Abstract
Objective
To assess whether rituximab (RTX) is associated with worse COVID-19 outcomes among patients with rheumatoid arthritis (RA).
Methods
We used the National COVID Cohort Collaborative (N3C), the largest US cohort of COVID-19 cases and controls, to identify patients with RA (International Classification of Diseases (ICD)-10 code, M05.X or M06.X). Key outcomes were COVID-19-related hospitalization, intensive care unit (ICU) admission, 30-day mortality, and World Health Organization (WHO) classification for COVID-19 severity. We used multivariable logistic regression models to assess the association between RTX use and the odds of COVID-19 outcomes compared with the use of conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs), adjusting for demographics, medical comorbidities, smoking status, body mass index, US region and COVID-19 treatments.
Results
A total of 69,549 patients met our eligibility criteria of which 22,956 received a COVID-19 positive diagnosis between 1/1/2020 and 9/16/2021. Median (IQR) age of the cohort was 63 (52–72) years, 76% of the cohort was female, 68% was non-Hispanic/Latinx White, and 73% was non-smokers. Prior to their first COVID-19 diagnosis, 364 patients were exposed to RTX. Compared to the use of csDMARDs, RTX use was associated with an increased odds of COVID-19-related hospitalization (adjusted odds ratio [aOR] 2.1, 95% confidence interval 1.5–3.0), ICU admission (aOR 5.2, 1.8–15.4) and invasive ventilation (aOR 2.7, 1.4–5.5). Results were confirmed in multiple sensitivity analyses.
Conclusion
Our findings can guide patients, providers, and policymakers regarding the increased risks associated with RTX use during the COVID-19 pandemic. These results can help risk stratification and prognosis-assessment.
Keywords: COVID-19, Rituximab, Rheumatoid arthritis, COVID-19 outcomes
Abbreviations
- N3C
National COVID Cohort Collaborative
- ICD-10-CM
International Classification of Diseases, Tenth Revision, Clinical Modification
- RA
Rheumatoid arthritis
- RTX
rituximab
- csDMARD
conventional synthetic non-biologic disease-modifying anti-rheumatic drug
- tsDMARD
targeted synthetic disease-modifying anti-rheumatic drug
- bDMARD
Biologic disease-modifying anti-rheumatic drug
Introduction
The COVID-19 pandemic has significantly impacted the lives of people worldwide. As the pandemic has progressed, a higher risk of incident COVID-19 infection or worse outcomes in certain immunocompromised subpopulations, such as those with rheumatoid arthritis (RA) [1,2] has been described. This is potentially related not only to their underlying disease but also due to the use of immunosuppressive medications used to treat RA [3,4]. Specifically, some studies have raised concerns regarding rituximab (RTX) exposure being associated with worse COVID-19 outcome [3], [4], [5], [6]. For example, Sparks et al., using the data from the global rheumatology alliance (GRA) that contained physician-reported survey data, found that people with RA who were treated with RTX or JAK inhibitor had worse COVID-19 severity (defined as hospitalization/death) than those on tumor necrosis factor inhibitors (TNFi) [5]. These studies have several limitations that limit the interpretation of their results. For example, most studies lacked a control group [5], had a small sample size of patients treated with RTX [4], or included patients with heterogeneous rheumatic diseases [6], which can have differential disease-associated risk of COVID-19 infection and related outcomes. Additionally, many important outcomes such as ICU-admission or 30-day mortality were not examined in earlier studies, likely due to the small sample sizes of RTX-treated RA patients.
Thus, an evidence gap exists in elucidating the potentially harmful role that RA treatment with RTX plays in COVID-19 infection risk and outcomes. Besides the innate immune system and T cells, B lymphocytes play a major role in the early stages of innate immune response to viral infections, including viral antigen processing, and building an immunologic memory [7]. Given that RTX inhibits this by eliminating B-cells for several months [8,9], RA patients exposed to RTX may have a heightened risk for worse COVID-19 outcomes. Therefore, the objective of our study was to evaluate the associations between baseline use of RTX in patients with RA and COVID-19 outcomes using a nationally sampled cohort of patients.
Methods
Study database and sample selection
The National COVID Cohort Collaborative (N3C), a centralized, harmonized, high-granularity electronic medical record (EMR) repository, is the largest nationally sampled US cohort of COVID-19 cases and controls to date [10]. N3C is comprised of members from the NIH Clinical and Translational Science Awards (CTSA) Program and its Center for Data to Health (CD2H), the IDeA Centers for Translational Research, the National Patient-Centered Clinical Research Network (PCORNet), the Observational Health Data Sciences and Informatics (OHDSI), TriNetX, and the Accrual to Clinical Trials (ACT) networks.
N3C includes de-identified data from outpatient, emergency room, and inpatient encounters and includes the health records of two COVID-19 negative controls for every COVID-19 positive patient, matched on demographic factors, from each participating data partner site [4]. COVID-19 positive patients are defined as individuals with any encounter on or after 1/1/2020 with 1) one of a set of a priori-defined SARS-CoV-2 laboratory tests, >95% of which are PCR-positive, 2) a “strong positive” diagnostic code, or 3) two “weak positive” diagnostic codes during the same encounter or date. The cohort definition is publicly available on GitHub [11]. For patients included in N3C, encounters in the same data partner site beginning on or after 1/1/2018 are also included to provide information about pre-existing health conditions (i.e., “lookback data”). For these analyses, the data from January 1, 2018, to those records available in the de-identified v45 database release (Release-v45–9–16–21) were used. De-identified data in N3C includes randomly shifted dates per patient of +/- 180 days and provides 3-digit zip codes.
Definition of the RA cohort and DMARD exposures
We defined patients with RA has having one or more International Classification of Disease, tenth revision, common modification (ICD-10-CM) codes for RA (Appendix 1), prior to their COVID-19 infection; alternate stricter definitions were tested in sensitivity analyses (see analysis section for details on each sensitivity analysis).
We categorized DMARDs as follows: [1] conventional DMARDs (csDMARDs) such as methotrexate, hydroxychloroquine, sulfasalazine, or leflunomide; [2] biologic DMARDs (bDMARDs) such as tumor necrosis factor inhibitors (TNFi-biologics: adalimumab, etanercept, infliximab, golimumab, or certolizumab), IL-6 inhibitors (tocilizumab or sarilumab), cytotoxic T lymphocyte-associated antigen immunoglobulin (CTLA4-Ig) abatacept; [3] targeted synthetic DMARDs (tsDMARDs) that include Janus Kinase inhibitors (JAKi), tofacitinib, baricitinib and upadacitinib; or [4] multiple DMARDs (more than one DMARD). We treated baseline use of RTX prior to the COVID-19 infection as the main exposure of interest. We separated RTX from the bDMARD category due to RTX's unique effects on B-cell function [8,9]. DMARD exposure is defined as having a DMARD-associated medication exposure date prior to the date of COVID-19 diagnosis. We calculated the time since the last documented RTX infusion before COVID-19 diagnosis and categorized it as: less than 30 days; 31–180 days; or greater than 180 days.
COVID-19 outcomes
Our study's primary COVID-19 outcomes included the following among COVID-19 positive patients: [1] COVID-19-related hospitalization; [2] intensive care unit (ICU) admission; [3] 30-day mortality; and [4] COVID-19 severe/fatal World Health Organization (WHO) classification level 7 or above [13]. Five levels of disease severity were defined, as in previous N3C analyses [14]: asymptomatic or mild disease with outpatient care only (WHO severity 1–2), mild disease requiring only an emergency department (ED) visit (WHO severity 3), moderate disease with COVID-19-related hospitalization but without invasive ventilation (WHO severity 4–6), severe disease with COVID-19-related hospitalization requiring invasive ventilation or extracorporeal membrane oxygenation (ECMO) treatment (WHO severity 7–9), and death (WHO severity 10).
Our secondary outcomes included the following for the first or index COVID-19-related hospitalization: [1] invasive ventilation; [2] acute kidney injury (AKI) in-hospital; [3] ICU mortality; [4] in-hospital mortality; [5] ICU length of stay; and [6] hospital length of stay.
Covariates
We extracted demographics (age, sex, and race/ethnicity), body mass index (BMI, classified as underweight, normal, overweight, and obese), smoking history (never vs. ever vs. unknown), and US region (West, Midwest, Northeast, South or unknown) from N3C. We identified comorbidities using the Deyo-Charlson index score (prior to the COVID-19 diagnosis) [12], a validated comorbidity measure, as detailed in Appendix 1. We utilized a modified Deyo-Charlson index, and excluded the rheumatic disease category to avoid redundancy, since all patients for these analyses had RA.
To account for the treatments received for COVID-19 infection, as they may have differentially affected COVID-19 outcomes, we created nine groups of COVID-19 treatments: group 1 (intravenous pressor support medications such as dopamine, dobutamine, epinephrine, epoprostenol); group 2 (intravenous use of hydrocortisone or dexamethasone); group 3 (chloroquine); group 4 (inhaled nitric oxide); group 5 (azithromycin); group 6 (oral steroids- methylprednisolone, prednisolone or prednisone); group 7 (antivirals such as remdesivir, ritonavir, lopinavir); group 8 (anakinra); and group 9 (intravenous immunoglobulin).
Statistical analysis
We provide the descriptive statistics for the cohort using median (interquartile range [IQR]) or categories (%) for continuous and categorical variables, respectively, stratified by COVID-19 status. We performed multivariable logistic regression models with Firth's penalized likelihood [13], that adjusted for demographics, BMI, smoking status, US region, and our modified Deyo-Charlson index as a binary variable (yes/no), to evaluate the association between baseline RTX use and our primary and secondary outcomes compared to baseline csDMARD use. Except for hospitalization, which usually occurs before administration of most of the listed COVID-19 treatments, we additionally adjusted the main models for all COVID-19 treatments. To assess the last-RTX-dose associated risk, we also evaluated the unadjusted associations between time since last RTX infusion and the odds of our primary outcomes.
We conducted the following sensitivity analyses to assess the robustness of our findings: [1] adjustment for modified Deyo-Charlson comorbidity score treating it as categorical variable (0, 1, 2 versus 3 or more); [2] adjustment for Deyo-Charlson comorbidity score with each comorbidity separately [3] limited the cohort to those with two or more ICD-10 codes for RA prior to the COVID-19 infection; [4] limited the cohort to those with one ICD-10 code for RA plus a DMARD, defined as a csDMARD, bDMARD (except RTX), RTX, or tsDMARD; [5] analysis where hydroxychloroquine was the reference category; [6] analysis where no DMARD was the reference category; and [7] analysis where TNFi-biologic was the reference category. Patients with missing data were excluded from analyses. All analyses were conducted in the N3C Enclave using R [14].
Results
Baseline patient characteristics
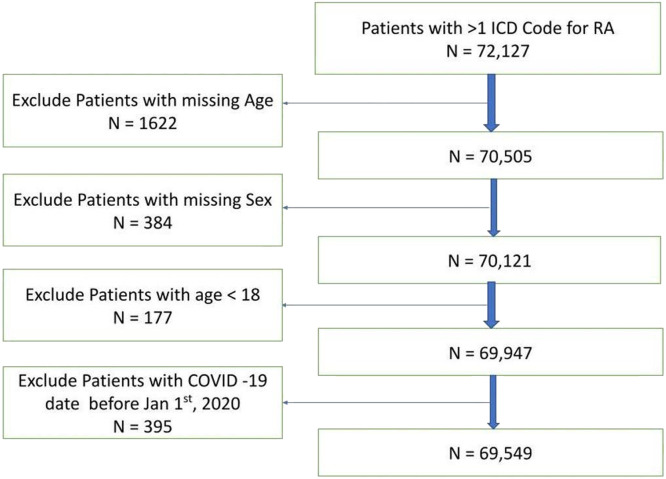
A total of 69,549 RA patients met our eligibility criteria (Fig. 1 ), of whom 22,956 (33%) were COVID-19 positive during the study period. The median age was 63 years (IQR, 52–72), 76% were females, 68% non-Hispanic/Latinx White and 73% non-smokers in our cohort (Table 1 ). Pulmonary disease was seen in 39% and hypertension in 60% of the patients. Overall, we observed 4219 (18.38%) hospitalizations, and 1079 (4.70%) deaths among those diagnosed with COVID-19.
Fig. 1.
Flow diagram showing the cohort selection
Legend: ICD International Classification of diseases; RA: Rheumatoid Arthritis.
Table 1.
Baseline characteristics of RA patients by COVID-19 status from US N3C cohort.
| Characteristic | COVID-19 Negative (n = 46,593) | COVID-19 Positive (n = 22,956) |
|---|---|---|
| Age (median [IQR]) | 64.00 [53.0, 73.0] | 61.00 [51.0, 71.0] |
| BMI (median [IQR]) | 28.94 [24.60, 34.39] | 29.90 [25.45, 35.44] |
| Weight (median [IQR]) | 79.47 [65.77, 96.16] | 81.69 [67.99, 98.90] |
| Deyo Charlson Index (median [IQR]) | 3.00 [2.00, 6.000] | 2.00 [1.00, 4.00] |
| Modified Deyo-Charlson (median [IQR]) | 2.00 [1.00 5.00] | 1.00 [0.00 3.00] |
| Males (%) | 11,369 (24.40) | 5464 (23.80) |
| Race/ethnicity (%) | ||
| Non-Hispanic/Latinx White | 32,402 (69.54) | 14,717 (64.11) |
| Non-Hispanic/Latinx Black | 7122 (15.29) | 3150 (13.72) |
| Hispanic/Latinx White | 1543 (3.31) | 915 (3.99) |
| Hispanic/Latinx Black | 115 (0.25) | 61 (0.27) |
| Asian | 837 (1.80) | 351 (1.53) |
| Pacific Islander | 43 (0.09) | 28 (0.12) |
| Other | 271 (0.58) | 0 (0.00) |
| Unknown | 4260 (9.14) | 3734 (16.27) |
| US Region (%) | ||
| Midwest | 13,609 (29.21) | 9375 (40.84) |
| Northeast | 7703 (16.53) | 3473 (15.13) |
| South | 10,741 (23.05) | 4088 (17.81) |
| West | 5124 (11.00) | 2053 (8.94) |
| Unknown | 9416 (20.21) | 3967 (17.28) |
| Smoking status (%) | ||
| Current or Former | 13,553 (29.09) | 4931 (21.48) |
| Non-smoker | 32,990 (70.80) | 17,709 (77.14) |
| Unknown | 50 (0.11) | 316 (1.38) |
| Payer (%) | ||
| Medicaid | 1109 (2.38) | 417 (1.82) |
| Medicare | 6661 (14.30) | 1960 (8.54) |
| None | 1458 (3.13) | 704 (3.07) |
| Other | 213 (0.46) | 78 (0.34) |
| Private | 3674 (7.89) | 1488 (6.48) |
| Unknown | 33,462 (71.82) | 18,300 (79.72) |
| Deyo Charlson Index Clinical comorbidities (%) | ||
| Myocardial infarction | 5093 (10.93) | 1482 (6.46) |
| Congestive heart failure | 9142 (19.62) | 3122 (13.60) |
| Peripheral vascular disease | 10,491 (22.52) | 3314 (14.44) |
| Stroke | 8163 (17.52) | 2542 (11.07) |
| Dementia | 1809 (3.88) | 697 (3.04) |
| Pulmonary | 19,623 (42.12) | 7305 (31.82) |
| Peptic ulcer disease | 2840 (6.10) | 821 (3.58) |
| Mild liver disease | 6982 (14.99) | 2407 (10.49) |
| Severe liver disease | 1182 (2.54) | 321 (1.40) |
| Diabetes | 15,040 (32.28) | 6555 (28.55) |
| Diabetes complications | 7311 (15.69) | 2994 (13.04) |
| Paralysis | 1118 (2.40) | 348 (1.52) |
| Renal disease | 8947 (19.20) | 3498 (15.24) |
| Cancer | 8214 (17.63) | 2677 (11.66) |
| Metastases | 1812 (3.89) | 394 (1.72) |
| HIV | 235 (0.50) | 92 (0.40) |
| Deyo Charlson Index Score | ||
| 0 | 10,531 (22.60) | 8111 (35.33) |
| 1 | 8922 (19.15) | 4589 (19.99) |
| 2 | 6538 (14.03) | 2849 (12.41) |
| 3 or more | 20,602 (44.22) | 7407 (32.27) |
| Non-Deyo Charlson comorbidities | ||
| Obesity | 14,546 (31.22) | 6446 (28.08) |
| Hypertension | 29,767 (63.89) | 12,294 (53.55) |
| CAD | 10,621 (22.80) | 3636 (15.84) |
| DMARDs for RA | ||
| Rituximab$ | 1357 (2.91) | 517$ (2.25) |
| csDMARD | 21,928 (47.06) | 10,453 (45.53) |
| TNFi-biologic | 7843 (16.83) | 3351 (14.60) |
| IL-6i | 1302 (2.79) | 615 (2.68) |
| Abatacept | 1831 (3.93) | 671 (2.92) |
| JAKi | 2718 (5.83) | 1265 (5.51) |
| Glucocorticoids | 30,097 (64.60) | 13,835 (60.27) |
| COVID-19 treatment groups | ||
| Hydrocortisone or Dexamethasone | <20* | 587 (2.56) |
| Chloroquine | 0 (0.00) | 463 (2.02) |
| Azithromycin | <20* | 586 (2.55) |
| Glucocorticoids1 | <20* | 1000 (4.36) |
| Antivirals# | <20* | 648 (2.82) |
| Anakinra | 0 (0.00) | 43 (0.19) |
| Intravenous Immunoglobulin | 74 (0.16) | 20 (0.09) |
| Selected COVID-19 outcomes amongst those COVID-19 positive | ||
| Hospitalization | – | 4219 (18.38) |
| Deaths | – | 1079 (4.70) |
| ICU admission | – | 193 (0.84) |
Abbreviations: BMI: body mass index; CAD: coronary artery disease; csDMARD: conventional synthetic disease modifying anti-rheumatic drug; H: Hispanic; HIV: human immunodeficiency virus; ICU: Intensive care unit; IL-6i: Interleukin 6 inhibitor; IQR: Interquartile range; JAKi: Janus Kinase inhibitor; NH: Non-Hispanic; RA: rheumatoid arthritis; US: United States.
Glucocorticoids: methylprednisolone, prednisolone, or prednisone.
Antivirals include remdesivir, lopinavir or lopinavir-ritonavir combination;.
Of these, 49 received RTX after COVID-19 infection and 104 received both before and after the COVID-19 infection.
Counts less than 20 can only be presented as <20 per N3C data and privacy requirements.
In the overall cohort, 43,932 (63.17%) of the patients were using oral glucocorticoids at baseline, 32,381 (46.56%) were on a csDMARD, 11,194 (16.10%) on a TNFi-biologic, 3983 (5.73%) on a JAKi and 1874 (2.69%) were on RTX. 364 (1.60%) patients were exposed to RTX prior to their first positive COVID-19 test, of whom 93 (26%) were hospitalized for COVID-19, 24 (6.6%) required invasive ventilation, and 29 (8%) died (Table 2 ).
Table 2.
Occurrence of COVID-19 outcomes among COVID-19 positive RA patients by rituximab exposure prior to the COVID infection from U.S. N3C cohort.
| Outcome | Non-exposed to RTX | Exposed to RTX |
|---|---|---|
| N = 22,439 | N = 364 | |
| Primary Outcomes | ||
| Hospitalization | 4085 (18.20) | 93 (25.55) |
| ICU admission | 182 (0.81) | <20⁎⁎ |
| 30-day mortality | 692 (3.08) | <20⁎⁎ |
| Severe/fatal COVID-19 (WHO severity 7–10)⁎⁎⁎ | 1046 (4.66) | 29 (7.97) |
| Invasive Ventilation | 582 (2.59) | 24 (6.59) |
| AKI in hospital | 1104 (4.92) | 28 (7.69) |
| ICU mortality | 72 (0.32) | <20⁎⁎ |
| In-hospital mortality | 652 (2.91) | <20⁎⁎ |
| ICU LOS (mean (SD)) | 15.41 (13.21) | 17.00 (10.24) |
| Hospital LOS (mean (SD)) | 10.26 (12.26) | 12.48 (13.73) |
Abbreviations: AKI: Acute kidney injury; ICU: Intensive care unit; LOS: Length of stay; RTX: Rituximab.
:<20: Counts less than 20 can only be presented as <20 per N3C data and privacy requirements.
COVID-19 severity definition used:
Mild: Outpatient World Health Organization (WHO) Severity 1–3
Mild-ED: Outpatient with Emergency department visit WHO Severity ∼3
Moderate: Hospitalized without invasive ventilation WHO Severity 4–6
Severe: Hospitalized with invasive ventilation or ECMO WHO Severity 7–9
Hospital Mortality or Discharge to Hospice WHO Severity 10.
Multivariable-adjusted association between baseline use of RTX and COVID-19 outcomes
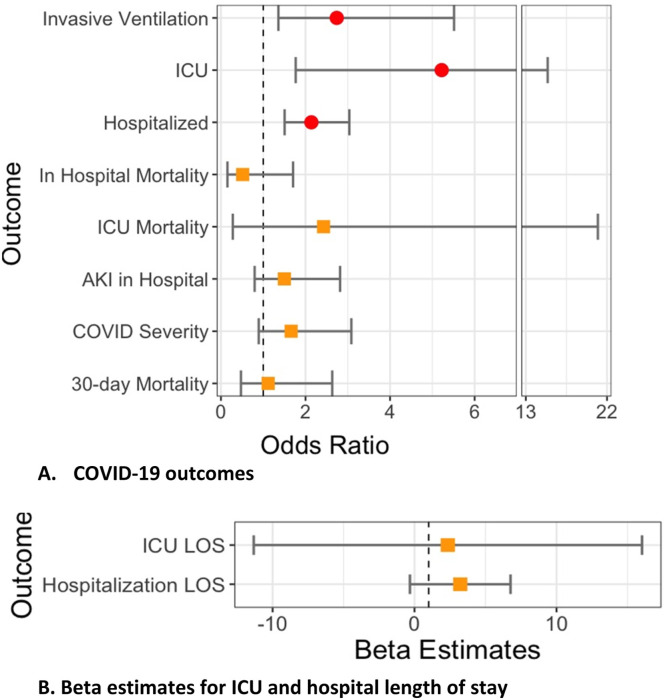
In multivariable-adjusted models, compared to the baseline use of csDMARDs, RTX use was associated with an increased odds of COVID-19-related hospitalization (adjusted odds ratio [aOR] 2.14, 95% CI 1.51–3.04); and, in those hospitalized, with increased odds of ICU admission (aOR 5.22, 95% CI 1.77–15.41) and invasive ventilation (aOR 2.74, 95% CI 1.36–5.51) (Table 3 and Fig. 2 ).
Table 3.
Association of baseline rituximab use and each COVID-19 outcome with csDMARD as the referent category from US N3C cohort.
| Primary Outcomes | Multivariable adjusted OR (95%CI)# |
|---|---|
| Hospitalization@ | 2.14 (1.51–3.04) |
| ICU admission | 5.22 (1.77–15.41) |
| 30-day mortality | 1.12 (0.47–2.63) |
| Severe/fatal COVID-19 (WHO severity 7–10)* | 1.66 (0.89–3.08) |
| Secondary Outcomes | |
| Invasive ventilation | 2.74 (1.36–5.51) |
| AKI in hospital | 1.50 (0.80–2.82) |
| ICU mortality | 2.43 (0.28–20.99) |
| In-hospital mortality | 0.80 (0.42–1.80) |
| Multivariable adjusted Beta Estimate (95% CI) | |
| ICU LOS | 2.35 (−11.33- 16.02) |
| Hospital LOS | 3.22 (−0.32 −6.77) |
Abbreviations: AKI: Acute kidney injury; CI: Confidence interval; csDMARD: conventional synthetic disease modifying anti-rheumatic drugs; ICU: Intensive care unit; LOS: Length of stay; OR: Odds Ratio.
COVID-19 severity definition used:
Mild: Outpatient World Health Organization (WHO) Severity 1–3
Mild-ED: Outpatient with Emergency department visit WHO Severity ∼3
Moderate: Hospitalized without invasive ventilation WHO Severity 4–6
Severe: Hospitalized with invasive ventilation or ECMO WHO Severity 7–9
Hospital Mortality or Discharge to Hospice WHO Severity 10.
Findings with p-values <0.05 are bolded in the table.
Hospitalization: adjusted for demographics, weight categories per BMI as normal vs. underweight, overweight, and obese, smoking status, US region, and modified Deyo-Charlson index.
Fig. 2.
Multivariable-adjusted association of baseline rituximab use and each COVID-19 outcome with csDMARD as the referent category from US N3C cohort.
Legend: Figure shows A) odds ratios and 95% confidence interval for association between rituximab use and COVID-19-outcomes; B) Beta estimates (95% CI) for association between rituximab and ICU and hospital length of stay.
AKI, acute kidney injury; CI: Confidence interval; ICU, intensive care unit; LOS: Length of stay.
Hospitalized*: adjusted for demographics, weight categories per BMI as normal vs. underweight, overweight, and obese, smoking status, US region, and modified Deyo-Charlson index.
All other outcomes: adjusted for above variables and all COVID-19 treatments
Circles (red) denote significant outcomes, orange squares denote non-significant outcomes.
Other variables associated with COVID-19-related hospitalization included being from the Midwest (aOR 1.28, 95% CI 1.09–1.49) or South (aOR 1.65, 95% CI 1.42–1.93) compared to the West, or being Black (aOR 1.89, 95% CI 1.72–2.08) or Hispanic/Latinx (aOR 1.25, 95% CI 1.04–1.50) compared to non-Hispanic/Latinx White.
Association between time since RTX and COVID-19 outcomes
Compared to less than 30 days since last RTX infusion, we observed lower 30-day mortality in those who had their infusions >180 days (unadjusted OR 0.25, 95% CI 0.06–0.98), but not for 31–180 days (Appendix 4). There were no statistically significant associations observed for COVID-19-related hospitalization or COVID-19 severity (Appendix 4).
Sensitivity analyses
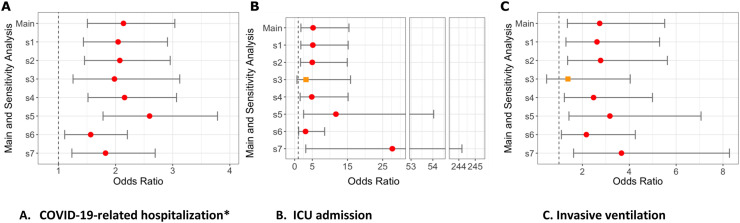
The results were similar in sensitivity analyses (Fig. 3 ). Compared to csDMARDs, RTX use was associated with increased odds of COVID-19-related hospitalization in models with: [1] Deyo-Charlson categorical (aOR 2.04, 95% CI 1.44–2.91); [2] Deyo-Charlson individual comorbidities (aOR 2.07, 95% CI 1.46–2.96); [3] 2 or more RA codes (aOR 1.98, 95% CI 1.26–3.12); [4] RA code plus DMARD (aOR 2.16, 95% CI 1.52–3.07) models. Compared to different referent category, results showed that RTX use was still associated with an increased odds of COVID-19-related hospitalization in comparison to: [5] HCQ (aOR 2.60, 95% CI 1.78–3.78); [6] no DMARD (aOR 1.56, 95% CI 1.11–2.21); and [7] TNFi-biologic (aOR 1.82, 95% CI 1.24–2.69). Similar results were seen for ICU admission and invasive ventilation (Fig. 3 and Appendices 5–11).
Fig. 3.
Sensitivity analyses (S1-S7) for odds of hospitalization, ICU admission or invasive ventilation with rituximab from US N3C cohort.
legend Figure shows odds ratios and 95% confidence interval for association between rituximab use and A) COVID-19-related Hospitalization; B) ICU admission, C) Invasive ventilation.
New S1, sensitivity analyses 1: Deyo-Charlson overall score (with RA excluded) as 0, 1, 2 vs. 3 or more.
New S2, sensitivity analyses 2: Each Deyo-Charlson comorbidity (with RA excluded).
New S3, sensitivity analyses 3: RA Case definition requires at least 2 ICD codes.
New S4, sensitivity analyses 4: RA Case definition requires >= 1 ICD code + any RA medication.
New S5, sensitivity analyses 5: Referent is Hydroxycholoroquine, nbDMARD variable except HCQ, i.e., Methotrexate, Leflunomide, or Sulfasalazine.
New S6, sensitivity analyses 6: Referent is No DMARD category.
New S7, sensitivity analyses 7: Referent is TNFi-biologic category.
Hospitalized*: adjusted for demographics, weight categories per BMI as normal vs. underweight, overweight, and obese, smoking status, US region, and modified Deyo-Charlson index.
All other outcomes: adjusted for above variables and all COVID-19 treatments.
Circles (red) denote significant outcomes, orange squares denote non-significant outcomes.
Discussion
In the largest U.S. retrospective cohort study to date among RA patients, as compared to csDMARDs, the baseline use of RTX is associated with increased odds of COVID-19 hospitalization, ICU admission, and invasive ventilation. More recent RTX use is associated with a higher risk of 30-day mortality compared to >180 days since the last infusion. These findings can guide patients, providers, and policymakers regarding the increased risks associated with RTX use during the COVID-19 pandemic.
Our findings of increased COVID-19-related hospitalization risk associated with RTX use are similar to those reported in patients on RTX for either rheumatic diseases or multiple sclerosis [4,[15], [16], [17]]. In contrast, our data on the association between RTX use and ICU admission and invasive ventilation are novel. A recent paper from our colleagues using the N3C data showed that RTX was associated with a higher risk of in-hospital death in people with rheumatologic conditions (HR 1.72, 95% CI 1.10–2.69) and as a cancer therapy (HR 2.57, 95% CI 1.86–3.56); this study examined outcomes regardless of underlying disease, but outcome assessment was limited to invasive mechanical ventilation and in-hospital death [3]. In a smaller study of patients with multiple sclerosis, RTX use was associated with a higher risk of serious illness and death [16]. Previous studies reported higher risk of incident and severe COVID-19 disease or longer hospital stay amongst those receiving RTX vs. not, but had several limitations in interpretation, since they: [1] combined heterogeneous RMD populations [15]; [2] were limited to single- or few-sites [4,16]; and/or [3] used patient or physician-reported data as primary data sources.
Our cohort included >95% PCR-confirmed cases of COVID-19, used robust definitions of COVID-19 outcomes, and adjusted for several key clinical outcomes (potential confounders) including in-hospital use of medications for COVID-19 such as antivirals and glucocorticoids – advances over previous studies. This reduces bias (ascertainment and confounding bias), potentially making these findings more accurate than the previous studies. To our knowledge, ours is among the first, multisite studies in the U.S. focused on RA, a single rheumatic disease population. We also present characteristics of a comparator group of patients with RA who tested negative for COVID-19, which provides an estimate of baseline risk in people with RA. The frequency of RTX use in our N3C cohort is similar to that reported in the US CORRONA registry [17], supporting the representativeness of our RA sample. Thus, our work has many strengths.
We acknowledge that some data related to RTX use and COVID-19 outcomes have reached contradictory or different conclusions than ours: [1] COVID-19-related hospitalization and more severe COVID-19 (that included mechanical ventilation) risk was increased in one study [5], but not in another study from the same author group [4]; [2] death risk was not increased in the French national study [15], but increased in a global [5] and single-center studies [4]. These differences are likely due to differences in comparators (no RTX vs. TNFi-biologic), disease of interest (multiple rheumatic diseases, multiple sclerosis vs. immune-mediated diseases), reference non-disease population (general population vs. other vs. none), study setting (France vs. global vs. single site), and time of the study in reference to the COVID-19 pandemic. Our study includes the largest COVID-19 US dataset, including the most recent period with Delta variant predominance, providing the much-needed additional clarity to this rapidly evolving field.
Our findings of a 3–5-fold higher odds of ICU admission and of invasive ventilation with RTX are noteworthy. This finding can be used by providers, patients, and policymakers, who can accurately assess the risk, prognosis, and allocate resources appropriately. The separation of these important outcomes is critical in understanding the RTX-associated risk. Each primary COVID-19 outcome we examined is associated with different prognosis, healthcare resources utilization, and patient outcome. Future studies are needed that can estimate this risk on an ongoing basis and keep providers, patients and policymakers informed. The French national study, like ours, did not find an association with an increased risk of death in the RTX -exposed vs. not-exposed group [15]. In contrast, in a single-site study, patients with immune-mediated disease treated with CD20 inhibitors (n = 114) had a higher risk of death (11% vs. 4%); adjusted HR 2.16; 95% CI: 1.03 to 4.54) than matched general population comparators. Previous studies did not adequately adjust for COVID-19 treatments, especially those used during COVID-19 hospitalization, as we did in our analyses.
Safety of RTX use, whether in rheumatology or other specialties, has been a historical concern for risk of immunosuppression, including for viral infections other than COVID-19, such as reactivation of hepatitis B [18,19], polyomavirus JC, and cytomegalovirus [20]. Such immune suppression with RTX may have implications for vaccine effectiveness too; some data suggests RTX use is also associated with a lower response to influenza vaccines [21,22]. For COVID-19, there are case reports of persistent COVID infection or delayed serological response to COVID vaccination among patients on RTX therapy [23,24]. These data raise the concern that RTX may directly influence adaptive immune responses, which are important for the control and clearance of viral infections [25]. Furthermore, RTX may have potentially long-lasting effects given that RTX eliminates B-cells for several months, and B-cells play a key role in the early stages of innate immune response to viral infections and building an immunologic memory [7].
Based on all data on RTX to date, including the current study, we suggest the following caution with its use in RA during the COVID-19 pandemic. For people currently on RTX who have not failed other biologics, an alternate medication may be a better option that may spare them the negative consequences associated with RTX use. For those with multiple biologic-failures, providers likely need to take a shared decision-making approach with the patient regarding risks and benefits of RTX continuation or re-dosing, using all current data. Due to an immunocompromised state, all RA patients, but especially those exposed to RTX, should be advised to get COVID-19 vaccinations and boosters as quickly as possible. In addition, RA patients, and, again, especially those exposed to RTX, should continue all precautionary measures recommended by CDC. Until more solutions emerge, these steps are needed to better protect patients with RA who continue RTX during the pandemic.
Despite the strengths, we note several limitations in our work. First, we identified patients using ICD-10-CM codes and thus, our patient groupings are subject to misclassification, with more true RA patients being assigned to the non-RA grouping (which likely biases our findings towards the null). However, in sensitivity analyses using two ICD-10-CM codes to identify RA, our findings were similar to the primary analyses. Similarly, there is a chance that we have under ascertained medication exposure in this cohort, especially if the patient was admitted at a facility where they do not normally receive healthcare; this would likely bias our results towards the null as well, making our current estimates conservative. We did not have information on RA disease activity, which may be associated with worse COVID outcomes or RTX exposure or both [6]; however, the magnitude of risk associated with these COVID-19 outcomes is likely small. We could not conduct adjusted analyses on the association between time since RTX and COVID outcomes due to the small number of outcomes when stratified by different durations since RTX infusion. Similarly, the sample size for patients exposed to rituximab who died was small. So, our analyses were limited in statistical power regarding outcomes such as mortality. In this study, we were unable to account for the use of anti-SARS-CoV-1 monoclonal antibodies, either as pre- or post-exposure prophylaxis as they were not yet approved. At the time of our study design, neither tocilizumab nor baricitinib treatments were yet approved for use for COVID-19, so our analyses did not adjust for their use. Our observational cohort study is at risk of residual or unmeasured confounding bias, despite our attempts at including known risk factors for COVID-19 outcomes that exist in an EMR repository.
In conclusion, RTX use in patients with RA is associated with worse COVID-19 outcomes (hospitalization, ICU admission and mechanical ventilation) compared to those on csDMARDs. These data help directly inform clinical practice and the care of patients exposed to RTX. These data also highlight the need for continued vigilance of patients on RTX, the need for COVID-19 vaccination/boosters, and continuation of the other preventive measures, including masking, social distancing and avoiding unnecessary travel. Future studies should examine if lymphopenia and decrease in CD19 cell count are the mediators of these significant associations noted between rituximab use and these outcomes
N3C attribution
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave covid.cd2h.org/enclave and supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the on-going development of this community resource (cite this https://doi.org/10.1093/jamia/ocaa196).
IRB
The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources.
Individual acknowledgements for core contributors
We gratefully acknowledge contributions from the following N3C core teams • Principal Investigators: Melissa A. Haendel*, Christopher G. Chute*, Kenneth R. Gersing, Anita Walden • Workstream, subgroup and administrative leaders: Melissa A. Haendel*, Tellen D. Bennett, Christopher G. Chute, David A. Eichmann, Justin Guinney, Warren A. Kibbe, Hongfang Liu, Philip R.O. Payne, Emily R. Pfaff, Peter N. Robinson, Joel H. Saltz, Heidi Spratt, Justin Starren, Christine Suver, Adam B. Wilcox, Andrew E. Williams, Chunlei Wu
-
•
Key liaisons at data partner sites
-
•
Regulatory staff at data partner sites
-
•
Individuals at the sites who are responsible for creating the datasets and submitting data to N3C
-
•
Data Ingest and Harmonization Team: Christopher G. Chute*, Emily R. Pfaff*, Davera Gabriel, Stephanie S. Hong, Kristin Kostka, Harold P. Lehmann, Richard A. Moffitt, Michele Morris, Matvey B. Palchuk, Xiaohan Tanner Zhang, Richard L. Zhu
-
•
Phenotype Team (Individuals who create the scripts that the sites use to submit their data, based on the COVID and Long COVID definitions): Emily R. Pfaff*, Benjamin Amor, Mark M. Bissell, Marshall Clark, Andrew T. Girvin, Stephanie S. Hong, Kristin Kostka, Adam M. Lee, Robert T. Miller, Michele Morris, Matvey B. Palchuk, Kellie M. Walters
-
•
Project Management and Operations Team: Anita Walden*, Yooree Chae, Connor Cook, Alexandra Dest, Racquel R. Dietz, Thomas Dillon, Patricia A. Francis, Rafael Fuentes, Alexis Graves, Julie A. McMurry, Andrew J. Neumann, Shawn T. O'Neil, Usman Sheikh, Andréa M. Volz, Elizabeth Zampino
-
•
Partners from NIH and other federal agencies: Christopher P. Austin*, Kenneth R. Gersing*, Samuel Bozzette, Mariam Deacy, Nicole Garbarini, Michael G. Kurilla, Sam G. Michael, Joni L. Rutter, Meredith Temple-O'Connor
-
•
Analytics Team (Individuals who build the Enclave infrastructure, help create codesets, variables, and help Domain Teams and project teams with their datasets): Benjamin Amor*, Mark M. Bissell, Katie Rebecca Bradwell, Andrew T. Girvin, Amin Manna, Nabeel Qureshi
-
•
Publication Committee Management Team: Mary Morrison Saltz*, Christine Suver*, Christopher G. Chute, Melissa A. Haendel, Julie A. McMurry, Andréa M. Volz, Anita Walden
-
•
Publication Committee Review Team: Carolyn Bramante, Jeremy Richard Harper, Wenndy Hernandez, Farrukh M Koraishy, Federico Mariona, Saidulu Mattapally, Amit Saha, Satyanarayana Vedula
Additional data partners who have signed DTA and data release pending
The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • Weill Medical College of
Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children's National (CTSA-CN) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Cincinnati Children's Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • Aurora Health Care — UL1TR002373: Wisconsin Network For Health Research • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • #N/A — UL1TR001445: Langone Health's Clinical and Translational Science Institute • Children's Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • HonorHealth — None (Voluntary) • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Arkansas Children's Hospital — UL1TR003107: UAMS Translational Research Institute
Availability of data and materials
To access patient-level data from the N3C consortium, institutions must have a signed Data Use Agreement executed with NCATS and principal investigators must complete mandatory training along with submitting a Data Use Request (DUR) to N3C. All code used for analyses can be found on GitHub. To request N3C data access follow instructions at https://covid.cd2h.org/onboarding.
Funding
RCP was supported by NIAID of the NIH (K23AI120855). JAS was supported by the resources and use of facilities at the Birmingham VA Medical Center, Birmingham, Alabama, USA. The funding body did not play any role in design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. NS was supported by the Rheumatology Research Foundation and the American Heart Association. TB was supported by the Bill and Melinda Gates Foundation (INV018455). ALO was supported by CTSA award No. UL1TR002649 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author contributions
NS, RCP and JS: Conception of the work
NS, JS: Study design
VM, CL, TB, KF, AO, JH: Data collection and analysis
All authors: Data interpretation
NS and JS: Drafting the article
All authors: Critical revision of the article and final approval of the submitted version
Declaration of Competing Interest
JAS has received consultant fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point communications; and the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in TPT Global Tech, Vaxart pharmaceuticals, Atyu biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals and Charlotte's Web Holdings, Inc. JAS previously owned stock options in Amarin, Viking and Moderna pharmaceuticals. JAS is on the speaker's bureau of Simply Speaking. JAS is a member of the executive of Outcomes Measures in Rheumatology (OMERACT), an organization that develops outcome measures in rheumatology and receives arms-length funding from 8 companies. JAS serves on the FDA Arthritis Advisory Committee. JAS is the chair of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. JAS previously served as a member of the following committees: member, the American College of Rheumatology's (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee and the co-chair of the ACR Criteria and Response Criteria subcommittee. Other authors have no conflicts to declare.
Acknowledgments
We thank Gabriella Tangkilisan of the Oregon Health Sciences Center for administrative support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.semarthrit.2022.152149.
Appendix. Supplementary materials
References
- 1.England B.R., Roul P., Yang Y., Kalil A.C., Michaud K., Thiele G.M., et al. Risk of COVID-19 in rheumatoid arthritis: a national veterans affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021;73(12):2179–2188. doi: 10.1002/art.41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topless R.K., Phipps-Green A., Leask M., Dalbeth N., Stamp L.K., Robinson P.C., et al. Gout, rheumatoid arthritis, and the risk of death related to coronavirus disease 2019: an analysis of the UK Biobank. ACR Open Rheumatol. 2021;3(5):333–340. doi: 10.1002/acr2.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen K.M., Bates B.A., Rashidi E.S., Olex A.L., Mannon R.B., Patel R.C., et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022;4(1):e33–e41. doi: 10.1016/S2665-9913(21)00325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel N.J., D'Silva K.M., Hsu T.Y., DiIorio M., Fu X., Cook C., et al. COVID-19 outcomes among users of CD20 inhibitors for immune-mediated diseases: a comparative cohort study. medRxiv. 2021 [Google Scholar]
- 5.Sparks J.A., Wallace Z.S., Seet A.M., Gianfrancesco M.A., Izadi Z., Hyrich K.L., et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis. 2021;80(9):1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strangfeld A., Schafer M., Gianfrancesco M.A., Lawson-Tovey S., Liew J.W., Ljung L., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current State of the Science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards J.C., Leandro M.J., Cambridge G. B lymphocyte depletion therapy with rituximab in rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30(2):393–403. doi: 10.1016/j.rdc.2004.01.006. viii. [DOI] [PubMed] [Google Scholar]
- 9.Edwards J.C., Szczepanski L., Szechinski J., Filipowicz-Sosnowska A., Emery P., Close D.R., et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 10.Bennett T.D., Moffitt R.A., Hajagos J.G., Amor B., Anand A., Bissell M.M., et al. The national COVID cohort collaborative: clinical characterization and early severity prediction. medRxiv. 2021 [Google Scholar]
- 11.https://github.com/National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition.
- 12.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80, (1):27–38.
- 14.Spark R. (R on Spark). 2020.
- 15.Avouac J., Drumez E., Hachulla E., Seror R., Georgin-Lavialle S., El Mahou S., et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3(6):e419–ee26. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esmaeili S., Abbasi M.H., Abolmaali M., Mojtahed M., Alavi S.N.R., Soleimani S., et al. Rituximab and risk of COVID-19 infection and its severity in patients with MS and NMOSD. BMC Neurol. 2021;21(1):183. doi: 10.1186/s12883-021-02218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrold L.R., John A., Best J., Zlotnick S., Karki C., Li Y., et al. Impact of rituximab on patient-reported outcomes in patients with rheumatoid arthritis from the US Corrona Registry. Clin Rheumatol. 2017;36(9):2135–2140. doi: 10.1007/s10067-017-3742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loomba R., Liang T.J. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riedell P., Carson K.R. A drug safety evaluation of rituximab and risk of hepatitis B. Expert Opin Drug Saf. 2014;13(7):977–987. doi: 10.1517/14740338.2014.918948. [DOI] [PubMed] [Google Scholar]
- 20.Gea-Banacloche J.C. Rituximab-associated infections. Semin Hematol. 2010;47(2):187–198. doi: 10.1053/j.seminhematol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Bedognetti D., Zoppoli G., Massucco C., Zanardi E., Zupo S., Bruzzone A., et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin's lymphoma patients treated with rituximab-containing regimens. J Immunol. 2011;186(10):6044–6055. doi: 10.4049/jimmunol.1004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westra J., van Assen S., Wilting K.R., Land J., Horst G., de Haan A., et al. Rituximab impairs immunoglobulin (Ig)M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients. Clin Exp Immunol. 2014;178(1):40–47. doi: 10.1111/cei.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benucci M., Quartuccio L., Li Gobbi F., Damiani A., Grossi V., Infantino M., et al. Persistence of rT-PCR-SARS-CoV-2 infection and delayed serological response, as a possible effect of rituximab according to the hypothesis of Schulze-Koops et al. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218590. [DOI] [PubMed] [Google Scholar]
- 24.Schulze-Koops H., Krueger K., Vallbracht I., Hasseli R., Skapenko A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2021;80(5):e67. doi: 10.1136/annrheumdis-2020-218075. [DOI] [PubMed] [Google Scholar]
- 25.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To access patient-level data from the N3C consortium, institutions must have a signed Data Use Agreement executed with NCATS and principal investigators must complete mandatory training along with submitting a Data Use Request (DUR) to N3C. All code used for analyses can be found on GitHub. To request N3C data access follow instructions at https://covid.cd2h.org/onboarding.