Abstract
The mechanisms used by Shiga toxin (Stx)-producing Escherichia coli to adhere to epithelial cells are incompletely understood. Two cosmids from an E. coli O157:H7 DNA library contain an adherence-conferring chromosomal gene encoding a protein similar to iron-regulated gene A (IrgA) of Vibrio cholerae (M. B. Goldberg, S. A. Boyko, J. R. Butterton, J. A. Stoebner, S. M. Payne, and S. B. Calderwood, Mol. Microbiol. 6:2407–2418, 1992). We have termed the product of this gene the IrgA homologue adhesin (Iha), which is encoded by iha. Iha is 67 kDa in E. coli O157:H7 and 78 kDa in laboratory E. coli and is structurally unlike other known adhesins. DNA adjacent to iha contains tellurite resistance loci and is conserved in structure in distantly related pathogenic E. coli, but it is absent from nontoxigenic E. coli O55:H7, sorbitol-fermenting Stx-producing E. coli O157:H−, and laboratory E. coli. We have termed this region the tellurite resistance- and adherence-conferring island. We conclude that Iha is a novel bacterial adherence-conferring protein and is contained within an E. coli chromosomal island of conserved structure. Pathogenic E. coli O157:H7 has only recently acquired this island.
Escherichia coli O157:H7 and other Shiga toxin (Stx)-producing E. coli (STEC) strains cause diarrhea, hemorrhagic colitis, and the hemolytic uremic syndrome. The mechanisms underlying the adherence of STEC to epithelial cells are only partly understood (35). The ability to adhere to epithelial cells is an important virulence trait, because adherence presumably enables enteric pathogens to deliver toxins efficiently to host organs, overcome peristaltic clearance, and gain access to host-derived nutrients.
Intimin is the best-characterized E. coli O157:H7 adherence molecule. Encoded by eae, intimin mediates the attaching and effacing lesion caused by enteropathogenic E. coli (EPEC) and many STEC serotypes (21) and is an important component of pathogenicity. However, cloned eae from EPEC and STEC do not confer the adherent phenotype upon laboratory E. coli (18, 25, 28). Moreover, though the cloned EPEC locus of enterocyte effacement, which includes eae, does confer the adherence phenotype on E. coli K-12 (27), the cloned E. coli O157:H7 locus of enterocyte effacement does not (12).
We describe an E. coli O157:H7 gene that renders laboratory E. coli adherent to epithelial cells and explore evolutionary aspects of its acquisition.
(These data were presented in part at the 3rd International Symposium and Workshop on Shiga Toxin-Producing Escherichia coli Infections, Baltimore, Md., 22 to 26 June 1997.)
MATERIALS AND METHODS
Bacteria.
The bacteria analyzed in this study are described in Table 1. The bacteria were inoculated directly from frozen stock (in Luria-Bertani [LB] broth–15% glycerol, maintained at −70°C) into LB broth (26). The cultures were grown overnight under standardized conditions (37°C; 14 to 16 h; stationary cultures) for adherence assays and protein preparations. The bacteria were grown in a shaking incubator (37°C; 14 to 24 h) for DNA preparations or matings. Ampicillin (200 mg/liter), nalidixic acid (20 mg/liter), or both were added if appropriate. Unless otherwise specified, E. coli O157:H7 strain 86-24 (37) and its DNA were used.
TABLE 1.
Bacteria analyzed in this study
| Strain(s) | Reference or source | Homology
|
||
|---|---|---|---|---|
| ihab | TAIc | Ihad | ||
| E. coli O157:H7a | ||||
| 25 E. coli O157:H7 strains from Seattle, Australia, and Colombia, including strain 86-24 | 37, 5; M. Samadpour, S. Mattar, Australia Government Analytical Laboratories | 4.7, 7.5e | + | 67 |
| E. coli O157:H7 (86-24nalR) | 6 | 4.7 | + | NT |
| E. coli O157:H7 (86-24nalR) (Δiha) | This paper | 2.7f | + | ± |
| Sorbitol-fermenting E. coli O157:H− (493-89, 5412-89, CB569, 514-91) | 22, 43; H. Karch, L. Beutin | − | NT | |
| E. coli O55:H7 (5A-D, TB156A, TB182A) | 41, 7 | − | NT | |
| E. coli O55:H7 (5E) | 41) | 2.9 | V | − |
| eae+ STEC distantly related to E. coli O157:H7 | ||||
| E. coli O26:NM (TB352A) | 7 | 4.7, 10.1 | + | 39 |
| E. coli O85:NM (TB334C) | 7 | 4.7, 10.1 | + | − |
| E. coli O126:H2 (TB285A) | 7 | 4.7, 10.1 | + | − |
| E. coli O103:H2 (UTI) | 36 | 4.7 | + | − |
| E. coli O111:HN (TB226A) | 7 | 4.7 | + | − |
| E. coli O103:H6 (TB154A) | 7 | − | NT | |
| eae-negative STEC distantly related to E. coli O157:H7 | ||||
| E. coli O104:H21 | 3; T. Damrow | 3.9, 10.1 | V | 56 |
| E. coli O113:H21 (CL15) | 11 | 7.9 | V | 56 |
| stx-negative EPEC distantly related to E. coli O157:H7 | ||||
| E. coli O15:NM (RDEC-1) | 8 | 4.7 | + | − |
| E. coli O111:NM (B171) | 32 | − | NT | |
| E. coli O119:H6 (659-79) | 23 | − | NT | |
| E. coli O127:H6 (E2348/69) | 23 | − | NT | |
| E. coli O142:H6 (E851/71) | 23 | − | NT | |
| E. coli O111:H− (2430-78) | 23 | 5.1 | + | − |
| Other | ||||
| E. coli ORN172(pSK+) | 42, this paper | − | − | |
| E. coli ORN172(pIHA) | 42, this paper | 2.2g | − | 78 |
| 20 E. coli strains from nondiarrheal human stools | S. Moseley | 3.6h | NT | NT |
Including derivatives and closely related strains.
Size (in kilobases) of BstXI fragment(s) detected by iha probe (the insert of pIha). −, iha homologue was not detected with this probe.
Characterization of BstXI fragments detected by TAI probe. +, TAI homologue detected. −, TAI probe detects BstXI fragments that are of different sizes, and fewer, than those detected in E. coli O157:H7; homology is considerably less intense, and the iha homologue is absent. V, one or more BstXI framgents are intense on a Southern blot using the TAI probe, but the pattern of bands detected by the TAI probe is different from that of TAI in E. coli O157:H7; iha homologue(s) is present. NT, not tested.
Size (in kilodaltons) of the largest OMP detected by α-Iha antibodies. NT, not tested. −, antigen not detected; ±, faint 67-kDa antigen detected in some OMP preparations.
In five E. coli O157:H7 strains, a 7.5-kb BstXI fragment is also detected by the iha probe.
Faint 2.7-kb band detected, representing homology of iha probe to intact 5′ and 3′ ends of truncated iha gene.
iha is cloned into pSK+ in this laboratory strain. Size reflects insert length, not BstXI fragment.
Only three of these isolates contain iha homologues.
Adherence assay.
HeLa or Madin-Darby bovine kidney (MDBK) cells were grown to confluence at 37°C in 5% CO2 in plastic flasks in minimal essential medium with 10% (vol/vol) heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin (100,000 IU/liter), and streptomycin (100 mg/liter). The cells were then trypsinized, diluted, added to four-chamber glass slides (Nunc, Naperville, Ill.), and reincubated. Two days later, the chambers were washed with sterile phosphate-buffered saline (PBS) and replenished with 0.6 ml of incubation medium (minimal essential medium, fetal calf serum [5% for HeLa cells or 10% for MDBK cells], 2 mM l-glutamine, nonessential amino acids, 0.5% d-mannose, and ampicillin [200 mg/liter] if appropriate). Twenty microliters of overnight LB broth cultures of bacteria, including positive (E. coli B171) and negative (E. coli ORN172) controls, was added to individual chambers. The slides were incubated (3 h; 37°C in 5% CO2), washed three times with PBS, covered with incubation medium (0.6 ml), incubated (2 h; 37°C in 5% CO2), washed 10 times with PBS, fixed (100% methanol; 5 min), Giemsa stained (60 min), and coverslipped.
A microscopist unaware of the identity of the bacteria being examined counted the total number of clusters (≥5 bacteria/cluster), bacteria, and cells in five separate fields in each chamber enumerated. Adherence indices (clusters per cell, bacteria per cluster, and bacteria per cell) were calculated. Comparisons were made between pairs of chambers assayed on the same day. The significance of differences in adherence indices was determined using the two-tailed Student t test or the Mann-Whitney rank sum test if tests for normal distribution and equal variances were passed or failed, respectively (14).
DNA preparation.
For cosmid cloning, DNA was prepared from bacteria lysed in ultracentrifuge tubes (9). For PCR amplifications and for Southern blotting, DNA was prepared from bacteria suspended in 50 mM Tris-HCl (pH 8.0) with 50 mM EDTA, to which sodium dodecyl sulfate (SDS) and proteinase K (Sigma, St. Louis, Mo.) (final concentrations, 1 and 0.04%, respectively) were added. Following incubation of the bacteria with SDS and proteinase K (65°C; 2 h), DNA was extracted (with phenol-chloroform) and precipitated (with ammonium acetate-ethanol). Plasmids were purified by CsCl density gradient centrifugation or alkaline lysis (26).
Cosmid library.
XbaI-digested plasmid Supercos (pSC) (Stratagene, La Jolla, Calif.) was treated with calf intestinal alkaline phosphatase (Boehringer Mannheim, Indianapolis, Ind.), phenol extracted, and digested with BamHI. The cosmid arms were ligated to a calf intestinal alkaline phosphatase-treated Sau3A partial digest of E. coli O157:H7 DNA, packaged with the Gigapack II XL system (Stratagene), transduced into E. coli NM554 (38), and tested for adherence.
Southern hybridization.
Two micrograms of BstXI-digested bacterial DNA was electrophoresed in 1% agarose–0.5× Tris-borate-EDTA, stained with ethidium bromide, transferred to a nylon membrane (Micron Separations, Westboro, Mass.), and UV cross-linked. The membranes were then immersed in hybridization buffer (10% polyethylene glycol, 7% SDS, and 1.5× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA, pH 7.7]) (26) (2 h; 65°C). Probes for eae, the large E. coli O157:H7 plasmid (pO157), and stx2 were derived from pCVD434 (18), pCVD419 (24), and pNN111-19 (30), respectively. Probes were also derived from the inserts of pIha, pSC(A-G6), and pSC(T-H12), described below. The fragments were labeled with the Megaprime DNA system (Amersham, Arlington Heights, Ill.) and [α-32P]dATP (New England Nuclear Research Products, Boston, Mass.) and added to the membranes. The membranes and probes were incubated overnight (65°C), washed twice (15 min; 65°C) in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) (26)–0.1% SDS and twice (15 min; 65°C) in 0.2× SSC–0.1% SDS and exposed to X-ray film in the presence of intensifying screens (−70°C).
Gene identification and sequencing strategy.
pSC(A-G6) segments were cloned into pSK+ and tested for their abilities to confer adherence on laboratory E. coli. An 8,040 bp KpnI adherence-conferring fragment was sequenced in both directions using the Taq DyeDeoxy cycle-sequencing kit and a model 373A DNA sequencer (Applied Biosystems, Foster City, Calif.). The sequences were compared to the National Center for Biotechnology Information Geninfo BLAST network server database (13). A 2,088-bp adherence-conferring open reading frame (ORF) was amplified using PCR and the primers 5′GGGGATCCAATTCTGGCATGCCGAGGCAGTGC3′ and 5′GGTCTAGATTCTCGTTGCCACTGTTCCGCCAGG3′ (the boldface nucleotides represent sequences derived from the 8,040-bp KpnI adherence-conferring fragment). These primers contain 5′ BamHI and XbaI sites, respectively. The primers produce an amplicon which includes the ORF, as well as 141 bp 5′ to its ATG start codon and 80 bp 3′ to its TGA stop codon. The amplicon was digested with BamHI and XbaI and cloned into corresponding sites in pSK+, resulting in a construct designated pIha.
Deletion mutant.
An in-frame deletion of the 2,088-bp ORF of interest was created in E. coli O157:H7nalR by PCR using the primer pairs 5′GGAGCGAGCTCGCCTTATCACGACTACGAATACCAGC3′ (primer A)-5′GGTAGGATCCCTCTGCAGCAGCTATGCTGCTGGC3′ and 5′GCTAGGATCCCAGACGGGATCATCAACAACAGGA3′-5′GCTATCTCGAGGGGACTTATCTGACCGGGCCCCTGG3′ (primer B) and E. coli O157:H7 DNA. These primers contain 5′ SacI, BamHI, BamHI, and XhoI sites, respectively; the boldface nucleotides were derived from the sequence of the 8,040-bp KpnI adherence-conferring fragment. The resulting 698- and 558-bp amplicons span the 5′ and 3′ ends of the gene of interest. These PCR products were digested with BamHI and then with SacI or XhoI, respectively, and separately cloned into pSK+. SacI-BamHI and BamHI-XhoI inserts were excised, purified, ligated to each other at their BamHI sites, and cloned into SacI-XhoI-digested pSK+, resulting in pSK+(Δiha). The insert of pSK+(Δiha) was excised and ligated into pCVD442 (10). The resulting pCVD442(Δiha) was transformed into E. coli SM10(λpir) (34).
E. coli SM10(λpir)(pCVD442(Δiha)) and E. coli O157:H7nalR were mated on LB agar at 37°C, and a transconjugant was selected by plating the mated bacteria on LB agar with ampicillin and nalidixic acid. This presumed merodiploid was then grown overnight (37°C) in LB broth without salt, plated onto LB agar containing 5% sucrose but no salt, and incubated (30°C). DNA from the resulting sucrose-resistant, ampicillin-sensitive, O157 antigen-expressing (confirmed with a latex particle agglutination test [Oxoid, Basingstoke, Hampshire, United Kingdom]) putative deletion mutant [designated E. coli O157:H7nalR(Δiha)] was analyzed on Southern blots and PCR amplified with primers A and B. The resulting amplicon was cloned into pSK+ and sequenced, to confirm that the 647 amino acids between Glu25 and Gln673 were replaced by a Gly and Ser, as intended.
Antibodies.
Polyclonal antibodies were raised in rabbits immunized with (C)YTWTRSEQRDGDNKG-COOH coupled to keyhole limpet hemocyanin via the Cys residue using the PolyQuik protocol (Zymed Laboratories, South San Francisco, Calif.). Affinity-purified antibodies to the Iha peptide (α-Iha antibodies) were produced by Zymed using a peptide-conjugated affinity matrix.
OMP and total bacterial protein (TBP) analysis.
Outer membrane proteins (OMPs) were prepared (1) from bacteria grown overnight (37°C in 100 ml of LB) to ascertain if the protein of interest localized to the cell envelope. Protein concentrations were determined with the Protein Assay Kit (Bio-Rad, Hercules, Calif.). For proteinase K susceptibility experiments (performed to establish if the molecule of interest possessed externally directed domains), two 100-ml cultures of bacteria were pooled, pelleted, washed once with 10 mM Tris-HCl (pH 8.0) (Tris), suspended in 2 ml of Tris with 1 mM EDTA, and divided into two equal aliquots. Twenty-five microliters of proteinase K (20 mg/ml) or water was added to each aliquot, and the tubes were then shaken (37°C; 1 h). Eight microliters of phenylmethylsulfonylfluoride (0.2 M) was then added to the samples, which were again shaken (37°C; 15 min), pelleted, washed twice (in Tris with 5 mM MgCl2), and suspended in 100 μl of Tris. Fifty microliters of suspended bacteria was added to 10 ml of Tris for OMP preparation. Triton-X (11.1 μl; 0.01%) in water was added to the remaining 50 μl, which was boiled (20 min) and iced. Then 12 μl of 10× DNase buffer and 2 μl of DNase (1 U/μl) (Promega, Madison, Wis.) were added to the tubes, which were incubated at room temperature (30 min). Then 5× loading buffer (32 μl) was added to each tube, and samples were stored at −20°C until they were studied.
OMPs in loading buffer (1 μg/lane) or TBPs equivalent to the bacteria in 35 μl [E. coli O157:H7 and E. coli O157:H7nalR(Δiha)] or 30 ml [E. coli ORN172(pSK+) and E. coli ORN172(pIha)] of overnight broth culture were separated in SDS–10% polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore, Bedford, Mass.) or Coomassie stained. The different volumes of TBP loaded reflect different levels of expression of maltose-binding protein (Mbp) observed in preliminary experiments (data not shown).
The membranes were blocked overnight at 4°C in antibody buffer (PBS with 0.05% [vol/vol] Tween 20) containing 5% nonfat dried milk and 0.02% sodium azide. The membranes were washed once and incubated overnight with α-Iha antibodies diluted 1:2,000 or with affinity-purified antibodies to Mbp (α-Mbp antibodies) (New England Biolabs, Beverly, Mass.) diluted 1:50,000 in antibody buffer. The blots were then washed three times in antibody buffer, incubated for 30 min with affinity-purified goat anti-rabbit immunoglobulin G (H+L) peroxidase conjugate (Boehringer Mannheim) diluted 1:2,000 in antibody buffer, and washed three times in antibody buffer. After the blocking, all washes and incubations were performed at room temperature. Bound antibodies were detected with SuperSignal chemiluminescent substrate, Western blotting (Pierce, Rockford, Ill.).
Nucleotide sequence accession number.
The sequence of the 8,040-bp KpnI adherence-conferring fragment has been entered into GenBank as submission AF126104.
RESULTS
Characterization of adherence-conferring cosmids and gene.
Two [pSC(A-G6) and pSC(T-H12)] of 2,200 cosmids constructed from E. coli O157:H7 DNA mediated the diffuse adherence to HeLa cells of transduced E. coli NM554. pSC(A-G6) and pSC(T-H12) each contain ca. 35 kbp of chromosomal DNA, and they overlap by ca. 15 kbp. Southern hybridization determined that neither cosmid contains eae or stx2. An 8,040-bp KpnI fragment from the overlap region of pSC(A-G6) cloned into pSK+ confers upon E. coli ORN172 the ability to adhere diffusely to epithelial cells (see below). This 8,040-bp KpnI fragment is present in E. coli O157:H7 (data not shown) DNA and is therefore not an artifact of cosmid construction.
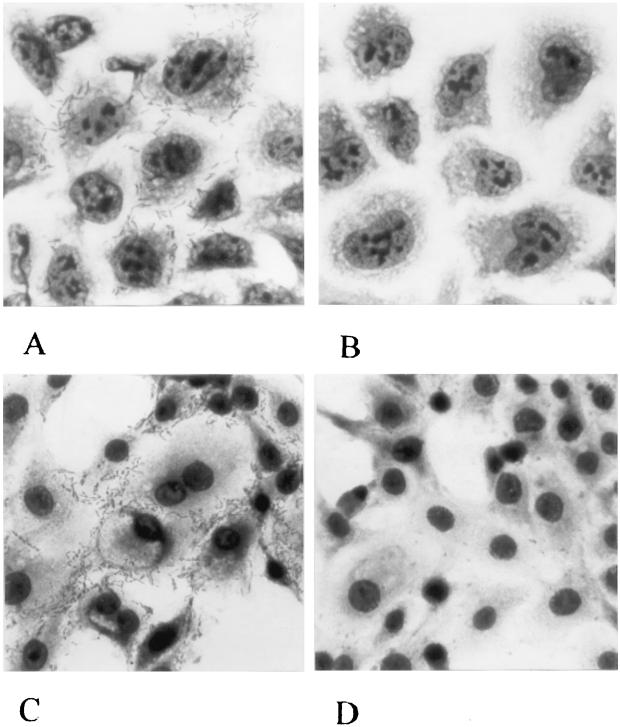
The 8,040-bp KpnI fragment (GenBank number AF126104) has a 45% G+C content and contains five ORFs of interest. Four ORFs are homologous to tellurite resistance genes of Alcaligenes sp. (19) and Serratia marcescens and are designated tlpA to -D (for tellurite resistance proteins). A fifth ORF of interest is 2,088 bp long and encodes a protein with a deduced mass of 76,494 da. An amplicon of this 2,088-bp ORF, when cloned into pSK+ (resulting in a construct designated pIha, described below) and transformed into E. coli ORN172, conferred upon that laboratory strain the ability to adhere diffusely to epithelial cells. In contrast, E. coli ORN172 that had been transformed with pSK+ did not adhere; the respective median (range) bacteria per cell were 4.9 (0.8 to 5.4) and 0.03 (0.00 to 0.06), P = 0.029 (Fig. 1A and B). E. coli ORN172(pIha) also adhered to MDBK cells (Fig. 1C and D).
FIG. 1.
Adherence of recombinants and controls to epithelial cells. HeLa (A and B) (×600) or MDBK (C and D) (×480) cells incubated with E. coli ORN172(pIha) (A and C) or E. coli ORN172(pSK+) (B and D).
This 2,088-bp adherence-conferring ORF from E. coli O157:H7 has a 52% G+C content. Of the amino acids this gene encodes, 53% can be matched exactly or conservatively (2) to amino acids in IrgA of Vibrio cholerae, encoded by iron-regulated gene A (irgA) (15) (Fig. 2). Six of seven amino acids composing a putative TonB box in IrgA can be identically or conservatively matched to amino acids in a potential TonB box in the E. coli O157:H7 IrgA homologue. Because of its similarity to this V. cholerae protein, we have designated the E. coli O157:H7 adherence-conferring protein the IrgA homologue adhesin (Iha), encoded by iha.
FIG. 2.
Deduced amino acid sequence of Iha and homology to V. cholerae IrgA. The boxed amino acids represent a probable TonB box. +, conservative amino acid substitution. The arrows indicate the borders of amino acids deleted from Iha in E. coli O157:H7nalR(Δiha). The shaded amino acids were used to immunize rabbits to induce α-Iha antibodies. The dashes represent BLAST program-generated gaps between amino acids to maintain alignment of the proteins.
E. coli O157:H7 iha lacks an upstream irgB homologue and a ferric uptake and regulation protein-binding site, unlike irgA in V. cholerae. A putative Shine-Dalgarno sequence (GGAG) is located 9 nucleotides upstream of the iha start codon. Of the 2,635 contiguous nucleotides starting at the 34th nucleotide from the A of the initiation codon of the irgA homologue of E. coli O157:H7, 99% are identical to ORF R4 (described as a putative exogenous ferric siderophore receptor) in a pathogenicity-associated island (PAI) of pyelonephritogenic E. coli strain CFT073 (20). Homology between the 5′ ends of the irgA homologue of E. coli O157:H7 and of ORF R4 of E. coli strain CFTO73 cannot be further assessed without additional sequence of this E. coli CFT073 PAI.
To determine if E. coli O157:H7 requires Iha to adhere to epithelial cells, we compared adherence indices for E. coli O157:H7nalR and its derivative, E. coli O157:H7nalR(Δiha). E. coli O157:H7nalR(Δiha) has sustained an in-frame deletion of 1,941 bp in iha, corresponding to the replacement of 647 amino acids with a Gly and a Ser. E. coli O157:H7nalR(Δiha) adheres to HeLa cells less well than does E. coli O157:H7nalR (0.13 ± 0.10 [standard deviation] clusters/cell and 8.8 ± 1.5 bacteria/cluster versus 0.25 ± 0.16 clusters/cell and 11.4 ± 4.3 bacteria/cluster), but not significantly so (respective t8df values, 1.306 and 1.391; respective P values, 0.23 and 0.20). In contrast, pSK+(Δiha), from which the same nucleotides in iha have been deleted, does not confer adherence on E. coli ORN172.
Conservation of iha and of surrounding DNA.
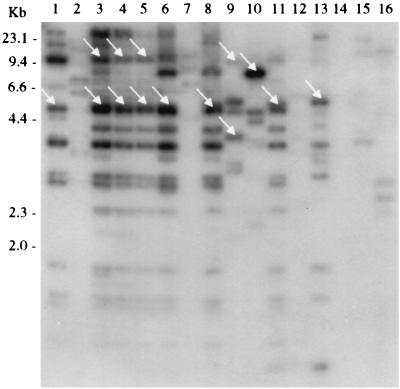
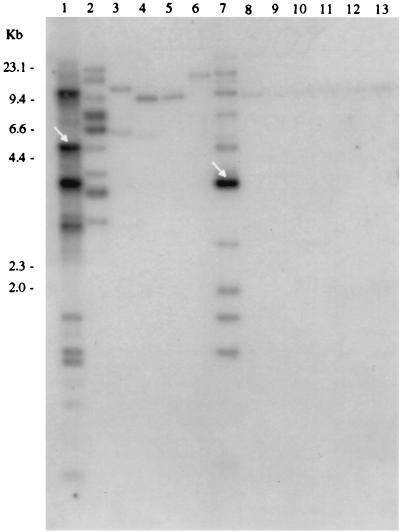
Twenty-five of 25 E. coli O157:H7 strains of diverse origins, 5 of 6 eae+ non-O157:H7 STEC strains isolated from patients in Seattle, and RDEC-1, a rabbit EPEC strain that probably evolved from an STEC progenitor (40), each contains a 4.7-kb BstXI DNA fragment that is homologous to the probe consisting of the insert of pIha (Table 1 and Fig. 3). Three non-O157:H7, eae+ STEC strains possess a second BstXI fragment of approximately 10.1 kb that is also detected by this iha probe (Fig. 3). Five of 25 E. coli O157:H7 strains also have a second BstXI fragment of approximately 7.5 kb that hybridizes to the iha probe (data not shown). eae-negative STEC O104:H21 and O113:H21 contain DNA that is homologous to the iha probe on 3.9- and 10.1-kb and 7.9-kb BstXI fragments, respectively (Fig. 3). One of five human EPEC strains distantly related to E. coli O157:H7 has a BstXI fragment detected by the iha probe that is slightly larger than the 4.7-kb iha-homologous BstXI fragment in E. coli O157:H7 (Fig. 3). None of four E. coli O157:H− strains (stx2+ pathogens closely related to E. coli O157:H7) and only one of seven E. coli O55:H7 strains (nontoxigenic EPEC closely related to E. coli O157:H7) tested contain iha-homologous DNA (Table 1 and Fig. 4). Only 3 of 20 commensal fecal E. coli strains have iha-homologous BstXI fragments (Table 1).
FIG. 3.
Conservation of TAI in E. coli strains from diverse lineages. Hybridization of the 35-kb TAI probe to BstXI-digested DNA from E. coli O157:H7 (lane 1), E. coli HB101 (lane 2), E. coli O26:NM (strain TB352A) (lane 3), E. coli O26:H2 (strain TB285A) (lane 4), E. coli O85:NM (strain TB334C) (lane 5), E. coli O103:H2 (UTI strain) (lane 6), E. coli O103:H6 (strain TB154A) (lane 7), E. coli O111:HN (strain TB226A) (lane 8), E. coli O104:H21 (lane 9), E. coli O113:H21 (strain CL15) (lane 10), E. coli O15:NM (strain RDEC-1) (lane 11), E. coli O111:NM (strain B171) (lane 12), E. coli O111:H− (strain 2430-78) (lane 13), E. coli O119:H6 (strain 659-79) (lane 14), E. coli O127:H6 (strain E2348-69) (lane 15), and E. coli O142:H6 (strain 581-71) (lane 16) is shown. The arrows indicate fragments detected when the membrane is probed with cloned iha.
FIG. 4.
Absence of TAI from E. coli strains closely related to E. coli O157:H7. Hybridization of the TAI probe to BstXI-digested DNA from E. coli O157:H7 (lane 1); E. coli HB101 (lane 2); E. coli O55:H7 strains 5A, 5B, 5C, 5D, 5E, TB156A, and TB182A (lanes 3 to 9, respectively); and E. coli O157:H− strains 493-89, 5412-89, CB569, and 514-91 (lanes 10 to 13, respectively) is shown. The arrows indicate fragments detected when membrane is probed with cloned iha.
The 35-kb insert of pSC(A-G6), which we have termed the tellurite resistance and adherence-conferring island (TAI), detects BstXI fragments of identical size in 21 of 21 E. coli O157:H7 strains tested (Fig. 3 and 4). Six of seven iha+ eae+ STEC and RDEC-1 strains and one human EPEC strain tested also contain TAI homologues (defined as DNA in which most or all of the TAI-homologous BstXI fragments are identical in size to TAI-homologous fragments in E. coli O157:H7 on Southern blots) (Fig. 3).
The TAI probe detects DNA in two iha+ eae-negative STEC strains and one iha+ eae+ E. coli O55:H7 strain (Fig. 4), but the fragments detected differ in number and size from TAI-homologous BstXI fragments in E. coli O157:H7. The TAI probe detects in some iha-negative E. coli strains, including E. coli HB101 (Fig. 3 and 4), BstXI fragments that are fewer in number, fainter, and of different sizes than the TAI-homologous BstXI fragments in E. coli O157:H7.
OMP and TBP analysis.
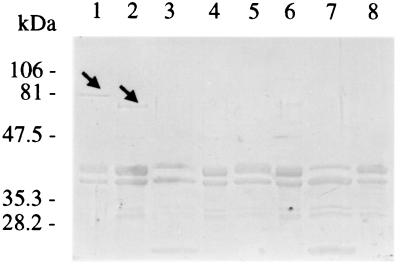
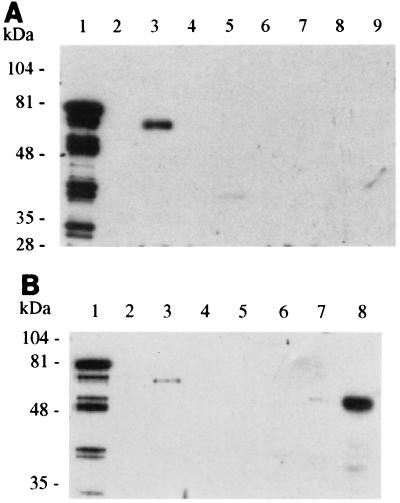
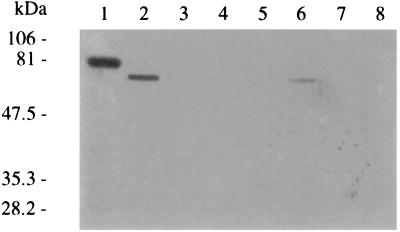
E. coli ORN172(pIha) expresses a 78-kDa OMP, the deduced mass of the iha-encoded protein. E. coli ORN172(pSK+) does not express a similar protein. A prominent 67-kDa band is seen in E. coli O157:H7 OMPs (Fig. 5). α-Iha antibodies detect a 78-kDa OMP in E. coli ORN172(pIha) and a 67-kDa OMP in E. coli 0157:H7 strain 86-24 (Fig. 6 and 7) and each of eight additional E. coli O157:H7 strains tested (data not shown), 56-kDa OMPs in eae-negative STEC O104:H21 and O113:H21 (Fig. 6B), and a faint 39-kDa OMP in E. coli O26:NM (Fig. 6A). α-Iha antibodies do not detect OMPs in other iha+ E. coli tested or in E. coli ORN172(pSK+) (Fig. 6 and 7). α-Iha antibodies detect multiple peptides smaller than 78 kDa in some (Fig. 6A and 6B), but not all (Fig. 7), E. coli ORN172(pIha) OMP preparations. A 67-kDa band is not seen in Coomassie-stained OMPs from E. colinalR(Δiha) (Fig. 5), but α-Iha antibodies detect a faint 67-kDa band in some (Fig. 7), but not all (Fig. 6A), of the OMPs prepared from this mutant.
FIG. 5.
Presence of Iha in OMPs of wild-type, mutant, and recombinant E. coli. Coomassie blue-stained OMPs of E. coli ORN172(pIha) (lanes 1 and 3), E. coli O157:H7 (lanes 2 and 4), E. coli ORN172(pSK+) (lanes 5 and 7), and E. coli O157:H7nalR(Δiha) (lanes 6 and 8) without (lanes 1, 2, 5, and 6) and with (lanes 3, 4, 7, and 8) proteinase K treatment. The arrows indicate probable Iha protein.
FIG. 6.
Detection of immunoreactive Iha in iha+ E. coli (A) OMPs from E. coli ORN172(pIha) (lane 1), E. coli ORN172(pSK+) (lane 2), E. coli O157:H7 (lane 3), E. coli O157:H7nalR(Δiha) (lane 4), E. coli O26:NM (strain TB352A) (lane 5), E. coli O26:H2 (strain TB285A) (lane 6), E. coli O85:NM (strain TB334C) (lane 7), E. coli O111:NM (strain TB226A) (lane 8), and E. coli O103:H2 (UTI strain) (lane 9) probed with α-Iha antibodies. (B) OMPs from E. coli ORN172(pIha) (lane 1), E. coli ORN172(pSK+) (lane 2), E. coli O157:H7 (lane 3), E. coli O55:H7 strain 5E (lane 4), E. coli O111:H− (strain 2430-78) (lane 5), E. coli O15:NM (strain RDEC-1) (lane 6), E. coli O104:H21 (lane 7), and E. coli O113:H21 (strain CL15) (lane 8) probed with α-Iha antibodies.
FIG. 7.
Effect of proteinase K on immunoreactive Iha. OMPs of E. coli ORN172(pIha) (lanes 1 and 3), E. coli O157:H7 (lanes 2 and 4), E. coli ORN172(pSK+) (lanes 5 and 7), and E. coli O157:H7nalR(Δiha) (lanes 6 and 8) without (lanes 1, 2, 5, and 6) and with (lanes 3, 4, 7, and 8) proteinase K treatment, probed with α-Iha antibodies.
We employed proteinase K digestion of whole bacteria to determine if Iha possesses proteinase-susceptible (i.e., externally directed) domains. Proteinase K digests the prominent 78- and 67-kDa and faint 67-kDa OMPs expressed by E. coli ORN172(pIha), E. coli O157:H7, and E. coli O157:H7nalR(Δiha), respectively, as seen on Coomassie blue-stained gels and protein immunoblots (Fig. 5 and 7). However, this treatment does not affect most other OMPs (Fig. 5) or periplasmic Mbp (data not shown). Hence, Iha has externally directed sites susceptible to proteinase digestion.
DISCUSSION
Iha, an OMP with externally directed domains, is the first E. coli O157:H7 protein to be described that is sufficient to confer the adherence phenotype upon nonadherent laboratory E. coli. Iha is homologous to a variety of bacterial iron acquisition proteins in the database but not to other known adhesins. However, the homology between Iha and IrgA is significant because an irgA::TnphoA mutant of V. cholerae colonizes infant mice less well than does its parent and is less virulent (16). Thus, of the many Iha-homologous proteins generated by the BLAST search, IrgA has a proposed function analogous to the adherence-conferring properties of Iha.
It is tempting to speculate that Iha homologues in organisms other than E. coli O157:H7 play a role in pathogenicity, especially in pathogens without eae. For example, ORF R4, an iha homologue found in E. coli CFT073 and other uropathogenic E. coli (17), might contribute to virulence by enhancing the adherence of these organisms. Also, eae-negative STEC, such as E. coli O104:H21 and E. coli O113:H21, which have caused epidemic (3, 31a) and sporadic (11) enteric human infections, are iha+ and have Iha antigen in their OMPs. Perhaps these eae-negative STEC utilize Iha or an Iha homologue for adherence and colonization purposes in lieu of intimin.
The possibility exists that iha does not encode an adhesin but instead encodes a protein that increases the expression of a cryptic adhesin in laboratory E. coli. Such a molecule might be analogous to Crl, a transcriptional activator of csgA, which encodes the curlin subunit enabling E. coli to bind fibronectin (4, 31). However, Crl is located predominantly in the cytoplasm, whereas Iha is a comparatively prominent E. coli OMP, as demonstrated by Coomassie blue staining and immunoblots. Furthermore, the susceptibility of Iha to proteinase K digestion following incubation of whole bacteria with this enzyme suggests that Iha possesses one or more externally exposed domains. These findings are all consistent with the role of Iha as an adhesin.
Technical difficulties precluded the performance of additional experiments that might have established more firmly the role of Iha as an adhesin. In particular, the demonstration that an antibody to an externally directed Iha epitope ablates or reduces epithelial cell adherence of E. coli O157:H7 or of E. coli ORN172(pIha) would help establish its role in adherence. However, the antibodies that we elicited by peptide immunization, after many unsuccessful attempts using other formulations of Iha as an immunogen, do not detect externally directed epitopes of this molecule, as they are expressed in E. coli O157:H7 or in laboratory E. coli (immunofluorescence data not shown). These α-Iha antibodies would not, therefore, be appropriate to use in adherence inhibition studies.
The smaller Mr of the antigen detected by α-Iha antibodies in E. coli O157:H7 OMPs, compared to the Mr of Iha deduced from the size of the adherence-conferring gene, suggests that full-length Iha might be cleaved in wild-type E. coli O157:H7. The array of immunoreactive proteins with Mrs smaller than the deduced size of Iha in some preparations of E. coli ORN172(pIha) OMPs also suggests that full-length Iha might be subject to either proteolytic cleavage or nonspecific degradation.
The lack of detectable Iha antigen in many non-O157:H7 iha+ E. coli suggests the possibility of serotype- and lineage-specific expression of Iha. E. coli O157:H7 differs from non-O157:H7 STEC in its array of virulence genes (33), and perhaps also in the ability to express proteins encoded by shared alleles, such as iha. In fact, a precedent for serotype- and lineage-specific in vitro protein expression exists in the case of the EHEC hemolysin, which is encoded by a gene on pO157 and on a similar plasmid in non-O157:H7 STEC (33) and which is variably expressed by pathogenic STEC. Alternatively, polymorphisms in expressed Iha homologues in non-O157:H7 STEC might interfere with the ability of α-Iha antibodies to detect these proteins.
Though Iha is sufficient to confer adherence, a virulence phenotype, upon nonadherent E. coli, we are cautious about designating Iha as a virulence factor. The role of Iha in the pathogenesis of E. coli O157:H7 might remain difficult to assign, because humans cannot be challenged with STEC or its derivatives. The possibility also exists that Iha facilitates the adherence of E. coli O157:H7 to epithelial cells in nonpathogenic milieus, such as animal gastrointestinal tracts. Indeed, iha confers upon laboratory E. coli the ability to adhere to MDBK cells, an epithelial line of bovine origin.
Our detection of a variably expressed OMP(s) that reacts with α-Iha antibodies raises the possibility that one or more yet to be characterized Iha homologues in E. coli O157:H7 also have adherence-conferring properties. Such a molecule might account for the residual and variable adherence of E. coli O157:H7 from which iha has been deleted. However, it should be noted that intimin, which clearly has a role in the intimate attachment of E. coli to epithelial cells, would probably be expressed by E. coli O157:H7nalR(Δiha) and could also have mediated this residual adherence.
The phylogenetic aspects of the acquisition of iha and TAI are noteworthy. The presence and conserved structure of TAI in multiple E. coli strains distantly related to E. coli O157:H7 suggest that this island transfers between organisms on a mobile element. Our data also suggest that E. coli O157:H7 acquired TAI relatively recently, i.e., after it acquired the O157 rfb cluster and the stx2-encoding bacteriophage in its evolution from the progenitor it shares with E. coli O55:H7. TAI acquisition therefore represents an additional differentiating event in the evolution of E. coli O157:H7 from E. coli O157:H− (39).
Our data provide a genetic explanation for the respective tellurite resistances and susceptibilities of E. coli O157:H7 and E. coli O157:H− (22, 43). Tellurite resistance loci and the Iha-homologous colicin I receptor are linked on plasmid R478 in S. marcescens. This plasmid confers adherence on E. coli J62 (29), possibly via this Iha homologue. However, in the E. coli CFT073 PAI, tellurite resistance loci are not found 3′ of an iha homologue (20), so the linkage between genes encoding tellurite resistance and iha homologues is not always conserved.
In summary, our work introduces Iha as a novel adherence-conferring molecule. In its evolution from the progenitor it shares with E. coli O55:H7, and in its differentiation from E. coli O157:H−, E. coli O157:H7 acquired TAI, and possibly other chromosomal islands, in addition to toxin-encoding bacteriophages and rfb loci. The role of Iha in animal and human colonization by STEC, the structure and function of the Iha homologues in a variety of bacteria, the mechanisms underlying Iha expression, and the mode of transfer of TAI between bacteria warrant further elucidation.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (AI-38419), U.S. Department of Agriculture (94-03953), and National Cattlemen's Beef Association grants.
We thank Steve L. Moseley for generous advice and encouragement; Stephen B. Calderwood, Michael S. Donnenberg, James B. Kaper, Beth Traxler, Thomas S. Whittam, and Shing-Erh Yen for helpful suggestions; and Christine A. Merrikin for secretarial assistance.
REFERENCES
- 1.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, Sutton A, Silver R P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Outbreak of acute gastroenteritis attributable to Escherichia coli serotype O104:H21—Helena, Montana, 1994. Morbid Mortal Weekly Rep. 1994;44:501–503. [PubMed] [Google Scholar]
- 4.Arnqvist A, Olsen A, Pfeifer J, Russell D G, Abrmark S. The Crl protein activates cryptic genes for curli formation and fibronectin binding in Escherichia coli HB101. Mol Microbiol. 1992;6:2443–2452. doi: 10.1111/j.1365-2958.1992.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 5.Bell B P, Goldoft M, Griffin P M, Davis M A, Gordon D C, Tarr P I, Bartleson C A, Lewis J H, Barrett T J, Wells J G, et al. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. JAMA. 1994;272:1349–1353. [PubMed] [Google Scholar]
- 6.Bilge S S, Vary J C, Jr, Dowell S F, Tarr P I. Role of the Escherichia coli O157:H7 O side chain in adherence and analysis of an rfb locus. Infect Immun. 1996;64:4795–4801. doi: 10.1128/iai.64.11.4795-4801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bokete T N, Whittam T S, Wilson R A, Clausen C R, O'Callahan C M, Moseley S L, Fritsche T R, Tarr P I. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. J Infect Dis. 1997;175:1382–1389. doi: 10.1086/516470. [DOI] [PubMed] [Google Scholar]
- 8.Cantey J R, Blake R K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977;135:454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- 9.Dauenhauer S A, Hull R A, Williams R P. Cloning and expression in Escherichia coli of Serratia marcescens genes encoding prodigiosin biosynthesis. J Bacteriol. 1984;158:1128–1132. doi: 10.1128/jb.158.3.1128-1132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P M. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott S J, Yu J, Kaper J B. The cloned locus of enterocyte effacement from enterohemorrhagic Escherichia coli O157:H7 is unable to confer the attaching and effacing phenotype upon E. coli K-12. Infect Immun. 1999;67:4260–4263. doi: 10.1128/iai.67.8.4260-4263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 14.Glantz S A. Primer of biostatistics. 4th ed. New York, N.Y: McGraw-Hill Health Professions; 1997. [Google Scholar]
- 15.Goldberg M B, Boyko S A, Butterton J R, Stoebner J A, Payne S M, Calderwood S B. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB-dependent proteins. Mol Microbiol. 1992;6:2407–2418. doi: 10.1111/j.1365-2958.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg M B, DiRita V J, Calderwood S B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990;58:55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyer D M, Kao J S, Mobley H L. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect Immun. 1998;66:4411–4417. doi: 10.1128/iai.66.9.4411-4417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jobling M G, Ritchie D A. The nucleotide sequence of a plasmid determinant for resistance to tellurium anions. Gene. 1988;66:245–258. doi: 10.1016/0378-1119(88)90361-7. [DOI] [PubMed] [Google Scholar]
- 20.Kao J S, Stucker D M, Warren J W, Mobley H L. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaper J B, Elliott S, Sperandio V, Perna N T, Mayhew G F, Blattner F R. Attaching-and-effacing intestinal histopathology and the locus of enterocyte effacement. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other shiga toxin-producing E. coli. Washington, D.C.: ASM Press; 1998. pp. 163–182. [Google Scholar]
- 22.Karch H, Janetzki-Mittmann C, Aleksic S, Datz M. Isolation of enterohemorrhagic Escherichia coli O157 strains from patients with hemolytic-uremic syndrome by using immunomagnetic separation, DNA-based methods, and direct culture. J Clin Microbiol. 1996;34:516–519. doi: 10.1128/jcm.34.3.516-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O'Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 24.Levine M M, Xu J G, Kaper J B, Lior H, Prado V, Tall B, Nataro J, Karch H, Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987;156:175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- 25.Louie M, de Azavedo J C, Handelsman M Y, Clark C G, Ally B, Dytoc M, Sherman P, Brunton J. Expression and characterization of the eaeA gene product of Escherichia coli serotype O157:H7. Infect Immun. 1993;61:4085–4092. doi: 10.1128/iai.61.10.4085-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 27.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 28.McKee M L, O'Brien A D. Truncated enterohemorrhagic Escherichia coli (EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect Immun. 1996;64:2225–2233. doi: 10.1128/iai.64.6.2225-2233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mignatti P, Pagani L, Perduca M, Romero E. R factor-mediated adhesiveness to mammalian cells in E. coli K12. Microbiologica. 1985;8:101–111. [PubMed] [Google Scholar]
- 30.Newland J W, Neill R J. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J Clin Microbiol. 1988;26:1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen A, Jonsson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;20:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 31a.Paton A W, Woodrow M C, Doyle R M, Lanser J A, Paton J C. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley L W, Junio L N, Libaek L B, Schoolnik G K. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun. 1987;55:2052–2056. doi: 10.1128/iai.55.9.2052-2056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 34.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. BioTechniques. 1983;1:784–791. [Google Scholar]
- 35.Tarr P I, Bilge S S. Intimin-independent adherence mechanisms of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. Washington, D.C.: ASM Press; 1998. pp. 157–162. [Google Scholar]
- 36.Tarr P I, Fouser L S, Stapleton A E, Wilson R A, Kim H H, Vary J C, Jr, Clausen C R. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coli O103:H2. N Engl J Med. 1996;335:635–638. doi: 10.1056/NEJM199608293350905. [DOI] [PubMed] [Google Scholar]
- 37.Tarr P I, Neill M A, Clausen C R, Newland J W, Neill R J, Moseley S L. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984–1987. J Infect Dis. 1989;159:344–347. doi: 10.1093/infdis/159.2.344. [DOI] [PubMed] [Google Scholar]
- 38.Wahl G M, Lewis K A, Ruiz J C, Rothenberg B, Zhao J, Evans G A. Cosmid vectors for rapid genomic walking, restriction mapping and gene transfer. Proc Natl Acad Sci USA. 1987;84:2160–2164. doi: 10.1073/pnas.84.8.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittam T S. Genetic population structure and pathogenicity in enteric bacteria. In: Baumberg S, Young J P W, Saunders S R, Wellington E M H, editors. Population genetics of bacteria. Cambridge, United Kingdom: Cambridge University Press; 1995. pp. 217–245. [Google Scholar]
- 40.Whittam T S, McGraw E A. Clonal analysis of EPEC serogroups. Rev Microbiol. 1996;27:7–16. [Google Scholar]
- 41.Whittam T W, Wolfe M L, Wachsmuth I K, Orskov F, Orskov I, Wilson R A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodall L D, Russell P W, Harris S L, Orndorff P E. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5 promoter. J Bacteriol. 1993;175:2770–2778. doi: 10.1128/jb.175.9.2770-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zadik P M, Chapman P A, Siddons C A. Use of tellurite for the selection of verocytotoxigenic Escherichia coli O157. J Med Microbiol. 1993;39:155–158. doi: 10.1099/00222615-39-2-155. [DOI] [PubMed] [Google Scholar]