Abstract
Background:
Early natural menopause has been regarded as a biomarker of reproductive and somatic aging. Cigarette smoking is the most detrimental factor for lung health and also an established risk factor for early menopause. Understanding the impact of early menopause on health outcomes in mid-life and older female smokers is important to develop preventive strategies.
Objective:
This study aimed to examine the associations of early menopause with multiple lung health and aging biomarkers, lung cancer risk, and all-cause and cause-specific mortality in post-menopausal females who were moderate or heavy smokers.
Study Design:
This study was conducted in post-menopausal females with natural (n=1038) or surgical (n=628) menopause from the Pittsburgh Lung Screening Study (PLuSS). PLuSS is a community-based research cohort of current and former smokers, screened with low-dose computed tomography and followed for lung cancer. Early menopause was defined as occurring before 45 years of age. Analyses were stratified by menopause types due to different biological and medical causes between natural and surgical menopause. Statistical methods include linear model, generalized linear model, linear mixed effects model, and time to event analysis.
Results:
The average age of the 1666 female smokers was 59.4 ± 6.7, with 91.2% of the population as non-Hispanic whites and 63.9% of the population as current smokers at baseline. Overall, 39% of women reported early menopause, including 19.1% of women with natural menopause and 71.3% of women with surgical menopause (P<0.001). Demographic variables did not differ between early and non-early menopause groups regardless of menopause type. Significant associations were identified between early natural menopause and higher risk of wheezing (odds ratio [OR]=1.65, P<0.01), chronic bronchitis (OR=1.73, P<0.01), and radiographic emphysema (OR=1.70, P<0.001), and lower baseline lung spirometry in an obstructive pattern (−104.8 ml/s for forced expiratory volume in the first second [FEV1] with P<0.01, −78.6 ml for forced vital capacity [FVC] with P=0.04, and −2.1% for FEV1/FVC ratio with P=0.01). Early natural menopause was also associated with a more rapid decline of FEV1/FVC ratio (−0.16 %/yr, P=0.01) and incident airway obstruction (OR=2.02, P=0.04). Early natural menopausal women also had a 40% increased risk for death (P=0.023), which was mainly driven by respiratory diseases (hazard ratio [HR]=2.32, P<0.001). Mediation analyses further identified over 33.3% of the magnitude of the associations between early natural menopause and all-cause and respiratory mortality was explained by baseline FEV1. Additional analyses in natural menopausal women identified that the associations between continuous smoking and subsequent lung cancer risk and cancer mortality were moderated by early menopause status, and females with early natural menopause who continued smoking had the worst outcomes (HRs>4.6, Ps<0.001). We did not find associations reported above in female smokers with surgical menopause.
Conclusion:
Early natural menopause is a risk factor for malignant and nonmalignant lung diseases and mortality in mid-life and older female smokers. These findings have strong public health relevance as preventive strategies including smoking cessation and chest computed tomography screening should target this population (i.e., female smokers with early natural menopause) to improve their post-menopausal health and wellbeing.
Keywords: early menopause, smoker, lung aging, lung cancer, mortality
Introduction
Natural cessation of menstruation, i.e., menopause, results from the decline in estrogen levels driven by ovarian aging 1 and initiates the non-reproductive phase of a woman’s life. Natural menopause commonly occurs in women between 45 and 55 years of age. The menopausal transition, during which endogenous production of estrogens is gradually taken over by the adipose tissue, lasts around 5 years 2, 3. Some tissues, including the lung, may contribute appreciably to local estrogen synthesis in the post-menopausal state 4–6. Surgical interventions can also cause or facilitate menopause and patients receiving oophorectomy often need subsequent hormone therapy (HT) to cope with estrogen withdrawal symptoms 7. Studies have identified many health conditions such as cardiovascular diseases and osteoporosis whose risk elevates dramatically in post-menopausal women and estrogen loss has been regarded as the key cause for post-menopausal symptoms and health conditions 8. With increasing life expectancy and greater exposure to estrogen associated with a modern reproductive pattern, i.e., earlier age at menarche, delayed reproduction, and lower fertility 9, it is important to delineate the impact of menopause timing and estrogen exposure on subsequent disease development, which is essential for health maintenance and disease prevention in older females.
Compromised lung health is associated with many aging-related morbidities and mortalities. Cigarette smoking remains the most common and detrimental factor for impairing lung health with the attributing loss of lung function not reversible after smoking cessation. Thus, characterization of host factors that contribute to the impairment of lung health in cigarette smokers is of translational importance because it is imperative for those susceptible subjects to adopt healthier behaviors. Studies from the European Community Respiratory Health Survey identified that: 1) the biochemically confirmed menopause transition (i.e., menstruation – transition – menopause) accelerated age-related decline of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC), however it is unknown whether this accelerated decline pattern would sustain post menopause; 2) post-menopausal women had higher risk of respiratory symptoms and lower FEV1 and FVC than menstruating women; and 3) the impact of menopause on lung function followed a restrictive pattern, i.e., stronger effect on FVC than FEV1 10, 11. Those studies provide evidence supporting immediate and simultaneous effects of waning estrogen accompanying menopause transition on FEV1 and FVC that result in an irreversible loss of lung function through the post-menopausal period. It is important to note that cigarette smoking is also the most established factor for early menopause and its intensity, duration, cumulative dose, and earlier initiation have been all associated with early menopause 12. Thus, it is critical to disentangle the relationship between smoking, menopause and its timing, and health effects. For example, a study in British women identified that menopause exaggerated the effect of cigarette smoking on lung function 13, supporting a potential synergistic effect of cigarette smoking and menopause on lung function.
In line with these findings regarding menopause and lung function, early age at natural menopause was reported associated with higher risk for hospitalization or death in patients with incident chronic obstructive pulmonary disease (COPD) and lower lung function in a restrictive pattern in the UK Biobank 14. A similar pattern affecting lung function for early natural menopause was also observed in the Tasmanian Longitudinal Health Study 15. Significant associations between early menopause and all-cause mortality were also consistently reported in the literature 16–21. In cause-specific mortality analyses, early menopause was associated with an increased mortality for cardiovascular diseases (CVD) but reduced mortality for breast, uterine, and ovary cancers 18, 19. It is hypothesized that early menopause could be a marker of accelerated reproductive and somatic aging (e.g., osteoporosis, compromised immune function, and suboptimal musculoskeletal function) that underlies the increased mortality in post-menopausal women 17, 22–25.
Given that evidence-based interventions for mid-life and older female current and former smokers may improve morbidity and mortality, we aimed to assess 1) the associations between early menopause and lung health biomarkers including respiratory symptoms, radiographic emphysema, bronchitis, and longitudinal spirometry; 2) the associations between early menopause and lung cancer risk, as well as all-cause and cause-specific mortality, in 1666 post-menopausal women from the Pittsburgh Lung Screening Study (PLuSS) who were moderate to heavy smokers. We hypothesized that early menopause is associated with worse lung health outcomes and higher lung cancer incidence and mortality in current and former smokers.
Methods
Study population
The PLuSS cohort is a community-based research cohort of current and former smokers, screened with low-dose computed tomography (CT) and followed for lung cancer 26. Between 2002 and 2005, 3,642 participants were enrolled into the PLuSS. Eligibility criteria were: 50–79 years old; smoked half pack cigarettes per day or more for at least 25 years; if quit, smoking cessation was no more than 10 years prior to enrollment; and no personal history of lung cancer. Upon study entry, participants completed a questionnaire, provided a blood sample, and underwent low-dose CT screening and spirometry. Participants underwent a repeat low-dose CT screening approximately one year later. The questionnaires covered demographics, smoking history, respiratory conditions and symptoms, comorbidities, and for women, menstrual and reproductive history. The current analysis includes female PluSS participants who reported natural (n=1038) or surgical (n=628) menopause status at study entry.
Menstrual and reproductive history
Menstrual and reproductive history were collected in baseline questionnaire through the following questions: Q27 “Are you still having menstrual periods? 1: No; 2: Yes”; Q28 “How old were you when you had your last period? 1, Less than 40 years; 2, 40–44 years; 3, 45–49 years; 4, 50–54 years; 5, 55 years or older”; Q29 “Did your periods stop because of natural menopause, surgery, radiation, or drug therapy? 1, Natural menopause; 2, Surgery; 3, Radiation; 4, Drug therapy”; Q38 “Sometimes women take female hormones such as estrogen or progesterone around the time of menopause. Have you ever used female hormones (tablets, pills, or creams) for menopause. 1: No; 2: Yes”. Early menopause was defined as age at menopause (AAM) <45 years.
Outcomes of interest
Variables from baseline questionnaire:
Self-reported respiratory symptoms (i.e., cough, phlegm, wheezing, and dyspnea), and physician diagnosed cardio-pulmonary comorbidities (i.e., coronary heart disease/heart attack, stroke, and coronary artery bypass or angioplasty), and chronic bronchitis.
Radiographic emphysema score from baseline CT scan:
Based on the National Emphysema Treatment Trial criteria, baseline chest CT scans (n=1606) were evaluated through visual scoring for emphysema presence and severity, with ordinal scores for no, trace, mild, moderate, and severe emphysema, the latter four categories roughly corresponding to emphysema affecting less than 10%, 10–25%, 25–50%, and higher than 50% of the lung, respectively 27. Because few subjects (n=20) had severe emphysema, this group was combined with moderate group for data analysis.
Spirometry and related outcomes:
All 1666 female smokers received spirometry at baseline. Spirometry was conducted in accordance with American Thoracic Society standards. Among the 1666 study subjects, 824 subjects had 2 or more spirometry tests due to their enrollment into PLuSS-X (n=389) and the PLuSS Supplemental Sample Collection (n=435). The PLuSS-X protocol was implemented in 2006 and ended in 2016 and enrolled 977 PLuSS men and women judged to be at the highest risk for lung cancer who underwent CT screening every other year and spirometry every year, and also yearly provided a blood and sputum sample (through 2016). Subjects eligible for PLuSS-X had to satisfy at least one of the following criteria: (a) 2.5% or greater 5-year cumulative lung cancer risk (Bach model); (b) moderate or severe structural emphysema on the baseline low-dose CT; (c) moderate or severe airflow obstruction on the baseline spirometry; (d) two or more first degree relatives had lung cancer diagnosis; and (e) moderate or high suspicion of lung nodule on both baseline and 1-year later repeated low-dose CT scan with delinquent diagnostic follow-up. Eligibility criteria for the PLuSS Supplemental Sample Collection (2011–2016) include PLuSS participant, no lung cancer detected, not lost to follow up (or death), and not already part of PLuSS-X. The PLuSS Supplemental Sample Collection aimed to 1) update questionnaire-based risk factor information, 2) collect and store blood, 3) collect and store sputum, 4) retest pulmonary function, 5) measure exhaled carbon monoxide, and 6) repeat a low dose CT for lung cancer screening among those not followed under the PLuSS-X protocol. We analyzed the distribution of several important variables between female smokers with ≥2 spirometry [n=824] versus those with one spirometry [n=842]. Percentage of surgical menopause is 35.6% in subjects with two or more spirometry tests versus 39.8% in those with only one spirometry test (P=0.08). Percentage of early menopause is 38.0% in subjects with two or more spirometry tests versus 40.0% in those with only one spirometry test (P=0.51). No statistically significant differences were identified for baseline values of age (P=0.57), smoking status (P=0.66), BMI (P=0.10), and spirometry (FEV1 [P=0.78], FVC [P=0.15], and FEV1/FVC ratio [P=0.12]) between these two groups. Airway obstruction was defined as FEV1/FVC ratio <70% according to the Global Initiative for Chronic Obstructive Lung Disease 28. Incident airway obstruction was defined as newly diagnosed airway obstruction among those (n=485) without airway obstruction at baseline.
LC incidence and Mortality:
Incident lung cancer was identified through CT screenings, National Death Index, obituary data or by self-report from study subjects or their next of kin. Pathology reports were reviewed to collect date, stage, and histology of lung cancer diagnosis. Mortality data was acquired from the National Death Index, obituary data, annual follow-up contact, and letters or calls from family members with May 2020 as the cut-off date. Death certificates were reviewed to collect dates and causes of death.
Longitudinal smoking status (LSS)
Participants were followed annually to update their smoking status and cancer diagnosis with May 2020 as the last follow-up date and were classified into continuous, former, and intermittent smokers as described in Supplemental Materials.
Statistical analysis
All analyses were conducted in natural and surgical menopause separately due to different biological and medical causes between natural and surgical menopause. Variables were summarized by early menopause status using statistics based on their nature (i.e., categorical vs continuous) and distribution (i.e., skewed vs normal) and were compared using parametric or non-parametric methods for continuous variables and Chi-square for categorical variables (Table 1).
Table 1.
Baseline variables and longitudinal smoking status in PLuSS female smokers by menopause type and early menopause status
| Variable | Natural menopause |
Surgical menopause |
||||||
|---|---|---|---|---|---|---|---|---|
| Sub-total | Early | Non-early | P1 | Sub-total | Early | Non-early | P1 | |
| N | 1038 | 198 | 840 | 628 | 448 | 180 | ||
| Age (yr, mean ± SD) | 59.3 ± 6.8 | 59.6 ± 7.4 | 59.2 ± 6.6 | 0.49 | 59.6 ± 6.5 | 59.6 ± 6.3 | 59.5 ± 6.9 | 0.76 |
| BMI (kg/m2, mean ± SD) | 28.2 ± 5.8 | 28.2 ± 5.9 | 28.2 ± 5.7 | 0.96 | 28.8 ± 6.1 | 29.0 ± 6.1 | 28.2 ± 6.2 | 0.16 |
| BMI group (n, %) | 0.95 | 0.077 | ||||||
| Normal/underweight | 333, 32.1 | 65, 32.8 | 268, 31.9 | 186, 29.6 | 121, 27.0 | 65, 36.1 | ||
| Overweight | 382, 36.8 | 71, 35.9 | 311, 37.0 | 225, 35.8 | 167, 37.3 | 58, 32.2 | ||
| Obesity | 323, 31.1 | 62, 31.3 | 261, 31.1 | 217, 34.6 | 160, 35.7 | 57, 31.7 | ||
| Current smoker (n, %) | 654, 63.0 | 128, 64.7 | 526, 62.6 | 0.60 | 410, 65.3 | 294, 65.6 | 116, 64.4 | 0.78 |
| Packyears (mean ± SD) | 45.6 ± 19.5 | 46.2 ± 18.3 | 45.5 ± 19.8 | 0.47 | 47.1 ± 20.4 | 47.5 ± 20.6 | 46.0 ± 19.9 | 0.26 |
| Ethnicity (n, %) | 0.037 | 0.97 | ||||||
| Non-Hispanic White | 959, 92.4 | 175, 88.4 | 784, 93.3 | 560, 89.2 | 399, 89.1 | 161, 89.4 | ||
| African American | 57, 5.5 | 15, 7.6 | 42, 5.0 | 60, 9.6 | 43, 9.6 | 17, 9.4 | ||
| Others2 | 22, 2.1 | 8, 4.0 | 14, 1.7 | 8, 1.3 | 6, 1.3 | 2, 1.1 | ||
| Ever HT (n, %) | 627, 60.5 | 123, 62.1 | 504, 60.1 | 0.60 | 501, 79.9 | 355, 79.4 | 146, 81.1 | 0.63 |
| Emphysema score (n, %) | 0.003 | 0.78 | ||||||
| None | 597, 59.8 | 97, 50.5 | 500, 62.0 | 368, 60.5 | 265, 61.1 | 103, 59.2 | ||
| Trace | 164, 16.4 | 30, 15.6 | 134, 16.6 | 114, 18.8 | 81, 18.7 | 33, 19.0 | ||
| Mild | 136, 13.6 | 36, 18.8 | 100, 12.4 | 82, 13.5 | 55, 12.7 | 27, 15.5 | ||
| Moderate and severe | 101, 10.1 | 29, 15.1 | 72, 8.9 | 44, 7.2 | 33, 7.6 | 11, 6.3 | ||
| FEV1 (L/s, mean ± SD) | 2.07 ± 0.58 | 1.92 ± 0.59 | 2.11 ± 0.57 | <.0001 | 2.00 ± 0.55 | 1.99 ± 0.55 | 2.03 ± 0.55 | 0.40 |
| FVC (L, mean ± SD) | 2.92 ± 0.65 | 2.77 ± 0.64 | 2.96 ± 0.64 | 0.0003 | 2.82 ± 0.63 | 2.81 ± 0.62 | 2.86 ± 0.65 | 0.38 |
| FEV1/FVC ratio (%, mean ± SD) | 70.4 ± 11.0 | 68.6 ± 12.5 | 70.8 ± 10.5 | 0.013 | 70.4 ± 10.5 | 70.2 ± 10.6 | 70.7 ± 10.3 | 0.61 |
| Cancer history (n, %) | 110, 10.6 | 19, 9.6 | 91, 10.8 | 0.61 | 101, 16.1 | 74, 16.5 | 27, 15.0 | 0.64 |
| Reproductive cancer history (n, %) | 10, 1.0 | 3, 1.5 | 7, 0.8 | 0.38 | 34, 5.4 | 25, 5.6 | 9, 5.0 | 0.77 |
| Longitudinal smoking status (n, %) | 0.24 | 0.66 | ||||||
| Continuous smoker | 370, 35.7 | 79, 39.9 | 291, 34.6 | 237, 37.7 | 169, 37.7 | 68, 37.8 | ||
| Former smoker | 446, 43.0 | 84 42.4 | 362, 43.1 | 266, 42.4 | 186, 41.5 | 80, 44.4 | ||
| Intermittent smoker | 222, 21.4 | 35, 17.7 | 187, 22.3 | 125, 19.9 | 93, 20.8 | 32, 17.8 | ||
BMI: body mass index; SD: standard deviation; HT: hormone therapy; FEV1: forced expiratory volume in first second; FVC: forced vital capacity.
One-way ANOVA for continuous variables and Chi-square test for categorical variables were used to obtain P values. Mantel-Haenszel Chi-Square was used for BMI group and radiographic emphysema.
Others include Hispanic, Asian, Pacific Islander, American Indian, or Alaskan Native.
A logistic model was used to assess the associations between early menopause (early vs non-early menopause) and HT (ever vs never) and respiratory symptoms (e.g., cough, phlegm, wheeze, and dyspnea) and lung comorbidities (e.g., chronic bronchitis, airway obstruction, and emphysema score) at baseline. A linear model was used to associate early menopause and HT with spirometry at baseline. Linear mixed-effects (LME) model with a subject-specific random intercept and slope was used to assess the effect of early menopause and HT on spirometry decline by including interaction terms with time-in-cohort (TIC). Cox proportional hazards models were used to assess the effect of early menopause and HT on lung cancer risk and mortality based on all-cause, lung cancer, all cancer, CVD, and respiratory diseases. When assessing disease-specific mortality, death due to other or unknown causes was treated as competing risk. Covariates adjusted in above models included age at enrollment, ethnicity (African American and Others with non-Hispanic white [NHW] as the reference), BMI group (BMI 25–30 and BMI ≥30 with BMI <25 as the reference), baseline smoking status (for baseline endpoints), LSS (former and intermittent smokers with continuous smokers as the reference and for long-term health outcomes), and baseline packyears. Height was additionally adjusted in models with spirometry as outcome. For endpoints associated with early menopause status with P values <0.05, we further assessed whether the associations with AAM groups follow a monotonically increasing or decreasing trend. AAM was coded as 1 for AAM>50yr, 2 for AAM 45–49yr, 3 for AAM 40–44yr, and 4 for AAM<40yr, and was included in the trend test modeling as a continuous variable. We further calculate the estimates and P values for individual AAM categories (i.e., AAM 45–49yr, AAM 40–44yr, and AAM<40yr) with AAM>50yr as the reference to better understand the monotonically increasing or decreasing trends. We are aware of the smaller sample size for age at natural menopause <40yr (n=41), thus we code the most common group, i.e., AAM>50 yr (n=471) as ‘1’ or reference group for the trend test. Because of concerns for multiple comparisons, we mainly focused on results with P values <0.01 for early menopause and/or those with P values for trend tests <0.05. Mediation analysis was conducted based on an established method 29 to explore the mediational effect of baseline spirometry on associations between early menopause and mortality. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study subject characteristics
The average age of the 1666 female smokers from the PLuSS cohort was 59.4 ± 6.7, with 91.2% of the population as NHWs and 7.0% as Blacks. 63.9% of the population was current smokers with an average packyears of 46.1 ± 19.8 at baseline. Overall, 39% of women in the sample reported early menopause, including 19.1% of women with natural menopause and 71.3% of women with surgical menopause (Table 1). Compared to females with natural menopause, females with surgical menopause had higher rate of HT use (79.9% vs 60.5%, P<0.001), higher percentage of African American (9.1% vs 5.2%, P<0.01), higher personal cancer history (16.1% vs 10.6%, P<0.01), and higher reproductive cancer history (5.4% vs 1.0%, P<0.001). Comparisons between early and non-early menopause groups did not identify any significant differences in demographic variables (age, BMI, smoking status and pack-years) regardless of menopause types.
Associations of early menopause with health conditions
Early menopause was associated with self-reported wheeze (OR=1.65, 95%CI=1.18–2.30, P<0.01), physician diagnosed chronic bronchitis (OR=1.73, 95%CI=1.19–2.53, P<0.01) and CVD (OR=1.87, 95%CI=1.13–3.11, P=0.02), radiographic emphysema (OR=1.70, 95%CI=1.25–2.31, P<0.001), and incident airway obstruction (OR=2.02, 95%CI=1.03–3.96, P=0.04) in females with natural menopause (Table 2), but not in females with surgical menopause except for physician diagnosed cardiovascular diseases ((OR=4.03, 95%CI=1.78–9.14, P<0.001, Supplemental Table 1). Trend tests using AAM groups were further conducted for these five endpoints in females with natural menopause and identified monotonically increasing risk for ever chronic bronchitis, radiographic emphysema, and CVD associated with younger AAM (Supplemental Table 2).
Table 2.
Early vs. non-early menopause and health conditions in females with natural menopause (n=1038)
| Variable (n, %) | Early (n=198) | Non-early (n=840) | OR (95%CI)1 | P |
|---|---|---|---|---|
| Baseline respiratory symptoms | ||||
| Phlegm | 101, 51.0 | 414, 49.3 | 1.07 (0.76, 1.49) | 0.71 |
| Cough | 91, 46.0 | 319, 38.0 | 1.37 (1.00, 1.89) | 0.05 |
| Wheeze | 79, 40.1 | 244, 29.1 | 1.65 (1.18, 2.30) | <0.01 |
| Dyspnea | 97, 49.0 | 369, 43.9 | 1.21 (0.87, 1.67) | 0.25 |
| Baseline cardio-pulmonary comorbidities | ||||
| Ever chronic bronchitis | 48, 24.2 | 131, 15.6 | 1.73 (1.19, 2.53) | <0.01 |
| Radiographic emphysema2 | 95, 49.5 | 306, 38.0 | 1.70 (1.25, 2.31) | <0.001 |
| Airway obstruction | 88, 44.4 | 319, 38.1 | 1.31 (0.94, 1.83) | 0.11 |
| Ever cardiovascular diseases3 | 26, 13.1 | 60, 7.1 | 1.87 (1.13, 3.11) | 0.01 |
| Incident airway obstruction4 | 22, 40.7 | 71, 27.4 | 2.02 (1.03, 3.96) | 0.04 |
OR: odds ratio
The associations were adjusted for baseline values of age, smoking status, packyears, overweight or obesity status (dummy variables with non-early BMI as the reference), ethnicity (two dummy variables for African American or other ethnicities with non-Hispanic white [NHW] as the reference), and history of hormone therapy.
Events for emphysema were summed as having radio-graphical evidence of emphysema (Trace, Mild, Moderate to Severe) at baseline CT screening. Ordered logistic regression was used for modeling emphysema risk under the proportional odds assumption.
Cardiovascular diseases were defined as having history of heart attack, stroke, or coronary artery bypass or angioplasty.
Incident airway obstruction was defined as newly diagnosed airway obstruction among those without airway obstruction at baseline. Analyzed among 54 and 259 females with early and non-early menopause, respectively.
Associations of early menopause with lung function at baseline and its decline overtime
Early menopause was associated with lower measurements of FEV1 (−104.8 ± 36.7 ml/s, P<0.01), FVC (−78.6 ± 38.2 ml, P=0.04), and FEV1/FVC ratio (−2.1 ± 0.8%, P=0.01) at baseline in females with natural menopause (Table 3), but not in those with surgical menopause (Supplemental Table 3). Monotonically decreasing levels of FEV1, FVC, and FEV1/FVC ratio at baseline were identified to be associated with younger AAM in females with natural menopause (Supplemental Table 4). A total of 3974 spirometry tests were obtained from 824 female smokers with longitudinal spirometry across an average of 9.6 years (Supplemental Table 5). We found early menopause was associated with accelerated decline of FEV1/FVC ratio by 44.4% (0.16/0.36) in females with natural menopause (Table 3). A monotonic pattern for more rapid decline of the FEV1/FVC ratio associated with younger AAM was identified though the P value for trend test is of borderline significance (P=0.06, Supplemental Table 4).
Table 3.
Associations between early menopause and spirometry in females with natural menopause (n=1038)
| Analysis | Estimate ± SE | P |
|---|---|---|
| Baseline spirometry1 | ||
| FEV1 (ml/s) | −104.8 ± 36.7 | <0.01 |
| FVC (ml) | −78.6 ± 38.2 | 0.04 |
| FEV1/FVC ratio (%) | −2.1 ± 0.8 | 0.01 |
| Decline of FEV1 (ml, est ± SE)2 | ||
| Early menopause | −131.6 ± 51.8 | 0.01 |
| TIC | −31.9 ± 3.6 | <0.001 |
| Early menopause*TIC | 0.83 ± 2.28 | 0.72 |
| Decline of FVC (ml, est ± SE)2 | ||
| Early menopause | −103.8 ± 54.4 | 0.05 |
| TIC | −32.5 ± 4.70 | <0.001 |
| Early menopause*TIC | 3.96 ± 2.95 | 0.18 |
| Decline of FEV1/FVC ratio (%, est ± SE)2 | ||
| Early menopause | −2.62 ± 1.15 | 0.02 |
| TIC | −0.36 ± 0.10 | <0.001 |
| Early menopause*TIC | −0.16 ± 0.06 | 0.01 |
SE: standard error; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; TIC: time in cohort.
Linear models were conducted to model the outcome of baseline spirometry in 1038 females. Covariates for adjustment included baseline values of age, smoking status, packyears, height, overweight or obesity status (dummy variables with non-early BMI as the reference), ethnicity (two dummy variables for African American or other ethnicities with NHWs as the reference), and history of hormone therapy.
Linear mixed-effects (LME) models were conducted among 103 females with early menopause and 428 females with non-early menopause with 2 or more spirometry tests. Covariates for adjustment included baseline values of age, packyears, height, overweight or obesity status (dummy variables with non-early BMI as the reference), ethnicity (two dummy variables for African American or other ethnicities with NHWs as the reference), and history of hormone therapy and LSS status (former and intermittent smokers with continuous smokers as the reference). Interaction terms of TIC with early menopause, hormone therapy, and LSS status were included in the LME models.
Associations of early menopause with lung cancer risk and mortality
During the follow-up period, a total of 170 incident lung cancer cases (10.2%) and 447 deaths (26.8%) were identified among the 1666 study participants. Early natural menopause was associated with a 40% increased risk for all-cause death with respiratory diseases (hazard ratio [HR]=2.32, 95%CI=1.52–3.52, P<0.001) as the major driver underlying this association (Table 4). Trend tests identified monotonically increasing all-cause and cancer- and respiratory disease-specific mortality associated with younger AAM in females with natural menopause (Supplemental Table 6). Among females with natural menopause, early menopause was associated with twice the risk for lung cancer-specific mortality (HR=1.94, 95%CI=1.05–3.58, P=0.03) and a trend towards increased lung cancer incidence (HR=1.51, 95%CI=0.93–2.44, P=0.09) (Table 4). Similar trends were also observed for lung cancer incidence (HR=1.56, 95%CI=0.92–2.66, P=0.09), lung cancer-specific (HR=1.97, 95%CI=0.87–4.47, P=0.11) and respiratory (HR=1.57, 95%CI=0.89–2.80, P=0.12) mortality in females with surgical menopause (Supplemental Table 7).
Table 4.
Early menopause and lung cancer risk and mortality in females with natural menopause (n=1038)
| Variable | Menopause | N | Event (n, %) | Person-years | HR (95%CI)1 | P |
|---|---|---|---|---|---|---|
| LC incidence | Early | 198 | 23, 11.6 | 2420.2 | 1.51 (0.93, 2.44) | 0.09 |
| Non-early | 840 | 67, 8.0 | 10931.3 | |||
| All-cause mortality | Early | 198 | 63, 31.8 | 2565.1 | 1.40 (1.05, 1.86) | 0.02 |
| Non-early | 840 | 200, 23.8 | 11490.8 | |||
| LC mortality | Early | 198 | 15, 7.6 | 2565.1 | 1.94 (1.05, 3.58) | 0.03 |
| Non-early | 840 | 34, 4.1 | 11490.8 | |||
| Cancer mortality | Early | 198 | 27, 13.6 | 2565.1 | 1.74 (1.11, 2.74) | 0.01 |
| Non-early | 840 | 67, 8.0 | 11490.8 | |||
| CVD mortality | Early | 198 | 21, 10.6 | 2565.1 | 1.38 (0.83, 2.29) | 0.21 |
| Non-early | 840 | 62, 7.4 | 11490.8 | |||
| Respiratory mortality | Early | 198 | 35, 17.7 | 2565.1 | 2.32 (1.52, 3.52) | <0.001 |
| Non-early | 840 | 65, 7.7 | 11490.8 |
LC: lung cancer; CVD: cardiovascular diseases; HR: hazard ratio; CI: confidence interval.
Cox regression was used to assess the impact of early menopause on risk for lung cancer incidence and all-cause and disease-specific mortality with adjustment for baseline values of age, packyears, overweight or obesity status (dummy variables with non-early BMI as the reference), ethnicity (two dummy variables for African American or other ethnicities with NHWs as the reference), and history of hormone therapy and LSS status (former and intermittent smokers with continuous smokers as the reference).
Mediation effect of baseline spirometry on the associations between early natural menopause and mortality
Because we observed significant associations between early menopause and baseline spirometry as well as mortality among those with natural menopause, and poor lung function is an established factor for mortality, mediation analyses were conducted to evaluate potential mediational effects by baseline spirometry on the associations between early menopause and mortality (Table 5). FEV1, FVC, and FEV1/FVC ratio all had significant mediational effects (all Pperm values <0.05). Large mediational effect sizes were observed for all-cause mortality (proportion mediated = 42.4%) and respiratory mortality (proportion mediated = 33.3%) with FEV1 as the strongest mediators.
Table 5.
Mediation effect of baseline spirometry on the associations between early menopause and mortality in females with natural menopause
| Outcome | Mediator | Early menopause-Outcome (β [SE], P)1 | Mediation effect | ||
|---|---|---|---|---|---|
|
| |||||
| Without mediator | With mediator | PM2 | Pperm3 | ||
| Mortality (All-cause) | FEV1 | 0.33 (0.15), 0.02 | 0.19 (0.15), 0.20 | 42.4% | <0.01 |
| FVC | 0.24 (0.15), 0.11 | 27.3% | <0.01 | ||
| FEV1/FVC | 0.25 (0.15), 0.09 | 24.2% | <0.01 | ||
| Mortality (LC) | FEV1 | 0.66 (0.31), 0.03 | 0.55 (0.32), 0.08 | 16.7% | <0.01 |
| FVC | 0.56 (0.32), 0.07 | 15.2% | <0.01 | ||
| FEV1/FVC | 0.63 (0.31), 0.04 | 4.5% | 0.013 | ||
| Mortality (Cancer) | FEV1 | 0.55 (0.23), 0.01 | 0.47 (0.23), 0.04 | 14.5% | <0.01 |
| FVC | 0.48 (0.23), 0.03 | 12.7% | <0.01 | ||
| FEV1/FVC | 0.51 (0.23), 0.02 | 7.3% | <0.001 | ||
| Mortality (Respiratory) | FEV1 | 0.84 (0.21), <0.001 | 0.56 (0.22), 0.01 | 33.3% | <0.01 |
| FVC | 0.68 (0.22), <0.01 | 19.0% | <0.01 | ||
| FEV1/FVC | 0.65 (0.22), <0.01 | 22.6% | <0.01 | ||
LC: lung cancer; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; SE: standard error; PM: proportion mediated.
Early menopause was modeled in relation to mortality with and without adjustment for potential mediators using Cox regression. Covariates for adjustment included baseline values of age, packyears, overweight or obesity status (dummy variables with non-early BMI as the reference), ethnicity (two dummy variables for African American or other ethnicities with NHWs as the reference), and history of hormone therapy and LSS status (former and intermittent smokers with continuous smokers as the reference).
Proportion mediated following the formula PM=100*(β - β’)/β, where β is total effect of early menopause and β’ is the effect estimate of early menopause when controlling for baseline spirometry.
Pperm is derived after permutation for 200 times. For associations with PM <10%, permutation was conducted for 1000 times to generate the Pperm.
Ever HT and the outcomes
Ever HT use as a binary variable was included in all above analyses that assessed the impacts of early menopause on health outcomes. In females with natural menopause, ever HT use (versus never HT use) was significantly associated with higher baseline FEV1 levels (58.0 ± 29.6 ml/s, P=0.05) and higher FEV1/FVC ratio (1.36 ± 0.66 %, P=0.03) at baseline. In females with surgical menopause, ever HT use (versus never HT use) was significantly associated with higher baseline FEV1 levels (107.6 ± 44.6 ml/s, P=0.01), higher FEV1/FVC ratio (1.89 ± 0.97 %, P=0.05), and lower rate of airway obstruction (OR=0.65, 95%CI=0.43–0.99, P=0.04) at baseline, and lower respiratory mortality (HR=0.61, 95%CI=0.37–0.99, P=0.04).
Interaction between early natural menopause and cigarette smoking or BMI group
We further assessed whether there were interactions between early natural menopause and BMI group or smoking status (baseline smoking status for baseline outcomes and LSS for longitudinal outcomes) on wheezing, bronchitis, emphysema, spirometry, lung cancer incidence, and all-cause, lung cancer-, all cancer- and respiratory mortality. All P values for interaction terms were >0.2 except for interactions between early natural menopause and LSS for affecting lung cancer incidence (P=0.06) and cancer mortality (P=0.01). Four combinations were formed including non-continuous smoking & non-early menopause, non-continuous smoking & early menopause, continuous smoking & non-early menopause, and continuous smoking & early menopause. Kaplan-Meier curve showed that females with early menopause who continued smoking had the highest lung cancer risk and cancer-specific mortality compared to other three combinations. With females with non-continuous smoking & non-early menopause as the reference, hazard ratios for lung cancer were 0.87 (95%CI=0.39–1.96, P=0.73) for non-continuous smoking & early menopause, 2.05 (95%CI=1.26–3.35, P<0.01) for continuous smoking & non-early menopause, and 4.65 (95%CI=2.55–8.49, P<0.001) for continuous smoking & early menopause. With females with non-continuous smoking & non-early menopause as the reference, hazard ratios for cancer mortality are 0.91 (95%CI=0.43–1.95, P=0.81) for non-continuous smoking & early menopause, 1.55 (95%CI=0.94–2.56, P=0.09) for continuous smoking & non-early menopause, and 4.61 (95%CI=2.63–8.07, P<0.001) for continuous smoking & early menopause.
Comment
Principal findings
In a cohort of mid-life and older post-menopausal female smokers, we found that early natural menopause was associated with worse lung health (i.e., chronic bronchitis, radiographic emphysema, and lower FEV1/FVC ratio), accelerated lung aging (i.e., incident airway obstruction and a more rapid decline of FEV1/FVC ratio), and higher all-cause and respiratory mortality. Females with early natural menopause who continued smoking had dramatically increased risk (>4.5-fold) for lung cancer and cancer-specific mortality. However, the above associations were not observed with surgical menopause.
Results in the context of what is known
This is the first time that early natural menopause was identified to be associated with more severe lung parenchymal destruction (radiographic emphysema). The impact of early natural menopause on lung spirometry is quite large as the differences of baseline spirometry measurements between early and non-early menopause were equivalent to age-related lung function decline in 2.2 (FVC), 3.1 (FEV1), and 6.2 (FEV1/FVC ratio) years. These equivalents were calculated as the differences between early and non-early natural menopause (Table 3, e.g., −2.1% for FEV1/FVC ratio) divided by the age-related decline rates (Supplemental Table 5, e.g., 0.34%/yr for FEV1/FVC ratio). We also identified that early natural menopause was associated with incident airway obstruction and a more rapid decline of FEV1/FVC ratio. In contrast to previous studies that identified a restrictive pattern (a stronger effect on FVC than FEV1) of menopause related effects on spirometry 10, 11, 15, we found the impact of early menopause on spirometry followed an obstructive pattern with larger effect on FEV1 than FVC which resulted in the identification of a significant association between early menopause and FEV1/FVC ratio. This difference is likely due to our study of moderate and heavy smokers. Thus, these findings provide strong evidence that early natural menopause is a risk factor for development of COPD in female smokers.
Time to event analyses identified a significant association between early menopause and all-cause mortality in female smokers with natural menopause. Cause-specific analyses further identified that lung cancer and respiratory diseases may be the causes of deaths driving this association. This is different from previous studies which showed CVD as the main driver for the association between early natural menopause and all-cause mortality 18, 19. Likewise, the different result could be due to our study of moderate and heavy smokers. Mediation analyses further identified over 36% of the magnitude of the associations between early natural menopause and all-cause and respiratory mortality could be explained by FEV1, further substantiating the importance of maintaining lung health in female smokers with early natural menopause.
The rates of early natural menopause were reported to be less than 10% in general populations 12. The early natural menopause rate in PluSS females who were moderate or heavy smokers is 19.1% which is consistent with a 2-fold increase in risk of early menopause in current smokers 12. Similarly, in a pilot study of smokers (n=318) from the Lovelace Smokers Cohort, early menopause occurred in 19.0% of the 179 subjects with natural menopause. However, although the associations between smoking and early menopause have been established 12, we did not identify any differences for smoking history between early and non-early menopause probably due to the enrollment of moderate and heavy smokers in the PLuSS cohort. A recent genome-wide association study in over 200K women of European ancestry identified 290 genetic determinants of natural ovarian aging assessed as age at natural menopause with DNA damage response as the top pathway implicated 30. Thus, the occurrence of early natural menopause in female smokers as a biomarker of ovarian aging could reflect the outcome of ovarian injury and repair under the chronic insults of toxicants in tobacco smoke 12. This is in consistency with the findings that cigarette smoking was associated with a prolonged and dose-dependent adverse effect on ovarian function 31, 32. Our identification of associations with lung health outcomes may further support early natural menopause as a biomarker predictive of aging of extra-ovarian organs such as lungs in female smokers potentially due to pan-organ host – environment interactions.
Interactions between cigarette smoking and menopause or early menopause have been reported. A study in British women identified that menopause exaggerated the effect of cigarette smoking on lung function 13. A recent meta-analysis of six cohort studies and five case-control studies also found convincing evidence supporting increased lung cancer risk associated with early menopause predominantly in smokers 33. We found that the impacts of continuous smoking on subsequent lung cancer risk and cancer mortality are moderated by early menopause status with more prominent associations found in females with early natural menopause. These studies suggested that females with early natural menopause were most vulnerable to cigarette smoking induced health effects including lung cancer. This could be due to early loss of lung health protection from estrogen or simply reflect lower inherent defense capacity against cigarette smoke induced injury. The later explanation is more favored because this interaction was only observed in females with natural menopause. Moreover, the significant interactions between early menopause and cigarette smoking identified in our studies and others 13, 33 do not support early natural menopause as merely a proxy for heavier smoking. This is also consistent with the fact that a substantial proportion of the variability (40 – 75%) in age at natural menopause is explained by genetic factors in twin and family studies (including population based) 34–37.
Clinical implications
Early menopause suggests a shorter lifetime “exposure” to endogenous hormones (e.g., estrogen) associated with female reproductive cycling. Age at baseline was comparable between early and non-early menopause groups in our study. Thus, our findings suggest that ovarian hormone production has a protective effect on lung health and mortality in moderate and heavy smokers at least in those with natural menopause. Two mechanisms are likely to explain the estrogen protection on lung health. First, reduced circulating estrogen accompanying the menopause transition results in osteoporosis and suboptimal musculoskeletal function which may mechanically weaken the expansion and contraction capacity of the thoracic cage during pulmonary ventilation 22–24. Second, female reproductive hormone metabolism, receptors, and signaling are active in various lung cells including bronchial epithelium, fibroblasts, and airway smooth muscle cells. Hormones (e.g., estrogen) associated with female reproductive cycling are important to maintain optimal lung physiology for lowering airway resistance (e.g., bronchodilation) 38, 39 and clearance defense (e.g., mucociliary clearance) 40, 41. Thus, premature loss of pulmonary protection from the natural cycling hormones in females due to early menopause may result in elevated vulnerability to adverse health outcomes induced by cigarette smoking. Our findings of ever HT use associated with greater baseline FEV1 levels in post-menopausal women regardless of menopause types partially support the “shorter lifetime estrogen exposure” hypothesis. HT use was also reported to be associated with slower decline rates of FEV1 and FVC in a duration-dependent manner 42. However, HT does not provide physiological cycling of sex steroids and does not entirely replicate the pre-menopausal state. Extended HT beyond coping with menopause symptoms has documented negative consequences, including reduced survival from lung cancer, and current guidelines recommend hormone replacement for a period of 2–5 years only 43–45.
The National Lung Screening Trial demonstrated that smoking cessation and annual low dose CT screening together offered the most reduction of lung cancer-specific mortality in heavy smokers 46–49. Our findings that females with early natural menopause who continued smoking had dramatically (>4.5-fold) increased lung cancer risk and cancer-specific mortality, suggesting that smoking cessation and lung cancer screening interventions should especially target this group. Smoking cessation is warranted both before menopause to prevent early menopause, and after menopause to prevent further health risks especially in females with early natural menopause.
Research implications
It is important to note that majority of the associations seen in female smokers with natural menopause were not observed in female smokers with surgical menopause in this study. However, sample size of female smokers with surgical menopause in the PLuSS is relatively smaller. Moreover, a subset of female smokers with surgical menopause may still have ovarian estrogen secretion due to receiving hysterectomy only. We expect that associations would differ in female smokers with bilateral oophorectomy versus hysterectomy with or without partial oophorectomy. Testing this hypothesis requires a large cohort of female smokers with detailed surgery information collected. Moreover, association analyses in female smokers with bilateral oophorectomy would further test the “shorter lifetime estrogen exposure” hypothesis.
Strengths and limitations
The PLuSS cohort enabled us to investigate impacts of early menopause on lung health and aging sequela in a large cohort of female current and former smokers. Moreover, this is the first time to report an association between early natural menopause and more severe lung parenchymal destruction and identify lung cancer and respiratory disease as the main driver for early menopause associated mortality in female smokers. An approximate 92.6% PLuSS participants are non-Hispanic whites and this is consistent with the fact that populations from southwestern Pennsylvania are predominantly non-Hispanic whites. Thus it is unknown how well our findings could be generalized to female smokers of Hispanic or Black ethnicity who consume less cigarettes but have higher lung disease susceptibility 50, 51. Although the 824 female smokers with 2 or more spirometry tests are not a random sample of the PLuSS cohort, similar distribution of key variables at baseline (e.g., menopause type, early menopause rate, age, smoking status, BMI, and spirometry) between these subjects and those with only baseline spirometry minimizes the selection bias and supports a high likelihood of generalization of the findings (i.e., early natural menopause is associated with incident airway obstruction and FEV1/FVC decline) to other female moderate and heavy smokers.
Conclusions
Our study provides evidence supporting early natural menopause as a risk factor for malignant and nonmalignant lung diseases and mortality in mid-life and older female smokers. Our studies have strong public health relevance as preventive strategies including smoking cessation and chest CT screening 46, 47 should target this population (i.e., female smokers with early natural menopause) to improve their post-menopausal health and wellbeing. More specifically, early natural menopause status should be considered when improving the criteria or risk assessment models for recommendation of CT screening of lung cancer in female smokers.
Supplementary Material
Figure 1. Interactions between early natural menopause and continuous smoking for affecting lung cancer incidence and cancer mortality.
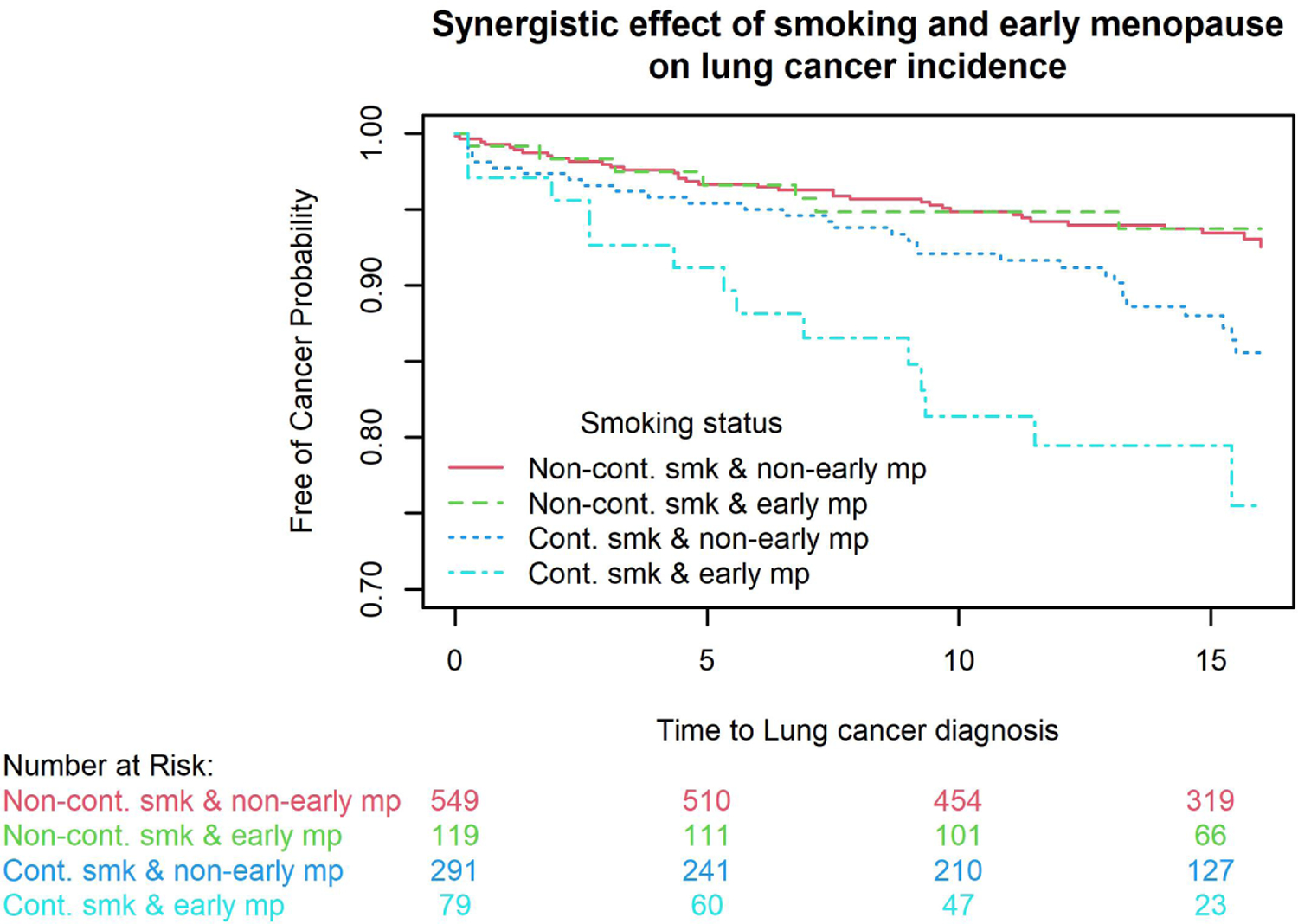
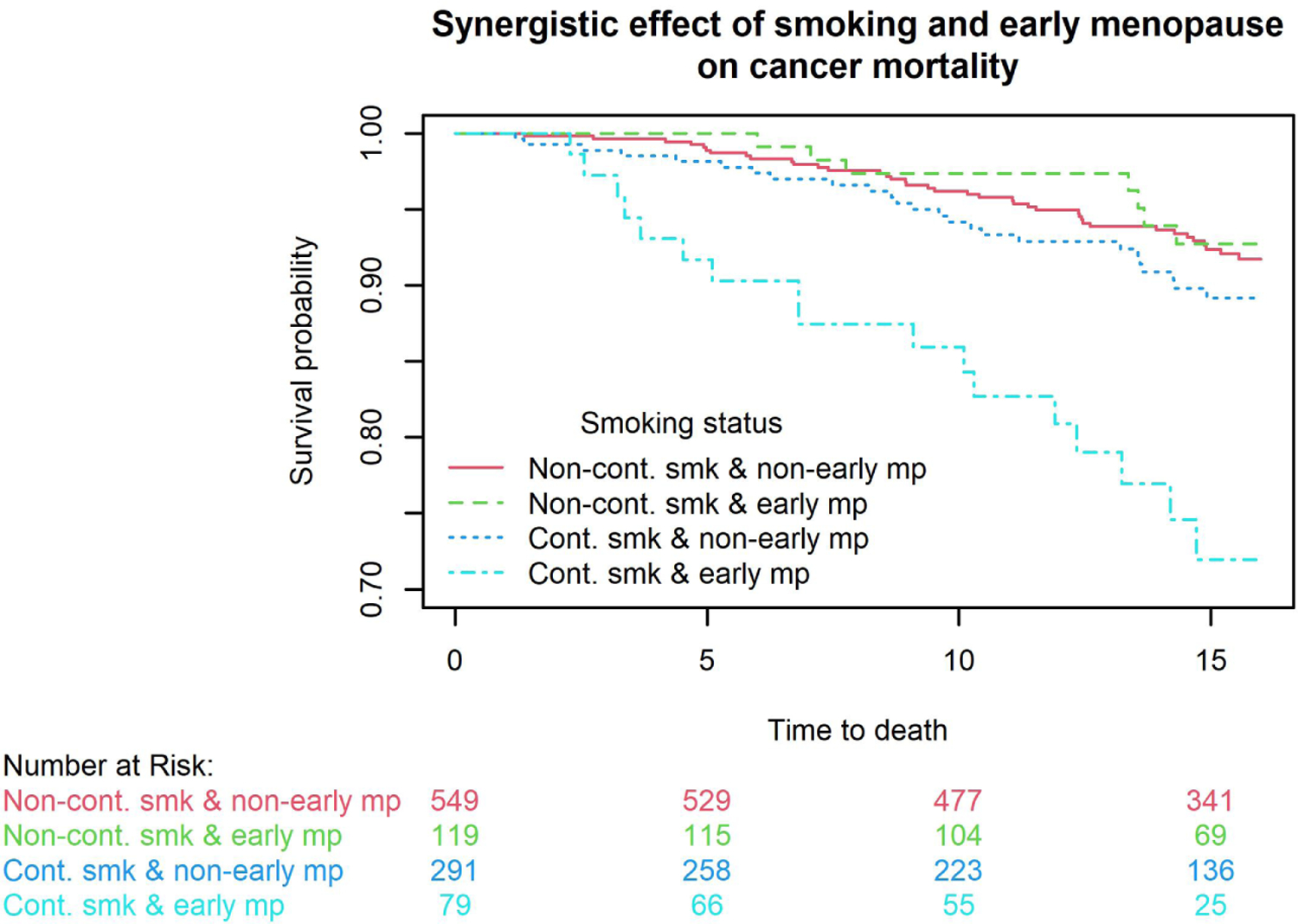
Kaplan-Meier curve showed that females with early menopause who continued smoking had the highest lung cancer risk (1A) and cancer mortality (1B) compared to other three combinations. With females with non-continuous smoking & non-early menopause as the reference, hazard ratios for lung cancer were 0.87 (95%CI=0.39–1.96, P=0.73) for non-continuous smoking & early menopause, 2.05 (95%CI=1.26–3.35, P<0.01) for continuous smoking & non-early menopause, and 4.65 (95%CI=2.55–8.49, P<0.001) for continuous smoking & early menopause. With females with non-continuous smoking & non-early menopause as the reference, hazard ratios for cancer mortality are 0.91 (95%CI=0.43–1.95, P=0.81) for non-continuous smoking & early menopause, 1.55 (95%CI=0.94–2.56, P=0.09) for continuous smoking & non-early menopause, and 4.61 (95%CI=2.63–8.07, P<0.001) for continuous smoking & early menopause.
Condensation.
Female smokers with early natural menopause have accelerated lung aging and should be targeted for smoking cessation and lung cancer screening
AJOG at a Glance.
A. Why was this study conducted?
Cigarette smoking is the most detrimental factor for lung health. Early natural menopause is a biomarker of reproductive and somatic aging. Understanding the impact of early menopause on health outcomes in mid-life and older female smokers is important to develop preventive strategies.
B. What are the key findings?
Early natural menopause was associated with worse lung health, accelerated lung aging, and higher all-cause and respiratory mortality. Females with early natural menopause who continued smoking had dramatically (>4.5-fold) increased risk for lung cancer and cancer-specific mortality.
C. What does this study add to what is already known?
The impacts of early menopause on lung health and aging sequela were rarely studied in female smokers. This study provides strong evidence supporting the importance of maintaining lung health in female smokers with early natural menopause. Moreover, female smokers with early natural menopause should be targeted for smoking cessation and lung cancer screening.
Acknowledgement
The authors thank Dr. Joel L. Weissfeld, previously at the Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, for his contribution in data management and analysis at the initial stage of this study.
Supported by National Cancer Institute grant P30 CA118100. PLuSS cohort has been funded by National Cancer Institute grants P50 CA090440 and P30 CA047904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author(s) report(s) no conflict of interest.
Reference
- 1.BROEKMANS FJ, SOULES MR, FAUSER BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 2009;30:465–93. [DOI] [PubMed] [Google Scholar]
- 2.DRATVA J, REAL FG, SCHINDLER C, et al. Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause 2009;16:385–94. [DOI] [PubMed] [Google Scholar]
- 3.MACSALI F, SVANES C, BJØRGE L, OMENAAS ER, REAL FG. Respiratory health in women: From menarche to menopause. Expert Review of Respiratory Medicine 2012;6:187–202. [DOI] [PubMed] [Google Scholar]
- 4.PENG J, MEIRELES SI, XU X, et al. Estrogen metabolism in the human lung: impact of tumorigenesis, smoke, sex and race/ethnicity. Oncotarget 2017;8:106778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SIMPSON ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol 2003;86:225–30. [DOI] [PubMed] [Google Scholar]
- 6.GEISLER J In the absence of systemic estrogen production, local increased activity of aromatase (CYP19) in adipocytes, macrophages, and other cell types can provide substantial estrogen to some tissues that repalces circulating hormone (Simpson, Geisler). For example, post-menopasual breast tissues often contains 10–20 times the amount of estradiol found in the circulation (Geisler). J Steroid Biochem Mol Biol 2003;86:245–53.14623518 [Google Scholar]
- 7.KEATING NL, CLEARY PD, ROSSI AS, ZASLAVSKY AM, AYANIAN JZ. Use of hormone replacement therapy by postmenopausal women in the United States. Annals of Internal Medicine 1999;130:545–53. [DOI] [PubMed] [Google Scholar]
- 8.DAVIS SR, BABER RJ. Treating menopause - MHT and beyond. Nat Rev Endocrinol 2022. [DOI] [PubMed] [Google Scholar]
- 9.AKTIPIS CA, ELLIS BJ, NISHIMURA KK, HIATT RA. Modern reproductive patterns associated with estrogen receptor positive but not negative breast cancer susceptibility. Evol Med Public Health 2014;2015:52–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.REAL FG, SVANES C, OMENAAS ER, et al. Lung function, respiratory symptoms, and the menopausal transition. J Allergy Clin Immunol 2008;121:72–80 e3. [DOI] [PubMed] [Google Scholar]
- 11.TRIEBNER K, MATULONGA B, JOHANNESSEN A, et al. Menopause Is Associated with Accelerated Lung Function Decline. Am J Respir Crit Care Med 2017;195:1058–65. [DOI] [PubMed] [Google Scholar]
- 12.ZHU D, CHUNG HF, PANDEYA N, et al. Relationships between intensity, duration, cumulative dose, and timing of smoking with age at menopause: A pooled analysis of individual data from 17 observational studies. PLoS Med 2018;15:e1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HAYATBAKHSH MR, NAJMAN JM, O’CALLAGHAN MJ, WILLIAMS GM, PAYDAR A, CLAVARINO A. Association between smoking and respiratory function before and after menopause. Lung 2011;189:65–71. [DOI] [PubMed] [Google Scholar]
- 14.TANG R, FRASER A, MAGNUS MC. Female reproductive history in relation to chronic obstructive pulmonary disease and lung function in UK biobank: a prospective population-based cohort study. BMJ Open 2019;9:e030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CAMPBELL B, BUI DS, SIMPSON JA, et al. Early Age at Natural Menopause Is Related to Lower Post-Bronchodilator Lung Function. A Longitudinal Population-based Study. Ann Am Thorac Soc 2020;17:429–37. [DOI] [PubMed] [Google Scholar]
- 16.JACOBSEN BK, HEUCH I, KVALE G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol 2003;157:923–9. [DOI] [PubMed] [Google Scholar]
- 17.LI S, ROSENBERG L, WISE LA, BOGGS DA, LAVALLEY M, PALMER JR. Age at natural menopause in relation to all-cause and cause-specific mortality in a follow-up study of US black women. Maturitas 2013;75:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MONDUL AM, RODRIGUEZ C, JACOBS EJ, CALLE EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol 2005;162:1089–97. [DOI] [PubMed] [Google Scholar]
- 19.OSSEWAARDE ME, BOTS ML, VERBEEK AL, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–62. [DOI] [PubMed] [Google Scholar]
- 20.ROMAN LAY AA, DO NASCIMENTO CF, DE OLIVEIRA DUARTE YA, PORTO CHIAVEGATTO FILHO AD. Age at natural menopause and mortality: A survival analysis of elderly residents of Sao Paulo, Brazil. Maturitas 2018;117:29–33. [DOI] [PubMed] [Google Scholar]
- 21.JANSEN SC, TEMME EH, SCHOUTEN EG. Lifetime estrogen exposure versus age at menopause as mortality predictor. Maturitas 2002;43:105–12. [DOI] [PubMed] [Google Scholar]
- 22.CHIDI-OGBOLU N, BAAR K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front Physiol 2018;9:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KARLAMANGLA AS, SHIEH A, GREENDALE GA. Hormones and bone loss across the menopause transition. Vitam Horm 2021;115:401–17. [DOI] [PubMed] [Google Scholar]
- 24.EL KHOUDARY SR, GREENDALE G, CRAWFORD SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause 2019;26:1213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.KUMRU S, GODEKMERDAN A, YILMAZ B. Immune effects of surgical menopause and estrogen replacement therapy in peri-menopausal women. J Reprod Immunol 2004;63:31–8. [DOI] [PubMed] [Google Scholar]
- 26.WILSON DO, WEISSFELD JL, FUHRMAN CR, et al. The Pittsburgh lung screening study (PLuSS): Outcomes within 3 years of a first computed tomography scan. American Journal of Respiratory and Critical Care Medicine 2008;178:956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WILSON DO, WEISSFELD JL, BALKAN A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. American Journal of Respiratory and Critical Care Medicine 2008;178:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Global startegy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report), 2021.
- 29.CAO X, LIN L, SOOD A, et al. Small airway wall thickening assessed by computerized tomography is associated with low lung function in Chinese carbon black packers. Toxicological Sciences 2020;178:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RUTH KS, DAY FR, HUSSAIN J, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature 2021;596:393–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VAN VOORHIS BJ, SYROP CH, HAMMITT DG, DUNN MS, SNYDER GD. Effects of smoking on ovulation induction for assisted reproductive techniques. Fertil Steril 1992;58:981–5. [PubMed] [Google Scholar]
- 32.VAN VOORHIS BJ, DAWSON JD, STOVALL DW, SPARKS AE, SYROP CH. The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol 1996;88:785–91. [DOI] [PubMed] [Google Scholar]
- 33.CHUNG HF, GETE DG, MISHRA GD. Age at menopause and risk of lung cancer: A systematic review and meta-analysis. Maturitas 2021;153:1–10. [DOI] [PubMed] [Google Scholar]
- 34.MURABITO JM, YANG Q, FOX C, WILSON PW, CUPPLES LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab 2005;90:3427–30. [DOI] [PubMed] [Google Scholar]
- 35.TRELOAR SA, DO KA, MARTIN NG. Genetic influences on the age at menopause. Lancet 1998;352:1084–5. [DOI] [PubMed] [Google Scholar]
- 36.SNIEDER H, MACGREGOR AJ, SPECTOR TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab 1998;83:1875–80. [DOI] [PubMed] [Google Scholar]
- 37.VAN ASSELT KM, KOK HS, PEARSON PL, et al. Heritability of menopausal age in mothers and daughters. Fertil Steril 2004;82:1348–51. [DOI] [PubMed] [Google Scholar]
- 38.TOWNSEND EA, SATHISH V, THOMPSON MA, PABELICK CM, PRAKASH YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol 2012;303:L923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SATHISH V, FREEMAN MR, LONG E, THOMPSON MA, PABELICK CM, PRAKASH YS. Cigarette Smoke and Estrogen Signaling in Human Airway Smooth Muscle. Cell Physiol Biochem 2015;36:1101–15. [DOI] [PubMed] [Google Scholar]
- 40.CHOI HJ, CHUNG YS, KIM HJ, et al. Signal pathway of 17beta-estradiol-induced MUC5B expression in human airway epithelial cells. American journal of respiratory cell and molecular biology 2009;40:168–78. [DOI] [PubMed] [Google Scholar]
- 41.JAIN R, RAY JM, PAN JH, BRODY SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. American journal of respiratory cell and molecular biology 2012;46:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.TRIEBNER K, ACCORDINI S, CALCIANO L, et al. Exogenous female sex steroids may reduce lung ageing after menopause: A 20-year follow-up study of a general population sample (ECRHS). Maturitas 2019;120:29–34. [DOI] [PubMed] [Google Scholar]
- 43.GANTI AK, SAHMOUN AE, PANWALKAR AW, TENDULKAR KK, POTTI A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol 2006;24:59–63. [DOI] [PubMed] [Google Scholar]
- 44.CHLEBOWSKI RT, WAKELEE H, PETTINGER M, et al. Estrogen Plus Progestin and Lung Cancer: Follow-up of the Women’s Health Initiative Randomized Trial. Clin Lung Cancer 2016;17:10–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CHLEBOWSKI RT, SCHWARTZ AG, WAKELEE H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 2009;374:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NATIONAL LUNG SCREENING TRIAL RESEARCH T, ABERLE DR, ADAMS AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.TANNER NT, KANODRA NM, GEBREGZIABHER M, et al. The Association between Smoking Abstinence and Mortality in the National Lung Screening Trial. Am J Respir Crit Care Med 2016;193:534–41. [DOI] [PubMed] [Google Scholar]
- 48.MOYER VA, FORCE USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330–8. [DOI] [PubMed] [Google Scholar]
- 49.FORCE USPST, KRIST AH, DAVIDSON KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962–70. [DOI] [PubMed] [Google Scholar]
- 50.LENG S, LIU Y, THOMAS CL, et al. Native American ancestry affects the risk for gene methylation in the lungs of Hispanic smokers from New Mexico. Am J Respir Crit Care Med 2013;188:1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DRANSFIELD MT, DAVIS JJ, GERALD LB, BAILEY WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med 2006;100:1110–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


