Abstract
AIM
To investigate the anti-inflammatory effects of the sesquiterpenes α-humulene and β-caryophyllene on pterygium fibroblasts.
METHODS
Primary cultures of pterygium fibroblasts were established. Third passage pterygium fibroblasts were exposed to α-humulene and β-caryophyllene separately and together. The cell viability was assessed by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay at 12, 24, 48, and 72h after exposure. The levels of the inflammatory cytokines interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α and IL-10 in the conditioned culture medium were assessed by enzyme-linked immunosorbent assay (ELISA) at 12, 24 and 48h after exposure. Data were statistically analyzed using Friedman repeated measures analysis of variances on ranks.
RESULTS
The 25 µmol/L β-caryophyllene induced significant decrease in the IL-6 production by pterygium fibroblasts 48h after the exposure (P=0.041). The levels of IL-1β, IL-8, IL-10, and TNF-α were very low and had no statistically significant variations after exposure to α-humulene, β-caryophyllene, or both compounds together.
CONCLUSION
The exposure to 25 µmol/L of β-caryophyllene significantly reduce the production of IL-6 by pterygium fibroblasts after 48h. This sesquiterpene may be a potential alternative adjuvant agent for the treatment of pterygium.
Keywords: pterygium, fibroblasts, β-caryophyllene, α-humulene, cytokines
INTRODUCTION
Pterygium is a benign triangular or trapezoidal lesion of the bulbar conjunctiva, composed by hyperplastic fibroblasts without atypia, which grows toward the cornea, infiltrating the corneal surface[1]. Despite being documented for centuries, the exact reasons for its etiology, pathogenesis and treatment remain unclear. Environmental stimuli, as UVB radiation exposure is considered the most acceptable etiological factor, inducing the production of pro-inflammatory cytokines by corneal limbal epithelial cells[2]–[3]. High levels of chronic inflammation, angiogenesis, degrading enzymes and anti-apoptotic mechanisms were confirmed in pterygium biopsies samples[4]–[5].
It was already proved that inflammation play a role in the development and severity of pterygium, inducing progressive proliferation of fibrovascular tissue[6]–[7]. Hence inflammation is a significant pathogenic factor, new anti-inflammatory therapies should be investigated.
Terpenes are natural compounds which proven bioactivities and can be used for new drugs development. The biological and pharmacological properties of these phytomolecules include antimicrobial, antifungal, antiviral, anti-inflammatory, antitumoral, among others[8]–[9]. Terpenes are structurally diversified and categorized according to the number of isopentenyl pyrophosphate units as hemiterpene (1 unit), monoterpene (2 units), sesquiterpene (3 units); diterpene (4 units), triterpene (6 units), tetraterpene (8 units) and polyterpenes (over 8 units)[10].
The sesquiterpenes β-caryophyllene and α-humulene have pronounced anti-inflammatory properties, being comparable to dexamethasone, by the regulation of inflammatory proteins expression[11]–[12]. Recent studies demonstrated the ability of β-caryophyllene to reduce pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), ameliorating chronic pathologies characterized by inflammation and oxidative stress, in particular metabolic and neurological diseases, in addition to analgesic, anesthetic, and anti-tumoral effects in vitro and in vivo[13]–[15].
The pathogenesis of pterygium is not completely understood, however there are the inflammatory and tumoral theories, which would be controlled by the action of the sesquiterpenes. As it was already demonstrated that inflammatory cytokines, including IL-6, IL-8, and vascular endothelial growth factor (VEGF) are elevated in the tears of the patients with pterygium[16]–[17] the present study investigated the effects of the sesquiterpenes β-caryophyllene and α-humulene (chemical structures in Figure 1A and 1B) on the production of inflammatory cytokines by pterygium fibroblasts, in order to determine if they can be used in the adjuvant treatment of pterygium.
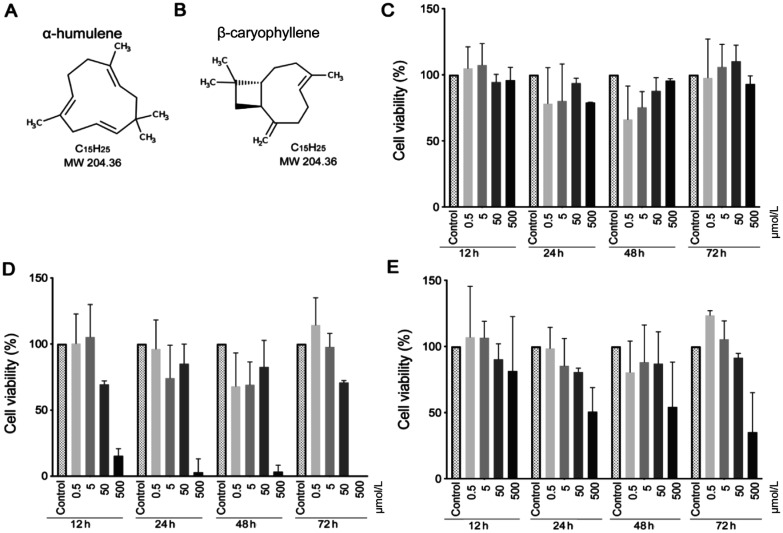
Figure 1. α-humulene and β-caryophyllene structures, and MTT cell viability study.
A, B: Molecular structures of α-humulene and β-caryophyllene; C, D: Cell viability of the pterygium fibroblasts measured by MTT assay after exposure to α-humulene and β-caryophyllene; E: Both compounds, analyzed for 12, 24, 48, and 72h. Error bars reveal the means ± standard deviation in triplicate tests (n=8).
MATERIALS AND METHODS
Ethical Approval
The study was approved by the Institutional Review Board and Research Ethics Committee of the Botucatu Medical School, Sao Paulo–Brazil (CEUA 1001/2013), and was conducted in accordance to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants. The study was registered at the Genetic Heritage Management Council of the Brazil Ministry of Environment (SISGEN).
This was an experimental in vitro study to evaluate the effects of two sesquiterpenes, β-caryophyllene and α-humulene, on primary pterygium fibroblasts in culture. Eight patients (age ranged from 49 to 67y, 63.3% female) had samples of Tenon's capsules of the body of primary pterygium collected during surgical exeresis. After the removal, the surgical specimens were placed in sterile microtubes and stored (for no longer than 3h) at 5°C until processing.
The exclusion criteria were recurrent lesions, previous ocular surgeries (e.g., glaucoma filtering surgery) or other ocular surface diseases (e.g., keratoconjunctivitis sicca).
β-caryophyllene and α-humulene
α-humulene (PubChem CID 5281520) and β-caryophyllene (PubChem CID 5281515) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). To make the stock solutions, α-humulene was dissolved in dimethilsulfoxide (DMSO) and β-caryophyllene in ethanol, as it is insoluble in water. Complete nutrient medium was added to the stock solutions to obtain the different concentrations used in the study.
Cell Cultures
The fibrovascular pterygium tissue samples were cut into approximately 1×1 mm2 fragments, washed with phosphate buffered saline solution (PBS) and transferred into tissue culture flasks containing Dulbecco's modified Eagle's culture medium: nutrient mixture F-12 (DMEM/F12; Gibco, Grand Island, NY, USA) supplemented with 15% fetal calf serum (FCS; Gibco, Grand Island, NY, USA), 0.01 U/mL human recombinant insulin (Sigma-Aldrich, Saint Louis, MO, USA), penicillin G (100 UI/mL), gentamycin (40 µg/mL) and amphotericin B (2 µg/mL; Sigma-Aldrich, Saint Louis, MO, USA). The cultures were maintained in humidified incubator with 5% CO2 at 37°C. Nutrient medium was added every 3 or 4d until the cultures reach 80% to 90% confluence, when the fibroblasts were detached with 0.25% trypsin- (EDTA) (Gibco, Grand Island, NY, USA) and subcultured until the third passage, when they were used in the study.
Cell Viability Study
Initially, the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay (MTT) was performed in order to establish the not cytotoxic and ideal anti-inflammatory concentration range of α-humulene and β-caryophyllene. Third passage 5×103 pterygium fibroblasts were seeded in each well of 96-well plates and maintained for 24h in humidified incubator with 5% CO2 at 37°C, for cell adherence. Subsequently, the cells were exposed in triplicates to 0.5, 5, 50, and 500 µmol/L of α-humulene, β-caryophyllene and both compounds. The controls contained only the vehicles of each compound with the same concentrations as the compared groups, at four exposure times: 12, 24, 48, and 72h. After each exposure time, the nutrient mediums in contact to the cells were removed and 0.5 mg/mL MTT solution (Sigma Aldrich-St Louis, MO, USA) was added to each well and the cells were incubated again at 37°C for 4h. The solution was removed and 200 µL of DMSO was added to each well. The optical density as the parameter of cell viability was measured at 570 nm in a spectrophotometer (Titertek Multiskan, Flow Laboratories, McLean, VA, USA) with reference wavelength of 630 nm to obtain the sample signal (OD570-OD630).
The cell viability was calculated using the equation: cell viability (%) = [(Asample - Ablank) / (Acontrol - Ablank) ×100%
Where A = absorbance in nm.
Study of Cytokines
The inflammatory cytokines were assessed by enzyme-linked immunosorbent assay (ELISA) from the conditioned culture medium of 5×103 pterygium fibroblasts/well seeded in 96-well, in triplicates, that were incubated for 24h with 5% CO2 at 37°C with complete nutrient medium. The cells were exposed to 0.25, 2.5, and 25 µmol/L of α-humulene, β-caryophyllene, both compounds and only the vehicles in the same tested concentration for the controls, during 0, 12, 24, and 48h. These concentrations were assessed previously by the MTT viability study as no cytotoxic for the pterygium fibroblasts.
Commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to quantify IL-1β, IL-6, IL-8, IL-10, and TNF-α levels in the studied samples. All assays were performed according to the manufacturer's instructions. The assays readers were performed in an ELISA automatic reader (Epoch-BioTek, Winooski, VT, USA), at wavelength of 492 nm. The concentrations of cytokines in the conditioned culture medium were calculated on the standard curve obtained with different concentrations of the recombinant human cytokines of interest and tests were performed to determine the inter and intra-assay variability.
Statistical Analysis
Data were statistically analyzed using Friedman repeated measures analysis of variances on ranks, if justified by the statistical probability (P<0.05). The differences between the control and other treatments were considered statistically significant if P<0.05 using Dunn's test.
RESULTS
Cell Viability Study
MMT test showed that the concentrations of 50 to 500 µmol/L considerably decreased the cell viability, mainly after 72h with β-caryophyllene (Figure 1C, 1D, 1E). Based on these results, to avoid the excessive cytotoxicity at high doses of sesquiterpenes, the optimal concentrations for the cytokines study were defined as 0.25, 2.5 and 25 µmol/L for each compound.
Cytokines Study
Initially the cytokine production was assessed in two primary cultures of pterygium fibroblasts, with measures at every four hours until 72h after exposure to the phytochemical compounds to determine the kinetic of the cytokines production to define the better evaluation time points. The experiment was repeated with another six primary cell cultures, with cytokine dosages at 0, 12, 24, and 48h after exposure to the tested sesquiterpenes.
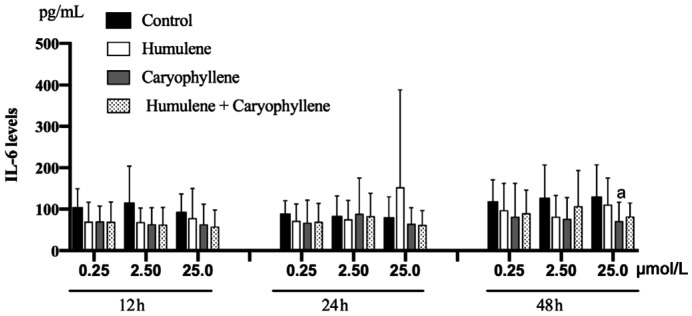
The IL-6 levels significantly decreased after 48h of the exposure to 25 µmol/L β-caryophyllene (P=0.041). There were no statistically significant changes in the remaining compounds and with the use of the other different concentrations (P>0.05, for all comparisons; Figure 2).
Figure 2. IL-6 levels obtained by ELISA after exposure to 0.25, 2.5, and 25 µmol/L α-humulene, β-caryophyllene and both compounds after 12, 24, and 48h of exposure.
IL-6 levels significantly decreased after 48h of exposure to 25 µmol/L caryophyllene (aP=0.041). There were no statistically significant changes in the remaining compounds and in their concentrations. Error bars reveal the means±standard deviation in triplicate tests (n=8).
The production of IL-1β, IL-8, IL-10, and TNF-α were very low, with no statistically significant changes in their production after exposure to the phytochemical compounds evaluated in this study (P>0.05, for all comparisons).
DISCUSSION
The current study, to our knowledge, is the first to evaluate the β-caryophyllene and α-humulene effects on pterygium fibroblasts assessing the production of inflammatory cytokines. The rationale behind the use of these sesquiterpenes in the treatment of pterygium is explained by the pterygium pathogenesis, which involves inflammation and cell proliferation, the known anti-inflammatory and anti-cancer effects of β-caryophyllene and α-humulene could justify the successful inhibition of the pterygium cell growth.
β-caryophyllene is a natural bicyclic sesquiterpene, present in various essential oils from medicinal plants, with anti-inflammatory activity through its agonist action on the receptor cannabinoid receptor 2 (CB2) and inflammatory mediators such as TNF-α[13]–[14]. However, in the nature, β-caryophyllene is frequently found associated to α-humulene[18]. The anti-inflammatory effect of α-humulene is related to its action on the pro-inflammatory cytokines TNF-α and IL-1β[11]. In addition, α-humulene induces intracellular depletion of glutathione and increases the production of reactive oxygen species, which may be responsible for its cytotoxicity to cancer cells, which increases significantly if α-humulene is combined with β-caryophyllene[18]. In contrast, β-caryophyllene alone does not inhibit cancer cell growth. Several cyclic hydrocarbons, including sesquiterpenes, have been shown to accumulate in the biological membrane bilayer, leading it to swell and resulting in an increased membrane fluidity and permeability, facilitating the passage of bioactive compounds through the cancer cells cytoplasmic membrane, consequently increasing the intracellular accumulation of anticancer agents, such as 5-fluorouracil and paclitaxel, potentiating their cytotoxicity[19]. This anti-cancer activity could explain the anti-proliferative effect on pterygium fibroblasts, since one of proposed pathogenesis mechanism postulates that this lesion can be a tumor. Due to this mechanism, we analyzed the effect of α-humulene and β-caryophyllene separately and together.
The MMT assay showed that α-humulene, β-caryophyllene and both compounds together had the same cytotoxic effect when used with the lowest concentrations (0.5 to 5 µmol/L), which are the in vitro safe concentration range. The 50 to 500 µmol/L concentrations were very high, showing important cytotoxic effect, mainly for β-caryophyllene (P<0.05). Our findings are in accordance to others, which verified the same non-cytotoxic concentration for α-humulene and β-caryophyllene in murine splenocytes[11]. However, we assessed the safe concentration of these sesquiterpenes by MTT viability assay on pterygium fibroblasts before the anti-inflammatory cytokines study, as different cells may involve others cytotoxicity mechanisms.
Our study confirmed that IL-6 is the main interleukin produced by pterygium fibroblasts being an abundant pro-inflammatory cytokine in inflamed corneas[20]. The exposure of pterygium fibroblasts to 25 µmol/L β-caryophyllene caused a statistically significant anti-inflammatory effect (P=0.041), decreasing IL-6 levels 48h after the exposure. This finding suggests that β-caryophyllene can have a significant anti-inflammatory effect but requires at least 48h to decrease the IL-6 levels, due to the proteins transcription required time. There was no statistical significance with the other compounds, concentrations and exposure times regarding the IL-6 production. Although we did not expose our cultures to UV irradiation, it was already observed that UVB irradiation on pterygium epithelial cells induces an increase in the expression of IL-6 and IL-8, resulting in a time-course-dependent induction of secretion above the constitutive levels of IL-6[21]. These observations suggest that IL-6 is one of the key cytokines involved in pterygium progression and recurrence, acting by mitogen-activated protein kinase (MAPK) and NF-κB pathways[22]. Recently, a growing number of reports has been described the multiple protective effects of β-caryophyllene in several disorders, mainly as anti-inflammatory agent due to its ability to inhibit the main inflammatory mediators, such as IL-1β, IL-6, TNF-α, NF-κB, cyclooxygenase 1 (COX-1), cyclooxygenase 2 (COX-2), and inducible nitric oxide synthase (iNOS)[13].
Our findings demonstrated the anti-inflammatory effect of β-caryophyllene on the IL-6 production in pterygium fibroblasts. Future studies are required to explore the therapeutic potential and clinical application of the β-caryophyllene for the treatment of pterygium.
A limitation of the study was the cytokine measures based on established primary cultures from only eight tissue samples obtained from unrelated patients. The successful establishment of primary cell cultures is time-consuming and requires technical skills. However, our findings are relevant because although the experimental model used presents disadvantages, such as limited cell-cycle divisions and expansion capacity, primary cell cultures show normal cell morphology and maintain many of the biological and functional features observed in vivo. Further studies can analyze β-caryophyllene oxide, in addition to β-caryophyllene and a-humulene, as these sesquiterpenes are frequently found associated in the nature, and there are relates that this compound can also contributes on immune system modulation[23].
In conclusion, our study demonstrated that 25 µmol/L β-caryophyllene showed significant anti-inflammatory effect on IL-6 production on pterygium fibroblasts, after 48h of exposure. This compound can represent a potential alternative adjuvant agent in the management of pterygium.
Acknowledgments
Foundations: Supported by Sao Paulo State Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP; No.2012/10032-4). Viveiros MMH was supported by a fellowship by Coordination for the Improvement of Higher Level Education Personnel (CAPES), Ministry of Education, Brazil (No.06460/2013).
Conflicts of Interest: Viveiros MMH, None; Silva MG, None; Costa JGM, None; Oliveira AG, None; Rubio C, None; Padovani CR, None; Rainho CA, None; Schellini SA, None.
REFERENCES
- 1.Shahraki T, Arabi A, Feizi S. Pterygium: an update on pathophysiology, clinical features, and management. Ther Adv Ophthalmol. 2021;13:25158414211020152. doi: 10.1177/25158414211020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanzeler ACV, Barbosa IAF, Duarte B, et al. Mechanisms and biomarker candidates in pterygium development. Arq Bras Oftalmol. 2019;82(6):528–536. doi: 10.5935/0004-2749.20190103. [DOI] [PubMed] [Google Scholar]
- 3.di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23(2):195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Josifovska N, Szabó DJ, Nagymihály R, Veréb Z, Facskó A, Eriksen K, Moe MC, Petrovski G. Cultivation and characterization of pterygium as an ex vivo study model for disease and therapy. Contact Lens Anterior Eye. 2017;40(5):283–292. doi: 10.1016/j.clae.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kim KW, Kim JC. Current approaches and future directions in the management of pterygium. Int J Ophthalmol. 2018;11(5):709–711. doi: 10.18240/ijo.2018.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.di Girolamo N, Wakefield D, Coroneo MT. UVB-mediated induction of cytokines and growth factors in pterygium epithelial cells involves cell surface receptors and intracellular signaling. Invest Ophthalmol Vis Sci. 2006;47(6):2430–2437. doi: 10.1167/iovs.05-1130. [DOI] [PubMed] [Google Scholar]
- 7.Aletras AJ, Trilivas I, Christopoulou ME, Drakouli S, Georgakopoulos CD, Pharmakakis N. UVB-mediated down-regulation of proteasome in cultured human primary pterygium fibroblasts. BMC Ophthalmol. 2018;18(1):328. doi: 10.1186/s12886-018-0987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho KS, Lim YR, Lee K, Lee J, Lee JH, Lee IS. Terpenes from forests and human health. Toxicol Res. 2017;33(2):97–106. doi: 10.5487/TR.2017.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M, Scherer R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24(13):2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.González-Burgos E, Gómez-Serranillos MP. Terpene compounds in nature: a review of their potential antioxidant activity. Curr Med Chem. 2012;19(31):5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 11.Ku CM, Lin JY. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013;141(2):1104–1113. doi: 10.1016/j.foodchem.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Legault J, Pichette A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol. 2007;59(12):1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 13.Scandiffio R, Geddo F, Cottone E, Querio G, Antoniotti S, Gallo MP, Maffei ME, Bovolin P. Protective effects of (E)-β-caryophyllene (BCP) in chronic inflammation. Nutrients. 2020;12(11):3273. doi: 10.3390/nu12113273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira-Tintino CDDM, Pessoa RT, Fernandes MNM, et al. Anti-inflammatory and anti-edematogenic action of the Croton campestris A. St.-Hil (Euphorbiaceae) essential oil and the compound β-caryophyllene in in vivo models. Phytomedicine. 2018;41:82–95. doi: 10.1016/j.phymed.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Haro-González JN, Castillo-Herrera GA, Martínez-Velázquez M, Espinosa-Andrews H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules. 2021;26(21):6387. doi: 10.3390/molecules26216387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Acker SI, Haagdorens M, Roelant E, Rozema J, Possemiers T, van Gerwen V, Tassignon MJ, de Groot V, Ní Dhubhghaill S, Koppen C, Zakaria N. Pterygium pathology: a prospective case-control study on tear film cytokine levels. Mediat Inflamm. 2019;2019:9416262. doi: 10.1155/2019/9416262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uthaithammarat L, Kasetsuwan N, Chongpison Y, Kasetsuwan P, Reinprayoon U, Nilyanimit P, Poovorawan Y. Lack of HPV in pterygium with no evidence of autoinoculation and the role of cytokines in pterygium with dry eye. Sci Rep. 2021;11:2842. doi: 10.1038/s41598-021-82114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antitumor activity of balsam fir oil: production of reactive oxygen species induced by α-humulene as possible mechanism of action. Planta Med. 2003;69(5):402–407. doi: 10.1055/s-2003-39695. [DOI] [PubMed] [Google Scholar]
- 19.Sikkema J, de Bont JA, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269(11):8022–8028. [PubMed] [Google Scholar]
- 20.Ghasemi H. Roles of IL-6 in ocular inflammation: a review. Ocular Immunol Inflamm. 2018;26(1):37–50. doi: 10.1080/09273948.2016.1277247. [DOI] [PubMed] [Google Scholar]
- 21.Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43(11):3430–3437. [PubMed] [Google Scholar]
- 22.Chao SC, Hu DN, Roberts J, Shen XL, Lee CY, Nien CW, Lin HY. Inhibition effect of curcumin on UVB-induced secretion of pro-inflammatory cytokines from corneal limbus epithelial cells. Int J Ophthalmol. 2017;10(6):827–833. doi: 10.18240/ijo.2017.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gullì M, Percaccio E, di Giacomo S, di Sotto A. Novel insights into the immunomodulatory effects of caryophyllane sesquiterpenes: a systematic review of preclinical studies. Appl Sci. 2022;12(5):2292. [Google Scholar]