Abstract
Objective:
To examine the association between lifetime lactation and risk and duration of frequent vasomotor symptoms (VMS).
Design:
Prospective cohort.
Setting:
USA, 1995-2008.
Sample:
2,356 parous midlife women in the Study of Women’s Health Across the Nation.
Methods:
Lifetime lactation was defined as the duration of breastfeeding across all births in months. We used generalized estimating equations to analyze risk of frequent VMS and Cox regression to analyze duration of frequent VMS in years.
Main Outcome Measures:
Frequent VMS (hot flashes and night sweats) were measured annually for 10 years, defined as occurring ≥6 days in the past 2 weeks.
Results:
Overall, 57.1% of women reported hot flashes and 43.0% reported night sweats during follow-up. Lifetime lactation was inversely associated with hot flashes plateauing at 12 months of breastfeeding (6 months: adjusted odds ratio [AOR] 0.85, 95% CI 0.75-0.96; 12 months: AOR 0.78, 95% CI 0.65-0.93), and was inversely associated with night sweats in a downward linear fashion (6 months: AOR 0.93, 95% CI 0.81-1.08; 18 months: AOR 0.82, 95% CI 0.67-1.02; 30 months: AOR 0.73, 95% CI 0.56-0.97). Lifetime lactation was associated with shorter duration of hot flashes and night sweats in a quadratic (bell-shaped) fashion. The association was strongest at 12-18 months of breastfeeding and significant for hot flashes (6 months: adjusted hazard ratio [AHR] 1.35, 95% CI 1.11-1.65; 18 months: AHR 1.54, 95% CI 1.16-2.03; 30 months: AHR 1.18, 95% CI 0.83-1.68).
Conclusions:
Longer lifetime lactation is associated with decreased risk and duration of frequent VMS.
Keywords: Hot Flashes, Sweating, Menopause, Breast Feeding, Middle Aged, Women’s Health, Prospective Studies
Tweetable Abstract:
Longer lifetime #breastfeeding is associated with decreased risk and duration of frequent #hotflashes and #nightsweats in midlife.
INTRODUCTION
Vasomotor symptoms (VMS) of hot flashes and night sweats are hallmark symptoms of menopause. VMS arise from dysfunction in the thermoregulatory system that coordinates the brain and peripheral vasculature regarding target body temperature range.1 Approximately 80% of women experience VMS during the menopausal transition, of which one third report frequent or bothersome symptoms.2,3 VMS negatively impact quality of life due to depressive symptoms, disruptions to sleep and daily life activities, and increased healthcare use.4,5 VMS are associated with higher risk for cardiometabolic diseases, with stronger effects observed when VMS are frequent or prolonged.6-9 Prevention of VMS through identification of modifiable risk factors is therefore paramount to improve women’s midlife health.
Breastfeeding has lasting beneficial effects on women’s endocrine and cardiovascular health, which generally operate in a dose-response manner with cumulative breastfeeding duration over the lifetime.10 Breastfeeding resets the extensive hypermetabolic changes from pregnancy and delays ovulation return through prolactin’s suppressive effect on circulating estrogen.11,12 Most women (>85%) form prenatal intentions to breastfeed for an average of 10-11 months,13-15 yet earlier than desired breastfeeding cessation is common due to lactation difficulties and inadequate support.16-18
Whether breastfeeding influences the menopausal transition has not been comprehensively explored. Existing research suggests that breastfeeding may be protective against early menopause before age 45,19-21 but has limited influence on age at natural menopause more broadly.22,23 The possible impact of breastfeeding on VMS has not been investigated; evidence on this relationship would elucidate the value of breastfeeding promotion as a preventative strategy for VMS. We therefore examined the association between lifetime lactation and the risk and duration of frequent VMS in midlife women.
METHODS
Data Source
We conducted a prospective cohort study using data from the Study of Women’s Health Across the Nation (SWAN), a multi-ethnic longitudinal study of 3,302 women.24 Beginning 1995-1997, women of Asian (Japanese and Chinese), Black, Hispanic, and White race/ethnicity were enrolled across 7 sites (Boston, Chicago, Detroit, Los Angeles, Newark, Pittsburgh, and Oakland). Women were eligible if they were aged 42-52 years, had an intact uterus and at least one ovary, experienced at least one menstrual period and had not used oral contraceptives or hormone replacement therapy (HRT) in the preceding 3 months, and were not pregnant. Data on physical and mental health, reproductive history, lifestyle, social determinants, and biomarkers were collected at baseline and annually for 15 years with clinical interviews, standardized questionnaires, physical examinations, and blood tests. Institutional review boards at each site approved the study protocol and all participants gave written, informed consent.
Analytic Sample
We accessed publicly available data from the baseline and first 10 follow-up visits (up to 2008).25 We included parous women with complete data on lifetime lactation (n=2,718), excluding those who reported hysterectomy with ovary conservation (n=77) or participated in fewer than 2 follow-up visits (n=285). The analysis for risk of VMS included 2,356 women. The analysis for duration of VMS was restricted to women who reported symptoms at least one during follow-up and were thus at risk for the event of VMS cessation, excluding those who reported surgical menopause (bilateral oophorectomy) before symptom onset or using HRT within 2 years before symptom onset, resulting in 1,096 women for hot flashes and 828 women for night sweats.
Patients and the public were not involved in the development of this secondary analysis study.
Lifetime Lactation Duration
At baseline, women answered retrospective questions on gravidity and breastfeeding duration in months for each live birth, with 0 representing no initiation of breastfeeding or breastfeeding for less than 1 month. We defined lifetime lactation as the sum of breastfeeding durations across all births, truncated at the 90th percentile (30 months) to reduce the influence of extreme values.26
Vasomotor Symptoms
At baseline and annual follow-up visits, women answered standardized questions on the frequency with which they experienced hot flashes and night sweats in the preceding 2 weeks: not at all, 1-5 days, 6-8 days, 9-13 days, or daily. We derived variables for hot flashes and night sweats separately, given their differential risk factors and effects on women’s quality of life.4,27 Frequent VMS were defined as those occurring ≥6 days in the past 2 weeks. Duration of VMS was defined as years elapsed between the visit dates of first and last report of VMS. VMS cessation was defined as two consecutive visits without HRT use or frequent VMS.28,29 To account for VMS reported at a single visit, we added a random standard normal deviate to the total duration for all observations. This technique was used instead of uniformly adding 0.5 years,28,29 to reduce the number of ties at 0.5 years duration.
Covariates
In order to estimate the total effect of lifetime lactation on VMS risk and duration (i.e., through all potentially causal pathways), we included potential confounders, but not mediators, as covariates;30 these included education, race/ethnicity, parity, smoking, and body mass index (BMI).31-36 Covariates were measured at baseline using questionnaires and clinical interviews. Educational level (college degree, less than college degree), race/ethnicity (Asian, Black, Hispanic, White), parity (1, 2, 3, 4+), and smoking (past/never, current) were self-reported. Height and weight were measured using standardized protocols to calculate BMI (kg/m2). Menopause was staged annually using self-reported bleeding patterns and surgical history (premenopausal, perimenopausal, natural postmenopausal, or surgical postmenopausal).
Statistical Analysis
For the association between lifetime lactation and risk of frequent VMS, we used generalized estimating equations (GEE) for binary repeated measures with an unstructured correlation structure to estimate odds ratios (OR) and 95% confidence intervals (CI). Models were separately constructed for hot flashes and night sweats. Lifetime lactation is often modelled as a continuous linear variable or reduced from its full distribution to categories of 3, 6, or 12-month intervals. These approaches may improve coefficient interpretability, but are often statistically inefficient and can distort the true relationship.37 To use all available data and permit flexible modelling of the relationships under study, we modelled lifetime lactation continuously using a restricted cubic spline with 3 knots at the recommended 10th, 50th, and 90th percentiles of the sample (Table S1).26 Multivariable models controlled for race/ethnicity, parity, smoking, education, and BMI as time-fixed covariates, and age as a time-varying covariate. Recognizing the sociodemographic barriers to HRT access and variability in how HRT use is handled in epidemiological analyses of VMS,38,39 our main analysis included visits where HRT use was reported to produce results reflective of the real world population average association between lifetime lactation and risk of frequent VMS.40
We conducted three sensitivity analyses for the GEE models. First, we censored (excluded) visits where HRT use was reported. Second, we restricted to women with a parity of 2 (the largest category). Third, we adjusted for menopause stage as a time-varying covariate.
For the association between lifetime lactation and duration of frequent VMS, we used Cox proportional hazards regression to estimate hazard ratios (HR) and 95% CIs. Models were constructed separately for hot flashes and night sweats and lifetime lactation was modelled using a restricted cubic spline with 3 knots (Table S1). The event was cessation of frequent VMS. An HR>1 indicated shorter duration of VMS compared to the reference group, which was lifetime lactation of 0 months. Women who did not experience VMS cessation were right censored at last completed study visit where frequent VMS were reported, HRT initiation, or end of follow-up, whichever came first. We additionally right censored women who experienced surgical menopause. Multivariable models controlled for race/ethnicity, parity, smoking, education, BMI, and age at first report of VMS as time-fixed covariates. Proportional hazards were assessed using smoothed plots and statistical tests of Schoenfeld residuals, time-by-covariate interactions, and log-log plots. Both models were stratified on race/ethnicity and adjusted for BMI categorized as under/normal weight (≤24.9 kg/m2), overweight (25.0-29.9 kg/m2), or obese (≥30 kg/m2), and models for night sweats were additionally stratified on education, given evidence of non-proportional hazards for these covariates.
Using the adjusted model, we calculated the predicted survival functions across all lifetime lactation values for a fixed covariate profile representing the average SWAN participant.26 From these functions, we plotted the median duration of frequent VMS according to lifetime lactation to support clinical interpretation of HRs.
Lastly, we conducted four sensitivity analyses for the Cox models. First, we used age as the underlying time scale.41 Second, we restricted to women with a parity of 2. Third, we adjusted for menopause stage at first report of VMS. Fourth, we used a competing risks framework to account for HRT. A competing risk is an event whose occurrence precludes the occurrence of the event of interest; in this setting, HRT use precludes naturally occurring VMS cessation and may be more appropriately modelled as a competing risk than through right-censoring. To explore this, we used Fine and Gray’s proportional subdistribution hazards model to estimate the subhazard ratio (SHR) and 95% CI, specifying HRT use within 2 years of the last report of VMS as a competing risk.
OR and HR results with a 95% CI that did not include the null value of 1 were considered statistically significant. All data cleaning and analyses were conducted in Stata MP V17.
RESULTS
Sample Characteristics
One third of women reported 0 months of lifetime lactation. Among those who reported ≥1 month of breastfeeding, the median lifetime lactation was 11 months (interquartile range, 4-22). Demographic and health gradients across lifetime lactation were evident for most characteristics, except for parity and HRT use (Table 1). Women with longer lifetime lactation were slightly more likely to be premenopausal at the end of follow-up. The proportions of women who reported never/past smoking, a college degree, and self-identified as White were higher in longer lactation groups, whereas the proportion with baseline obesity decreased. Overall, 57.1% of women (n = 1,346) reported frequent hot flashes and 43.0% (n = 1,012) reported frequent night sweats during follow-up.
Table 1.
Sample characteristics (N=2,356)
| Lactation 0 months |
Lactation 1-5 months |
Lactation 6-11 months |
Lactation 12-17 months |
Lactation 18-23 months |
Lactation ≥24 months |
|
|---|---|---|---|---|---|---|
| Number of participants, n | 797 | 454 | 333 | 253 | 141 | 378 |
| Baseline age, median (IQR) | 46 (44-48) | 46 (44-48) | 46 (43-48) | 46 (44-48) | 46 (44-48) | 45 (43-47) |
| Parity | ||||||
| 1 | 25.8 | 30.2 | 22.8 | 12.6 | 5.0 | 6.6 |
| 2 | 39.0 | 40.3 | 51.1 | 50.2 | 49.6 | 29.6 |
| 3 | 21.5 | 18.3 | 17.4 | 24.1 | 29.1 | 35.7 |
| 4+ | 13.7 | 11.2 | 8.7 | 13.0 | 16.3 | 28.0 |
| Type of menopause | ||||||
| None (pre-menopausal) | 20.5 | 20.0 | 25.2 | 26.1 | 26.2 | 26.7 |
| Natural | 72.9 | 74.4 | 70.3 | 68.8 | 70.2 | 70.6 |
| Surgical | 6.6 | 5.5 | 4.5 | 5.1 | 3.5 | 2.6 |
| Self-identified race/ethnicity | ||||||
| Asian | 9.5 | 24.2 | 20.4 | 23.3 | 24.8 | 19.8 |
| Black | 45.8 | 31.3 | 23.4 | 19.0 | 19.9 | 13.0 |
| Hispanic | 9.0 | 6.2 | 8.7 | 7.5 | 3.5 | 6.6 |
| White | 35.6 | 38.3 | 47.4 | 50.2 | 51.8 | 60.6 |
| Baseline BMI category | ||||||
| Under/normal weight | 29.8 | 44.3 | 44.2 | 44.8 | 48.9 | 45.2 |
| Overweight | 27.9 | 25.4 | 27.9 | 30.6 | 24.1 | 31.1 |
| Obese | 42.3 | 30.3 | 27.9 | 24.6 | 27.0 | 23.7 |
| Baseline smoking | ||||||
| Never or past | 75.2 | 82.1 | 88.9 | 89.7 | 90.1 | 91.0 |
| Current | 24.8 | 17.9 | 11.1 | 10.3 | 9.9 | 9.0 |
| Educational level | ||||||
| College degree | 22.5 | 38.2 | 49.8 | 50.8 | 58.3 | 55.7 |
| Less than college degree | 77.5 | 61.8 | 50.2 | 49.2 | 41.7 | 44.3 |
| HRT during follow-up | 27.2 | 30.4 | 33.0 | 27.7 | 21.3 | 29.4 |
| VMS during follow-up | ||||||
| Frequent hot flashes | 61.4 | 63.0 | 58.9 | 51.0 | 41.8 | 49.5 |
| Frequent night sweats | 48.2 | 47.8 | 42.3 | 39.1 | 32.6 | 33.1 |
BMI, body mass index. IQR, interquartile range. HRT, hormone replacement therapy. VMS, vasomotor symptoms.
Lifetime Lactation and Risk of Frequent VMS
Prevalence of hot flashes generally decreased as lifetime lactation increased (Table 1), with the highest prevalence in the 0 month group (61.4%, n = 489) and the lowest prevalence in the 18-23 month group (41.8%, n = 59). In the GEE models, lifetime lactation was associated frequent hot flashes in a non-linear fashion (Figure 1). This association was attenuated toward the null following adjustment for covariates, but remained statistically significant across nearly all lifetime lactation values. Compared to 0 months of lactation, duration of lifetime lactation was inversely associated with odds of hot flashes up to approximately 12 months after which we observed a plateau (6 months: AOR 0.85, 95% CI 0.75-0.96; 12 months: AOR 0.78, 95% CI 0.65-0.93).
Figure 1.
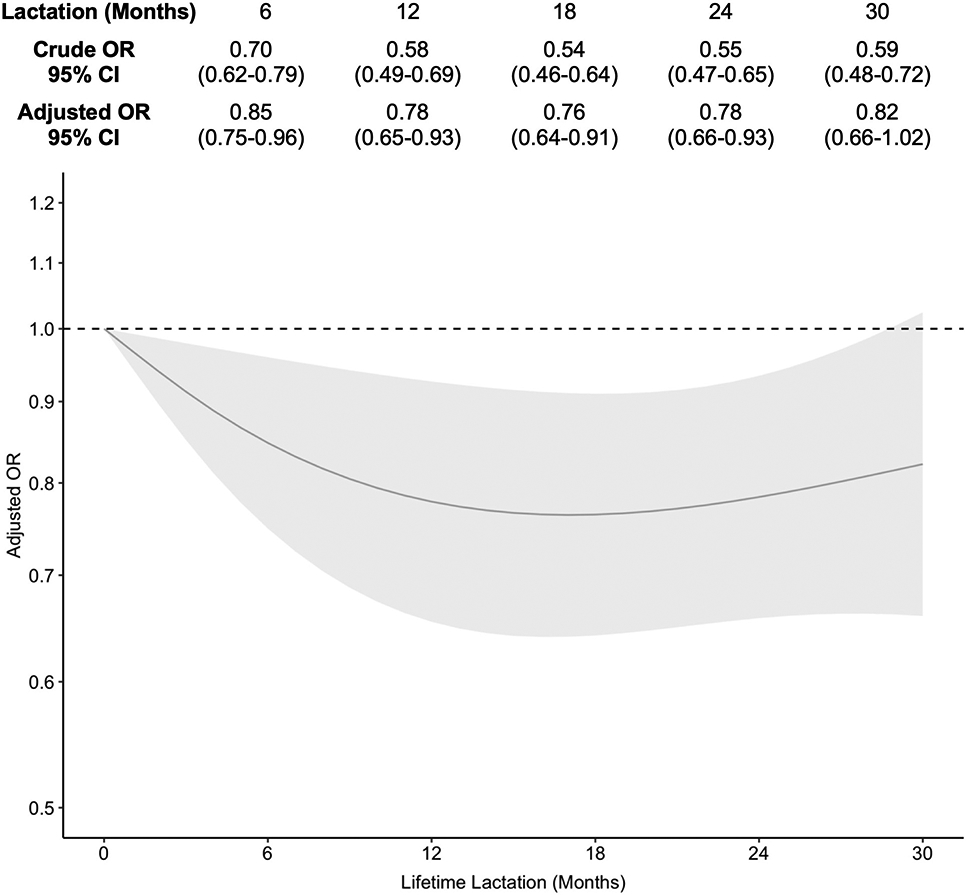
Association of lifetime lactation and frequent hot flashes [Caption: OR, odds ratio. CI, confidence interval. Adjusted models controlled for education, race/ethnicity, parity, smoking, and body mass index at baseline as time-fixed covariates, and age as a time-varying covariate.]
Prevalence of night sweats generally decreased as lifetime lactation increased (Table 1), with the highest prevalence in the 0 month group (48.2%, n = 384) and the lowest prevalence in the 18-23 month group (32.6%, n = 46). In the GEE models, lifetime lactation was associated with frequent night sweats in a linear fashion (Figure 2). This association was attenuated toward the null following adjustment for covariates; 95% CIs shifted to enclose the null for lifetime lactation values at 1 through 18 months, however the downward trend remained intact. Compared to 0 months of lactation, duration of lifetime lactation was inversely associated with odds of night sweats (6 months: AOR 0.93, 95% CI 0.81-1.08; 18 months: AOR 0.82, 95% CI 0.67-1.02; 30 months: AOR 0.73, 95% CI 0.56-0.97).
Figure 2.
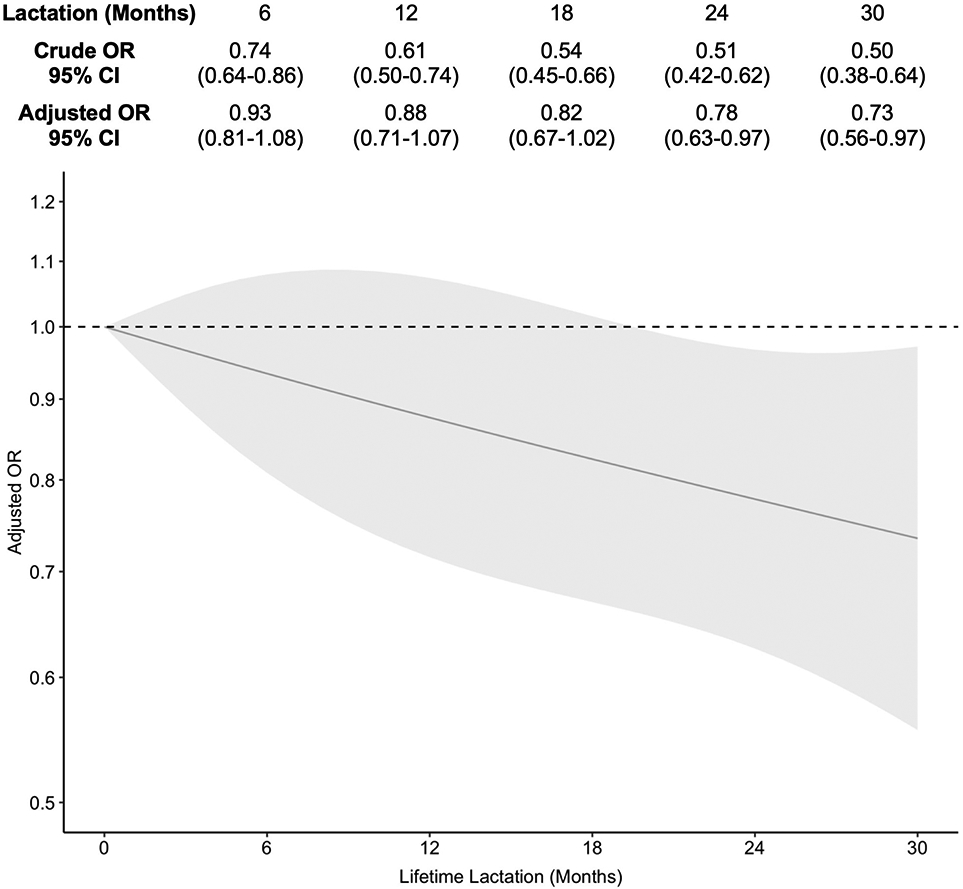
Association of lifetime lactation and frequent night sweats [Caption: OR, odds ratio. CI, confidence interval. Adjusted models controlled for education, race/ethnicity, parity, smoking, and body mass index at baseline as time-fixed covariates, and age as a time-varying covariate.]
Results were robust to sensitivity analyses censoring visits with HRT, adjusting for menopause stage, and restricting to women with a parity of 2 (Figure S1 and S2).
Lifetime Lactation and Frequent VMS Duration
In the Cox models, lifetime lactation was associated with duration of frequent hot flashes in a quadratic (bell-shaped) fashion (Figure 3). This association was unchanged following adjustment for covariates. Compared to 0 months of lactation, duration of lactation was associated with shorter duration of hot flashes up to approximately 12 months where we observed a plateau until 18 months, followed by a decrease in the magnitude of this association trending towards the null (6 months: AHR 1.35, 95% CI 1.11-1.65; 18 months: AHR 1.54, 95% CI 1.16-2.03; 30 months: AHR 1.18, 95% CI 0.83-1.68). Median duration of frequent hot flashes for the average participant was 6.6 years at 0 months of lactation, which decreased as lifetime lactation increased to as low as 4.5 years at 8 through 20 months of lactation (Figure S3).
Figure 3.
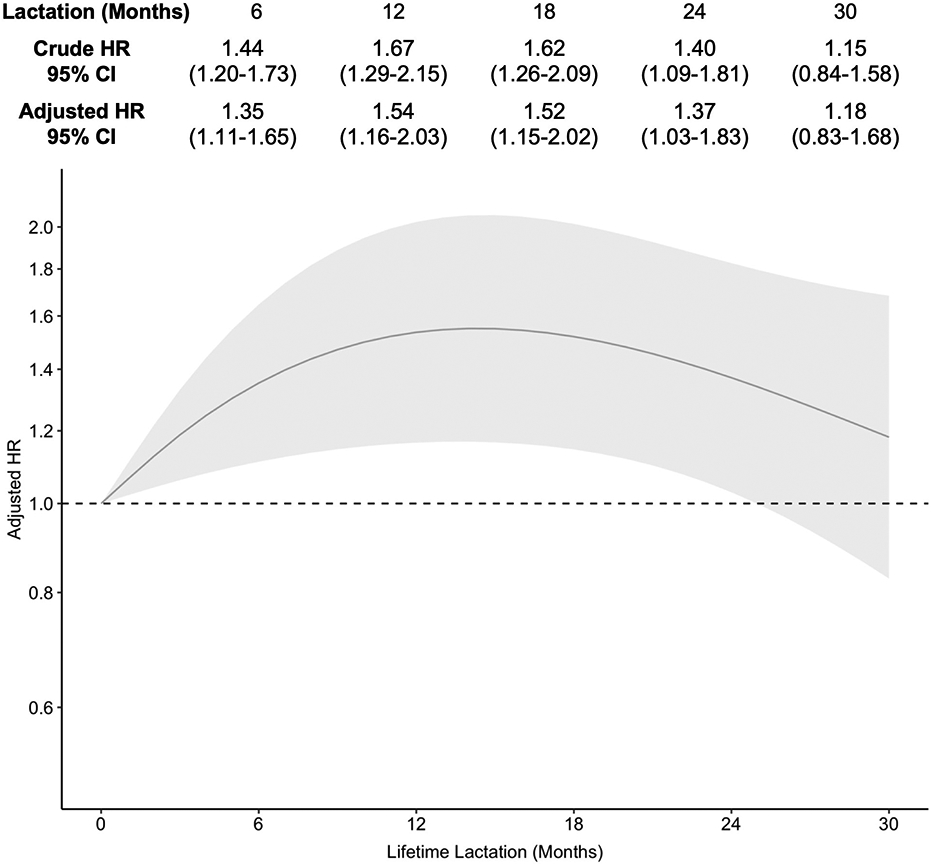
Association of lifetime lactation and duration of frequent hot flashes [Caption: HR, odds ratio. CI, confidence interval. Adjusted models controlled for education, race/ethnicity, parity, smoking, and body mass index at baseline and age at first report of hot flashes. An HR>1 indicated shorter duration of frequent hot flashes compared to the reference group, which was lifetime lactation of 0 months.]
Lifetime lactation was associated with duration of frequent night sweats in a similar quadratic (bell-shaped) fashion (Figure 4). This association was attenuated following adjustment for covariates; 95% CIs shifted to enclose the null for lifetime lactation values at 1 through 20 months, however the bell-shaped trend remained intact. Compared to 0 months of lactation, duration of lactation was associated with shorter duration of night sweats up to approximately 12 months where we observed a plateau until 18 months, followed by a decrease in the magnitude of this association trending towards (and reaching) the null; at no point was this association statistically significant (6 months: AHR 1.19, 95% CI 0.93-1.52; 18 months: AHR 1.22, 95% CI 0.88-1.68; 30 months: AHR 1.01, 95% CI 0.66-1.55). Median duration of frequent night sweats for the average participant was 4.3 years at 0 months of lactation, which decreased as lifetime lactation increased to as low as 3.5 years at 4 through 24 months of lactation (Figure S4).
Figure 4.
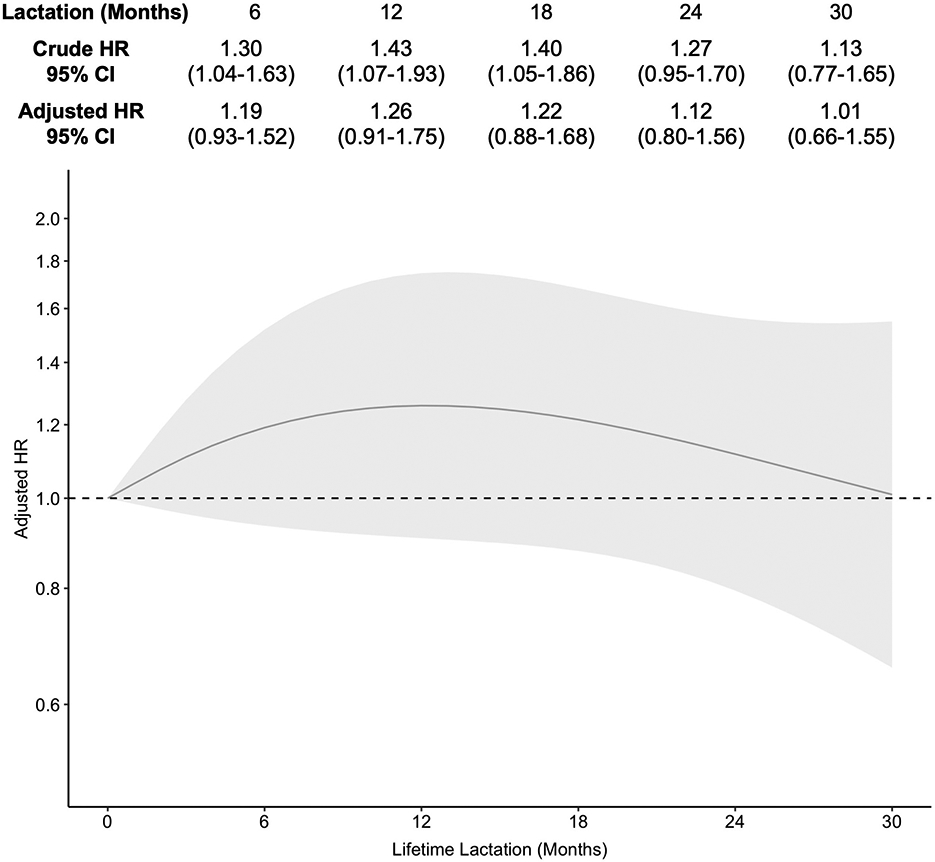
Association of lifetime lactation and duration of frequent night sweats [Caption: HR, odds ratio. CI, confidence interval. Adjusted models controlled for education, race/ethnicity, parity, smoking, and body mass index at baseline and age at first report of night sweats. An HR>1 indicated shorter duration of frequent night sweats compared to the reference group, which was lifetime lactation of 0 months.]
Results were robust to sensitivity analyses using age as the time scale, restricting to women with a parity of 2, adjusting for menopause stage at first report of VMS, and using a subdistribution hazards model to account for competing risks from HRT (Figures S5 and S6). Of note, the association between lifetime lactation and duration of night sweats was statistically significant at 1 through 18 months in the subdistribution hazards model (6 months: SHR 1.28, 95% CI 1.01-1.61; 12 months: SHR 1.40, 95% CI 1.03-1.91; 18 months: SHR 1.38, 95% CI 1.01-1.87).
DISCUSSION
Main Findings
In this longitudinal dose-response analysis of the SWAN cohort, we found that lifetime lactation may be modestly protective against frequent VMS during midlife. While the shape of this association varied by VMS type, it was generally observed that longer lifetime lactation was linked to reduced likelihood of frequent hot flashes and night sweats. Women with lifetime lactation around 12 months had roughly 20% lower relative odds of both VMS types after adjusting for demographic and lifestyle factors. Among women who experienced frequent VMS, lifetime lactation was associated with VMS duration in a bell-shaped fashion with the shortest duration of hot flashes and night sweats observed at 12 to 18 months of lactation. This association remained significant for hot flashes, but not night sweats, after adjustment. On average, women reporting lifetime lactation between approximately 6 to 20 months experienced a 2.1-year reduction in hot flash duration and 0.8- year reduction in night sweat duration across 10 years of prospective follow-up. Lactation-related reductions in risk and duration of frequent VMS were similar in magnitude to those observed for other lifestyle and behavioural factors, such as smoking and obesity, that are emphasized for VMS prevention in clinical practice guidelines.42
While benefits of breastfeeding for maternal cardiometabolic health and cancer risk are widely acknowledged,10,43 evidence related to menopausal health is limited. Findings from a recent comprehensive review on risk factors for VMS suggest that breastfeeding has been overlooked entirely to date.44 Our study addressed this knowledge gap by demonstrating a clinically important reduction in risk and duration of frequent VMS with cumulative months of lifetime lactation and highlights a potential role for breastfeeding promotion in primary prevention of VMS.
Strengths and Limitations
A key limitation is the possibility that our results are attributable to confounding. Breastfeeding behaviours are highly socially patterned due to structural socioeconomic barriers and cultural factors. For example, Black American women report the lowest breastfeeding rates among any racial group,31 and nuanced barriers to breastfeeding include historical trauma related to wet nursing during slavery as well as inadequate and racially biased healthcare support.45 Breastfeeding disparities have also been observed in women with low socioeconomic status.46-48 VMS vary across these demographic groups and according to lifestyle behaviours such as smoking.36,44 We controlled for race/ethnicity, education, smoking, parity, and BMI at study baseline, and lactation effects were attenuated but not eliminated. However, these characteristics can change over the life course and may have differed during the reproductive years, resulting in some residual confounding.
We lacked data on birth spacing, oral contraceptive use, and breastfeeding intensity. Breastfeeding can continue during conception of a subsequent pregnancy and initiation of oral contraceptives following birth, and can vary in intensity from exclusive in the first 6 months to partial breastfeeding up to 2 years and beyond; all of which markedly influence reproductive hormones and maternal physiology. Unmeasured confounding from these factors could explain the non-linear relationships we observed, specifically the plateauing or attenuation of effects with longer lifetime lactation.
Breastfeeding was measured through retrospective self-report. Recall of breastfeeding duration is generally valid and reliable several decades after birth,49,50 yet recall bias or memory error is possible. SWAN participants lost to follow-up have tended to be less healthy, smokers, less educated, or self-identify as Black or Hispanic.51 This may have given rise to selection bias since these factors are associated with shorter breastfeeding duration and higher risk of VMS, in which case we under-estimated the true association; however, we adjusted for several of these factors.52 For women who reported VMS at the first or last study visit, exact start and end dates of VMS are unknown thus our estimates of median duration are likely conservative.
Finally, we acknowledge statistical limitations pertaining to precision and type 1 error. The 95% CIs we observed ranged from a null or very small protective effect to a large protective effect, and larger replication studies are needed to determine the true effect size for the impact of lifetime lactation on frequent VMS with greater precision. Given the large number of models and point estimates reported in our main and sensitivity analyses, it is possible that some significant results are attributable to type 1 error.
Interpretation
The pathophysiology of VMS is not well understood. Leading hypotheses describe how declining estrogen levels around menopause narrow the thermoneutral zone and interfere with peripheral vasculature response to body temperature fluctuations.53,54 Increasing evidence has highlighted a group of hypothalamic neurons–kisspeptin, neurokinin B and dynorphin (KNDy neurons)–which hypertrophy with waning levels of estrogen and gonadotropins and contribute to the generation of VMS.55-57 Importantly, gradual cessation of VMS suggests that the thermoregulatory system eventually adapts to menopausal changes and restores normal function.
Our results showed that duration of lifetime lactation could have a significant and positive impact on the female body’s capacity to adapt to midlife changes in the thermoneutral zone. Lactation bears similarities to menopause in that both are hypoestrogenic states. During lactation, high levels of prolactin exert an antagonistic effect on estrogen production,12 and women have reported hypoestrogenic symptoms like VMS and vaginal dryness during the early postpartum.58,59 It is possible that breastfeeding acclimates the thermoregulatory system and KNDy neurons to uncharacteristically low levels of estrogen in advance of menopause, which renders a more robust adaptation to similar circumstances during midlife.
Lactation also facilitates sustained improvements in vascular function and anthropometry. Midlife women who breastfed exhibit better subclinical vascular health and lower BMI than women who did not, even after accounting for cardiovascular and social risk factors.60,61 These differences are thought to partly underpin the dose-response reductions in cardiovascular morbidity observed in women who have breastfed.43,62 Breastfeeding may therefore improve vascular reactivity in the context of thermoregulation, reducing the onset and persistence of VMS in midlife. Additional studies on the impact of lactation and hypoestrogenic exposures on VMS and that explore mediation through vascular and anthropometric health markers would help corroborate these interpretations.
CONCLUSIONS
Breastfeeding is associated with reduced risk and duration of frequent VMS in midlife. Protective effects were strongest around 12 months of lifetime lactation and were pronounced for hot flashes. VMS are a significant concern for women with far-reaching impacts on quality of life and health service use, thus primary prevention strategies are needed. Scaling up healthcare policies and practices shown to improve population breastfeeding rates may help reduce the burden of VMS.
Supplementary Material
FUNDING
The Study of Women’s Health Across the Nation (SWAN) was supported by grants (U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495) from the National Institutes of Health, Department of Health and Human Services, National Institute on Aging, National Institute of Nursing Research, and Office of Research on Women’s Health. NVS is supported by a Canadian Institutes of Health Research Canada Graduate Scholarship Doctoral Award. EAB is supported by a Canadian Institutes of Health Research Early Career Investigator Award.
Footnotes
DISCLOSURES OF INTEREST
AKS has received honoraria from Pfizer and Bio-Syent, and has received grant funding from Pfizer. PDF is a shareholder and consultant and receives payment in convertible notes from ViTAA medical devices. EAB has received speaking fees from Searchlight Pharmaceuticals. NVS has no disclosures to report.
DETAILS OF ETHICS APPROVAL
Institutional review boards at each site in the SWAN Study approved the study protocol and all participants gave written, informed consent. In accordance with the Canadian Tri-Council Policy Statement Article 2.2, this secondary analysis using publicly available data was exempted from ethical review and approval.
REFERENCES
- 1.Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch Womens Ment Health. 2007;10(6):247–57. [DOI] [PubMed] [Google Scholar]
- 2.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Granger AL, Fehnel SE, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11(1):32–43. [DOI] [PubMed] [Google Scholar]
- 3.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58(4):348–58. [DOI] [PubMed] [Google Scholar]
- 6.Herber-Gast GCM, Mishra GD. Early severe vasomotor menopausal symptoms are associated with diabetes. Menopause. 2014;21(8):855–60. [DOI] [PubMed] [Google Scholar]
- 7.Herber-Gast GCM, Mishra GD, Van Der Schouw YT, Brown WJ, Dobson AJ. Risk factors for night sweats and hot flushes in midlife: Results from a prospective cohort study. Menopause. 2013;20(9):953–9. [DOI] [PubMed] [Google Scholar]
- 8.Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: A systematic review and meta-analysis. PLoS One. 2016;11(6):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu D, Chung HF, Dobson AJ, Pandeya N, Anderson DJ, Kuh D, et al. Vasomotor menopausal symptoms and risk of cardiovascular disease: a pooled analysis of six prospective studies. Am J Obstet Gynecol. 2020;223(6):898.e1–898.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury R, Sinha B, Sankar M, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015;104(Supplement 467):99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuebe AM, Rich-Edwards JW. The reset hypothesis: Lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strauss J, Barbieri R. Yen & Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 7th Ed. Philadelphia, PA: Elsevier Saunders; 2014. [Google Scholar]
- 13.Ballesta-Castillejos A, Gómez-Salgado J, Rodríguez-Almagro J, Ortiz-Esquinas I, Hernández-Martínez A. Factors that influence mothers’ prenatal decision to breastfeed in Spain. Int Breastfeed J. 2020;15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutsiv O, Pullenayegum E, Foster G, Vera C, Giglia L, Chapman B, et al. Women’s intentions to breastfeed: A population-based cohort study. BJOG. 2013;120(12):1490–8. [DOI] [PubMed] [Google Scholar]
- 15.Nelson JM, Li R, Perrine CG, Scanlon KS. Changes in mothers’ intended duration of breastfeeding from the prenatal to neonatal periods. Birth. 2018;45(2):178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CRL, Dodds L, Legge A, Bryanton J, Semenic S. Factors influencing the reasons why mothers stop breastfeeding. Can J Public Heal. 2014;105(3):179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Fein SB, Chen J, Grummer-Strawn LM. Why mothers stop breastfeeding: Mothers’ self-reported reasons for stopping during the first year. Pediatrics. 2008;122(Suppl 2):69–76. [DOI] [PubMed] [Google Scholar]
- 18.Gianni ML, Bettinelli ME, Manfra P, Sorrentino G, Bezze E, Plevani L, et al. Breastfeeding difficulties and risk for early breastfeeding cessation. Nutrients. 2019/September/25. 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Kartsonaki C, Guo Y, Lv J, Gan W, Chen Z-M, et al. Factors related to age at natural menopause in China: results from the China Kadoorie Biobank. Menopause. 2021;28(10):1130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SH, Kim CS, Lee KS, Kim H, Yim SV, Lim YJ, et al. Premenopausal factors influencing premature ovarian failure and early menopause. Maturitas. 2007;58(1):19–30. [DOI] [PubMed] [Google Scholar]
- 21.Langton CR, Whitcomb BW, Purdue-Smithe AC, Sievert LL, Hankinson SE, Manson JE, et al. Association of parity and breastfeeding with risk of early natural menopause. JAMA Netw Open. 2020;3(1):e1919615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whelan EA, Sandler DP, Mcconnaughey DR, Weinberg CR. Menstrual and reproductive characteristics and age at natural menopause. Am J Epidemiol. 1990;131(4):625–32. [DOI] [PubMed] [Google Scholar]
- 23.Nagel G, Altenburg HP, Nieters A, Boffetta P, Linseisen J. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas. 2005;52(3–4):337–47. [DOI] [PubMed] [Google Scholar]
- 24.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, et al. SWAN: a multi- center, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. San Diego, CA: Academic Press; 2000. p. 175–88. [Google Scholar]
- 25.Sutton-Tyrrell K, Selzer F, Sowers M, Neer R, Powell L, Gold E, et al. Study of Women’s Health Across the Nation (SWAN) Series [Public Dataset]. Inter-university Consortium for Political and Social Research [distributor]; 2019. doi: 10.3886/ICPSR28762.v5 (Baseline); doi: (Visit 1); doi: (Visit 2); doi: (Visit 3); doi: (Visit 4); doi: (Visit 5); doi: (Visit 6); doi: (Visit 7); doi: (Visit 8); doi: (Visit 9); doi: (Visit 10). https://www.icpsr.umich.edu/icpsrweb/ICPSR/series/00253 [DOI] [Google Scholar]
- 26.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 3rd Ed. New York, NY: Springer-Verlag; 2015. [Google Scholar]
- 27.Duffy OK, Iversen L, Hannaford PC. Factors associated with reporting classic menopausal symptoms differ. Climacteric. 2013;16(2):240–51. [DOI] [PubMed] [Google Scholar]
- 28.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schisterman E, Cole S, Platt R. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li R, Perrine CG, Anstey EH, Chen J, Macgowan CA, Elam-Evans LD. Breastfeeding Trends by Race/Ethnicity among US Children Born from 2009 to 2015. JAMA Pediatr. 2019;173(12):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JA. Predictors of Breastfeeding Duration: Evidence From a Cohort Study. Pediatrics. 2006;117(4):e646–55. [DOI] [PubMed] [Google Scholar]
- 33.Thulier D, Mercer J. Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs. 2009;38:259–68. [DOI] [PubMed] [Google Scholar]
- 34.Stuebe A, Rich-Edwards J, Willett W, Manson J, Michels K. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–10. [DOI] [PubMed] [Google Scholar]
- 35.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes Control. 2007;18(5):517–23. [DOI] [PubMed] [Google Scholar]
- 36.Avis NE, Crawford SL, Green R. Vasomotor Symptoms Across the Menopause Transition: Differences Among Women. Obstet Gynecol Clin North Am. 2018;45(4):629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat Med. 2006;25(1):127–41. [DOI] [PubMed] [Google Scholar]
- 38.Lawlor DA, Smith GD, Ebrahim S. Socioeconomic position and hormone replacement therapy use: Explaining the discrepancy in evidence from observational and randomized controlled trials. Am J Public Health. 2004;94(12):2149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: A meta-analysis. J Gen Intern Med. 2008;23(9):1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cusimano MC, Chiu M, Ferguson SE, Moineddin R, Aktar S, Liu N, et al. Association of bilateral salpingo-oophorectomy with all cause and cause specific mortality: Population based cohort study. BMJ. 2021;375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiébaut ACM, Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: A simulation study. Stat Med. 2004;23(24):3803–20. [DOI] [PubMed] [Google Scholar]
- 42.Shifren JL, Gass MLS, Kagan R, Kaunitz AM, Liu JH, Pinkerton JAV., et al. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21(10):1038–62. [DOI] [PubMed] [Google Scholar]
- 43.Tschiderer L, Seekircher L, Kunutsor S, Peters S, O’Keeffe L, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: Systematic review and meta-analysis involving data from 8 studies and 1 192 700 parous women. J Am Heart Assoc. 2022;10:e022746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costanian C, Zangiabadi S, Bahous SA, Deonandan R, Tamim H. Reviewing the evidence on vasomotor symptoms: the role of traditional and non-traditional factors. Climacteric. 2020;23(3):213–23. [DOI] [PubMed] [Google Scholar]
- 45.Green VL, Killings NL, Clare CA. The Historical, Psychosocial, and Cultural Context of Breastfeeding in the African American Community. Breastfeed Med. 2021;16(2):116–20. [DOI] [PubMed] [Google Scholar]
- 46.Kehler HL, Chaput KH, Tough SC. Risk factors for cessation of breastfeeding prior to six months postpartum among a community sample of women in Calgary, Alberta. Can J Public Heal. 2009;100(4):376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moss KM, Dobson AJ, Tooth L, Mishra GD. Which australian women do not exclusively breastfeed to 6 months, and why? J Hum Lact. 2021;37(2):390–402. [DOI] [PubMed] [Google Scholar]
- 48.Mitra AK, Khoury AJ, Hinton AW, Carothers C. Predictors of breastfeeding intention among low-income women. Matern Child Health J. 2004;8(2):65–70. [DOI] [PubMed] [Google Scholar]
- 49.Li R, Scanlon KS, Serdula MK. The Validity and Reliability of Maternal Recall of Breastfeeding Practice. Nutr Rev. 2005;63(4):103–10. [DOI] [PubMed] [Google Scholar]
- 50.Natland ST, Andersen LF, Nilsen TIL, Forsmo S, Jacobsen GW. Maternal recall of breastfeeding duration twenty years after delivery. BMC Med Res Methodol. 2012;12(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, et al. Factors related to age at natural menopause: Longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. 2018;97(4):407–16. [DOI] [PubMed] [Google Scholar]
- 53.Rossmanith WG, Ruebberdt W. What causes hot flushes? the neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol Endocrinol. 2009;25(5):303–14. [DOI] [PubMed] [Google Scholar]
- 54.Charkoudian N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin Proc. 2003;78(5):603–12. [DOI] [PubMed] [Google Scholar]
- 55.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: A novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Modi M, Dhillo WS. Neurokinin 3 Receptor Antagonism: A Novel Treatment for Menopausal Hot Flushes. Neuroendocrinology. 2019;109(3):242–8. [DOI] [PubMed] [Google Scholar]
- 57.Reame NK. More promising news (mostly) on manipulating neurokinin B activity as a nonhormonal treatment of hot flashes. Menopause. 2020;27(4):375–6. [DOI] [PubMed] [Google Scholar]
- 58.Rowland M, Foxcroft L, Hopman W, Patel R. Breastfeeding and sexuality immediately post partum. Can Fam Physician. 2005;51:1366–7. [PMC free article] [PubMed] [Google Scholar]
- 59.Thurston RC, Luther JF, Wisniewski SR, Eng H, Wisner KL. Prospective evaluation of nighttime hot flashes during pregnancy and postpartum. Fertil Steril. 2013;100(6):1667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McClure CK, Catov JM, Ness RB, Schwarz EB. Lactation and maternal subclinical cardiovascular disease among premenopausal women. Am J Obstet Gynecol. 2012;207(1):46.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bobrow KL, Quigley MA, Green J, Reeves GK, Beral V. Persistent effects of women’s parity and breastfeeding patterns on their body mass index: Results from the Million Women Study. Int J Obes. 2013;37(5):712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stuebe AM, Schwarz EB, Grewen K, Rich-Edwards JW, Michels KB, Foster EM, et al. Duration of lactation and incidence of maternal hypertension: A longitudinal cohort study. Am J Epidemiol. 2011;174(10):1147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


