Dear Editor,
We present three anatomical forms of premacular hemorrhage with niveau formation: hemorrhage in posterior precortical vitreous pocket (intrapocket hemorrhage), sub-internal limiting membrane (ILM) hemorrhage, and premacular retrocortical hemorrhage. Niveau formation is the formation of a horizonal boundary between fluid and blood cells when they accumulate in a closed space, because blood cells with higher specific gravity settle in a standing position. Differentiating between the three forms of hemorrhage is important for understanding the pathophysiology of premacular hemorrhage and selecting treatment.
Premacular hemorrhage may cause sudden loss of vision when the accumulation of blood is localized in the macular region. Various causes have been reported, such as retinal arterial macroaneurysm, Valsalva retinopathy, trauma, Terson syndrome, proliferative diabetic retinopathy, retinal vein occlusion, Eales' disease, pregnancy and hematologic disorders[1]–[7]. Different treatment approaches have been used, including observation[1], neodymium-doped yttrium aluminum garnet (Nd:YAG) laser hyaloidotomy or membranotomy[2]–[5], and vitrectomy[6]–[7], but no clear guidelines are available. One reason is that the different anatomical forms of premacular hemorrhage are not differentiated before treatment; namely, hemorrhage in the posterior precortical vitreous pocket, hemorrhage behind the ILM, and hemorrhage between the ILM and the posterior vitreous cortex.
The vitreous of the human eye has a physiological liquefaction cavity before the macula. Worst[8] observed the vitreous by injecting Indian ink into an autopsy eye without retina, choroid or sclera. He reported for the first time that there was a space called the “bursa premacularis” in front of the macula. Kishi and Shimizu[9] stained the gel component of autopsy eyes with fluorescein and showed a space in front of the macula without posterior vitreous detachment (PVD), which was attached to the macula. They called this structure “posterior precortical vitreous pocket” (vitreous pocket) to differentiate it from bursa premacularis. From this anatomical relationship, it became possible to explain the pathogenic mechanism of vitreous macular diseases[10]–[11]. When triamcinolone was injected into the vitreous pocket during vitreous surgery, a disc-shaped glossy surface was observed, and the vitreous pocket was described as a liquefied lacuna surrounded by dense networks of fibrils[12]. Recently, a combination of ultra-widefield swept-source optical coherence tomography (OCT) and 3-dimensional structural imaging has been reported to be useful in visualizing the complex structure of the vitreous. These technologies are powerful tools that can be used to clarify the normal evolution of the vitreous, pathological changes of the vitreous, and implications of vitreous changes in various vitreoretinal diseases[13].
This study was conducted in accordance with the ethical principles of Declaration of Helsinki. Written informed consent for publication of patient information and images was given by the patients. The study was approved by the Ethics Committee of the Nihon University School of Medicine (No.161001).
CASE 1
A 50-year-old man diagnosed with Valsalva retinopathy had best corrected visual acuity (BCVA) of 20/400. Examination revealed a hemispherical hemorrhage 6 mm in diameter within the vitreous pocket centered over the macula. The pocket was surrounded by fibril networks, giving the surface of the intrapocket hemorrhage a shiny appearance. Bleeding in the pocket showed niveau formation. Since the vitreous pocket is almost a closed space, no spreading of bleeding to surrounding tissue was found, except for the hemorrhage in the connecting channel leading to Cloquet's canal (Figure 1A). On OCT, no retinal compression was seen as the hemorrhage was on the retina. Because of the thin anterior wall of the vitreous pocket (Figure 1B), the hemorrhage was denser in the inferior portion due to gravity. A space corresponding to the vitreous pocket posterior wall was visible. Four days later, the intrapocket hemorrhage was displaced inferiorly (Figure 1C) and the hemorrhage was absorbed spontaneously following formation of PVD (Figure 1D), and BCVA improved to 20/16.
Figure 1. Intrapocket hemorrhage in case 1.

A: Fundus photograph shows an intrapocket hemorrhage with niveau formation. There is no spreading of hemorrhage to surrounding tissue (*), except for the hemorrhage in the connecting channel leading to Cloquet's canal (arrow). B: On OCT, no retinal compression (arrow) and the thin anterior wall of the vitreous pocket (arrowhead) can be seen. The hemorrhage is denser in the inferior portion due to gravity. C: The hemorrhage is displaced inferiorly. D: OCT confirms posterior vitreous detachment. Hemorrhage is identified in the vitreous (*) but not in posterior vitreous cortex. OCT: Optical coherence tomography.
CASE 2
A 47-year-old healthy female presented with decreased BCVA to 20/25 in her left eye associated with Valsalva retinopathy. Examination revealed a hemispherical hemorrhage beneath the ILM with a shiny surface showing niveau formation (Figure 2A). PVD was apparently absent on OCT. Since the ILM is not a closed structure, spreading of bleeding to surrounding tissue was observed (Figure 2B). On OCT, the sub-ILM hemorrhage was found to compress the retina, and the hemorrhage had uniform density throughout the whole depth because bleeding was beneath the ILM. The hemorrhage was absorbed after 3mo (Figure 2C), but BCVA remained unchanged at 20/25 due to cells depositing in the sub-ILM space of the fovea as shown on OCT (Figure 2D).
Figure 2. Sub-ILM hemorrhage in case 2.
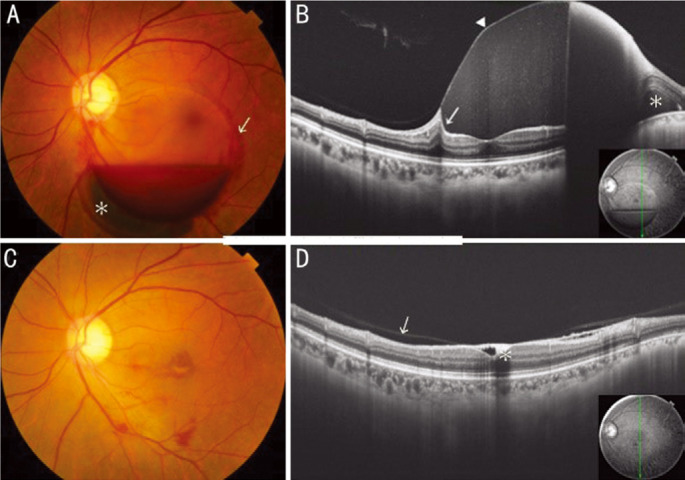
A: Fundus photograph shows a sub-ILM hemorrhage with niveau formation and subretinal hemorrhage (*). Spreading of hemorrhage to surrounding tissue is observed (arrow). B: On OCT, the hemorrhage compresses the retina (arrow) and has uniform density because of the ILM (arrowhead). Subretinal hemorrhage (*) is confirmed. C: Hemorrhage was absorbed after 3mo. D: On OCT, cells depositing on sub-ILM space (*) at the fovea and partial posterior vitreous detachment (arrow) can be seen. OCT: Optical coherence tomography, ILM: Internal limiting membrane.
CASE 3
A 52-year-old man with premacular retrocortical hemorrhage associated with proliferative diabetic retinopathy presented with left BCVA of 20/400. Examination showed a hemispherical hemorrhage with niveau formation. Since the retrocortical space is not a closed structure, spreading of bleeding to surrounding tissue was observed (Figure 3A). Bleeding spread nasally beyond the optic disc, accompanied by vitreous hemorrhage. On OCT, the retina was not compressed by the premacular retrocortical hemorrhage (Figure 3B). Vitreous surgery and cataract surgery were performed, and retinal photocoagulation was also used (Figure 3C). After 5mo, BCVA recovered to 20/32, and OCT showed no macular edema (Figure 3D).
Figure 3. Premacular retrocortical hemorrhage in case 3.

A: Fundus photograph shows a premacular retrocortical hemorrhage with niveau formation. Bleeding spreads nasally beyond the optic disc, accompanied by vitreous hemorrhage. Spreading of hemorrhage to surrounding tissue is observed (*). B: On OCT, the hemorrhage does not compress the retina (arrow), and posterior vitreous (arrowhead) can be seen. C: Fundus photograph at 6mo after vitrectomy. D: OCT shows no macular edema. OCT: Optical coherence tomography.
Table 1 summaries the differentiation between the three forms of premacular hemorrhage with niveau formation. In intrapocket hemorrhage, bleeding occurs in the vitreous pocket[9]; therefore, the hemorrhage is approximately 6 mm in diameter and centered on the macula, coinciding with the vitreous pocket[12]. Since bleeding occurs in the vitreous pocket, the retina is not compressed by the hemorrhage, and the space corresponding to vitreous pocket posterior wall can be seen. Intrapocket hemorrhage is recognized as a large hemispherical hemorrhage with a shiny surface accompanied by niveau formation. This form of hemorrhage occurs in association with conditions such as Valsalva retinopathy in the absence of PVD. Since the vitreous pocket is almost a closed space, no spreading of bleeding to surrounding tissue was found, except for the hemorrhage in the connecting channel leading to Cloquet's canal. Because bleeding occurs below the thin anterior wall of the vitreous pocket, the hemorrhage is denser in the inferior region due to gravity.
Table 1. Fundus findings and OCT findings of three forms of premacular hemorrhage with niveau formation.
| Parameters | Intrapocket hemorrhage | Sub-ILM hemorrhage | Premacular retrocortical hemorrhage |
| Site of hemorrhage | Inside vitreous pocket | Below ILM | Between ILM and posterior vitreous cortex |
| PVD | Absent Hemorrhage disappears with PVD |
Unrelated Hemorrhage remains after PVD |
Partial Hemorrhage disappears with PVD |
| Fundus findings | Hemispheric bleeding centered on the macula Size of vitreous pocket: about 6 mm in diameter Bleeding spreading to surrounding (-) Smooth surface (+) |
Hemispheric hemorrhage not centered on the macula Various sizes: 1/4 to several disc diameters Bleeding spreading to surrounding (+) Smooth surface (+) |
Irregular or hemispherical bleeding not centered on the macula Various sizes: 1/4 to several disc diameters Bleeding spreading to surrounding (+) Smooth surface (+/-) |
| OCT findings | Denser lower portion by gravity due to the thin vitreous pocket anterior wall Bleeding compresses retina (-) |
Uniform density from upper to lower portion due to bleeding beneath ILM Bleeding compresses the retina (+) |
Denser at the site of PVD Bleeding compresses retina (-) |
OCT: Optical coherence tomography; ILM: Internal limiting membrane; PVD: Posterior vitreous detachment.
The occurrence of sub-ILM hemorrhage is unrelated to the status of PVD. The size of hemorrhage ranges from 1/4 to several disc diameters. Large hemispherical hemorrhages show a shiny surface with niveau formation, but the location and size of the hemorrhage do not coincide with the vitreous pocket. As the ILM is not a closed structure, bleeding spreads slightly to surrounding tissue. Because bleeding occurs behind the rigid ILM, there is no density gradient in the hemorrhage due to gravity. Furthermore, since the ILM is a part of the retinal tissue, compression of the retina by the hemorrhage is clearly depicted in the peripheral region. Hemorrhage may be associated with subretinal or intraretinal hemorrhage.
Premacular retrocortical hemorrhage is caused by bleeding between the posterior vitreous cortex and ILM, associated with conditions such as proliferative diabetic retinopathy, in the presence of partial PVD. Although this hemorrhage may show niveau formation, bleeding spreads nasally beyond the optic disc, accompanied by vitreous hemorrhage. The hemorrhage has a flat irregular appearance and may spread irregularly. There is no compression of the retina by the hemorrhage.
Regarding treatment, although intrapocket hemorrhage may be absorbed spontaneously accompanying PVD formation, YAG laser treatment is selected when spontaneous absorption does not occur. An observation period of around one month would be appropriate. For the treatment of sub-ILM hemorrhage, observation, YAG laser and vitrectomy are used[1]–[7]. Waiting for spontaneous absorption is recommended for small hemorrhage. For large hemorrhages, many cases have been successfully treated by YAG laser, but multiple retinal holes after treatment have been reported[4]. Large hemorrhage may lead to proliferative vitreoretinopathy-like changes on the inner retinal surface of the ILM if surgery is delayed[6]. Many cases require treatment with anti-vascular endothelial growth factor therapy, vitrectomy, or photocoagulation for underlying diseases such as proliferative diabetic retinopathy.
Differentiation between intrapocket hemorrhage, sub-ILM hemorrhage, and premacular retrocortical hemorrhage by fundus photography and OCT is important for understanding the pathophysiology of premacular hemorrhages, which may help decide treatment approach. In this short report, we have clearly demonstrated the features of the three forms of macular hemorrhage in three cases. However, in clinical practice, differentiation of the premacular hemorrhages may not be straightforward due to morphological variations and differences in clinical background. More detailed clinical and imaging studies on a larger number of cases are required, and the effects on retinal tissues should be examined.
Acknowledgments
Conflicts of Interest: Kitagawa Y, None; Shimada H, None; Kawamura A, None; Kaneko H, None; Tanaka K, None; Nakashizuka H, None.
REFERENCES
- 1.Celik Dulger S, Ozdal PC, Teke MY. Valsalva retinopathy: long-term results and management strategies. Eur J Ophthalmol. 2021;31(4):1953–1960. doi: 10.1177/1120672120936175. [DOI] [PubMed] [Google Scholar]
- 2.Babu N, Kohli P, Rajan RP, Ramasamy K. Inverse drainage Nd:YAG membranotomy for pre-macular hemorrhage. Eur J Ophthalmol. 2022:112067212211022. doi: 10.1177/11206721221102258. [DOI] [PubMed] [Google Scholar]
- 3.Hua R, Liu LM, Hu YD, Zhou Y, Chen L. Combine intravitreal bevacizumab with Nd:YAG laser hyaloidotomy for Valsalva pre-macular haemorrhage and observe the internal limiting membrane changes: a spectralis study. Int J Ophthalmol. 2013;6(2):242–245. doi: 10.3980/j.issn.2222-3959.2013.02.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurung RL. Multiple retinal holes secondary to Valsalva retinopathy. Case Rep Ophthalmol Med. 2018;2018:8950682. doi: 10.1155/2018/8950682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allam K, AlMutairi N, Ellakwa AF, Abdelkader E. Nd:YAG laser therapy for non-resolving premacular subhyaloid hemorrhage in Saudi patients. Saudi J Ophthalmol. 2019;33(1):61–65. doi: 10.1016/j.sjopt.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain RN, Stappler T, Hiscott P, Wong D. Histopathological changes and clinical outcomes following intervention for sub-internal limiting membrane haemorrhage. Ophthalmologica. 2020;243(3):217–223. doi: 10.1159/000502442. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa Y, Kawamorita A, Shimada H, Nakashizuka H. Treatment of macular hemorrhage in retinal arterial microaneurysm: anatomic site-oriented therapy. Jpn J Ophthalmol. 2019;63(2):186–196. doi: 10.1007/s10384-019-00653-y. [DOI] [PubMed] [Google Scholar]
- 8.Worst JG. Cisternal systems of the fully developed vitreous body in the young adult. Trans Ophthalmol Soc U K (1962) 1977;97(4):550–554. [PubMed] [Google Scholar]
- 9.Kishi S, Shimizu K. Posterior precortical vitreous pocket. Arch Ophthalmol. 1990;108(7):979–982. doi: 10.1001/archopht.1990.01070090081044. [DOI] [PubMed] [Google Scholar]
- 10.Kishi S, Shimizu K. Oval defect in detached posterior hyaloid membrane in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1994;118(4):451–456. doi: 10.1016/s0002-9394(14)75795-2. [DOI] [PubMed] [Google Scholar]
- 11.Kishi S, Hagimura N, Shimizu K. The role of the premacular liquefied pocket and premacular vitreous cortex in idiopathic macular hole development. Am J Ophthalmol. 1996;122(5):622–628. doi: 10.1016/s0002-9394(14)70480-5. [DOI] [PubMed] [Google Scholar]
- 12.Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Three-dimensional depiction of the vitreous pocket using triamcinolone acetonide. Eur J Ophthalmol. 2009;19(6):1102–1105. doi: 10.1177/112067210901900638. [DOI] [PubMed] [Google Scholar]
- 13.Ohno-Matsui K, Takahashi H, Mao Z, Nakao N. Determining posterior vitreous structure by analysis of images obtained by AI-based 3D segmentation and ultrawidefield optical coherence tomography. Br J Ophthalmol. 2021:bjophthalmol-2021-320131. doi: 10.1136/bjophthalmol-2021-320131. [DOI] [PubMed] [Google Scholar]


