Abstract
Background:
Alcohol use problems are associated with serious medical, mental health and socioeconomic consequences. Yet even when patients are identified in healthcare settings, most do not receive treatment, and use of pharmacotherapy is rare. This study will test the effectiveness of the Alcohol Telemedicine Consult (ATC) Service, a novel, personalized telehealth intervention approach for primary care patients with alcohol use problems.
Methods:
This cluster-randomized pragmatic trial, supplemented by qualitative interviews, will include adults with a primary care visit between 9/10/21-3/10/23 from 16 primary care clinics at two large urban medical centers within Kaiser Permanente Northern California, a large, integrated healthcare system. Clinics are randomized to the ATC Service (intervention), including alcohol pharmacotherapy and SBIRT (screening, MI (Motivational Interviewing)-based brief intervention and referral to addiction treatment) delivered by clinical pharmacists, or Usual Care (UC) arm that provides systematic alcohol SBIRT. Primary outcomes include a comparison of the ATC and UC arms on 1) implementation outcomes (alcohol pharmacotherapy prescription rates, specialty addiction treatment referrals); and 2) patient outcomes (medication fills, addiction treatment initiation, alcohol use, healthcare services utilization) over 1.5 years. A general modeling approach will consider clustering of patients/providers, and a random effects model will account for intra-class correlations across patients within providers and across clinics. Qualitative interviews with providers will examine barriers and facilitators to implementation.
Discussion:
The ATC study examines the effectiveness of a pharmacist-provided telehealth intervention that combines pharmacotherapy and MI-based consultation. If effective, the ATC study could affect treatment models across the spectrum of alcohol use problems.
Keywords: alcohol use problems, primary care, alcohol pharmacotherapy, telemedicine, brief intervention, pharmacist
1. Introduction
1.1. Scientific background and rationale
Alcohol use problems, ranging from unhealthy alcohol use, i.e., drinking over the recommended safe limits, to alcohol use disorders (AUDs), are associated with serious medical conditions [1–3] and increased healthcare utilization and costs [4, 5]. They adversely affect acute and chronic medical conditions [6, 7], and increase the risk for major depression [8] and other psychiatric disorders [9]. They can hinder seeking preventive health services [10], and increase avoidable emergency department (ED) visits and hospitalizations [11, 12]. Adverse effects on families and communities include domestic violence, unemployment and legal problems [13]. Alcohol use problems may also contribute to other substance use disorders, including opioid use disorder, and elevated opioid overdose risk [14].
Ample evidence shows that many patients who might benefit from alcohol treatment do not receive it [15]. Reasons include lack of transportation, treatment availability, childcare [10, 16, 17], motivation [18], information about specialty alcohol treatment and its effectiveness [19], and the stigma associated with treatment [20, 21]. For many patients, primary care physicians (PCPs) are their main contact with the health system. Yet PCPs are often not able to adequately address alcohol use (e.g., they lack specific training or time due to competing priorities or the confidence to address and treat substance use) [22–24].
The most notable effort to address alcohol use problems in primary care has been Screening, Brief Intervention, and Referral to Treatment (SBIRT) [25, 26]. Evidence for the efficacy and effectiveness of SBIRT and its components is mixed. Brief interventions can help some patients reduce unhealthy alcohol use [27, 28], but for more severe alcohol problems, including AUDs, a brief intervention may be insufficient [29–31]. However, referral to specialty addiction treatment has been a weak link in the SBIRT model; a recent meta-analysis found that brief interventions did not increase receipt of treatment [30], even in settings where treatment is readily available. Instead, providing PCPs with additional resources and other clinical staff may be options in these situations. Increased use of alcohol pharmacotherapy in primary care could address this need. Three medications are approved for alcohol use treatment by the Food and Drug Administration in the United States (U.S.): naltrexone, acamprosate and disulfiram [32]. Though widely available for many years, they remain underused [33], despite the large body of research that supports their safety and efficacy in alcohol use reduction [15, 34, 35]. The U.S. National Institute on Alcohol Abuse and Alcoholism and the Substance Abuse and Mental Health Administration has published guidelines for using naltrexone and acamprosate, with and without adjunctive psychosocial treatment, including in general medical settings [36, 37]. The evidence is growing regarding the efficacy and safety of other medications, such as gabapentin [38, 39] and topiramate [40]. In spite of this pharmacotherapy armamentarium, medications are still rarely prescribed for alcohol problems [41, 42].
Efforts to increase use of alcohol pharmacotherapy have been few [43], and the early evaluation studies found minimal or no increases [44–46]. One promising strategy is to expand the role of pharmacists in primary care-based interventions. Clinical pharmacists are increasingly serving patients for many health conditions, and are trained and involved in alcohol use treatment in several settings: detoxifications in jails [47], post-treatment discharge care [48], and opportunistic screening and brief intervention in community pharmacies [49], demonstrating potential to further expand their roles. To date, however, no rigorous, large-scale trials of interventions using clinical pharmacists to deliver alcohol pharmacotherapy in primary care in a real-world healthcare system have been conducted.
Our approach, the Alcohol Telemedicine Consult (ATC) service is an innovative approach comprising motivational interviewing (MI)-based counseling, medication management, and facilitated referral to specialty treatment, delivered to primary care patients via virtual consultation with clinical pharmacists. The cluster-randomized, pragmatic trial will be conducted in 16 primary care clinics at two medical centers of a large, U.S. integrated healthcare delivery system with a highly diverse membership and systematic, integrated screening for unhealthy alcohol use in primary care [50, 51]. This trial was informed by a successful pilot feasibility study that demonstrated high acceptability among both PCPs and patients with a positive effect on naltrexone prescribing [52]. The trial is designed to examine the effectiveness of this virtual consultation intervention which provides flexible support to PCPs and their patients via a brief, pharmacist-led telemedicine intervention. We will compare the ATC and Usual Care (UC) arms, examining factors associated with ATC implementation through the following specific aims:
Aim 1, Implementation Outcomes:
Compare alcohol pharmacotherapy prescription rates and specialty addiction treatment referrals over 1.5 years.
Aim 2, Patient Outcomes:
Compare alcohol pharmacotherapy fills, specialty addiction treatment initiation, alcohol use (quantity/frequency), and health services utilization over 1.5 years.
Aim 3, characteristics associated with ATC implementation:
Examine provider characteristics (i.e., gender, race/ethnicity, panel size, years of experience, addiction medicine expertise) which are associated with ATC implementation outcomes using electronic health record (EHR) data, and conduct semi-structured qualitative interviews with PCPs to explore how the elements of ATC (video consult/telephone/email) facilitate its implementation.
We hypothesize that the ATC arm will have higher alcohol pharmacotherapy prescription and fill rates, higher rates of referral to and initiation of specialty addiction treatment, lower unhealthy alcohol use (heavy drinking days (four+ (for women)/five+ (for men) drinks/day); five+ heavy drinking days/90 days; heavy drinking weeks (seven+ (for women)/14+ (for men) drinks/week)) at follow-up visits, and lower emergency department (ED) and inpatient services utilization than the UC arm.
2. Methods: Participants, Interventions and Outcomes
2.1. Study setting
The study will be conducted in two Kaiser Permanente Northern California (KPNC) medical centers—Oakland and San Francisco—which collectively serve ~250,000 adult patients.
A KPNC-wide alcohol SBIRT initiative has been implemented in adult primary care since June 2013. Medical assistants screen all patients, using the National Institute on Alcohol Abuse and Alcoholism evidence-based screening questions, embedded in the EHR [53].
2.2. Eligibility and randomization
Adult primary care clinics (n=16) are randomized to the ATC (intervention) or UC arm (Figure 1). Randomization is stratified and blocked by facility (eight per arm) to ensure balance between the study arms within facilities and across the sample of clinics. Other clinic characteristics (e.g., distance from addiction treatment program) will be adjusted for statistically rather than used for matching, since matching would prevent us from examining their direct effects on outcomes in any future secondary analyses.
Figure 1:
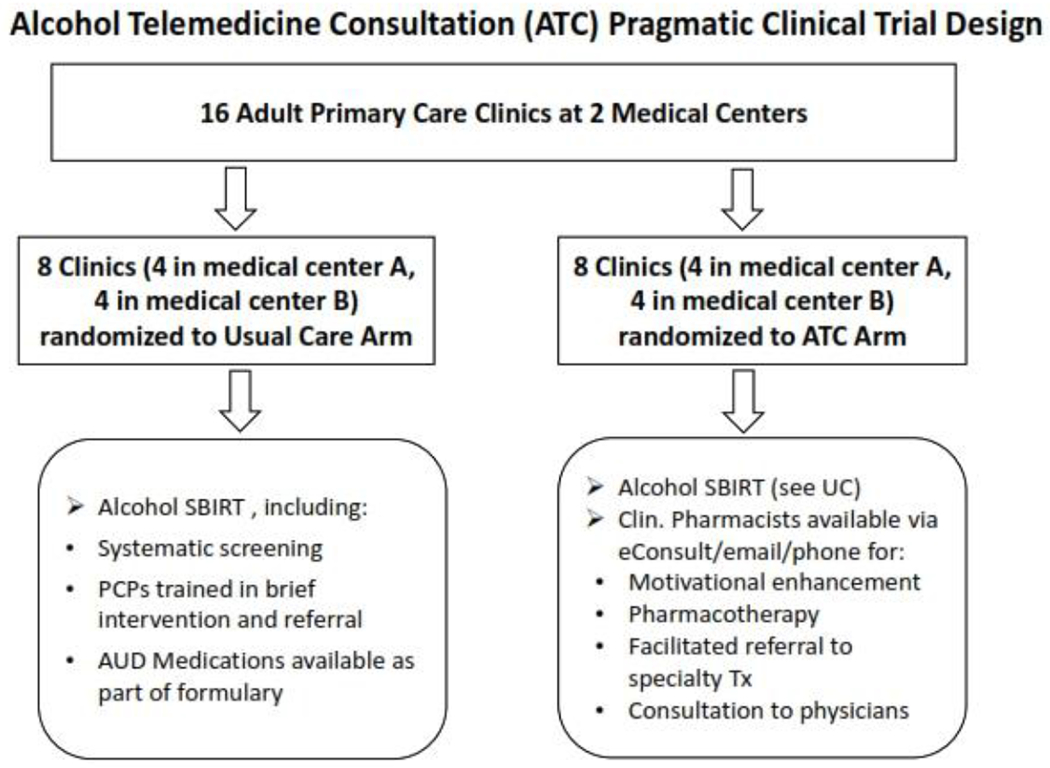
Cluster randomization of primary care clinics
Intervention arms: ATC Service (intervention) versus Usual Care (UC)
PCPs in the intervention arm will use an EHR-based electronic referral tool to request outreach or directly schedule appointments with a clinical pharmacist. For more immediate response, physicians may use the health system’s EHR-based secure messaging system.
ATC pharmacists will receive focused training in addiction medicine, including screening, assessment and treatment of alcohol use problems, management of alcohol pharmacotherapies, Motivational Interviewing (MI), observations of specialty treatment, case presentations, and review and discussion of materials with the research team.
Pharmacists will provide patients with:
MI-based counseling to increase problem awareness and help identify reasons for and strategies to change.
Psychoeducation about available pharmacotherapies, specialty treatment, and community support programs.
Alcohol pharmacotherapy prescribing, including labs and follow-ups.
Facilitated referral to specialty treatment, and instructions to follow-up.
Documentation in the EHR.
In addition to direct patient care, provide intervention-arm PCPs with advice regarding patient-specific treatment options.
The ATC intervention will include a one-hour educational session offered to PCPs in the intervention arm, focusing on epidemiology of unhealthy drinking, ATC intervention rationale and referral criteria, an introduction to available pharmacotherapies, and instructions for accessing the service. Clinical pharmacists will provide coverage of the ATC service during regular primary care clinic hours.
Each clinic in the intervention arm will receive periodic refresher trainings by study staff that will also provide materials for training of new physicians.
The UC arm consists of treatment as usual, including standardized and systematic alcohol screening as part of the “rooming” process conducted by medical assistants, and brief interventions and referrals to addiction treatment delivered by PCPs [51]. PCPs can prescribe the same alcohol pharmacotherapy as in the ATC arm.
2.3. Data sources and outcome measures
Electronic Health Record (EHR).
KPNC’s EHR system integrates data on patient enrollment, demographics, health services utilization (including medications), International Classification of Diseases (ICD-10) diagnostic codes [54], procedures, laboratory, and vital signs, linked by a unique membership number. We will use the EHR to identify the study cohort and to extract implementation outcomes, patient-reported alcohol use, and patient demographic and clinical characteristics. Outpatient utilization and hospitalization data will be extracted from the EHR and administrative databases. Service utilization and pharmacy data outside of KPNC are captured in billing/claims databases [55, 56]. They will be used to measure alcohol pharmacotherapy prescriptions and fills [57, 58]. We will use associated diagnostic codes to examine whether medications were used for alcohol use problems. Inpatient and outpatient prescription data are captured for nearly 100% of enrollees and capture over 95% of medication fills [59], including National Drug Codes, standard drug class codes, dates of prescription/dispensing, strength/frequency/quantity, and days of supply. Table 1 shows the EHR variables for each outcome and study aim.
Table 1:
Variables and related outcomes for each study aim
| Domain | Variables |
|---|---|
| Implementation Outcomes (Aim 1) | •Alcohol Pharmacotherapy Prescribing Rate: calculated as number of patients in the physician’s panel ordered a prescription in each of the 12 months post-ATC intervention implementation divided by the total number of patients in the PCP’s panel who have screened positive for unhealthy alcohol use, a brief intervention or an AUD diagnosis in the same time period. •Addiction Treatment Referral Rate: calculated as number of patients who are referred to specialty addiction treatment in each of the 12 months post-ATC intervention implementation period divided by the total number of patients in the panel who have screened positive for unhealthy alcohol use, a brief intervention or an AUD diagnosis in the same time period. |
| Primary Patient Outcomes (Aim 2) | •Alcohol Pharmacotherapy Fills: for patients with a primary care encounter and a positive unhealthy alcohol use screening, a brief intervention or an AUD diagnosis within six months prior to through the primary care encounter, a dichotomous indicator of whether the patient fills a medication from a KPNC pharmacy within 30 days of that index PCP encounter. •Addiction Treatment Initiation Rate: for the same cohort, an indicator of whether the patient initiates specialty treatment within 14 days post the index PCP encounter. Using Health Effectiveness Data Information System (HEDIS) measures, treatment initiation is defined as one addiction medicine visit within 14 days of the index visit [60]. |
| Secondary Patient Outcomes (Aim 2) | •Alcohol Use: drinking days/week, # drinks/week, # days exceeding four+/five+ (“heavy drinking day”) in past 90 days, indicator of any five+ heavy drinking days in past 90 days (as a proxy for alcohol dependence) [61]. •Health Service Utilization: Summarized (total, and by type of service) by 12-month intervals beginning one year prior through two years post the index encounter. |
| Predictors and Potential Confounders (All 3 Aims) | •Provider-level Factors: gender, race/ethnicity, panel size, years of experience, addiction medicine expertise •Patient-level Factors: age, gender, race/ethnicity, language preference, treatment history, medical and mental health comorbidities (in the one year prior to, and 30 days following the index visit) (primary and multiple secondary diagnoses are recorded at every visit) [62, 63], and insurance type (Commercial, Medicaid) at time of the index encounter. |
Qualitative Interviews.
The study team will conduct interviews with physicians (n~30) from the ATC intervention arm to explore barriers and facilitators, and patient needs. Interview topics will include feasibility and logistical challenges, perceived need for and benefits of the intervention, and perspectives on the challenges and opportunities of providing treatment in primary care. Interviews will be 20-to 30-minutes long and conducted by experienced qualitative interviewers [52, 64, 65]
ATC Care Data.
During the course of the intervention, the ATC consultant will document clinical notes and process measures such as ATC modality (video/telephone/email), duration of encounter, and clinical characteristics.
2.4. Duration of ATC intervention
Length of ATC intervention will be flexible and individually tailored. While there is no specific limit to number of appointments, the intervention is designed to be brief (e.g., ranging from one to five appointments). For patients prescribed a medication, the intervention will be considered complete when the patient is on a stable dose (side effects are tolerable and the medication is helping meet reduction/abstinence goals). Analyses will include data about average/range of number of appointments and total time spent by pharmacists.
2.5. Sample size and recruitment
We will recruit/randomize clinics to the ATC and UC arms, not individual patients. Block randomization will be performed as described earlier (Section Eligibility and Randomization). We will identify adults who had a primary care visit between 9/10/21-3/10/23. We will follow the cohort through 3/10/2024 to allow a 12-month administrative censored follow-up period. Based on prior studies [52, 66], and preliminary analyses, we project to identify an initial cohort of ~300,000 patients, and 60% of them will have follow-up outcomes.
3. Methods: Data Collection, Management & Analysis
3.1. Data collection
Data will be collected and entered into the patients’ EHR during the course of regular clinical care, by clinical staff. An ATC-specific clinical note template will be used to increase completeness of EHR data.
3.2. Data management
Study data will be held in strict confidence, collected as part of usual medical care and reside in the health system’s secure clinical and administrative databases. Study data will be stored on KPNC Division of Research’s secure, firewall-protected servers. During data extraction, identifying information will be available only to the study programmer and only in non-readable, electronic formats. Once data extraction is complete, all identifying information will be immediately removed from the new combined dataset.
Key informant interviews will be conducted via KPNC’s secure, HIPAA-compliant teleconferencing system. Interviewees’ identifying information will be stored separately from recordings. No identifying data will be used in any report or publication from this study.
3.3. Missing data
While missing data from EHRs are likely, we expect missingness to be low given KPNC’s mature EHR. However, analyses will include examination of patterns and extent of missingness across treatment arms. If necessary, we will impute missing values assuming missing at random- (MAR) and use established imputation methods [67, 68], including relevant covariates such as demographics and comorbidity index (i.e. the Charlson index) to increase accuracy [69], and conduct sensitivity analyses.
3.4. Data analyses
Preliminary analyses will be used to determine the distribution of outcome variables and covariates of interest. For continuous measures we will use univariate analyses to obtain descriptive statistics and check for departures from normality. We will use simple t-tests and analysis of variance techniques to assess effects of covariates on these outcomes. For proportions, we will use chi-squared tests from frequency distributions and bivariate contingency tables by treatment arm, to determine if proportions are significantly different.
3.5. General modeling approach
Our general modeling approach will take into consideration the clustering of patients within providers and providers within clinics as appropriate (see Figure 2). We will use a mixed model (also known as random effects model or hierarchical model) [70] that consists of both fixed and random effects to account for the intra-class correlations (ICCs) across patients within providers and across clinics.
Figure 2.
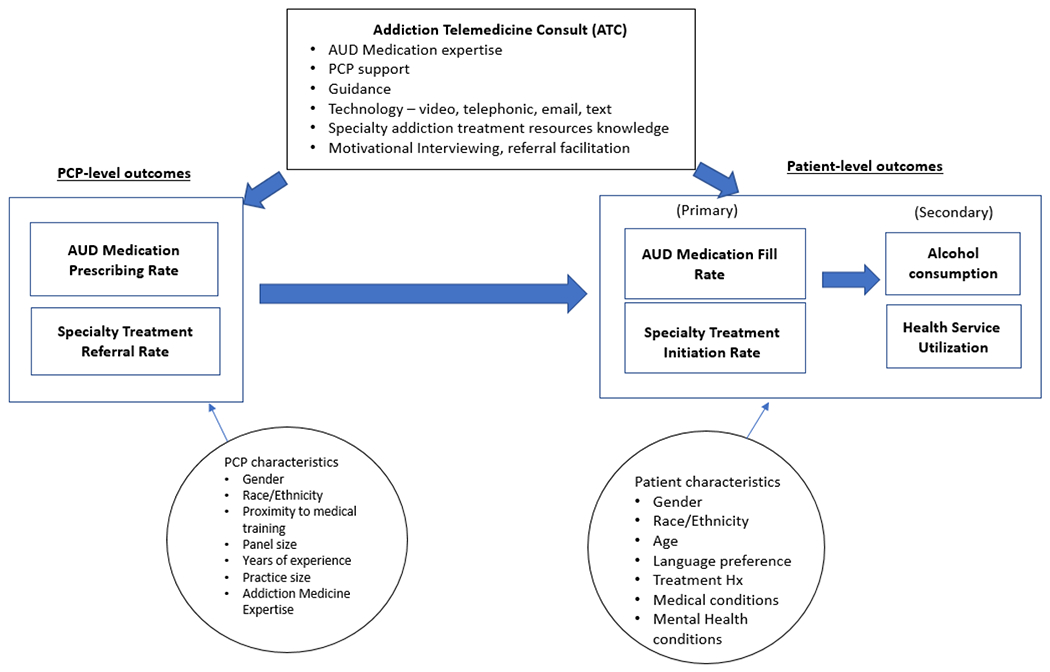
Analytic Model
To examine the difference in implementation outcomes between ATC and UC arms over 1.5 years, the ATC implementation date will be the index date for these analyses. For each 12-month-post-implementation-period, we will use the EHR to obtain the number of adults who have either screened positive on the alcohol screening, received a brief intervention or an AUD diagnosis (denominator). We will also summarize (by provider) the number of patients among these who have a medication order within the same time window (numerator). The prescription rate for each PCP is the ratio of these two metrics. Medications issued will be assigned to the provider who initiates the ATC consult. We will compare the annual prescribing rates for PCPs in the two arms using the linear mixed model accounting for correlation between providers within clinics. The key covariate is the indicator variable denoting the treatment arms.
To examine patient outcomes, we will use the first primary care encounter in the post-implementation-period when eligibility criteria is met as the index date. We will use a dichotomous measure of whether a prescription is filled within 30 days of this index date and create indicator variables for the presence of comorbidity any time in the prior year through the 30-day index post-index period; this time window is based on the premise that the presence of comorbidity and the addition of the ATC might trigger an alcohol pharmacotherapy prescription that might not have been issued otherwise. We will fit a mixed effects logistic regression model to compare likelihood of filling a prescription for patients in the two arms, adjusting for age and gender, race/ethnicity, language preference and the presence of alcohol-related medical conditions [58]; of particular interest is the sign and magnitude of the coefficient of the race/ethnicity/language variables as these will help identify disparities.
For examining drinking outcomes, we will include patients with a return visit within two years after index PCP encounter (~60%). We will use # of days exceeding four+/five+ drinks (“heavy drinking day”) in past 90 days as our primary outcome. The treatment indicator variable will be the key covariate of interest. We will examine changes in these values between the patient’s consecutive screenings controlling for time between visits and accounting for the nesting of patients within providers within clinics.
We will compare the various measures of utilization between the two arms over 1.5 years. For each year from one year prior to 1.5 years post the index primary care encounter, we will create dichotomous indicators for having any ED visit and any inpatient stay and examine effects of ATC by fitting mixed effects logistic regression models. We will also examine the counts of ED, primary care, psychiatry department and total outpatient visits for each patient over the 1.5 years post index PCP encounter as the outcome measures, and fit mixed models using negative binomial distribution which can be accommodated in the mixed model framework.
3.6. Qualitative analyses
We will conduct qualitative analyses of interview data to examine barriers and facilitators of alcohol pharmacotherapy and the ATC model more broadly. These data will provide context for understanding factors that may predict higher rates of medication prescription and inform possible programmatic/policy changes to implement the intervention in this health system and others.
Interviews will be recorded and transcribed. We will use a content analysis approach [71] to generate insights from participant responses. We will use NVivo qualitative analysis software for coding [72]. Code frequencies and inter-rater reliability will be examined, and Kappa coefficients calculated. Qualitative data will help understand the clinicians’ experiences and attitudes towards alcohol pharmacotherapy, treatment of alcohol use problems in primary care and ATC barriers and facilitators. We will also examine provider responses to the use of specific elements (e.g., EHR-based referral, direct-booking, access to pharmacist notes) of the intervention. This will provide insight on which element of the intervention worked best under different circumstances. In this embedded approach, data integration will also occur at the interpretation phase with a triangulation method, by identifying varying levels of agreement [73].
The sample size for the study is expected to have adequate power for conducting statistical analyses. Power calculation for 3-level cluster randomized designs [74] accounted for the number of clusters (clinics) nc, the number of subjects (providers) within clusters ns and the number of replications (individuals) within providers ni [74]. When randomization is at the highest level (e.g. clinic) but analyses is a lower level (e.g. provider-level for Aim 1 and patient-level for Aim 2), the required sample size is calculated as the product of the sample size in the absence of clustering, and two variance inflation factors (or the design effect) [75] to account for clustering [76, 77]. We used a conservative estimate of 10% ICC at both provider and clinic levels in all calculations and assume a significance level of .05 for all hypotheses tests. Each arm consists of eight clinics and approximately 12 PCPs per clinic with an average panel size of 1100 patients (conservative estimate). Given our clinic and provider sample sizes, for tests of hypotheses in Aim 1, we will have a power of .84 to detect a small-to-medium effect size of .40 standard deviation in the outcome. Our pilot study results suggest larger effects and we expect to have adequate power to detect differences between the ATC and UC arm. For examining drinking and utilization outcomes at the patient level, we anticipate that 60% of the initial sample (N=660 patients/provider) will have a return visit during the study period. Using excessive drinking days as an example in these three-level models, we will have .92 power to detect a difference of .2 standard deviations in # of excessive drinking days between the ATC and UC arm. Other patient level outcomes will have similar power.
3.7. Data monitoring
The study protocol has been approved by the KPNC Institutional Review Board (IRB Number: 1564446). The study involves minimal risk to participants, and procedures are in place for clinical consultation in case of adverse events.
3.8. Ethics and dissemination
Regular updates/progress reports will be submitted to the IRB as well as any protocol modifications. Since we will only analyze EHR data collected during clinical procedures, no direct participant recruitment is involved, and we were granted a waiver of informed written consent. Regarding the qualitative key informant interviews, a waiver of signed informed written consent was granted as well, as their verbal agreement will be accepted as implied consent. To ensure confidentiality, all names will be removed from research records; no identifying information will be used in any report or publication. Data will only be presented in the aggregate. Data are kept under password protection on the Division of Research network.
3.9. Dissemination and resource sharing
The study team plans on disseminating the study results via multiple routes, i.e. publishing outcomes in peer-reviewed international journals, presenting findings at conferences, regional meetings and KPNC-internal seminars. If proven feasible, acceptable and successful, there is potential for much broader adoption within KPNC; i.e., across medical centers.
The following approach will be used to maximize the utility of the analytic datasets that will be developed through the project in compliance with the National Institutes of Health (NIH) Data Sharing Policy. The analytical datasets will include patient and provider level data from the KPNC EHR, administrative and clinical databases. External investigators can contact the study PI to initiate a request for study data to support new study proposals or manuscripts. Approved requests will consider data sharing agreements between KPNC and NIH.
4. Discussion
The innovative telehealth intervention, ATC, aims to overcome barriers to treatment of alcohol use problems– including patient-, provider-, and systems-level limitations, and to provide evidence-based interventions such as MI and alcohol pharmacotherapy provided by clinical pharmacists in a primary care setting. The trial intervention was tested in a pilot study and showed feasibility, acceptability, and promising results regarding medication prescription and treatment initiation rates; but used addiction medicine consultants rather than pharmacists [52]. Feedback from physicians and stakeholders informed the current study approach that combines technological and clinical innovation with scientific rigor, offering the most promising treatment interventions to as many primary care patients as possible. The study is population-based and will include patients typically excluded from traditional randomized clinical trials because of selection/consent criteria.
Thus, this pragmatic trial will yield data of high external validity at the implementation level, i.e., prescriptions/referrals to specialty treatment, and the patient level, i.e., medication fills/treatment initiation/healthcare utilization. It will also yield information about barriers and facilitators to treating alcohol use problems in primary care. The richness of quantitative and qualitative data from a large, diverse sample in a real-world primary care setting will yield an important contribution to the clinical community and healthcare systems. Specifically, the study will provide insight into how clinical pharmacists can be integrated into treatment of alcohol use problems in primary care.
The pragmatic trial has several limitations. Contamination between the ATC and UC arms is possible (e.g., physicians in UC might learn from colleagues about the benefits of the ATC and attempt to access it) but unlikely, as using the EHR-based ATC intervention referral system requires them to note the clinic they are referring from, enabling the study team to identify these attempts. Another limitation is that, as a pragmatic trial, analyses will be reliant on accurate documentation of alcohol use for some study outcomes. While there are no formal fidelity checks to monitor accurate data entry, KPNC’s alcohol screening initiative involves robust staff trainings with periodic boosters. Additionally, some KPNC members might fill prescriptions at an outside pharmacy. However, prior studies have found that over 90% of KPNC prescriptions are filled within KPNC [59], likely due to favorable pricing, and most transactions outside of KPNC are captured in the outside claims database. Another potential limitation is that the adherence measure is based on prescribing/dispensing, and this measure cannot capture patient noncompliance with taking medications [78]. However, adherence based on electronic pharmacy data has been shown to be associated with patient self-report of adherence [79], and has been used extensively [80, 81]. Finally, the study occurs in a private, integrated, not-for-profit healthcare system with an insured membership. With adaptation, the centralized ATC model could potentially be used by other systems, such as rural county health systems with little access to specialty addiction treatment, and thus findings will be broadly generalizable.
In summary, the ATC study will examine a pioneering approach that combines MI-based counseling, alcohol pharmacotherapy management and facilitated referrals to specialty treatment – all delivered via live consultation with clinical pharmacists. This intervention approach, if found to be effective, has potential for scalability, expanded access to evidence-based care for alcohol use problems, and cost savings, as many patients never seek specialty care, but are seen by their PCP. This trial has the potential to provide groundbreaking insights and impetus for the use of telemedicine to provide safe, effective pharmacotherapy for alcohol use problems and successful referral to specialty addiction treatment as a standard practice in primary care settings and could profoundly affect how we treat the spectrum of alcohol use problems.
Most primary care patients with alcohol problems do not receive treatment
Evidence-based pharmacotherapy for alcohol use problems is rare in primary care
The ATC is an innovative approach to addressing alcohol problems in primary care
Pharmacists may enhance primary care’s capacity to care for alcohol problems
If effective, ATC has potential for large-scale dissemination and implementation
Acknowledgements:
Funding for the study was received by the NIH/NIAAA (R01 AA028211; PI Dr. Stacy Sterling, Kaiser Permanente Northern California). Dr. Satre was supported by K24 AA025703.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration: This study has been registered on ClinicalTrials.gov (NCT05252221).
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Praud D, Rota M, Rehm J, et al. , Cancer incidence and mortality attributable to alcohol consumption, Int. J. Cancer 138(6) (2016) 1380–7. [DOI] [PubMed] [Google Scholar]
- 2.Ricci C, Wood A, Muller D, et al. , Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study, BMJ 361 (2018) k934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood AM, Kaptoge S, Butterworth AS, et al. , Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies, Lancet 391(10129) (2018) 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams R, Alexander G, Armstrong I, et al. , Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK, Lancet 391(10125) (2018) 1097–1107. [DOI] [PubMed] [Google Scholar]
- 5.Mundt MP, Zakletskaia LI, Prevention for college students who suffer alcohol-induced blackouts could deter high-cost emergency department visits, Health Aff. (Millwood) 31(4) (2012) 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global status report on alcohol and health 2018, 2018. https://www.who.int/publications/i/item/9789241565639#:~:text=Global%20status%20report%20on%20alcohol%20and%20health%202018.,three%20quarters%20of%20these%20deaths%20were%20among%20men.
- 7.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Alcohol use and your health, Last reviewed 14 April 2022. https://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm.
- 8.McHugh RK, Weiss RD, Alcohol use disorder and depressive disorders, Alc. Res 40(1) (2019) e1097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo-Carniglia A, Keyes KM, Hasin DS, et al. , Psychiatric comorbidities in alcohol use disorder, Lancet Psychiatry 6(12) (2019) 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services, Office of the Surgeon General, Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health, U.S. Department of Health & Human Services, Washington, DC, 2016. [PubMed] [Google Scholar]
- 11.Miquel L, Manthey J, Rehm J, et al. , Risky alcohol use: the impact on health service use, Eur. Addict. Res 24(5) (2018) 234–244. [DOI] [PubMed] [Google Scholar]
- 12.Verelst S, Moonen PJ, Desruelles D, et al. , Emergency department visits due to alcohol intoxication: characteristics of patients and impact on the emergency room, Alcohol Alcohol 47(4) (2012)433–8. [DOI] [PubMed] [Google Scholar]
- 13.Stockwell T, Andreasson S, Cherpitel C, et al. , The burden of alcohol on health care during COVID-19, Drug Alcohol Rev. 40(1) (2021) 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen KB, Nordentoft M, Hjorthoj C, Association between alcohol and substance use disorders and psychiatric service use in patients with severe mental illness: a nationwide Danish register-based cohort study, Psychol. Med 48(15) (2018) 2592–2600. [DOI] [PubMed] [Google Scholar]
- 15.Pettinati HM, O’Brien CP, Rabinowitz AR, et al. , The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking, J. Clin. Psychopharmacol 26(6) (2006) 610–25. [DOI] [PubMed] [Google Scholar]
- 16.McCollister KE, French MT, Pyne JM, et al. , The cost of treating addiction from the client’s perspective: results from a multi-modality application of the Client DATCAP, Drug Alcohol Depend. 104(3) (2009) 241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ait-Daoud N, Blevins D, Khanna S, et al. , Women and addiction: an update, Med. Clin. North Am 103(4) (2019) 699–711. [DOI] [PubMed] [Google Scholar]
- 18.Le Berre AP, Vabret F, Cauvin C, et al. , Cognitive barriers to readiness to change in alcohol-dependent patients, Alcohol. Clin. Exp. Res 36(9) (2012) 1542–9. [DOI] [PubMed] [Google Scholar]
- 19.May C, Nielsen AS, Bilberg R, Barriers to treatment for alcohol dependence, Journal of Alcohol and Drug Research 19 (2019) Article ID 236083. [Google Scholar]
- 20.Sattler S, Escande A, Racine E, et al. , Public stigma toward people with drug addiction: a factorial survey, J. Stud. Alcohol Drugs 78(3) (2017) 415–425. [DOI] [PubMed] [Google Scholar]
- 21.Wakeman SE, Rich JD, Barriers to medications for addiction treatment: how stigma kills, Subst. Use Misuse 53(2) (2018) 330–333. [DOI] [PubMed] [Google Scholar]
- 22.Aira M, Kauhanen J, Larivaara P, et al. , Factors influencing inquiry about patients’ alcohol consumption by primary health care physicians: qualitative semi-structured interview study, Fam. Pract 20(3) (2003) 270–5. [DOI] [PubMed] [Google Scholar]
- 23.Rahm AK, Boggs JM, Martin C, et al. , Facilitators and barriers to implementing Screening, Brief Intervention, and Referral to Treatment (SBIRT) in primary care in integrated health care settings, Subst. Abus 36(3) (2015) 281–8. [DOI] [PubMed] [Google Scholar]
- 24.Rosario F, Santos MI, Angus K, et al. , Factors influencing the implementation of screening and brief interventions for alcohol use in primary care practices: A systematic review using the COM-B system and Theoretical Domains Framework, Implement. Sci 16(1) (2021) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell A, Anderson P, Newbury-Birch D, et al. , The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews, Alcohol Alcohol 49(1) (2014) 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaner EF, Beyer FR, Muirhead C, et al. , Effectiveness of brief alcohol interventions in primary care populations, Cochrane Database Syst. Rev (2018) CD004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertholet N, Daeppen JB, Wietlisbach V, et al. , Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis, Arch. Intern. Med 165(9) (2005) 986–95. [DOI] [PubMed] [Google Scholar]
- 28.Fleming MF, Brief interventions and the treatment of alcohol use disorders: current evidence, Recent Dev. Alcohol 16 (2003) 375–90. [PubMed] [Google Scholar]
- 29.Cucciare MA, Coleman EA, Timko C, A conceptual model to facilitate transitions from primary care to specialty substance use disorder care: a review of the literature, Prim. Health Care Res. Dev 16(5) (2015) 492–505. [DOI] [PubMed] [Google Scholar]
- 30.Glass JE, Hamilton AM, Powell BJ, et al. , Specialty substance use disorder services following brief alcohol intervention: a meta-analysis of randomized controlled trials, Addiction 110(9) (2015) 1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitz R, Alcohol screening and brief intervention in primary care: Absence of evidence for efficacy in people with dependence or very heavy drinking, Drug Alcohol Rev. 29(6) (2010) 631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winslow BT, Onysko M, Hebert M, Medications for Alcohol Use Disorder, Am. Fam. Physician 93(6) (2016) 457–65. [PubMed] [Google Scholar]
- 33.Worley J, Review of evidence-based strategies to treat alcohol use disorder, J. Psychosoc. Nurs. Ment. Health Serv 59(12) (2021) 7–11. [DOI] [PubMed] [Google Scholar]
- 34.Garbutt JC, Kranzler HR, O’Malley SS, et al. , Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial, JAMA 293(13) (2005) 1617–25. [DOI] [PubMed] [Google Scholar]
- 35.Jonas DE, Amick HR, Feltner C, et al. , Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis, JAMA 311(18) (2014) 1889–900. [DOI] [PubMed] [Google Scholar]
- 36.Center for Substance Abuse Treatment, Incorporating alcohol pharmacotherapies into medical practice, Substance Abuse and Mental Health Services Administration (Treatment Improvement Protocol (TIP) Series 49. HHS Publication No. (SMA) 09-4380, Rockville, MD, 2009. [PubMed] [Google Scholar]
- 37.Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism, Medication for the treatment of alcohol use disorder: A brief guide, Substance Abuse and Mental Health Services Administration, Rockville, MD, 2015. [Google Scholar]
- 38.Anton RF, Latham P, Voronin K, et al. , Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial, JAMA Intern. Med 180(5) (2020) 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnette EM, Nieto SJ, Grodin EN, et al. , Novel agents for the pharmacological treatment of alcohol use disorder, Drugs 82(3) (2022) 251–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manhapra A, Chakraborty A, Arias AJ, Topiramate pharmacotherapy for alcohol use disorder and other addictions: a narrative review, J Addict Med 13(1) (2019) 7–22. [DOI] [PubMed] [Google Scholar]
- 41.Han B, Jones CM, Einstein EB, et al. , Use of medications for alcohol use disorder in the US: Results from the 2019 National Survey on Drug Use and Health, JAMA Psychiatry 71(5) (2021) 640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joudrey PJ, Kladney M, Cunningham CO, et al. , Primary care engagement is associated with increased pharmacotherapy prescribing for alcohol use disorder (AUD), Addict. Sci. Clin. Pract 14(1) (2019) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams EC, Matson TE, Harris AHS, Strategies to increase implementation of pharmacotherapy for alcohol use disorders: a structured review of care delivery and implementation interventions, Addict. Sci. Clin. Pract 14(1) (2019) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford JH 2nd, Abraham AJ, Lupulescu-Mann N, et al. , Promoting adoption of medication for opioid and alcohol use disorders through system change, J Stud Alcohol Drugs 78(5) (2017) 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris AHS, Brown R, Dawes M, et al. , Effects of a multifaceted implementation intervention to increase utilization of pharmacological treatments for alcohol use disorders in the US Veterans Health Administration, J. Subst. Abuse Treat 82 (2017) 107–112. [DOI] [PubMed] [Google Scholar]
- 46.Ornstein SM, Miller PM, Wessell AM, et al. , Integration and sustainability of alcohol screening, brief intervention, and pharmacotherapy in primary care settings, J. Stud. Alcohol Drugs 74(4) (2013) 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muradian IK, Aminzadeh A, Lin CH, et al. , Clinical pharmacist’s role in an alcohol detox unit in a correctional setting, J. Pharm. Pract 34(4) (2021) 592–595. [DOI] [PubMed] [Google Scholar]
- 48.Smith A, Hansen J, Colvard M, Impact of a pharmacist-led substance use disorder transitions of care clinic on postdischarge medication treatment retention, J. Subst. Abuse Treat 130(8) (2021) 108440. [DOI] [PubMed] [Google Scholar]
- 49.Hattingh HL, Tait RJ, Pharmacy-based alcohol-misuse services: current perspectives, Integ. Pharm. Res. Pract 7(7) (2017) 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palzes VA, Weisner C, Chi FW, et al. , The Kaiser Permanente Northern California Adult Alcohol Registry, an electronic health records-based registry of patients with alcohol problems: Development and implementation, JMIR Med. Inform 8(7) (2020) e19081.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sterling SA, Palzes VA, Lu Y, et al. , Associations between medical conditions and alcohol consumption levels in an adult primary care population, JAMA Netw. Open 3(5) (2020) e204687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leibowitz A, Satre DD, Lu W, et al. , A telemedicine approach to increase treatment of alcohol use disorder in primary care: A pilot feasibility study, J. Addict. Med 15(1) (2021) 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Institute on Alcohol Abuse and Alcoholism, Helping patients who drink too much: a clinician’s guide, 2005, updated 2007. [Google Scholar]
- 54.World Health Organization, International Statistical Classification of Diseases and Related Health Problems: 10th Revision (ICD-10), 5th ed., World Health Organization, Geneva, Switzerland, 2016. [Google Scholar]
- 55.Parthasarathy S, Weisner C, Hu TW, et al. , Association of outpatient alcohol and drug treatment with health care utilization and cost: revisiting the offset hypothesis, J. Stud. Alcohol Drugs 62(1) (2001) 89–97. [DOI] [PubMed] [Google Scholar]
- 56.Ray GT, Collin F, Lieu T, et al. The cost of health conditions in a health maintenance organization, Med. Care Res. Rev 57(1) (2000) 92–109. [DOI] [PubMed] [Google Scholar]
- 57.Ray GT, Mertens JR, Weisner C, The excess medical cost and health problems of family members of persons diagnosed with alcohol or drug problems, Med. Care 45(2) (2007) 116–22. [DOI] [PubMed] [Google Scholar]
- 58.Mertens JR, Lu YW, Parthasarathy S, et al. , Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls, Arch. Intern. Med 163(20) (2003)2511–7. [DOI] [PubMed] [Google Scholar]
- 59.Selby JV, Smith DH, Johnson ES, et al. , Kaiser Permanente Medical Care Program., in: Strom BL (Ed.), Pharmacoepidemiology, Wiley, New York, 2005, pp. 241–59. [Google Scholar]
- 60.CareSource, Hedis Measure: Initiation and Engagement of Alcohol and Other Drug Abuse or Dependence Treatment (IET), 2021. https://www.caresource.com/documents/ga-mcd-iet-hedis-measure/.
- 61.Smith PC, Schmidt SM, Allensworth-Davies D, et al. , Primary care validation of a single-question alcohol screening test, J. Gen. Intern. Med 24(7) (2009) 783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization, International classification of diseases: [9th] ninth revision, basic tabulation list with alphabetic index, 1978. https://apps.who.int/iris/handle/10665/39473
- 63.World Health Organization, ICD-10 : International statistical classification of diseases and related health problems: Tenth revision, 2nd ed., 2004. https://apps.who.int/iris/handle/10665/42980.
- 64.Sterling S, Jones A, Hinman A, et al. , A qualitative study of the barriers to and facilitators of implementation of Screening, Brief Intervention and Referral to Treatment for risky substance use for adolescents in pediatric primary care, Health Care Systems Research Network, Minneapolis, MN, 2018. [Google Scholar]
- 65.Kvale S, Brinkmann S, Interviews: Learning the Craft of Qualitative Research Interviewing, 2nd ed., Sage, Thousand Oaks, CA, 2009. [Google Scholar]
- 66.Lu Y, Chi FW, Parthasarathy S, et al. , Patient and provider factors associated with receipt and delivery of brief interventions for unhealthy alcohol use in primary care, Alcohol. Clin. Exp. Res 45(10) (2021) 2179–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayati Rezvan P, Lee KJ, Simpson JA, The rise of multiple imputation: a review of the reporting and implementation of the method in medical research, BMC Med. Res. Methodol 15 (2015) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. , Missing data and multiple imputation in clinical epidemiological research, Clin. Epidemiol 9 (2017) 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.empty, 2022. [Google Scholar]
- 70.Bryk AS, Raudenbush SW, Hierarchical Linear Models: Applications and Data Analysis Methods, Sage, Newbury Park, 1992. [Google Scholar]
- 71.Hsieh HF, Shannon SE, Three approaches to qualitative content analysis, Qual. Health Res 15(9) (2005) 1277–88. [DOI] [PubMed] [Google Scholar]
- 72.QSR International, NVivo 10 [software program], Version 10, 2012. [Google Scholar]
- 73.O’Cathain A, Murphy E, Nicholl J, Three techniques for integrating data in mixed methods studies, BMJ 341 (2010) c4587. [DOI] [PubMed] [Google Scholar]
- 74.Teerenstra S, Moerbeek M, van Achterberg T, et al. , Sample size calculations for 3-level cluster randomized trials, Clin. Trials 5(5) (2008) 486–95. [DOI] [PubMed] [Google Scholar]
- 75.Cohen J, Statistical Power Analysis for the Behavioral Sciences, 2nd ed., Lawrence Erlbaum Associates, Inc., Hillsdale, NJ, 1988. [Google Scholar]
- 76.Barrientos-Gutierrez T, Gimeno D, Mangione TW, et al. , Drinking social norms and drinking behaviours: a multilevel analysis of 137 workgroups in 16 worksites, Occup. Environ. Med 64(9) (2007) 602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cosby RH, Howard M, Kaczorowski J, et al. , Randomizing patients by family practice: sample size estimation, intracluster correlation and data analysis, Fam. Pract 20(1) (2003) 77–82. [DOI] [PubMed] [Google Scholar]
- 78.Andrade SE, Kahler KH, Frech F, et al. , Methods for evaluation of medication adherence and persistence using automated databases, Pharmacoepidemiol Drug Saf. 15(8) (2006) 565–74; discussion 575-7. [DOI] [PubMed] [Google Scholar]
- 79.McHorney CA, Victor Spain C, Alexander CM, et al. , Validity of the adherence estimator in the prediction of 9-month persistence with medications prescribed for chronic diseases: a prospective analysis of data from pharmacy claims, Clin. Ther 31(11) (2009) 2584–607. [DOI] [PubMed] [Google Scholar]
- 80.Adams AS, Uratsu C, Dyer W, et al. , Health system factors and antihypertensive adherence in a racially and ethnically diverse cohort of new users, JAMA Intern. Med 173(1) (2013) 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirkman MS, Rowan-Martin MT, Levin R, et al. , Determinants of adherence to diabetes medications: findings from a large pharmacy claims database, Diabetes Care 38(4) (2015) 604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


