Abstract
Objective:
To assess the effect of Nystatin on Candida albicans and Streptococcus mutans duo-species biofilms using an in vitro cariogenic biofilm model.
Design:
Biofilms were formed on saliva-coated hydroxyapatite discs under high sugar challenge (1% sucrose and 1% glucose), with inoculation of 105CFU/ml S. mutans and 103CFU/ml C. albicans. Between 20–68 hours, biofilms were treated with 28,000 IU Nystatin solution, 5 minutes/application, 4 times/day, to mimic the clinical application. Biofilm’s three-dimensional structure was assessed using multi-photon confocal microscopy. The expression of C. albicans and S. mutans virulence genes was assessed via real-time PCR. Duplicate discs were used in 3 independent repeats. t-test and Mann-Whitney U test were used to compare outcomes between treatment and control group.
Results:
Nystatin treatment eliminated C. albicans in biofilms at 44 hours. Nystatin-treated group had a significant reduction of biofilm dry-weight and reduced S. mutans abundance by 0.5 log CFU/ml at 44 and 68 hours (p<0.05). Worth noting that biomass distribution across the vertical layout was altered by Nystatin treatment, resulting in less volume on the substrate layers in Nystatin-treated biofilms compared to the control. Reduction of microcolonies size and volume was also observed in Nystatin-treated biofilms (p<0.05). Nystatin-treated biofilms formed unique halo-shaped microcolonies with reduced core EPS coverage. Furthermore, Nystatin-treated biofilms had significant down-regulations of S. mutans gtfD and atpD genes (p<0.05).
Conclusions:
Nystatin application altered the formation and characteristics of C. albicans and S. mutans duo-species biofilms. Therefore, developing clinical regimens for preventing or treating dental caries from an antifungal perspective is warranted.
Keywords: Fungi, Bacteria, Antifungal, Streptococcus mutans, Biofilm
Introduction
Candida albicans is the most common fungal human pathogen causing localized and systemic infections in humans (Talapko et al., 2021). Young children often have a history of fungal infection in their early life; for example, the medical records of 196 1–3 years old American children indicated past histories of diaper rash (7%), oral candidiasis (6%), anemia (2%), and facial rash (2%) (Jabra-Rizk et al., 2007). The oral carriage of candida organisms is reported to be 45–65% in healthy children and 45% in neonates (Patil et al., 2015). During a routine oral exam, a study revealed that 66% of those 196 children had fungal growth in the samples collected from the mid-dorsum of the tongue, with most of the isolates being C. albicans (Jabra-Rizk et al., 2007).
Intriguingly, previous studies reported that the prevalence of C. albicans in children with early childhood caries (ECC) was higher than in caries-free children (Xiao et al., 2018). The carriage of C. albicans was also positively associated with the carriage of Streptococcus mutans in the saliva and plaque samples of children with ECC (Xiao et al., 2018). A review by Koo and his colleagues highlighted a synergistic interaction between C. albicans and S. mutans mediated by S. mutans-derived glucosyltransferases (GTFs) (Koo et al., 2018). With the presence of sucrose, the GtfB enzyme binds to the cell surface of C. albicans and involves synthesizing exopolysaccharides (EPS), mainly insoluble α-glucan (Hwang et al., 2017). EPS mediates the binding interaction between C. albicans and S. mutans and further promotes interspecies biofilm formation (Koo et al., 2018). Furthermore, the presence of C. albicans and S. mutans leads to more severe dental caries in an animal model (Falsetta et al., 2014) and is associated with a higher prevalence of caries in children (Eidt et al., 2020). Due to the cross-kingdom interaction in the context of dental caries, an antifungal regimen might be able to control S. mutans, thus preventing ECC via controlling oral yeast infections.
The antifungal medications for treating oral candidiasis include two main groups -polyenes and azole (Scheibler et al., 2018). Fluconazole, one of the Azoles, is a broadly used antifungal agent to treat systemic fungal infections (Govindarajan et al., 2022). A recent study assessed the effect of Fluconazole and povidone-iodine on the disruption of C. albicans-S. mutans duo-species biofilms; this study provided compelling evidence that targeting bacterial EPS is a critical factor for enhanced antifungal drug efficacy in mixed kingdom biofilms (Kim et al., 2018). Among the polyene group, Nystatin is the most commonly used medication to treat oral candidiasis (Garcia-Cuesta et al., 2014). Moreover, Nystatin is an antimicrobial agent with both fungicidal and fungistatic properties (Scheibler et al., 2018).
The topical use of Nystatin is considered the most common route of administration in dentistry, as systemic exposure is minimal (Lyu et al., 2016; Pappas et al., 2016). The commonly recommended dose for Nystatin topical use is 200,000–600,000 IU (four times a day, qid) for children and adults, and 100,000–200,000 IU qid for newborns and infants (Lyu et al., 2016).Treatment duration varies from 1 or 2 to 4 weeks (Lyu et al., 2016). Nystatin is well tolerated because of its poor absorption in the gastrointestinal tract (Garcia-Cuesta et al., 2014). In addition, Nystatin has minimal side effects (such as Nausea and vomiting) and a low risk of hepatotoxicity, compared to Fluconazole, in which hepatotoxicity was reported in rare cases due to its systemic exposure (Garcia-Cuesta et al., 2014).
To our knowledge, no studies have assessed the effect of Nystatin on the interaction of S. mutans and C. albicans in vitro and in vivo. Therefore, our immediate goal was to assess the effectiveness of using Nystatin to inhibit the formation of C. albicans-S. mutans duo-species biofilms using an established in vitro model, which would provide rationale for conducting further studies using multi-species biofilm in vitro model, in situ model and in clinical trials.
Materials and Methods
The overall Study design is demonstrated in Fig. 1; detailed study methods are described below. All assays were repeated in three independent experiments.
Fig. 1. Schematic study design.
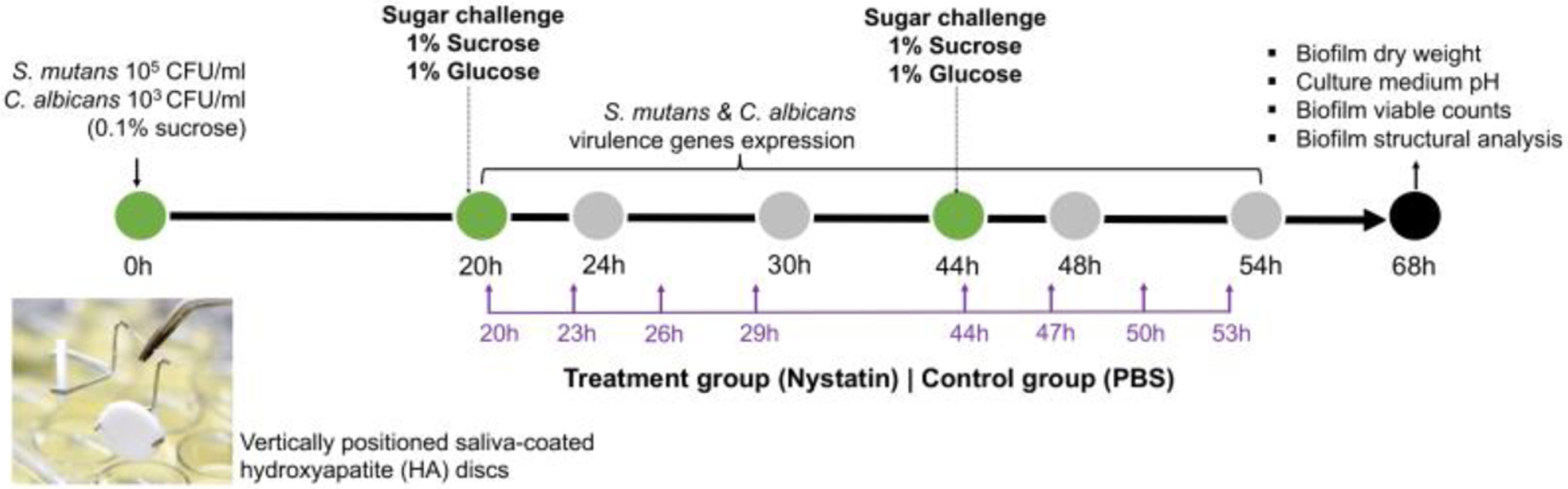
We used a duo species biofilm model to evaluate the effect of antifungal medications on S. mutans and C. albicans duo-species biofilm formation. Culture medium was changed once/daily at 20 and 44 hours (marked as green dot). Biofilms grew under 0.1% sucrose condition in the first 20hours, then were subjected to sugar challenge with 1% sucrose and 1% glucose. Nystatin (treatment group) and PBS (control group) applications were administered 4 times/day, 5 minutes/application, between 20–68 hours (marker in purple).
Bacterial strains and growth conditions
In this study, S. mutans UA159 (ATCC 700610), a proven virulent cariogenic dental pathogen (Ajdic et al., 2002), and C. albicans SC5314 were used to form duo-species biofilms. We selected C. albicans in addition to S. mutans because this fungus is frequently detected in the supragingival plaque of children with dental caries (Xiao et al., 2018).
Using a batch culture approach, saliva-coated hydroxyapatite (sHA) discs were utilized to mimic the formation of biofilms according to the “ecological plaque-biofilm” concept (Marsh, 2003), as described by Xiao et al. (Xiao et al., 2012). The bacterial strains were recovered from frozen stock on blood agar (TSA with Sheep Blood, Thermo Scientific™ R01202) and were incubated for 48 hours (5% CO2, 37°C). Then, 3–5 colonies of each species were inoculated into 10 ml of broth for overnight incubation (5% CO2, 37°C). C. albicans cells were grown in YPD broth (BD Difco™, 242820), and S. mutans cells were grown in TSBYE broth (3% Tryptic Soy, 0.5%Yeast Extract Broth, BD Bacto™ 286220 and Gibco™ 212750) with 1% glucose at 37°C and 5% CO2.
On the following day, 0.5 ml of the overnight starters were added to an individual glass tube containing fresh broth and incubated for 3–4 hours to the mid-exponential phase until an optimal optical density OD600 of 0.80 for C. albicans and 1.00 for S. mutans was reached. These two bacterial suspensions were then mixed to produce an inoculum containing S. mutans (105 CFU/ml) and C. albicans (103 CFU/ml) to mimic the clinical carriage of S. mutans and C. albicans in children with early childhood caries. It is also critical for the reproducibility of our model (Koo et al., 2010).
Duo-species biofilm model
Duo-species biofilms were formed on saliva-coated hydroxyapatite discs (1.25 cm diameter, Clarkson Chromatography Products, Inc., South Williamsport, PA) (Koo et al., 2005; Koo et al., 2010), which were vertically positioned using custom-made disc holders, in order to imitate the position of natural teeth. The mixed population of S. mutans and C. albicans were inoculated in 2.8 ml of TSBYE with 0.1% (w/v) sucrose and incubated at 37°C and 5% CO2. During the first 20 h, the organisms were grown undisturbed to allow initial biofilm formation and mimic the clinical condition that C. albicans and S. mutans already present in the oral cavity. At 20 h of biofilm growth, the biofilms were transferred to TSBYE that contains 1% (w/v) sucrose or 1% (w/v) glucose to introduce a cariogenic challenge, while an additional set of biofilms were grown in TSBYE with 0.1% (w/v) sucrose. The culture medium was replaced once daily (every 24 h) until the end of the experimental period (68 h). Duplicate discs were used in 3 independent repeats for accuracy and reproducibility, a total of 6 discs were used for each biofilm condition (n=6).
The culture medium pH was measured daily using a standard pH electrode. The biofilms were analyzed at specific time points using confocal imaging/fluorescence and biochemical assays (Fig. 1).
Nystatin Treatment
2.8 ml of 10,000 unit/mL Nystatin suspension in DPBS (Sigma-Aldrich, BioReagent, Saint Louis, MO, USA) was used for each treatment. The biofilms formed on the sHA discs were treated in Nystatin suspension for 5 minutes, four times/day (detailed treatment regimen see Fig. 1). After each treatment, HA discs were dip-washed in sterile Dulbecco’s phosphate-buffered saline (DPBS) to remove excess agents, and then transferred back to the culture medium. DPBS solution was also used as a topical treatment for the control group.
Viable cells in biofilms
The biofilms were removed from the discs and resuspended in sterile 0.89% (w/v) NaCl solution. The solution was homogenized by sonication (30-sec pulse at an output of 7W; Branson Sonifier 150, Branson Ultrasonics, Danbury, CT) without killing bacterial species from sonication (Koo et al., 2010). The homogenized suspension was plated on blood agar using an automated EddyJet Spiral Plater (IUL, SA, Barcelona, Spain) to determine the colony-forming units (CFU), which reflect the number of viable cells. The two species were differentiated by observation of colony morphology in conjunction with microscopic examination of cells from selected colonies (Guggenheim et al., 2001). The homogenized biofilm suspension was also used for the measurement of dry weight detailed previously (Zeng et al., 2019).
Laser scanning confocal fluorescence microscopy (LCSFM) imaging of biofilm matrix
We examined the three-dimensional (3D) structure of intact biofilms by directly incorporating fluorescent markers during the synthesis of the extracellular polysaccharide (EPS) matrix using LCSFM (Klein et al., 2009; Klein et al., 2011). Hence, 1 μM Alexa Fluor® 647-labeled dextran conjugate (molecular weight, 10 kDa; absorbance/fluorescence emission maxima of 647/668 nm; Molecular Probes, Invitrogen Corp., Carlsbad, CA) was added to the culture medium starting from the biofilm formation to the biofilm development for EPS visualization. This fluorescent marker does not stain the bacterial cells used in our study using a specific concentration (Klein et al., 2009). Both bacterial and fungal species in the biofilms were labeled by SYTO® 9 green, fluorescent nucleic acid stain (485/498 nm; Molecular Probes) following a standard protocol (Klein et al., 2009; Klein et al., 2011). The images were taken using Olympus FV 1000 two-photon laser scanning microscope (Olympus, Tokyo, Japan) that contains a 10X (0.45 numerical aperture) or 25X LPlan N (1.05 numerical aperture) water immersion objective lens(Koo et al., 2010). Each HA disc with biofilm was scanned at five random positions selected at the microscope stage (Xiao & Koo, 2010), and confocal image series were generated. A total of 10 image stacks were obtained for each biofilm condition.
Computational analyses of the confocal biofilm images
We analyzed the confocal images with a specific software that simultaneously visualizes and quantifies EPS and bacterial cells within intact biofilms (Klein et al., 2011). Therefore, Amira 5.0.2 (Mercury Computer Systems Inc., Chelmsford, MS) was used to visualize the morphology and 3D architecture of both structural components of biofilms (EPS and bacteria) as detailed previously (Klein et al., 2011; Xiao & Koo, 2010). The quantitative analysis of the images was carried out using COMSTAT and DUOSTAT scripts (http://www.imageanalysis.dk). We used COMSTAT to calculate the biomass of bacteria and EPS components, the number, thickness, and layer distribution of biofilms. In addition, COMSTAT quantifies the size (volume, diameter, and height) of surface-attached and floating microcolonies, as detailed elsewhere (Heydorn et al., 2000; Xiao & Koo, 2010). We used DUOSTAT to calculate the co-localization of EPS and bacterial cells within the 3D biofilm structure (Klein et al., 2011).
Real-time PCR
Real-time PCR was performed to assess the expression of C. albicans and S. mutans virulence genes. We collected biofilms from four discs at six points, 20, 24, 30, 44, 48, and 54 h, for both the control group and the Nystatin-treated group (Fig. 5–6). The discs were immersed in RNAlater (AppliedBiosystems/Ambion, Austin, TX, USA) for 1 hour, followed by biofilms removal with a spatula. RNA extraction and purification were completed using MasterPure complete DNA and RNA purification kit (Epicentre, Lucigen, WI, USA). The raw RNA product was then quantified using NanoDrop One Microvolume UV-Vis Spectrophotometer (Thermo Scientific™, Wilmington, DE, USA). Depletion of rRNA was performed using Ribozero rRNA Removal Kit (Illumina, San Diego, CA, USA).
Fig. 5. Expression of C. albicans virulence genes.
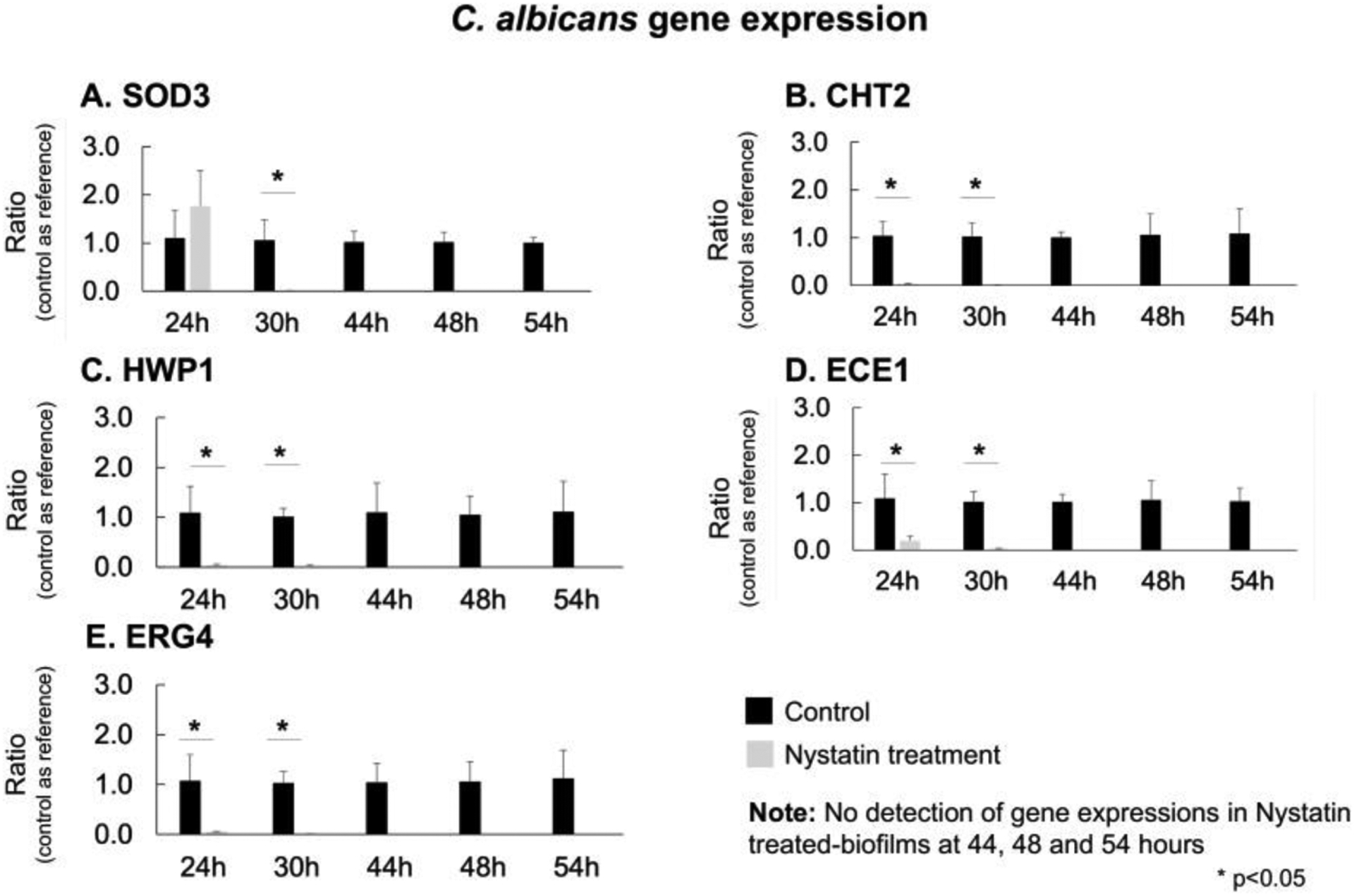
The real-time PCR was performed to assess C. albicans virulence genes. CHT2, HWP1, ECE1, and ERG4 were significantly down-regulated (p<0.05, t-test) immediately following the first Nystatin application at 24h. SOD3 gene was significantly down-regulated (p<0.05, t-test) after 30 hours. At 44 hours which is after 4 applications of Nystatin, the expressions of all C. albicans virulence genes were not detectable.
Fig. 6. Expression of S. mutans virulence genes.
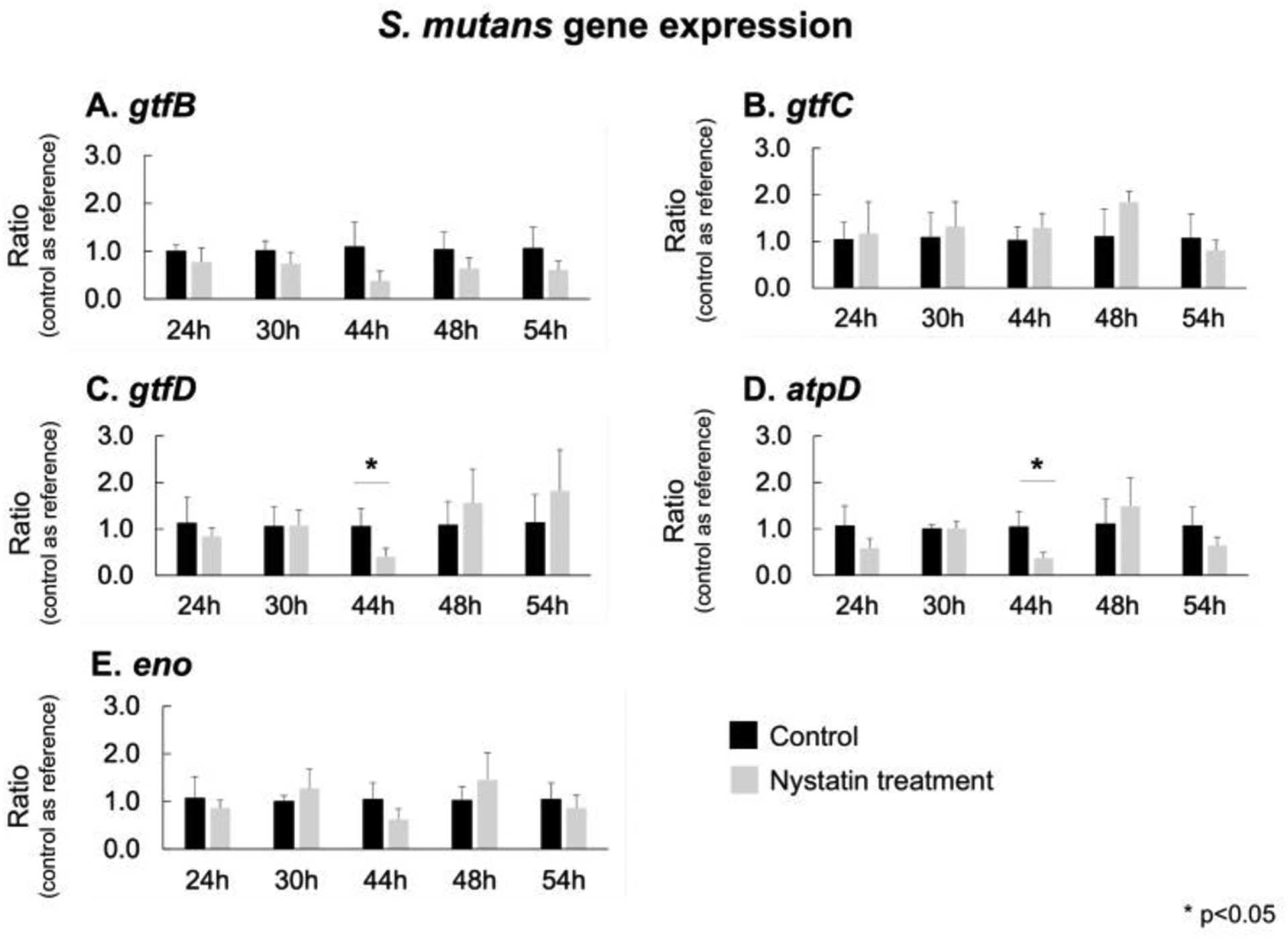
The real-time PCR was performed to assess S. mutans virulence genes. The biofilms treated with Nystatin had down-regulations of gtfB, and eno genes, compared to the control group, despite no statistically significant differences were detected. The expression of S. mutans gtfD and atpD was significantly down-regulated (p<0.05, t-test) in the Nystatin-treated biofilms than the control group at 44 hours when 4 Nystatin applications had been administered (p<0.05, t-test)
The cDNA synthesis was completed from 0.2 mg of purified RNA with the Bio-Rad iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA). Amplification of S. mutans and C. albicans genes was conducted using Applied Biosystems™ PowerTrack™ SYBR Green Master Mix and a QuantStudio™ 3 Real-Time PCR System (Thermo FisherScientific, USA). For S. mutans and C. albicans genes comparative calculation, we used reference genes: gyrA for S. mutans (Zeng & Burne, 2013) and ACT1 for C. albicans. For C. albicans virulence genes, the hyphae-specific genes (HWP1, and ECE1), manganese-dependent superoxide dismutase (SOD3) (Martchenko et al., 2004), chitinase gene (CHT2) (McCreath et al., 1995), and ergosterol Biosynthesis gene (ERG4) were analyzed. For S. mutans virulence genes, the target genes were Gtf genes (gtfB, gtfC, and gtfD) responsible for cellular adhesion (Ooshima et al., 2001), enolase gene (eno), and ATPase gene (atpD). Methods were detailed previously (Zeng et al., 2022). The genes and primers used are listed in Table S1.
Statistical analysis
The CFU value for S. mutans and C. albicans were converted to a natural log for statistical analysis. The normality of numerical values (CFU natural log value, biomass of bacteria and EPS, number and size of microcolonies, pH value of the culture medium, and gene expression ratio) was assessed. t-test was performed to compare the difference between the control and Nystatin treatment group for normally distributed data, and Mann-Whitney U test was used to assess the differences for non-normal data. Repeated ANOVA was used to assess the S. mutans virulence genes expression between different time points among the control and Nystatin-treated group. Statistical analyses were performed using IBM SPSS.
Results
The effect of Nystatin on duo-species biofilm was assessed using a duo-species biofilm model (Fig. 1). Nystatin treatment eliminated C. albicans after 44 hours of biofilm formation compared to the control group (Fig. 2A). Intriguingly, the Nystatin-treated biofilms formed in a higher sugar condition (1% sucrose) had a significant reduction of dry weight (p<0.05) (Fig. 3B). The 1% sucrose sugar condition reflected a high cariogenic challenge. In addition, the abundance of S. mutans was reduced by 0.5 log CFU/ml at 44 and 68 hours in the Nystatin-treated group with 1% sucrose (p<0.05).
Fig. 2. Effect of Nystatin on S. mutans and C. albicans viability in duo-species biofilms.
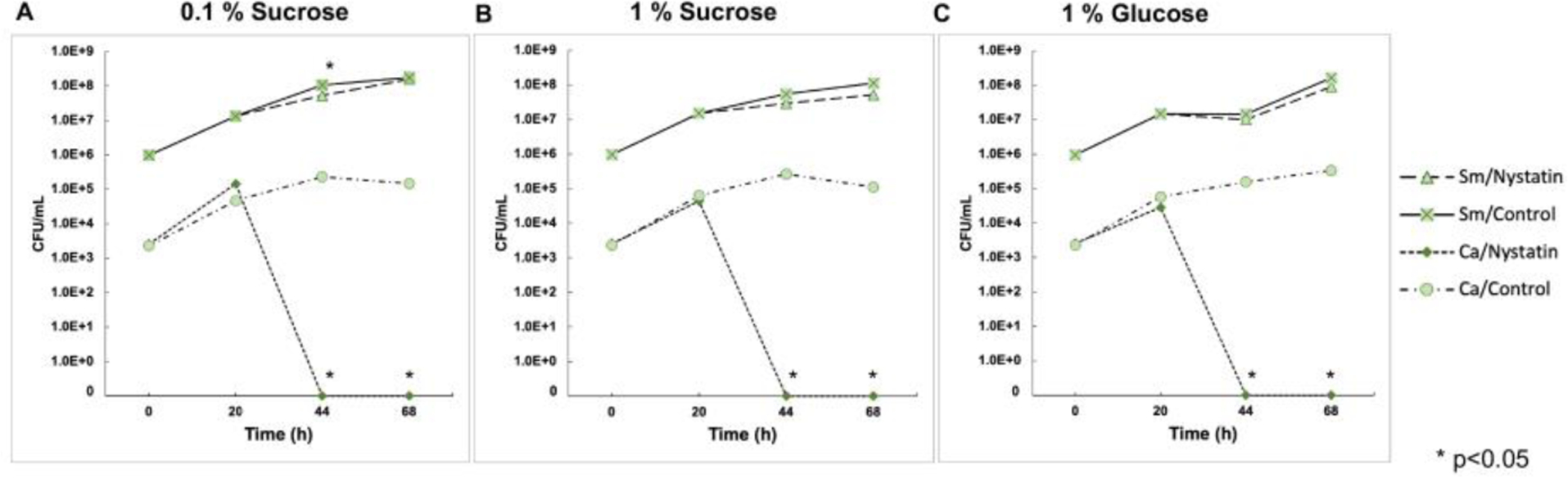
Viable cells of S. mutans and C. albicans were assessed using colony forming unit (CFU). (A) 0.1% sugar condition, (B) 1% sucrose condition, and (C) 1% glucose condition. Viable C. albicans were undetected after 44 hours of biofilm formation in all sugar conditions. The abundance of S. mutans was reduced by 0.5 log CFU/ml at 44 and 68 hours in the Nystatin-treated group with 1% sucrose, (p=0.03, t-test).
Fig. 3. Morphogenesis and 3D architecture of S. mutans and C. albicans duo-species biofilms treated by Nystatin.
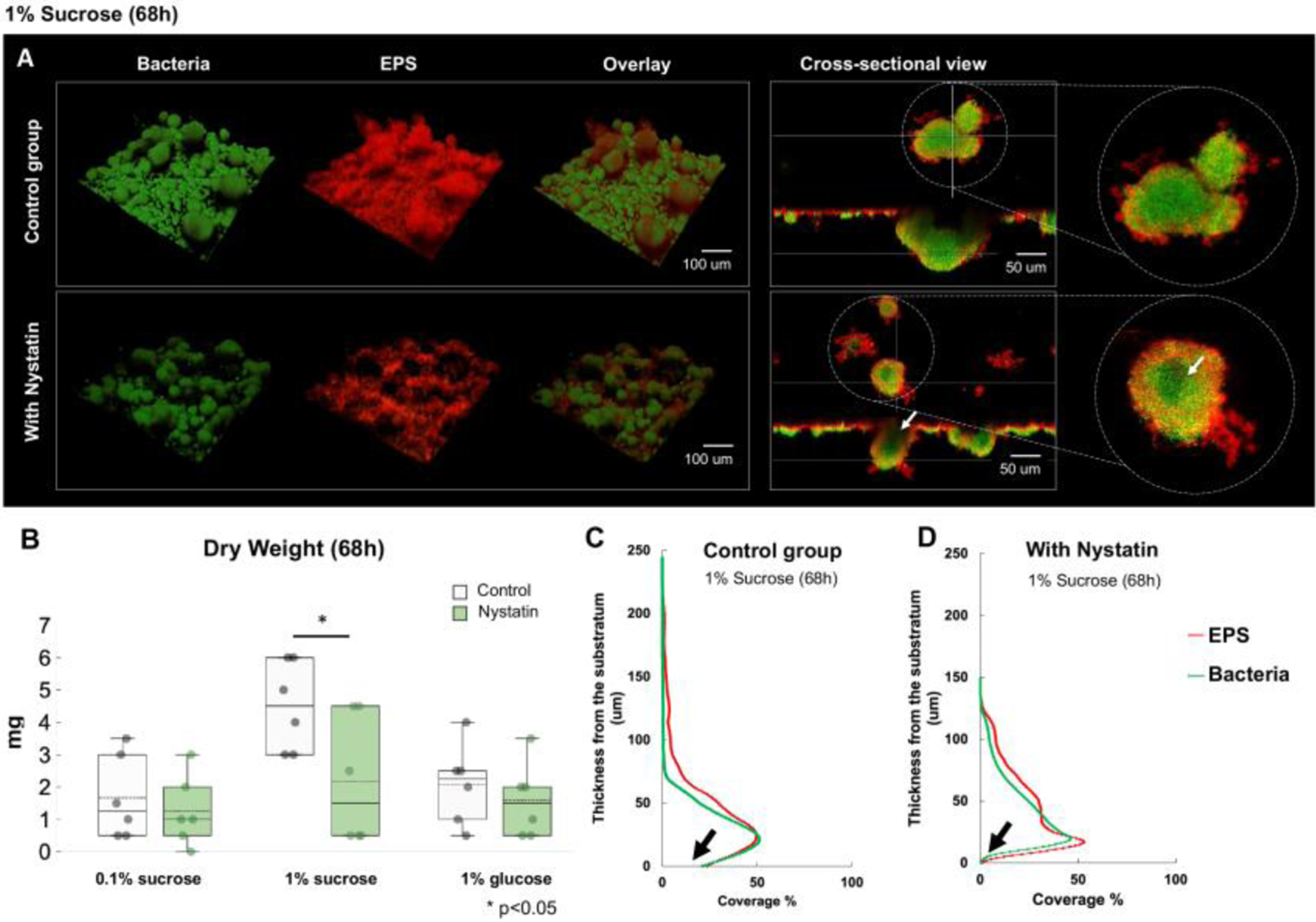
(A) Morphogenesis and 3D architecture of 68h Nystatin-treated biofilms were visualized. Altered biofilm structures were seen, with smaller and hollow microcolonies. White arrow indicates inner aspect of microcolony. (B) Significant reduction of biofilm dry weight following Nystatin treatment was seen in 1% sucrose condition, comparing to the control group, (p=0.04, t-test). (C-D) Layer distribution of the 68h biofilms formed in 1% sucrose showed that the Nystatin-treated biofilms had thinner layers and with less biomass at the substrate layer (marked with black arrows).
Inhibitory effect of Nystatin on biofilm formation
We observed the biofilm closely using a two-photon laser confocal microscope to explore the Nystatin treatment’s effect further. COMSTAT and DUOSTAT were used to determine the biofilm’s three-dimensional structure parameters (biomass of bacteria and EPS, thickness/layer distribution of biofilms, and formation of surface-attached and floating microcolonies). The biomass and average thickness of bacteria and EPS was lower in the treatment group than in the control group (Fig. 3A). Biofilms formed in the control group had a dense EPS layer covering the sHA disc. Worth noting that the biomass distribution across the vertical biofilm layout was altered by Nystatin treatment, resulting in less volume on the substrate layers of the Nystatin group compared to the control group (Fig. 3C and D).
The biofilms treated with Nystatin formed unique halo-shaped microcolonies with reduced core EPS coverage (red) (Fig. 3A). We also observed smaller microcolonies (green) in the Nystatin-treated group, which can be seen clearly in the cross-sectional view. Reduction of the number and size of floating and attached microcolonies in the Nystatin treatment group was also confirmed from the quantitative assessment via COMSTAT (Table 1). The amount of bacteria co-localization by EPS was quantified using DUOSTAT, which confirms the findings of the representative confocal images.
Table 1.
Quantitative assessment of microcolonies in mixed species biofilms
| Parameters | Control group | Nystatin group | P-value |
|---|---|---|---|
| Substate-attached microcolonies | |||
| Number | 7.3±3.7 | 1.8±1.7 | 0.04* |
| Area (um2) ×103 | 2.1±2.6 | 0.2±0.1 | 0.13 |
| Volume (um3) ×106 | 1.9±1.3 | 3.7±1.6 | 0.89 |
| Floating microcolonies | |||
| Number | 235.4±142.2 | 200.8±47.2 | 0.48 |
| Diameter (um) | 42.9±8.7 | 36.2±3.2 | 0.04* |
| Volume (um3) ×103 | 6.9±7.8 | 8.9±9. | 0.62 |
Values are represented as means ±SD
p<0.05
Changes in culture media pH
The culture media pH was measured at three different time points during the biofilm experiment. The acidity of the spent culture medium was significantly lower in the Nystatin-treated group compared to the control group at 44 and 68 hours under 0.1% sucrose sugar conditions (Fig. 4A). However, the acidity of the control and treatment group was similar at 44 and 68 hours under 1% sucrose and 1% glucose sugar conditions (Fig. 4B–C).
Fig. 4. pH value in culture media.
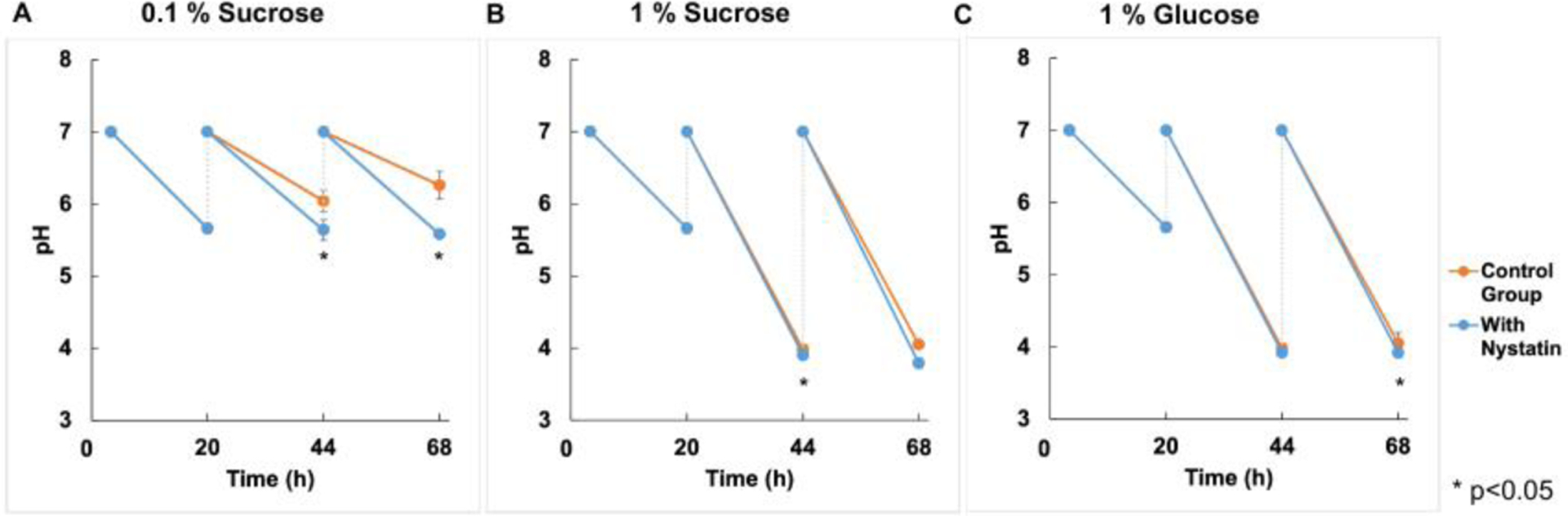
(A) The pH value of culture medium was significantly lower in the Nystatin treated group comparing to the control group at 44 and 68 hours under 0.1% sucrose sugar conditions, (p<0.001, t-test). (B-C) No significant reduction was noted in 1% sucrose and 1% glucose conditions.
Expression of virulence genes
We performed real-time PCR to assess C. albicans and S. mutans virulence genes. For C. albicans, virulence genes (CHT2, HWP1, ECE1, and ERG4) were significantly down-regulated (p<0.05) immediately following the first Nystatin application at 24h; SOD3 gene was significantly down-regulated (p<0.05) after 30 hours (see Fig. 5). At 44 hours which is after 4 applications of Nystatin, expression was not detection for all C. albicans virulence genes, which is consistent with our results in (Fig. 2) where no viable C. albicans cells were cultured at 44h biofilms in the Nystatin treatment group.
The biofilms treated with Nystatin had down-regulations of S. mutans gtfB, and eno genes compared to the control group, despite no statistically significant differences being between the control and treatment group (Fig. 6). However, at 44 hours, and 4 Nystatin applications, gtfD and atpD were down-regulated and statistical difference (p<0.05) was noted.
We further assessed the dynamic expression of S. mutans virulence genes in control and Nystatin-treated biofilms. No significant change was seen in the dynamic expression of gtfB, atpD, and eno genes in both the control and Nystatin-treatment group. However, there was a significant change (p<0.05) in the dynamic expression of gtfD in the control group (Fig. 7A) and a significant change in the dynamic expression of gtfC in the Nystatin-treated biofilms (Fig. 7B).
Fig. 7. Dynamic changes of S. mutans virulence genes in control and Nystatin-treated S. mutans and C. albicans duo-species biofilms.
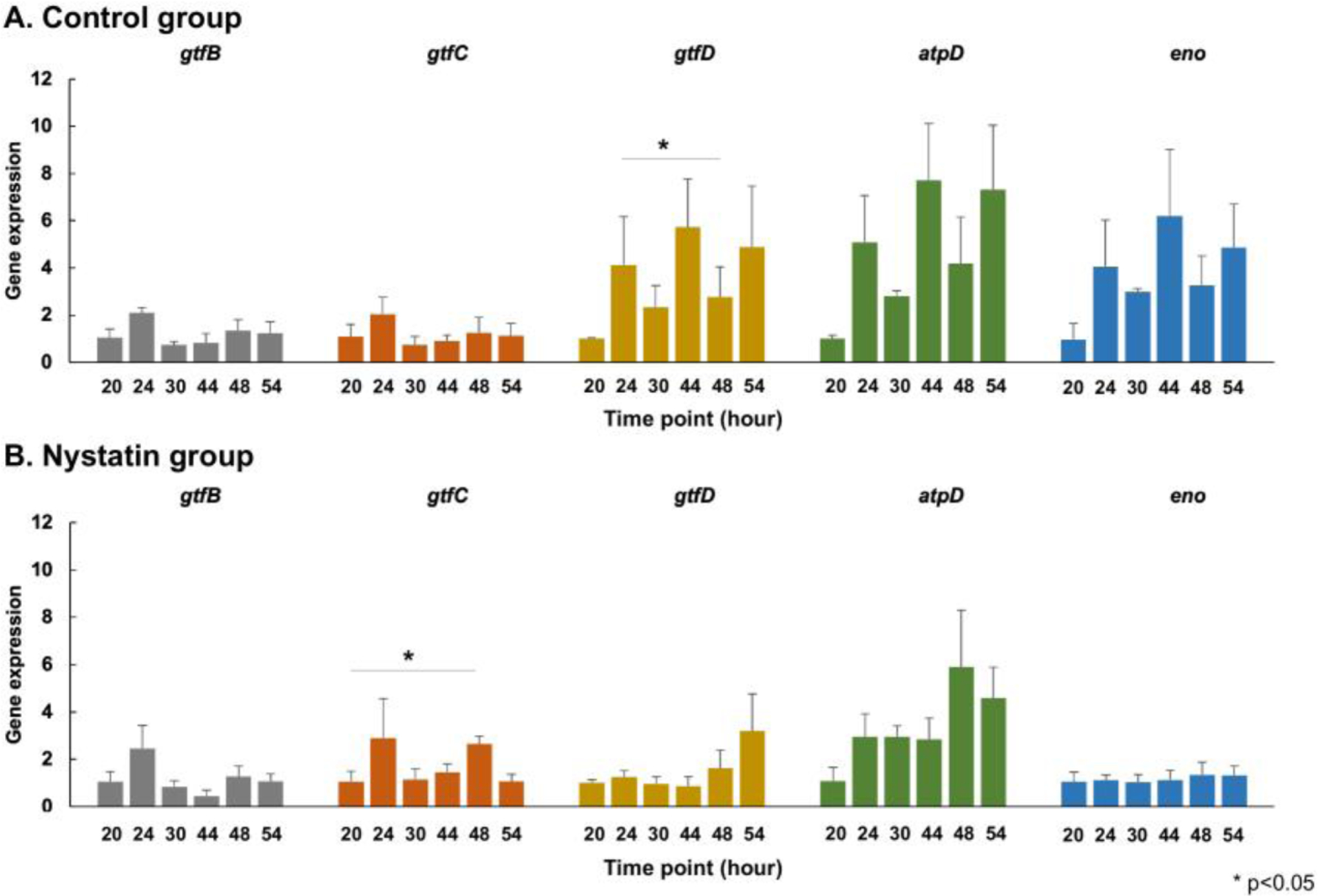
The dynamic expressions of S. mutans virulence genes in the control (A) and Nystatin-treated biofilms (B) were assessed. No significant change was seen in the dynamic expression of gtfB, atpD, and eno genes in both the control and Nystatin-treatment group. However, there was a significant change in the dynamic expression of gtfD in the control group and a significant change in the dynamic expression of gtfC in the Nystatin-treated biofilms. (p<0.05, repeated ANOVA).
Discussion
Given that limited studies have assessed the effect of an antifungal regimen on inhibiting C. albicans and S. mutans biofilms, our study sheds new light on C. albicans-S. mutans duo-species biofilm control from a fungal perspective. In our biofilm model, Nystatin eliminated C. albicans after 44 hours of biofilm formation, with a significant down-regulation of several virulence genes immediately following Nystatin application. For instance, the hyphae-specific genes (HWP1 and ECE1) were significantly down-regulated at 30 h and the expression of HWP1 and ECE1 was not detectable after 44 h. The expression of other C. albicans genes, such as manganese-dependent superoxide dismutase (SOD3) (Martchenko et al., 2004), chitinase gene (CHT2) (McCreath et al., 1995), and ergosterol Biosynthesis gene (ERG4) was also undetected after 4 applications of Nystatin at 44 h.
The reduction of C. albicans in the biofilms was associated with a 0.5 log reduction in S. mutans viable count (CFU/ml). Less S. mutans viable cells in the Nystatin-treated biofilms could be partially explained by the down-regulation of eno (acid production) and atpD (acid tolerance) (Jung et al., 2022). Furthermore, a significant reduction in biofilm dry weight was seen in the Nystatin-treated group compared to the control group under the most cariogenic sugar condition (1% sucrose). Nystatin-treated biofilms had reduced biomass and modified biofilm architecture that is less virulent, with fewer and smaller substrate attached microcolonies. The gtfB is associated with insoluble glucan synthesis and the formation of the backbone of cariogenic biofilms (Koo et al., 2010). Despite a statistically non-significant difference in gtfB/C expression in the Nystatin-treated biofilms compared to the control group, a trend of down-regulation of gtfB expression after the Nystatin application was seen. The GtfD enzyme produces soluble glucans and collaborates with the other Gtf enzymes (GtfB and GtfC) to promote adherence of bacterial cells and biofilm formation in the oral cavity (Matsumoto-Nakano, 2018). We also see a statistically significant reduction of gtfD expression at 44h (after 4 applications of Nystatin). Despite the positive reduction of S. mutans in relation to the C. albicans reduction, it is worth noting 0.5 log reduction of S. mutans is below the desirable 3 log10 reduction for considering a product for being clinically relevant, according to the American Society of Microbiology (Petersen et al., 2007).
Moreover, Nystatin-treated biofilm formed unique halo-shaped microcolonies with reduced EPS in the core of the microcolonies. Since the microcolony structure in biofilms is often associated with oral biofilm virulence, such as acting as barriers to environmental challenges (sheer force, application of antimicrobial agents) and forming an acidic environment that leads to more severe demineralization of enamel surfaces (Xiao et al., 2017; Xiao et al., 2012), further understanding of the Nystatin-treatment related halo-shaped microcolonies should be conducted in the future.
A recent study (Kim et al., 2018) examined the effect of using an antifungal regimen (Fluconazole) on duo-species biofilm in vitro and in vivo, reporting a significant reduction in S. mutans and C. albicans viable count compared to the control group, however, Fluconazole did not eliminate the viable cells of C. albicans. Our study observed a complete elimination of C. albicans viable cells, which might be due to the washing action of Nystatin suspension compared to the systemically taken antifungal medication fluconazole. In addition, the significant reduction in S. mutans after the topical application with fluconazole correlates with our findings as Nystatin reduced the abundance of S. mutans as well. In addition, C. albicans serve as a surface for S. mutans to bind and form biofilms. Hwang and his colleagues report that mannans located on the outer surface of C. albicans cell wall mediate the binding of S. mutans-derived enzyme GtfB, enhance glucan-matrix production and modulate bacterial-fungal association within biofilms (Hwang et al., 2017; Hwang et al., 2015). Reduction of C. albicans in the biofilm system could reduce S. mutans binding due to the abovementioned S. mutans-C. albicans binding interactions.
A noteworthy finding is that we observed a significant reduction in culture media pH in the Nystatin-treated group with 0.1% sucrose, compared to the control group, at 44 and 68 hours (Fig. 4A). This finding is in line with a previous study by Willem et al., where growing C. albicans in duo-species biofilms (C. albicans and S. mutans) led to a higher culture medium pH at 24 hours compared to S. mutans single-species biofilms (Willems et al., 2016). When grown alone or in combination, the metabolic productions of S. mutans and C. albicans deserve further investigation to better understand their synergistic relation.
In addition to Nystatin tested in our study, other antifungal agents to be tested include caspofungin. Caspofungin belongs to the echinocandin family and has a unique mechanism of action by inhibiting the synthesis of b(1–3) glucan, a critical component of fungal cell walls (Katzung et al., 2015). In an in vitro study investigating the effect of caspofungin on C. albicans biofilms, the results suggested that the therapeutic dosage of this agent showed potent activity against C. albicans biofilms (Bachmann et al., 2002). Due to its potent effect and unique mechanism of action merits further investigation of caspofungin on other biofilm-associated diseases such as dental caries. Furthermore, recent advanced probiotic research revealed a remarkable inhibitory effect of Lactobacillus spp. on S. mutans and C. albicans duo-species biofilm formation (Zeng et al., 2022). Conjunction of using probiotics and antifungal medication could lead to simultaneous and optimal control of S. mutans and C. albicans.
Studying multispecies biofilm in vitro is critical to understanding oral infectious disease and related management. However, limitations exist from using in vitro models. For example, although our biofilm model mimicked a high caries risk condition and supplied a high sugar challenge, our model did not introduce a flow cell setting, which does not mimic clinical shear force, salivation, etc. In addition, this study tested the wild type of S. mutans and C. albicans, which does not reflect the full spectrum of clinical isolates virulence; further studies are needed to assess the responses of clinical isolates. Furthermore, the current study focused on assessing C. albicans; future studies could expand the attention and assessment of the cariogenic role of other Candida species, such as C. dubliniensis. Future studies are also required to analyze Nystatin’s effect in animal models and clinical trials.
Conclusions
Nystatin altered the formation and characteristics of C. albicans and S. mutans duo-species biofilms. Future studies that investigate the effect of Nystatin on C. albicans-S. mutans multi-species biofilms via in vitro/in situ models and clinical trials are critical to providing further rationale for developing clinical regimens for preventing or treating dental caries from an antifungal perspective.
Supplementary Material
Acknowledgement
This study was supported by the NIH National Institute of Dental and Craniofacial Research grant K23DE027412 and R01DE031025. The funding agencies had no role in the study design, data collection, analyses, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Ethics
An ethics statement was not required for this study type, no human or animal subjects or materials were used.
Conflict of Interest Statement
The authors declared no potential conflicts of interest.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References:
- Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, & Ferretti JJ (2002, Oct 29). Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A, 99(22), 14434–14439. 10.1073/pnas.172501299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, Graybill JR, & Lopez-Ribot JL (2002, Nov). In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother, 46(11), 3591–3596. 10.1128/AAC.46.11.3591-3596.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidt G, Waltermann EDM, Hilgert JB, & Arthur RA (2020, Nov). Candida and dental caries in children, adolescents and adults: A systematic review and meta-analysis. Arch Oral Biol, 119, 104876. 10.1016/j.archoralbio.2020.104876 [DOI] [PubMed] [Google Scholar]
- Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, & Koo H (2014, May). Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun, 82(5), 1968–1981. 10.1128/IAI.00087-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cuesta C, Sarrion-Perez MG, & Bagan JV (2014, Dec). Current treatment of oral candidiasis: A literature review. J Clin Exp Dent, 6(5), e576–582. 10.4317/jced.51798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Bistas KG, Ingold CJ, & Aboeed A (2022). Fluconazole. In StatPearls. https://www.ncbi.nlm.nih.gov/pubmed/30725843 [PubMed] [Google Scholar]
- Guggenheim B, Giertsen E, Schupbach P, & Shapiro S (2001, Jan). Validation of an in vitro biofilm model of supragingival plaque. J Dent Res, 80(1), 363–370. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11269730 [DOI] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, & Molin S (2000, Oct). Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology, 146 (Pt 10), 2395–2407. 10.1099/00221287-146-10-2395 [DOI] [PubMed] [Google Scholar]
- Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, & Koo H (2017, Jun). Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog, 13(6), e1006407. 10.1371/journal.ppat.1006407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang G, Marsh G, Gao L, Waugh R, & Koo H (2015, Sep). Binding Force Dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J Dent Res, 94(9), 1310–1317. 10.1177/0022034515592859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabra-Rizk MA, Torres SR, Rambob I, Meiller TF, Grossman LK, & Minah G (2007, Summer). Prevalence of oral Candida species ina North American pediatric population. J Clin Pediatr Dent, 31(4), 260–263. 10.17796/jcpd.31.4.820968206675v577 [DOI] [PubMed] [Google Scholar]
- Jung HY, Cai JN, Yoo SC, Kim SH, Jeon JG, & Kim D (2022, Feb 7). Collagen Peptide in a Combinatorial Treatment with Lactobacillus rhamnosus Inhibits the Cariogenic Properties of Streptococcus mutans: An In Vitro Study. Int J Mol Sci, 23(3). 10.3390/ijms23031860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung BG, Trevor AJ, & Kruidering-Hall M (2015). Pharmachology: Examination & Board Review (11th ed.). [Google Scholar]
- Kim D, Liu Y, Benhamou RI, Sanchez H, Simon-Soro A, Li Y, Hwang G, Fridman M, Andes DR, & Koo H (2018, Jun). Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J, 12(6), 1427–1442. 10.1038/s41396-018-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MI, Duarte S, Xiao J, Mitra S, Foster TH, & Koo H (2009, Feb). Structural and molecular basis of the role of starch and sucrose in Streptococcus mutans biofilm development. Appl Environ Microbiol, 75(3), 837–841. https://doi.org/AEM.01299-08 [pii] 10.1128/AEM.01299-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MI, Xiao J, Heydorn A, & Koo H (2011, Jan 25). An analytical tool-box for comprehensive biochemical, structural and transcriptome evaluation of oral biofilms mediated by mutans streptococci. J Vis Exp (47). 10.3791/2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Andes DR, & Krysan DJ (2018, Dec). Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog, 14(12), e1007342. 10.1371/journal.ppat.1007342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA, Rosalen PL, & Park YK (2005, Nov). Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res, 84(11), 1016–1020. 10.1177/154405910508401109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Xiao J, Klein MI, & Jeon JG (2010, Jun). Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol, 192(12), 3024–3032. 10.1128/JB.01649-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu X, Zhao C, Yan ZM, & Hua H (2016). Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther, 10, 1161–1171. 10.2147/DDDT.S100795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD (2003, Feb). Are dental diseases examples of ecological catastrophes? Microbiology, 149(Pt 2), 279–294. 10.1099/mic.0.26082-0 [DOI] [PubMed] [Google Scholar]
- Martchenko M, Alarco AM, Harcus D, & Whiteway M (2004, Feb). Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell, 15(2), 456–467. 10.1091/mbc.e03-03-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Nakano M (2018, Feb). Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev, 54(1), 22–29. 10.1016/j.jdsr.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreath KJ, Specht CA, & Robbins PW (1995, Mar 28). Molecular cloning and characterization of chitinase genes from Candida albicans. Proc Natl Acad Sci U S A, 92(7), 2544–2548. 10.1073/pnas.92.7.2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooshima T, Matsumura M, Hoshino T, Kawabata S, Sobue S, & Fujiwara T (2001, Jul). Contributions of three glycosyltransferases to sucrose-dependent adherence of Streptococcus mutans. J Dent Res, 80(7), 1672–1677. 10.1177/00220345010800071401 [DOI] [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, & Sobel JD (2016, Feb 15). Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis, 62(4), e1–50. 10.1093/cid/civ933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S, Rao RS, Majumdar B, & Anil S (2015). Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front Microbiol, 6, 1391. 10.3389/fmicb.2015.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PJ, Jones CH, & Bradford PA (2007, Nov). In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn Microbiol Infect Dis, 59(3), 347–349. 10.1016/j.diagmicrobio.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Scheibler E, da Silva RM, Leite CE, Campos MM, Figueiredo MA, Salum FG, & Cherubini K (2018, May). Stability and efficacy of combined nystatin and chlorhexidine against suspensions and biofilms of Candida albicans. Arch Oral Biol, 89, 70–76. 10.1016/j.archoralbio.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Talapko J, Juzbasic M, Matijevic T, Pustijanac E, Bekic S, Kotris I, & Skrlec I (2021, Jan 22). Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J Fungi (Basel), 7(2). 10.3390/jof7020079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems HM, Kos K, Jabra-Rizk MA, & Krom BP (2016, Jul). Candida albicans in oral biofilms could prevent caries. Pathog Dis, 74(5). 10.1093/femspd/ftw039 [DOI] [PubMed] [Google Scholar]
- Xiao J, Hara AT, Kim D, Zero DT, Koo H, & Hwang G (2017, Jun). Biofilm three-dimensional architecture influences in situ pH distribution pattern on the human enamel surface. Int J Oral Sci, 9(2), 74–79. 10.1038/ijos.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, Castillo DA, Campbell F, Davis J, Herzog K, Billings R, Kopycka-Kedzierawski DT, Hajishengallis E, & Koo H (2018). Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries Res, 52(1–2), 102–112. 10.1159/000481833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR 3rd, Heydorn A, & Koo H (2012). The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog, 8(4), e1002623. 10.1371/journal.ppat.1002623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, & Koo H (2010, Jun). Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J Appl Microbiol, 108(6), 2103–2113. 10.1111/j.1365-2672.2009.04616.x [DOI] [PubMed] [Google Scholar]
- Zeng L, & Burne RA (2013, Feb). Comprehensive mutational analysis of sucrose-metabolizing pathways in Streptococcus mutans reveals novel roles for the sucrose phosphotransferase system permease. J Bacteriol, 195(4), 833–843. 10.1128/JB.02042-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Fadaak A, Alomeir N, Wu TT, Rustchenko E, Qing S, Bao J, Gilbert C, & Xiao J (2022). Lactobacillus plantarum Disrupts S. mutans-C. albicans Cross-Kingdom Biofilms. Front Cell Infect Microbiol, 12, 872012. 10.3389/fcimb.2022.872012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Nikitkova A, Abdelsalam H, Li J, & Xiao J (2019, Feb). Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch Oral Biol, 98, 9–16. 10.1016/j.archoralbio.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


