425, 82072498), the Key Research and Development Program of Anhui Province (No. 202004j07020013 and 2022e07020017), the Natural Science Foundation of Anhui Province (Grant No. 2108085QH319), the Fundamental Research Funds for the Central Universities (Grant No. WK9110000173), the Scientific research project approved by Anhui Provincial Health Commission (Grant No. AHWJ2021b079), the Natural Science Foundation of Jiangsu Province (BE2020666), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and Special Project of Diagnosis and Treatment Technology for Key Clinical Diseases in Suzhou (LCZX202003).
Contributor Information
Chen Zhu, Email: zhuchena@ustc.edu.cn.
Weiwei Zhang, Email: zhangxueweiwei@sina.com.
Dechun Geng, Email: szgengdc@suda.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Patel S., Srivastava S., Singh M.R., Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 4.Mogoşanu G.D., Grumezescu A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014;463(2):127–136. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Eleftheriadou I., Tentolouris N., Argiana V., Jude E., Boulton A.J. Methicillin-resistant Staphylococcus aureus in diabetic foot infections. Drugs. 2010;70(14):1785–1797. doi: 10.2165/11538070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Schaper N.C., Van Netten J.J., Apelqvist J., Lipsky B.A., Bakker K. Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes/metabolism research and reviews. 2016;32(Suppl 1):7–15. doi: 10.1002/dmrr.2695. [DOI] [PubMed] [Google Scholar]
- 7.Nather A., Cao S., Chen J.L.W., Low A.Y. Prevention of diabetic foot complications. SMJ (Singap. Med. J.) 2018;59(6):291–294. doi: 10.11622/smedj.2018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brem H., Sheehan P., Rosenberg H.J., Schneider J.S., Boulton A.J. Evidence-based protocol for diabetic foot ulcers. Plast. Reconstr. Surg. 2006;117(7 Suppl):193S–209S. doi: 10.1097/01.prs.0000225459.93750.29. ; discussion 210S-211S. [DOI] [PubMed] [Google Scholar]
- 9.Dumville J.C., Lipsky B.A., Hoey C., Cruciani M., Fiscon M., Xia J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2017;6(6):Cd011038. doi: 10.1002/14651858.CD011038.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kido D., Mizutani K., Takeda K., Mikami R., Matsuura T., Iwasaki K., Izumi Y. Impact of diabetes on gingival wound healing via oxidative stress. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson H.N., Hardman M.J. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9) doi: 10.1098/rsob.200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng L., Du C., Song P., Chen T., Rui S., Armstrong D.G., Deng W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/8852759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakroborty D., Goswami S., Basu S., Sarkar C. Catecholamines in the regulation of angiogenesis in cutaneous wound healing. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 2020;34(11):14093–14102. doi: 10.1096/fj.202001701R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okonkwo U.A., DiPietro L.A. Diabetes and wound angiogenesis. Int. J. Mol. Sci. 2017;18(7) doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theocharidis G., Veves A. Autonomic nerve dysfunction and impaired diabetic wound healing: the role of neuropeptides. Auton. Neurosci. : basic & clinical. 2020;223 doi: 10.1016/j.autneu.2019.102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellechea A., Bai S., Dangwal S., Theocharidis G., Nagai M., Koerner S., Cheong J.E., Bhasin S., Shih T.Y., Zheng Y., Zhao W., Zhang C., Li X., Kounas K., Panagiotidou S., Theoharides T., Mooney D., Bhasin M., Sun L., Veves A. Topical application of a mast cell stabilizer improves impaired diabetic wound healing. J. Invest. Dermatol. 2020;140(4):901–911. doi: 10.1016/j.jid.2019.08.449. e11. [DOI] [PubMed] [Google Scholar]
- 17.Moura L.I., Dias A.M., Carvalho E., de Sousa H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater. 2013;9(7):7093–7114. doi: 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y., Li Z., Li Q., Yang L., Liu H., Yan R., Xiao L., Liu H., Wang J., Yang B., Lin Q. Transparent conductive supramolecular hydrogels with stimuli-responsive properties for on-demand dissolvable diabetic foot wound dressings. Macromol. Rapid Commun. 2020;41(24) doi: 10.1002/marc.202000441. [DOI] [PubMed] [Google Scholar]
- 19.Chen K., Wang F., Liu S., Wu X., Xu L., Zhang D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020;148:501–509. doi: 10.1016/j.ijbiomac.2020.01.156. [DOI] [PubMed] [Google Scholar]
- 20.Deng H., Yu Z., Chen S., Fei L., Sha Q., Zhou N., Chen Z., Xu C. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115565. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi M., Noruzi E.B., Sheykhsaran E., Ebadi B., Kariminezhad Z., Molaparast M., Mehrabani M.G., Mehramouz B., Yousefi M., Ahmadi R., Yousefi B., Ganbarov K., Kamounah F.S., Shafiei-Irannejad V., Kafil H.S. Carbohydrate polymer-based silver nanocomposites: recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2020;231 doi: 10.1016/j.carbpol.2019.115696. [DOI] [PubMed] [Google Scholar]
- 22.Su T., Zhang M., Zeng Q., Pan W., Huang Y., Qian Y., Dong W., Qi X., Shen J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021;6(3):579–588. doi: 10.1016/j.bioactmat.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi X., Pan W., Tong X., Gao T., Xiang Y., You S., Mao R., Chi J., Hu R., Zhang W., Deng H., Shen J. ε-Polylysine-stabilized agarose/polydopamine hydrogel dressings with robust photothermal property for wound healing. Carbohydr. Polym. 2021;264 doi: 10.1016/j.carbpol.2021.118046. [DOI] [PubMed] [Google Scholar]
- 24.He X., Hou J.T., Sun X., Jangili P., An J., Qian Y., Kim J.S., Shen J. NIR-II photo-amplified sonodynamic therapy using sodium molybdenum bronze nanoplatform against subcutaneous Staphylococcus aureus infection. Adv. Funct. Mater. 2022;32(38) doi: 10.1002/adfm.202203964. [DOI] [Google Scholar]
- 25.Bai Q., Han K., Dong K., Zheng C., Zhang Y., Long Q., Lu T. Potential applications of nanomaterials and technology for diabetic wound healing. Int. J. Nanomed. 2020;15:9717–9743. doi: 10.2147/ijn.S276001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cascone S., Lamberti G. Hydrogel-based commercial products for biomedical applications: a review. Int. J. Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118803. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y., Cheng Y., Chen J., Ding J., Li M., Li C., Wang J.C., Chen X. Injectable hydrogel-microsphere construct with sequential degradation for locally synergistic chemotherapy. ACS Appl. Mater. Interfaces. 2017;9(4):3487–3496. doi: 10.1021/acsami.6b15245. [DOI] [PubMed] [Google Scholar]
- 28.Wen X., Zhang Y., Chen D., Zhao Q. Reversible shape-shifting of an ionic strength responsive hydrogel enabled by programmable network anisotropy. ACS Appl. Mater. Interfaces. 2022;14(35):40344–40350. doi: 10.1021/acsami.2c11693. [DOI] [PubMed] [Google Scholar]
- 29.Andrade F., Roca-Melendres M.M., Llaguno M., Hide D., Raurell I., Martell M., Vijayakumar S., Oliva M., Schwartz S., Jr., Durán-Lara E.F., Rafael D., Abasolo I. Smart and eco-friendly N-isopropylacrylamide and cellulose hydrogels as a safe dual-drug local cancer therapy approach. Carbohydr. Polym. 2022;295:119859. doi: 10.1016/j.carbpol.2022.119859. [DOI] [PubMed] [Google Scholar]
- 30.Deng M., Wu Y., Ren Y., Song H., Zheng L., Lin G., Wen X., Tao Y., Kong Q., Wang Y. Clickable and smart drug delivery vehicles accelerate the healing of infected diabetic wounds. J. Contr. Release : official journal of the Controlled Release Society. 2022;350:613–629. doi: 10.1016/j.jconrel.2022.08.053. [DOI] [PubMed] [Google Scholar]
- 31.Gu J., Zhao G., Yu J., Xu P., Yan J., Jin Z., Chen S., Wang Y., Zhang L.W., Wang Y. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnol. 2022;20(1):372. doi: 10.1186/s12951-022-01561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu B., Han X., Zou D., Luo X., Liu L., Wang J., Maitz M.F., Yang P., Huang N., Zhao A. Catechol-chitosan/polyacrylamide hydrogel wound dressing for regulating local inflammation. Mater Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieto C., Vega M.A., Rodríguez V., Pérez-Esteban P., Martín Del Valle E.M. Biodegradable gellan gum hydrogels loaded with paclitaxel for HER2+ breast cancer local therapy. Carbohydr. Polym. 2022;294:119732. doi: 10.1016/j.carbpol.2022.119732. [DOI] [PubMed] [Google Scholar]
- 34.Mohanty A.R., Ravikumar A., Peppas N.A. Recent advances in glucose-responsive insulin delivery systems: novel hydrogels and future applications. Regenerative biomaterials 9. 2022 doi: 10.1093/rb/rbac056. rbac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger K., Coclite A.M. Glucose-responsive boronic acid hydrogel thin films obtained via initiated chemical vapor deposition. Biomacromolecules. 2022 doi: 10.1021/acs.biomac.2c00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng P., Cheng L., Han H., Li J., Ma C., Huang H., Zhou J., Feng J., Huang Y., Lv Y., Huang H., Wang Y., Hou L., Chen Y., Li G. A pH/H(2) O(2)/MMP9 time-response gel system with sparc(high) tregs derived extracellular vesicles promote recovery after acute myocardial infarction. Advanced healthcare materials. 2022 doi: 10.1002/adhm.202200971. [DOI] [PubMed] [Google Scholar]
- 37.Sharma A., Panwar V., Salaria N., Ghosh D. Protease-responsive hydrogel, cross-linked with bioactive curcumin-derived carbon dots, encourage faster wound closure. Biomaterials advances. 2022;139 doi: 10.1016/j.bioadv.2022.212978. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh S., Davis J., Roros A., Zhou C., He N., Gao W., Khan S., Genzer J., Menegatti S. ACS applied materials & interfaces; 2022. Nonwoven Membranes with Infrared Light-Controlled Permeability. [DOI] [PubMed] [Google Scholar]
- 39.He P.P., Du X., Cheng Y., Gao Q., Liu C., Wang X., Wei Y., Yu Q., Guo W. Thermal-responsive MXene-DNA hydrogel for near-infrared light triggered localized photothermal-chemo synergistic cancer therapy. Small. 2022 doi: 10.1002/smll.202200263. [DOI] [PubMed] [Google Scholar]
- 40.Vítková L., Musilová L., Achbergerová E., Kolařík R., Mrlík M., Korpasová K., Mahelová L., Capáková Z., Mráček A. Formulation of magneto-responsive hydrogels from dually cross-linked polysaccharides: synthesis, tuning and evaluation of rheological properties. Int. J. Mol. Sci. 2022;23(17) doi: 10.3390/ijms23179633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haghighattalab M., Kajbafzadeh A., Baghani M., Gharehnazifam Z., Jobani B.M., Baniassadi M. Silk fibroin hydrogel reinforced with magnetic nanoparticles as an intelligent drug delivery system for sustained drug release. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.891166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasan M.M., Johnson C.L., Dunn A.C. Soft contact mechanics with gradient-stiffness surfaces. Langmuir : the ACS journal of surfaces and colloids. 2022;38(31):9454–9465. doi: 10.1021/acs.langmuir.2c00296. [DOI] [PubMed] [Google Scholar]
- 43.Chang S., Wang S., Liu Z., Wang X. Advances of stimulus-responsive hydrogels for bone defects repair in tissue engineering. Gels (Basel, Switzerland) 2022;8(6) doi: 10.3390/gels8060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H., Huang C., Kong X., Ma J., Ren P., Chen J., Zhang X., Luo H., Chen G. Nanoarchitectonics of cartilage-targeting hydrogel microspheres with reactive oxygen species responsiveness for the repair of osteoarthritis. ACS Appl. Mater. Interfaces. 2022 doi: 10.1021/acsami.2c12703. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Tian S., Huang L., Li Y., Lu Y., Li H., Chen G., Meng F., Liu G.L., Yang X., Tu J., Sun C., Luo L. Reactive oxygen species-responsive and Raman-traceable hydrogel combining photodynamic and immune therapy for postsurgical cancer treatment. Nat. Commun. 2022;13(1):4553. doi: 10.1038/s41467-022-32160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y., Fang K., Hu X., Yang J., Jiang Z., Feng L., Li R., Rao Y., Shi S., Dong C. NIR-light-controlled G-quadruplex hydrogel for synergistically enhancing photodynamic therapy via sustained delivery of metformin and catalase-like activity in breast cancer. Mater Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding M., Fan Y., Lv Y., Liu J., Yu N., Kong D., Sun H., Li J. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. 2022;149:334–346. doi: 10.1016/j.actbio.2022.06.041. [DOI] [PubMed] [Google Scholar]
- 48.Wu C., Pu Y., Zhang Y., Liu X., Qiao Z., Xin N., Zhou T., Chen S., Zeng M., Tang J., Pi J., Wei D., Sun J., Luo F., Fan H. A bioactive and photoresponsive platform for wireless electrical stimulation to promote neurogenesis, advanced healthcare materials. 2022. [DOI] [PubMed]
- 49.Lei H., Fan D. A combination therapy using electrical stimulation and adaptive, conductive hydrogels loaded with self-assembled nanogels incorporating short interfering RNA promotes the repair of diabetic chronic wounds, advanced science. 2022. (Weinheim, Baden-Wurttemberg, Germany) [DOI] [PMC free article] [PubMed]
- 50.He X., Dai L., Ye L., Sun X., Enoch O., Hu R., Zan X., Lin F., Shen J. A vehicle-free antimicrobial polymer hybrid gold nanoparticle as synergistically therapeutic platforms for Staphylococcus aureus infected wound healing. Adv. Sci. 2022;9(14) doi: 10.1002/advs.202105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian Y., Zheng Y., Jin J., Wu X., Xu K., Dai M., Niu Q., Zheng H., He X., Shen J. Immunoregulation in diabetic wound repair with a photoenhanced glycyrrhizic acid hydrogel scaffold. Adv. Mater. 2022;34(29) doi: 10.1002/adma.202200521. [DOI] [PubMed] [Google Scholar]
- 52.Kasiński A., Zielińska-Pisklak M., Oledzka E., Sobczak M. Smart hydrogels - synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020;15:4541–4572. doi: 10.2147/ijn.S248987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizzo F., Kehr N.S. Recent advances in injectable hydrogels for controlled and local drug delivery. Advanced healthcare materials. 2021;10(1) doi: 10.1002/adhm.202001341. [DOI] [PubMed] [Google Scholar]
- 54.Zeng Y., Hoque J., Varghese S. Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater. 2019;93:152–168. doi: 10.1016/j.actbio.2019.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broughton G., 2nd, Janis J.E., Attinger C.E. Wound healing: an overview. Plast. Reconstr. Surg. 2006;117(7 Suppl) doi: 10.1097/01.prs.0000222562.60260.f9. 1e-S-32e-S. [DOI] [PubMed] [Google Scholar]
- 56.Menke N.B., Ward K.R., Witten T.M., Bonchev D.G., Diegelmann R.F. Impaired wound healing. Clin. Dermatol. 2007;25(1):19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Li J., Chen J., Kirsner R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Campos A.C., Groth A.K., Branco A.B. Assessment and nutritional aspects of wound healing. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11(3):281–288. doi: 10.1097/MCO.0b013e3282fbd35a. [DOI] [PubMed] [Google Scholar]
- 59.Bansal S., Siddarth M., Chawla D., Banerjee B.D., Madhu S.V., Tripathi A.K. Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol. Cell. Biochem. 2012;361(1–2):289–296. doi: 10.1007/s11010-011-1114-9. [DOI] [PubMed] [Google Scholar]
- 60.Davis F.M., Kimball A., Boniakowski A., Gallagher K. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr. Diabetes Rep. 2018;18(1):2. doi: 10.1007/s11892-018-0970-z. [DOI] [PubMed] [Google Scholar]
- 61.Schreml S., Szeimies R.M., Karrer S., Heinlin J., Landthaler M., Babilas P. The impact of the pH value on skin integrity and cutaneous wound healing. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2010;24(4):373–378. doi: 10.1111/j.1468-3083.2009.03413.x. [DOI] [PubMed] [Google Scholar]
- 62.Cui T., Yu J., Wang C.F., Chen S., Li Q., Guo K., Qing R., Wang G., Ren J. Micro-gel ensembles for accelerated healing of chronic wound via pH regulation. Adv. Sci. 2022;9(22) doi: 10.1002/advs.202201254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L., Ouyang M., Zhang Y., Zhang L., Huang Z., He L., Lei Y., Zou Z., Feng F., Yang R. The fluorescence imaging and precise suppression of bacterial infections in chronic wounds by porphyrin-based metal-organic framework nanorods. J. Mater. Chem. B. 2021;9(38):8048–8055. doi: 10.1039/d1tb01649k. [DOI] [PubMed] [Google Scholar]
- 64.Wallace L.A., Gwynne L., Jenkins T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019;10(11):719–735. doi: 10.4155/tde-2019-0066. [DOI] [PubMed] [Google Scholar]
- 65.Sim P., Strudwick X.L., Song Y., Cowin A.J., Garg S. Influence of acidic pH on wound healing in vivo: a novel perspective for wound treatment. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider L.A., Korber A., Grabbe S., Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res. 2007;298(9):413–420. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 67.Schreml S., Meier R.J., Wolfbeis O.S., Landthaler M., Szeimies R.M., Babilas P. 2D luminescence imaging of pH in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108(6):2432–2437. doi: 10.1073/pnas.1006945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dunnill C., Patton T., Brennan J., Barrett J., Dryden M., Cooke J., Leaper D., Georgopoulos N.T. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017;14(1):89–96. doi: 10.1111/iwj.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang J., Chen L., Gu Z., Wu J. Red jujube-incorporated gelatin methacryloyl (GelMA) hydrogels with anti-oxidation and immunoregulation activity for wound healing. J. Biomed. Nanotechnol. 2019;15(7):1357–1370. doi: 10.1166/jbn.2019.2815. [DOI] [PubMed] [Google Scholar]
- 70.Srivastava S.K., Yadav U.C., Reddy A.B., Saxena A., Tammali R., Shoeb M., Ansari N.H., Bhatnagar A., Petrash M.J., Srivastava S., Ramana K.V. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem. Biol. Interact. 2011;191(1–3):330–338. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kunkemoeller B., Bancroft T., Xing H., Morris A.H., Luciano A.K., Wu J., Fernandez-Hernando C., Kyriakides T.R. Elevated thrombospondin 2 contributes to delayed wound healing in diabetes. Diabetes. 2019;68(10):2016–2023. doi: 10.2337/db18-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen C.Y., Abell A.M., Moon Y.S., Kim K.H. An advanced glycation end product (AGE)-receptor for AGEs (RAGE) axis restores adipogenic potential of senescent preadipocytes through modulation of p53 protein function. J. Biol. Chem. 2012;287(53):44498–44507. doi: 10.1074/jbc.M112.399790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demidova-Rice T.N., Hamblin M.R., Herman I.M. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv. Skin Wound Care. 2012;25(7):304–314. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sen C.K. Wound healing essentials: let there be oxygen, Wound repair and regeneration. official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(1):1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. The World Allergy Organization journal. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan F., Tanaka M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2017;19(1) doi: 10.3390/ijms19010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davoodi P., Lee L.Y., Xu Q., Sunil V., Sun Y., Soh S., Wang C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018;132:104–138. doi: 10.1016/j.addr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Sun Y., Nan D., Jin H., Qu X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020;81 doi: 10.1016/j.polymertesting.2019.106283. [DOI] [Google Scholar]
- 79.Su K., Tan L., Liu X., Cui Z., Zheng Y., Li B., Han Y., Li Z., Zhu S., Liang Y., Feng X., Wang X., Wu S. Rapid photo-sonotherapy for clinical treatment of bacterial infected bone implants by creating oxygen deficiency using sulfur doping. ACS Nano. 2020;14(2):2077–2089. doi: 10.1021/acsnano.9b08686. [DOI] [PubMed] [Google Scholar]
- 80.Jain A.K., Jain S. Instrumented stabilization in spinal tuberculosis. Int. Orthop. 2012;36(2):285–292. doi: 10.1007/s00264-011-1296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lui Y.S., Sow W.T., Tan L.P., Wu Y., Lai Y., Li H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019;92:19–36. doi: 10.1016/j.actbio.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Y., Zhang J., Song J., Yang J., Du Z., Zhao W., Guo H., Wen C., Li Q., Sui X., Zhang L. A multifunctional pro-healing zwitterionic hydrogel for simultaneous optical monitoring of pH and glucose in diabetic wound treatment. Adv. Funct. Mater. 2019;30(6) doi: 10.1002/adfm.201905493. [DOI] [Google Scholar]
- 83.Mirani B., Pagan E., Currie B., Siddiqui M.A., Hosseinzadeh R., Mostafalu P., Zhang Y.S., Ghahary A., Akbari M. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Advanced healthcare materials. 2017;6(19) doi: 10.1002/adhm.201700718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao W.C., Lilienthal S., Kahn J.S., Riutin M., Sohn Y.S., Nechushtai R., Willner I. pH- and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 2017;8(5):3362–3373. doi: 10.1039/c6sc04770j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu H., Shi X., Wu D., Kahsay Khshen F., Deng L., Dong A., Wang W., Zhang J. Injectable, biodegradable, thermosensitive nanoparticles-aggregated hydrogel with tumor-specific targeting, penetration, and release for efficient postsurgical prevention of tumor recurrence. ACS Appl. Mater. Interfaces. 2019;11(22):19700–19711. doi: 10.1021/acsami.9b01987. [DOI] [PubMed] [Google Scholar]
- 86.Masteiková R., Chalupová Z., Sklubalová Z. Stimuli-sensitive hydrogels in controlled and sustained drug delivery. Medicina. 2003;39(Suppl 2):19–24. [PubMed] [Google Scholar]
- 87.Qu J., Zhao X., Ma P.X., Guo B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017;58:168–180. doi: 10.1016/j.actbio.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 88.McLister A., McHugh J., Cundell J., Davis J. New developments in smart bandage technologies for wound diagnostics. Adv. Mater. 2016;28(27):5732–5737. doi: 10.1002/adma.201504829. [DOI] [PubMed] [Google Scholar]
- 89.Wang T., Zheng Y., Shi Y., Zhao L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv Transl Res. 2019;9(1):227–239. doi: 10.1007/s13346-018-00609-8. [DOI] [PubMed] [Google Scholar]
- 90.Nayak A.K., Hasnain M.S., Nanda S.S., Yi D.K. Hydroxyapatite-alginate based matrices for drug delivery. Curr. Pharmaceut. Des. 2019;25(31):3406–3416. doi: 10.2174/1381612825666190906164003. [DOI] [PubMed] [Google Scholar]
- 91.Liu X., Dou G., Li Z., Wang X., Jin R., Liu Y., Kuang H., Huang X., Yang X., Yang X., Liu S., Wu M., Guo H., Ding F., Xu H., Liu S., Jin Y., Xuan K. Hybrid biomaterial initiates refractory wound healing via inducing transiently heightened inflammatory responses. Adv. Sci. 2022;9(21) doi: 10.1002/advs.202105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng Y., Zhu C., Tao L. Stimuli-responsive multifunctional phenylboronic acid polymers via multicomponent reactions: from synthesis to application. Macromol. Rapid Commun. 2021;42(18) doi: 10.1002/marc.202100022. [DOI] [PubMed] [Google Scholar]
- 93.Perera M.M., Ayres N. Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym. Chem. 2020;11(8):1410–1423. doi: 10.1039/c9py01694e. [DOI] [Google Scholar]
- 94.Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., Xu H., Lei B., Mao C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liang Y., Li M., Yang Y., Qiao L., Xu H., Guo B. pH/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano. 2022;16(2):3194–3207. doi: 10.1021/acsnano.1c11040. [DOI] [PubMed] [Google Scholar]
- 96.Lee J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018;22:27. doi: 10.1186/s40824-018-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang X., Li Z., Yang P., Duan G., Liu X., Gu Z., Li Y. Polyphenol scaffolds in tissue engineering. Mater. Horiz. 2021;8(1):145–167. doi: 10.1039/d0mh01317j. [DOI] [PubMed] [Google Scholar]
- 98.Jia Y., Zhang X., Yang W., Lin C., Tao B., Deng Z., Gao P., Yang Y., Cai K. A pH-responsive hyaluronic acid hydrogel for regulating the inflammation and remodeling of the ECM in diabetic wounds. J. Mater. Chem. B. 2022;10(15):2875–2888. doi: 10.1039/d2tb00064d. [DOI] [PubMed] [Google Scholar]
- 99.Zhu Y.J., Chen F. pH-responsive drug-delivery systems. Chem. Asian J. 2015;10(2):284–305. doi: 10.1002/asia.201402715. [DOI] [PubMed] [Google Scholar]
- 100.He L., Zuo Q., Xie S., Huang Y., Xue W. Intelligent hydrogels for drug delivery system. Recent Pat. Drug Deliv. Formulation. 2011;5(3):265–274. doi: 10.2174/187221111797200533. [DOI] [PubMed] [Google Scholar]
- 101.Klouda L. Thermoresponsive hydrogels in biomedical applications: a seven-year update. Eur. J. Pharm. Biopharm. : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2015;97(Pt B):338–349. doi: 10.1016/j.ejpb.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 102.Chen C.H., Kuo C.Y., Chen S.H., Mao S.H., Chang C.Y., Shalumon K.T., Chen J.P. Thermosensitive injectable hydrogel for simultaneous intraperitoneal delivery of doxorubicin and prevention of peritoneal adhesion. Int. J. Mol. Sci. 2018;19(5) doi: 10.3390/ijms19051373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fan R., Tong A., Li X., Gao X., Mei L., Zhou L., Zhang X., You C., Guo G. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int. J. Nanomed. 2015;10:7291–7305. doi: 10.2147/ijn.S89066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sapino S., Chirio D., Peira E., Abellán Rubio E., Brunella V., Jadhav S.A., Chindamo G., Gallarate M. Ocular drug delivery: a special focus on the thermosensitive approach. Nanomaterials. 2019;9(6) doi: 10.3390/nano9060884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sponchioni M., Capasso Palmiero U., Moscatelli D. Thermo-responsive polymers: applications of smart materials in drug delivery and tissue engineering, Materials science & engineering. C, Materials for biological applications. 2019;102:589–605. doi: 10.1016/j.msec.2019.04.069. [DOI] [PubMed] [Google Scholar]
- 106.Lei N., Gong C., Qian Z., Luo F., Wang C., Wang H., Wei Y. Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale. 2012;4(18):5686–5693. doi: 10.1039/c2nr30731f. [DOI] [PubMed] [Google Scholar]
- 107.Wang W., Song H., Zhang J., Li P., Li C., Wang C., Kong D., Zhao Q. An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J. Contr. Release : official journal of the Controlled Release Society. 2015;203:57–66. doi: 10.1016/j.jconrel.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 108.He D., Zhao A.S., Su H., Zhang Y., Wang Y.N., Luo D., Gao Y., Li J.A., Yang P. An injectable scaffold based on temperature-responsive hydrogel and factor-loaded nanoparticles for application in vascularization in tissue engineering. J. Biomed. Mater. Res., Part A. 2019;107(10):2123–2134. doi: 10.1002/jbm.a.36723. [DOI] [PubMed] [Google Scholar]
- 109.Dai L., Liu R., Hu L.-Q., Wang J.-H., Si C.-L. Self-assembled PEG–carboxymethylcellulose nanoparticles/α-cyclodextrin hydrogels for injectable and thermosensitive drug delivery. RSC Adv. 2017;7(5):2905–2912. doi: 10.1039/c6ra25793c. [DOI] [Google Scholar]
- 110.Asai D., Fukuda T., Morokuma K., Funamoto D., Yamaguchi Y., Mori T., Katayama Y., Shibayama K., Nakashima H. Injectable polypeptide hydrogel depot system for assessment of the immune response-inducing efficacy of sustained antigen release alone. Macromol. Biosci. 2019;19(10) doi: 10.1002/mabi.201900167. [DOI] [PubMed] [Google Scholar]
- 111.Xu Q., He C., Ren K., Xiao C., Chen X. Thermosensitive polypeptide hydrogels as a platform for ROS-triggered cargo release with innate cytoprotective ability under oxidative stress. Advanced healthcare materials. 2016;5(15):1979–1990. doi: 10.1002/adhm.201600292. [DOI] [PubMed] [Google Scholar]
- 112.Singh N.K., Nguyen Q.V., Kim B.S., Lee D.S. Nanostructure controlled sustained delivery of human growth hormone using injectable, biodegradable, pH/temperature responsive nanobiohybrid hydrogel. Nanoscale. 2015;7(7):3043–3054. doi: 10.1039/c4nr05897f. [DOI] [PubMed] [Google Scholar]
- 113.Yoshida Y., Kawahara K., Inamoto K., Mitsumune S., Ichikawa S., Kuzuya A., Ohya Y. Biodegradable injectable polymer systems exhibiting temperature-responsive irreversible sol-to-gel transition by covalent bond formation. ACS Biomater. Sci. Eng. 2017;3(1):56–67. doi: 10.1021/acsbiomaterials.6b00581. [DOI] [PubMed] [Google Scholar]
- 114.Feng Z., Zhao J., Li Y., Xu S., Zhou J., Zhang J., Deng L., Dong A. Temperature-responsive in situ nanoparticle hydrogels based on hydrophilic pendant cyclic ether modified PEG-PCL-PEG. Biomater. Sci. 2016;4(10):1493–1502. doi: 10.1039/c6bm00408c. [DOI] [PubMed] [Google Scholar]
- 115.Phan V.H.G., Thambi T., Kim B.S., Huynh D.P., Lee D.S. Engineering highly swellable dual-responsive protein-based injectable hydrogels: the effects of molecular structure and composition in vivo. Biomater. Sci. 2017;5(11):2285–2294. doi: 10.1039/c7bm00707h. [DOI] [PubMed] [Google Scholar]
- 116.Cao M., Wang Y., Hu X., Gong H., Li R., Cox H., Zhang J., Waigh T.A., Xu H., Lu J.R. Reversible thermoresponsive peptide-PNIPAM hydrogels for controlled drug delivery. Biomacromolecules. 2019;20(9):3601–3610. doi: 10.1021/acs.biomac.9b01009. [DOI] [PubMed] [Google Scholar]
- 117.Liu D., Wu Q., Zhu Y., Liu Y., Xie X., Li S., Lin H., Chen W., Zhu F. Co-delivery of metformin and levofloxacin hydrochloride using biodegradable thermosensitive hydrogel for the treatment of corneal neovascularization. Drug Deliv. 2019;26(1):522–531. doi: 10.1080/10717544.2019.1609623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi K., Xue B., Jia Y., Yuan L., Han R., Yang F., Peng J., Qian Z. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. 2019;12(6):1389–1399. doi: 10.1007/s12274-019-2342-7. [DOI] [Google Scholar]
- 119.Xie W., Gao Q., Guo Z., Wang D., Gao F., Wang X., Wei Y., Zhao L. Injectable and self-healing thermosensitive magnetic hydrogel for asynchronous control release of doxorubicin and docetaxel to treat triple-negative breast cancer. ACS Appl. Mater. Interfaces. 2017;9(39):33660–33673. doi: 10.1021/acsami.7b10699. [DOI] [PubMed] [Google Scholar]
- 120.Gil M.S., Thambi T., Phan V.H.G., Kim S.H., Lee D.S. Injectable hydrogel-incorporated cancer cell-specific cisplatin releasing nanogels for targeted drug delivery. J. Mater. Chem. B. 2017;5(34):7140–7152. doi: 10.1039/c7tb00873b. [DOI] [PubMed] [Google Scholar]
- 121.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006;58(15):1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 122.Roy D., Brooks W.L., Sumerlin B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013;42(17):7214–7243. doi: 10.1039/c3cs35499g. [DOI] [PubMed] [Google Scholar]
- 123.Chen C., Wang Y., Zhang H., Zhang H., Dong W., Sun W., Zhao Y. Responsive and self-healing structural color supramolecular hydrogel patch for diabetic wound treatment. Bioact. Mater. 2022;15:194–202. doi: 10.1016/j.bioactmat.2021.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ho E., Lowman A., Marcolongo M. Synthesis and characterization of an injectable hydrogel with tunable mechanical properties for soft tissue repair. Biomacromolecules. 2006;7(11):3223–3228. doi: 10.1021/bm0602536. [DOI] [PubMed] [Google Scholar]
- 125.Ohya S., Matsuda T. Poly(N-isopropylacrylamide) (PNIPAM)-grafted gelatin as thermoresponsive three-dimensional artificial extracellular matrix: molecular and formulation parameters vs. cell proliferation potential, Journal of biomaterials science. Polymer edition. 2005;16(7):809–827. doi: 10.1163/1568562054255736. [DOI] [PubMed] [Google Scholar]
- 126.Alexander A., Ajazuddin, Khan J., Saraf S., Saraf S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2014;88(3):575–585. doi: 10.1016/j.ejpb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 127.Zhu Y., Hoshi R., Chen S., Yi J., Duan C., Galiano R.D., Zhang H.F., Ameer G.A. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Contr. Release : official journal of the Controlled Release Society. 2016;238:114–122. doi: 10.1016/j.jconrel.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 128.Klouda L., Mikos A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rafael D., Melendres M.M.R., Andrade F., Montero S., Martinez-Trucharte F., Vilar-Hernandez M., Duran-Lara E.F., Schwartz S., Jr., Abasolo I. Thermo-responsive hydrogels for cancer local therapy: challenges and state-of-art. Int. J. Pharm. 2021;606:120954. doi: 10.1016/j.ijpharm.2021.120954. [DOI] [PubMed] [Google Scholar]
- 130.Francesko A., Petkova P., Tzanov T. Hydrogel dressings for advanced wound management. Curr. Med. Chem. 2018;25(41):5782–5797. doi: 10.2174/0929867324666170920161246. [DOI] [PubMed] [Google Scholar]
- 131.Jiang Y., Zhang X., Zhang W., Wang M., Yan L., Wang K., Han L., Lu X. Infant skin friendly adhesive hydrogel patch activated at body temperature for bioelectronics securing and diabetic wound healing. ACS Nano. 2022 doi: 10.1021/acsnano.2c00662. [DOI] [PubMed] [Google Scholar]
- 132.Yang M., He S., Su Z., Yang Z., Liang X., Wu Y. Thermosensitive injectable chitosan/collagen/beta-glycerophosphate composite hydrogels for enhancing wound healing by encapsulating mesenchymal stem cell spheroids. ACS Omega. 2020;5(33):21015–21023. doi: 10.1021/acsomega.0c02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Y., Yang H.Y., Lee D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Contr. Release : official journal of the Controlled Release Society. 2021;330:151–160. doi: 10.1016/j.jconrel.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 134.Yang H.M., Park C.W., Lee K.W. Enhanced surface decontamination of radioactive Cs by self-generated, strippable hydrogels based on reversible cross-linking. J. Hazard Mater. 2019;362:72–81. doi: 10.1016/j.jhazmat.2018.08.064. [DOI] [PubMed] [Google Scholar]
- 135.Lei H., Fan D. Conductive, adaptive, multifunctional hydrogel combined with electrical stimulation for deep wound repair. Chem. Eng. J. 2021;421 doi: 10.1016/j.cej.2021.129578. [DOI] [Google Scholar]
- 136.Li Y., Wang X., Fu Y.N., Wei Y., Zhao L., Tao L. Self-adapting hydrogel to improve the therapeutic effect in wound-healing. ACS Appl. Mater. Interfaces. 2018;10(31):26046–26055. doi: 10.1021/acsami.8b08874. [DOI] [PubMed] [Google Scholar]
- 137.Yang R., Liu X., Ren Y., Xue W., Liu S., Wang P., Zhao M., Xu H., Chi B. Injectable adaptive self-healing hyaluronic acid/poly (γ-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021;127:102–115. doi: 10.1016/j.actbio.2021.03.057. [DOI] [PubMed] [Google Scholar]
- 138.Wang H., Zheng X. Theoretical study of macrocyclic host molecules: from supramolecular recognition to self-assembly. Phys. Chem. Chem. Phys. : Phys. Chem. Chem. Phys. 2022;24(32):19011–19028. doi: 10.1039/d2cp02152h. [DOI] [PubMed] [Google Scholar]
- 139.Zhou W., Duan Z., Zhao J., Fu R., Zhu C., Fan D. Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact. Mater. 2022;17:1–17. doi: 10.1016/j.bioactmat.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao C., Lv Q., Wu W. Application and prospects of hydrogel additive manufacturing. Gels (Basel, Switzerland) 2022;8(5) doi: 10.3390/gels8050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cho H., Blatchley M.R., Duh E.J., Gerecht S. Acellular and cellular approaches to improve diabetic wound healing. Adv. Drug Deliv. Rev. 2019;146:267–288. doi: 10.1016/j.addr.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 142.Sharifzadeh G., Hosseinkhani H. Biomolecule-responsive hydrogels in medicine. Advanced healthcare materials. 2017;6(24) doi: 10.1002/adhm.201700801. [DOI] [PubMed] [Google Scholar]
- 143.Wu Q., Wang L., Yu H., Wang J., Chen Z. Organization of glucose-responsive systems and their properties. Chem. Rev. 2011;111(12):7855–7875. doi: 10.1021/cr200027j. [DOI] [PubMed] [Google Scholar]
- 144.Yang J., Zeng W., Xu P., Fu X., Yu X., Chen L., Leng F., Yu C., Yang Z. Glucose-responsive multifunctional metal-organic drug-loaded hydrogel for diabetic wound healing. Acta Biomater. 2022;140:206–218. doi: 10.1016/j.actbio.2021.11.043. [DOI] [PubMed] [Google Scholar]
- 145.Gao L., Chen Q., Gong T., Liu J., Li C. Recent advancement of imidazolate framework (ZIF-8) based nanoformulations for synergistic tumor therapy. Nanoscale. 2019;11(44):21030–21045. doi: 10.1039/c9nr06558j. [DOI] [PubMed] [Google Scholar]
- 146.Zhang C., Hong S., Liu M.D., Yu W.Y., Zhang M.K., Zhang L., Zeng X., Zhang X.Z. pH-sensitive MOF integrated with glucose oxidase for glucose-responsive insulin delivery. J. Contr. Release : official journal of the Controlled Release Society. 2020;320:159–167. doi: 10.1016/j.jconrel.2020.01.038. [DOI] [PubMed] [Google Scholar]
- 147.Cao J., Li X., Tian H. Metal-organic framework (MOF)-Based drug delivery. Curr. Med. Chem. 2020;27(35):5949–5969. doi: 10.2174/0929867326666190618152518. [DOI] [PubMed] [Google Scholar]
- 148.Zhang S., Ding F., Liu Y., Ren X. Glucose-responsive biomimetic nanoreactor in bacterial cellulose hydrogel for antibacterial and hemostatic therapies. Carbohydr. Polym. 2022;292 doi: 10.1016/j.carbpol.2022.119615. [DOI] [PubMed] [Google Scholar]
- 149.Shi M., Du Z., Qi Y., Li W., Hu H., Lin X., Wang S., Tang Z., Zhou M. Wound microenvironment-responsive glucose consumption and hydrogen peroxide generation synergistic with azithromycin for diabetic wounds healing. Theranostics. 2022;12(6):2658–2673. doi: 10.7150/thno.64244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gu Z., Aimetti A.A., Wang Q., Dang T.T., Zhang Y., Veiseh O., Cheng H., Langer R.S., Anderson D.G. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7(5):4194–4201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ravaine V., Ancla C., Catargi B. Chemically controlled closed-loop insulin delivery. J. Contr. Release : official journal of the Controlled Release Society. 2008;132(1):2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 152.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. : Cailiao Baohu. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang J., Ye Y., Yu J., Kahkoska A.R., Zhang X., Wang C., Sun W., Corder R.D., Chen Z., Khan S.A., Buse J.B., Gu Z. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12(3):2466–2473. doi: 10.1021/acsnano.7b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Y., Wang J., Yu J., Wen D., Kahkoska A.R., Lu Y., Zhang X., Buse J.B., Gu Z. Bioresponsive microneedles with a sheath structure for H(2) O(2) and pH cascade-triggered insulin delivery. Small. 2018;14(14) doi: 10.1002/smll.201704181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu X., Fu X., Yang J., Chen L., Leng F., Yang Z., Yu C. Glucose/ROS cascade-responsive ceria nanozymes for diabetic wound healing. Mater Today Bio. 2022;15 doi: 10.1016/j.mtbio.2022.100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Z., Li Y., Li W., Lian W., Kemell M., Hietala S., Figueiredo P., Li L., Mäkilä E., Ma M., Salonen J., Hirvonen J.T., Liu D., Zhang H., Deng X., Santos H.A. Close-loop dynamic nanohybrids on collagen-ark with in situ gelling transformation capability for biomimetic stage-specific diabetic wound healing. Mater. Horiz. 2019;6(2):385–393. doi: 10.1039/c8mh01145a. [DOI] [Google Scholar]
- 157.Ahmed Saeed Al-Japairai K., Mahmood S., Hamed Almurisi S., Reddy Venugopal J., Rebhi Hilles A., Azmana M., Raman S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020;587 doi: 10.1016/j.ijpharm.2020.119673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang G., Xu B., Zhu J., Zhang Y., Liu T., Song G. Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomedical Physics & Engineering Express. 2019;5(4) doi: 10.1088/2057-1976/ab3202. [DOI] [Google Scholar]
- 159.Ballerstadt R., Evans C., McNichols R., Gowda A. Concanavalin A for in vivo glucose sensing: a biotoxicity review. Biosens. Bioelectron. 2006;22(2):275–284. doi: 10.1016/j.bios.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 160.Wang B., Ma R., Liu G., Liu X., Gao Y., Shen J., An Y., Shi L. Effect of coordination on the glucose-responsiveness of PEG-b-(PAA-co-PAAPBA) micelles. Macromol. Rapid Commun. 2010;31(18):1628–1634. doi: 10.1002/marc.201000164. [DOI] [PubMed] [Google Scholar]
- 161.Guo Q., Wu Z., Zhang X., Sun L., Li C. Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological pH. Soft Matter. 2014;10(6):911–920. doi: 10.1039/c3sm52485j. [DOI] [PubMed] [Google Scholar]
- 162.Nishiyabu R., Kubo Y., James T.D., Fossey J.S. Boronic acid building blocks: tools for self assembly. Chem. Commun. 2011;47(4):1124–1150. doi: 10.1039/c0cc02921a. [DOI] [PubMed] [Google Scholar]
- 163.Lee S.Y., Yang M., Seo J.H., Jeong D.I., Hwang C., Kim H.J., Lee J., Lee K., Park J., Cho H.J. Serially pH-modulated hydrogels based on boronate ester and polydopamine linkages for local cancer therapy. ACS Appl. Mater. Interfaces. 2021;13(2):2189–2203. doi: 10.1021/acsami.0c16199. [DOI] [PubMed] [Google Scholar]
- 164.Xia F., Ge H., Hou Y., Sun T., Chen L., Zhang G., Jiang L.J.A.M. Multiresponsive surfaces change between superhydrophilicity and superhydrophobicity. 2007;19(18):2520–2524. [Google Scholar]
- 165.Meng H., Zheng J., Wen X., Cai Z., Zhang J., Chen T.J.M.r.c. pH-and sugar-induced shape memory hydrogel based on reversible. Phenylboronic Acid–Diol Ester Bonds. 2015;36(6):533–537. doi: 10.1002/marc.201400648. [DOI] [PubMed] [Google Scholar]
- 166.Zhao W., Zhang H., He Q., Li Y., Gu J., Li L., Li H., Shi J. A glucose-responsive controlled release of insulin system based on enzyme multilayers-coated mesoporous silica particles. Chem. Commun. 2011;47(33):9459–9461. doi: 10.1039/c1cc12740c. [DOI] [PubMed] [Google Scholar]
- 167.Xu Z., Liu G., Huang J., Wu J. Novel glucose-responsive antioxidant hybrid hydrogel for enhanced diabetic wound repair. ACS Appl. Mater. Interfaces. 2022;14(6):7680–7689. doi: 10.1021/acsami.1c23461. [DOI] [PubMed] [Google Scholar]
- 168.Moran H.B.T., Turley J.L., Andersson M., Lavelle E.C. Immunomodulatory properties of chitosan polymers. Biomaterials. 2018;184:1–9. doi: 10.1016/j.biomaterials.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 169.Zhu J., Jiang G., Hong W., Zhang Y., Xu B., Song G., Liu T., Hong C., Ruan L. Rapid gelation of oxidized hyaluronic acid and succinyl chitosan for integration with insulin-loaded micelles and epidermal growth factor on diabetic wound healing, Materials science & engineering. C, Materials for biological applications. 2020;117 doi: 10.1016/j.msec.2020.111273. [DOI] [PubMed] [Google Scholar]
- 170.Narayanaswamy R., Torchilin V.P. Hydrogels and their applications in targeted drug delivery. Molecules. 2019;24(3) doi: 10.3390/molecules24030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leu A., Leach J.K. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm. Res. (N. Y.) 2008;25(5):1222–1229. doi: 10.1007/s11095-007-9508-9. [DOI] [PubMed] [Google Scholar]
- 172.Gorustovich A.A., Roether J.A., Boccaccini A.R. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences, Tissue engineering. Part B, Reviews. 2010;16(2):199–207. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 173.Rahaman M.N., Day D.E., Bal B.S., Fu Q., Jung S.B., Bonewald L.F., Tomsia A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miguez-Pacheco V., Hench L.L., Boccaccini A.R. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1–15. doi: 10.1016/j.actbio.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 175.Xu H., Lv F., Zhang Y., Yi Z., Ke Q., Wu C., Liu M., Chang J. Hierarchically micro-patterned nanofibrous scaffolds with a nanosized bio-glass surface for accelerating wound healing. Nanoscale. 2015;7(44):18446–18452. doi: 10.1039/c5nr04802h. [DOI] [PubMed] [Google Scholar]
- 176.Zhu S., Dai Q., Yao L., Wang Z., He Z., Li M., Wang H., Li Q., Gao H., Cao X. Engineered multifunctional nanocomposite hydrogel dressing to promote vascularization and anti-inflammation by sustained releasing of Mg2+ for diabetic wounds. Compos. B Eng. 2022;231 doi: 10.1016/j.compositesb.2021.109569. [DOI] [Google Scholar]
- 177.Zhao L., Niu L., Liang H., Tan H., Liu C., Zhu F. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl. Mater. Interfaces. 2017;9(43):37563–37574. doi: 10.1021/acsami.7b09395. [DOI] [PubMed] [Google Scholar]
- 178.Zhao F., Wu D., Yao D., Guo R., Wang W., Dong A., Kong D., Zhang J. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomater. 2017;64:334–345. doi: 10.1016/j.actbio.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 179.Shi W., Hass B., Kuss M.A., Zhang H., Ryu S., Zhang D., Li T., Li Y.L., Duan B. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr. Polym. 2020;233 doi: 10.1016/j.carbpol.2019.115803. [DOI] [PubMed] [Google Scholar]
- 180.Hou S., Wang X., Park S., Jin X., Ma P.X. Rapid self-integrating, injectable hydrogel for tissue complex regeneration. Advanced healthcare materials. 2015;4(10):1491–1495. doi: 10.1002/adhm.201500093. 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hu D.-N., Ju X.-J., Pu X.-Q., Xie R., Wang W., Liu Z., Chu L.-Y. Injectable temperature/glucose dual-responsive hydrogels for controlled release of insulin. Ind. Eng. Chem. Res. 2021;60(22):8147–8158. doi: 10.1021/acs.iecr.1c01277. [DOI] [Google Scholar]
- 182.Wang C., Lin B., Zhu H., Bi F., Xiao S., Wang L., Gai G., Zhao L. Recent advances in phenylboronic acid-based gels with potential for self-regulated drug delivery. Molecules. 2019;24(6) doi: 10.3390/molecules24061089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Du G., Hathout R.M., Nasr M., Nejadnik M.R., Tu J., Koning R.I., Koster A.J., Slütter B., Kros A., Jiskoot W., Bouwstra J.A., Mönkäre J. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Contr. Release : official journal of the Controlled Release Society. 2017;266:109–118. doi: 10.1016/j.jconrel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 184.Mönkäre J., Pontier M., van Kampen E.E.M., Du G., Leone M., Romeijn S., Nejadnik M.R., O'Mahony C., Slütter B., Jiskoot W., Bouwstra J.A. Development of PLGA nanoparticle loaded dissolving microneedles and comparison with hollow microneedles in intradermal vaccine delivery. Eur. J. Pharm. Biopharm. : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2018;129:111–121. doi: 10.1016/j.ejpb.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 185.Rege N.K., Phillips N.F.B., Weiss M.A. Development of glucose-responsive 'smart' insulin systems. Curr. Opin. Endocrinol. Diabetes Obes. 2017;24(4):267–278. doi: 10.1097/med.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang J., Wang Z., Yu J., Kahkoska A.R., Buse J.B., Gu Z. Glucose-responsive insulin and delivery systems: innovation and translation. Adv. Mater. 2020;32(13) doi: 10.1002/adma.201902004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Matsumoto A., Tanaka M., Matsumoto H., Ochi K., Moro-Oka Y., Kuwata H., Yamada H., Shirakawa I., Miyazawa T., Ishii H., Kataoka K., Ogawa Y., Miyahara Y., Suganami T. Synthetic "smart gel" provides glucose-responsive insulin delivery in diabetic mice. Sci. Adv. 2017;3(11) doi: 10.1126/sciadv.aaq0723. eaaq0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen X., Wang L., Yu H., Li C., Feng J., Haq F., Khan A., Khan R.U. Preparation, properties and challenges of the microneedles-based insulin delivery system. J. Contr. Release : official journal of the Controlled Release Society. 2018;288:173–188. doi: 10.1016/j.jconrel.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 189.Yang S., Wu F., Liu J., Fan G., Welsh W., Zhu H., Jin T. Phase-transition microneedle patches for efficient and accurate transdermal delivery of insulin. Adv. Funct. Mater. 2015;25(29):4633–4641. doi: 10.1002/adfm.201500554. [DOI] [Google Scholar]
- 190.Zhu H., Mah Jian Qiang J., Wang C.G., Chan C.Y., Zhu Q., Ye E., Li Z., Loh X.J. Flexible polymeric patch based nanotherapeutics against non-cancer therapy. Bioact. Mater. 2022;18:471–491. doi: 10.1016/j.bioactmat.2022.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Guo Z., Liu H., Shi Z., Lin L., Li Y., Wang M., Pan G., Lei Y., Xue L. Responsive hydrogel-based microneedle dressing for diabetic wound healing. J. Mater. Chem. B. 2022;10(18):3501–3511. doi: 10.1039/d2tb00126h. [DOI] [PubMed] [Google Scholar]
- 192.Tang R.Z., Liu Z.Z., Gu S.S., Liu X.Q. Multiple local therapeutics based on nano-hydrogel composites in breast cancer treatment. J. Mater. Chem. B. 2021;9(6):1521–1535. doi: 10.1039/d0tb02737e. [DOI] [PubMed] [Google Scholar]
- 193.Yang X.-X., Chen Y.-L., Feng P.-F., Wang C.-C., Li X.-K., Liu L.-L., Tang Y. Hierarchically porous MOF-based microneedles for glucose-responsive infected diabetic wound treatment. Mater. Chem. Front. 2022;6(6):680–688. doi: 10.1039/d1qm01512e. [DOI] [Google Scholar]
- 194.Jung J.H., Jin S.G. Microneedle for transdermal drug delivery: current trends and fabrication. Journal of pharmaceutical investigation. 2021;51(5):503–517. doi: 10.1007/s40005-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290(16):2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Donnelly L.A., Morris A.D., Frier B.M., Ellis J.D., Donnan P.T., Durrant R., Band M.M., Reekie G., Leese G.P. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet. Med. : a journal of the British Diabetic Association. 2005;22(6):749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 197.Zhou K., Wang Y., Huang X., Luby-Phelps K., Sumer B.D., Gao J. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem. 2011;50(27):6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Luo F.-Q., Chen G., Xu W., Zhou D., Li J.-X., Huang Y.-C., Lin R., Gu Z., Du J.-Z. Microneedle-array patch with pH-sensitive formulation for glucose-responsive insulin delivery. Nano Res. 2021;14(8):2689–2696. doi: 10.1007/s12274-020-3273-z. [DOI] [Google Scholar]
- 199.D'Autréaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 200.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 201.Krause K.H. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2007;42(4):256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 202.Paravicini T.M., Touyz R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006;71(2):247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 203.Ye H., Zhou Y., Liu X., Chen Y., Duan S., Zhu R., Liu Y., Yin L. Recent advances on reactive oxygen species-responsive delivery and diagnosis system. Biomacromolecules. 2019;20(7):2441–2463. doi: 10.1021/acs.biomac.9b00628. [DOI] [PubMed] [Google Scholar]
- 204.Xu Z., Han S., Gu Z., Wu J. Advances and impact of antioxidant hydrogel in chronic wound healing. Advanced healthcare materials. 2020;9(5) doi: 10.1002/adhm.201901502. [DOI] [PubMed] [Google Scholar]
- 205.Abudula T., Colombani T., Alade T., Bencherif S.A., Memić A. Injectable lignin-co-gelatin cryogels with antioxidant and antibacterial properties for biomedical applications. Biomacromolecules. 2021;22(10):4110–4121. doi: 10.1021/acs.biomac.1c00575. [DOI] [PubMed] [Google Scholar]
- 206.Qu J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 207.Zhao H., Huang J., Li Y., Lv X., Zhou H., Wang H., Xu Y., Wang C., Wang J., Liu Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120286. [DOI] [PubMed] [Google Scholar]
- 208.Chengwei W., Yihao L., Xiaoxiao Y., Wentao L., Xianhao Z., Ya R., Changru Z., Han Y., Weiqing K., Jinwu W., Haoyi N. In-situ forming hydrogel incorporated with reactive oxygen species responsive and antibacterial properties for diabetic infected chronic wound healing. Chem. Eng. J. 2022;450 doi: 10.1016/j.cej.2022.138077. [DOI] [Google Scholar]
- 209.Talebian S., Mehrali M., Taebnia N., Pennisi C.P., Kadumudi F.B., Foroughi J., Hasany M., Nikkhah M., Akbari M., Orive G., Dolatshahi-Pirouz A. Self-healing hydrogels: the next paradigm shift in tissue engineering? Adv. Sci. 2019;6(16) doi: 10.1002/advs.201801664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hu C., Zhang F., Long L., Kong Q., Luo R., Wang Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Contr. Release : official journal of the Controlled Release Society. 2020;324:204–217. doi: 10.1016/j.jconrel.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 211.Zhang Y., Tao L., Li S., Wei Y. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules. 2011;12(8):2894–2901. doi: 10.1021/bm200423f. [DOI] [PubMed] [Google Scholar]
- 212.Wang Y., Wu Y., Long L., Yang L., Fu D., Hu C., Kong Q., Wang Y. Inflammation-responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment. ACS Appl. Mater. Interfaces. 2021;13(28):33584–33599. doi: 10.1021/acsami.1c09889. [DOI] [PubMed] [Google Scholar]
- 213.Xu Z., Liu G., Liu P., Hu Y., Chen Y., Fang Y., Sun G., Huang H., Wu J. Hyaluronic acid-based glucose-responsive antioxidant hydrogel platform for enhanced diabetic wound repair. Acta Biomater. 2022;147:147–157. doi: 10.1016/j.actbio.2022.05.047. [DOI] [PubMed] [Google Scholar]
- 214.Wu Y., Wang Y., Long L., Hu C., Kong Q., Wang Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Contr. Release : official journal of the Controlled Release Society. 2022;341:147–165. doi: 10.1016/j.jconrel.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 215.Ravanti L., Kähäri V.M. Matrix metalloproteinases in wound repair (review) Int. J. Mol. Med. 2000;6(4):391–407. [PubMed] [Google Scholar]
- 216.Chang M., Nguyen T.T. Strategy for treatment of infected diabetic foot ulcers. Acc. Chem. Res. 2021;54(5):1080–1093. doi: 10.1021/acs.accounts.0c00864. [DOI] [PubMed] [Google Scholar]
- 217.Gao M., Nguyen T.T., Suckow M.A., Wolter W.R., Gooyit M., Mobashery S., Chang M. Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc. Natl. Acad. Sci. U. S. A. 2015;112(49):15226–15231. doi: 10.1073/pnas.1517847112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li N., Luo H.C., Ren M., Zhang L.M., Wang W., Pan C.L., Yang L.Q., Lao G.J., Deng J.J., Mai K.J., Sun K., Yang C., Yan L. Efficiency and safety of β-CD-(D(3))(7) as siRNA carrier for decreasing matrix metalloproteinase-9 expression and improving wound healing in diabetic rats. ACS Appl. Mater. Interfaces. 2017;9(20):17417–17426. doi: 10.1021/acsami.7b02809. [DOI] [PubMed] [Google Scholar]
- 219.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Turk B.E., Huang L.L., Piro E.T., Cantley L.C. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat. Biotechnol. 2001;19(7):661–667. doi: 10.1038/90273. [DOI] [PubMed] [Google Scholar]
- 221.Tu Y., Zhu L.J.S.P.N. 2016. Matrix Metalloproteinase-Sensitive Nanocarriers; pp. 83–116. [Google Scholar]
- 222.Dai Z., Yao Q., Zhu L. MMP2-Sensitive PEG-lipid copolymers: a new type of tumor-targeted P-glycoprotein inhibitor. ACS Appl. Mater. Interfaces. 2016;8(20):12661–12673. doi: 10.1021/acsami.6b03064. [DOI] [PubMed] [Google Scholar]
- 223.Liu J., Chen Z., Wang J., Li R., Li T., Chang M., Yan F., Wang Y. Encapsulation of curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl. Mater. Interfaces. 2018;10(19):16315–16326. doi: 10.1021/acsami.8b03868. [DOI] [PubMed] [Google Scholar]
- 224.Shao Z., Yin T., Jiang J., He Y., Xiang T., Zhou S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2022;20:561–573. doi: 10.1016/j.bioactmat.2022.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sonamuthu J., Cai Y., Liu H., Kasim M.S.M., Vasanthakumar V.R., Pandi B., Wang H., Yao J. MMP-9 responsive dipeptide-tempted natural protein hydrogel-based wound dressings for accelerated healing action of infected diabetic wound. Int. J. Biol. Macromol. 2020;153:1058–1069. doi: 10.1016/j.ijbiomac.2019.10.236. [DOI] [PubMed] [Google Scholar]
- 226.Guo C., Wu Y., Li W., Wang Y., Kong Q. Development of a microenvironment-responsive hydrogel promoting chronically infected diabetic wound healing through sequential hemostatic, antibacterial, and angiogenic activities. ACS Appl. Mater. Interfaces. 2022;14(27):30480–30492. doi: 10.1021/acsami.2c02725. [DOI] [PubMed] [Google Scholar]
- 227.Wang S., Zheng H., Zhou L., Cheng F., Liu Z., Zhang H., Wang L., Zhang Q. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett. 2020;20(7):5149–5158. doi: 10.1021/acs.nanolett.0c01371. [DOI] [PubMed] [Google Scholar]
- 228.Wei Q., Arami H., Santos H.A., Zhang H., Li Y., He J., Zhong D., Ling D., Zhou M. Intraoperative assessment and photothermal ablation of the tumor margins using gold nanoparticles. Adv. Sci. 2021;8(5) doi: 10.1002/advs.202002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang T., Li G., Miao Y., Lu J., Gong N., Zhang Y., Sun Y., He Y., Peng M., Liu X., Liang X.J., Fan H. Magnetothermal regulation of in vivo protein corona formation on magnetic nanoparticles for improved cancer nanotherapy. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121021. [DOI] [PubMed] [Google Scholar]
- 230.Lü B., Chen Y., Li P., Wang B., Müllen K., Yin M. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat. Commun. 2019;10(1):767. doi: 10.1038/s41467-019-08434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yang X., Zhang C., Deng D., Gu Y., Wang H., Zhong Q. Multiple stimuli-responsive MXene-based hydrogel as intelligent drug delivery carriers for deep chronic wound healing. Small. 2022;18(5) doi: 10.1002/smll.202104368. [DOI] [PubMed] [Google Scholar]
- 232.Guo S., Dipietro L.A. Factors affecting wound healing. J. Dent. Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Burgess J.L., Wyant W.A., Abdo Abujamra B., Kirsner R.S., Jozic I. Diabetic wound-healing science. Medicina. 2021;57(10) doi: 10.3390/medicina57101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Knipe J.M., Peppas N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regenerative biomaterials. 2014;1(1):57–65. doi: 10.1093/rb/rbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nair L.S., Laurencin C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007;32(8–9):762–798. doi: 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- 236.Guo B., Finne-Wistrand A., Albertsson A.-C. Degradable and electroactive hydrogels with tunable electrical conductivity and swelling behavior. Chem. Mater. 2011;23(5):1254–1262. doi: 10.1021/cm103498s. [DOI] [Google Scholar]
- 237.Liang Y., He J., Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021 doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 238.Veiseh O., Tang B.C., Whitehead K.A., Anderson D.G., Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug Discov. 2015;14(1):45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mo R., Jiang T., Di J., Tai W., Gu Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014;43(10):3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 240.Schulman R.C., Moshier E.L., Rho L., Casey M.F., Godbold J.H., Mechanick J.I. Association of glycemic control parameters with clinical outcomes in chronic critical illness. Endocr. Pract. : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2014;20(9):884–893. doi: 10.4158/ep13324.Or. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.