Abstract
Context
The COVID-19 pandemic placed the issue of resource utilization front and center. Our comprehensive cancer center developed a Goals of Care Rapid Response Team (GOC RRT) to optimize resource utilization balanced with goal-concordant patient care.
Objectives
Primary study objective was to evaluate feasibility of the GOC RRT by describing the frequency of consultations that occurred from those requested. Secondary objectives included adherence to consultation processes in terms of core team member participation and preliminary efficacy in limiting care escalation.
Methods
We conducted a retrospective chart review of patients referred to GOC RRT (3/23/2020–9/30/2020). Analysis was descriptive. Categorical variables were compared with Fisher's exact or Chi-Square tests and continuous variables with Mann-Whitney U tests.
Results
A total of 89 patients were referred. Eighty-five percent (76 of 89) underwent a total of 95 consultations. Median (range) patient age was 61 (49, 69) years, 54% (48 of 89) male, 19% (17 of 89) Hispanic, 48% (43/89) White, 73% (65 of 89) married/partnered and 66% (59 of 89) Christian. Hematologic malignancies and solid tumors were evenly balanced (53% [47/89] vs. 47% [42 of 89, P = 0.199]). Most patients (82%, 73 of 89) had metastatic disease or relapsed leukemia. Seven percent (6 of 89) had confirmed COVID-19. Sixty-nine percent (61 of 89) died during the index hospitalization. There was no statistically significant difference in demographic or clinical characteristics among groups (no consultation, 1 consultation, >1 consultation). Core team members were present at 64% (61 of 95) of consultations. Care limitation occurred in 74% (56 of 76) of patients.
Conclusion
GOC RRT consultations were feasible and associated with care limitation. Adherence to core team participation was fair.
Key Words: Goals of care, goal-concordant care, advance care planning, care limitation, family meetings
Key-Message
This descriptive study finds that implementation of a Goals of Care Rapid Response Team for supporting provision of goal concordant care is feasible for critically ill patients at a comprehensive cancer center. Preliminary outcomes suggest that these consultations are associated with goal-concordant care limitation.
Introduction
The COVID-19 pandemic placed the issue of adequacy of patient care resources front and center. It is well established that patients, families and providers often have an optimistic bias regarding prognosis and of the outcome of available treatments.1 Accordingly, patients and families may request care that is unlikely to provide medical benefit or that is inconsistent with their values and goals for care, leading to undue suffering and financial insolvency. Under ideal circumstances, goals of care (GOC) discussions are a continuum of earlier stage discussions of advance care planning (ACP) that take place over the course of time with a trusted provider. Communication of the outcome of these conversations to key stakeholders that include patients, family members, surrogate decision makers and health care professionals is vital for the provision of goal concordant patient care.1
For numerous reasons well described elsewhere, these conversations often do not occur in a timely fashion or at all, leading to care that may not be aligned with patient preferences.2 , 3 When conversations occur, they frequently do not include key stakeholders and may result in an incomplete or inaccurate understanding of the patient's values and goals for care, prognosis and care outcomes.1 Moreover, preferences may change over time.4 , 5 Advance directives, which serve as imperfect proxies for ACP, are present in only a minority of adults.1 , 6 At our institution, despite increasing efforts over the years, quality improvement projects focused on selecting and preparing a medical decision maker have not yielded a significant increase in scanned Medical Power of Attorney documents available in the electronic health record (EHR) from a low baseline frequency.7 , 8
At the onset of the COVID-19 pandemic our institution created a Goals of Care Rapid Response Team (GOC RRT). Developed in response to anticipated increase in critical illness and resource limitations, the goal was to promote rapid and effective alignment between care provided and patient preference. The GOC RRT was for all cancer patients regardless of COVID status, to support best practice in communication for GOC discussions9, 10, 11, 12 in critically ill patients who did not have GOC conversations documented in the electronic health record (EHR). Designed to focus on patients deemed to be at imminent risk for transfer to the intensive care unit (ICU) or at high likelihood for transition to a higher level of care, the GOC RRT was deployed to clarify GOC after receiving approval from the patient's oncologist.
The intent of the GOC RRT consultation was to promote patient and family understanding of the patient's medical situation, prognosis and treatment options by discussion with a trusted oncologist, while ensuring the medical team's understanding of the patient's GOC. The process was underpinned by supportive care expertise in facilitating communication. It was anticipated that the GOC consultation would lead to higher rates of “do not resuscitate status,” “no escalation” in level of care and transition to symptom-oriented supportive care and hospice care.
GOC RRT consultations typically took place within 24 hours of request and often within 3 to 4 hours. For patients, invited members included the patient (if able to participate), designated medical power of attorney (MPOA) or legally authorized medical decision maker per state hierarchy and others, as desired by the patient or medical decision maker. For the medical team, invited members were the patient's primary medical oncologist, responsible inpatient oncologist or hospitalist, critical care provider, supportive (palliative) care GOC provider, social worker, ethicist and chaplain. Designated core team members were Clinical Ethics, Medical Oncology, Supportive Care and Social Work.
The primary study objective was to evaluate feasibility of the GOC RRT by describing the frequency of consultations that occurred from those requested. Secondary objectives were to describe adherence to consultation processes in terms of core team member participation and to explore preliminary efficacy in limiting care escalation.
Methods
This study was conducted as a retrospective chart review of patients referred for GOC RRT consultation from 3/23/2020-9/30/2020. Patients with a poor prognosis at risk for deterioration and escalation of care were identified from daily review of patients on the Medical Emergency Rapid Intervention Team (MERIT) list, those in the ICU and patients receiving high flow oxygen or BiPAP. The MERIT evaluates patients experiencing non-emergent clinical deterioration such as change in vital signs, chest pain, symptoms of sepsis or change in mental status. Patients without an ACP note documented in the EHR were referred for GOC RRT, after approval of the primary medical oncologist.
Care limitation was defined as at least one of the following: 1. Change in location to lower intensity level (i.e., from ICU to regular nursing [medical/surgical] unit or acute palliative care unit), 2. Change in resuscitation status from full code to do not resuscitate and 3. Withdrawal of life sustaining therapy. Analysis was descriptive. Categorical variables were compared with Fisher's exact or Chi-Square tests and continuous variables with Mann-Whitney U tests. P-value significance level was less than 0.05.
Results
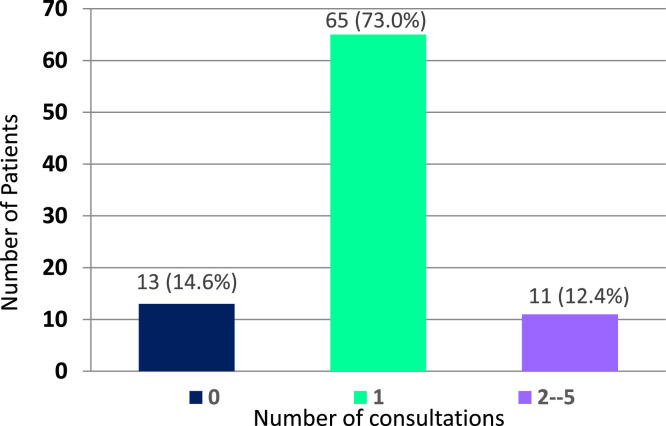
Over the 6-month study period, 89 patients were referred. Eighty-five percent (76 of 89) underwent a total of 95 consultations. Twelve percent (11 of 89) had multiple consultations (range 2 to 5; Fig. 1 ). Reasons referred patients did not undergo GOC RRT consultation are noted in Table 1 .
Fig. 1.
Consultation completion status of patients referred for GOC RRT (N=89).
Table 1.
Reasons Patients did not Undergo GOC RRT Consultation (N=13).
| N | % | |
|---|---|---|
| Primary oncologist felt there had been adequate discussion with patient/family | 5 | 38.5% |
| Family decided to de-escalate care | 5 | 38.5% |
| Other | 3 | 23.1% |
Other: Family declined consultation (n=1), wrong consultation type placed-wanted supportive care assisted GOC consult (n=1), no information 1 (n=1).
For completed consultations, all core team disciplines were present in 68% (52 of 76) for the first consultation and in 64% (61 of 95) for all consultations. Medical Oncology was present in 83% (63 of 76) of first consultations, Supportive Care in 96% (73 of 76), Clinical Ethics in 87% (66 of 76) and Social Work in 96% (73 of 76).
Patients’ demographic and clinical characteristics are noted in Tables 2 and 3 . Median (range) patient age was 61 (49, 69) years, 54% (48 of 89) male, 19% (17 of 89) Hispanic, 48% (43 of 89) White, 73% (65 of 89) married/partnered and 66% (59 of 89) of the Christian faith. Hematologic malignancies and solid tumors were evenly balanced (53% [47 of 89] vs. 47% [42 of 89, P = 0.199]). Most patients (82%, 73 of 89) had metastatic disease or relapsed leukemia. Seven percent (6 of 89) had confirmed COVID-19.
Table 2.
Demographic Characteristics of Patients REFERRED for GOC RRT Consultations (N=89).
| Characteristic | Total Patients Referred for GOC RRT Consultation (N=89) | Patients’ GOC RRT Consultation Status |
|||
|---|---|---|---|---|---|
| Consultation Completed (N=76) | Consultation Not Completed (N=13) | P value | |||
| Age, years Median (Range) | 61 (27, 86) | 60 (27, 84) | 66 (38, 86) | 0.193 | |
| Sex N (%) | Female | 41 (46.1%) | 34 (44.7%) | 7 (53.8%) | 0.543 |
| Male | 48 (53.9%) | 42 (55.3%) | 6 (46.2%) | ||
| Marital status N (%) | Married, Significant Other | 65 (73.0%) | 0.742 | ||
| Divorced, Single, Widowed | 24 (27.0%) | 20 (26.3%) | 4 (30.8%) | ||
| Religion N (%) | Catholic | 19 (21.3%) | 14 (18.4%) | 5 (38.5%) | 0.411 |
| Christian (not Catholic) | 40 (44.9%) | 36 (47.4%) | 4 (30.8%) | ||
| None | 7 (7.9%) | 6 (7.9%) | 1 (7.7%) | ||
| Other | 23 (25.8%) | 20 (26.3%) | 3 (23.1%) | ||
| Ethnicity N (%) | Hispanic or Latino | 17 (19.1%) | 15 (19.7%) | 2 (15.4%) | 1.00 |
| Not Hispanic or Latino | 70 (78.7%) | 59 (77.6%) | 11 (84.6%) | ||
| Patient declined to specify and Unknown | 2 (2.2%) | 2 (2.6%) | 0 (0.0%) | ||
| Race N (%) | Asian | 11 (12.4%) | 8 (10.5%) | 3 (23.1%) | 0.243 |
| Black or African American | 16 (18.0%) | 12 (15.8%) | 4 (30.8%) | ||
| White or Caucasian | 43 (48.3%) | 39 (51.3%) | 4 (30.8%) | ||
| Other | 19 (21.3%) | 17 (22.4%) | 2 (15.4%) | ||
Table 3.
Clinical Characteristics of Patients REFERRED for GOC RRT Consultation (N=89).
| Characteristic | Total Patients Referred for GOC RRT Consultation (N=89) | Patients’ GOC RRT Consultation Status |
|||
|---|---|---|---|---|---|
| Consultation Completed (N=76) | Consultation Not Completed (N=13) | P value | |||
| Tumor diagnosis N (%) | Solid tumor and non-cancer diagnoses | 42 (47.2%) | 38 (50.0%) | 4 (30.8%) | 0.199 |
| Acute leukemia, MDS, lymphoma, myelofibrosis, myeloma, amyloidosis | 47 (52.8%) | 38 (50.0%) | 9 (69.2%) | ||
| Disease status N (%) | Localized, locally advanced and non-cancer diagnoses | 12 (13.5%) | 11 (14.5%) | 1 (7.7%) | 0.132 |
| Metastatic cancer or leukemia in ≥1 relapse | 73 (82.0%) | 63 (82.9%) | 10 (76.9%) | ||
| Without evidence of cancer ≥1 year | 4 (4.5%) | 2 (2.6%) | 2 (15.4%) | ||
| COVID status at referral N (%) | Confirmed diagnosis | 6 (6.7%) | 5 (6.6%) | 1 (7.7%) | 0.573 |
| Not detected | 75 (84.3%) | 65 (85.5%) | 10 (76.9%) | ||
Hospitalization characteristics of the referred population are in Table 4 . Median (range) hospital length of stay (LOS) for referred patients was 9 days (0-243). 69% (61 of 89) died while hospitalized. At discharge, 20% (18 of 89) were hospitalized on the Acute Palliative Care Unit. There were no statistically significant differences in demographic, clinical or hospitalization characteristics between patients referred for consultation by completion status (not completed, 1 or ≥1). Care limitation occurred in 74% (56 of 76) of patients who underwent consultation (Table 5 ). Of these patients, 9% (7 of 76) had a living will and 24% (18 of 76) had a medical power of attorney (MPOA) available in the EHR. All patients who had a living will also had a MPOA.
Table 4.
Hospitalization Characteristics of Patients REFERRED for GOC RRT Consultations (N=89).
| Characteristic | GOC RRT Consultation Completion Status |
|||||
|---|---|---|---|---|---|---|
| Total (N=89) | 0 (N=13) | 1 (N=65) | >1 (N=11) | P valuea | P valueb | |
| 1st MDACC visit to hospital admission (months) | ||||||
| Median (Range) | 9 (0–243) | 11 (0–185) | 9 (0–169) | 3 (0–243) | 0.584 | 0.400 |
| Hospital length of stay (days) | ||||||
| Median (range) | 14 (1–373) | 9 (3–81) | 14 (1–373) | 20 (4–131) | 0.444 | 0.412 |
| Discharge disposition N (%) | ||||||
| Expired | 61 (68.5%) | 10 (76.9%) | 42 (64.6%) | 9 (81.8%) | 0.973 | 0.834 |
| Home without hospice | 15 (16.9%) | 2 (15.4%) | 12 (18.5%) | 1 (9.1%) | ||
| Home with hospice | 8 (9.0%) | 1 (7.7%) | 6 (9.2%) | 1 (9.1%) | ||
| Other | 5 (5.6%) | 0 (0.0%) | 5 (7.7%) | 0 (0.0%) | ||
| Hospital discharge service N (%) | ||||||
| Supportive Care | 18 (20.2%) | 4 (30.8%) | 14 (21.5%) | 0 (0.0%) | 0.161 | 0.200 |
| Other Services | 71 (79.8%) | 9 (69.2%) | 51 (78.5%) | 11 (100.0%) | ||
For comparison of all 3 groups (0 vs. 1 vs. >1).
For comparison of 1 vs. >1 GOC RRT consultation.
Table 5.
GOC RRT Consultation Outcomes.
| Variables | Measures | Total (N=76) | GOC RRT Number of Incidences |
Pa | |
|---|---|---|---|---|---|
| 1 (N=65) | 2-5 (N=11) | ||||
| Outcome of GOC RRT per patient, N (%) | Care limitation | 56 (73.7%) | 49 (75.4%) | 7 (63.6%) | 00.466 |
| All others | 20 (26.3%) | 16 (24.6%) | 4 (36.4%) | ||
| Change of location, N (%) | APSCU, RNF, hospice | 16 (21.1%) | 15 (23.1%) | 1 (9.1%) | 0.440 |
| All others | 60 (78.9%) | 50 (76.9%) | 10 (90.9%) | ||
| Change in resuscitation status from FULL CODE to Do Not Resuscitate (DNR), N (%) | Yes | 50 (65.8%) | 44 (67.7%) | 6 (54.5%) | 0.496 |
| All others | 26 (34.2%) | 21 (32.3%) | 5 (45.5%) | ||
| Did patient have any withdrawal of an LST? N (%) | Yes | 16 (21.1%) | 14 (21.5%) | 2 (18.2%) | 1.00 |
| No | 60 (78.9%) | 51 (78.5%) | 9 (81.8%) | ||
P<.05 is significant.
Discussion
The GOC RRT consultation was a novel intervention designed and established by the palliative care team with a different structure and format from a traditional supportive/palliative care consultation. The GOC RRT included a palliative care specialist, clinical ethicist, primary and/or inpatient oncology attending/hospitalist, intensivist and critical care social worker.13 , 14 The palliative care specialist actively participated in all aspects of the meeting; the ethicist facilitated these meetings. Clinical ethicists, like palliative care specialists, are experienced in facilitating difficult conversations in emotionally charged circumstances. Typically, their focus is on identification and analysis of ethical questions and values clarification in the context of uncertainty, supporting collaborative decision making among the patient, family and medical team. The nature of their intervention is episodic, as relates to the meeting,15, 16, 17 while palliative care provides ongoing clinical care. They can be highly complementary to one another.
The GOC RRT was intended to be distinct from palliative care consultations. Benefits were two-fold. At our and most institutions, supportive/palliative care consultation are discretionary and requires a provider order. Keeping supportive/palliative care consultation distinct from GOC RRT consultations allowed the supportive/palliative care team to avoid the negative associations of mandatory consultations. Simultaneously, the GOC RRT process allowed the supportive care team to introduce themselves and the care offered in a supportive and beneficial light. For many patients, the GOC RRT was their first introduction to supportive/palliative care and often served as the impetus for supportive care consultation, thus enabling the supportive care team to establish an ongoing relationship of care with the patient and family. While clinical ethicists can provide ongoing support with decision making in the short term, they cannot provide the full spectrum of symptom management, emotional and spiritual support that the supportive/palliative care team provides.
The GOC RRT was not intended to replace ongoing efforts to introduce ACP and GOC conversations earlier in the disease trajectory or to replace supportive/palliative care consultations. The goal was to support real time conversations for patients who had not yet established GOC, prioritized by need. These conversations typically do not take place early in the health care continuum or at all.1 , 7 , 8 When they do, often they are not conducted in a manner that is meaningful to the patient's current situation; additionally, patients’ preferences may change.1 , 2 The GOC RRT process was to supplement processes supporting downstream GOC discussion and was targeted at critically ill individuals without documented GOC discussions. The GOC RRT has continued at our institution as we have moved to the endemic phase of COVID-19, as one more tool to provide goal-aligned patient care.
While designed as a one-time intervention, in 15% of patients, it was repeated. GOC RRT consultations frequently served as a source of new supportive care referrals for patient, family and provider support. The sudden onset of the pandemic generated great distress among clinicians who were not well prepared to conduct serious illness conversations with their patients. The GOC RRT was widely available to help them conduct such conversations in a safe manner with the presence of the supportive care and ethics teams, as many had limited experience conducting such conversations. Future research is needed to explore if these clinicians have now adopted these conversations into regular practice.
Our findings show that GOC RRT consultations were feasible: 85% of referrals underwent consultation. Consultations appeared to reach the population of interest. These patients were critically ill, as evidenced by 67% in-hospital mortality, did not have documented GOC conversations in the EHR and were largely hospitalized in the intensive care unit, a focus of high resource utilization and patient/caregiver distress. While most patients did not have COVID-19, they were impacted by the pandemic with fear of infection, visitation restrictions and potential resource limitation.
Lack of differences in demographic, clinical and hospitalization characteristics by consultation completion status may speak to lack of perceived need in consultation non-completers. In the majority, the oncologist felt there had been enough discussion about prognosis and goals of care or the patient and/or family had already made a choice to de-escalate care.
At a high level, adherence to GOC RRT consultation processes was fair. Potential reasons for variable participation by discipline include logistics, competing responsibilities and discomfort with GOC discussions. It was more challenging for patients’ primary oncologists to accommodate last minute changes in clinic schedules, as compared to on-site ICU, social work, supportive care and clinical ethics providers. At times, the ethicist was not able to participate when two GOC RRTs were happening simultaneously, as there was only one dedicated ethicist. For hospitalists and inpatient oncologists, time challenges were paramount, given the labor-intensive nature of the process.
Unsolicited provider feedback suggested that many found the process to be beneficial by providing a unified message from the medical team, reducing medically non-beneficial care and illustrating how difficult it is for clinicians, patients and families to make these kind of care decisions. This feedback is consistent with that reported by palliative care teams who worked predominantly with COVID-positive patients.18 , 19 Future research should evaluate contribution of this model to provider wellbeing, as well as to reasons for provider discomfort/engagement with participation, ideal mode of encounter (in person, virtual or hybrid) and participation for only part of the meeting for some medical team members, as ways to increase participation and optimize resource allocation, especially in smaller institutions.
Consultation outcomes suggest that the process did limit care escalation. The most common measure of goal-concordant care limitation was change in resuscitation status from full code to do not resuscitate in 66%, with change in location and withdrawal of life sustaining treatment less common, each at 21%. Despite the low frequency of COVID-19 positivity in our population, the regional prevalence of COVID-19 positivity at the time likely influenced the salience of these GOC conversations. If confirmed to promote goal-concordant care, this approach may benefit critically ill patients regardless of their COVID-19 status as we enter the endemic phase of this illness.
Study limitations include its retrospective nature, conduct in a single institution and the specialized nature of our setting as a comprehensive cancer center. Our patients may differ in the intensity of preferred treatment from that pursued by patients in more community-based settings. Furthermore, as a tertiary cancer center, we may have resources more easily available for the rapid deployment of this human resource-intense intervention than available in other settings. If prospective studies confirm GOC RRT consult efficacy in promoting goal-concordant care in critically ill patients with cancer, research could be extended to different settings and to different populations.
This preliminary study raises many questions for future study. Can these results be reproduced and how do outcomes compare to those of similar patients who do not undergo the process? If results are reproducible, can the intervention be modified to achieve comparable results with fewer resources? What is the role of facilitated communication incorporating expert communication skills on outcomes? A pre-pandemic ICU based study among critically ill patient with advanced medical illness found less use of invasive ICU procedures and shorter ICU length of stay based on a goal-concordant care model incorporating time-limited trials. This model was based on palliative care-led communication skills training designed to teach clinicians the requisite components and skills needed to lead effective family meetings.20 Our institution is currently deploying communication skills training throughout the organization.
On a more basic level is the age-old question: Can similar results be achieved by conversations in the outpatient setting earlier in the disease trajectory or is the stimulus of a potentially life-threatening event critical for individuals to recognize the salience of these discussions to their own life course? Many authors have deemed ACP in the traditional sense a failure, with a move to preparing patients and surrogate decision makers for “in-the-moment” decision making.21, 22, 23, 24 At a minimum, GOC RRT consultations supplemented pre-existing processes for ACP and GOC discussions. It is yet unknown if they might contribute to new models for establishing GOC. Certainly, knowledge of impact on the emotional wellbeing of the patients, their family members and their survivors is critical, as is the impact on the complicated bereavement for survivors whose family members die. All these issues and more are fodder for future research to evaluate if moving to a “Just- in-Time” model of ACP and GOC planning would lead to improved outcomes with respect to goal concordant care from the perspective of patients, families, providers and/or the health care system.
Disclosures and Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors do not have any competing interests to disclose.
The authors express their gratitude to Brittany Cullen for her expert administrative support of this manuscript.
References
- 1.Institute of Medicine (IOM) National Academies Press; Washington DC: 2015. Dying in America: improving quality and honoring individual preferences near the end of life. [PubMed] [Google Scholar]
- 2.Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV. Medical care inconsistent with patients' treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc. 2002;50:496–500. doi: 10.1046/j.1532-5415.2002.50116.x. [DOI] [PubMed] [Google Scholar]
- 3.Khandelwal N, Curtis JR, Freedman VA, et al. How often is end-of-life care in the U.S. inconsistent with patients' goals of care? J Palliat Med. 2017;20:1400–1404. doi: 10.1089/jpm.2017.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabbarian LJ, Maciejewski RC, Maciejewski PK, et al. The stability of treatment preferences among patients with advanced cancer. J Pain Symptom Manage. 2019;57:1071–1079. doi: 10.1016/j.jpainsymman.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auriemma CL, Nguyen CA, Bronheim R, et al. Stability of end-of-life preferences: a systematic review of the evidence. JAMA Intern Med. 2014;174:1085–1092. doi: 10.1001/jamainternmed.2014.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auriemma CL, O’Donnell H, Klaiman T, et al. How traditional advance directives undermine advance care planning: if you have it in writing, you do not have to worry about it. JAMA Intern Med. 2022;182:682–684. doi: 10.1001/jamainternmed.2022.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhukovsky DS, Soliman PT, Mathew B, et al. Systematic approach to selecting and preparing a medical power of attorney in the gynecologic oncology center. J Oncol Pract. 2019;15:e1092–e1097. doi: 10.1200/JOP.19.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhukovsky DS, Haider A, Williams JL, et al. An integrated approach to selecting a prepared medical decision-maker. J Pain Symptom Manag. 2021;61:1305–1310. doi: 10.1016/j.jpainsymman.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 9.McMahan RD, Knight SJ, Fried TR, Sudore RL. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manag. 2013;46:355–365. doi: 10.1016/j.jpainsymman.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Back A, Tulsky JA, Arnold RM. Communication skills in the age of COVID-19. Ann Intern Med. 2020;172:759–760. doi: 10.7326/M20-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried TR, Redding CA, Robbins ML, et al. Stages of change for the component behaviors of advance care planning. J Am Geriatr Soc. 2010;58:2329–2336. doi: 10.1111/j.1532-5415.2010.03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis JR, Kross EK, Stapleton RD. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel oronavirus 2019 (COVID-19) JAMA. 2020;323:1771–1772. doi: 10.1001/jama.2020.4894. [DOI] [PubMed] [Google Scholar]
- 13.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 14.Yang GM, Neo SH, Lim SZ, Krishna LK. Effectiveness of hospital palliative care teams for cancer inpatients: a systematic review. J Palliat Med. 2016;19:1156–1165. doi: 10.1089/jpm.2016.0052. [DOI] [PubMed] [Google Scholar]
- 15.Carter BS, Wocial LD. Ethics and palliative care: which consultant and when? Am J Hosp Palliat Care. 2012;29:146–150. doi: 10.1177/1049909111410560. [DOI] [PubMed] [Google Scholar]
- 16.Aulisio MP, Arnold RM. Role of the ethics committee: helping to address value conflicts or uncertainties. Chest. 2008;134:417–424. doi: 10.1378/chest.08-0136. [DOI] [PubMed] [Google Scholar]
- 17.American Society for . Bioethics and Humanities. Core competencies for health care ethics consultation. 2nd ed. American Society of Bioethics and Humanities; Chicago, Illinois, United States of America: 2011. [Google Scholar]
- 18.Schockett E, Ishola M, Wahrenbrock T, et al. The impact of integrating palliative medicine into COVID-19 critical care. J Pain Symptom Manag. 2021;62:153–158. doi: 10.1016/j.jpainsymman.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thery L, Vaflard P, Vuagnat P, et al. Advanced cancer and COVID-19 comorbidity: medical oncology-palliative medicine ethics meetings in a comprehensive cancer centre. BMJ Support Palliat Care. 2021;bmjspcare-2021-002946 doi: 10.1136/bmjspcare-2021-002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang DW, Neville TH, Parrish J, et al. Evaluation of time-limited trials among critically ill patients with advanced medical illnesses and reduction of nonbeneficial ICU treatments. JAMA Intern Med. 2021;181:786–794. doi: 10.1001/jamainternmed.2021.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison RS, Meier DE, Arnold RM. What's wrong with advance care planning? JAMA. 2021;326:1575–1576. doi: 10.1001/jama.2021.16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudore RL, Fried TR. Redefining the “Planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256–261. doi: 10.1059/0003-4819-153-4-201008170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison RS. Advance directives/care planning: clear, simple, and wrong. J Palliat Med. 2020;23:878–879. doi: 10.1089/jpm.2020.0272. [DOI] [PubMed] [Google Scholar]
- 24.Periyakoil VS, Gunten CFV, Arnold R, et al. Caught in a loop with advance care planning and advance directives: how to move forward? J Palliat Med. 2022;25:355–360. doi: 10.1089/jpm.2022.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]