Abstract
Metastatic malignant melanoma (MM) represents a highly aggressive cancer associated with overall poor prognosis. Various anatomic sites can be affected, including the oral cavity and the oropharynx. It may mimic other entities by assuming a variety of clinical appearances and exhibiting a plethora of microscopic variations. Herein, we present a case of a 63-year-old male with a MM metastasizing to the base of tongue, which developed 5 years after the original diagnosis and treatment of cutaneous MM of the chest and heralded its relapse; subsequently, neurological symptoms developed as a result of metastasis to the brain. Diagnostic challenges were encountered, as the tongue lesion clinically masqueraded as a pedunculated reactive lesion and microscopically displayed unusual rhabdoid and neuroendocrine features. Tumor cells expressed S-100, HMB-45, Melan-A, and SOX-10, while most cells with rhabdoid morphology were also positive for myogenin and Myo-D1. Chromogranin and synaptophysin positivity was further noticed in a subset of cells, suggestive of focal neuroendocrine differentiation. Molecular investigation revealed mutations for the BRAF V600E gene. Divergent differentiation of tumor cells may cause diagnostic pitfalls necessitating thorough immunohistochemical analysis. The presence of rhabdoid features and neuroendocrine differentiation are very uncommon, while their co-existence is extremely rare. Better characterization of such microscopic variations in MMs with evaluation of their potential biologic significance is warranted.
Keywords: Malignant melanoma, Metastasis, Base of tongue, Rhabdoid features, Neuroendocrine differentiation
Introduction
Metastatic malignant melanoma (ΜΜ) is a highly aggressive malignancy associated with overall poor prognosis [1]. In the head and neck (HN) area (excluding the skin), primary mucosal MM represents the most common subtype, usually affecting the sinonasal tract (66%) and oral cavity (25%) [1]; in the latter location, the global age-standardized rate of primary mucosal MM does not exceed 0.01 per 100,000 persons-years with the palate (47%) and gingiva (27.6%) being the most frequent sites of involvement [2]. Metastasis of MM to this region occurs rarely with an estimated incidence of 0.6% [3]. The oropharynx, followed by the larynx, is the most common HN site of metastasis from cutaneous MM in the upper aerodigestive tract [4]. It is worth to mention that the presence of metastatic disease in these sites is indicative of disseminated disease and poor prognosis [4].
Metastatic ΜΜ is quite often amelanotic and has a propensity to microscopically mimic other neoplasms by displaying diverse histomorphologic variations [5], many of which are still of unspecified clinical significance. For instance, the rhabdoid phenotype, although infrequent in primary MMs, is relatively more common in metastatic tumors [6], which anyhow display poor outcome. Furthermore, cases of primary and metastatic MMs with aberrant immunophenotypic expression of several non-melanocytic, including neuroendocrine, markers have been described in the literature and may cause diagnostic challenges, while their biologic and prognostic role is questionable [5, 7, 8]; for example, in a recent study evaluating the aberrant expression of neuroendocrine markers in 308 primary and metastatic MMs, no correlation with prognosis was observed [8].
Herein, we present a case of cutaneous MM metastatic to the base of tongue, clinically masquerading as a pedunculated reactive lesion and microscopically exhibiting unusual rhabdoid and neuroendocrine features.
Case Report
A 63-year-old Caucasian male presented for evaluation of a painless mass on the tongue of approximately 5 months duration. The patient reported that the lesion had recently increased in size and changed in color. His current medical history was significant for hypertension and hypothyroidism, both controlled with medications (valsartan/hydrochlorothiazide and thyroxine, respectively); also, he was a heavy smoker (67.5 pack years). Further, the patient was diagnosed 5 years ago with cutaneous MM on the right chest (nodular melanoma, Clark’s level II), which was completely surgically removed, along with excision of the sentinel lymph nodes that were negative. Then, the patient was placed under follow-up by his oncologist with absence of any clinical or imaging signs of relapse for the next 3.5 years; however, he had failed to return for subsequent follow-up (for the last 1.5 years).
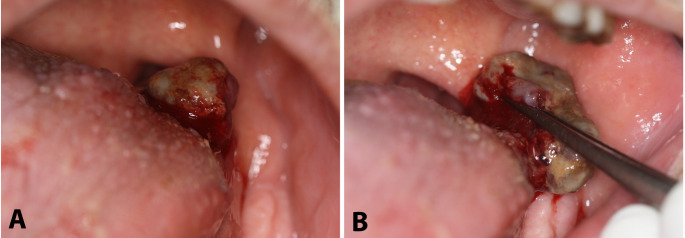
On clinical examination, an exophytic, pedunculated, partially ulcerated, friable hemorrhagic mass of soft consistency involving the left base of the tongue was observed; on palpation, neither induration nor presence of a deeper submucosal component were detected (Fig. 1a and b). No palpable cervical lymph nodes were noticed. The differential diagnosis mainly included pyogenic granuloma or other reactive soft tissue lesions; the possibility of a primary or metastatic malignancy with an unusual clinical appearance was also considered. Due to the exophytic morphology, friable consistency, and presence of a narrow peduncle, an excisional biopsy was performed. Intraoperatively, no extension of the lesion below the level of the peduncle was noticed.
Fig. 1.
Clinical examination. a and b) Exophytic, pedunculated, partially ulcerated, friable hemorrhagic mass of soft consistency involving the left base of tongue
On gross examination, a brownish, somewhat lobulated, solid mass of soft consistency measuring 2.5 × 1.5 × 1.5 cm was observed; the cut surfaces were brown to tan (Fig. 2a and b). Histopathologically, the connective tissue was diffusely occupied by epithelioid neoplastic cells with pleomorphism, prominent eosinophilic nucleoli, and atypical mitotic activity, organized in solid sheets (Fig. 3a and b). A brown pigment (consistent with melanin granules) was recognized diffusely within the cytoplasm of the tumor cells (Fig. 3b). Nests of large polygonal rhabdoid cells with eccentric nuclei and abundant eosinophilic cytoplasm (Fig. 3c and d), as well as cells with plasmacytoid features, were detected. Occasionally, small cells forming clusters or rosette-like structures were discerned (Fig. 3e). Despite the proximity of the tumor cells to the overlying epithelium, proliferation of malignant melanocytes along the junction of the epithelium and connective tissue (“junctional activity”) was absent (Fig. 3f).
Fig. 2.

Gross examination. a) Partially ulcerated and somewhat lobulated mass of brownish color measuring 2.5 × 1.5 × 1.5 cm. b) Brown to tan cut surfaces of the solid tumor mass
Fig. 3.
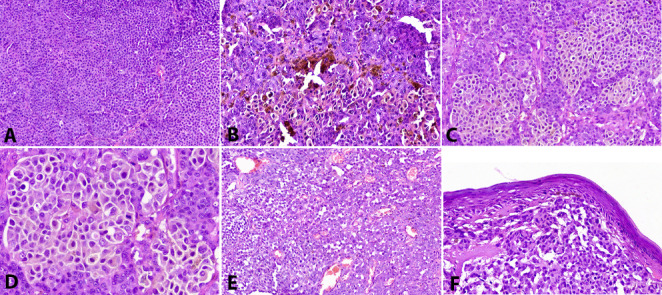
Histopathologic examination. a) Diffuse infiltration by malignant cells organized in solid sheets. b) Diffuse melanin pigmentation within the cytoplasm of tumor cells, which show predominant epithelioid features, pleomorphism, prominent eosinophilic nucleoli, and atypical mitotic activity. c and d) Large polygonal rhabdoid cells with eccentric nuclei and abundant eosinophilic cytoplasm forming nests. e) Clusters of small cells with neuroendocrine features. f) Absence of junctional activity. Hematoxylin and eosin, initial magnification 200x
Immunohistochemical analysis showed that the tumor cells were intensely and diffusely positive for melanocytic markers S-100 (Fig. 4a), HMB-45 (Fig. 4b), Melan-A (Fig. 4c), and SOX-10 (Fig. 4d). A subpopulation of cells with rhabdoid morphology were positive for myogenin (Fig. 4e) and Myo-D1 (Fig. 4f), while desmin was not expressed. Focal expression of synaptophysin (Fig. 4 g) and chromogranin (Fig. 4 h) was suggestive of the neuroendocrine differentiation of a minority of the tumor cell population, while CD56 was negative.
Fig. 4.

Immunohistochemical analysis. Diffuse and intense positivity of tumor cells for S-100 (a), HMB-45 (b), Melan-A (c), and SOX-10 (d). Cells with rhabdoid morphology expressed myogenin (e) and Myo-D1 (f). Focally, a minority of cells were positive for synaptophysin (g) and chromogranin (h), suggestive of neuroendocrine differentiation
The microscopic evaluation - in accordance with the patient’s previous history - rendered a final diagnosis of metastatic MM to the base of tongue; the presence of rhabdoid features and focal neuroendocrine differentiation was also acknowledged. Unfortunately, due to unavailability of the microscopic slides of the original skin lesion, direct comparison of the histopathologic features of the two tumors, including the possible presence of rhabdoid and/or prominent neuroendocrine differentiation in the primary cutaneous MM, was not possible. Further molecular analysis of the mucosal tumor revealed mutations for the BRAFV600E gene.
On the 2-week follow-up appointment, complete healing was noted with no evidence of any residual lesion in the left base of tongue or anywhere else in the mouth (Fig. 5). However, the patient reported that, approximately one week after his first appointment, he was hospitalized due to a fainting episode; a brain computed tomography (CT) showed the presence of a well-defined multilobular lesion in the left frontal lobe with a maximum diameter of 24.5 mm, exhibiting a central hypodense (necrotic) area and perilesional edema. The aforementioned findings were consistent with brain metastasis of probable cutaneous MM origin. Subsequently, magnetic resonance imaging (MRI) of the HN confirmed the presence of nodular alterations in the brain parenchyma, consistent with metastatic disease; there was no evidence of any residual tumor in the base of tongue or elsewhere and cervical lymph nodes were within normal limits. The patient was referred to his oncologist for further staging and treatment; however, he was unfortunately lost to follow-up.
Fig. 5.
Complete healing with no evidence of any residual lesion in the left base of tongue
Discussion
Cutaneous MM may spread via hematogenous or lymphatic routes through three major pathways of metastasis: in almost half of the cases, metastatic disease develops in the regional lymph nodes, whereas less common types include “satellite/in-transit metastases” and direct distant metastases [9]. Interestingly, it has been reported that patients with primary cutaneous MM affecting the trunk developed distant metastasis more commonly compared to patients with other primary cutaneous sites [10]. Similarly, in the case presented here, our patient with primary cutaneous MM of the chest developed distant metastases to the base of the tongue and, based on imaging findings, to the brain parenchyma. It is noteworthy that these metastatic lesions were detected due to clinical manifestations developing around the same time and almost five years since the original diagnosis, while no evidence of relapse had been observed until the last follow-up. The time period from the diagnosis of a primary MM to the onset of metastatic disease depends on the type of metastasis. Direct distant metastases have been reported to occur after a median period of 25 months after the initial diagnosis, later than direct regional lymph node metastases (16 months) and direct satellite/in-transit metastases (17 months) [9]. Interestingly, the time of distant metastases development is independent of the metastatic pathway, indicating that hematogenous spread may take place irrespective of lymph node involvement [9]. Specifically, in the oral and oropharyngeal area, this time period varies with a reported median time of 3 years [4]. Unfortunately, in the present case, the lack of proper follow-up for the last 1.5 years may have contributed to the late discovery of the metastatic spreading.
Oral metastases are relatively rare; however, they may represent the first sign of a primary undetected malignancy in up to 23% of cases. According to large epidemiological studies, a male predilection is observed, while the jawbones are more commonly affected than the oral soft tissues [11, 12]. The clinical appearance of the tongue lesion of our patient was rather deceptive and indicative more of a soft tissue tumor of benign, likely reactive, origin, due to its exophytic and pedunculated morphology and lack of a deeper invasive component. Nonetheless, malignancy could not be ruled out, since some clinical features, including the relatively large size, ulceration, and hemorrhage, could be related to either a reactive lesion, such as pyogenic granuloma, or a primary or metastatic malignant tumor. Regarding the latter, metastatic disease in the oral cavity and oropharynx has indeed been reported to mimic reactive lesions and MM is not an exception to this rule, especially when the tongue and/or gingiva are affected and melanin pigmentation is not present [11, 13]. In this context, several cases of MM metastatic to the tongue have been reported in the literature. Markman et al. in 2018 [14] described 2 cases of MM metastasis to the tongue, noticing their similarity with reactive lesions, as well as the late onset of metastatic disease to the tongue. Furthermore, the authors performed a literature review on MM cases metastasizing to the tongue; the majority of patients were in their 6th or 7th decade of life and all metastatic tumors originated from cutaneous MMs with a mean time of 4.9 years from initial diagnosis to metastasis development, consistent with the present case [14]. In our case, an excisional biopsy was performed, due to the ease of complete removal just below the level of a narrow stalk, taking also into account that the hemorrhagic and friable consistency of the lesion could hamper an incisional biopsy procedure, possibly resulting in fragmented and inadequate tissue for histopathologic examination. A similar potential problem is also encountered in other malignant neoplasms in the oropharyngeal area, leading to the performance of multiple biopsies in order to render a final diagnosis [15, 16].
The tendency of MM to mimic other lesions is not limited to its clinical manifestations, as this neoplasm, both as primary and metastatic tumor, has a propensity to microscopically resemble other neoplasms by exhibiting morphological and/or immunophenotypic diversity [5]. Histopathologically, this divergent differentiation/dedifferentiation may include spindle, epithelioid, plasmacytoid, schwannian, and/or rhabdoid morphology; in addition, MMs have been reported to immunohistochemically express several non-melanocytic proteins [5, 17]. In a recent study, Agaimy et al. [17] described several cases of the so called “dedifferentiated MMs”, i.e. MMs with histological and/or immunohistochemical transition from a conventional to an atypical tumoral component. Moreover, a significant number of such cases exhibit loss of classic melanocytic markers, further adding to the diagnostic conundrum, as several undifferentiated/pleomorphic sarcomas or carcinomas might be included in the differential diagnosis [17]. On the other hand, the demonstration of melanocytic origin, either via immunohistochemical positivity for melanocytic markers and/or the presence of melanin, does not exclude the possibility of occult soft tissue tumors with melanocytic differentiation, such as melanocytic epithelioid Malignant Peripheral Nerve Sheath Tumors (eMPNST) [18]. In such equivocal cases, the inclusion of a broad panel of immunohistochemical markers for MM, such as HMB-45 which is negative in melanocytic eMPNST, as well as molecular investigation for MM specific BRAF mutations, are essential steps to establish a final diagnosis of MM [19].
In the present case, the final diagnosis of MM was rather straightforward, based on the following: (1) the vast majority of the tumor cells displayed typical MM histopathology with predominance of epithelioid and, less so, plamacytoid cells and a characteristic presence of melanin pigment, while the “dedifferentiated component” (rhabdoid morphology and/or neuroendocrine differentiation) represented a restricted area of the lesion, (2) there was retention of the immunohistochemical positivity of multiple markers of melanocytic origin, and (3) a MM-compatible gene mutation (BRAFV600E); all these findings lead to an unequivocal diagnosis of MM.
Another question about the MM case presented here revolved around its primary or metastatic origin. The previous history of cutaneous MM, diagnosed and managed 5 years ago, readily raised the strong possibility that the mucosal tumor represented a metastasis from the cutaneous primary. The fact that, at almost the same time with the detection of the mucosal tumor, a brain metastasis was also discovered, further strengthened the scenario of disseminated metastatic disease, the primary source of which was likely to be the original cutaneous tumor. As mentioned before, cutaneous MMs are known to metastasize even many years after the original diagnosis, including metastatic spread to the tongue [14]; in one large study, median time to distant metastasis from cutaneous MM was 40.5 months for women and 33 months for men [10]. Even though the possibility that the mucosal MM could represent an independent primary tumor could not be excluded with absolute certainty, the presence of intact surface epithelium and, mainly, the lack of junctional activity and/or intraepithelial involvement strongly supported its metastatic origin [20]. There were several other findings that also favored the diagnosis of metastasis of the cutaneous tumor, including the location of the lesion, with the tongue being one of the most common sites for MM metastasis [14, 20], the presence of BRAFV600E mutation, which is rare in primary mucosal MMs of the HN in contrast to MMs of cutaneous origin [21, 22], as well as the clinical features of the lesion, i.e. the exophytic pedunculated morphology resembling a reactive lesion [1, 11, 14]. On the other hand, the unavailability of the microscopic slides of the original tumor did not allow us to directly compare the histopathologic features of the cutaneous and mucosal tumors and to assess whether the divergent differentiation seen in the metastatic tumor was also present in the primary tumor.
Despite the predominance of typical microscopic and immunohistochemical MM features, the unusual findings of rhabdoid morphology with expression of skeletal muscle markers, as well as the focal positivity for neuroendocrine markers, was in accord with phenotypic heterogeneity of MMs. To assess the frequency of these phenotypic diversions among mucosal MMs of the HN, we conducted a comprehensive review of the pertinent English-language literature on primary and metastatic MMs affecting the mucosal surfaces of the HN and demonstrating (a) rhabdoid features and/or (b) neuroendocrine differentiation (Table 1a and 1b) [22–27]. Tumors (primary or metastatic) involving other (non-mucosal) sites of the HN, such as skin, lymph nodes, or salivary glands, were not included. Moreover, primary and metastatic MMs with co-existence of neuroendocrine and rhabdoid features regardless of their anatomic location were also recorded (Table 2) [28–32]. Large epidemiologic studies lacking sufficient individual clinicopathologic data were excluded.
Table 1.
a. Primary and metastatic mucosal malignant melanomas of the head and neck* with rhabdoid morphology
| Year | Author | No of cases | Age (years) | Gender | Site of involvement | Primary/Metastatic | Symptoms | Melanin pigment | Immunohistochemical profile | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | Chraybi et al. [22] | 3 | 80 | Male | Nasal cavity and cavum | Primary | Anosmia and nasal obstruction | Present |
Melanocytic: Melan-A +, HMB-45 + Rhabdoid: NP |
NR | NR |
| 68 | Female | Nasal cavity and ethmoid sinus | Primary | Epistaxis and nasal obstruction | Present |
Melanocytic: Melan-A +, HMB-45 + Rhabdoid: NP |
NR | NR | |||
| 58 | Female | Nasal cavity | Primary | Epistaxis | Present |
Melanocytic: Melan-A +, HMB-45 + Rhabdoid: NP |
NR | NR | |||
| 2015 | Smith et al. [23] | 1 | 70 | Male | Sphenoid sinus | Primary | Headache, lightheadedness, lassitude, blurry vision, diplopia | NR |
Melanocytic: S-100 +, Melan-A +, HMB-45 +, tyrosinase + Rhabdoid: desmin +, Myo-D1 -, myogenin - |
Surgery and radiation therapy (ipilumamab for metastatic disease) | DOD 7 months post-surgery due to multifocal metastases |
| 2021 | Present case | 1 | 63 | Male | Base of tongue |
Metastatic (Primary: Cutaneous malignant melanoma of the chest, 5 years ago) |
Discomfort | Present |
Melanocytic: S-100 +, Melan-A +, HMB-45 +, SOX-10 + Rhabdoid: Myo-D1 +, myogenin +, desmin - |
NR | One week after presentation diagnosed with brain metastasis (due to fainting) |
NR: not reported; DOD: died of disease; LN: lymph nodes; NP: not performed
*Tumors involving non-mucosal head and neck sites, such as skin, lymph nodes, and salivary glands, were not included
Table 1.
b. Primary and metastatic mucosal malignant melanomas of the head and neck* with neuroendocrine differentiation
| Year | Author | No of cases | Age (years) | Gender | Site of involvement | Primary/Metastatic | Symptoms | Melanin pigment | Immunohistochemical profile* | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | Coli et al. [24] | 1 | 60 | Male | Nasal cavity | Primary | Epistaxis and nasal obstruction | Almost totally absent |
Melanocytic: S-100 +, Melan-A +, HMB-45 + Neuroendocrine: synaptophysin +, NSE +, chromogranin - |
Surgery, radiation therapy | No recurrence 3 years post treatment |
| 2005 | Eyden et al. [25] | 1 | 67 | Male | Inferior turbinate | Primary | NR | Absent | Melanocytic: S-100 +, Melan-A +, HMB-45 +, tyrosinase + Neuroendocrine: synaptophysin +, chromogranin +, CD-56 + | NR | LN metastasis |
| 2011 | Lee et al. [26] | 4 | 74 | Female | Paranasal sinus | Primary | Nasal obstruction | Almost totally absent |
Melanocytic: S-100 +, Melan-A +, HMB-45 + Neuroendocrine: chromogranin +, synaptophysin - |
NS | Multiple liver metastases |
| 79 | Male | Nasal cavity | Primary | Epistaxis | Present |
Melanocytic: S-100+, Melan-A+, HMB-45+ Neuroendocrine: synaptophysin +, chromogranin + |
NS | Neck LN metastasis | |||
| 54 | Female | Nasal cavity and paranasal sinuses | Primary | Epistaxis and nasal obstruction | Almost totally absent |
Melanocytic: S-100 +, Melan-A +, HMB-45 + Neuroendocrine: chromogranin +, synaptophysin - |
NS | Neck LN metastasis and local spread | |||
| 72 | Female | Nasal cavity and paranasal sinus | Primary | Epistaxis and nasal obstruction | Almost totally absent |
Melanocytic: S-100 +, Melan-A +, HMB-45 + Neuroendocrine: chromogranin +, synaptophysin - |
NS | Multiple local recurrences and tracheal metastases | |||
| 2020 | Kaur et al. [27] | 1 | 63 | Female | Nasopharyngeal space | Primary | Epistaxis | Absent | Melanocytic: S-100 +, Melan-A +, HMB-45 +, SOX-10 + Neuroendocrine: synaptophysin +, chromogranin +, CD56 + | Surgery, radiation therapy | Recurrence 7 months later |
| 2021 | Present case | 1 | 63 | Male | Base of tongue |
Metastatic (Primary: Cutaneous malignant melanoma of the chest, 5 years ago) |
Discomfort | Present | Melanocytic: S-100 +, Melan-A +, HMB-45 +, SOX-10 +, Neuroendocrine: synaptophysin +, chromogranin +, CD56 - | NR | One week after presentation diagnosed with brain metastases (due to fainting) |
NR: not reported; LN: lymph node; NS: not specified
*Tumors involving non-mucosal head and neck sites, such as skin, lymph nodes, and salivary glands, were not included
Table 2.
Primary and metastatic malignant melanomas of any location with co-existence of rhabdoid features and neuroendocrine differentiation
| Year | Author | No of cases | Age (years) | Gender | Site of involvement | Primary/ Metastatic |
Melanin pigment | Immunohistochemical markers | Treatment | Follow -up |
|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | Abbott et al. [28] | 1 | 62 | Male | Lung |
Possibly metastatic (of unknown primary) |
Absent |
Melanocytic: S-100 +, Melan-A -, HMB-45 -, tyrosinase - Neuroendocrine: CD56 +, synaptophysin - Rhabdoid: desmin - |
NR | NR |
| 2014 | Shenjere et al. [29] | 1 | 51 | Female | Cervix | Primary | Present |
Melanocytic: S-100 +, Melan-A +, HMB-45 + Neuroendocrine: CD56 +, desmin + Rhabdoid: myogenin + |
Surgery and chemotherapy | Widespread pelvis disease 10 months post treatment |
| 2015 | Fernandez-Vega et al. [30] | 1 | 80 | Male | Forehead and scalp | Primary | Absent |
Melanocytic: S-100 +, SOX-10 +, p75NGFR +, Melan-A -, HMB-45 - Neuroendocrine: CD56 +, NSE +, synaptophysin - Rhabdoid: desmin +, Myo-D1 - |
Surgery and radiation therapy | DOD 2 months after treatment |
| 2016 | Prieto-Torres et al. [31] | 1 | 69 | Female | Scapula | Primary | Absent |
Melanocytic: S-100 +, SOX-10 +, Melan-A -, HMB-45 -, MITF - Neuroendocrine: synaptophysin + Rhabdoid: desmin +, Myo-D1 -, myogenin -, myoglobin - |
Surgery | No recurrence or metastatic disease after 14 months |
| 2019 | Murakami et al. [32] | 1 | 78 | Male | Forehead | Primary | Absent |
Melanocytic: S-100 +, Melan-A +, HMB-45 + Neuroendocrine: CD56 +, NSE + Rhabdoid: desmin - |
Surgery | No recurrence 24 months after treatment |
| 2021 | Present case | 1 | 63 | Male |
Base of tongue |
Metastatic (Primary: Cutaneous malignant melanoma of the chest, 5 years ago) |
Present |
Melanocytic: S-100 +, Melan-A +, HMB-45 +, SOX-10 + Neuroendocrine: synaptophysin +, chromogranin +, CD56 – Rhabdoid: MyoD1 +, myogenin +, desmin - |
NR | One week after presentation diagnosed with brain metastases (due to fainting) |
NR: not reported; DOD: died of disease
The descriptive term “rhabdoid” has been designated to cells with eosinophilic cytoplasm, eccentrically placed nuclei, and globular hyaline inclusions, bearing resemblance to rhabdomyoblasts, and was initially described in childhood renal neoplasms [33]. Since their first description, they have been observed in several soft tissue neoplasms, while their presence in primary or metastatic MM has been well-documented [6, 34]. The rhabdoid appearance that MM cells might exhibit has been attributed to the inclusion of paranuclear whorls of intermediate filaments, such as desmin or vimentin or changes in the endoplasmic reticulum [5]. The presence of this morphology is mainly observed in metastatic and/or recurrent forms of MM and rarely in primary MMs [5, 6]. Nevertheless, there is a considerable discrepancy regarding the terminology used to refer to MMs with rhabdoid cells; several authors have used interchangeably the terms “rhabdomyoblastic/rhabdomyosarcomatous” for tumors expressing striated muscle markers, with the most reliable being desmin and myogenin [35]. Other authors prefer the term “MM with rhabdoid features/rhabdoid MM”, relying solely on the morphological presence of rhabdoid cells, regardless of their immunophenotype [22, 36]. In this review, we decided to include cases with morphological description of rhabdoid cells, even if muscle markers were negative or not thoroughly assessed.
Our review revealed only 4 cases of primary mucosal MMs with rhabdoid morphology in the HN area (Table 1a) [22, 23]. Noticeably, the present case is the first metastatic MM with rhabdoid features to affect a HN mucosal site reported up to this date. Along with our patient, the mean age of patients with HN mucosal MM with rhabdoid morphology was 67.8 years (median age: 68 years, age range: 58–80 years) with a 3:2 male to female ratio. With the exception of our case, the nasal cavity and paranasal sinuses were involved, causing variable symptomatology, such as nasal obstruction and epistaxis. Microscopically, melanin pigment was frequently present. In terms of immunohistochemistry, desmin was detected in one case [23] and myogenin and Myo-D1 in the present case. Notwithstanding, Chraybi et al. [22] did not investigate the expression of any myogenic marker in the studied rhabdoid tumors. In the only case with available treatment and follow-up information [23], the patient was treated with surgery and radiotherapy; however, multifocal metastatic disease was discovered 4 months later and, despite ipilimumab administration, the patient succumbed to the disease 7 months post-surgery.
Another feature of morphological heterogeneity in the case presented here was the presence of small cells forming clusters or rosette-like structures, similar to those seen in neoplasms of neuroendocrine origin. This prompted further immunohistochemical evaluation of neuroendocrine markers, revealing areas of expression of synapthophysin and chromogranin in tumor cells, consistent with focal neuroendocrine differentiation.
There is a rarity of studies reporting on MMs with neuroendocrine features, while the exact mechanism of acquisition of a neuroendocrine immunophenotype by MM cells has not been elucidated [5]. However, it is well established that normal melanocytes possess neuroendocrine properties, owing to their neural crest origin, while cases of MMs producing neurotransmitters and hormones, reminiscent of neuroendocrine/carcinoid syndromes, have been reported in the literature [37–39].
Expression of at least one pertinent marker is mandatory to confirm neuroendocrine differentiation of tumor cells; among these markers, synaptophysin represents the most sensitive and specific, while CD56 the least specific one [40]. Although variable results have been reported, synaptophysin seems to be the most frequently and reliably positive marker to reveal neuroendocrine features in MMs [24, 25, 41, 42]. Regarding mucosal MMs of the HN possessing a neuroendocrine phenotype (Table 1b) [24–27], 7 additional cases have been reported to this date in the literature, all of which were primary tumors. Therefore, our case represents the first metastatic mucosal MM of the HN with neuroendocrine features to be described up to this date. Including our case, the mean age of patients with HN mucosal MM with neuroendocrine differentiation was 66.5 years (median age: 65 years, age range: 54–79 years), while no gender predilection was noticed. The nasal cavity with or without the involvement of paranasal sinuses was the most common site to be affected, frequently causing epistaxis and nasal obstruction; one case was located in the nasopharynx, while our case was the first one to affect the oropharynx and specifically the base of tongue. Most authors did not provide sufficient information regarding the applied therapeutic methods in those patients, while all but one case [24] developed recurrence and/or metastasis. It is worth to mention that the majority of tumors were amelanotic or presented with very limited melanin pigment, while synaptophysin and chromogranin were the most commonly used markers for the identification of cells displaying neuroendocrine characteristics, exhibiting positivity in 5/8 and 7/8 cases, respectively. In addition to these cases, Thompson et al. [42] reported that in a large series of 115 sinonasal tract MMs, 13 (13.3%) were positive for synaptophysin, while none of them exhibited chromogranin reactivity; further, positivity for NSE and CD-56 was present in 49 (46.2%) and 8 (7.5%) cases, respectively.
Sensitivity of melanocytic markers (Melan-A or MART-1, HMB-45, SOX10, tyrosinase, and MITF), as well as S-100 protein, varies among different morphological types of MM [1]. In mucosal HN cases of MM with rhabdoid or neuroendocrine features (Table 1), these markers usually retained their positivity. However, it is prudent to perform a broad panel of markers of melanocytic differentiation, when uncommon morphological features are encountered.
Even though this phenomenon might be rather underreported, cases of simultaneous rhabdoid and neuroendocrine differentiation in primary or metastatic MMs are scarce in the literature and, to this date, no such cases have ever been described in the HN region. The available data of previously published cases in other anatomic locations are summarized in Table 2 [28–32]. Our review yielded only 5 additional cases involving several areas of the human body, the skin of the forehead appearing to be the most common site [30, 32]. Including our case, MMs with co-existent rhabdoid and neuroendocrine features showed a male predilection (4:2 male to female ratio) with a mean age of 67.2 years (median age: 66 years, age range: 51–80 years). Four out of 6 tumors were primary, hence the current case is only the second reported metastatic MM combining such peculiar features to be described up to this date. The majority (4/6) of the tumors were amelanotic, while loss of classic melanocytic markers may not be an unusual phenomenon in such cases. Based on our findings, Melan-A and HMB-45 were negative in 3 out of 6 these MMs [28, 30, 31], while loss of tyrosinase [28] and MITF [31] were also reported. In contrast to our case, CD56 positivity revealed the neuroendocrine differentiation of MM cells in 4 cases [28–30, 32], while desmin was the striated muscle marker most frequently expressed by cells with rhabdoid morphology (3/6 tumors) [29–31]. In terms of treatment strategy, surgical excision was performed in all 4 cases with available information [29–32], followed by adjuvant chemotherapy [29] or radiation therapy [30] in two of them. Follow-up data were available in only 4 cases [29–32], with poor prognosis (widespread disease or death of disease) in 2 of them [29, 30].
Conclusions
Divergent differentiation of MM cells may cause diagnostic pitfalls necessitating thorough immunohistochemical analysis. Uncommon histopathologic variations of MMs such as rhabdoid phenotype and neuroendocrine differentiation require better characterization, including evaluation of their potential biologic role and prognostic significance.
Acknowledgements
None.
Authors’ contributions
All authors contributed to the study conception and design. All authors commented on previous versions and contributed to the preparation of the last version of the manuscript. All authors read and approved the final manuscript. Conceptualization: [Konstantinos Tzanavaris], [Efstathios Pettas], [Nikolaos G. Nikitakis]; Methodology: [Konstantinos Tzanavaris], [Efstathios Pettas], [Grigorios Thermos], [Maria Georgaki], [Nikolaos G. Nikitakis]; Formal analysis and investigation: [Konstantinos Tzanavaris], [Efstathios Pettas], [Maria Georgaki], [Nikolaos G. Nikitakis]; Writing - original draft preparation: [Konstantinos Tzanavaris], [Efstathios Pettas], [Grigorios Thermos]; Writing - review and editing: [Evangelia Piperi], [Nikolaos G. Nikitakis]; Supervision: [Nikolaos G. Nikitakis]
Funding
The authors have no funding or other financial relationship related to this manuscript. The authors did not receive support from any organization for the submitted work. No funds, grants, or other support was received.
Availability of data and material (data transparency)
Department of Oral Medicine & Pathology and Hospital Dentistry, School of Dentistry, National and Kapodistrian University of Athens, Athens, Greece.
Code Availability (software application or custom code)
Not Applicable.
Declarations
Conflicts of interest/Competing interests
All authors declare that they have no conflict of interest to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki.
Consent to participate
No informed consent to participate was necessary due to retrospective nature of the case and since only intra-oral images and pathology slides were used.
Consent for publication
Informed consent for the anonymous use of data is obtained as a standard procedure.
Footnotes
This work was presented at the 2021 American Academy of Oral and Maxillofacial Pathology (AAOMP) Annual Virtual Meeting & Continuing Education Program (May 22–25, 2021).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Konstantinos Tzanavaris, Email: kontzanavaris@gmail.com.
Efstathios Pettas, Email: stathis.pettas12@gmail.com.
Grigorios Thermos, Email: greg.thermos@gmail.com.
Maria Georgaki, Email: mar1georgaki@gmail.com.
Evangelia Piperi, Email: liapiperi@dent.uoa.gr.
Nikolaos G. Nikitakis, Email: nnikitakis1@yahoo.com
References
- 1.Williams MD. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Mucosal Melanomas. Head Neck Pathol. 2017;11:110–7. doi: 10.1007/s12105-017-0789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sortino-Rachou AM, Cancela Mde C, Voti L, Curado MP. Primary oral melanoma: population-based incidence. Oral Oncol. 2009;45:254–8. doi: 10.1016/j.oraloncology.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Henderson LT, Robbins KT, Weitzner S. Upper Aerodigestive Tract Metastases in Disseminated Malignant Melanoma. Arch Otolaryngol Neck Surg. 1986;112:659–63. doi: 10.1001/archotol.1986.03780060071011. [DOI] [PubMed] [Google Scholar]
- 4.Mifsud M, Padhya TA. Metastatic melanoma to the upper aerodigestive tract: A Systematic Review of the Literature. Laryngoscope. 2014;124:1143–9. doi: 10.1002/lary.24436. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119–29. doi: 10.1111/j.1365-2559.2007.02823.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang ES, Wick MR, Swanson PE, Dehner LP. Metastatic Malignant Melanoma with “Rhabdoid” Features. Am J Clin Pathol. 1994;102:426–31. doi: 10.1093/ajcp/102.4.426. [DOI] [PubMed] [Google Scholar]
- 7.Feely C, Theaker J. Epithelial markers in primary sinonasal mucosal melanoma. Histopathology. 2004;45:96–8. doi: 10.1111/j.1365-2559.2004.01824.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Lai Y, Zhang M, Li Z. Prognostic significance of the aberrant expression of neuroendocrine markers in melanomas. Diagn Pathol. 2021;16:78. doi: 10.1186/s13000-021-01135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, Garbe C. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147:62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 10.Mervic L. Time course and pattern of metastasis of cutaneous melanoma differ between men and women. PLoS ONE. 2012;7:e32955. doi: 10.1371/journal.pone.0032955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity - Pathogenesis and analysis of 673 cases. Oral Oncol. 2008;44:743–52. doi: 10.1016/j.oraloncology.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Oliver-Puigdomènech C, González-Navarro B, Polis-Yanes C, Estrugo-Devesa A, Jané-Salas E, López-López J. Incidence rate of metastases in the oral cavity: a review of all metastatic lesions in the oral cavity. Med Oral Patol Oral Cir Bucal. 2021;26:e619-25. doi: 10.4317/medoral.24625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares CD, Carlos R, Andrade BAB, Cunha JLS, Agostini M, Romañach MJ, Hernandez-Guerrero JC, Mosqueda-Taylor A, Almeida OP, Jorge J. Oral Amelanotic Melanomas: Clinicopathologic Features of 8 Cases and Review of the Literature. Int J Surg Pathol. 2021;29:263–72. doi: 10.1177/1066896920946435. [DOI] [PubMed] [Google Scholar]
- 14.Markman RL, Rosa GAB, Cardili L, Simonato LE, Brandão TB, Ribeiro ACP. Tongue metastasis of cutaneous melanoma: Report of two cases and literature review. J Clin Exp Dent. 2018;10:e1130-4. doi: 10.4317/jced.54980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolgar JA, Triantafyllou A. Pitfalls and procedures in the histopathological diagnosis of oral and oropharyngeal squamous cell carcinoma and a review of the role of pathology in prognosis. Oral Oncol. 2009;45:361–85. doi: 10.1016/j.oraloncology.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Lam MT, O’Sullivan B, Gullane P, Huang SH. Challenges in establishing the diagnosis of human papillomavirus-related oropharyngeal carcinoma. Laryngoscope. 2016;126:2270–5. doi: 10.1002/lary.25985. [DOI] [PubMed] [Google Scholar]
- 17.Agaimy A, Stoehr R, Hornung A, Popp J, Erdmann M, Heinzerling L, Hartmann A. Dedifferentiated and undifferentiated melanomas: Report of 35 new cases with literature review and proposal of diagnostic criteria. Am J Surg Pathol. 2021;45:240–54. doi: 10.1097/PAS.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 18.Tanas MR, Rubin BP. Malignant neuroectodermal tumor with melanocytic and rhabdomyoblastic differentiation. Rare Tumors. 2009;1:e26. doi: 10.4081/rt.2009.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaspard M, Lamant L, Tournier E, et al. Evaluation of eight melanocytic and neural crest-associated markers in a well-characterised series of 124 malignant peripheral nerve sheath tumours (MPNST): useful to distinguish MPNST from melanoma? Histopathology. 2018;73:969–82. doi: 10.1111/his.13740. [DOI] [PubMed] [Google Scholar]
- 20.Billings KR, Wang MB, Sercarz JA, Fu YS. Clinical and pathologic distinction between primary and metastatic mucosal melanoma of the head and neck. Otolaryngol Head Neck Surg. 1995;112:700–6. doi: 10.1016/S0194-5998(95)70179-6. [DOI] [PubMed] [Google Scholar]
- 21.López F, Rodrigo JP, Cardesa A, Triantafyllou A, Devaney KO, Mendenhall WM, Haigentz M, Jr, Strojan P, Pellitteri PK, Bradford CR, Shaha AR, Hunt JL, de Bree R, Takes RP, Rinaldo A, Ferlito A. Update on primary head and neck mucosal melanoma. Head Neck. 2016;38:147–55. doi: 10.1002/hed.23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chraybi M, Abd Alsamad I, Copie-Bergman C, Baia M, André J, Dumaz N, Ortonne N. Oncogene abnormalities in a series of primary melanomas of the sinonasal tract: NRAS mutations and cyclin D1 amplification are more frequent than KIT or BRAF mutations. Hum Pathol. 2013;44:1902–11. doi: 10.1016/j.humpath.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SM, Schmitt AC, Carrau RL, Iwenofu OH. Primary sinonasal mucosal melanoma with aberrant diffuse and strong desmin reactivity: a potential diagnostic pitfall! Head Neck Pathol. 2015;9:165–71. doi: 10.1007/s12105-014-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coli A, Giacomini PG, Bigotti G, Ferraro S, Alessandrini M, Del Vecchio M, Massi G. Aberrant neurofilament protein and synaptophysin expression in malignant melanoma of the nasal cavity. Histopathology. 2004;44:193–5. doi: 10.1111/j.1365-2559.2004.01784.x. [DOI] [PubMed] [Google Scholar]
- 25.Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402–9. doi: 10.1111/j.1365-2559.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Torres FX, McLean SA, Chen R, Lee MW. Immunophenotypic heterogeneity of primary sinonasal melanoma with aberrant expression of neuroendocrine markers and calponin. Appl Immunohistochem Mol Morphol. 2011;19:48–53. doi: 10.1097/PAI.0b013e3181ee8dcb. [DOI] [PubMed] [Google Scholar]
- 27.Kaur K, Kakkar A, Rastogi S, Sharma MC. Sinonasal amelanotic melanoma with neuroendocrine differentiation: a diagnostic conundrum. Ultrastructural Pathol. 2020;44:249–54. doi: 10.1080/01913123.2020.1740367. [DOI] [PubMed] [Google Scholar]
- 28.Abbott JJ, Amirkhan RH, Hoang MP. Malignant melanoma with a rhabdoid phenotype: histologic, immunohistochemical, and ultrastructural study of a case and review of the literature. Arch Pathol Lab Med. 2004;128:686–8. doi: 10.5858/2004-128-686-MMWARP. [DOI] [PubMed] [Google Scholar]
- 29.Shenjere P, Fisher C, Rajab R, Patnaik L, Hazell S, Thway K. Melanoma with rhabdomyosarcomatous differentiation: two further cases of a rare pathologic pitfall. Int J Surg Pathol. 2014;22:512–9. doi: 10.1177/1066896914531817. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Vega I, Santos-Juanes J, Fresno-Forcelledo MF. Primary amelanotic rhabdoid melanoma of the forehead. Br J Dermatol. 2016;174:1156–8. doi: 10.1111/bjd.14382. [DOI] [PubMed] [Google Scholar]
- 31.Prieto-Torres L, Alegría-Landa V, Llanos C, Córdoba A, Kutzner H, Requena L. Cutaneous Malignant Melanoma with Rhabdoid Morphology and Smooth Muscle Differentiation: A Challenging Histopathologic Diagnosis. Am J Dermatopathol. 2017;39:397–403. doi: 10.1097/DAD.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 32.Murakami T, Ogata D, Arai E, Tsuchida T. Case of primary hypomelanotic rhabdoid melanoma on the forehead. J Dermatol. 2019;46:e278–9. doi: 10.1111/1346-8138.14838. [DOI] [PubMed] [Google Scholar]
- 33.Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney: A distinctive renal tumor of children. Hum Pathol. 1981;12:646–57. doi: 10.1016/S0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- 34.Borek BT, McKee PH, Freeman JA, Maguire B, Brander WL, Calonje E. Primary Malignant Melanoma With Rhabdoid Features: A Histologic and Immunocytochemical Study of Three Cases. Am J Dermatopathol. 1998;20:123–7. doi: 10.1097/00000372-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Franchi A, Flucke U, Thompson LDR. Rhabdomyosarcoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Sootweg P, editors. World Health Organization classification of head and neck tumours. 4. Lyon: IARC; 2017. pp. 36–8. [Google Scholar]
- 36.Campbell K, Kumarapeli AR, Gokden N, Cox RM, Hutchins L, Gardner JM. Metastatic melanoma with dedifferentiation and extensive rhabdomyosarcomatous heterologous component. J Cutan Pathol. 2018;45:360–4. doi: 10.1111/cup.13122. [DOI] [PubMed] [Google Scholar]
- 37.Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–3. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horai T, Nishihara H, Hattori S, Md RT. Malignant melanoma producing serotonin. Cancer. 1979;43:294–8. doi: 10.1002/1097-0142(197901)43:1<294::AID-CNCR2820430142>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 39.Jahng AW, Liao SS. Successful Palliation with Octreotide of a Neuroendocrine Syndrome from Malignant Melanoma. J Pain Symptom Manage. 2006;32:191–5. doi: 10.1016/j.jpainsymman.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Thompson LDR, Bell D, Bishop JA. Neuroendocrine carcinomas. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Sootweg P, editors. World Health Organization classification of head and neck tumours. 4. Lyon: IARC; 2017. pp. 21–3. [Google Scholar]
- 41.Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033–42. doi: 10.1038/modpathol.2015.62. [DOI] [PubMed] [Google Scholar]
- 42.Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Department of Oral Medicine & Pathology and Hospital Dentistry, School of Dentistry, National and Kapodistrian University of Athens, Athens, Greece.
Not Applicable.