Abstract
Chronic restraint stress is known to cause significant alterations of mitochondrial biology. However, its effects on effort-based behavior and the sensitivity of these effects to treatments that restore mitochondrial function have not been assessed. Based on the hypothesis that the behavioral consequences of this stressor should be more severe for an energy demanding activity than for an energy procuring activity, we compared the effects of chronic restraint stress on the performance of male mice trained to use a running wheel or to nose poke for a food reward in an operant conditioning cage. In accordance with our hypothesis, we observed that exposure of mice to 2-hour daily restraint sessions for 14 to 16 days during the light phase of the cycle reliably decreased voluntary wheel running but had no effect on working for food in a fixed ratio 10 schedule of food reinforcement or in a progressive ratio schedule of food reinforcement. This dissociation between the two types of behavioral activities could reflect an adaptive response to the constraint imposed by chronic restraint stress on mitochondria function and its negative consequences on energy metabolism. To determine whether it is the case, we administered mesenchymal stem cells intranasally to chronically restrained mice to repair the putative mitochondrial dysfunction induced by chronic restraint stress. This intervention had no effect on wheel running deficits. Assessment of mitochondrial gene expression in the brain of mice submitted to chronic restraint stress revealed an increase in the expression of genes involved in mitochondrial biology that showed habituation with repetition of daily sessions of restraint stress. These original findings can be interpreted to indicate that chronic restraint stress induces behavioral and mitochondrial adjustments that contribute to metabolic adaptation to this stressor and maintain metabolic flexibility.
Introduction
Physical restraint is a commonly used procedure to induce acute or chronic stress in rodents and study its effects on anxiety- and depression-like behavior (Pare and Glavin 1986, Glavin, Pare et al. 1994, Buynitsky and Mostofsky 2009). It is used extensively to study the interactions among the hypothalamic-pituitary-adrenal axis, sympathetic nervous system, and the immune response to infection (Sheridan, Dobbs et al. 1998). The very first episode of physical restraint usually induces intense struggling associated with activation of main neural and endocrine systems. These responses show habituation with repetition of daily bouts of restraint (Konarska, Stewart et al. 1989). The systematic use of chronic restraint to study stress-induced behavioral and physiological disorders has pushed researchers to neglect the possibility that the changes observed in animals submitted to this procedure are adaptive rather than maladaptive. This is particularly apparent in the effects of physical restraint on energy metabolism and mitochondrial function. The energy demand that is imposed on the organism by physical restraint is met by an increased mobilization of energy substrates permitted by catecholamines and glucocorticoids. However, this needs to be accompanied by coordinated changes in shape and function of mitochondria, the energy powerhouses of cells (Picard, McEwen et al. 2018). Therefore, it not surprising that physical restraint enhances mitochondrial function at least acutely, when measured by oxygen consumption rate (Picard and McEwen 2018). In contrast, chronically prolonged physical restraint is associated with decreased mitochondrial energy production capacity and alterations in mitochondria morphology both in peripheral cells (Picard and McEwen 2018) and in the brain (Weger, Alpern et al. 2020). Whether this is sufficient to impact behavior by compromising energy metabolism is unknown.
From an energetic perspective it can be hypothesized that the organism is able to adapt to physical restraint as long as the coordinated interplay between stress hormones and mitochondria takes place. The failure of this coordinated process would be the signature of pathology. In other terms, maladaptive or pathological responses to chronic restraint stress should be associated with and possibly even be the consequence of mitochondrial dysfunction.
The present set of experiments was initiated to test the hypothesis that a moderate form of chronic restraint should lead to a set of coordinated behavioral and mitochondrial adaptive responses to the increased energy demands it is associated with. At the behavioral level, chronic restraint should reduce investment in energy consuming activities while sparing energy procuring activities. To test this possibility, we compared wheel running, an energy-dependent activity, to food-motivated behavior, an energy procuring activity, that we have already shown to be differentially affected by the increased energy demand imposed on the organism by tumor growth in mice (Grossberg, Vichaya et al. 2018). At the mitochondrial level, we hypothesized that the expected deficit in wheel running induced by chronic restraint should not be alleviated by interventions that repair damaged mitochondria as long as mitochondria are not damaged and can still sustain the increased energy demand induced by metabolic stress. To test this possibility, we used an intervention represented by intranasal administration of mesenchymal stem cells. Mesenchymal stem cells have emerged as potential treatments for cellular repair and regeneration thanks to their ability to release immunomodulatory factors, micro-vesicles, and microRNAs, and to transfer healthy mitochondria to damaged cells (Donega, Nijboer et al. 2014, Paliwal, Chaudhuri et al. 2018, Fu, Liu et al. 2019, Li, Gong et al. 2019). We have already demonstrated that intranasal administration of mesenchymal stem cells alleviates the decreased oxygen consumption rate of mitochondria in synaptosomes collected from the brain of mice treated with the chemotherapeutic agent cisplatin and restores cognitive function probably via transfer of healthy mitochondria (Boukelmoune, Chiu et al. 2018, Chiu, Boukelmoune et al. 2018, Alexander, Seua et al. 2021).
The present set of experiments shows that chronic restraint stress decreases voluntary wheel running activity but does not impair the effort necessary to engage in food-motived behavior. This dissociation does not appear to be dependent on mitochondrial dysfunction as intranasal administration of mesenchymal stem cells does not alleviate the impairment in wheel running induced by chronic restraint. It is more likely to reflect adaptive behavioral responses to the metabolic constraints imposed by chronic restraint stress.
1. Animals and methods
1.1. Animals
The experiments were carried out on C57BL/6J mice (Stock No: 000664) bought from Jackson Labs. All mice were aged 12 weeks prior to the start of the experiments. They were singly housed in a temperature- and humidity-controlled environment with a 12-hr light/dark cycle and given ad libitum access to rodent chow (PicoLab Rodent Diet 20– 5053) and water unless otherwise stated. All animal protocols followed recommendations of the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas MD Anderson Cancer Center. Only male mice were used in the present experiments as the objective was not to investigate possible sex differences in the response to chronic restraint stress but to determine whether coordinated behavioral and metabolic adjustments take place in response to this form of stress.
1.2. Physical restraint
Mice were submitted to a repeated schedule of physical restraint in small mouse restraint tubes (MH-100, IBI Scientific, Dubuque, Iowa). Mice were inserted into the restraint tubes tail-first before adjustment of the nose restraint at a length allowing each mouse to fully extend in the tube. Mice were restrained in the same tube and at the same length each day. Mice were restrained in their home cage for 2 hours per day, during which their breathing was monitored. Restraint tubes were washed with non-scented soap and water after use. Unless specified otherwise, restraint was repeated daily for 12–16 consecutive days.
1.3. Behavior
- Food-motivated behavior:
After one-week habituation to single housing, mice were weighed and food restricted with a limited amount of food to maintain their body weights to 80–90% of their original baseline, unless otherwise specified. Operant conditioning chambers were housed within a sound attenuating cabinet (ENV-022MD, Med Associates, Fairfax VT) and were divided into two compartments, each with a nose-poke response unit and a reward unit (ENV-307W, Med Associates, Fairfax VT). Mice were trained to nose-poke to obtain 20 mg chocolate pellets (Bio-Serv, Flemington NJ) in daily 20-min sessions according to either a fixed ratio 10 schedule of food reinforcement (10 consecutive nose pokes for 1 chocolate pellet) or a progressive ratio schedule of food reinforcement in which the number of nose pokes to obtain a reward increased gradually within a 45-min session according to a rate of PR = 5e(R*0.2) – 5 (Grossberg et al., 2018). Mice were trained in the progressive ratio schedule of food reinforcement for one week before being submitted to chronic restraint. During the time of chronic restraint, they were tested 3 times a week 1 or 2 days apart in 40-min sessions. Performance was measured by the total number of nose pokes per session and by the breakpoint, i.e., the last ratio achieved before mice paused their responding for longer than 3 min.
- Voluntary wheel running:
After one-week habituation to single housing, mice were given continuous access to low-profile wireless running wheels (ENV-047, Med-Associates, Fairfax VT) in their home cage. Mice were allowed to train on the running wheels until they reached a stable baseline for 10–12 days before being submitted to chronic restraint. Wheel running behavior was measured by the number of wheel rotations over time and exported in 1-hour bins for analysis using wheel manager software (SOF-860).
1.4. Tissue collection and biochemical analyses
At the end of each experiment, mice were euthanized by CO2 inhalation. Blood was collected via cardiac puncture in EDTA washed 1mL syringes and stored in ice-chilled tubes until centrifugation (1,500g for 15 minutes at 4 degrees). Mice were perfused intracardially with physiological saline before collection of livers and brains which were immediately snap frozen in liquid nitrogen. Plasma and organ tissues were stored in ultracold (−80°) freezers. Frozen tissues were pulverized using a liquid nitrogen cooled mortar and pestle.
Quantitative real-time polymerase chain reaction (qRT-PCR):
RNA was extracted from powdered livers and brains using Trizol reagent (Invitrogen, Carlsbad CA) per manufacturer’s instruction. Extracted RNA was quality tested using micro-spot analysis with the Take3 plate system (Biotek, Winooski VT) per manufacturer’s instructions. cDNA was transcribed from RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems by Life Technologies, Waltham MA). PCR reactions were run on a CFX384 (BioRad, Hercules CA) plate reader using TaqMan Mastermix with the gene expression assays from Integrated DNA Technologies (IDT, Newark NJ). We selected Nfkb1 and Rela to assess activation of nuclear factor kappa B (Liu, Zhang et al. 2017), Il1, Tnf, Il6, Il10, Ifna, Ifnb and Ifng to assess activation of the cytokine network (Medzhitov 2021, Walker, Basisty et al. 2022), and Oas1a and Cxcl10 to assess involvement of a viral response in addition to type I interferon (Casanova and Abel 2021). We assessed mitochondrial biology by the expression of genes involved in mitochondrial regulation of energy metabolism (Sirt3), mitochondrial biogenesis and liver gluconeogenesis (Ppargc1a), mitochondrial toxicity (Snca), mitophagy (Pink1), mitochondrial fission (Dnm1l) and fusion (Mfn1, Mfn2), and stability of the mitochondrial genome (Tfam) (Scarpulla 2008, Adebayo, Singh et al. 2021, Malpartida, Williamson et al. 2021). Gdf15 is a mitokine produced by cells undergoing mitochondrial and endoplasmic reticulum stress (Breit, Brown et al. 2021). Gene expression of Tdo2 was used as a marker of glucocorticoid activity (Soichot, Vaast et al. 2013). Primer sequences are listed in Supplemental Table 1. Assays were conducted in duplicate with Rps3 and Eif3 as housekeeping genes. Relative expression was calculated with the ΔΔCt method and results were normalized to respective control groups.
1.5. Statistical analysis
All data are presented as mean +/− standard error of the mean (SEM). Data were analyzed with SPSS (version 26) and graphed using Prism 8 (GraphPad). One- or two-way analyses of variances (ANOVAs) with repeated measures on the time factor when needed were performed, with post hoc Bonferroni-adjusted t tests when applicable. Differences between groups were considered significant when p< 0.05.
1.6. Experimental design
Experiment I. Effects of chronic restraint on voluntary wheel running activity
Voluntary wheel running is expensive in terms of energy metabolism. Its performance depends on several factors including the amount of mitochondrial biogenesis in skeletal muscles and brain (Davis, Murphy et al. 2009). Because of the ability of chronic restraint to induce alterations in mitochondrial biology at the periphery and in the brain (Picard, McEwen et al. 2018), the present experiment was carried out to test the hypothesis that the constraint imposed by the stress response to chronic restraint on energy metabolism should be reflected by a decreased ability of stressed mice to engage in energy demanding activities. Mice were trained to run in a wheel for 2 weeks before being submitted or not to 14 consecutive daily chronic restraint sessions (n=8/group). Body weights were measured daily immediately before restraint.
Experiment II. Effects of chronic restraint on food-motivated behavior
The experiments on food-motivated behavior were carried out to determine whether the energy metabolic constraints of the stress response to chronic restraint also impair behavioral activities – nose poking in this particular case - that are not only less demanding in energy than wheel running but also procure energy. Mice maintained at 85% of their body weight were trained for 2–3 weeks on increasing fixed ratio (FR) schedules of food reinforcement requiring 1 then 5 and 10 consecutive nose pokes for each reward. Each daily session lasted 20 min. After stabilization of their performance in FR10 which was achieved after 2–3 weeks, they were submitted or not to daily chronic restraint for 14 days and tested for FR10 performance 3 times a week spaced one or two days apart. On days of behavioral testing FR10 sessions were run before the chronic restraint procedure. Each experimental group included 5 mice.
Experiment III. Role of food restriction in the resistance of food motivated behavior to chronic restraint
Food restriction or more exactly caloric restriction has well known mitigating effects on inflammatory responses (Matsuzaki, Kuwamura et al. 2001, Horrillo, Sierra et al. 2011) which could account for the resilience of food-restricted mice to the effects of chronic restraint on their food-motivated behavior. To test for this possibility, food-restricted mice were first trained to nose-poke for a chocolate pellet according to a fixed ratio 10 and then returned to a regimen of food ad libitum or maintained at about 85% of their body weight while being submitted to daily chronic restraint or no restraint for 20 days according to a 2 (ad libitum vs. food restricted) x 2 (restraint vs. no-restraint) factorial design (n=5/group). Food restriction was reinstated at the end of the chronic restraint period for those mice that were given food ad libitum and the total number of nose pokes was measured during the next 6 days during daily 20-min sessions.
Experiment IV. Effects of chronic restraint on performance in a progressive ratio schedule of food reinforcement
The fixed ratio schedule of food reinforcement has relatively low sensitivity to interventions especially when performance is maintained by schedules with a low ratio requirement (Sanger 1987). To modulate sensitivity of food motivated behavior to the effects of chronic restraint, we carried out an additional experiment based on performance in a progressive ratio schedule of food reinforcement (Hodos 1961). In a progressive ratio, the number of consecutive nose pokes needed to obtain a chocolate pellet progressively increases after each reward. Eventually, the mouse will reach a ratio at which the effort for getting a chocolate pellet is excessive in view of the rewarding value of the chocolate pellet. The ratio at which the mouse stops responding is defined as the breakpoint. This breakpoint serves as a measure of the motivation to obtain the reinforcer (Olarte-Sanchez, Valencia-Torres et al. 2015).
Food-restricted mice (n=7) were first trained to nose-poke for a chocolate pellet according to a fixed ratio 1 that was increased to a fixed ratio of 5 before being shifted to the progressive ratio schedule using the series 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95 and so on, derived from the following equation: response ratio = (5e(R x 0.2))-5 where R is the number of chocolate pellets already earned plus 1 (Sharma, Hryhorczuk et al. 2012, Grossberg, Vichaya et al. 2018). Progressive ratio sessions lasted 45 min. The breakpoint was calculated as the number of nose pokes that the mice needed to make for the last chocolate pellet before they paused for >=3 min. After mice achieved a stable performance in the progressive ratio schedule of food reinforcement, half of them were submitted to daily sessions of restraint for 14 days. During this time mice were tested in the progressive ratio schedule only 3 times a week.
Experiment V. Effects of intranasal injection of mesenchymal stem cells on wheel running deficit induced by chronic restraint
This experiment was carried out to determine whether intranasal injection of mesenchymal stem cells that treats mitochondrial dysfunction (Boukelmoune, Chiu et al. 2018, Alexander, Seua et al. 2021, Boukelmoune, Laumet et al. 2021, Gomzikova, James et al. 2021) alleviates deficits in wheel running induced by chronic restraint. Single housed mice were initially trained to run in a wheel for 13 days. Then they were submitted or not to 2 h of chronic restraint per day for 14 days and two 2-day cycles of intranasal injection of mesenchymal stem cells according to a 2 (+/− chronic restraint) x 2 (+/− mesenchymal stem cells) factorial design. Mice which failed to meet the baseline wheel running cutoff of 25,000 counts per night were excluded from the analysis, so that the number of mice for each group was 6–8. The protocol for culture of murine mesenchymal stem cells and their intranasal administration has already been described (Boukelmoune, Chiu et al. 2018). Briefly, murine mesenchymal stem cells were cultured in 5% CO2 at 37 °C in DMEM/F12 medium with GlutaMax-I, containing 10% MSC-qualified fetal bovine serum and 5 μg/mL gentamycin (all from GIBCO, Carlsbad, CA, USA). Cells were harvested using TrypLE-express (GIBCO). MSCs were positive for MSC-associated antigens CD29, CD44, CD73, CD105, CD106, Sca-1, and negative for hematopoietic markers CD11b and CD45. Before administration of mesenchymal stem cells, mice received 3 μl of hyaluronidase in PBS in each nostril (100 U per mouse, Sigma-Aldrich) to temporarily increase the permeability of the mucosa lining the nasal cavity. Mesenchymal stem cells or PBS were given to mice 30 min later, as two doses of 3 μl in each nostril, for a total of 12 μl and 1 × 106 cells per mouse per day for 2 consecutive days. The first series of treatment was given on the 7th and 8th day following the initiation of chronic restraint stress. The second series of mesenchymal stem cell treatment was identical to the first one but given on the 13th and 14th day of chronic restraint stress. Recovery was monitored for 10 days after the end of chronic restraint stress that took place on Day 14.
Experiment VI. Effects of physical restraint on mitochondrial biology and inflammation
In view of the lack of effect of intranasal administration of mesenchymal stem cells on deficits in wheel running induced by chronic restraint, the objective of this experiment was to determine whether the physical restraint regimen that was used in the present experiments was able to induces signs of mitochondrial dysfunction at the periphery and in the brain. In order to assess the time course of the mitochondrial response to restraint, 13-week old mice were submitted to no restraint or 2 h of chronic restraint stress per day from 1:00 to 3:00 PM for either 1 day, 7 days or 14 days after 1 week of individual housing. Body weight was measured every other day. All mice were euthanized on the same day to minimize variability. Mice were anesthetized by exposure to 2% CO2 24 h after the last session of restraint. Brains and livers were collected after intracardiac perfusion with phosphate buffer saline and snap frozen. Expression of genes coding for inflammation and mitochondrial dysfunction was assessed in the liver and brain by qRT-PCR. Each experimental group included 5 mice.
2. Results
Experiment I. Chronic restraint stress decreases voluntary wheel running
Experiment I was designed to determine whether chronic restraint stress impairs voluntary wheel running. The experimental design of this experiment is represented in Fig. 1A. Body weight of chronically restrained mice expressed as percentage of their initial body weight decreased during the first half of the chronic restraint procedure (Fig. 1B). However. this change did not reach significance due to high inter-individual differences in each group (treatment effect F(1,14)=1.02, NS; treatment x time interaction F(12,168)= 0.88, NS). In contrast, chronic restraint stress decreased wheel running activity expressed as percent of baseline (Fig. 1C) (treatment effect F(1,14)=16.5, p=0.001, treatment x time interaction F(13,182)=2.23, p=0.01). This decrease in wheel running activity did not persist after cessation of the chronic restraint stress procedure. These results indicate that chronic restraint stress decreases wheel running activity during the stress period but does not affect the physical condition of mice, at least based on their body weight.
Fig. 1 –
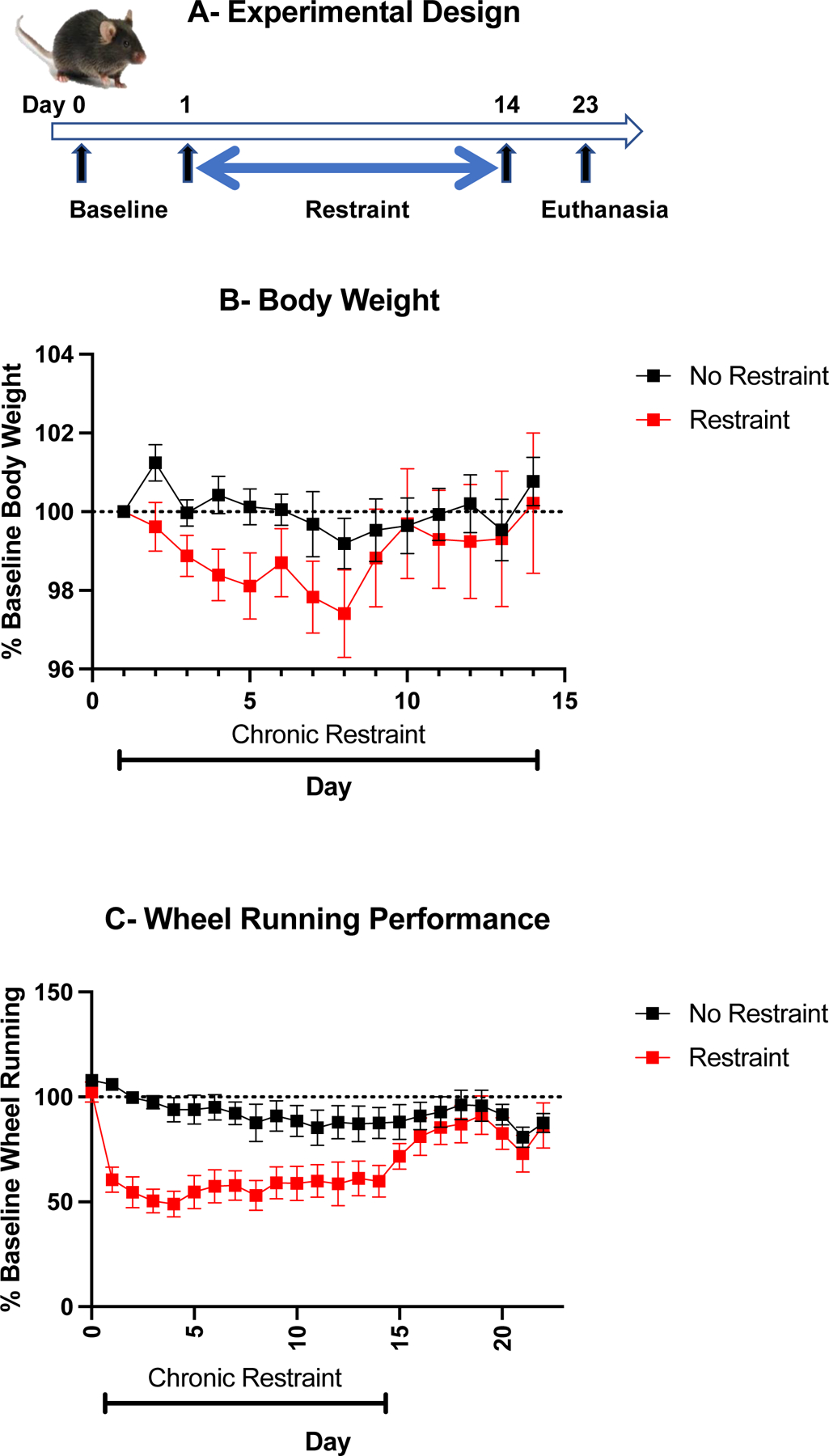
Chronic restraint stress decreases wheel running activity. (A) Experimental design. (B) Body weight expressed as percent of baseline. (C) Wheel running activity measured by number of total wheel revolutions during the night expressed as percent of baselin. Mean +/− standard errors of the mean, n=8 mice/group
Experiment II. Chronic restraint stress does not alter performance in a fixed ratio-10 schedule of food reinforcement
The experimental design of this experiment is summarized in Fig. 2A. In mice maintained at 85% of their initial body weight, a 2-way ANOVA (+/− restraint x 6 time points) on body weight expressed as percentage of baseline with repeated measures on the time factor showed that chronic restraint stress had no detrimental effect on body weight (restraint factor, F(1,8)=0.04, NS); restraint x time interaction F(5,40)=1.21, NS) (Fig. 2B). A 2-way ANOVA (+/− restraint x 7 time points) on the total number of nose pokes per session expressed as percentage of baseline with repeated measures on the time factor showed that the number of nose pokes did not differ between groups and was not modified by chronic restraint stress (restraint factor F(1,8)=1.07, NS; restraint x day F(5,40)=0.19, NS) (Fig. 2C). These results indicate that chronic restraint stress does not alter nose poking in a FR10 schedule of food reinforcement
Fig. 2 –
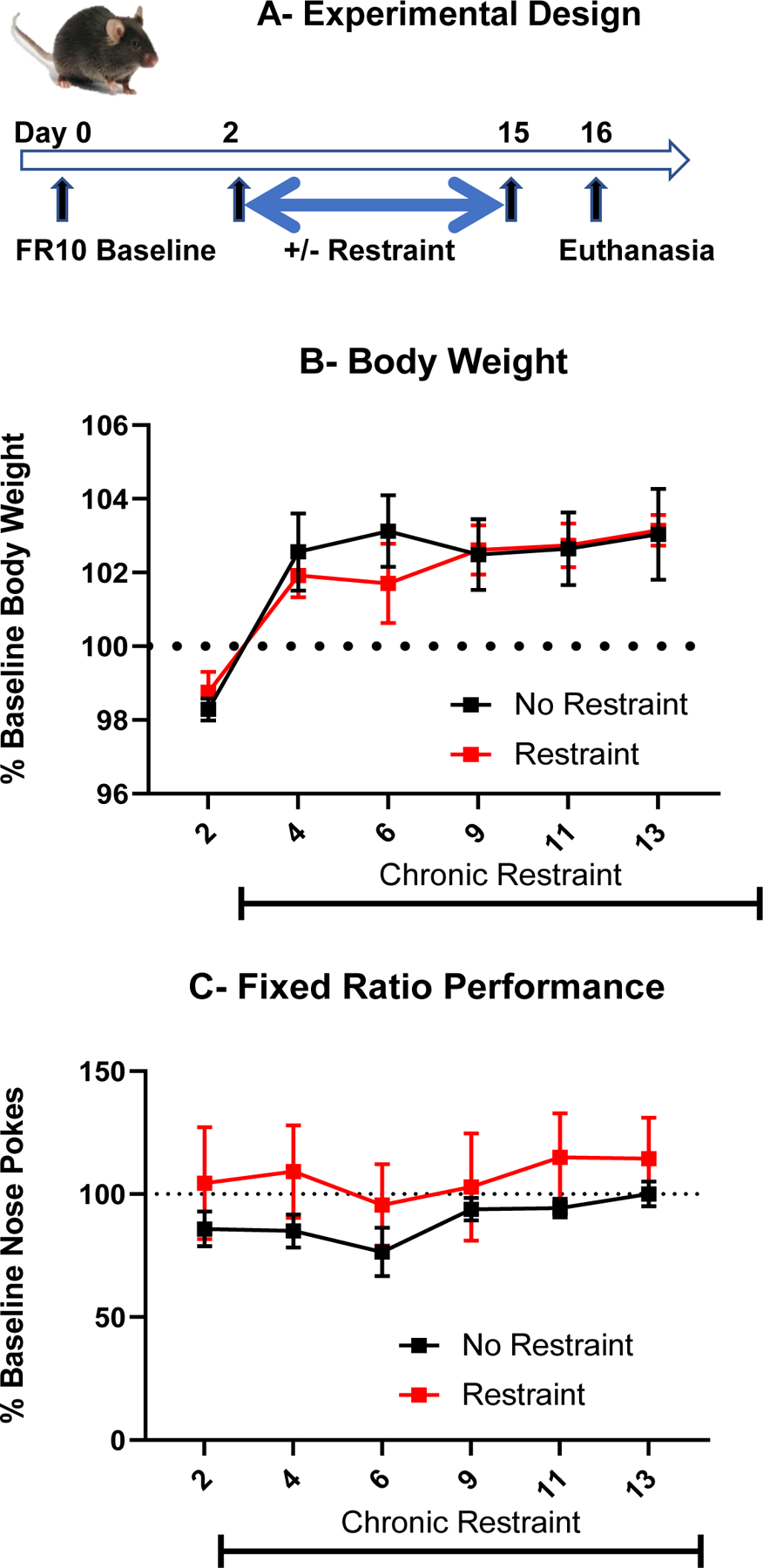
Chronic restraint stress does not alter food-motivated behavior. (A) Experimental design. (B) Body weight expressed as percent of baseline. (C) Food-motivated behavior measured by number of nose-pokes emitted during a 20 min session in mice trained to nose poke for 1 chocolate pellet according to a fixed ratio 10 schedule of food reinforcement before and during chronic restraint stress and expressed as percent of baseline.
Experiment III. Food restriction during chronic restraint is not responsible for the resistance of food-motivated behavior to chronic restraint stress
This experiment was designed to determine whether food restriction during chronic restraint stress makes mice resilient to the effect of restraint on food-motivated behavior. The experimental design of this experiment is represented in Fig. 3A. Nose poking performance was measured only after exposure to chronic restraint stress as fully sated mice were no longer engaging in nose poking for food.
Fig. 3 –
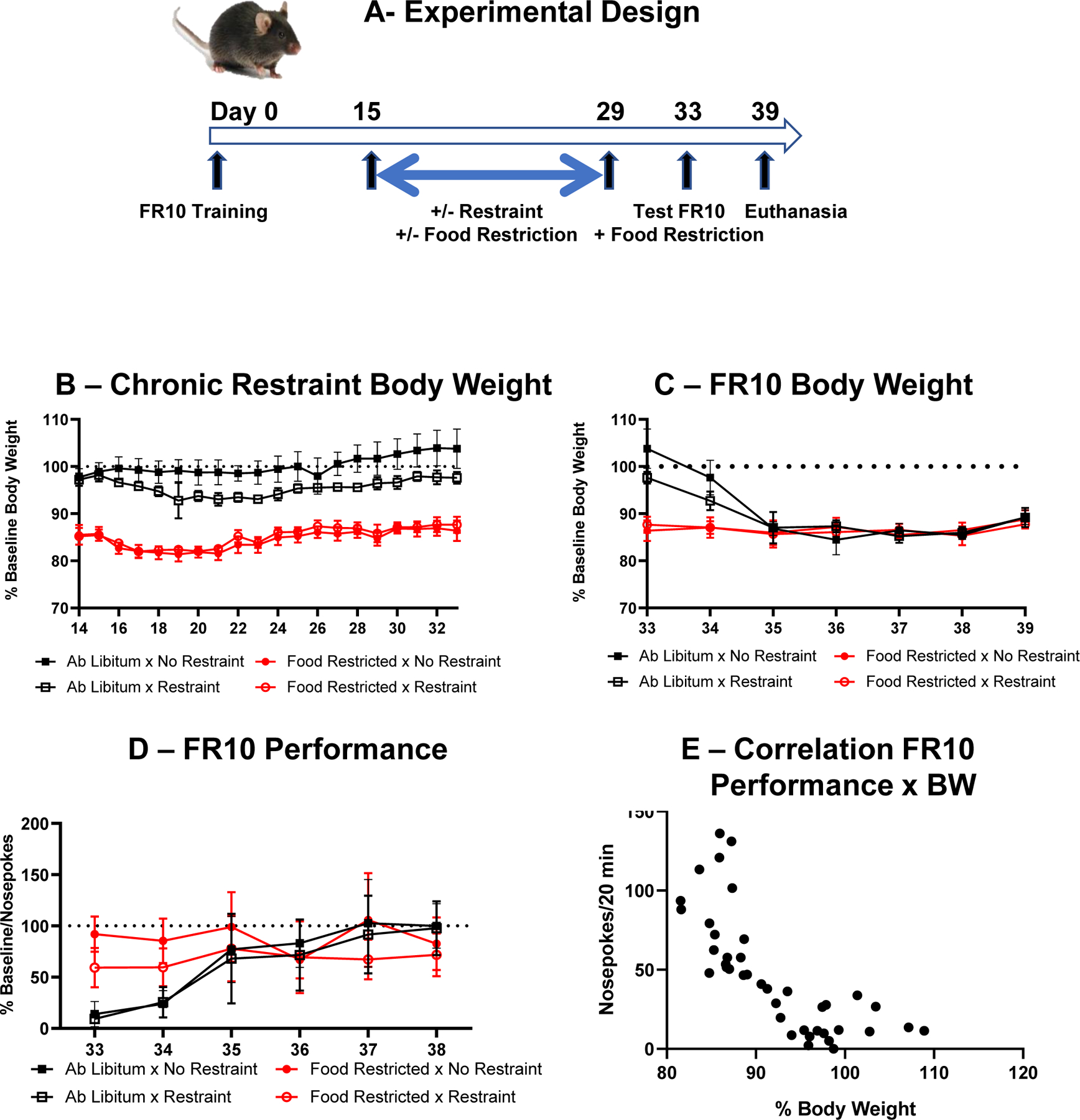
Food restriction does not account for the lack of effect of chronic restraint stress on food motived behavior. (A) Experimental design. (B) Effects of chronic restraint stress on body weight expressed as percent of baseline in mice submitted or not to food restriction. (C) Residual effects of chronic restraint stress on body weight expressed as percent of body weight in mice submitted or not to food restriction during chronic restraint stress and submitted again to food restriction from Day 33. (D) Effects of chronic restraint stress on FR10 performance expressed as percent of baseline in mice submitted or not to food restriction during the days of chronic restraint stress and submitted again to food restriction from Day 33. (E) Relationship between number of nose pokes during the FR10 session and body weight expressed as percent of baseline during the four daily sessions run on Day 33 to Day 36. Each point represents an individual mouse from the two groups that received food ad libitum during Day 15 to Day 29 and were submitted again to food restriction from Day 33.
The effects of chronic restraint stress on body weight during chronic restraint and after cessation of chronic restraint are represented in Fig. 3B and 3C respectively. A 3-way ANOVA (+/− restraint x feeding schedule x 20 time points) on body weight expressed as percent of baseline with repeated measures on the time factor shows a significant effect of feeding schedule (F(1,16)=387, p<0.001), restraint (F(1,16)=8.60, p=0.01) and a significant restraint x feeding schedule interaction (F(1,16)=16.2, p=0.001) indicating that body weight of non-food restricted mice submitted to chronic restraint stress decreased in response to restraint with the intensity of this effect varying according to time (restraint x feeding schedule x day interaction F(19,304)=3.86, p<0.001) (Fig. 3B). A 3-way ANOVA (+/− restraint x +/− feeding schedule x 7 time points) on body weight expressed as percent of baseline and measured during the 7 days following chronic restraint stress, with repeated measures on the time factor, shows a significant 3-way interaction (F(6,98)=5.58, p<0.001). Mice given food ad libitum during chronic restraint stress required 2 days after reinstatement of food restriction to reach 87% of their initial body weight (Fig. 3C).
Fig. 3D represents the residual effects of chronic restraint stress on FR10 performance. A 3-way ANOVA (+/− restraint x +/− feeding schedule x 6 time points) on the total number of nose pokes expressed as percent of baseline (average of last 3 days of training), with repeated measures on the time factor, shows a feeding schedule x time interaction (F(5,80)=14.5, p<0.001). Nose-poking in mice submitted to chronic restraint stress while fed ad libitum was significantly lower during the first 2 days of reinstatement of food restriction than that of mice submitted to chronic restraint stress while food restricted (p<0.001).
Fig. 3E represents the relationship between FR10 performance and body weight expressed as percentage of baseline. Each point represents percent body weight and FR10 performance for each mouse which was fed ad libitum during chronic restraint stress on each day of the period during which they were resubmitted to food restriction and FR10 testing (days 33 to 36 corresponding to the period during which their body weight went down from an average of 100 percent to 87 percent). The curvilinear regression between the number of nose pokes per session and percent body weight was highly significant (r = −0.81, p<0.001).
Taken together, the results of Experiment III indicate that food restriction rather than chronic restraint stress drives the performance of mice submitted to a fixed ratio of food reinforcement. This confirms that chronic restraint stress has no effect on food-motivated behavior in this task.
Experiment IV. Chronic restraint stress does not alter performance on a progressive ratio schedule of food reinforcement
This experiment was designed to determine whether the lack of effect of chronic restraint stress on food-motivated behavior was due to the relative lack of sensitivity of performance in the FR10 schedule of food reinforcement. The experimental design of this experiment is summarized in Fig. 4A. A 2-way ANOVA (+/− restraint x 6 time points) on body weight expressed as percent of baseline, with repeated measures on the time factor, showed that chronic restraint stress had no effect on body weights that were maintained around 85% of the free feeding body weight during the experiment (Fig. 4B) (restraint factor, F(1,5)=0.06, NS; restraint x time interaction, F(5,25)=0.65, NS). A 2-way ANOVA (+/− restraint x 7 time points) on the total number of nose pokes when mice reached the breakpoint, did not show any effect of the restraint and time factor nor their interaction (Fig. 4C) (restraint factor, F(1,5)=0.03, NS; time, F(5,25)=0.70, NS; restraint x day interaction, F(5,25)=2.02, NS). These results indicate that chronic restraint stress does not affect behavioral performance in a progressive ratio schedule of food reinforcement.
Fig. 4 –
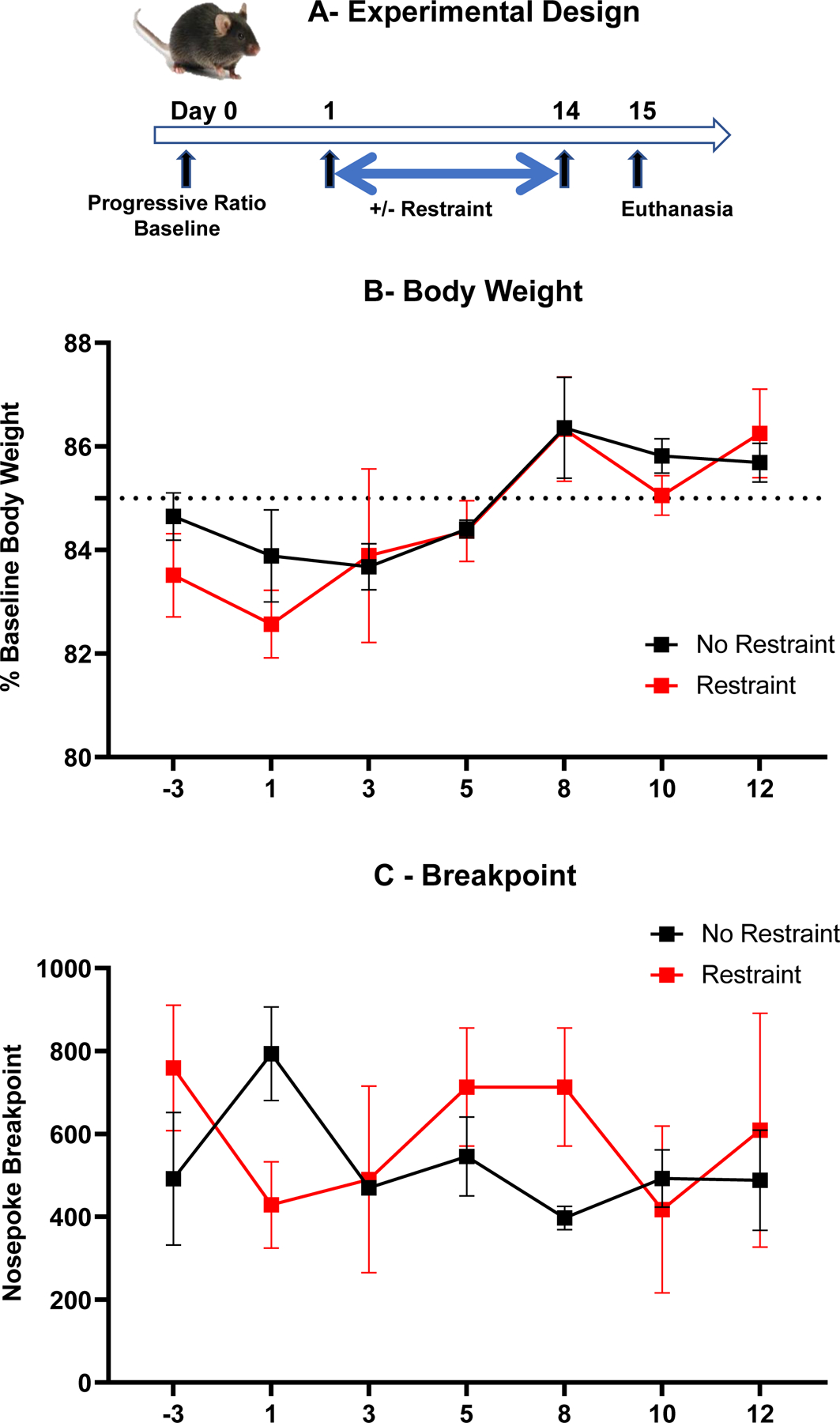
Chronic restraint stress does not alter performance in a progressive ratio schedule of food reinforcement. (A) Experimental design. (B) Body weight changes expressed as percent of baseline over time. (C) Total number of nose pokes per session when mice reached the breakpoint.
Experiment V. Intranasal administration of mesenchymal stem cells does not attenuate the wheel running deficit induced by chronic restraint stress
The objective of this experiment was to determine whether intranasal administration of mesenchymal stem cells that has been shown to alleviate behavioral consequences of mitochondrial dysfunction attenuates the detrimental effects of chronic restraint stress on wheel running. For this purpose, mice trained to run in a wheel were submitted or not to 14 daily sessions of chronic restraint stress. They were injected intranasally with MSC or the placebo twice at 24 h intervals beginning on day 7 and 13 of this stress procedure according to a 2 (+/− chronic restraint) x 2 (+/− MSC) factorial design with n=6–8/group. The experimental design is summarized in Fig. 5A. Body weights were available for all the mice only at baseline and on day 7 and 13 of treatment with MSC (Fig. 5B) and were analyzed accordingly. Chronic restraint stress and MSC had no effect on body weight expressed as percent of baseline (restraint: F(1,23)=0.6; MSC: F(1,23)=2.01) and there was no influence of time alone or in interaction with the main factors (time F(1,23)=0.54; time x restraint F(1,23)=0.43; time x MSC=0.37; time x restraint x MSC F(1,23)=1.38). Wheel running activity expressed as percent of baseline is represented in Fig. 5C. As expected, chronic restraint stress decreased wheel running activity (restraint F(1,23)=37.8, p<0.001). Mice treated with MSCs had a tendency to run more than mice receiving the placebo (MSC F(1,23)=4.02, p<0.10) but this difference was only present in mice that were not submitted to chronic restraint stress (restraint x MSC F(1,23)=4.06 p<0.10). However, this trend was not dependent on time as the interaction of time and MSC and of time x treatment x MSC did not reach significance (F(21,483)=0.75 and 0.44 respectively) whereas the effect of time and its interaction with restraint were significant (F(21,483)=5.73 p<0.001 and 4.47 p<0.001 respectively).
Fig. 5 –
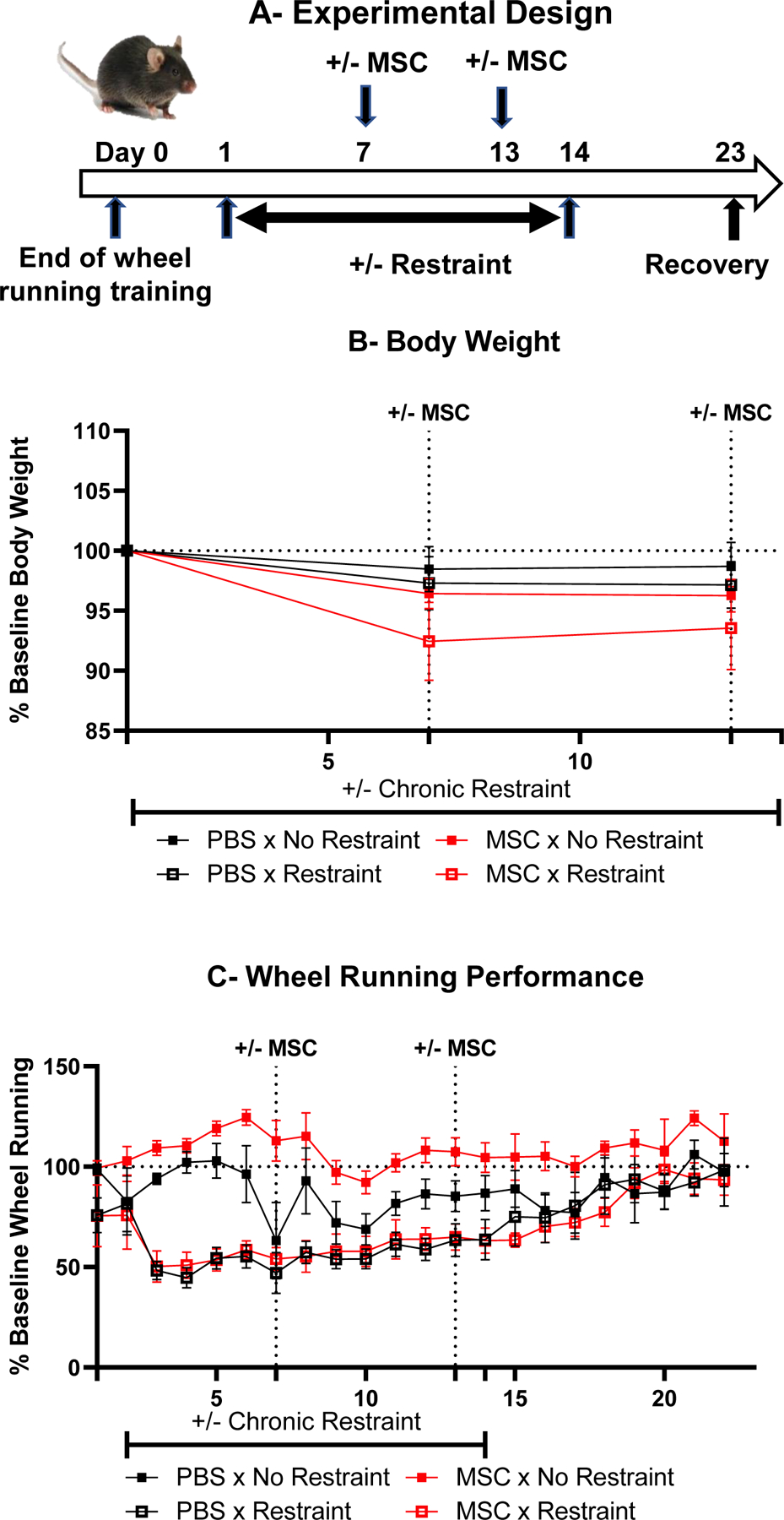
Intranasal administration of mesenchymal stem cells does not alleviate the negative effects of chronic restraint stress on wheel running activity in mice. (A) Experimental design. (B) Body weight changes expressed as percent of baseline over time. (C) Wheel running activity measured by number of total wheel revolutions during the night, expressed as percent of baseline.
These results indicate that administration of mesenchymal stem cells did not alter the time course of the effect of chronic restraint stress on wheel running activity both during the period of chronic restraint and after it.
Experiment VI. Effects of acute versus chronic restraint stress on inflammation and mitochondrial biology
Experiment VI was designed to determine whether physical restraint alters the expression of genes involved in mitochondrial biology and in inflammatory responses in the liver and brain. The experimental design of this experiment is represented in Fig. 6A. A 4 (4 experimental groups corresponding to no restraint, 1-day, 7-day and 14-day restraint) x 7 time points ANOVA on body weight changes over time (Fig. 6B) revealed a significant group factor (F(3,16)=4.99, p<0.05) and a significant group x time interaction (F(18,96)=4.06, p<0.001). Mice restrained for 7 or 14 days showed decreased body weight, with its maximum between 7 and 14 days for the 14-day restraint group.
Fig. 6 -.
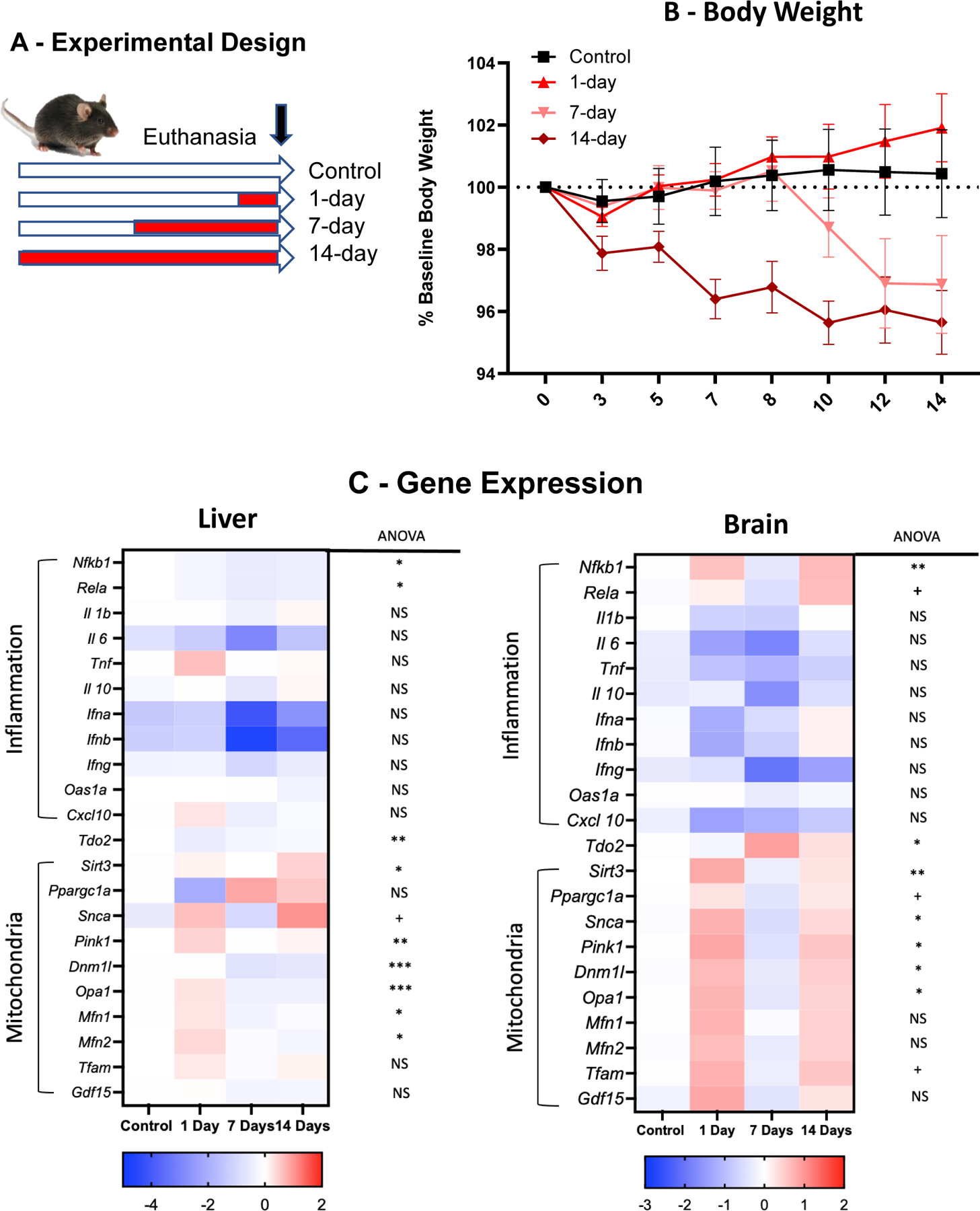
Effects of different durations of chronic restraint on body weight and gene expression in the liver and brain. (A) Experimental design. (B) Effects of restraint on body weight expressed as percent of baseline. Mice were weighed before physical restraint and 24 h after for mice submitted to acute restraint or every other day for mice submitted to 7- or 14-day chronic restraint. * p<0.05 compared to control mice. (C) Heat map of liver and brain gene expression for inflammatory and mitochondrial genes. Values represent averages of individual log2 transform of relative expression.
The effects of restraint on expression of inflammatory and mitochondrial genes in the livers and brains collected 24 after the last restraint session are presented in Fig. 6C with the results of the statistical analysis provided in Supplementary Table II. In the liver, the gene expression of inflammatory genes tended to decrease although this variation was not significant except for Nfkb1 and Rel5 after 7 and 14 sessions. In contrast, the expression of genes involved in mitochondrial biology tended to increase in response to one session of restraint and to decrease in response to 7 and 14 sessions. The increase was significant for Pink1 on day 1 and Sirt3 after 14 sessions of restraint. The decrease was significant for Opa1, Dnm1l and Mfn2 after 7 and 14 sessions of restraint and for Mfn1 after 7 sessions. In the brain expression of Nfkb1 and Rel5 was increased after 1 and 14 sessions. Gdf15 was also increased after 1 session of restraint. The decreased expression of brain cytokines did not reach significance. Expression of mitochondrial genes in the brain increased significantly after 1 session of restraint for Sirt3, Snca, Pink1, Dnm1l, and Opa1 but not for Mfn1 and Mfn2. This increase was no longer significant after 14 sessions of restraint although the overall change was in the same direction.
Taken together, the results of this experiment show that physical restraint decreases body weight and enhance mitochondrial biology in both the brain and liver. The effects on mitochondria were mainly present after 1 session of restraint and showed some evidence of habituation after 14 sessions of restraint.
Discussion
The results of the present series of experiments show that chronic restraint stress decreases voluntary wheel running but has no effect on food-motivated behavior measured in various schedules of food reinforcement. The dissociation between wheel running and food-motivated behavior was not due to the lack of sensitivity of fixed ratio performance as using a progressive ratio schedule of food reinforcement provided the same result. This dissociation between wheel running and food-motivated behavior was not caused by the food restriction schedule to which mice were submitted for measuring their food-motivated behavior. It indicates that chronic restraint does not impair wheel running by decreasing motivation. This dissociation has not been described before as most studies on the effects of chronic restraint on behavior focus on depression-like behavior measured by immobility in the forced swim test or the tail suspension test or by decreased sucrose preference. Although this last effect is usually interpreted as evidence of anhedonia, this interpretation is not compatible with the observation that chronically restrained rats increase their preference for comfort food under the influence of glucocorticoids (Pecoraro, Reyes et al. 2004). A possible explanation for this phenomenon is that the increase in ingestion of palatable preferred food attenuates the HPA axis response to stress (Foster, Warne et al. 2009). It could be speculated that the ability of mice to obtain a highly palatable and preferred food in the form of chocolate pellets would play the same role and therefore mice rewarded with chocolate pellets would be protected from the impairing effects of restraint stress on behavior. However, before further speculating on alternative explanations for the dissociation between the respective sensitivity of voluntary wheel running and food-motivated behavior to chronic restraint, it was important to check that food restriction per se during the chronic restraint period was not responsible for the lack of effect of chronic restraint stress on food-motivated behavior. This was done by comparing mice food restricted at 85% of their body weight and mice allowed food ad libitum during the period of chronic restraint stress. Food restriction was re-instated at the end of this period. Mice that were restrained while being fed ad libitum displayed a lower rate of nose poking than before initiation of chronic restraint. However, this was not due to their higher sensitivity to chronic restraint stress compared to mice with food ad libitum but to the fact they were not fully motivated as they had not yet reached 85% of their initial bodyweight. When this criterion was reached, nose poking performance no longer differed between mice exposed to chronic restraint stress while food-restricted and mice exposed to the same stress while being fed ad libitum. The other possibility represented by the relative lack of sensitivity of ratio schedules of food reinforcement with low ratio values to interventions was discarded by confirming that chronic restraint stress did not impair performance in a progressive ratio schedule of food reinforcement.
The selective decrease in nightly wheel running could be due to alterations in the sleep-wake cycle induced by restraint stress. Physical restraint of mice for 1 h during the light phase was shown to decrease rapid eye movement (REM) sleep after its cessation with a rebound during the dark phase of the cycle (Meerlo, Easton et al. 2001). There was little or no effect on non-REM sleep. The same effect was observed in rats when physical restraint was applied at the beginning of the dark phase or during the light phase (Rampin, Cespuglio et al. 1991, Cespuglio, Marinesco et al. 1995, Gargiulo, Jasodanand et al. 2021). However, this increase in REM sleep during the recovery phase showed rapid habituation with repetition of daily sessions of restraint stress, which explains why it is only studied in acute conditions (Rampin, Cespuglio et al. 1991, Cespuglio, Marinesco et al. 1995, Rachalski, Alexandre et al. 2009, Gargiulo, Jasodanand et al. 2021). As there was no evidence of habituation in the decrease in wheel running observed in mice submitted to chronic restraint stress, we can conclude that stress-induced alterations in sleep-wake cycle are unlikely to account for this effect of restraint stress. However, a definite answer to this question would still require a comparison of the time course of the effects of chronic restraint stress on the sleep-wake cycle with its effects on wheel running activity.
An alternative hypothesis for the dissociation between voluntary wheel running and food motivation is the possibility that the caloric restriction that is imposed to mice to keep them sufficiently motivated to work for a food reinforcement increases resilience by preserving mitochondrial function in face of excessive oxidative stress (Lanza, Zabielski et al. 2012). It is also possible that in a situation of compromised energy metabolism due to alteration in mitochondrial biology it is more important to engage in energy-procuring behavioral activities than in energy-consuming behavioral activities. The advantage of this last hypothesis is that it is testable by providing mice with sufficient energy before they engage in wheel running and by decreasing the caloric value of the reward in schedule of food motivation.
In more general terms, our behavioral results point to the importance of metabolic flexibility defined as an adaptive response of the organism’s physiology to maintain energy homeostasis by matching fuel availability and demand to availability of nutrients, physical activity, and environmental fluctuations (Smith, Soeters et al. 2018). This notion appears to be more appropriate to account for the diversity of behavioral responses to chronic restraint stress than the notion of stress that evokes an invariant non-specific response to the derangement of homeostasis.
Mitochondria are known to fulfill a crucial role in determining cellular, tissue, and systemic metabolic flexibility (Smith, Soeters et al. 2018). The mere occurrence of adaptive behavioral responses to the metabolic constraint imposed by chronic restraint stress on the organism is an indication that the mitochondrial alterations that are induced possibly by the chronic restraint schedule used in the present study are not sufficient to precipitate a state of metabolic inflexibility (Picard, McEwen et al. 2018). To test this possibility, we used an intervention already validated to repair brain injury in various traumatic conditions. This intervention consisted of administering mesenchymal stem cells intranasally. Such a treatment has already been shown to attenuate brain injury after neonatal stroke (van Velthoven, Sheldon et al. 2013), boost neurogenesis (Donega, van Velthoven et al. 2013), and reverse chemotherapy-induced cognitive deficits by replacing damaged mitochondria in neurons by healthy mitochondria (Alexander, Seua et al. 2021, Boukelmoune, Laumet et al. 2021). As administration of mesenchymal stem cells was unable to attenuate the decrease in wheel running induced by chronic restraint and to speed up recovery, in contrast to what was observed in situations of brain damage, we assessed the time course of mitochondrial alterations in response to chronic restraint stress. In addition to the brain in which most of the mitochondrial alterations in response to chronic restraint have been described (Picard and McEwen 2018), we selected the liver as a peripheral organ because it is an important organ for energy metabolism homeostasis due to its involvement in the lactate-glucose cycle used by skeletal muscles during physical exercise (Knudsen, Bienso et al. 2015). As we wanted to assess the effects of chronic restraint on both mitochondria biology and inflammation as a possible triggering factor for mitochondrial damage, we used real time PCR to measure expression of the main genes of interest in both organs collected at different time points during the chronic restraint procedure.
Most of the effects of chronic restraint stress were observed in the brain. Physical restraint increased the expression of genes coding for proteins involved in fission and fusion of mitochondria and in mitophagy. These changes were more marked in mice exposed to only 1 session of physical restraint than to 14 sessions, and they are consistent with what has been already reported in the literature even if the emphasis is usually on mitochondrial respiration rate (Picard and McEwen 2018).
Despite increased expression of brain Nfkb1and Rela, two genes coding for subunits of NFκB, there was no evidence for recruitment of the cytokine network by chronic restraint stress. It is generally agreed that chronic restraint stress induces low grade inflammation at the periphery and in the brain, with this inflammation being responsible for the depression-like behavior and the cognitive dysfunction that can be evidenced in chronically stressed animals (Himmerich, Fischer et al. 2013, Guo, Guo et al. 2014, Han, Yeo et al. 2015, Tan, Wang et al. 2017, Miller, Apple et al. 2019). However, this statement needs to be nuanced as there are large variations between laboratories in the protocols for chronic restraint stress and in the strain, age, and sex of the animals that are submitted to it. To blur even more the picture, most studies do not consider the time course of the immune response to restraint and just assess the immune response to chronic restraint stress at the end of the experiment once all behavioral tests have been completed. In addition, the peripheral inflammatory response is easier to evidence when it is measured by circulating levels of inflammatory cytokines rather than by gene expression of cytokines in specific brain areas. This is probably due to the fact that macrophage-dependent production of inflammatory cytokines is constrained by the potent activation of the hypothalamic-pituitary adrenal axis induced by physical restraint (Goujon, Parnet et al. 1995, Buynitsky and Mostofsky 2009). In addition, there is evidence that non-immune cells are involved in the production of stress-induced circulating cytokines such as IL-18 which is released from its precursor molecule in the adrenal gland in response to superoxide-mediated activation of caspase-1 (Sekiyama, Ueda et al. 2005). This might extend to other inflammatory cytokines such as IL-1α/β and TNF as they are produced by chromaffin cells within the adrenal medulla (Douglas, Sreenivasan et al. 2010), possibly via pituitary adenylate cyclase-activating polypeptide (PACAP) signaling (Eiden, Emery et al. 2018).
Concerning HPA axis activation, we did not measure circulating levels of corticosterone in the present study as this hormonal response to chronic restraint is already amply documented (Marin, Cruz et al. 2007, Delgado-Morales, del Rio et al. 2012, Son, Yang et al. 2019). As corticosterone induces Tdo2, we used this marker as a proxy for activation of the HPA axis. However, there was only limited evidence of induction of Tdo2, which could be due to the fact that the response of Tdo2 to increased corticosterone is short-lived as it peaks at 2 hours after restraint and is no longer apparent 24 h later (Gibney, Fagan et al. 2014, Dostal, Carson Sulzer et al. 2017). The importance of HPA axis activation in the effects of chronic restraint stress is further illustrated by the already reported observation that the body weight loss that is induced by chronic restraint stress is attenuated in adrenalectomized rats exposed to 3 h of restraint for 3 days and it reoccurs in restrained adrenalectomized rats injected with corticosterone (Scherer, Holmes et al. 2011).
The present study has a number of limitations. The main one is we did not specify the exact nature of the metabolic deficiency that leads mice running in a wheel to limit their activity while still engaging in an effort in order to get access to energy rich food pellets. This clearly requires further investigation on the metabolic consequences of chronic restraint stress. Another limitation is the lack of comparison of the relatively mild schedule of chronic restraint stress we used to a more severe schedule that could have resulted in frank pathological alterations in behavior and mitochondrial function. This would have allowed us to delineate the transition from metabolic flexibility to metabolic inflexibility. Other limitations are represented by the consideration of alterations in gene expression of cytokines and mitochondria biology at the organ rather than at the cellular level. Concerning the use of mesenchymal stem cells, we did not verify that the cells that were injected intranasally reached their target in the brain and we did not check the consequences of this intervention on brain mitochondria. However, we have shown that MSC when injected nasally, do not enter the brain parenchyma but can be detected in the meningeal compartment to resolve peripheral and brain mitochondrial function. (Boukelmoune, Chiu et al. 2018, Chiu, Boukelmoune et al. 2018, Boukelmoune, Laumet et al. 2021).
Despite these limitations, it can be concluded that the present experiments demonstrate for the first time a dissociation between the effects of chronic restraint stress on voluntary wheel running and those on food-motivated behavior. This dissociation is likely due to the constraints on energy metabolism caused by chronic restraint stress, and it attests that metabolic flexibility and mitochondria biology are not irreversibly affected by the procedure of chronic restraint stress used in the present study. This is confirmed by the lack of effect of intranasal administration of mesenchymal stem cells on the decrease in wheel running activity induced by chronic restraint stress, which is in general agreement with the lack of drastic alterations in the gene expression of mitochondria genes in the brain of chronically restrained mice.
Supplementary Material
Highlights.
Chronic restraint stress decreased wheel running but had no effect on food-motivated behavior in male mice
Intranasal administration of mesenchymal stem cells to repair possible mitochondrial dysfunction induced by chronic restraint stress did not alleviate wheel running deficits
Acute restraint stress enhanced expression of brain mitochondrial genes but this effect showed evidence of habituation in mice submitted to chronic restraint stress
These findings indicate that chronic restraint stress induces behavioral and mitochondrial responses that facilitate adaptation to the metabolic constraints of this procedure
Funding;
This research was supported by the National Institute of Health (R01 CA193522, R01 NS073939) and an MD Anderson Cancer Center Support Grant (P30 CA016672)
Footnotes
Declaration of Competing Interest: The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adebayo M, Singh S, Singh AP and Dasgupta S (2021). “Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis.” FASEB J 35(6): e21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JF, Seua AV, Arroyo LD, Ray PR, Wangzhou A, Heibeta-Luckemann L, Schedlowski M, Price TJ, Kavelaars A and Heijnen CJ (2021). “Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits.” Theranostics 11(7): 3109–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JF, Seua AV, Arroyo LD, Ray PR, Wangzhou A, Heiss-Luckemann L, Schedlowski M, Price TJ, Kavelaars A and Heijnen CJ (2021). “Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits.” Theranostics 11(7): 3109–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukelmoune N, Chiu GS, Kavelaars A and Heijnen CJ (2018). “Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin.” Acta Neuropathol Commun 6(1): 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukelmoune N, Laumet G, Tang Y, Ma J, Mahant I, Singh SK, Nijboer C, Benders M, Kavelaars A and Heijnen CJ (2021). “Nasal administration of mesenchymal stem cells reverses chemotherapy-induced peripheral neuropathy in mice.” Brain Behav Immun 93: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit SN, Brown DA and Tsai VW (2021). “The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe?” Annu Rev Physiol 83: 127–151. [DOI] [PubMed] [Google Scholar]
- Buynitsky T and Mostofsky DI (2009). “Restraint stress in biobehavioral research: Recent developments.” Neurosci Biobehav Rev 33(7): 1089–1098. [DOI] [PubMed] [Google Scholar]
- Casanova JL and Abel L (2021). “Mechanisms of viral inflammation and disease in humans.” Science 374(6571): 1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespuglio R, Marinesco S, Baubet V, Bonnet C and el Kafi B (1995). “Evidence for a sleep-promoting influence of stress.” Adv Neuroimmunol 5(2): 145–154. [DOI] [PubMed] [Google Scholar]
- Chiu GS, Boukelmoune N, Chiang ACA, Peng B, Rao V, Kingsley C, Liu HL, Kavelaars A, Kesler SR and Heijnen CJ (2018). “Nasal administration of mesenchymal stem cells restores cisplatin-induced cognitive impairment and brain damage in mice.” Oncotarget 9(85): 35581–35597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Murphy EA, Carmichael MD and Davis B (2009). “Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance.” Am J Physiol Regul Integr Comp Physiol 296(4): R1071–1077. [DOI] [PubMed] [Google Scholar]
- Delgado-Morales R, del Rio E, Gomez-Roman A, Bisagno V, Nadal R, de Felipe C and Armario A (2012). “Adrenocortical and behavioural response to chronic restraint stress in neurokinin-1 receptor knockout mice.” Physiol Behav 105(3): 669–675. [DOI] [PubMed] [Google Scholar]
- Donega V, Nijboer CH, van Tilborg G, Dijkhuizen RM, Kavelaars A and Heijnen CJ (2014). “Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury.” Exp Neurol 261: 53–64. [DOI] [PubMed] [Google Scholar]
- Donega V, van Velthoven CT, Nijboer CH, Kavelaars A and Heijnen CJ (2013). “The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment.” J Cereb Blood Flow Metab 33(5): 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal CR, Carson Sulzer M, Kelley KW, Freund GG and McCusker RH (2017). “Glial and tissue-specific regulation of Kynurenine Pathway dioxygenases by acute stress of mice.” Neurobiol Stress 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SA, Sreenivasan D, Carman FH and Bunn SJ (2010). “Cytokine interactions with adrenal medullary chromaffin cells.” Cell Mol Neurobiol 30(8): 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden LE, Emery AC, Zhang L and Smith CB (2018). “PACAP signaling in stress: insights from the chromaffin cell.” Pflugers Arch 470(1): 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF and Dallman MF (2009). “Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint.” Endocrinology 150(5): 2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Liu G, Halim A, Ju Y, Luo Q and Song AG (2019). “Mesenchymal Stem Cell Migration and Tissue Repair.” Cells 8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargiulo AT, Jasodanand V, Luz S, O’Mara L, Kubin L, Ross RJ, Bhatnagar S and Grafe LA (2021). “Sex differences in stress-induced sleep deficits.” Stress 24(5): 541–550. [DOI] [PubMed] [Google Scholar]
- Gibney SM, Fagan EM, Waldron AM, O’Byrne J, Connor TJ and Harkin A (2014). “Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour.” Int J Neuropsychopharmacol 17(6): 917–928. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Pare WP, Sandbak T, Bakke HK and Murison R (1994). “Restraint stress in biomedical research: an update.” Neurosci Biobehav Rev 18(2): 223–249. [DOI] [PubMed] [Google Scholar]
- Gomzikova MO, James V and Rizvanov AA (2021). “Mitochondria Donation by Mesenchymal Stem Cells: Current Understanding and Mitochondria Transplantation Strategies.” Front Cell Dev Biol 9: 653322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon E, Parnet P, Laye S, Combe C, Kelley KW and Dantzer R (1995). “Stress downregulates lipopolysaccharide-induced expression of proinflammatory cytokines in the spleen, pituitary, and brain of mice.” Brain Behav Immun 9(4): 292–303. [DOI] [PubMed] [Google Scholar]
- Grossberg AJ, Vichaya EG, Christian DL, Molkentine JM, Vermeer DW, Gross PS, Vermeer PD, Lee JH and Dantzer R (2018). “Tumor-Associated Fatigue in Cancer Patients Develops Independently of IL1 Signaling.” Cancer Res 78(3): 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Guo Z, Yang X, Sun L, Wang S, Yingge A, He X and Ya T (2014). “The Alterations of IL-1Beta, IL-6, and TGF-Beta Levels in Hippocampal CA3 Region of Chronic Restraint Stress Rats after Electroacupuncture (EA) Pretreatment.” Evid Based Complement Alternat Med 2014: 369158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A, Yeo H, Park MJ, Kim SH, Choi HJ, Hong CW and Kwon MS (2015). “IL-4/10 prevents stress vulnerability following imipramine discontinuation.” J Neuroinflammation 12: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmerich H, Fischer J, Bauer K, Kirkby KC, Sack U and Krugel U (2013). “Stress-induced cytokine changes in rats.” Eur Cytokine Netw 24(2): 97–103. [DOI] [PubMed] [Google Scholar]
- Hodos W (1961). “Progressive ratio as a measure of reward strength.” Science 134(3483): 943–944. [DOI] [PubMed] [Google Scholar]
- Horrillo D, Sierra J, Arribas C, Garcia-San Frutos M, Carrascosa JM, Lauzurica N, Fernandez-Agullo T and Ros M (2011). “Age-associated development of inflammation in Wistar rats: Effects of caloric restriction.” Arch Physiol Biochem 117(3): 140–150. [DOI] [PubMed] [Google Scholar]
- Knudsen JG, Bienso RS, Hassing HA, Jakobsen AH and Pilegaard H (2015). “Exercise-induced regulation of key factors in substrate choice and gluconeogenesis in mouse liver.” Mol Cell Biochem 403(1–2): 209–217. [DOI] [PubMed] [Google Scholar]
- Konarska M, Stewart RE and McCarty R (1989). “Habituation of sympathetic-adrenal medullary responses following exposure to chronic intermittent stress.” Physiol Behav 45(2): 255–261. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR 3rd, Dasari S, Walrand S, Short KR, Johnson ML, Robinson MM, Schimke JM, Jakaitis DR, Asmann YW, Sun Z and Nair KS (2012). “Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis.” Cell Metab 16(6): 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Gong Z, Shultz LD and Ren G (2019). “Mesenchymal stem cells: From regeneration to cancer.” Pharmacol Ther 200: 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D and Sun SC (2017). “NF-kappaB signaling in inflammation.” Signal Transduct Target Ther 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpartida AB, Williamson M, Narendra DP, Wade-Martins R and Ryan BJ (2021). “Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy.” Trends Biochem Sci 46(4): 329–343. [DOI] [PubMed] [Google Scholar]
- Marin MT, Cruz FC and Planeta CS (2007). “Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats.” Physiol Behav 90(1): 29–35. [DOI] [PubMed] [Google Scholar]
- Matsuzaki J, Kuwamura M, Yamaji R, Inui H and Nakano Y (2001). “Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice.” J Nutr 131(8): 2139–2144. [DOI] [PubMed] [Google Scholar]
- Medzhitov R (2021). “The spectrum of inflammatory responses.” Science 374(6571): 1070–1075. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Easton A, Bergmann BM and Turek FW (2001). “Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice.” Am J Physiol Regul Integr Comp Physiol 281(3): R846–854. [DOI] [PubMed] [Google Scholar]
- Miller ES, Apple CG, Kannan KB, Funk ZM, Plazas JM, Efron PA and Mohr AM (2019). “Chronic stress induces persistent low-grade inflammation.” Am J Surg 218(4): 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olarte-Sanchez CM, Valencia-Torres L, Cassaday HJ, Bradshaw CM and Szabadi E (2015). “Quantitative analysis of performance on a progressive-ratio schedule: effects of reinforcer type, food deprivation and acute treatment with Delta(9)-tetrahydrocannabinol (THC).” Behav Processes 113: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal S, Chaudhuri R, Agrawal A and Mohanty S (2018). “Regenerative abilities of mesenchymal stem cells through mitochondrial transfer.” J Biomed Sci 25(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP and Glavin GB (1986). “Restraint stress in biomedical research: a review.” Neurosci Biobehav Rev 10(3): 339–370. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A and Dallman MF (2004). “Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress.” Endocrinology 145(8): 3754–3762. [DOI] [PubMed] [Google Scholar]
- Picard M and McEwen BS (2018). “Psychological Stress and Mitochondria: A Systematic Review.” Psychosom Med 80(2): 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS, Epel ES and Sandi C (2018). “An energetic view of stress: Focus on mitochondria.” Front Neuroendocrinol 49: 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachalski A, Alexandre C, Bernard JF, Saurini F, Lesch KP, Hamon M, Adrien J and Fabre V (2009). “Altered sleep homeostasis after restraint stress in 5-HTT knock-out male mice: a role for hypocretins.” J Neurosci 29(49): 15575–15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampin C, Cespuglio R, Chastrette N and Jouvet M (1991). “Immobilisation stress induces a paradoxical sleep rebound in rat.” Neurosci Lett 126(2): 113–118. [DOI] [PubMed] [Google Scholar]
- Sanger D (1987). Effects of drugs on schedule-controlled behavior. Experimental Psyhcopharmacology. Concepts and Methods AJ G and Clifton DCT, New Jersey, Humana Press: 213–261. [Google Scholar]
- Scarpulla RC (2008). “Transcriptional paradigms in mammalian mitochondrial biogenesis and function.” Physiol Rev 88(2): 611–638. [DOI] [PubMed] [Google Scholar]
- Scherer IJ, Holmes PV and Harris RB (2011). “The importance of corticosterone in mediating restraint-induced weight loss in rats.” Physiol Behav 102(2): 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiyama A, Ueda H, Kashiwamura S, Nishida K, Kawai K, Teshima-kondo S, Rokutan K and Okamura H (2005). “IL-18; a cytokine translates a stress into medical science.” J Med Invest 52 Suppl: 236–239. [DOI] [PubMed] [Google Scholar]
- Sharma S, Hryhorczuk C and Fulton S (2012). “Progressive-ratio responding for palatable high-fat and high-sugar food in mice.” J Vis Exp(63): e3754. [DOI] [PMC free article] [PubMed]
- Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D and Glaser R (1998). “Stress-induced neuroendocrine modulation of viral pathogenesis and immunity.” Ann N Y Acad Sci 840: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Soeters MR, Wust RCI and Houtkooper RH (2018). “Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease.” Endocr Rev 39(4): 489–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soichot M, Vaast A, Vignau J, Guillemin GJ, Lhermitte M, Broly F and Allorge D (2013). “Characterization of functional polymorphisms and glucocorticoid-responsive elements in the promoter of TDO2, a candidate gene for ethanol-induced behavioural disorders.” Alcohol Alcohol 48(4): 415–425. [DOI] [PubMed] [Google Scholar]
- Son H, Yang JH, Kim HJ and Lee DK (2019). “A Chronic Immobilization Stress Protocol for Inducing Depression-Like Behavior in Mice.” J Vis Exp(147) [DOI] [PubMed]
- Tan S, Wang Y, Chen K, Long Z and Zou J (2017). “Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice.” Biol Pharm Bull 40(8): 1260–1267. [DOI] [PubMed] [Google Scholar]
- van Velthoven CT, Sheldon RA, Kavelaars A, Derugin N, Vexler ZS, Willemen HL, Maas M, Heijnen CJ and Ferriero DM (2013). “Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke.” Stroke 44(5): 1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Basisty N, Wilson DM 3rd and Ferrucci L (2022). “Connecting aging biology and inflammation in the omics era.” J Clin Invest 132(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger M, Alpern D, Cherix A, Ghosal S, Grosse J, Russeil J, Gruetter R, de Kloet ER, Deplancke B and Sandi C (2020). “Mitochondrial gene signature in the prefrontal cortex for differential susceptibility to chronic stress.” Sci Rep 10(1): 18308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


