Abstract
Objectives:
The optimal frequency and modality of sarcoma surveillance imaging are uncertain, and current practices vary substantially. While efforts to develop evidence-based guidelines are ongoing, patient perspectives regarding surveillance imaging have not been reported. The primary goal of this study was to pilot the novel Sarcoma Surveillance Survey to assess patient concerns regarding sarcoma surveillance.
Methods:
In this single-center, cross-sectional study, patients receiving surveillance imaging after surgical sarcoma treatment were administered the 10-item Sarcoma Surveillance Survey, the validated Appraisal Scale, measuring positive and negative emotional reactions to imaging, and the Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety Short Form 8a as a measure of anxiety.
Results:
Patients expressed highest levels of concern about cost and radiation exposure associated with surveillance, and most (87.6%) did not express a preference for more or less frequent imaging. Younger patients and those living further away from the imaging center were more concerned about cost of surveillance. Female patients had higher levels of concern compared to males regarding radiation, IV contrast, and overall levels of concern about surveillance. Higher levels of anxiety were correlated with preference for more frequent imaging (rs=0.274, p=0.027) and higher overall level of concern about surveillance (rs=0.259, p=0.037). Higher negative appraisal scores were also correlated with higher overall concerns (rs= 0.323; p=0.012).
Conclusions:
Patient perspectives should be considered when developing sarcoma surveillance strategies. Identifying patients with greater anxiety and concerns regarding imaging may create opportunities for improved surveillance practices as well as counseling and survivorship interventions.
Keywords: Cancer, oncology, PROMIS, sarcoma, surveillance, survey, survivorship
1. Background
Sarcomas are a rare and highly variable form of malignancy. While many patients are curable, approximately 35% succumb to pulmonary metastatic disease in the first 5 years.1 The most common site of sarcoma metastasis is the lungs, making pulmonary surveillance an important aspect of sarcoma management.2,3 However, the optimal modality and frequency of surveillance remains disputed. Under current guidelines, patients undergo between one and six chest examinations per year for a minimum of 10 years, with an estimated mean of 16 studies per patient over the course of follow-up.4 Pulmonary surveillance in sarcoma patients can be performed with either x-ray or computed tomography (CT). CT is more sensitive than x-ray; however, it exposes patients to higher levels of ionizing radiation, incurs greater costs, and has a higher risk of false-positive results leading to a costly and potentially invasive additional workup.5,6 Moreover, the optimal frequency of pulmonary imaging has not been determined. Shorter interval surveillance may detect disease earlier but is more costly in terms of direct imaging expense as well as patient, physician, and caregiver time.7,8 More intense surveillance with CT and higher imaging frequency has not been demonstrated to improve survival.9 However, as treatments for metastatic soft tissue sarcoma improve, early identification of limited metastatic disease and use of ablation or stereotactic x-ray therapy could allow for long-term disease-free intervals.10,11 Thus, in certain cases, early detection of metastases with more sensitive or frequent imaging may allow for interventions that impact short-term survivorship.
The sarcoma community has recognized the need for evidence-based surveillance protocols, which are currently lacking. In 2017, the Musculoskeletal Tumor Society (MSTS) identified this need as the organization’s single highest research priority.12 Ongoing efforts are being devoted to determine the ideal method and frequency of sarcoma surveillance imaging with a focus on oncologic outcomes.13 However, to our knowledge, patients’ attitudes, preferences, and concerns regarding surveillance imaging have not yet been examined. The value of patient-centered medicine is becoming increasingly recognized, and shared decision-making has become a standard of care. Accordingly, patient perspectives and priorities should be considered when creating and implementing management guidelines.
The primary objective of this study was to develop and pilot the Sarcoma Surveillance Survey to assess patients’ concerns and perceptions related to pulmonary surveillance imaging as well as their preferences for frequency of follow-up. The secondary objective was to examine associations between the novel Sarcoma Surveillance Survey results and previously validated measures of both anxiety and patient’s positive and negative emotional appraisals of surveillance imaging. Our ultimate goal is for the Sarcoma Surveillance Survey to be used to gather data that can be incorporated into the development of surveillance guidelines as well as help patients and providers choose between high- and low-intensity imaging protocols on an individual patient basis.
2. Materials and Methods
2.1. Study design and population
This single-center, cross-sectional study was approved by the Institutional Review Board at our institution, and patients provided informed written consent for involvement. At the time of recruitment, all patients were at least 18 years of age, had completed surgical treatment for sarcoma between 01/01/2014 and 01/01/2019, and were receiving surveillance imaging. Only patients with non-metastatic and non-recurrent sarcoma were included. One patient had a diagnosis of high-risk gastrointestinal stromal tumor and was followed with an identical surveillance protocol to the other patients. Patients with low grade tumors underwent chest surveillance imaging every 6 months for five years after surgery and then annually. Patients with high grade tumors underwent chest surveillance imaging every 3 months for the first two years after surgery, followed by every 6 months until five years, and annually thereafter. Sarcoma patients with follow-up clinic appointments for surveillance imaging between 03/2019 and 09/2019 were approached during their clinic appointment and invited to participate.
2.2. Survey Measures
Ten, novel Sarcoma Surveillance Survey items were developed to assess patient concerns about surveillance imaging, such as radiation exposure, cost and transportation. Two items asked about patients’ preferences regarding more- and less-frequent imaging. Response options use a 5-point Likert scale (0 = strongly disagree to 4 = strongly agree). Prior to administering the Sarcoma Surveillance Survey items to the entire study sample, five patients completed cognitive interviews regarding the survey items. In this process, respondents were questioned about their views on clarity of language, item structure, and survey length.14 Additionally, Sarcoma Surveillance Survey items were reviewed by a fellowship-trained orthopedic oncologist and a medical oncologist. Items were amended based on feedback from the cognitive interviews and clinicians before administering the Sarcoma Surveillance Survey to the study sample.
We also included the Appraisal Scale,15 a validated measure that assesses positive and negative emotions related to a stressor. The Appraisal Scale consists of 15 items on a 5-point scale (0 = not at all to 4 = a great deal). In a separate study of patients with cancer, the Appraisal Scale was shown to assess two appraisal constructs: whether the stressor of uncertainty after a gynecological cancer diagnosis was appraised positively as a challenge to overcome (seven items) or negatively as a threat (eight items). Higher total scores on each scale indicate greater challenge or greater threat appraisals of uncertainty.16 In our study, respondents were asked to “indicate the extent to which you have each of these feelings about receiving follow-up CT scans or x-rays to monitor change in your condition.” Patients who did not complete all of the Appraisal Scale questions were excluded from analysis of the Appraisal Scale data. The Appraisal Scale assessed patients’ positive and negative emotional responses towards sarcoma surveillance, while the Sarcoma Surveillance Survey was designed to provide more specific information about specific aspects of surveillance that were concerning to patients. The validated Patient-Reported Outcomes Measurement Information System (PROMIS) Short Form Emotional Distress – Anxiety 8a survey for adults was used as a measure of anxiety.17 This 8-item PROMIS anxiety measure (hereafter Anxiety 8a) uses a 5-point scale ranging from Never (1) to Always (5), which assesses the degree to which patients have experienced fear, anxiety, and tension within the preceding 7 days. Raw scores are transformed to a standardized T-score, with a mean of 50 and standard deviation of 10 for analysis.18
These measures were administered to patients in the medical or orthopedic oncology office via a web-based Research Electronic Data Capture (REDCap)19 database programmed on an iPad. Measures were administered to patients during the office visit prior to discussion of imaging results with the provider in order to prevent the introduction of bias when reflecting on general attitudes towards surveillance imaging. Patients also provided demographic data and included items about their gender, race/ethnicity, level of education, and employment status. Patient age, tumor grade, distance to the Cancer Center, and time in surveillance were determined from the medical record. Time in surveillance was calculated as the number of days between the first imaging appointment after tumor resection and the date of study participation.
2.3. Statistical Analysis
Descriptive statistics, including medians (interquartile range [IQR]), and frequencies (%) were used to summarize the survey results and patient demographics. Sarcoma Surveillance Survey item values were combined for analysis to denote three levels of agreement: disagree (strongly disagree/disagree), neutral, and agree (agree/strongly agree). Dichotomized grouping variables also were created for patient age (<65 vs. ≥65 years old), education (≤ high school diploma/equivalent vs. > high school education), and employment (employed at least part time vs. student/unemployed/retired). Responses to each Sarcoma Surveillance Survey item were compared with the previously validated Appraisal and Anxiety 8a measures. In addition to examining individual Sarcoma Surveillance Survey items, similar items were combined, based on face validity, into two multi-item measures of concern by summing responses to individual items: items 1, 2, and 3 were combined into a “logistical concerns” measure, and items 5, 6, and 8 were combined into a “medical concerns” measure. Responses to item 4 (cost) and item 7 (claustrophobia) were not significantly associated with any other survey item, and were therefore considered independently. An 8-item measure of overall concerns also was calculated by summing responses to Sarcoma Surveillance Survey questions 1 through 8. The two items measuring a preference for more- or less-frequent imaging were analyzed separately. Internal-consistency reliability for these multi-item Sarcoma Surveillance measures was evaluated using Cronbach’s alpha. Cronbach’s alpha for the logistical concerns measure was 0.75, for the medical concerns measure was 0.73, and for the 8-item overall concerns measure was 0.69.
Correlations between each of the Sarcoma Surveillance items and multi-item scales and each of time in surveillance, the Appraisal scales, and Anxiety 8a scores were analyzed using Spearman’s rank-order tests. Differences in Sarcoma Surveillance responses between different patient demographic groups were analyzed with Kruskal-Wallis tests.
3. Results
3.1. Participants
Sixty-five patients meeting inclusion criteria who consented to participate were included in the study. All patients were being followed with CTs, except one who was being followed with chest x-ray. This patient had expressed a desire to reduce the number of follow-up scans and appointments, and so a surveillance strategy with chest x-ray was chosen. Our sample was primarily female (52.3%). The majority of patients self-identified as white (92.3%), while the remaining participants self-identified as Black or African American (7.7%). More than two-thirds of our participants were reported having at least a high school diploma or equivalent, and a similar proportion were under 65 years of age. Approximately half of the patients were employed at least part time. When examining distance from home to the cancer center where patients received follow-up imaging, 41.5% lived more than 50 miles away. The majority of patients had extremity sarcoma (89.2%), and the most common histological subtypes were liposarcoma (13.8%), chondrosarcoma (13.8%), undifferentiated pleomorphic sarcoma (12.3%), and angiosarcoma (10.8%). Most patients had high-grade sarcoma, with 18.5% of patients having Grade 2 sarcoma and 67.7% of patients having Grade 3 sarcoma (Table 1).
Table 1.
Patient characteristics
| Characteristics | N=65 N (%) |
|---|---|
| . Male | 31 (47.7) |
| . Female | 34 (52.3) |
| Age (years) | |
| . < 65 | 45 (69.2) |
| . ≥ 65 | 20 (30.8) |
| Race/ethnicity | |
| . White | 60 (92.3) |
| . Black or African American | 5 (7.7) |
| Employment | |
| . Employed | 32 (49.2) |
| . Not employed | 33 (50.8) |
| Education | |
| . High school or equivalent or less | 21 (32.3) |
| . More than high school or equivalent | 44 (67.7) |
| Tumor grade | |
| . Grade 1 | 9 (13.8) |
| . Grade 2 | 12 (18.5) |
| . Grade 3 | 44 (67.7) |
| Tumor location | |
| . Trunk | 7 (10.8) |
| . Extremity | 58 (89.2) |
| Tumor histology | |
| . Liposarcoma | 9 (13.8) |
| . Chondrosarcoma | 9 (13.8) |
| . Undifferentiated pleomorphic sarcoma | 8 (12.3) |
| . Angiosarcoma | 7 (10.8) |
| . Osteosarcoma | 7 (10.8) |
| . Myxofibrosarcoma | 6 (9.2) |
| . Leiomyosarcoma | 5 (7.7) |
| . Malignant peripheral nerve sheath tumor | 4 (6.2) |
| . Other | 10 (15.4) |
| Distance from cancer center (miles) | |
| . < 50 | 38 (58.5) |
| . ≥ 50 | 27 (41.5) |
| Median [IQR] | |
| Positive Appraisals of surveillance imaginga | 14.0 [7.5, 17.0] |
| Negative Appraisals of surveillance imaginga | 3.0 [1.0, 8.0] |
| PROMIS Anxiety Short Form 8a (T-score) | 52.3 [43.2, 59.4] |
| Time in surveillance (days) | 385 [183, 837] |
N=65; IQR, interquartile range.
Five patients did not complete the Appraisal Scale, thus n=60.
3.2. Sarcoma Surveillance Survey
Patients were most concerned about cost and radiation exposure associated with surveillance imaging; 30.7% of patients agreed that they were concerned about the cost of their follow-up imaging, and 35.4% of patients agreed that they were concerned about radiation exposure from follow-up imaging. Additionally, 24.6% of patients agreed that they were concerned about the possibility of needing additional tests if their imaging findings are concerning for cancer, while 20.0% agreed that they had concerns about the IV contrast used during surveillance imaging. Patients generally did not have a strong preference for either more- or less-frequent follow-up imaging, with only 12.4% of patients favoring more-frequent imaging and 12.4% favoring less-frequent imaging. Lower levels of concern were expressed regarding disruption to one’s own or to family’s or caregivers’ schedules caused by surveillance imaging and regarding transportation to imaging appointments (Table 2; Supplemental Figure 1).
Table 2.
Responses to the Sarcoma Surveillance Survey
| Sarcoma Surveillance Survey Item | N (%) |
|---|---|
| 1. Getting follow-up imaging is disruptive to my daily schedule | |
| . Strongly disagree | 29 (44.6) |
| . Disagree | 21 (32.3) |
| . Neither agree nor disagree | 4 (6.2) |
| . Agree | 8 (12.3) |
| . Strongly agree | 3 (4.6) |
| 2. Getting follow-up imaging is disruptive to my family’s/caregiver’s schedules | |
| . Strongly disagree | 30 (46.2) |
| . Disagree | 18 (27.7) |
| . Neither agree nor disagree | 10 (15.4) |
| . Agree | 4 (6.2) |
| . Strongly agree | 3 (4.6) |
| 3. I have concerns about transportation to get to my follow-up imaging | |
| . Strongly disagree | 39 (60.0) |
| . Disagree | 15 (23.1) |
| . Neither agree nor disagree | 4 (6.2) |
| . Agree | 6 (9.2) |
| . Strongly agree | 1 (1.5) |
| 4. I am concerned about the cost of my follow-up imaging | |
| . Strongly disagree | 21 (32.3) |
| . Disagree | 14 (21.5) |
| . Neither agree nor disagree | 10 (15.4) |
| . Agree | 14 (21.5) |
| . Strongly agree | 6 (9.2) |
| 5. I have concerns about the radiation exposure from my follow-up imaging | |
| . Strongly disagree | 14 (21.5) |
| . Disagree | 15 (23.1) |
| . Neither agree nor disagree | 13 (20.0) |
| . Agree | 17 (26.2) |
| . Strongly agree | 6 (9.2) |
| 6. I have concerns about having to use an IV contrast for my follow-up imaging | |
| . Strongly disagree | 22 (33.8) |
| . Disagree | 18 (27.7) |
| . Neither agree nor disagree | 12 (18.5) |
| . Agree | 9 (13.8) |
| . Strongly agree | 4 (6.2) |
| 7. I am concerned about being in a small space and feeling “closed in” during my follow-up imaging | |
| . Strongly disagree | 38 (58.5) |
| . Disagree | 14 (21.5) |
| . Neither agree nor disagree | 5 (7.7) |
| . Agree | 4 (6.2) |
| . Strongly agree | 4 (6.2) |
| 8. I have concerns about needing additional tests (such as biopsy) if my imaging were to show something that might be cancer | |
| . Strongly disagree | 20 (30.8) |
| . Disagree | 14 (21.5) |
| . Neither agree nor disagree | 15 (23.1) |
| . Agree | 7 (10.8) |
| . Strongly agree | 9 (13.8) |
| 9. I prefer more frequent follow-up imaging | |
| . Strongly disagree | 21 (32.3) |
| . Disagree | 17 (26.2) |
| . Neither agree nor disagree | 19 (29.2) |
| . Agree | 4 (6.2) |
| . Strongly agree | 4 (6.2) |
| 10. I prefer to have less frequent follow-up imaging | |
| . Strongly disagree | 19 (29.2) |
| . Disagree | 16 (24.6) |
| . Neither agree nor disagree | 22 (33.8) |
| . Agree | 4 (6.2) |
| . Strongly agree | 4 (6.2) |
3.3. Associations of Sarcoma Surveillance Survey results with patient demographics and time since initial treatment
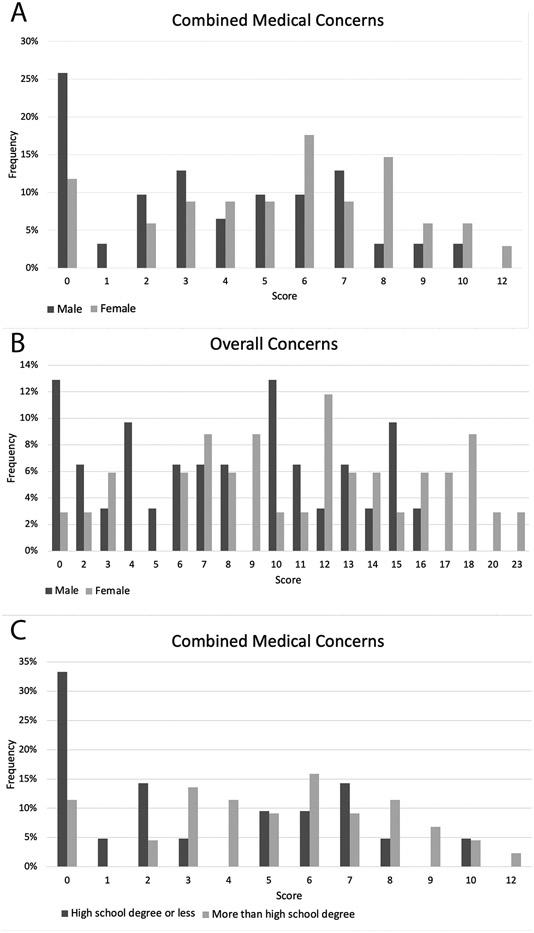
Several differences between patient groups were observed in patient’s concerns about surveillance. Patients under 65 reported higher levels of concern about cost of follow-up imaging, with 40.0% of patients under 65 agreeing that they were concerned about cost versus 10% of patients 65 years of age and over. We also observed gender differences in concern about the need for additional tests if imaging were positive (29.4% of women reported concerns versus 19.4% of men). Women also reported higher scores on both the combined medical concerns and overall concerns measures (Figure 1A-B). Patients with higher education levels reported greater concern about having to use IV contrast for follow-up imaging, as 22.7% of patients with more than a high school diploma or equivalent agreed with this statement versus 14.3% of patients with less education. Patients with higher education levels also had higher combined medical concerns scores (Figure 1C). Patients living greater than 50 miles from the cancer center expressed greater concern about the cost of follow-up imaging, with 40.7% of patients greater than 50 miles away agreeing with this statement compared to 23.7% of patients living within 50 miles. In this relatively small sample, the Sarcoma Surveillance Survey did not identify any associations between patient concerns and either race or histologic sarcoma grade.
Figure 1.
Differences were observed in (A) scores on the combined medical concerns measure between male and female patients, (B) scores on the overall concerns measure between male and female patients, and (C) scores on the combined medical concerns measure between patients with a high school diploma/equivalent or less education and those with more education
The median [IQR] length of surveillance was 385 [83, 837] days. Longer time since initial sarcoma treatment was negatively correlated with concerns about surveillance disrupting the patient’s schedule (rs= −0.27; p=0.031) and family’s/caregivers’ schedules (rs= −0.31; p=0.01). A negative correlation was also observed between patients’ logistical concerns and length of surveillance (rs=−0.32; p=0.009).
3.4. Correlations between Sarcoma Surveillance Survey, the Appraisal Scale, and Anxiety 8a
Patients reported greater positive than negative emotional appraisals of surveillance imaging: the median [IQR] score for the Appraisal Scale’s positive emotions scale was 14 [7.5, 17.0], and for the negative emotions scale was 3 [1.0, 8.0]. The median Anxiety 8a score among all participants was 52.3 [43.2, 59.4], which is comparable to the PROMIS measure’s normative score. Table 3 shows the correlations between the Sarcoma Surveillance Survey items and scales and each of the Anxiety 8a, and positive and negative Appraisal scales. Anxiety 8a scores were weakly but positively correlated with preference for more frequent follow-up imaging (rs= 0.274) and with overall level of concern about follow-up imaging (rs= 0.259) on the Sarcoma Surveillance Survey. Anxiety 8a scores were strongly correlated with the negative appraisal scale, indicating surveillance was perceived more negatively as a threat (rs = 0.748). The positive appraisal scale was negatively correlated with concern about surveillance disrupting the patient’s schedule (rs= −0.274). The negative appraisal scale was positively correlated with concern about surveillance disrupting the patient’s schedule (rs= 0.341) and the family’s/caregiver’s schedules (rs= 0.280), the concern about needing additional tests if imaging were positive (rs= 0.283), the logistical concerns measure (rs= 0.277) and overall concerns (rs= 0.323) on the Sarcoma Surveillance Survey.
Table 3.
Spearman rho correlations between each Sarcoma Surveillance Survey item and multi-item concerns scales and the Anxiety 8a and Appraisal measures
| Correlation coefficients, rs | |||
|---|---|---|---|
| Outcome Measure | Anxiety 8a N=65 |
Positive Appraisala N=60 |
Negative Appraisala N=60 |
| Positive Appraisal scale (challenge, benefit) | −0.254 | 1.00 | −0.331 ** |
| Negative Appraisal scale (threat, harm) | 0.748 ** | −0.331 ** | 1.00 |
| Getting follow-up imaging is disruptive to my daily schedule | 0.204 | −0.274 ** | 0.341 * |
| Getting follow-up imaging is disruptive to my family’s/caregiver’s schedules | 0.154 | −0.196 | 0.280 ** |
| I have concerns about transportation to get to my follow-up imaging | 0.058 | −0.066 | 0.005 |
| I am concerned about the cost of my follow-up imaging | 0.207 | 0.078 | 0.145 |
| I have concerns about the radiation exposure from my follow-up imaging | 0.235 | 0.014 | 0.230 |
| I have concerns about having to use an IV contrast for my follow-up imaging | 0.054 | −0.185 | 0.073 |
| I am concerned about being in a small space and feeling “closed in” during my follow-up imaging | 0.190 | −0.106 | 0.056 |
| I have concerns about needing additional tests (such as biopsy) if my imaging were to show something that might be cancer | 0.201 | −0.224 | 0.283 ** |
| I prefer more frequent follow-up imaging | 0.274 * | −0.025 | 0.134 |
| I prefer to have less frequent follow-up | −0.209 | −0.090 | −0.057 |
| Combined Logistical Concerns | 0.184 | −0.220 | 0.277 * |
| Combined Medical Concerns | 0.174 | −0.175 | 0.250 |
| Overall Level of Concern | 0.259 * | −0.200 | 0.323 ** |
Coefficients in bold font indicate significant correlations between the two measures.
Five patients did not complete the Appraisal Scale, thus n=60.
P<0.05.
P<0.001.
4. Discussion
The lungs are the most common site of metastasis for sarcoma, and detection of and intervention on pulmonary metastases may have short-term impact on mortality in bone and soft tissue sarcomas.3,20 Despite this potential length of survival benefit, the optimal method and frequency of pulmonary surveillance remains a topic of debate. Compared to chest x-ray, chest CT can detect smaller lung nodules; however, the clinical relevance of this sensitivity has been questioned. While some studies demonstrate a survival advantage with the use of chest CT for sarcoma surveillance,21,22 a randomized controlled trial by Puri et al. demonstrated no survival benefit from CT compared with chest x-ray.9 Additionally, the increased sensitivity of CT carries an increased risk of false-positive results,23 higher levels of radiation,4,5 higher cost, and potential complications from biopsy procedures, including pneumothorax and bleeding.24,25 Clinical trials examining the effect of different surveillance protocols on detection of systemic recurrence and overall survival are ongoing,13,26 and international guidelines do not provide clear directives on optimal surveillance frequency or modality in sarcoma patients. The National Comprehensive Cancer Network guidelines for bone27 and soft tissue28 sarcoma state a preference for chest CT for pulmonary surveillance but note that this has not been shown to improve outcomes, while the most recent guidelines from the European Society for Medical Oncology and the MSTS endorse a range of surveillance options.27-31 Given the lack of definitive guidelines, physicians must exercise clinical judgement in choosing follow-up strategies that take into account factors such as tumor grade, location, and time since surgical treatment. Physicians should also consider issues affecting patient quality of life, including logistical challenges, cost, and anxiety related to surveillance, which are not addressed in published guidelines.27-31
Patient outcomes and input are increasingly being incorporated into orthopedic clinical practice, and to create truly comprehensive protocols for sarcoma surveillance, patient views must be integrated into the development of these protocols. In this study, we developed and administered a survey to examine patient views towards surveillance imaging after surgical sarcoma treatment. The ten novel items developed for the Sarcoma Surveillance Survey provide insight into which aspects of surveillance imaging are concerning to patients and query patients about their preference for more or less frequent imaging to help physicians incorporate patient views into individualized strategies for pulmonary surveillance. When queried about concerns relating to surveillance imaging, patients expressed highest levels of concern about the associated cost and radiation exposure and about the need for additional tests if imaging were positive. Using Monte Carlo simulations, a 2009 study reported an estimated 29,000 future, radiation-related incident cancers that could have been associated with CT scans performed in 2007.5 However, the future cancer risk associated specifically with sarcoma surveillance has not been quantified. Concern expressed by patients about cost and radiation exposure also mirror concerns conveyed by the members of the MSTS; in a survey of 118 musculoskeletal oncologists, greater than 75% cited limiting radiation exposure and 45.2% cited cost savings as reasonable justifications to reduce the number of CT scans performed for pulmonary surveillance.32 The majority of physicians who responded to this and to another MSTS survey33 also reported that their patients expressed concern regarding radiation exposure from surveillance imaging. Given the high levels of concern about radiation exposure, cost, and need for potentially invasive procedures associated with surveillance imaging, particularly chest CT,5 physicians should closely examine the use of chest CT in their surveillance protocols. Separating patients based on risk for metastasis and using separate protocols for high- and low-risk patients, with fewer routine CTs for the low-risk group, would potentially reduce costs and radiation exposure and be noninferior in terms of detecting metastases.
Patients’ scores on the positive and negative Appraisal scales indicate that patients regarded surveillance imaging more positively than negatively. There were several correlations between more negative appraisals and greater concerns on the Sarcoma Surveillance Survey, including greater concern about disrupting one’s own and family’s/caregiver’s schedules, concern about needing additional tests if imaging were positive, and greater logistical and overall concerns. These correlations with the validated Appraisal Scale contribute to the validity of the novel Sarcoma Surveillance Survey. While the Appraisal Scale assesses patients’ positive and negative emotional reactions towards sarcoma surveillance, the Sarcoma Surveillance Survey provides information about particular aspects of surveillance that are concerning to patients. The Sarcoma Surveillance Survey identifies specific sarcoma surveillance-related concerns, which can be used in a clinical setting to inform decisions regarding surveillance for the larger population of patients with sarcoma.
In our sample, higher Anxiety 8a scores were positively correlated with higher perceived threat (i.e., negative appraisals) related to surveillance imaging. Higher anxiety was positively correlated with greater overall concerns regarding surveillance imaging as measured by the Sarcoma Surveillance Survey 8-item overall concerns scale. In addition, patients with higher anxiety also reported greater preference for more frequent surveillance imaging. This might be in response to heightened concerns about sarcoma recurrence, which can lead to hypervigilance and increased health care utilization among cancer survivors.34 The sarcoma community has recognized the importance of reducing anxiety related to cancer care, and an ongoing clinical trial aims to determine how patient anxiety is affected by limited versus intensive surveillance protocols.26 Our findings suggest that the Anxiety 8a could be a useful tool to screen and identify patients who are anxious and provide early counseling to reduce anxiety and mitigate against undue resource utilization, including survivors’ desires for more frequent sarcoma surveillance. Utilizing the Sarcoma Surveillance Survey in combination with a measure of anxiety, such as the Anxiety 8a, could contribute to improving quality of life of sarcoma survivors by adding to assessment of patients’ emotional states and ensuring that anxiety related to sarcoma surveillance is addressed.
We also observed various patient factors that were associated with surveillance-related concerns. Female patients had higher levels of concern about needing additional tests, medical concerns, and overall concerns about surveillance imaging. We also observed greater medical concerns, including reservations about use of IV contrast, regarding follow-up imaging among patients with a greater levels of education. In addition, patients who were younger and lived further away from the cancer center at which they received their follow-up imaging were more concerned about cost associated with imaging. Physicians should inquire about these concerns and be prepared to discuss the risks and benefits of imaging frequency and modalities. Following such conversations, physicians may choose to offer high- or low-intensity surveillance strategies based not only on clinical guidelines, but with consideration of individual patient’s concerns. Additionally, we found that patients having been under surveillance longer had lower levels of concern about disrupting their own and family’s or caregivers’ schedules. Although it was beyond the scope of this study, patient attitudes towards surveillance may shift during the course of follow-up. As such, providers may wish to administer the Sarcoma Surveillance Survey periodically over the course of surveillance to determine if any new concerns or preferences have developed.
There are several limitations of this study. This was a small, cross-sectional study in predominately white patients, conducted at a single institution, which also was a National Cancer Institute-designated Comprehensive Cancer Center. Thus, we cannot generalize the results of this study to the larger population of sarcoma patients. The varied patient population with small numbers of individual sarcoma histologies precluded a detailed analysis of patient concerns by histologic subtype. Rates and patterns of recurrence and, subsequently, surveillance imaging recommendations vary for different sarcoma subtypes. A goal of future large studies using the Sarcoma Surveillance Survey should be to evaluate differences in patient concerns and anxiety based on histologic subtype, as these concerns may differ based on risk of local and systemic recurrence. Additionally, the nature of a survey study introduces response bias, though after patients were shown how to navigate the survey, they answered the questions in isolation to reduce social desirability bias. Our aims were to develop a new measure about patient concerns regarding sarcoma surveillance imaging and to administer the measure in a limited sample of patients. While the newly developed items were reviewed by experts in the field of musculoskeletal oncology and by a group of patients prior to administration, studies with larger samples of sarcoma patients, both orthopedic and non-orthopedic, at multiple time points during surveillance, are needed to gather additional evidence of construct and discriminant validity of the Sarcoma Surveillance Survey.
5. Conclusions
In the absence of definitive, evidence-based protocols for surveillance after sarcoma treatment, individual physicians often determine the frequency and modality of imaging. The Sarcoma Surveillance Survey may be a useful tool for eliciting patient concerns about surveillance imaging, and administered in conjunction with a measure of anxiety, can help identify patients who may be struggling with emotional distress over the course of surveillance. This information should be considered when developing surveillance guidelines and can be used to assist with shared decision-making on the individual level. Additional research with more diverse and representative patient populations are needed to further evaluate the validity of the Sarcoma Surveillance Survey and increase the generalizability of our findings.
Supplementary Material
Supplementary Figure 1. Patient responses to the Sarcoma Surveillance Survey questions.
Acknowledgments
We would like to thank Jingqin Luo, Lee Rhea, and Yu Tao for their work on the statistical analysis, Stephanie Myles for her help with protocol development and IRB submission, Kirsten Brouillet for study coordination, and Carrie Heineman for administrative assistance.
Funding
Research reported in this publication was supported in part by a grant from The Foundation for Barnes-Jewish Hospital. Additional funding was provided by the Washington University Institute of Clinical and Translational Sciences (ICTS) grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) for services rendered through the Health Behavior, Communication and Outreach Core, an affiliated resource of the ICTS and the Siteman Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to report.
Data Availability
Data from this study are available upon request.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA: a cancer journal for clinicians. 1998;48(1):6–29. [DOI] [PubMed] [Google Scholar]
- 2.Predina JD, Puc MM, Bergey MR, et al. : Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol 2011;6:913–919. [DOI] [PubMed] [Google Scholar]
- 3.Horan TA, Santiago FF, Araujo LM: The benefit of pulmonary metastectomy for bone and soft tissue sarcomas. Int Surg 85:185–189. [PubMed] [Google Scholar]
- 4.Beitler AL, Virgo KS, Johnson FE, Gibbs JF, Kraybill WG. Current follow-up strategies after potentially curative resection of extremity sarcomas: results of a survey of the members of the society of surgical oncology. Cancer. 2000;88:(4): 777–785. [PubMed] [Google Scholar]
- 5.Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:(22): 2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007;357(22):2277–84 [DOI] [PubMed] [Google Scholar]
- 7.Goel A, Christy ME, Virgo KS, Kraybill WG, Johnson FE. Costs of follow-up after potentially curative treatment for extremity soft-tissue sarcoma. Int J Oncol. 2004;25:(2): 429–435. [PubMed] [Google Scholar]
- 8.Cipriano C, Griffin AM, Ferguson PC, Wunder JS: Developing an evidence-based followup schedule for bone sarcomas based on local recurrence and metastatic progression. Clin Orthop Relat Res 2017;475:830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puri A, Gulia A, Hawaldar R, Ranganathan P, Badwe RA: Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clin Orthop Relat Res 2014;472:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirbe AC, Jennings J, Saad N, Giardina JD, Tao Y, Luo J, Berry S, Toeniskoetter J, Van Tine BA. A Phase II Study of Tumor Ablation in Patients with Metastatic Sarcoma Stable on Chemotherapy. Oncologist. 2018. Jul;23(7):760–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann BC, Nagda SN, Kolker JD, Levin WP, Weber KL, Berman AT, Staddon A, Hartner L, Hahn SM, Glatstein E, Simone CB 2nd. Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: A potential alternative to resection. J Surg Oncol. 2016. Jul;114(1):65–9 [DOI] [PubMed] [Google Scholar]
- 12.Schneider PJ, Evaniew N, McKay P, Ghert M. Moving Forward Through Consensus: A Modified Delphi Approach to Determine the Top Research Priorities in Orthopaedic Oncology. Clin Orthop Relat Res. 2017. Dec;475(12):3044–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Surveillance After Extremity Tumor Surgery (SAFETY) trial: protocol for a pilot study to determine the feasibility of a multi-centre randomised controlled trial. BMJ Open. 2019. Sep 18;9(9):e029054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty PC, Willis GB. Research synthesis: The practice of cognitive interviewing. Public Opinion Quarterly. 2007. Jan 1;71(2):287–311 [Google Scholar]
- 15.Folkman S, Lazarus RS. If it changes it must be a process: study of emotion and coping during three stages of a college examination. Journal of Personality and Social Psychology 1985;48:150–170 [DOI] [PubMed] [Google Scholar]
- 16.Mishel MH, Sorenson DS. Uncertainty in gynecological cancer: a test of the mediating functions of mastery and coping. Nursing Research 1991;40:161–171. [PubMed] [Google Scholar]
- 17.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, & Cella D. Item Banks for Measuring Emotional Distress from the Patient-Reported Outcomes Measurement Information System (PROMIS): Depression, Anxiety, and Anger. Assessment, 2011;18(3), 263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanmer J, Jensen RE, Rothrock N. A reporting checklist for Health Measures’ patient-reported outcomes: ASCQ-Me, Neuro-QoL, NIH Toolbox, and PROMIS. Journal of patient-reported outcomes. 2020. Dec;4(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009. Apr;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Predina JD, Puc MM, Bergey MR, et al. : Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol 2011;6:913–919. [DOI] [PubMed] [Google Scholar]
- 21.Cho HS, Park IH, Jeong WJ, Han I, Kim HS. Prognostic value of computed tomography for monitoring pulmonary metastases in soft tissue sarcoma patients after surgical management: a retrospective cohort study. Annals of surgical oncology. 2011;18(12):3392–8. [DOI] [PubMed] [Google Scholar]
- 22.Paioli A, Rocca M, Cevolani L, et al. : Osteosarcoma follow-up: Chest X-ray or computed tomography? Clin Sarcoma Res 2017;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croswell JM, Baker S, Marcus PM. Cumulative incidence of false-positive test results in lung cancer screening: a randomized trial. Ann Intern MED. 2010;152:(W176-W180): 505–512. [DOI] [PubMed] [Google Scholar]
- 24.Choo JY, Park C, Lee NK. Percutaneous transthoracic needle biopsy of small (</=1 cm) lung nodules under C-arm cane-beam CT virtual navigation guidance. Eur Radiol. 2013;23: 712–719. [DOI] [PubMed] [Google Scholar]
- 25.Ng YL, Patsios D, Robertts H. CT-Guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10mm or less. Clin Radiol. 2008;63: 272–277. [DOI] [PubMed] [Google Scholar]
- 26.Roland CR. Evaluating the Impact of Limited Compared With Intense Post-Operative Surveillance on Patient-Reported Outcomes in Patients With Stage II-III Soft Tissue Sarcoma of the Trunk and Extremities. ClinicalTrials.gov identifier: NCT04751409. https://clinicaltrials.gov/ct2/show/NCT04751409 [Google Scholar]
- 27.Biermann JS, Chow W, Reed DR, et al. : NCCN guidelines insights: Bone cancer, version 2.2017. J Natl Compr Canc Netw 2017;15:155–167. [DOI] [PubMed] [Google Scholar]
- 28.von Mehren M, Randall RL, Benjamin RS, et al. : Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:536–563. [DOI] [PubMed] [Google Scholar]
- 29.ESMO/European Sarcoma Network Working Group: Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 30.ESMO/European Sarcoma Network Working Group: Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii113–iii123. [DOI] [PubMed] [Google Scholar]
- 31.American Academy of Orthopaedic Surgeons: Appropriate Use Criteria. 2015. http://www.orthoguidelines.org/go/auc/
- 32.Ries Z, Gibbs CP, Scarborough MT, Miller BJ: Pulmonary surveillance strategies following sarcoma excision vary among orthopedic oncologists: A survey of the Musculoskeletal Tumor Society. Iowa Orthop J 2016;36:109–116. [PMC free article] [PubMed] [Google Scholar]
- 33.Greenberg DD, Crawford B: Surveillance strategies for sarcoma: Results of a survey of members of the Musculoskeletal Tumor Society. Sarcoma 2016;2016:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vachon E, Krueger E, Champion VL, Haggstrom DA, Cella D, Cohee AA. The impact of fear of cancer recurrence on healthcare utilization among long-term breast cancer survivors recruited through ECOG-ACRIN trials. Psycho-Oncology. 2020. Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Patient responses to the Sarcoma Surveillance Survey questions.
Data Availability Statement
Data from this study are available upon request.