Abstract
AIM
To evaluate the clinical efficacy of topical 0.05% cyclosporine nano-emulsion in the treatment of dry eye syndrome (DES) with meibomian gland dysfunction (MGD).
METHODS
This prospective study included 64 patients with DES and MGD who were randomly assigned to three groups: Group 1 (n=24, conventional cyclosporine), Group 2 (n=21, nano-emulsion cyclosporine), and Group 3 (n=19, control). Lid margin telangiectasia (LMT), meibomian gland secretion (MGS), conjunctival injection (CI), corneal staining (CS), tear break-up time (TBUT), Schirmer test I (STI), Ocular Surface Disease Index (OSDI), and lipid layer thickness (LLT) was evaluated at 4, 8, and 12wk of treatment.
RESULTS
In Group 3 (control), LMT, CS, and CI improved after 8wk, MGS, TBUT after 12wk of treatment. In Group 1 (conventional cyclosporine), LMT, MGS, and TBUT improved significantly after 4wk, whereas CS, CI, STI, and LLT improved significantly after 8wk, and OSDI at 12wk. In Group 2 (nano-cyclosporine), LMT, MGS, CS, CI, TBUT, and OSDI significantly improved after 4wk, and STI after 8wk. Especially, LLT was significantly higher than other groups after 4wk.
CONCLUSION
Cyclosporine and nano-cyclosporine shows significant improvement in DES with MGD than the control group. In contrast, the nano-cyclosporine group shows more statistically improved CI and CS at 4wk, especially LLT at 4, 8, and 12wk compared to the conventional cyclosporine group.
Keywords: anti-inflammatory treatment, cyclosporine, dry eye disease, meibomian gland dysfunction
INTRODUCTION
Meibomian gland dysfunction (MGD) is a common ocular condition and a major cause of evaporative dry eye syndrome (DES) characterized by a deficiency of the tear film lipid layer, instability of the tear film, and short tear film break-up time (TBUT)[1]–[4]. Management of eyelids through cleansing and warm massage of the eyelids is a traditional treatment method for MGD[5], which improves meibomian gland function and helps release secretions[4]. Conventional treatments options for MGD include lid hygiene, warm compression, using artificial tear, topical erythromycin, topical corticosteroids, and oral doxycycline tetracycline are used recently[6]–[7].
MGD is frequently reported to be highly related to evaporative DES, but it is also known to increase persistent inflammation of the ocular surface[8]. Since inflammation contributes to the pathogenesis of MGD, previous studies related to the use of cyclosporine drops for the treatment of dry eyes with MGD are investigated[9]–[12]. As though the evaporative dry eye related to MGD has less incidence than the aqueous deficient dry eye, it should be required whether the cyclosporine can be useful in managing MGD combined with eyelid inflammation. Therefore, we are going to investigate the therapeutic efficacy between cyclosporine nano-emulsion and conventional cyclosporine eye drops in dry eye with MGD.
Even though the main objective of the treatment is to reduce ocular discomfort, the use of conventional cyclosporine eye drops may induce ocular stinging and burning sensation. Moreover, cyclosporine bioavailability and adherence to the treatment tend to decrease because of the burning and stinging sensations experienced after its topical use[10]. A total of 17% of patients with dry eye treated with cyclosporine discontinued the application of topical cyclosporine eye drops in one study[13]. Advances in technology and understanding of disorders at the cellular/molecular level led to an increase in the application of nanotechnology in eye drops. Cyclosporine nano-emulsion could be expected to have the ability to increase the precorneal residence time and ocular bioavailability. Also, cyclosporine nano-emulsion have more effective in suppressing the inflammation of the conjunctiva, cornea, and eyelid compared with conventional cyclosporine eye drops. As nanoparticles contained with drug material are deposited in the ocular surface tissue, the drug is continuously more released compared to the conventional particle of cyclosporine. In general, cyclosporine emulsion formulation is thermodynamically unstable, and the size of the dispersed particles ranges from 50 to 1000 nm. Nano-emulsion technology has been recently introduced to prepare a novel cyclosporine ophthalmic solution to overcome the limitations of traditional cyclosporine emulsion formulations. Various forms of nano-technology eye drop are being used in DES treatment[14].
Therefore, the purpose of this study was to investigate the effect of 0.05% nano-emulsion cyclosporine formulation on the meibomian gland, ocular surface, and dry eye symptoms, and lipid layer thickness (LLT) when compared to those of traditional cyclosporine or sodium hyaluronate for the treatment of DES with MGD.
SUBJECTS AND METHODS
Ethical Approval
All procedures performed in this study were under the ethical standards of the institutional and/or national research committee and the Declaration of Helsinki and its later amendments or comparable ethical standards. The study protocol was approved by the Institutional Review Board of the Pusan National University Hospital (Protocol code 1510-011-045). This study is registered with the Korean clinical research information service (KCT0006923).
Study Design
A randomized, double-masked interventional study was performed at one referral eye center from March 2018 to December 2018.
Inclusion and Exclusion Criteria
The patients exhibited one or more clinically significant signs of MGD, such as meibomian gland capping or dropout, lid telangiectasia, redness, irregularity. The above signs are known to most common clinically observable pathognomic signs of MGD[15]. And patients have similar symptoms of DES, such as ocular irritation, glare, dryness, excessive tears, itching, pain, and fluctuating visual acuity. Before commencing the study, the participants of the clinical trial were informed of their participation in the clinical trial after preliminary examination.
Patients with ocular surface disease, previous ocular surgery, glaucoma, contact lens use, and systemic immune-related disease, receiving cyclosporine treatment for more than a year, and pregnant and lactating women were excluded from the study.
Randomization, Masking, and Sample Size Calculation
A target sample size of 18 participants per arm was calculated based on having 90% power, at a 2-tailed significance level of 0.05, to detect a 30% change from baseline in Ocular Surface Disease Index (OSDI). An additional 12 participants per group were included to allow for up to 40% participant attrition, giving a recruitment target of 30 per group (90 participants in total).
The subjects assigned to Group 1 were treated with preservative-free 0.1% sodium hyaluronate eye drops (Hyal Q®, Il-Dong Inc., Korea) four times daily and 0.05% topical cyclosporine eye drops (Restasis®, Allergan Inc., USA) twice daily. The subjects assigned to Group 2 were treated with a combination of preservative-free 0.1% sodium hyaluronate eye drops four times daily and 0.05% topical nano-emulsion cyclosporine eye drops (Nano-cyclosporine; Cyporin N, Taejoon, Korea) twice daily. The control group (Group 3) was treated with preservative-free 0.1% sodium hyaluronate eye drops four times daily. All subjects were instructed to maintain eyelid hygiene, including eyelid warm massage for 10min and cleansing using Blephaclean wipes (Blephaclean®, Thea Inc., France).
Outcome Measures
Patients were followed up during four visits to the hospital at the following time points: 4wk (visit 2), 8wk (visit 3), and 12wk (visit 4) after the first visit (visit 1). All patient ocular examinations were evaluated by a single ophthalmologist. There were three main categories of evaluation: complications related to MGD, ocular surface, and dry eye. Lid margin telangiectasia (LMT) and meibomian gland secretion (MGS) were observed for MGD evaluation. LMT was scored based on the degree of capillary dilatation around the meibomian gland in the upper eyelid: 0, without capillary dilatation; 1, with mild dilatation; 2, with moderate dilatation; and 3, with severe capillary dilatation[16]. For MGS measurement, the upper lid margin was compressed with a sterile swab to collect the meibum. After partial quantitative analysis of meibum, it was classified into four stages: grade 0 to grade 3[17]. Ocular surface complications were evaluated by observing corneal staining (CS) and conjunctival injection (CI). CS is the evaluation of the degree of corneal staining using slit-lamp examination after staining with fluorescein paper (Haag-Streit AG, Köniz, Switzerland). As suggested by the National Eye Institute[18], the cornea is divided into five zones, which are graded according to the degree of staining, and then presented as the sum of the scores. The total score ranges from 0 to 15 points. CI is observed during the slit lamp examination and is expressed as five grades using the Efron grading scale[19]. Dry eye problems were assessed using OSDI, TBUT, and LLT. The OSDI is calculated based on 12 questions related to DES, which are used to assess changes in symptoms[20]. The score ranges from 0 to 100, and the higher the score, the more severe the symptoms. TBUT is assessed by measuring the time duration in seconds at which a dry spot appears in the tear film under slit lamp blue filter illumination after exposure of the lower conjunctival sac to fluorescein paper (Haag-Streit AG, Köniz, Switzerland). The test was conducted three times consecutively, and the mean value was used. The Schirmer test I (STI) was performed using a filter paper strip (Eagle Vision, Memphis, TN, USA). The strip was placed inside the lower eyelid to avoid corneal stimulation and left for 5min. Then, the filter paper was removed, and the amount of wetting was measured on a millimeter scale. Average LLT measurements were obtained using a Lipiview Ocular Surface Interferometer (TearScience Inc, Morrisville, NC, USA).
Statistical Analysis
Statistical analysis was performed using the SPSS software (version 20 for Windows; SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to compare the effects between the three groups at the time course of statistical changes in the value while controlling the effects of the baseline values. And paired t-test was used to compare the mean changes within each group with that of the baseline after treatment. Statistical significance was set at P<0.05.
RESULTS
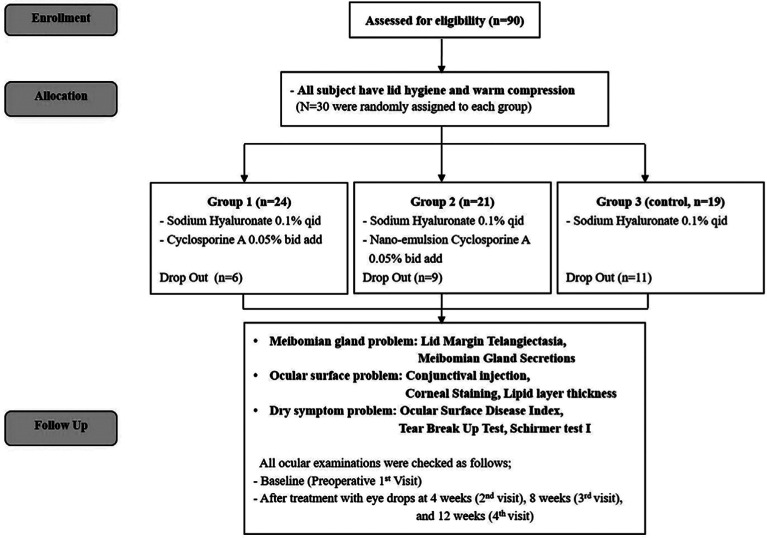
At the institution of Pusan National University Hospital, 90 subjects were recruited and met the eligibility criteria of the clinical trial, 26 subjects did not complete the study. Six were in Group 1, 9 were in Group 2, and 11 were in Group 3; in 26 participants, 14 subjects were lost to follow up after the first visit, and 8 subjects were lost to follow up after 4wk, and 4 subjects were discontinued eye drops due to patient withdrawal. In the 26 dropout participants, any drug side effects, or adverse reactions were not reported. A total of 64 subjects completed the study. They were randomly assigned to different groups: 24 subjects in Group 1, 21 in Group 2, and 19 in Group 3 (Figure 1).
Figure 1. Flow chart of the study protocol.
Among the 64 subjects, 18 were male and 46 were female, with a mean age of 58.9±14.6y. There were statistically insignificant differences among the three groups (P=0.06). The results of the comparison between Groups 1, 2, and 3 are shown in Table 1. There were no statistically significant differences between the three groups at 4, 8, and 12wk after treatment. However, all three groups showed improvement in the score of MGD, ocular surface, and dry eye complications compared to the baseline values before treatment initiation.
Table 1. Baseline demographics and ocular examination results of all patients in the three groups.
| Parameters | Group 1 | Group 2 | Group 3 | P |
| Sex (M/F) | 7/17 | 2/19 | 9/10 | 0.250 |
| Age, y | 58.2±14.4 | 57.2±15.7 | 64.1±9.9 | 0.060 |
| Lid margin telangiectasia (LMT) | 1.75±1.33 | 1.48±1.33 | 1.89±0.94 | 0.649 |
| Meibomian gland secretion (MGS) | 1.25±0.99 | 1.52±1.08 | 1.53±0.96 | 0.521 |
| Corneal staining (CS) | 0.83±0.76 | 0.67±0.86 | 1.00±0.75 | 0.259 |
| Conjunctival injection (CI) | 1.17±0.76 | 1.19±0.87 | 1.37±0.60 | 0.679 |
| Tear break up time (TBUT), s | 5.32±2.06 | 4.84±1.23 | 5.59±1.94 | 0.347 |
| Schirmer test I (STI), mm | 4.76±3.62 | 4.82±2.63 | 4.85±3.88 | 0.749 |
| Ocular Surface Disease Index (OSDI) | 47.69±15.89 | 45.34±22.93 | 45.45±18.69 | 0.320 |
| Lipid layer thickness (LLT), nm | 47.69±15.89 | 45.34±22.93 | 45.45±18.69 | 0.277 |
mean±SD
Lid Margin Telangiectasia
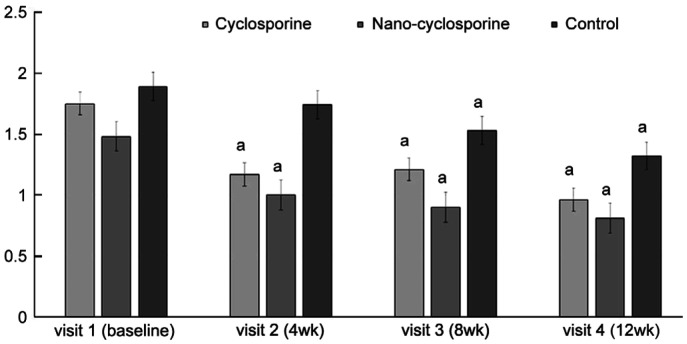
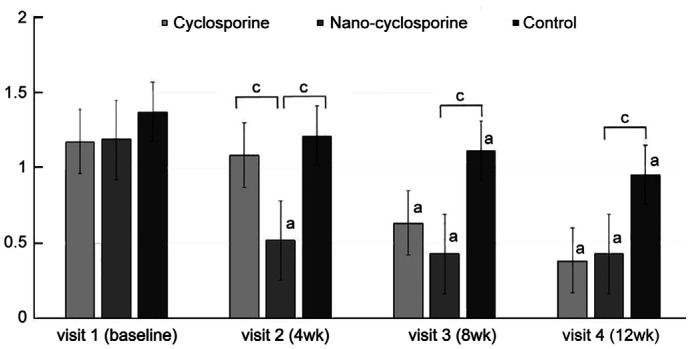
The LMT for evaluation of MGD complications was significantly improved at 4, 8, and 12wk of treatment in Group 1 compared with that before treatment (P=0.007, 0.009, and 0.004, respectively). In Group 2, a statistically significant improvement was observed at 4, 8, and 12wk of treatment (P=0.002, 0.004, and <0.001, respectively). Moreover, in the control group (Group 3), statistically significant improvement was observed at 8 and 12wk after treatment (P=0.005 and <0.001, respectively), But no statistically significant intergroup differences were observed (Figure 2).
Figure 2. Changes in lid margin telangiectasia from baseline to 12wk after treatment in the three groups.
Statistically significant improvements from baseline were observed in Group 1 and Group 2 at 4, 8, and 12wk. Statistically significant improvement from baseline was observed in Group 3 at 8 and 12wk. aP<0.05 compared with baseline analyzed by compared t-test.
Meibomian Gland Secretion
There was a statistically significant improvement in MGS, a parameter related to MGD complications, in Group 1 at 4, 8, and 12wk of treatment (P=0.026, 0.018, and 0.010, respectively) compared with that before treatment. In Group 2, a statistically significant improvement was observed in MGS at 4, 8, and 12wk of treatment (P=0.02, <0.001, and <0.001, respectively). In Group 3, there was a statistically significant improvement in MGS at 12wk of treatment (P=0.005). After 8 and 12wk of treatment, the MGS of Group 2 significantly decreases than that of Group 3 (P=0.032, 0.020). And Group 1 showed a statistically significant decrease rather to Group 3 at 12wk of treatment (Figure 3).
Figure 3. Changes in meibomian gland secretion from baseline to 12wk in the three groups.
Statistically significant improvements from baseline were observed in Group 1 and Group 2 at 4, 8, and 12wk. Statistically significant improvement from baseline was observed in the control group at 12wk. After 8wk, Group 2 showed a significant difference compared to Group 3. At the 12wk, Group 1 and 2 showed significant differences compare to Group 3. aP<0.05 compared to baseline analyzed by compared t-test. cP<0.05 analyzed by one-way ANOVA.
Corneal Staining
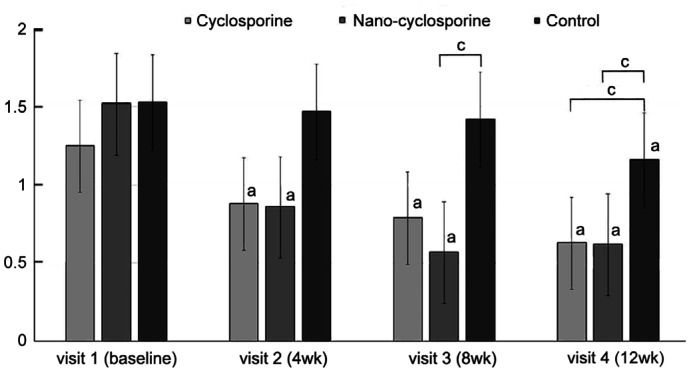
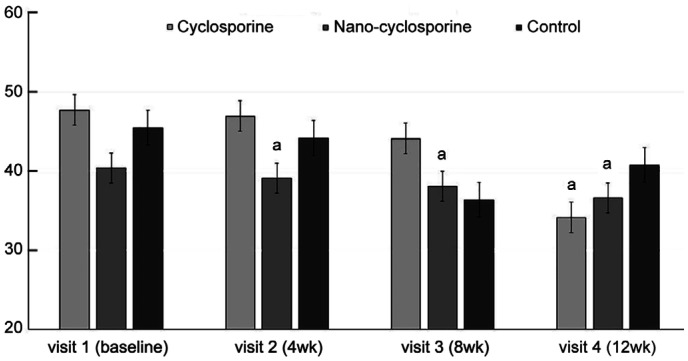
There was a significant improvement in CS, the first parameter used to assess ocular surface complications, in Group 1 at 4, 8, and 12wk compared with that at baseline (P=0.017, 0.023, and 0.011, respectively). In Group 2, a statistically significant improvement was observed in CS at 4, 8, and 12wk of treatment (P=0.048, 0.042, and 0.010, respectively). Statistically, significant improvement was observed in CS in the control group at 8 and 12wk of treatment (P=0.030 and <0.001, respectively). After 4 and 8wk, Group 2 showed significant decreases rather than Group 1 and Group 3 (P=0.048, 0.005 and 0.022, 0.012; Figure 4).
Figure 4. Changes in corneal staining from baseline to 12wk in the three groups.
Statistically significant improvements from baseline were observed in Group 1 and Group 2 at 4, 8, and 12wk. Statistically significant improvement from baseline was observed in Group 3 at 8 and 12wk. Group 2 showed statistically significantly lower than those of other groups at 4 and 8wk of treatment. aP<0.05 compared with baseline analyzed by compared t-test. cP<0.05 analyzed by one-way ANOVA.
Conjunctival Injection
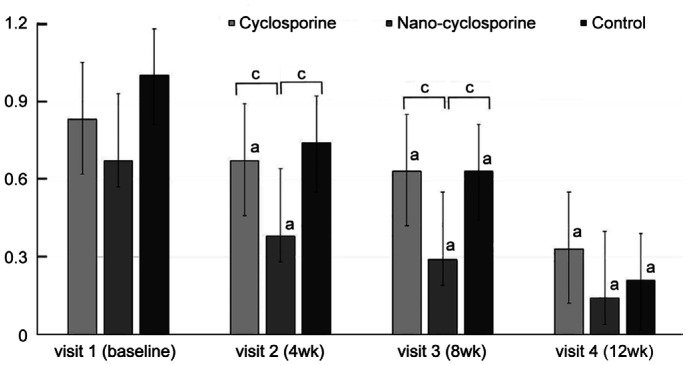
Significant improvement was observed in CI, the second parameter related to ocular surface complications, in Group 1 and Group 3 at 8 and 12wk of treatment compared with that at baseline (Group 1, P=0.002 and <0.001; Group 3, P=0.021 and 0.002; respectively). In Group 2, significant improvement was observed in CI at 4, 8, and 12wk of treatment (P=0.030, 0.028, and <0.001, respectively) compared with that at baseline. Group 2 showed significantly decreases compared to Group 1 at 4wk (P=0.034), and Group 3 at 4, 8, and 12wk of treatment (P=0.050, 0.041, and 0.011; Figure 5).
Figure 5. Changes in conjunctival injection from baseline to 12wk in the three groups.
Statistically significant improvements from baseline were observed in Group 2 at 4, 8, and 12wk. Statistically significant improvements from baseline were observed in Group 1 and Group 3 at 8 and 12wk. Group 2 showed statistically significantly lower than that of Group 1 and 3 at 4wk, and that of Group 3 at 8 and 12wk of treatment. aP<0.05 compared with baseline analyzed by compared t-test. cP<0.05 analyzed by one-way ANOVA.
Tear Film Break-up Time
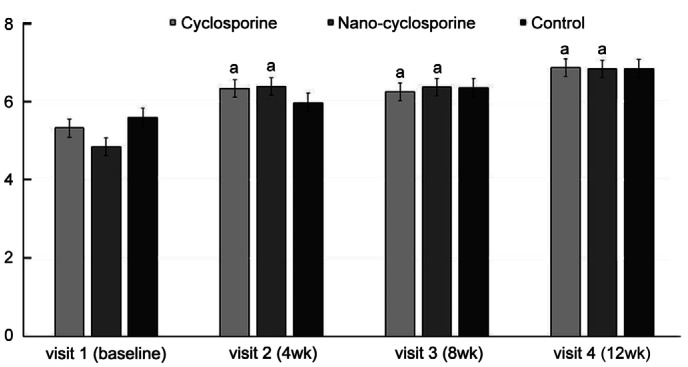
Significant improvement was observed in TBUT, a parameter used for the evaluation of dry eye complications in Groups 1 and 2, at 4, 8 and 12wk of treatment compared with that at baseline (Group 1, P=0.034, 0.028, and 0.020; Group 2, P=0.021, 0.011, and <0.001; respectively). Moreover, no significant difference was observed in TBUT in the control group compared to that at baseline and no statistically significant intergroup differences were observed (Figure 6).
Figure 6. Changes in tear film break-up time from baseline to 12wk in the three groups.
Statistically significant improvements from baseline were observed in Group 1 and Group 2 at 4, 8, and 12wk. aP<0.05 compared with baseline analyzed by compared t-test.
Schirmer Test
Groups 1 and 2 showed significant improvement at 8 and 12wk of treatment compared with that of Group 3 (Group 1, P=0.041 and 0.028; Group 2, P=0.037 and 0.019; respectively). Moreover, no significant difference was observed in the control group and between intergroup (Figure 7).
Figure 7. Changes in Schirmer test I from baseline to 12wk in the three groups.
Statistically significant improvements from baseline were observed in Group 1 and Group 2 at 8 and 12wk. aP<0.05 compared with baseline analyzed by compared t-test.
Ocular Surface Disease Index Score
Group 2 showed a significant improvement in OSDI at 4, 8, and 12wk of treatment (P=0.009, 0.004, and 0.002, respectively), whereas in Group 1, there was a significant improvement only at 12wk of treatment (P=0.020). No significant difference was observed in the OSDI of the control group after treatment for 12wk compared with that before treatment and in the intergroup (Figure 8).
Figure 8. Changes in Ocular Surface Disease Index score from baseline to 12wk in the three groups.
Statistically significant improvements from baseline were observed in Group 2 at 4, 8, and 12wk and in Group 1 at 12wk. aP<0.05 compared with baseline analyzed by compared t-test.
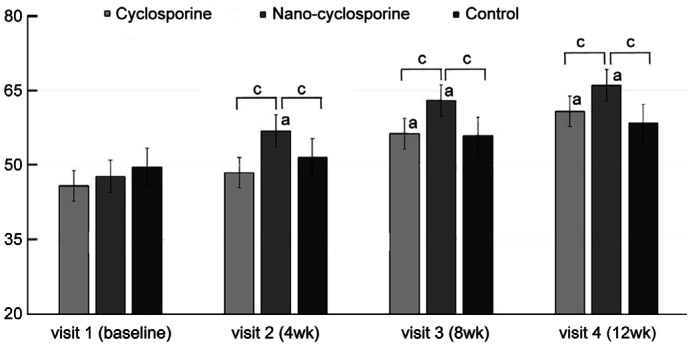
Lipid Layer Thickness
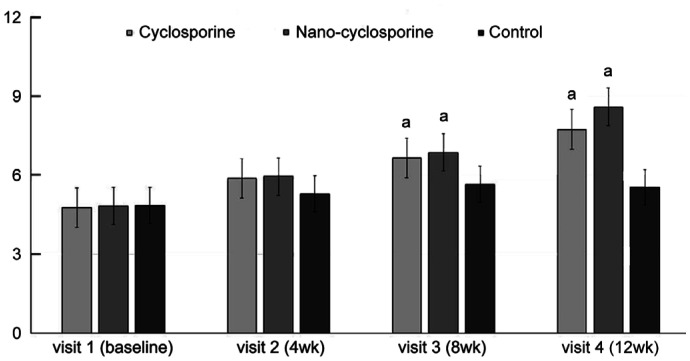
LLT differences between the three groups were not significant at baseline (P=0.277). After treatment, Group 2 showed a significant improvement in LLT at 4, 8, and 12wk of treatment (P<0.001 in all). Group 1 showed a significant improvement of LTT in 8 and 12wk after eye drops treatment (P<0.001 and P=0.002, respectively). Compared with other groups, the LLT of Group 2 showed statistically significant increases at 4, 8, and 12wk of treatment (P=0.002, 0.035, and 0.021, respectively). Group 2 has larger value of LLT than Group 1 (P<0.001, P=0.001, and 0.019, respectively), and Group 3 after 4, 8, and 12wk (P=0.007, 0.018, and 0.004; Figure 9).
Figure 9. Changes in lipid layer thickness from baseline to 12wk in the three groups.
In Group 2 statistically significant improvements from baseline were observed at 4, 8, and 12wk. Statistically significant improvements from baseline were observed in Group 1 at 8 and 12wk. Group 2 showed a significantly higher than other groups at 4, 8, and 12wk of treatment. aP<0.05 compared with baseline analyzed by compared t-test. bP<0.05 analyzed by one-way ANOVA.
DISCUSSION
MGD is characterized by meibomian gland orifice blockage or other abnormalities, which result in inflammation of the meibomian gland and a decrease in lipid secretion, leading to increased evaporation of tears and instability of the tear film. As a result, the ocular surface epithelium is damaged, leading to DES[1]–[4]. Therefore, it is crucial to evaluate MGD in patients with DES. Several studies have reported that the chronic inflammatory state of MGD is associated with an elevation of inflammatory cytokines in tears, such as epidermal growth factor, interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α)[21]–[23]. Such elevation of inflammatory cytokine levels propagates MGD, and inflammation occurs on the ocular surface as well as the eyelid margins through expansion of capillaries, resulting in abnormal meibomian gland secretion[24]. Therefore, the 2011 Tear Film & Ocular Society (TFOS) recommends the initiation of anti-inflammatory treatment in case of occurrence of grade 3 MGD, severe telangiectasia, or occlusion of the meibomian gland orifice[25]–[26]. Cyclosporine inhibits inflammatory cytokine production and T cell activity and acts on the conjunctiva and lacrimal glands to reduce inflammation and increase tear production. Several studies have reported the use of cyclosporine eye drops to improve the symptoms of DES and other inflammatory diseases of the ocular surface[27]–[29].
The particle size of a nano-emulsion ranges from 10 to 100 nm, and it is considered a thermodynamically stable liquid dispersion, whereas that of cyclosporine emulsion ranges from 50 to 1000 nm[30]–[31]. In addition, the bioavailability of cyclosporine and adherence to the treatment with emulsion formulation of cyclosporine tend to be lower than those of nano-emulsion cyclosporine. To overcome the limitations of emulsion formulations, nano-emulsion technology has been introduced to prepare a novel cyclosporine ophthalmic solution. However, only a few studies have focused on the effects of nano-emulsion cyclosporine in improving the symptoms of DES associated with MGD.
The purpose of this study was to investigate the improvement of MGD-related symptoms and decrease in inflammation of the eyelids after using 0.05% Nano-cyclosporine in patients with MGD. Other previous study shows Nano-cyclosporine eyedrops had improved pharmacologic properties relative to conventional cyclosporine in an animal model[32]. Compared with conventional cyclosporine, the Nano-cyclosporine was able to act faster because of smaller and homogenous particle size, and a longer precorneal residence time, and ocular bioavailability. It is thought that reducing particle size and improving the homogeneity of nanotechnology in eye drops could suppress the inflammatory reaction of the ocular surface more effectively. In Groups 1 and 2, LMT and MGS, which reflect the degree of inflammation of the meibomian gland, were significantly improved at 4, 8, and 12wk, whereas in the control group, LMT improved at 8 and 12wk and MGS improved at 12wk of treatment. These results suggest that the groups treated with cyclosporine showed a faster reduction in the meibomian gland inflammation than that in the control group. The control group also showed some improvement, which might be due to the effects of conventional treatment strategies (maintenance of eyelid hygiene and eyelid warm massage), which were practiced by all three groups.
CS and CI, which indicate inflammatory conditions of the ocular surface, showed improvement at 4, 8, and 12wk in Groups 1 and 2. In the control group, CI and CS significantly improved after 8 and 12wk, respectively. CI and LMT are associated with increased interleukin-17 (IL-17) levels in tears. Activation of T-cells in patients with DES and MGD leads to an increase in IL-17 level, which results in increased production of vascular endothelial growth factor (VEGF) and angiogenic chemokines, resulting in CI and LMT[33]–[34]. Improvement of CI in Group 2 treated with nano-emulsion cyclosporine formulation compared to that in Group 1 and the control group could be attributed to improved medication compliance. Nano-emulsion cyclosporine formulation is non-toxic and non-irritant and can be easily applied to the eye[35]. Moreover, it produces quick effects. Because of its non-irritating nature, irritation, burning sensation, foreign body sensation, and blurred vision are significantly reduced during topical instillation of cyclosporine nano-emulsion formulation into the eye[36]–[37]. However, nano-emulsion cyclosporine has a higher solubilization capacity and more thermodynamic stability than unstable dispersions of suspensions[38]. Overall, nano-emulsion cyclosporine has been suggested to have better permeability and efficacy, which might be attributable to the early improvement of CI in Group 2[36]–[37]. Higher permeability of cyclosporine nano-emulsion at the conjunctival surface of the eye than that of cyclosporine emulsion may have beneficial effects[38].
Regarding dry eye symptoms, there was a significant improvement in TBUT in the cyclosporine groups compared with that in the control group from baseline to 12wk of treatment. In addition, STI showed statistically significant improvement in the cyclosporine groups compared with that in the control group at 8 and 12wk of treatment. This indicates that cyclosporine increases tear volume by controlling inflammation of the lacrimal gland and improves tear film stability by increasing mucin secretion and the number and density of conjunctival goblet cells[10],[25].
OSDI is an index reflecting subjective symptoms related to DES; Group 2 showed improvement after 4wk, whereas Group 1 showed improvement after 12wk, which might be related to medication compliance and patient's feeling while using eye drops. Cyclosporine eye drops showed faster efficacy in relieving inflammation of the meibomian gland and ocular surface than that of the control. Moreover, nano-emulsion cyclosporine eye drops presented less ocular sensation discomfort than those of emulsion cyclosporine eye drops.
After treatment, Nano-cyclosporine showed a significant improvement in LLT at 4, 8, and 12wk of treatment. Conventional cyclosporine showed a significant improvement of LTT in 8 and 12wk after eye drops treatment. Compared with control and conventional cyclosporine, the use of nano-emulsion cyclosporine eye drops led to an early improvement LLT than conventional cyclosporine or hyaluronate. The efficacy of cyclosporine on LLT has been limitedly investigated. In a previous study, significant improvement in LLT after 3mo of using conventional cyclosporine was reported[39]. This study also showed significant changes occurred after 8wk of treatment with conventional cyclosporine, In contrast, the Nano-cyclosporine group showed statistically significant change in LLT after 4wk. The statistically significant change in LLT was also confirmed compared to the other two groups.
The limitations of this study are as follows: First, the present study does not classify DES associated with MGD using stepwise classification. Depending on the stage of the disease, the extent and rate of response to treatment may differ, which was not considered. Second, the study was conducted to eliminate the influence of eyelid health management on the improvement of symptoms and signs in all three groups to eliminate ethical problems in the control group. Third, patient compliance to all treatments performed in the trial was not investigated. However, despite these limitations, the purpose of this study was to investigate the effectiveness of 0.05% nano-emulsion cyclosporine eye drops in patients with MGD through a prospective study of disease improvement by measuring the reduction of eyelid inflammation.
In conclusion, similar to the findings of several studies, the present study demonstrated that dry eye symptoms and ocular surface inflammation improved by the use of 0.05% cyclosporine eye drops than hyaluronate. And the use of Nano-cyclosporine eye drops led to an early improvement CS, CI, and LLT than conventional cyclosporine or hyaluronate. Therefore, Nano-emulsion cyclosporine formulation has the potential to control inflammation caused by dry eye with MGD, particularly regarding efficacy and compliance of cyclosporine application.
Acknowledgments
Foundation: Supported by Biomedical Research Institute Grant (No.20200026), Pusan National University Hospital.
Conflicts of Interest: Jo YJ, None; Lee JE, None; Lee JS, None.
REFERENCES
- 1.Wolffsohn JS, Travé Huarte S, Jones L, Craig JP, Wang MTM, TFOS ambassadors Clinical practice patterns in the management of dry eye disease: a TFOS international survey. Ocul Surf. 2021;21:78–86. doi: 10.1016/j.jtos.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Shimazaki J. Definition and diagnostic criteria of dry eye disease: historical overview and future directions. Invest Ophthalmol Vis Sci. 2018;59(14):DES7–DES12. doi: 10.1167/iovs.17-23475. [DOI] [PubMed] [Google Scholar]
- 3.Amano S. Meibomian gland dysfunction: recent progress worldwide and in Japan. Invest Ophthalmol Vis Sci. 2018;59(14):DES87–DES93. doi: 10.1167/iovs.17-23553. [DOI] [PubMed] [Google Scholar]
- 4.Ngo W, Gann D, Nichols JJ. Impact of the 2011 International Workshop on Meibomian Gland Dysfunction on clinical trial attributes for meibomian gland dysfunction. Ocul Surf. 2020;18(1):27–30. doi: 10.1016/j.jtos.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Soh Qin R, Tong Hak Tien L. Healthcare delivery in meibomian gland dysfunction and blepharitis. Ocul Surf. 2019;17(2):176–178. doi: 10.1016/j.jtos.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Villani E, Marelli L, Dellavalle A, Serafino M, Nucci P. Latest evidences on meibomian gland dysfunction diagnosis and management. Ocul Surf. 2020;18(4):871–892. doi: 10.1016/j.jtos.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Sridharan K, Sivaramakrishnan G. Therapies for meibomian gland dysfunction: a systematic review and meta-analysis of randomized controlled trials. Open Ophthalmol J. 2017;11:346–354. doi: 10.2174/1874364101711010346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T. Inflamed obstructive meibomian gland dysfunction causes ocular surface inflammation. Invest Ophthalmol Vis Sci. 2018;59(14):DES94–DES101. doi: 10.1167/iovs.17-23345. [DOI] [PubMed] [Google Scholar]
- 9.Kam WR, Liu Y, Ding J, Sullivan DA. Do cyclosporine A, an IL-1 receptor antagonist, uridine triphosphate, rebamipide, and/or bimatoprost regulate human meibomian gland epithelial cells? Invest Ophthalmol Vis Sci. 2016;57(10):4287–4294. doi: 10.1167/iovs.16-19937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boboridis KG, Konstas AGP. Evaluating the novel application of cyclosporine 0.1% in ocular surface disease. Expert Opin Pharmacother. 2018;19(9):1027–1039. doi: 10.1080/14656566.2018.1479742. [DOI] [PubMed] [Google Scholar]
- 11.Perry HD, Donnenfeld ED. Topical 0.05% cyclosporin in the treatment of dry eye. Expert Opin Pharmacother. 2004;5(10):2099–2107. doi: 10.1517/14656566.5.10.2099. [DOI] [PubMed] [Google Scholar]
- 12.de Paiva CS, Pflugfelder SC, Ng SM, Akpek EK. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst Rev. 2019;9(9):CD010051. doi: 10.1002/14651858.CD010051.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang-Liu DD, Acheampong A. Ocular pharmacokinetics and safety of ciclosporin, a novel topical treatment for dry eye. Clin Pharmacokinet. 2005;44(3):247–261. doi: 10.2165/00003088-200544030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CH, Wang PY, Lin IC, Huang H, Liu GS, Tseng CL. Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci. 2018;19(9):2830. doi: 10.3390/ijms19092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarei-Ghanavati S, Nooghabi MJ, Zamani G. Comparison of the effect of tea tree oil shampoo with regular eyelid shampoo in meibomian gland dysfunction treatment. Am J Ophthalmol. 2021;229:45–51. doi: 10.1016/j.ajo.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 17.Arita R, Suehiro J, Haraguchi T, Maeda S, Maeda K, Tokoro H, Amano S. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2013;97(6):725–729. doi: 10.1136/bjophthalmol-2012-302668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellegrini M, Bernabei F, Moscardelli F, Vagge A, Scotto R, Bovone C, Scorcia V, Giannaccare G. Assessment of corneal fluorescein staining in different dry eye subtypes using digital image analysis. Transl Vis Sci Technol. 2019;8(6):34. doi: 10.1167/tvst.8.6.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begley C, Caffery B, Chalmers R, Situ P, Simpson T, Nelson JD. Review and analysis of grading scales for ocular surface staining. Ocul Surf. 2019;17(2):208–220. doi: 10.1016/j.jtos.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Ayyildiz T, Sezgin FM. The effect of ocular demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(Suppl 1):S39–S41. doi: 10.1097/ICL.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 21.Nicolle P, Liang H, Reboussin E, Rabut G, Warcoin E, Brignole-Baudouin F, Melik-Parsadaniantz S, Baudouin C, Labbe A, Reaux-Le Goazigo A. Proinflammatory markers, chemokines, and enkephalin in patients suffering from dry eye disease. Int J Mol Sci. 2018;19(4):E1221. doi: 10.3390/ijms19041221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205.e1. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudouin C, Irkeç M, Messmer EM, Benítez-Del-Castillo JM, Bonini S, Figueiredo FC, Geerling G, Labetoulle M, Lemp M, Rolando M, Van Setten G, Aragona P, ODISSEY European Consensus Group Members Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96(2):111–119. doi: 10.1111/aos.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, Lemp MA, Sullivan DA. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HY, Lee JE, Oh HN, Song JW, Han SY, Lee JS. Clinical efficacy of combined topical 0.05% cyclosporine A and 0.1% sodium hyaluronate in the dry eyes with meibomian gland dysfunction. Int J Ophthalmol. 2018;11(4):593–600. doi: 10.18240/ijo.2018.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prabhasawat P, Tesavibul N, Mahawong W. A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea. 2012;31(12):1386–1393. doi: 10.1097/ICO.0b013e31823cc098. [DOI] [PubMed] [Google Scholar]
- 27.Periman LM, Mah FS, Karpecki PM. A review of the mechanism of action of cyclosporine A: the role of cyclosporine A in dry eye disease and recent formulation developments. Clin Ophthalmol. 2020;14:4187–4200. doi: 10.2147/OPTH.S279051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byun YS, Rho CR, Cho K, Choi JA, Na KS, Joo CK. Cyclosporine 0.05% ophthalmic emulsion for dry eye in Korea: a prospective, multicenter, open-label, surveillance study. Korean J Ophthalmol. 2011;25(6):369–374. doi: 10.3341/kjo.2011.25.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Yoon TJ, Kim KH. Cinical effect of restasis® eye drops in mild dry eye syndrome. J Korean Ophthalmol Soc. 2009;50(10):1489. [Google Scholar]
- 30.Thakur A, Walia MK, Kumar SL. Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: a review. Pharmacophore. 2013;4(1):15–25. [Google Scholar]
- 31.Čerpnjak K, Zvonar A, Gašperlin M, Vrečer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63(4):427–445. doi: 10.2478/acph-2013-0040. [DOI] [PubMed] [Google Scholar]
- 32.Bang SP, Yeon CY, Adhikari N, Neupane S, Kim H, Lee DC, Son MJ, Lee HG, Kim JY, Jun JH. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS One. 2019;14(11):e0224805. doi: 10.1371/journal.pone.0224805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7(1):38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci. 2011;26(7):938–944. doi: 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sing AJF, Graciaa A, Lachaise J, Brochette P, Salager JL. Interactions and coalescence of nanodroplets in translucent O/W emulsions. Colloids Surf A Physicochem Eng Asp. 1999;152(1-2):31–39. [Google Scholar]
- 36.Aksungur P, Demirbilek M, Denkbaş EB, Vandervoort J, Ludwig A, Ünlü N. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: cellular toxicity, uptake, and kinetic studies. J Control Release. 2011;151(3):286–294. doi: 10.1016/j.jconrel.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Park CH, Kim MK, Kim EC, Kim JY, Kim TI, Kim HK, Song JS, Yoon KC, Lee DH, Lee HK, Chung TY, Choi CY, Kim HS. Efficacy of topical cyclosporine nanoemulsion 0.05% compared with topical cyclosporine emulsion 0.05% and diquafosol 3% in dry eye. Korean J Ophthalmol. 2019;33(4):343–352. doi: 10.3341/kjo.2018.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66(2):227–243. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Kang MS, Shin J, Kwon JM, Huh J, Lee JE. Efficacy of 0.05% cyclosporine A on the lipid layer and meibomian glands after cataract surgery: a randomized, double-masked study. PLoS One. 2021;16(1):e0245329. doi: 10.1371/journal.pone.0245329. [DOI] [PMC free article] [PubMed] [Google Scholar]