Abstract
This study is the first to examine spectrum-wide (1 to 250 Hz) differences in electroencephalogram (EEG) power between eyes open (EO) and eyes closed (EC) resting state conditions in 486 children. The results extend the findings of previous studies by characterizing EEG power differences from 30 to 250 Hz between EO and EC across childhood. Developmental changes in EEG power showed spatial and frequency band differences as a function of age and EO/EC condition. A 60-electrode system was used to record EEG at 4, 5, 7, 9, and 11 years of age. Specific findings were: 1) the alpha peak shifts from 8 Hz at 4 years to 9 Hz at 11 years, 2) EC results in increased EEG power (compared to EO) at lower frequencies but decreased EEG power at higher frequencies for all ages, 3) the EEG power difference between EO vs EC changes from positive to negative within a narrow frequency band which shifts towards higher frequencies with age, from 9 to 12 Hz at 4 years to 32 Hz at 11 years, 4) at all ages EC is characterized by an increase in lower frequency EEG power most prominently over posterior regions, 5) at all ages, during EC, decreases in EEG power above 30 Hz are mostly over anterior regions of scalp. This report demonstrates that the simple challenge of opening and closing the eyes offers the potential to provide quantitative biomarkers of phenotypic variation in brain maturation by employing a brief, minimally invasive protocol throughout childhood.
1. Introduction
In humans, as in all primates, more cortical surface area is devoted to visual processing than to any other sensory modality. As a result, it is not surprising that profound differences in brain activity occur depending on whether the eyes are open or closed. Such differences in brain activity can be measured with electroencephalography (EEG) or functional magnetic resonance imaging (fMRI) (eg. Nunez, 2001; Xu, 2014). Thus, the “task” of opening or closing the eyes has emerged as a practical, noninvasive, and sensitive measure of brain function (Barry, 2007).
Traditionally, the signature brain state change during the eyes closed (EC) condition has been the sustained increase in EEG power in the alpha band (8 to 12 Hz depending on participant’s age), most prominent over posterior scalp regions (Berger H, 1929; Nunez PL, 2006). This increase in alpha activity during EC is thought to reflect increased global synchronization of neuronal activity, with desynchronization and decreased alpha activity accompanying the eyes open (EO) condition (Klimesch, 1999). Alpha desynchronization may be mediated by cortical and thalamo-cortical interactions from increased visual stimulation and increased autonomic arousal (Barry, Clarke, Johnstone, & Brown, 2009; Bellato, Arora, Kochhar, Hollis, & Groom, 2020).
EEG recordings during EO/EC conditions have been studied in relation to a number of developmental outcomes and clinical diagnoses. For example, EEG alpha power during resting state has been related to cognitive (Bell & Fox, 1992; 1994) and socioemotional (Dawson et al., 2001; Diego et al., 2006; Hane et al., 2010; Fox, 1991) processes starting in infancy. Similarly, activity in the theta range has been longitudinally related to intelligence (Jones et al., 2020). In adolescents, alpha power during EC was reduced in individuals with autism spectrum disorder and attention deficit hyperactivity disorder compared to typically developing controls (Bellato et al., 2020). Another study in adults found an association between autism traits, including social rigidity, and alpha power in the parietal scalp region during the EC condition (Carter Leno, Tomlinson, Chang, Naples, & McPartland, 2018). In adults with schizophrenia, auditory steady state responses were dependent upon EO/EC state (Griskova-Bulanova, Dapsys, Maciulis, & Arnfred, 2013).
Other studies have focused on characterization of EO/EC in healthy individuals. Most of those studies have been conducted in cohorts of adults (Barry, Clarke, Johnstone, Magee, & Rushby, 2007; Chapman, Armington, & Bragdon, 1962; Geller et al., 2014; Glass & Kwiatkowski, 1970; Xu et al., 2014). To our knowledge, only three studies investigated EEG measures during an EO/EC paradigm in children. Barry and colleagues showed that in 8 to 10-year-old children there were significant differences in EEG activity from 1.5 to 25 Hz in the EO vs EC conditions using a 19-electrode system (Barry et al., 2009). Mason et al. (Mason et al., 2022) recently reported developmental changes from 2 to 30 Hz in the EO condition only. Johnstone and coauthors recorded EEG with a single electrode device in 7 to 12-year-old participants and similarly found significant frontal EO vs EC differences from 0.5 to 25 Hz (Johnstone et al., 2020). Thus, differential EEG activity during the EO vs EC conditions can provide markers of neurobehavioral disorders and maturation.
Here we explore EEG spectral changes between the EO and EC conditions to elucidate differences in neuronal networks across development. The purpose of this study was to examine spectrum-wide differences (i.e. not limited to the alpha band and inclusive of the gamma band) in EEG power between the EO and EC conditions in a cross-sectional study of young children using a high density (60 electrode) EEG system. EEG was recorded as part of an ongoing follow-up of participants originally enrolled in the Prenatal Alcohol in SIDS and Stillbirth Safe Passage cohort (Dukes et al., 2014). These follow-up assessments occurred as part of the PASS-ECHO (Environment Influences on Child Health Outcomes) study in South Dakota (Gillman et al., 2018). Results from children assessed at 4, 5, 7, 9, and 11 years of age are presented.
2. Method
Participants
Data were collected as part of the Environmental Influences on Childhood Outcomes (ECHO) longitudinal study in South Dakota (PASS-ECHO). This study assesses multiple factors that influence health outcomes in children from fetal, infancy (1 month, 1 year), early childhood (2, 3, 4, 5 years) to late childhood (7, 9, and 11 years of age). Demographics are shown in Table 1. EEG studies were conducted at the Avera Center for Pediatric and Community Research (CPCR), in Sioux Falls and Rapid City, SD. Recordings were collected from September 2018 through March 2020. Informed consents to collecting brain activity using EEG were procured as part of consent for the main study. Written informed consent was obtained from the parents of all participants. Institutional Review Board approval was obtained from New York State Psychiatric Institute, Columbia University, Avera Health and the Western Institutional Review Board. All research was performed in accordance with the relevant guidelines/regulations of those institutions. In total, across all ages, 549 children were studied. Exclusion criteria for enrollment in the ECHO study were: parent unable to provide informed consent; health care provider advises against participation; major neurologic or developmental deficit, diagnosed by a pediatrician according to parental report.
Table 1:
Demographic information of study participants
| Age | Mean Age (years) | IQR of Age (years) | N total | N male |
|---|---|---|---|---|
| 4 | 4.2 | 0.3 | 92 | 48 (52.17%) |
| 5 | 5.2 | 0.2 | 144 | 69 (47.92%) |
| 7 | 7.2 | 0.3 | 138 | 69 (50.00%) |
| 9 | 9.3 | 0.2 | 60 | 29 (48.33%) |
| 11 | 11.2 | 0.3 | 52 | 29 (55.78%) |
EEG Recording
Baseline EEG was collected cross-sectionally at 4, 5, 7, 9, and 11 years of age. Children were seated in front of a computer screen and were asked to fixate on a central crosshair. The research assistant ensured the child remained calm with minimal distractions. EEG was then collected for up to 3 minutes using a 64-channel Geodesic Sensor Net System (EGI,Inc., Eugene, OR). The nets had the four face channels (E61-E64) removed to measure heart rate and respiration. Children were asked to keep their eyes open for 30 sec and then closed for 30 sec. There were 3 EO/EC repetitions. EEG data were referenced to the single vertex electrode, Cz, and sampled at 500 Hz. Impedances were checked prior to collection and recording was started when they reached values below 50 kOhms. EEG data were exported from Netstation to raw binary format and imported into MATLAB for pre-processing to remove artifacts
EEG Processing
As a first step, raw data were filtered to remove line noise. A 16,000-point finite-impulse response 4 Hz wide notch filter was applied at the line noise frequency (60 Hz) and its first three harmonics (120 Hz, 180 Hz, 240 Hz), with 36 to 100 dB power reduction within the notches. In the second step, ECG artifact was removed from each electrode/channel, using a recently developed method that mimics ballistocardiogram removal from EEG recorded during MRI. The technique is described in detail in supplementary materials.
EEG power spectra were calculated for each 30 second epoch in the two condition and then averaged within the EO/EC conditions. Spectra were computed using the Welch method, averaging over fast Fourier transforms (FFTs) taken on 1 second segments. Data were demeaned and a Hanning window was applied prior to computing the FFT for each second. To minimize effects of eye-movement artifact, data from the four leads closest to the eyes were removed. Leads and epochs contaminated by movement-related or other sources of electrical artifact were identified using multiple criteria on a second-by-second basis to data from each lead (Isler et al., 2010). Such criteria were: standard deviation of voltage less than 40 μV (to remove noisy leads) and greater than 0.000001 (10−6) μV (to remove disconnected leads); sample-to-sample change less than 25 μV (to screen out sudden movements); absolute value of voltage less than 100 μV (to screen out blinks and slow movements); log-log spectral slope of EEG power between 20 and 120 Hz less than −0.1 (to screen for muscle artifact which has a white noise spectrum). If more than nine leads had artifact during any one second, that second was excluded from subsequent analyses. Remaining data were re-referenced to the average over all leads at each sample. Finally, base 10 log power was the average of the squared FFT’s over the accepted seconds, requiring at least 15 acceptable seconds per 30-second epoch for each lead as a minimum inclusion threshold. Finally, to be included in the analyses a participant must have had acceptable data in both eye conditions.
To determine whether our results were dependent upon the EEG artifact screening described above, we also used an independent data preprocessing methodology, namely the Maryland Analysis of Developmental EEG (MADE) pipeline (Debnath et al., 2020; Leach et al., 2020). MADE is specifically designed to preprocess EEG signals from pediatric populations in order to remove environmental noise, blinks, eye movements, and generic discontinuities. MADE utilizes customized EEGLAB (Delorme et al., 2004) function plugins throughout the steps of the pipeline. MADE implements ICA (Jung et al., 2000) to subtract the artifact-related activity from the EEG signal. Specifically, data were high pass filtered at 0.3Hz and then low pass filtered at 49Hz. Artifact-laden channels were identified and removed using the EEGLAB plug-in FASTER (Nolan et al., 2010). To further remove ocular and muscle artifacts, ICA was performed on an identical copy of the dataset. In order to improve ICA decomposition, this copied dataset was high pass filtered at 1 Hz and segmented into 1s epochs. Then, noisy segments of the data and EMG-like activity were rejected using a voltage threshold of +/−1000 μV and spectral threshold (range −100 dB to +30 dB) within the 20–40 Hz frequency band. If a channel had an identified artifact in more than 20% of the epochs, that channel was removed from both the ICA copied dataset and the original dataset. ICA was then run on the copied dataset, and ICA weights were subsequently applied back to the original dataset (Debener et al., 2010). Artifactual ICs were then removed from the original dataset by an automated process that included using the Adjusted-ADJUST algorithm (Leach et al., 2020). EEG data were then segmented into 2-s epochs with 1-s (50%) overlap for both EO and EC conditions. After ICA artifact removal and epoching, a two-step procedure for identifying residual artifacts was employed. First, any epochs where ocular channel (E1, E5, E10, and E17) voltages exceeded ±150 μV were rejected to remove residual ocular activity not removed through ICA. Second, for any epoch in which non-ocular channel voltages exceeded ±125 μV, these channels were interpolated at the epoch level. If greater than 10% of the channels (not considering globally rejected channels) exceeded ±125 μV in the epoch, the epoch was rejected. All missing channels were interpolated using a spherical spline interpolation and then the data were referenced to the average of all the electrodes.
Also, to investigate whether we would obtain the same, or very similar results, if we only used the first epoch in each condition rather than averaging over epochs, we repeated all analyses using just the first epoch in each condition.
For portraying topographies of EEG power, we computed band power for seventeen 3 Hz wide bands from 1 to 49 Hz, with an additional 3 Hz wide band centered at 100 Hz. These band-averages were used to illustrate spectral differences between eye conditions in a more compact format.
Statistical Analyses
To assess whether EO vs EC state power differences were significant, power spectra were first averaged over all electrode locations for each condition and age group separately. At each age, averaged spectra were then tested for eye state differences with paired t-tests at each frequency. Subsequently, to determine the spatial underpinnings of the averaged spectra, paired t-tests between EC and EO were performed at each electrode location. Effects of multiple comparisons were controlled for using a False Discovery Rate (FDR) of 10% (Genovese et al., 2002). Two-dimensional spatial maps were then used to show the t-statistic for each location where the p-value was less than the critical p-value determined by FDR. FDR was applied across frequencies for spatially averaged spectra and across electrodes for spatial maps.
Moreover, via generalized linear models we investigated the relationship between differences in EO vs EC and sociodemographic variables known to impact the EEG signal in different domains. The independent outcome of each model was the difference score between EO vs EC (i.e., EO – EC). Different models were run investigating such score at multiple frequencies; 8, 20, and 100 Hz. Models included the following predictors: participants’ age at assessment (4, 5, 7, 9, 11 years of age); participants’ biological sex (boys, girls); participants’ race (White, African American, American Indian or Alaska Native, Other); maternal education (no high school, some high school, completed high school, beyond high school); maternal depression (none, mild, major); maternal marital status (yes, no); maternal monthly income (<$3,500/month, ≥$3,500/month). A missing indicator variable was included for each predictor (Groenwold, R. H., 20xx). The reference category of each predictor is reported in the Results section.
3. Results
Table 1 summarizes the number of participants assessed at each age.
There were significant differences in EEG power in the EO versus the EC conditions across considerable portions of the spectrum for all ages. At low frequencies (below 15 Hz), there was higher power in the EC condition than in the EO condition. In contrast, at higher frequencies (above 20 Hz) there was lower power in the EC condition than in the EO condition. Frequencies at which there were no significant differences between conditions were limited to a spectral band where this transition occurs. This band was near to and just above the well-known alpha peak. These spectral features are exemplified in Figure 1, which displays the power difference between the two conditions (EO-EC) of the spatially-averaged spectra for participants from 4 (top panel) to 11 (bottom panel) years of age. In this representation, we defined the transition frequency as the intersection of the spectral difference and the zero (blue horizontal) line. For frequencies below the transition frequency, the difference (EO-EC) is negative, thus, on average, the power associated with the EC condition is greater than that during EO, while the opposite is found for frequencies above the transition frequency.
Figure 1.
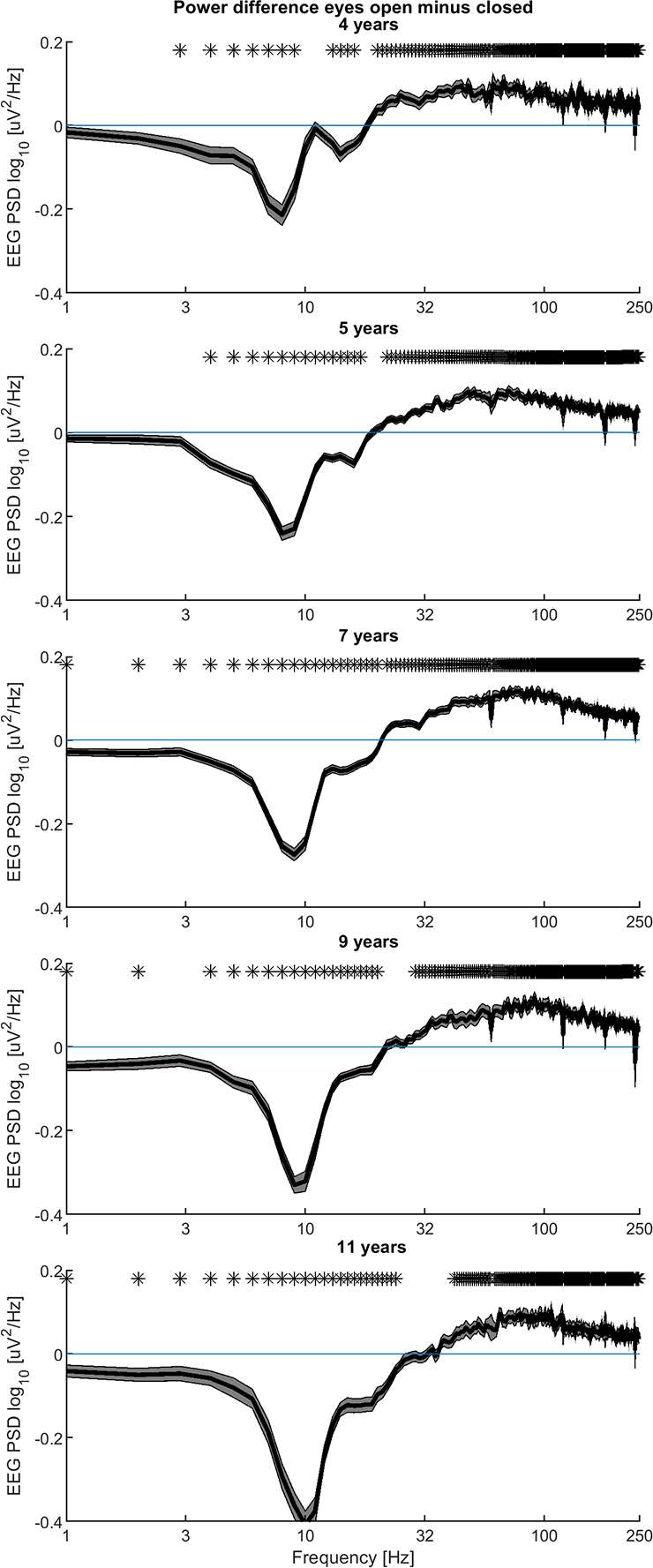
The contrast between eyes open and eyes closed conditions in participants of all ages. Differences (EO-EC) between spatially-averaged EEG power spectral density amplitudes are shown with mean values (solid line) and ± standard error (shading). Paired t-tests at each frequency were applied. Asterisks identify frequencies where differences remained significant after applying a 10% false discovery rate.
To ensure that high frequency differences in spatially averaged spectra were not driven by micro-saccades, which primarily contaminate the frontal leads, spectra were separately averaged only within parietal regions which should not contain microsaccade related activity. These parietal results were nearly identical to those presented in Figure 1 (see Supplemental Figure 1).
Overall, we found that EO-EC decreases in EEG power become more pronounced with age, while EO-EC increases in EEG power change little with age. Additionally, at 4 years of age, the transition frequency is spread over a narrow band from 9 to 15 Hz, while at 11 years of age it is more discrete, approximately 32 Hz. This change in transition frequency parallels changes in the alpha peak which at 4 years of age occurs at 8 Hz and at 11 years of age occurs at 9 Hz. The change in transition frequency is also associated with a much larger difference (~ two-fold larger) between the EO and EC conditions.
To illustrate these changes with age, Figure 2 shows EO/EC differences at all five ages and with a focus on three specific frequency bands; 7 to 9 Hz (in close proximity to the alpha band), 19 to 21 Hz (a range of frequencies close to the transition band), and 99 to 101 Hz. As described in Method, we computed band power for seventeen 3 Hz wide bands from 1 to 49 Hz and an additional band centered at 100 Hz. However, the three bands shown in Figure 2 were chosen because they are representative of the results found at different frequency ranges: the 8 Hz topography is similar to frequency bands less than ~ 17 Hz; the 20 Hz topography is similar to frequency bands between 17 and 30 Hz; the 100 Hz topography is similar to all bands above ~ 30 Hz (see Figure 4 for topographies). The panels in Figure 2 are labeled with the central frequencies of these bands. At 8 Hz (left panel), for all ages, EO power is significantly lower than power during the EC condition. The magnitude of these differences shows minimal change as a function of age. On the other hand, the sign of the differences in the middle panel of Figure 2, shows how the transition frequency varies with age. At 4 and 5 years of age, in the frequency interval 19–21 Hz, power in the EO condition is higher than for EC. At 7 and 9 years of age the difference between the two conditions is not significant and by 11 years of age the EO condition has significantly lower power. For higher frequency bands EO power is significantly greater than EC power for all ages (see 100 Hz example, right panel of Figure 2).
Figure 2.
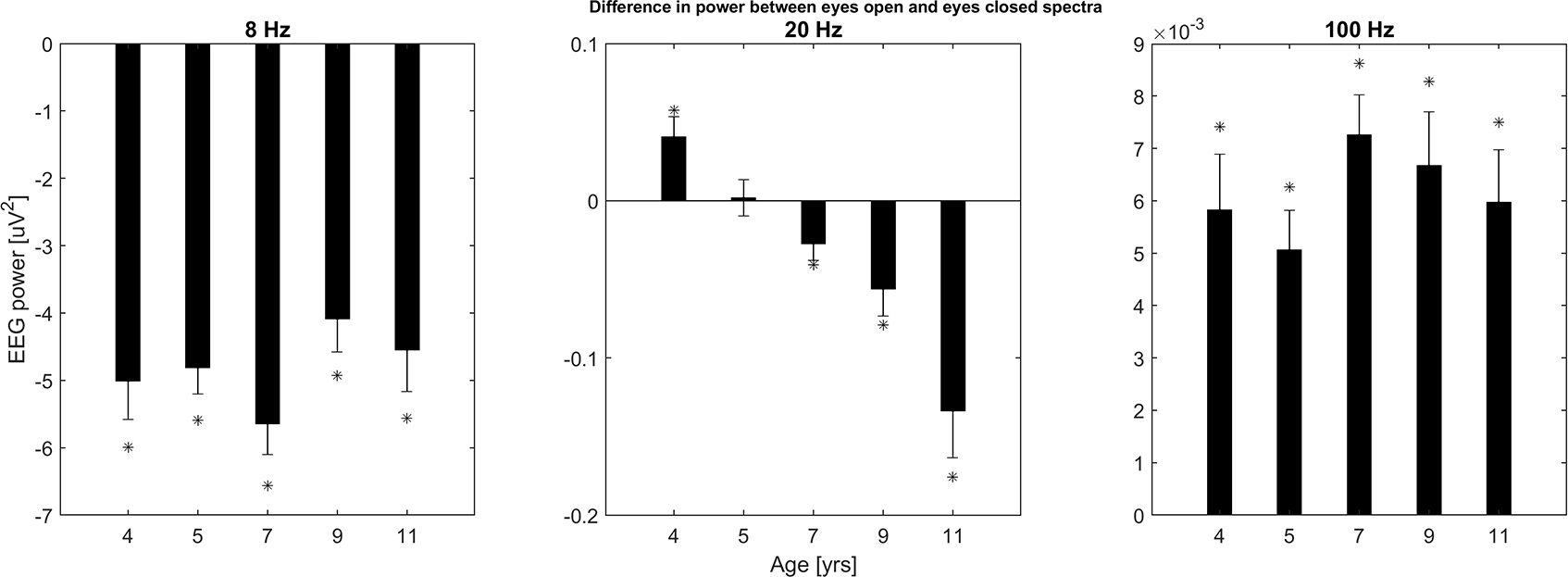
Developmental changes of differences between spatially-averaged spectral bands (EO - EC) are shown with bar plots at each of the five ages. Bands were 3 Hz wide, (subpanels, labeled by center frequency, 8, 20, and 100 Hz). Bands with significant EC vs EO differences are marked with asterisks, all of which remained significant after applying a Bonferroni adjustment to alpha.
Figure 4.
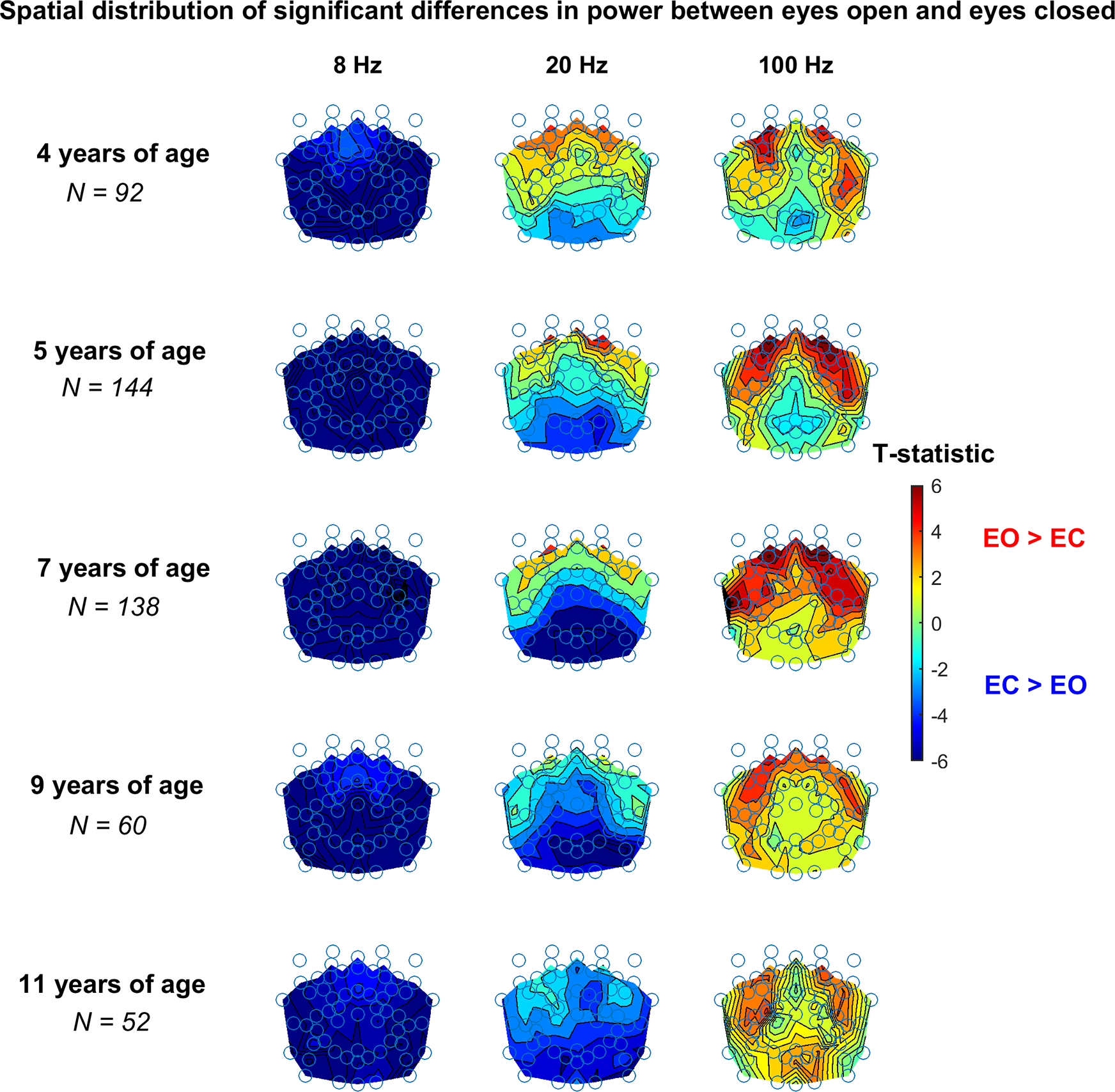
Spatial dependence of EO - EC spectral band differences, colored according to the associated T-statistic (transparent portion of the color bar indicates not significant T-statistic values) for 3 Hz wide bands.
To provide additional evidence for the results in Figure 2, paired observation plots showing single subject data are shown in Figure 3 for all of the bar plots in Figure 2.
Figure 3.
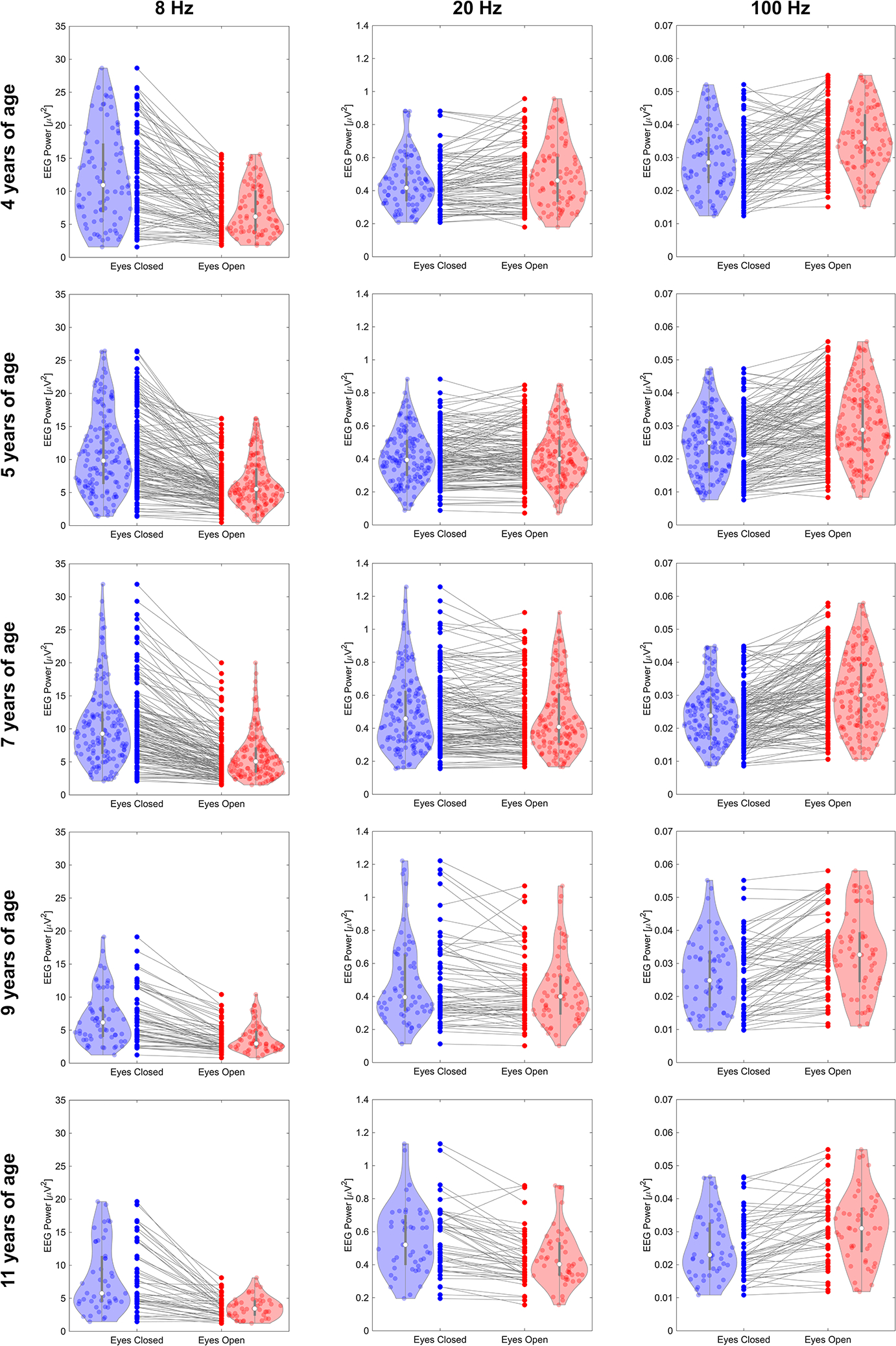
Paired observation plots and violin plots displaying the distributions of the effects tested in Table 2.
The above-described results were obtained by averaging power over all electrodes. To determine if there is spatial variation in those results, we examined EC vs EO differences at each electrode location. As in Figure 2, power was averaged in 3 Hz wide bins for 3 frequency bins (8, 20 and 100 Hz) for the different ages. As displayed in the left panel of Figure 4, at all years of age the overall greater power seen in the EC condition at 8 Hz is a widespread phenomenon not exclusively associated with the posterior area of the brain. However, the largest effects occur over posterior areas.
The variation with age in the EO versus EC differences in the band 19–21 Hz (middle panel) is accompanied by changes in spatial location of the EO vs EC differences. Specifically, at 4 years of age the power associated with EO is greater than EC (frequencies above the transition band) and spatially confined in the frontal regions of the brain. On the other hand, the posterior regions of the brain are responsible for the greater power of EC at 9 and 11 years of age. The electrode configurations for the remaining age groups are associated with intermediate spatial patterns.
At 100 Hz (right panel), the greater power seen in the EO condition is driven by frontal, parietal, and temporal regions. Differences at 100 Hz (and above, not shown) are associated with a spatial organization consistent with those of lower frequencies from 30 to 50 Hz, supporting evidence for the frontal, parietal, and temporal distribution of power differences above the transition frequency.
Table 2 displays median p-values and effect sizes (Cohen’s d) for significant electrode locations in Figure 4.
Table 2:
Median p-values and effect sizes of electrode locations in Figure 4 where p-values were less than 0.05.
| 8 Hz | 20 Hz | 100 Hz | ||||
|---|---|---|---|---|---|---|
| Age | p-value | effect size | p-value | effect size | p-value | effect size |
| 4 | 9.5×10−8 | −0.61 | 7.6×10−4 | 0.36 | 1.8×10−4 | 0.40 |
| 5 | 1.4×10−14 | −0.69 | 6.9×10−4 | −0.26 | 4.4×10−5 | 0.35 |
| 7 | 7.3×10−15 | −0.71 | 1.3×10−4 | −0.25 | 2.0×10−6 | 0.40 |
| 9 | 2.4×10−7 | −0.71 | 4.3×10−5 | −0.58 | 2.9×10−4 | 0.46 |
| 11 | 1.9×10−6 | −0.76 | 6.2×10−4 | −0.51 | 0.001 | 0.49 |
Furthermore, we independently tested the association between differences in EO and EC power at 8, 20, and 100 Hz and other factors known to have an impact on EEG as described in the Statistical Analyses section.
At 8 Hz, participants aged 7, 9, and 11 compared to the reference group (4 years of age) had a greater difference in EO and EC, β (mean ± std) = 1.5151 ± 0.5816, p-value = 0.0095; β = 2.1457 ± 0.6996, p-value = 0.0023; β = 1.6385 ± 0.7602, p-value = 0.0317, respectively. The group of participants of 5 years of age was not found different compared to the reference. Girls exhibited a more pronounced difference in EO and EC power compared to boys, β = 1.2151 ± 0.3983, p-value = 0.0024. Any level high school education and beyond was associated with EO-EC at 8 Hz, some high school β = 6.2025 ± 2.3217, p-value = 0.0078; completed high school β = 4.9881 ± 2.2536, p-value = 0.0273; beyond high school β = 5.2867 ± 2.2113, p-value = 0.0172. At 20 Hz, participants aged 5, 7, 9, and 11 compared to the reference group (4 years of age) showed a decrease in EO and EC differences, β = −0.0389 ± 0.0150, p-value = 0.0099; β = −0.07017 ± 0.0145, p-value <0.0001; β = −0.08845 ± 0.0176, p-value <0.0001; β = −0.1664 ± 0.0191, p-value <0.0001, respectively. Lastly, at 100 Hz, no differences by age were found. Any level high school education and beyond was negatively associated with EO-EC at this frequency, some high school β = −0.0134 ± 0.0049, p-value = 0.0369; completed high school β = −0.0130 ± 0.0048, p-value = 0.0069; beyond high school β = −0.0121 ± 0.0047, p-value = 0.0100.
Table 3 reports the estimates of each predictor and associated missing indicator for the above-described analyses.
Table 3:
Association of sex, age, education, race, depression, marital status, and income with EEG power (EO-EC) at 8, 20, and 100 Hz. NA codes for absence of missing data for a given predictor.
| 8 Hz | 20 Hz | 100 Hz | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| β (CI) | p-value | β (CI) | p-value | β (CI) | p-value | |
|
| ||||||
|
Sex
|
||||||
| Male | [reference] | |||||
| Female | 1.22 (0.43, 2.00) | <0.01 | 8×10−3 (−0.01, 0.03) | 0.45 | 1×10−4 (−2×10−3, 2×10−3) | 0.88 |
| Missing | NA | NA | NA | NA | NA | NA |
|
| ||||||
|
Age
|
||||||
| 4 | [reference] | |||||
| 5 | 0.73 (−0.46, 1.91) | 0.23 | −0.04 (−0.07, −0.01) | <0.01 | −9×10−4 (−3×10−3, 2×10−3) | 0.51 |
| 7 | 1.52 (0.37, 2.66) | <0.01 | −0.07 (−0.10, −0.04) | <1×10−5 | 8×10−4 (−2×10−3, 3×10−3) | 0.54 |
| 9 | 2.15 (0.77, 3.52) | <0.01 | −0.09 (−0.12, −0.05) | <1×10−6 | 3×10−4 (−3×10−3, 3×10−3) | 0.83 |
| 11 | 1.64 (0.14, 3.13) | 0.03 | −0.17 (−0.20, −0.13) | <1×10−15 | 5×10−5 (−3×10−3, 3×10−3) | 0.98 |
| Missing | NA | NA | NA | NA | NA | NA |
|
| ||||||
|
Education
|
||||||
| Any Primary | [reference] | |||||
| Some High | 6.20 (1.64, 10.77) | <0.01 | −0.06 (−0.18, 0.05) | 0.27 | −0.01 (−0.02, −6×10−4) | 0.04 |
| Completed High | 4.99 (0.56, 9.42) | 0.03 | −0.06 (−0.17, 0.05) | 0.31 | −0.01 (−0.02, −4×10−3) | <0.01 |
| Beyond High | 5.29 (0.94, 9.63) | 0.02 | −0.06 (−0.16, 0.05) | 0.31 | −0.01 (−0.02, −3×10−3) | 0.01 |
| Missing | NA | NA | NA | NA | NA | NA |
|
| ||||||
|
Race
|
||||||
| American Indian or Alaska Native | [reference] | |||||
| White | −0.32 (−1.75, 1.10) | 0.66 | −0.01 (−0.05, 0.02) | 0.42 | 3×10−4 (−3×10−3, 3×10−3) | 0.83 |
| Other | −2.16 (−3.98, −0.34) | 0.02 | −1×10−3 (−0.05, 0.04) | 0.96 | 1×10−3 (−3×10−3, 5×10−3) | 0.59 |
| Missing | NA | NA | NA | NA | NA | NA |
|
| ||||||
|
Depression
|
||||||
| Normal | [reference] | |||||
| Probable | 1.14 (−1.46, 3.75) | 0.39 | −0.01 (−0.08, 0.05) | 0.65 | 9×10−4 (−5×10−3, 6×10−3) | 0.75 |
| Major | −1.63 (−5.11, 1.86) | 0.36 | −0.05 (−0.13, 0.04) | 0.29 | −1×10−3 (−9×10−3, 6×10−3) | 0.76 |
| Missing | NA | NA | NA | NA | NA | NA |
|
| ||||||
| Married | ||||||
| No | [reference] | |||||
| Yes | −0.02 (−1.39, 1.34) | 0.97 | 0.01 (−0.02, 0.05) | 0.51 | 2×10−3 (−9×10−4, 5×10−3) | 0.18 |
| Missing | NA | NA | NA | NA | NA | NA |
|
| ||||||
|
Income
|
||||||
| $250–$3500 | [reference] | |||||
| >$3500 | −0.16 (−1.02, 0.70) | 0.71 | −4×10−3 (−0.03, 0.02) | 0.69 | −2×10−4 (−2×10−3, 2×10−3) | 0.82 |
| Missing | 1.16 (−3.85, 6.18) | 0.65 | −0.02 (−0.17, 0.13) | 0.76 | 0.01 (−7×10−4, 0.02) | 0.07 |
Moreover, an additional set of analyses were conducted removing the subset of preterm infants in the cohort under investigation (preterm birth was defined as delivery at a gestational age < 370/7 weeks). The results illustrated in the previous sections remained unchanged after excluding a total of 67 preterm participants.
4. Discussion
This study reports spectrum-wide (2–250 Hz) differences in EEG power between EO and EC conditions in a cross-sectional study of children at 4, 5, 7, 9, and 11 years of age. The principal results are that: 1) the alpha band spectral peak increases from 8 Hz at 4 years of age to 9 Hz at 11 years of age, 2) eye closure results in increased power at lower frequencies (below ~ 15 Hz) but decreased at higher frequencies (above ~ 20 Hz), 3) the change in sign for the difference between EO and EC conditions occurs in a narrow band of ‘transitional’ frequencies, 4) the transitional frequencies change across childhood, from 9 to 12 Hz at 4 years of age to a center frequency of 32 Hz at 11 years of age, 5) eye closure increases lower frequency power most prominently over posterior regions, and 6) reduced power at higher frequencies with eye closure is most prominent over anterior regions.
In addition, we found that, in general, using only the first epoch in each condition replicates the results of averaging over epochs in each condition (see Supplemental Figures 3 through 6). This suggests that the amount of data needed to observe the described eye closure effect is minimal. This characteristic may be important to some deployments of the EO/EC paradigm. Furthermore, we found that results using the MADE pre-processing pipeline were nearly identical to results presented above for frequencies below 50 Hz where MADE applies a low pass filter (see Supplemental Figures 7 through 9), demonstrating that the developmental EO versus EC phenomena reported here are robust to artifact-screening methodologies.
The earliest normative studies of EEG power during eye closure focused solely on the alpha band (Chapman, Shelburne, & Bragdon, 1970). Subsequent work with adults explored frequency bands outside of alpha, such as the beta band (14 to 30 Hz) (Barry et al., 2007; Glass & Kwiatkowski, 1970). Those studies found increased beta power in the eyes closed condition. Barry et. al. (Barry et al., 2009) studied children aged 8 to 12 and found a similar increase in beta power with eye closure. Our findings for the older children are consistent with these adult findings; however, we found that at earlier ages beta power decreases with eye closure. Recently, Johnstone et. al. (Johnstone et. al, 2020) reported a developmental increase in frontal alpha power in children from 7 to 12 years old, but no developmental change in beta power. However, similar to Barry et. al., they defined the beta band as 12.5 to 25 Hz. In contrast to both the Barry and Johnstone studies, we examined 3 Hz wide bands across the same beta range and found mixed results that were dependent upon both age and frequency (Figure 2 and Supplemental Figure 2).
To our knowledge, this is the first study in children to test for EEG differences induced by eye closure in the gamma band and higher frequencies (above 30 Hz) using scalp EEG. Decreased power during eyes closed occurred over the entire range of high frequencies from 30 to 250 Hz. Interestingly, one study of eye closure used EEG data from adult patients with intracranial electrodes implanted for seizure localization (Geller et al., 2014). Convergent with our results, they reported that eye closure resulted in widespread increased power at lower frequencies but decreased power over a broad frequency range above 30 Hz. Further, the higher frequency effects were limited to occipital regions and two focal frontal areas.
Barry et al (Barry et al., 2007) interpreted their power spectra findings as follows: widespread increases in power at lower frequencies with eye closure reflects a decrease in global arousal, while more localized higher frequency power reflects cortical activation and visual processing. This view has some support in the results reported by Geller 2014 (Geller et al., 2014) discussed previously. Furthermore, a recent study of eye closure using fMRI network analyses showed that networks active during EC had higher global efficiency, while networks active during EO had higher local clustering (Xu et al., 2014). In subsequent work, we plan to study if the same network measures, applied to our scalp EEG data, are consistent with their results. Additional studies have demonstrated associations between increased alpha power during the EC condition and increased autism traits in typically developing adults (Carter Leno et al., 2018) suggesting a potential relationship between global arousal and behavioral rigidity.
Taken together, the results of both our and prior EO/EC studies are reminiscent of metabolic signatures of brain activation found to accompany changes between cortical up and down states in sleep and anesthesia (He et al., 2008). These similarities suggest parallels between how cortical up and EO states, and conversely cortical down and EC states, bias the brain toward greater, or lesser, receptivity to external stimuli (Kohn et al., 2009).
One limitation of our study, especially regarding the transition frequencies and spatial location of differences between EO and EC, is that these current results were cross-sectional. Characterizing individual trajectories over time would be important for validating the hypothesis of that variation in transition frequencies with age are related to neurobehavioral functions. Further studies that would link these differences to neurodevelopmental measures are expected to offer a likely biomarker of neurodevelopmental risk.
A limitation of our very high frequency results is their origin in scalp EEG, which is predominately susceptible to muscle artifact at those frequencies. However, muscle artifact has a flat spectrum (Nunez, 2006), and the lack of any clear break in the log-log slopes of our power spectra argue against that explanation. In future work we plan to investigate whether the electrocardiogram (ECG), independently of EEG and the central nervous system, shows evidence of autonomic arousal in response to the EO versus EC condition.
Typically, investigations aimed at providing viable markers of brain function and maturation require long periods of data acquisition and/or equipment that allows precise linkage of stimuli presentation and cortical activity thus making these approaches problematic for large cohort studies. In contrast, this current report demonstrates that the simple challenge lasting less than 3 minutes of opening and closing the eyes can provide important information about the maturation of brain functional activity and can be accomplished with a very brief, minimally demanding protocol. In conclusion, the present work demonstrates EO/EC elicits changes in EEG spectra not confined to lower frequencies and which change as function of age during childhood.
Supplementary Material
Financial Support:
This research was supported by grants UH3OD023279 and T32MH016434 awarded the National Institutes of Health.
Footnotes
Conflict of Interest Statement:
None of the authors have potential conflicts of interest to be disclosed.
References
- Barry RJ, Clarke AR, Johnstone SJ, & Brown CR (2009). EEG differences in children between eyes-closed and eyes-open resting conditions. Clin Neurophysiol, 120(10), 1806–1811. 10.1016/j.clinph.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, & Rushby JA (2007). EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol, 118(12), 2765–2773. 10.1016/j.clinph.2007.07.028 [DOI] [PubMed] [Google Scholar]
- Berger H (1929) Uber das elektroenzephalorgamm des menschen. Arch Psychiatr Nervenk, 87:527–570. [Google Scholar]
- Bellato A, Arora I, Kochhar P, Hollis C, & Groom MJ (2020). Atypical Electrophysiological Indices of Eyes-Open and Eyes-Closed Resting-State in Children and Adolescents with ADHD and Autism. Brain Sci, 10(5). 10.3390/brainsci10050272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter Leno V, Tomlinson SB, Chang SA, Naples AJ, & McPartland JC (2018). Resting-state alpha power is selectively associated with autistic traits reflecting behavioral rigidity. Sci Rep, 8(1), 11982. 10.1038/s41598-018-30445-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RM, Armington JC, & Bragdon HR (1962). A quantitative survey of kappa and alpha EEG activity. Electroencephalogr Clin Neurophysiol, 14, 858–868. 10.1016/0013-4694(62)90136-0 [DOI] [PubMed] [Google Scholar]
- Chapman RM, Shelburne SA Jr., & Bragdon HR (1970). EEG alpha activity influenced by visual input and not by eye position. Electroencephalogr Clin Neurophysiol, 28(2), 183–189. 10.1016/0013-4694(70)90186-0 [DOI] [PubMed] [Google Scholar]
- Debener S, Thorne J, Schneider TR, & Viola FC (2010). Using ICA for the analysis of multi-channel EEG data. In Ullsperger M & Debener S (Eds.), Simultaneous EEG and fMRI (pp. 121–135). New York, NY: Oxford University Press. [Google Scholar]
- Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, & Fox NA (2020) The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology, 57(6), e13580. 10.1111/psyp.13580 [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004) EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GDV, . . . Network, P. R. (2014). The Safe Passage Study: Design, Methods, Recruitment, and Follow-Up Approach. Paediatric and Perinatal Epidemiology, 28(5), 455–465. 10.1111/ppe.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AS, Burke JF, Sperling MR, Sharan AD, Litt B, Baltuch GH, . . . Kahana MJ. (2014). Eye closure causes widespread low-frequency power increase and focal gamma attenuation in the human electrocorticogram. Clin Neurophysiol, 125(9), 1764–1773. 10.1016/j.clinph.2014.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, and Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the False Discovery Rate. NeuroImage 15, 870–878. 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gillman Matthew W., and Blaisdell Carol J. (2018) Environmental Influences on Child Health Outcomes, a research program of the NIH. Current opinion in pediatrics 30(2), 260–262. 10.1097/MOP.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A, & Kwiatkowski AW (1970). Power spectral density changes in the EEG during mental arithmetic and eye-opening. Psychol Forsch, 33(2), 85–99. 10.1007/BF00424979 [DOI] [PubMed] [Google Scholar]
- Groenwold RH, White IR, Donders ART, Carpenter JR, Altman DG, & Moons KG (2012). Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ, 184(11), 1265–1269. 10.1503/cmaj.110977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griskova-Bulanova I, Dapsys K, Maciulis V, & Arnfred SM (2013). Closed eyes condition increases auditory brain responses in schizophrenia. Psychiatry Res, 211(2), 183–185. 10.1016/j.pscychresns.2012.04.004 [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, & Raichle ME (2008) Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. PNAS, 105(41): 16039–16043. 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler JR, Martien KM, Grieve PG, Stark RI, Herbert MR. (2010) Reduced functional connectivity in visual evoked potentials in children with autism spectrum disorder. Clin Neurophysiol 121, 2035–2043. 10.1016/j.clinph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Jiang H, Sun L, Rogers JM, Valderrama J, Zhang D (2020) Development of Frontal EEG Differences Between Eyes-Closed and Eyes-Open Resting Conditions in Children: Data From a Single-Channel Dry-Sensor Portable Device. Clin EEG Neurosci, Jul 31;1550059420946648. 10.1177/1550059420946648. [DOI] [PubMed] [Google Scholar]
- Jones EJH, Goodwin A, Orekhova E, Charman T, Dawson G, Webb SJ, Johnson MH (2020) Infant EEG theta modulation predicts childhood intelligence. Sci Rep. 2020 Jul 8;10(1):11232. 10.1038/s41598-020-67687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, & Sejnowski TJ (2000) Removing electroencephalographic artifacts by blind source separation. Psychophysiology, 37(2), 163–178. [PubMed] [Google Scholar]
- Klimesch W (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev, 29(2–3), 169–195. 10.1016/s0165-0173(98)00056-3 [DOI] [PubMed] [Google Scholar]
- Kohn A, Amin Zandvakili A & Smith MA (2009) Correlations and brain states: from electrophysiology to functional imaging. Curr Opin Neurobiol. 19(4):434–438 10.1016/j.conb.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach SC, Morales S, Bowers ME, Buzzell GA, Debnath R, Beall D, & Fox NA. (2020) “Adjusting ADJUST: Optimizing the ADJUST Algorithm for Pediatric Data Using Geodesic Nets.” Psychophysiology 57: e13566. 10.1111/psyp.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason LM, Barry RJ, & Clarke AR (2022). Age-related changes in the EEG in an eyes-open condition: I. Normal development. Intl.l J. of Psychophysiology 172: 40–45. 10.1016/j.ijpsycho.2021.11.005. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, & Silberstein RB (2001). Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. Jul;13(3):125–64. 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL SR (2006). Electric fields of the brain: The Neurophysics of EEG, 2nd Edition. New York, NY: Oxford University Press. [Google Scholar]
- Xu P, Huang R, Wang J, Van Dam NT, Xie T, Dong Z, . . . Luo YJ. (2014). Different topological organization of human brain functional networks with eyes open versus eyes closed. Neuroimage, 90, 246–255. 10.1016/j.neuroimage.2013.12.060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


