Abstract
Sinonasal malignancies constitute 3% of head and neck cancers, with squamous cell carcinoma (SCC) the most common histology. Neuroendocrine carcinomas (NEC) are rare, with a subset showing neuroendocrine carcinoma and a non-neuroendocrine component. The pathogenesis of these combined tumors is largely unknown, and TP53 driver mutations may play a role. A database search for combined NEC was performed across two institutions (UNM and UCSF) spanning 15 years. Excluding NUT midline carcinoma, 3 cases met inclusion criteria. All were morphologically NEC + SCC and underwent a comprehensive immunohistochemical evaluation. Tumors demonstrated two components histologically: moderately to poorly differentiated SCC and high-grade NEC. Divergent differentiation was confirmed with lineage-specific markers. Only one patient received neoadjuvant chemotherapy prior to surgery, with a remarkable response (a marked decrease in the size of the primary lesion and resolution of liver metastases). Immunohistochemical staining for p53 was increased in 2 of 3 cases (both components), suggesting a role in the carcinogenesis of these tumors. Aberrant expression of beta-catenin was not identified. One case tested positive for p16, which can be seen in high grade NECs due to inactivation of Rb gene. Additionally, both cases with a small cell NEC component expressed PD-L1, suggesting that immunotherapy may be an effective treatment. Findings in this study support the role of p53 mutation in a subset of combined NEC + SCC of the sinonasal tract. Recognition of this rare entity is essential for optimal management of these aggressive neoplasms.
Keywords: Neuroendocrine, Combined, Squamous cell, Carcinoma, TP53, p53
Introduction
Cancers of the sinonasal tract are rare, representing only 3% of head and neck cancers and less than 1% of all cancers [1–3]. Histologic diagnosis of sinonasal tract malignancies is extremely challenging due to their diverse histological profile including epithelial, hematolymphoid, neuroectodermal, melanocytic, and mesenchymal tumor types. The challenge is further compounded by the recent description of newer entities like NUT midline carcinoma, HPV-related multiphenotypic sinonasal carcinoma, SWI/SNF Complex‑Deficient Sinonasal Carcinoma, biphenotypic sinonasal sarcoma, and adamantinoma-like Ewing sarcoma [4]. More than half of all sinonasal tumors are epithelial, with squamous cell carcinoma the most common, followed by adenocarcinoma, adenoid cystic carcinoma, melanoma, and olfactory neuroblastoma [5].
These cancers are more common in men and typically present late at a locally advanced stage contributing to a poor prognosis [1–3]. Limited treatment options exist for advanced disease, and surgery and radiotherapy are not always curative. Prognosis is dependent on the patient’s age and stage of the disease. Turner and Reh [5] reported stage-specific 5-year relative survival for sinonasal cancers, ranging from 81 for localized tumors to 49% for regional disease, and 28% for metastatic cancers.
Neuroendocrine carcinoma (NEC, a terminology used for head and neck neuroendocrine tumors) is rare in the sinonasal tract when compared with the larynx and salivary gland [6, 7]. NECs are currently classified as well-differentiated, moderately differentiated, and poorly differentiated NECs, the latter is further classified into small cell neuroendocrine carcinoma (SmCC) and large cell neuroendocrine carcinoma (LCNEC) [7–9].
Combined sinonasal tract tumors with a neuroendocrine and a non-neuroendocrine histomorphology are exceedingly uncommon and remain a diagnostic conundrum. Combined tumors occur more frequently in the larynx, representing approximately 10% of all neuroendocrine carcinomas [10–12]. Only a few cases have been reported in the sinonasal tract, typically a combination of adenocarcinoma and neuroendocrine carcinoma [13–16]. Rare case reports of combined small cell and squamous cell carcinoma (NEC + SCC) exist [7, 11] in literature. Although cases of “combined” or “mixed” neoplasms composed of neuroendocrine and non-neuroendocrine components have been described, this nomenclature is controversial. It is currently not included in the current World Health Organization (WHO) classification.
The aim of this study was to analyze the clinicopathologic and immunohistochemical profile of NEC + SCC tumors of the sinonasal tract and identify possible driver mutations and potential treatment targets.
Methodology
After obtaining approval from the institutional review board at the University of New Mexico and the University of California San Francisco, a database search for combined neuroendocrine carcinoma and non-neuroendocrine carcinoma was performed across the two institutions spanning 15 years. Cases of NUT midline carcinoma (a tumor defined by overexpression of NUT protein that can demonstrate overlapping histomorphology with NEC + SCC) were excluded. Three cases met inclusion criteria which was defined as 1. Sinonasal malignant tumors; 2. Morphologic evidence of two cell types (Neuroendocrine differentiation: Morphologically defined as presence of monotonous regular cells with round or oval nuclei, salt and pepper chromatin and moderate eosinophilic granular cytoplasm supported by IHC in the form of positive expression for at least two of the neuroendocrine markers: Synaptophysin, chromogranin, CD56, INSM1; and non-neuroendocrine differentiation in the form ofsquamous cell carcinoma, latter defined by morphology and/or IHC markers); 3. Each component should comprise at least 30% of the tumor [13]. Presence of focal NE and/or squamous differentiation were excluded from the study; 4. Negative expression for NUT protein.
The age, sex, and histopathologic characteristics of the tumors were recorded. A comprehensive immunohistochemical panel was additionally performed. All immunohistochemistry was performed at Tricore Laboratories, Albuquerque, New Mexico using the Ventana/Roche Benchmark.
The PD-L1 (NeoGenomics, 22C3 pharmDx assay) protein expression was determined by using Combined Positive Score (CPS), which is calculated as follows:
The specimen is considered to have positive expression for PD-L1 if CPS ≥ 1 [17].
For p53, a positive nuclear staining of > 20% tumor cells with moderate to strong intensity was considered as overexpression [18]. The Ki67 proliferative index was evaluated by counting the number of Ki67-positive cells in a total of 500 neoplastic cells in areas of high nuclear labeling (“hot spots”) and expressed as percentage [19]. The number of mitoses (cases 2 and 3) was counted in 10 high power fields which approximated mitotic rate per 2 mm2 area [9, 20, 21].
Table 1 lists the antibodies utilized in this study and their clones and sources All antibodies were received pre-diluted and ready to use (RTU).
Table 1.
Summary of antibodies employed in this study
| Antibody | Clones | Source |
|---|---|---|
| CK 5/6 | XM26 | Leica Biosystems, Buffalo Grove, IL, USA |
| p63 | BC4A4 | Biocare Medical, Pacheco, CA, USA |
| P40 | BC28 | Biocare Medical, Pacheco, CA, USA |
| Synaptophysin | SP11 | Richard-Allen Scientific/Thermo Fisher Scientific, Kalamazoo, MI, USA |
| Chromogranin | LK2H10 | Ventana Medical Systems, Oro Valley, AZ, USA |
| INSM1 | A8 | Santa Cruz Biotechnology, Dallas, TX, USA |
| CD56 | MRQ-42 | Cell Marque, Rocklin, CA, USA |
| NUT protein | C52B1 | Cell signaling, Danvers, MA, USA |
| INI-1 | BAF-47 | BD Biosciences, Miami, FL |
| p53 | DO-7 | Dako/Agilent, Santa Clara, CA, USA |
| Beta-catenin | MAB 14 | BD transduction, San Jose, CA, USA |
| p16 | EGH4 | Roche Ventana Medical Systems, Oro Valley, AZ, USA) |
| hrHPV in situ hybridization | RNAscope HPV-HR18 probe | Leica Bond 3 platform |
| Ki-67 | 30–9 | Roche Ventana Medical Systems, Oro Valley, AZ, USA |
| EBER in situ hybridization | N/A | Roche Ventana, Tucson, AZ |
N/A not applicable
Results
Based on the inclusion criteria (as mentioned in methods section), three cases were included in this study. All cases were morphologically NEC + SCC, a diagnosis supported by histology and the comprehensive immunohistochemical evaluation detailed below. All three tumors arose in the maxillary sinus with an age range of 48–83 years, and M:F ratio of 2:1. A summary of the clinical and histologic parameters is presented in Table 2. Table 3 reports the immunohistochemical profile of the three cases.
Table 2.
Clinical and histological features of 3 cases of NEC + SCC
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (years) | 48 | 83 | 69 |
| Sex | F | M | M |
| Location of tumor | Maxillary sinus with liver metastasis | Maxillary sinus | Maxillary sinus |
| Histology | Squamous cell carcinoma + small cell carcinoma (intermixed) | Squamous cell carcinoma + Large cell NEC (discrete nests juxtaposed) | Squamous cell carcinoma + small cell carcinoma (discrete nests juxtaposed) |
| Diagnosis on biopsy vs resection | Biopsy | Biopsy | Resection |
| Treatment offered | Neoadjuvant chemotherapy (with marked response) followed by resection) | Resection followed by chemo-radiation therapy | Resection followed by radiation and chemotherapy |
|
Proportion of SCC (%); Proportion of NEC (%) |
98:2 (proportion as present in post chemotherapy resection specimen) | 70:30 | 50:50 |
| LVI | Positive | Negative | Negative |
| PNI | Positive (Extensive) | Positive | Negative |
| Necrosis | Positive | Positive | Negative |
| Mitotic count (/2 mm2) | N/A (NEC component in the post chemotherapy specimen had crush artifact and constituted < 1% of total tumor mass); FNA specimen showing NEC was too scant to estimate Mitotic count | 15 | 10 |
| Lymph node metastasis/distant metastasis | None (s/p neoadjuvant chemotherapy) | Negative at the time of presentation; developed distant metastasis 6 months post-surgery | None |
| Follow up | Patient is without recurrence over a 14-month follow-up | Patient recurred 6 months post-surgery and was placed on palliative care | Patient was diagnosed with high grade squamous dysplasia two years post-surgery, that was excised. He is alive and symptom-free 14 years after his initial diagnosis |
Table 3.
Immunohistochemical results for 3 cases of NEC + SCC
| Antibody | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| CK 5/6 | Positive in squamous component | Positive in squamous component | Positive in squamous component |
| p63 | Positive in squamous component | Positive in squamous component | Positive in squamous component |
| P40 | Positive in squamous component | Not performed | Not performed |
| Synaptophysin | Negative | Positive in neuroendocrine component | Positive in neuroendocrine component |
| Chromogranin | Positive in neuroendocrine component | Negative | Negative |
| CD56 | Positive in neuroendocrine component | Positive in neuroendocrine component | Positive in neuroendocrine component |
| INSM1 | Patchy positive in NEC | Not performed | Not performed |
| NUT protein | Negative | Negative | Negative |
| CD99 | Negative | Negative | Negative |
| INI-1 | Intact expression | Intact expression | Intact expression |
|
p53 (% cells showing positive nuclear expression) |
Increased (90%) | Increased (90%) | Negative (Null type) |
|
Beta-catenin (Expression, localization) |
Positive, membranous | Positive, membranous | Positive, membranous |
| p16 | Negative | Negative | Positive (both SCC and NEC components) |
| hrHPV ISH | N/A | N/A | Negative |
| Ki-67 (SCC and NEC components) | High ~ 50% and 90% | High ~ 70% (both components) | Higher in SCC component (10% and 3%) |
| EBER ISH | Negative | Not performed | Not performed |
|
PD-L1 (Expression, CPS) |
Positive, 40 | Negative, 0 | Positive, 10 |
hrHPV ISH high risk Human papilloma virus in situ hybridization, EBER ISH EBV-encoded RNA in-situ hybridization
Case 1
Clinical
A 48-year-old female with a past medical history significant for a 75-pack-year history of smoking presented to the with a complaint of right-sided facial swelling. Symptoms had been present for 3 months and were associated with pain radiating to the right eye, and the swelling had been gradually enlarging over that time. Physical exam findings included a tender, non-mobile, ill-defined, firm bulge over the right maxilla and extending from the nasolabial fold across the right cheek from the right eyelid to the angle of the mouth. No palpable lymphadenopathy was appreciated. Extraocular muscles were functional, and gaze was intact. Her smile was asymmetric with right upper lip ptosis, and V2/V3 distribution facial numbness was noted.
Maxillofacial computed tomography (CT) with contrast revealed a large mass (4.1 × 3.7 × 3.8 cm) centered within the right maxillary sinus eroding through the adjacent bone with extension into the right middle meatus, subcutaneous tissue, the floor of the right orbit, and posterior wall of the right maxillary sinus (Fig. 1A). Positron emission tomography (PET) showed the mass to be hypermetabolic with equivocal lymph nodes. Notably, a hypermetabolic liver mass was also identified on PET scan.
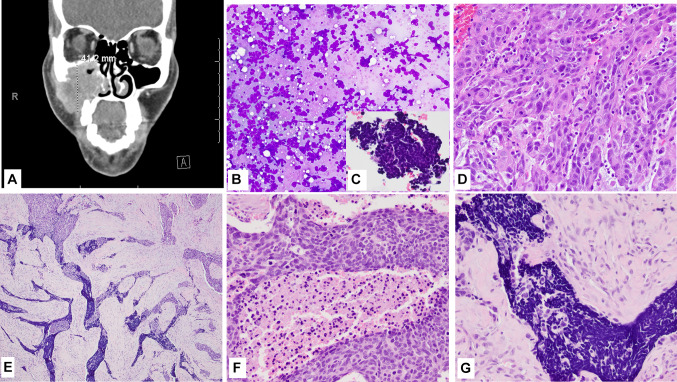
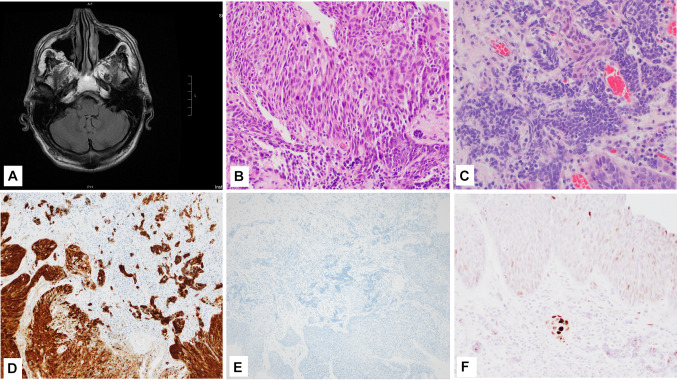
Fig. 1.
Case 1 (Clinical and morphology) A CT with contrast shows a 4.1 cm mass in the right maxillary sinus; B, C Fine needle aspirate smear showed small blue cell morphology, Diff-Quick (C Inset shows the small blue cell clusters in the cell block, H&E); D Presurgical biopsy shows a moderately differentiated squamous cell carcinoma, H&E; E Histologic section from the resection specimen (post neo-adjuvant therapy) showing both squamous cell and neuroendocrine components, H&E; F High power view of the more basaloid appearing squamous cell component in the resection specimen, H&E; G High power view of the small cell NEC component in the resection specimen, H&E. Magnification in B is ×10; C is ×40; D, F, G is ×20; and E is ×4
Fine Needle Aspiration Biopsy
An ultrasound-guided fine-needle aspiration (FNA) of the maxillary lesion was performed under local anesthesia, and smears were prepared and stained with Diff-Quik, and the needle rinsed into Thin Prep collection media. Based on the cytomorphology of the smears (Fig. 1B), cell block (Fig. 1C, inset), and positive expression of chromogranin, CD56 and Ki-67 ~ 100%, while negative expression for CK5-p40, a diagnosis of sinonasal neuroendocrine neoplasm (SNEC) was rendered.
Surgical Biopsy
The patient subsequently underwent surgical biopsy of the right maxillary mass with concurrent core biopsy of the liver mass. A 1.0 × 0.5 × 0.3 cm fragment of grey-tan tissue from the right maxilla submitted for intraoperative diagnosis via frozen section showed invasive squamous cell carcinoma (Fig. 1D). Microscopic analysis of the permanent sections revealed neoplastic cells, which were strongly and diffusely positive for markers of squamous differentiation (p40 andCK5) with no convincing morphologic or immunohistochemical evidence of neuroendocrine differentiation. The core biopsy of the liver was focally positive for high-grade neuroendocrine neoplasm with a Ki-67 proliferation index of > 80%, supporting the diagnosis of a high-grade neuroendocrine carcinoma.
Neoadjuvant Chemotherapy
Given the results of the FNAB and subsequent biopsies, the patient was discussed in the multidisciplinary head and neck tumor conference. Based on the presence of a high-grade component of NEC, the recommendation was to administer neoadjuvant systemic chemotherapy. Patient received 6 cycles of chemotherapy with carboplatin, etoposide, and atezolizumab. A surveillance PET scan obtained 3 months into treatment showed excellent treatment effect in both the maxillary sinus tumor and liver lesion, but new uptake in cervical and mediastinal lymph nodes prompting excisional biopsy of a right level II cervical lymph node.
Surgery
The primary maxillary sinus tumor had decreased in size with neoadjuvant therapy, and subsequent imaging rendered it resectable. In addition, imaging showed resolution of patient’s liver metastases. The patient underwent partial maxillectomy and level 1 selective neck dissection with extensive sampling of adjacent structures.
Histopathology
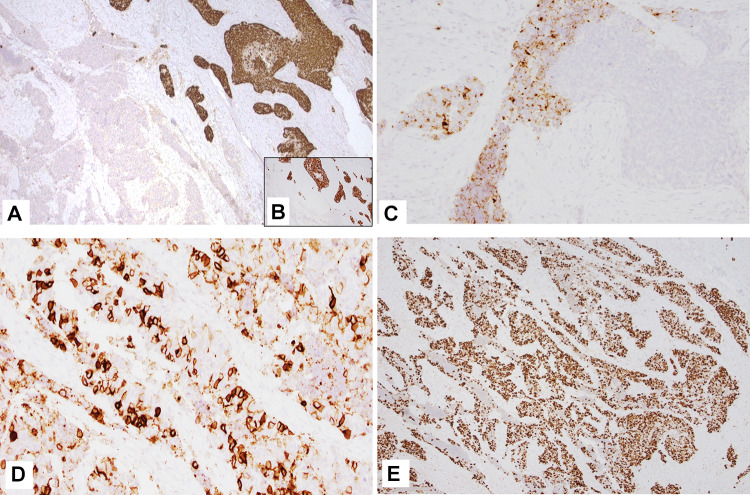
Grossly, the right facial mass was received in several fragments, including skeletal muscle, bone, and adipose tissue. Sectioning of the largest fragment (3.5 cm) revealed an indurated, white, fibrous lesion with stellate borders abutting the maxillary bone. Several areas were concerning for gross extension into adipose tissue and skeletal muscle. Microscopically, the bulk of the tumor consisted of nests of poorly differentiated, non-keratinizing squamous cell carcinoma (CK5, p40, and p63 positive), accounting for ~ 98% of the resected tumor. Tumor extensively infiltrated the maxillary sinus and invaded the maxillary bone. Foci of small blue cells with marked crush artifact were seen closely intermingled with the squamous component (Fig. 1E–G). These foci were negative for squamous markers but were positive for CD56 and showed patchy weak staining with chromogranin (Fig. 2A–D), and INSM1. Ki-67 proliferation index was higher in the NEC component than SCC component, 90% and 60% respectively. Extensive perineural invasion, lymphovascular invasion, and necrosis were present. P53 showed markedly increased expression (Fig. 2E); p16 was negative.
Fig. 2.
Case 1 (IHC) A CK5/6-p40 (cocktail stain) and p63 (B, inset) stain the squamous cell carcinoma component; C and D Chromogranin and CD56 (respectively) stain the small cell NEC component; E Tumor showed high expression of p53. Magnification in A, B and E is ×4 while C and D is ×10
Interestingly, when compared to the previously biopsied tissue, the squamous component in the resection specimen appeared non-keratinizing/basaloid (possible treatment effect, Fig. 1F). The high-grade NEC/small cell carcinoma component had shown the most remarkable response and constituted ~ 2% of the resected tumor (NEC component was seen in one of ten slides from the sampled tumor, constituting about 20% of the viable tumor in one slide, thereby estimated to be 2% of total sampled volume). Other treatment-related changes (scarring, calcification, and multinucleated giant cell reaction were present. The cervical lymph nodes were negative for tumor; pathologic staging was ypT2N0.
A diagnosis of combined squamous cell and high-grade neuroendocrine carcinoma/small cell carcinoma was rendered based on histomorphology and immunohistochemical stains.
Outcome/Follow Up
The patient recovered well from surgery, and maintenance atezolizumab was initiated along with and postoperative radiation therapy to the right maxillary sinus and neck as well as definitive radiation therapy to the liver metastasis. The patient is doing well with no evidence of recurrence over a follow-up period of 14 months.
Case 2
Clinical
An 83-year-old male with a 40-pack year smoking history presented with sinusitis, congestion, and increasing left-sided facial pain. A dental evaluation was concerning for infection. Computerized tomography of the sinuses showed opacification of the left maxillary sinus with invasion of the medial maxillary wall and anterior maxilla without apparent erosion of the palate (Fig. 3A).
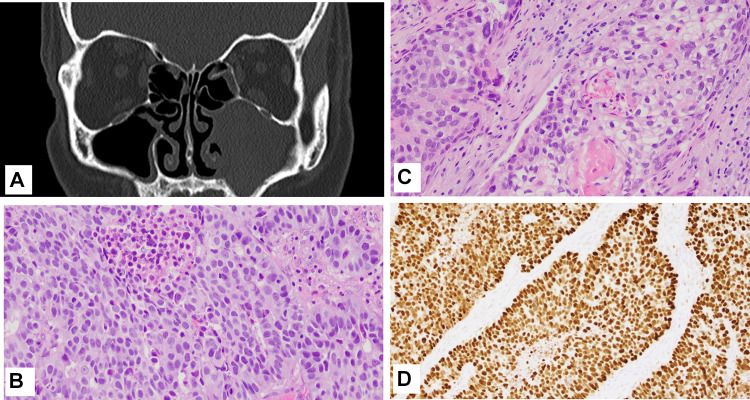
Fig. 3.
Case 2 A Maxillofacial CT showed opacification of the left maxillary sinus; B High power view of the moderately differentiated SCC component, H&E; C High power view of the large cell NEC component; D p53 expression. Magnification in B and C is ×20 and D is ×10
Surgical Biopsy
He underwent sinus surgery at an outside facility for excision biopsy that revealed mixed neuroendocrine carcinoma and squamous carcinoma. The pathologic specimen was reviewed as part of the continuity of care and immunohistochemical stains performed on the specimen revealed a component of squamous cell carcinoma (positive for pan-keratin and p63) and a neuroendocrine component (positive for CD56).
Surgery
The patient underwent left inferior maxillectomy and left selective neck dissection.
Pathology
Gross examination of the specimen showed a mucosal-based firm, tan-white mass (3.0 × 2.4 × 1.6 cm) within the maxillary sinus. Microscopic evaluation revealed a tumor with two components with distinct histomorphology. One component was composed of solid nests and trabeculae, occasionally forming rosettes, of medium to large-sized tumor cells with high nuclear to cytoplasmic ratios, coarse chromatin, and single prominent nucleoli, and brisk mitotic activity (15 mitosis per 2 mm2). These features were consistent with a high-grade/large cell neuroendocrine carcinoma. The second component showed squamous differentiation with tumor cells containing eosinophilic cytoplasm and focal keratinization, consistent with moderately differentiated squamous cell carcinoma (Fig. 3B, C).
The two components were closely juxtaposed with each other. Immunohistochemical stains for synaptophysin and CD56 were positive in the neuroendocrine component, and negative in the SCC component while CK5/6 and p63 stained the SCC component and were negative in NEC component (Fig. 4A–D), supporting the diagnosis of a combined squamous cell carcinoma and neuroendocrine carcinoma. The Ki-67 proliferation index was 80% and 60% in NEC and SCC components respectively%. Necrosis was present. P16 was negative in both components, while P53 showed markedly increased expression (Fig. 3D).
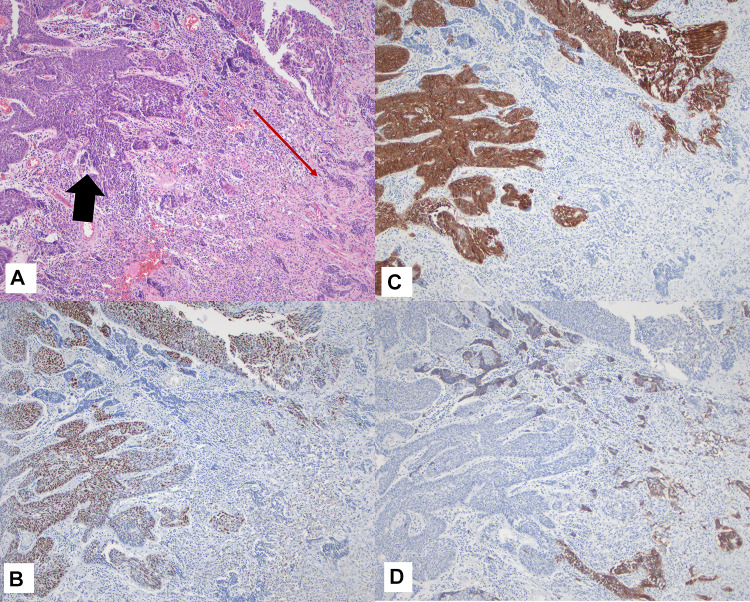
Fig. 4.
Case 2 A Combined SCC and NEC juxtaposed, H&E (Black arrows: SCC, Red arrow: NEC); B and C p63 and CK5/6 expression in SCC component (respectively); D Synaptophysin (weak) expression in NEC. Magnification in A–D is ×10
Diagnosis of combined squamous cell and high-grade/large cell neuroendocrine carcinoma was rendered based on histomorphology and immunohistochemical stains.
Chemoradiation Therapy
The patient completed radiation therapy along with 3 months of chemoradiation after surgery. He became PEG dependent during chemoradiation therapy.
Outcome/Follow Up
MRI of the skull base and neck (6 months post-surgery) showed enhancing soft tissue mass surrounding the left maxillary resection cavity involving the adjacent parotid gland and concerning for recurrent tumor versus posttreatment changes. Biopsy from this area was positive for squamous cell carcinoma with invasion into bone. A PET-CT showed bilateral pulmonary nodules, and fine-needle aspiration of the lung showed keratinizing squamous cell carcinoma without a neuroendocrine component. At this point, the patient was referred to palliative care.
Case 3
Clinical
A 69-year-old male presented with complaints of chronic sinusitis, epistaxis, significant weight loss, and a left nasal mass. Computerized tomography showed a soft tissue abnormality (possible polyp) involving the posterior nasal cavity. He was referred for a surgical biopsy of the mass; however, he had massive intraoperative bleeding, which required cauterization. He subsequently coughed up a “sausage-like” mass which was sent to pathology for analysis. Pathologic evaluation, including histomorphology and immunohistochemical stains, was consistent with a sinonasal neuroendocrine carcinoma. Surgical resection, including medial maxillectomy and possible ethmoidectomy, was recommended.
Pre-operative Imaging
CT confirmed residual tumor involving the left inferior turbinate and medial wall of the left maxillary sinus and eroding medially into the left maxillary sinus (Fig. 5A).
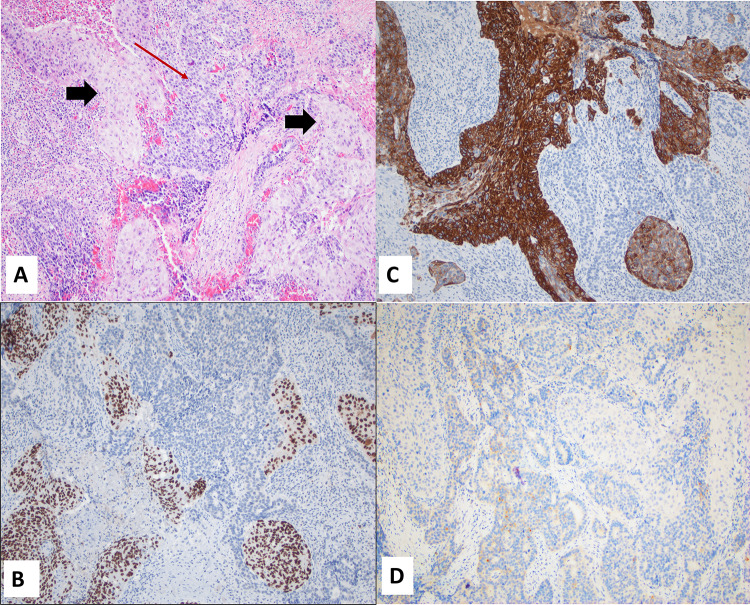
Fig. 5.
Case 3 A A post biopsy CT showed residual tumor involving the left inferior turbinate and medial wall of the left maxillary sinus; B High power view of the SCC component, H&E; C High power view of the small cell NEC component, H&E; D p16 expression; E HPV hr ISH; F p53 expression. Magnification in B, C, and F is ×20 while D and E is ×10
Surgery
The patient underwent a left endoscopic medial maxillectomy and left partial septectomy with image guidance, revealing a 2.0 × 2.0 cm tumor.
Pathology
Gross examination of the specimen revealed a tan-brown, irregular fragment of fibro-membranous tissue measuring 5.3 × 4.5 × 1.1 cm with a 0.5 × 0.4 × 0.2 cm white-tan, raised polypoid nodule. Sections from both the initial biopsy and the resection specimen revealed two distinct histomorphologic patterns. The dominant pattern was non-keratinizing, moderately differentiated squamous cell carcinoma, and this component involved the surface mucosa and invaded into the underlying submucosa. Additionally present was a distinct focal area of small nests and infiltrating single cells, immediately adjacent to and underlying the squamous cell carcinoma. This focus had cells with scant cytoplasm and hyperchromatic nuclei with nuclear molding and numerous mitotic Figs. (10/2 mm2), suggestive of neuroendocrine differentiation (Fig. 5B, C).
The presence of a lesion with two distinct histologic appearances prompted immunohistochemical studies. The squamous cell carcinoma was positive for CK5/6 and p63 (negative in NEC component), while CD56 and synaptophysin highlighted the neuroendocrine component (negative in SCC component), supporting the diagnosis of mixed sinonasal squamous cell carcinoma and neuroendocrine carcinoma/small cell carcinoma (Fig. 6A–D). The Ki-67 proliferation index in the scant residual tumor foci in the resected specimen were 10% and 3% in SCC and NEC components respectively. Unfortunately, at the time of this study, the initial biopsy tissue containing significant NEC component was not available for IHC evaluation.. P16 was diffusely positive in both NEC and SCC components, however In situ hybridization study for high risk HPV was negative (Fig. 5D, E respectively). P53 IHC showed staining of focal stromal cells while tumor cells stained negative (null type), (Fig. 5F). No necrosis was identified. Post-surgery, he was referred for chemotherapy and radiation therapy, which he completed at an outside facility.
Fig. 6.
Case 3 A Combined SCC and NEC juxtaposed, H&E (Black arrows: SCC, Red arrow: NEC); B and C p63 and CK5/6 expression in SCC component (respectively); D Synaptophysin (weak) expression in NEC. Magnification in A–D is ×10
Outcome/Follow-Up
The patient was diagnosed with recurrent high-grade squamous dysplasia of the nasal septum 2 years post-surgery, which was excised. He is alive and symptom-free 14 years after his initial diagnosis.
All 3 tumors showed membranous staining for beta-catenin without evidence of any aberrant nuclear expression (Fig. 7B). PD-L1 was expressed in cases 1 and 3 with a combined positive score of 40 and 10, respectively (Representative image of case 1 shown in Fig. 7A).
Fig. 7.
A Representative image of PD-L1 expression (Case 1); B Representative image of Beta-catenin (Case 1). Magnification in both A and B is ×10
Discussion
Combined tumors with both a neuroendocrine and a non-neuroendocrine component are exceedingly rare in the sinonasal tract. Pathologic diagnosis of combined tumors at this site is challenging and requires expertise in sinonasal anatomy and the histology of sinonasal tumors. Sinonasal tumors have overlapping clinical, radiologic, and histopathologic features, and a multidisciplinary approach to diagnosis and treatment planning is warranted. Furthermore, treatment options for advanced disease are limited, and surgery and radiotherapy are not always curative. Mixed adeno-neuroendocrine carcinomas (MANECs) are well reported in the gastrointestinal tract and are considered a specific entity by WHO [22, 23]. MANEC in the intestinal tract is defined as a neoplasm in which each component represents at least 30% of the lesion. In the head and neck region, mixed tumors occur more frequently in the larynx, where they represent approximately 10% of all neuroendocrine carcinomas [10–12].
Combined tumors with NEC and Non-NEC components have been described in the sinonasal tract with only 7 cases in the literature (Table 4, [8, 12–16]). The majority (5/7) of these cases were composed of an intestinal-type adenocarcinoma (ITAC) with a neuroendocrine tumor, while two cases had SCC as the exocrine component. The most common neuroendocrine component reported amongst these cases was the small cell NEC (5/7 cases), while one case showed large cell NEC [12]. Only one case showed an atypical carcinoid/well differentiated SNEC as the NEC component [15].
Table 4.
Comparative summary of cases of combined NEC and exocrine carcinoma reported in literature
| Serial number | Author, year | Number of cases (n) | NEC component | Non-NEC component | Molecular data | Treatment offered | Recurrence/metastasis |
|---|---|---|---|---|---|---|---|
| 1. | Bonato et al. [14] | 1 | Well diff NEC/atypical carcinoid | ITAC | N/A | Surgery and radiation | Patient recurred twice. Last recurrence was intracranial, which patient did not survive |
| 2. | Jain et al. [15] | 2 | SCNEC (both cases) |
1. ITAC; Poorly differentiated adenocarcinoma with signet ring cells 2. ITAC (papillary architecture) |
N/A |
1. Surgical debulking and chemoradiation 2. Debulking surgery |
1. No follow up provided 2. Patient died 4 months after surgery |
| 3. | Huang et al. [13] | 1 | SCNEC | Adenosquamous carcinoma | EGFR neg | Total maxillectomy and adjuvant chemotherapy | Regional recurrence/LN metstasis 2 months post-surgery; Distant metastasis (lung) 6 months post-surgery. Patient died 8 months post diagnosis |
| 4. | La Rosa et al. [12] | 1 | SCNEC | ITAC | TP53, | Endoscopic endonasal tumor resection, and radiotherapy | Patient was free of disease until 26 months post-surgery, when he developed bone metastasis and died |
| 5. | Barham et al. [7] | 1 | SCNEC | Moderately differentiated SCC | N/A | Medial maxillectomy and chemotherapy | Patient recurred 7 months post-surgery with multiple bone metastasis |
| 6. | Franchi et al. [11] | 1 | LCNEC | Well differentiated SCC | TP53 | Maxillectomy. Post operative radiation was planned, but not completed due to complications | Recurrence free interval: 20 months post-operatively |
| 7. | Current study | 3 |
1.SCNEC 2.LCNEC 3.SCNEC |
1. Moderately differentiated SCC 2. Moderately differentiated SCC 3. Non-keratinizing SCC |
N/A |
1. Neo-adjuvant chemotherapy followed by maxillectomy and selective neck dissection 2. Maxillectomy and selective neck dissection, and chemoradiation 3. Medial maxillectomy and partial septectomy, and chemoradiation |
1. Recurrence free interval:14 months post-surgery 2. Patient recurred both locally and with distant (lung) metastasis and was referred to palliative care 3. Patient had local recurrence in form of high-grade squamous dysplasia, which was excised. He is symptom free 14 years post his initial diagnosis |
SCNEC small cell neuroendocrine carcinoma, LCNEC large cell neuroendocrine carcinoma, SCC squamous cell carcinoma, ITAC intestinal type adenocarcinoma
All three cases in our study showed squamous cell carcinoma closely intermingled with high-grade neuroendocrine carcinoma: small cell NEC in two cases and large cell NEC in one case. The differential diagnosis of these tumors should include NUT midline carcinoma, a recently described high-grade malignancy that can show undifferentiated tumor cells with areas of abrupt keratinization [24]. The possibility of NUT midline carcinoma was excluded in all three cases by immunohistochemical staining for NUT protein. Another high-grade tumor, sinonasal undifferentiated carcinoma (SNUC), is a consideration for the cases presented here but was excluded by the presence of squamous differentiation, an exclusion criterion for diagnosis of SNUC [25, 26]. In addition, two other entities recently described in the sinonasal tract: Sinonasal teratocarcinosarcoma (SNTCS), and Adamantinoma-like Ewing sarcoma (ALES) were considered as differentials as squamous differentiation and blue round cell components can be seen in both tumor types. Absence of mesenchymal elements in all three cases helped rule out the possibility of SNTCS, while negative expression for CD99 negated the possibility of ALES. Further, our study tested all three cases for possible role of CTNNB1 mutations in these tumors and found no aberrant expression of beta-catenin [27].
Barham et al. [8] proposed two possible hypotheses for the origin of these tumors: either the tumor arises from a common pluripotent stem cell with subsequent divergent differentiation; or two separate tumors arise synchronously in the same region through two independent molecular processes, also known as a “collision tumor.” Some of the poorly differentiated sinonasal carcinomas (previously included under SNUC), can show loss of SMARCB1 and SMARCA4 proteins, which are part of SWI/SNF chromatin remodelling complex. In their normal state, SMARCB1 and SMARCA4 play an important role in chromatin remodeling, and cell proliferation [28, 29]. Tumors with aberrations in these two proteins show complete loss of staining with SMARCB1/INI-1 and SMARCA4 immunostains, respectively. These tumors primarily show small blue cell like morphology with variable staining for neuroendocrine markers and can resemble SNUC or non-keratinizing SCC. All three cases in our study showed intact nuclear expression for INI-1. (Table 3). SMARCA4 stain could not be performed due to lack of adequate funds for send out testing. Although, this can be considered a potential drawback of this study, given the presence of small blue cell population in two of our cases (case 1 and case 3) and the recent inclusion and emphasis on SWI/SNF complex aberrations in sinonasal tract malignancies. However, we do want to emphasize the fact that all the cases include in this study showed significant proportion of squamous cell carcinoma (supported by both morphology and IHC) in contrast to primarily small blue cell like morphology as described in the SWI/SNF mutated tumors.
Sinonasal neuroendocrine carcinomas/SNEC are a distinct group of sinonasal neoplasms associated with poor survival (5-year survival rates of 5–20%), and high recurrence and metastatic rates [28, 30]. Diagnosis of SNEC requires the conventional histology of neuroendocrine carcinomas in the form of diffuse sheets or solid nests of malignant cells with small cell or large cell morphology, abundant mitoses and apoptotic bodies, nuclear molding, salt and pepper chromatin; and staining with at least one neuroendocrine IHC markers (synaptophysin, chromogranin, CD56 or NSE) [9]. A recent study showed that the insulinoma-associated protein 1 (INSM1) may serve as a potential diagnostic biomarker for neuroendocrine carcinoma in head and neck [31]. Case 1 in our study showed patchy expression of INSM1 (both in the initial FNA sample as well as in the residual NEC in resection specimen), while synaptophysin was negative. INSM1 was not performed on cases 2 and 3.
The role of TP53 mutation has been studied by a few researchers in sinonasal cancers with a reported incidence of 30–75% [12, 32]. TP53 mutation has been reported in several tumors, such as urinary bladder, lung, and esophageal carcinomas [11]. The literature regarding the role of TP53 mutation in combined neuroendocrine carcinomas is limited. In their study of combined small cell lung carcinomas, Pelosi et al. reported a TP53 codon V274F mutation in exon 8 shared by both the small cell neuroendocrine carcinoma and the sarcomatous component. Their finding suggested a possible clonal evolution of a p53-mutated common ancestor lesion [33]. In contrast, Hiraki et al. [34] and Franchi et al. [12] reported TP53 mutation in only the small cell carcinoma component and not the non-small cell carcinoma component in their respective studies. The latter study utilized p53 immunohistochemistry to validate their findings further and found overexpression of P53 restricted to the neuroendocrine component.
Our study found increased immunohistochemical expression of the cell cycle regulator, p53 in two of three cases. While case 2 showed uniformly increased expression amongst the two components, case 1 showed higher expression in the squamous component as compared to the residual neuroendocrine component in the post chemotherapy resection specimen. Our findings are more in line with the findings of Pelosi et al. [33] and suggest the role of amplification of the p53 locus as a possible contributor to tumorigenesis in combined SCC + NEC. In addition, our findings open the possibility of p53 gene therapy in these rare and aggressive tumors [18].
The role of high-risk human papillomavirus (HrHPV) is well established in head and neck squamous cell carcinomas, particularly those of the oropharynx, where they account for up to 80% of cases [3]. Various studies have identified HPV in tumors of the sinonasal tract, but with widely divergent results [3, 35, 36]. Bishop et al. [3] reported that one-third of all sinonasal squamous cell carcinomas were positive for high-risk HPV and most common in non-keratinizing and basaloid subgroups. In addition, they observed a trend towards a better clinical outcome in the HPV + sinonasal cancers. The latter observation is supported by Cohen et al. [35], who reported improved overall survival (OS) and disease-free survival (DFS) in HPV + sinonasal carcinomas. In our study, one case tested positive for p16 (Case 3, Fig. 5D), which could be secondary to RB gene inactivation, a finding commonly seen in high grade neuroendocrine carcinomas [37]. HPV association was ruled out in this case with a negative hrHPV in situ hybridization (ISH) study (Fig. 5E).
Retinoblastoma gene (RB) is a tumor suppressor, that plays a pivotal role in the negative control of cell cycle and in tumor progression [38]. Inactivation of RB is seen in some human cancers and in most small-cell lung carcinoma (SCLC) cell lines [37]. As Rb normally suppresses p16 transcription, lack of the protein leads to marked overexpression of p16. A study by Jiromaru et al. [39] suggested the combined use of p16 and Rb immunohistochemistry as a reliable, cost-effective method to predict HR-HPV infection in oropharyngeal squamous cell carcinomas. Immunohistochemistry for Rb was not performed in the current study.
Beta-Catenin, a cadherin-associated membrane protein, is involved in the regulation of cell-to-cell adhesion as well as gene transcription and is part of the Wnt-signaling pathway. Aberrant expression of β-catenin secondary to mutation in CTNNB1 gene is a well-known event in tumorigenesis and tumor progression [40]. Nuclear accumulation of β-catenin due to mutational activation is considered possible central event in the pathogenesis of sinonasal glomangiopericytomas [41]. In addition, the nuclear accumulation of β-catenin is also seen in nasopharyngeal angiofibromas. Our study is the first study to investigate role of β-catenin in combined NEC + SCC cases of the sinonasal tract. None of the three cases in this series showed aberrant expression of β-catenin.
The primary treatment modality for sinonasal SCC is surgery combined with radiotherapy, with the option of chemoradiation (CRT). Despite improvements in surgery, radiotherapy, and systemic therapy, the prognosis for sinonasal SCC remains poor [41]. Similar to other sinonasal cancers, complete surgical resection is the mainstay of treatment in SNECs too. A single case report [42] has suggested a possible role for neoadjuvant chemotherapy and concurrent CRT in SNECs. Interestingly, one of our cases (case 1) was treated with neoadjuvant chemotherapy and showed excellent tumor response in the NEC component. Subsequent resection of the maxillary mass showed > 95% squamous cell component with a tiny residual component of small cell NEC comprising ~ 1% and complete resolution of liver metastases. This highlights the importance of recognizing the possibility of combined tumors in sinonasal tract (especially in discordant biopsy results as seen in case 1 of this study) and exploring role of neo-adjuvant chemotherapy for the high-grade NEC component which shows excellent response to chemotherapy. However, it should be noted that focal expression of neuroendocrine markers can be seen in otherwise conventional SCC and adenocarcinoma, which should not be diagnosed as SNEC in the absence of morphologic features of NEC [43].
Limited data regarding treatment outcomes is available in combined SCC + NEC due to the rarity of these tumors in the sinonasal tract. Immunotherapy with specific monoclonal antibodies against the programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) immune checkpoint pathway is a treatment option for some advanced-stage cancers. Pembrolizumab, an antibody targeting PD-1, was approved by the FDA in 2016 for head and neck squamous cell carcinoma and non-small cell lung cancer [44].
The role of PD-L1 in sinonasal tumors is quite limited. Only two studies in the literature evaluate the role of PD-L1 in sinonasal cancers, namely Riobello et al. and Quan et al. [44, 45]. In their study of 53 sinonasal SCCs and 126 intestinal-type adenocarcinomas (ITACs), Riobellow et al. reported membranous PD-L1 staining of tumor cells in 34% of the SCC and 17% of the ITAC samples. In addition, they found PD-L1 positivity on infiltrating immune cells in 45% of the sinonasal SCC samples and 33% of the ITAC samples. The researchers did not identify a correlation between PD-L1 expression and clinicopathological parameters [44]. In contrast, Quan et al. [45] found a significant correlation of PD-L1 expression with poor differentiation and tumor-infiltrating lymphocytes (TILs), thereby suggesting the PD-1/PD-L1 pathway may be a target in sinonasal SCC. Immune checkpoint inhibitors like atezolizumab (PD-L1 inhibitor) have recently shown promising role as first-line agents in combination with platinum based-etoposide chemotherapy in patients with extensive stage small cell lung carcinoma [46]. In the current study, the two cases with a small cell NEC component expressed PD-L1 with a combined positive score of 40 and 10 (case 1 and case 3, respectively), suggesting a role for immunotherapy in the treatment of these rare, aggressive neoplasms.
To summarize, all three combined NEC + SCC tumors in our study demonstrated two components histologically: moderately to poorly differentiated SCC and high-grade NEC. Divergent differentiation was confirmed with lineage-specific markers. Only 1 patient received neoadjuvant chemotherapy prior to surgery, with marked decrease in the size of the primary lesion and resolution of liver metastases. This finding highlights the importance of identifying combined sinonasal carcinomas (especially in cases with discrepant FNA and biopsy results) translating to use of neoadjuvant chemotherapy for the high-grade NEC component. Our immunohistochemical analysis revealed increased expression of the cell cycle regulator p53 in two of three cases, suggesting that the upregulation of p53/mutation in TP53 may contribute to tumorigenesis at this site.
In addition, two cases were PD-L1 positive. Although evidence regarding the role of PD-L1 in sinonasal tumors is limited, increasing evidence in the literature suggests that a proportion of these patients may benefit from therapy with immune checkpoint inhibitors. To the best of our knowledge, our study is the first case series to perform and report a comprehensive immunohistochemical panel (including β-catenin and PD-L1 expression) on this extremely rare type of tumors. These findings add to the limited literature available on this vanishingly rare entity in the sinonasal tract. We emphasize the importance of recognizing these tumors for optimal patient management. Our findings of increased p53 and PD-L1 expression in a subset of cases opens the door for further investigation into the pathogenesis and potential new targets for therapeutic intervention in these aggressive tumors.
Author Contributions
SA designed and executed this study and wrote the final manuscript. MG helped with drafting the case presentation section of the manuscript. AVZ revised the manuscript.
Funding
Departmental funds (UNM) utilized.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
No disclosures.
Ethical Approval
It was obtained from University of New Mexico and University of San Francisco.
Research Involving Humans and/or Animals
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
No patient’s personal data/identifiers are disclosed; hence, no consent was necessary.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Youlden DR, Cramb SM, Peters S, et al. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 2013;37(6):770–779. doi: 10.1016/j.canep.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Dutta R, Dubal PM, Svider PF, et al. Sinonasal malignancies: a population-based analysis of site-specific incidence and survival. Laryngoscope. 2015;125(11):2491–2497. doi: 10.1002/lary.25465. [DOI] [PubMed] [Google Scholar]
- 3.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop JA. Newly described tumor entities in Sinonasal Tract Pathology. Head Neck Pathol. 2016;10(1):23–31. doi: 10.1007/s12105-016-0688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34(6):877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 6.Mills SE. Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol. 2002;15:264–278. doi: 10.1038/modpathol.3880522. [DOI] [PubMed] [Google Scholar]
- 7.Chen DA, Mandell-Brown M, Moore SF, et al. “Composite” tumor-mixed squamous cell and small-cell anaplastic carcinoma of the larynx. Otolaryngol Head Neck Surg. 1986;95:99–103. doi: 10.1177/019459988609500119. [DOI] [PubMed] [Google Scholar]
- 8.Barham HP, Said S, Ramakrishnan VR. Colliding tumor of the paranasal sinus. Allergy Rhinol. 2013;4(1):e13–e16. doi: 10.2500/ar.2013.4.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Ordonez B, Bishop JA, Gnepp DR, Hunt JL, Thompson LDR. Neuroendocrine tumors. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumors. 4. Lyon: IARC; 2017. pp. 95–98. [Google Scholar]
- 10.Jaiswal VR, Hoang MP. Primary combined squamous and small cell carcinoma of the larynx: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:1279–1282. doi: 10.5858/2004-128-1279-PCSASC. [DOI] [PubMed] [Google Scholar]
- 11.Davies-Husband CR, Montgomery P, Premachandra D, et al. Primary, combined, atypical carcinoid and squamous cell carcinoma of the larynx: a new variety of composite tumour. J Laryngol Otol. 2010;124:226–229. doi: 10.1017/S0022215109991228. [DOI] [PubMed] [Google Scholar]
- 12.Franchi A, Rocchetta D, Palomba A, et al. Primary combined neuroendocrine and squamous cell carcinoma of the maxillary sinus: report of a case with immunohistochemical and molecular characterization. Head Neck Pathol. 2015;9(1):107–113. doi: 10.1007/s12105-013-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Rosa S, Furlan D, Franzi F, et al. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr pathol. 2016;27(4):284–311. doi: 10.1007/s12022-016-9432-9. [DOI] [PubMed] [Google Scholar]
- 14.Huang SF, Chuang WY, Cheng SD, et al. A colliding maxillary sinus cancer of adenosquamous carcinoma and small cell neuroendocrine carcinoma—a case report with EGFR copy number analysis. World J Surg Oncol. 2010;20(8):92. doi: 10.1186/1477-7819-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonato M, Frigerio B, Capella C, et al. Composite enteric-type adenocarcinoma-carcinoid of the nasal cavity. Endocr Pathol. 1993;4:40–47. doi: 10.1007/BF02914488. [DOI] [PubMed] [Google Scholar]
- 16.Jain R, Gramigna V, Sanchez-Marull R, et al. Composite intestinal-type adenocarcinoma and small cell carcinoma of sinonasal tract. J Clin Pathol. 2009;62:634–637. doi: 10.1136/jcp.2009.065433. [DOI] [PubMed] [Google Scholar]
- 17.PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria: Dako, Agilent Pathology Solutions; 2019.
- 18.Laura LL, Menander K, Sobol RE, Zumstein LA, French M, Bocangel D, Roth J, Chada S. Abnormal overexpression of p53 is a predictive molecular biomarker of Advexin (adenoviral p53) efficacy in recurrent squamous cell carcinoma of the head and neck (SCCHN) Clin Cancer Res. 2006;12(19):B52. [Google Scholar]
- 19.Turri-Zanoni M, Maragliano R, Battaglia P, et al. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017;74:21–29. doi: 10.1016/j.oraloncology.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97(6):1488–1498. doi: 10.1002/cncr.11196. [DOI] [PubMed] [Google Scholar]
- 21.Burton AL, Egger ME, Gilbert JE, et al. Assessment of mitotic rate reporting in melanoma. Am J Surg. 2012;204(6):969–974. doi: 10.1016/j.amjsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa Y, Kikuchi M, Imai Y, et al. Successful treatment of a mixed neuroendocrine-nonneuroendocrine neoplasm of the colon with metastases to the thyroid gland and liver. Case Rep Otolaryngol. 2020;13:5927610. doi: 10.1155/2020/5927610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al., editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. pp. 13–14. [Google Scholar]
- 24.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejaz A, Wenig BM. Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv Anat Pathol. 2005;12(3):134–143. doi: 10.1097/01.pap.0000163958.29032.56. [DOI] [PubMed] [Google Scholar]
- 26.Smith SR, Som P, Fahmy A, et al. A clinicopathological study of sinonasal neuroendocrine carcinoma and sinonasal undifferentiated carcinoma. Laryngoscope. 2000;110:1617–1622. doi: 10.1097/00005537-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Thompson LDR, Bishop JA. Update from the 5th Edition of the World Health Organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Head Neck Pathol. 2022 doi: 10.1007/s12105-021-01406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechner M, Liu J, Lund VJ. Novel biomarkers in sinonasal cancers: from bench to bedside. Curr Oncol Rep. 2020;22(10):106. doi: 10.1007/s11912-020-00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agaimy A, Koch M, Lell M, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38:1274–1281. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babin E, Rouleau V, Vedrine PO, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol. 2006;120:289–297. doi: 10.1017/S0022215106000594. [DOI] [PubMed] [Google Scholar]
- 31.Rooper LM, Bishop JA, Westra WH. INSM1 is a sensitive and specific marker of neuroendocrine differentiation in head and neck tumors. Am J Surg Pathol. 2018;42:665–671. doi: 10.1097/PAS.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 32.Holmila R, Bornholdt J, Suitiala T, et al. Profile of TP53 gene mutations in sinonasal cancer. Mutat Res. 2010;686:9–14. doi: 10.1016/j.mrfmmm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Pelosi G, Sonzogni A, Galetta D, et al. Combined small cell carcinoma of the lung with quadripartite differentiation of epithelial, neuroendocrine, skeletal muscle, and myofibroblastic type. Virchows Arch. 2011;458:497–503. doi: 10.1007/s00428-010-1011-8. [DOI] [PubMed] [Google Scholar]
- 34.Hiraki A, Ueoka H, Yoshino T, et al. Synchronous primary lung cancer presenting with small cell carcinoma and non-small cell carcinoma: diagnosis and treatment. Oncol Rep. 1999;6:75–80. [PubMed] [Google Scholar]
- 35.Cohen E, Coviello C, Menaker S, et al. P16 and human papillomavirus in sinonasal squamous cell carcinoma. Head Neck. 2020;42(8):2021–2029. doi: 10.1002/hed.26134. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita Y, Hasegawa M, Deng Z, et al. Human papillomavirus infection and immunohistochemical expression of cell cycle proteins pRb, p53, and p16(INK4a) in sinonasal diseases. Infect Agent Cancer. 2015;10:23. doi: 10.1186/s13027-015-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gouyer V, Gazzéri S, Bolon I, et al. Mechanism of retinoblastoma gene inactivation in the spectrum of neuroendocrine lung tumors. Am J Respir Cell Mol Biol. 1998;18(2):188–196. doi: 10.1165/ajrcmb.18.2.3008. [DOI] [PubMed] [Google Scholar]
- 38.Du W, Searle JS. The rb pathway and cancer therapeutics. Curr Drug Targets. 2009;10(7):581–589. doi: 10.2174/138945009788680392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiromaru R, Yamamoto H, Yasumatsu R, et al. p16 overexpression and Rb loss correlate with high-risk HPV infection in oropharyngeal squamous cell carcinoma. Histopathology. 2021;79(3):358–369. doi: 10.1111/his.14337. [DOI] [PubMed] [Google Scholar]
- 40.Lasota J, Felisiak-Golabek A, Aly FZ, et al. Nuclear expression and gain-of-function β-catenin mutation in glomangiopericytoma (sinonasal-type hemangiopericytoma): insight into pathogenesis and a diagnostic marker. Mod Pathol. 2015;28(5):715–720. doi: 10.1038/modpathol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robin TP, Jones BL, Gordon OM, et al. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer. 2017;123(16):3040–3049. doi: 10.1002/cncr.30686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosokawa S, Okamura J, Takizawa Y, et al. Long-term survival of a patient with primary small cell neuroendocrine carcinoma of the maxillary sinus: a case report. J Oral Maxillofac Surg. 2013;71:e248–e252. doi: 10.1016/j.joms.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 43.Thompson LDR, Bell D, Bishop JA. Neuroendocrine carcinomas. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumors. 4. IARC: Lyon; 2017. pp. 21–22. [Google Scholar]
- 44.Riobello C, Vivanco B, Reda S, et al. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck. 2018;40(4):818–827. doi: 10.1002/hed.25067. [DOI] [PubMed] [Google Scholar]
- 45.Quan H, Yan L, Wang S, et al. Clinical relevance, and significance of programmed death-ligand 1 expression, tumor-infiltrating lymphocytes, and p16 status in sinonasal squamous cell carcinoma. Cancer Manag Res. 2019;11:4335–4345. doi: 10.2147/CMAR.S201568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esposito G, Palumbo G, Carillio G, et al. Immunotherapy in small cell lung cancer. Cancers. 2020;12(9):2522. doi: 10.3390/cancers12092522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.