Abstract
Background:
The United States has persistently high rates of preterm birth and low birthweight, and is characterized by significant racial disparities in these rates. Innovative group prenatal care models, like CenteringPregnancy, have been proposed as a potential approach to improve rates of preterm birth and low birthweight and to reduce disparities in these pregnancy outcomes.
Objectives:
This study aimed to test whether participation in group prenatal care would reduce rates of preterm birth and low birthweight compared to individual prenatal care, and whether group prenatal care would reduce the racial disparity in these rates between Black and White patients.
Study Design:
This is a randomized controlled trial among medically low-risk pregnant patients at a single study site. Eligible patients were stratified by self-identified race and ethnicity and then randomly allocated 1:1 between group and individual prenatal care. The primary outcomes were preterm birth <37 weeks gestational age and low birthweight <2500 grams. The primary analysis was performed according to the intent-to-treat principle. Secondary analyses were performed according to the as-treated principle using modified intent-to-treat and per compliance approaches. Analysis of effect modification by race and ethnicity was planned.
Results:
A total of 2350 participants were enrolled, with 1176 assigned to group prenatal care and 1174 assigned to individual prenatal care. The study population included 952 (40.5%) Black, 502 (21.4%) Hispanic, 863 (36.8%) White, and 31 (1.3%) “Other race or ethnicity”. Group prenatal care did not reduce the rate of preterm birth (10.4% vs. 8.7%, OR 1.22, 95% CI 0.92–1.63, p=0.17) or low birthweight (9.6% vs. 8.9%, OR 1.08, 95% CI 0.80–1.45, p=0.62) when compared to individual prenatal care. In subgroup analysis, greater attendance in prenatal care was associated with lower rates of preterm birth and low birthweight. This effect was most pronounced for rates of low birthweight for Black participants in group care; intent-to treat 12.5% (51/409), modified intent-to-treat 11.5% (36/313), and per compliance 8.3% (20/240). While LBW rates were significantly higher for Black participants compared to White participants seen in individual care (aOR 2.00, 95% CI 1.14 – 3.50) the difference was not significant for Black participants in group care compared to their White counterparts (aOR 1.58, 95% CI 0.74 – 3.34).
Conclusions:
There was no significant difference in overall rates of preterm birth or low birthweight between group and individual prenatal care. With increased participation in group prenatal care, we observed lower rates of preterm birth and low birthweight for Black participants. The role for group care models in reducing racial disparities in these birth outcomes requires further study.
Keywords: Prenatal care, group prenatal care, CenteringPregnancy, racial equity, health disparities, preterm birth, low birthweight
Condensation:
GPNC does not decrease overall rates of PTB or LBW, but increased participation in GPNC is associated with improved outcomes for Black participants.
Introduction
Preterm birth (PTB) occurs at high rates in the United States, where 10.2% of pregnancies are delivered before 37 weeks gestational age.1 Large racial disparities persist in these rates, with Black patients experiencing a 14.4% rate of PTB compared to 9.3% in White patients in 2019.1,2 PTB is the second leading cause of newborn death and disability, and infants born both preterm and with a low birthweight (LBW; <2500 grams) can face medical and neurodevelopmental challenges.3–9 In this way, the racial disparities arising during pregnancy, amplified further by environmental and sociodemographic factors after birth, have the potential to impact the burden of chronic disease and economic achievement across the lifespan.4,10
The dominant model of individual prenatal care (IPNC) includes approximately 13 visits scheduled with increasing frequency throughout pregnancy.11–13 Patients who enter care late or attend fewer than the recommended number of visits have an increased risk of PTB, infant mortality, and pre-eclampsia.14–16 The IPNC model, however, has significant limitations.17,18 Typically, IPNC visits are short, can feel hurried, and do not necessarily allow adequate time for healthcare providers to share all the anticipatory guidance pregnant individuals need and want.19,20 When obstetric practices rotate patients through multiple providers, the lack of continuity can feel impersonal and decrease satisfaction and trust in the clinical recommendations.19,20 Within this traditional structure of IPNC, not only are there persistent racial disparities in birth outcomes but also in the delivery of health care itself. Examples of healthcare disparities in outpatient prenatal care impacting Black patients include lower rates of influenza vaccination, lower comprehension of prenatal genetic testing options, and lower rates of postpartum depression screening.21–24
Group prenatal care (GPNC) potentially addresses many shortcomings of IPNC by bundling physical assessment with family and peer support as well as provider-led, patient-centered health education.25 There is a promising yet inconsistent evidence base that supports GPNC as a mechanism to improve birth outcomes and narrow the racial disparity in adverse outcomes.26–27 In two randomized clinical trials and several retrospective cohort studies, participation in GPNC was associated with decreased rates of LBW and PTB for Black patients.26,28–33 The prospective studies, however, were underpowered to show differences in PTB by race, and the retrospective studies were limited by potential confounding from selection bias and small sample sizes.
To provide more definitive evidence, we sought to test two primary hypotheses: 1) participation in GPNC will reduce rates of PTB and LBW; and 2) participation in GPNC will reduce the racial disparities for PTB and LWB between Black and White participants when compared to traditional IPNC.
Materials and Methods
Participants, Design and Setting
The study was conducted at a single practice site and was approved by the Institutional Review Board (Pro00043994). The study was registered with ClinicalTrials.gov on December 29, 2015 (NCT02640638). The full study protocol has been previously published.35
Patients were screened for study eligibility by medical record review prior to their first appointment for prenatal care. Inclusion criteria included age 14 – 45 years, singleton pregnancy, entry to care <20 6/7 weeks gestational age, and gestational age <24 0/7 weeks at enrollment. Following longstanding internal practice protocols consistent with the CenteringPregnancy GPNC model, exclusion criteria included medical or pregnancy complications that would preclude prenatal care delivery by a nurse practitioner. Examples include pregestational diabetes, chronic hypertension on medications, any disease requiring chronic immunosuppression (such as solid organ transplant) and severe obesity with body mass index >50 kg/m2. Patients anticipating a planned preterm delivery for reasons such as a history of myomectomy or classical uterine incision were excluded, as well as those with a plan for history indicated cerclage. Finally, patients with medical, social or behavioral conditions that would preclude group participation such as active tuberculosis, current incarceration, or severe uncontrolled psychiatric illness, were also excluded. Patients could only participate in the study with one pregnancy to maintain independence.
Patients were approached by trained research staff at the time of either their first prenatal care appointment or during their dating ultrasound appointment. Research staff described all study procedures, including a detailed description of the GPNC model of care and study incentives. After research staff obtained written informed consent, participants were randomly assigned in a 1:1 allocation to GPNC or IPNC, stratified by self-reported maternal race and ethnicity using REDCap (Research Electronic Data Capture) electronic data capture tools (see Appendix A).34
At the time of enrollment, participants completed a survey instrument, also in REDCap, which included psychosocial measures, assessment of maternal health behavior and other baseline demographic questions and detailed information about race and ethnicity. This gave the participants a private opportunity to self-identify a more a nuanced description of their race and ethnicity, including validated questions from the US Federal Government 1997 Office of Management and Budget standards on race and ethnicity used by the US Census Bureau. These questions allowed participants to select multiple categories for race and ethnicity and included open-ended descriptions for a more thorough and comprehensive reporting of participants’ race and ethnicity.35–36
Interventions
The IPNC arm received prenatal care following the schedule of visits recommended by the American College of Obstetricians and Gynecologists.12,37 The GPNC arm received CenteringPregnancy curriculum (Centering Healthcare Institute, Boston MA), and the study site was able to deliver the intervention in English and Spanish. This trademarked model includes a formal training and annual certification process to assure consistency in implementation.25,38 Groups of 8–12 pregnant patients due to deliver in the same month are scheduled for ten 2-hour GPNC sessions. During the first 30 minutes, a healthcare provider conducts a brief physical assessment in the group space. The remaining 90 minutes are spent in facilitated discussion. The curriculum is comprehensive, and topics include family planning and childbirth preparation, stress management, newborn care, and parenting skills. GPNC participants are allowed to have additional individual prenatal care visits in addition to the 10 scheduled GPNC sessions as needed.
Study Outcomes
Antepartum, delivery, postpartum and neonatal outcomes were abstracted from the electronic medical record (Epic Systems Incorporated, Verona, WI). Medical records of participants who transferred out of the practice were requested and included in the final analysis. Abstractors were independent of the medical team but were not blinded to the study group assignment.
Our primary outcomes were PTB (delivery <37 weeks gestational age), and LBW (infant birthweight <2500 grams). Both variables were dichotomized for the two primary analyses. The best obstetrical estimate of gestational age was used in all cases, and all participants had ultrasound confirmation of pregnancy dating <20 weeks gestational age as standard of care.39,40
Sample Size Determination
Based on a previously published rate for PTB of 13% in our practice, we estimated a sample size of 2712 (n= 1356 in each treatment group) which would have 90% power to detect an odds ratio of 0.70 in preterm birth (GPNC vs. IPNC), at a two-sided significance of 0.05.31,41 This was obtained using the methods for sample size calculation through the logistic regression with two binary covariates and implemented by PASS software (NCSS, LLC, UT, USA). More information about the sample size and power calculations can be found in the published study protocol.35 We anticipated a 15% attrition rate and therefore sought to recruit 3160 patients (n=1580 in each treatment group).
Statistical Analysis
Descriptive statistics included mean, standard deviation, frequency, and percentages. Between arm comparisons were made using t-tests for continuous variables and chi-square or Fisher’s exact test for categorical variables. Logistic regressions were employed to test the first hypothesis concerning GPNC main effect adjusting for race.
For the second hypothesis examining reduction in racial disparities, we tested an interaction effect between the group assignment (i.e., GPNC vs. IPNC) and race and ethnicity (i.e., Black, Hispanic, and White) for outcomes of PTB and LBW using logistic regression models. We excluded the “other” racial group in this second hypothesis testing due to its small sample size.
The primary analysis was performed according to the intent-to-treat principle, but two additional analytic samples were planned. First, the modified intent-to-treat (mITT) sample included participants who attended one or more sessions or visits in their assigned treatment arm (GPNC and IPNC). Second, the per-compliance (PC) sample included participants with five or more sessions or visits in their assigned treatment arm (GPNC and IPNC) and excluded participants who crossed over from IPNC to GPNC. This five-visit threshold was based on previously published studies which have also used this threshold as an indicator of adequate exposure to the GPNC treatment.29,30,42–45
Because the mITT and PC samples no longer represented a randomized pool of participants due to exclusions, we additionally adjusted for the baseline variables that were significantly different (p<0.01) between care models in each sample at baseline. All analyses were performed using SAS version 9.4 (SAS Institute Inc. Cary, NC) and statistical significance was declared for a two-sided p-value <0.05 except where indicated.
Results
We screened 9181 patients for study eligibility. Of these, 3818 were ineligible, 3019 eligible patients declined to participate, and 2350 were enrolled and randomized (43.8% of eligible patients enrolled, Figure 1). The most common reasons for screen failure and non-randomization were late entry to prenatal care (n=1758), complex medical co-morbidities precluding care from nurse practitioners (n=824) and pregnancy loss prior to study enrollment (n=287). After randomization, one set of twins was identified in each study arm and were excluded from further analysis.
Figure 1:
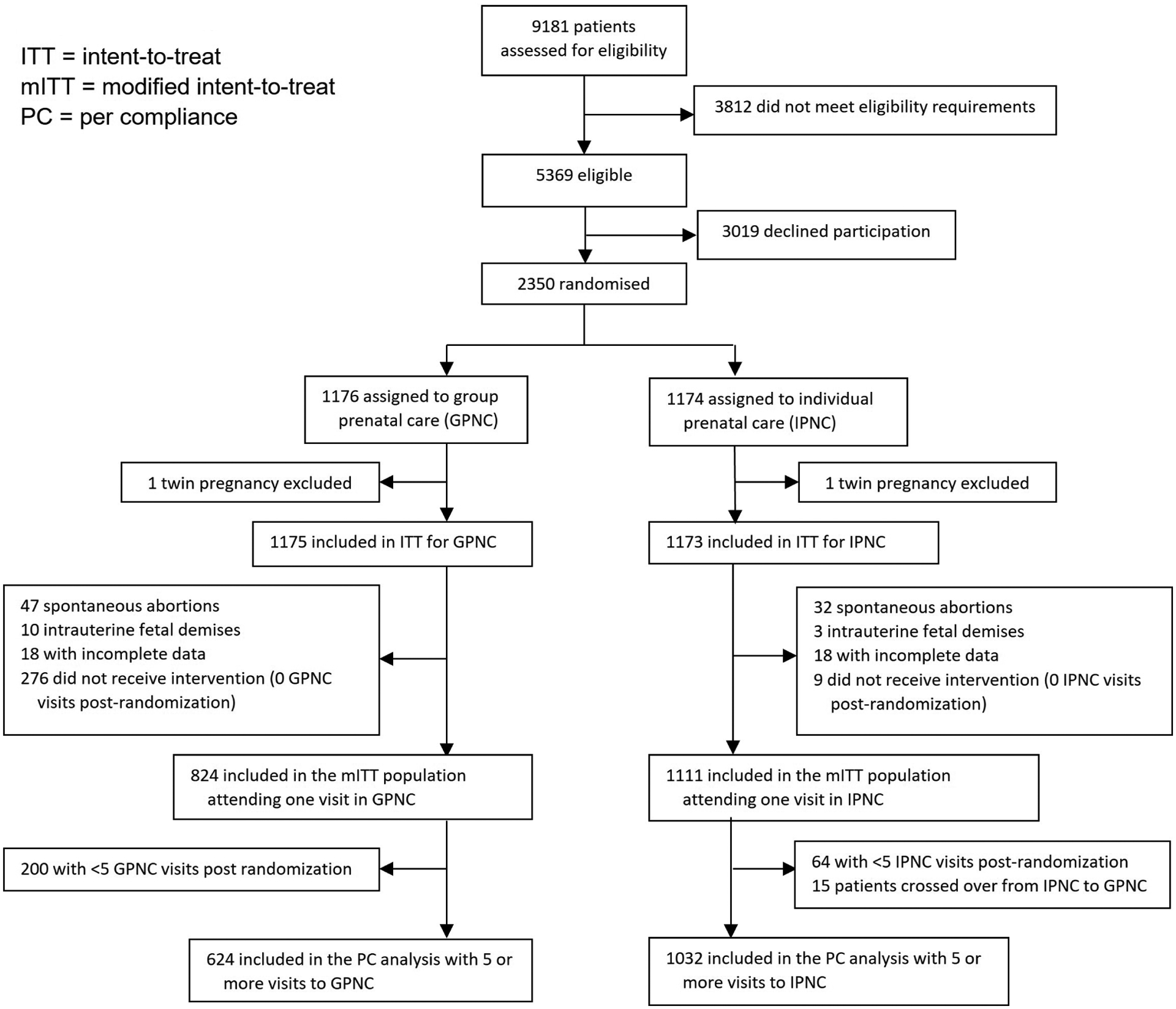
Trial Profile.
This figure contains the CONSORT flow diagram of the progress through the phases of a parallel randomized trial of two groups, including enrollment, intervention allocation, follow-up, and data analysis
Study enrollment began February 24, 2016 and ended early on March 16, 2020 when the COVID-19 pandemic restricted our ability to safely continue in-person GPNC sessions. At that time, 172 participants were actively enrolled, including 85 in the GPNC assignment. Of these, 39 could not complete the 5 visits minimum in GPNC due to the pandemic.
Of the 2348 participants in this study, 1175 were assigned to GPNC and the 1173 assigned to IPNC. Detailed baseline characteristics are shown in Table 1 and were balanced between the GPNC and IPNC treatment arms. Table 1 includes demographic characteristics and risk factors for PTB across all three analytic samples: ITT, mITT and PC. Overall, 35.8% (n=841) of participants reported Black race, 19.7% (n=462) reported Hispanic ethnicity, 35.2% (n=826) reported White race, and 31 (1.3%) reported Other race or ethnicity. The mean age was 25 (SD±5.4) years, and 44.5% (n=1044) participants were primiparous. Most participants (96.5%, n=2028) were Medicaid eligible at delivery, 25.8% (n=575) had not completed a high school degree, 23.7% (n=434) were married and 66.6% (n=1504) of pregnancies were unplanned.
Table 1.
Demographic characteristics and risk factors by group prenatal care (GPNC) compared to individual prenatal care (IPNC) in three analytic samples; intent-to-treat, modified intent-to-treat, and per compliance in the Cradle study.
| Intent-to-Treat | Modified Intent-to-Treata | Per Complianceb | ||||
|---|---|---|---|---|---|---|
| GPNC | IPNC | GPNC | IPNC | GPNC | IPNC | |
| Demographic Characteristics | 1175 | 1173 | 824 (70.1) | 1111 (94.7) | 624 (53.1) | 1032 (88.0) |
| Other | 16 (1.4) | 15 (1.3) | 10 (1.2) | 13 (1.2) | 10 (1.6) | 13 (1.3) |
| Maternal age, (mean ± SD) | 25.3 ± 5.42 | 25.0 ± 5.32 | 25.4 ± 5.5 | 25.0 ± 5.3 | 25.6 ± 5.5c | 25.0 ± 5.3 |
| Language, English % | 987 (84.5) | 994 (85.0) | 681 (83.4) | 938 (84.7) | 504 (81.4) | 867 (84.3) |
| Education, High school or above % | 830 (75.1) | 819 (73.3) | 581 (75.3) | 766 (72.4) | 447 (76.5) | 712 (72.5) |
| Employment, Employed full or part-time % | 591 (54.0) | 593 (53.9) | 422 (55.2) | 556 (53.3) | 325 (56.2) | 520 (53.7) |
| ≥50,000 | 30 (3.8) | 34 (4.3) | 20 (3.6) | 34 (4.5) | 15 (3.5) | 32 (4.6) |
| Marital status, Married % | 212 (23.9) | 222 (23.5) | 175 (24.4) | 220 (23.4) | 154 (26.2) | 214 (23.6) |
| Medicaid eligible, % | 998 (96.2) | 1029 (96.8) | 763 (96.2) | 1025 (96.9) | 585 (95.6) | 966 (96.9) |
| No insurance any time within past year, % | 563 (49.1) | 579 (50.8) | 391 (49.0) | 543 (50.3) | 297 (49.2) | 506 (50.5) |
| Pregnancy Intention, % | 386 (34.0) | 368 (32.8) | 269 (34.0) | 353 (33.2) | 205 (34.2) | 328 (33.3) |
| Gestational age at entry to prenatal care, weeks (mean ±SD) | 9.21 ± 3.46 | 9.38 ± 3.47 | 9.2 ± 3.3 | 9.4 ± 3.4 | 9.1 ± 3.2 | 9.3 ± 3.4 |
| Gestational age study enrollment, weeks (mean ±SD) | 12.11 ± 3.95 | 12.24 ± 3.98 | 12.0 ± 3.8 | 12.3 ± 4.0 | 12.0 ± 3.8 | 12.2 ± 3.9 |
| Parity, Nulliparous, % | 522 (44.4) | 522 (44.5) | 356 (43.2) | 494 (44.5) | 260 (41.7) | 465 (45.1) |
| Obese (30+) | 474 (40.3) | 446 (38.0) | 327 (39.7) | 420 (37.8) | 241 (38.6) | 397 (38.5) |
| Risk Factors for Preterm Birth | ||||||
| History of prior LEEP/Cervical survey, % | 14 (1.2) | 11 (0.9) | 10 (1.2) | 10 (0.9) | 6 (1.0) | 9 (0.9) |
| Muellerian uterine anomaly, % | 0 (0.0) | 5 (0.4) | 0 (0.0)a | 5 (0.5) | 0 (0.0) | 4 (0.4) |
| Pregnancy conceived by ART, % | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Smoking during pregnancy, % | 196 (17.2) | 220 (19.3) | 127 (15.9) | 207 (19.1) | 83 (13.8)c | 194 (19.3) |
| Drinking alcohol during pregnancy, % | 52 (4.6) | 43 (3.8) | 34 (4.2) | 39 (3.6) | 24 (4.0) | 35 (3.5) |
| Previous preterm birth, %b | 106 (16.2) | 120 (18.4) | 64 (13.7)c | 113 (18.3) | 47 (12.9)c | 102 (18.0) |
| Previous hypertension, % | 122 (10.4) | 98 (8.4) | 82 (10.0) | 95 (8.6) | 55 (8.8) | 90 (8.7) |
| Bacterial vaginosis | 101 (8.6) | 111 (9.5) | 71 (8.6) | 110 (9.9) | 56 (9.0) | 104 (10.1) |
| Any vaginal bleeding in pregnancy, % | 95 (8.1) | 99 (8.5) | 52 (6.3) | 92 (8.3) | 40 (6.4) | 83 (8.0) |
| Cerclage placed during pregnancy, % | 13 (1.1) | 15 (1.3) | 9 (1.1) | 14 (1.3) | 6 (1.0) | 14 (1.4) |
| Cervical shortening (≤25 mm), % | 16 (1.6) | 19 (1.9) | 9 (1.2) | 18 (1.8) | 8 (1.4) | 17 (1.8) |
Patients attended ≥1 visit in their assigned treatment arm.
Patients attended ≥5 visits in their assigned treatment arm.
Significance of P < 0.05 for Group Prenatal Care participants compared to Individual Care.
Previous preterm birth percentages utilize multiparous patients only; nulliparous participants are excluded from this analysis
The distribution of GPNC sessions attended by participants in the GPNC study arm is shown in Figure 2. Participants in GPNC attended a mean of 4.7 (SD±3.6) GPNC sessions and were also permitted to attend individual prenatal care visits as needed. On average, participants in both arms attended a mean of 12 (SD±3.3) total prenatal care visits; this mean includes both individual and group visits for patients assigned to GPNC.
Figure 2:
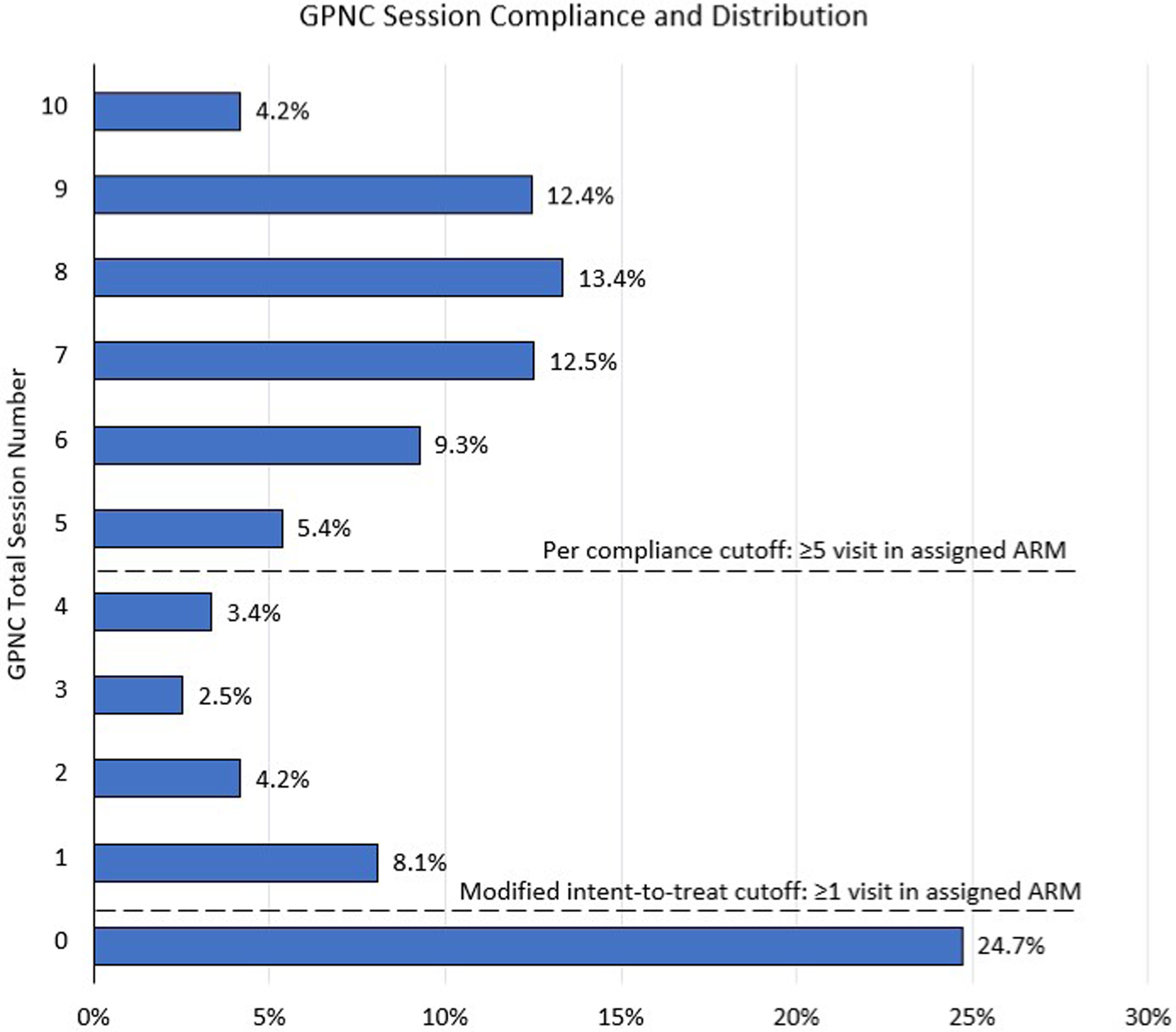
Group Prenatal Care Session Compliance and Distribution
This figure contains a graph showing compliance for group prenatal care (GPNC) session and the session number cutoff for the modified intent-to-treat and per compliance analytic groups.
Intent-to-treat Outcomes
For our ITT analysis, there were no statistically significant differences between GPNC and IPNC for PTB (10.4% vs. 8.7%; OR 1.22, 95% CI 0.92 – 1.63) or LBW (9.6% vs. 8.9%; OR 1.08, 95% CI 0.80 – 1.45) (Table 2). Similarly, in models including interaction terms, we did not observe any statistically significant differences between GPNC and IPNC in PTB or LBW within any race or ethnicity (Table 2). Black participants had higher rates of PTB compared to White participants in both GPNC and IPNC but the differences did not reach statistical significance in either GPNC (11.4% vs. 10.8%, OR 1.07, 95% CI 0.70 – 1.65) or IPNC (10.2% vs 7.7%, OR 1.37, 95% CI 0.85–2.19) (Figure 3). The pattern was different for LBW where Black participants had significantly greater rates in IPNC compared to White participants (12.7% vs 6.4%, OR=2.13, 95% CI 1.29 – 3.50) but not in GPNC (12.5% vs. 8.9%, OR=1.45, 95% CI 0.91 – 2.30) (Figure 4).
Table 2:
Comparison of preterm birth (<37 weeks) and low birthweight (<2500 grams) by race and ethnicity for patients in group prenatal care (GPNC) and individual prenatal care (IPNC).
| Preterm Birth, <37 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | GPNC | IPNC | OR (95%CI) | p value | ||||
| Overall | (211/2219) | (114/1099) | (97/1120) | 1.22 (0.92 – 1.63) | 0.17 | |||
| Black | (97/897) | (51/ 446) | (46/ 451) | 1.14 (0.75 – 1.73) | 0.55 | |||
| White | (75/817) | (43/ 400) | (32/ 417) | 1.45 (0.90 – 2.34) | 0.13 | |||
| Hispanic | (39/477) | (20/ 238) | (19/ 239) | 1.06 (0.55 – 2.05) | 0.86 | |||
| Low Birthweight, <2500 grams | ||||||||
| All | GPNC | IPNC | OR (95%CI) | p value | ||||
| Overall | (192/2072) | (99/1028) | (93/1044) | 1.08 (0.80 – 1.45) | 0.62 | |||
| Black | (103/818) | (51/ 409) | (52/409) | 0.98 (0.65 – 1.48) | 0.92 | |||
| White | (58/759) | (33/ 369) | (25/ 390) | 1.43 (0.84 – 2.46) | 0.19 | |||
| Hispanic | (30/468) | (14/ 235) | (16/ 233) | 0.86 (0.41 – 1.80) | 0.69 | |||
Figure 3:
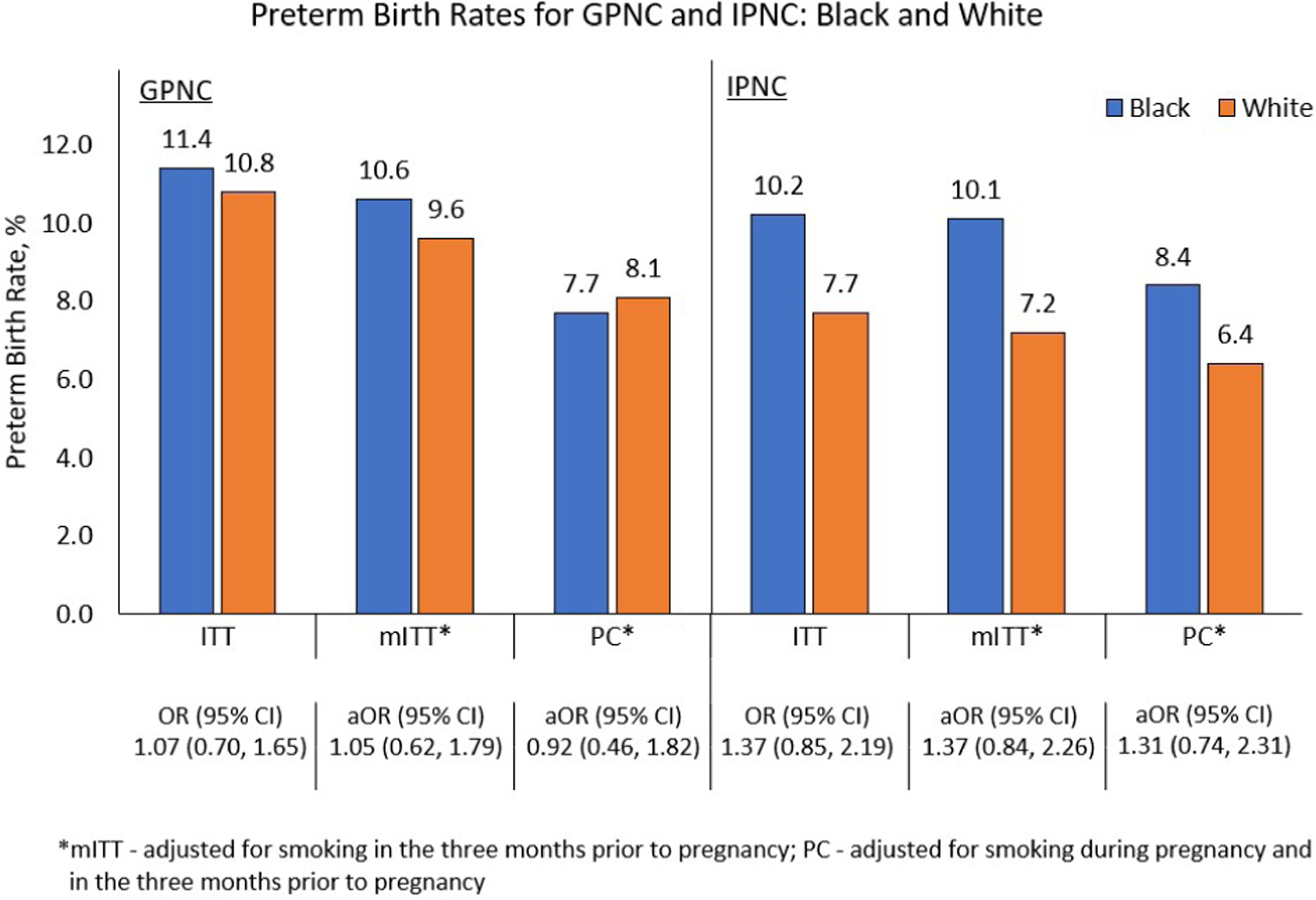
Preterm birth rate comparisons between Black and White participants
Preterm birth rates for group prenatal care (GPNC) and individual prenatal care (IPNC) for the comparisons between race and ethnicity for the intent-to-treat (ITT), modified intent-to-treat (mITT), and per compliance (PC) analytic groups.
Figure 4:
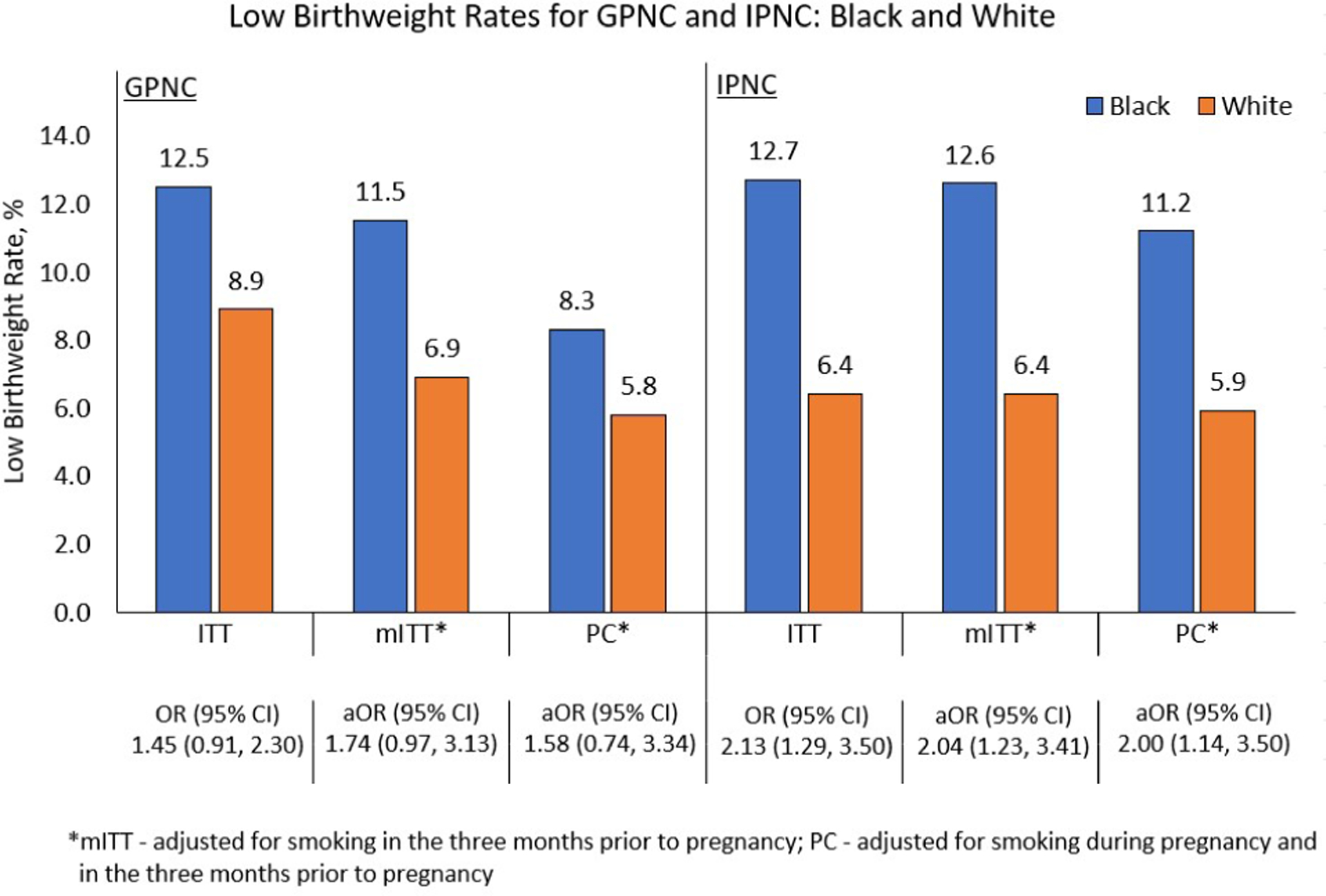
Low birthweight rate comparisons between Black and White participants
Low birthweight rates for group prenatal care (GPNC) and individual prenatal care (IPNC) for the comparisons between race and ethnicity for the intent-to-treat (ITT), modified intent-to-treat (mITT), and per compliance (PC) analytic groups.
Modified Intent-to-treat and Per Compliance Analyses
Our two additional pre-specified analytic samples are included on the consort diagram (Figure 1) and the distribution of GPNC visits for participants in that treatment arm is described in Figure 2. Our mITT analytic group included all participants who attended ≥1 visit in their assigned treatment arm (this included 70% of GPNC ITT and 95% of IPNC ITT). After exclusions for pregnancy losses, incomplete outcome data and no attendance of any visits in their assigned treatment arm, 824 participants were included in the mITT analysis for the GPNC study arm and 1111 in the IPNC study arm. For the mITT analytic group, participants assigned to the GPNC study arm attended a median of 7 (range 1 – 10) GPNC sessions. For our PC analytic group, we excluded participants from both study arms who attended <5 visits in their assigned treatment group. Additionally, there were 15 (0.6%) participants who were randomized to IPNC but later requested GPNC. These participants were allowed to attend their preferred model of prenatal care, considered crossovers, and excluded for the PC analysis. We did not consider any crossovers from GPNC to IPNC since the CenteringPregnancy GPNC model allows patients to schedule IPNC visits as needed in addition to their GPNC sessions. Our final PC analytic group included 624 in our GPNC study arm (53% of GPNC ITT) and 1032 in the IPNC study arm (88% of ITT), and participants assigned to the GPNC study arm in the PC analytic group attended a median of 8 (range 5 – 10) GPNC sessions.
Most demographic characteristics remained balanced between the mITT and PC analytic samples (Table 1). Logistic regression models were adjusted for smoking in the 3 months prior to pregnancy in our mITT analytic samples and smoking during pregnancy as well as in the 3 months prior to pregnancy for our PC analytic group.
In comparing rates of PTB and LBW by treatment group within these two analytic samples, results were similar to the ITT analysis. There were no significant differences for the overall mITT sample for PTB (10.0% GPNC vs. 8.5% IPNC; aOR 1.16, 95% CI 0.84 – 1.60) or LBW (8.8% GPNC vs. 8.8% IPNC; aOR 0.98, 95% CI 0.70 – 1.38). We also did not find significant differences in the PC sample for PTB (7.7% vs. 7.2%; aOR 1.08, 95% CI 0.73 – 1.60) or LBW (6.5% vs. 7.7%; aOR 0.84, 95% CI 0.55 – 1.27).
Comparisons in PTB and LBW by treatment group for Black and White participants
There were no significant differences in PTB between Black and White participants in either mITT or PC samples in either treatment arm (Figure 3). Although the aOR for PTB between Black and White participants was not statistically significant in the GPNC arm, when evaluated across analytic groups the aOR for PTB monotonically decreased and even reversed, favoring Black participants with decreasing rates from the ITT (aOR=1.07, 95% CI 0.70 – 1.65) to the mITT (aOR=1.05, 95% CI 0.62 – 1.79) to the PC (aOR=0.92, 95% CI 0.46 – 1.82) sample (Figure 3). In comparison, for the IPNC arm, the aOR for PTB was consistently higher across the samples for ITT (aOR=1.37, 95% CI 0.85 – 2.19), mITT (aOR=1.37, 95% CI 0.84 – 2.26), and PC (aOR=1.31, 95% CI 0.74 – 2.31) (Figure 3).
Comparisons in LBW between Black and White participants by treatment group showed more consistent findings. For LBW, Black participants in the IPNC arm in ITT, mITT and PC analytic groups all showed significantly greater LBW rates compared to White participants: ITT aOR 2.13, 95% CI 1.29–3.50; mITT aOR 1.37, 95% CI 0.84–2.26; PC aOR 1.31, aOR 0.74–2.31 (Figure 4). In contrast, LBW rates were not significantly higher for Black participants compared to White participants in the GPNC arm in any sample: ITT aOR 1.45, 95% CI (0.91–2.30); mITT aOR 1.74, 95% CI (0.97–3.13); PC aOR 1.58 (0.74–3.34) (Figure 4).
Comment
Principal Findings.
There was no significant difference overall in PTB or LBW between GPNC and IPNC. The study was underpowered to fully evaluate the interaction effect of GPNC on birth outcomes by race due to lower-than-expected rates of our primary outcomes and our inability to achieve our prespecified sample size due to the COVID-19 pandemic. The results suggest that increased engagement with group prenatal care is associated with improved outcomes for Black participants.
Results in the Context of What is Known.
Our findings are consistent with two prospective studies, two meta-analyses, and a 2015 Cochrane review which showed no difference in overall rates of PTB for patients in GPNC compared with IPNC.26,28,29,46–48 Our finding that increased participation in GPNC is associated with improved outcomes is consistent with other reports, both prospective and retrospective, in which authors noted the dose-response correlation between increased attendance at GPNC sessions and reductions in LBW and PTB.23, 28–29
Our finding that GPNC may be more impactful for rates of LBW and PTB in Black participants, particularly with greater levels of attendance at group sessions, is consistent with previously published large retrospective cohort studies.21,28,49
Clinical Implications.
Improving racial equity in birth outcomes is one of the most important clinical challenges facing obstetric care providers. While GPNC was not explicitly developed to reduce racial disparities in health care, the model has elements that may serve to address some root causes of unconscious or implicit bias in the healthcare setting.50 For example, the essential elements of CenteringPregnancy GPNC include limiting group size to optimize interaction and facilitating groups to be interactive.25 The group setting provides a unique environment which improves patient-provider communication and fosters relationship building.18,19,51 Improving patient-provider communication and fostering shared decision-making have been proposed as approaches to overcoming provider implicit bias and building patient satisfaction and trust in the healthcare encounter.50 GPNC also provides opportunity for clinical encounters to address social determinants of health.52–54
Although the results presented here highlight the potential value of GPNC, they also illustrate the need for ongoing innovation to the GPNC model. As part of our study design, we attempted to address anticipated barriers to participants’ willingness and ability to participate in GPNC. Dedicated research staff educated participants about GPNC during study recruitment, often including tours of our group care spaces. Participants were provided with a list of all ten GPNC sessions at study enrollment, to help avoid potential scheduling conflicts. The study paid for onsite professional childcare during group sessions because the CenteringPregnancy model discourages children from attend group sessions with their parents. Attendance at group sessions was also incentivized with a pack of newborn diapers at every session (approximately $15/each retail value) and a gift card after attending 5 sessions. Even in this ideal environment, nearly 20% of participants randomized to GPNC did not attend a single session. Other RCTs using GPNC models have reported similarly high rates of non-compliance with assigned group sessions, and this may be an indication that the GPNC model is not a “one size fits all” strategy for reinventing prenatal care.29
Implementation of GPNC can be difficult, requiring changes in physical space, patient-provider interactions, and logistics of clinical flow.56,57 However, there are few other clinical interventions available that potentially address racial disparities in birth outcomes that affect millions of pregnant persons each year. Clinicians and systems, particularly in areas of high health disparities, may determine that adopting this model is worth the effort.58–70
Research Implications.
There are still unanswered questions related to the magnitude of and the mechanism by which GPNC may improve racial equity in birth outcomes. There is potential, however, for GPNC to address root causes of racial disparities in health and healthcare at the system, community, provider, and patient levels.71 Future projects would benefit from the inclusion of qualitative evaluation of patient and provider experiences in the GPNC setting to explore these and other potential mechanisms for improvements in birth outcomes and other measures.
Additional work also remains to be done investigating the effect of GPNC session attendance on pregnancy outcomes. A deeper investigation of the impact of engagement with prenatal care on birth outcomes and racial disparities may be warranted.
Strengths and Limitations.
This study has several strengths. Our randomized study design minimizes selection bias and confounding, as does our success in recruiting a diverse study population. We lost very few patients from follow-up and had outcome data on 2312 (98.5%) of study participants.
The fact that the clinical team providing prenatal care had extensive experience with the CenteringPregnancy GPNC model predating the clinical trial by many years is both a strength and potentially a limitation. Our site has been continuously certified by the Centering Healthcare Institute since 2009, indicating a high degree of fidelity to the model. Prior to initiation of the study, four of our most experienced nurse practitioners had each facilitated more than 50 groups, representing >2000 GPNC sessions and >4000 clinical hours leading groups. During the study, all nurse practitioners provided care in both the GPNC and IPNC model of care. The unique experience and skills the clinical team learned through years of experience with GPNC likely carry over to IPNC encounters, and potentially improve or otherwise bias care provided in the IPNC setting. This could potentially have contributed to our lower-than-expected rates of PTB and LBW in the IPNC arm.
The most significant limitation of this study was inadequate power to compare the intervention or the outcomes between racial groups as planned. This power limitation resulted from three inter-related challenges: early termination of the trial due to the COVID-19 pandemic, lower-than-expected enrollment and attendance, and unexpectedly lower rates of adverse outcomes in comparison to the general population.
The most obvious limitation of the study was the interruption of patient recruitment and GPNC treatment protocols by the COVID-19 pandemic which prevented us from reaching proposed study recruitment targets. This was compounded by a lower-than-expected rate of study enrollment (43%) for eligible patients which predated the pandemic. Based on previously published RCT of GPNC, we anticipated higher rates of study enrollment (52%−67%).28,46 When recruitment targets began lagging behind expectations, our study team conducted targeted short surveys with 107 patients who declined study enrollment and who were willing to explain why (unpublished data collected April, 2018). Most commonly, patients did not want to be randomized due to preferences for either IPNC (70%) or GPNC (13%); others cited scheduling conflicts (21%) including unpredictable work schedules, transportation challenges (11%) and a preference not to be part of a research trial (19%) as reasons for non-enrollment. Our struggles with recruitment mirror those many practices have with recruiting patients into GPNC, and this is another structural barrier to its widespread adoption.72,73
In addition to challenges with recruitment, the study was also limited by lower-than-expected patient attendance at GPNC sessions. This includes not only those (n=277; 23.6%) who did not attend a single assigned GPNC session but also the very low numbers (n= 52; 4.4%) who were able to attend all 10 assigned sessions. In 2019, our research team published an interim analysis exploring the reasons patients attended fewer than 5 sessions or visits in their assigned treatment arm.55 At the time of the administration of our second survey between 30–40 weeks gestational age, study staff collected self-reported reasons for non-adherence during short interviews of 301 enrolled participants in both the GPNC (n=207) and the IPNC (n=94) arms. Scheduling conflicts (23.2%), dislike of the GPNC model (16.4%) and leaving the practice (34.3%) were the most common reasons for non-adherence cited by participants in the GPNC study arm. While participants randomized to IPNC reported leaving the practice (34.0%), pregnancy loss (12.8%) and moving out of the area (11.7%) as the most common reasons for their inability to attend >5 scheduled IPNC visits. An essential element of the CenteringPregnancy GPNC model is that group members, including facilitators and support people, are consistent across sessions. This requires more rigid scheduling in comparison to IPNC visits and is an important structural barrier to attendance for patients who may not have flexibility in work or school schedules, dependable transportation or childcare.
We anticipated attrition, and therefore prespecified two analytic samples based on patient participation thresholds to allow evaluation of outcomes for participants attending meaningful numbers of sessions or visits. The relatively lower compliance with the assigned treatment in the GPNC arm of our study (70% in the mITT analytic group and 53% in the PC analytic group) led us to differentially exclude more patients from the GPNC arm in sub-group analysis; though we adjusted our logistic regression models to account for differences, this methodology still introduces the risk of bias.
The most important limitation of this study is that our observed rate of PTB of 9.5% was lower than the predicted rates we used in the power calculations and substantially lower than rates observed in our community. For Medicaid-eligible patients delivering during the same timeframe as this study (April 2016 – September 2020) in the single suburban county that our practice primarily serves, the rate of PTB was 13.4% (1824/13,630) and the rate of LBW was 10.9% (1485/13,630). Rates were higher for Black patients who demonstrated a 15.9% (687/4326) rate of PTB and 15.3% (662/4326) rate of LBW according to vital statistics from the University of South Carolina Institute for Families in Society (Sarah Gareau, PhD, e-mail communication, February 2022). Both our lower-than-expected rates for our primary outcomes as well as our failure to reach our planned recruitment targets mean that findings must be interpreted with caution.
Generalizability of findings is limited by our study population, which was drawn from a single obstetric practice serving mainly a low-income population. Generalizability is also limited by the number of patients who were ineligible for study enrollment due to late entry to prenatal care and medical complications of pregnancy.
Conclusions.
Participation in GPNC did not lead to differences in PTB or LBW in comparison to traditional IPNC. We found improvements in rates of PTB and LBW with increased attendance for Black participants in GPNC, but our study was underpowered to conclude that GPNC definitively decreases the racial disparity in these birth outcomes.
Recently, there have been calls for a redesign of prenatal care to improve outcomes, to add flexibility, to incorporate virtual visits, to adapt to patient preferences, and create new opportunities for education and social support.25,71,74 This moment of introspection provides an opportunity to evaluate the current structure of prenatal health care and its contribution to racial disparities in obstetric practice.75 Willingness to explore innovative practice models such as GPNC is part of the necessary work to achieve racial equity in birth outcomes.
Supplementary Material
AJOG at a Glance:
A. Why was the study conducted?
The study utilized a randomized study design to examine the impact of group prenatal care on rates of preterm birth and low birthweight, as well as the longstanding racial disparities in these birth outcomes.
B. What are the key findings?
Overall, group prenatal care is associated with similar pregnancy outcomes to traditional prenatal care.
Increasing participation in group prenatal care may reduce rates of preterm birth and low birthweight, an effect which was more pronounced for Black participants.
C. What does this study add to what is already known?
This study supports findings from several large retrospective cohort studies and two meta-analyses indicating that group prenatal care does not reduce overall rates of preterm birth and low birthweight compared to individual prenatal care.
Acknowledgments:
We thank the research team in the Department of Obstetrics and Gynecology at Prisma Health for their dedication in the management and implementation of the study, with special acknowledgement to Patti Parker and Alice O’Handley. We would also like to acknowledge Dr. Jessica Smith and Ana LaBoy from the Georgia Health Policy Center at the Andrew Young School of Policy Studies at Georgia State University who provided valuable guidance and support throughout the project. Research reported in this publication was supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number R01HD082311. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support:
Research reported in this publication was supported by the United States Department of Health and Human Services through the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number R01HD082311. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders did not participation in the study design, data collection, analysis or interpretation, writing the report or in the decision to submit the article for publication.
Appendix: Determination of patient race and ethnicity
To stratify patients by race and ethnicity, at enrollment patients were asked by study personnel to choose one option from a combined list of race and ethnicity, including options for Black, Hispanic, White, Mixed Race and Other. Subsequently, patients privately completed a survey instrument which included a broader range of race and ethnicity categories, and patients were allowed to choose more than one option. We felt that the responses provided directly by the patients in the survey were a more accurate reflection of race and ethnicity and therefore these were used in our final analysis for assignment of race and ethnicity. Supplemental Table A demonstrates the differences between the race and ethnicity assigned by study staff at randomization (used for stratification) and the final assignments of race and ethnicity we determined after reviewing survey responses (used for analysis).
The study team made additional decisions regarding the assignment of race and ethnicity for our final analytic groups. Because the United States has a significant history of racial discrimination, we determined that patients who identified as Black and other races or ethnicity should be grouped with patients reporting Black only rather than creating a separate multiracial group. For similar reasons, patients who identified as Latina, Hispanic, or Spanish origin were grouped into Hispanic, even if they selected White as a second racial category. Patients who identified only as White, were assigned as White. All other categories were combined into "Other groups combined." For patients who selected prefer not to answer, their response to the single option question verbally asked by study staff at randomization was used.
Supplemental Table B reflects the differences between the patient’s self-reported race and ethnicity on survey 1 and the final assignments our study team used for evaluation in this manuscript. In general, there was a high fidelity between the two assignments with only 150 (6.4%) of patients shifting categories for final analysis. Of these, 78 (52.0%) were originally assigned to the multiracial category which was redistributed to Black, Hispanic, White or Other as appropriate.
Although we chose to use the term “Hispanic” ethnicity in our study, we appreciate that many other terms have also been used to describe people living in the United States of Spanishs-peaking or Latin-American heritage. We also realize that this category includes patients from a wide variety of geographic locations and countries of origin. In our study population, 243 (46.9%) participants identified as Mexican, Mexican American, 91 (17.6%) identified as Guatemalan, Salvadoran, or Honduran, 7 (1.4%) identified as Nicaraguan or Costa Rican, 56 (10.8%) identified as Colombian, 100 (19%) identified multiple countries of origin or other and 21 (4.1%) preferred not to answer that question.
The duration of residence in the United States varied widely for these patients, with 185 (36.6%) reporting living in the United States all their lives. The remaining 313 (61.9%) were immigrants, with 183 (36.2%) reporting living in the United States for more than 10 years and 130 (25.7%) reported living in the United States less than 10 years. Of these, 25 (4.9%) were very recent immigrants and had been living in the United States for less than one year prior to study enrollment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors report conflict of interest
ClinicalTrials.gov Identifier: NCT02640638. Registered with ClinicalTrials.gov December 29, 2015. Study recruitment began February 24, 2016
URL of ClincialTrials.gov registration site: https://clinicaltrials.gov/ct2/show/NCT02640638?term=NCT02640638&draw=2&rank=1
Paper presentation: The findings were presented as an oral presentation at the Society for Maternal Fetal Medicine’s 42nd Annual Pregnancy Meeting on Thursday, February 3, 2022 in Orlando, Florida. Abstract #2. Inclusive dates for this meeting were January 30 – February 5, 2022.
Contributor Information
Amy H CROCKETT, Division of Maternal Fetal Medicine Department of Obstetrics and Gynecology Prisma Health/University of South Carolina School of Medicine Greenville, 890 West Faris Road, Suite 470, Greenville SC 29605.
Liwei CHEN, Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, 650 Charles E Young Drive South, Room 76-051A, Los Angeles CA 90095.
Emily C HEBERLEIN, Georgia Health Policy Center, Andrew Young School of Policy Studies, Georgia State University, 55 Park Place NE, 8th Floor, Atlanta GA 30303.
Jessica L BRITT, Department of Obstetrics and Gynecology, Prisma Health, 890 West Faris Road, Suite 470, Greenville SC 29605.
Ms. Sarah COVINGTON-KOLB, Center for Community Health Alignment, Arnold School of Public Health, University of South Carolina, 120 Research Drive, Columbia SC 29203.
Mr. Brian WITRICK, Department of Public Health Sciences, College of Behavioral, Social and Health Sciences, Clemson University, 503 Edwards Hall, Clemson SC 29634.
Ms. Emily DOHERTY, Department of Public Health Sciences, College of Behavioral, Social and Health Sciences, Clemson University, 503 Edwards Hall, Clemson SC 29634.
Lu ZHANG, Department of Public Health Sciences, College of Behavioral, Social and Health Sciences, Clemson University, 503 Edwards Hall, Clemson SC 29634.
Ann BORDERS, Department of Obstetrics and Gynecology, NorthShore University HealthSystem, 2650 Ridge Ave, Walgreen Building Suite 1507, Evanston IL 60201.
Lauren KEENAN-DEVLIN, Department of Obstetrics and Gynecology, NorthShore University HealthSystem, 2650 Ridge Ave, Walgreen Building Suite 1507, Evanston IL 60201.
Ms. Britney SMART, Department of Obstetrics and Gynecology, NorthShore University HealthSystem, 2650 Ridge Ave, Walgreen Building Suite 1507, Evanston IL 60201.
Moonseong HEO, Department of Public Health Sciences, College of Behavioral, Social and Health Sciences, Clemson University, 605 Grove Road, Suite 205, Greenville SC 29605.
Data Sharing:
Deidentified study data will be available publicly on the NICHD/DASH Data and Specimen Hub (https://dash.nichd.nih.gov/) in October 2026, five years after study completion. Prior to that time, researchers with a methodologically sound proposal can direct inquiries to amy.crockett@prismahealth.org to gain access to the study protocol, informed consent forms, deidentified data, data dictionaries and the analytic plan. Requestors will need to sign a data access agreement.
References
- 1.Hamilton BE, Martin JA, Osterman MJK. Births: Provisional Data for 2019. Natl Vital Stat Rep. 2020. [Google Scholar]
- 2.Heberlein E, Smith J, Willis C, Hall W, Covington-Kolb S, Crockett A. The effects of CenteringPregnancy group prenatal care on postpartum visit attendance and contraception use. Contraception. 2020;102(1):46–51. [DOI] [PubMed] [Google Scholar]
- 3.Ely DM, Driscoll AK. Infant Mortality in the United States, 2018: Data From the Period Linked Birth/Infant Death File. Natl Vital Stat Rep. 2020;69(7):1–18. [PubMed] [Google Scholar]
- 4.Barfield WD. Public Health Implications of Very Preterm Birth. Clin Perinatol. 2018;45(3):565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen SJ, Sommerfelt K, Markestad T. Early motor development of premature infants with birthweight less than 2000 grams. Acta Paediatr. 2000;89(12):1456–1461. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. [DOI] [PubMed] [Google Scholar]
- 7.de Jong M, Verhoeven M, van Baar AL. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: a review. Semin Fetal Neonatal Med. 2012;17(3):163–169. [DOI] [PubMed] [Google Scholar]
- 8.Chehade H, Simeoni U, Guignard JP, Boubred F. Preterm Birth: Long Term Cardiovascular and Renal Consequences. Curr Pediatr Rev. 2018;14(4):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955. [DOI] [PubMed] [Google Scholar]
- 10.Behrman RE, Butler AS, Institute of Medicine (U.S.). Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm birth : causes, consequences, and prevention. Washington, D.C.: National Academies Press; 2007. [PubMed] [Google Scholar]
- 11.In: WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: 2016. [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for perinatal care. Eighth edition. ed. Elk Grove Village, IL Washington, DC: American Academy of Pediatrics; The American College of Obstetricians and Gynecologists; 2017. [Google Scholar]
- 13.Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. 2001;116(4):306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debiec KE, Paul KJ, Mitchell CM, Hitti JE. Inadequate prenatal care and risk of preterm delivery among adolescents: a retrospective study over 10 years. Am J Obstet Gynecol. 2010;203(2):122 e121–126. [DOI] [PubMed] [Google Scholar]
- 15.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(16):1661–1667. [DOI] [PubMed] [Google Scholar]
- 16.Partridge S, Balayla J, Holcroft CA, Abenhaim HA. Inadequate prenatal care utilization and risks of infant mortality and poor birth outcome: a retrospective analysis of 28,729,765 U.S. deliveries over 8 years. Am J Perinatol. 2012;29(10):787–793. [DOI] [PubMed] [Google Scholar]
- 17.Downe S, Finlayson K, Tuncalp O, Gulmezoglu AM. Provision and uptake of routine antenatal services: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2019;6:CD012392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heberlein EC, Picklesimer AH, Billings DL, Covington-Kolb S, Farber N, Frongillo EA. Qualitative Comparison of Women’s Perspectives on the Functions and Benefits of Group and Individual Prenatal Care. J Midwifery Womens Health. 2016;61(2):224–234. [DOI] [PubMed] [Google Scholar]
- 19.Novick G Women’s experience of prenatal care: an integrative review. J Midwifery Womens Health. 2009;54(3):226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sword W, Heaman MI, Brooks S, et al. Women’s and care providers’ perspectives of quality prenatal care: a qualitative descriptive study. BMC Pregnancy Childbirth. 2012;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichelberger KY, Doll K, Ekpo GE, Zerden ML. Black Lives Matter: Claiming a Space for Evidence-Based Outrage in Obstetrics and Gynecology. Am J Public Health. 2016;106(10):1771–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold LD, Luong L, Rebmann T, Chang JJ. Racial disparities in U.S. maternal influenza vaccine uptake: Results from analysis of Pregnancy Risk Assessment Monitoring System (PRAMS) data, 2012–2015. Vaccine. 2019;37(18):2520–2526. [DOI] [PubMed] [Google Scholar]
- 23.Bryant AS, Norton ME, Nakagawa S, et al. Variation in Women’s Understanding of Prenatal Testing. Obstet Gynecol. 2015;125(6):1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidebottom A, Vacquier M, LaRusso E, Erickson D, Hardeman R. Perinatal depression screening practices in a large health system: identifying current state and assessing opportunities to provide more equitable care. Arch Womens Ment Health. 2021;24(1):133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rising SS, Kennedy HP, Klima CS. Redesigning prenatal care through CenteringPregnancy. J Midwifery Womens Health. 2004;49(5):398–404. [DOI] [PubMed] [Google Scholar]
- 26.Carter EB, Temming LA, Akin J, et al. Group Prenatal Care Compared With Traditional Prenatal Care: A Systematic Review and Meta-analysis. Obstet Gynecol. 2016;128(3):551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACOG Committee Opinion No. 731: Group Prenatal Care. Obstet Gynecol. 2018;131(3):e104–e108. [DOI] [PubMed] [Google Scholar]
- 28.Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 Pt 1):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ickovics JR, Earnshaw V, Lewis JB, et al. Cluster Randomized Controlled Trial of Group Prenatal Care: Perinatal Outcomes Among Adolescents in New York City Health Centers. Am J Public Health. 2016;106(2):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. The effects of CenteringPregnancy group prenatal care on gestational age, birth weight, and fetal demise. Matern Child Health J. 2014;18(4):801–809. [DOI] [PubMed] [Google Scholar]
- 31.Picklesimer AH, Billings D, Hale N, Blackhurst D, Covington-Kolb S. The effect of CenteringPregnancy group prenatal care on preterm birth in a low-income population. Am J Obstet Gynecol. 2012;206(5):415 e411–417. [DOI] [PubMed] [Google Scholar]
- 32.Crockett AH, Heberlein EC, Smith JC, Ozluk P, Covington-Kolb S, Willis C. Effects of a Multi-site Expansion of Group Prenatal Care on Birth Outcomes. Matern Child Health J. 2019;23(10):1424–1433. [DOI] [PubMed] [Google Scholar]
- 33.Carter EB, Barbier K, Sarabia R, Macones GA, Cahill AG, Tuuli MG. Group versus traditional prenatal care in low-risk women delivering at term: a retrospective cohort study. J Perinatol. 2017;37(7):769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Crockett AH, Covington-Kolb S, Heberlein E, Zhang L, Sun X. Centering and Racial Disparities (CRADLE study): rationale and design of a randomized controlled trial of centeringpregnancy and birth outcomes. BMC Pregnancy Childbirth. 2017;17(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallman KK, Hodgdon J. Race and ethnic standards for Federal statistics and administrative reporting. Stat Report. 1977(77–110):450–454. [PubMed] [Google Scholar]
- 37.Peahl AF, Howell JD. The evolution of prenatal care delivery guidelines in the United States. Am J Obstet Gynecol. 2021;224(4):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massey Z, Rising SS, Ickovics J. CenteringPregnancy group prenatal care: Promoting relationship-centered care. J Obstet Gynecol Neonatal Nurs. 2006;35(2):286–294. [DOI] [PubMed] [Google Scholar]
- 39.Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring Gestational Age in Vital Statistics Data: Transitioning to the Obstetric Estimate. Natl Vital Stat Rep. 2015;64(5):1–20. [PubMed] [Google Scholar]
- 40.Committee Opinion No 700: Methods for Estimating the Due Date. Obstet Gynecol. 2017;129(5):e150–e154. [DOI] [PubMed] [Google Scholar]
- 41.Scan Project Team. South Carolina Community Assessment Network. Updated February 15, 2022. Accessed February 15, 2022. https://apps.dhec.sc.gov/Health/scan/scan/index.aspx
- 42.Cunningham SD, Lewis JB, Shebl FM, et al. Group Prenatal Care Reduces Risk of Preterm Birth and Low Birth Weight: A Matched Cohort Study. J Womens Health (Larchmt). 2019;28(1):17–22. [DOI] [PubMed] [Google Scholar]
- 43.Gareau S, Lopez-De Fede A, Loudermilk BL, et al. Group Prenatal Care Results in Medicaid Savings with Better Outcomes: A Propensity Score Analysis of CenteringPregnancy Participation in South Carolina. Matern Child Health J. 2016;20(7):1384–1393. [DOI] [PubMed] [Google Scholar]
- 44.Heberlein E, Smith J, Willis C, Hall W, Covington-Kolb S, Crockett A. The effects of CenteringPregnancy group prenatal care on postpartum visit attendance and contraception use. Contraception. 2020;102(1):46–51. [DOI] [PubMed] [Google Scholar]
- 45.Crockett AH, Heberlein EC, Smith JC, Ozluk P, Covington-Kolb S, Willis C. Effects of a Multi-site Expansion of Group Prenatal Care on Birth Outcomes. Matern Child Health J. 2019;23(10):1424–1433. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy HP, Farrell T, Paden R, et al. A randomized clinical trial of group prenatal care in two military settings. Mil Med. 2011;176(10):1169–1177. [DOI] [PubMed] [Google Scholar]
- 47.Catling CJ, Medley N, Foureur M, et al. Group versus conventional antenatal care for women. Cochrane Database Syst Rev. 2015(2):CD007622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Wang Y, Wu Y, Chen X and Bai J. Effectiveness of the CenteringPregnancy program on maternal and birth outcomes: A systematic review and meta-analysis. Int J Nurs Stud. 2021. 120:103981. [DOI] [PubMed] [Google Scholar]
- 49.Cunningham SD, Lewis JB, Shebl FM, et al. Group Prenatal Care Reduces Risk of Preterm Birth and Low Birth Weight: A Matched Cohort Study. J Womens Health (Larchmt). 2019;28(1):17–22. [DOI] [PubMed] [Google Scholar]
- 50.Hagiwara N, Elston Lafata J, Mezuk B, Vrana SR, Fetters MD. Detecting implicit racial bias in provider communication behaviors to reduce disparities in healthcare: Challenges, solutions, and future directions for provider communication training. Patient Educ Couns. 2019;102(9):1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novick G, Reid AE, Lweis J, Kershaw TS, Rising SS, Ickovics JR. Group prenatal care: model fidelity and outcomes. Am J Obstet Gynecol. 2013;209(2):112e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang E, Glazer KB, Howell EA, Janevic TM. Social Determinants of Pregnancy-Related Mortality and Morbidity in the United States: A Systematic Review. Obstet Gynecol. 2020;135(4):896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hogan VK, Rowley D, Bennett T, Taylor KD. Life course, social determinants, and health inequities: toward a national plan for achieving health equity for African American infants--a concept paper. Matern Child Health J. 2012;16(6):1143–1150. [DOI] [PubMed] [Google Scholar]
- 54.Taylor LA, Tan AX, Coyle CE, et al. Leveraging the Social Determinants of Health: What Works? PLoS One. 2016;11(8):e0160217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francis E, Johnstone MB, Covington-Kolb S, Witrick B, Griffin SF, Sun X, Crockett A, Chen L. Group prenatal care attendance and women’s characteristics associated with low attendance: Results from Centering and Racial Disparities (CRADLE Study). Matern Child Health J. 2019;23(10):1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novick G, Womack JA, Sadler LS. Beyond Implementation: Sustaining Group Prenatal Care and Group Well-Child Care. J Midwifery Womens Health. 2020;65(4):512–519. [DOI] [PubMed] [Google Scholar]
- 57.Van De Griend KM, Billings DL, Frongillo EA, Hilfinger Messias DK, Crockett AH, Covington-Kolb S. Core strategies, social processes, and contextual influences of early phases of implementation and statewide scale-up of group prenatal care in South Carolina. Eval Program Plann. 2020;79:101760. [DOI] [PubMed] [Google Scholar]
- 58.Heberlein EC, Frongillo EA, Picklesimer AH, Covington-Kolb S. Effects of Group Prenatal Care on Food Insecurity during Late Pregnancy and Early Postpartum. Matern Child Health J. 2016;20(5):1014–1024. [DOI] [PubMed] [Google Scholar]
- 59.Chae SY, Chae MH, Kandula S, Winter RO. Promoting improved social support and quality of life with the CenteringPregnancy((R)) group model of prenatal care. Arch Womens Ment Health. 2017;20(1):209–220. [DOI] [PubMed] [Google Scholar]
- 60.Cunningham SD, Grilo S, Lewis JB, et al. Group Prenatal Care Attendance: Determinants and Relationship with Care Satisfaction. Matern Child Health J. 2017;21(4):770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hale N, Picklesimer AH, Billings DL, Covington-Kolb S. The impact of Centering Pregnancy Group Prenatal Care on postpartum family planning. Am J Obstet Gynecol. 2014;210(1):50 e51–57. [DOI] [PubMed] [Google Scholar]
- 62.Heberlein EC, Picklesimer AH, Billings DL, Covington-Kolb S, Farber N, Frongillo EA. The comparative effects of group prenatal care on psychosocial outcomes. Arch Womens Ment Health. 2016;19(2):259–269. [DOI] [PubMed] [Google Scholar]
- 63.Heberlein EC, Picklesimer AH, Billings DL, Covington-Kolb S, Farber N, Frongillo EA. Qualitative Comparison of Women’s Perspectives on the Functions and Benefits of Group and Individual Prenatal Care. J Midwifery Womens Health. 2016;61(2):224–234. [DOI] [PubMed] [Google Scholar]
- 64.Ickovics JR, Reed E, Magriples U, Westdahl C, Schindler Rising S, Kershaw TS. Effects of group prenatal care on psychosocial risk in pregnancy: results from a randomised controlled trial. Psychol Health. 2011;26(2):235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu R, Chao MT, Jostad-Laswell A, Duncan LG. Does CenteringPregnancy Group Prenatal Care Affect the Birth Experience of Underserved Women? A Mixed Methods Analysis. J Immigr Minor Health. 2017;19(2):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kweekel L, Gerrits T, Rijnders M, Brown P. The Role of Trust in CenteringPregnancy: Building Interpersonal Trust Relationships in Group-Based Prenatal Care in The Netherlands. Birth. 2017;44(1):41–47. [DOI] [PubMed] [Google Scholar]
- 67.Patberg E, Young M, Archer S, et al. Postpartum Contraceptive Use and Other Reproductive Health Outcomes Among CenteringPregnancy Group Prenatal Care Participants. J Womens Health (Larchmt). 2021;30(7):990–996. [DOI] [PubMed] [Google Scholar]
- 68.Novick G, Sadler LS, Kennedy HP, Cohen SS, Groce NE, Knafl KA. Women’s experience of group prenatal care. Qual Health Res. 2011;21(1):97–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennedy HP, Farrell T, Paden R, et al. “I wasn’t alone”--a study of group prenatal care in the military. J Midwifery Womens Health. 2009;54(3):176–183. [DOI] [PubMed] [Google Scholar]
- 70.Hill I, Cross-Barnet C, Courtot B, Benatar S, Thornburgh S. What do women in Medicaid say about enhanced prenatal care? Findings from the national Strong Start evaluation. Birth. 2019;46(2):244–252. [DOI] [PubMed] [Google Scholar]
- 71.Peahl AF, Smith RD, Moniz MH. Prenatal care redesign: creating flexible maternity care models through virtual care. Am J Obstet Gynecol. 2020;223(3):389 e381–389 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berman R, Weber Yorga K, Sheeder J. Intention to Paticipate in Group Prenatal Care: Moving Beyond Yes or No. Health Promot Pract. 2018;21(1):123–132. [DOI] [PubMed] [Google Scholar]
- 73.McDonald SD, Sword W, Eryuzlu LN, Neupane B, Beyena J, Biringer AB. Why Are Half of Women Interested in Participating in Group Prenatal Care? Matern Child Health J. 2015;20(1):97–105. [DOI] [PubMed] [Google Scholar]
- 74.Mehta PK, Carter T, Vinoya C, Kangovi S, Srinivas SK. Understanding High Utilization of Unscheduled Care in Pregnant Women of Low Socioeconomic Status. Womens Health Issues. 2017;27(4):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson A Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666–668. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified study data will be available publicly on the NICHD/DASH Data and Specimen Hub (https://dash.nichd.nih.gov/) in October 2026, five years after study completion. Prior to that time, researchers with a methodologically sound proposal can direct inquiries to amy.crockett@prismahealth.org to gain access to the study protocol, informed consent forms, deidentified data, data dictionaries and the analytic plan. Requestors will need to sign a data access agreement.


