Abstract
Purpose of Review
Obesity is a chronic disease, a major public health problem due to its association with non-communicable diseases and all-cause mortality. Indeed, people with obesity are at increased risk for a variety of obesity-related disorders including hypertension, dyslipidemia, type 2 diabetes mellitus, cardiovascular disease, and several cancers. Many popular diets with very different macronutrient composition, including the Mediterranean diet (MD), have been used, proposed, and studied for prevention and management of obesity. In particular, MD has been the subject of countless studies over the years and now boasts a large body of scientific literature. In this review, we aimed to update current knowledge by summarizing the most recent evidence on the effect of MD on obesity and obesity-related disorders.
Recent Findings
The negative effects of obesity are partly reversed by substantial weight loss that can be achieved with MD, especially when low-calorie and in combination with adequate physical activity. In addition, the composition of MD has been correlated with an excellent effect on reducing dyslipidemia. It also positively modulates the gut microbiota and immune system, significantly decreasing inflammatory mediators, a common ground for many obesity-related disorders.
Summary
People with obesity are at increased risk for a variety of medical disorders including hypertension, dyslipidemia, type 2 diabetes mellitus, and cardiovascular disease. Therefore, there is an inevitable need for measures to manage obesity and its related disorders. At this point, MD has been proposed as a valuable nutritional intervention. It is characterized by a high consumption of vegetables, fruit, nuts, cereals, whole grains, and extra virgin olive oil, as well as a moderate consumption of fish and poultry, and a limited intake of sweets, red meat, and dairy products. MD proves to be the healthiest dietary pattern available to tackle obesity and prevent several non-communicable diseases, including cardiovascular disease and type 2 diabetes.
Keywords: Mediterranean diet, Obesity, Obesity-related disorders, Cardiovascular diseases, Type 2 diabetes, Dyslipidemia
Introduction
The World Health Organization defines obesity as abnormal or excessive fat accumulation that portends increased risk to health status [1]. Despite this relatively simplistic definition, it is a chronic disease, a major public health problem that adversely effects all aspects of mental and physical health. Obesity has a growing worldwide prevalence irrespective of age, sex, race, or socioeconomic status [2]. Nearly a third of the world population is now suffering of overweight or obesity, which corresponds to one-fold increase since 1980 [3].
People with obesity are at increased risk for a variety of medical disorders including hypertension, dyslipidemia, type 2 diabetes mellitus, cardiovascular disease (CVD), and several cancers [4–6], all of which render obesity to be consistently associated with increased mortality [7, 8]. Therefore, there is inevitable need for measures to manage obesity and its related disorders. At this point, Mediterranean diet (MD) has been proposed to serve as a valuable nutritional intervention [9]. It is characterized by a high intake of vegetables, fruits, nuts, cereals, whole grains, and extra-virgin olive oil, as well as a moderate consumption of fish and poultry, and a limited intake of sweets, red meat, and dairy products [10]. Indeed, the adherence to MD dietary pattern is characterized by high intake of monounsaturated fat and fiber, and low in saturated fat with a balanced ratio of omega-6/omega-3 essential fatty acids [11]. The adherence to MD dietary pattern has been showed to be protective against the occurrence of several diseases, in particular obesity and CVD [12]. In particular, two previously conducted landmark randomized controlled trials (RCT) provided sobering evidence concerning the potential of MD for weight control and CVD prevention, which is not available for any other dietary pattern [13, 14]. Furthermore, Mediterranean dietary pattern compared to other diets has been reported as having proved to be the most effective in prevention of obesity and obesity-related diseases [15].
In this paper, we aimed to review the current knowledge regarding the effect of MD on obesity and obesity-related disorders.
Mediterranean Diet and Body Composition
There is no single definition of what constitutes MD, but it generally consists of little amounts of red meat, low to moderate amounts of fish, poultry, and large quantities of fruit, vegetables, whole grains, and pulses with unrestricted olive oil as an important source of monounsaturated fatty acids (MUFA) [16]. This diet is generally considered to be relatively high in fat, and as such many health professionals may be reluctant to recommend it to individuals with overweight or obesity as high fat diets are perceived to promote weight gain. Contrary to that popular belief, epidemiological studies have described an inverse association of adherence to MD with Body Mass Index (BMI) and weight gain [17, 18]. Moreover, higher adherence to MD is associated with increased likelihood of weight loss maintenance [19]. However, most of those studies did not include assessments of physical activity or capture total energy intake, which could serve as significant confounders. The evidence from interventional studies on MD suggest that the weight effect depends more on energy content rather than macronutrient composition [20]. However, even when not energy restricted, this diet is not associated with weight gain. In the largest RCT on MD conducted to date, 7447 individuals were randomized to MD supplemented with olive oil, MD supplemented with nuts, or a low-fat diet and followed for a median of 4.8 years [21]. The fat content in the two MD arms accounted for 42% of daily energy. Calorie restriction was not required in either intervention arm and physical activity was not encouraged, despite high prevalence of overweight and obesity in the study population. At the end of follow-up, participants in each of the three groups had slightly reduced body weight and increased waist circumference (WC). In comparison with low-fat diet, neither arm of ad libitum MD demonstrated significant difference in body weight: −0.410 kg (95% CI −0.830 to 0.01; p = 0.056) for MD supplemented with olive oil and −0.016 kg (95% CI −0.453 to 0.421; p = 0.942) for MD supplemented with nuts. There was evidence that MD was associated with less gain of central adiposity as shown by the adjusted difference in WC after 5 years of −0.466 cm (95% CI −1.109 to 0.176; p = 0.154) in MD with olive oil and −0.923 cm (95% CI −1.604 to −0.241; p = 0.008) in the nut group, compared with low-fat diet group. In conclusion, high fat, unrestricted calories MD was associated with little weight changes and less central adiposity compared with low-fat diet long term [21]. A systematic meta-analysis of 16 RCTs (n = 3436) assessing MD interventions of duration between 4 weeks and 24 months concluded that consumption of MD is associated with a greater weight loss compared to control diets and that the weight loss is more significant when energy restriction and/or increased physical activity are recommended as part of the intervention [22]. Another systematic analysis assessed the effects of calorie-restricted MD on weight loss in individuals with overweight and obesity after 12 months or longer [23]. Five RCTs were included (n = 998). MD was somewhat superior in producing weight loss compared to low-fat diets (range of mean weight loss −4.1 to −10.1 kg vs. −2.9 to −5.0 kg) but (similar to) same as low-carbohydrate diet and the American Diabetes Association (ADA) diet. The effects of MD on BMI and WC were similar to that on body weight reduction [23]. The current recommendation for lifestyle management of subjects with type 2 diabetes and overweight or obesity is to achieve and sustain a weight loss of ≥ 5% a target which is often difficult to reach in clinical practice [24]. A meta-analysis incorporating 19 weight-loss intervention study groups (n = 2711) in type 2 diabetes demonstrated that energy restricted Mediterranean style diet combined with 175 min of physical activity weekly was one of only two interventions achieving the recommended 5% weight loss at 12 months [25]. This was associated with significant improvement in metabolic parameters [25]. Emerging evidence suggests that MD can reduce central adiposity and visceral fat, both of which have been associated with the risk of type 2 diabetes and CVD [26]. In cross-sectional studies, adherence to MD has been shown to be inversely associated with abdominal adiposity [17, 27, 28]. The beneficial effect of MD on reducing central adiposity and visceral fat could be related to its high content of polyunsaturated fatty acids (PUFA) and MUFA and low intake of saturated fatty acids (SFA) [11]. It has long been known that visceral adipose tissue comprises predominantly SFA, whereas subcutaneous fat has deposits of PUFA and MUFA [29]. In line with this hypothesis, a short cross-over study in patients with obesity (n = 11), individuals with insulin resistance demonstrated that an isocaloric MD rich in extra-virgin olive oil prevented central body fat accumulation compared with a low-fat diet without effect on body weight [30]. Reduction in visceral adipose tissue has been reported in two interventional trials of MD after 2 months [31, 32]. Contrary to these findings, a small RCT of ad libitum MD (n = 35) compared to a low-fat diet (n = 31) for 6 months demonstrated that the former was associated with reduced subcutaneous adipose tissue but not visceral adipose tissue or other body composition parameters in patients with overweight or obesity post coronary event [33]. However, the participants with more sustained adherence to MD had significantly lower WC (−2.81 cm, p = 0.01). No change in body weight, and a trend for reduction in total body fat, was observed despite the tendency for increased total energy intake in the MD group [33]. An intervention with calorie-restricted protein-enriched MD of 8 weeks’ duration has been shown to result in significant reduction in weight (−16.7%), visceral fat (−27.4%), and fat mass (−28.1%) with preservation of fat free mass (FFM) in men with obesity (n = 37) awaiting laparoscopic sleeve gastrectomy [32]. Another short-intervention study of 6 weeks’ duration demonstrated that hypocaloric MD was superior in reducing body fat mass and preserving FFM compared to high-protein diets in young, sedentary individuals [34••]. Preservation of FFM may be of particular importance in preserving short- and long-term benefits of weight loss given that FFM has been associated with decreased basal metabolic rate and the risk of developing sarcopenic obesity [35]. A meta-analysis of 50 studies including an overall population of nearly half a million subjects concluded that MD had beneficial effects on the risk of metabolic syndrome and its individual components, including WC (mean difference −0.42 cm; 95% CI −0.81 to −0.02) [36]. Importantly, larger effects were seen in trials conducted in Mediterranean countries, possibly due to better availability of the required food produce, although other confounders such as genetic or environmental factors may have played a role [36]. A systematic review looked specifically at the effects of MD on central obesity outcomes. The analysis of 18 interventional trials (7186 total subjects and 5168 subjects assigned to MD) concluded that MD could diminish abdominal adiposity as evidenced by reduction in WC, waist-hip ratio, or visceral fat [37]. The most consistent reductions were observed in WC and visceral fat, although only two studies reported the effects on the latter. The reduction in central adiposity, albeit not universal, was observed irrespective of whether energy restriction was recommended or not as part of the intervention. Interestingly, four of the five studies that did not observe improvement in measures of central adiposity were conducted in non-Mediterranean populations. It remains unclear if MD is more effective in reducing central adiposity compared to other dietary interventions given that MD showed superior effects only in three studies included in the analysis [37•]. In conclusion, MD is an effective tool in reducing body weight, particularly when energy restricted and in combination with increased exercise. Reassuringly, even when not energy-restricted it is not associated with weight gain in the short or long term.
MD has the potential to reduce abdominal adiposity, in particular metabolically detrimental visceral fat, independently of weight loss, and can be recommended as a healthy diet choice to individuals with obesity and overweight, particularly at risk of cardiovascular and metabolic disease. MD may be more effective in Southern European populations due to better availability of specific food produce, cultural and other factors.
Mediterranean Diet and Type 2 Diabetes
The International Diabetes Federation (IDF) estimated just over 20 years ago that 151 million adults were affected by T2D worldwide [38]. This has increased to 463 million in 2019, suggesting a tripling of the global burden over this period [38].
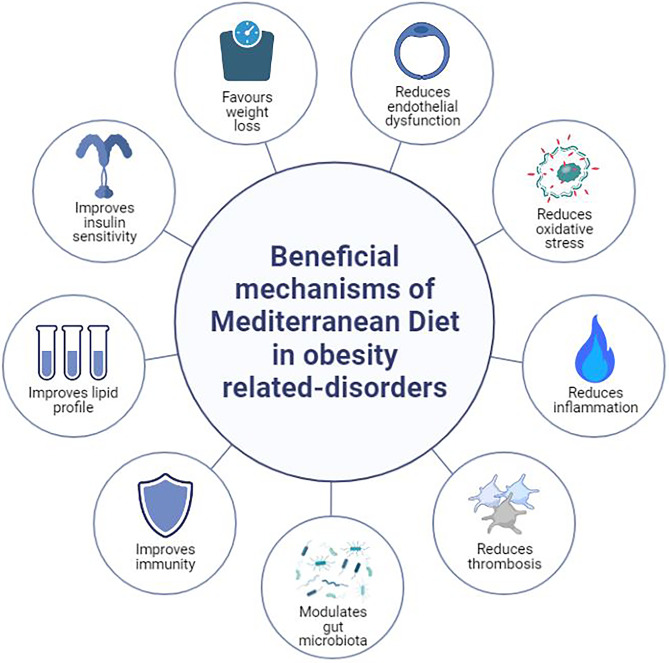
Lifestyle measures remain the cornerstone for type 2 diabetes treatment as recommended by several scientific societies, including the ADA and the European Association for the Study of Diabetes (EASD) [39, 40]. In the latest (2021) ADA guidelines, recommendations for medical nutrition therapy emphasize the implementation of a Mediterranean-style eating pattern to improve both glucose and lipid metabolism, thus minimizing individual’s cardiovascular risk [39]. Such an eating pattern is characterized by reduced consumption of saturated and trans-fat, as well as an increased intake of dietary PUFA n-3, viscous fiber, and plant sterols/stanols. The plant-based components of MD (e.g., vegetables, fruits, whole grains, and nuts) contain polyphenols that have been shown to reduce insulin resistance and improve cardiometabolic risk factors [41]. Olive oil and low-to-moderate alcohol intake (especially red wine) also contribute to the benefits of MD via their polyphenol content [42, 43]. Overall, potential mechanisms underlying the beneficial effects of MD include improvements in oxidative stress, inflammation, thrombosis, insulin sensitivity, lipid profile, endothelial dysfunction, and gut microbiota [44–46] (Fig. 1).
Fig. 1.
The beneficial mechanisms of Mediterranean diet in obesity-related disorders
Mediterranean Diet and Type 2 Diabetes Prevention
Type 2 diabetes is considered one of the major obesity-related comorbidities. The core pathophysiologic defect which is at the base of obesity is insulin resistance in muscle and liver, predicting the onset of type 2 diabetes in susceptible subjects [47]. Although current dietary recommendations focused on weight loss and overall dietary quality, to date, in subjects with obesity, there is no consensus on the optimal macronutrient composition of the diet for long-term management of type 2 diabetes [48]. Previous meta-analyses showed that adherence to MD could prevent type 2 diabetes development by 19–23% [49, 50]. In a more recent cohort study (n = 25,317 female participants from the Women’s Health Study), a higher MD intake was related to a 30% relative risk decrease in type 2 diabetes incidence during a 20-year follow-up [51]. Furthermore, an inverse association between adherence to MD and the prevalence of metabolic syndrome and prediabetes has been reported [52, 53].
Mediterranean Diet and Cardiometabolic Risk Factors
In patients with type 2 diabetes, MD can beneficially affect glycemic control and cardiovascular risk [54, 55]. In this context, a cross-sectional study among 500 patients with type 2 diabetes investigated the impact of MD on glycated hemoglobin (HbA1c). Mean HbA1c was 8.57 (±standard deviation, SD 1.94), 7.63 (±1.32), and 6.47 (±0.7) % in patients with low, moderate, and high adherence to MD, respectively [56]. In another randomized trial (n = 215 newly diagnosed patients with type 2 diabetes), HbA1c was significantly reduced by 1.2 and 0.9% at year 1 and 4, respectively, in patients on MD [57]. Fasting plasma glucose, serum insulin levels, and Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) were also significantly decreased. As a consequence, significantly less patients needed antidiabetic drug therapy both at year 1 and year 4 (hazard ratio, HR 0.70, 95% CI 0.59 to 0.90) [57]. Similar results were reported in a systematic review of 20 RCTs (> 6 months duration, n = 2223 patients with type 2 diabetes) showing greater decreases in body weight and HbA1c levels, as well as delayed requirement for antidiabetic drugs in patients with type 2 diabetes following a MD compared with those on other low-fat or low-carbohydrate diets [58].
Apart from improvements in glucose metabolism and body weight, MD can beneficially affect other cardiovascular risk factors, including lipids as triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), and blood pressure (BP), in patients with type 2 diabetes [59]. For example, among 2568 patients with type 2 diabetes, those with a high MD score had significantly lower LDL-C (101.5 ± 31.2 vs. 105.1 ± 31.9 mg/dL), TG (146.7 ± 71.0 vs. 156.2 ± 78.6 mg/dL), systolic BP (133.3 ± 23.7 vs. 135.3 ± 14.9 mmHg), and diastolic BP (78.6 ± 8.5 vs. 80.7 ± 8.7 mmHg), as well as higher HDL-C (46.8 ± 12.4 vs. 45.3 ± 11.6 mg/dL) than those with a low MD score [60]. Such findings were confirmed in a network meta-analysis (10 RCTs, n = 921 patients with type 2 diabetes) reporting that MD was superior to a low-fat diet in reducing HbA1c (mean difference −0.45%; 95% CI −0.55 to −0.34), fasting plasma glucose (mean difference −1.24 mmol/L; 95% CI −1.57 to −0.91), weight (mean difference −1.18 kg; 95% CI −1.99 to −0.37), WC (mean difference −0.73 cm; 95% CI −1.26 to −0.19), and TG (mean difference −0.21 mmol/L; 95% CI −0.27 to −0.16), as well as in increasing HDL-C (mean difference 0.07 mmol/L; 95% CI 0.04 to 0.11) [61]. Similarly, in another meta-analysis (9 RCTs, n = 1178 type 2 diabetes patients), MD led to greater decreases in HbA1c (mean difference −0.30%; 95% CI −0.46 to −0.14), fasting plasma glucose (mean difference −0.72 mmol/L; 95% CI −1.24 to −0.21), fasting insulin (M mean difference −0.55 μU/mL; 95% CI −0.81 to −0.29), weight (mean difference −0.29 kg; 95% CI −0.55 to −0.04), BMI (mean difference −0.29 kg/m2; 95% CI −0.46 to −0.12), TG (mean difference −0.29 mmol/L; 95% CI −0.47 to −0.10), systolic BP (mean difference −1.45 mmHg; 95% CI −1.97 to −0.94), and diastolic BP (mean difference −1.41 mmHg; 95% CI −1.84 to −0.97), as well as greater increases in HDL-C (mean difference 0.06 mmol/L; 95% CI 0.02 to 0.10) compared with control diets [62]. These cardiometabolic effects of MD in patients with type 2 diabetes were also summarized in a previous systematic review [63].
Mediterranean Diet and Diabetic Microvascular Complications
Low adherence to MD was also linked to impaired renal function and health-related quality of life in patients with type 2 diabetes and chronic kidney disease [64, 65]. Among women with type 2 diabetes, moderate and high MD scores were related to significantly reduced rates of diabetic nephropathy by 62% (OR 0.38; 95% CI 0.20 to 0.73) and 86% (OR 0.14; 95% CI 0.06 to 0.33), respectively, compared with a low MD score [66]. Of note, increases in MD score by 1-point were associated with 10% lower risk of CKD (mean follow-up 20.6 ± 7.0 years) as shown in a meta-analysis (13 studies, n = 27,618 individuals) [67]. Furthermore, implementation of MD was associated with decreased rate of incident CKD during mean follow-up of 24 years among 12,155 participants (aged 45–64 years) from the Atherosclerosis Risk in Communities Study [68]. Even when CKD has been developed, MD can exert nephroprotection. For example, in a cross-sectional analysis of the German Chronic Kidney Disease Study (n = 2813 patients with CKD), a high MD score correlated with higher estimated glomerular filtration rate (eGFR) (β-coefficient 0.932, p = 0.007) [64]. Overall, MD has been shown to prevent CKD, as well as decrease renal function decline and improve survival in patients with CKD [69].
Increased adherence to MD has been associated with lower risk of developing diabetic retinopathy (HR 0.34; 95% CI 0.13 to 0.89; p = 0.001 for trend) among 3614 patients with type 2 diabetes (aged 55–80 years) from the PREvención con DIeta MEDiterránea (PREDIMED) study [70]. Similar results have been reported in other studies [71–73].
Few evidence supports a link between adherence to MD and protection against diabetic neuropathy development [72], but further research is needed to elucidate such associations. Similarly, there is data showing that MD may preserve cognitive function and prevent dementia [74, 75], even in patients with type 2 diabetes [76].
Mediterranean Diet and Diabetic Macrovascular Complications
A previous umbrella review of meta-analyses found that high adherence to MD was associated with a reduced risk of overall mortality, CVD, coronary heart disease (CHD), myocardial infarction (MI), overall cancer incidence, type 2 diabetes, and neurodegenerative diseases [77]. Furthermore, MD was reported to protect against worse outcomes (heart failure hospitalization, unstable angina, stroke, recurrent MI, all cause or cardia death) up to 46 months following an MI [78]. Similarly, data from the GISSI-Prevenzione clinical trial showed that MD significantly decreased all-cause death in 11,323 patients with MI [79]. Furthermore, among 23,232 participants of the European Prospective Investigations into Cancer and Nutrition (EPIC) study, followed up for 17 years, stroke risk was significantly reduced with a greater adherence to MD (HR 0.78; 95% CI 0.65 to 0.93) [80]. Low adherence to MD was also related to a higher incident of stroke compared with moderate (HR 1.32; 95% CI 1.05 to 1.66) and high adherence (HR 1.28; 95% CI 1.00 to 1.63) among 30,239 participants of the REasons for Geographic And Racial Differences in Stroke (REGARDS) study, followed up for 6.5 years [81]. MD was shown to protect against peripheral artery disease (PAD) development in the PREDIMED study (n = 7435 participants, median follow-up: 4.8 years) [82].
In patients with type 2 diabetes, following MD led to significant reductions in CVD incidence (RR 0.62; 95% CI 0.5 to 0.78) [83]. A recent meta-analysis of 38 cohort studies and 3 RCTs (including patients with type 2 diabetes) found that adherence to MD was associated with significantly lower incidences of CHD (RR 0.73; 95% CI 0.62 to 0.86), MI (RR 0.73; 95% CI 0.61 to 0.88), and stroke (RR 0.80; 95% CI 0.71 to 0.90), as well as of CHD mortality (RR 0.83; 95% CI 0.75 to 0.92), stroke mortality (RR 0.87; 95% CI 0.80 to 0.96), and total CVD death (RR 0.79; 95% CI 0.77 to 0.82) [84]. A higher MD score was also linked to a significantly lower risk (by 66%) of PAD incidence (OR 0.44; 95% CI 0.24 to 0.83) in patients with type 2 diabetes (n = 944) [85]. Similarly, increased intake of fish and shellfish was reported to marginally decrease PAD risk (HR per additional gram/week 0.99; 95% CI 0.99 to 1.00, p = 0.051) in 1112 patients with type 2 diabetes followed for a median of 19.7 years in the Malmö Diet and Cancer study [86]. Nevertheless, further clinical data is needed in patients with type 2 diabetes to establish the effects of MD on cardiovascular morbidity and mortality.
In sum, in patients with type 2 diabetes, MD may minimize the risk of diabetic micro and macrovascular complications, although further evidence is required. Therefore, implementation of MD is recommended for both prevention and treatment of prediabetes and type 2 diabetes [55, 87, 88].
Mediterranean Diet and Non-alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) has been associated with type 2 diabetes and further increase of cardiovascular risk [89–92]. NAFLD is regarded as the hepatic manifestation of metabolic syndrome [93]. Currently, the global NAFLD prevalence is reported to be 25%, being highest in the Middle East (32%) and South America (31%) followed by Asia (27%), the USA (24%), Europe (23%), and Africa with the lowest prevalence (14%) [94].
MD has been proposed as an effective nutrition therapy for patients with type 2 diabetes and/or NAFLD, representing the first-line treatment for these metabolic diseases [93, 95•, 96]. In this context, MD was shown to improve biochemical and histological features of NAFLD [97, 98]. Therefore, implementation of MD may protect liver structure and function in patients with type 2 diabetes. Of interest, in patients with of NAFLD, it has been reported the enlargement of spleen which is the central organ in regulating the inflammation-related immune response depicting the so called liver-spleen axis [99]. Healthy dietary patterns, including MD, have been reported to improve immune and inflammatory responses by both reducing NAFLD and improving spleen function [100].
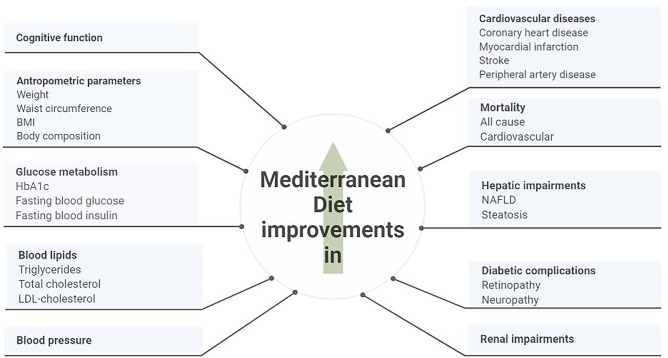
Overall, adherence to MD exerts several health benefits by improving cardiometabolic risk factors, including glucose and lipid metabolism, obesity indexes and NAFLD (Fig. 2).
Fig. 2.
The beneficial effects of adherence to MD on cardiometabolic factors and diabetic complications in patients with type 2 diabetes
Mediterranean Diet and Dyslipidemia
Dyslipidemia is a primary cause of the atherosclerotic cardiovascular disease (ASCVD) [101]. In particular, the most atherogenic form has been associated with type 2 diabetes and insulin resistance conditions [102]. Dyslipidemia is characterized by elevated serum levels of LDL-C and TG and low levels of HDL-C [103]. In 2008, according to the WHO Global Health Observatory, the prevalence of a plasma total cholesterol level ≥ 190 mg/dl was highest in Europe (54% for both sexes), followed by North and South America (48% for both sexes), while Africa and South-East Asia had the lowest prevalence (22.6% and 29.0%, respectively) [104]. Between 1980 and 2018, globally, little or no change in total and non-HDL plasma cholesterol was observed, but several regions experienced significant changes in some lipid parameters: high-income countries, which had the highest plasma cholesterol levels in 1980, experienced a substantial reduction in plasma cholesterol levels, while low- and middle-income countries experienced large increases in both plasma cholesterol and plasma triglycerides [105].
In recent years, several studies have investigated the role of diet and dyslipidemia. MD is a dietary pattern recommended for cardiovascular prevention and has been promoted by the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) 2019 guidelines for the management of dyslipidemia in addition to other lifestyle changes (Table 1) [103]. Ancel Keys designed MD almost five decades ago, and it has been recognized as one of the healthiest dietary patterns [16]. In addition, it has been negatively related to various chronic diseases, such as CVD [106], cancer [107], obesity [108], type 2 diabetes [88], and other metabolic conditions [109].
Table 1.
Treatment targets/goals 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines [103]
| Treatment | Recommendation |
|---|---|
| Smoking | No use in any form of tobacco |
| Diet | Diet low in saturated fat with whole grain products, vegetables, fruit, and fish |
| Physical activity | 30–60 min moderately vigorous physical activity most days |
| Bodyweight | BMI 20–25 kg/m2, and WC < 94 cm and < 80 cm (women) |
| Blood pressure | < 140/90 mmHg |
BMI body mass index, WC waist circumference
Specifically for patients that suffer from hyperlipidemia, MD advocates for low intake of SFA, less than < 7% in patients with hypercholesterolemia, and high consumption of PUFA and micronutrients, including dietary vitamins and minerals, that rises the plasma antioxidant capacity [110]. Also, MD favors the restriction of milk and dairy product consumption and limited intake of meat and meat-derived products [16]. Moreover, high plant-based food intake, such as whole grains, vegetables, and fruits, is highly advisable. MD suggests the consumption of seafood, regular consumption of olive oil, and increased physical activity. Finally, MD recommends reducing simple sugar intake and eliminating alcohol. This dietary pattern is high on food groups such as fruits and vegetables, fibers, olive oil, fish, and red wine, that are rich sources of several bioactive compounds, including antioxidants like carotenoids, flavonoids, resveratrol, and other polyphenolic compounds [16].
Studies have proven that consumption of foods causes modifications in the gut microbiota leading to an increase of beneficial bacteria such as Lactobacillus, Bifidobacterium, and Prevotella, and a reduction of harmful bacteria like Clostridium [111]. This effect is positive for prevention and treatment of chronic diseases like obesity [112], dyslipidemia [113], and inflammation [114]. MD is also considered a high fiber intake pattern, which can act on gut microbiota by modulating its composition and the production of metabolites that regulate immune function [115]. MD leads to an increase in the number of intestinal bacterial species responsible for producing short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, essential for proper functioning in preventing metabolic diseases [115]. Also, high consumption of PUFA n-3 from fish and vegetable sources and an adequate PUFA n-6/ PUFA n-3 ratio from MD [116] promotes a better metabolic profile compared to other dietary patterns that are high on PUFA n-6 and favors a higher production of proinflammatory substances and that increases the risk of chronic diseases like ASCVD [117]. Dietary fiber has become a key mediator of the communication between the brain and the gut [118]. SCFAs exert their beneficial effects directly by contributing to modulation of host health through a range of tissue-specific mechanisms related to gut barrier function, glucose homeostasis, immunomodulation, and appetite regulation [118]. Evidence on this effect has emerged from the modulation of gut microbial composition through administration of prebiotics, defined as a non-digestible food ingredient that stimulates the growth and/or activity of one or a limited number of beneficial bacteria in the colon [119].
In addition to the gut microbiota modulation effects, the beneficial impact of MD could also be due to immune system modulation [100, 120]. In an intervention study conducted by Llorente-Cortés et al. in 2010 on a population with a high risk of ASCVD (n = 49), it was found that after 3 months, subjects who followed MD integrated with virgin olive oil or nuts showed not only a reduction in interleukin-6 and soluble intercellular adhesion molecule-1, significant inflammation mediators in the adhesion of leukocytes to the endothelial surface, but also a reduction in activation of biomarkers related to the atherosclerotic process [120]. There was a reduction of the proinflammatory ligand CD40 and the adhesion molecule CD49d on T lymphocytes and monocytes after both MD [120].
Different researchers have studied the relationship between SFA consumption, ASCVD risk and increased LDL-C levels [121], while recent clinical studies support the fact that SFA do not increase the risk of ASCVD. The relationship between SFA intake and cardiovascular mortality has been discussed previously, and there was no higher risk of ASCVD events in individuals with high consumption of SFA than those with low consumption [121]. It has been concluded that ASCVD risk may be more influenced by the dietary source of SFA, mainly giving dairy and meat products more focus [121]. Therefore, a higher intake of SFA from meat products is related to the development of ASCVD, and that lower ASCVD risk is linked to a higher intake of SFA from dairy sources. However, the literature is still controversial regarding the relationship between meat and dairy products intake and the effect on lipid profile [88, 121, 122]. The Prospective Urban and Rural Epidemiology study was a large study that helped understand the connection between macronutrient intake and mortality, concluding that SFA intake does not influence mortality rate [123]. At the same time, high consumption of carbohydrates has been associated with a higher mortality risk for CVD [123].
Other studies have investigated the relationship between SFA intake and cardiovascular mortality and did not observe an increased risk of ASCVD events in subjects with high consumption of SFA compared with those with low consumption [124–126]. Consequently, ASCVD risk may be influenced by the dietary source of SFA, mainly represented by dairy and meat products. Meat consumption is a dietary risk factor for atherogenic dyslipidemia [126]. de Oliveira et al. in the Multi-Ethnic Study of Atherosclerosis (n = 5209) reported that a higher intake of SFA from meat products is related to the development of ASCVD; on the other hand, a lower ASCVD risk has been correlated to a higher intake of SFA from dairy products [125]. However, studies are controversial regarding the relationship between meat and dairy products intake and the effect on lipid profile [125, 126].
In 2021, Formisano et al. evaluated the influence of different eating habits on the lipid profile of 106 patients suffering from different types of dyslipidemia [126]. They concluded that a high intake of dairy products was associated with hyperlipidemia (higher levels of total cholesterol and HDL-C), while a diet with an excessive amount of meat products caused a form of dyslipidemia (higher total cholesterol and TG levels and lower HDL-C levels) [126]. Therefore, dietary recommendations should specify between SFA other than suggesting general reduction in SFA intake.
Adherence is primordial for treatment of chronic diseases [88, 122, 127]. Some reports examine the adherence to MD and have shown that individuals with obesity and with low adherence to MD presented worse anthropometric measurements and metabolic profile compared with subjects who were good sleepers and with an average adherence, independently of age and gender [88, 122, 127]. Also, the effect on gut microbiota includes the maintenance of presence of Prevotella bacteria and other Firmicutes according to the degree of adherence to MD [127–129]. On the contrary, low adherence to MD has been associated with high levels of urinary trimethylamine N-oxide, which is related to increased cardiovascular risk [130, 131]. Of interest, through inflammatory processes, TMAO would have a potential role in different chronic non-communicable diseases, including obesity [132], CVD [133], type 2 diabetes [134], NAFLD [134], and inflammatory diseases [135]. Also, obesity is associated with reduced spontaneous and stimulated growth hormone secretion and basal insulin-like growth factor I levels [136] which has been associated with increased risk of ASCVD [137]. The degree of adherence to MD and protein grams intake was one of the most predictive factors of growth hormone status in obesity, showing an association between adherence to MD and the clinical alterations of cardiometabolic status [136].
Moreover, another critical nutrient that MD considers is vitamin D, and the evidence suggests that vitamin D deficiency may represent a significant risk factor [138–140]. There is a close relationship between vitamin D and the cardiovascular system by vitamin D receptors in vital tissues like endothelium, smooth muscle, and myocardium [138–140]. Therefore, low vitamin D status has been associated with increased BP [139, 140], dyslipidemia [139, 140], impaired insulin metabolism [138], sleep disturbances [141, 142], thus increasing the risk of cardiovascular atherosclerosis [139, 140]. It has been studied that hypovitaminosis D might increase the cardiovascular risk in hypopituitarism patients, and it is a powerful predictor of prevalence of dyslipidemia and hypertension in individuals [138, 140]. Hypovitaminosis D is commonly reported in patients with obesity due to several mechanisms [143, 144]. Of interest, very recently in a cross-sectional, observational study, it was reported that high adherence to MD was associated with low BMI in 617 individuals, probably through the antioxidant and anti-inflammatory effects synergistically exerted by either high vitamin D levels or high adherence to MD on body weight [145••].
Mediterranean Diet and Cancer
To date, the important role of prevention in several cancer settings is well known, and diet has a good place among these prevention strategies. A meta-analysis including 2,130,753 participants concluded that greater adherence to MD was associated with a significantly lower risk of cancer mortality (RR 0.86; 95% CI 0.81 to 0.91), colorectal cancer (RR 0.82; 95% CI 0.75 to 0.88), breast cancer (RR 0.92; 95% CI 0.87 to 0.96), gastric cancer (RR 0.72; 95% CI 0.60 to 0.86), liver cancer (RR 0.58; 95% CI 0.46 to 0.73), head and neck cancer (RR 0.49; 95% CI 0.37 to 0.66), and prostate cancer (RR 0.96; 95% CI 0.92 to 1.00) [146]. In addition, data on the benefits of MD against incident cancers were reported in the EPIC trial (n = 9669 incident cancers in men and 21,062 in women) [147]. Evidence of protection was strongest for colorectal, gastric, and breast cancers, especially after exclusion of alcohol from the score [147].
Breast cancer has increased by more than 20% worldwide since 2008 and is the leading cause of cancer in women [148]. Data from the PREDIMED study showed that after a mean follow-up of 4.8 years, the observed rates of breast cancer were lower in the intervention groups with EVOO or with nuts than in the control group with a low-fat diet. After multivariate adjustment, the group supplementing MD with EVOO had a significantly lower risk of developing breast cancer than the control group; for every 5% additional calories from EVOO, the risk was 28% lower (95% CI, 0.57 to 0.90) [148]. A case–control study of 2396 women aged 25–74 years found that MD was associated with a 35% reduction in breast cancer risk [149]. The Four Corners Breast Cancer Study showed that Hispanic (n = 757 cases and 867 controls) and non-Hispanic (n = 1524 cases and 1598 controls) women who adopted MD had a lower risk of breast cancer [150]. Two prospective studies of 91,779 American women [151] and 65,374 French women [152] confirmed a protective association between adherence to MD and breast cancer incidence. The protective effect of MD on breast cancer risk was associated with a reduction in circulating estrogen levels and increased intake of carotenoids, which are known antioxidants that reduce oxidative stress. The protective associations were greater in women with negative progesterone and estrogen receptor [152].
A lower risk of colon cancer has been associated with dietary patterns that are higher in vegetables, legumes, fruits, whole grains, fish, lean meats, low-fat dairy products, moderate alcohol consumption, and lower consumption of red and/or processed meats, sugar-sweetened beverages, and saturated fats [153]. In contrast, diets containing greater amounts of red/processed meat, sugars (i.e., desserts, sugar-sweetened beverages, and sweets), potatoes, and chips are associated with an increased risk of colorectal cancer [153]. Data from the Italian EPIC study involving 42,275 participants aged 25–70 years who did not have cancer at baseline found that increased adherence to MD was associated with an 8–11% lower risk of colorectal cancer in men and women [154]. The protective effect was observed mainly for distal colon and rectal cancer, whereas it was lower for proximal colon cancer [154].
In conclusion, all the above results suggest that adherence to MD may contribute to the reduction of various cancers and also of overall cancer-related mortality. Nevertheless, further research is needed to determine which foods and nutrients are most effective for this outcome (Table 2).
Table 2.
Meta-analyses of studies regarding the effects of Mediterranean diet on obesity-associated disorders
| Source | No. and type of studies | Subjects | Aim | Main findings |
|---|---|---|---|---|
| Esposito et al. [22] | 16 RCTs | 3436 subjects | To evaluate the effect of MD on body weight | MD had a significant effect on weight (95% CI −2.86 to −0.64) and BMI (95% CI −0.93 to −0.21). The effect of MD on body weight was greater in association with energy restriction (mean difference, −3.88 kg, 95% CI −6.54 to −1.21 kg), increased physical activity (−4.01 kg, 95% CI −5.79 to −2.23 kg), and follow-up longer than 6 months (−2.69 kg, 95% CI −3.99 to −1.38 kg) |
| Franz et al. [25] | 11 RCTs | 6754 adults with overweight or obesity and T2DM |
To evaluate the outcomes on HbA1c, lipid (total cholesterol, LDL-C, HDL-C, and TG) and BP (systolic and diastolic) from lifestyle weight-loss interventions resulting in weight losses greater than or less than 5% at 12 months To evaluate the weight and metabolic outcomes from differing amounts of macronutrients in weight-loss interventions |
2 study groups reported a weight loss of ≥ 5%: a Mediterranean-style diet implemented in newly diagnosed adults with T2DM, and an intensive lifestyle intervention implemented in the Look AHEAD trial. Both included regular physical activity and frequent contact with health professionals and reported significant beneficial effects on HbA1c, lipids, and blood pressure |
| Kastorini et al. [36] | 50 RCTs (35 clinical trials, 2 prospective and 13 cross-sectional) | 534,906 subjects | To meta-analyze epidemiological studies and clinical trials that have assessed the effect of MD on metabolic syndrome as well as its components | Adherence to MD was associated with reduced risk of metabolic syndrome (log HR −0.69; 95% CI −1.24 to −1.16). Results from clinical studies revealed the protective role of MD on components of metabolic syndrome, like WC (mean difference −0.42 cm; 95% CI −0.82 to −0.02), HDL-C (mean difference 1.17 mg/dl; 95% CI 0.38 to 1.96), TG (mean difference −6.14 mg/dl; 95% CI −10.35 to −1.93), systolic (mean difference −2.35 mm Hg; 95% CI −3.51 to −1.18) and diastolic BP (mean difference −1.58 mm Hg; 95% CI −2.02 to −1.13), and glucose (mean difference −3.89 mg/dl; 95% CI −5.84 to −1.95), whereas results from epidemiological studies also confirmed those of clinical trials |
| Koloverou et al. [49] | 10 prospective studies (1 clinical trial, 9 prospective and 7 cross-sectional) | 136,846 subjects | To meta-analyze prospective studies that have evaluated the effect of MD on the development of T2DM | Higher adherence to MD was associated with 23% reduced risk of developing T2DM (combined RR for upper vs lowest available centile: 0.77; 95% CI 0.66 to 0.89). Subgroup analyses based on region, health status of participants and number of confounders controlling for, showed similar results |
| Schwingshackl et al. [50] | 1 RCT and 8 prospective cohort studies | 122,810 subjects | To meta-analyze the effects of MD adherence on the risk of T2DM | For highest vs lowest adherence to MD score, the pooled RR for T2DM was 0.81 (95% CI 0.73 to 0.90). Sensitivity analysis including only long-term studies confirmed the results of the primary analysis (pooled RR 0.75; 95% CI 0.68 to 0.83) |
| Pan et al. [61] | 10 RCTs | 921 subjects with T2DM | To comprehensively compare the differences between major dietary patterns in improving glycemic control, cardiovascular risk, and weight loss for patients with T2DM | Compared to low-fat diet, MD showed beneficial effects in glycemic control (HbA1c 95% CI –0.55 to –0.34; fasting plasma glucose 95% CI –1.57 to –0.91; weight loss 95% CI –1.99 to –0.37; WC 95% CI –1.26 to –0.19), and cardiovascular risk factors (HDL-C 95% CI 0.04 to 0.11; total cholesterol 95% CI –0.26 to –0.08; TG 95% CI –0.27 to –0.16) |
| Huo et al. [62] | 9 RCTs | 1178 subjects with T2DM | To explore the effects of MD on glycemic control, weight loss and cardiovascular risk factors in T2DM patients | Compared with control diets, MD led to greater reductions in HbA1c (mean difference, −0.30; 95% CI −0.46 to −0.14), fasting plasma glucose (−0.72 mmol/l; 95% CI −1.24 to −0.21), fasting insulin (−0.55 μU/ml; 95% CI −0.81 to −0.29), BMI (−0.29 kg/m2; 95% CI −0.46 to −0.12) and body weight (−0.29 kg; 95% CI −0.55 to −0.04). Likewise, concentrations of total cholesterol and TG were decreased (−0.14 mmol/l; 95% CI −0.19 to −0.09 and −0.29 mmol/l; 95% CI −0.47 to −0.10, respectively), and HDL-C was increased (0.06 mmol/l; 95% CI 0.02 to 0.10). In addition, MD was associated with a decline of 1.45 mm Hg (95% CI −1.97 to −0.94) for systolic and 1.41 mm Hg (95% CI −1.84 to −0.97) for diastolic BP |
| Hansrivijit et al. [67] | 4 prospective studies | 8467 subjects ≥ 18 years of age without CKD | To assess the association between MD adherence and CKD prevention | With the mean follow-up duration of 20.6 ± 7.0 years, the pooled OR for CKD was 0.901 (95% CI 0.868 to 0.935) for each 1-point increment of MD scale. The incidence of CKD was 0.026 events per person-year (95% CI 0.008 to 0.045) |
| Becerra-Tomás et al. [84] | 3 RCTs and 38 prospective cohort studies |
Adults with type 1 diabetes or T2DM |
To evaluate the effect of MD on the prevention of CVD incidence and mortality |
Meta-analyses of RCTs revealed a beneficial effect of MD on total CVD (RR: 0.62; 95% CI 0.50 to 0.78) and total myocardial infarction (RR: 0.65; 95% CI 0.49 to 0.88) incidence Meta-analyses of prospective cohort studies, which compared the highest vs lowest categories of MD adherence, revealed an inverse association with total CVD mortality (RR 0.79; 95% CI 0.77 to 0.82), CHD incidence (RR 0.73; 95% CI 0.62 to 0.86), CHD mortality (RR 0.83; 95% CI 0.75 to 0.92), stroke incidence (RR 0.80; 95% CI 0.71 to 0.90), stroke mortality (RR 0.87; 95% C: 0.80 to 0.96) and myocardial infarction incidence (RR 0.73; 95% CI 0.61 to 0.88) |
| Schwingshackl et al. [146] | 2 RCTs, 51 cohort studies and 30 case–control studies | 2,130,753 subjects | To evaluate the effects of adherence to MD on risk of overall cancer mortality, risk of different types of cancer, and cancer mortality and recurrence risk in cancer survivors | Greater adherence to MD was associated with a significantly lower risk of cancer mortality (RR 0.86; 95% CI 0.81 to 0.91), colorectal cancer (RR 0.82; 95% CI 0.75 to 0.88), breast cancer (RR 0.92; 95% CI 0.87 to 0.96), gastric cancer (RR 0.72; 95% CI 0.60 to 0.86), liver cancer (RR 0.58; 95% CI 0.46 to 0.73), head and neck cancer (RR 0.49; 95% CI 0.37 to 0.66), and prostate cancer (RR 0.96; 95% CI 0.92 to 1.00) |
RCT randomized controlled trial, MD Mediterranean diet, CI confidence interval, BMI body mass index, T2DM type 2 diabetes mellitus, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, BP blood pressure, AHEAD Action for Health in Diabetes, HR hazard ratio, WC waist circumferences, RR risk ratio, CKD chronic kidney disease, CVD cardiovascular disease, CHD coronary heart disease
Conclusion
The obesity pandemic is associated with high risk of morbidity and mortality from different non-communicable diseases. Of interest, the negative effects of obesity are reversed in part with substantial weight loss. The composition of MD has been related to an excellent effect on reducing dyslipidemia. Additionally, it positively modulates the gut microbiota and immune system, significantly decreasing inflammation mediators, common ground for many obesity-related disorders. MD is the healthiest dietary pattern available to prevent several non-communicable diseases, including cardiovascular disease and type 2 diabetes.
Author Contribution
Conceptualization: Giovanna Muscogiuri and Luigi Barrea; Literature search: Ludovica Verde, Cem Sulu, Niki Katsiki, Maria Hassapidou, Evelyn Frias-Toral, Gabriela Cucalón and Agnieszka Pazderska; Original draft preparation: Ludovica Verde, Cem Sulu, Niki Katsiki, Maria Hassapidou, Evelyn Frias-Toral, Gabriela Cucalón and Agnieszka Pazderska; Writing–review and editing: Volkan Demirhan Yumuk, Ludovica Verde, Luigi Barrea and Giovanna Muscogiuri; Supervision: Giovanna Muscogiuri, Annamaria Colao and Luigi Barrea.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Compliance with Ethical Standards
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Giovanna Muscogiuri and Ludovica Verde contributed equally to this work.
This article is part of the Topical Collection on Metabolism
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giovanna Muscogiuri, Email: giovanna.muscogiuri@unina.it.
Luigi Barrea, Email: luigi.barrea@unina.it.
References
- 1.Organization WH. Obesity and overweight. Fact sheet N 311. 2012. http://www.who.int/mediacentre/factsheets/fs311/en/. Last accessed 21 Jan 2022.
- 2.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Network GBoDC . Global Burden of Disease Study 2015 (GBD 2015) Obesity and Overweight Prevalence 1980–2015. IHME Seattle; 2017. [Google Scholar]
- 4.Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138(4):345–355. doi: 10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose–response meta-analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527–2545. doi: 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valencia WM, Stoutenberg M, Florez H. Weight loss and physical activity for disease prevention in obese older adults: an important role for lifestyle management. Curr Diab Rep. 2014;14(10):539. doi: 10.1007/s11892-014-0539-4. [DOI] [PubMed] [Google Scholar]
- 7.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, De Gonzalez AB, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 10.Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12):2274–2284. doi: 10.1017/S1368980011002515. [DOI] [PubMed] [Google Scholar]
- 11.Marventano S, Kolacz P, Castellano S, Galvano F, Buscemi S, Mistretta A, et al. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? Int J Food Sci Nutr. 2015;66(6):611–622. doi: 10.3109/09637486.2015.1077790. [DOI] [PubMed] [Google Scholar]
- 12.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92(5):1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41(2):377–385. doi: 10.1093/ije/dyq250. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-González MA, Buil-Cosiales P, Corella D, Bulló M, Fitó M, Vioque J, et al. Cohort profile: design and methods of the PREDIMED-Plus randomized trial. Int J Epidemiol. 2019;48(2):387–388. doi: 10.1093/ije/dyy225. [DOI] [PubMed] [Google Scholar]
- 15.Romaguera D, Norat T, Mouw T, May AM, Bamia C, Slimani N, et al. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J Nutr. 2009;139(9):1728–1737. doi: 10.3945/jn.109.108902. [DOI] [PubMed] [Google Scholar]
- 16.Trichopoulou A. Mediterranean diet as intangible heritage of humanity: 10 years on. Nutr Metab Cardiovasc Dis. 2021;31(7):1943–1948. doi: 10.1016/j.numecd.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Villegas A, Bes-Rastrollo M, Martinez-Gonzalez MA, Serra-Majem L. Adherence to a Mediterranean dietary pattern and weight gain in a follow-up study: the SUN cohort. Int J Obes. 2006;30(2):350–358. doi: 10.1038/sj.ijo.0803118. [DOI] [PubMed] [Google Scholar]
- 18.Schroder H, Marrugat J, Vila J, Covas MI, Elosua R. Adherence to the traditional mediterranean diet is inversely associated with body mass index and obesity in a spanish population. J Nutr. 2004;134(12):3355–3361. doi: 10.1093/jn/134.12.3355. [DOI] [PubMed] [Google Scholar]
- 19.Poulimeneas D, Anastasiou CA, Santos I, Hill JO, Panagiotakos DB, Yannakoulia M. Exploring the relationship between the Mediterranean diet and weight loss maintenance: the MedWeight study. Br J Nutr. 2020;124(8):874–880. doi: 10.1017/S0007114520001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JY. Optimal diet strategies for weight loss and weight loss maintenance. J Obes Metab Syndr. 2021;30(1):20–31. doi: 10.7570/jomes20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Fito M, Chiva-Blanch G, et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):e6–e17. doi: 10.1016/S2213-8587(19)30074-9. [DOI] [PubMed] [Google Scholar]
- 22.Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9(1):1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- 23.Mancini JG, Filion KB, Atallah R, Eisenberg MJ. Systematic review of the Mediterranean diet for long-term weight loss. Am J Med. 2016;129(4):407–415. doi: 10.1016/j.amjmed.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S100–S110. doi: 10.2337/dc21-S008. [DOI] [PubMed] [Google Scholar]
- 25.Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115(9):1447–1463. doi: 10.1016/j.jand.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Prasad DS, Kabir Z, Dash AK, Das BC. Abdominal obesity, an independent cardiovascular risk factor in Indian subcontinent: a clinico epidemiological evidence summary. J Cardiovasc Dis Res. 2011;2(4):199–205. doi: 10.4103/0975-3583.89803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoli S, Leone A, Vignati L, Bedogni G, Martinez-Gonzalez MA, Bes-Rastrollo M, et al. Adherence to the Mediterranean diet is inversely associated with visceral abdominal tissue in Caucasian subjects. Clin Nutr. 2015;34(6):1266–1272. doi: 10.1016/j.clnu.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Gonzalez MA, Garcia-Arellano A, Toledo E, Salas-Salvado J, Buil-Cosiales P, Corella D, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS ONE. 2012;7(8):e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calder PC, Harvey DJ, Pond CM, Newsholme EA. Site-specific differences in the fatty acid composition of human adipose tissue. Lipids. 1992;27(9):716–720. doi: 10.1007/BF02536031. [DOI] [PubMed] [Google Scholar]
- 30.Paniagua JA, de la Sacristana AG, Romero I, Vidal-Puig A, Latre JM, Sanchez E, et al. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care. 2007;30(7):1717–1723. doi: 10.2337/dc06-2220. [DOI] [PubMed] [Google Scholar]
- 31.Buscemi S, Verga S, Tranchina MR, Cottone S, Cerasola G. Effects of hypocaloric very-low-carbohydrate diet vs. Mediterranean diet on endothelial function in obese women. Eur J Clin Invest. 2009;39(5):339–347. doi: 10.1111/j.1365-2362.2009.02091.x. [DOI] [PubMed] [Google Scholar]
- 32.Schiavo L, Scalera G, Sergio R, De Sena G, Pilone V, Barbarisi A. Clinical impact of Mediterranean-enriched-protein diet on liver size, visceral fat, fat mass, and fat-free mass in patients undergoing sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(5):1164–1170. doi: 10.1016/j.soard.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Mayr HL, Itsiopoulos C, Tierney AC, Kucianski T, Radcliffe J, Garg M, et al. Ad libitum Mediterranean diet reduces subcutaneous but not visceral fat in patients with coronary heart disease: a randomised controlled pilot study. Clin Nutr ESPEN. 2019;32:61–69. doi: 10.1016/j.clnesp.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 34.•• Feidantsis K, Methenitis S, Ketselidi K, Vagianou K, Skepastianos P, Hatzitolios A, et al. Comparison of short-term hypocaloric high-protein diets with a hypocaloric Mediterranean diet: effect on body composition and health-related blood markers in overweight and sedentary young participants. Nutrition. 2021;91–92. In this randomised controlled trial, the authors demonstrated that among the three low-calorie diets (low-calorie Mediterranean diet and two high-protein diets, with and without whey protein supplementation), only the Mediterranean diet induced positive changes in body composition and metabolic profile in overweight and sedentary individuals. [DOI] [PubMed]
- 35.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68(7):375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 36.Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57(11):1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 37.Bendall CL, Mayr HL, Opie RS, Bes-Rastrollo M, Itsiopoulos C, Thomas CJ. Central obesity and the Mediterranean diet: a systematic review of intervention trials. Crit Rev Food Sci Nutr. 2018;58(18):3070–3084. doi: 10.1080/10408398.2017.1351917. [DOI] [PubMed] [Google Scholar]
- 38.Federation ID . IDF diabetes atlas. 1. Brussels, Belgium: International Diabetes Federation; 2019. [Google Scholar]
- 39.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S125–S150. doi: 10.2337/dc21-S010. [DOI] [PubMed] [Google Scholar]
- 40.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in Type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guasch-Ferre M, Merino J, Sun Q, Fito M, Salas-Salvado J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxid Med Cell Longev. 2017;2017:6723931. doi: 10.1155/2017/6723931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsiki N, Perez-Martinez P, Lopez-Miranda J. Olive oil intake and cardiovascular disease prevention: "seek and you shall find". Curr Cardiol Rep. 2021;23(6):64. doi: 10.1007/s11886-021-01496-1. [DOI] [PubMed] [Google Scholar]
- 43.Katsiki N, Tziomalos K, Mikhailidis DP. Alcohol and the cardiovascular system: a double-edged sword. Curr Pharm Des. 2014;20(40):6276–6288. doi: 10.2174/1381612820666140620125741. [DOI] [PubMed] [Google Scholar]
- 44.Ditano-Vazquez P, Torres-Pena JD, Galeano-Valle F, Perez-Caballero AI, Demelo-Rodriguez P, Lopez-Miranda J, et al. The fluid aspect of the mediterranean diet in the prevention and management of cardiovascular disease and diabetes: the role of polyphenol content in moderate consumption of wine and olive oil. Nutrients. 2019;11(11):2833. doi: 10.3390/nu11112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotsis E, Anagnostis P, Mariolis A, Vlachou A, Katsiki N, Karagiannis A. Health benefits of the Mediterranean Diet: an update of research over the last 5 years. Angiology. 2015;66(4):304–318. doi: 10.1177/0003319714532169. [DOI] [PubMed] [Google Scholar]
- 46.Ismael S, Silvestre MP, Vasques M, Araujo JR, Morais J, Duarte MI, et al. A pilot study on the metabolic impact of Mediterranean diet in type 2 diabetes: is gut microbiota the key? Nutrients. 2021;13(4):1228. doi: 10.3390/nu13041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muscogiuri G, Barrea L, Caprio M, Ceriani F, Chavez AO, El Ghoch M, et al. Nutritional guidelines for the management of insulin resistance. Crit Rev Food Sci Nutr. 2021;1–14. [DOI] [PubMed]
- 48.Barrea L, Vetrani C, Caprio M, El Ghoch M, Frias-Toral E, Mehta RJ, et al. Nutritional management of type 2 diabetes in subjects with obesity: an international guideline for clinical practice. Crit Rev Food Sci Nutr. 2021;1–13. [DOI] [PubMed]
- 49.Koloverou E, Esposito K, Giugliano D, Panagiotakos D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism. 2014;63(7):903–911. doi: 10.1016/j.metabol.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Schwingshackl L, Missbach B, Konig J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr. 2015;18(7):1292–1299. doi: 10.1017/S1368980014001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad S, Demler OV, Sun Q, Moorthy MV, Li C, Lee IM, et al. Association of the Mediterranean diet with onset of diabetes in the women's health study. JAMA Netw Open. 2020;3(11):e2025466. doi: 10.1001/jamanetworkopen.2020.25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortega E, Franch J, Castell C, Goday A, Ribas-Barba L, Soriguer F, et al. Mediterranean diet adherence in individuals with prediabetes and unknown diabetes: the Di@bet.es Study. Ann Nutr Metab. 2013;62(4):339–346. doi: 10.1159/000346553. [DOI] [PubMed] [Google Scholar]
- 53.Viscogliosi G, Cipriani E, Liguori ML, Marigliano B, Saliola M, Ettorre E, et al. Mediterranean dietary pattern adherence: associations with prediabetes, metabolic syndrome, and related microinflammation. Metab Syndr Relat Disord. 2013;11(3):210–216. doi: 10.1089/met.2012.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin-Pelaez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients. 2020;12(8):2236. doi: 10.3390/nu12082236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pascual Fuster V, Perez Perez A, Carretero Gomez J, Caixas Pedragos A, Gomez-Huelgas R, Perez-Martinez P. Executive summary: updates to the dietary treatment of prediabetes and type 2 diabetes mellitus. Endocrinol Diabetes Nutr (Engl Ed) 2021;68(4):277–287. doi: 10.1016/j.endien.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Mirahmadizadeh A, Khorshidsavar H, Seif M, Sharifi MH. Adherence to medication, diet and physical activity and the associated factors amongst patients with type 2 diabetes. Diabetes Ther. 2020;11(2):479–494. doi: 10.1007/s13300-019-00750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Carvalho GB, Dias-Vasconcelos NL, Santos RKF, Brandao-Lima PN, da Silva DG, Pires LV. Effect of different dietary patterns on glycemic control in individuals with type 2 diabetes mellitus: a systematic review. Crit Rev Food Sci Nutr. 2020;60(12):1999–2010. doi: 10.1080/10408398.2019.1624498. [DOI] [PubMed] [Google Scholar]
- 58.Papamichou D, Panagiotakos DB, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis. 2019;29(6):531–543. doi: 10.1016/j.numecd.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Tosatti JAG, Alves MT, Gomes KB. The role of the Mediterranean dietary pattern on metabolic control of patients with diabetes mellitus: a narrative review. Adv Exp Med Biol. 2021;1307:115–128. doi: 10.1007/5584_2020_513. [DOI] [PubMed] [Google Scholar]
- 60.Vitale M, Masulli M, Calabrese I, Rivellese AA, Bonora E, Signorini S, et al. Impact of a Mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: a real-life study. Nutrients. 2018;10(8):1067. doi: 10.3390/nu10081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan B, Wu Y, Yang Q, Ge L, Gao C, Xun Y, et al. The impact of major dietary patterns on glycemic control, cardiovascular risk factors, and weight loss in patients with type 2 diabetes: a network meta-analysis. J Evid Based Med. 2019;12(1):29–39. doi: 10.1111/jebm.12312. [DOI] [PubMed] [Google Scholar]
- 62.Huo R, Du T, Xu Y, Xu W, Chen X, Sun K, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200–1208. doi: 10.1038/ejcn.2014.243. [DOI] [PubMed] [Google Scholar]
- 63.Sleiman D, Al-Badri MR, Azar ST. Effect of mediterranean diet in diabetes control and cardiovascular risk modification: a systematic review. Front Public Health. 2015;3:69. doi: 10.3389/fpubh.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heindel J, Baid-Agrawal S, Rebholz CM, Nadal J, Schmid M, Schaeffner E, et al. Association between dietary patterns and kidney function in patients with chronic kidney disease: a cross-sectional analysis of the German Chronic Kidney Disease Study. J Ren Nutr. 2020;30(4):296–304. doi: 10.1053/j.jrn.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Picard K, Senior PA, Adame Perez S, Jindal K, Richard C, Mager DR. Low Mediterranean Diet scores are associated with reduced kidney function and health related quality of life but not other markers of cardiovascular risk in adults with diabetes and chronic kidney disease. Nutr Metab Cardiovasc Dis. 2021;31(5):1445–1453. doi: 10.1016/j.numecd.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Jayedi A, Mirzaei K, Rashidy-Pour A, Yekaninejad MS, Zargar MS, Akbari Eidgahi MR. Dietary approaches to stop hypertension, mediterranean dietary pattern, and diabetic nephropathy in women with type 2 diabetes: a case-control study. Clin Nutr ESPEN. 2019;33:164–170. doi: 10.1016/j.clnesp.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 67.Hansrivijit P, Oli S, Khanal R, Ghahramani N, Thongprayoon C, Cheungpasitporn W. Mediterranean diet and the risk of chronic kidney disease: a systematic review and meta-analysis. Nephrology (Carlton) 2020;25(12):913–918. doi: 10.1111/nep.13778. [DOI] [PubMed] [Google Scholar]
- 68.Hu EA, Steffen LM, Grams ME, Crews DC, Coresh J, Appel LJ, et al. Dietary patterns and risk of incident chronic kidney disease: the Atherosclerosis Risk in Communities study. Am J Clin Nutr. 2019;110(3):713–721. doi: 10.1093/ajcn/nqz146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chauveau P, Aparicio M, Bellizzi V, Campbell K, Hong X, Johansson L, et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33(5):725–735. doi: 10.1093/ndt/gfx085. [DOI] [PubMed] [Google Scholar]
- 70.Diaz-Lopez A, Babio N, Martinez-Gonzalez MA, Corella D, Amor AJ, Fito M, et al. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: a post hoc analysis of a randomized trial. Diabetes Care. 2015;38(11):2134–2141. doi: 10.2337/dc15-1117. [DOI] [PubMed] [Google Scholar]
- 71.Dow C, Mancini F, Rajaobelina K, Boutron-Ruault MC, Balkau B, Bonnet F, et al. Diet and risk of diabetic retinopathy: a systematic review. Eur J Epidemiol. 2018;33(2):141–156. doi: 10.1007/s10654-017-0338-8. [DOI] [PubMed] [Google Scholar]
- 72.Ghaemi F, Firouzabadi FD, Moosaie F, Shadnoush M, Poopak A, Kermanchi J, et al. Effects of a Mediterranean diet on the development of diabetic complications: a longitudinal study from the nationwide diabetes report of the National Program for Prevention and Control of Diabetes (NPPCD 2016–2020) Maturitas. 2021;153:61–67. doi: 10.1016/j.maturitas.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Wong MYZ, Man REK, Fenwick EK, Gupta P, Li LJ, van Dam RM, et al. Dietary intake and diabetic retinopathy: a systematic review. PLoS ONE. 2018;13(1):e0186582. doi: 10.1371/journal.pone.0186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siervo M, Shannon OM, Llewellyn DJ, Stephan BC, Fontana L. Mediterranean diet and cognitive function: from methodology to mechanisms of action. Free Radic Biol Med. 2021;176:105–117. doi: 10.1016/j.freeradbiomed.2021.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Soldevila-Domenech N, Forcano L, Vintro-Alcaraz C, Cuenca-Royo A, Pinto X, Jimenez-Murcia S, et al. Interplay between cognition and weight reduction in individuals following a Mediterranean diet: three-year follow-up of the PREDIMED-Plus trial. Clin Nutr. 2021;40(9):5221–5237. doi: 10.1016/j.clnu.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 76.Kossler T, Weber KS, Wolwer W, Hoyer A, Strassburger K, Burkart V, et al. Associations between cognitive performance and Mediterranean dietary pattern in patients with type 1 or type 2 diabetes mellitus. Nutr Diabetes. 2020;10(1):10. doi: 10.1038/s41387-020-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72(1):30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 78.Tuttle KR, Shuler LA, Packard DP, Milton JE, Daratha KB, Bibus DM, et al. Comparison of low-fat versus Mediterranean-style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial) Am J Cardiol. 2008;101(11):1523–1530. doi: 10.1016/j.amjcard.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 79.Barzi F, Woodward M, Marfisi RM, Tavazzi L, Valagussa F, Marchioli R, et al. Mediterranean diet and all-causes mortality after myocardial infarction: results from the GISSI-Prevenzione trial. Eur J Clin Nutr. 2003;57(4):604–611. doi: 10.1038/sj.ejcn.1601575. [DOI] [PubMed] [Google Scholar]
- 80.Paterson KE, Myint PK, Jennings A, Bain LKM, Lentjes MAH, Khaw KT, et al. Mediterranean diet reduces risk of incident stroke in a population with varying cardiovascular disease risk profiles. Stroke. 2018;49(10):2415–2420. doi: 10.1161/STROKEAHA.117.020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsivgoulis G, Psaltopoulou T, Wadley VG, Alexandrov AV, Howard G, Unverzagt FW, et al. Adherence to a Mediterranean diet and prediction of incident stroke. Stroke. 2015;46(3):780–785. doi: 10.1161/STROKEAHA.114.007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruiz-Canela M, Estruch R, Corella D, Salas-Salvado J, Martinez-Gonzalez MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311(4):415–417. doi: 10.1001/jama.2013.280618. [DOI] [PubMed] [Google Scholar]
- 83.Kahleova H, Salas-Salvado J, Rahelic D, Kendall CW, Rembert E, Sievenpiper JL. Dietary patterns and cardiometabolic outcomes in diabetes: a summary of systematic reviews and meta-analyses. Nutrients. 2019;11(9):2209. doi: 10.3390/nu11092209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becerra-Tomas N, Blanco Mejia S, Viguiliouk E, Khan T, Kendall CWC, Kahleova H, et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: a systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60(7):1207–1227. doi: 10.1080/10408398.2019.1565281. [DOI] [PubMed] [Google Scholar]
- 85.Ciccarone E, Di Castelnuovo A, Salcuni M, Siani A, Giacco A, Donati MB, et al. A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with Type 2 diabetes. J Thromb Haemost. 2003;1(8):1744–1752. doi: 10.1046/j.1538-7836.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- 86.Lilja E, Bergwall S, Sonestedt E, Gottsater A, Acosta S. The association between dietary intake, lifestyle and incident symptomatic peripheral arterial disease among individuals with diabetes mellitus: insights from the Malmo Diet and Cancer study. Ther Adv Endocrinol Metab. 2019;10:2042018819890532. doi: 10.1177/2042018819890532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Milenkovic T, Bozhinovska N, Macut D, Bjekic-Macut J, Rahelic D, Velija Asimi Z, et al. Mediterranean diet and type 2 diabetes mellitus: a perpetual inspiration for the scientific world. A Review. Nutrients. 2021;13(4):1307. doi: 10.3390/nu13041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muscogiuri G, Barrea L, Di Somma C, Altieri B, Vecchiarini M, Orio F, et al. Patient empowerment and the Mediterranean diet as a possible tool to tackle prediabetes associated with overweight or obesity: a pilot study. Hormones (Athens) 2019;18(1):75–84. doi: 10.1007/s42000-018-0090-9. [DOI] [PubMed] [Google Scholar]
- 89.Athyros VG, Tziomalos K, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: an update. World J Gastroenterol. 2015;21(22):6820–6834. doi: 10.3748/wjg.v21.i22.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katsiki N, Athyros VG, Karagiannis A, Wierzbicki AS, Mikhailidis DP. Should we expand the concept of coronary heart disease equivalents? Curr Opin Cardiol. 2014;29(4):389–395. doi: 10.1097/HCO.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 91.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65(8):1109–1123. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Katsiki N, Perez-Martinez P, Anagnostis P, Mikhailidis DP, Karagiannis A. Is nonalcoholic fatty liver disease indeed the hepatic manifestation of metabolic syndrome? Curr Vasc Pharmacol. 2018;16(3):219–227. doi: 10.2174/1570161115666170621075619. [DOI] [PubMed] [Google Scholar]
- 93.Gastaldelli A. Fatty liver disease: the hepatic manifestation of metabolic syndrome. Hypertens Res. 2010;33(6):546–547. doi: 10.1038/hr.2010.60. [DOI] [PubMed] [Google Scholar]
- 94.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 95.Bakaloudi DR, Chrysoula L, Kotzakioulafi E, Theodoridis X, Chourdakis M. Impact of the level of adherence to Mediterranean diet on the parameters of metabolic syndrome: a systematic review and meta-analysis of observational studies. Nutrients. 2021;13(5):1514. doi: 10.3390/nu13051514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pugliese N, Plaz Torres MC, Petta S, Valenti L, Giannini EG, Aghemo A. Is there an 'ideal' diet for patients with NAFLD? Eur J Clin Invest. 2021;52(3):e13659. doi: 10.1111/eci.13659. [DOI] [PubMed] [Google Scholar]
- 97.Abenavoli L, Di Renzo L, Boccuto L, Alwardat N, Gratteri S, De Lorenzo A. Health benefits of Mediterranean diet in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2018;12(9):873–881. doi: 10.1080/17474124.2018.1503947. [DOI] [PubMed] [Google Scholar]
- 98.Saeed N, Nadeau B, Shannon C, Tincopa M. Evaluation of dietary approaches for the treatment of non-alcoholic fatty liver disease: a systematic review. Nutrients. 2019;11(12):3064. doi: 10.3390/nu11123064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barrea L, Di Somma C, Muscogiuri G, Tarantino G, Tenore GC, Orio F, et al. Nutrition, inflammation and liver-spleen axis. Crit Rev Food Sci Nutr. 2018;58(18):3141–3158. doi: 10.1080/10408398.2017.1353479. [DOI] [PubMed] [Google Scholar]
- 100.Barrea L, Muscogiuri G, Frias-Toral E, Laudisio D, Pugliese G, Castellucci B, et al. Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Crit Rev Food Sci Nutr. 2021;61(18):3066–3090. doi: 10.1080/10408398.2020.1792826. [DOI] [PubMed] [Google Scholar]
- 101.Descamps OS, Verhaegen A, Demeure F, Langlois M, Rietzschel E, Mertens A, et al. Evolving concepts on the management of dyslipidaemia. Acta Clin Belg. 2020;75(1):80–90. doi: 10.1080/17843286.2019.1702823. [DOI] [PubMed] [Google Scholar]
- 102.Filippatos T, Tsimihodimos V, Pappa E, Elisaf M. Pathophysiology of diabetic dyslipidaemia. Curr Vasc Pharmacol. 2017;15(6):566–575. doi: 10.2174/1570161115666170201105425. [DOI] [PubMed] [Google Scholar]
- 103.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 104.WHO. Noncommunicable diseases: risk factors. The Global Health Observatory. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-risk-factors. Last accessed 21 Jan 2022.
- 105.Coll N. Repositioning of the global epicentre of non-optimal cholesterol. Nature. 2020;582(7810):73–77. doi: 10.1038/s41586-020-2338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Migliaccio S, Brasacchio C, Pivari F, Salzano C, Barrea L, Muscogiuri G, et al. What is the best diet for cardiovascular wellness? A comparison of different nutritional models. Int J Obes Suppl. 2020;10(1):50–61. doi: 10.1038/s41367-020-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laudisio D, Castellucci B, Barrea L, Pugliese G, Savastano S, Colao A, et al. Mediterranean diet and breast cancer risk: a narrative review. Minerva Endocrinol (Torino) 2021;46(4):441–452. doi: 10.23736/S2724-6507.20.03266-6. [DOI] [PubMed] [Google Scholar]
- 108.Barrea L, Pugliese G, Laudisio D, Colao A, Savastano S, Muscogiuri G. Mediterranean diet as medical prescription in menopausal women with obesity: a practical guide for nutritionists. Crit Rev Food Sci Nutr. 2021;61(7):1201–1211. doi: 10.1080/10408398.2020.1755220. [DOI] [PubMed] [Google Scholar]
- 109.Barrea L, Arnone A, Annunziata G, Muscogiuri G, Laudisio D, Salzano C, et al. Adherence to the Mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS) Nutrients. 2019;11(10):2278. doi: 10.3390/nu11102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tosti V, Bertozzi B, Fontana L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. 2018;73(3):318–326. doi: 10.1093/gerona/glx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 112.Barrea L, Muscogiuri G, Annunziata G, Laudisio D, Pugliese G, Salzano C, et al. From gut microbiota dysfunction to obesity: could short-chain fatty acids stop this dangerous course? Hormones (Athens) 2019;18(3):245–250. doi: 10.1007/s42000-019-00100-0. [DOI] [PubMed] [Google Scholar]
- 113.Marzullo P, Di Renzo L, Pugliese G, De Siena M, Barrea L, Muscogiuri G, et al. From obesity through gut microbiota to cardiovascular diseases: a dangerous journey. Int J Obes Suppl. 2020;10(1):35–49. doi: 10.1038/s41367-020-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 2020;17(20):7618. doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, et al. Influence of Mediterranean diet on human gut microbiota. Nutrients. 2020;13(1):7. doi: 10.3390/nu13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14(4):245–254. doi: 10.2174/1871530314666140922153350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwingshackl L, Morze J, Hoffmann G. Mediterranean diet and health status: active ingredients and pharmacological mechanisms. Br J Pharmacol. 2020;177(6):1241–1257. doi: 10.1111/bph.14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 119.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Llorente-Cortes V, Estruch R, Mena MP, Ros E, Gonzalez MA, Fito M, et al. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis. 2010;208(2):442–450. doi: 10.1016/j.atherosclerosis.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Visioli F, Poli A. Fatty acids and cardiovascular risk. Evidence, lack of evidence, and diligence. Nutrients. 2020;12(12):3782. doi: 10.3390/nu12123782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barrea L, Tarantino G, Somma CD, Muscogiuri G, Macchia PE, Falco A, et al. Adherence to the Mediterranean diet and circulating levels of sirtuin 4 in obese patients: a novel association. Oxid Med Cell Longev. 2017;2017:6101254. doi: 10.1155/2017/6101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S, Group PI-W The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J. 2009;158(1):1–7. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 124.Antoniazzi L, Arroyo-Olivares R, Bittencourt MS, Tada MT, Lima I, Jannes CE, et al. Association of dietary components with dyslipidemia and low-grade inflammation biomarkers in adults with heterozygous familial hypercholesterolemia from different countries. Eur J Clin Nutr. 2019;73(12):1622–1625. doi: 10.1038/s41430-019-0529-3. [DOI] [PubMed] [Google Scholar]
- 125.de Oliveira Otto MC, Mozaffarian D, Kromhout D, Bertoni AG, Sibley CT, Jacobs DR, Jr, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2012;96(2):397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Formisano E, Pasta A, Cremonini AL, Di Lorenzo I, Sukkar SG, Pisciotta L. Effects of a Mediterranean diet, dairy, and meat products on different phenotypes of dyslipidemia: a preliminary retrospective analysis. Nutrients. 2021;13(4):1161. doi: 10.3390/nu13041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Laudisio D, et al. Sleep Quality in obesity: does adherence to the Mediterranean diet matter? Nutrients. 2020;12(5):1364. doi: 10.3390/nu12051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Antoniazzi L, Arroyo-Olivares R, Bittencourt MS, Tada MT, Lima I, Jannes CE, et al. Adherence to a Mediterranean diet, dyslipidemia and inflammation in familial hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2021;31(7):2014–2022. doi: 10.1016/j.numecd.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 129.Barrea L, Altieri B, Muscogiuri G, Laudisio D, Annunziata G, Colao A, et al. Impact of Nutritional Status on Gastroenteropancreatic Neuroendocrine Tumors (GEP-NET) Aggressiveness. Nutrients. 2018;10(12):1854. doi: 10.3390/nu10121854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, et al. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. 2018;10(12):1971. doi: 10.3390/nu10121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barrea L, Annunziata G, Muscogiuri G, Laudisio D, Di Somma C, Maisto M, et al. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: also a matter of sex? Nutrition. 2019;62:7–17. doi: 10.1016/j.nut.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 132.Barrea L, Muscogiuri G, Annunziata G, Laudisio D, de Alteriis G, Tenore GC, et al. A new light on vitamin D in obesity: a novel association with trimethylamine-N-Oxide (TMAO) Nutrients. 2019;11(6):1310. doi: 10.3390/nu11061310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thomas MS, Fernandez ML. Trimethylamine N-Oxide (TMAO), Diet and cardiovascular disease. Curr Atheroscler Rep. 2021;23(4):12. doi: 10.1007/s11883-021-00910-x. [DOI] [PubMed] [Google Scholar]
- 134.Leon-Mimila P, Villamil-Ramirez H, Li XS, Shih DM, Hui ST, Ocampo-Medina E, et al. Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. 2021;47(2):101183. doi: 10.1016/j.diabet.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barrea L, Muscogiuri G, Pugliese G, de Alteriis G, Maisto M, Donnarumma M, et al. Association of trimethylamine N-Oxide (TMAO) with the clinical severity of Hidradenitis Suppurativa (Acne Inversa) Nutrients. 2021;13(6):1997. doi: 10.3390/nu13061997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muscogiuri G, Barrea L, Laudisio D, Di Somma C, Pugliese G, Salzano C, et al. Somatotropic axis and obesity: is there any role for the Mediterranean diet? Nutrients. 2019;11(9):2228. doi: 10.3390/nu11092228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lombardi G, Di Somma C, Grasso LF, Savanelli MC, Colao A, Pivonello R. The cardiovascular system in growth hormone excess and growth hormone deficiency. J Endocrinol Invest. 2012;35(11):1021–1029. doi: 10.3275/8717. [DOI] [PubMed] [Google Scholar]
- 138.Altieri B, Grant WB, Della Casa S, Orio F, Pontecorvi A, Colao A, et al. Vitamin D and pancreas: the role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr. 2017;57(16):3472–3488. doi: 10.1080/10408398.2015.1136922. [DOI] [PubMed] [Google Scholar]
- 139.Muscogiuri G, Barrea L, Altieri B, Di Somma C, Bhattoa HP, Laudisio D, et al. Calcium and vitamin D supplementation. Myths and realities with regard to cardiovascular risk. Curr Vasc Pharmacol. 2019;17(6):610–617. doi: 10.2174/1570161117666190408165805. [DOI] [PubMed] [Google Scholar]
- 140.Savanelli MC, Scarano E, Muscogiuri G, Barrea L, Vuolo L, Rubino M, et al. Cardiovascular risk in adult hypopituitaric patients with growth hormone deficiency: is there a role for vitamin D? Endocrine. 2016;52(1):111–119. doi: 10.1007/s12020-015-0779-3. [DOI] [PubMed] [Google Scholar]
- 141.Muscogiuri G, Barrea L, Annunziata G, Di Somma C, Laudisio D, Colao A, et al. Obesity and sleep disturbance: the chicken or the egg? Crit Rev Food Sci Nutr. 2019;59(13):2158–2165. doi: 10.1080/10408398.2018.1506979. [DOI] [PubMed] [Google Scholar]
- 142.Muscogiuri G, Barrea L, Scannapieco M, Di Somma C, Scacchi M, Aimaretti G, et al. The lullaby of the sun: the role of vitamin D in sleep disturbance. Sleep Med. 2019;54:262–265. doi: 10.1016/j.sleep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 143.Barrea L, Savastano S, Di Somma C, Savanelli MC, Nappi F, Albanese L, et al. Low serum vitamin D-status, air pollution and obesity: a dangerous liaison. Rev Endocr Metab Disord. 2017;18(2):207–214. doi: 10.1007/s11154-016-9388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Savastano S, Barrea L, Savanelli MC, Nappi F, Di Somma C, Orio F, et al. Low vitamin D status and obesity: role of nutritionist. Rev Endocr Metab Disord. 2017;18(2):215–225. doi: 10.1007/s11154-017-9410-7. [DOI] [PubMed] [Google Scholar]
- 145.Barrea L, Muscogiuri G, Laudisio D, Pugliese G, de Alteriis G, Colao A, et al. Influence of the Mediterranean diet on 25- hydroxyvitamin D levels in adults. Nutrients. 2020;12(5):1439. doi: 10.3390/nu12051439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9(10):1063. doi: 10.3390/nu9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Couto E, Boffetta P, Lagiou P, Ferrari P, Buckland G, Overvad K, et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br J Cancer. 2011;104(9):1493–1499. doi: 10.1038/bjc.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Toledo E, Salas-Salvado J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1752–1760. doi: 10.1001/jamainternmed.2015.4838. [DOI] [PubMed] [Google Scholar]
- 149.Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr. 2009;89(4):1145–1154. doi: 10.3945/ajcn.2008.26915. [DOI] [PubMed] [Google Scholar]
- 150.Murtaugh MA, Sweeney C, Giuliano AR, Herrick JS, Hines L, Byers T, et al. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the Four-Corners Breast Cancer Study. Am J Clin Nutr. 2008;87(4):978–984. doi: 10.1093/ajcn/87.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Link LB, Canchola AJ, Bernstein L, Clarke CA, Stram DO, Ursin G, et al. Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am J Clin Nutr. 2013;98(6):1524–1532. doi: 10.3945/ajcn.113.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cottet V, Touvier M, Fournier A, Touillaud MS, Lafay L, Clavel-Chapelon F, et al. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol. 2009;170(10):1257–1267. doi: 10.1093/aje/kwp257. [DOI] [PubMed] [Google Scholar]
- 153.WCRF. Eat wholegrains, vegetables, fruit and beans. https://www.wcrf.org/dietandcancer/eat-wholegrains-vegetables-fruit-and-beans/. Last accessed 21 Jan 2022.
- 154.Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28(4):317–328. doi: 10.1007/s10654-013-9795-x. [DOI] [PubMed] [Google Scholar]