Abstract
As the field of cellular and genetic therapies transitions from a scientific concept to a clinical reality, it has become evident that there are several conflicting or imprecise nomenclatures to describe these novel therapeutic products. The lack of uniformity and accuracy in the terminology often creates regulatory, educational, administrative, and billing quagmires. Standardization of the nomenclature for these therapeutic products is essential for a harmonized regulatory and developmental framework, development of training paradigms and educational programs, equitable and rational decisions about accessibility, and consistency in the billing and coding structures used for reimbursement. In this manuscript, we propose an updated framework as a foundation for categorizing these cell-based and genetically modified therapies.
Keywords: Cellular therapy, gene therapy, immune effector cell therapy
Introduction
Given the novelty and complexity of current and emerging cellular and gene therapies (CGTs), it is understandable that there are many examples of conflicting or imprecise definitions and descriptions of these products. Within weeks of the commercial availability of chimeric antigen receptor T-cell (CAR-T) therapy in the United States, it had been described in governmental, scientific, payer and patient-facing materials in a plethora of ways: a “living drug”, a prescription cancer treatment, an immunotherapy, a cellular therapy, immune effector cell therapy, and a gene therapy, among others.1–5
The rapid growth of CGTs in the last few years suggests that these therapies are now a permanent part of the clinical landscape and therefore necessitates seeking a widespread definitional understanding among clinicians, scientists, patients, and other stakeholders regarding what constitutes a cellular therapy or gene therapy. While the purpose for which each stakeholder categorizes these therapeutic interventions varies – ranging anywhere from scientific presentations, clinical decision-making, coding healthcare encounters or seeking insurance authorizations – the first step towards harmonizing the categorization of these therapies is the development of an accurate and uniform taxonomy.
In 2013, a task force established by the American Society for Blood and Marrow Transplantation (ASBMT) (now known as the American Society for Transplantation and Cellular Therapy [ASTCT]) authored a document that established standardized terminology for stem cell transplantation and related cellular product infusions.6 Since that time, the introduction of CAR-T therapy7–9 and the development of genetically modified hematopoietic stem and progenitor cells (HSPCs) for patients with primary immunodeficiency disorders,10, 11 inborn errors of metabolism,12, 13 and hemoglobinopathies14–16 have created the need for additional clarification and purposeful categorization.
Novel CGT products include aspects of hematopoietic cell transplantation (HCT), cellular therapy, genetically modified cellular products and gene therapy. To this end, the taxonomy needs to be revised to add precision and provide standardization for clinical, research, regulatory, and billing purposes to facilitate communication regarding novel CGT products. A comparison of terms used by the United States (US) Food and Drug Administration (FDA), the Foundation for the Accreditation of Cellular Therapy (FACT), and the Centers for Medicare & Medicaid Services (CMS) illustrates the potential for ambiguity – and confusion in some cases – among both scientific and layperson stakeholders. A uniform nomenclature and categorization for these novel therapies should capture their therapeutic intent, the specific processes surrounding their administration as well as genetic manipulation (if any). Such a nomenclature will unify some of these therapeutic platforms, clarify the terminology, and provide clear definitions for the different approaches by which human cells are manipulated and administered for therapeutic benefit. This clarification will facilitate a clear regulatory framework; harmonize supervision and accreditation processes; standardize long-term follow-up for efficacy and risk assessment (potentially improving cross-product comparisons); develop useful training resources and patient education materials; and develop equitable and rational decisions for coverage, coding, and reimbursement.
As a first step, and in alignment with our expertise, we limit our scope to therapeutics involving cells of hematopoietic origin. Due to the primary focus of the ASTCT on cells of hematopoietic origin, the following discussion specifically excludes neuronal, cardiac, musculoskeletal, and all other non-hematopoietic cellular therapies or gene transfer directed to non-hematopoietic organs.
Stakeholders and Nomenclature Discrepancies
In the US, the FDA sets the regulatory framework for clinical trials and approval of therapeutic agents like CGTs. Specifically, the FDA Center for Biologics Evaluation and Research (CBER) regulates cellular therapy products and human gene therapy products as biologics, as well as some devices related to cellular and gene therapy. Even within the FDA, however, the terminology applied to CGT varies. Tisagenlecleucel, a CAR-T product, was referred to as “the first gene therapy [in] the United States”, a “cell-based gene therapy”, and a “genetically-modified autologous T-cell immunotherapy” all within the same FDA press release announcing its marketing approval in 2017.4 Further, according to the FDA, cellular therapy products “include cellular immunotherapies, cancer vaccines, and other types of both autologous and allogeneic cells for certain therapeutic indications, including hematopoietic stem cells and adult and embryonic stem cells.” Human gene therapy, on the other hand, seeks to “modify or manipulate the expression of a gene or to alter the biologic properties of living cells for therapeutic use.”17 In a recent proposed guidance on the development of CAR-T products, the FDA called these products “human gene therapy products.”18 A comparison of the above definitions reveals a considerable overlap that reflects the ambiguities in the field.
FACT sets standards and accredits clinical sites that conduct cellular therapy trials, treat patients with cellular therapies and/or produce the CGT products used in these trials. FACT was co-founded by the International Society for Cell and Gene Therapy (ISCT) and the ASBMT (now the ASTCT) in 1996 to establish and maintain standards for clinical and laboratory practice in cellular therapy. Currently, more than 90% of all eligible facilities in the US are FACT accredited; the standards promulgated by FACT are also used in Canada, many European countries, Australia, and New Zealand. The scope of the standards established by FACT and its European counterpart JACIE (Joint Accreditation Committee—ISCT and EBMT) includes hematopoietic progenitor cells (HPCs), nucleated and mononuclear cells derived from hematopoietic tissues, immune effector cells (IECs), and genetically modified cells.19 While FACT defines these cellular products precisely, FACT definitions do not always align with those used by the FDA. The terms used by both FDA and FACT are accurate, but the complexities, nuances, redundancy in terminology and lack of reference to a hierarchical framework can lead to confusion among various new stakeholders as these products enter more mainstream clinical practice.
All payers and providers in the US are required to report therapies and services furnished to patients using the official code sets set out in the Health Insurance Portability and Accountability Act (HIPAA) standards (Code of Federal Regulations, 45 CFR § 160.102 and 45 CFR § 162.1000–1011). The code sets mandated for use include the International Classification of Diseases (ICD) diagnosis and procedure codes and the Health Care Procedural Coding System (HCPCS), which includes the American Medical Association’s Common Procedural Coding (CPT). In the US, these code sets are inextricably linked to the coverage and reimbursement methodologies for payers. Therefore, accurate representation of the services provided by healthcare providers by using terms that are descriptively clear, precise and in alignment with coding convention is essential for payer reimbursement as well.
Lastly, a review of how the ICD coding managers have grappled with the coding of cellular and gene therapies demonstrates why clear terminology will assist payers, providers and coders to correctly identify and report the provision of gene therapies. The ICD-10 Coordination and Maintenance Committee (C&M Committee)20, a Federal interdepartmental committee of representatives from CMS and the Center for Disease Control and Prevention’s (CDC) National Center for Health Statistics (NCHS) hosts bi-annual meetings to discuss stakeholder requests for procedure and diagnosis code additions, revisions, and deletions. On October 1, 2015 CMS, which oversees the ICD-10 procedure coding system (PCS), implemented a New Technology coding table (Section X) to allow for specific identification of procedures associated with new technologies and therapies, including the infusion and/or administration of most cellular and gene therapies approved thus far.21 The ICD-10 C&M committee meetings maintain a public record of the changes proposed by multiple stakeholders to these New Technology codes within Section X, as well as within other tables throughout the ICD-10-PCS system. For example, the September 2021 C&M Committee meeting transcript reveals a lengthy discussion of whether a new procedure code should be added for ex vivo gene edited hematopoietic stem cell therapies, and whether the code should be unique to the specific gene being modified. This merely provides an illustrative example of a how a novel CGT product can include aspects of gene therapy, gene editing, hematopoietic stem cell transplant, and cellular therapy; furthermore, it is not yet clear whether the same overall procedure and technology but in the context of a different genetic disease would require a separate coding category.22 The coding nomenclature employed by CMS differs from those of FDA and FACT, which further complicates the accurate and precise communication surrounding these therapeutic products. As a result, patients, clinicians, scientists, regulators, payers, and the press use many of these same terms in different combinations which can lead to differences in coverage, coding, and reimbursement ultimately impacting patient access. In this manuscript, we propose a framework addressing some of these concerns.
Methodology
The ASTCT Cell Therapy Committee formed a subcommittee and tasked it with considering the need for classifications of CGTs and propose recommendations, if needed. This sub-committee included physician-scientists with HSCT and CGT expertise (AS, SG, NNS, FO, and MVM) and health policy consultants (SF, JS, and AR). Focused small group discussions comprising the subcommittee members explored CGT product scenarios related to product type, mode of manipulation, route of administration, therapeutic intent, and the use of pretreatment techniques such as preparative chemotherapy.
Based on themes identified during the focus group discussions, a survey instrument (Supplementary Appendix) was developed to probe a wider audience.
Survey instrument and questionnaire:
The subcommittee distributed the survey to 53 members of the ASTCT Cell Therapy Committee, Committee on Education, and Committee on Practice Guidelines. These Committees comprise clinicians and scientists who are experienced in developing and administering CGT products.
First, these individuals were presented with a sorting exercise to categorize 16 cellular and gene therapy products, which represented a combination of near-term investigational and FDA-approved products. The goal was to ascertain differences in how physicians and scientists categorize these products. Responders were asked to assign each of the 16 chosen products to one of four classes: genetically modified cellular therapy – not inclusive of immune effector cell (IEC) therapy (abbreviated as ‘GMCT–not IEC’), IEC therapy, or gene therapy. In this definition, “cellular therapy” refers to the administration of whole, live cells without genetic modification (e.g., infusion of cord blood cells). “IEC therapy,” as a sub-category of cellular therapy, refers to the administration of cells that have differentiated into a form capable of modulating or effecting an immune response (e.g., CAR T-cells). The differentiation between GMCT-not IEC and IEC therapy classification choices was meant to assess if respondents felt genetic modification of a cellular product warranted taxonomic separation from either ‘cellular therapy’ or ‘gene therapy’. A focusing of the classification to IEC, from a broader category of cellular therapy, was due to the fact that none of the products listed were unmanipulated cellular products such as whole blood or platelets. If responders did not believe a product belonged to any of the three delineated classes, they could assign it to an “other” category.
After completing the sorting exercise, survey respondents answered a series of single-election (i.e. exclusive) questions designed to elicit their perspectives on the prevalent classifications and nomenclature of various CGT products. The analysis also reviewed additional free-text comments provided by respondents.
Survey Results:
Eighteen responses were received from a total of 53 potential respondents to whom the survey link was sent, reflecting a 34% response rate. In the sorting exercise, gene-edited HPC products were considered to be GMCT-not IEC (42%) or gene therapy (50%). When asked to categorize the specific example of an autologous ex vivo genetically manipulated hematopoietic stem cell product, the answers were highly variable. Ten respondents (59%) respondents stated that these products should be classified as gene therapy; five (29%) classified them as GMCT-not IECT; and 2 (12%) considered this to be cellular therapy. At the same time, when asked as a direct question, 14 respondents (82%) agreed with the statement that GMCT-not IEC products are those containing whole live cells that have undergone ex vivo genetic modification prior to being administered. However subsequently, 17 (94%) of the 18 respondents indicated “that administration of an ex vivo genetically modified cellular products should be considered a hematopoietic stem cell transplant” – i.e. a process including administration of a preparative regimen, hematopoietic cell infusion, supportive care, and monitoring associated with administration of these products. These survey results demonstrate definitional discrepancies and inconsistences that are present even among experts in the field.
Analyses of responses to the rest of the survey was limited to the 17 respondents who answered all the remaining questions. Fifteen respondents (88%) agreed with the statement that CGT could be defined based on the type of product administered. Most respondents (82%, n=14) considered in vivo gene editing to be gene therapy. Fourteen respondents (82%) agreed that cellular therapy could be divided into two subcategories based on treatment purpose: 1) cellular therapy for lympho-hematopoietic reconstitution (such as hematopoietic cell transplantation), and 2) IEC therapy. Each of the two cellular therapy subcategories were then presented to respondents with further qualifications according to the mode of manipulation: selected or unselected; cultured/stimulated or not cultured/stimulated; and gene-modified or not gene-modified. Fourteen respondents (82%) specifically categorized the infusion of T and NK cells as IEC therapy. Fifteen (88%) defined the infusion of hematopoietic cells after a conditioning regimen as “hematopoietic stem cell transplantation” (HSCT).
Consensus Definitions
Based on the survey results and after discussion among experts in the field, two broad models for classification of CGT emerged. One model discretely separated the therapies based on what type of product is administered to an individual (Figure 1a). Under this model, if a cellular product – genetically modified or not – is administered to an individual, it would constitute cellular therapy. In contrast, only in those circumstances where a drug (typically nucleic acid within a vector) is being administered for introducing a genetic change directly into an individual, it would be considered gene therapy. The second framework involved overlap between the cellular and gene therapies based on the site of genetic modification (ex vivo or in vivo) and is more congruent with the current understanding of the field (Figure 1b). Under this framework, genetically modified cellular products would fall under both gene therapy as well as cellular therapy encompassing the similarities and complexities that these products share with the two parent categories. We adopted the latter to preserve the historical context of how these therapies have been developed and classified. We do note that the latter acknowledges the scientific context of the therapeutic product rather than purely prioritizing how it is administered clinically.
Figure 1:
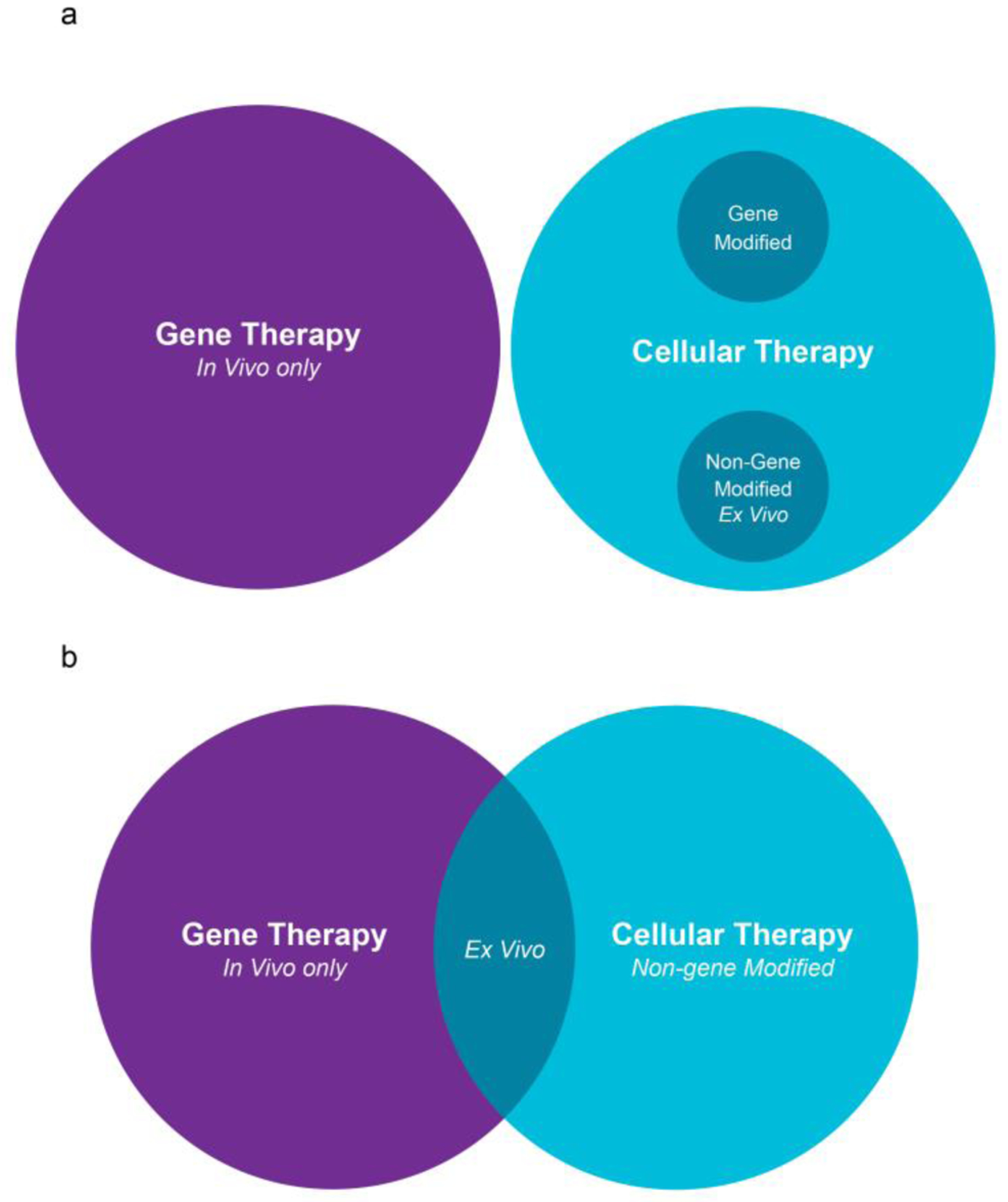
Two models of classification of cellular and gene therapies.
Further, to build upon areas of consensus and address the discrepancies identified by the survey, we propose to update the definitions for CGT that are currently used in clinical practice or are in development. These revised definitions have been updated with consideration of (1) the type of cellular product being administered, (2) the purpose and intent of the cellular infusion, and (3) the preparatory regimen and the post-infusion clinical requirements for the care of the patient that are associated with the cell product (or gene therapy product) infusion, and (4) the site of genetic manipulation (ex vivo or in vivo).
Cellular therapy is a broad term that encompasses both the infusion of a cellular product for the purposes of hematopoietic reconstitution and the infusion of a cellular product intended to have a direct immunologic impact (without any expectation of multi-lineage hematopoietic reconstitution). The term “gene therapy” has traditionally been used to describe an ex vivo or in vivo therapy whereby nucleic acids (RNA or DNA) are introduced into target cells (ex vivo) or tissues (in vivo) by a delivery vector (a virus, nanoparticle or via electroporation). We recognize that there is an overlap in the definitions of genetic and cellular therapies (as depicted in Figure 1b), wherein some cellular products infused for the purpose of hematopoietic or immune reconstitution may in fact have been genetically modified and fit the definition of a “gene therapy”. We also note that there is an increasing trend to differentiate genetic therapies that introduce a transgene (e.g., using a viral or non-viral vector), currently called “gene therapy” from therapeutic cellular products involving nuclease-mediated genetic manipulation, which are increasingly being called “genome editing.” It is likely that as modes of delivery, genetic materials, and technologies evolve, the definitions or gene therapy may have to be revised. Hence, we recommend that the term “genetically modified cellular therapy” (GMCT) may specifically be used when whole live cells that have undergone ex vivo genetic modification are administered to a patient. It is pertinent to point out that clinically, ex vivo GMCT may procedurally be similar to non-genetically modified cellular therapy, hence in an hierarchical framework we have kept it under both parent categories: under “gene therapy” to align it with the potential risks and benefits inherent to administration of genetically modified products, and the need for longer follow up as mandated by the current regulatory frameworks; as well as “cellular therapy” to highlight the similarities with administration of non-genetically modified cells. GMCT may be composed of HSPC, IEC or other blood cell lineages.
An HSPC product is one whose infusion is intended to restore hematopoiesis in the recipients and reconstitute their immune system. The infused product is expected to engraft eventually and lead to multi-lineage reconstitution. Using previously established definitons,6 Hematopoietic cell transplantation (HCT) should be used to describe the episode of infusion of a cellular product that contains HSPCs, such as bone marrow, peripheral blood–derived stem cells (PBSCs), and progenitor cells, or umbilical cord blood. The source of the HSPCs can be allogeneic, autologous, or rarely syngeneic. The HSPC product may be unmanipulated (e.g., unmanipulated bone marrow or PBSCs), minimally manipulated (e.g., by RBC depletion of the bone marrow or PBSC product), or highly selected (e.g., a CD34+-selected PBSC product or a TCR-α/β–depleted PBSC product). The HSPCs can be genetically modified (e.g., by transduction with a lentivirus or by genome editing with a DNA nuclease such as CRISPR-Cas9) in which case they fall under GMCT. These HSPCs may further be grown in culture and stimulated with cytokines or other agents (as in a nicotinamide-stimulated and expanded umbilical cord blood unit). Further, the recipient may or may not have received a preparative regimen prior to such infusion. If administered, the preparative regimen may range in intensity from myeloablative (intended to produce profound and long-lasting pancytopenia that is usually irreversible without HSPC infusion) to reduced-intensity regimens (with which cytopenias are prolonged but are expected to resolve spontaneously with autologous recovery) and finally to nonmyeloablative regimens, which are intended to be solely immunoablative (allowing engraftment of donor cells while producing minimal bone marrow myelosuppression or cytopenia).23, 24
In response to the discrepancies revealed in our own survey responses and the ICD-10 C&M committee meetings regarding how to categorize infusion of autologous ex vivo genetically manipulated HSPCs, we propose that these episodes of care be considered an autologous HCT using a genetically modified cellular product.
FACT defines “immune effector cell therapy” (IECT) as therapy with cells from the human body that have differentiated into a form capable of modulating or effecting an immune response. After discussion, our subcommittee chose to uphold the FACT definition. In IECT, cells that are found in the body (such as B cells, dendritic cells, natural killer cells, and T cells) are collected, modified into a therapeutic product, and then administered to a patient. These cellular therapy products are part of a new pillar of cancer treatment—immunotherapy—which uses a patient’s own immune system to attack tumors. FACT used this definition to determine which cellular products are subject to inspections and audits that assess compliance with FACT standards.
An IECT is one in which the cellular product is infused solely to exploit its immunologic properties, in contrast to HCT, where HSPCs are given to restore hematopoiesis in a patient in addition to reconstitute their immune system. An IEC product is not expected to contribute to multi-lineage hematopoiesis, even though it may engraft permanently in the host. Instead, it may either hasten immunologic recovery or provide specific immune properties to the recipient. The latter may include antiviral properties (e.g., virus-specific T cells), anti-tumor properties (e.g., antigen-directed CAR-T cells), or immune modulator properties (e.g., regulatory T cells or mesenchymal cells). These cells may be unselected (e.g., in donor lymphocyte infusions) or selected (e.g., CD45RA-depleted T cells); cytokine-stimulated or unstimulated; autologous or allogeneic; and genetically modified or unmodified. Based on our consensus definitions, a product such as cord-blood derived NK cells (non-CAR bearing) would be considered cellular therapy – not genetically-modified IEC. Similarly, CAR T cells such as tisagenlecleucel would be considered ex vivo genetically modified cellular therapy.
Summary
As gene and gene-modified cellular therapeutics are becoming a permanent part of the clinical landscape, appropriate categorization is necessary for clinicians and the healthcare system to use standardized nomenclature that captures scientific nuance and clinical administration logistics appropriately. Over the course of several hours of meetings, including the development and implementation of a survey, and further refinements in the course of preparation of this manuscript, we recognize that it will be challenging for all stakeholders to transition from the current plethora of terminology to a standardized nomenclature. Our sorting exercise for placing FDA-approved therapeutics and other investigational therapies into various categories highlighted ambiguities and inconsistencies that are present even among experts in the field, with the greatest area of variability was in the use of HSPC-based products with genetic modification. Despite the discrepancies, areas of agreement emerged from this exercise. First, administration of lymphocytes without genetic modification is clearly considered to be IEC therapy. Second, in vivo gene editing is strictly considered to be gene therapy. Third, infusion of hematopoietic stem cells with the intent of hematopoietic and immune reconstitution is considered to be an HCT.
As practicing physician-scientists with expertise in CGT-based therapies, working alongside health policy experts, we sought to identify areas of conflict in the current CGT nomenclature and to provide a new contextual framework to classify CGT approaches in an inclusive, clear and non-redundant fashion. The new framework we propose should be sufficiently robust to accommodate novel therapies emerging from future technological advances, though we acknowledge that additional consideration and conversation is likely.
In conclusion, the rapidly growing field of CGT is undergoing a transition from the bench to many bedsides; accordingly, the molecular or manufacturing-based terminology that was initially used to describe the product, has given way to a need for standardized terminology that captures clinical use and administration of these therapeutic products by healthcare organizations communicating with payors and regulators. These new, precise definitions and standardized nomenclature are meant to acknowledge historical context and will serve several purposes. First, and most importantly, these will foster an efficient, harmonized, and streamlined regulatory and developmental framework within which patients receive these novel therapies. Additional benefits include the development of training paradigms and educational programs; equitable and rational decisions about accessibility; and consistency in the billing and coding structures used for reimbursement. Considering the cost of these novel agents, this latter aspect is an essential component of delivering these promising approaches to individuals in greatest need. We recognize that no classification system is perfect. Despite the limited responses, our survey showed a considerable degree of variability when respondents were asked to sort currently available novel CGT therapies. This observation and the issues discussed above both provide a very strong impetus to align all interested parties around consensus definitions. The importance of standardization is nonetheless critical to the field and the proposed framework provides a foundation to build upon. We invite and look forward to additional dialogue with a broader set of stakeholders.
Supplementary Material
Figure 2:
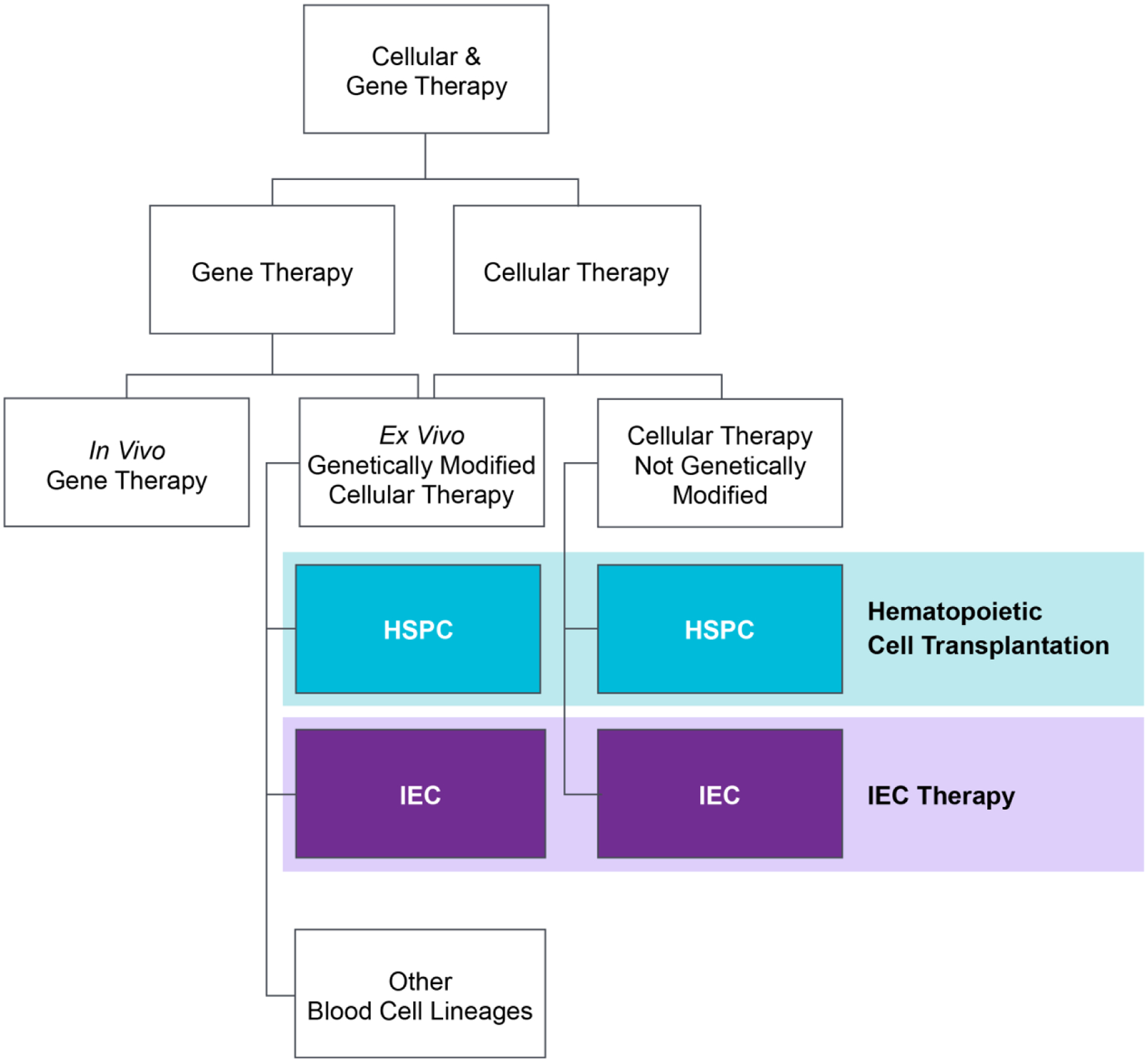
Proposed schematic of hierarchy and classification of CGT products.
Table 1:
Proposed framework of cellular and gene therapy products.
| Term | Definition | Therapeutic Intent | Procedural Details | Examples |
|---|---|---|---|---|
| Gene therapy | In vivo or ex vivo delivery of genetic material or agents intended to produce a genetic modification in the target cells. | Genetic modification in the target cells to achieve a specific goal. | Introducing a transgene by using a viral or non-viral vector, or nuclease-mediated genetic manipulation. | Examples classified by in vivo or ex vivo below: |
| In Vivo Gene Therapy | Delivery of genetic material or agents intended to produce a genetic modification in the target cells directly into the human body. | Introduction of new genetic material or modification of the genome in target cells to affect a functional or phenotypic change. | May involve administration of the genetic material directly into the target organ or into the blood stream with homing to the target organ. | Voretigene neparvovec (Luxturna); onasemnogene abeparvovec (Zolgensma) |
| Ex Vivo Genetically modified cellular therapy (GMCT) | Infusion of whole live cells that have undergone ex vivo genetic modification. | Hematopoietic reconstitution or immune reconstitution or to provide specific immunologic characteristics. | Ex vivo genetic modification of live cells followed by reinfusion into the patient. | See below under specific categories: |
| Genetically modified - Hematopoietic cell transplantation (HCT) | Infusion of a cellular product that contains HSPCs. | Restoration of hematopoiesis and immunity, as well as effecting a phenotype change based on the genetic modification (production of a new protein or a change in production of an existing protein in blood cells). | Often involves administration of a preparative regimen that leads to varying degrees of cytopenia and immunosuppression. | Lentivirus transduced: Lentiglobin/Betibeglogene autotemcel (beti-cel), Libmeldy/OTL-200 (autologous CD34+ cells encoding the ARSA gene). CRISPR-Cas9 edited: CTX001/Exagamglogene autotemcel, VOR33 (Vor Biopharma) |
| Genetically modified - Immune effector cell (IEC) therapy | Infusion of a cellular product consisting of immunologically active cells that are unlikely to engraft and result in multi-lineage reconstitution. | Leveraging the immunologic properties of the cellular product by modifying the targeting the capability of the effector cells. | May involve administration of lymphodepleting chemotherapy before infusion. | Retrovirus/Lentivirus transduced: Tisagenlecleucel (Kymriah), Afamitresgene autoleucel (afami-cel, ADP-A2M4; Adaptimmune). CRISPR-Cas9 or TALEN edited: ALLO-501 (Allogene) |
| Hematopoietic cell transplantation (HCT) | Infusion of a cellular product that contains HSPCs. | Restoration of hematopoiesis and immunity. | Often involves administration of a preparative regimen that leads to varying degrees of cytopenia and immunosuppression. |
Examples based on degree of selection: Unmanipulated bone marrow or PBSC vs highly selected CD34+ selected PBSC product, TCR-α/β depleted PBSC graft. |
| Immune effector cell (IEC) therapy | Infusion of a cellular product consisting of immunologically active cells that are unlikely to result in multi-lineage reconstitution. | Leveraging the immunologic properties of the cellular product. | May involve administration of lymphodepleting chemotherapy before infusion. |
Examples based on degree of selection: Unselected: Donor lymphocyte infusion. Selected: CD45RA depleted T cells. |
Highlights.
There is a need to standardize the nomenclature of cellular and genetic therapies.
We performed focus groups and surveys to arrive at consensus definitions.
There is an overlap in the definitions of genetic and cellular therapies.
Administration of cells that can modulate an immune response is IEC therapy.
Infusion of a genetically modified HSCs constitutes an HCT.
Acknowledgements:
A.S. is partially supported by ASH Scholar Award and acknowledges the training he received at the ASTCT Clinical Research Training Course. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the Warren Grant Magnuson Clinical Center. (ZIA BC 011823, N.N.S.) The authors thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript.
Disclosures and Conflicts of Interests
A.S. is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for ex vivo HSPC genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals (NCT03745287), by Novartis (NCT04443907) and by Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trials, which includes salary support paid to A.S. institution. A.S. has received consultant fee from Spotlight Therapeutics, Medexus Inc. and Vertex Pharmaceuticals. He has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education.
M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital (some licensed to Promab) and University of Pennsylvania (some licensed to Novartis); serves on the Board of Directors of 2Seventy Bio, and holds Equity in 2SeventyBio, Century Therapeutics, Neximmune, Oncternal, and TCR2 and has served as a consultant for multiple companies involved in cell therapies.
S.G. is named in multiple patents in the field of chimeric antigen receptor T cells and chimeric antigen receptor macrophages, and is a co-founder of Carisma Therapeutics (a genetically engineered macrophage cell therapy company) and Interius Biotherapeutics (an in vivo cell & gene therapy company).
J.S. is the President of Nimitt Consulting, which acts as a reimbursement advisor to ASTCT and has a fiduciary role in educating biotechnology and pharmaceutical companies about United States coding and provider reimbursement mechanisms. S.F. and A.R. are contracted employees of Nimitt Consulting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government (N.N.S).
References
- 1.National Cancer Institute. CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. Vol 2022. Bethesda, MD: 2022. [Google Scholar]
- 2.Grady D Cell Therapy Shows Promise for Acute Type of Leukemia. The New York Times. New York City, NY: The New York Times Company; 2013. [Google Scholar]
- 3.Novartis Pharmaceuticals Corporation. KYMRIAH® (tisagenlecleucel) | Official Patient Website. Vol 20222021. [Google Scholar]
- 4.US Food and Drug Administration. FDA approval brings first gene therapy to the United States. Press Announcements. Vol 2022. Silver Spring, MD: US Food and Drug Administration; 2017. [Google Scholar]
- 5.Foundation for the Accreditation of Cellular Therapy, The Joint Accreditation Committee – ISCT and EBMT. Standards for Immune Effector Cells. First Edition ed: The Foundation for the Accreditation of Cellular Therapy; 2018. [Google Scholar]
- 6.LeMaistre CF, Farnia S, Crawford S, McGuirk J, Maziarz RT, Coates J, Irwin D, Martin P, Gajewski JL. Standardization of terminology for episodes of hematopoietic stem cell patient transplant care. Biol Blood Marrow Transplant. 2013;19:851–857. [DOI] [PubMed] [Google Scholar]
- 7.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, Dionisio F, Calabria A, Giannelli S, Castiello MC, Bosticardo M, Evangelio C, Assanelli A, Casiraghi M, Di Nunzio S, Callegaro L, Benati C, Rizzardi P, Pellin D, Di Serio C, Schmidt M, Von Kalle C, Gardner J, Mehta N, Neduva V, Dow DJ, Galy A, Miniero R, Finocchi A, Metin A, Banerjee PP, Orange JS, Galimberti S, Valsecchi MG, Biffi A, Montini E, Villa A, Ciceri F, Roncarolo MG, Naldini L. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S, Belohradsky BH, Leiva L, Sorensen R, Debre M, Casanova JL, Blanche S, Durandy A, Bushman FD, Fischer A, Cavazzana-Calvo M. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, Canale S, Benedicenti F, Vallanti G, Biasco L, Leo S, Kabbara N, Zanetti G, Rizzo WB, Mehta NA, Cicalese MP, Casiraghi M, Boelens JJ, Del Carro U, Dow DJ, Schmidt M, Assanelli A, Neduva V, Di Serio C, Stupka E, Gardner J, von Kalle C, Bordignon C, Ciceri F, Rovelli A, Roncarolo MG, Aiuti A, Sessa M, Naldini L. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. [DOI] [PubMed] [Google Scholar]
- 13.Sessa M, Lorioli L, Fumagalli F, Acquati S, Redaelli D, Baldoli C, Canale S, Lopez ID, Morena F, Calabria A, Fiori R, Silvani P, Rancoita PM, Gabaldo M, Benedicenti F, Antonioli G, Assanelli A, Cicalese MP, Del Carro U, Sora MG, Martino S, Quattrini A, Montini E, Di Serio C, Ciceri F, Roncarolo MG, Aiuti A, Naldini L, Biffi A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil JA, Hongeng S, Magrin E, Schiller GJ, Payen E, Semeraro M, Moshous D, Lefrere F, Puy H, Bourget P, Magnani A, Caccavelli L, Diana JS, Suarez F, Monpoux F, Brousse V, Poirot C, Brouzes C, Meritet JF, Pondarre C, Beuzard Y, Chretien S, Lefebvre T, Teachey DT, Anurathapan U, Ho PJ, von Kalle C, Kletzel M, Vichinsky E, Soni S, Veres G, Negre O, Ross RW, Davidson D, Petrusich A, Sandler L, Asmal M, Hermine O, De Montalembert M, Hacein-Bey-Abina S, Blanche S, Leboulch P, Cavazzana M. Gene Therapy in Patients with Transfusion-Dependent beta-Thalassemia. N Engl J Med. 2018;378:1479–1493. [DOI] [PubMed] [Google Scholar]
- 15.Ribeil JA, Hacein-Bey-Abina S, Payen E, Magnani A, Semeraro M, Magrin E, Caccavelli L, Neven B, Bourget P, El Nemer W, Bartolucci P, Weber L, Puy H, Meritet JF, Grevent D, Beuzard Y, Chretien S, Lefebvre T, Ross RW, Negre O, Veres G, Sandler L, Soni S, de Montalembert M, Blanche S, Leboulch P, Cavazzana M. Gene Therapy in a Patient with Sickle Cell Disease. N Engl J Med. 2017;376:848–855. [DOI] [PubMed] [Google Scholar]
- 16.Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, Foell J, de la Fuente J, Grupp S, Handgretinger R, Ho TW, Kattamis A, Kernytsky A, Lekstrom-Himes J, Li AM, Locatelli F, Mapara MY, de Montalembert M, Rondelli D, Sharma A, Sheth S, Soni S, Steinberg MH, Wall D, Yen A, Corbacioglu S. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia. N Engl J Med. 2021;384:252–260. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Cellular & Gene Therapy Products. Vaccines, Blood & Biologics. Vol 2022. Silver Spring, MD: US Food and Drug Administration; 2021. [Google Scholar]
- 18.Center for Biologics Evaluation and Research, US Food and Drug Administration. Guidance Document: Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products. Vol 2022. Silver Spring, MD: US Food and Drug Administration; 2022. [Google Scholar]
- 19.Foundation for the Accreditation of Cellular Therapy, The Joint Accreditation Committee – ISCT and EBMT. Hematopoietic Cellular Therapy Accreditation Manual. 8.2 ed: The Foundation for the Accreditation of Cellular Therapy. [Google Scholar]
- 20.US Centers for Medicare & Medicaid Services. ICD-10 Coordination and Maintenance Committee Meetings. Vol 2022. Baltimore, MD: US Centers for Medicare & Medicaid Services; 2022. [Google Scholar]
- 21.US Centers for Medicare & Medicaid Services. Using the ICD-10-PCS New Technology Section Codes. Vol 2022. Baltimore, MD: US Centers for Medicare & Medicaid Services; 2022. [Google Scholar]
- 22.Centers for Medicare & Medicaid Services. ICD-10 Coordination and Maintenance Committee Meeting. Baltimore, MD: Centers for Medicare & Medicaid Services; 2021. [Google Scholar]
- 23.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, Blaise D, Lowski R, Horowitz M. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, Sandmaier B. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


