Abstract
The prevalence of diabetic retinopathy (DR), and associated morbidity is high in the Asia-Pacific region. Emerging evidence suggests a potential role for fenofibrate in the prevention of progression of DR, especially in patients with cardiovascular risk, and pre-existing mild-to-moderate DR. Fenofibrate has also been found to reduce maculopathy, and the need for laser treatment in these patients. Considering these benefits of fenofibrate, a group of experts from the fields of endocrinology and ophthalmology convened in May 2017, to discuss on the the mechanism of action, and clinical efficacy of fenofibrate in DR. The findings from key clinical studies on fenofibrate in DR were reviewed by the experts, and consensus statements were derived to define the role of fenofibrate in the prevention and treatment of DR. The statements were rated based on the GRADE criteria. An algorithm was also developed for the screening and treatment of DR in patients with type 2 diabetes (T2D), and the place of fenofibrate was defined in the algorithm. The expert recommendations, and the algorithm provided in this review will serve as a guide to the clinicians to reconsider the adjunctive use of fenofibrate for preventing the progression of DR in selected T2D patients.
Keywords: diabetic retinopathy, fenofibrate, consensus recommendations
INTRODUCTION
Diabetic retinopathy (DR) is a major cause of blindness worldwide and a complication of diabetes mellitus (DM). It was reported that DR accounted for 1.25% of moderate to severe visual impairment and 1.07% of blindness[1]. The prevalence of DR-related blindness and vision impairment was higher in the elderly population worldwide. It was estimated that the prevalence of any DR among diabetic patients was at 34.6%, whereas diabetic macular oedema was 6.8%, while the vision impairment in DR was 10.2%[2]. In separate regional studies conducted in the Asia-Pacific region, 1 in 10 patients with diabetes were noted to have DR[3]–[5]. In the Singapore Malay Eye study, the overall prevalence rates of DR, diabetic macular edema (DME) and vision threatening diabetic retinopathy (VTDR) among Malay adults with diabetes in Singapore were noted to be 35%, 5.7%, and 9.0%, respectively[5]. The prevalence of DR reported in Selangor, Malaysia is 8.9%[6]. In addition to these high prevalence rates, the morbidity associated with DR is high. DR has been recognised as an important cause of blindness[1]. DR is reported to be one of the chronic complications of diabetes and a leading cause of blindness in the industrialized world[7]. Considering these estimates, the current clinical need of the hour should be directed towards early detection, prevention and treatment of DR.
Although there are several proven strategies for the prevention and treatment of DR, emerging evidence suggests a potential role of fenofibrate for this indication. A recent systematic review of 13 clinical studies suggested a significant role of early initiation of fenofibrate therapy for the prevention of microvascular complications in patients with type 2 diabetes (T2D). The review reported a significant reduction in the rate of progression of early DR by about 30%–40% with fenofibrate therapy in patients with T2D and pre-existing mild DR at baseline[8]. These findings suggest the need for a deeper understanding into the potential mechanisms of protection by fenofibrate in patients with DR.
Mechanism of Action of Fenofibrate in Diabetic Retinopathy
Fenofibrate is a derivative of fenofibric acid, clinically proven for its lipid-modifying effects in the treatment of dyslipidaemia. The lipid-modifying actions of fenofibrate are mediated by the activation of peroxisome proliferator activated receptor type-alpha (PPAR-α). Activation of PPAR-α by fenofibrate results in the activation of lipoprotein lipase, which increases lipolysis and eliminates triglycerides from the plasma. Furthermore, this may lead to a reduction in very low-density lipoproteins (VLDLs) and low-density lipoproteins (LDLs) and an increase in high-density lipoproteins (HDLs)[9]. Although several mechanisms have been proposed to explain the actions of fenofibrate in DR, they may be broadly categorised into lipid-related and non-lipid-related mechanisms[10].
Lipid-Related Actions of Fenofibrate in Diabetic Retinopathy
Upregulation of apolipoprotein A1
Apolipoprotein A1 (apoA1) is an independent protective factor in DR. Lower levels of apoA1 have been found to be associated with increased progression to proliferative DR in patients with long-term T2D[11]. Furthermore, in a recent cross-sectional study in patients with diabetes, a significant negative correlation has been reported between apoA1 levels and severity of retinopathy[12]. An in-depth review of the underlying mechanisms reveals that apoA1 is localised at several locations throughout the retina, including the neural retina and retinal pigment epithelium (RPE), and it serves as an important factor for the intraretinal reverse transport of lipids, thus preventing deposition of oxidised lipids in the retina[13]. In addition, it is a potent scavenger of reactive oxygen species[14]. Thus, apoA1 may have a protective role by preventing the retina from oxidative stress and lipotoxicity. Fenofibrate is known to upregulate the expression of genes coding for apoA1 in the liver, macrophages and fibroblast cells[15]; hence, it may have a protective effect in DR.
Lowering of lipoprotein-associated phospholipase A2
Lipoprotein-associated phospholipase A2 (LpPLA2) has been noted to have a prominent inflammatory potential[16]. It has also been reported to liberate arachidonic acid, the substrate for prostaglandins, which exerts a pro-angiogenic effect by inducing the production of vascular endothelial growth factor (VEGF) in vascular endothelial cells. Inhibition of PLA2 has been shown to block the production of pro-angiogenic prostaglandins and decrease retinal neovascularisation[17]. Fenofibrate has been found to lower LpPLA2[18], which may be responsible for its anti-inflammatory and anti-angiogenic effects in DR.
Non-Lipid-Related Actions of Fenofibrate in Diabetic Retinopathy
Anti-apoptotic activity
Fenofibrate has been found to prevent apoptotic cell death in human retinal endothelial cells through PPAR-α-independent and adenosine monophosphate (AMP)-activated protein kinase (AMPK)-dependent pathways[19]. Fenofibrate has also been found to downregulate stress-mediated signalling and induce survival pathways in RPE cells[20].
Antioxidant and anti-inflammatory activity
Several actions have been proposed to contribute to the antioxidant and anti-inflammatory activities of fenofibrate in DR. Fenofibrate has been found to inhibit the activity of nuclear factor (NF)-κB and significantly downregulate the expression of proinflammatory cytokines, resulting in anti-inflammatory effects in DME[21]. A part of the anti-inflammatory effects of fenofibrate may also be due to its ability to improve adipocytokines in patients with elevated triglyceride levels[22]. In experimental models, adiponectin has been found to protect against retinal vessel injury by modulating tumour necrosis factor-alpha (TNF-α) inflammatory responses[23].
Protection against blood retinal barrier breakdown
Breakdown of the blood retinal barrier (BRB), due to disruption of tight junctions with subsequent leakage, is the main factor implicated in DME[24]. Fenofibrate has been found to prevent the disorganisation of tight junction proteins and subsequent hyperpermeability in RPE cells by suppression of AMPK activation[25].
Reduction in retinal vascular permeability
Fenofibric acid downregulates the overexpression of basement membrane components (fibronectin and collagen IV) responsible for the increase in the permeability in RPE cells[26].
Anti-angiogenic effect
Fenofibrate may have a potential role in the inhibition of angiogenesis and neovascularisation by inhibiting the upregulation of VEGF in RPE cells[27].
Improvement in endothelial function
Fenofibric acid has been found to induce endothelium-dependent vasodilation by stimulating endothelial nitric oxide production in retinal arterioles in experimental models, suggesting a potential role in improving the retinal microvasculature in DR[28]. The effects of fenofibrate on various retinal components such as RPE, basement membrane, neuroretina and endothelial cells in experimental and human models have been summarised in Figure 1[19]–[21],[25]–[29]. To date, the use of intravitreal injections of anti-VEGF are accepted as standard of care for progression of DR. However, to best of our knowledge and review of literature, the efficacy and safety are not well documented for some patients. Considering these potential protective effects of fenofibrate in DR, a group of experts from the fields of endocrinology and ophthalmology convened to explore the alternative therapies of fenofibrate as an alternative intervention for progression DR. This review will assess the efficacy and safety of fenofibrate for patients with DR and develop an algorithm for the screening and treatment of DR in patients with T2D and define the place of fenofibrate in the algorithm.
Figure 1. Effects of fenofibrate in diabetic retinopathy.
METHODOLOGY
A total of 11 experts, including four endocrinologists, six ophthalmologists and one drug controller authority, formed the working group for the meeting. The experts summarised evidence published by primary trials and grey literature. The following databases was searched from the inception to the present: PubMed, EMBASE, CINAHI, Web of Science, CENTRAL, WHO trial registry and all relevant articles from the reference lists. This review only included participants who were clinically diagnosed with DR without any restriction on ethic group, gender and age. This review included studies that used fenofibrate as an intervention alone in any forms against control treatment except fenofibrate if any. The review included reported outcomes such as the progression of DR, vision loss, development of DME, retina related complications, quality of life, and any adverse events. After identifying all the eligibility studies, the clinical efficacy of fenofibrate in DR was presented and reviewed. A set of consensus statements was derived to define the role of fenofibrate in the prevention and treatment of DR. These statements were graded by the experts based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The GRADE system classifies the quality of evidence as high (grade A), moderate (grade B) or low (grade C) based on factors such as study design, consistency of the results and the directness of the evidence. The recommendations are classified by the GRADE approach as strong (grade 1) or weak (grade 2) based on the balance between benefits, risks, burdens and to some extent cost, and the degree of confidence in the estimates of benefits, risks and burdens[30]. The rating of the statements was followed by the development of an algorithm by the expert panel for the screening and treatment of DR in patients with T2D, along with identifying the place of fenofibrate in the algorithm.
Clinical Efficacy of Fenofibrate in Diabetic Retinopathy
Four studies were presented and reviewed by the experts during the meeting: The action to control cardiovascular risk in diabetes (ACCORD)-Eye lipid trial, fenofibrate intervention and event lowering in diabetes (FIELD) trial, including the FIELD-ophthalmology sub-study, retrospective cohort study by Morgan et al[31] and the latest ACCORD follow-on (ACCORDION) study.
ACCORD-Eye Lipid Trial
Briefly, ACCORD was a randomised, multicentre, double 2×2 factorial design trial conducted to determine if intensive control of the three important risk factors, that is, hyperglycaemia, dyslipidaemia and elevated blood pressure (BP), can help reduce cardiovascular disease (CVD) event rates in high-risk patients with T2D. All the eligible participants were enrolled into the ACCORD glycaemia trial (n=10 251) and were randomised to receive either intensive [glycated haemoglobin (HbA1c) <6.0%] or standard glycaemic control (HbA1c 7.0%–7.9%)[32]. Of the 10 251 randomised participants, 5518 participants with moderate dyslipidaemia were further randomised in a double-blind manner in the ACCORD lipid trial to receive either fenofibrate 160 mg/d (dose adjusted based on renal function) or matching placebo, along with open-label simvastatin therapy titrated to 40 mg/d[32]–[33]. The remaining 4733 participants were enrolled into the ACCORD BP trial and were randomised to either intensive or standard BP control groups[32].
The ACCORD-Eye study was a prospective sub-study of ACCORD to assess the effects of the three treatment plans evaluated in ACCORD on the progression of DR. All ACCORD participants without proliferative DR treated with laser and/or vitrectomy at baseline and who were enrolled after the initiation of the ACCORD-Eye study were considered eligible[34]–[35]. The primary endpoint of ACCORD-Eye was progression of DR by ≥3 steps on Early Treatment Diabetic Retinopathy Study (ETDRS) scale or progression to proliferative DR requiring photocoagulation and/or vitrectomy. The secondary outcomes included 1) change in visual acuity at 4y versus baseline (including moderate vision loss, legal blindness or severe vision loss), 2) rate of cataract extraction, 3) rate of photocoagulation and/or vitrectomy, 4) development or progression of macular oedema[34].
ACCORD-eye lipid trial: prevention of progression of diabetic retinopathy
Baseline and 4-year follow-up data were available for 2856 participants in the ACCORD-Eye trial. Out of the 2856 patients in the ACCORD-Eye trial, 1593 patients were in the ACCORD-Eye lipid trial, with 806 participants in the fenofibrate group and 787 in the placebo group. Close to half of the participants in both the treatment arms in the ACCORD-Eye lipid trial had non-proliferative diabetic retinopathy (NPDR) at baseline.
The primary outcome of the rate of progression of DR at 4y was significantly lower in the fenofibrate group (52 of 806; 6.5%) versus the placebo group (80 of 787; 10.2%) [adjusted odds ratio (OR), 0.60; 95% confidence interval (CI), 0.42–0.87; P=0.006]. In the subgroup analysis of the primary endpoint, there was no significant interaction between the effect of fenofibrate in reducing the progression of DR and baseline lipid levels; the benefit of fenofibrate was noted to be independent of baseline HDL-cholesterol and triglyceride levels. Although there was a significant nominal interaction between treatment effect and baseline LDL-cholesterol levels (P=0.04), this was not significant after adjustment for multiple comparisons. There was also no significant interaction between the effect of fenofibrate in reducing the progression of DR and the duration of diabetes or intensity of glycaemic control. However, there was a significant interaction between the effect of fenofibrate in reducing the progression of DR and retinopathy status at baseline; the benefit of fenofibrate was significantly higher in patients with DR at baseline than in patients without DR at baseline [relative risk reduction (RRR): 51% vs 7.5%, respectively; P=0.03][35]. There was no significant difference between the fenofibrate group and the placebo group with respect to any of the secondary outcomes, including moderate vision loss, changes in visual acuity, changes in macular oedema or the rates of cataract surgery[36]–[37].
FIELD Trial
The multicentre, randomised, double-blind, placebo-controlled FIELD trial evaluated the effects of fenofibrate versus placebo on morbidity and mortality from coronary heart disease (CHD) in patients with T2D. Eligibility criteria included age between 50 and 75y, T2D, risk of CHD, initial plasma total cholesterol (TC) concentration of 3.0–6.5 mmol/L plus a TC/HDL-cholesterol ratio of 4.0 or more, or a plasma triglyceride concentration >1.0 mmol/L and no requirement of lipid-modifying treatment at study entry[31],[38]–[39]. After a 16-week run-in period, 9795 participants were randomly assigned to receive micronized fenofibrate 200 mg once daily or matching placebo with a background of usual care in the final active study period. Patients were followed up at every 4–6mo for a period of 5y. The primary endpoint was the combined incidence of first non-fatal myocardial infarction (MI) or CHD death. The occurrence of laser treatment for any retinopathy, macular oedema or proliferative retinopathy without macular involvement was a prespecified tertiary endpoint of the study[31],[38]–[39].
FIELD tertiary endpoint results: reduction in the need for laser therapy
In the FIELD study, fenofibrate therapy was associated with a significantly lesser requirement for first laser treatment for any retinopathy versus placebo (3.4% vs 4.9%, respectively; hazard ratio (HR): 0.69, 95%CI: 0.56–0.84; P=0.0002). This correlated to an absolute risk reduction (ARR) of 1.5% (0.7–2.3). The need for first laser therapy for any maculopathy and proliferative retinopathy was also significantly low with fenofibrate therapy compared with placebo (ARR: 1.1% and 0.7%; P=0.002 and 0.015, respectively). Patients with a history of retinopathy had a larger ARR than patients without a history of retinopathy [number needed to treat (NNT): 17 vs 90, respectively]. The cumulative laser treatment events were also lower for any retinopathy in the fenofibrate group versus the placebo group (3.4% vs 4.9%, respectively; RRR: 37%, 95%CI: 19–51; P=0.0003). The RRR in the need for laser treatments with fenofibrate treatment was 36% in those with any maculopathy (95%CI: 14–52; P=0.003) and 38% in those with proliferative retinopathy (95%CI: 11–57; P=0.009)[31].
FIELD-Ophthalmology Sub-study
Patients at 22 of the 63 FIELD study sites participated in this ophthalmology sub-study. The objective of this sub-study was to determine the effect of fenofibrate therapy on the cumulative incidence of DR. A total of 1012 participants without any evidence of proliferative DR, severe NPDR or clinically significant macular oedema or a history of/indication for laser therapy at screening were recruited in this sub-study[31].
Retinopathy was assessed at baseline, 2y, 5y and at the end of study. Retinopathy and macular oedema were graded based on the ETDRS criteria. The primary endpoint was progression of DR, defined as at least a 2-step increase in ETDRS grade after 2y or more of follow-up. The occurrence or progression of macular oedema and of hard exudates, and the occurrence of laser treatment, were some of the secondary end points of the study. The development of significant retinal pathology, including any of a 2-step progression of retinopathy grade, new macular oedema or laser treatment was a post-hoc exploratory composite endpoint of the study[31].
Reduction in progression of diabetic retinopathy, need for laser therapy and incidence of diabetic macular edema
The rate of progression of DR did not differ significantly between the fenofibrate and placebo groups. However, this was significantly lower with fenofibrate therapy versus placebo in patients with pre-existing DR (3.1% vs 4.6%, respectively; P=0.004). Furthermore, there was a significant difference in the treatment effect within the two subgroups with and without pre-existing retinopathy (P=0.019). These findings are consistent with the results from the ACCORD-Eye lipid trial. Significantly fewer patients on fenofibrate therapy versus placebo in the FIELD ophthalmology sub-study received one or more laser treatments (5 vs 23 patients, respectively; HR: 0.21, 95%CI: 0.08–0.54; P=0.0004). This is also in line with the ACCORD-Eye results. Additionally, in the FIELD ophthalmology sub-study, the incidence of macular oedema was low in patients treated with fenofibrate compared with those treated with placebo. The fenofibrate group was also associated with a lower risk of composite endpoint than the placebo group (HR: 0.66, 95%CI: 0.47–0.94; P=0.022)[31].
Retrospective Cohort Study
A retrospective matched cohort study was conducted by Morgan et al[31] to compare the progression of DR in individuals with T2D treated with fibrates with that in matched non-exposed control subjects. Fenofibrate was used by 44.5% of the study patients. Fenofibrate was the fibrate with the longest duration of use (mean duration, 2.8y). Data for the study were collected from the United Kingdom Clinical Practice Research Datalink, derived from nearly 700 primary care practices throughout the United Kingdom[40].
Patients with T2D and a history of fibrate exposure but without evidence of DR before the date of first fibrate exposure or diabetes presentation (n=5038) were considered eligible for the fibrate-exposed cohort. More than half (3176; 63%) of the subjects were randomly matched to one non-exposed control subject; of these subjects, 2599 (81.8%) were matched without any missing BP or HbA1c values. The primary endpoint was first-recorded diagnosis of DR and the secondary outcome was all-cause mortality or first DR. The mean follow-ups for fibrate-treated participants and matched control subjects were 5.1 and 5.0y, respectively[40].
Reduction in the risk of development of new diabetic retinopathy
Newly detected DR was noted in 489 (15.4%) patients exposed to fibrates versus 569 (17.9%) patients not exposed to fibrates, corresponding to a significant reduction in newly detected DR (33.4 vs 40.4 events/1000 person-years, respectively; P=0.002) and in death or DR (50.6 vs 60.2 events/1000 person-years, respectively; P<0.001). The study concluded that treatment with fibrates may be associated with reduced progression to newly diagnosed DR in individuals with T2D[40].
ACCORDION Study
Surviving participants from the ACCORD main study who had fundus photographs at baseline were invited to participate in the ACCORDION study and have additional photographs taken 8y after initial ACCORD randomisation (i.e., 3–5y after the end of the ACCORD main trial). The objective of the ACCORDION-Eye study was to assess the effects of the treatment plans evaluated in ACCORD on the progression of DR during 8y of follow-up. The primary outcome was progression of DR by ≥3 steps on the ETDRS scale based on fundus photographs at year 8 versus baseline. The secondary outcome was the effect of these medical strategies on moderate vision loss at year 8 versus baseline.
Effects after discontinuation of fenofibrate therapy
The data of 1310 participants were analysed in the ACCORDION-Eye study: 1268 patients had eye examinations at baseline, 4y and 8y; an additional 42 participants had only baseline examinations and returned for the examination at year 8. Of these 1310 participants, 762 participants were in the lipid trial. The rate of progression of DR at year 8 was 11.8% in the fenofibrate group versus 10.2% in the placebo group (adjusted OR: 1.13; 95%CI: 0.71–1.79; P=0.60). Although the effect of fenofibrate on DR in the ACCORD-Eye study was beneficial, it did not persist for long in the ACCORDION study, indicating that continued use of fenofibrate is needed to maintain the clinical benefit. Furthermore, there were no significant interactions between fenofibrate treatment and any of the prespecified characteristics in subgroup analyses in the ACCORDION-Eye lipid trial, except for baseline retinopathy (nominal P=0.01)[40].
Guideline Views
In addition to the literature support, several endocrinology and ophthalmology guidelines support a role of fenofibrate in targeting microvascular complications and reducing the progression of DR in patients with T2D[41]–[46].
Consensus Statements
The consensus statements derived by the expert panel on the role of fenofibrate in the prevention and treatment of DR, based on the available literature, along with the rating of the statements based on the GRADE criteria, have been shown in Table 1.
Table 1. Evidence-based consensus statements with rating of the recommendations for defining the role of fenofibrate in the prevention and treatment of DR.
| Consensus statements | References in support | Rating of the recommendation |
| Fenofibrate is effective in reducing the risk of progression of DR in patients with T2D with high cardiovascular risk and pre-existing mild to moderate NPDR | Chew 2011[35]; Keech 2007[39] | 1A |
| Fenofibrate is effective in reducing the need for first laser treatment for DR and maculopathy in patients with T2D with high cardiovascular risk and pre-existing mild-to-moderate NPDR | Keech 2007[39] | 1A |
| Fenofibrate is effective in reducing the risk of development of new DR | Morgan 2013[31] | No strong evidence/prospective data; no recommendation at this point |
| The effect of fenofibrate in reducing the risk of progression of DR in patients with T2D and high cardiovascular risk is independent of baseline triglyceride levels | Chew 2010[35]; Keech 2007[39] | 2A |
| The effect of fenofibrate in reducing the risk of progression of DR in patients with T2D and high cardiovascular risk is independent of baseline high-density lipoprotein cholesterol levels | Chew 2010[35]; Keech 2007[39] | 2A |
| The effect of fenofibrate in reducing the risk of progression of DR in patients with T2D and high cardiovascular risk is independent of baseline low-density lipoprotein cholesterol levels | Chew 2010[35]; Keech 2007[39] | 2A |
| The effect of fenofibrate in reducing the risk of progression of DR in patients with T2D and high cardiovascular risk is independent of the duration of diabetes and intensity of glycaemic control | Chew 2010[35]; Keech 2007[39] | 2A |
| The effect of fenofibrate in reducing the risk of progression of DR in patients with T2D and high cardiovascular risk is dependent on baseline retinopathy status | Chew 2010[35]; Keech 2007[39] | 1A |
| Continued treatment with fenofibrate is important to sustain its clinical benefits on lipid parameters and to reduce the risk of progression of DR | ACCORDION study 2016[40] | 2A |
DR: Diabetic retinopathy; T2D: Type 2 diabetes; NPDR: Non-proliferative diabetic retinopathy.
DISCUSSION
Medical strategies for the treatment of DR, other than those targeting glycaemic and BP control, remain to be explored. Fenofibrate, a lipid-lowering agent, has been extensively studied for its potential protective effects in various models of DR. Apart from its lipid-modifying effects, the protective effects of fenofibrate in DR have been attributed to its antioxidative, anti-inflammatory, anti-apoptotic and anti-angiogenic potential, along with its effects in improving endothelial function and reducing retinal hyperpermeability[19]–[21],[25]–[28].
Data from FIELD and the ophthalmology sub-study of FIELD provide firm evidence on the efficacy of fenofibrate in significantly reducing the need for laser treatment for DR, maculopathy or proliferative retinopathy in patients with T2D and high CVD risk. The ophthalmology sub-study also provides support for a role of fenofibrate in the reduction of macular oedema and lesser progression of DR; the reduction in progression of DR with fenofibrate was more significant in patients with a history of retinopathy versus those without a history of retinopathy[31]. This latter finding is consistent with the ACCORD-Eye lipid study results, which concluded that addition of fenofibrate to statin therapy significantly slows down the progression of DR in patients with T2D and high CVD risk compared with statin therapy alone. These benefits of fenofibrate therapy in the ACCORD-Eye lipid study were significantly greater in patients with NPDR at baseline than in patients without any DR at baseline[35].
Another important clinical aspect that needs a mention is that fenofibrate therapy does not exhibit any legacy effect in preventing the progression of DR. Therefore, continued use of fenofibrate may be needed to maintain its clinical benefits for preventing the progression of DR[41].
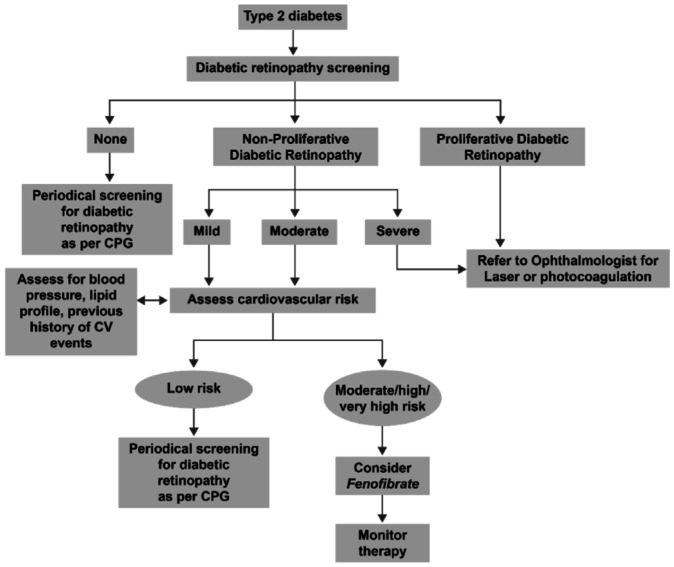
Despite the proven protective potential and clinical benefits from the FIELD, FIELD-ophthalmology and ACCORD-Eye studies, fenofibrate is not routinely prescribed for the treatment of DR. Ophthalmologists and clinicians should reconsider the adjunctive use of fenofibrate for preventing the progression of DR in their patients with T2D with high CVD risk and mild-to-moderate NPDR, irrespective of their baseline lipid levels, intensity of glycaemic control or duration of diabetes. The algorithm developed by the expert panel for the screening of DR in T2D patients, and for considering these patients for fenofibrate therapy for preventing the progression of DR, has been shown in Figure 2. Studies in future to establish the exact mechanism of action of fenofibrate in DR and clinical studies on fenofibrate with progression of DR as the primary endpoint will further help in updating the place of fenofibrate in this algorithm and defining other patient subsets that can benefit from this new treatment platform.
Figure 2. Algorithm for considering patients with T2D for fenofibrate therapy for preventing DR progression.
CPG: Clinical practice guidelines; CV: Cardiovascular; T2D: Type 2 diabetes; DR: Diabetic retinopathy.
Acknowledgments
Conflicts of Interest: Ngah NF, None; Muhamad NA, None; Abdul Aziz RA, None; Mohamed SO, None; Ahmad Tarmizi NA, None; Adnan A, None; Asnir ZZ, None; Hussein Z, None; Siew HF, None; Mohamad M, None; Lodz NA, None; Valayatham V, None.
REFERENCES
- 1.Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 2.Sabanayagam C, Wong TY. Diabetic retinopathy and cardiovascular disease. S. Karger AG. 2019 [Google Scholar]
- 3.Hu YD, Teng WP, Liu LM, Chen K, Liu L, Hua R, Chen J, Zhou Y, Chen L. Prevalence and risk factors of diabetes and diabetic retinopathy in Liaoning Province, China: a population-based cross-sectional study. PLoS One. 2015;10(3):e0121477. doi: 10.1371/journal.pone.0121477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care. 2014;2(1):e000005. doi: 10.1136/bmjdrc-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, Lim SC, Tai ES, Mitchell P. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11):1869–1875. doi: 10.1016/j.ophtha.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Ngah NF, Muhamad NA, Asnir ZZ, et al. Descriptive assessment on diabetic retinopathy screening in an awareness programme in Malaysia. Int J Ophthalmol. 2020;13(11):1808–1813. doi: 10.18240/ijo.2020.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatwadekar AD, Shughoury A, Belamkar A, Ciulla TA. Genetics of diabetic retinopathy, a leading cause of irreversible blindness in the industrialized world. Genes. 2021;12(8):1200. doi: 10.3390/genes12081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N, Zou C, Zhao SZ, Wang YZ, Han CJ, Zheng Z. Fenofibrate exerts protective effects in diabetic retinopathy via inhibition of the ANGPTL3 pathway. Invest Ophthalmol Vis Sci. 2018;59(10):4210–4217. doi: 10.1167/iovs.18-24155. [DOI] [PubMed] [Google Scholar]
- 9.Simó R, Roy S, Behar-Cohen F, Keech A, Mitchell P, Wong TY. Fenofibrate: a new treatment for diabetic retinopathy. Molecular mechanisms and future perspectives. Curr Med Chem. 2013;20(26):3258–3266. doi: 10.2174/0929867311320260009. [DOI] [PubMed] [Google Scholar]
- 10.Hu A, Luo Y, Li T, Guo XB, Ding XY, Zhu XB, Wang XQ, Tang SB. Low serum apolipoprotein A1/B ratio is associated with proliferative diabetic retinopathy in type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):957–962. doi: 10.1007/s00417-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 11.Gao L, Zhang YJ, Wang XM, Dong HL. Association of apolipoproteins A1 and B with type 2 diabetes and fasting blood glucose: a cross-sectional study. BMC Endocr Disord. 2021;21(1):59. doi: 10.1186/s12902-021-00726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006;12:1319–1333. [PubMed] [Google Scholar]
- 13.Robbesyn F, Augé N, Vindis C, Cantero AV, Barbaras R, Negre-Salvayre A, Salvayre R. High-density lipoproteins prevent the oxidized low-density lipoprotein-induced epidermal[corrected]growth factor receptor activation and subsequent matrix metalloproteinase-2 upregulation. Arterioscler Thromb Vasc Biol. 2005;25(6):1206–1212. doi: 10.1161/01.ATV.0000164805.73558.80. [DOI] [PubMed] [Google Scholar]
- 14.Georgila K, Vyrla D, Drakos E. Apolipoprotein A-I (ApoA-I), immunity, inflammation and cancer. Cancers (Basel) 2019;11(8):E1097. doi: 10.3390/cancers11081097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuior EV, Gafencu AV. Apolipoprotein C1:its pleiotropic effects in lipid metabolism and beyond. Int J Mol Sci. 2019;20(23):E5939. doi: 10.3390/ijms20235939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang FB, Wang K, Shen JH. Lipoprotein-associated phospholipase A2:the story continues. Med Res Rev. 2020;40(1):79–134. doi: 10.1002/med.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giurdanella G, Lupo G, Gennuso F, Conti F, Furno DL, Mannino G, Anfuso CD, Drago F, Salomone S, Bucolo C. Activation of the VEGF-A/ERK/PLA2 axis mediates early retinal endothelial cell damage induced by high glucose: new insight from an in vitro model of diabetic retinopathy. Int J Mol Sci. 2020;21(20):7528. doi: 10.3390/ijms21207528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du YP, Liu Y, Guan YH. The efficacy of fenofibrate in the treatment of diabetic retinopathy with nephropathy. Chin J Endemiol Prev Treat. 2018;33:82–84. [Google Scholar]
- 19.Hsu YJ, Lin CW, Cho SL, Yang WS, Yang CM, Yang CH. Protective effect of fenofibrate on oxidative stress-induced apoptosis in retinal-choroidal vascular endothelial cells: implication for diabetic retinopathy treatment. Antioxidants. 2020;9(8):712. doi: 10.3390/antiox9080712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandala A, Armstrong A, Girresch B, Zhu JY, Chilakala A, Chavalmane S, Chaudhary K, Biswas P, Ogilvie J, Gnana-Prakasam JP. Fenofibrate prevents iron induced activation of canonical Wnt/β-catenin and oxidative stress signaling in the retina. NPJ Aging Mech Dis. 2020;6:12. doi: 10.1038/s41514-020-00050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh KK, Quon MJ, Lim S, Lee Y, Sakuma I, Lee YH, Han SH, Shin EK. Effects of fenofibrate therapy on circulating adipocytokines in patients with primary hypertriglyceridemia. Atherosclerosis. 2011;214(1):144–147. doi: 10.1016/j.atherosclerosis.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression. Circ Res. 2009;104(9):1058–1065. doi: 10.1161/CIRCRESAHA.109.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu ZJ, Sun Y, Cakir B, Tomita Y, Huang S, Wang ZX, Liu CH, Cho SS, Britton W, Kern TS, Antonetti DA, Hellström A, Smith LEH. Targeting neurovascular interaction in retinal disorders. Int J Mol Sci. 2020;21(4):1503. doi: 10.3390/ijms21041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudraraju M, Narayanan SP, Somanath PR. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol Res. 2020;161:105115. doi: 10.1016/j.phrs.2020.105115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trudeau K, Roy S, Guo W, Hernández C, Villarroel M, Simó R, Roy S. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci. 2011;52(9):6348–6354. doi: 10.1167/iovs.11-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simó R, Simó-Servat O, Bogdanov P, Hernández C. Neurovascular unit: a new target for treating early stages of diabetic retinopathy. Pharmaceutics. 2021;13(8):1320. doi: 10.3390/pharmaceutics13081320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omae T, Nagaoka T, Tanano I, Kamiya T, Yoshida A. Fenofibrate, an anti-dyslipidemia drug, elicits the dilation of isolated porcine retinal arterioles: role of nitric oxide and AMP-activated protein kinase. Invest Ophthalmol Vis Sci. 2012;53(6):2880–2886. doi: 10.1167/iovs.11-8841. [DOI] [PubMed] [Google Scholar]
- 28.Ruan Y, Jiang SB, Musayeva A, Gericke A. Oxidative stress and vascular dysfunction in the retina: therapeutic strategies. Antioxidants. 2020;9(8):761. doi: 10.3390/antiox9080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Liang WT, Zhou KL, Wassel RA, Ridge ZD, Ma JX, Wang B. Therapeutic effects of fenofibrate nano-emulsion eye drops on retinal vascular leakage and neovascularization. Biology. 2021;10(12):1328. doi: 10.3390/biology10121328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ACCORD Study Group. Buse JB, Bigger, et al. Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12A):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Morgan CL, Owens DR, Aubonnet P, Carr ES, Jenkins-Jones S, Poole CD, Currie CJ. Primary prevention of diabetic retinopathy with fibrates: a retrospective, matched cohort study. BMJ Open. 2013;3(12):e004025. doi: 10.1136/bmjopen-2013-004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsberg HN, Bonds DE, Lovato LC, Crouse JR, Elam MB, Linz PE, O'Connor PJ, Leiter LA, Weiss D, Lipkin E, Fleg JL, ACCORD Study Group Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12A):56i–67i. doi: 10.1016/j.amjcard.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Chew EY, Ambrosius WT, Howard LT, Greven CM, Johnson S, Danis RP, Davis MD, Genuth S, Domanski M, ACCORD Study Group Rationale, design, and methods of the action to control cardiovascular risk in diabetes eye study (ACCORD-EYE) Am J Cardiol. 2007;99(12A):103i–111i. doi: 10.1016/j.amjcard.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 34.ACCORD Study Group, ACCORD Eye Study Group. Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chew EY, Ambrosius WT. Update of the ACCORD eye study. N Engl J Med. 2011;364(2):188–189. doi: 10.1056/NEJMc1011499. [DOI] [PubMed] [Google Scholar]
- 36.Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, Ambrosius WT, Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121(12):2443–2451. doi: 10.1016/j.ophtha.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Investigators FIELDS. The need for a large-scale trial of fibrate therapy in diabetes: the rationale and design of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study.[ISRCTN64783481] Cardiovasc Diabetol. 2004;3:9. doi: 10.1186/1475-2840-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott R, Best J, Forder P, Taskinen MR, Simes J, Barter P, Keech A, FIELD Study Investigators Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: baseline characteristics and short-term effects of fenofibrate [ISRCTN64783481] Cardiovasc Diabetol. 2005;4:13. doi: 10.1186/1475-2840-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keech AC, Mitchell P, Summanen PA, et al. FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 40.Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and the Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the action to control cardiovascular risk in diabetes (ACCORD) follow-on study. Diabetes Care. 2016;39(7):1089–1100. doi: 10.2337/dc16-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ministry of Health Singapore Clinical Practice Guidelines for diabetes mellitus 2014. [Accessed on 2 May 2017]. https://www.moh.gov.sg/content/dam/moh_web/HPP/Doctors/cpg_medical/current/2014/diabetes_mellitus/cpg_Diabetes%20Mellitus%20Booklet%20-%20Jul%202014.pdf.
- 42.Catapano AL, Graham I, de Backer G, et al. ESC Scientific Document Group 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 43.International Diabetes Federation global guideline for type 2 diabetes 2012. [Accessed on 2 May 2017]. https://www.idf.org/sites/default/files/IDF%20T2DM%20Guideline.pdf.
- 44.RACPG general practice management of type 2 diabetes 2016-18. [Accessed on 2 May 2017]. https://static.diabetesaustralia.com.au/s/fileassets/diabetes-australia/5d3298b2-abf3-487e-9d5e-0558566fc242.pdf.
- 45.Ministry of Health Singapore Clinical Practice Guidelines for screening of diabetic retinopathy 2011. [Accessed on 2 May 2017]. http://www.moh.gov.my/penerbitan/CPG2017/6601.pdf.
- 46.The Royal College of Ophthalmologists Diabetic Retinopathy Guidelines 2012. [Accessed on 2 May 2017]. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdf.