Abstract
Here we provide the ‘Global Spectrum of Plant Form and Function Dataset’, containing species mean values for six vascular plant traits. Together, these traits –plant height, stem specific density, leaf area, leaf mass per area, leaf nitrogen content per dry mass, and diaspore (seed or spore) mass – define the primary axes of variation in plant form and function. The dataset is based on ca. 1 million trait records received via the TRY database (representing ca. 2,500 original publications) and additional unpublished data. It provides 92,159 species mean values for the six traits, covering 46,047 species. The data are complemented by higher-level taxonomic classification and six categorical traits (woodiness, growth form, succulence, adaptation to terrestrial or aquatic habitats, nutrition type and leaf type). Data quality management is based on a probabilistic approach combined with comprehensive validation against expert knowledge and external information. Intense data acquisition and thorough quality control produced the largest and, to our knowledge, most accurate compilation of empirically observed vascular plant species mean traits to date.
Subject terms: Biodiversity, Biogeography, Macroecology
| Measurement(s) | plant trait |
| Technology Type(s) | various |
| Factor Type(s) | none |
| Sample Characteristic - Organism | Tracheophyta |
| Sample Characteristic - Environment | natural environment |
| Sample Characteristic - Location | global |
Background & Summary
Plant traits are the morphological, chemical, physiological or phenological properties of individuals1. They determine how plants as primary producers capture, process and store resources, how they respond to their abiotic and biotic environment and disturbances, and how they affect other trophic levels and the fluxes of water, carbon and energy through ecosystems2–8.
Despite the overwhelming diversity of plant forms and life histories on Earth, single plant organs, such as leaves, stems, or seeds, show comparatively few essential trait combinations9. Evidence for recurrent trait syndromes beyond the level of single organs has been rare, restricted geographically or taxonomically, and often contradictory. Díaz et al.9 addressed this question by analyzing the worldwide variation in six major traits critical to growth, survival and reproduction, namely: plant height (H), stem specific density (SSD), leaf area (LA), leaf mass per area (LMA), leaf nitrogen content per dry mass (Nmass) and diaspore (seed or spore) mass (SM). Díaz et al.9 found that occupancy of the six-dimensional trait space is highly constrained, and is captured in a two-dimensional global spectrum of plant form and function, indicating strong correlation and trade-offs among traits. These results provide a foundation and baseline for studies of plant evolution, comparative plant and ecosystems ecology, and predictive modelling of future vegetation based on continuous variation in essential plant functional dimensions.
Here we provide the trait dataset that served as basis for the analysis of the global spectrum of plant form and function presented in Díaz et al.9 –the ‘Global Spectrum of Plant Form and Function Dataset’ (short here ‘Global Spectrum Dataset’). The dataset is predominantly based on trait records compiled in the TRY database10,11 and provides trait values corresponding –to the extent possible–to mature and healthy plants grown under natural conditions within the species distribution range. The dataset provides species mean values for the six plant traits mentioned above plus leaf dry matter content, used for the imputation of stem specific density. The dataset covers >46,000 of the approximately 391,000 vascular plant species known to science12. Despite the rapid development of large plant trait datasets, the Global Spectrum Dataset stands out in terms of coverage and reliability. First, it provides quantitative information for a very high number of species, including about 5% of them with ‘complete coverage’ (all six traits). Second, it represents a unique combination of probabilistic outlier detection and comprehensive validation of trait values against expert knowledge and external information for data quality assurance. Third, it contains the attribution of data to original references, even if datasets contributed to TRY had been assembled from multiple original sources.
The quantitative trait data are enhanced by higher-level taxonomic information, based on the Angiosperm Phylogeny APG III (http://www.mobot.org/MOBOT/research/APweb/) and categorical traits, based on the ‘TRY – Categorical Traits Dataset’13, enriched by field data and various literature sources. This information facilitates stratification of species and quantitative traits according to phylogenetic and morpho-functional criteria.
The present dataset results from the integration of trait measurements from many datasets received via TRY and additional, partly unpublished, data. The data come from largely independent studies, that address a wide variety of questions at different scales, and using different measurement methods, units and terminologies14. The development of the dataset therefore faced three challenges: (1) to derive a dataset of species mean values covering all six traits with the aim of being representative of vascular plant species worldwide; (2) to detect erroneous trait records (due to errors in sampling, measurement, unit conversion, etc.); and (3) to ensure that correctly measured extreme values of traits in nature were not mistakenly identified as outliers and therefore excluded from the dataset. To deal with these challenges, we collected as many trait observations as possible. The dataset was developed over a period of six years (2009–2015) with continuous addition of new trait records as data became available. The final dataset is based on almost 1 million trait records, which can be traced back to ca. 2,500 references (see file: ‘References_original_sources.xlsx’). We identified outliers and potential errors based on a probabilistic approach10 combined with validation by domain experts and external information.
These combined efforts of data acquisition, integration and quality control resulted in the most comprehensive and probably most accurate dataset for species mean traits of vascular plants published so far.
Methods
Selection of plant traits
There is an extensive literature summarized in Díaz et al.9 and Pérez-Harguindeguy et al.6 supporting the key importance of the six core traits chosen – H, SSD, LA, LMA, Nmass and SM – to growth, survival and reproduction. Díaz et al.9 went further by showing that, together, these traits capture the essence of plant form and function at the broad scale: a two-dimensional space, with one major dimension reflecting the size of whole plants and its organs, and the other representing a balance between leaf construction cost against growth potential, captures roughly three-quarters of total trait variation. The core quantitative traits were complemented with the categorical traits: woodiness, growth form, succulence, adaptation to terrestrial or aquatic habitats, nutrition type, and leaf type.
Definition of traits
In the following section we provide the names and definitions used for the continuous traits in the original publication of the global spectrum9, plus the names and definitions used in the Thesaurus Of Plant Characteristics (TOP)14. The detailed rationale, ecological meaning and key references for each of them can be found in the methods section of Díaz et al.9 and in Garnier et al.7. For the categorical traits we provide names, definition where available, and the categories used in the database. Traits were mostly measured following the protocols and definitions specified in the ‘New Handbook for Standardised Measurement of Plant Functional Traits Worldwide’6 (http://www.nucleodiversus.org). In the case of data from the LEDA database, measurements followed the protocols developed in the context of the LEDA project16 (https://www.leda-traitbase.org). In the case of published datasets individual measurement protocols are available in the original publications listed in Table S1.
Plant height (H) (unit: m)
Adult plant height, i.e. typical height of the upper boundary of the main photosynthetic tissues at maturity (TOP: vegetative plant height; the plant height considering the highest vegetative component).
Stem specific density (SSD) (unit: mg mm−3)
Stem dry mass per unit of stem fresh volume (TOP: stem specific density; the ratio of the mass of the stem or a unit thereof assessed after drying to its volume assessed without drying). SSD is much more commonly measured on woody species (particularly trees), than on non-woody species. Therefore, gaps in SSD for non-woody species were filled by estimates derived from leaf dry matter content (see Data Imputation below).
Leaf area (LA) (unit: mm2)
One-sided surface area of an individual lamina (TOP: leaf lamina area; the area of the leaf lamina in the one-sided projection; in case of compound leaves the area of a leaflet lamina).
Leaf mass per area (LMA) (unit: g m−2)
Leaf dry mass per unit of lamina surface area (TOP: leaf mass per area, the ratio of the dry mass of a leaf to its area).
Leaf nitrogen per mass (Nmass) (unit: mg g−1)
Leaf nitrogen content per unit of lamina dry mass (leaf total N) (TOP: leaf nitrogen content per leaf dry mass; the ratio of the quantity of nitrogen in the leaf or component thereof, i.e. leaf lamina or leaflet, per respective unit dry mass).
Diaspore mass (SM) (unit: mg)
Dry mass of an individual seed or spore plus any additional structures that assist dispersal and do not easily detach (TOP: seed dry mass; mass of an individual seed or spore assessed after drying; seed dry mass). Spore mass of pteridophytes, rarely reported in the literature, was estimated from published values of diaspore diameter and density (see Data Imputation below).
Leaf dry matter content (LDMC) (unit: g g−1)
The ratio of the dry mass of the leaf or component thereof, i.e. leaf lamina, to the corresponding water saturated fresh mass. In addition to the six focal traits, we compiled LDMC for herbaceous plants to calculate missing values for SSD (see Data Imputation below).
Adaptation to terrestrial or aquatic habitats
On the basis of the type of habitat in which the species naturally grows. Categories: aquatic, aquatic/semiaquatic, semiaquatic, terrestrial.
Woodiness
A feature of the whole plant defining the occurrence and distribution of wood along the stem. Categories: woody, non-woody, semi-woody (woody at base of stem(s) only).
Growth form
Growth form is mainly determined by woodiness and the direction and extent of growth, and any branching of the main shoot axis or axes. Categories: bamboo graminoid, climber, fern, herbaceous graminoid, herbaceous non-graminoid, herbaceous non-graminoid/shrub, succulent, shrub, shrub/tree, tree, other.
Succulence
Succulence characterizes plants with parts that are thickened, fleshy, and engorged, usually to retain water in conditions where climate or soil characteristics strongly limit water availability to plants. This criterion aims to provide more detailed information to the succulent growth form whenever available. Categories: leaf and stem succulent, leaf rosette and stem succulent, leaf rosette succulent, leaf rosette succulent (tall), leaf succulent, stem succulent, stem succulent (short), stem succulent (tall), succulent.
Nutrition type
Nutrition type here refers to whether the major source of energy and nutrients for the plant is photosynthesis, animals, dead material or other plants. Parasitism categories: hemiparasitic, holoparasitic, independent, parasitic. Carnivory categories: carnivorous, detritivorous.
According to the ‘New Handbook for Standardised Measurement of Plant Functional Traits Worldwide’6 succulence and nutrition type are part of growth form. We here treat them separately for simplicity and to avoid combined categories.
Leaf type
A classification of presence/absence of photosynthetic active leaves and their basic forms. Categories: broadleaved, needleleaved, scale-shaped, scale-shaped/needleleaved, photosynthetic stem.
Definition of representative trait records
The six core quantitative traits certainly show intraspecific variation, amongst others caused by different ontogenetic stages and growth conditions. The dataset, focused on mean trait values for species rather than intraspecific variation, was intended to represent species mean trait values for mature and healthy (not obviously unhealthy) plants grown under natural conditions within the species distribution range. Leaf traits were intended to represent young but fully expanded and healthy leaves from the light exposed top canopy. Trait records not conforming to these requirements, i.e. records from plants grown in laboratories under experimental conditions and records measured on juvenile plants, were excluded from the dataset. This decision was made based on the respective metadata in the TRY database (see below).
Data sources
The vast majority of quantitative trait data was provided by the TRY Plant Trait Database10 (https:// www.try-db.org, TRY version 2.0 accessed July 2010, updated by TRY version 3.0 accessed May 2015). This dataset was supplemented by a small number of published data not included in TRY and original unpublished data contributed by W. J. Bond, J. H. C. Cornelissen, S. Díaz, L. Enrico, M. T. Fernandez-Piedade, L. D. Gorné, D. Kirkup, M. Kleyer, N. Salinas, E.-D. Schulze, K. Thompson, and R. Urrutia-Jalabert.
Categorical traits were derived from the TRY Categorical Traits Dataset (https://www.try-db.org/TryWeb/Data.php#3), enhanced by field data and various literature sources.
The datasets contributing via TRY to the quantitative traits are described in Supplementary Table S1, which contains data from refs. 4,16–233 and the following unpbublished datasets: French Weeds Trait Database; Photosynthesis and Leaf Characteristics Database; South African Woody Plants Database (ZLTP); Tundra Plant Traits Database; Leaf N-Retention Database; Traits for Herbaceous Species from Andorra; Leaf Characteristics of Pinus sylvestris and Picea abies; Plant Coastal Dune Traits (France, Aquitaine); Dispersal Traits Database; LABDENDRO Brazilian Subtropical Forest Traits Database; Growth and Herbivory of Juvenile Trees; Cold Tolerance, Seed Size and Height of North American Forest Tree Species; Harze Trait Intravar: SLA; LDMC and Plant Height for Calcareous Grassland Species in South Belgium; Functional Traits for Restoration Ecology in the Colombian Amazon; Komati Leaf Trait Data; Baccara - Plant Traits of European Forests; Traits of Bornean Trees Database; Meadow Plant Traits: Biomass Allocation, Rooting depth; New South Wales Plant Traits Database; Traits for Herbaceous Species from Andorra; Catalonian Mediterranean Shrubland Trait Database; The Netherlands Plant Height Database; Plant Traits from Spanish Mediterranean Shrublands; Crown Architecture Database; Maxfield Meadow, Rocky Mountain Biological Laboratory – LMA; Herbaceous Plants Traits From Southern Germany; Leaf Area, Dry Mass and SLA Dataset; Herbaceous Leaf Traits Database Old Field New York; Plant Functional Traits From the Province of Almeria, Spain; Traits for Common Grasses and Herbs in Spain; Midwestern and Southern US Herbaceous Species Trait Database; Overton/Wright New Zealand Database; San Lorenzo Epiphyte Leaf Traits Database.
The reference for each individual trait record contributing via TRY to the Global Spectrum Dataset before exclusion of non-representative trait records, errors and duplicates is documented in the data file ‘References.xlsx’.
Data integration and quality management
Semantic integration of terminologies from different datasets
Ecological studies are carried out for a large number of different questions at different scales and researchers often work independently and with little coordination among them. This results in idiosyncratic datasets using heterogeneous terminologies14. The first step was therefore a semantic integration of terminologies. The core traits were standardized according to the definitions and measurement protocols provided in the Thesaurus Of Plant Characteristics (TOP)14 and the ‘New Handbook for Standardised Measurement of Plant Functional Traits Worldwide’6,15. The metadata for plant and organ maturity (juvenile, mature), health (healthy, not healthy), growth conditions (natural conditions, experimental conditions), and sun- versus shade-grown leaves were harmonized across datasets.
Consolidation of taxonomy
Species names were standardized and attributed to families according to The Plant List (http://www.theplantlist.org), the commonly accepted list for vascular plants at the time of publication of Díaz et al.9, using TNRS234,235, complemented by manual standardization by experts. Attribution of families to higher-rank groups was made according to APG III (2009) (http://www.mobot.org/MOBOT/research/APweb/).
Conversion and correction of units, and exclusion of errors
Different datasets often used different units for the same trait. After conversion to the standardized unit per trait, differences among datasets - sometimes in the order of magnitude - became obvious. These differences could often be traced back to errors in the original units and were corrected. Obvious errors (e.g. impossible trait values like LMA < 0 g/m2) were excluded from the dataset.
Data imputation
To improve the number of species with values for all six core traits, trait records for stem SSD, LMA, Nmass and SM were complemented by trait values derived from records of related traits:
- Imputation of SSD
Trait records for SSD are available for a very large number of woody species, but only for very few herbaceous species. To incorporate this fundamental trait in the analyses by Díaz et al.9, we complemented SSD of herbaceous species using an estimation based on leaf dry matter content (LDMC), a much more widely available trait, and its close correlation to stem dry matter content (StDMC, the ratio of stem dry mass to stem water-saturated fresh mass). StDMC is a good proxy of SSD in herbaceous plants with a ratio of approximately 1:1199, despite substantial differences in stem anatomy among botanical families236, including those between non-monocotyledons and monocotyledons (where sheaths were measured). We used a data set of 422 herbaceous species collected in the field across Europe and Israel, and belonging to 31 botanical families, to parameterize linear relationships of StDMC to LDMC. The slopes of the relationship were significantly higher for monocotyledons than for other angiosperms (F = 12.3; P < 0.001, from a covariance analysis); within non-monocotyledons, the slope for Fabaceae was higher than that for species from other families (F = 4.5; P < 0.05, from a covariance analysis). We thus used three different equations to predict SSD for 1963 herbaceous species for which LDMC values were available in TRY (Table 1): one for monocotyledons, one for Fabaceae, and a third one for other non-monocotyledons. Estimated data are flagged.
Table 1.
Summary statistics for model I regressions between LDMC and StDMC (dependent variable) for the whole data set and various subsets of species.
| Dataset | Slope (SE) | Intercept (SE) | r2 | N |
|---|---|---|---|---|
| All species | 0.698 (0.042) | 0.058 (0.011) | 0.398*** | 422 |
| Monocots | 0.888 (0.071) | 0.027 (0.022) | 0.467*** | 181 |
| Fabaceae | 0.692 (0.129) | 0.048 (0.033) | 0.367*** | 52 |
| All dicots except Fabaceae | 0.524 (0.076) | 0.096 (0.017) | 0.203*** | 188 |
Coefficients in italics were used in linear regressions to predict StDMC from LDMC in various subsets of herbaceous species for which LDMC values were available in the TRY database. Units for StDMC and LDMC are g g−1, giving SSD estimates in equivalent units of mg mm−3. Note that in Díaz et al.9, the coefficients of the regressions are given for LDMC and StDMC values expressed in mg g−1, yielding SSD estimates in g cm−3.
- Imputation of LMA
Trait records for SLA (leaf area per leaf dry mass) were converted to LMA (leaf dry mass per leaf area): LMA = 1/SLA.
- Imputation of Nmass
Trait records for leaf nitrogen content per leaf area (Narea) were converted to records of leaf nitrogen content per leaf dry mass (Nmass) if records for LMA were available for the same observation (leaf): Nmass = Narea/LMA.
- Imputation of SM
To be able to include trait data for pteridophytes in the analyses in Díaz et al.9, diaspore mass values were estimated based on published data for spore radius (r). We assumed that spores would be approximately spherical, with volume = (4/3)πr3, and that their density would be 0.5 mg mm−3 (refs. 237–240). Although these assumptions were imprecise, we are confident they result in spore masses within the right order of magnitude and several orders of magnitude smaller than seed mass of spermatophytes. Most data were from Page237, data for Sadleria pallida were from Lloyd238, for Pteridium aquilinum from Conway239, and for Diphasiastrum spp from Stoor et al.240.
Probabilistic outlier detection
The hierarchical taxonomic classification of plants into families, genera and species has been shown to be highly informative with respect to the probability of trait values241–243. We therefore used it to conduct outlier detection at each of these levels.
The six core traits provided in the Global Spectrum Dataset are approximately normally distributed on a logarithmic scale10. We therefore assume that on log-scale, traits sample from normal distributions. In the context of a normal distribution the density distribution is symmetric to the mean with 99.73% (99.99%) of data to be expected within the range of mean +/− 3 standard deviations, and 99.99% of data within +/− 4 standard deviations. Using these wide confidence intervals ensures that extreme values that correspond to truly extreme values of traits in nature are not mistakenly identified as outliers and therefore excluded from the dataset.
The z-score indicates how many standard deviations a record is away from the mean:
Trait values with absolute z-scores >4 (>3) have a probability of less than 0.1% (0.3%) to be true values of the normal distribution. These trait values are most probably caused by errors not yet detected for these individual records, e.g., wrong unit, decimal error of trait value, wrong species (e.g. by mistake attributing a herb species name to a height measured on a tree), problems related to the trait definition or non-representative growth or measurement conditions. We acknowledge however that our z-score cutoff choice is an arbitrary one.
In many cases the number of trait values per taxon (e.g. a given species) was too small for a representative sample and did not provide a reliable estimate of the standard deviation (see Fig. 1). To circumvent this problem, we used the average standard deviation of trait values at the given taxonomic level, e.g., species, genus, family or all vascular plants. This average is an approximation of the standard deviation to be expected for an individual taxon, if a sufficient number of observations would be available (Fig. 1)10.
Fig. 1.
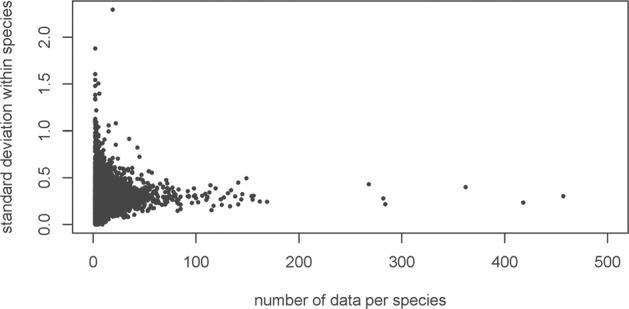
Scatterplot indicating the relation of standard deviation within species and sample size on the example of SLA data (1/LMA) derived from the TRY database version 1 (Kattge et al.10, Fig. S1).
This probability-based data quality assessment on the different levels of the taxonomic hierarchy is routinely conducted within the TRY database for all traits with more than 1000 records. The z-score values for each trait record are made available on the TRY website and the highest absolute value is provided with each data release.
Trait values with an absolute z-score >4 (more than 4 standard deviations from at least one taxon mean) were excluded from the dataset unless their retention could be justified from external sources. Trait records with an absolute z-score 3 to 4 (3 to 4 standard deviations from at least one taxon mean) were checked by domain experts among the authors for plausibility, and retained or excluded accordingly.
Exclusion of duplicate trait records
Duplicate trait records were identified on the basis of the following criteria: same species (after standardization of taxonomy), similar trait values (accounting for rounding errors after semantic integration, unit conversion and data complementation), and no information on different measurement locations or dates.
Calculation of species mean trait values
The resulting dataset was used to calculate species mean trait values, without further stratification along, e.g., datasets or measurement sites. As trait distributions of the six core traits have been shown to be log-normal9, the mean species trait values were calculated after log-transformation of the trait values (geometric mean).
Addition of categorical traits
Data for the categorical traits were added and, if in doubt, checked against expert knowledge and independent external information from specialized websites in the Internet.
Final validation of taxonomy and mean trait values
Taxonomy was finally checked once more manually against the Plant List and APGIII. The ten most extreme species mean values of each trait (smallest and largest) were checked manually for reliability against external sources. Finally, outliers of species mean traits – after categorization of species according to the categorical traits and in bi- and multivariate trait space – were validated against external sources (see Díaz et al.9 Fig. 2, Extended Data Fig. 3, and Extended Data Fig. 4).
Fig. 2.
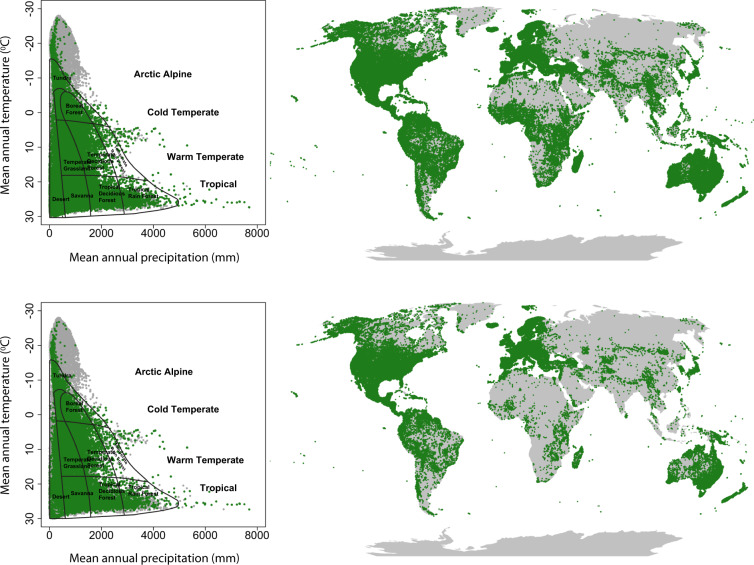
Climatic and geographical coverage of the dataset. Green points, occurrences according to the Global Biodiversity Information Facility (GBIF) (http://www.gbif.org) of species with information on at least one core trait (upper panels) and all six core traits (lower panels). Right panels show distribution in the global map (Robinson projection); grey: land surface. Maps are based on the R package ‘maps’, accessed at The Comprehensive R Archive Network (https://cran.r-project.org/web/packages/maps/index.html). Left panels show distribution in major climatic regions of the world; grey: MAP and MAT as in Climate Research Unit (CRU) CL v.1.0 0.5 degree climatology (http://www.cru.uea.ac.uk/data, ref. 245); Biome classification according to Whittaker246. This figure is reproduced from ref. 9 with permission.
Data Records
The dataset is available under a CC-BY license at the TRY File Archive (https://www.try-db.org/TryWeb/Data.php):
Díaz, S. et al. The global spectrum of plant form and function: enhanced species-level trait dataset. TRY File Archive 10.17871/TRY.81 (2022)244
The dataset consists of two data files
Species_mean_traits.xlsx
References.xlsx
Species_mean_traits.xlsx
The file provides mean trait values of plants grown under natural conditions for 46,047 species (including a small number of genus level classifications, sub-species and local varieties). Species names and mean trait values are complemented by taxonomic hierarchy (genus, family and phylogenetic group), the number of trait records contributing to each mean trait value and by categorical traits. Values of all six traits were available for 2,214 species. In total the dataset contains 476,932 entries for quantitative and categorical trait records and higher-level taxonomy (92,159 entries for quantitative traits, 200,585 entries for categorical traits, and 184,188 entries for higher-level taxonomy).
The quantitative species-level trait information is based on about 1 million trait records (see Table S1), measured on >500,000 plant individuals (number of different Observations in References (see below)). One trait record reported in the datasets is often based on several replicated measurements from different representative individuals at a site. The New Handbook for Standardised Measurement of Plant Functional Traits Worldwide6 recommends measurements on 10 to 25 individual plants or leaves, depending on the trait. Therefore in the cases that followed this or related protocols, a trait record in the original database probably represents the site-specific mean trait value for a given species. Reporting only the site-specific mean trait value was standard procedure in older publications and aggregated databases, assuming a common approach to replicated measurements on different individuals. More recent datasets tend to provide all individual measurements, among other reasons because this allows better treatment of intraspecific trait variation.
The present dataset was derived from 157 datasets (Table S1). Trait records can be traced to ca. 2500 original publications (see References_original_sources.xlsx). All species are complemented with higher-level taxonomic information; 92.5% and 84.8% of species are attributed to categories according to woodiness and basic growth-form, respectively. The raw data are available via the TRY Database (https://www.try-db.org/TryWeb/Home.php).
References.xlsx
This file contains the references of all trait data, which contributed to the core traits of the Global Spectrum Dataset via the TRY database. If datasets contributed to TRY were already compiled from original publications, the table also provides the references of these original publications. The references are linked to the data in the species mean trait dataset via species unique identifiers and trait names.
The sum of replicates in the species mean trait table is about 100,000 trait records less than the sum of 979,924 trait records in References and Supplementary Table S1, because the species mean trait table contains mean trait values and information on number of trait records only for those species-trait combinations that were retained after data cleaning and imputation.
Technical Validation
The dataset has a global coverage in geographic and climate space (Fig. 2, also Díaz et al.9 Extended Data Fig. 1), however with known gaps9–11. The numbers of species characterized per trait are similar to the TRY Database version 5, published in 201911. This indicates the efficiency of data collection and curation for the Global Spectrum Dataset. All species mean trait values (Table 2) are within the ranges published in Kattge et al.10. Histograms of trait frequency distributions are provided in Fig. 3. The coverage of species per trait with respect to woodiness is presented in Fig. 4. The dataset has so far been used in Díaz et al.9, where the data show a high internal consistency in bi- and multivariate analyses: known bivariate relationships were well reproduced (Díaz et al.9 Extended Data Figs. 3 and 4) and individual species were located in the first axes of the principal component analysis in positions expected from general knowledge about these species (Díaz et al.9 Fig. 2).
Table 2.
Number of species and range of variation of species mean traits, geographic distributions and climatic conditions in the Global Spectrum Dataset.
| No. of species | Mean | Range | Mean & median n per species | |||
|---|---|---|---|---|---|---|
| Min. | Max. | |||||
| H | 24704 | 1.62 | 0.001 | to | 90 | 9.5 (2) |
| SSD | 11350 | 0.47 | 0.06 | to | 1.39 | 8.9 (2) |
| LA | 12164 | 1336 | 0.8 | to | 2.79E6 | 10 (3) |
| LMA | 10486 | 72.4 | 4.9 | to | 1507 | 15.4 (5) |
| Nmass | 8689 | 19.2 | 2.48 | to | 68.98 | 9.3 (3) |
| SM | 24766 | 2.65 | 5.15E-6 | to | 2.05E7 | 8.6 (6) |
| Latitude | 55 S | to | 83.17 N | |||
| Altitude | −59 | to | 5249 | |||
| MAT | −27.22 | to | 29.97 | |||
| MAP | <5 | to | 7693 | |||
No. of species: number of species characterized; Mean: geometric mean of species traits; Range: lowest and highest species mean trait values; Mean & median n per species: mean and median (in brackets) number of trait records per species; H: adult plant height (m); SSD: stem specific density (mg mm−3); LA: leaf area (mm2); LMA: leaf mass per area (g m−2); Nmass: N content per unit leaf mass (mg g−1); SM: diaspore (seed or spore) mass (mg); Latitude in degrees; Altitude in m; MAT: Mean annual temperature (°C); MAP: Mean annual precipitation (mm). Mean annual temperature and precipitation refer to CRU0.5 degree climatology. Modified from ref. 9 with permission.
Fig. 3.
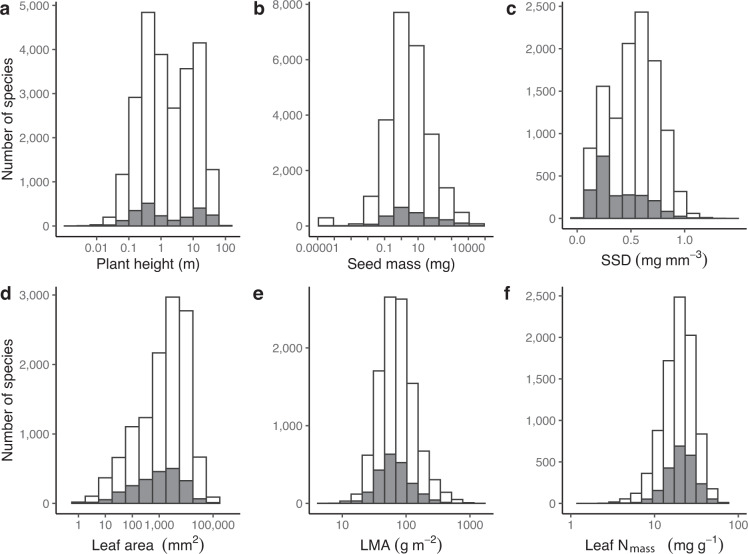
Frequency distributions of species for the six core traits. Grey: species with all six traits; white: species with at least one trait. (a) Plant height, (b) Seed mass, (c) SSD: stem dry mass per stem fresh volume (stem specific density), (d) Leaf area, (e) LMA: leaf dry mass per leaf area, (f) Leaf Nmass: leaf nitrogen content per leaf dry mass (leaf nitrogen concentration).
Fig. 4.
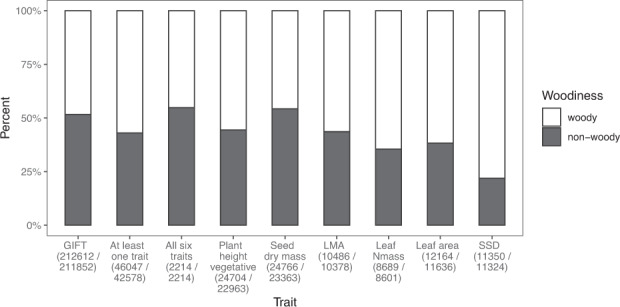
The coverage of species per trait with respect to woodiness (woody versus non-woody incl. semi-woody). The coverage in the GIFT database247,248 a comprehensive baseline of plant growth form, is included for external comparison (see ref. 11 for more details). In parentheses: the number of species with data for the trait and the number of species for which woodiness could be determined.
Usage Notes
In case the dataset is used in publications, both this paper and Díaz et al.9 should be cited.
The six quantitative traits compiled here (plus LDMC) are among the best-covered quantitative traits in the TRY database. However, as is typical for these kinds of observational data, the numbers of records per species are unevenly distributed: few species mean trait values are based on a large number of records, while a large fraction of the species mean estimates is based on only a few or a single trait record(s) (see difference between mean and median number of trait records per species and trait in Table 2, the number of trait records per species mean is also indicated in the dataset file ‘Species_mean_traits.xlsx’). The representativeness of these mean values should be taken with caution, because the trait measurements have to be treated as samples from the variation of traits within species, which – for some traits – can be substantial10. However, as mentioned above, one trait record is often based on several trait measurements on characteristic individuals and therefore represents a species per site-specific mean value. In the context of large-scale analyses the variation within species has been shown to be considerably smaller than the variation between species10.
Supplementary information
Acknowledgements
The study has been supported by the TRY initiative on plant traits (https://www.try-db.org). TRY is an initiative of the Max Planck Institute for Biogeochemistry, bioDISCOVERY/Future Earth (ICSU), the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig and Núcleo DiverSus (CONICET- Universidad Nacional de Córdoba, Argentina). The Global Spectrum of Plant Form and Function study has been supported by the European BACI project (Towards a Biosphere Atmosphere change Index, EU grant ID 640176), and grants to SD by FONCyT, CONICET, Universidad Nacional de Córdoba, the Inter-American Institute for Global Change Research, and The Newton Fund (NERC UK – CONICET ARG). VO thanks RSF (#19-14-00038p).
Author contributions
P.A., B.A., O.A., M.B., D.B., C.B., Mi.B., B.B., W.B., B.B.-L., K.B., S.B., B.B., Ch.B., G.C., F.C., J.C.-B., B.C., F.S.C., J.C., J.H.C.C., W.C., J.C., D.C., M.D., Ad.A., Fd.V., A.D., S.D., J.D., M.D., T.D., B.E., L.E.,J.F., J.i.F., F.F.-M., M.T.F.P., B.F., K.F., H.F., E.F., C.F.-D., G.F., S.G., R.G., E.G., A.N.G., A.G.-M., W.G., N.G., G.G., A.G.G., W.H., S.H., M.H., W.H., B.H., T.H., Th.H., S.H., P.H., R.J., H.J., S.J., J.K., D.K., M.K., F.K., N.K., K.K., I.K., Hi.K., E.L., V.L., D.L., S.L., M.L., F.L., J.L., F.L., A.M., P.M., B.M., P.M., M.M., J.M., R.M., V.M., A.T.M., J.M., R.M., Ma.M., U.N., K.Ö., Y.O., J.O., W.O., A.P., J.G.P., B.P., Jo.P., S.P., V.P., H.P., Je.P., C.P., Q.R., P.B.R., C.R., L.S., N.S., B.S., Br.S., M.S.-L., E.D.S., F.S., S.N.S., S.S., B.S., A.S., E.S., N.S., M.S., E.S., N.S., K.T., A.T., R.U.J., F.V., Pv.B., K.V., Bv.H., Z.W., E.W., M.W., C.W., I.J.W., S.J.W., J.W., B.Y., A.E.Z., B.Z. contributed plant trait data. P.W., H.K. and C.K. provided additional categorical traits. G.B. and J.K. integrated the contributed datasets, excluded non-representative trait values and duplicates, and applied the probabilistic outlier detection. S.D., J.H.C.C., E.G., J.K., K.T., M.K., S.L., D.K., J.D., A.T.M., A.E.Z., P.B.R., H.P., C.W., J.K., I.J.W., G.B., M.W. and H.P. additionally checked data manually for errors. M.D.M., S.T. and St.D. checked data for normality and ranges. E.G. established the equations to derive SSD from LDMC in herbaceous plants. S.D., J.K., G.B., V.G., V.F. and L.D.G. consolidated taxonomy. V.G., L.D.G., A.G. and S.D. added categorical trait values. S.D. and J.K. wrote the draft of the data descriptor. All authors contributed to the final version of the data descriptor.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Code availability
Does not apply.
Competing interests
The authors declare no competing interests.
Footnotes
Retired: Regis Miller.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sandra Díaz, Jens Kattge.
Deceased: Bernard Amiaud, Fritz Schweingruber.
Contributor Information
Sandra Díaz, Email: sandra.diaz@unc.edu.ar.
Jens Kattge, Email: jkattge@bgc-jena.mpg.de.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-022-01774-9.
References
- 1.Violle C, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. doi: 10.1111/j.2007.0030-1299.15559.x. [DOI] [Google Scholar]
- 2.Aerts R, Chapin FS. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Advances in Ecological Research, Vol 30. 2000;30:1–67. [Google Scholar]
- 3.Grime, J. P. Plant Strategies, Vegetation Processes, and Ecosystem Properties., (John Wiley & Sons, 2001).
- 4.Diaz S, et al. The plant traits that drive ecosystems: Evidence from three continents. Journal of Vegetation Science. 2004;15:295–304. doi: 10.1111/j.1654-1103.2004.tb02266.x. [DOI] [Google Scholar]
- 5.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- 6.Pérez-Harguindeguy N, et al. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany. 2013;61:167. doi: 10.1071/bt12225. [DOI] [Google Scholar]
- 7.Garnier, E., Navas, M.-L. & Grigulis, K. Plant Functional Diversity. (Oxford University Press, 2016).
- 8.Pausas JG, Bradstock RA, Keith DA, Keeley JE. Plant functional traits in relation to fire in crown-fire ecosystems. Ecology. 2004;85:1085–1100. doi: 10.1890/02-4094. [DOI] [Google Scholar]
- 9.Díaz S, et al. The global spectrum of plant form and function. Nature. 2016;529:167–171. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- 10.Kattge J, et al. TRY – a global database of plant traits. Global Change Biology. 2011;17:2905–2935. doi: 10.1111/j.1365-2486.2011.02451.x. [DOI] [Google Scholar]
- 11.Kattge J, et al. TRY plant trait database – enhanced coverage and open access. Global Change Biology. 2020;26:119–188. doi: 10.1111/gcb.14904. [DOI] [PubMed] [Google Scholar]
- 12.Royal Botanic Gardens, Kew. The State of the World’s Plants Report - 2016. (Royal Botanic Gardens, Kew, 2016).
- 13.Kattge, J. et al. TRY - Categorical Traits Dataset. Data from: TRY - a global database of plant traits. TRY File Archivehttps://www.try-db.org/TryWeb/Data.php - 3 (2012).
- 14.Garnier E, et al. Towards a thesaurus of plant characteristics: an ecological contribution. Journal of Ecology. 2016;105:298–309. doi: 10.1111/1365-2745.12698. [DOI] [Google Scholar]
- 15.Cornelissen JHC, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335. doi: 10.1071/bt02124. [DOI] [Google Scholar]
- 16.Kleyer M, et al. The LEDA Traitbase: a database of life-history traits of the Northwest European flora. Journal of Ecology. 2008;96:1266–1274. doi: 10.1111/j.1365-2745.2008.01430.x. [DOI] [Google Scholar]
- 17.Adler PB, Milchunas DG, Lauenroth WK, Sala OE, Burke IC. Functional traits of graminoids in semi-arid steppes: a test of grazing histories. Journal of Applied Ecology. 2004;41:653–663. doi: 10.1111/j.0021-8901.2004.00934.x. [DOI] [Google Scholar]
- 18.Adler, P. B. A comparison of livestock grazing effects on sagebrush steppe, USA, and Patagonian steppe, Argentina. PhD thesis, Colorado State University, (2003).
- 19.Atkin OK, Westbeek MHM, Cambridge ML, Lambers H, Pons TL. Leaf Respiration in Light and Darkness (A Comparison of Slow- and Fast-Growing Poa Species. Plant Physiology. 1997;113:961–965. doi: 10.1104/pp.113.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell C, et al. Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytologist. 2007;176:375–389. doi: 10.1111/j.1469-8137.2007.02183.x. [DOI] [PubMed] [Google Scholar]
- 21.Atkin OK, Schortemeyer M, McFarlane N, Evans JR. The response of fast- and slow-growing Acacia species to elevated atmospheric CO2: an analysis of the underlying components of relative growth rate. Oecologia. 1999;120:544–554. doi: 10.1007/s004420050889. [DOI] [PubMed] [Google Scholar]
- 22.Loveys BR, et al. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Global Change Biology. 2003;9:895–910. doi: 10.1046/j.1365-2486.2003.00611.x. [DOI] [Google Scholar]
- 23.Bahn, M. et al. in Land-use changes in European mountain ecosystems. ECOMONT- Concept and Results (eds A. Cernusca, U. Tappeiner, & N. Bayfield) 247-255 (Blackwell Wissenschaft, Berlin, 1999).
- 24.Wohlfahrt G, et al. Inter-specific variation of the biochemical limitation to photosynthesis and related leaf traits of 30 species from mountain grassland ecosystems under different land use. Plant, Cell and Environment. 1999;22:1281–1296. doi: 10.1046/j.1365-3040.1999.00479.x. [DOI] [Google Scholar]
- 25.Wilson KB, Baldocchi DD, Hanson PJ. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiology. 2000;20:565–578. doi: 10.1093/treephys/20.9.565. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Baldocchi DD. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiology. 2003;23:865–877. doi: 10.1093/treephys/23.13.865. [DOI] [PubMed] [Google Scholar]
- 27.Baraloto C, et al. Decoupled leaf and stem economics in rain forest trees. Ecology Letters. 2010;13:1338–1347. doi: 10.1111/j.1461-0248.2010.01517.x. [DOI] [PubMed] [Google Scholar]
- 28.Baraloto C, et al. Functional trait variation and sampling strategies in species-rich plant communities. Functional Ecology. 2010;24:208–216. doi: 10.1111/j.1365-2435.2009.01600.x. [DOI] [Google Scholar]
- 29.Blonder B, et al. The leaf-area shrinkage effect can bias paleoclimate and ecology research. American Journal of Botany. 2012;99:1756–1763. doi: 10.3732/ajb.1200062. [DOI] [PubMed] [Google Scholar]
- 30.Blonder B, et al. Testing models for the leaf economics spectrum with leaf and whole-plant traits in Arabidopsis thaliana. AoB Plants. 2015;7:plv049. doi: 10.1093/aobpla/plv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blonder B, Violle C, Enquist BJ. Assessing the causes and scales of the leaf economics spectrum using venation networks in Populus tremuloides. Journal of Ecology. 2013;101:981–989. doi: 10.1111/1365-2745.12102. [DOI] [Google Scholar]
- 32.Blonder B, Violle C, Bentley LP, Enquist BJ. Venation networks and the origin of the leaf economics spectrum. Ecology Letters. 2010;14:91–100. doi: 10.1111/j.1461-0248.2010.01554.x. [DOI] [PubMed] [Google Scholar]
- 33.Bond-Lamberty B, Wang C, Gower ST. Aboveground and belowground biomass and sapwood area allometric equations for six boreal tree species of northern Manitoba. Canadian Journal of Forest Research. 2002;32:1441–1450. doi: 10.1139/x02-063. [DOI] [Google Scholar]
- 34.Bond-Lamberty B, Wang C, Gower ST, Norman J. Leaf area dynamics of a boreal black spruce fire chronosequence. Tree Physiology. 2002;22:993–1001. doi: 10.1093/treephys/22.14.993. [DOI] [PubMed] [Google Scholar]
- 35.Bond-Lamberty B, Wang C, Gower ST. The use of multiple measurement techniques to refine estimates of conifer needle geometry. Canadian Journal of Forest Research. 2003;33:101–105. doi: 10.1139/x02-166. [DOI] [Google Scholar]
- 36.Brown KA, et al. Assessing Natural Resource Use by Forest-Reliant Communities in Madagascar Using Functional Diversity and Functional Redundancy Metrics. PLoS ONE. 2011;6:e24107. doi: 10.1371/journal.pone.0024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burrascano S, et al. Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecology. 2015;16:244–253. doi: 10.1556/168.2015.16.2.12. [DOI] [Google Scholar]
- 38.Butterfield BJ, Briggs JM. Regeneration niche differentiates functional strategies of desert woody plant species. Oecologia. 2010;165:477–487. doi: 10.1007/s00442-010-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byun C, de Blois S, Brisson J. Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. Journal of Ecology. 2012;101:128–139. doi: 10.1111/1365-2745.12016. [DOI] [Google Scholar]
- 40.Campetella G, et al. Patterns of plant trait–environment relationships along a forest succession chronosequence. Agriculture, Ecosystems & Environment. 2011;145:38–48. doi: 10.1016/j.agee.2011.06.025. [DOI] [Google Scholar]
- 41.Cavender-Bares J, Keen A, Miles B. Phylogenetic structure of floridian plant communities depends on taxonomic and spatial scale. Ecology. 2006;87:S109–S122. doi: 10.1890/0012-9658(2006)87[109:psofpc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Cerabolini BEL, et al. Can CSR classification be generally applied outside Britain? Plant Ecology. 2010;210:253–261. doi: 10.1007/s11258-010-9753-6. [DOI] [Google Scholar]
- 43.Pierce S, Brusa G, Sartori M, Cerabolini BEL. Combined use of leaf size and economics traits allows direct comparison of hydrophyte and terrestrial herbaceous adaptive strategies. Annals of Botany. 2012;109:1047–1053. doi: 10.1093/aob/mcs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornelissen JHC, et al. Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Functional Ecology. 2004;18:779–786. doi: 10.1111/j.0269-8463.2004.00900.x. [DOI] [Google Scholar]
- 45.Quested HM, et al. Decomposition of sub-arctic plants with differenting nitogen economies: a functional role for hemiparasites. Ecology. 2003;84:3209–3221. doi: 10.1890/02-0426. [DOI] [Google Scholar]
- 46.Cornelissen JHC, Diez PC, Hunt R. Seedling Growth, Allocation and Leaf Attributes in a Wide Range of Woody Plant Species and Types. The Journal of Ecology. 1996;84:755. doi: 10.2307/2261337. [DOI] [Google Scholar]
- 47.Cornelissen JHC, Werger MJA, CastroDiez P. vanRheenen, J. W. A. & Rowland, A. P. Foliar nutrients in relation to growth, allocation and leaf traits in seedlings of a wide range of woody plant species and types. Oecologia. 1997;111:460–469. doi: 10.1007/s004420050259. [DOI] [PubMed] [Google Scholar]
- 48.Cornelissen JHC, et al. Functional traits of woody plants: correspondence of species rankings between field adults and laboratory-grown seedlings? Journal of Vegetation Science. 2003;14:311. doi: 10.1658/1100-9233(2003)014[0311:ftowpc]2.0.co;2. [DOI] [Google Scholar]
- 49.Castro-Díez P, Puyravaud JP, Cornelissen JHC, Villar-Salvador P. Stem anatomy and relative growth rate in seedlings of a wide range of woody plant species and types. Oecologia. 1998;116:57–66. doi: 10.1007/s004420050563. [DOI] [PubMed] [Google Scholar]
- 50.Cornelissen JHC. A triangular relationship between leaf size and seed size among woody species: allometry, ontogeny, ecology and taxonomy. Oecologia. 1999;118:248–255. doi: 10.1007/s004420050725. [DOI] [PubMed] [Google Scholar]
- 51.Cornelissen JHC. An Experimental Comparison of Leaf Decomposition Rates in a Wide Range of Temperate Plant Species and Types. The Journal of Ecology. 1996;84:573. doi: 10.2307/2261479. [DOI] [Google Scholar]
- 52.Cornwell WK, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 53.Preston KA, Cornwell WK, DeNoyer JL. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. New Phytologist. 2006;170:807–818. doi: 10.1111/j.1469-8137.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 54.Cornwell WK, Schwilk DW, Ackerly DD. A trait-based test for habitat filtering: Convex hull volume. Ecology. 2006;87:1465–1471. doi: 10.1890/0012-9658(2006)87[1465:attfhf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Ackerly DD, Cornwell WK. A trait-based approach to community assembly: partitioning of species trait values into within- and among-community components. Ecology Letters. 2007;10:135–145. doi: 10.1111/j.1461-0248.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 56.Cornwell WK, Ackerly DD. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs. 2009;79:109–126. doi: 10.1890/07-1134.1. [DOI] [Google Scholar]
- 57.Craine JM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytologist. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 58.Craine JM, et al. Functional consequences of climate change-induced plant species loss in a tallgrass prairie. Oecologia. 2011;165:1109–1117. doi: 10.1007/s00442-011-1938-8. [DOI] [PubMed] [Google Scholar]
- 59.Craine JM, Towne EG, Ocheltree TW, Nippert JB. Community traitscape of foliar nitrogen isotopes reveals N availability patterns in a tallgrass prairie. Plant and Soil. 2012;356:395–403. doi: 10.1007/s11104-012-1141-7. [DOI] [Google Scholar]
- 60.Tucker SS, Craine JM, Nippert JB. Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere. 2011;2:art48. doi: 10.1890/es11-00023.1. [DOI] [Google Scholar]
- 61.Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC. Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology. 2005;86:12–19. doi: 10.1890/04-1075. [DOI] [Google Scholar]
- 62.Craven D, et al. Between and within-site comparisons of structural and physiological characteristics and foliar nutrient content of 14 tree species at a wet, fertile site and a dry, infertile site in Panama. Forest Ecology and Management. 2007;238:335–346. doi: 10.1016/j.foreco.2006.10.030. [DOI] [Google Scholar]
- 63.Craven D, et al. Seasonal variability of photosynthetic characteristics influences growth of eight tropical tree species at two sites with contrasting precipitation in Panama. Forest Ecology and Management. 2011;261:1643–1653. doi: 10.1016/j.foreco.2010.09.017. [DOI] [Google Scholar]
- 64.Bragazza L. Conservation priority of Italian Alpine habitats: a floristic approach based on potential distribution of vascular plant species. Biodiversity and Conservation. 2009;18:2823–2835. doi: 10.1007/s10531-009-9609-3. [DOI] [Google Scholar]
- 65.Dainese M, Bragazza L. Plant traits across different habitats of the Italian Alps: a comparative analysis between native and alien species. Alpine Botany. 2012;122:11–21. doi: 10.1007/s00035-012-0101-4. [DOI] [Google Scholar]
- 66.de Araujo, A. C. et al. LBA-ECO CD-02 C and N Isotopes in Leaves and Atmospheric CO2, Amazonas, Brazil. Data set. Available on-line [http://daac.ornl.gov] from Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, U.S.A. (2011).
- 67.Royal Botanical Gardens KEW. Seed Information Database (SID). Version 7.1. Available from: http://data.kew.org/sid/ (accessed May 2011). (2008).
- 68.Domingues TF, Berry JA, Martinelli LA, Ometto JPHB, Ehleringer JR. Parameterization of Canopy Structure and Leaf-Level Gas Exchange for an Eastern Amazonian Tropical Rain Forest (Tapajós National Forest, Pará, Brazil) Earth Interactions. 2005;9:1–23. doi: 10.1175/ei149.1. [DOI] [Google Scholar]
- 69.Domingues TF, Martinelli LA, Ehleringer JR. Ecophysiological traits of plant functional groups in forest and pasture ecosystems from eastern Amazônia, Brazil. Plant Ecology. 2007;193:101–112. doi: 10.1007/s11258-006-9251-z. [DOI] [Google Scholar]
- 70.Domingues TF, et al. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant, Cell & Environment. 2010;33:959–980. doi: 10.1111/j.1365-3040.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- 71.Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and Growth Form Variation in the Scaling of Nitrogen and Phosphorus in the Seed Plants. The American Naturalist. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- 72.Fagúndez J, Izco J. Seed morphology of the European species of Erica L. sect. Arsace Salisb. ex Benth. (Ericaceae) Acta Botanica Gallica. 2010;157:45–54. doi: 10.1080/12538078.2010.10516188. [DOI] [Google Scholar]
- 73.Han W, Fang J, Guo D, Zhang Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytologist. 2005;168:377–385. doi: 10.1111/j.1469-8137.2005.01530.x. [DOI] [PubMed] [Google Scholar]
- 74.He J-S, et al. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytologist. 2006;170:835–848. doi: 10.1111/j.1469-8137.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 75.He J-S, et al. Leaf nitrogen:phosphorus stoichiometry across Chinese grassland biomes. Oecologia. 2007;155:301–310. doi: 10.1007/s00442-007-0912-y. [DOI] [PubMed] [Google Scholar]
- 76.Bocanegra K, Fernández F, Galvis J. Grupos funcionales de arboles en bosques secundarios de la region Bajo Calima (Buenaventura, Colombia) Boletín Científico. Centro de Museos. Museo de Historia Natural. 2015;19:17–40. doi: 10.17151/bccm.2015.19.1.2. [DOI] [Google Scholar]
- 77.Fitter AH, Peat HJ. The Ecological Flora Database. The Journal of Ecology. 1994;82:415. doi: 10.2307/2261309. [DOI] [Google Scholar]
- 78.Frenette-Dussault C, Shipley B, Léger J-F, Meziane D, Hingrat Y. Functional structure of an arid steppe plant community reveals similarities with Grime’s C-S-R theory. Journal of Vegetation Science. 2011;23:208–222. doi: 10.1111/j.1654-1103.2011.01350.x. [DOI] [Google Scholar]
- 79.Kichenin E, Wardle DA, Peltzer DA, Morse CW, Freschet GT. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Functional Ecology. 2013;27:1254–1261. doi: 10.1111/1365-2435.12116. [DOI] [Google Scholar]
- 80.Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology. 2010;98:362–373. doi: 10.1111/j.1365-2745.2009.01615.x. [DOI] [Google Scholar]
- 81.Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: what is the link with other resource economics traits. New Phytologist. 2010;186:879–889. doi: 10.1111/j.1469-8137.2010.03228.x. [DOI] [PubMed] [Google Scholar]
- 82.Gallagher RV, Leishman MR. A global analysis of trait variation and evolution in climbing plants. Journal of Biogeography. 2012;39:1757–1771. doi: 10.1111/j.1365-2699.2012.02773.x. [DOI] [Google Scholar]
- 83.Garnier E, et al. Assessing the Effects of Land-use Change on Plant Traits, Communities and Ecosystem Functioning in Grasslands: A Standardized Methodology and Lessons from an Application to 11 European Sites. Annals of Botany. 2007;99:967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pakeman RJ, Lepš J, Kleyer M, Lavorel S, Garnier E. Relative climatic, edaphic and management controls of plant functional trait signatures. Journal of Vegetation Science. 2009;20:148–159. doi: 10.1111/j.1654-1103.2009.05548.x. [DOI] [Google Scholar]
- 85.Pakeman RJ, et al. Impact of abundance weighting on the response of seed traits to climate and land use. Journal of Ecology. 2008;96:355–366. doi: 10.1111/j.1365-2745.2007.01336.x. [DOI] [Google Scholar]
- 86.Fortunel C, et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology. 2009;90:598–611. doi: 10.1890/08-0418.1. [DOI] [PubMed] [Google Scholar]
- 87.Gillison AN, Carpenter G. A generic plant functional attribute set and grammar for dynamic vegetation description and analysis. Functional Ecology. 1997;11:775–783. doi: 10.1046/j.1365-2435.1997.00157.x. [DOI] [Google Scholar]
- 88.Hill, M. O., Preston, C. D. & Roy, D. B. PLANTATT - attributes of British and Irish Plants: status, size, life history, geography and habitats. (Huntingdon: Centre for Ecology and Hydrology, 2004).
- 89.Green, W. USDA PLANTS Compilation, version 1, 09-02-02. (http://bricol.net/downloads/data/PLANTSdatabase/) NRCS: The PLANTS Database (http://plants.usda.gov, 1 Feb 2009). National Plant Data Center: Baton Rouge, LA 70874-74490 USA (2009).
- 90.Guerin GR, Wen H, Lowe AJ. Leaf morphology shift linked to climate change. Biology Letters. 2012;8:882–886. doi: 10.1098/rsbl.2012.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gutiérrez AG, Huth A. Successional stages of primary temperate rainforests of Chiloé Island, Chile. Perspectives in Plant Ecology, Evolution and Systematics. 2012;14:243–256. doi: 10.1016/j.ppees.2012.01.004. [DOI] [Google Scholar]
- 92.Han W, et al. Floral, climatic and soil pH controls on leaf ash content in China’s terrestrial plants. Global Ecology and Biogeography. 2011;21:376–382. doi: 10.1111/j.1466-8238.2011.00677.x. [DOI] [Google Scholar]
- 93.Chen Y, Han W, Tang L, Tang Z, Fang J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography. 2011;36:178–184. doi: 10.1111/j.1600-0587.2011.06833.x. [DOI] [Google Scholar]
- 94.Meng T-T, et al. Responses of leaf traits to climatic gradients: adaptive variation versus compositional shifts. Biogeosciences. 2015;12:5339–5352. doi: 10.5194/bg-12-5339-2015. [DOI] [Google Scholar]
- 95.Prentice IC, et al. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. New Phytologist. 2010;190:169–180. doi: 10.1111/j.1469-8137.2010.03579.x. [DOI] [PubMed] [Google Scholar]
- 96.He T, Pausas JP, Belcher CM, Schwilk DW, Lamont BB. Fire-adapted traits of Pinus arose in the fiery Cretaceous. New Phytologist. 2012;194:751–759. doi: 10.1111/j.1469-8137.2012.04079.x. [DOI] [PubMed] [Google Scholar]
- 97.He T, Lamont BB, Downs KS. Banksias born to burn. New Phytologist. 2011;191:184–196. doi: 10.1111/j.1469-8137.2011.03663.x.. [DOI] [PubMed] [Google Scholar]
- 98.Hickler, T. Plant functional types and community characteristics along environmental gradients on Öland’s Great Alvar (Sweden) Master thesis, University of Lund, Sweden, (1999).
- 99.Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecological Monographs. 2012;82:205–220. doi: 10.1890/11-0416.1. [DOI] [Google Scholar]
- 100.Vergutz, L., Manzoni, S., Porporato, A., Novais, R. F. & Jackson, R. B. A Global Database of Carbon and Nutrient Concentrations of Green and Senesced Leaves Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, USA, 10.3334/ORNLDAAC/1106 (2012).
- 101.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 102.Kattge J, Knorr W, Raddatz T, Wirth C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Global Change Biology. 2009;15:976–991. doi: 10.1111/j.1365-2486.2008.01744.x. [DOI] [Google Scholar]
- 103.Kirkup D, Malcolm P, Christian G, Paton A. Towards a Digital African Flora. Taxon. 2005;54:457. doi: 10.2307/25065373. [DOI] [Google Scholar]
- 104.Koike F. Plant traits as predictors of woody species dominance in climax forest communities. Journal of Vegetation Science. 2001;12:327–336. doi: 10.2307/3236846. [DOI] [Google Scholar]
- 105.Koike, F., Clout, M., Kawamichi, M., De Poorter, M. & Iwatsuki, K. Assessment and Control of Biological Invasion Risks. (Cambridge, UK and Shoukadoh Book Sellers, Kyoto, Japan, and IUCN, Gland, Switzerland, 2006).
- 106.Kraft NJB, Ackerly DD. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecological Monographs. 2010;80:401–422. doi: 10.1890/09-1672.1. [DOI] [Google Scholar]
- 107.Kraft NJB, Valencia R, Ackerly DD. Functional Traits and Niche-Based Tree Community Assembly in an Amazonian Forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- 108.Kühn I, Durka W, Klotz S. BiolFlor - a new plant-trait database as a tool for plant invasion ecology. Diversity and Distribution. 2004;10:363–365. doi: 10.1111/j.1366-9516.2004.00106.x. [DOI] [Google Scholar]
- 109.Otto, B. Merkmale von Samen, Früchten, generativen Germinulen und generativen Diasporen. In: Klotz, S., Kühn, I. & Durka, W. [eds.]: BIOLFLOR - Eine Datenbank zu biologisch-ökologischen Merkmalen der Gefäßpflanzen in Deutschland. Schriftenreihe für Vegetationskunde 38. Bundesamt für Naturschutz, Bonn (2002).
- 110.Kurokawa H, Nakashizuka T. Leaf herbivory and decomposability in a Malaysian tropical rain forest. Ecology. 2008;89:2645–2656. doi: 10.1890/07-1352.1. [DOI] [PubMed] [Google Scholar]
- 111.Guy AL, Mischkolz JM, Lamb EG. Limited effects of simulated acidic deposition on seedling survivorship and root morphology of endemic plant taxa of the Athabasca Sand Dunes in well-watered greenhouse trials. Botany. 2013;91:176–181. doi: 10.1139/cjb-2012-0162. [DOI] [Google Scholar]
- 112.Mishkolz, J. M. Selecting and evaluating native forage mixtures for the mixed grass prairie. (University of Saskatchewan, Saskatoon, SK., 2013).
- 113.Laughlin DC, Leppert JJ, Moore MM, Sieg CH. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Functional Ecology. 2009;24:493–501. doi: 10.1111/j.1365-2435.2009.01672.x. [DOI] [Google Scholar]
- 114.Laughlin DC, Fulé PZ, Huffman DW, Crouse J, Laliberté E. Climatic constraints on trait-based forest assembly. Journal of Ecology. 2011;99:1489–1499. doi: 10.1111/j.1365-2745.2011.01885.x. [DOI] [Google Scholar]
- 115.Fyllas NM, et al. Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences. 2009;6:2677–2708. doi: 10.5194/bg-6-2677-2009. [DOI] [Google Scholar]
- 116.Baker TR, et al. Do species traits determine patterns of wood production in Amazonian forests. Biogeosciences. 2009;6:297–307. doi: 10.5194/bg-6-297-2009. [DOI] [Google Scholar]
- 117.Patiño S, et al. Branch xylem density variations across the Amazon Basin. Biogeosciences. 2009;6:545–568. doi: 10.5194/bg-6-545-2009. [DOI] [Google Scholar]
- 118.Louault F, Pillar VD, Aufrère J, Garnier E, Soussana JF. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. Journal of Vegetation Science. 2005;16:151–160. doi: 10.1111/j.1654-1103.2005.tb02350.x. [DOI] [Google Scholar]
- 119.Malhado ACM, et al. Spatial distribution and functional significance of leaf lamina shape in Amazonian forest trees. Biogeosciences. 2009;6:1577–1590. doi: 10.5194/bg-6-1577-2009. [DOI] [Google Scholar]
- 120.Manning P, Houston K, Evans T. Shifts in seed size across experimental nitrogen enrichment and plant density gradients. Basic and Applied Ecology. 2009;10:300–308. doi: 10.1016/j.baae.2008.08.004. [DOI] [Google Scholar]
- 121.Fry EL, Power SA, Manning P. Trait-based classification and manipulation of plant functional groups for biodiversity-ecosystem function experiments. Journal of Vegetation Science. 2013;25:248–261. doi: 10.1111/jvs.12068. [DOI] [Google Scholar]
- 122.Everwand G, Fry EL, Eggers T, Manning P. Seasonal Variation in the Capacity for Plant Trait Measures to Predict Grassland Carbon and Water Fluxes. Ecosystems. 2014;17:1095–1108. doi: 10.1007/s10021-014-9779-z. [DOI] [Google Scholar]
- 123.Medlyn BE, Jarvis PG. Design and use of a database of model parameters from elevated [CO2] experiments. Ecological Modelling. 1999;124:69–83. doi: 10.1016/s0304-3800(99)00148-9. [DOI] [Google Scholar]
- 124.Medlyn BE, et al. Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant, Cell & Environment. 1999;22:1475–1495. doi: 10.1046/j.1365-3040.1999.00523.x. [DOI] [Google Scholar]
- 125.Medlyn BE, et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytologist. 2001;149:247–264. doi: 10.1046/j.1469-8137.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 126.Meir P, et al. Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant, Cell and Environment. 2002;25:343–357. doi: 10.1046/j.0016-8025.2001.00811.x. [DOI] [Google Scholar]
- 127.Carswell FE, et al. Photosynthetic capacity in a central Amazonian rain forest. Tree Physiology. 2000;20:179–186. doi: 10.1093/treephys/20.3.179. [DOI] [PubMed] [Google Scholar]
- 128.Meir P, Levy PE, Grace J, Jarvis PG. Photosynthetic parameters from two contrasting woody vegetation types in West Africa. Plant Ecology. 2007;192:277–287. doi: 10.1007/s11258-007-9320-y. [DOI] [Google Scholar]
- 129.Mencuccini M. The ecological significance of long-distance water transport: short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant, Cell and Environment. 2003;26:163–182. doi: 10.1046/j.1365-3040.2003.00991.x. [DOI] [Google Scholar]
- 130.Messier J, McGill BJ, Lechowicz MJ. How do traits vary across ecological scales? A case for trait-based ecology. Ecology Letters. 2010;13:838–848. doi: 10.1111/j.1461-0248.2010.01476.x. [DOI] [PubMed] [Google Scholar]
- 131.Milla R, Reich PB. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Annals of Botany. 2011;107:455–465. doi: 10.1093/aob/mcq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Minden V, Kleyer M. Testing the effect-response framework: key response and effect traits determining above-ground biomass of salt marshes. Journal of Vegetation Science. 2011;22:387–401. doi: 10.1111/j.1654-1103.2011.01272.x. [DOI] [Google Scholar]
- 133.Minden V, Andratschke S, Spalke J, Timmermann H, Kleyer M. Plant trait–environment relationships in salt marshes: Deviations from predictions by ecological concepts. Perspectives in Plant Ecology, Evolution and Systematics. 2012;14:183–192. doi: 10.1016/j.ppees.2012.01.002. [DOI] [Google Scholar]
- 134.Moles AT, Falster DS, Leishman MR, Westoby M. Small-seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. Journal of Ecology. 2004;92:384–396. doi: 10.1111/j.0022-0477.2004.00880.x. [DOI] [Google Scholar]
- 135.Moles AT, et al. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences. 2005;102:10540–10544. doi: 10.1073/pnas.0501473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lavergne S, Muenke NJ, Molofsky J. Genome size reduction can trigger rapid phenotypic evolution in invasive plants. Annals of Botany. 2009;105:109–116. doi: 10.1093/aob/mcp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences. 2007;104:3883–3888. doi: 10.1073/pnas.0607324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Givnish TJ, Montgomery RA, Goldstein G. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. American Journal of Botany. 2004;91:228–246. doi: 10.3732/ajb.91.2.228. [DOI] [PubMed] [Google Scholar]
- 139.Moretti M, Legg C. Combining plant and animal traits to assess community functional responses to disturbance. Ecography. 2009;32:299–309. doi: 10.1111/j.1600-0587.2008.05524.x. [DOI] [Google Scholar]
- 140.Niinemets U. Global-Scale Climatic Controls of Leaf Dry Mass per Area, Density, and Thickness in Trees and Shrubs. Ecology. 2001;82:453. doi: 10.2307/2679872. [DOI] [Google Scholar]
- 141.Niinemets Ü. Research review. Components of leaf dry mass per area - thickness and density - alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytologist. 1999;144:35–47. doi: 10.1046/j.1469-8137.1999.00466.x. [DOI] [Google Scholar]
- 142.Ciocarlan, V. The illustrated Flora of Romania. Pteridophyta et Spermatopyta. 1141 (Editura Ceres, 2009).
- 143.Sanda, V., Bita-Nicolae, C. D. & Barabas, N. The flora of spontane and cultivated cormophytes from Romania. (Editura “Ion Borcea”, Bacau, 2003).
- 144.Onoda Y, et al. Global patterns of leaf mechanical properties. Ecology Letters. 2011;14:301–312. doi: 10.1111/j.1461-0248.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- 145.Ordoñez JC, et al. Plant Strategies in Relation to Resource Supply in Mesic to Wet Environments: Does Theory Mirror Nature? The American Naturalist. 2010;175:225–239. doi: 10.1086/649582. [DOI] [PubMed] [Google Scholar]
- 146.Ordoñez, J. C. et al. Leaf habit and woodiness regulate different leaf economy traits at a given nutrient supply. Ecology, 100413130925016, 10.1890/09-1509 (2010). [DOI] [PubMed]
- 147.Pahl AT, Kollmann J, Mayer A, Haider S. No evidence for local adaptation in an invasive alien plant: field and greenhouse experiments tracing a colonization sequence. Annals of Botany. 2013;112:1921–1930. doi: 10.1093/aob/mct246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Paula S, et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology. 2009;90:1420–1420. doi: 10.1890/08-1309.1. [DOI] [Google Scholar]
- 149.Paula S, Pausas JG. Burning seeds: germinative response to heat treatments in relation to resprouting ability. Journal of Ecology. 2008;96:543–552. doi: 10.1111/j.1365-2745.2008.01359.x. [DOI] [Google Scholar]
- 150.Peco B, de Pablos I, Traba J, Levassor C. The effect of grazing abandonment on species composition and functional traits: the case of dehesa grasslands. Basic and Applied Ecology. 2005;6:175–183. doi: 10.1016/j.baae.2005.01.002. [DOI] [Google Scholar]
- 151.Ogaya R, Peñuelas J. Comparative field study of Quercus ilex and Phillyrea latifolia: photosynthetic response to experimental drought conditions. Environmental and Experimental Botany. 2003;50:137–148. doi: 10.1016/s0098-8472(03)00019-4. [DOI] [Google Scholar]
- 152.Ogaya R, Penuelas J. Contrasting foliar responses to drought in Quercus ilex and Phillyrea latifolia. Biologia Plantarum. 2006;50:373–382. doi: 10.1007/s10535-006-0052-y. [DOI] [Google Scholar]
- 153.Ogaya R, Peñuelas J. Tree growth, mortality, and above-ground biomass accumulation in a holm oak forest under a five-year experimental field drought. Plant Ecology. 2006;189:291–299. doi: 10.1007/s11258-006-9184-6. [DOI] [Google Scholar]
- 154.Ogaya R, Peñuelas J. Changes in leaf δ13C and δ15N for three Mediterranean tree species in relation to soil water availability. Acta Oecologica. 2008;34:331–338. doi: 10.1016/j.actao.2008.06.005. [DOI] [Google Scholar]
- 155.Sardans J, Peñuelas J, Ogaya R. Drought-induced changes in C and N stoichiometry in a Quercus ilex Mediterranean forest. Forest Science. 2008;54:513–522. [Google Scholar]
- 156.Sardans, J., Peñuelas, J., Prieto, P. & Estiarte, M. Changes in Ca, Fe, Mg, Mo, Na, and S content in a Mediterranean shrubland under warming and drought. Journal of Geophysical Research113, 10.1029/2008jg000795 (2008).
- 157.Penñelas J, et al. Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Global Change Biology. 2009;16:2171–2185. doi: 10.1111/j.1365-2486.2009.02054.x. [DOI] [Google Scholar]
- 158.Peñuelas J, et al. Higher Allocation to Low Cost Chemical Defenses in Invasive Species of Hawaii. Journal of Chemical Ecology. 2010;36:1255–1270. doi: 10.1007/s10886-010-9862-7. [DOI] [PubMed] [Google Scholar]
- 159.Pierce S, Brusa G, Vagge I, Cerabolini BEL. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Functional Ecology. 2013;27:1002–1010. doi: 10.1111/1365-2435.12095. [DOI] [Google Scholar]
- 160.Pierce S, Ceriani RM, De Andreis R, Luzzaro A, Cerabolini B. The leaf economics spectrum of Poaceae reflects variation in survival strategies. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology. 2007;141:337–343. doi: 10.1080/11263500701627695. [DOI] [Google Scholar]
- 161.Pierce S, Luzzaro A, Caccianiga M, Ceriani RM, Cerabolini B. Disturbance is the principal α-scale filter determining niche differentiation, coexistence and biodiversity in an alpine community. Journal of Ecology. 2007;95:698–706. doi: 10.1111/j.1365-2745.2007.01242.x. [DOI] [Google Scholar]
- 162.Müller SC, Overbeck GE, Pfadenhauer J, Pillar VD. Plant Functional Types of Woody Species Related to Fire Disturbance in Forest–Grassland Ecotones. Plant Ecology. 2006;189:1–14. doi: 10.1007/s11258-006-9162-z. [DOI] [Google Scholar]
- 163.Pillar VD, Sosinski EE. An improved method for searching plant functional types by numerical analysis. Journal of Vegetation Science. 2003;14:323–332. doi: 10.1111/j.1654-1103.2003.tb02158.x. [DOI] [Google Scholar]
- 164.Duarte LdS, Carlucci MB, Hartz SM, Pillar VD. Plant dispersal strategies and the colonization of Araucaria forest patches in a grassland-forest mosaic. Journal of Vegetation Science. 2007;18:847–858. doi: 10.1111/j.1654-1103.2007.tb02601.x. [DOI] [Google Scholar]
- 165.Blanco C, Sosinski E, Santos B, Silva M, Pillar V. On the overlap between effect and response plant functional types linked to grazing. Community Ecology. 2007;8:57–65. doi: 10.1556/comec.8.2007.1.8. [DOI] [Google Scholar]
- 166.Overbeck GE, Müller SC, Pillar VD, Pfadenhauer J. Fine-scale post-fire dynamics in southern Brazilian subtropical grassland. Journal of Vegetation Science. 2005;16:655. doi: 10.1658/1100-9233(2005)016[0655:fpdisb]2.0.co;2. [DOI] [Google Scholar]
- 167.Overbeck GE, Pfadenhauer J. Adaptive strategies in burned subtropical grassland in southern Brazil. Flora - Morphology, Distribution, Functional Ecology of Plants. 2007;202:27–49. doi: 10.1016/j.flora.2005.11.004. [DOI] [Google Scholar]
- 168.Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- 169.Powers JS, Tiffin P. Plant functional type classifications in tropical dry forests in Costa Rica: leaf habit versus taxonomic approaches. Functional Ecology. 2010;24:927–936. doi: 10.1111/j.1365-2435.2010.01701.x. [DOI] [Google Scholar]
- 170.Price CA, Enquist BJ. Scaling of mass and morphology in Dicotyledonous leaves: an extension of the WBE model. Ecology. 2007;88:1132–1141. doi: 10.1890/06-1158. [DOI] [PubMed] [Google Scholar]
- 171.Price CA, Enquist BJ, Savage VM. A general model for allometric covariation in botanical form and function. Proceedings of the National Academy of Sciences. 2007;104:13204–13209. doi: 10.1073/pnas.0702242104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Willis, C. G. et al. Phylogenetic community structure in Minnesota oak savanna is influenced by spatial extent and environmental variation. Ecography, no-no, 10.1111/j.1600-0587.2009.05975.x (2009).
- 173.Reich PB, Oleksyn J, Wright IJ. Leaf phosphorus influences the photosynthesis–nitrogen relation: a cross-biome analysis of 314 species. Oecologia. 2009;160:207–212. doi: 10.1007/s00442-009-1291-3. [DOI] [PubMed] [Google Scholar]
- 174.Reich PB, et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecology Letters. 2008;11:793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 175.Cavender-Bares J, Sack L, Savage J. Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiology. 2007;27:611–620. doi: 10.1093/treephys/27.4.611. [DOI] [PubMed] [Google Scholar]
- 176.Coomes DA, Heathcote S, Godfrey ER, Shepherd JJ, Sack L. Scaling of xylem vessels and veins within the leaves of oak species. Biology Letters. 2008;4:302–306. doi: 10.1098/rsbl.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cornwell WK, Bhaskar R, Sack L, Cordell S, Lunch CK. Adjustment of structure and function of Hawaiian Metrosideros polymorpha at high vs. low precipitation. Functional Ecology. 2007;21:1063–1071. doi: 10.1111/j.1365-2435.2007.01323.x. [DOI] [Google Scholar]
- 178.Dunbar‐Co S, Sporck, Margaret J, Sack L. Leaf Trait Diversification and Design in Seven Rare Taxa of the Hawaiian Plantago Radiation. International Journal of Plant Sciences. 2009;170:61–75. doi: 10.1086/593111. [DOI] [Google Scholar]
- 179.Hao G-Y, Sack L, Wang A-Y, Cao K-F, Goldstein G. Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Functional Ecology. 2010;24:731–740. doi: 10.1111/j.1365-2435.2010.01724.x. [DOI] [Google Scholar]
- 180.Hoof J, Sack L, Webb DT, Nilsen ET. Contrasting Structure and Function of Pubescent and Glabrous Varieties of Hawaiian Metrosideros polymorpha (Myrtaceae) at High Elevation. Biotropica. 2007;0:070606001740001-???. doi: 10.1111/j.1744-7429.2007.00325.x. [DOI] [Google Scholar]
- 181.Martin RE, Asner GP, Sack L. Genetic variation in leaf pigment, optical and photosynthetic function among diverse phenotypes of Metrosideros polymorpha grown in a common garden. Oecologia. 2006;151:387–400. doi: 10.1007/s00442-006-0604-z. [DOI] [PubMed] [Google Scholar]
- 182.Nakahashi CD, Frole K, Sack L. Bacterial Leaf Nodule Symbiosis in Ardisia (Myrsinaceae): Does it Contribute to Seedling Growth Capacity. Plant Biology. 2005;7:495–500. doi: 10.1055/s-2005-865853. [DOI] [PubMed] [Google Scholar]
- 183.Quero JL, et al. Relating leaf photosynthetic rate to whole-plant growth: drought and shade effects on seedlings of four Quercus species. Functional Plant Biology. 2008;35:725. doi: 10.1071/fp08149. [DOI] [PubMed] [Google Scholar]
- 184.Sack L. Responses of temperate woody seedlings to shade and drought: do trade-offs limit potential niche differentiation. Oikos. 2004;107:110–127. doi: 10.1111/j.0030-1299.2004.13184.x. [DOI] [Google Scholar]
- 185.Sack L, Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology. 2006;87:483–491. doi: 10.1890/05-0710. [DOI] [PubMed] [Google Scholar]
- 186.Sack L, Tyree MT, Holbrook NM. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist. 2005;167:403–413. doi: 10.1111/j.1469-8137.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- 187.Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell and Environment. 2003;26:1343–1356. doi: 10.1046/j.0016-8025.2003.01058.x. [DOI] [Google Scholar]
- 188.Sack L, Melcher PJ, Liu WH, Middleton E, Pardee T. How strong is intracanopy leaf plasticity in temperate deciduous trees. American Journal of Botany. 2006;93:829–839. doi: 10.3732/ajb.93.6.829. [DOI] [PubMed] [Google Scholar]
- 189.Scoffoni C, Pou A, Aasamaa K, Sack L. The rapid light response of leaf hydraulic conductance: new evidence from two experimental methods. Plant, Cell & Environment. 2008;31:1803–1812. doi: 10.1111/j.1365-3040.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 190.Sandel, B., Corbin, J. D. & Krupa, M. Using plant functional traits to guide restoration: a case study in California coastal grassland. Ecosphere2, 10.1890/ES10-00175.1 (2011).
- 191.Scherer-Lorenzen M, Schulze E, Don A, Schumacher J, Weller E. Exploring the functional significance of forest diversity: A new long-term experiment with temperate tree species (BIOTREE. Perspectives in Plant Ecology, Evolution and Systematics. 2007;9:53–70. doi: 10.1016/j.ppees.2007.08.002. [DOI] [Google Scholar]
- 192.Schweingruber, F. H. & Landolt, W. The Xylem Database. (Swiss Federal Research Institute WSL, 2005).
- 193.Schweingruber FH, Poschlod P. Growth rings in herbs and shrubs: Life span, age determination and stem anatomy. Forest, Snow and Landscape Research. 2005;79:195–415. [Google Scholar]
- 194.Sheremetev, S. N. Herbs on the soil moisture gradient (water relations and the structural-functional organization). (KMK Scientific Press Ltd, Moscow, 2005).
- 195.Shiodera S, Rahajoe JS, Kohyama T. Variation in longevity and traits of leaves among co-occurring understorey plants in a tropical montane forest. Journal of Tropical Ecology. 2008;24:121–133. doi: 10.1017/s0266467407004725. [DOI] [Google Scholar]
- 196.Shipley B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Functional Ecology. 2002;16:682–689. doi: 10.1046/j.1365-2435.2002.00672.x. [DOI] [Google Scholar]
- 197.Meziane D, Shipley B. Interacting components of interspecific relative growth rate: constancy and change under differing conditions of light and nutrient supply. Functional Ecology. 1999;13:611–622. doi: 10.1046/j.1365-2435.1999.00359.x. [DOI] [Google Scholar]
- 198.McKenna MF, Shipley B. Interacting determinants of interspecific relative growth: Empirical patterns and a theoretical explanation. Écoscience. 1999;6:286–296. doi: 10.1080/11956860.1999.11682529. [DOI] [Google Scholar]
- 199.Shipley B, Vu T-T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytologist. 2002;153:359–364. doi: 10.1046/j.0028-646x.2001.00320.x. [DOI] [Google Scholar]
- 200.Shipley B, Parent M. Germination Responses of 64 Wetland Species in Relation to Seed Size, Minimum Time to Reproduction and Seedling Relative Growth Rate. Functional Ecology. 1991;5:111. doi: 10.2307/2389561. [DOI] [Google Scholar]
- 201.Shipley B, Lechowicz MJ. The functional co-ordination of leaf morphology, nitrogen concentration, and gas exchange in40 wetland species. Écoscience. 2000;7:183–194. doi: 10.1080/11956860.2000.11682587. [DOI] [Google Scholar]
- 202.Pyankov VI, Kondratchuk AV, Shipley B. Leaf structure and specific leaf mass: the alpine desert plants of the Eastern Pamirs, Tadjikistan. New Phytologist. 1999;143:131–142. doi: 10.1046/j.1469-8137.1999.00435.x. [DOI] [Google Scholar]
- 203.Meziane D, Shipley B. Interacting determinants of specific leaf area in 22 herbaceous species: effects of irradiance and nutrient availability. Plant, Cell & Environment. 1999;22:447–459. doi: 10.1046/j.1365-3040.1999.00423.x. [DOI] [Google Scholar]
- 204.Shipley B. Structured Interspecific Determinants of Specific Leaf Area in 34 Species of Herbaceous Angiosperms. Functional Ecology. 1995;9:312. doi: 10.2307/2390579. [DOI] [Google Scholar]
- 205.Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Functional Ecology. 2006;20:21–30. doi: 10.1111/j.1365-2435.2006.01080.x. [DOI] [Google Scholar]
- 206.Vile, D. Significations fonctionnelle et ecologique des traits des especes vegetales: exemple dans une succession post-cultural mediterraneenne et generalisations PhD thesis, Université de Sherbrooke, Sherbrooke (Quebec), (2005).
- 207.Auger, S. L’importance de la variabilité interspécifique des traits fonctionnels par rapport à la variabilité intraspécifique chez les jeunes arbres en forêt mature Msc thesis, Université de Sherbrooke, Sherbrooke (Quebec) (2012).
- 208.Auger S, Shipley B. Inter-specific and intra-specific trait variation along short environmental gradients in an old-growth temperate forest. Journal of Vegetation Science. 2012;24:419–428. doi: 10.1111/j.1654-1103.2012.01473.x. [DOI] [Google Scholar]
- 209.Soudzilovskaia NA, et al. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proceedings of the National Academy of Sciences. 2013;110:18180–18184. doi: 10.1073/pnas.1310700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Elumeeva TG, et al. Long-term vegetation dynamic in the Northwestern Caucasus: which communities are more affected by upward shifts of plant species? Alpine Botany. 2013;123:77–85. doi: 10.1007/s00035-013-0122-7. [DOI] [Google Scholar]
- 211.Spasojevic MJ, Suding KN. Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes. Journal of Ecology. 2012;100:652–661. doi: 10.1111/j.1365-2745.2011.01945.x. [DOI] [Google Scholar]
- 212.Swaine, E. K. Ecological and evolutionary drivers of plant community assembly in a Bornean rain forest PhD thesis, University of Aberdeen, (2007).
- 213.Zheng, W. Silva Sinica: Volume 1-4. (China Forestry Publishing House, Beijing 1983).
- 214.Pan Y, Cieraad E, van Bodegom PM. Are ecophysiological adaptive traits decoupled from leaf economics traits in wetlands. Functional Ecology. 2019;33:1202–1210. doi: 10.1111/1365-2435.13329. [DOI] [Google Scholar]
- 215.Douma JC, Bardin V, Bartholomeus RP, van Bodegom PM. Quantifying the functional responses of vegetation to drought and oxygen stress in temperate ecosystems. Functional Ecology. 2012;26:1355–1365. doi: 10.1111/j.1365-2435.2012.02054.x. [DOI] [Google Scholar]
- 216.van Bodegom PM, Sorrell BK, Oosthoek A, Bakker C, Aerts R. Separating the effects of partial submergence and soil oxygen demand on plant physiology. Ecology. 2008;89:193–204. doi: 10.1890/07-0390.1. [DOI] [PubMed] [Google Scholar]
- 217.Bakker C, Van Bodegom PM, Nelissen HJM, Ernst WHO, Aerts R. Plant responses to rising water tables and nutrient management in calcareous dune slacks. Plant Ecology. 2006;185:19–28. doi: 10.1007/s11258-005-9080-5. [DOI] [Google Scholar]
- 218.Bakker C, Rodenburg J, Van Bodegom PM. Effects of Ca- and Fe-rich seepage on P availability and plant performance in calcareous dune soils. Plant and Soil. 2005;275:111–122. doi: 10.1007/s11104-005-0438-1. [DOI] [Google Scholar]
- 219.Adriaenssens, S. Dry deposition and canopy exchange for temperate tree species under high nitrogen deposition PhD thesis, Ghent University, Ghent, Belgium, (2012).
- 220.Von Holle B, Simberloff D. Testing Fox’s assembly rule: does plant invasion depend on recipient community structure? Oikos. 2004;105:551–563. doi: 10.1111/j.0030-1299.2004.12597.x. [DOI] [Google Scholar]
- 221.Williams M, Shimabokuro YE, Rastetter EB. LBA-ECO CD-09 Soil and Vegetation Characteristics, Tapajos National Forest, Brazil, Dataset. Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, USA. 2012 doi: 10.3334/ORNLDAAC/1104. [DOI] [Google Scholar]
- 222.Wirth, C. & Lichstein, J. W. in Old-Growth Forests 81–113 (Springer Berlin Heidelberg, 2009).
- 223.Fonseca CR, Overton JM, Collins B, Westoby M. Shifts in trait-combinations along rainfall and phosphorus gradients. Journal of Ecology. 2000;88:964–977. doi: 10.1046/j.1365-2745.2000.00506.x. [DOI] [Google Scholar]
- 224.McDonald PG, Fonseca CR, Overton JM, Westoby M. Leaf-size divergence along rainfall and soil-nutrient gradients: is the method of size reduction common among clades? Functional Ecology. 2003;17:50–57. doi: 10.1046/j.1365-2435.2003.00698.x. [DOI] [Google Scholar]
- 225.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 226.Wright IJ, et al. Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: evidence from comparisons across 20 sites. New Phytologist. 2005;169:309–319. doi: 10.1111/j.1469-8137.2005.01590.x. [DOI] [PubMed] [Google Scholar]
- 227.Wright IJ, et al. Relationships Among Ecologically Important Dimensions of Plant Trait Variation in Seven Neotropical Forests. Annals of Botany. 2006;99:1003–1015. doi: 10.1093/aob/mcl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wright JP, Sutton-Grier A. Does the leaf economic spectrum hold within local species pools across varying environmental conditions. Functional Ecology. 2012;26:1390–1398. doi: 10.1111/1365-2435.12001. [DOI] [Google Scholar]
- 229.Wright, S. J. et al. Functional traits and the growth-mortality tradeoff in tropical trees. Ecology, 100514035422098, 10.1890/09-2335 (2010). [DOI] [PubMed]
- 230.Yguel B, et al. Phytophagy on phylogenetically isolated trees: why hosts should escape their relatives. Ecology Letters. 2011;14:1117–1124. doi: 10.1111/j.1461-0248.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- 231.Zanne, A. E. et al. in Data from: Towards a worldwide wood economics spectrum. Dataset, 10.5061/dryad.234 (Dryad, 2009). [DOI] [PubMed]
- 232.Chave J, et al. Towards a worldwide wood economics spectrum. Ecology Letters. 2009;12:351–366. doi: 10.1111/j.1461-0248.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 233.Wang H, et al. The China Plant Trait Database: toward a comprehensive regional compilation of functional traits for land plants. Ecology. 2011;99(2):500–500. doi: 10.1002/ecy.2091. [DOI] [PubMed] [Google Scholar]
- 234.Boyle, B. et al. The taxonomic name resolution service: an online tool for automated standardization of plant names. BMC Bioinformatics14, 10.1186/1471-2105-14-16 (2013). [DOI] [PMC free article] [PubMed]
- 235.The Taxonomic Name Resolution Service [Internet]. iPlant Collaborative. Version 4.0 [Accessed: Sep 2015]. Available from: http://tnrs.iplantcollaborative.org.
- 236.Büntgen U, Psomas A, Schweingruber FH. Introducing wood anatomical and dendrochronological aspects of herbaceous plants: applications of the Xylem Database to vegetation science. Journal of Vegetation Science. 2014;25:967–977. doi: 10.1111/jvs.12165. [DOI] [Google Scholar]
- 237.Page, C. N. The ferns of Britain and Ireland. (Cambridge Univ. Press, 1997).
- 238.Lloyd RM. Spore morphology of the Hawaiian genus Sadleria (Blechnaceae) Am. Fern J. 1976;66:1–7. doi: 10.2307/1546364. [DOI] [Google Scholar]
- 239.Conway E. Spore production in bracken (Pteridium aquilinum (L.) Kuhn) J. Ecol. 1957;45:273–284. doi: 10.2307/2257089. [DOI] [Google Scholar]
- 240.Stoor AM, Boudrie M, Jéröme C, Horn K, Bennert HW. Diphasiastrum oellgaardii (Lycopodiaceae, Pteridophyta), a new lycopod species from Central Europe and France. Feddes Repert. 1996;107:149–157. doi: 10.1002/fedr.19961070302. [DOI] [Google Scholar]
- 241.Shan, H. et al. Trait prediction using hierarchical probabilistic matrix factorization. In J. Langford (Ed.)Proceedings of the International Conference for Machine Learning (ICML). Edinburgh: International Conference on Machine Learning, 1303–1310 (2012).
- 242.Fazayeli F, Banerjee, A., Kattge, J., Schrodt, F. & Reich, P. B. Uncertainty Quantified Matrix Completion using Bayesian Hierarchical Matrix Factorization. 13th International Conference on Machine Learning and Applications (ICMLA), Detroit, USA December 3–6, 10.1109/ICMLA.2014.56 (2014).
- 243.Schrodt, F. et al. BHPMF – a hierarchical Bayesian approach to gap-filling and trait prediction for macroecology and functional biogeography. Global Ecology and Biogeography, 10.1111/geb.12335 (2015).
- 244.Díaz S, 2022. The global spectrum of plant form and function: enhanced species-level trait dataset. TRY File Archive. [DOI] [PMC free article] [PubMed]
- 245.New, M., Hulme, M. & Jones, P. Representing Twentieth-Century Space–Time Climate Variability. Part I: Development of a 1961–90 Mean Monthly Terrestrial Climatology. Journal of Climate12, 829–856, 10.1175/1520-0442(1999)012<0829:RTCSTC>2.0.CO;2 (1999).
- 246.Whittaker, R. J. Communities and Ecosystems. (Macmillan, 1975).
- 247.Weigelt, P., König, C. & Kreft, H. The Global Inventory of Floras and Traits (GIFT) database. Available: http://gift.uni-goettingen.de (2018).
- 248.Weigelt P, König C, Kreft H. GIFT – A Global Inventory of Floras and Traits for macroecology and biogeography. Journal of Biogeography. 2020;47(1):16–43. doi: 10.1111/jbi.13623. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Díaz S, 2022. The global spectrum of plant form and function: enhanced species-level trait dataset. TRY File Archive. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Does not apply.