Abstract
Dr-fimbriated Escherichia coli capable of invading epithelial cells recognizes human decay-accelerating factor (DAF) as its cellular receptor. The role of extracellular domains and the glycosylphosphatidylinositol anchor of DAF in the process of internalization of Dr+ E. coli was characterized in a cell-cell interaction model. Binding of Dr+ E. coli to the short consensus repeat 3 domain of DAF expressed by Chinese hamster ovary cells was critical for internalization to occur. Deletion of short consensus repeat 3 domain or replacement of Ser165 by Leu in this domain, or the use of a monoclonal antibody to this region abolished internalization. Replacing the glycosylphosphatidylinositol anchor of DAF with the transmembrane anchor of membrane cofactor protein or HLA-B44 resulted in abolition or reduction of internalization respectively. Cells expressing glycosylphosphatidylinositol-anchored DAF but not the transmembrane-anchored DAF internalized Dr+ E. coli through a glycolipid pathway, since the former cells were more sensitive to inhibition by methyl-β-cyclodextrin, a sterol-chelating agent. Electron microscopic studies revealed that the intracellular vacuoles containing the internalized Dr+ E. coli were morphologically distinct between the anchor variants of DAF. The cells expressing glycosylphosphatidylinositol-anchored DAF contained a single bacterium in tight-fitting vacuoles, while the cells expressing transmembrane-anchored DAF contained multiple (two or three) bacteria in spacious phagosomes. This finding suggests that distinct postendocytic events operate in the cells expressing anchor variants of DAF. We provide direct evidence for the DAF-mediated internalization of Dr+ E. coli and demonstrate the significance of the glycosylphosphatidylinositol anchor, which determines the ability and efficiency of the internalization event.
Urinary tract infections (UTI) are among the most common bacterial infections in humans, and Escherichia coli is the predominant etiologic agent (14). About 10 to 20% of women and 12% of men experience symptomatic UTI at some point in their lives (14, 19). The characteristic feature of UTI is its marked tendency to recur. About 25% of women with a first episode of UTI have a second episode within 6 months (8). An ascending route of infection is the most common pathogenic mechanism and involves the spontaneous ascent of bacteria from the urethra to the bladder and to the kidneys. Adhesions of uropathogenic E. coli are specific lectin-like structures which are expressed by the bacteria and enable them to attach to and colonize the uroepithelium and to initiate infectious processes (14). Epidemiological studies of clinical isolates from UTI and diarrhea suggest that children and pregnant women are predisposed to infection by the E. coli-expressing Dr family of adhesins. As many as 50% of isolates from children with protracted diarrhea and 25 to 50% of isolates from children with cystitis express Dr adhesins (1, 10, 25), in contrast to 10 to 15% of isolates from adults, especially young healthy women, with cystitis (37, 39). E. coli Dr adhesins are associated with 30% of cases of pyelonephritis in pregnant women, particularly during the third trimester of gestation (26). The expression of Dr adhesins has been associated with a twofold-increased risk of a second episode of UTI (9). The members of the Dr family of adhesins, including Dr, Dr-II, AFA I, AFA III, and F1845, have a similar genetic organization and recognize decay-accelerating factor (DAF or CD55) as their cellular receptor (27, 31).
DAF is a complement-regulatory protein which protects host tissues from damage by the autologous complement system by inhibiting the formation and accelerating the decay of C3 and C5 convertases (24). DAF has a wide tissue distribution; for example, it is present on epithelial surfaces of the gastrointestinal mucosa, exocrine glands, renal pelvis, ureter, bladder, cervix, and uterine mucosa (22). Using an indirect-immunofluorescence technique, we also demonstrated that the purified Dr fimbria binds to these sites, including the renal epithelium in the urinary tract (28). DAF is a 70-kDa glycoprotein and consists of five domains, i.e., four short consensus repeats (SCR1 to SCR4) followed by a serine-threonine-rich (ST-rich) domain, and is attached to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor (24). The other membrane-bound complement receptors of the regulators of complement activation family are membrane cofactor protein (MCP or CD46), and complement receptors 1 and 2 (CR1 or CD35, CR2 or CD21). Recent reports suggest that these complement receptors are targeted as cellular receptors by several bacteria, viruses, and parasites (2, 23, 33). E. coli expressing the Dr family of adhesins was the first described example of a pathogen that targets DAF as its cellular receptor (29). Of particular interest, Dr+ E. coli and other pathogens recognizing DAF are known to cause chronic infections; for example, echoviruses are associated with chronic fatigue syndrome (38) and coxsackieviruses are implicated in the etiology of chronic dilated cardiomyopathy (21), for which the pathogenic mechanism is unclear. Pathogens interacting with the ubiquitously expressed DAF may exploit them for colonization and/or internalization purposes, which may in turn lead to chronicity.
High-density receptor sites for Dr+ E. coli are present in the renal interstitium, a site histologically unavailable to bacteria that infect by an ascending route. We hypothesized that following colonization of the renal epithelium, Dr+ E. coli must invade the epithelial cells to reach deeper tissues (interstitium) rich in receptor sites. Indeed, in a previous report, we demonstrated that Dr+ E. coli was able to invade HeLa epithelial cells (11). We postulated that colonization of the renal interstitium creates a functional basis for the development of chronic infection. The invasive Dr+ E. coli strains were also able to set up a chronic infection in C3H/HeJ mice, resulting in tubulointerstitial nephritis (12). These studies proved that Dr fimbriae are essential virulence factors in the pathogenesis of experimental chronic pyelonephritis. To provide direct evidence for the DAF-mediated internalization of Dr+ E. coli and to molecularly characterize the role of extracellular domains and GPI anchorage of DAF in the pathogenesis of infection, we developed a recombinant model of cell-cell interaction (30). This model consists of well-characterized laboratory cells expressing recombinant molecules of the bacterial ligand (Dr) and the cellular receptor (DAF). Recombinant Chinese hamster ovary (CHO) cells expressing human DAF or its extracellular domain deletion mutants were allowed to interact with a laboratory E. coli K-12 strain expressing Dr fimbriae or its fimbrial mutant to characterize the domains involved in the internalization event. The significance of the GPI-anchored receptor interaction with the pathogen is poorly understood. Hence, using GPI-anchored DAF as a model, we molecularly characterized the role of the GPI anchorage of DAF in the internalization of the interacting bacterium and demonstrated that the GPI anchor is essential for efficient entry, in contrast to the transmembrane (TM) anchors tested.
MATERIALS AND METHODS
Bacteria and recombinant cell lines. (i) Bacterial strains.
The E. coli EC901 strain containing plasmid pBJN406 (BN 406) and its transposon mutant with a mutation in the dra E adhesin subunit (BN 17) were grown on Luria-agar plates containing 25 μg of chloramphenicol per ml. The Dr+ E. coli strain BN 406 and the Dr− E. coli strain BN 17 were collected from plates, suspended in 2% α-methylmannose plus phosphate-buffered saline (PBS), and adjusted to an optical density at 600 nm (OD600) of 2.0. Appropriate amounts of this suspension were added to F12 medium for the invasion studies. The minimal hemagglutination titer (MHT) for Dr+ E. coli was determined using 3% (vol/vol) human group O erythrocytes in 2% α-methylmannose plus PBS. Acceptable MHTs for Dr+ E. coli at different concentrations were 1.5 × 108 CFU/ml = 1/64 titer and 5 × 107 CFU/ml = 1/16 titer.
(ii) CHO cell transfectants expressing human DAF or its domain deletion mutants.
CHO cell transfectant clones that stably express DAF cDNA or various DAF deletion constructs and anchor variants have been previously reported (4, 20). Briefly, CHO cells were transfected with the appropriate recombinant molecule cloned into the expression vector SFFVneo and transfected into CHO cells by lipofection. Positive cells were selected by their resistance to the neomycin analogue G418. Clones were selected by several rounds of sorting by flow cytometry with a polyclonal serum, and limiting dilution was used to produce subclones. Surface expression levels of DAF were analyzed by flow cytometry, as described elsewhere (20). Briefly, mean channel fluorescence was quantitatively compared to assess the relative expression of DAF, using one DAF transfectant as an arbitrary standard. Flow cytometric analysis of DAF levels was performed with monoclonal antibody (MAb) IA10 for DAF followed by fluorescein isothiocyanate-labeled anti-mouse immunoglobulin G, allowing a semiquantitative determination of DAF expression levels. We then isolated a set of DAF clones that express a range of DAF levels (24, 48, and 148%) relative to clone DAF-GPI, whose value was arbitrarily set to 100%. The construction of transmembrane versions of DAF was also described previously (20). DAF/HLA-TM consists of the amino terminus of DAF through amino acid 257 (SCR1 or SCR4) and the carboxyl terminus of HLA-B44 (the membrane-proximal domain with the TM anchor and cytoplasmic tail) from amino acid 66 through the stop codon, representing the four SCR units of DAF on almost the entire HLA-B44 molecule. DAF/MCP-TM consists of the amino terminus of DAF through amino acid 304 (SCR1 to SCR4 and the ST-rich region) and the carboxyl terminus of MCP (TM anchor and cytoplasmic tail) from amino acid 270 through 350, representing the entire extracellular domain of DAF fused with the TM anchor and the cytoplasmic tail of MCP. The recombinant CHO cells were maintained in Geneticin (G418) at 25 μg/ml of medium.
Binding and internalization assay.
Recombinant CHO cells expressing DAF, its domain deletion mutants, or its chimeric TM forms were seeded in 24-well plates at 1.25 × 10–5 cells/well and allowed to grow to subconfluency for 24 h. The monolayer was washed once with F12 medium, and about 500 μl of bacterial suspension in 2% α-methylmannose plus PBS adjusted to a final OD600 of 0.1 or 0.4 was added. After incubation for 3 h at 37°C under 5% CO2, the monolayer was washed twice with F12 plus gentamicin (200 μg/ml) and incubated in the same medium for an additional 1 h to kill the extracellular bacteria and select for the internalized bacteria. The monolayer was then washed twice with F12 medium alone, lysed in 100 μl of lysis buffer, and reconstituted to a final volume of 500 μl with double-distilled H2O. About 25 μl of the lysate was plated on Luria agar plates and incubated overnight at 37°C under 5% CO2. The following day, the colonies were counted and the results were expressed in CFU per well. Each cell line was tested in triplicate. In a parallel binding assay, individual monolayers grown on coverslips were incubated with bacterial suspensions at the above concentration for 1 h at 37°C under 5% CO2. Following incubation, the unbounded bacteria were removed by washing three times with PBS; the monolayer was fixed with ice-cold methanol for 10 min, air dried, and stained with 1:20-diluted Giemsa to visualize bound bacteria. Using a Nikon light microscope (Eclipse 600), 50 individual CHO cells in random fields were viewed under ×60 magnification to count the number of bacteria binding to each cell. The results are expressed as the average number of bacteria binding per cell. Internalization into domain mutants occurred essentially as in the above invasion assay, except that the bacterial suspension was adjusted to an OD600 of 0.1. Internalization into domain mutants is expressed as a percentage of the invasion into the DAF+ cell. DAF anti-SCR3 mouse MAb IH4 and DAF-anti SCR2 mouse MAb BRIC110 were used for inhibition of internalization.
Internalization of purified fimbriae.
Dr fimbrial protein was isolated by heat shock treatment, ammonium sulfate precipitation, and size exclusion chromatography, as described previously (13). All incubations were carried out with the monolayer of recombinant CHO cells expressing DAF at 4°C, unless otherwise noted. About a 1:50 dilution of recombinant Dr fimbriae was incubated for 30 min, washed once, and blocked with 1% bovine serum albumin for 10 min. Rabbit polyclonal anti-Dr antibody was incubated at a 1:20 dilution and washed twice; goat anti-rabbit immunoglobulin G (Sigma) gold conjugate (10 nm) at a 1:20 dilution was incubated for 30 min. After two washes, the cell lines were shifted to 37°C for different times such as 15 min, 1 h, and 3 h. Finally, the samples were prepared for electron microscopy. The recombinant Dr fimbriae were omitted in the control experiment.
Electron microscopy.
For transmission electron microscopy (TEM), 2.5 ml of recombinant cells were grown in 10-cm2 culture dishes at 2.5 × 105 cells/ml, 2.5 ml of a bacterial suspension adjusted to an OD600 of 0.4 was inoculated for 3 h, and the sample was prepared for electron microscopy as described previously (11).
RESULTS
DAF mediates the internalization of Dr+ E. coli.
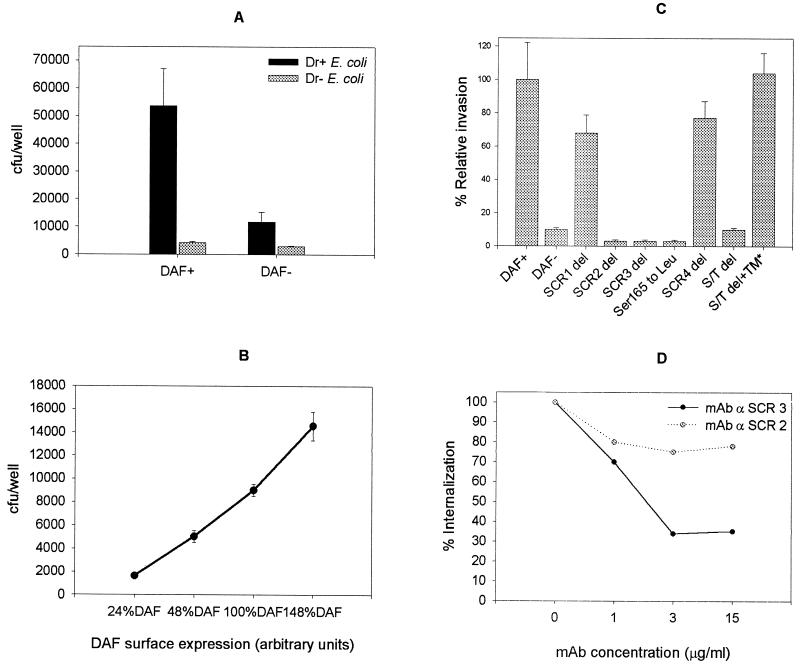
In a previous study, we showed that Dr+ E. coli was able to internalize into a cultured HeLa epithelial cell line in a gentamicin protection assay. Since HeLa cells express a variety of other surface proteins in addition to the abundant DAF expression, we developed a recombinant cell-cell interaction model to demonstrate that human DAF was sufficient and a key factor in causing internalization of Dr+ E. coli. The ability of the recombinant CHO cells expressing human DAF to internalize Dr+ E. coli was tested in a gentamicin protection assay. Nonphagocytic CHO cells expressing human DAF internalized Dr+ but not Dr− E. coli (P ≤ 0.02) (Fig. 1A). Although a low level of internalization of Dr+ E. coli into control CHO cells transfected with the vector only (DAF−) was observed, the difference between the internalization rates into DAF+ and DAF− cells was statistically significant (P ≤ 0.04) (Fig. 1A). The ability of other gram-negative invasive bacteria such as Shigella flexneri and Salmonella enterica serovar Typhimurium to invade recombinant CHO DAF+ and DAF− cells was tested under similar conditions of Dr+ E. coli internalization. Although S. flexneri and S. enterica serovar Typhimurium invaded CHO DAF+ cells at rates 4- and 1.5-fold higher, respectively, than did Dr+ E. coli, they also invaded DAF− cells to an equal extent to their invasion of the DAF+ cells, suggesting that they do not employ DAF as their cellular receptor. To test if the internalization rate of Dr+ E. coli increased proportionally with the amount of surface DAF expressed, we used recombinant CHO cells expressing different levels of DAF in the internalization assay. The clones were selected by flow cytometry, as described in Materials and Methods. A gradual increase in uptake of Dr+ E. coli into recombinant CHO cells, proportional to the amount of surface DAF expressed, was noticed. This finding indicates that the internalization event was a function of DAF receptor density (Fig. 1B).
FIG. 1.
The interaction of Dr+ E. coli with the SCR3 domain of human DAF leads to internalization of the bacterium. The ability of recombinant CHO cells expressing human DAF or its domain deletion mutants to internalize laboratory K-12 E. coli strains expressing Dr fimbriae (Dr+ E. coli) or its mutant (Dr− E. coli) was tested in a gentamicin protection assay. (A and B) Dr+ E. coli but not Dr− E. coli (P < 0.02) cells were internalized into CHO DAF+ but not DAF− cells (P < 0.04) (A) with a gradual increase in internalization with respect to surface DAF expression (B). (C) Extracellular domains SCR2 and SCR3, specifically Ser165 in SCR3, but not SCR1 and SCR4 are important for internalization, while the ST-rich region plays a nonspecific spacing role in the internalization process. (D) Antibodies to the SCR3 domain inhibit internalization, while antibodies to the SCR2 domain are less effective. Each bar in panels A and C represents the mean and standard deviation from three independent experiments, with each assay done in triplicate. S/T del+TM* is the same construct as DAF/HLA-TM.
Binding epitopes on SCR2 and SCR3 are essential for internalization of Dr+ E. coli into CHO DAF+ cells.
DAF is composed of four short consensus repeats (SCR1 to SCR4), an ST-rich region, and a GPI anchor. DAF also carries the Dr (a)+ antigen of the Cromer blood group antigen, which is recognized by the Dr adhesins (29). To understand the contribution of different extracellular domains to the process of DAF-mediated internalization, various DAF deletion constructs were used. Internalization into SCR deletion mutants of DAF indicated that Dr+ E. coli required binding domains SCR3 and SCR2 but not SCR1 or SCR4 for bacterial uptake (Fig. 1C). To further characterize the epitopes recognized by Dr fimbriae on the SCR2 and SCR3 region, we performed inhibition studies with mouse anti-DAF anti-SCR2 and anti-SCR3 MAb. Mouse MAb IH4, which recognizes the SCR3 domain of DAF, inhibited internalization in a dose-dependent fashion, while MAb to SCR2 did not have a significant effect on internalization; this suggests that SCR3 domain plays a critical role in the internalization process (Fig. 1D). The SCR3 domain was further mapped by changing Ser165 to Leu in the SCR3 domain, corresponding to Dra to the Drb allelic polymorphism, which completely abolished both binding and internalization of Dr+ E. coli. Deletion of the ST-rich region (DAFΔS/T) abolished invasion, but internalization was restored when the mutant construct was fused with the membrane-proximal region and the TM anchor and cytoplasmic tail of the HLA-B44 molecule (DAF/HLA-TM); this suggests that the ST-rich region may play a nonspecific role in projecting the DAF molecule to make it accessible to the pathogen (Fig. 1C).
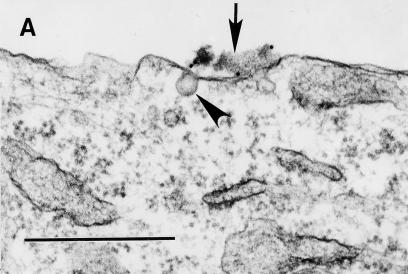
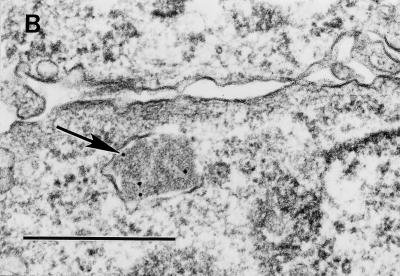
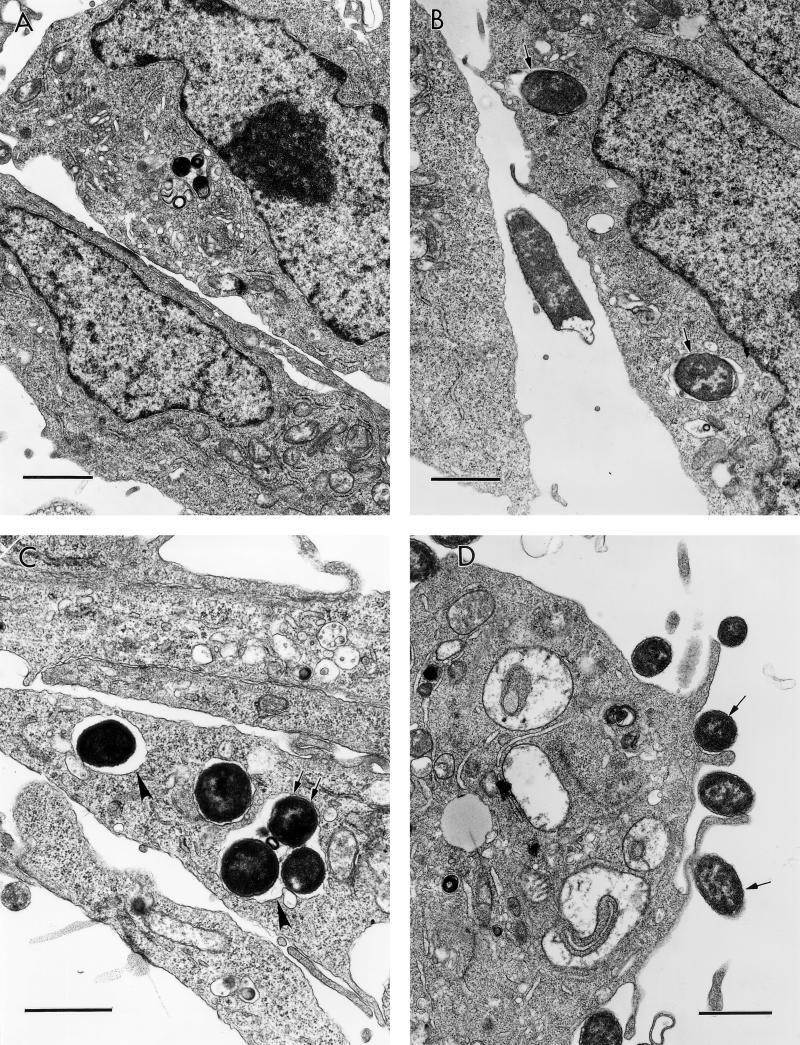
Purified Dr fimbriae induce noncoated microinvaginations in recombinant CHO DAF+ cells, leading to self-internalization.
We have recently shown that initial contact of Dr+ E. coli with HeLa cells caused the clustering of DAF and cytoskeleton rearrangement around the bound bacterium (13). These findings were also reproducible in our recombinant cell-cell interaction system (data not shown) and led us to hypothesize that clustering of DAF initiated by attachment of Dr adhesin may trigger signaling events, which may be transmitted through a GPI anchor, resulting in uptake of the bacterium. It has also been shown that GPI-anchored proteins endocytose preferentially through noncoated microinvaginations of the plasma membrane. To further evaluate the mechanism of entry, we performed TEM evaluation of early cell membrane events induced by interaction of GPI-anchored DAF with Dr fimbriae purified from recombinant E. coli BN 406. Filamentous Dr fimbrial protein alone induced microinvaginations of the plasma membrane, leading to internalization of the fimbriae by noncoated pits as early as 15 min postinfection. Similarly, latex beads coated with Dr fimbriae triggered microinvaginations in the plasma membrane and were able to internalize into the CHO cells expressing human DAF (Fig. 2A to D). This is the first example of a fimbrial protein capable of interacting with complement-regulatory protein and inducing self-uptake by a nonphagocytic mammalian cell.
FIG. 2.
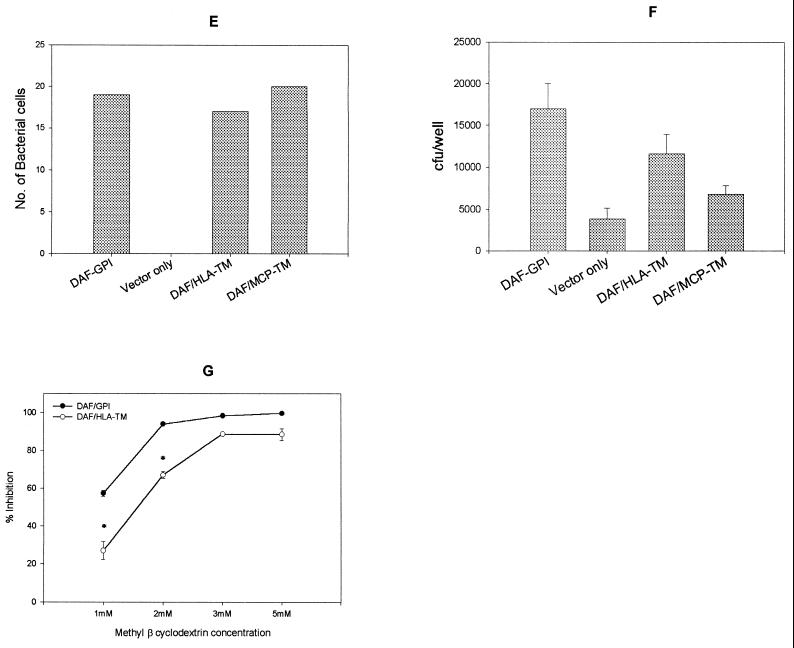
The GPI anchor of DAF allows efficient internalization of purified Dr fimbriae and Dr+ E. coli through a glycolipid pathway. (A to D) Binding of purified Dr fimbriae (arrow) or latex beads coated with Dr fimbriae (arrow) to GPI-anchored DAF causes noncoated microinvaginations (arrowheads), leading to internalization. (E) Dr+ E. coli binds at comparable levels to the recombinant CHO cells expressing DAF-GPI and its chimeric TM anchor constructs. (F) Dr+ E. coli is taken up more efficiently into GPI-anchored DAF than into the TM fusion forms DAF/HLA-TM (P ≥ 0.10) and DAF/MCP-TM (P ≤ 0.004). Each bar represents the mean and standard error of the mean from 10 independent experiments, with each assay done in duplicate. (G) Endocytosis mediated by the GPI-anchored but not the TM version of DAF can be specifically inhibited by the sterol-chelating agent methyl-β-cyclodextrin, suggesting that the two pathways are distinct (∗, P ≤ 0.02). Error bars are the standard error of the mean from three independent experiments. Scale bars, 0.5 μm.
The GPI anchor of DAF allows the most efficient internalization of Dr+ E. coli into CHO cells by the glycolipid pathway.
The anchorage of a receptor often plays a key role in cellular responses to external stimuli. Binding of a pathogen (bacteria) to the extracellular domains of a receptor exploits the host cell signaling cascades and cytoskeletal rearrangements for efficient entry into the cell (7). To understand the efficiency of endocytosis mediated by a GPI anchor and to evaluate its role in mediating the internalization of Dr+ E. coli, we used chimeric fusion forms of DAF that differed in their anchors. We used recombinant CHO cells expressing wild-type DAF with the GPI anchor (DAF-GPI) and chimeric forms of DAF with extracellular domains of DAF fused with the TM anchor and cytoplasmic tails of MCP (DAF/MCP-TM), a closely related complement receptor, or HLA/B44 (DAF/HLA-TM). The GPI and TM fusions had comparable levels of surface DAF expression and equal Dr fimbria binding capacity, as detected by an enzyme-linked immunosorbent assay (data not shown). Further, both the growth rate and the total cellular DAF content detected by Western blotting were comparable among these cell lines. Although the DAF/MCP-TM construct bound Dr+ E. coli to an equal extent to the DAF-GPI and DAF/HLA-TM constructs (Fig. 2E), internalization into the DAF/MCP-TM construct was significantly reduced in comparison to that into DAF-GPI (P ≤ 0.004). The DAF/HLA-TM construct (P ≥ 0.10) permitted lower internalization than did the GPI control; however, the reduced internalization did not reach statistical significance (Fig. 2F). This implied that TM anchor versions of DAF were unable to mediate internalization or less efficient in doing so; the TM anchor of MCP was the least effective and was significantly reduced in its ability to internalize the bound pathogen. It has been suggested that GPI-anchored proteins endocytose through a cholesterol-rich lipid microdomain pathway, a process distinct from that of endocytosis of TM-anchored proteins (5, 16). To test the involvement of cholesterol-rich domains, we evaluated the ability of methyl-β-cyclodextrin, a sterol-chelating agent, to selectively inhibit the internalization mediated by DAF-GPI. Our results indicated that DAF-GPI appeared more sensitive to inhibition of internalization by a sterol-chelating agent than was the TM form tested and suggest the involvement of lipid-rich microdomains in the uptake of Dr+ E. coli (Fig. 2G).
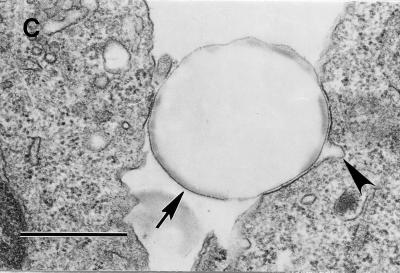
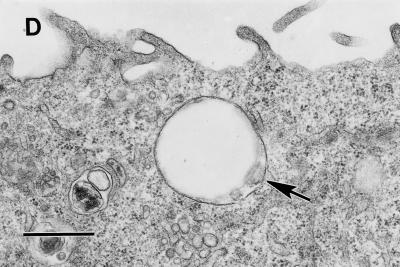
Intracellular vacuoles containing internalized bacteria in the cells expressing GPI and TM-anchored DAF are morphologically distinct.
To provide evidence for the operation of different endocytic pathways among the TM- and GPI-anchored forms of DAF and to analyze the nature of the intracellular compartments entrapping the internalized E. coli, we undertook TEM studies. As early as 1 h following bacterium-CHO cell interaction, most of the intracellular vacuoles in the DAF GPI cells entrapped a single bacterium while about 80% of the internalized bacteria in the DAF/HLA-TM cells were found in multiples (two or three) in each intracellular vacuole. This finding suggests that different endocytic mechanisms or postendocytic events operate in these anchor variants. Numerous extracellularly bound bacteria were seen in DAF/MCP-TM cells, while rarely were any intracellular bacteria found in this cell line (Fig. 3), which supports our findings from the gentamicin protection assay (Fig. 2F).
FIG. 3.
Postendocytic events among the GPI-anchored and TM-anchored DAF direct bacteria to morphologically distinct vacuoles. (A) Dr+ E. coli organisms do not bind or internalize into control CHO cells transfected with vector only (DAF− cells). (B and C) Bound and single internalized Dr+ E. coli organisms (arrow) are seen in tight-fitting phagosomes in the GPI-anchored DAF+ cells (B), while multiple Dr+ E. coli organisms (arrow) are seen in spacious phagosomes (arrowhead) in the DAF/HLA-TM cells (C). (D) Numerous Dr+ E. coli organisms bind but do not internalize into DAF/MCP-TM cells. Scale bars, 1.0 μm.
DISCUSSION
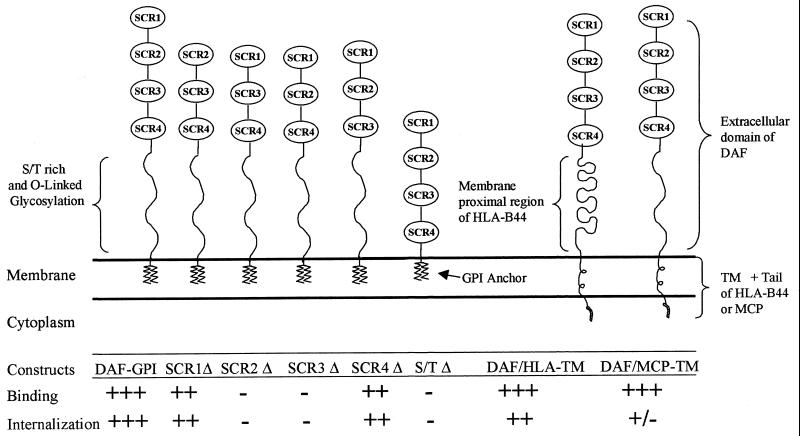
The present study provides direct evidence that DAF serves as a receptor for bacterial entry. By using a cell-cell interaction model consisting of CHO cells expressing human DAF and its mutants interacting with laboratory K-12 E. coli strain expressing Dr fimbriae, we characterized the role of extracellular domains and anchorage of DAF in the internalization of the bacterial pathogen (Fig. 4). The recombinant system enabled us to dissect the receptor-ligand interaction into two discrete but interrelated functional stages, attachment and internalization.
FIG. 4.
Role of DAF domains and anchorage in the binding and internalization of Dr+ E. coli. The recombinant constructs depicted are a hypothetical representation of CHO cells expressing complete human DAF, its extracellular domain deletion mutants, or chimeric DAF molecules (partly adapted from reference 3). The description of constructs is as follows (from left to right): DAF-GPI, CHO cells expressing full-length DAF; SCR1Δ, deletion of the SCR1 domain; SCR2Δ, deletion of the SCR2 domain; SCR3Δ, deletion of the SCR3 domain; SCR4Δ, deletion of the SCR4 domain; S/T Δ, deletion of the ST-rich region; DAF/HLA-TM, extracellular domain of DAF fused with the membrane-proximal region, TM anchor, and cytoplasmic tail of HLA-B44; and DAF/MCP-TM, extracellular domain of DAF fused with the TM anchor and cytoplasmic tail of MCP. The rate of Dr+ E. coli binding and internalization into these recombinant CHO cells is denoted as follows: +++, high; ++, medium; +, low; +/−, very low; −, none. The constructs are not drawn to scale.
Most of the pathogens targeting DAF, including Dr+ E. coli, coxsackieviruses B1, B3, and B5, and echovirus 7, require SCR2 and SCR3 for binding (3, 30, 34). The few exceptions to this scenario are human enteroviruses EV70 and CAV21, which require sequences in SCR1 (15). Molecular modeling of DAF, based on structurally and functionally homologous serum protein factor H, suggests that SCRs are in a helical arrangement with a positively charged surface on SCR2 and SCR3 essential for binding to ligands (convertases and pathogen structures) (17). Based on crystallographic studies, it is evident that these domains may combine to form the Dr binding epitope (18). This is also supported by our findings that although the SCR2 deletion mutant abolished internalization, inhibition with an anti-SCR2 MAb did not effectively block binding or internalization. Currently we are characterizing the Dr-binding epitope on the proposed SCR2 and SCR3 groove to identify the key amino acids involved in the binding and internalization event.
Internalization assays on recombinant CHO DAF+ and control DAF− cells provided direct evidence that human DAF plays a key role in the internalization of Dr+ E. coli into mammalian cells. The evidence for this is deduced from three different approaches: (i) Dr+ E. coli is internalized into CHO DAF+ cells but not into control CHO cells transfected with vector only; (ii) a dose-dependent increase in the uptake of Dr+ E. coli with an increase in the receptor density was found; and (iii) this initial contact of the bacterium with DAF is essential but not sufficient to mediate internalization, since DAF/MCP-TM, in comparison with the DAF-GPI construct, allowed equal binding of Dr+ E. coli but lost its ability to internalize the Dr+ E. coli. The background internalization observed in CHO cells transfected with vector only (DAF−) might represent a random uptake event in response to the interaction of the bacterium with the CHO cell. This is supported by electron microscopic findings which failed to show any Dr+ E. coli organisms specifically interacting with DAF− cells or Dr− E. coli organisms interacting with the DAF+ cells in control experiments.
Our knowledge of apical cellular receptors mediating the entry of bacterial pathogens into host cells is limited. Although integrins are hypothesized to serve as receptors for some of the well-studied enteropathogens such as Shigella, Salmonella, and Yersinia, the basolateral distribution of this molecule on M cells makes the receptor-ligand interaction a secondary or late event. A concerted effort to identify primary receptor(s) on the apical side of M cells is under way. Here we report the identification of a primary receptor (DAF) available on the apical side of the epithelial cells lining the mucosa, which is the initial site of bacterial entry. It is likely that other pathogens use an apical receptor for the initial encounter, but these receptors are unknown, and the Dr-DAF model may serve as a molecular model of the apical receptor-mediated entry.
Interestingly, viruses binding to DAF require coreceptors for their entry (6). Coxsackievirus A21 uses DAF as an attachment receptor but requires intercellular adhesion molecule 1 (ICAM-1) for internalization (35). Interestingly, the entry of coxsackievirus A21 has recently been shown to be mediated by cross-linked DAF (36). In contrast, the multiple adhesins on the Dr fimbriae of E. coli provide multiple binding sites, which enable the bacteria to use DAF as a primary receptor by initially preclustering DAF and subsequently triggering events leading to the entry. This paradigm is also supported by our findings that polystyrene beads coated with the recombinant Dr adhesin are internalized by CHO DAF+ cells.
DAF is the first example of a GPI-anchored protein serving as a cellular receptor for E. coli and several viruses known to cause chronic infections; the pathogenic significance of this finding is unknown. The significance of the GPI anchorage in different cellular events has been an object of intense investigation for several years. It is also noteworthy that the GPI anchor of DAF has been used as a model system to study the endocytosis and intracellular trafficking of the CD4 molecule (16). We designed experiments to determine the importance of the GPI anchorage in the pathogenic interaction. The DAF-GPI form and its chimeric TM constructs (DAF/HLA-TM and DAF/MCP-TM) were tested for their efficiency of uptake of the interacting pathogen. Interestingly, we found that although binding is essential to initiate the internalization process, the ability to internalize the bound bacteria and the efficiency of internalization are controlled by the anchorage of the receptor. These findings may be explained by the ability of the receptor's anchor to effectively relay the extracellular signal to the intracellular milieu, triggering cellular responses that result in uptake of the bacterium. Using a TEM approach, we characterized the endocystosis process operating in these cell lines. We found that the number of internalized bacteria and the nature of the vacuole containing these bacteria were morphologically distinct, suggesting different endocytosis events operating among the anchor variants.
Dr adhesin is an unique example of a virulence factor that is clinically associated with chronic and recurrent infections, as further demonstrated in our experimental model of chronic interstitial nephritis. The present study supports our hypothesis that diverse pathogens such as bacteria and viruses target DAF, a GPI-anchored molecule, for cellular entry to achieve chronicity. Bacteria, viruses, and parasites associated with chronic and/or recurrent infections have evolved an ingenious mechanism to exploit the structural and functional ability of DAF to internalize into cells through a lipid-rich microdomain pathway. This represents a form of complement evasion, since the organisms parasitize a complement-regulatory molecule, such as DAF, for uptake into cells. The selective process of entry mediated by GPI-anchored DAF may increase the chance that the organism will gain access to intracellular sites, thereby representing an evolutionary advantage for the pathogens associated with chronic infections.
ACKNOWLEDGMENTS
We thank David Niesel, Rafeul Alum, and Petri Urvil for helpful comments and discussions. We also appreciate the editorial assistance of Mardelle Susman.
This work was supported by National Institutes of Health grant DK42029 to B.J.N. and a James McLaughlin Foundation Fellowship fund to R.S.
REFERENCES
- 1.Arthur M, Johnson C E, Rubin R H, Arbeit R D, Campanelli C, Kim C, Steinbach S, Agarwal M, Wilkinson R, Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989;57:303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson J P, Krych M, Nickells M, Birmingham D, Subramanian V B, Clemenza L, Alvarez J, Liszewski K. Complement receptors and regulatory proteins: immune adherence revisited and abuse by microorganisms. Clin Exp Immunol. 1994;97(Suppl. 2):1–3. doi: 10.1111/j.1365-2249.1994.tb06251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarkson N A, Kaufman R, Lublin D M, Ward T, Pipkin P A, Minor P D, Evans D J, Almond J W. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J Virol. 1995;69:5497–5501. doi: 10.1128/jvi.69.9.5497-5501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyne K E, Hall S E, Thompson S, Arce M A, Kinoshita T, Fujita T, Anstee D J, Rosse W, Lublin D M. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992;149:2906–2913. [PubMed] [Google Scholar]
- 5.Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans D J, Almond J W. Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol. 1998;6:198–202. doi: 10.1016/s0966-842x(98)01263-3. [DOI] [PubMed] [Google Scholar]
- 7.Finlay B B, Cossart P. Exploitation of Mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 8.Foxman B. Recurring urinary tract infections: incidence and risk factors. Am J Public Health. 1990;80:331–333. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foxman B, Zhang L, Tallman P, Palin K, Rode C, Bloch C, Gillespie B, Marrs C F. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 10.Giron J A, Jones T, Millan-Velasco F, Castro-Munoz E, Zarate L, Fry J, Frankel G, Moseley S L, Baudry B, Kaper J B. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991;163:507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- 11.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki B. Dr fimbria operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion of the HeLa epithelial cell line. J Infect Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 12.Goluszko P, Moseley S, Truong L D, Kaul A, Willi Ford J R, Selvarangan R, Nowicki S, Nowicki B. Development of experimental model of chronic pyelonephritis with E. coli 075:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J Clin Investig. 1997;99:1–11. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goluszko P, Selvarangan R, Popov V, Pham T, Wen J W, Singhal J. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing the Dr family of adhesins. Infect Immun. 1999;67:3989–3997. doi: 10.1128/iai.67.8.3989-3997.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnauchow T M, Dawe S, Lublin D M, Dimock K. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J Virol. 1998;72:9380–9383. doi: 10.1128/jvi.72.11.9380-9383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller G A, Siegel M W, Caras I W. Endocytosis of glycophospholipid-anchored and transmembrane forms of CD4 by different endocytic pathways. EMBO J. 1992;11:863–874. doi: 10.1002/j.1460-2075.1992.tb05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuttner-Kondo L, Medof M E, Brodbeck W, Shoham M. Molecular modeling and mechanism of action of human decay-acceleratingfactor. Protein Eng. 1996;9:1143–1149. doi: 10.1093/protein/9.12.1143. [DOI] [PubMed] [Google Scholar]
- 18.Lea S, Powell R, Evans D. Crystallization and preliminary X-ray diffraction analysis of a biologically active fragment of CD55. Acta Crystallogr Ser D. 1999;55:1198–1200. doi: 10.1107/s0907444999001638. [DOI] [PubMed] [Google Scholar]
- 19.Lipski B A. Urinary tract infections in men: epidemiology, pathophysiology, diagnosis and treatment. Ann Intern Med. 1989;110:138–150. doi: 10.7326/0003-4819-110-2-138. [DOI] [PubMed] [Google Scholar]
- 20.Lublin D M, Coyne K E. Phospholipid anchored and transmembrane versions of either decay accelerating factor or membrane co-factor protein show equal efficiency in protection from complement mediated cell damage. J Exp Med. 1991;174:35–44. doi: 10.1084/jem.174.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martino T A, Liu P, Sole M J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ Res. 1995;74:182–188. doi: 10.1161/01.res.74.2.182. [DOI] [PubMed] [Google Scholar]
- 22.Medof M E, Walter E I, Rutgers J L, Knowles D M, Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987;165:848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moulds J M, Nowicki S, Moulds J J, Nowicki B J. Human blood groups: incidental receptors for viruses and bacteria. Transfusion. 1996;36:362–374. doi: 10.1046/j.1537-2995.1996.36496226154.x. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson-Weller A, Wang C E. Structure and function of decay accelerating factor CD55. J Lab Clin Med. 1994;123:485–491. [PubMed] [Google Scholar]
- 25.Nowicki B, Svanborg E C, Hull R, Hull S. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect Immun. 1989;57:446–451. doi: 10.1128/iai.57.2.446-451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowicki B, Martens M, Hart A, Nowicki S. Gestational age dependent distribution of E. coli fimbriae in pregnant patients with pyelonephritis. Ann N Y Acad Sci. 1994;730:290–291. doi: 10.1111/j.1749-6632.1994.tb44268.x. [DOI] [PubMed] [Google Scholar]
- 27.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated E. coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowicki B, Truong L, Moulds J, Hull R. Presence of Dr receptor in normal human tissues and the possible role in the pathogenesis of ascending urinary tract infections. Am J Pathol. 1988;133:1–4. [PMC free article] [PubMed] [Google Scholar]
- 29.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by E. coli recombinant Dr adhesin in a model of a cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pham T, Kaul A, Hart A, Goluszko P, Moulds J, Nowicki S, Lublin D M, Nowicki B J. dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect Immun. 1995;63:1663–1668. doi: 10.1128/iai.63.5.1663-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pham T, Goluszko P, Popov V, Nowicki, D. S, Nowicki B J. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect Immun. 1997;65:4309–4318. doi: 10.1128/iai.65.10.4309-4318.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seya T. Human regulator of complement activation (RCA) gene family proteins and their relationship to microbial infection. Microbiol Immunol. 1995;39:295–305. doi: 10.1111/j.1348-0421.1995.tb02205.x. [DOI] [PubMed] [Google Scholar]
- 34.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackieviruses B1, B3, and B5 use decay-accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafren D R, Dorahy D J, Ingham R A, Burns G F, Barry R D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafren D R. Virus cell entry induced by cross-linked decay-accelerating factor. J Virol. 1998;72:9407–9412. doi: 10.1128/jvi.72.11.9407-9412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton A, Moseley S, Stamm W E. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991;163:773–779. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- 38.Yousef G E, Bell E J, Mann G F, Murugesan V, Smith D G, McCartney R A, Mowbray J F. Chronic enterovirus infection in patients with post-viral fatigue syndrome. Lancet. 1988;i:146–150. doi: 10.1016/s0140-6736(88)92722-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Foxman B, Tallman P, Cladera E, Le Bouguenec C, Marrs C F. Distribution of drb genes coding for Dr binding adhesins among uropathogenic and fecal Escherichia coli isolates and identification of new subtypes. Infect Immun. 1997;65:2011–2018. doi: 10.1128/iai.65.6.2011-2018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]