Abstract
Aims/hypothesis
Serum sex hormone-binding globulin (SHBG) has been proposed to act as a hepatokine that contributes to the extrahepatic complications observed in non-alcoholic fatty liver disease (NAFLD). However, it remains uncertain whether serum SHBG mediates the association between intrahepatic lipids (IHL) and type 2 diabetes. Therefore, we studied whether, and to what extent, serum SHBG mediates the association between IHL content and type 2 diabetes.
Methods
We used cross-sectional data from the Maastricht Study (n=1554), a population-based cohort study with oversampling of individuals with type 2 diabetes. Type 2 diabetes status was assessed by oral glucose tolerance test, and IHL content was measured using 3T Dixon MRI. Mediation analyses were performed to assess the role of serum SHBG in mediating the association between IHL content and type 2 diabetes.
Results
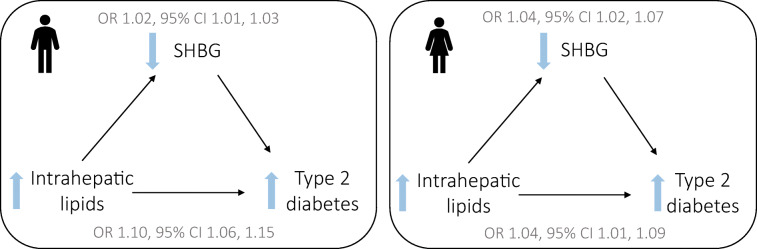
IHL content was significantly associated with type 2 diabetes in women and men (OR 1.08 [95% CI 1.04, 1.14] and OR 1.12 [95% CI 1.08, 1.17], respectively). Serum SHBG significantly mediated the association between IHL content and type 2 diabetes. The contribution of serum SHBG was higher in women (OR 1.04 [95% CI 1.02, 1.07]; proportion mediated 50.9% [95% CI 26.7, 81.3]) than in men (OR 1.02 [95% CI 1.01, 1.03]; proportion mediated 17.2% [95% CI 9.6, 27.6]). Repeat analyses with proxies of type 2 diabetes and adjustment for covariates did not substantially affect the results.
Conclusions/interpretation
In this large-scale population-based cohort study, serum SHBG was found to be a mediator of the association between IHL content and type 2 diabetes. These findings extend our understanding of the potential mechanisms by which NAFLD is a risk factor for type 2 diabetes, and further elaborate on the role of SHBG as a hepatokine.
Graphical abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-022-05790-7
Keywords: Hepatokine, Mediation, Non-alcoholic fatty liver disease, Sex hormone-binding globulin, Type 2 diabetes
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a serious health concern that affects approximately 25% of the global population [1]. It encompasses a spectrum of histological abnormalities that result from excess storage of intrahepatic lipids (IHL) [2]. NAFLD is a precursor for several hepatic complications including liver failure and hepatocellular carcinoma, and is also a risk factor for the development of various extrahepatic complications, such as type 2 diabetes and cardiovascular disease [3, 4].
It has been proposed that hepatokines (liver-derived proteins that have systemic metabolic effects) may, in part, mediate the association between IHL accumulation and extrahepatic complications [5, 6]. Serum sex hormone-binding globulin (SHBG) has emerged as a hepatokine [7, 8]. SHBG is a glycoprotein that is synthesised in the liver under the regulation of several transcription factors including hepatocyte nuclear factor 4α, constitutive androstane receptor, peroxisome proliferator-activated receptor γ and chicken ovalbumin upstream promotor transcription factor [9–11]. In turn, these transcription factors are affected by several metabolic, hormonal, inflammatory and nutritional factors. Experimental studies have shown that de novo lipogenesis, the principal pathway resulting in accumulation of IHL [12], downregulates hepatocyte nuclear factor 4α, resulting in reduced SHBG expression [13]. We have previously extended these experimental data by showing that de novo lipogenesis, assessed using stable isotopes [12], is inversely associated with serum SHBG levels in humans [14].
Of interest, Mendelian randomisation studies have shown that genetically predicted low serum SHBG levels are associated with a higher risk of type 2 diabetes [15, 16]. This effect appears to be attributable to SHBG itself, i.e. independent of the effects of SHBG on free testosterone levels [17].
However, it remains to be elucidated to what extent serum SHBG mediates the association between IHL accumulation and type 2 diabetes. Therefore, the aim of the present study was to assess whether, and to what extent, serum SHBG has a mediating role in the association between IHL content and type 2 diabetes in a population-based cohort.
Methods
Study population
The Maastricht Study is a population-based cohort study with oversampling of individuals with type 2 diabetes. The study design and rationale have been extensively described previously [18]. In brief, the Maastricht Study focuses on the aetiology, pathophysiology, complications and comorbidities of type 2 diabetes, and involves extensive phenotyping of all participants. All individuals between 40 and 75 years of age living in the southern part of the Netherlands were eligible for participation. Participants were recruited through mass media campaigns and via mailings from the municipal registries and the regional Diabetes Patient Registry.
The present study includes cross-sectional data from 3340 participants in whom serum SHBG levels were measured and who completed baseline measurements between November 2010 and December 2017. Quantification of IHL content was implemented from December 2013 onwards. Participants were excluded from the current analyses if they were diagnosed with other types of diabetes (n=41) or had missing data for IHL content (n=1161) or covariates (n=584). This resulted in a study population of 1554 participants (see electronic supplementary material [ESM] Fig. 1), of whom 369 had type 2 diabetes.
The Maastricht Study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (permit 131088-105234-PG). All participants gave written informed consent prior to participation.
Outcome: type 2 diabetes
All participants underwent a standardised 2 h 75 g oral glucose tolerance test after an overnight fast, except for individuals using insulin and/or individuals with a fasting capillary glucose ≥ 11.1 mmol/l. For these individuals, information about fasting glucose levels, use of glucose-lowering medication, and history of other types of diabetes was used to assess type 2 diabetes status [18]. Diabetes was defined according to the WHO 2006 diagnostic criteria as a fasting plasma glucose ≥ 7.0 mmol/l and/or a 2 h plasma glucose ≥ 11.1 mmol/l [19].
Exposure: IHL content
IHL content was quantified by Dixon MR imaging using a 3.0 Tesla MRI system (MAGNETOM Prismafit, Siemens, Germany) with body matrix and supine radiofrequency coils. After a scout scan, transverse two-dimensional T2-weighted true fast imaging with steady-state free precession (T2w TRUFI) images of the liver were obtained using the following variables: voxel size: 1.2 × 1.2 × 5.0 mm3, repetition time 422 ms, echo time 1.65 ms, flip angle 60°, number of signal averages 1, parallel imaging (GRAPPA) factor 2. Next, transverse two-dimensional turbo spin echo Dixon MR images were obtained of the liver during a breath-hold using the following variables: voxel size: 2.0 × 2.0 × 6.0 mm3, number of slices 4, repetition time 500 ms, echo time 31 ms, turbo factor 5, number of signal averages 1, parallel imaging (GRAPPA) factor 3 [20]. Three regions of interest (ROIs) in the liver were drawn on the T2w TRUFI images by trained observers, taking care to position the ROIs in artifact-free regions and to avoid positioning the ROIs on visible structures, such as vessels and bile ducts. Subsequently, these ROIs were copied to the water and fat Dixon MR images to calculate the IHL percentage, expressed as the ratio CH2/H2O × 100%. Hepatic steatosis has been defined as an IHL content >5.56% when expressed as CH2/(H2O + CH2) [21], which corresponds to a cut-off of 5.89% (0.0556/(1–0.0556)) when IHL is expressed as CH2/H2O, as was done in this study.
The Dixon MRI method was validated in 36 participants with a broad range of IHL content, and calibrated against the results obtained using 3T proton magnetic resonance spectroscopy (1H-MRS), i.e. the gold standard for non-invasively quantifying IHL [22]. After calibration, the intraclass correlation coefficient between Dixon MRI and 1H-MRS was 0.989 (95% CI 0.979, 0.994).
Mediator: serum SHBG levels
Serum SHBG levels were measured using a human SHBG DuoSet solid-phase sandwich ELISA (R&D Systems, USA) according to the manufacturer’s instructions. The intra- and interassay coefficients of variation for serum SHBG were 2.8% and 5.1%, respectively. The DuoSet ELISA was validated against a chemiluminescent immunometric assay (Immulite XPi, Siemens, Germany) in eight samples. The intraclass correlation coefficient was 0.974 (95% CI 0.862, 0.995).
Measurement of covariates
All participants completed questionnaires regarding age, sex, educational level (low, medium or high), smoking status (never, former or current smoker), use of alcohol (g/day) and menopausal status (postmenopausal status was defined as a most recent menstrual period more than 12 months prior to the time of assessment) [18]. Use of medication was assessed through medication interviews. Anthropometric measurements including weight, height, waist circumference and office systolic and diastolic blood pressure were measured during physical examination. BMI was calculated as weight (kg) divided by height (m) squared [18]. Daily total physical activity levels were measured during 8 consecutive days using activPAL3 physical activity monitors (PAL Technologies, UK) and expressed as minutes of stepping activity per day [23]. Fasting levels of glucose, insulin, HbA1c and lipid profile (total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides) were measured in venous blood samples [18]. Insulin sensitivity was estimated using the Matsuda insulin sensitivity index [24]. Adherence to the Dutch dietary guidelines was assessed based on the Dutch Healthy Diet (DHD) index consisting of 15 components and based on food frequency questionnaires [25]. In the Maastricht Study, the DHD index consists of 14 components (DHD-14). The coffee component was not included as it is based on the type of coffee consumed, which the food frequency questionnaires were unable to distinguish between [26]. Furthermore, as we included alcohol consumption as a separate covariate in the regression models (see below), the DHD index in the present study was reported as the DHD-13 (DHD-14 minus the alcohol component).
Statistical analyses
Continuous data are presented as mean ± SD, or as median (IQR) in the case of a non-normal distribution. Categorical data are presented as percentages. Non-normally distributed variables were log10-transformed prior to further analyses.
Univariate regression analyses were performed to study the associations between (1) IHL content and serum SHBG; (2) serum SHBG and type 2 diabetes (crude and adjusted for IHL content); and (3) IHL content and type 2 diabetes. All associations were explored for an interaction with sex. Regression coefficients are presented as unstandardised β coefficients.
Mediation analyses were then performed to investigate whether the association between IHL content and type 2 diabetes status was mediated by serum SHBG, as illustrated in the directed acyclic graph in ESM Fig. 2. We also tested for an effect of an interaction between the exposure and the mediator on the outcome. The mediation analyses were adjusted for the following confounders: model 1 was adjusted for age; model 2 was additionally adjusted for (proxies of) lifestyle: BMI, alcohol intake, DHD-13, level of education and total physical activity; model 3 was additionally adjusted for menopausal status and use of oestrogen-containing medication in women. Furthermore, given the oversampling of participants with type 2 diabetes, analyses were repeated after taking the higher prevalence of type 2 diabetes into consideration using case–control mediation analyses [27]. The analyses were then repeated using a binary exposure, i.e. hepatic steatosis yes/no. Lastly, to further test the robustness of our findings, the analyses were repeated with adjustment for waist circumference instead of BMI (model 2), and analyses were repeated with proxies of type 2 diabetes as the outcome variable, i.e. HbA1c and the Matsuda index.
For all mediation analyses, the regression-based approach [28, 29] was used to estimate the natural direct and natural indirect effects. The 95% CIs were estimated using non-parametric bootstrapping with the percentile method. The proportion mediated (%) was estimated as ORDirect × (ORIndirect – 1)/(ORDirect × ORIndirect – 1) × 100 in the case of a binary outcome [30], or as βIndirect/βTotal × 100 in the case of a continuous outcome [31]. All results were considered statistically significant at a p value<0.05, except for interaction terms where a less stringent p value threshold was considered statistically significant (p<0.10).
Statistical analyses were performed using SPSS version 27.0 for Windows (IBM, USA) and R statistical software version 4.0.1 (R Foundation for Statistical Computing, Austria) with the CMAverse package [32].
Results
Study population
Table 1 shows the characteristics of the overall study population and the population stratified according to type 2 diabetes status. The overall population had a mean age of 60 ± 8 years and 47.9% were female, of whom the majority (79.2%) were postmenopausal. Only a small number of women (4.0%) used oestrogen-containing medication. The overall population had a median IHL content of 3.5% (IQR 2.1–6.5) and a median serum SHBG level of 35.5 nmol/l (IQR 25.3–49.8). Although Table 1 data were not tested for statistical significance, participants with type 2 diabetes were more often male, tended to be older, and generally had a poorer metabolic profile (i.e. higher BMI, waist circumference, systolic blood pressure and serum triglycerides, and lower HDL-cholesterol and Matsuda index). Furthermore, participants with type 2 diabetes had a higher median IHL content and lower serum SHBG levels.
Table 1.
Characteristics of the overall study population and the population stratified according to type 2 diabetes status
| Variable | Overall (n=1554) |
Individuals without type 2 diabetes (n=1185) |
Individuals with type 2 diabetes (n=369) |
|---|---|---|---|
| Age, years | 60 ± 8 | 59 ± 8 | 62 ± 8 |
| Sex, % women | 47.9 | 53.8 | 29.0 |
| Postmenopausal, % of women | 79.2 | 78.3 | 84.1 |
| Use of oestrogen-containing medication, % of women | 4.0 | 3.8 | 5.6 |
| Education level low/medium/high, % | 30.3/28.8/40.9 | 27.3/28.4/44.2 | 39.8/30.1/30.1 |
| Smoking, never/former/current, % | 38.0/50.2/11.8 | 39.0/49.3/11.7 | 34.8/53.0/12.2 |
| DHD index (DHD-13)a | 77.2 ± 13.9 | 78.0 ± 13.9 | 74.8 ± 13.7 |
| Alcohol, g/day | 9.0 (2.0–19.0) | 9.8 (2.7–19.5) | 5.8 (0.5–16.0) |
| Physical activity, min/day | 120.8 (93.6–148.7) | 125.5 (101.1–152.5) | 100.9 (74.6–135.8) |
| BMI, kg/m2 | 26.6 ± 4.1 | 25.8 ± 3.7 | 29.0 ± 4.3 |
| Waist circumference, cm | 94.3 ± 12.5 | 91.3 ± 11.0 | 103.8 ± 12.5 |
| Office systolic blood pressure, mmHg | 134 ± 17 | 132 ± 17 | 140 ± 16 |
| Office diastolic blood pressure, mmHg | 76 ± 10 | 76 ± 10 | 77 ± 9 |
| Total cholesterol, mmol/l | 5.3 ± 1.2 | 5.6 ± 1.1 | 4.5 ± 1.0 |
| HDL-cholesterol, mmol/l | 1.6 ± 0.5 | 1.7 ± 0.5 | 1.3 ± 0.4 |
| LDL-cholesterol, mmol/l | 3.1 ± 1.0 | 3.3 ± 1.0 | 2.4 ± 0.9 |
| Triglycerides, mmol/l | 1.2 (0.9–1.7) | 1.1 (0.8–1.5) | 1.5 (1.1–2.1) |
| Use of lipid-modifying medication, % | 32.3 | 19.7 | 72.9 |
| HbA1c, % | 5.6 (5.4–6.0) | 5.4 (5.3–5.7) | 6.7 (6.2–7.4) |
| HbA1c, mmol/mol | 38.0 (35.0–42.0) | 36.0 (34.0–39.0) | 50.0 (44.5–57.0) |
| Fasting glucose, mmol/l | 5.5 (5.0–6.3) | 5.3 (4.9–5.7) | 7.5 (6.8–8.6) |
| Fasting insulin, mmol/l | 59.1 (41.8–87.8) | 55.3 (40.0–77.0) | 83.2 (51.3–126.0) |
| Matsuda index | 3.6 (2.1–5.3) | 4.1 (2.6–5.8) | 2.0 (1.3–3.0) |
| Use of glucose-lowering medication, % | 17.9 | 0.0 | 75.3 |
| IHL content, % | 3.5 (2.1–6.5) | 2.9 (1.9–5.1) | 5.2 (3.5–10.7) |
| Hepatic steatosis, % yes | 29.9 | 22.0 | 55.0 |
| Serum SHBG, nmol/l | 35.5 (25.3–49.8) | 38.7 (27.6–54.2) | 26.4 (19.6–37.0) |
Data are presented as mean ± SD or median (IQR) unless otherwise indicated
aDutch Healthy Diet (DHD-14) index minus alcohol component (DHD-13)
Univariate regression analyses
Univariate regression analyses were performed to study the association between (1) IHL content and serum SHBG levels (i.e. exposure–mediator); (2) IHL content and type 2 diabetes status (i.e. exposure–outcome); and (3) serum SHBG levels and type 2 diabetes status (i.e. mediator–outcome). As there was a statistically significant interaction effect of the dependent variable and sex on the outcome for all three associations (p=0.001, 0.040 and < 0.001, respectively), all analyses were subsequently stratified according to sex. The sex-stratified population characteristics are presented in ESM Table 1.
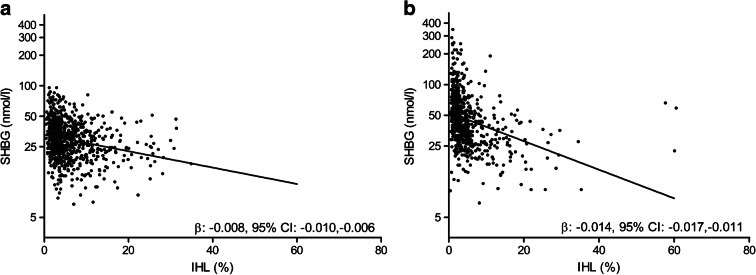
There was a statistically significant inverse association between IHL content and serum SHBG levels in men and women (β −0.008 [95% CI −0.010, −0.006] and β −0.014 [95% CI −0.017, −0.011], respectively) (Fig. 1). The strength of association was stronger in women, and this remained after exclusion of premenopausal women and women using oestrogen-containing medication (β −0.015 [95% CI −0.018, −0.012]). Furthermore, there was a statistically significant association between IHL content and type 2 diabetes status in men and women (OR 1.12 [95% CI 1.08, 1.17] and OR 1.08 [95% CI 1.04, 1.14], respectively). Of note, these associations represent the total effect estimates in the mediation analyses (i.e. exposure–outcome). Lastly, there was a statistically significant inverse association between serum SHBG and type 2 diabetes status in men and women (OR 0.96 [95% CI 0.95, 0.98] and OR 0.98 [95% CI 0.97, 0.99], respectively). Adjustment for IHL content did not affect these associations (OR 0.97 [95% CI 0.96, 0.98] and OR 0.98 [95% CI 0.97, 0.99], respectively). Furthermore, for all analyses, adjustment for age, BMI, alcohol intake, DHD-13, level of education, physical activity, and menopausal status and use of oestrogen-containing medication in women, did not substantially affect the results (p<0.025 for all analyses; data not shown).
Fig. 1.
Association between IHL content and serum SHBG (logarithmic scale) in men (a) and women (b). The black line represents the line of best fit
Mediation analyses
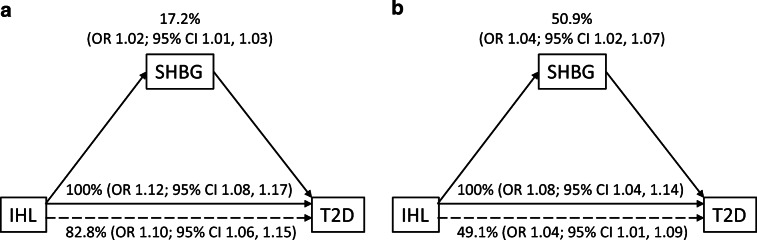
We performed mediation analyses to assess whether, and to what extent, the relationship between IHL content and type 2 diabetes status was mediated by serum SHBG levels. There was no interaction effect of the exposure and the mediator on the outcome in both men and women. We found that the association between IHL content and type 2 diabetes was statistically significantly mediated by serum SHBG in men (OR 1.02 [95% CI 1.01, 1.03]) and women (OR 1.04 [95% CI 1.02, 1.07]) (Fig. 2). In men, serum SHBG was estimated to mediate 17.2% (95% CI 9.6, 27.6) of the association between IHL content and type 2 diabetes, while the proportion mediated was 50.9% (95% CI 26.7, 81.3) in women. The mediation effect of serum SHBG remained statistically significant after adjustment for age (model 1), BMI, alcohol intake, DHD-13, level of education and total physical activity (model 2), and menopausal status and use of oestrogen-containing medication in women (model 3) (Table 2).
Fig. 2.
Crude association between IHL content and type 2 diabetes (T2D) mediated by serum SHBG in men (a) and women (b). Solid horizontal arrows represent the total effect, i.e. the association between IHL content and type 2 diabetes status. Dashed horizontal arrows indicate the direct effect, i.e. the association between IHL content and type 2 diabetes status that is not attributable to serum SHBG
Table 2.
Mediation effect of serum SHBG on the association between IHL content and type 2 diabetes
| Model | Men (n=810) | Women (n=744) | ||
|---|---|---|---|---|
| OR (95% CI) | Proportion mediated (95% CI)a | OR (95% CI) | Proportion mediated (95% CI)a | |
| Crude | ||||
| Total effectb | 1.12 (1.08, 1.17) | 1.08 (1.04, 1.14) | ||
| Direct effectb | 1.10 (1.06, 1.15) | 1.04 (1.01, 1.09) | ||
| Indirect effectb | 1.02 (1.01, 1.03) | 17.2 (9.6, 27.6) | 1.04 (1.02, 1.07) | 50.9 (26.7, 81.3) |
| Model 1 | ||||
| Total effect | 1.13 (1.09, 1.17) | 1.08 (1.05, 1.13) | ||
| Direct effect | 1.10 (1.06, 1.14) | 1.04 (1.01, 1.09) | ||
| Indirect effect | 1.03 (1.02, 1.04) | 24.6 (15.6, 36.0) | 1.04 (1.02, 1.07) | 48.5 (24.0, 80.4) |
| Model 2 | ||||
| Total effect | 1.08 (1.05, 1.13) | 1.04 (1.00, 1.09) | ||
| Direct effect | 1.07 (1.03, 1.11) | 1.02 (0.98, 1.07) | ||
| Indirect effect | 1.01 (1.01, 1.02) | 17.7 (8.3, 32.8) | 1.02 (1.00, 1.04) | 42.6 (2.5, 254.1) |
| Model 3 | ||||
| Total effect | 1.04 (1.00, 1.10) | |||
| Direct effect | 1.02 (0.98, 1.07) | |||
| Indirect effect | 1.02 (1.01, 1.04) | 55.9 (−72.38, 337.2) | ||
Model 1 was adjusted for age; model 2 was additionally adjusted for BMI, alcohol intake, DHD-13, level of education and total physical activity; model 3 was additionally adjusted for menopausal status and use of oestrogen-containing medication in women
aThe proportion mediated (%) is calculated as ORDirect × (ORIndirect − 1)/(ORDirect × ORIndirect − 1) × 100
bTotal effect represents association between IHL content and type 2 diabetes status; direct effect represents association between IHL and type 2 diabetes status not attributable to serum SHBG; indirect effect represents association between IHL and type 2 diabetes attributable to serum SHBG (mediation)
Additional analyses
The mediation analyses were repeated after accounting for the oversampling of participants with type 2 diabetes in the Maastricht Study; this did not substantially affect the results (ESM Table 2). In addition, the analyses were repeated using a binary exposure, i.e. hepatic steatosis (yes/no). This did not materially affect the results, although statistical significance was lost in model 2 for men (p=0.16; ESM Table 3). In addition, the mediation analyses were repeated after adjustment for waist circumference instead of BMI (model 2); this did not materially change the results (ESM Table 4). Finally, mediation analyses were repeated using proxies for type 2 diabetes (i.e. HbA1c and the Matsuda index) as the dependent variable. In both men and women, serum SHBG remained a statistically significant mediator in the association between IHL content and both HbA1c and the Matsuda index in the crude and fully adjusted models (ESM Tables 5 and 6).
Discussion
In the present study, serum SHBG partially mediated the association between the IHL content and type 2 diabetes status. The mediating role of serum SHBG in the association between IHL content and type 2 diabetes was more substantial in women than in men. Similar results were found when the analyses were repeated using proxies of type 2 diabetes (i.e. HbA1c and the Matsuda index) and when adjusted for confounders.
The importance of hepatokines in the pathogenesis of extrahepatic disease, in particular type 2 diabetes, is increasingly recognised [5, 6, 33]. Nevertheless, this is the first study that has assessed the mediation effect of serum SHBG in the association between IHL content and type 2 diabetes. The current findings corroborate the hypothesis that SHBG may have a role not only as carrier protein for testosterone and a biomarker of metabolic disease, but also as a hepatokine affecting type 2 diabetes [16, 17, 33]. Experimental studies have shown that de novo lipogenesis, which is one of the primary pathways contributing to the accumulation of IHL [12], downregulates hepatocyte nuclear factor 4α and subsequently serum SHBG levels [13], a finding that we recently extrapolated to humans [14, 34]. Mendelian randomisation studies have shown that genetically predicted low serum SHBG levels are causally associated with an increased risk of type 2 diabetes, independent of the effects of SHBG on free testosterone levels [15–17, 35]. However, the exact biological mechanism by which serum SHBG influences type 2 diabetes is poorly understood, and experimental studies into the mechanism of action are scarce and sometimes contradictory [36–38]. These studies are complicated by the fact that wild-type rodents do not express hepatic SHBG, which limits the extrapolation to humans. Therefore, further research to unravel the exact mechanisms through which SHBG exerts its effects is needed.
It is likely that there are several pathways that mediate the association between IHL content and type 2 diabetes, of which serum SHBG is merely one. Insulin resistance and excess (hepatic) glucose production are other well-known mediators [39]. The accumulation of IHL contributes to an excess of circulating fatty acid metabolites in peripheral tissues, which are involved in the pathogenesis of insulin resistance [6, 39]. In addition, the carbohydrate regulatory element binding protein, which is one of the principal transcription factors that regulate de novo lipogenesis [40], activates glucose-6-phosphatase and thereby contributes to increased hepatic glucose production [41]. It is likely that these pathways are largely responsible for the remaining direct effect of IHL content on type 2 diabetes that was observed in this study.
We observed a relatively high estimated proportion of mediation by serum SHBG in the association between IHL content and type 2 diabetes. This may be an indication of the biological relevance of serum SHBG in the pathogenesis of type 2 diabetes. Indeed, the indirect effects of our mediation analyses suggest that a single percentage point increase in IHL content is associated with 8–12% higher odds of type 2 diabetes overall and 2–4% higher odds of type 2 diabetes when mediated via serum SHBG. Given that the median IHL content in this study population was 3.5% (IQR 2.1–6.5) (Table 1), the current findings are likely to be clinically relevant. Nevertheless, in some analyses, there was a considerable uncertainty in the estimated proportion mediated, particularly when the direct effect (i.e. the effect of IHL content on type 2 diabetes that was not attributable to serum SHBG) was not statistically significant. This may indicate methodological limitations of the estimated proportion mediated, and the results should therefore be interpreted with care [42]. Furthermore, the high estimated proportion mediated may partially be the result of potential bi-directionality of the associations. Although it is assumed that there is a causal association between IHL content, serum SHBG and type 2 diabetes (an assumption that is supported by experimental and genetic studies [13, 15, 16]) we cannot exclude the possibility that the associations are in fact bi-directional. Indeed, experimental studies have found that transgenic SHBG mice show reduced IHL content and improved glucose homeostasis [36, 37, 43], although these findings have not yet been extrapolated to humans. In addition, hyperinsulinaemia and hyperglycaemia, which are characteristic of type 2 diabetes [44], stimulate de novo lipogenesis and consequently IHL accumulation [45]. As a result of the bi-directionality of these associations, the observed estimates should be regarded as the maximum mediation effects.
There was a noticeable difference in the mediation effect of serum SHBG between men and women, with a higher contribution observed in women. Of interest, previous observational studies have reported similar sexually dimorphic associations between IHL content and serum SHBG [7], in line with the results of the univariate regression analyses in this study [8, 15]. Moreover, genetic studies have reported that variants in the glucokinase regulator gene GCKR, which are associated with higher rates of de novo lipogenesis and IHL content [46], have a stronger, inverse association with serum SHBG in women than in men [47]. However, the biological mechanisms that account for these sex differences remain poorly understood and deserve further investigation.
This study has several strengths. By using data from the Maastricht Study, we were able to obtain a large cohort of individuals with oversampling of participants with type 2 diabetes. The extensive phenotyping allowed for adjustment for many well-defined confounders, such as use of oestrogen-containing medication and physical activity, as assessed using an accelerometer. Furthermore, IHL content was quantified using state-of-the-art methodology (i.e. Dixon MRI). This study also had several limitations. First, as a result of the cross-sectional nature of the data, we cannot draw conclusions on causality. Although experimental and genetic studies support the assumptions of causality in this study, we cannot exclude the possibility that, as mentioned above, the associations are bi-directional. Furthermore, although we adjusted for the most important confounders, we cannot exclude the possibility that there may be residual confounding. For instance, other unmeasured confounders such as endogenous oestrogens, thyroid hormone or adipokines, which all affect IHL content, serum SHBG levels and type 2 diabetes, may confound the current mediation analyses. Adjustment for these unmeasured confounders may reduce the strength of the observations. Furthermore, we cannot exclude the possibility that other hepatokines, which are also associated with IHL content [33], also mediate the association between IHL content and type 2 diabetes status [48]; this deserves further investigation. Lastly, the participants in the current study were primarily of European descent, aged between 40 and 75 years, which resulted in a relatively low number of premenopausal women. Caution should therefore be exercised when extrapolating to other groups.
In conclusion, in a large-scale population-based cohort study, we show that serum SHBG is a mediator in the association between IHL content and type 2 diabetes. The mediation effect was larger in women. These findings extend our knowledge on the potential mechanisms that link NAFLD with type 2 diabetes, and emphasise the importance of serum SHBG as a hepatokine. Furthermore, the current data support the concept that increasing serum SHBG (by reduction of IHL content) may be used as a means to reduce type 2 diabetes risk.
Supplementary information
(PDF 582 KB)
Acknowledgments
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
PIHGS analysed the data and drafted the article. PIHG, MCGJB, OV and CDAS contributed to the conception and design of the study, MCGJB, MPHvdW, MMJvG, MEK, JFAJ, CGS and CDAS contributed to the acquisition of data. MCGJB, OV, MPHvdW, MMJvG, MEK, JFAJ, CGS and CDAS revised the manuscript. All authors approved the final version of the manuscript. MCGJB is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- DHD
Dutch Healthy Diet
- IHL
Intrahepatic lipid
- NAFLD
Non-alcoholic fatty liver disease
- ROI
Region of interest
- SHBG
Sex hormone-binding globulin
Funding
This study was supported by the EASD/Sanofi. The Maastricht Study was supported by the European Regional Development Fund via OPZuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), the Stichting de Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), the School for Cardiovascular Diseases (CARIM, Maastricht, the Netherlands), the School for Public Health and Primary Care (Care and Public Health Research Institute, Maastricht, the Netherlands), the School for Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), the Stichting Annadal (Maastricht, the Netherlands), the Health Foundation Limburg (Maastricht, the Netherlands), and unrestricted grants from Janssen-Cilag (Tilburg, the Netherlands), Novo Nordisk Farma (Alphen aan den Rijn, the Netherlands), Sanofi-Aventis Netherlands (Gouda, the Netherlands) and Medtronic (Tolochenaz, Switzerland).
Data availability
Data are available upon reasonable request and with permission from the Maastricht Study management team.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75(18):3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus — mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 4.Brouwers MCGJ, Simons N, Stehouwer CDA, Isaacs A. Non-alcoholic fatty liver disease and cardiovascular disease: assessing the evidence for causality. Diabetologia. 2020;63(2):253–260. doi: 10.1007/s00125-019-05024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iroz A, Couty J-P, Postic C. Hepatokines: Unlocking the multi-organ network in metabolic diseases. Diabetologia. 2015;58(8):1699–1703. doi: 10.1007/s00125-015-3634-4. [DOI] [PubMed] [Google Scholar]
- 6.Stefan N, Häring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9(3):144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 7.Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Upala S. Testosterone, sex hormone-binding globulin and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Ann Hepatol. 2017;16(3):382–394. doi: 10.5604/16652681.1235481. [DOI] [PubMed] [Google Scholar]
- 8.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 9.Jänne M, Hammond GL. Hepatocyte nuclear factor-4 controls transcription from a TATA-less human sex hormone-binding globulin gene promoter. J Biol Chem. 1998;273(51):34105–34114. doi: 10.1074/jbc.273.51.34105. [DOI] [PubMed] [Google Scholar]
- 10.Saez-Lopez C, Brianso-Llort L, Torres-Torronteras J, Simó R, Hammond GL, Selva DM. Resveratrol increases hepatic SHBG expression through human constitutive androstane receptor: A new contribution to the french paradox. Sci Rep. 2017;7(1):12284. doi: 10.1038/s41598-017-12509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selva DM, Hammond GL. Peroxisome-proliferator receptor gamma represses hepatic sex hormone-binding globulin expression. Endocrinology. 2009;150(5):2183–2189. doi: 10.1210/en.2008-1289. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/jci23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest. 2007;117(12):3979–3987. doi: 10.1172/JCI32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons PIHG, Valkenburg O, Telgenkamp I, et al. Relationship between de novo lipogenesis and serum sex hormone binding globulin in humans. Clin Endocrinol. 2021;95(1):101–106. doi: 10.1111/cen.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–258. doi: 10.1038/s41591-020-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons PIHG, Valkenburg O, Stehouwer CDA, Brouwers MCGJ. Sex hormone-binding globulin: biomarker and hepatokine? Trends Endocrinol Metab. 2021;32(8):544–553. doi: 10.1016/j.tem.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Schram MT, Sep SJ, van der Kallen CJ, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29(6):439–451. doi: 10.1007/s10654-014-9889-0. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (2006) Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. World Health Organization, Geneva, Switzerland
- 20.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 21.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 22.van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256(1):159–168. doi: 10.1148/radiol.10091790. [DOI] [PubMed] [Google Scholar]
- 23.Vandercappellen EJ, Henry RMA, Savelberg H, et al. Association of the Amount and Pattern of Physical Activity With Arterial Stiffness: The Maastricht Study. J Am Heart Assoc. 2020;9(20):e017502. doi: 10.1161/jaha.120.017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 25.van Lee L, Geelen A, van Huysduynen EJCH, de Vries JHM, van’t Veer P, Feskens EJM. The Dutch Healthy Diet index (DHD-index): an instrument to measure adherence to the Dutch Guidelines for a Healthy Diet. Nutr J. 2012;11(1):49. doi: 10.1186/1475-2891-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maasen K, Eussen SJPM, Scheijen JLJM et al (2021) Higher habitual intake of dietary dicarbonyls is associated with higher corresponding plasma dicarbonyl concentrations and skin autofluorescence: the Maastricht Study. Am J Clin Nutr. 10.1093/ajcn/nqab329 [DOI] [PubMed]
- 27.VanderWeele TJ, Tchetgen Tchetgen EJ. Mediation Analysis With Matched Case-Control Study Designs. Am J Epidemiol. 2016;183(9):869–870. doi: 10.1093/aje/kww038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. 2014;25(5):749–761. doi: 10.1097/ede.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanderWeele TJ. Mediation Analysis: A Practitioner's Guide. Annu Rev Public Health. 2016;37(1):17–32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 31.VanderWeele TJ. Policy-relevant proportions for direct effects. Epidemiology (Cambridge, Mass) 2013;24(1):175–176. doi: 10.1097/EDE.0b013e3182781410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi B, Choirat C, Coull BA, VanderWeele TJ, Valeri L. CMAverse: A Suite of Functions for Reproducible Causal Mediation Analyses. Epidemiology. 2021;32(5):e20–e22. doi: 10.1097/ede.0000000000001378. [DOI] [PubMed] [Google Scholar]
- 33.Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13(9):509–520. doi: 10.1038/nrendo.2017.56. [DOI] [PubMed] [Google Scholar]
- 34.Simons PIHG, Valkenburg O, Telgenkamp I et al (2022) Serum sex hormone-binding globulin levels are reduced and inversely associated with intrahepatic lipid content and saturated fatty acid fraction in adult patients with glycogen storage disease type 1a. J Endocrinol Investig. 10.1007/s40618-022-01753-2 [DOI] [PMC free article] [PubMed]
- 35.Le TN, Nestler JE, Strauss JF, 3rd, Wickham EP., 3rd Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab. 2012;23(1):32–40. doi: 10.1016/j.tem.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saez-Lopez C, Barbosa-Desongles A, Hernandez C, et al. Sex hormone-binding globulin reduction in metabolic disorders may play a role in NAFLD development. Endocrinology. 2017;158(3):545–559. doi: 10.1210/en.2016-1668. [DOI] [PubMed] [Google Scholar]
- 37.Saez-Lopez C, Villena JA, Simó R, Selva DM. Sex hormone-binding globulin overexpression protects against high-fat diet-induced obesity in transgenic male mice. J Nutr Biochem. 2020;85:108480. doi: 10.1016/j.jnutbio.2020.108480. [DOI] [PubMed] [Google Scholar]
- 38.Sofer Y, Nevo N, Vechoropoulos M, et al. Human sex hormone-binding globulin does not provide metabolic protection against diet-induced obesity and dysglycemia in mice. Endocr Connect. 2018;7(1):91–96. doi: 10.1530/ec-17-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targher G, Byrne CD. Nonalcoholic fatty liver disease: A novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab. 2013;98(2):483–495. doi: 10.1210/jc.2012-3093. [DOI] [PubMed] [Google Scholar]
- 40.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45(3):199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M-S, Krawczyk SA, Doridot L, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest. 2019;126(11):4372–4386. doi: 10.1172/JCI81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16(2):93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 43.Sáez-López C, Salcedo-Allende MT, Hernandez C, Simó-Servat O, Simó R, Selva DM. Sex hormone-binding globulin expression correlates with acetyl-coenzyme a carboxylase and triglyceride content in human liver. J Clin Endocrinol Metab. 2019;104(5):1500–1507. doi: 10.1210/jc.2018-00740. [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1(1):15019. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 45.Sanders FWB, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev. 2016;91(2):452–468. doi: 10.1111/brv.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santoro N, Caprio S, Pierpont B, Van Name M, Savoye M, Parks EJ. Hepatic de novo lipogenesis in obese youth is modulated by a common variant in the GCKR gene. J Clin Endocrinol Metab. 2015;100(8):E1125–E1132. doi: 10.1210/jc.2015-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coviello AD, Haring R, Wellons M, et al. A genome-wide association meta-analysis of circulating sex hormone–binding globulin reveals multiple loci implicated in sex steroid hormone regulation. PLoS Genet. 2012;8(7):e1002805. doi: 10.1371/journal.pgen.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen-Cody SO, Potthoff MJ. Hepatokines and metabolism: Deciphering communication from the liver. Mol Metab. 2021;44:101138. doi: 10.1016/j.molmet.2020.101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 582 KB)
Data Availability Statement
Data are available upon reasonable request and with permission from the Maastricht Study management team.