Abstract
Histomorphometry seems to provide more rigid quantitative elements for histological analysis and to bring less subjectivity to the diagnosis of oral lichen planus lesions (OLP). This study aimed to verify the association between white and red lesions and histomorphometric characteristics of OLP lesions. This retrospective cross-sectional study assessed 48 hematoxylin- and eosin-stained histological sections from incisional biopsies obtained from OLP cases. A single previously calibrated evaluator performed the light microscopy analyses to evaluate morphological and morphometric parameters. Analyses of associations among variables were performed using the Fisher’s exact test. Morphometric variables were assessed using the Mann–Whitney non-parametric test. Comparisons among the three groups (age range) were performed using the Kruskal–Wallis test. In this study, 81.2% of the participants were women aged < 50 years. Keratosis, acanthosis, and inflammatory infiltrates were noted in 10.4, 10.4, and 37.5% of moderate/severe cases, respectively. Inflammatory infiltrate (52.1%), papillary projections (54.2%), saw teeth (12.5%), basal layer degeneration (39.6%), and Civatte bodies (68.8%) were also observed. There was no significant association between lesion type and clinicopathological variables (p > 0.05) or between lesion type and histological (p > 0.05) and morphometric variables (p > 0.05). Furthermore, the morphometric variables analyzed did not differ between white and red lesions (p > 0.05) or in their associations with clinicopathological variables (p > 0.05). The results of this investigation showed no associations between white and red OLP lesions and the histomorphometric characteristics evaluated.
Keywords: Oral lichen planus, Morphological and microscopic findings, Epithelium, Inflammation
Introduction
Oral lichen planus (OLP) is a chronic inflammatory disease that is considered a potentially malignant oral disorder (PMOD) [1]. A systematic review published in 2020 showed that this condition had a global prevalence of 1.01%, with marked geographical differences, with Europe having the highest prevalence and India having the lowest prevalence [2]. OLP has a predilection for women, affects adults between their fifth and sixth decades of life, and most often occurs in the buccal mucosa [3–5]. The etiopathogenesis of OLP is unknown. However, immunological mechanisms may be crucial in its development, particularly cellular immunity. Specific and non-antigen-specific mechanisms may be involved, resulting in host immune responses, such as the accumulation of T cells in the superficial lamina propria and their migration to the epithelial tissue [6]. Thus, OLP is likely an immunologically mediated condition [7].
Some studies have classified OLP lesions into two broad groups: white lesions, formed by reticular, papular, and plaque types, and red lesions, comprising erosive, atrophic, and bullous forms [8, 9]. Boñar-Alvarez et al. also clinically classified such lesions as “mixed” when concomitant white and red lesions are present [10].
A multidisciplinary approach to OLP is essential. In many cases, the first and often the only manifestation of the disease occurs in the oral cavity. A dental surgeon is often the first professional to detect or diagnose OLP. The patient is then referred to other professionals to exclude manifestations in other anatomical locations [7].
In 1978, the World Health Organization (WHO) [11] proposed the following histological criteria for OLP: basal layer degeneration, presence of orthokeratosis or parakeratosis, presence of Civatte bodies, and a subepithelial inflammatory infiltrate. In 2016, the American Academy of Oral and Maxillofacial Pathology suggested updating these criteria for the diagnosis of OLP lesions to include the presence of a subepithelial inflammatory infiltrate band, basal layer degeneration, lymphocytic exocytosis, absence of epithelial dysplasia, and absence of verrucous changes in the epithelial structure [12].
Histomorphometry is a structural technique that quantitatively obtains information from cellular and tissue sections. Its objectivity may find applications in the differential diagnosis and prognosis of injuries. [13]. In a histomorphometry-based study of OLP lesions, Lopéz-Jornet et al. [14] analyzed the epithelial–conjunctive tissue junction referring to the epithelial papillae and measured several parameters. The authors reported that this metric characterization of the epithelial papillae may be clinically important for the local topical treatment of OLP because such measures reflect the relationship between the epithelial tissue and the underlying connective tissue, thus providing information on the anatomical and physiological characteristics of such lesions [14].
A previous study also applied morphometric techniques to evaluate the correlation between apoptosis and epithelial thickness in reticular and erosive lesions of OLP, in which the epithelial thickness varied according to the intensity of apoptosis and the clinical form. The authors of the study concluded that the clinical differences among OLP lesions probably resulted from biological and histological variations [15].
Thus, the use of more objective criteria in the histopathological characterization of OLP may allow for the identification of potentially more aggressive lesions, such as those with malignant potential. Although clinical follow-up of patients diagnosed with OLP is extremely important, the purpose of the present study was strictly laboratory, with the analysis of histological sections obtained from the patients.
This study aimed to describe the morphometric characteristics of these lesions and investigate the association between OLP lesions and histophotometric parameters.
Materials and Methods
This retrospective, cross-sectional study analyzed data obtained from histological reports, biopsied material, and histological sections from patients diagnosed with OLP filed at the Laboratory of Pathological Anatomy of the Bahiana School of Medicine and Public Health (Salvador, Bahia, Brazil) between 2008 and 2018 and the Laboratory of Oral Pathology at the State University of Feira de Santana (Feira de Santana, Bahia, Brazil) between 2005 and 2019.
The study protocol, in compliance with CNS Resolution 466/12, was reviewed by the Human Research Ethics Committee and approved with opinion number 3.189.846.
A non-probabilistic convenience sample was used, which included reports, biopsies, and respective histological sections of patients seen at the aforementioned services with clinical suspicion of OLP confirmed by histopathological examination. Cases histopathologically diagnosed with lichenoid reactions; patients without a final diagnosis and/or epithelial dysplasia, cancer, or other lesions included in the differential diagnosis with OLP; and those whose slides could not be reassessed, for example, due to the presence of fungi, were excluded, resulting in a final sample of 48 cases.
Data were collected and recorded in a form prepared by the researchers specifically for this study. Based on the histopathological reports, the following variables were verified: patient sex and age, clinical form of OLP, affected site, and smoking habits. Existing hematoxylin and eosin (H&E)-stained slides, prepared from biopsied material sampled during routine histological procedures, were analyzed by light microscopy. The histological sections were analyzed at the Laboratory of Oral Biochemistry of the Health Sciences Institute of the Federal University of Bahia, using the Motic BA410 microscope (Quimis, São Paulo, Brazil) and Motic Images Plus 2.0 software (Motic Asia, Hong Kong, China).
From the H&E-stained histological sections, color (8-bit RGB) images with a standard size of 1296 × 972 mm were captured at 10× magnification. All morphological analyses were performed in triplicate using three different regions of the same histological section, with a minimum interval of 1 week between analyses. The evaluations were performed by the same previously trained and calibrated evaluator (ACBS) who was blinded to the case data. The evaluator was trained by a professional (GBM) experienced in analyzing histological sections of oral lesions.
Photomicrographs were scored for morphological parameters, including keratosis, acanthosis, and subepithelial inflammatory infiltrate as absent/mild (1), mild/moderate (2), and moderate/severe (3). Subepithelial bands of the inflammatory infiltrate, epithelial papillary projections, papillary projections on saw teeth, degeneration of the epithelial basal layer, and Civatte bodies were categorized as present (P) or absent (A). Civatte bodies were also confirmed by examination of the histological slide at 40× magnification, after visualization of the photomicrographs obtained at 10× magnification.
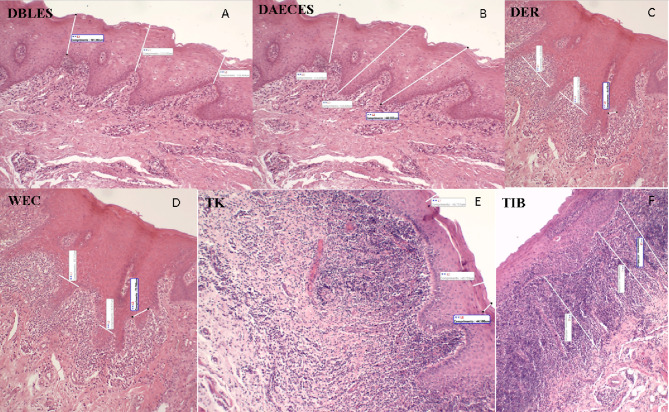
The morphometric parameters analyzed were measured in micrometers in the same photomicrographs and included the following: (1) epithelial papillary projections, based on the distance from the basal layer to the epithelial surface (DBLES), distance from the apex of the epithelial crest to the epithelial surface (DAECES), the width of the epithelial crest (WEC), and the distance between the epithelial ridges (DER), as described by Lopéz-Jornet et al. [14], and measurements of (2) the thickness of the subepithelial inflammatory infiltrate band (TIB), (3) the thickness of keratin (TK), and (4) the extent and depth of the ulcer (EDU; Figs. 1, 2). Morphometric analysis was performed at three different points in each photomicrograph using linear measurement tools in Motic Images Plus 2.0 software (Motic Asia, Hong Kong, Asia). The analyzed regions included areas with epithelial and conjunctive tissues that were representative of the histopathological features of the OLP lesion.
Fig. 1.
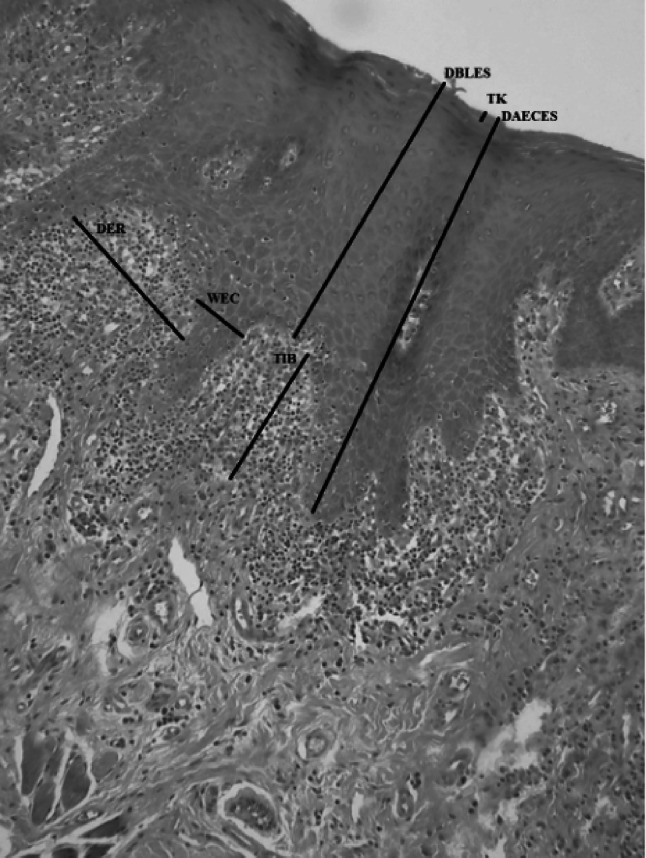
Photomicrograph showing the different histophotometric parameters used in this study (black lines). DBLES distance from the basal layer to the epithelial surface, DAECES distance from the apex of the epithelial crest to the epithelial surface, WEC width of epithelial crest, DER distance between epithelial ridges, TIB thickness of the inflammatory infiltrate band, TK thickness of keratin (H&E, 100×).
Source: LBO (2018–2019)
Fig. 2.
Photomicrographs with representation of the morphometric measurements taken in the study (white lines). A: DBLES; B: DAECES; C: DER; D: WEC; E: TK; F: TIB (H&E, 100×).
Source: LBO (2018–2019)
Statistical analyses were performed using the R program with a 5% significance level. Quantitative variables are expressed as medians and quartiles, whereas categorical variables are expressed as frequencies and percentages. Analyses of associations between variables were performed using the Fisher’s exact test. Since the data were asymmetrically distributed, non-parametric tests were used for comparisons between the two groups (type of injury, sex, and smoking). Morphometric variables were assessed using the Mann–Whitney non-parametric test. Comparisons among the three groups (age range) were performed using the Kruskal–Wallis test.
Results
Table 1 shows the clinical lesion type (white or red) according to the patients’ demographic variables, lesion location, and smoking habits. Eight of 48 cases studied (16.7%) were classified as red lesions, and 39 of 48 (83.3%) as white lesions.
Table 1.
Analysis of the associations between lesion type (white or red) and demographic variables in 48 cases (UEFS/EBMSP; 2018–2019)
| Variable | Category | Total sample | Injury group | p-value | |
|---|---|---|---|---|---|
| White | Red | ||||
| Frequencya (%) | Frequencyb (%) | ||||
| Gender | Female | 39 (81.2%) | 32 (82.0%) | 7 (18.0%) | 1.00 |
| Male | 9 (18.8%) | 8 (88.9%) | 1 (11.1%) | ||
| Age (years) | Below de 50 | 24 (50.0%) | 19 (79.2%) | 5 (20.8%) | 1.00 |
| Between 50 e 60 | 13 (27.1%) | 11 (84.6%) | 2 (15.4%) | ||
| More than 60 | 9 (18.8%) | 8 (88.9%) | 1 (11.1%) | ||
| NI | 2 (4.2%) | 2 (100.0%) | 0 (0.0%) | ||
| Lesion location | Buccal mucosa | 28 (58.3%) | 24 (85.7%) | 4 (14.3%) | 0.71 |
| Tongue | 12 (25.0%) | 10 (83.3%) | 2 (16.7%) | ||
| Buccal mucosa and tongue | 1 (2.1%) | 1 (100.0%) | 0 (0.0%) | ||
| Others | 7 (14.6%) | 5 (71.4%) | 2 (28.6%) | ||
| Smoking habit | Absent | 21 (43.8%) | 19 (90.5%) | 2 (9.5%) | 0.57 |
| Present | 9 (18.8%) | 7 (77.8%) | 2 (22.2%) | ||
| NI | 18 (37.5%) | 14 (77.8%) | 4 (22.2%) | ||
aPercentage in columns
bPercentage in lines
Source: own authorship; EBMSP: Laboratory of Pathological Anatomy of the Bahiana School of Medicine and Public Health; UEFS: Laboratory of Oral Pathology at the State University of Feira de Santana
Table 2 shows the distributions of cases according to the lesion location. The buccal mucosa was the most commonly affected site (28 of 48 cases, 58.3%), followed by the sides of the tongue (9 of 48 cases, 18.8%) and the back of the tongue (3 of 48 cases, 6.2%).
Table 2.
Descriptive analysis of the sites affected by OLP lesions according to the clinical group (n = 48) (UEFS/EBMSP; 2018–2019)
| Sites affected | Total sample | Group | |
|---|---|---|---|
| White | Red | ||
| Frequency (%) | |||
| Buccal mucosa | 28 (58.3%) | 24 (60.0%) | 4 (50.0%) |
| Buccal mucosa and tongue | 1 (2.1%) | 1 (2.5%) | 0 (0.0%) |
| Lateral edge of the tongue | 9 (18.8%) | 7 (17.5%) | 2 (25.0%) |
| Back of tongue | 3 (6.2%) | 3 (7.5%) | 0 (0.0%) |
| Between the lingual edges and mouth floor | 1 (2.1%) | 1 (2.5%) | 0 (0.0%) |
| Gum | 2 (4.2%) | 1 (2.5%) | 1 (12.5%) |
| Interdental papilla | 1 (2.1%) | 1 (2.5%) | 0 (0.0%) |
| Alveolar ridge | 1 (2.1%) | 0 (0.0%) | 1 (12.5%) |
| PM groove bottom region | 1 (2.1%) | 1 (2.5%) | 0 (0.0%) |
| Buccal region of the alveolar ridge | 1 (2.1%) | 1 (2.5%) | 0 (0.0%) |
| Total | 48 (100.0%) | 40 (100.0%) | 8 (100.0%) |
Source: own authorship; EBMSP: Laboratory of Pathological Anatomy of the Bahiana School of Medicine and Public Health; UEFS: Laboratory of Oral Pathology at the State University of Feira de Santana
The morphological findings observed in the studied samples are shown in Table 3 and Fig. 3. There were no significant associations between the clinical lesion group and the histological variables (p > 0.05).
Table 3.
Analysis of the associations between the clinical lesion group and histological variables (n = 48 cases) (UEFS/EBMSP; 2018–2019)
| Variable | Category | Total sample | Clinical group | p-value | |
|---|---|---|---|---|---|
| White | Red | ||||
| Frequencya (%) | Frequencyb (%) | ||||
| Keratosis | Absent/light | 21 (43.8%) | 16 (76.2%) | 5 (23.8%) | 0.54 |
| Light/moderate | 22 (45.8%) | 19 (86.4%) | 3 (13.6%) | ||
| Moderate/severe | 5 (10.4%) | 5 (100.0%) | 0 (0.0%) | ||
| Acanthosis | Absent/light | 18 (37.5%) | 14 (77.8%) | 4 (22.2%) | 0.53 |
| Light/moderate | 25 (52.1%) | 22 (88.0%) | 3 (12.0%) | ||
| Moderate/severe | 5 (10.4%) | 4 (80.0%) | 1 (20.0%) | ||
| Inflammatory infiltrate | Absent/light | 12 (25.0%) | 9 (75.0%) | 3 (25.0%) | 0.57 |
| Light/moderate | 18 (37.5%) | 16 (88.9%) | 2 (11.1%) | ||
| Moderate/severe | 18 (37.5%) | 15 (83.3%) | 3 (16.7%) | ||
| Inflammatory infiltrate range | Absent | 23 (47.9%) | 19 (82.6%) | 4 (17.4%) | 1.00 |
| Present | 25 (52.1%) | 21 (84.0%) | 4 (16.0%) | ||
| Papillary projections | Absent | 22 (45.8%) | 18 (81.8%) | 4 (18.2%) | 1.00 |
| Present | 26 (54.2%) | 22 (84.6%) | 4 (15.4%) | ||
| Saw teeth | Absent | 42 (87.5%) | 36 (85.7%) | 6 (14.3%) | 0.26 |
| Present | 6 (12.5%) | 4 (66.7%) | 2 (33.3%) | ||
| Basal layer degeneration | Absent | 29 (60.4%) | 25 (86.2%) | 4 (13.8%) | 0.70 |
| Present | 19 (39.6%) | 15 (79.0%) | 4 (21.0%) | ||
| Civatte bodies | Absent | 15 (31.2%) | 10 (66.7%) | 5 (33.3%) | 0.09 |
| Present | 33 (68.8%) | 30 (90.9%) | 3 (9.1%) | ||
aPercentage in columns
bPercentage in lines
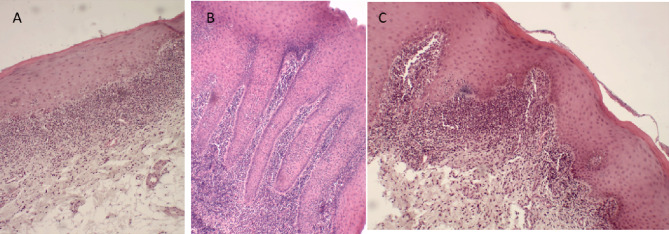
Fig. 3.
Photomicrographs showing the diversity of the histopathological characteristics of the OLP lesions analyzed. A: Keratinized stratified squamous epithelium showing disorganization of the basal layer and a band of inflammatory infiltrate with severe intensity in the region of the lamina propria. B: Superficial epithelium showing acanthosis with digitiform papillary projections and involvement in its own lamina as well as an intense inflammatory infiltrate. C: Keratinized squamous epithelium showing irregular papillary projections at the epithelial–conjunctive interface, with focal areas of degeneration of the basal layer and moderate-to-severe inflammatory infiltrate (H&E, 100×).
Source: LBO (2018–2019)
Table 4 shows the results of the analysis of morphometric variables according to the clinical lesion group in the total sample; no significant differences were observed (p > 0.05). All values referring to the extent and depth of the ulcer were zero, as an ulcer was not identified in the analyzed lesions. Thus, these values are not listed in the table.
Table 4.
Analysis of morphometric variables (μm) according to the lesion clinical group (n = 48) (UEFS/EBMSP; 2018–2019)
| Variable | Clinical group | IQR-1 | Median | IQR-3 | p-value |
|---|---|---|---|---|---|
| DBLES | White | 208.61 | 252.3 | 305.09 | 0.77 |
| Red | 208.18 | 243.1 | 303.18 | ||
| General | 208.61 | 252.2 | 305.09 | ||
| DAECES | White | 0.00 | 201.5 | 439.83 | 0.80 |
| Red | 10.17 | 164.6 | 381.12 | ||
| General | 0.00 | 201.5 | 436.37 | ||
| WEC | White | 0.00 | 92.9 | 116.62 | 0.57 |
| Red | 4.72 | 34.9 | 94.84 | ||
| General | 0.00 | 74.9 | 116.62 | ||
| DER | White | 0.00 | 40.0 | 100.02 | 0.18 |
| Red | 0.00 | 12.2 | 43.96 | ||
| General | 0.00 | 33.8 | 85.81 | ||
| TIB | White | 47.13 | 94.8 | 171.89 | 0.96 |
| Red | 21.46 | 77.7 | 312.57 | ||
| General | 44.16 | 94.8 | 182.33 | ||
| TK | White | 21.09 | 31.1 | 40.89 | 0.66 |
| Red | 13.760 | 27.7 | 51.89 | ||
| General | 20.68 | 30.3 | 43.19 |
DBLES distance from the basal layer to the epithelial surface, DAECES distance from the apex of the epithelial crest to the epithelial surface, WEC width of epithelial crest, DER distance between epithelial ridges, TIB thickness of the inflammatory infiltrate band, TK thickness of keratin
Source: own authorship; EBMSP: Laboratory of Pathological Anatomy of the Bahiana School of Medicine and Public Health; UEFS: Laboratory of Oral Pathology at the State University of Feira de Santana
In addition, there were no significant differences in morphometric variables among patients, stratified according to sex, age group, and smoking habits (p > 0.05; Table 5).
Table 5.
Median values of morphometric variables (μm) as a function of demographic variables and smoking habits (n = 48) (UEFS/EBMSP; 2018–2019)
| Variables | DBLES | DAECES | WEC | DER | TIB | TK | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR1-IQR3 values) | p-value | Median (IQR1-IQR3 values) | p-value | Median (IQR1-IQR3 values) | p-value | Median (IQR1-IQR3 values) | p-value | Median (IQR1-IQR3 values) | p-value | Median (IQR1-IQR3 values) | p-value | |
| Gender | ||||||||||||
| Female | 263.45 (220.80–311.25) | 0.07 | 212.77 (0.00–419.59) | 0.97 | 92.60 (0.00–116.62) | 0.74 | 34.71 (0.00–89.31) | 0.97 | 93.35 (36.56–192.74) | 0.75 | 30.37 (19.86–45.44) | 0.22 |
| Male | 224.41 (166.70–246.11) | 196.51 (43.13–442.11) | 55.33 (18.48–94.21) | 26.31 (10.01–83.92) | 119.26 (67.79–158.96) | 26.34 (21.24–34.23) | ||||||
| Age | ||||||||||||
| < 50 | 232.69 (209.40–289.38) | 0.32 | 314.82 (15.26–435.36) | 0.50 | 97.94 (7.08–123.87) | 0.50 | 52.48 (0.00–106.75) | 0.45 | 105.05 (39.74–269.30) | 0.51 | 31.73 (18.10–49.16) | 0.67 |
| 50–60 | 263.45 (192.34–328.67) | 152.85 (0.00–441.27) | 32.29 (0.00–113.37) | 34.71 (0.00–59.84) | 93.35 (48.86–132.01) | 30.37 (26.34–42.94) | ||||||
| > 50 | 270.50 (224.02–316.93) | 166.23 (0.00–196.51) | 60.72 (0.00–106.61) | 20.92 (0.00–26.31) | 96.15 (0.00–156.95) | 29.33 (22.50–31.80) | ||||||
| No information | 432.99 (330.96–535.01) | 224.41 (112.20–336.61) | 46.30 (23.15–69.45) | 20.27 (10.13–30.40) | 186.48 (126.10–246.86) | 38.06 (37.93–38.18) | ||||||
| Smoking habit | ||||||||||||
| Absent | 291.10 (231.94–308.77) | 0.10 | 202.75 (20.34–377.77) | 0.67 | 106.61 (9.44–129.88) | 0.21 | 39.61 (0.00–106.63) | 0.77 | 93.35 (48.04–132.01) | 0.62 | 29.83 (20.42–38.19) | 0.89 |
| Present | 246.11 (150.72–276.04) | 196.51 (43.13–212.77) | 55.33 (18.48–86.77) | 32.95 (14.78–54.90) | 67.79 (0.00–158.96) | 31.80 (25.22–38.84) | ||||||
| No information | 228.55 (180.73–262.19) | 304.96 (0.00–447.13) | 65.06 (0.00–114.65) | 31.73 (0.00–83.85) | 132.18 (46.26–267.03) | 32.18 (19.72–43.31) | ||||||
DBLES distance from the basal layer to the epithelial surface, DAECES distance from the apex of the epithelial crest to the epithelial surface, WEC width of epithelial crest, DER distance between epithelial ridges, TIB thickness of the inflammatory infiltrate band, TK thickness of keratin
Source: own authorship; EBMSP: Laboratory of Pathological Anatomy of the Bahiana School of Medicine and Public Health; UEFS: Laboratory of Oral Pathology at the State University of Feira de Santana
Discussion
Histopathological analysis of suspected OLP lesions provides a more accurate diagnosis. This is relevant because this condition is clinically similar to other lesions of the oral cavity and is considered PMOD. Therefore, incisional biopsies should be performed in all patients with clinical presentations that are suggestive of OLP. However, especially in cases with classic disease patterns, these procedures are not routinely performed in dental clinics, as demonstrated in the present study, which analyzed a relatively low number of histological sections from patients from two traditional educational institutions in the state of Bahia, over a period of 10–14 years.
According to the literature, there seems to be a low correlation among examiners between clinical and histopathological factors concerning OLP lesions. Thus, there is relative subjectivity in the diagnosis of OLP [16]. Therefore, this study aimed to assess the objectivity of histopathological analysis based on morphometric techniques to allow a better distinction between the different clinical types of OLP (white and red lesions). Few studies have used histomorphometry to describe and distinguish between white and red lesions in OLP, or between OLP and other oral lesions. Therefore, this study is important. The findings of this study may enhance diagnosis and contribute to existing knowledge relating to the natural history of OLP. This distinction is critical because red lesions are associated with aggressive clinical manifestations and have the greatest potential for becoming malignant [17]. In 2020, Guan et al. [18] reported that the ulcerative clinical type of OLP was more conducive to the progression to squamous cell carcinoma and that the malignant transformation in such lesions was related to chronic inflammation [18]. There were some study limitations, including the sample used in this study has the characteristic of being a transversal cut, clinical follow-up of patients previously biopsied was not possible, and no histomorphometric differences were observed between the studied groups to support different clinical behaviors between the white and red lesions of OLP. More studies on this topic are needed.
Classically, OLP mainly affects the buccal mucosa [4, 5, 19]. Consistent with the findings of previous studies, we also observed a predominance of female patients (38 of 48, 81.2%) samples analyzed were from females, and buccal mucosal lesions (28 of 48 cases, 58.3%). The second most affected site was the tongue (12 of 48 cases, 25%) and most were white lesions (39 of 48, 83.3%). However, unlike the findings of previous studies [3, 5], the < 50 years age group showed the highest frequency of OLP, (24 of 48 cases, 50%) in our study. As demographic information was obtained from histopathological reports, it was not possible to identify the reasons for this discordance relating to age. One possible explanation may be that these patients had a more stressful and faster pace of life than the general population, leading to an earlier appearance of diseases.
Previous studies have reported the predisposing factors associated with OLP injuries. Barbosa et al. [3] reported that smoking, systemic diseases such as diabetes and hepatitis C infection, and anxiety and depression levels were not associated with OLP onset or progression. Although smoking [20, 21] and certain medications, such as anxiolytics and antidepressants, have been associated with the atrophic-erosive clinical type of OLP, further studies are needed to investigate these relationships [10]. In this study, no significant differences were observed between smoking and white and red lesions or between morphometric patterns and such clinical groups, suggesting that such habits are not associated with the appearance of OLP lesions, which is consistent with the findings of previous studies [3, 10, 22]. However, it was not possible to assess this information in all analyzed reports, which may have influenced the results. Regardless of these results, smoking should be recorded in all clinical studies of PMOD, as it is the main risk factor for the development of oral cancer. High anxiety and depression levels also increase the risk of malignant transformation in OLP lesions [23, 24]; however, the aim of the present study was not to evaluate the associations between psychological disorders and OLP.
This study grouped reticular and plaque OLP lesion types into white lesions for analysis, which comprised the most frequent clinical group in this study, corresponding to 83.3% (39 of 48 cases). Thus, white lesions may have had higher median DBLES, DAECES, and TK values, demonstrating greater epithelial thickness in white lesions than in red lesions, which are characterized by atrophy and/or absence of epithelial tissue [10]. Red lesions comprised 17.7% (9 of 48 cases) of the total sample and included erosive and atrophic clinical types, which are associated with symptomatic lesions of OLP [25], with more aggressive manifestations and greater potential for malignancy [16].
Ulcers, a characteristic of red lesions with epithelial absence, were not observed in any of the histological sections evaluated. A close relationship between the presence of these ulcers and/or epithelial atrophy and the presence of a more significant range of inflammatory infiltrates has been described [10, 26]. These findings are contrary to those reported by Boñar-Alvarez et al. [10], who observed a higher frequency of red lesions (49.2%), accompanied by reticular (40.7%) and mixed forms (10.2%), and a histopathological association between the presence of the ulcer and red lesions was observed in all cases (p = 0.05). The severity of the inflammatory infiltrates does not depend on the clinical type of OLP; epithelial atrophy and ulcerations occur due to the qualitative factors of the inflammatory infiltrates, since the exacerbation of these lesions is associated with both the presence and amount of plasma cells in the inflammatory infiltrates [10, 26, 27]. Owing to the predominance of white lesions in the present study, a reduced number of ulcers was expected. However, ulcers were also not found in the red lesions. The lack of ulcerated regions in the samples may be attributed to the incisional biopsy technique of removing tissues on the margins of ulcerated lesions for analysis, avoiding areas of necrosis and excess fibrin coverage. However, biopsies for the diagnosis of immune lesions can be performed directly on the lesion, perilesional tissue, or even at a distance, using direct immunofluorescence tests for diagnostic confirmation, as immune deposits can be degraded in areas of greatest inflammation and cause false-negative results. Distant areas of the lesion also provide greater sample options in cases where obtaining tissue is difficult. As OLP is considered an immunologically mediated disease [1], these approaches can be used when performing biopsies, as there is no difference in the sensitivity of direct immunofluorescence in relation to the biopsy region in OLP lesions [28].
Since 1978, the WHO [11] has recommended histopathological criteria for the diagnosis of OLP lesions. Of the 48 cases of OLP analyzed in this study, 10.4% (5 of 48 cases) showed a moderate-to-severe keratin layer, confirming previously reported findings [8, 10, 29], despite the use of different methodologies. Among other histological findings discussed in the literature, the appearance of a granular layer in the oral mucosa together with an inflammatory infiltrate band helps in the diagnosis of OLP [29], especially in keratinized areas that form the masticatory mucosa, gums, and tongue as they tend to develop keratosis [8] as a protective mechanism.
In this study, papillary projections occurred frequently in white lesions, which may be related to the level of acanthosis in these lesions, with the intensity varying from mild to moderate. However, papillary projections on saw teeth were absent in 87.5% (42 of 48) of the samples, consistent with the observations of Navas-Alfaro et al. [29], showing that this pattern occurred more frequently in cutaneous lesions of lichen planus than in those of OLP.
As previously mentioned, the small changes in the pattern of inflammatory response between the clinical types of OLP described in the literature include a continuous band of inflammatory infiltrates immediately underlying the epithelial tissue in white lesions, deeper involvement of the connective tissue, and a higher number of plasma cells, mainly lymphocytes, associated with epithelial ulcerations, in red lesions [10]. In the present study, 37.5% (18 of 48) of the total sample presented with moderate-to-severe inflammatory infiltrates, consistent with the findings reported by Lopéz-Jornet et al. [14], and 52.1% of cases exhibited an inflammatory infiltrate band immediately underlying the epithelium, which was observed more frequently in white lesions because only half of the red lesions showed this histopathological characteristic. Regardless of the clinical presentation, evidence shows that the band-type inflammatory pattern is most frequently observed in different clinical types of OLP injuries [8, 10].
Civatte bodies were identified in 68.8% (33 of 48) of the cases in the present study, consistent with the findings reported by Bascones et al. [30], who observed this histological feature in 93.7% of cases, suggesting its diagnostic relevance in OLP. Despite the high frequency of Civatte bodies, the authors noted that they were present in small quantities. Thus, the authors concluded that the number of epithelial cells that underwent apoptosis was relatively small, and that they were mainly located in the basal layer. Apoptosis occurring in OLP lesions may influence their malignant potential [30]. Interestingly, degeneration of the basal layer was observed in 39.6% (19 of 48) of the total sample in the present study, which is a relatively low value compared to the reported prevalence of Civatte bodies. The quantification and identification of the presence of Civatte bodies could help justify this finding.
Brant et al. [15] evaluated the correlation between epithelial structure and the frequency of apoptosis in OLP lesions and reported a higher frequency of apoptosis in erosive lesions than in reticular lesions, which had greater epithelial thickness, suggesting that the thickness of this epithelium varies according to the clinical form and apoptosis intensity. In the present study, it was not possible to evaluate this association because the total sample size was disproportionate between white and red lesions.
The morphological information measured in this study revealed no statistically significant differences between the histological parameters analyzed and the types of clinical lesions. However, histopathological analysis revealed that degeneration of the basal layer and presence of Civatte bodies had a moderate to severe intensity of inflammatory infiltrates, regardless of the clinical classification. Brant et al. [26] reported a close relationship between the presence of subepithelial inflammatory infiltrate and the occurrence of apoptosis, which could explain the potential for malignancy of OLP lesions. However, further studies are required to confirm this relationship.
Morphometric assessments were used in this study to provide a more objective analysis for the measurement of histological characteristics, such as different epithelial measurements and the inflammatory infiltrates in the lamina propria, which would allow a more reliable verification of differences between white and red lesions in OLP. The median values of these measurements were not significantly different between the groups.
Lopéz-Jornet et al. [14] reported statistically significant associations between the presence of inflammatory infiltrates and epithelial papilla width between white and red lesions (p = 0.03). Although the measurement of epithelial papillae is difficult and lacks standardization, morphometric characterization of these factors may be important for clinical treatment [14].
In this study, we observed no differences among the measurements made on epithelial papillae and variables such as the types of lesions analyzed and patient sex, age, and smoking status (p > 0.05). However, white lesions showed higher median epithelial papillae and keratin layer values than red lesions did. Such histological findings are characteristic of many white lesions affecting the oral cavity, owing to the greater thickness of the epithelial tissue due to different factors.
There is no consensus regarding OLP’s potential for malignancy [31]. However, in a recent systematic review, the rate of malignant transformation of this disease was 1.1% [17].
The biological basis that justified the accomplishment of this study is related to the differentiated and normally more aggressive behaviors of the red lesions. Current literature shows that this clinical form of OLP tends to become more malignant than white lesions. This may be associated with some histopathological aspects observed in red lesions, including an atrophic epithelium or its absence (ulcer), the lack and/or slowness of the epithelial repair process, which makes this tissue more susceptible to aggression and characterizes the chronicity of these lesions, as well as the presence of a more significant inflammatory infiltrate band. Future morphometric analysis of histopathological parameters may further elucidate the possible differences between the white and red lesions of OLP. Our results revealed no significant differences between the measurements compared. Differences may be related to other histopathological parameters not included in our study, or more related to the molecular biological aspects. Therefore, further studies that more accurately describe the cellular changes should be performed.
This study was limited by the sample size and the lack of homogeneity between white and red lesions. Additional studies performing histomorphometric analysis of OLP lesions are needed to better characterize the clinical forms of the disease, investigate their biological behaviors, and identify important variations within the same clinical type to refine the diagnosis and predict the clinical behaviors of this condition. Our findings suggest that additional research is required, including validation of histomorphometric parameters in OLP lesions at different locations.
Conclusion
The results of the present study did not show an association between white and red OLP lesions and their histomorphometric characteristics. Further studies using morphometry are needed to investigate the histopathological differences between these lesions, which may define their different biological behaviors.
Acknowledgements
We would like to thank à FAPESB for the scholarship which allowed the authors to develop this study.
Author Contributions
Study concepts: ACBS, ALPVP, VSF, GBM; Study design: ACBS, ALPVP, VSF, GBM; Data acquisition: ACBS, ALPVP, GBM; Quality control of data and algorithms: GBM; Data analysis and interpretation: ACBS, ARAPM, SRAR, GBM; Manuscript preparation: ACBS, GBM. Manuscript editing: GBM; Manuscript review: ACBS, ARAPM, SRAR, GBM.
Funding
Not applicable.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
Not applicable.
Ethical Approval
Applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Warnakulasuriya S, Johnson NW, Van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–580. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Moles MA, Warnakulasuriya S, González-Ruiz I, González-Ruiz L, Avén A, Lenouvel D, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Oncol. 2020;106:104688. doi: 10.1016/j.oraloncology.2020.104688. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa NG, Silveira EJD, Lima ENA, Oliveira PT, Soares MSM, Medeiros AMC. Factors associated with clinical characteristics and symptoms in a case series of oral lichen planus. Int J Dermatol. 2015;54:e1–e6. doi: 10.1111/ijd.12485. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho CHP, Santos BRM, Vieira CC, Lima ENA, Santos PPA, Freitas RA. An epidemiological study of immune-mediated skin diseases affecting the oral cavity. An Bras Dermatol. 2011;86(5):905–909. doi: 10.1590/S0365-05962011000500007. [DOI] [PubMed] [Google Scholar]
- 5.Lima SL, De Arruda JA, Abreu LG, Mesquita RA, Ribeiro-Rotta RF, Mendonça EF, et al. Clinicopathologic data of individuals with oral lichen planus: a Brazilian case series. J Clin Exp Dent. 2019;11(12):e1109–e1119. doi: 10.4317/jced.56379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogueira PA, Carneiro S, Ramos-e-Silva M. Oral lichen planus: an update on its pathogenesis. Int J Dermatol. 2015;54:1005–1010. doi: 10.1111/ijd.12918. [DOI] [PubMed] [Google Scholar]
- 7.Cassol-Spanemberg J, Blanco-Carrión A, Rodríguez-deRivera-Campillo ME, Estrugo-Devesa A, Jané-Salas E, López-López J. Cutaneous, genital and oral lichen planus: a descriptive study of 274 patients. Med Oral Patol Oral Cir Bucal. 2019;24(1):e1–e7. doi: 10.4317/medoral.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberdi-Navarro J, Marichalar-Mendia X, Lartitegui-Sebastián M-J, Gainza-Cirauqui M-L, Echebarria-Goikouria M-A, Aguirre-Urizar J-M. Histopathological Characterization of the oral lichenoid disease subtypes and the relation with the clinical data. Med Oral Patol Oral Cir Bucal. 2017;22(3):e307–e313. doi: 10.4317/medoral.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishnan A, Balan A, Kumar NR, Haris PS, Bindu P. Malignant potential of oral lichen planus an analysis of literature over the past 20 years. Int J Appl Dent Sci. 2016;2:1–5. [Google Scholar]
- 10.Boñar-Alvarez AP, Sayáns MP, Garcia-Garcia A, Chamorro-Petronacci C, Gándara-Vila P, Luces-González R, et al. Correlation between clinical and pathological features oral lichen planus. Medicine. 2019;98:8. doi: 10.1097/MD.0000000000014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Collaborating Centre for Precancerous Lesions Definition of leukoplasia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518–539. doi: 10.1016/0030-4220(78)90383-3. [DOI] [PubMed] [Google Scholar]
- 12.Cheng YL, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Max Pathol. 2016;122:332–354. doi: 10.1016/j.oooo.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesiah SS, Kale AD, Hallikeremath SR, Kotrashetti VS. Histomorphometric analysis of nuclear and cellular volumetric alterations in oral lichen planus, lichenoid lesions and normal oral mucosa using image analysis software. Indian J Dent Res. 2013;24(2):277. doi: 10.4103/0970-9290.116678. [DOI] [PubMed] [Google Scholar]
- 14.Lopéz-Jornet P, Camacho-Alonso F, Molia-Miñano F. Quantitative analysis of epitelial papillae in patients with oral lichen planus. JEADV. 2009;23:692–696. doi: 10.1111/j.1468-3083.2009.03160.x. [DOI] [PubMed] [Google Scholar]
- 15.Brant JMC, Vasconcelos AC, Rodrigues LV. Role of apoptosis in erosive and reticular oral lichen planus exhibiting variable epithelial thickness. Braz Dent J. 2008;19(3):179–185. doi: 10.1590/S0103-64402008000300001. [DOI] [PubMed] [Google Scholar]
- 16.Van der Meij EH, Van der Waal I. Lack of clinicopathologiccorrelation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med. 2003;32:507–512. doi: 10.1034/j.1600-0714.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 17.Giuliani M, Troiano G, Cordaro M, et al. Rate of malignant transformation of oral lichen planus: a systematic review. Oral Dis. 2019;25(3):693–709. doi: 10.1111/odi.12885. [DOI] [PubMed] [Google Scholar]
- 18.Guan G, Mei L, Polonowita A, Hussaini H, Seo B, Rich AM. Malignant transformation in oral lichen planus and lichenoid lesions: a 14 year longitudinal retrospective cohort study of 829 patients in New Zealand. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(4):411–418. doi: 10.1016/j.oooo.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Feldmeyer L, Cazzaniga S, Borradori L, Beltraminelli H. Oral lichen planus and oral lichenoid lesions—na analysis of clinical and histophatological features. J Eur Acad Dermatol Venereol. 2020;34(2):e104–e107. doi: 10.1111/jdv.15981. [DOI] [PubMed] [Google Scholar]
- 20.Aghbari SMH, Abushouk AI, Attia A, Elmaraezy A, Menshawy A, Ahmed MS. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92–102. doi: 10.1016/j.oraloncology.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Casparis S, Borm JM, Tektas S, Corsalini M, Gioco G, Lo Muzio L, et al. Oral lichen planus (OLP), oral lichenoid lesions (OLL), oral dysplasia, and oral cancer: retrospective analysis of clinicopathological data from 2002–2011. Oral Maxillofac Surg. 2015;19:149–156. doi: 10.1007/s10006-014-0469-y. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho MFMS, Cavalieri D, Nascimento S, Lourenço TGB, Ramos DVR, Pasqualin DC, et al. cytokines levels and salivary microbiome play a potential role in oral lichen planus diagnosis. Sci Rep. 2019;9:18137. doi: 10.1038/s41598-019-54615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zucoloto ML, Shibakura MEW, Pavanin JV, Garcia FT, da Silva Santos PS, Maciel AP. Severity of oral lichen planus and oral lichenoid lesions is associated with anxiety. Clin Oral Investig. 2019;23:4441–4448. doi: 10.1007/s00784-019-02892-2. [DOI] [PubMed] [Google Scholar]
- 24.Manczyk B, Golda J, Biniak A, Reszelewska K, Mazur B, Zajac K, et al. Evaluation of depression, anxiety and stress levels in patients with oral lichen planus. J Oral Sci. 2019;61(3):391–397. doi: 10.2334/josnusd.18-0113. [DOI] [PubMed] [Google Scholar]
- 25.Cox T, Woodhead J, Nelson BL. Reticular oral lichen planus. Head Neck Pathol. 2020;14(1):192–194. doi: 10.1007/s12105-018-0983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brant JM, Aguiar MCF, Grandinetti HA, Rodrigues LV, Vasconcelos AC. A comparative study of apoptosis in reticular and erosive oral lichen planus. Braz Dent J. 2012;23:564–569. doi: 10.1590/S0103-64402012000500016. [DOI] [PubMed] [Google Scholar]
- 27.Anitua E, Piñas L, Alkhraisat MH. Histopathological features of oral lichen planus and its response to corticosteroid therapy: a retrospective study. Medicine. 2019;98(51):e18321. doi: 10.1097/MD.0000000000018321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sano SM, Quarracino MC, Aguas SC. Sensitivity of direct immunofluorescence in oral diseases. Study of 125 cases. Med Oral Patol Oral Cir Bucal. 2008;5:287–291. [PubMed] [Google Scholar]
- 29.Navas-Alfaro SE, Fonseca EC, Guzman-Silva MA, Rochael MC. Análise histopatológica comparativa entre líquen plano oral e cutâneo. J Bras Patol Med Lab. 2003;39(4):351–360. doi: 10.1590/S1676-24442003000400013. [DOI] [Google Scholar]
- 30.Bascones-Ilundain C, Gonzalez-Moles MA, Esparza-Gómez G, Gil-Montoya JA, Bascones-Martínez A. Importance of apoptotic mechanisms in inflammatory infiltrate of oral lichen planus lesions. Anticancer Res. 2006;26:357–362. [PubMed] [Google Scholar]
- 31.Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci World J. 2014;14:1–22. doi: 10.1155/2014/742826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.