Abstract
Interferon consensus sequence binding protein (ICSBP)-deficient mice display enhanced susceptibility to intracellular pathogens. At least two distinct immunoregulatory defects are responsible for this phenotype. First, diminished production of reactive oxygen intermediates in macrophages results in impaired intracellular killing of microorganisms. Second, defective early interleukin-12 (IL-12) production upon microbial challenge leads to a failure in gamma interferon (IFN-γ) induction and subsequently in T helper 1 immune responses. Here, we investigated the role of ICSBP in resistance against the extracellular bacterium Yersinia enterocolitica. ICSBP−/− mice failed to produce IL-12 and IFN-γ, but also IL-4, after Yersinia challenge. In addition, granuloma formation was highly disturbed in infected ICSBP−/− mice, leading to multiple necrotic abscesses in affected organs. Consequently, ICSBP−/− mice rapidly succumbed to acute Yersinia infection. In vitro treatment of spleen cells from ICSBP−/− mice with recombinant IL-12 (rIL-12) or rIL-18 in combination with a second stimulus resulted in IFN-γ induction. In experimental therapy of infected ICSBP−/− mice, we observed that administration of rIL-12 induced IFN-γ production which was associated with improved resistance to Yersinia. In contrast, treatment with rIL-18 failed to enhance endogenous IFN-γ production but nevertheless reduced bacterial burden in ICSBP−/− mice. Although cytokine therapy with rIL-12 or rIL-18 ameliorated the course of Yersinia infection in ICSBP−/− mice, both cytokines failed to completely restore impaired immunity. Taken together, the results indicate that the transcription factor ICSBP is essential for efficient host immune defense against Yersinia. These results are important for understanding the complex host immune responses in bacterial infections.
Interferon (IFN) consensus sequence binding protein (ICSBP) (19) belongs to the IFN regulatory factor (IRF) family of mammalian transcription factors (see for a review reference 47). Proteins of the IRF family bind to the IFN-stimulated response element (ISRE) and control transcription of genes with ISREs within their promoter regions (57). The IRF family plays an important role in the regulation of both type I (IFN-α/β) and type II (IFN-γ) IFN-inducible genes. ICSBP is exclusively expressed in hematopoietically derived cells and predominantly induced by IFN-γ (25, 55). Analyses of recently generated ICSBP knockout (ICSBP−/−) mice have permitted insights into the in vivo role of ICSBP (34). These mice exhibit a chronic myelogenous leukemia (CML)-like syndrome and display enhanced susceptibility to a variety of intracellular pathogens including Listeria monocytogenes, Leishmania major, and Toxoplasma gondii (22, 28, 34, 64). ICSBP−/− mice fail to develop T helper 1 (Th1)-driven immune responses due to a primary defect in interleukin-12 (IL-12) p40 induction and, as a consequence, IFN-γ-dependent host resistance (28, 34, 64). Furthermore, ICSBP−/− mice show reduced and delayed oxidative burst, whereas nitric oxide (NO) production is normal (22). Th2 immune responses, however, are not affected in these mice. In addition, ICSBP modulates survival of myeloid cells by regulating expression of apoptosis-related genes (26).
Yersinia enterocolitica is enteropathogenic for humans and rodents. The bacteria cross the intestinal epithelial barrier by translocating through M cells, spread into the lamina propria, and colonize preferentially the underlying Peyer's patches (2, 4, 14, 29, 30). Virulence plasmid (pYV)-harboring strains are able to migrate from the Peyer's patches to the mesenteric lymph nodes and deeper organs such as the spleen, liver, and lungs, where they multiply extracellularly and lead to the formation of multiple necrotic abscesses (2, 4, 30, 69). In contrast, nonvirulent strains lacking the pYV (pCD1 in Y. pestis) plasmid are contained within granulomas, resulting in a lower rate of infection before rapid clearance of the bacteria (45, 71, 74).
Successful control and elimination of Y. enterocolitica depends on both innate and adaptive immunity. Neutrophils and macrophages are involved in partial restriction of bacterial replication in the early phase of primary infection in mice (4, 15, 31, 62). Furthermore, despite Y. enterocolitica being an extracellular pathogen, it is well established that T-cell-mediated and IFN-γ-dependent immune mechanisms are essential for resistance (1, 5). Consequently, adoptive transfer of Yersinia-specific CD4+ Th1 cell clones into athymic T-cell-deficient nude mice confers resistance against this pathogen (6). Previous studies have shown that C57BL/6 mice, which produce high levels of IFN-γ, are resistant to Y. enterocolitica, whereas BALB/c mice, which secrete only small quantities of IFN-γ, are susceptible to Yersinia infection (1). Furthermore, neutralization or genetic deletion of the cytokine tumor necrosis factor alpha (TNF-α), IFN-γ, IL-12, or IL-18 abrogates resistance to Yersinia infection (3, 7, 10). Based on these results, it is conceivable that antigen-presenting cells such as dendritic cells and macrophages become activated during contact with microbes and start producing IL-12 and IL-18. These cytokines strongly induce the expression of IFN-γ in natural killer (NK) cells and CD4+ Th1 cells. Most likely, IFN-γ produced by these cells synergizes with macrophage-produced TNF-α to activate microbicidal mechanisms such as reactive oxygen intermediates and reactive nitrogen intermediates in macrophages.
Although ICSBP has been shown to be essential for immunity to intracellular pathogens, nothing is known about its requirement for immunity to extracellular pathogens, in particular Y. enterocolitica. The aim of this study was to investigate (i) whether ICSBP−/− mice exhibit an altered susceptibility to Yersinia, (ii) which defense mechanisms against Yersinia depend on the coordinate expression of the transcription factor ICSBP, and (iii) whether administration of recombinant cytokines restores impaired immunity in ICSBP−/− mice. The experiments described herein argue for an essential role of ICSBP in resistance against Y. enterocolitica.
MATERIALS AND METHODS
Mice.
Young ICSBP−/− mice, 6 to 10 weeks old, on a C57BL/6 × 129/Sv or C57BL/6 background were used for all experiments, as they do not display the severe CML-like disease which develops in aged animals (34). Control C57BL/6 × 129/Sv or C57BL/6 mice were purchased from Charles River Wiga (Sulzfeld, Germany). All animals were housed in specific-pathogen-free conditions in negative-pressure cabinets.
Bacteria, experimental infection, and in vivo administration of cytokines.
Plasmid (pYV)-harboring Y. enterocolitica WA-314 (serotype O:8) was used for intravenous (i.v.) infection as previously described (33). In brief, Y. enterocolitica was passaged in mice, cultivated at 26°C in Luria broth, harvested during the log phase, aliquoted, and frozen at −80°C. Surface expression of YadA was tested by agglutination using anti-YadA polyclonal antiserum. For experimental infection, freshly thawed bacteria diluted in sterile phosphate-buffered saline (PBS; pH 7.4) to obtain the indicated dose were injected i.v. in a total volume of 100 μl. The actual number of bacteria administered was determined by plating serial dilutions of the inoculum on Mueller-Hinton agar. Mice were weighed before infection and every day after infection. On the indicated days after infection, the spleen and liver were taken out and homogenized. Bacterial titers were determined by plating out serial 10-fold dilutions of organ suspensions on Mueller-Hinton agar. The limit of detectable CFU was 25 (log 1025 = 1.4). Mice were treated by intraperitoneal (i.p.) administration of PBS, murine recombinant IFN-γ (rIFN-γ) (a gift from Bender, Vienna, Austria), murine rIL-12 (kindly provided by M. Gately), and murine rIL-18 (kindly provided by H. Okamura) over 5 days starting 1 day prior to infection.
Histology and immunohistology.
Histological and immunohistological examinations were performed as previously described (4). For histological examinations, the liver and spleen were excised, fixed in 4% buffered formalin, embedded in paraffin, cut, and stained. For immunohistological analysis, the tissues were embedded in Tissue-Tek O.C.T. compound (Nunc, Roskilde, Denmark), snap-frozen in liquid nitrogen, and stored at −80°C. Frozen sections were prepared and double immunostainings were performed. Nonspecific binding sites were blocked by incubation of the sections with PBS containing 25% sheep serum. Then sections were incubated with rabbit anti-Y. enterocolitica O:8 antibodies (diluted 1:100) followed by alkaline phosphatase-conjugated goat anti-rabbit antibody diluted 1:100. Substrate solution (9.8 ml of Tris buffer [pH 8.2] containing 10 ml of levamisole, 20 mg of naphthol-AS-MX-phosphate, and 10 mg of fast red salt) was incubated for 20 min. Then an indirect three-stage immunoperoxidase method (peroxidase-antiperoxidase [PAP]) including 3,3-diaminobenzidine tetrahydrochloride acid (Sigma, Deisenhofen, Germany) as indicator was used for detection of immunolabeling with anti-Mac-1 (5C6) antibodies (hybridoma cell culture supernatant diluted 1:10). Peroxidase-conjugated mouse F(ab′)2 fragment anti-rat immunoglobulin G (diluted 1:100; Dianova, Hamburg, Germany) was used as secondary antibody, and rat PAP complex (diluted 1:100; Dianova) was used as tertiary antibody. After incubation with substrate solution, the sections were counterstained with Mayer's hematoxylin, mounted, and assessed microscopically by two independent investigators. Isotype-matched irrelevant antibodies were used as controls and revealed no staining signal.
Cell preparation, culture conditions, and in vitro stimulation of cells.
Single-cell suspensions were prepared from spleen for tissue culture. In brief, erythrocytes were lysed with ammonium chloride lysing buffer (0.15 M NH4Cl [pH 7.2]), and the remaining cells were washed three times with Hanks balanced salt solution and resuspended in Click/RPMI 1640 cell culture medium supplemented with 10% heat-inactivated fetal calf serum, streptomycin (10 μg/ml), penicillin (100 U/ml), 2 mM l-glutamine, 10 mM HEPES, and 50 μM 2-mercaptoethanol (Biochrom, Berlin, Germany). For in vitro studies, 106 cells per ml were cultured for 48 h at 37°C in a humidified 5% CO2 atmosphere. For determination of cytokine production, cells were incubated in the presence of medium alone, concanavalin A, heat-killed Yersinia (HKY), rIL-12, and rIL-18 as indicated.
Determination of cytokine production in cell culture supernatants and sera.
IFN-γ levels were determined by using a capture enzyme-linked immunoadsorbent assay (ELISA). Briefly, ELISA microtiter plates (Greiner, Frickenhausen, Germany) were coated with anti-IFN-γ monoclonal antibody (MAb) (AN-18.17.24). After blocking of nonspecific binding sites, sera or supernatants were added to the wells and incubated overnight. After several wash steps, biotin-labeled anti-IFN-γ MAb (R4-6A2) was added. Finally, an avidin-biotin-alkaline phosphatase complex (Strept ABC-AP kit; DAKO, Glostrup, Denmark) was added. For signal development, the wells were incubated with p-nitrophenyl phosphate disodium (Sigma), and the optical density was determined at wavelengths of 405 and 490 nm with an ELISA reader. The level of IFN-γ from spleen cell culture was determined from the straight-line portion of the standard curve by using recombinant murine IFN-γ.
TNF-α and IL-12 (p40 and p70) levels were determined by a capture ELISA using (i) anti-TNF-α MAb (G281-2626) and biotin-labeled anti-TNF-α MAb (MP6XT3) and (ii) anti-IL-12 MAb (C17.8) and biotin-labeled anti-IL-12 MAb (C15.6) (Pharmingen, Hamburg, Germany), respectively, as described above for IFN-γ ELISA.
IL-4 was measured using a bioassay employing IL-4-dependent CTS4 cells. Single-cell suspensions of splenocytes derived from infected ICSBP+/+ and ICSBP−/− mice were cultured under conditions described above; after 48 h, supernatants were collected and assayed for IL-4 activity. Then 5 × 103 CTS4 cells were added to serial dilutions of supernatants. Cells were incubated for 24 h in an humidified 37°C, 5% CO2 incubator and pulsed with [3H]thymidine for an additional 24 h. [3H]thymidine incorporation was measured by liquid scintillation counting. The limit of detection was 5 U of IL-4 per ml.
Purification of RNA, cDNA synthesis, and RT-PCR analysis of cytokine mRNA.
Approximately 100 mg of tissue was homogenized in 1 ml of TRIzol (Gibco-Life Technologies, Karlsruhe, Germany), and total RNA was isolated by a single-step method as described elsewhere (13). Reverse transcription (RT) was performed by mixing 20 μg of RNA in 10 μl of diethyl pyrocarbonate-treated double-distilled H2O with 0.5 μg of oligo(dT) (Gibco). This solution was incubated for 10 min at 65°C. Then 10 μl of a solution containing 4 μl of 5× reverse transcriptase buffer (100 mM Tris-HCl [pH 8.3], 150 mM KCl, 6 mM MgCl2) (Gibco), 40 U of RNasin (Promega, Mannheim, Germany), 20 mM dithiothreitol (Gibco), and 2 mM deoxynucleoside triphosphates was added, and tubes were incubated for 60 min at 37°C. Finally, tubes were heated to 90°C for 5 min, and 180 μl of distilled H2O was added to the reaction mixture. Samples were stored at −20°C until further use. Five microliters of cDNA prepared as described above was added to 50 μl of a solution consisting of 1 U of AmpliTaq or AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.), 200 μM deoxynucleoside triphosphates, 200 nM sense and antisense primers, and 5 μl of 10× PCR buffer (50 mM KCl, 10 mM Tris-HCl [pH 8.3], 1.5 mM MgCl2; Perkin-Elmer). As indicated in Table 1, 21 to 40 PCR cycles consisting of denaturation (30 s at 94°C), annealing (45 s at 60°C), and amplification (60 s at 72°C) were carried out on a DNA thermal cycler (GeneAmp PCR System 9600; Perkin-Elmer). PCR amplification was started by an initial denaturation step (5 min at 94°C) and completed by a final amplification step (7 min at 72°C); 20 μl of the PCR product was mixed with 5 μl of 5× gel loading buffer and subjected to electrophoresis on a 2% agarose gel. PCR products were visualized by staining with ethidium bromide. The sequences of sense and antisense primers used in this study are shown in Table 1.
TABLE 1.
PCR primers used in this study
| Product | Primer sequence (5′-3′) | Product size (bp) | No. of PCR cycles |
|---|---|---|---|
| β-Actin | 348 | 21 | |
| Sense | TGG AAT CCT GTG GCA TCC ATG AAA C | ||
| Antisense | TAA AAC GCA GCT CAG TAA CAG TCC G | ||
| IL-12 p35 | 310 | 35a | |
| Sense | GGC TAC TAG AGA GAC TTC TTC C | ||
| Antisense | GTG AAG CAG GAT GCA GAG CTT C | ||
| IL-12 p40 | 345 | 30 | |
| Sense | GTG AAG CAC CAA ATT ACT CCG G | ||
| Antisense | GCT TCA TCT GCA AGT TCT TGG G | ||
| IFN-γ | 460 | 30 | |
| Sense | TGA ACG CTA CAC ACT GCA TCT TGG | ||
| Antisense | CGA CTC CTT TTC CGC TTC CTG AG | ||
| IL-18 | 436 | 25 | |
| Sense | ACT GTA CAA CCG CAG TAA TAC GG | ||
| Antisense | AGT GAA CAT TAC AGA TTT ATC CC | ||
| TNF-α | 307 | 30 | |
| Sense | GGC AGG TCT ACT TTG GAG TCA TTG C | ||
| Antisense | ACA TTC GAG GCT CCA GTG AAT TCG G | ||
| IL-4 | 399 | 35a | |
| Sense | ATG GGT CTC AAC CCC CAG CTA GT | ||
| Antisense | GCT CTT TAG GCT TTC CAG GAA GTC | ||
| IL-10 | 237 | 35a | |
| Sense | ACC TGG TAG AAG TGA TGC CCC AGG CA | ||
| Antisense | CTA TGC AGT TGA TGA AGA TGT CAA A | ||
| IL-12 receptor β1 | 365 | 40a | |
| Sense | GGC CAG GAG CGC TGC CG | ||
| Antisense | ATG CTC CCA CAA ATG TCA CC | ||
| IL-12 receptor β2 | 423 | 40a | |
| Sense | AAA CAA TGT TTT TCT GAC AAT CG | ||
| Antisense | CCA ATT ACT CCA ACT TCC TCC | ||
| IL-15 | 602 | 30 | |
| Sense | GCC AGC TCA TCT TCA ACA | ||
| Antisense | TAA GTC TGA GAC GAG CTC TTT |
AmpliTaq Gold polymerase (Perkin-Elmer) was used.
Statistics.
Statistical analysis of data was carried out using the Student t test. P < 0.05 was considered statistically significant. All experiments have been repeated at least once and revealed comparable results.
RESULTS
ICSBP−/− mice succumb to acute infection with Y. enterocolitica.
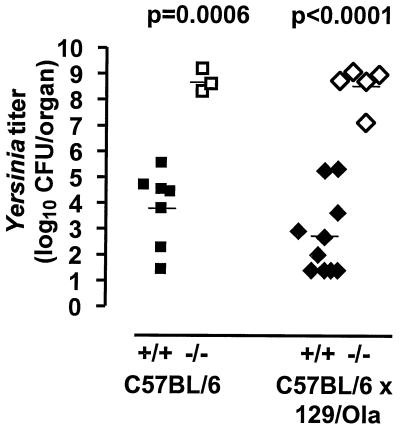
To assess the role of ICSBP in resistance against extracellularly located Yersinia, wild-type (wt) and gene-deficient mice were infected i.v. with 3 × 103 CFU of a virulent strain. This dose corresponds to 0.1 median lethal dose in C57BL/6 mice. Since differences in the genetic background of inbred mice affect immunity against Yersinia, ICSBP−/− mice on a randomly mixed C57BL/6 × 129/Ola background and ICSBP−/− mice on a C57BL/6 background, which is normally resistant to Yersinia, were tested in comparison to the corresponding wt mice. All wt mice survived and appeared healthy, whereas 7 out of 15 ICSBP−/− mice (5 out of 10 mice on a C57BL/6 × 129/Ola background and 2 out of 5 mice on a C57BL/6 background) succumbed to infection. Surviving ICSBP−/− animals were severely compromised and developed a wasting-like syndrome with weight loss within 3 to 4 days after infection (data not shown). These mice showed multiple abscesses in the spleen and liver, whereas only marginal changes were found in wt mice. ICSBP−/− mice exhibited significantly higher bacterial numbers in spleens compared to wt animals (Fig. 1). These data indicate that immune mechanisms contributing to resistance against Yersinia are regulated by the transcription factor ICSBP. For all further experiments, ICSBP+/+ and ICSBP−/− mice on a C57BL/6 background were used.
FIG. 1.
ICSBP−/− mice are highly susceptible to extracellular Yersinia. Bacterial counts in spleens of ICSBP+/+ (black symbols) and ICSBP−/− (open symbols) mice on different genetic backgrounds (squares, C57BL/6; rhombi, C57BL/6 × 129/Ola) are shown. Mice were infected i.v. with 3 × 103 CFU of Y. enterocolitica serotype O:8. On day 4 after infection, bacterial counts were determined. Each symbol represents one mouse.
Impaired IL-12 and IFN-γ production of ICSBP−/− mice in response to Yersinia.
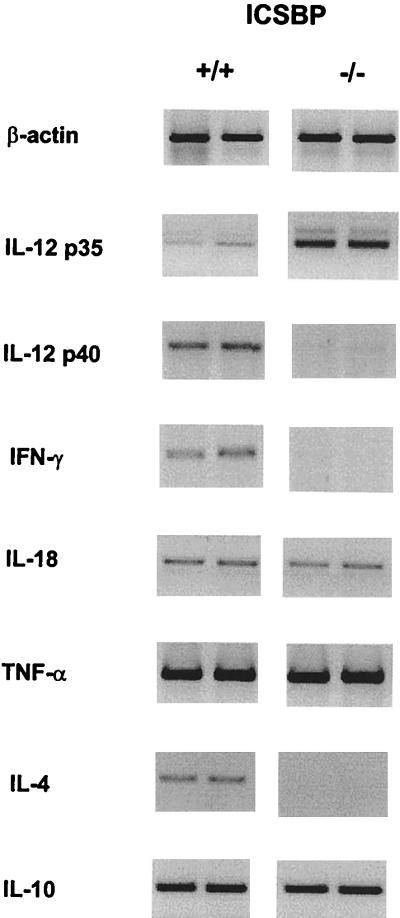
To determine whether the enhanced susceptibility to Y. enterocolitica in ICSBP−/− mice correlates with an altered pattern in cytokine production, gene expression was analyzed in Yersinia-infected ICSBP+/+ and ICSBP−/− mice by RT-PCR. IL-12 p40 and IFN-γ mRNA expression was markedly reduced, whereas IL-12 p35 mRNA was upregulated in ICSBP−/− mice (Fig. 2). In contrast to infections with intracellular L. major (28) or T. gondii (64), IL-4 mRNA was not detectable in ICSBP−/− mice after infection with Y. enterocolitica. Furthermore, mRNA expression levels of IL-10, IL-18, and TNF-α (Fig. 2), as well as IL-15 and IL-12 receptor β1 and β2 (data not shown), did not differ between ICSBP+/+ and ICSBP−/− mice.
FIG. 2.
mRNA expression of cytokines in ICSBP+/+ and ICSBP−/− mice. Primer sequences and number of PCR cycles are listed in Table 1. Gene expression in the liver was determined by RT-PCR on day 4 after infection. Two representative results are shown for ICSBP+/+ and ICSBP−/− mice.
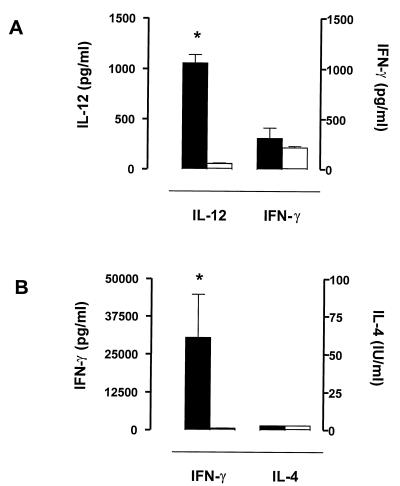
Cytokine levels in sera of infected mice were measured by ELISA. IL-12 levels were higher in infected ICSBP+/+ mice than in ICSBP−/− mice (Fig. 3A). Although we did not detect high levels of IFN-γ in sera of ICSBP+/+ mice on day 4 after infection with Yersinia (Fig. 3A), spleen cells of these mice showed substantial IFN-γ production after in vitro restimulation with HKY (Fig. 3B). In contrast, spleen cells of infected ICSBP−/− mice failed to produce IFN-γ after in vitro restimulation (Fig. 3B). Both ICSBP−/− and ICSBP+/+ splenocytes did not produce IL-4 in vitro (Fig. 3B). Previous work (1, 8) showed that BALB/c mice did not produce IL-4 upon Yersinia infection, indicating that susceptibility to this pathogen is caused by a defect in IFN-γ-mediated immune responses rather than by a switch to Th2 immune responses. These data suggest that ICSBP is required for sufficient IL-12 and IFN-γ production in response to Yersinia.
FIG. 3.
Cytokine production of infected ICSBP+/+ and ICSBP−/− mice. (A) IL-12 and IFN-γ levels in sera of ICSBP+/+ (black bars) and ICSBP−/− (open bars) mice on day 4 after Yersinia infection. (B) IFN-γ and IL-4 production by HKY (10 μg/ml)-restimulated splenocytes from ICSBP+/+ (black bars) and ICSBP−/− (open bars) mice 4 days after Yersinia infection. Spleen cells were isolated as described in Materials and Methods, and cytokines were measured after 48 h in culture supernatants. ∗ indicates statistically significant differences (P < 0.05).
ICSBP−/− mice reveal a failure in granuloma formation after challenge with Yersinia.
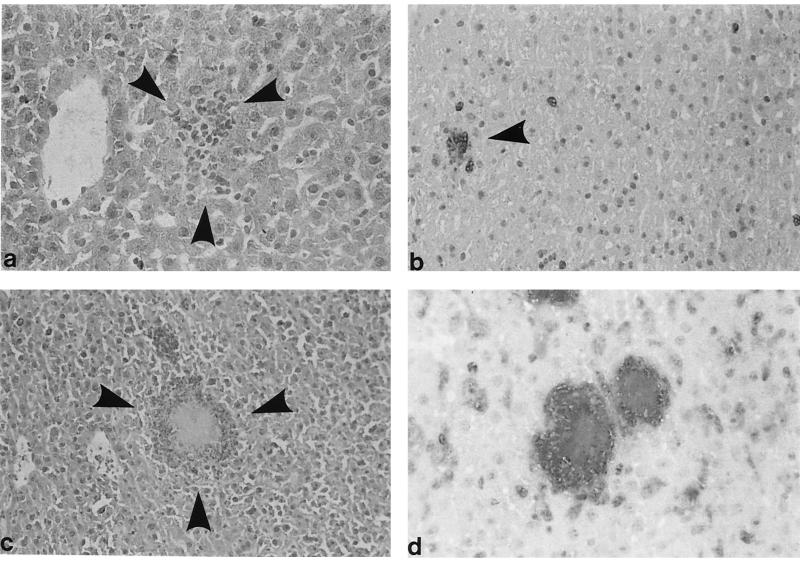
To assess whether ICSBP deficiency affects granuloma formation, histological and immunohistological examinations of the liver and spleen after Yersinia infection were performed. ICSBP+/+ mice exhibited well-demarcated, small granuloma-like lesions, whereas ICSBP−/− mice showed an extensive and protracted tissue destruction with multiple necrotic abscesses (Fig. 4). While Yersinia was hardly detectable by immunohistological analysis in wt mice, large numbers of bacteria were observed in ICSBP−/− animals. Yersinia was located either extracellularly in areas of necrosis or phagocytosed in macrophages or Kupffer cells but not in hepatocytes.
FIG. 4.
Histological and immunohistological examination of liver tissue from ICSBP+/+ (a and b) and ICSBP−/− (c and d) mice 4 days after i.v. infection. Hematoxylin-eosin stains (a and c) or immunohistological stainings (b and d) of frozen sections with anti-Mac-1 antibodies (PAP method) and anti-Y. enterocolitica antibodies (APAAP method) are shown. Each photograph is a representative view of the entire section and for all mice studied. (a) Small granuloma-like lesion (arrowheads) in the liver of ICSBP+/+ mice; hematoxylin-eosin stain. (b) Corresponding immunohistological staining with anti-Mac-1 and anti-Yersinia antibodies. The granuloma-like lesion (arrowhead) is composed of Mac-1+ cells. Few single Mac-1+ cells are visible. (c) Necrotic lesion in the liver of an ICSBP−/− mouse. (d) Masses of Yersinia bacilli within the lesions and in Kupffer cells; no clear demarcation of the lesion by Mac-1+ cells. Many Mac-1+ cells are scattered throughout the liver tissue.
Mac-1+ mononuclear phagocytes were mainly found in granuloma of ICSBP+/+ mice. In contrast, Yersinia-induced micro- and macroabscesses in ICSBP−/− mice lacked a distinct demarcation by Mac-1+ cells. Moreover, many of these cells were scattered in liver and spleen tissues of infected ICSBP−/− mice. These results show that ICSBP is critical for granuloma formation, an important step for the control of infections caused by Yersinia.
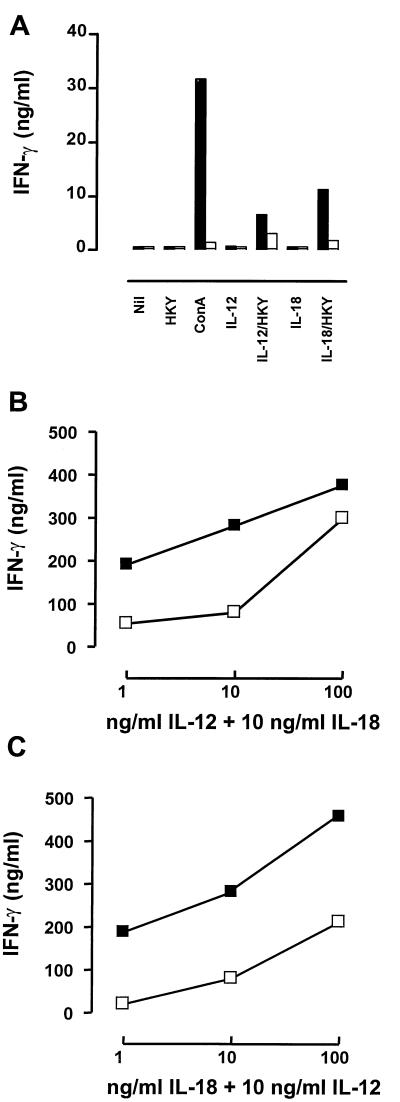
Spleen cells from ICSBP−/− mice are able to produce IFN-γ after treatment with rIL-12 or rIL-18 in combination with a second stimulus.
Previous studies have shown that splenic lymphocytes from ICSBP−/− mice are able to produce IFN-γ upon appropriate stimulation (28, 34, 64, 77). To investigate whether cytokine treatment using strong IFN-γ inducers such as IL-12 or IL-18 can restore impaired IFN-γ-dependent resistance against Yersinia in ICSBP−/− mice, IFN-γ production of naive spleen cells was determined following in vitro stimulation. Both rIL-12 and rIL-18 given alone failed to induce IFN-γ (Fig. 5A). These cytokines, however, combined with HKY resulted in low levels of IFN-γ production in spleen cells of ICSBP−/− mice (Fig. 5A). Moreover, combined stimulation with rIL-12 and rIL-18 caused a strong dose-dependent increase in IFN-γ production in spleen cells from both ICSBP+/+ and ICSBP−/− mice (Fig. 5B and C). Although IFN-γ production was still considerably lower in ICSBP−/− mice than in wt animals, these results indicated that the defect of ICSBP−/− mice to produce IFN-γ was only in part intrinsic and might be curable by exogenous delivery of IFN-γ-inducing cytokines.
FIG. 5.
IFN-γ production in spleen cells from uninfected ICSBP+/+ and ICSBP−/− mice after treatment with various stimuli. Supernatants of cultures were collected after 48 h and analyzed by ELISA as described in Materials and Methods. (A) Splenocytes from ICSBP+/+ (black bars) and ICSBP−/− (open bars) mice treated with medium alone, HKY (10 μg/ml), concanavalin A (ConA; 3 μg/ml), IL-12 (10 ng/ml), IL-12 (10 ng/ml) plus HKY (10 μg/ml), IL-18 (10 ng/ml), or IL-18 (10 ng/ml) plus HKY (10 μg/ml). (B) IFN-γ production in spleen cells of ICSBP+/+ (black bars) and ICSBP−/− (open bars) mice after treatment with various doses of IL-12 in combination with a constant dose of IL-18 (10 ng/ml). (C) IFN-γ production by splenocytes from ICSBP+/+ (black bars) and ICSBP−/− (open bars) mice after administration of different amounts of IL-18 in combination with a constant dose of IL-12 (10 ng/ml).
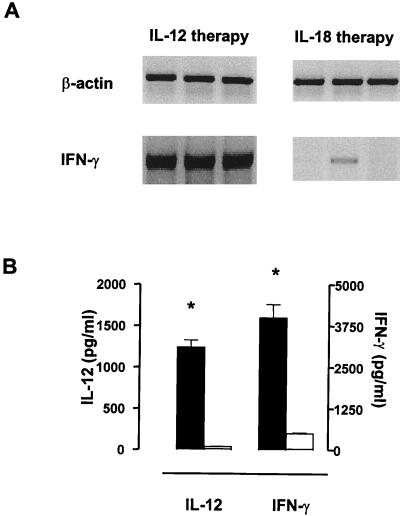
Administration of rIL-12, but not rIL-18, augments IFN-γ synthesis in Yersinia-infected ICSBP−/− mice.
We have demonstrated that either rIL-12 or rIL-18 in combination with a second stimulus induces IFN-γ in splenocytes of ICSBP−/− mice. Therefore, IFN-γ production in Yersinia-infected ICSBP−/− mice was examined after treatment with rIL-12 or rIL-18. In these experiments, Y. enterocolitica served as a second stimulus for IFN-γ induction. As shown in Fig. 6A, rIL-12 amplified IFN-γ mRNA expression in Yersinia-infected ICSBP−/− mice, whereas rIL-18 stimulated only little, if any, IFN-γ production. In addition, significantly higher protein levels for both IL-12 and IFN-γ were detected in sera of ICSBP−/− mice after treatment with rIL-12 compared to those after administration of rIL-18 (Fig. 6B). However, we cannot exclude that a dose higher than 1 μg per day or a different application scheme for rIL-18 may induce IFN-γ in ICSBP−/− mice. The protein levels for IL-12 in treated mice were mainly due to injected rIL-12.
FIG. 6.
rIL-12 induces IFN-γ production in Yersinia-infected ICSBP−/− mice. (A) IFN-γ mRNA expression 4 days after Yersinia infection and experimental therapy with IL-12 (1 μg/day) or IL-18 (1 μg/day) in ICSBP−/− mice. mRNA expression of β-actin serves as control. Results are shown for three separate ICSBP+/+ and ICSBP−/− mice. (B) Serum protein levels on day 4 in ICSBP−/− mice after IL-12 (black bars) or IL-18 (open bars) treatment. ∗ indicates statistically significant differences (P < 0.05).
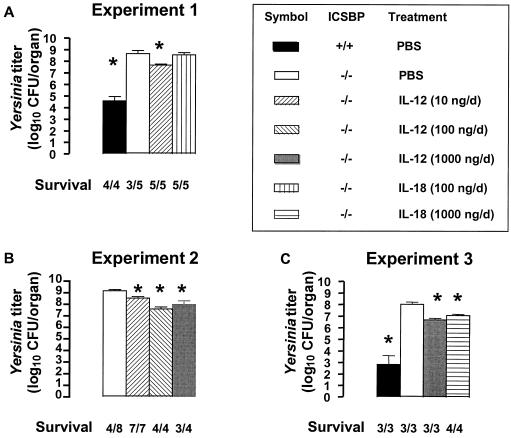
Recent studies have shown that administration of 10 to 100 ng of rIL-12 per day dramatically reduces bacterial numbers in susceptible BALB/c mice, whereas rIL-18 (20 ng to 4 μg per day) has no therapeutic effect on Yersinia infection (7, 10). To test whether IFN-γ induction restored antibacterial resistance, we investigated the role of rIL-12 or rIL-18 in the course of Yersinia infection in ICSBP−/− mice. Administration of rIL-12 improved survival of Yersinia-infected ICSBP−/− mice but reduced bacterial load in spleen and livers of ICSBP−/− mice only about 10-fold (Fig. 7). We observed only minimal differences in the effect of rIL-12 between ICSBP−/− mice infected with a medium dose (3 × 103 CFU) (Fig. 7A) or a low dose (3 × 102 CFU) (Fig. 7B and C) of Yersinia. Administration of 1 μg of rIL-18 per day, but not lower doses, decreased Yersinia counts in ICSBP−/− mice (Fig. 7A and C). Semiquantitative analysis of immunohistological examinations revealed that treatment by both rIL-12 and rIL-18 leads to a reduction of Yersinia and necrotic lesions and to a small increase of Mac1+ cells in infected organs (Table 2).
FIG. 7.
Bacterial counts in ICSBP−/− mice after i.v. Yersinia infection and cytokine therapy. Mice were treated i.p. for 5 days with a single dose of cytokine or PBS. Treatment was started 1 day before infection. Data are shown for three independent experiments. (A) Experiment 1. Mice were inoculated with 3 × 103 CFU of Y. enterocolitica and sacrificed on day 4 after infection. (B) Experiment 2. Mice were inoculated with 3 × 102 CFU of Y. enterocolitica and sacrificed on day 5 after infection. (C) Experiment 3. Mice were inoculated with 3 × 102 CFU of Y. enterocolitica and sacrificed on day 4 after infection. ∗ indicates statistically significant differences (P < 0.05) compared to ICSBP−/− mice treated with PBS (white bars). The ratio of surviving to infected mice is shown for each group.
TABLE 2.
Assessment of tissue destruction after Yersinia infection in ICSBP−/− micea
| Treatment | ICSBP phenotype | Yersinia infiltration | Necrosis | No. of Mac1+ cells |
|---|---|---|---|---|
| PBS | +/+ | 0/+ | 0/+ | 10–20 |
| −/− | ++++ | +++ | 100–120 | |
| IL-12 | −/− | 0/+ | 0/++ | 120–130 |
| IL-18 | −/− | ++ | 0/+ | 90–120 |
Yersinia infiltration and extent of necrosis were evaluated semiquantitatively. Mac1+ cells were counted in centrilobular areas of each high-power field. Similar results have been obtained in two independent experiments.
Administration of murine rIFN-γ has been shown to decrease bacterial load in susceptible BALB/c mice (1). However, experimental therapy with rIFN-γ (105 IU/day) diminished bacterial counts in affected organs of ICSBP−/− mice only about 10-fold (data not shown). In addition, experimental therapy by a combination of rIL-12 (10 ng/day) and rIL-18 (100 ng/day) failed to reduce bacterial load more efficiently than the corresponding dose of rIL-12 given alone (data not shown). Again, we cannot exclude that a more drastic reduction of Yersinia titers could be achieved by a combined IL-12 and IL-18 therapy using higher doses of both cytokines. However, the doses of recombinant cytokines used in this study are comparable to those employed by others (24, 51, 56, 64). Thus, experimental cytokine therapy ameliorated the course of Yersinia infection in ICSBP−/− mice but failed to completely restore immunity.
DISCUSSION
The aim of this study was to analyze the role of the mammalian transcription factor ICSBP in host resistance to the extracellular bacterium Y. enterocolitica. ICSBP−/− mice rapidly succumbed to acute microbial infection and failed to produce sufficient amounts of IL-12 and IFN-γ after bacterial challenge. In contrast to infection with intracellular pathogens, we could not observe a shift toward Th2 immune responses in Yersinia-infected ICSBP−/− mice (28, 64). Moreover, granuloma formation, which is a hallmark of protective immune responses against Yersinia, was highly disturbed in these mice. In contrast to our in vitro data, rIL-12 but not rIL-18 restored Yersinia-triggered IFN-γ production in infected ICSBP−/− mice. Although treatment of ICSBP−/− mice with rIL-12 or rIL-18 improved survival and reduced bacterial load, both cytokines failed to completely restore impaired immunity.
Resistance to Yersinia depends on the coordinate expression of the cytokines IFN-γ, IL-12, IL-18, and TNF-α (1, 7–10). Neutralization of any of these cytokines abrogates clearance of bacteria in infected mice (3, 7, 10). In addition, studies of Yersinia infection in mice deficient for IL-12 p40, IL-18, IFN-γ receptor, or TNF receptor p55 have confirmed the crucial role of these cytokines (9). Although all T-lymphocyte subpopulations are required for optimum protection against Yersinia, IFN-γ production by CD4+ T cells is indispensable to promote bacterial clearance (1).
In contrast to previous studies characterizing the phenotype of ICSBP−/− mice using the intracellular pathogens L. major and T. gondii (28, 64), we could not detect any IL-4 production or Th2 dominance in these mice during Yersinia infection. In fact, IL-4 mRNA expression levels were even lower in ICSBP−/− mice than in ICSBP+/+ mice. These findings are consistent with a previous report showing that Yersinia-susceptible BALB/c mice express less IL-4 mRNA than Yersinia-resistant C57BL/6 mice (1, 8). Although it is well established that IFN-γ is crucial for resistance to Yersinia, the reason for this observation is unclear. Thus, the role of Th2 cytokines such as IL-4 or IL-10 in Yersinia infection remains to be investigated.
The obvious defect of ICSBP−/− mice in control of extracellular Yersinia is at least partially due to the inability of their antigen-presenting cells to produce IL-12 and, as a consequence, to confer sufficient T-cell-mediated activation of macrophages by IFN-γ. However, in vitro stimulation of splenic T cells from ICSBP−/− mice showed that these cells are able to produce IFN-γ under appropriate stimulation. Moreover, the apparent defect of ICSBP−/− mice to secrete IFN-γ after Yersinia infection could be restored by exogenous delivery of rIL-12. Although administration of rIL-12 ameliorated the course of bacterial infection, it did not result in complete restoration of resistance against Yersinia. These data suggest that additional, probably IL-12- and IFN-γ-independent defense mechanisms are also regulated by the transcription factor ICSBP.
Like IL-12, IL-18 is a potent inducer of IFN-γ and important for NK cell activity and Th1 immune responses (48, 58, 72). In contrast to a recent publication, we did not observe a regulatory effect of ICSBP on the expression of IL-18 in infected mice (39). Interestingly, rIL-18 failed to enhance IFN-γ synthesis in infected ICSBP−/− mice but nevertheless reduced bacterial replication similarly to IL-12 therapy. Although the doses of rIL-18 used in this study are comparable to those employed by others (24, 51), we cannot definitively exclude that a higher dose of this cytokine amplifies IFN-γ production and protects more efficiently against Yersinia, as shown for infections caused by Cryptococcus neoformans (38). However, our data indicate that endogenous IL-12 is required for IL-18-mediated induction of measurable quantities of IFN-γ in infected mice. Whether IL-18 is involved in IFN-γ-independent immune responses remains to be investigated.
The mechanisms by which ICSBP regulates gene transcription appear to be complex. Previous in vitro studies have shown that ICSBP is a negative regulator of several IFN-responsive genes such as the major histocompatibility complex class I genes (46, 75, 76). In contrast, recent analyses implicate ICSBP as an activator of gene transcription for IL-12 p40 and cytochrome b558 heavy-chain gene (CYBB), the gene encoding gp91phox, a subunit of the phagocyte respiratory burst oxidase catalytic unit (21, 28, 64). This dichotomy in ICSBP function is not completely unexpected since other transcription factors have been shown to act positively or negatively depending on the context. ICSBP forms complexes with other IRF family members such as IRF-1 and IRF-2 that strongly bind to ISREs (12, 67, 68). Interestingly, the ability of IRF-1−/− mice to produce IL-12 is severely compromised (40, 73). Although several major differences exist between IRF-1−/− and ICSBP−/− mice, these data suggest that a heterodimer composed of IRF-1 and ICSBP regulates IL-12 p40 expression. Furthermore, the Ets family transcription factor PU.1, which is exclusively expressed in myeloid and B cells, cooperates with ICSBP in gene expression (20, 21). Thus, regulation of ICSBP-mediated gene expression and suppression depends heavily on the proper balance of transcription factors bound to this molecule.
The data presented here argue for a crucial role of ICSBP in resistance to Yersinia. ICSBP is involved in signal transduction events downstream from the IFN-γ receptor enhancing IL-12 p40 and CYBB gene expression. Since macrophages of ICSBP−/− mice are capable of responding to IFN-γ and producing NO, ICSBP affects only a subset of IFN-γ-inducible genes (21, 22, 28, 34, 64). IL-12 p40 mRNA expression has been detected in mice 1 day after i.v. infection with Yersinia (7). However, it remains to be investigated whether IL-12 p40 synthesis in infected mice is directly induced by microbial products of invading Yersinia or requires endogenous IFN-γ. The former would argue for a second receptor involved in IL-12 p40 induction after Yersinia infection. In this model, ICSBP regulates both the IFN-γ-dependent and IFN-γ-independent, but Yersinia-dependent, pathway of IL-12 p40 synthesis. We have shown that IL-12 is essential for resistance against Yersinia by triggering IFN-γ production in NK cells and CD4+ T cells (7). Therefore, one mechanism of ICSBP to confer resistance against Yersinia is based on its requirement for IL-12 p40 induction and, as a consequence, IFN-γ production.
Interestingly, virulence plasmid-containing strains of Yersinia are capable of suppressing the cytokine production of their host during infection. Thus, it is tempting to speculate whether bacterial factors function by blocking ICSBP or disrupting signal transduction pathways which regulate the induction and activation of transcription factors of the IRF and Ets family. The 70-kb virulence plasmid (pYV/pCD1) of Yersinia encodes a contact-dependent type III secretion system (see for reviews reference 16 to 18 and 35). pYV+/pCD1+-harboring strains are able to abrogate the generic inflammatory response in mice by downregulating IFN-γ and TNF-α (44). Priming by injection of proinflammatory cytokines before infection or passive immunization with antiserum against LcrV (or V antigen) later facilitates the inflammatory response and granuloma formation, thereby preventing lethality (44). In addition, recombinant LcrV inhibits synthesis of IFN-γ and TNF-α in mice challenged with avirulent, i.e., plasmid-cured, Yersinia, suggesting that this virulence factor prevents inflammation (45). Furthermore, recent findings have shown that suppression of TNF-α by an LcrV-containing fusion protein requires the presence of activated T cells and does not depend on cell-to-cell contact, indicating that this effect is mediated by an as yet unknown soluble factor (66). However, cytokine suppression by virulence plasmid-containing Yersinia has also been attributed to the action of type III secreted virulence factors (referred to as Yersinia outer proteins, or Yops), especially YopP (YopJ in Y. pestis and Y. pseudotuberculosis). This protein perturbs a multiplicity of signaling pathways including inhibition of the extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase pathways and inhibition of the NF-κB pathway (11, 50, 52, 53, 59, 60, 65). The interruption of these signaling pathways inhibits expression of TNF-α and IL-8 and induces apoptosis in the infected target cell (11, 42, 43, 52, 59, 61, 65).
Two models of LcrV function are currently discussed (23, 54, 63). In the first model, Yop effector proteins are translocated by an LcrV-independent mechanism. LcrV is exported from the bacterium to directly prevent the inflammatory response of the host. In the second model, LcrV is involved in virulence protein translocation into the host cell. This would argue for an indirect effect of LcrV on cytokine suppression since the cytotoxins themselves inhibit the inflammatory response.
In view of the above, targeting of ICSBP or signal transduction pathways upstream from ICSBP by a Yersinia virulence protein would impair IL-12 p40 synthesis. Whether IFN-γ inhibition by Yersinia is secondary to IL-12 suppression remains to be investigated. In addition, intracellular trafficking of a Yersinia factor through the nuclear pore into the nucleus is a prerequisite for the direct interaction with the transcription factor ICSBP. The only Yersinia virulence protein known to do so is YopM, a strongly acidic protein containing multiple leucine-rich repeat motifs (70). However, its intracellular target and mode of action has not been identified.
Beside the defects in cytokine production, impaired macrophage effector functions may also contribute to the increased susceptibility of ICSBP−/− mice to infectious agents (37, 41). A recent study of mice deficient for IRF-1, IRF-2, and ICSBP in resistance to the intracellular bacterium L. monocytogenes demonstrated that the oxidative burst was delayed and reduced in ICSBP−/− mice, whereas NO production was normal (22). Furthermore, it has been shown that PU.1, IRF-1, and ICSBP together increase gp91phox protein expression, a subunit of a membrane-bound flavocytochrome involved in the regulation of the oxidative burst (21). The absence of gp91phox protein leads to chronic granulomatous disease, a disorder of host defense (49). In addition, the fact that ICSBP−/− mice display a CML-like disorder further supports the crucial role of ICSBP for the development and function of cells of the myeloid lineage such as macrophages and neutrophils (34). T lymphocytes, however, seem to be only slightly affected in these mice. It has been shown that adoptively transferred splenic T cells of ICSBP−/− mice were able to promote elimination of Listeria in RAG2−/− mice which lack functional B and T cells (22).
As previously suggested, other mechanisms involved in resistance against bacterial pathogens might also be regulated by the transcription factor ICSBP (22). For example, iron metabolism is a primitive but crucial defense mechanism against microorganisms, and iron overload syndromes are associated with severe systemic or septicemic infection with Y. enterocolitica (27, 36). Moreover, Yersinia spp., which have an absolute requirement for iron, developed sophisticated mechanisms like the siderophore system to acquire iron from their host (32). Interestingly, increased iron load was measured in sera of uninfected ICSBP mice compared to wt animals (J. Hein, R. Gruber, and I. B. Autenrieth, unpublished data). Whether and how ICSBP is involved in iron withholding and whether this parameter affects Yersinia infection in ICSBP−/− mice remain to be investigated.
In conclusion, we showed that the transcription factor ICSBP confers resistance against the extracellular bacterium Y. enterocolitica. Further studies are required to elucidate additional molecular defects of ICSBP−/− mice resulting in their increased susceptibility to Yersinia.
ACKNOWLEDGMENTS
We thank Maurice K. Gately and Haruki Okamura for providing reagents and Ralf Schulte and Heinrich Körner for critical reading.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 217).
REFERENCES
- 1.Autenrieth I B, Beer M, Bohn E, Kaufmann S H, Heesemann J. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect Immun. 1994;62:2590–2599. doi: 10.1128/iai.62.6.2590-2599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Firsching R. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth I B, Kempf V, Sprinz T, Preger S, Schnell A. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect Immun. 1996;64:1357–1368. doi: 10.1128/iai.64.4.1357-1368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autenrieth I B, Tingle A, Reske-Kunz A, Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect Immun. 1992;60:1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autenrieth I B, Vogel U, Preger S, Heymer B, Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohn E, Autenrieth I B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J Immunol. 1996;156:1458–1468. [PubMed] [Google Scholar]
- 8.Bohn E, Heesemann J, Ehlers S, Autenrieth I B. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect Immun. 1994;62:3027–3032. doi: 10.1128/iai.62.7.3027-3032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn E, Schmitt E, Bielfeldt C, Noll A, Schulte R, Autenrieth I B. Ambiguous role of interleukin-12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect Immun. 1998;66:2213–2220. doi: 10.1128/iai.66.5.2213-2220.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, Heesemann J, Autenrieth I B. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 11.Boland A, Cornelis G R. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bovolenta C, Driggers P H, Marks M S, Medin J A, Politis A D, Vogel S N, Levy D E, Sakaguchi K, Appella E, Coligan J E. Molecular interactions between interferon consensus sequence binding protein and members of the interferon regulatory factor family. Proc Natl Acad Sci USA. 1994;91:5046–5050. doi: 10.1073/pnas.91.11.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Clark M A, Hirst B H, Jepson M A. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlan J W. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 19.Driggers P H, Ennist D L, Gleason S L, Mak W H, Marks M S, Levi B Z, Flanagan J R, Appella E, Ozato K. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1990;87:3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenbeis C F, Singh H, Storb U. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 1995;9:1377–1387. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- 21.Eklund E A, Jalava A, Kakar R. PU.1, interferon regulatory factor 1, and interferon consensus sequence-binding protein cooperate to increase gp91(phox) expression. J Biol Chem. 1998;273:13957–13965. doi: 10.1074/jbc.273.22.13957. [DOI] [PubMed] [Google Scholar]
- 22.Fehr T, Schoedon G, Odermatt B, Holtschke T, Schneemann M, Bachmann M F, Mak T W, Horak I, Zinkernagel R M. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J Exp Med. 1997;185:921–931. doi: 10.1084/jem.185.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fields K A, Nilles M L, Cowan C, Straley S C. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun. 1999;67:5395–5408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujioka N, Akazawa R, Ohashi K, Fujii M, Ikeda M, Kurimoto M. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J Virol. 1999;73:2401–2409. doi: 10.1128/jvi.73.3.2401-2409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fultz M J, Vogel S N. Analysis of the antagonist effect of IFN-alpha on IFN-gamma-induced interferon consensus sequence binding protein messenger RNA in murine macrophages. J Inflamm. 1998;48:28–39. [PubMed] [Google Scholar]
- 26.Gabriele L, Phung J, Fukumoto J, Segal D, Wang I M, Giannakakou P, Giese N A, Ozato K, Morse H C. Regulation of apoptosis in myeloid cells by interferon consensus sequence-binding protein. J Exp Med. 1999;190:411–422. doi: 10.1084/jem.190.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallant T, Freedman M H, Vellend H, Francombe W H. Yersinia sepsis in patients with iron overload treated with deferoxamine. N Engl J Med. 1986;314:1643. doi: 10.1056/NEJM198606193142515. [DOI] [PubMed] [Google Scholar]
- 28.Giese N A, Gabriele L, Doherty T M, Klinman D M, Tadesse-Heath L, Contursi C, Epstein S L, Morse H C. Interferon (IFN) consensus sequence-binding protein, a transcription factor of the IFN regulatory factor family, regulates immune responses in vivo through control of interleukin 12 expression. J Exp Med. 1997;186:1535–1546. doi: 10.1084/jem.186.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grützkau A, Hanski C, Hahn H, Riecken E O. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanski C, Naumann M, Grützkau A, Pluschke G, Friedrich B, Hahn H, Riecken E O. Humoral and cellular defense against intestinal murine infection with Yersinia enterocolitica. Infect Immun. 1991;59:1106–1111. doi: 10.1128/iai.59.3.1106-1111.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heesemann J, Hantke K, Vocke T, Saken E, Rakin A, Stojiljkovic I, Berner R. Virulence of Yersinia enterocolitica is closely associated with siderophore production, expression of an iron-repressible outer membrane polypeptide of 65,000 Da and pesticin sensitivity. Mol Microbiol. 1993;8:397–408. doi: 10.1111/j.1365-2958.1993.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 33.Heesemann J, Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983;155:761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch K P, Gabriele L, Waring J F, Bachmann M F, Zinkernagel R M, Morse H C, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 35.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurado R L. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25:888–895. doi: 10.1086/515549. [DOI] [PubMed] [Google Scholar]
- 37.Kantakamalakul W, Politis A D, Marecki S, Sullivan T, Ozato K, Fenton M J, Vogel S N. Regulation of IFN consensus sequence binding protein expression in murine macrophages. J Immunol. 1999;162:7417–7425. [PubMed] [Google Scholar]
- 38.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-gamma production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 39.Kim Y M, Kang H S, Paik S G, Pyun K H, Anderson K L, Torbett B E, Choi I. Roles of IFN consensus sequence binding protein and PU.1 in regulating IL-18 gene expression. J Immunol. 1999;163:2000–2007. [PubMed] [Google Scholar]
- 40.Lohoff M, Ferrick D, Mittrücker H W, Duncan G S, Bischof S, Röllinghoff M, Mak T W. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity. 1997;6:681–689. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 41.Marecki S, Atchison M L, Fenton M J. Differential expression and distinct functions of IFN regulatory factor 4 and IFN consensus sequence binding protein in macrophages. J Immunol. 1999;163:2713–2722. [PubMed] [Google Scholar]
- 42.Monack D M, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson N, Marks M S, Driggers P H, Ozato K. Interferon consensus sequence-binding protein, a member of the interferon regulatory factor family, suppresses interferon-induced gene transcription. Mol Cell Biol. 1993;13:588–599. doi: 10.1128/mcb.13.1.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen H, Hiscott J, Pitha P M. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 48.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 49.Orkin S H. Molecular genetics of chronic granulomatous disease. Annu Rev Immunol. 1989;7:277–307. doi: 10.1146/annurev.iy.07.040189.001425. [DOI] [PubMed] [Google Scholar]
- 50.Orth K, Palmer L E, Bao Z Q, Stewart S, Rudolph A E, Bliska J B, Dixon J E. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 1999;285:1920–1923. doi: 10.1126/science.285.5435.1920. [DOI] [PubMed] [Google Scholar]
- 51.Osaki T, Peron J M, Cai Q, Okamura H, Robbins P D, Kurimoto M, Lotze M T, Tahara H. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol. 1998;160:1742–1749. [PubMed] [Google Scholar]
- 52.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 53.Palmer L E, Pancetti A R, Greenberg S, Bliska J B. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect Immun. 1999;67:708–716. doi: 10.1128/iai.67.2.708-716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettersson J, Holmstrom A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg A, Wolf-Watz H. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 55.Politis A D, Sivo J, Driggers P H, Ozato K, Vogel S N. Modulation of interferon consensus sequence binding protein mRNA in murine peritoneal macrophages. Induction by IFN-gamma and down-regulation by IFN-alpha, dexamethasone, and protein kinase inhibitors. J Immunol. 1992;148:801–807. [PubMed] [Google Scholar]
- 56.Qureshi M H, Zhang T, Koguchi Y, Nakashima K, Okamura H, Kurimoto M, Kawakami K. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur J Immunol. 1999;29:643–649. doi: 10.1002/(SICI)1521-4141(199902)29:02<643::AID-IMMU643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 57.Reid L E, Brasnett A H, Gilbert C S, Porter A C, Gewert D R, Stark G R, Kerr I M. A single DNA response element can confer inducibility by both alpha- and gamma-interferons. Proc Natl Acad Sci USA. 1989;86:840–844. doi: 10.1073/pnas.86.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 59.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Kohler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-kappaB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, Liautard J P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. Correlation with its inhibitory effect on tumor necrosis factor-alpha production. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 61.Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schesser K, Spiik A K, Dukuzumuremyi J M, Neurath M F, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt A, Röllinghoff M, Beuscher H U. Suppression of TNF by V antigen of Yersinia spp. involves activated T cells. Eur J Immunol. 1999;29:1149–1157. doi: 10.1002/(sici)1521-4141(199904)29:04<1149::aid-immu1149>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 67.Sharf R, Azriel A, Lejbkowicz F, Winograd S S, Ehrlich R, Levi B Z. Functional domain analysis of interferon consensus sequence binding protein (ICSBP) and its association with interferon regulatory factors. J Biol Chem. 1995;270:13063–13069. doi: 10.1074/jbc.270.22.13063. [DOI] [PubMed] [Google Scholar]
- 68.Sharf R, Meraro D, Azriel A, Thornton A M, Ozato K, Petricoin E F, Larner A C, Schaper F, Hauser H, Levi B Z. Phosphorylation events modulate the ability of interferon consensus sequence binding protein to interact with interferon regulatory factors and to bind DNA. J Biol Chem. 1997;272:9785–9792. doi: 10.1074/jbc.272.15.9785. [DOI] [PubMed] [Google Scholar]
- 69.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skrzypek E, Cowan C, Straley S C. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol Microbiol. 1998;30:1051–1065. doi: 10.1046/j.1365-2958.1998.01135.x. [DOI] [PubMed] [Google Scholar]
- 71.Straley S C, Cibull M L. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL−Yersinia pestis in BALB/c mice. Infect Immun. 1989;57:1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 73.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, Suzuki G, Mitsuyama M, Shin E H, Kojima S, Taniguchi T, Asano Y. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 74.Une T, Nakajima R, Brubaker R R. Roles of V antigen in promoting virulence in Yersinia. Contrib Microbiol Immunol. 1987;9:179–185. [PubMed] [Google Scholar]
- 75.Weisz A, Kirchhoff S, Levi B Z. IFN consensus sequence binding protein (ICSBP) is a conditional repressor of IFN inducible promoters. Int Immunol. 1994;6:1125–1131. doi: 10.1093/intimm/6.8.1125. [DOI] [PubMed] [Google Scholar]
- 76.Weisz A, Marx P, Sharf R, Appella E, Driggers P H, Ozato K, Levi B Z. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J Biol Chem. 1992;267:25589–25596. [PubMed] [Google Scholar]
- 77.Wu C Y, Maeda H, Contursi C, Ozato K, Seder R A. Differential requirement of IFN consensus sequence binding protein for the production of IL-12 and induction of Th1-type cells in response to IFN-gamma. J Immunol. 1999;162:807–812. [PubMed] [Google Scholar]