Dear Editor,
Cefuroxime is a second-generation cephalosporin and a member of the β-lactam family of antibiotics. Intracameral cefuroxime injection (1 mg/0.1 mL) during cataract surgery was found to be beneficial in reducing the risk of postoperative endophthalmitis[1]–[3]. At the recommended dose of 1.0 mg in 0.1 mL, cefuroxime presents no ocular toxicity and is well tolerated[4]–[6]. Acute macular edema with retinal detachment after cataract surgery is very rare[7].
In China, there is no manufactured product for intracameral injection like Aprokam, therefore, there is a need for compounding for each surgical case. At Qingdao Eye Hospital, we used a balanced salt solution (BSS) containing cefuroxime (1 mg/1 mL) during phacoemulsification (phaco) surgery instead of a postoperative intracameral injection. The use of cefuroxime in cataract surgery has been approved by the Hospital Pharmacy Committee and Ethics Committee. Here, we report four cases of toxicity to the macula after using BSS containing small doses cefuroxime (1 mg/1 mL) for phaco and pars plana vitrectomy (PPV) combined surgery by mistake. The publication of this study was approved by the Research Ethics Committee of Qingdao Eye Hospital and written informed consent was obtained from every patient.
Four patients were found through retrospective investigation to have severe macular edema with extensive serous retinal detachment and postoperative vision loss on the first day after an uneventful phaco and PPV combined surgery performed between October and November 2017 (Table 1).
Table 1. Preoperative conditions and surgical information.
| No. | Sex | Age (y) | Diagnosis | Eye | Pre-operation |
Length of operation (min) | Type of IOL | ||
| BCVA | IOP (mm Hg) | Axial length (mm) | |||||||
| 1 | M | 54 | Lens subluxation & secondary glaucoma | OS | 0.6 | 35 (21) | 23.22 | 45 | AMO AR40e |
| 2 | M | 61 | Lens subluxation & secondary glaucoma | OD | 0.3 | 56 (11) | 23.26 | 45 | AMO AR40e |
| 3 | F | 67 | Cataract & macular epiretinal membrane | OD | 0.2 | 15 | 31.00 | 25 | B&L ADAPT-AO |
| 4 | F | 72 | Cataract & macular epiretinal membrane | OD | 0.4 | 9 | 24.13 | 35 | B&L ADAPT-AO |
BCVA: Best-corrected visual acuity; M: Male; F: Female; IOP: Intraocular pressure.
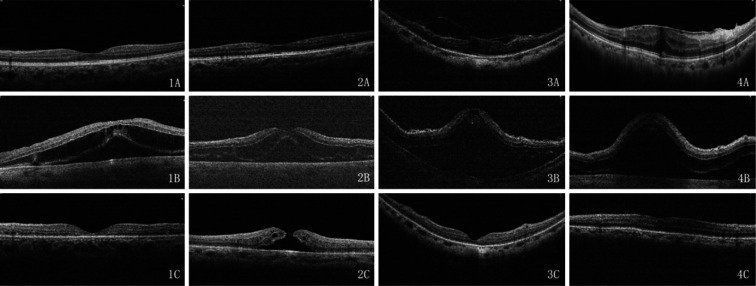
Case 1 had lens subluxation and secondary glaucoma for 2mo. Intraocular pressure (IOP) was up to 35 mm Hg and was controlled to 21 mm Hg using brinzolamide/timolol eye drops (Azarga, Alcon, Ireland) before surgery, however, the cup-disc ratio was about 0.6. Case 2 had lens subluxation and secondary glaucoma for three days due to ocular contusion. IOP was up to 56 mm Hg and was controlled to 11 mm Hg with methazolamide before surgery. Preoperative optical coherence tomography (OCT) examination of the two patients showed that the macula was normal (Figure 1: 1A, 2A). Cases 3 and 4 had cataracts and macular epiretinal membrane with normal IOP. The OCT showed that the epiretinal membrane pulled the macular forming edema (Figure 1: 3A, 4A).
Figure 1. The four patients' OCT examination on pre-operation (A), 1st day post operation (B), and 9th/10th day post operation (C).
OCT: Optical coherence tomography.
The patients who had lens subluxation accepted phaco/PPV/intraocular lens (IOL) suspension surgery. The patients who had macular epiretinal membrane accepted phaco/PPV/membrane peeling surgery. BSS containing cefuroxime sodium was used in the phaco step and continued to be used in subsequent operations by mistake. Ordinarily, 750 mg of cefuroxime sodium injectable powder is reconstituted with 7.5 mL of normal saline (sodium chloride, 0.9%), then 5 mL of the resulting solution is injected into a bottle of 500 mL BSS, leaving a final cefuroxime concentration of nearly 1 mg/1 mL. Triamcinolone acetonide was injected to visualize the vitreous gel and posterior hyaloid during the PPV and to reduce the inflammatory reaction at the end of the operation.
The first day after the operation, the visual acuity of each patient was worse than preoperative and IOP was normal (Table 2). Fundus examination showed no foveal reflection in the macula. Diffuse retinal edema affected most of the posterior pole. No significant abnormality was found in the peripheral retina. The OCT exam showed that macular edema mostly manifested at the outer nuclear layer and shallow serous retinal detachment around the macula (Figure 1: 1B-4B). Routine eye drops were given to the patients, including prednisolone acetate, gatifloxacin, and pranoprofen, but the dosage of prednisolone acetate increased from q.i.d. to once every 2h for the macular edema.
Table 2. Postoperative vision and IOP.
| No. | 1st day |
9th or 10th day |
Follow-up |
||||
| VA | IOP | VA | IOP | VA | IOP | Length | |
| 1 | 0.2 | 17 | 0.02 | 13 | 0.02 | 19 | 11mo |
| 2 | FC/30 cm | 14 | 0.12 | 40 | 0.1 | 14 | 10mo |
| 3 | 0.1 | 11 | 0.5 | 16 | 0.5 | 15 | 12mo |
| 4 | 0.1 | 9 | 0.3 | 13 | 0.3 | 15 | 12mo |
IOP: Intraocular pressure; VA: Visual acuity; FC: Finger counting.
After nine or 10d, all the patients' macular edema was absorbed. The macular retina was scanned by OCT, and the image showed that the retinal thickness of the fovea significantly decreased and the integrated ellipsoid zone was discontinuous or disappeared from the outer retina (Figure 1: 1C-4C).
After nine days, the case 2 patient's vision recovered to 0.12 and the IOP rose to 40 mm Hg. Brinzolamide eye drops (Azopt, Alcon, Ireland) and bimatoprost/timolol maleate eye drops (Ganfort, Allergan, Ireland) were given to control the IOP to 8-10 mm Hg. OCT showed that macular edema at the outer layer was absorbed and formed a macular hole that resembled a crater (Figure 1: 2C). The case 3 patient's vision recovered to 0.3 on the 2nd day after surgery and then to 0.5 by the 10th day after surgery. The fellow eye had phaco and IOL implanted 1wk later with visual acuity of 0.6.
Intracameral cefuroxime sodium at a concentration of 1 mg/0.1 mL has been shown to be efficacious and safe[1]. Intravitreal injection of 0.1-1 mg cefuroxime in uninflamed rabbit eyes produced intravitreal levels close to the minimum inhibitory concentration for most ocular pathogens up to 24h after drug administration. Mild histopathological changes were seen, however, electroretinography showed no definite changes suggestive of retinal toxicity in rabbit eyes[8]. Some reports have shown a long-term deleterious effect on the retina from injection of cefuroxime sodium in high concentration[4],[9].
Our patients showed severe macular edema and shallow serous retinal detachment on the first day post-operation, and the edema gradually decreased for about 10d. The macular lesions in cases 1 and 2 were more significant. Their surgery times were 10 or 20min longer than those of cases 3 and 4. The surgery time of case 3 was the shortest and the damage was the least. However, the amount of cefuroxime sodium that actually flowed through the vitreous cavity and was absorbed by the retina cells during surgery could not be calculated. Compared to the time that cefuroxime sodium is retained in the eye after surgery, the time difference of 10-20min may be meaningless.
After the routine phaco surgery, part of the intracameral antibiotic diffused in the vitreous cavity. The intravitreal diffusion of an intracameral drug is difficult to predict because it is based on lens status[10], the stage of liquefaction of the vitreous, and intraocular inflammation[11]. At the end of our patient's surgery, the vitreous cavity was filled with BSS containing cefuroxime (1 mg/1 mL), so according to the vitreous volume of 5 mL, the cefuroxime sodium in the eye was approximately 5 mg. Case 3 had high myopia, with eye axis of 31.00 mm. The cefuroxime sodium dose in her vitreous cavity must have been more than 5 mg, which was the highest, but macular damage was not the most serious. Therefore, the dose difference of cefuroxime sodium in the eye may not be a determinant of the degree of macular damage.
Shahar et al[12] showed that cefuroxime sodium can cause damage to rabbit retinal glial cells, especially Müller cells. High IOP can also cause damage to the retinal nerve fiber layer and loss of glial cells. The preoperative IOP of cases 1 and 2 was significantly elevated. We consider that the double effects of drug toxicity and high IOP may be responsible for the significant thinning of the nerve fibers in the macular region and even the formation of macular holes in case 2. At the end of the operation, we routinely inject 2 mg triamcinolone acetonide into the vitreous cavity to reduce the inflammatory reaction. We presume that glucocorticoids have no effect on reducing the toxic damage of cefuroxime sodium.
Cefuroxime sodium should be avoided entering the vitreous cavity to prevent irreversible damage to the macular structure. The high IOP may have a synergistic effect with the retinal toxicity of cefuroxime sodium, which significantly increases the damage.
Acknowledgments
Conflicts of Interest: Jing LL, None; Ruan T, None; Li DF, None; Li J, None.
REFERENCES
- 1.ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978–988. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Bowen RC, Zhou AX, Bondalapati S, et al. Comparative analysis of the safety and efficacy of intracameral cefuroxime, moxifloxacin and vancomycin at the end of cataract surgery: a Meta-analysis. Br J Ophthalmol. 2018;102(9):1268–1276. doi: 10.1136/bjophthalmol-2017-311051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun JJ, Guo Z, Li HL, Yang BX, Wu XM. Acute infectious endophthalmitis after cataract surgery: epidemiological characteristics, risk factors and incidence trends, 2008-2019. Infect Drug Resist. 2021;14(1):1231–1238. doi: 10.2147/IDR.S304675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delyfer MN, Rougier MB, Leoni S, Zhang QH, Dalbon F, Colin J, Korobelnik JF. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. J Cataract Refract Surg. 2011;37(2):271–278. doi: 10.1016/j.jcrs.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 5.Ma B, Liu Y, Liu SR, Luo M. Evaluation of the effect of intracameral cefuroxime on macular and subfoveal choroidal thickness and macular sensitivity in diabetic patients after cataract surgery. J Cataract Refract Surg. 2017;43(2):201–206. doi: 10.1016/j.jcrs.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Wei Y, Li H, Zheng S, Wu X. Macular toxicity of low-concentration cefuroxime during cataract surgery in vitrectomized eyes. Ophthalmic Res. 2022:2022. doi: 10.1159/000526449. [DOI] [PubMed] [Google Scholar]
- 7.Besozzi G, di Salvatore A, Cardillo D, Finzi A, Pinackatt JS, Baldi A, Monfardini A, Forioli V, Frisina R, Parolini B. Intracameral cefuroxime in combined pars plana vitrectomy and phacoemulsification: a study of safety. Clin Ophthalmol. 2018;12:1567–1570. doi: 10.2147/OPTH.S170751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koul S, Philipson A, Philipson BT, Kock E, Nylén P. Intraocular levels of cefuroxime in uninflamed rabbit eyes. Acta Ophthalmol (Copenh) 1990;68(4):455–465. doi: 10.1111/j.1755-3768.1990.tb01676.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamal-Salah R, Osoba O, Doyle E. Ocular toxicity after inadvertent intracameral injection of high dose of cefuroxime during cataract surgery: a case series. Retin Cases Brief Rep. 2019;13(3):269–272. doi: 10.1097/ICB.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 10.Shaarawy A, Meredith TA, Kincaid M, Dick J, Aguilar E, Ritchie DJ, Reichley RM. Intraocular injection of ceftazidime. Effects of inflammation and surgery. Retina. 1995;15(5):433–438. [PubMed] [Google Scholar]
- 11.Koul S, Philipson A, Philipson BT, Arvidson S. Intraocular levels of cefuroxime in inflamed rabbit eyes. Eur J Ophthalmol. 1993;3(2):61–65. doi: 10.1177/112067219300300202. [DOI] [PubMed] [Google Scholar]
- 12.Shahar J, Zemel E, Perlman I, Loewenstein A. Physiological and toxicological effects of cefuroxime on the albino rabbit retina. Invest Ophthalmol Vis Sci. 2012;53(2):906–914. doi: 10.1167/iovs.11-8053. [DOI] [PubMed] [Google Scholar]