Abstract
Background:
Internalizing psychopathologies (IPs) are highly comorbid and exhibit substantial overlap, such as aberrant affective reactivity. Neural reactivity to emotional images, measured via the Late Positive Potential (LPP) event-related potential (ERP) component, has been utilized to index affective reactivity in IPs. The LPP is often examined in isolation with a specific disorder, ignoring overlap between IPs. The current study examined how transdiagnostic IP symptom dimensions relate to neural affective reactivity in a highly comorbid patient sample.
Methods:
Participants (N = 99) completed a battery of IP symptom assessments as well as a target categorization task while viewing pleasant, unpleasant, and neutral images during electroencephalography recording. ERPs to each image valence were averaged from 400 to 1,000 ms following picture onset at pooled centroparietal and occipital electrodes to calculate the LPP. A principal components analysis performed on the IP symptom measures resulted in two factors: affective distress/misery and fear-based anxiety.
Results:
Fear-based anxiety was associated with enhanced LPP reactivity to unpleasant, but not pleasant, images. Distress/misery was related to attenuated average LPP reactivity across images.
Conclusions:
Results revealed a dissociable effect of IP symptom factors in a transdiagnostic sample such that enhanced reactivity to negative images was specific to enhanced fear-based anxiety symptoms while distress/misery symptoms predicted blunted affective reactivity. Neural affective reactivity may serve as an objective biological marker to elucidate the nature of psychological concerns in individuals with comorbid IPs.
Keywords: EEG/Evoked Potentials, Anxiety/Anxiety disorders, Biological Markers, Depression
Introduction
Internalizing psychopathologies (IPs) are the most prevalent mental disorders (Kessler, Chiu, Demler, & Walters, 2005), which contribute to significant global economic burden (Greenberg, Fournier, Sisitsky, Pike, & Kessler, 2015) and are highly comorbid (Brown, Campbell, Lehman, Grisham, & Mancill, 2001; Kessler et al., 2005). Given that IPs encompass a spectrum of psychopathology, a closer examination of distinctions within IPs may further understanding of pathophysiology and mechanisms separating disorders at the phenotypic level. Studies employing factor analytic approaches have identified two empirically-supported IP factors (Kendler, Prescott, Myers, & Neale, 2003; Slade & Watson, 2006; Vollebergh et al., 2001), distress/misery (i.e., MDD, PDD, GAD) and fear-based anxiety (i.e., PD, SAD, SP). Elucidating the etiology and mechanisms contributing to these discrete IP factors may inform targeted interventions to support movement towards precision medicine in the treatment of psychiatric disorders.
While emotion dysregulation has been proposed as a transdiagnostic mechanism underlying IPs, evidence suggests distinct patterns of reactivity, which distinguish fear-based anxiety and distress/misery disorders. Disorders characterized by fear-based anxiety (e.g., SP, SAD) are associated with enhanced neural activation in corticolimbic regions (e.g., amygdala, insula) during the processing of threat-relevant stimuli (e.g., Etkin & Wager, 2007). Patterns of emotion reactivity have been less consistent in patients with distress/misery disorders. Investigations of depression alone have been guided by three disparate theories proposed to explain deficits in emotion processing (Rottenberg, Gross, & Gotlib, 2005). The positive attenuation hypothesis suggests that reactivity is blunted to positive stimuli while the negative potentiation hypothesis posits enhanced reactivity to negative stimuli. Meanwhile, the emotion context insensitivity (ECI) hypothesis proposes blunted reactivity to all stimuli.
To characterize relations between IPs and emotional reactivity at the neurophysiological level, researchers have used event-related potentials (ERPs), derived from electroencephalogram (EEG) data. In particular, the late positive potential (LPP), which is a slow, positive-going ERP component that emerges 300 ms after stimulus onset and is potentiated by emotional stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Schupp et al., 2000). The LPP is associated with activation in cortical (e.g., parietal, occipital cortices) and subcortical (e.g., amygdala, striatum) regions supporting emotion processing (Sabatinelli, Keil, Frank, & Lang, 2013; Sabatinelli, Lang, Keil, & Bradley, 2007). The LPP is hypothesized to reflect allocation of attention to motivationally significant stimuli (Bradley, 2009).
The LPP has been employed to characterize affective reactivity in IPs. Regarding distress/misery diagnoses, in depressed individuals, studies demonstrate blunted LPP to unpleasant stimuli (Foti, Olvet, Klein, & Hajcak, 2010; MacNamara, Kotov, & Hajcak, 2016; Weinberg, Perlman, Kotov, & Hajcak, 2016). Furthermore, reduced reactivity to pleasant stimuli has been reported in depressed samples (Klawohn, Burani, Bruchnak, Santopetro, & Hajcak, 2020; Weinberg et al., 2016). A blunted LPP across emotional stimuli is evident in those with a family history of distress/misery disorders (Kujawa, Hajcak, Torpey, Kim, & Klein, 2012; Nelson, Perlman, Hajcak, Klein, & Kotov, 2015) and may be evident at subclinical levels of depression symptoms (Benning & Ait Oumeziane, 2017) while blunted LPP to pleasant stimuli prospectively predicts depression symptoms (Sandre, Bagot, & Weinberg, 2019). In contrast, generalized anxiety disorder has often been associated with increased reactivity to aversive stimuli (MacNamara & Hajcak, 2010; MacNamara et al., 2016; however, Weinberg & Hajcak, 2011a). Meanwhile, studies demonstrate reduced LPP to threatening faces in combat-exposed veterans with PTSD (DiGangi et al., 2017; MacNamara, Post, Kennedy, Rabinak, & Phan, 2013); however, other work suggests enhanced LPPs to negative images in those with greater posttraumatic stress symptoms (Lobo et al., 2014).Thus, studies employing categorical diagnoses or disorder-specific symptoms provide mixed results across the distress/misery factor.
Research has also examined the LPP in fear-based anxiety disorders. Across DSM-IV anxiety disorders, one study reported no modulation of the LPP to rewarding or threatening images (Weinberg et al., 2016). In contrast, in a transdiagnostic sample, higher social anxiety symptoms predicted greater LPP to negative images while greater panic symptoms predicted less differentiation in reactivity between image valences (MacNamara, Jackson, Fitzgerald, Hajcak, & Phan, 2019; see also, Weinberg & Sandre, 2018). Social anxiety is associated with an enhanced LPP to threatening faces (Kujawa, MacNamara, Fitzgerald, Monk, & Phan, 2015; Moser, Huppert, Duval, & Simons, 2008) and negative images (Kinney, Burkhouse, & Klumpp, 2019). In individuals with phobias, studies suggest enhanced LPPs specific to feared stimuli (Flykt & Caldara, 2006; Leutgeb, Schäfer, & Schienle, 2009; Norberg, Peira, & Wiens, 2010). Furthermore, an enhanced LPP is evident in individuals with a family history of fear-based anxiety (Nelson et al., 2015).
Inconsistent LPP-IP relations may be partially explained by comorbidities which obscure disorder-specific effects. Few studies have examined the influence of comorbid diagnoses on LPP relations. Previous work suggests comorbid MDD obscures LPP-GAD relations (MacNamara et al., 2016) while other studies demonstrate no influence of comorbid diagnoses (Klawohn et al., 2020; MacNamara & Proudfit, 2014; Weinberg et al., 2016). Further, despite substantial comorbidity between IPs, prior studies have utilized categorical diagnoses or dimensional approaches tied to categorical diagnoses (e.g., using IMAS Generalized Anxiety scale). The current study sought to fill this gap by employing a patient sample with substantial IP comorbidity to examine IP-LPP relations using a transdiagnostic dimensional approach. Given inconsistent findings with individual dimensional subscales, this study utilized data reduction techniques to identify dimensions across broad and disorder-specific IP measures, expanding upon previous studies utilizing one picture valence, unselected undergraduate samples, and categorical diagnoses. Consistent with previous studies utilizing this sample, we predicted that two IP symptom dimensions would emerge: fear-based anxiety and distress/misery (Burkhouse, Gorka, Afshar, & Phan, 2017; Gorka, Burkhouse, Afshar, & Phan, 2017). We hypothesized that fear-based anxiety symptoms would be associated with hyper-reactivity to negative images (e.g., Kinney et al., 2019; Lobo et al., 2014). Meanwhile, consistent with the ECI (Rottenberg et al., 2005), distress/misery symptoms would be associated with valence-independent blunted reactivity.
Method
Participants
Participants included 99 patients from a treatment study investigating the efficacy of psychotherapy compared to selective serotonin reuptake inhibitors (SSRI). Consistent with the RDoC initiative, we enrolled a transdiagnostic sample with a variety of IPs. Patients, ages 18–65, were enrolled in the study if they had an IP, based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1995) administered by trained masters- and doctoral-level clinicians. Patients were required to score ≥ 23 on the Depression Anxiety Stress Scales (Lovibond & Lovibond, 1995) and ≤ 60 on the Global Assessment of Functioning. Exclusion criteria included psychotic or manic symptoms, active suicidal ideation, SSRI contraindications or resistance, cognitive impairment, primary obsessive compulsive or substance use disorder, ongoing treatment, obstacles to providing informed consent, or positive pregnancy test. The University of Illinois at Chicago Institutional Review Board approved the study, and informed consent was obtained from all participants. All participants were compensated for their time, and all procedures complied with the Declaration of Helsinki. The current study included data from participants with complete baseline data (i.e., prior to the initiation of treatment).
Measures
Symptom Measures
Participants completed a battery of internalizing symptom measures. The total score was used in analyses unless otherwise specified.
The Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959) is a 14-item scale administered by a clinician to assess anxiety symptom severity over the past week.
The Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960) is a 21-item scale administered by a clinician to assess depressive symptom severity over the past week.
The Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) is a 21-item self-report scale measuring depressive symptoms over the past week.
The Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988) is a 21-item self-report scale assessing anxiety symptoms over the past month.
The State-Trait Anxiety Inventory- Trait Subscale (STAI-T; Spielberger, Gorusch, & Lushene, 1990) is a 20-item subscale of the STAI which assesses trait anxiety.
The Overall Depression Severity and Impairment Scale (ODSIS; Bentley, Gallagher, Carl, & Barlow, 2014) consists of five items measuring depressive symptom severity and functional impairment over the past week.
The Overall Anxiety Severity and Impairment Scale (OASIS; Norman, Hami Cissell, Means-Christensen, & Stein, 2006) consists of five items measuring anxiety symptom severity and functional impairment over the past week.
The Depression Anxiety Stress Scales (DASS-21; Lovibond & Lovibond, 1995) comprises 21 items which form the self-report depression, anxiety, and stress subscales. Subscales are calculated by summing their respective seven items.
The Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990) is a 16-item questionnaire that measures trait worry.
The Ruminative Response Scale (RRS; Treynor, Gonzalez, & Nolen-Hoeksema, 2003) contains 22 items characteristic of depressive rumination.
The Inventory of Depression and Anxiety Symptoms-II (IDAS-II; Watson et al., 2012) comprises 99 items concerning internalizing symptoms in the past two weeks. Items are summed to create subscales: general depression, dysphoria, lassitude, insomnia, suicidality, appetite gain, appetite loss, ill-temper, well-being, panic, social anxiety, claustrophobia, euphoria, mania, traumatic intrusions, traumatic avoidance, and tendencies for checking, ordering, and cleaning. Subscales measuring bipolar disorder and obsessive-compulsive behaviors were excluded since they formed aspects of the exclusionary criteria. The general depression subscale was removed for overlapping items with other subscales.
The Panic Disorder Severity Scale (PDSS; Shear et al., 1997) includes seven items which assess the frequency, intensity, and impairment related to panic attacks and panic disorder.
The Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987) contains 24 items assessing the presence of social anxiety symptoms across social interactions and performance situations. Items are summed to create two subscales: total anxiety and total avoidance.
Data Reduction
Measures/subscales were submitted to a principal components analysis (PCA) using direct oblimin rotation to identify transdiagnostic symptom dimensions and reduce the number of measures. Given that the aim was to replicate two previously identified factors in this sample, distress/misery and fear-based anxiety, a two-factor solution was specified. The two factors accounted for 37.93% of the variance, 20.31% (λ = 5.69) and 17.62% (λ = 4.93), respectively. Factor scores were exported using the regression-based approach and utilized in subsequent analyses. The first factor, distress/misery, included measures of depressive symptoms and trait anxiety while the second factor, fear-based anxiety, was composed of measures related to chronic arousal and fear (Table 1).
Table 1.
Factor Loadings for Principal Components Analysis with Direct Oblimin Rotation of Internalizing Symptom Measures/Subscales
| Measure/Subscale | Distress/Misery | Fear-Based Anxiety | α | M(SD) |
|---|---|---|---|---|
| IDAS Dysphoria | .81 | .17 | .67 | 31.76 (5.54) |
| ODSIS Total | .76 | −.18 | .91 | 8.79 (4.52) |
| BDI Total | .75 | −.09 | .84 | 26.06 (8.48) |
| DASS Depression | .75 | −.28 | .88 | 11.52 (4.94) |
| RRS Total | .69 | −.03 | .88 | 58.90 (11.55) |
| IDAS Wellbeing | −.66 | .21 | .87 | 15.23 (5.05) |
| IDAS Lassitude | .63 | −.04 | .77 | 17.91 (5.37) |
| HAM-D Total | .59 | .06 | .60 | 12.45 (4.26) |
| STAI-T Total | .58 | .13 | .77 | 56.89 (7.10) |
| IDAS Suicidality | .48 | −.08 | .77 | 7.87 (2.96) |
| IDAS Appetite Loss | .34 | .18 | .91 | 6.43 (3.29) |
| IDAS Insomnia | .28 | .19 | .83 | 15.22 (6.27) |
| BAI Total | .10 | .74 | .86 | 18.93 (9.46) |
| DASS Anxiety | −.23 | .73 | .77 | 7.82 (4.43) |
| PDSS Total | .12 | .71 | .87 | 5.73 (4.89) |
| IDAS Panic | −.02 | .71 | .73 | 13.31 (4.37) |
| IDAS Social | −.27 | .69 | .83 | 14.77 (5.51) |
| IDAS Claustrophobia | −.09 | .62 | .85 | 6.64 (3.13) |
| HAM-A Total | .39 | .61 | .71 | 17.40 (6.44) |
| LSAS Total Anxiety | −.32 | .60 | .91 | 33.79 (13.49) |
| OASIS Total | .20 | .59 | .81 | 9.48 (3.60) |
| PSWQ Total | .02 | .53 | .87 | 64.44 (8.64) |
| LSAS Total Avoidance | −.18 | .52 | .90 | 29.56 (14.26) |
| DASS Stress | .16 | .47 | .68 | 13.35 (3.58) |
| IDAS Intrusions | .27 | .30 | .63 | 7.72 (2.94) |
| IDAS Temper | .18 | .20 | .83 | 10.96 (4.62) |
| IDAS Avoidance | .16 | .18 | .82 | 8.95 (3.60) |
| IDAS Appetite Gain | −.00 | .08 | .79 | 6.56 (3.10) |
Note. Factor loadings ≥ |.45| are in boldface. IDAS = Inventory of Depression and Anxiety Symptoms-II; ODSIS = Overall Depression Severity and Impairment Scale; BDI = Beck Depression Inventory-II; DASS = Depression Anxiety Stress Scales; RRS = Ruminative Response Scale; HAM-D = Hamilton Depression Rating Scale; STAI-T = State-Trait Anxiety Inventory- Trait Subscale; BAI = Beck Anxiety Inventory; PDSS = Panic Disorder Severity Scale; HAM-A = Hamilton Anxiety Rating Scale; LSAS = Liebowitz Social Anxiety Scale; OASIS = Overall Anxiety Severity and Impairment Scale; PSWQ = Penn State Worry Questionnaire.
Emotional Interrupt Task
Participants completed an emotional interrupt task (Mitchell, Richell, Leonard, & Blair, 2006; Weinberg & Hajcak, 2011b) during EEG collection. Sixty IAPS images (Lang, Bradley, & Cuthbert, 2008), comprised of 20 pleasant, 20 neutral, and 20 unpleasant images, were selected for this task (see Supplement). The task included 180 trials, with 60 trials for each picture valence, presented in random order. Each trial began with a fixation cross (800 ms) followed by the presentation of a task-irrelevant IAPS image (pre-target picture) for 1000 ms. Participants were then shown a target (i.e., circle or square) for 150 ms before the same IAPS image that was shown prior to the target presentation appeared again for 400 ms (post-target picture). Trials were followed by an inter-trial interval, which varied randomly from 1500 to 2000 ms, during which the screen was blank. Participants were asked to identify the target shape by clicking the corresponding mouse button.
Electroencephalography Recording
Continuous EEG was recorded during the task using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands) on the 10/20 system. Thirty‐four electrode sites were used (including FCz and Iz), along with one electrode placed on each mastoid. Electrooculogram (EOG) was recorded from four additional facial electrodes to measure eye blinks and eye movements: 1 cm above and below the right eye to measure vertical eye movements (VEOG) and 1 cm beyond each eye to measure horizontal eye movements (HEOG). The EEG signal was pre-amplified at the electrode to improve the signal-to-noise ratio. The data were digitized at 24-bit resolution with a least significant bit (LSB) value of 31.25 nV and a sampling rate of 1,024 Hz, using a low‐pass fifth order sinc filter with a −3dB cutoff point at 204.8 Hz.
Electroencephalography Data Analyses
Off-line data analyses were performed using Brain Vision Analyzer 2 software (Brain Products, Gilching, Germany). EEG data were re-referenced to the average of the two mastoids and band-pass filtered (0.01Hz and 30Hz). Eye corrections were performed according to standard procedures (Gratton, Coles, & Donchin, 1983). Data were segmented from 200 ms before pre-target image onset and continuing for 1,200 ms. Semiautomated artifact analysis was used to identify a voltage step of more than 50μV between sample points, a voltage difference of 300μV within a trial, and a maximum difference of less than 0.50μV within 100 ms intervals. Trials were inspected visually for any remaining artifacts and removed when appropriate. Baseline correction was performed using 200 ms pre-stimulus. ERPs, for trials in which the target was correctly identified, were averaged for each image type 400 to 1,000 ms after pre-target picture onset using pooled centroparietal sites (P3, P4, Pz, PO3, PO4, CP1, and CP2) consistent with centroparietal poolings used in previous research (e.g., DiGangi et al., 2017; Kinney et al., 2019). Participants were required to have an overall accuracy ≥ 50% to be included.
Data Analysis
All statistical analyses were conducted with IBM SPSS Statistics, version 26.0 (IBM, Armonk, NY). Internalizing factors were entered simultaneously as covariates of interest in an rmANCOVA predicting LPP to each image valence; Greenhouse-Geiser correction was applied for violations of sphericity. The main effect of image valence was examined utilizing t-tests for estimated marginal means with Bonferroni correction for multiple comparisons. To parse interactions between image valence and factors, residual scores were calculated by regressing reactivity to neutral images on LPP to pleasant and unpleasant images, respectively, and saving the unstandardized residual, isolating the variance specific to image valence not accounted for by the neutral image. In prior work (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017), residual scores have been shown to isolate component specific variance. Both IP symptom dimensions were then simultaneously entered as predictors of the LPP residuals in separate linear regressions. Behavioral data analyses are available in Supplemental Material.
Results
Descriptive statistics for the sample are displayed in Table 2. LPP waveforms are presented in Figure 1. Bivariate correlations between age, gender, and LPP indicated female gender was significantly associated with enhanced LPP to pleasant pictures, r(97) = .26, p = .01. Age correlated with reduced LPP to unpleasant images, r(97) = −.25, p =.01. None of the other correlations reached significance.
Table 2.
Demographics and Clinical Characteristics of the Sample
| Demographics | M (SD) |
|---|---|
| Age (years) | 27.37 (8.75) |
| Education (years) | 16.24 (3.15) |
| Number of current IP diagnoses (average) | 2.52 (1.17) |
| EEG Task Variables | M (SD) |
| Unpleasant Image LPP (µV) | 3.36 (3.74) |
| Pleasant Image LPP (µV) | 2.71 (3.34) |
| Neutral Image LPP (µV) | −.45 (3.16) |
| n (%) | |
| Gender (female) | 73 (73.74) |
| Race | |
| White | 62 (62.63) |
| Black | 16 (16.16) |
| Asian | 11 (11.11) |
| Multiracial or other | 10 (10.10) |
| Ethnicity (Hispanic/Latinx) | 21 (21.21) |
| Primary IP Diagnosis | |
| Generalized Anxiety Disorder | 34 (34. 34) |
| Major Depressive Disorder | 30 (30.30) |
| Social Anxiety Disorder | 24 (24.24) |
| Panic Disorder | 2 (2.02) |
| PTSD | 2 (2.02) |
| Dysthymia | 1 (1.01) |
| Current IP Diagnosis | |
| Generalized Anxiety Disorder | 62 (62.63) |
| Major Depressive Disorder | 61 (61.62) |
| Social Anxiety Disorder | 61 (61.62) |
| Panic Disorder | 21 (21.21) |
| Specific Phobia | 19 (19.19) |
| PTSD | 13 (13.13) |
| Dysthymia | 5 (5.05) |
| Obsessive Compulsive Disorder | 3 (3.03) |
| Agoraphobia | 3 (3.03) |
| Panic Disorder + Agoraphobia | 1 (1.01) |
Note. IP= Internalizing Psychopathology. EEG = Electroencephalogram. LPP = Late Positive Potential. PTSD = Posttraumatic Stress Disorder.
Figure 1.

Waveforms and topographical maps for pleasant, unpleasant, and neutral images. Waveforms and topographical maps depicting the late positive potential (LPP) to unpleasant (a), pleasant (b), and neutral images (c) calculated 400–1,000 ms after image onset at a pooling of P3, P4, PZ, PO3, PO4, CP1, and CP2.
There was a significant main effect of image valence, F(1.87, 179.06) = 71.62, p < .001,ηp2= .43. LPP to pleasant and unpleasant images differed significantly from reactivity to neutral images (p’s <.001); however, reactivity did not significantly differ between pleasant and unpleasant images (p =.09). Results revealed an interaction between image valence and fear-based anxiety symptoms, F(1.87, 179.06)= 3.31, p = .04, ηp2= .03. To parse the interaction, the fear-based anxiety factor was entered into two separate linear regressions, controlling for the distress/misery factor, predicting the residual LPP to pleasant and unpleasant images, respectively. Greater fear-based anxiety symptoms predicted enhanced residual LPP to unpleasant images, β = 0.24, t(97) = 2.43, p =.02; the association between fear-based anxiety and residual LPP to pleasant images was not significant, β = 0.05, t(97) = 0.50, p =.62 (Figure 2). Given significant bivariate correlations, age and sex were entered as covariates of no interest in the corresponding linear regressions predicting residual LPPs, and results were maintained.
Figure 2.
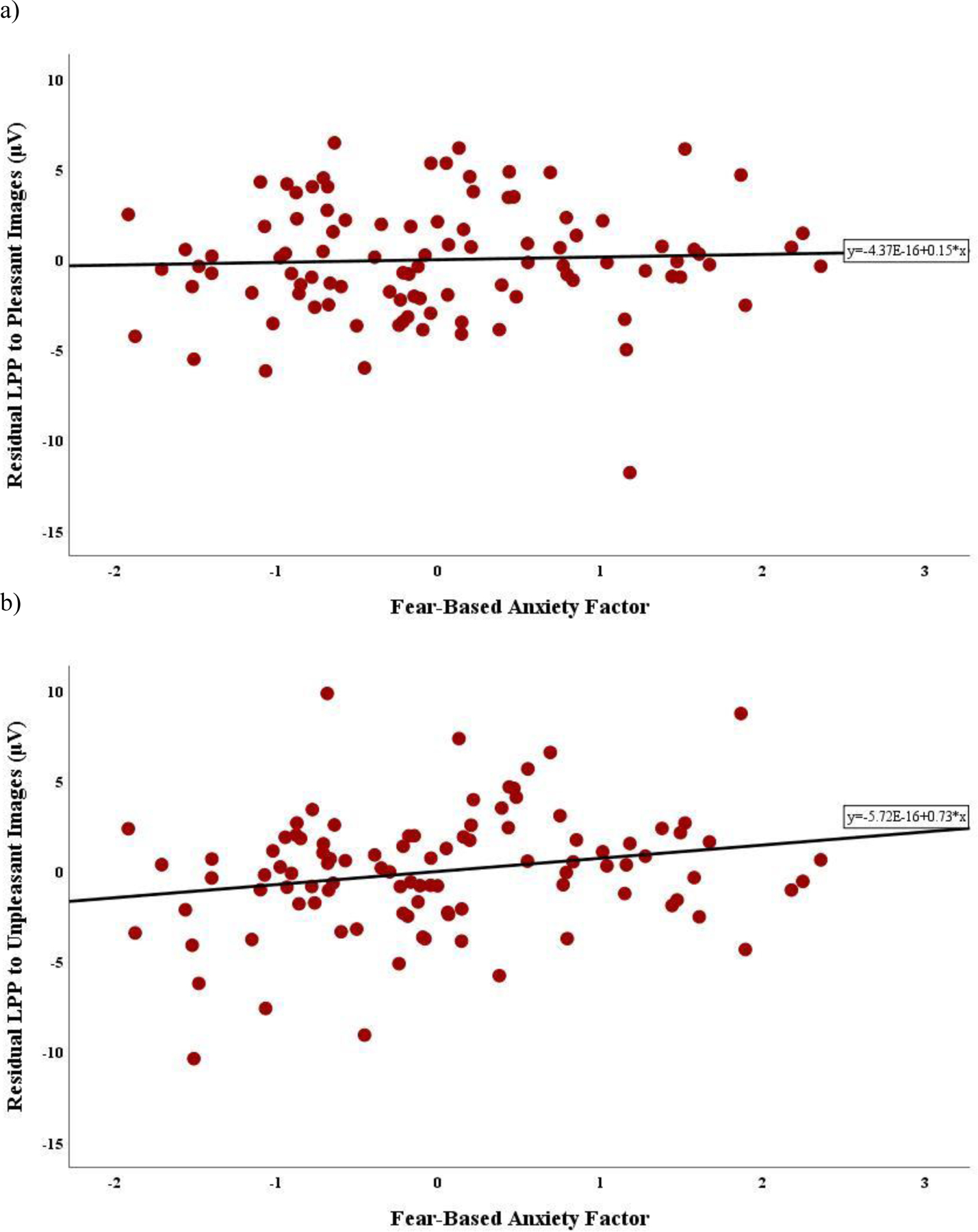
Relations between the fear-based anxiety factor and late positive potential (LPP) residual to pleasant and unpleasant images. Scatterplots depicting the bivariate association between LPP residuals for (a) pleasant and (b) unpleasant images and the fear-based anxiety factor.
Results also revealed a main effect of distress/misery, F(1, 96) = 10.85, p = .001,ηp2= .10. LPP to pleasant, unpleasant, and neutral images were averaged to create an index of average reactivity. Distress/misery symptoms were entered into a linear regression predicting average LPP across images, controlling for the fear-based anxiety factor, which revealed that greater distress/misery symptoms were associated with an attenuated LPP across images, β = −0.32, t(97) = −3.29, p =.001 (Figure 3). There were no other significant main effects or interactions.
Figure 3.

Relation between the distress/misery factor and average late positive potential (LPP) across all images. Scatterplot depicting the bivariate association between average LPP and the distress/misery factor.
Discussion
The current study employed a transdiagnostic, highly comorbid sample to examine relations between IP factors and neural reactivity to affective images, assessed via the LPP. Building on previous research, we employed a dimension reduction technique to consolidate broad and disorder-specific measures to dimensional IP symptom factors, fear-based anxiety and distress/misery. Results indicated that greater fear-based anxiety symptoms only associated with enhanced LPP to unpleasant images. In contrast, greater distress/misery symptoms corresponded to an attenuated LPP across image valences. Despite differences in the sample and measures, these findings are consistent with prior research showing that risk for distress disorders is associated with an attenuated LPP to all stimuli, whereas risk for fear disorders is associated with an enhanced LPP to unpleasant stimuli (Nelson et al., 2015). Taken together, these findings contribute to the identification of potential neural mechanisms implicated in dimensional models of psychopathology (Watson et al., 2022) by demonstrating that within a heterogenous sample of patients with IPs, an enhanced LPP to negative stimuli may be an objective, neurophysiological indicator of fear-based anxiety symptoms while an attenuated LPP indexes the distress/misery factor.
Associations between the distress/misery factor and global deficits in reactivity support initial hypotheses. Partially consistent with the ECI model (Rottenberg et al., 2005), which suggests that elevated distress/misery symptoms associate with valence-independent blunted reactivity, we demonstrated a blunted LPP to pleasant and unpleasant images related to the distress/misery factor. Interestingly, findings also exhibited blunted reactivity to neutral images, consistent with Nelson et al., 2015, representing overgeneralized or global disengagement from all stimuli. The ECI model suggests that a pathological form of an evolutionary adaptation to engage defensive motivational systems to aversive stimuli may underlie emotion dysregulation in depression (Rottenberg et al., 2005). Thus, these findings potentially extend disengagement as a protective mechanism to neutral stimuli, demonstrating inflexibility in reactivity across stimuli valence in individuals characterized by increased distress/misery symptoms. Despite emerging support for alterations in reactivity to neutral stimuli in IPs, studies often employ reactivity to neutral stimuli as a baseline or comparison; thus, future research should investigate reactivity to neutral stimuli independently to better characterize affective functioning (Bylsma, 2021). Over-engagement of defensive motivational systems may be evident in blunted reactivity at the electrocortical level and symptoms such as anhedonia at the phenotypic level. Indeed, the distress/misery factor was characterized by depressive symptomatology (e.g., low positive affect) and related constructs (e.g., rumination) as well as trait anxiety. In previous studies, low positive affect, rather than heightened negative affect (MacNamara et al., 2019; Weinberg & Sandre, 2018), is related to blunted LPP, reflecting this pathological disengagement from motivationally salient stimuli at the self-report and neurophysiological level (Weinberg & Sandre, 2018). Further, individuals exhibiting elevated trait anxiety may ruminate, which may be reflected in their affective reactivity as an inability to engage with stimuli due to worries. Thus, the distress/misery factor may capture profiles of IP symptomatology characterized by withdraw, reflected in valence-independent blunted reactivity, which may be represented by transdiagnostic constructs such as rumination and anhedonia at the phenotypic level.
Consistent with previous studies (e.g., Lobo et al., 2014; MacNamara et al., 2019), LPP to unpleasant stimuli was potentiated in individuals with greater fear-based anxiety symptoms. An enhanced LPP related to greater fear-based anxiety symptoms may reflect chronic fear which manifests as heightened arousal to negative stimuli. Alternatively, heightened LPP to negative stimuli may be interpreted as pathological engagement with threatening stimuli in anxious psychopathology (MacNamara & Hajcak, 2010). Studies examining fear-based IPs have often focused on LPP to unpleasant images (e.g., Kinney et al., 2019; Leutgeb et al., 2009). A small but consistent body of literature has demonstrated the absence of a significant relationship between LPP to pleasant images and fear-based anxiety psychopathology (e.g., DiGangi et al., 2017; Weinberg et al., 2016), suggesting that enhanced LPP may be specific to negative images, representing a neural signature for fear-based IPs.
Findings from the current study exhibit dissociable relations between the LPP and IP factors, highlighting the importance of considering comorbidity. In comparison to studies examining individual diagnoses, few studies have examined comorbidity in the context of the LPP. One study found that GAD and enhanced LPP relations emerged when controlling for comorbid depression (MacNamara et al., 2016), indicating that MDD may obscure altered neural reactivity. In contrast, studies have failed to demonstrate an effect of comorbid diagnoses (Klawohn et al., 2020; MacNamara & Proudfit, 2014). However, utilization of one image valence and categorical diagnoses hinders integrating inconsistent findings. The current study suggests the importance of considering transdiagnostic IP symptoms instead of categorical comorbid diagnoses given differential effects of IP factors. Considering the limitations of the current nosological system, findings from the current study corroborate distinctions between fear and distress factors at the electrocortical level, providing support for a dimensional conceptualization of psychopathology (e.g., Hierarchical Taxonomy of Psychopathology (HiTOP)) to prevent problems arising from comorbidity, diagnostic overlap, and polythetic criteria (Watson et al., 2022).
Strengths of this study include the use of a transdiagnostic, comorbid sample, and broad and disorder-specific IP measures. However, there were limitations which should be addressed. In contrast to previous studies using a smaller subset of this sample (Burkhouse et al., 2017; Gorka et al., 2017), broad measures of anxiety symptoms (e.g., HAM-A) loaded onto the fear-based anxiety factor, which may also be inconsistent with studies suggesting that GAD loads onto the distress/misery factor (e.g., Vollebergh et al., 2001). Comorbid diagnoses were the norm rather than the exception in this sample; thus, the fear-based anxiety factor may have captured individuals with more than one anxiety psychopathology (e.g., GAD and PD). Broad anxiety measures may reflect chronic physiological arousal across GAD and fear-related IPs. Further, given that anxiety-related measures predominantly loaded onto the fear-based anxiety factor, the distress/misery factor was comprised mostly of depression measures and related constructs. Thus, the distress/misery factor may be better identified as a depression factor; however, it is recommended that future studies replicate these findings to better ascertain the factor dimensions. Next, the sample size is relatively small for a PCA, and the factors only accounted for approximately 38% of the variance in the sample, suggesting that other important symptom dimensions may exist. Furthermore, the sample was predominantly anxious, so the study was not powered to test diagnostic differences in the LPP. Future studies would benefit from recruiting a diagnostically distributed sample to examine if dimensional versus categorical approaches are more sensitive to individual differences in the LPP. Finally, the sample size and composition precluded examination of age and sex as moderators of LPP-IP dimension relations. Thus, future studies should examine whether results are consistent across both genders and with increasing age.
Conclusion
Results from the current study emphasize the LPP as a potential objective biomarker of altered affective reactivity in IPs. Findings suggest that the LPP may serve as a correlate of fear and distress symptoms, which has important implications for the translation of models of dimensional psychopathology to clinical settings. For instance, individual differences in the LPP may inform treatment selection (e.g., exposure for individuals with greater LPP to unpleasant images) and the order in which symptoms are addressed, such as behavioral activation first for individuals with greater distress/misery symptoms exhibiting blunted LPP. Furthermore, the LPP may be particularly well-suited as an objective and transdiagnostic assessment of treatment progress for novel treatments targeting transdiagnostic mechanisms such as emotion dysregulation (Wilamowska et al., 2010). Taken together, the current study demonstrates increased LPP to unpleasant images as an objective biomarker of fear-based anxiety symptoms and attenuated affective reactivity as an indicator of distress/misery symptoms in a comorbid, transdiagnostic sample.
Supplementary Material
Acknowledgements:
We would like to thank Abigail Ayemoba and Saranya Menon for their help in preparing the data for analyses. This work was supported by National Institute of Mental Health grant R01MH101497 (to KLP), and Center for Clinical and Translational Science (CCTS) UL1RR029879. KLB is supported by National Institute of Mental Health grant [MH113793]. MG is supported by National Institute of Mental Health training grant [5T32MH067631].
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II San Antonio, TX: Psychological Corporation. [Google Scholar]
- Benning SD, & Ait Oumeziane B (2017). Reduced positive emotion and underarousal are uniquely associated with subclinical depression symptoms: Evidence from psychophysiology, self-report, and symptom clusters. Psychophysiology, 54(7), 1010–1030. 10.1111/psyp.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley KH, Gallagher MW, Carl JR, & Barlow DH (2014). Development and validation of the Overall Depression Severity and Impairment Scale. Psychological Assessment, 26(3), 815–830. 10.1037/a0036216 [DOI] [PubMed] [Google Scholar]
- Bradley MM (2009). Natural selective attention: Orienting and emotion. Psychophysiology, 46(1), 1–11. 10.1111/j.1469-8986.2008.00702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, & Mancill RB (2001). Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology, 110(4), 585–599. 10.1037/0021-843X.110.4.585 [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Gorka SM, Afshar K, & Phan KL (2017). Neural reactivity to reward and internalizing symptom dimensions. Journal of Affective Disorders, 217, 73–79. 10.1016/j.jad.2017.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM (2021). Emotion context insensitivity in depression: Toward an integrated and contextualized approach. Psychophysiology, 58(2), e13715. 10.1111/psyp.13715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- DiGangi JA, Burkhouse KL, Aase DM, Babione JM, Schroth C, Kennedy AE, … Phan KL (2017). An electrocortical investigation of emotional face processing in military-related posttraumatic stress disorder. Journal of Psychiatric Research, 92, 132–138. 10.1016/j.jpsychires.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1995). Structured clinical interview for DSM–IV Axis I disorders—Patient edition (SCID–I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Flykt A, & Caldara R (2006). Tracking fear in snake and spider fearful participants during visual search: A multi-response domain study. Cognition and Emotion, 20, 1075–1091. 10.1080/02699930500381405 [DOI] [Google Scholar]
- Foti D, Olvet DM, Klein DN, & Hajcak G (2010). Reduced electrocortical response to threatening faces in major depressive disorder. Depression & Anxiety, 27(9), 813–820. 10.1002/da.20712 [DOI] [PubMed] [Google Scholar]
- Gorka SM, Burkhouse KL, Afshar K, & Phan KL (2017). Error-related brain activity and internalizing disorder symptom dimensions in depression and anxiety. Depression and Anxiety, 34(11), 985–995. 10.1002/da.22648 [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, & Kessler RC (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). The Journal of Clinical Psychiatry, 76(2), 155–162. 10.4088/JCP.14m09298 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. British Journal of Medical Psychology, 32, 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, & Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, & Neale MC (2003). The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry, 60(9), 929. 10.1001/archpsyc.60.9.929 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62(6), 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney KL, Burkhouse KL, & Klumpp H (2019). Self-report and neurophysiological indicators of emotion processing and regulation in social anxiety disorder. Biological Psychology, 142, 126–131. 10.1016/j.biopsycho.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawohn J, Burani K, Bruchnak A, Santopetro N, & Hajcak G (2020). Reduced neural response to reward and pleasant pictures independently relate to depression. Psychological Medicine, 1–9. 10.1017/S0033291719003659 [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, & Klein DN (2012). Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry, 53(2), 207–215. 10.1111/j.1469-7610.2011.02461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. 10.1007/s10802-015-0029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8 University of Florida, Gainesville, FL. [Google Scholar]
- Leutgeb V, Schäfer A, & Schienle A (2009). An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology, 82(3), 293–300. 10.1016/j.biopsycho.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Liebowitz MR (1987). Social phobia. Modern Problems of Pharmacopsychiatry, 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Lobo I, David IA, Figueira I, Campagnoli RR, Volchan E, Pereira MG, & de Oliveira L (2014). Brain reactivity to unpleasant stimuli is associated with severity of posttraumatic stress symptoms. Biological Psychology, 103, 233–241. 10.1016/j.biopsycho.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Lovibond SH, & Lovibond PF (1995). Manual for the Depression Anxiety Stress Scale (2nd ed.). Sydney: Psychology Foundation. [Google Scholar]
- MacNamara A, & Hajcak G (2010). Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression & Anxiety, 27(3), 234–243. 10.1002/da.20679 [DOI] [PubMed] [Google Scholar]
- MacNamara A, Jackson TB, Fitzgerald JM, Hajcak G, & Phan KL (2019). Working memory load and negative picture processing: Neural and behavioral associations with panic, social anxiety, and positive affect. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(2), 151–159. 10.1016/j.bpsc.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Kotov R, & Hajcak G (2016). Diagnostic and symptom-based predictors of emotional processing in generalized anxiety disorder and major depressive disorder: An event-related potential study. Cognitive Therapy & Research, 40(3), 275–289. 10.1007/s10608-015-9717-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Post D, Kennedy AE, Rabinak CA, & Phan KL (2013). Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biological Psychology, 94(2), 441–449. 10.1016/j.biopsycho.2013.08.009 [DOI] [PubMed] [Google Scholar]
- MacNamara A, & Proudfit GH (2014). Cognitive load and emotional processing in generalized anxiety disorder: Electrocortical evidence for increased distractibility. Journal of Abnormal Psychology, 123(3), 557–565. 10.1037/a0036997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy, 28(6), 487–495. 10.1016/0005-7967(90)90135-6 [DOI] [PubMed] [Google Scholar]
- Mitchell D, Richell R, Leonard A, & Blair R (2006). Emotion at the expense of cognition: Psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology, 115, 559–566. 10.1037/0021-843X.115.3.559 [DOI] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, & Simons RF (2008). Face processing biases in social anxiety: An electrophysiological study. Biological Psychology, 78(1), 93–103. 10.1016/j.biopsycho.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Hajcak G, Klein DN, & Kotov R (2015). Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychological Medicine, 45(12), 2545–2556. 10.1017/S0033291715000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg J, Peira N, & Wiens S (2010). Never mind the spider: Late positive potentials to phobic threat at fixation are unaffected by perceptual load. Psychophysiology, 47(6), 1151–1158. 10.1111/j.1469-8986.2010.01019.x [DOI] [PubMed] [Google Scholar]
- Norman SB, Hami Cissell S, Means-Christensen AJ, & Stein MB (2006). Development and validation of an Overall Anxiety Severity and Impairment Scale (OASIS). Depression and Anxiety, 23(4), 245–249. 10.1002/da.20182 [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, & Gotlib IH (2005). Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology, 627–639. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Keil A, Frank DW, & Lang PJ (2013). Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biological Psychology, 92(3), 513–519. 10.1016/j.biopsycho.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, & Bradley MM (2007). Emotional perception: Correlation of functional MRI and event-related potentials. Cerebral Cortex, 17(5), 1085–1091. 10.1093/cercor/bhl017 [DOI] [PubMed] [Google Scholar]
- Sandre A, Bagot RC, & Weinberg A (2019). Blunted neural response to appetitive images prospectively predicts symptoms of depression, and not anxiety, during the transition to university. Biological Psychology, 145, 31–41. 10.1016/j.biopsycho.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, & Lang PJ (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–261. 10.1111/1469-8986.3720257 [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, … Papp LA (1997). Multicenter collaborative Panic Disorder Severity Scale. American Journal of Psychiatry, 154(11), 1571–1575. 10.1176/ajp.154.11.1571 [DOI] [PubMed] [Google Scholar]
- Slade T, & Watson D (2006). The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychological Medicine, 36(11), 1593–1600. 10.1017/S0033291706008452 [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorusch RL, & Lushene RE (1990). Manual for the State-Trait Anxiety Inventory (self-evaluation questionnaire) Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. 10.1023/A:1023910315561 [DOI] [Google Scholar]
- Vollebergh WA, Iedema J, Bijl RV, de Graaf R, Smit F, & Ormel J (2001). The structure and stability of common mental disorders: The NEMESIS study. Archives of General Psychiatry, 58(6), 597–603. 10.1001/archpsyc.58.6.597 [DOI] [PubMed] [Google Scholar]
- Watson D, Levin-Aspenson HF, Waszczuk MA, Conway CC, Dalgleish T, Dretsch MN, … Workgroup HU (2022). Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry, 21(1), 26–54. 10.1002/wps.20943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2011a). Electrocortical evidence for vigilance-avoidance in generalized anxiety disorder. Psychophysiology, 48(6), 842–851. 10.1111/j.1469-8986.2010.01149.x [DOI] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2011b). The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience, 23(10), 2994–3007. 10.1162/jocn.2011.21630 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Perlman G, Kotov R, & Hajcak G (2016). Depression and reduced neural response to emotional images: Distinction from anxiety, and importance of symptom dimensions and age of onset. Journal of Abnormal Psychology, 125(1), 26–39. 10.1037/abn0000118 [DOI] [PubMed] [Google Scholar]
- Weinberg A, & Sandre A (2018). Distinct associations between low positive affect, panic, and neural responses to reward and threat during late stages of affective picture processing. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(1), 59–68. 10.1016/j.bpsc.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Wilamowska ZA, Thompson‐Hollands J, Fairholme CP, Ellard KK, Farchione TJ, & Barlow DH (2010). Conceptual background, development, and preliminary data from the unified protocol for transdiagnostic treatment of emotional disorders. Depression and Anxiety, 27(10), 882–890. 10.1002/da.20735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


