Abstract
Starting in 2002, regulations and legislative amendments in Germany focused on the non-smoker protection with several measures to reduce exposure to secondhand tobacco smoke (SHS). The present work aimed to evaluate the relationship between polycyclic aromatic hydrocarbons (PAHs) and SHS exposure and to determine to which extent enforced non-smoking regulations and smoking bans affected the exposure of the non-smoking population in Germany since their implementation in the early 2000s until today. For this purpose, cotinine and selected monohydroxylated PAHs (OH-PAHs) were analyzed by means of (UP)LC-MS/MS in 510 24-h-urine samples of the Environmental Specimen Bank collected over a time span of 24 years from 1995 to 2019. Median urinary cotinine levels were found to steadily and significantly decline by 82% from 1995 to 2019. A significant decrease of urinary 3-hydroxybenzo[a]pyrene (19%), 1-OH-pyrene (39%), 1-naphthol (66%), 1- (17%), 2- (25%), and 3-OH-phenanthrene (22%) was also observed throughout the same time span. The decline in urinary levels of cotinine and several OH-PAHs can most likely be attributed to smoking bans and regulations limiting SHS and PAH exposure. This study therefore emphasizes the relevance of human biomonitoring to investigate the exposure of humans to chemicals of concern, assess the effectiveness of regulatory measures, and help policies to enforce provisions to protect public health.
Keywords: Secondhand tobacco smoke, Passive smoking, Polycyclic aromatic hydrocarbons, Human biomonitoring, Public health, HBM4EU
Graphical abstract
Highlights
-
•
HBM study assessing exposure to SHS and OH-PAHs over a 24-year period in Germany .
-
•
Descending trend of cotinine and OH-PAH in urine from 1995 to 2019.
-
•
Decline is attributed to regulations in Germany limiting SHS and PAH exposure.
-
•
Increasing trend for 2-naphthol indicating elevated exposure specific to 2-naphthol.
-
•
Study underlines the importance of nationwide HBM to monitor chemicals of concern and the success of implemented regulations.
1. Introduction
Until the early 2000s, secondhand tobacco smoke (SHS) was one of the most common indoor air pollutants worldwide (Musk and de Klerk, 2003; Öberg et al., 2011). SHS is a complex mixture of more than 4500 compounds in the vapor phase and the particulate matter comprising amongst others tobacco alkaloids, amines, alkanes, numerous polycyclic aromatic hydrocarbons (PAHs), aldehydes, carbonyls, and metals (Jaakkola and Jaakkola, 1997; Narkowicz et al., 2013; Talhout et al., 2011). It is described to exhibit adverse health effects (IARC, 2002; Narkowicz et al., 2013; Reardon, 2007; Schick and Glantz, 2005; Torres et al., 2018) and some of its compounds are described to be toxic (e.g., acrolein) or carcinogenic (e.g., benzene). In many parts of the world, the growing awareness of health impairment from SHS required the need for comprehensive legislation in order to protect non-smokers from exposure (McNabola and Gill, 2009; WHO, 2009). Countries such as the Unites States, France, Italy, the Netherlands, Sweden, Scotland, Spain and England have introduced policies in order to reduce SHS exposure by banning smoking in workplaces and public places (McNabola and Gill, 2009; WHO, 2009). In Germany, regulations and legislative amendments focused on the non-smoker protection since the early 2000's (Federal Non-smokers Protection Act, 2007; Kohler and Minkner, 2014; Kuntz et al., 2017; Schaller and Mons, 2018), with the first workplace smoking bans enacted in 2002 (ArbStättV, 2002). Tobacco tax increase (Schaller and Mons, 2018) and the ban on smoking in federal facilities and public transport in 2007 (Federal Non-smokers Protection Act, 2007; Kohler and Minkner, 2014) were further measures to reduce exposure to SHS.
Human biomonitoring (HBM) offers an ideal tool to assess the effects of these regulatory actions on human exposure and to tailor further pollutant reduction measures, as it can quantify actual exposure at both the individual and population level by determining a set of specific biomarkers covering all exposure sources and pathways (Angerer et al., 2007). The European Human Biomonitoring Initiative (HBM4EU), a joint effort of 30 countries, the European Environment Agency and the European Commission, targets PAHs as one of their priority substances to evaluate the exposure of humans on a European level in order to close data gaps and, on the longer run, capture time trends.
Within the framework of HBM4EU, the present work aimed to evaluate whether enforced non-smoking regulations and smoking bans affected the SHS exposure of the general population in Germany since their implementation in 2002 until today. The biomarker of choice for assessing the internal SHS exposure is cotinine (Cot) (Avila-Tang et al., 2012; Benowitz, 1996), the major metabolite of the most abundant alkaloid in tobacco leaf, nicotine (Benowitz, 1996; Benowitz and Jacob, 1993; Djordjevic and Doran, 2009; US Department of Health and Human Services, 2010). Other biomarkers associated with SHS exposure are monohydroxylated polycyclic aromatic hydrocarbons (OH-PAHs). The evidence for a relationship between SHS and PAH metabolite excretion has already been demonstrated by Murawski et al. where a comparison in the population-representative German Environmental Survey for Children and Adolescents (GerES II, 1990–1992, GerES IV, 2003–2006, GerES V 2014–2017) revealed decreasing concentrations (Murawski et al., 2020), underlining the need for a comprehensive OH-PAH monitoring. PAHs are formed during the incomplete combustion of organic matter (Hoseini et al., 2018; IARC, 2010; Rodgman and Perfetti, 2006). Exposure of the general population is caused by several environmental factors. Vehicle exhaust, tobacco smoke, smoke from woodstoves and fireplaces are main contributors to PAH exposure via ambient air. Moreover, consumption of processed food, especially fried, grilled or smoked food, are major sources of PAH intake, while other foodstuff may also have significant levels of PAH due to environmental contamination (Hoseini et al., 2018; IARC, 2010; Rodgman and Perfetti, 2006; Srogi, 2007). Once absorbed by the human body, PAH are converted into hydroxylated metabolites followed by a glucuronidation and excretion primarily in urine (Farzan et al., 2016). By analyzing a profile of selected OH-PAHs, the exposure to relevant PAHs, such as benzo[a]pyrene (BaP), one of the best-studied and carcinogenic PAHs (DFG, 2012; IARC, 2010), pyrene, naphthalene, or phenanthrene can be assessed (Farzan et al., 2016).
To evaluate the exposure to SHS and PAH in the non-smoking population, Cot and the following OH-PAHs were determined in 24-h-urine samples from the German Environmental Specimen Bank (ESB) collected by 510 individuals from 1995 to 2019: 3-hydroxybenzo[a]pyrene (3-OH-BaP), 1-OH-pyrene (1-OH-Pyr), 1-naphthol (1-Nap), 2-naphthol (2-Nap), 1-OH-phenanthrene (1-OH-Phe), 2-OH-phenanthrene (2-OH-Phe), 3-OH-phenanthrene (3-OH-Phe), 4-OH-phenanthrene (4-OH-Phe), and 9-OH-phenanthrene (9-OH-Phe). The ESB archives biological samples with the objective to monitor the time trend of human exposure towards environmental pollutants (Kolossa-Gehring et al., 2012). Samples stored in the ESB are well characterized in terms of sex, body weight, height, and age as well as 24-h urine volumes and creatinine concentrations used for normalization, and allow thus retrospective investigation of time trends of various toxicants and environmental chemicals in Germany. By combining data from Cot and OH-PAHs from the well-characterized urine samples of the ESB, the conducted human biomonitoring study shall add important information on the exposure of the German population to SHS, the correlation between PAHs and SHS exposure, as well as on the effectiveness of legislative amendments for non-smoker protection.
2. Materials and method
2.1. Study population
The 24-h-urine samples analyzed in this study were collected and stored in the Environmental Specimen Bank (ESB) in the years 1995, 2000, 2005, 2010, 2014, 2015, 2017, 2018, and 2019. Samples were provided by the German Environmental Agency (Umweltbundesamt, UBA) for analysis. In total, 599 urine samples (male to female split 50/50) were analyzed for their Cot and OH-PAH concentration in this study. Due to limited sample volume, 3-OH-BaP was not analyzed in 2014 and 2018. Smokers were excluded from the evaluation based on self-reporting and urinary Cot concentration. Cot is a long-established biomarker indicative of smoking (Benowitz, 1996; Benowitz and Jacob, 1993; Djordjevic and Doran, 2009; US Department of Health and Human Services, 2010), for which a cut-off value of 50 μg/L urine has been established in the past to differentiate active smokers from non-smokers (Benowitz et al., 2009; Stragierowicz et al., 2013). Due to governmental smoking bans in the recent past, lower limits of 3 μg/L cotinine in serum have been published based on human biomonitoring data, which are equivalent to about 15 μg/L cotinine in urine (Benowitz et al., 2009). For that reason, study subjects having Cot levels >15 μg/L were omitted from further analysis. This was done under the premise of excluding possible passive smokers with levels between 15 and 50 μg/L. However, only 2.5% of all study samples and 0–4 subjects per year had levels in this range, making them negligible for statistical evaluation. As a result, a total of 510 urine samples were considered for data evaluation. Characteristics on the study population are summarized in Table A1 (Appendix). Information on the samples, sampling procedures and storage conditions are described elsewhere (Pluym et al., 2020; Schettgen et al., 2020). The study protocol of the ESB has been approved by the ethics committee of the Medical Association Westfalen-Lippe, the Medical Faculty of the University of Münster and (since 2012) by the ethical committee of the Medical Association of the Saarland.
2.2. Analytical methods
2.2.1. Determination of cotinine
Quantification of cotinine in human urine was conducted according to Landmesser et al. (2019) with minor modifications. In brief, 20 μL of 50 ng/mL cotinine-d3 as internal standard (IS) and 4 μL of 6 M sodium hydroxide (NaOH) were added to 100 μL urine sample. 1 mL ethyl acetate was added for liquid-liquid extraction and the sample was extracted for 30 min on a multi-vortex mixer. The mixture was subsequently centrifuged for 15 min (3000 rpm, 4 °C). 850 μL of the organic supernatant were transferred into a vial and evaporated to dryness for 7 min 200 μL acetonitrile (ACN) were added to reconstitute the sample. Samples were analyzed by means of LC-MS/MS with an Acquity UPLC I-Class PLUS System coupled to a Xevo TQ-S Triple Quadrupol (Waters GmbH, Eschborn, Germany). Details on the analytical method applied can be found in the Appendix. The method was fully validated according to FDA guidelines (Bioanalytical Method Validation Guidance for Industry) (FDA, 2018). The linear calibration ranged from 0.2 to 1000 μg/L (LLOQ 0.2 μg/L), intra- and inter-day precisions were found to be <8% (CV < 17% for levels < 3x LLOQ) and the method accuracy rates were between 93 and 108% throughout the calibration range. For cotinine in urine, a mean matrix suppression of 33% was observed, which was fully compensated by the deuterated internal standard.
2.2.2. Determination of OH-PAHs
The quantification of the following OH-PAHs was conducted according to Ramsauer et al. (2011) with minor modifications. In brief, 1 mL 1 M acetate buffer (pH 5.1) was added to 5 mL urine sample. The following amounts of IS were spiked to the sample: 0.5 ng 13C4-1-/4-OH-Phe, 0.5 ng 13C6-2-/3-OH-Phe, and 2.5 ng 13C6–OH-Pyr, 30 ng 13C6-1-/2-Nap. The pH of the mixture was adjusted to 5.1 ± 0.1 using 2 M hydrochloric acid. For enzymatic hydrolysis, 20 μL of glucuronidase (0.09 U)/arylsufatase (0.28 U) were added to the mixture and incubated overnight at 37 °C. The sample was centrifuged for 10 min (3000 g, 10 °C) before solid-phase extraction (SPE). SPE was conducted with Chromabond® Easy cartridges (6 mL, 200 mg, Macherey-Nagel, Düren, Germany). The cartridges were conditioned with 3 mL methanol and 3 mL ultrapure water. After addition of the sample hydrolysate, cartridges were washed using 3 mL water/methanol (95/5, v/v) and dried under nitrogen (0.5 bar, 20 min). Analytes were eluted with 2 × 3 mL dichloromethane. The eluate was evaporated to dryness, reconstituted in 100 μL acetonitrile/water (50/50, v/v) and samples were analyzed by means of LC-MS/MS with a Shimadzu LC-20AB (Shimadzu Germany, Duisburg, Germany) coupled to a Qtrap 6500+ (Sciex, Framingham, United States). Details on the analytical method can be found in the Appendix.
Linear calibration ranged from 0.05 to 150 μg/L (LLOQ 0.05 μg/L) for 1-, 2-Nap, 0.001–2.0 μg/L (LLOQ 0.001 μg/L) for 1-OH-, 2-OH-, 3-OH-, 4-OH-, 9-OH-Phe, and 0.01–2.0 μg/L (LLOQ 0.01 μg/L) for 1-OH-Pyr, respectively. The method was fully validated according to FDA guidelines (Bioanalytical Method Validation Guidance for Industry) (FDA, 2018). Details on method validation can be found elsewhere (Ramsauer et al., 2011).
2.2.3. Determination of 3-OH-BaP
The quantification of 3-OH-BaP was performed as previously described by means of LC-MS/MS after enzymatic hydrolysis and SPE (Rögner et al., 2021). Linear calibration ranged from 50 to 3321 pg/L (LLOQ 50 pg/L).
2.3. Sample analysis and data evaluation
To avoid batch effects, all study samples were first randomized and subsequently processed and analyzed in batches consisting of study samples, calibrators, blanks, and QC samples at low, medium, and high levels. More than 5% of unknown samples per analytical run (or at least 6 QC samples, two QC samples for each level) were randomly interspersed across the analytical runs as QC samples covering the expected range of analyte concentrations. Deviation from the target values were evaluated for accepting calibrators and to verify the calibration range. In order to monitor the validity of the measurements, acceptance criteria as set forth in the FDA Guidance for Bioanalytical Method Validation (FDA, 2018) were used.
The total amount excreted in 24 h (pg/24 h, μg/24 h) was used for data evaluation in the time trend analysis as the data corrected for urinary 24 h volume are considered the most robust compared to other normalization approaches (Lermen et al., 2019). In addition, urinary concentrations in pg/L and μg/L, respectively, are provided in the Appendix (Table A3) for better comparability with other studies. Concentrations below LLOQ were set to LLOQ/2 for calculation of the amount excreted in 24 h (Hornung and Reed, 1990).
Descriptive analysis including geometric means (GM), confidence intervals (CI) of the GM, medians, and 95th (P95) percentiles were calculated, where appropriate. Normal distribution was tested using Shapiro-Wilk and D'Agostino-K squared test. Since values for the analytes investigated were not normally distributed, the non-parametric Mann-Whitney U test (comparison of two groups) was used to determine statistical significance with a significance level set to α = 0.05, unless otherwise stated. Correlations were evaluated with the non-parametric Spearman rank correlation analysis. Statistical analysis was conducted in OriginPro (2020b) (Version 9.7.5.184, OriginLab Corporation, Northampton, United States). Geometric mean (GM) values are discussed in this manuscript unless otherwise stated.
3. Results
3.1. Cotinine
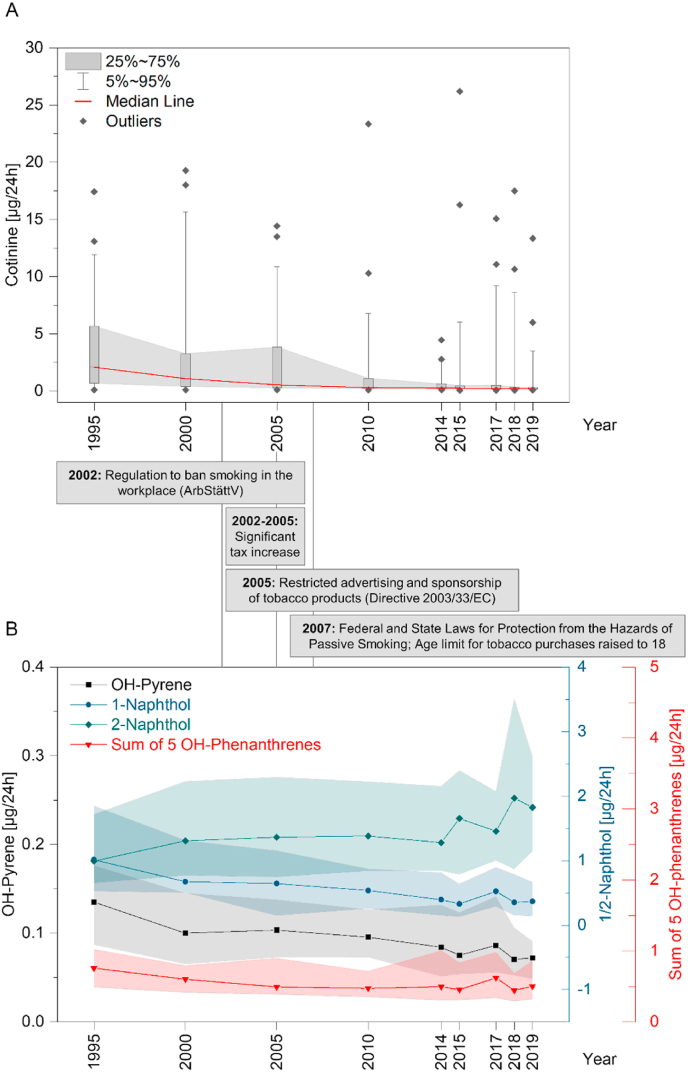
The daily excretion of cotinine (μg/24 h) distinctly decreased over the considered time period from 1995 to 2019. A summary of the urinary Cot excretion for each year is given in Table 1. From 1995 to 2000, Cot excretion decreased in the GM from 1.6 μg/24 h to 1.1 μg/24 h and further declined to 0.8 μg/24 h in 2005 and 0.4 μg/24 h in 2010. In the following years, Cot levels decreased only slightly to 0.3 μg/24 h (GM) in 2019 (Fig. 1A), which corresponds to an overall decrease of 82% relative to the levels measured in 1995.
Table 1.
Summary statistics for urinary daily excretion of cotinine (μg/24 h) by sampling year.
| Sampling year | 1995 | 2000 | 2005 | 2010 | 2014 | 2015 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|
| N total | 55 | 57 | 58 | 55 | 57 | 58 | 57 | 59 | 54 |
| N > LLOQ | 45 (82%) | 47 (82%) | 36 (62%) | 26 (47%) | 26 (46%) | 20 (34%) | 16 (28%) | 15 (25%) | 13 (24%) |
| GM | 1.6 | 1.1 | 0.8* | 0.4*** | 0.4*** | 0.3*** | 0.4*** | 0.3*** | 0.3*** |
| CI1 | 0.5–2.7 | <LLOQ-2.2 | <LLOQ-2.0 | – | – | – | – | – | – |
| Median1 | 2.1 | 1.1 | 0.5 | – | – | – | – | – | – |
| P95 | 11.9 | 15.6 | 10.9 | 6.7 | 2.6 | 6.0 | 9.2 | 8.6 | 3.5 |
| Max | 17.4 | 19.3 | 14.4 | 23.4 | 4.5 | 26.2 | 15.1 | 17.5 | 13.3 |
GM: geometric mean; CI: 95% confidence interval of geometric mean, P95: 95th percentile; Max: Maximum; Significance test: Mann-Whitney-U test, compared to 1995: *p < 0.05, **p < 0.01, ***p < 0.001. 1 CI and median not calculated, if number of samples below LLOQ >50%.
Fig. 1.
A) Box-and-whisker plot of cotinine in urine [μg/24 h] from 1995 until 2019. Box-and-whisker plot represent medians (red horizontal line) with 25% and 75% percentiles (boxes), 5% and 95% percentiles (whiskers), and outliers (diamonds). B) Time trend for 1-OH-Pyr (black), 1-Nap, 2-Nap, and the sum of 1-/2-/3-/4-/9-OH-Phe [μg/24 h] from 1995 until 2019. Plot represent medians (symbol + line) with 25% and 75% percentiles (shaded area). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Cot levels were significantly lower (Mann-Whitney U Test, p < 0.05) since 2005 until 2019 when compared to the first sampling year in 1995. In 2010 and 2014, Cot levels significantly declined compared to the respective previous year. By 2019, a further decreasing but non-significant trend was observed. The decrease in Cot exposure over time is also reflected in the number of quantifiable samples. While the majority of study samples showed quantifiable values in 1995 (82%), only 24% of the samples collected in 2019 were above the LLOQ (Table 1).
Comparison of the Cot excretion between sexes revealed slightly higher median values in males compared to females (Appendix, Table A4). However, differences were not significant in four of the nine years evaluated. Taking into account the decreasing number of Cot levels > LLOQ through the years for the respective sexes, these differences appear not to be representative. It should be noted that the total number of participants excluded from further evaluation on the basis of Cot cut-off values was evenly distributed across the years analyzed, with a general downward trend in self-reported smoking among ESB subjects (Umweltprobenbank, 2022).
3.2. Hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs)
General statistics for the daily urinary excretion of all OH-PAHs are given in Table 2. It should be noted that samples of the years 2014 and 2018 were not analyzed for 3-OH-BaP. A decline in urinary 3-OH-BaP levels was observed over time, with a GM of 70.9 pg/24 h in 1995 that decreased continuously until 2017, when a GM of 52.5 pg/24 h was determined. In 2019, 3-OH-BaP levels slightly increased to 57.4 pg/24 h. Compared to the reference year 1995, the decrease in the years 2010, 2015, and 2017 was significant (Mann-Whitney U Test, p < 0.05). As for Cot, the 19% decrease in 3-OH-BaP levels from 1995 to 2019 is also reflected by the increasing number of levels that were determined < LLOQ over the years (Table 2). In 1995, 61% of the samples showed levels above LLOQ, which steadily decreased over time. In 2019, only 15% of the samples were quantifiable for 3-OH-BaP.
Table 2.
Descriptive statistics for urinary daily excretion of OH-PAHs by sampling year.
| Sampling year | 1995 | 2000 | 2005 | 2010 | 2014 | 2015 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|---|---|---|---|
| N total | 55 | 57 | 58 | 55 | 57 | 58 | 57 (56 for 3-OH-BaP) | 59 | 54 (53 for 3-OH-BaP) | |
| 3-OH-BaP [pg/24 h] | N > LLOQ | 31 (56%) | 22 (39%) | 20 (34%) | 10 (18%) | – | 10 (17%) | 7 (13%) | – | 8 (15%) |
| GM | 70.9 | 63.5 | 70.6 | 56.5* | – | 56.0* | 52.5* | – | 57.4 | |
| CI1 | 70.4–71.4 | – | – | – | – | – | – | – | – | |
| Median | 81.5 | – | – | – | – | – | – | – | – | |
| P95 | 170.2 | 229.2 | 347.8 | 155.9 | – | 162.7 | 126.1 | – | 150.4 | |
| Max | 244.3 | 335.3 | 376.1 | 202.8 | – | 183.4 | 174.5 | – | 321.0 | |
| 1-OH-Pyr [μg/24 h] | N > LLOQ | 52 (95%) | 48 (84%) | 48 (83%) | 51 (93%) | 46 (81%) | 54 (93%) | 51 (89%) | 54 (92%) | 49 (91%) |
| GM | 0.12 | 0.10* | 0.11 | 0.09** | 0.08** | 0.08*** | 0.10* | 0.08*** | 0.07*** | |
| CI1 | <LLOQ-0.62 | <LLOQ-0.66 | <LLOQ-0.62 | <LLOQ-0.53 | <LLOQ-0.71 | <LLOQ-0.60 | <LLOQ-0.65 | <LLOQ-0.57 | <LLOQ-0.54 | |
| Median | 0.14 | 0.10 | 0.10 | 0.10 | 0.08 | 0.08 | 0.09 | 0.07 | 0.07 | |
| P95 | 0.25 | 0.37 | 0.49 | 0.23 | 0.43 | 0.28 | 0.48 | 0.30 | 0.17 | |
| Max | 0.30 | 1.26 | 0.55 | 0.45 | 0.90 | 0.60 | 0.53 | 0.32 | 0.43 | |
| 1-Nap [μg/24 h] | N > LLOQ | 50 (91%) | 53 (93%) | 43 (74%) | 45 (82%) | 41 (72%) | 40 (69%) | 45 (79%) | 44 (75%) | 38 (70%) |
| GM | 0.92 | 0.75 | 0.50** | 0.47*** | 0.41*** | 0.32*** | 0.43*** | 0.35*** | 0.31*** | |
| CI1 | 0.13–1.70 | 0.08–1.42 | <LLOQ-1.33 | <LLOQ-1.14 | <LLOQ-1.23 | <LLOQ-1.12 | <LLOQ-1.15 | <LLOQ-1.13 | <LLOQ-1.03 | |
| Median | 1.02 | 0.67 | 0.65 | 0.54 | 0.40 | 0.33 | 0.52 | 0.35 | 0.37 | |
| P95 | 3.94 | 3.74 | 2.58 | 2.03 | 2.43 | 4.30 | 2.00 | 1.72 | 1.66 | |
| Max | 5.13 | 5.97 | 5.99 | 2.54 | 14.22 | 5.48 | 3.87 | 11.25 | 2.38 | |
| 2-Nap [μg/24 h] | N > LLOQ | 55 (100%) | 57 (100%) | 58 (100%) | 55 (100%) | 57 (100%) | 57 (98%) | 57 (100%) | 58 (98%) | 54 (100%) |
| GM | 1.10 | 1.42 | 1.49* | 1.44* | 1.33 | 1.44* | 1.52* | 1.68** | 1.84*** | |
| CI1 | 0.53–1.66 | 0.78–2.05 | 0.89–2.10 | 0.89–1.98 | 0.82–1.85 | 0.78–2.09 | 0.94–2.09 | 1.02–2.34 | 1.25–2.42 | |
| Median | 0.99 | 1.31 | 1.36 | 1.38 | 1.28 | 1.66 | 1.46 | 1.97 | 1.82 | |
| P95 | 5.65 | 11.42 | 10.98 | 5.47 | 4.55 | 6.39 | 7.35 | 6.25 | 9.61 | |
| Max | 9.83 | 12.32 | 14.58 | 27.42 | 6.01 | 11.65 | 9.99 | 13.42 | 19.40 | |
| 1-OH-Phe [μg/24 h] | N > LLOQ | 55 (100%) | 57 (100%) | 58 (100%) | 55 (100%) | 57 (100%) | 58 (100%) | 57 (100%) | 59 (100%) | 54 (100%) |
| GM | 0.23 | 0.22 | 0.20* | 0.18** | 0.19* | 0.19* | 0.22 | 0.17** | 0.19* | |
| CI1 | <LLOQ-0.80 | <LLOQ-0.78 | <LLOQ-0.73 | <LLOQ-0.59 | <LLOQ-0.82 | <LLOQ-0.72 | <LLOQ-0.78 | <LLOQ-0.71 | <LLOQ-0.77 | |
| Median | 0.27 | 0.21 | 0.18 | 0.18 | 0.17 | 0.18 | 0.23 | 0.17 | 0.18 | |
| P95 | 0.75 | 0.89 | 0.78 | 0.39 | 0.96 | 0.63 | 1.01 | 0.64 | 0.74 | |
| Max | 1.24 | 1.35 | 1.53 | 0.52 | 1.89 | 0.76 | 1.57 | 1.63 | 1.66 | |
| 2-OH-Phe [μg/24 h] | N > LLOQ | 55 (100%) | 57 (100%) | 58 (100%) | 55 (100%) | 57 (100%) | 58 (100%) | 57 (100%) | 59 (100%) | 54 (100%) |
| GM | 0.09 | 0.09 | 0.09 | 0.07** | 0.07 | 0.07 | 0.08 | 0.07** | 0.06** | |
| CI1 | <LLOQ-0.61 | <LLOQ-0.63 | <LLOQ-0.60 | <LLOQ-0.49 | <LLOQ-0.64 | <LLOQ-0.60 | <LLOQ-0.62 | <LLOQ-0.59 | <LLOQ-0.59 | |
| Median | 0.09 | 0.09 | 0.08 | 0.07 | 0.07 | 0.07 | 0.08 | 0.06 | 0.06 | |
| P95 | 0.23 | 0.32 | 0.37 | 0.16 | 0.26 | 0.24 | 0.31 | 0.26 | 0.22 | |
| Max | 0.27 | 0.66 | 0.66 | 0.25 | 0.46 | 0.33 | 0.39 | 0.38 | 0.41 | |
| 3-OH-Phe [μg/24 h] | N > LLOQ | 55 (100%) | 57 (100%) | 58 (100%) | 55 (100%) | 57 (100%) | 58 (100%) | 57 (100%) | 59 (100%) | 54 (100%) |
| GM | 0.12 | 0.12 | 0.11 | 0.08*** | 0.09* | 0.09* | 0.12 | 0.09** | 0.10** | |
| CI1 | <LLOQ-0.67 | <LLOQ-0.69 | <LLOQ-0.59 | <LLOQ-0.54 | <LLOQ-0.66 | <LLOQ-0.64 | <LLOQ-0.69 | <LLOQ-0.64 | <LLOQ-0.59 | |
| Median | 0.14 | 0.11 | 0.11 | 0.08 | 0.10 | 0.09 | 0.11 | 0.08 | 0.10 | |
| P95 | 0.38 | 0.58 | 0.41 | 0.20 | 0.28 | 0.36 | 0.50 | 0.35 | 0.24 | |
| Max | 0.39 | 0.96 | 0.50 | 0.50 | 0.89 | 0.38 | 0.98 | 0.49 | 0.42 | |
| 4-OH-Phe [μg/24 h] | N > LLOQ | 53 (96%) | 56 (98%) | 56 (97%) | 55 (100%) | 53 (93%) | 51 (88%) | 54 (95%) | 55 (93%) | 51 (94%) |
| GM | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | |
| CI1 | <LLOQ-0.72 | <LLOQ-0.78 | <LLOQ-0.79 | <LLOQ-0.66 | <LLOQ-0.84 | <LLOQ-0.90 | <LLOQ-0.87 | <LLOQ-0.68 | <LLOQ-0.80 | |
| Median | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | |
| P95 | 0.21 | 0.17 | 0.24 | 0.20 | 0.27 | 0.24 | 0.33 | 0.08 | 0.24 | |
| Max | 0.47 | 1.09 | 0.86 | 0.26 | 0.74 | 2.92 | 0.76 | 0.47 | 0.41 | |
| 9-OH-Phe [μg/24 h] | N > LLOQ | 54 (98%) | 57 (100%) | 52 (90%) | 54 (98%) | 57 (100%) | 57 (98%) | 57 (100%) | 57 (97%) | 53 (98%) |
| GM | 0.13 | 0.12 | 0.08* | 0.09* | 0.11 | 0.08** | 0.13 | 0.08** | 0.11 | |
| CI1 | <LLOQ-0.90 | <LLOQ-0.69 | <LLOQ-0.94 | <LLOQ-0.72 | <LLOQ-0.94 | <LLOQ-0.76 | <LLOQ- 0.79 | <LLOQ-0.84 | <LLOQ-0.89 | |
| Median | 0.15 | 0.12 | 0.09 | 0.10 | 0.11 | 0.08 | 0.12 | 0.07 | 0.10 | |
| P95 | 0.95 | 0.40 | 0.39 | 0.34 | 1.26 | 0.38 | 0.72 | 0.47 | 0.96 | |
| Max | 1.32 | 0.72 | 0.77 | 0.52 | 3.29 | 0.61 | 1.18 | 1.80 | 1.60 | |
| Sum of 1-/2-/3-/4-/9-OH-Phe [μg/24 h] | GM | 0.65 | 0.61 | 0.57 | 0.49** | 0.55 | 0.51* | 0.63 | 0.47** | 0.55 |
| CI1 | 0.09–1.22 | 0.07–1.16 | 0.06–1.07 | 0.06–0.92 | <LLOQ-1.18 | <LLOQ-1.05 | 0.07–1.19 | <LLOQ-1.01 | <LLOQ-1.10 | |
| Median | 0.76 | 0.60 | 0.49 | 0.47 | 0.49 | 0.45 | 0.62 | 0.44 | 0.50 | |
| P95 | 1.96 | 2.63 | 2.03 | 1.04 | 2.60 | 1.61 | 2.63 | 1.84 | 1.83 | |
| Max | 2.55 | 3.38 | 3.59 | 1.79 | 5.60 | 4.51 | 3.41 | 4.04 | 3.84 | |
GM: geometric mean; CI: 95% confidence interval of geometric mean, P95: 95th percentile; Max: Maximum; Significance test: Mann-Whitney-U test, compared to 1995: *p < 0.05, ** p < 0.01, ***p < 0.001. 1 CI and median not calculated, if number of samples below LLOQ >50%.
For OH-Pyr, 1-Nap, 1-, 2-, 3-, 4-, 9-OH-Phe, a decreasing time trend was observed from 1995 to 2019, with the exception of the year 2017, when a slight increase in values of the aforementioned PAHs was observed (Table 2). The most distinct decrease was observed for 1-Nap and 1-OH-Pyr. Here the daily excretion in 2019 only accounted for 34% and 61%, respectively, of those determined for the year 1995 (Fig. 1B). The decrease from 1995 to 2019 was highly significant for 1-OH-Pyr (p < 0.001) and 1-Nap (p < 0.001). For phenanthrene metabolites, the levels decreased by 10–25% from 1995 to 2019. Differences were significantly lower (Mann-Whitney U, p < 0.05) in 2019 referred to 1995 for 1-OH-Phe (p < 0.05), 2-OH-Phe (p < 0.01), and 3-OH-Phe (p < 0.01). Although a moderate decline in urinary 4- and 9-OH-Phe was observed, results were not significant (p > 0.05). For better comparability with other published data, which reported the sum of OH-Phes, data for the five OH-Phes was evaluated both individually (Table 2) and as sum (Table 2 and Fig. 1B). For the sum of OH-Phes, the levels decreased by 16% from 1995 to 2019. This decline was significant (Mann-Whitney U, p < 0.05) in 2010, 2015, and 2018.
In contrast to the before mentioned OH-PAHs, 2-Nap showed an opposite trend with steadily increasing levels by a factor of 1.7 (p < 0.001) from 1995 until 2019 (Table 2, Fig. 1B). No sex-specific differences were found for any of the OH-PAHs investigated (Mann-Whitney U test, p > 0.05, data not shown).
3.3. Correlation of cotinine with OH-PAHs
To investigate the relation of OH-PAH excretion and SHS exposure, data from Cot and OH-PAH were correlated using the Spearman rank correlation analysis (Table A5 in Appendix). A moderate but significant correlation with Cot was observed for 1-OH-Pyr, 1-Nap, as well as all of the single OH-Phes (p < 0.05, Table A5 in Appendix). The sum of all five OH-Phes and the individual metabolites 1-, 2-, 3-, 9-OH-Phe showed similar correlations to Cot in the range of r = 0.27 to 0.41 while 4-OH-Phe had the lowest Spearman r of only 0.17. In contrast, 3-OH-BaP and 2-Nap exposure did not correlate with urinary Cot (Spearman r = 0.07). To evaluate the effect of the high number of samples below LLOQ, the correlation was additionally calculated for samples with quantifiable Cot excretion rates only, which confirmed the results (data not shown).
4. Discussion
The growing awareness of health impairment from SHS has necessitated comprehensive legislation in many parts of the world to actively protect non-smokers from exposure to SHS (McNabola and Gill, 2009; WHO, 2009). In Germany, regulations and legislative amendments have focused on non-smoker protection since 2002 (Federal Non-smokers Protection Act, 2007; ArbStättV, 2002; Kohler and Minkner, 2014; Kuntz et al., 2017; Schaller and Mons, 2018). These include direct regulations, such as the ban on smoking in federal facilities and public transport (Federal Non-smokers Protection Act, 2007; Kohler and Minkner, 2014), and regulations that focus on reducing the proportion of smokers in the general population, such as tax measures, which have a more indirect effect on reducing SHS exposure (Federal Non-smokers Protection Act, 2007; ArbStättV, 2002; Kohler and Minkner, 2014; Kuntz et al., 2017; Schaller and Mons, 2018). Therefore, the objective of the present study was to investigate the possible impact of governmental regulations on the SHS exposure from 1995 to 2019 as well as the correlation between PAH and SHS exposure.
4.1. Cotinine
From 1995 to the last sampling year considered, 2019, urinary Cot levels have steadily and significantly decreased. Already in the year 2000, Cot levels decreased by a factor of 1.5 (p > 0.05) and continued to decline significantly to 51% of the levels determined in 1995 by 2005 (p < 0.05, Fig. 1A). Evidently, this trend appears within the time frame of the first strong actions against smoking with the national smoking ban at the workplace in 2002 (ArbStättV, 2002) and significant tobacco tax increases from 2002 to 2005 (Deutsches Krebsforschungszentrum, 2014; Schaller and Mons, 2018). The considerable decrease in SHS exposure until 2010 to approximately 28% compared to 1995 may be attributed to the State Non-Smoker Protection Acts (Schaller and Mons, 2018), which, for instance, banned smoking in gastronomy, and the Federal Law for Protection from the Hazards of Passive Smoking (Federal Non-smokers Protection Act, 2007), both coming into force in 2007. The latter included various changes and amendments to the existing legislation, as well as a decisive article imposing a ban on smoking in federal facilities and public transport (Federal Non-smokers Protection Act, 2007; Kohler and Minkner, 2014). Cot excretion decreased moderately in the further years until 2019 to levels of 0.3 μg/24 h, which corresponds to only 18% of the Cot levels determined in 1995. The aforementioned regulations and further preventive legislation such as additional tobacco tax increases and the prohibition of advertising for cigarette products (Schaller and Mons, 2018) are regarded as major factors in the general decrease in smoking rates (Hampsher et al., 2021; Kuntz et al., 2018), as reflected in tobacco sales that declined by approximately one-third between 1991 and 2016 (Schaller and Mons, 2018), and in the total number of self-reported smokers among all ESB participants over the years (Umweltprobenbank, 2022). Certainly, the reduced smoking prevalence over the considered time span (Hampsher et al., 2021) additionally contributes to reductions in SHS exposure. In this context, it should be noted that Kuntz and Lampert have also observed a general decrease in smoking and passive smoke exposure from 2003 to 2006 compared to 2009–2012 (Kuntz and Lampert, 2016). Additional control might be achieved by sales ban to youths <18 years. Minor tobacco tax increase, the adjustment of Non-Smoker Protection Law of single states (e.g. Bavaria, Northern Rhine Westphalia), and warning on tobacco products in the next years were further measures which may have contributed to reduced SHS exposure (Schaller and Mons, 2018).
A similar impact of smoking regulation policies on SHS exposure of the population have also been observed in other countries, such as Korea, where levels decreased by 54% from 2009 to 2011 (Park et al., 2015). Additionally, in the U.S. population the exposure of non-smokers to SHS significantly decreased by 86% from 1988 to 1991 to 2001–2002 as a result of extensive efforts made by the public health community to reduce the exposure to SHS (Pirkle et al., 2006).
4.2. Hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs)
Elevated PAH exposure caused by smoking has already been proven for various OH-PAHs such as 3-OH-BaP (Aquilina et al., 2010; Rögner et al., 2021), 1-OH-Pyr (Hagedorn et al., 2009; Jacob et al., 1999; Scherer et al., 2000), naphthols (Preuss et al., 2003, 2004; St Helen et al., 2012; Yang et al., 1999), and OH-phenantrenes (Heudorf and Angerer, 2001; Jacob et al., 1999; St Helen et al., 2012). For that reason, a set of different OH-PAHs has been investigated, to monitor the time trend and to investigate the correlation between PAH and SHS exposure.
With the exception of 2-Nap, 4-, and 9-OH-Phe, all OH-PAHs showed a significant decrease within the investigated time span from 1995 to 2019. The most distinct decline was found for the biomarkers most commonly used to assess PAH exposure, 1-Nap and 1-OH-Pyr (Castano-Vinyals et al., 2004; Li et al., 2008; Merlo et al., 1998; Srogi, 2007). In 2019, urinary excretion of 1-Nap and 1-OH-Pyr, respectively, were only 34% and 61% of the value measured in 1995 (Fig. 1B). Both metabolites showed a moderate but significant correlation with Cot excretion. Hence, urinary 1-Nap and 1-OH-Pyr can be related to SHS exposure despite the presence of confounding sources such as wood smoke for 1-Nap or dietary patterns which are frequently associated with 1-OH-Pyr exposure (Abdel-Shafy and Mansour, 2016; Gao et al., 2018; Kato et al., 2004; Kim et al., 2013; Li et al., 2010; Nethery et al., 2012; St Helen et al., 2012). These observations are in accordance with previous findings from the U.S. National Health and Nutrition Examination Survey (NHANES) in children and adolescents showing a correlation between SHS and elevated exposure to Pyr and Nap (Huang et al., 2006; Kim et al., 2014). In case of 3-OH-BaP, the levels decreased by 19% from 1995 to 2019, which is also reflected by the increasing number of levels that were determined < LLOQ over the years. However, data on statistical analysis should be taken with caution, as only 28% of the 24-h urine samples analyzed for 3-OH-BaP could be quantified above the LLOQ (50 fg/mL).
This is in agreement with Rögner et al. (2021), whose sensitive method to quantify 3-OH-BaP demonstrated that levels in non-smokers could hardly be quantified. That could also explain the non-existent correlation of 3-OH-BaP and Cot in the herein investigated population of non-smokers. For the OH-Phes, levels moderately decreased by 10–25% from 1995 to 2019, with only weak to moderate correlation with Cot.
The overall decrease in OH-PAH excretion from 1995 to 2019 in general correlates well with a reduced Cot exposure. However, this correlation cannot be attributed solely to a reduced SHS exposure as reductions in other PAH exposure sources are likely to contribute to a major extent to the observed trends over time. Next to SHS, PAH exposure of the general population is mainly caused by several environmental factors such as indoor air pollution evoked by burning domestic fuels for heating or cooking, and traffic emissions infiltrating indoor air (Hansen et al., 2006; Sochacka-Tatara et al., 2018). Additionally, the ambient air from industries and traffic (Freire et al., 2009; Hansen et al., 2006) as well as the intake of processed food (e.g., fried and smoked food) were identified to impact urinary PAH metabolite concentration. Especially the consumption of barbequed food is reported to contribute to urinary PAH metabolite exposure (Alghamdi et al., 2015; Dobraca et al., 2018; Hoseini et al., 2018; IARC, 2010; Rodgman and Perfetti, 2006; Srogi, 2007). The consumption of chocolate is also a potential dietary source of PAHs (Bansal and Kim, 2015; Murawski et al., 2020).
The exposure from the above-mentioned sources could be affected by recent EU regulations specifying maximum levels of PAHs in foodstuffs in 2006 (1881/2006/EC) and 2011 (835/2011/EU) as well as in air (EC, 2004b, EC, 2004a) in 2005. Although these regulations mainly focused on benzo[a]pyrene, they certainly resulted in reductions of PAHs in general, leading to a decline not only in 3-OH-BaP, but also for the other OH-PAHs studied. Further directives and regulations, that aimed at minimizing environmental PAH input to media air, soil, and water (850/2004/EC, 2004a, 2004b), but also PAH emission from combustion plants and industrial installations (2001/80/EC, 2001) may have additionally contributed to the observed decline in PAH exposure. A reason or source for the increased PAH exposure in 2017, which was not observed for Cot and therefore cannot be explained by SHS exposure, could not be deduced. The higher levels in 2017 cannot be attributed to outliers either, as their exclusion still resulted in higher levels compared to the other years investigated. This supports the hypothesis, that other sources than SHS, e.g., diet or air pollution, are main contributors to PAH exposure in non-smokers (Aquilina et al., 2010; Scherer et al., 2000).
In contrast to the aforementioned OH-PAHs, steadily increasing levels were observed for 2-Nap from 1995 to 2019. Thereby, the 2-Nap concentration significantly increased by a factor of 1.7 (p < 0.001). This ascending trend cannot be explained by SHS exposure, as Cot, 3-OH-Bap, and the other OH-PAHs studied clearly decreased over time. Aquilina et al. already demonstrated that gas-phase naphthalene concentrations did not correlate with urinary 2-Nap, which might explain this countervailing trend (Aquilina et al., 2010). A similar trend of increasing 2-Nap and decreasing 1-Nap levels has been previously observed by Jung et al. between 2001 and 2012 in young children living in New York City. They, however, also observed an increase in 1-OH-Pyr. Jung et al. explained the decline in 1-Nap levels by a decrease in carbaryl use in the residential environment. In contrast, higher 2-Nap concentrations were attributed to an increase in ambient emissions (Jung et al., 2014). Another explanation for the observed effect might be that different levels of exposure to naphthalene cause different routes of naphthalene metabolism, which was proposed by Thai et al. (de Oliveira et al., 2014; Thai et al., 2020) and recently reported by Scherer et al. (2022). The cause for the opposing trend of 1- and 2-Nap in the studied population remains unclear as several of the aforementioned factors may lead to the observed differences. However, the most reasonable cause for the countervailing trend and especially the increasing 2-Nap values might be the rise in 2-Nap exposure or exposure to precursor compounds specifically metabolizing to 2-Nap (Farzan et al., 2016; Griffiths and Hawkins, 1977; Jung et al., 2014; Meeker et al., 2007; Preuss et al., 2003). This, however, would implicate an increased exposure to chemicals of concern and potentially as yet unidentified sources for 2-Nap exposure that need further investigation.
4.3. Comparison with other national surveys
Concurrent data on Cot and PAH metabolites were assessed by the NHANES (CDC, 2019), the Canadian Health Measures Survey (CHMS) (Health Canada, 2017, 2021), and previous German Environmental Surveys (GerES) in adults (Becker et al., 2002, 2003). It should be noted that GerES data include only cycles II and III, and investigated fewer OH-PAHs, indicating the data gap that was addressed by the present study. For better comparison of national surveys, median values are discussed in the following section. An overview of the data comparison is presented in Table 3.
Table 3.
Median levels of Cot and OH-PAHs in different studies.
| 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cotinine [μg/L] | present study (GER) | 1.40 | 0.60 | 0.35 | <LLOQ1 | <LLOQ1 | <LLOQ1 | <LLOQ1 | <LLOQ1 | <LLOQ1 | |||||||||||||||||||||
| NHANES (USA)2 | 0.30 | <LOD3 | 0.25 | 0.20 | 0.20 | 0.15 | 0.10 | 0.09 | |||||||||||||||||||||||
| CHMS (CAN) | <LOD4 | <LOD4 | <LOD4 | ||||||||||||||||||||||||||||
| GerES (GER) | <LLOQ5 | <LLOQ5 | |||||||||||||||||||||||||||||
| 1-OH-Pyr [ng/g creatinine] | present study (GER) | 92 | 68 | 71 | 72 | 69 | 62 | 66 | 55 | 50 | |||||||||||||||||||||
| NHANES (USA) | 80 | 86 | 113 | 117 | 119 | 146 | |||||||||||||||||||||||||
| CHMS (CAN) | 99 | 88 | 78 | ||||||||||||||||||||||||||||
| GerES (GER) | 80 | 80 | |||||||||||||||||||||||||||||
| 1-Nap [μg/g creatinine] | present study (GER) | 0.81 | 0.50 | 0.40 | 0.40 | 0.31 | 0.30 | 0.37 | 0.25 | 0.25 | |||||||||||||||||||||
| NHANES (USA) | 2.1 | 1.79 | 1.98 | 1.69 | 1.51 | 1.28 | |||||||||||||||||||||||||
| CHMS (CAN) | 1.2 | 0.99 | 0.68 | ||||||||||||||||||||||||||||
| GerES (GER) | |||||||||||||||||||||||||||||||
| 2-Nap [μg/g creatinine] | present study (GER) | 0.71 | 0.88 | 0.82 | 0.97 | 1.17 | 1.30 | 1.07 | 1.50 | 1.17 | |||||||||||||||||||||
| NHANES (USA) | 1.94 | 2.56 | 3.08 | 3.44 | 3.33 | 4.32 | 4.77 | ||||||||||||||||||||||||
| CHMS (CAN) | 3.40 | 4.10 | 3.80 | ||||||||||||||||||||||||||||
| GerES (GER) | |||||||||||||||||||||||||||||||
| 1-OH-Phe [ng/g creatinine] | present study (GER) | 175 | 144 | 118 | 133 | 116 | 140 | 168 | 121 | 132 | |||||||||||||||||||||
| NHANES (USA) | 87 | 141 | 140 | 137 | 130 | 134 | 104 | ||||||||||||||||||||||||
| CHMS (CAN) | 14 | 14 | 13 | ||||||||||||||||||||||||||||
| GerES (GER) | 400 | 270 | |||||||||||||||||||||||||||||
| 2-OH-Phe [ng/g creatinine] | present study (GER) | 62 | 57 | 54 | 57 | 52 | 60 | 63 | 42 | 42 | |||||||||||||||||||||
| NHANES (USA) | 53 | 52 | 57 | 60 | 62 | 62 | |||||||||||||||||||||||||
| CHMS (CAN) | 62 | 56 | 51 | ||||||||||||||||||||||||||||
| GerES (GER) | |||||||||||||||||||||||||||||||
| 3-OH-Phe [ng/g creatinine] | present study (GER) | 103 | 78 | 68 | 61 | 71 | 73 | 88 | 58 | 67 | |||||||||||||||||||||
| NHANES (USA) | 87 | 100 | 88 | 91 | 68 | 63 | |||||||||||||||||||||||||
| CHMS (CAN) | 79 | 82 | 71 | ||||||||||||||||||||||||||||
| GerES (GER) | 250 | 190 | |||||||||||||||||||||||||||||
| 4-OH-Phe [ng/g creatinine] | present study (GER) | 21 | 24 | 16 | 23 | 21 | 26 | 24 | 17 | 17 | |||||||||||||||||||||
| NHANES (USA) | 23 | 27 | 22 | ||||||||||||||||||||||||||||
| CHMS (CAN) | 22 | 19 | 18 | ||||||||||||||||||||||||||||
| GerES (GER) | |||||||||||||||||||||||||||||||
| 9-OH-Phe [ng/g creatinine] | present study (GER) | 96 | 83 | 59 | 74 | 84 | 65 | 98 | 52 | 80 | |||||||||||||||||||||
| NHANES (USA) | |||||||||||||||||||||||||||||||
| CHMS (CAN) | 32 | 32 | 32 | ||||||||||||||||||||||||||||
| GerES (GER) | |||||||||||||||||||||||||||||||
1 LLOQ = 0.2 μg/L; 2 calculated from serum cotinine levels [μg/L] according to Benowitz et al. (2009); 3 LOD = 0.015 ng/mL; 4 LOD in urine 1.0 μg/L and 1.1 μg/L; 5 LLOQ = 4 μg/L.
Data from NHANES (CDC, 2019), CHMS (Health Canada, 2017, 2021), and GerES (Becker et al., 2002, 2003; Murawski et al., 2020).
In case of Cot, data of non-smokers were used for the assessment because NHANES and CHMS differentiate between the smoking status for this biomarker. NHANES only provided serum Cot levels that were estimated to be converted to urinary levels according to Benowitz et al. (2009). Nevertheless, urinary Cot excretion in our study was slightly higher in the early 2000s compared to NHANES; however, a decreasing trend was also observed in the U.S. population over time (CDC, 2019). In CHMS and GerES, urinary Cot excretion in the non-smoking population in all survey cycles was below the LOD (1.0 μg/L and 1.1 μg/L, respectively) (Health Canada, 2021) and LLOQ (4 μg/L) (Becker et al., 2002, 2003), respectively, and therefore could not be evaluated.
NHANES and CHMS do not differentiate between smokers and non-smokers for OH-PAH evaluation in the data set of adults, which is why data of the total population are presented (Table 3). In contrast, data of never-smokers are discussed for GerES.
Levels for 1-OH-Pyr were in the same range in all four surveys. However, in contrast to the Canadian and German population, increasing levels of 1-OH-Pyr were observed from 2003 to 2004 to 2013–2014 in the U.S. Median 1-Nap and 2-Nap levels were somewhat lower in the present study compared to NHANES and CHMS. The decrease for 1-Nap was observed across all surveys except GerES, for which data were not available. In general, 1-Nap and 2-Nap levels were about 4 times higher in the US and Canada. The conspicuous upward trend for 2-Nap was observed across all surveys and also by Jung et al. between 2001 and 2012 among young children living in New York City (Jung et al., 2014), indicating increasing exposure specifically to 2-Nap or chemicals metabolized to 2-Nap that require further investigation.
Levels for 1-, 2-, 3-, and 4-OH-Phe were comparable in the German, Canadian and U.S. population, with a decreasing trend in NHANES and CHMS, as observed in the current study. For 9-OH-Phe, no data is provided by NHANES and data from CHMS was approximately 3-times lower compared to the present study with no apparent time trend. In case of GerES, no data were collected for 2-, 4-, and 9-OH-Phe. For 1-, and 3-OH-Phe, levels were generally higher than in the other surveys, however, the descending trend was observed across the two cycles (Becker et al., 2002, 2003). A comparable and substantial decrease in phenanthrene and pyrene exposure in children and adolescents in Germany (aged 3–17) since the 1990s was recently reported by Murawski et al. They showed that the number of smokers in the household as well as the children's presence in rooms at home where people smoke, significantly impacted urinary 1-OH-Pyr, 1-Nap, and 2-Nap of the non-smoking children (Murawski et al., 2020), which is in agreement with our findings that passive smoking contributes to PAH exposure.
The HBM of 3-OH-BaP has only been conducted in CHMS so far. However, 3-OH-BaP concentrations were below LOD in cycle 2 (2009–2011), cycle 3 (2012–2013) and cycle 4 (2014–2015) (CDC, 2019; Health Canada, 2017; Health Canada, 2021).
To the best of our knowledge, this is the first study systematically determining the exposure to benzo[a]pyrene (DFG, 2012; IARC, 2010) in a large HBM campaign over a long time period of 24 years. The excreted amount of 3-OH-BaP observed for non-smokers in this study is in accordance with the literature (Lafontaine et al., 2006; Rögner et al., 2021; Sarkar et al., 2010).
The comparability between different national surveys is challenging due to different factors that affect the results. Some of these factors should be mentioned briefly: the sampling year does not always overlap; cohorts differ in terms of age, ethnicity, socio economic status; the sampling area varies in terms of exposure to several contaminants; the smoking prevalence varies among different populations, countries and time points and is not always considered during evaluation; the non-smoker status classification in these studies is often based on self-reports that are not confirmed by exposure markers, which might add higher variability in case of non-compliance; LOD and LLOQ differ between the methods, which affects the treatment of values below LOD or LLOQ. These aspects limit the overall comparison between different surveys, but they still provide valuable insights regarding time trends in the exposure to SHS and PAHs.
5. Conclusion
This is the first HBM study systematically assessing the urinary excretion of Cot and nine different OH-PAHs, including 3-OH-BaP, in Germany over a time period of 24 years. Although the study population is geographically constrained, very homogeneous in terms of age and does not reflect the whole population of Germany, the current study allows for exposure trend analysis and complements HBM data within the HBM4EU program. With the exception of 2-Nap, a descending trend was observed for all biomarkers analyzed in urine from 1995 to 2019. The decline for Cot can most likely be attributed to regulations and legislative amendments that focused on non-smoker protection in Germany and positively affected SHS exposure in the non-smoking population. Reduced PAH exposure presumably resulted from several concomitant factors including reduced SHS exposure, less industrial air pollution, and regulations limiting PAHs in air and food. Although the demonstrated decrease in exposure towards SHS, and thus PAHs, is positive, further reductions in PAH exposure are warranted due to their carcinogenic properties and the variety of PAH exposure sources, e.g., food and indoor air, that have a higher impact on overall general exposure and thus a greater regulatory importance.
The present study underlines the importance of Europe wide HBM to cover exposure by transboundary air pollution as well as nationwide HBM to monitor the exposure to chemicals of concern and to assess the effects of regulatory measures with respect to public health. The continuing high prevalence of smoking requires additional efforts to protect the non-smoking population from SHS and HBM will serve as an important tool to monitor the success of future measures for smoke-free indoor and outdoor environments.
Reasons for the increase in 2-Nap levels are yet to be unveiled, but indicate novel or increasing exposures to 2-Nap and chemicals metabolized to 2-Nap, respectively. Future representative datasets (to be expected from GerES VI) will help to put this time trend analysis in relation to the population representative exposure of people living in Germany.
Author contributions
Conceptualization, T.W. and M.K.; Formal Analysis, T.B.; Investigation, T.B.; Data Curation, T.W.; Writing – Original Draft Preparation, T.B.; Writing – Review & Editing, N.P., G.S., M.S. and T.W.; Visualization, T.B.; Supervision, N.P.; Project Administration, T.W.; Funding Acquisition, M.K.
Information on funding and research on human subjects
This study was funded by the Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV), with a part of the analyses being funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 733032 HBM4EU (www.hbm4eu.eu).
The study protocol of the ESB has been approved by the ethics committee of the Medical Association Westfalen-Lippe, the Medical Faculty of the University of Münster and (since 2012) by the ethical committee of the Medical Association of the Saarland.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The study protocol of the ESB has been approved by the ethics committee of the Medical Association Westfalen-Lippe, the Medical Faculty of the University of Münster and (since 2012) by the ethical committee of the Medical Association of the Saarland.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nikola Pluym reports financial support was provided by Horizon 2020 European Innovation Council Fast Track to Innovation.
Acknowledgments
This study was funded by the Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV), with a part of the analyses being funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 733032 HBM4EU (www.hbm4eu.eu). We would like to acknowledge the work of the ESB teams of Fraunhofer IBMT and the University Hospital Münster for their work in acquiring, handling and storing of human samples and measuring of the physiological parameters for the German ESB. We would also like to thank N. Roegner, M. Latawiec, J. Taucher, for performing the OH-PAH analyses, and J. Mütze and J. Kugele for the bioanalysis of cotinine.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.114638.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abdel-Shafy H.I., Mansour M.S.M. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt. J. Petrol. 2016;25:107–123. [Google Scholar]
- Act F.N.-s.P. In: I. Federal Non-smokers Protection Act. Bundesgesetzblatt, editor. 2007. Gesetz zur Einführung eines Rauchverbotes in Einrichtungen des Bundes und öffentlichen Verkehrsmitteln (Bundesnichtraucherschutzgesetz - BNichtrSchG) pp. 1595–1597. Bonn. [Google Scholar]
- Alghamdi M.A., et al. Urinary metabolites of polycyclic aromatic hydrocarbons in Saudi Arabian schoolchildren in relation to sources of exposure. Environ. Res. 2015;140:495–501. doi: 10.1016/j.envres.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Angerer J., et al. Human biomonitoring: State of the art. Int. J. Hyg Environ. Health. 2007;210:201–228. doi: 10.1016/j.ijheh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Aquilina N.J., et al. Environmental and biological monitoring of exposures to PAHs and ETS in the general population. Environ. Int. 2010;36:763–771. doi: 10.1016/j.envint.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ArbStättV . ArbStättV; 2002. Arbeitsstättenverordnung. § 5 Nichtraucherschutz. [Google Scholar]
- Deutsches Krebsforschungszentrum Tabaksteuererhöhungen und Rauchverhalten in Deutschland. Aus der Wissenschaft - für die Politik, Heidelberg. 2014:1–4. [Google Scholar]
- DFG . In: MAK Collection: Occupational Toxicants, Part 1. Commission M., editor. Wiley-VCH Verlag, Deutsche Forschungsgemeinschaft; Heidelberg: 2012. Benzo[a]pyrene. [Google Scholar]
- FDA . Food and Drug Administration; 2018. Bioanalytical Method Validation - Guidance for Industry. [Google Scholar]
- Avila-Tang E., et al. Assessing secondhand smoke using biological markers. Tobac. Control. 2012;22:164–171. doi: 10.1136/tobaccocontrol-2011-050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V., Kim K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015;84:26–38. doi: 10.1016/j.envint.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Becker K., et al. Umwelt-Survey 1998 Band III: human-Biomonitoring Stoffgehalte in Blut und Urin der Bevölkerung in Deutschland. WaBoLu-Hefte. 2002;1/02 [Google Scholar]
- Becker K., et al. German Environmental Survey 1998 (GerES III): environmental pollutants in the urine of the German population. Int. J. Hyg Environ. Health. 2003;206:15–24. doi: 10.1078/1438-4639-00188. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L., Jacob P., 3rd Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin. Pharmacol. Therapeut. 1993;53:316–323. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L., et al. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob. Res. 2009;11:954–960. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano-Vinyals G., et al. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup. Environ. Med. 2004;61:e12. doi: 10.1136/oem.2003.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2019. Fourth National Report on Human Exposure to Environmental Chemicals. Vol. 2019. https://www.cdc.gov/exposurereport/index.html. [Google Scholar]
- de Oliveira B.F., et al. A curated review of recent literature of biomarkers used for assessing air pollution exposures and effects in humans. J. Toxicol. Environ. Health, Part A B. 2014;17:369–410. doi: 10.1080/10937404.2014.976893. [DOI] [PubMed] [Google Scholar]
- Djordjevic M.V., Doran K.A. Nicotine content and delivery across tobacco products. Handb. Exp. Pharmacol. 2009:61–82. doi: 10.1007/978-3-540-69248-5_3. [DOI] [PubMed] [Google Scholar]
- Dobraca D., et al. Urinary biomarkers of polycyclic aromatic hydrocarbons in pre- and peri-pubertal girls in Northern California: predictors of exposure and temporal variability. Environ. Res. 2018;165:46–54. doi: 10.1016/j.envres.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2001/80/EC . Directive 2001/80/EC of the European Parliament and of the Council of 23 October 2001 on the Limitation of Emissions of Certain Pollutants into the Air from Large Combustion Plants. vol. 44. Official Journal of the European Union; 2001. pp. 1–21. [Google Scholar]
- 850/2004/EC . Official Journal of the European Union; 2004. Regulation (EC) No 850/2004 of the European Parliament and of the Council of 29 April 2004 on Persistent Organic Pollutants and Amending Directive 79/117/EEC; pp. 7–49. vol. 47. [Google Scholar]
- 2004/92/EC . Commision Decision of 21 January 2004 on Emergency Measures Regarding Chilli and Chilli Products. vol. 47. Official Journal of the European Union; 2004. pp. 52–54. [Google Scholar]
- 1881/2006/EC . Official Journal of the European Union; 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; pp. 5–24. vol. 49. [Google Scholar]
- 835/2011/EU . Official Journal of the European Union; 2011. Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs; pp. 4–8. vol. 54. [Google Scholar]
- Farzan S.F., et al. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003–2008. Environ. Res. 2016;144:149–157. doi: 10.1016/j.envres.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C., et al. Urinary 1-hydroxypyrene and PAH exposure in 4-year-old Spanish children. Sci. Total Environ. 2009;407:1562–1569. doi: 10.1016/j.scitotenv.2008.10.068. [DOI] [PubMed] [Google Scholar]
- Gao P., et al. Human exposure to polycyclic aromatic hydrocarbons: metabolomics perspective. Environ. Int. 2018;119:466–477. doi: 10.1016/j.envint.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Griffiths J., Hawkins C. Synthesis and photochemical stability of 1-phenylazo-2-naphthol dyes containing insulated singlet oxygen quenching groups. J. Appl. Chem. Biotechnol. 1977;27:558–564. [Google Scholar]
- Hagedorn H.W., et al. Urinary excretion of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in nonsmokers and in smokers of cigarettes with different ISO tar yields. J. Anal. Toxicol. 2009;33:301–309. doi: 10.1093/jat/33.6.301. [DOI] [PubMed] [Google Scholar]
- Hampsher S., et al. 2021. Smoking Cessation in Germany: Drivers and Barriers. Available at: SSRN: https://ssrn.com/abstract=3977282 November 19, 2021. [Google Scholar]
- Hansen Å.M., et al. Urinary 1-hydroxypyrene in children living in city and rural residences in Denmark. Sci. Total Environ. 2006;363:70–77. doi: 10.1016/j.scitotenv.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Health Canada . Published by authority of the Minister of Health; Ottawa: 2017. Fourth Report on Human Biomonitoring of Environmental Chemicals in Canada. [Google Scholar]
- Health Canada . Published by authority of the Minister of Health; Ottawa: 2021. Sixth Report on Human Biomonitoring of Environmental Chemicals in Canada. [Google Scholar]
- Heudorf U., Angerer J. Urinary monohydroxylated phenanthrenes and hydroxypyrene - the effects of smoking habits and changes induced by smoking on monooxygenase-mediated metabolism. Int. Arch. Occup. Environ. Health. 2001;74:177–183. doi: 10.1007/s004200000215. [DOI] [PubMed] [Google Scholar]
- Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5:46–51. [Google Scholar]
- Hoseini M., et al. Environmental and lifestyle factors affecting exposure to polycyclic aromatic hydrocarbons in the general population in a Middle Eastern area. Environ. Pollut. 2018;240:781–792. doi: 10.1016/j.envpol.2018.04.077. [DOI] [PubMed] [Google Scholar]
- Huang W., et al. Levels of 1-hydroxypyrene and other monohydroxy polycyclic aromatic hydrocarbons in children: a study based on U.S. reference range values. Toxicol. Lett. 2006;163:10–19. doi: 10.1016/j.toxlet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- IARC IARC monographs on the evaluation of carcinogenic risks to humans: some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC (Int. Agency Res. Cancer) Monogr. Eval. Carcinog. Risks Hum. 2002;82 [PMC free article] [PubMed] [Google Scholar]
- IARC Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC (Int. Agency Res. Cancer) Monogr. Eval. Carcinog. Risks Hum. 2010;92 [PMC free article] [PubMed] [Google Scholar]
- Jaakkola M.S., Jaakkola J.J.K. Assessment of exposure to environmental tobacco smoke. Eur. Respir. J. 1997;10:2384–2397. doi: 10.1183/09031936.97.10102384. [DOI] [PubMed] [Google Scholar]
- Jacob J., et al. Profile of urinary phenanthrene metabolites in smokers and non-smokers. Biomarkers. 1999;4:319–327. doi: 10.1080/135475099230705. [DOI] [PubMed] [Google Scholar]
- Jung K.H., et al. Time trends of polycyclic aromatic hydrocarbon exposure in New York City from 2001 to 2012: assessed by repeat air and urine samples. Environ. Res. 2014;131:95–103. doi: 10.1016/j.envres.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., et al. Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiology. Biomark. Prevent. 2004;13:1005–1012. [PubMed] [Google Scholar]
- Kim K.-H., et al. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Kim H.W., et al. Synergistic interaction between polycyclic aromatic hydrocarbons and environmental tobacco smoke on the risk of obesity in children and adolescents: the U.S. National Health and Nutrition Examination Survey 2003-2008. Environ. Res. 2014;135:354–360. doi: 10.1016/j.envres.2014.08.032. [DOI] [PubMed] [Google Scholar]
- Kohler S., Minkner P. Smoke-free laws and direct democracy initiatives on smoking bans in Germany: a systematic review and quantitative assessment. Int. J. Environ. Res. Publ. Health. 2014;11:685–700. doi: 10.3390/ijerph110100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolossa-Gehring M., et al. Environmental surveys, specimen bank and health related environmental monitoring in Germany. Int. J. Hyg Environ. Health. 2012;215:120–126. doi: 10.1016/j.ijheh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Kuntz B., Lampert T. Smoking and passive smoke exposure among adolescents in Germany. Dtsch. Ärztebl. Int. 2016;113:23–30. doi: 10.3238/arztebl.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz B., et al. Robert Koch-Institut; 2017. Trends in Tobacco Sales in Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz B., et al. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz; 2018. [Time Trends of Occupational Differences in Smoking Behaviour of Employed Men and Women in Germany : Results of the 1999-2013 Microcensus] [DOI] [PubMed] [Google Scholar]
- Lafontaine M., et al. 3-Hydroxybenzo[a]pyrene in urine of smokers and non-smokers. Toxicol. Lett. 2006;162:181–185. doi: 10.1016/j.toxlet.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Landmesser A., et al. Biomarkers of exposure specific to E-vapor products based on stable-isotope labeled ingredients. Nicotine Tob. Res. 2019;21:314–322. doi: 10.1093/ntr/nty204. [DOI] [PubMed] [Google Scholar]
- Lermen D., et al. Trends in characteristics of 24-h urine samples and their relevance for human biomonitoring studies - 20 years of experience in the German Environmental Specimen Bank. Int. J. Hyg Environ. Health. 2019;222:831–839. doi: 10.1016/j.ijheh.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Li Z., et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Li Z., et al. Assessment of non-occupational exposure to polycyclic aromatic hydrocarbons through personal air sampling and urinary biomonitoring. J. Environ. Monit. 2010;12:1110–1118. doi: 10.1039/c000689k. [DOI] [PubMed] [Google Scholar]
- McNabola A., Gill L.W. The control of environmental tobacco smoke: a policy review. Int. J. Environ. Res. Publ. Health. 2009;6:741–758. doi: 10.3390/ijerph6020741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J.D., et al. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J. Expo. Sci. Environ. Epidemiol. 2007;17:314–320. doi: 10.1038/sj.jes.7500502. [DOI] [PubMed] [Google Scholar]
- Merlo F., et al. Urinary excretion of 1-hydroxypyrene as a marker for exposure to urban air levels of polycyclic aromatic hydrocarbons. Cancer Epidemiol. Biomarkers Prev. 1998;7:147–155. [PubMed] [Google Scholar]
- Murawski A., et al. Polycyclic aromatic hydrocarbons (PAH) in urine of children and adolescents in Germany - human biomonitoring results of the German Environmental Survey 2014-2017 (GerES V) Int. J. Hyg Environ. Health. 2020;226 doi: 10.1016/j.ijheh.2020.113491. [DOI] [PubMed] [Google Scholar]
- Musk A.W., de Klerk N.H. History of tobacco and health. Respirology. 2003;8:286–290. doi: 10.1046/j.1440-1843.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- Narkowicz S., et al. Environmental tobacco smoke: exposure, health effects, and analysis. Crit. Rev. Environ. Sci. Technol. 2013;43:121–161. [Google Scholar]
- Nethery E., et al. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. J. Expo. Sci. Environ. Epidemiol. 2012;22:70–81. doi: 10.1038/jes.2011.32. [DOI] [PubMed] [Google Scholar]
- Öberg M., et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- Park J.-H., et al. Decline in non-smoking workers' urine cotinine levels after increased smoking regulation in Korea. Ann. Occup. Environ. Med. 2015;27:17. doi: 10.1186/s40557-015-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle J.L., et al. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988-2002. Environ. Health Perspect. 2006;114:853–858. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluym N., et al. Biomonitoring data on young adults from the Environmental Specimen Bank suggest a decrease in the exposure to the fragrance chemical 7-hydroxycitronellal in Germany from 2000 to 2018. Int. J. Hyg Environ. Health. 2020;227 doi: 10.1016/j.ijheh.2020.113508. [DOI] [PubMed] [Google Scholar]
- Preuss R., et al. Naphthalene - an environmental and occupational toxicant. Int. Arch. Occup. Environ. Health. 2003;76:556–576. doi: 10.1007/s00420-003-0458-1. [DOI] [PubMed] [Google Scholar]
- Preuss R., et al. Pilot study on the naphthalene exposure of German adults and children by means of urinary 1- and 2-naphthol levels. Int. J. Hyg Environ. Health. 2004;207:441–445. doi: 10.1078/1438-4639-00313. [DOI] [PubMed] [Google Scholar]
- Ramsauer B., et al. A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in urine of non-smokers and smokers. Anal. Bioanal. Chem. 2011;399:877–889. doi: 10.1007/s00216-010-4355-7. [DOI] [PubMed] [Google Scholar]
- Reardon J.Z. Environmental tobacco smoke: respiratory and other health effects. Clin. Chest Med. 2007;28:559–573. doi: 10.1016/j.ccm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Rodgman A., Perfetti T.A. The composition of cigarette smoke: a catalogue of the polycyclic aromatic hydrocarbons. Beitraege Tabakforsch. Int. 2006;22:13–69. [Google Scholar]
- Rögner N., et al. A sensitive LC–MS/MS method for the quantification of 3-Hydroxybenzo[a]pyrene in urine-exposure assessment in smokers and users of potentially reduced-risk products. Separations. 2021;8:171. [Google Scholar]
- Sarkar M., et al. Evaluation of biomarkers of exposure in adult cigarette smokers using Marlboro Snus. Nicotine Tob. Res. 2010;12:105–116. doi: 10.1093/ntr/ntp183. [DOI] [PubMed] [Google Scholar]
- Schaller K., Mons U. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz; 2018. [Tobacco Control in Germany and Worldwide] [DOI] [PubMed] [Google Scholar]
- Scherer G., et al. Biomonitoring of exposure to polycyclic aromatic hydrocarbons of nonoccupationally exposed persons. Cancer Epidemiol. Biomark. Prev. 2000;9:373–380. [PubMed] [Google Scholar]
- Scherer G., et al. Assessment of the exposure to polycyclic aromatic hydrocarbons in users of various tobacco/nicotine products by suitable urinary biomarkers. Arch. Toxicol. 2022;96(11):3113–3126. doi: 10.1007/s00204-022-03349-4. [DOI] [PubMed] [Google Scholar]
- Schettgen T., et al. N-methylmalonamic acid (NMMA) as metabolite of methylisothiazolinone and methylchloroisothiazolinone in 24-h urine samples of the German Environmental Specimen Bank from 2000 to 2017 - exposure and time trends. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125743. [DOI] [PubMed] [Google Scholar]
- Schick S., Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tobac. Control. 2005;14:396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochacka-Tatara E., et al. Urinary polycyclic aromatic hydrocarbon metabolites among 3-year-old children from Krakow, Poland. Environ. Res. 2018;164:212–220. doi: 10.1016/j.envres.2018.02.032. [DOI] [PubMed] [Google Scholar]
- Srogi K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ. Chem. Lett. 2007;5:169–195. doi: 10.1007/s10311-007-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G., et al. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem. Res. Toxicol. 2012;25:952–964. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragierowicz J., et al. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. BioMed Res. Int. 2013 doi: 10.1155/2013/386784. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhout R., et al. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Publ. Health. 2011;8:613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai P.K., et al. Analysis of urinary metabolites of polycyclic aromatic hydrocarbons and cotinine in pooled urine samples to determine the exposure to PAHs in an Australian population. Environ. Res. 2020;182 doi: 10.1016/j.envres.2019.109048. [DOI] [PubMed] [Google Scholar]
- Torres S., et al. Biomarkers of exposure to secondhand and thirdhand tobacco smoke: recent advances and future perspectives. Int. J. Environ. Res. Publ. Health. 2018;15 doi: 10.3390/ijerph15122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umweltprobenbank. Umweltprobenbank des Bundes; 2022. https://www.umweltprobenbank.de/en/documents/investigations/results?genders=0&measurement_params=10088&specimen_types=10004. [Google Scholar]
- US Department of Health and Human Services . National Library of Medicine Cataloging in Publication; Rockville, MD, USA: 2010. How Tobacco Smoke Causes Disease: the Biology and Behavioral Basis for Smoking-Attributable Disease- A Report of the Surgeon General. [Google Scholar]
- WHO . 2009: Implementing Smoke-free Environments: Executive Summary. World Health Organization; 2009. WHO report on the global tobacco epidemic. [Google Scholar]
- Yang M., et al. A study for the proper application of urinary naphthols, new biomarkers for airborne polycyclic aromatic hydrocarbons. Arch. Environ. Contam. Toxicol. 1999;36:99–108. doi: 10.1007/s002449900447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.