Abstract
Background
Lymph node metastasis (LNM) is a well-known prognostic factor in Oral Squamous Cell Carcinoma(OSCC). A biological marker that predicts the Lymph Node Metastasis (LNM) in OSCC cases is the need of the hour. A Disintegrin And Metalloproteinases (ADAMs), a family of proteins that exhibit a metalloproteinase domain play a pivotal role in the pathogenesis of tumor growth and metastasis. This study aims to evaluate whether ADAM 10 can be used as a predictor of lymph node metastasis in OSCC using immunohistochemistry.
Method
A total of 90 samples that were categorized into 3 groups were included in the present study. Group I consisted of 30 samples of the normal oral mucosa, and Group II consisted of 30 samples of OSCC without lymph node metastasis. Group III consisted of 30 samples of OSCC with lymph node metastasis. Esophageal Squamous Cell Carcinoma was used as external positive control. Immunohistochemical expression of ADAM10 in their corresponding stained sections was assessed and staining intensity was calculated.
Results
ADAM10 immunoreactivity was considered positive when located in cytoplasm or membrane or both. This method is similar to that used by Bamane et al. for OSCC cases. The mean value of the Staining Index score “AxB” was highest in Group III (7.90), followed by Group II (3.13) and least in Group I (0.27). These values were statistically significant.
Conclusion
Considering the findings of a higher percentage of ADAM10 positive cells, higher staining intensity, and higher staining index, the overexpression of ADAM10 can be used as an independent marker for OSCC patients to predict the lymph node metastasis.
Keywords: ADAM10, Immunohistochemistry, Oral squamous cell carcinoma, Metastasis, Prognosis, Predictor
Introduction
Oral cancer is the 11th most common malignancy in the world. In 2012, 369,200 new cases were reported worldwide. The highest incidence of these cancers is reported in south and southeast Asia [1]. Demographics and health surveys report that tobacco use in all countries of Southeast Asia exceeded 50% among men aged 15–49 years. In southeast Asia, smokeless tobacco is often used as one of the ingredients of betel quid. According to recent GLOBOCAN data, Asia accounts for the largest proportion of oral cancer cases (64.2%) with a mortality rate of 73.3% [2]. The 5-year survival rate in patients with early-stage oral cancer is 82% and in patients with advanced stages only 27%. This declining survival rate in advanced stages is attributed to the complications associated with the disease. The most serious prognostic factor is metastasis [3]. The main pathway of metastasis of a solid tumor is via regional lymphatic channels. Lymph node metastasis (LNM) in many solid cancers including Oral Squamous Cell Carcinoma (OSCC) is a well-known and clinically accepted prognostic factor [4]. Staging by the TNM classification system is widely and routinely used for determining the extent of disease. Although 20–30% of clinically N0 patients will show occult metastasis on elective neck dissection, a considerably large number of patients will remain pathologically N0 and therefore are subjected to unnecessary neck dissection [5]. Many histological features have been tested over the last two decades to predict LN metastasis that includes nuclear pleomorphism, lymphoplasmacytic response, the pattern of invasion (POI), tumor nest, tumor thickness, tumor depth, lymphatic invasion, intravascular and perineural invasion and grade of the tumor. In a study, the only significant association was seen between cervical LN metastasis exists with high grade of differentiation [6]. Various imaging techniques like color doppler ultrasound, Magnetic Resonance Imaging, Computed tomography, Lymphoscintigraphy / Sentinel lymph node (LN) Mapping and molecular techniques such as immunohistochemistry, Polymerase chain reaction have led to the definition of unique markers like VEGF-C and/or VEGD-D, ADAMs to predict and visualize the growth of tumor cells in the lymph nodes [7].
A disintegrin and metalloproteinases (ADAMs) are recently discovered family of proteins with sequence similarity to the reprolysin family of snake venoms that exhibit a metalloproteinase domain. ADAMs are involved in biological events such as cell adhesion, cell fusion, cell migration, membrane protein shedding and proteolysis [8]. Literature search shows that ADAM10 plays a pivotal role in the pathogenesis of tumor growth and metastasis [9]. The aim of this study is to evaluate whether ADAM10 can be used as a predictor of lymph node metastasis in OSCC using immunohistochemistry.
Material & Methods
The study was approved by Institutional Ethics Committee, Government Dental College and Hospital. The formalin fixed paraffin embedded (FFPE) blocks were obtained from the archives of Dept of Pathology, RST, Regional Cancer Center, Nagpur. The study included histopathologically diagnosed 30 cases of OSCC without lymph node metastasis and 30 cases of OSCC with lymph node metastasis, 30 samples of normal oral mucosa and 10 samples of esophageal carcinoma which constituted the external positive control. In the study by Bo ma et al., it was concluded that ADAM10 was overexpressed in human ESCC tissues in vivo and was positively associated with lymph node metastasis contributing to tumor progression[10]. Many studies showed that ADAM-10 over expression was associated with cell invasion and metastasis in patients with esophageal cancer. According to the manufacturer’s recommendation, known esophageal squamous cell carcinoma samples showing good ADAM10 expression acted as external positive control. One positive control was included for each immunohistochemical cohort. This Positive control was observed for the presence of a coloured end product (DAB chromogen, brown coloured end product) at the site of target antigen. The presence of brown colored cytoplasmic staining was interpreted as positive staining, indicating proper performance of kit reagent (Fig. 1).
Fig. 1.

IHC stained photomicrograph of External Positive Control of Oesophageal C arcinoma (10 × magnification)
Inclusion Criteria
The study focused on the paraffin embedded biopsy specimens including (1) Surgically operated cases with complete clinical data. (2) Histopathologically confirmed cases of primary Oral Squamous Cell Carcinoma without lymph node metastasis. (3) Histopathologically confirmed cases of primary Oral Squamous Cell Carcinoma with lymph node metastasis.
Exclusion Criteria
(1) Patients receiving prior Radiotherapy and Chemotherapy. (2) Record of clinical data is inadequate.
Two Sects. (4 μm) of each specimen from all study groups, were prepared and stained immunohistochemically. Immunohistochemical staining was performed with Ready to use supersensitive antibodies which had been optimally diluted for use with these reagents and did not require further dilutions. FFPE (3 mm) were mounted on silane-coated glass slides and dewaxed in xylene. Rehydration was done with ethanol gradient washes and then the slides were boiled in 0.1 M citrate buffer (ph 6.0) for 15 min using a microwave. Subsequently, sections were exposed to 0.3% hydrogen peroxide for inhibiting endogenous peroxidase activity (10 min, room temperature). The specimens were incubated with the above primary antibody at room temperature for 60 min. The Envision kit (Dako Cytomation, Glostrup, Denmark) was used for detection at room temperature for 30 min).
Stained anti-ADAM10 sections were observed under research microscope with computer assisted image-analyser.
The counting of positive cells (ADAM 10 in stained sections) was performed by counting in five randomly selected, most representative microscopic fields showing positivity at magnification of 40 × and average was calculated to get the final value of percentage of positive cells per section. The result was presented as the mean number of ADAM 10 positive cells per section “A”. Staining intensity was calculated at different magnifications “B”. The product of “AxB” gives the Staining index [11]. Similar method was used by Bamane et al. for OSCC cases [12] and others [13–15]. This method is considered to be a “gold-standard” in evaluating immunohistochemical index. (16)The data on demographic parameters like age and gender is expressed in terms of numbers and percentage for each study group. Also the mean and standard deviation were obtained for age of patients in both the groups. Frequencies were obtained for site distribution in each group. The Data obtained was compiled and was subjected to statistical analysis. Descriptive statistics like frequency & percentage of subjects as per groups has been depicted. (Table 1).
Table 1.
Distribution and intergroup comparison of study groups according to age and gender
| Mean age (N = 90) | I | II | III | Total | F value | P value |
|---|---|---|---|---|---|---|
| 34.6 | 48.7 | 45.9 | 90 | 16.071 | 0.000** | |
| Gender (N = 90) | M = 17(5.6%) | M = 23(76.6%) | M = 16(53.3%) | 56(62.2%) | Chi square value | 0.131# |
| F = 13(43.3%) | F = 7(23.3%) | F = 14(46.6%) | ||||
| 34(37.7%) | 4.065 |
Bold values represent total number of patients for ease of interpretation by the readers
M Male, F female
Comparison of numerical variables among different groups was done using Kruskall Wallis ANOVA followed by pair-wise comparison using Mann Whitney U test. Comparison of observations grades / nominal / qualitative data with that of groups has been done using chi square test. For all the statistical tests, p < 0.05 was considered to be statistically significant.
Observations and Results
A total of 90 samples which were categorized in three groups were included in the present study. Group I consisted of 30 samples of normal oral mucosa, Group II consisted of 30 samples of OSCC without lymph node metastasis. Group III consisted of 30 samples of OSCC with lymph node metastasis. 10 samples of esophageal carcinoma were used as external positive control. Two sections were prepared from each block using microtome LEICA RM 2145. One section was stained with Hematoxylin & Eosin and the other was mounted on a silane coated slide and was stained immunohistochemically to assess the expression of ADAM10. Absence of non-specific staining in negative control confirmed the specificity of primary antibody. Vascular endothelial cells acted as the internal positive control. Immunostaining was assessed at the basal layers of normal mucosa (Figs. 2, 3).
Fig. 2.
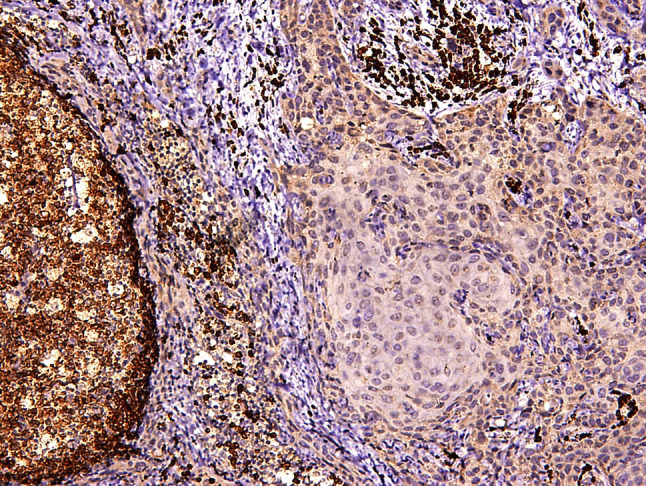
IHC stained photomicrograph of Metastatic Lymph Node showing islands of tumor cells in sheet the perifollicular area. (10 × magnification)
Fig. 3.

IHC stained photomicrograph of Internal positive control— Endothelial cells (arrow)
Immunohistochemical expression of ADAM10 in their corresponding stained sections was assessed in OSCC (group II) and (group III) in the dysplastic epithelium, infiltrated islands of malignant epithelial cells within stroma by evaluation of staining intensity & percentage of positive (ADAM10) cells. ADAM10 immunoreactivity was considered positive when located in cytoplasm or membrane or both. This method is similar to that used by Bamane et al. for OSCC cases[12–15]. All the Haematoxylin–Eosin and immunohistochemically stained sections were evaluated by two observers to eliminate inter-observer bias and any disagreement if any were resolved with the help of a third observer. The counting of ADAM10 positive cells was done using computer assisted image Analyser.
The OSCC patients mainly belong to fifth decade of life. There was an obvious male predominance in OSCC cases with M: F ratio of 1.8:1. (Table 1). Majority of cases of OSCC involved alveolobuccal complex. Within the group II and III also most cases were seen involving alveolobuccal complex. Most of the OSCC cases belonged to T3 tumor size in Group III and T1 tumor size in Group II. In OSCC without LN metastasis group majority of patients belonged to Stage I whereas in OSCC with LN metastasis group majority belonged to Stage IVa. (Table 2). Both OSCC groups (Group II and Group III) showed 100% positivity for ADAM10 expression and there was statistically highly significant difference in two groups of OSCC when compared to normal mucosa and also between the two groups of OSCC. The mean value of percentage of ADAM10 positive cells/ HPF “A” was found to be highest in Group III (2.77) as compared to Group II (1.77) and minimal for Group I (0.77). Similarly, the mean value of Staining Intensity “B” was highest in Group III (2.80), followed by Group II (1.67) and least for Group I (0.27). Also, the mean value of Staining Index score “AxB” was highest in Group III (7.90), followed by Group II (3.13) and least in Group I (0.27). (Table 3) On Kruskal Wallis test the difference was statistically highly significant (p < 0.01) (Table 4).
Table 2.
Distribution of study groups according to site, Bryne’s Grading, Primary Tumor Size, TNM staging
| Site | II | III | Total | Chi square | P value |
|---|---|---|---|---|---|
| ABC = 17 | ABC = 15 (50%) | 60 | 0.835 | 0.841# | |
| (56.6%) | BM = 5(16.6%) | ||||
| BM = 6(20%) | Lip = 2(6.6%) | ||||
| Lip = 1(3.3%) | Tongue = 8(26.6%) | ||||
| Tongue = 6(20%) | |||||
| Histopathological Diagnosis (Bryne’s grading) | WDSCC = 14 (46.6%) | WDSCC = 14 (46.6%) | 60 | 0.000 | 1.000# |
| MDSCC = 10 (33.3%) | MDSCC = 10 (33.3%) | ||||
| PDSCC = 6 (20%) | PDSCC = 6 (20%) | ||||
| Tumor Size | T1 = 12(40%) | T1 = 4 | 60 | 8.132 | 0.043* |
| T2 = 8(26.6%) | T2 = 6 | ||||
| T3 = 8(26.6%) | T3 = 18 | ||||
| T4a = 2(6.6%) | T4a = 2 | ||||
| TNM Staging | Stage I = 13 (43.3%) | Stage I = 0 | 60 | 17.875 | 0.000** |
| Stage II = 8 (26.6%) | Stage II = 0 | ||||
| Stage III = 7 (23.6%) | Stage III = 11 (36.6%) | ||||
| Stage IVa = 2 (6.6%) | Stage IVa = 19 (63.3%) |
Bold values represent total number of patients for ease of interpretation by the readers
ABC Alveolobuccal complex, BM Buccal mucosa, WDSCC Well differentiated squamous cell carcinoma, MDSCC Moderately differentiated squamous cell carcinoma, PDSCC Poorly differentiated squamous cell carcinoma, * = significant, ** = highly significant, # = non-significant
Table 3.
Comparison of interpretation of staining index between among the three groups
| Groups | IHC staining index | ||||||
|---|---|---|---|---|---|---|---|
| Absent | Low | Moderate | High | Total | Chi square value | p value | |
| Group I Normal Mucosa | 24(80%) | 6(20%) | 0 | 0 | 30 | 128.155 | 0.000** |
| Group II OSCC w/o metastasis | 0 | 15(50%) | 13(43.3%) | 2(6.67%) | 30 | ||
| Group III OSCC with metastasis | 0 | 1(3.33%) | 2(6.66%) | 27(90%) | 30 | ||
| Total | 24(26.6%) | 22(24.4%) | 15(16.6%) | 29(32.2%) | 90 | ||
Bold values represent total number of patients for ease of interpretation by the readers
Table 4.
Comparison of mean values of staining index “A”, staining intensity “B” and IHC staining index “C” amongst the three groups
| Groups (n = 30) | Mean | Std. Deviation | Std. Error | Chi square value | p value of KW ANOVA | |
|---|---|---|---|---|---|---|
| Percentage of ADAM 10 positive cells/HPF "A" | Group I | 0.20 | 0.407 | 0.074 | 72.669 | 0.000** |
| Group II | 1.77 | 0.568 | 0.104 | |||
| Group III | 2.77 | 0.504 | 0.092 | |||
| Staining Intensity "B" | Group I | 0.27 | 0.583 | 0.106 | 69.690 | 0.000** |
| Group II | 1.67 | 0.606 | 0.111 | |||
| Group III | 2.80 | 0.484 | 0.088 | |||
| IHC Staining Index Score "AXB" | Group I | 0.27 | 0.583 | 0.106 | 72.667 | 0.000** |
| Group II | 3.13 | 2.013 | 0.367 | |||
| Group III | 7.90 | 2.057 | 0.376 |
Discussion
Oral cancer is one of the ten most common cancers in the world, with a delayed clinical detection, a poor prognosis, lack of specific biomarkers for the disease and expensive therapeutic alternatives [17].
Presence of regional lymph node metastasis is the most important prognostic factor in OSCC [18]. The outcome of metastasis is so grim that the 5-year survival rate of patients with lymph node metastasis is only 25–40%, compared to approximately 90% of patients without lymph node metastasis[19]. The urgent need for molecular markers to predict lymph node metastasis in OSCC patients cannot be over emphasized. ADAM (A Disintegrin And Metalloprotease) is one such member of this family. They serve in homeostatic functions like control of membrane fusion, as well as processes such as muscle development, fertilization, and cell fate determination. Additionally, pathologies such as inflammation and cancer also involve ADAMs family members. ADAM9, ADAM10, and ADAM17, are considered to be the key players during genesis, development, and metastasis of cancers[20]. ADAM10 is involved in events like cell adhesion, cell fusion, cell migration, membrane protein shedding and proteolysis. It cleaves type 4 collagen. ADAMs are intially synthesized in inactive form known as pro-ADAMs. They are activated by furins or MMPs. Secondly, ADAMs shed growth factors, this alters cell signaling on surface of cancer cells. The outcome of this is increased cell proliferation. Thirdly, using their disintegrin and cystein rich domains, ADAMs bind to integrins and syndecans helping the cell to digest their substrates. Fourth, ADAMs regulate signals of cell proliferation through integrins. And finally, owing to their proteolytic activity, ADAMs have a pivotal role in cancer development and metastasis. They cleave extracellular matrix molecules to establish a secondary site of growth [7]. By the virtue of its biological properties, ADAM10 has the propensity to play a pivotal role in regulating the process of lymph node metastasis in OSCC. the objective of our study was to evaluate the expression of ADAM10 as a predictor of lymph node metastasis in OSCC using immunohistochemistry.
The study comprised of a total of 90 samples, which were categorized under three groups: Group I- 30 cases of Normal Mucosa Group II- 30 cases of OSCC without lymph node metastasis Group III- 30 cases of OSCC with lymph node metastasis.
All groups were evaluated for the Immunohistochemical analysis of ADAM 10 antigen. Immunostaining was assessed by evaluation of staining intensity and percentage of ADAM10 positive cells. In the present study, all 30 samples of normal mucosa, showed mainly cytoplasmic ADAM10 immunoexpression of Absent to Low staining index confined to basal and at places to the suprabasal layers. (Fig. 4). In an immunohistochemical study done by Stasikowska et al. [8]. the author demonstrated low immunostaining of 19 normal mucosa samples which were used as controls. Another study by Jones et al. [9]. also showed that normal mucosa had lower immunostaining (24.2 ± 4.1, n = 61) as compared to the OSCC specimens (41.2 ± 5.1, n = 50) examined.
Fig. 4.

IHC expression of ADAM 10 with low staining index in normal mucosa (GroupI) (10 × magnification)
Group II consisted of 30 cases of OSCC without metastasis. The expression of ADAM 10 was positive in all cases of OSCC specimens that were examined as a part of this group. ADAM10 staining showed mainly cytoplasmic positivity. Slight membranous positivity was also seen (Fig. 5A, 5B). Out of the 30 cases of Group II, 2 cases showed high ADAM10 staining index, 13 cases showed moderate and 15 cases showed low ADAM10 staining index. (Table 3).
Fig. 5.

A IHC stained photomicrograph showing low to moderate ADAM 10 staining in the the tumor island. (Group II) (10 × magnification). B IHC stained photomicrograph of ADAM 10 staining showing predominantly cytoplasmic and slightly membranous staining in the tumor cells. (Group II) (40 × magnification)
This study group consisted of 30 cases of OSCC with metastasis. The expression of ADAM10 was positive in all cases of OSCC specimens that were examined as a part of this group. ADAM10 staining showed predominantly cytoplasmic expression. Some membranous positivity was also noticed. Out of 30 cases of Group III, 27 cases showed high ADAM10 Staining index, 2 cases showed moderate ADAM10 staining index and only1 case showed low ADAM10 staining index. (Table 3).
Mean staining index of OSCC with metastasis was higher than that of OSCC without metastasis with highly statistically significant difference. This higher ADAM10 expression may be suggestive of its potential role in metastatic process. (Table 4) Our findings seen in OSCC groups were consistent with the findings of Statikowska et al. [8].
Bao X et al. [21] in their study evaluated the expression of ADAM10 in metastatic oral cancer tissues. They found a significant relation between elevated ADAM10 expression and metastasis (Fig. 6).
Fig. 6.
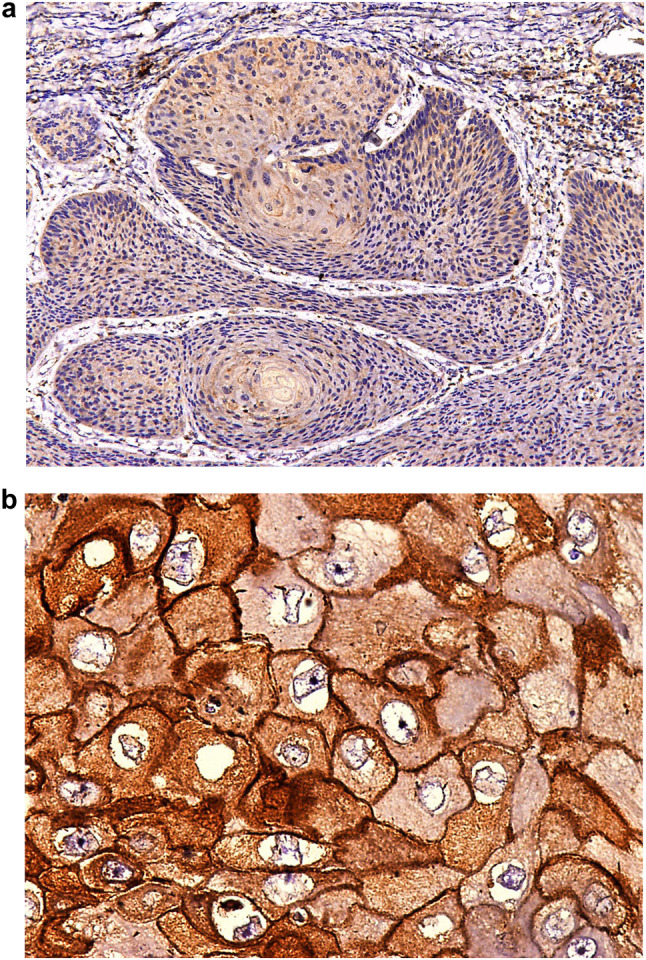
A IHC stained photomicrograph showing High ADAM 10 staining in the tumor islands in the connective tissue predominantly in cytoplasm with slight membranous staining (10 × magnification). B IHC stained Photomicrograph showing High ADAM 10 staining predominantly in the cytoplasm and slightly in the membrane(40 × magnification)
However, in contrast to our findings, Jones et al. [9] found that ADAM10 was significantly reduced in cases of OSCC with metastatic disease compared to those without metastasis.
Ko et al. [22] and Etemad moghadam et al. mentioned in their paper that although metastatic OSCC cases do show elevated ADAM10 expression than the non-malignant matched tissues, they were unable to identify the relevance between increased ADAM10 expression and lymph node involvement. Howsoever, these authors do acknowledge the role of ADAM10 as a type IV collagenase and has capacity to digest basement membrane and facilitate metastasis [22].
A little work is also done on development of selective inhibitors of ADAM10. Two low molecular weight compounds termed G1254023X (GSK) and INCB8765 (Incyte) are described in the literature. No work is done to develop ADAM10 inhibitors for OSCC. This opens up a field of opportunities for clinical trials to be conducted for same [23].
Conclusion
Metastasis of Oral Squamous Cell Carcinoma to regional lymph nodes renders the tumor notorious and unmanageable. The role of ADAM10 (A Disintegrin And Metalloprotease) in OSCC is evaluated by some researchers but contrasting results are obtained. To the best of our knowledge, our study is one amongst the very few studies that aimed to evaluate role of ADAM10 as a predictor of lymph node metastasis in OSCC by comparing its expression in cases of OSCC with LN metastasis and cases of OSCC without LN metastasis. In this study we observed that percentage of positive cells, staining intensity and staining index of OSCC was higher than that of normal tissue and this difference was statistically highly significant. This indicates that ADAM10 is associated with malignant transformation and invasion. The mean staining index of cases of OSCC with LN metastasis was higher than the cases of OSCC without LN metastasis. Likewise, the mean percentage of ADAM10 positive cells and the mean staining intensity was also higher in LN metastasis positive group as compared to LN metastasis negative group. This may be indicative of its role in LN metastasis. Therefore, considering the findings of higher percentage of ADAM10 positive cells, higher staining intensity and higher staining index, the overexpression of ADAM10 can be used as an independent marker for OSCC patients to predict the lymph node metastasis.
Limitations
To reduce the discrepancy, studies with a larger sample size and a wider geographical area may provide differing results owing to the demographic and epidemiologic differences. Study of various other members of the ADAM family are needed to reach at definitive conclusions. Interplay of epigenetic factors like history of habit abuse, occupation, lifestyle habits, socioeconomic status were not considered in this study. Immunohistochemistry is a very technique sensitive staining procedure. Hence, the requirements of a well-established laboratory set up, experienced and skilled personnel are required. Human error should be taken into consideration. Although studies assessing association between neo-angiogenesis and ADAM10 expression are plentiful, researches assessing expression of ADAM10 with lymph node metastasis and lymphatic vessel density are only handful. This opens scope for new research arenas. Expression of ADAM10 at the tumor invasive front may provide more fruitful conclusions. If possible, long term follow up of patients by assessing their survival curves can provide clues about use of ADAM10 as a prognostic marker also.
Acknowledgements
Authors wish to acknowledge Dr. B.K. Sharma, Jt. Director RST Regional Cancer Center Nagpur, Dr. Manisha Mishra HOD, Dept of Pathology, RST Regional cancer center Nagpur, to allow us to use the paraffin embedded blocks for the study.
Author Contributions
Dr RJ and Dr SG formulated the manuscript, and data collection.
Funding
This research did not receive any specific grant from funding agencies in public, commercial or not-for-profit sectors.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of Interest
None.
Ethical Approval
Ethical clearance was obtained from ethics committee, GDCH Nagpur. Ethical clearance number: GDCHN/SS/7511/2018. The manuscript is not submitted to any other journal. Submitted work is original and is not split up. There is no falsification or manipulation or plagiarism. Proper acknowledgement is given.
Consent to Participate
The authors declare that they have no conflict of interest.
Consent to Publish
Since archival tissue blocks were used in the study, informed consent was not necessary.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Riya Jain, Email: riyasjain25@gmail.com.
Suchitra Gosavi, Email: gosavisr@gmail.com.
Deepak Sethia, Email: sethia.d@gmail.com.
Akshay Trimuke, Email: atrimukhe1@gmail.com.
Mayuri Salunke, Email: mayurisalunke1995@gmail.com.
References
- 1.Ghantous Y, Abu EI. Global incidence and risk factors of oral cancer. Harefuah. 2017;156(10):645–649. [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Iype EM, Pandey M, Mathew A, Thomas G, Sebastian P, Nair MK. Oral cancer among patients under the age of 35 years. J Postgrad Med. 2001;47(3):171–6. [PubMed] [Google Scholar]
- 4.Cho J, Hyun SH, Choi N, Kim M, Padera TP, Choi JY, et al. Significance of lymph node metastasis in cancer dissemination of head and neck cancer. Translational Oncology. 2015;8(2):119–125. doi: 10.1016/j.tranon.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10(3):160–167. doi: 10.1002/hed.2890100304. [DOI] [PubMed] [Google Scholar]
- 6.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98(2):413–423. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007;98(5):621–628. doi: 10.1111/j.1349-7006.2007.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stasikowska-Kanicka O, Wągrowska-Danilewicz M, Kulicka P, Danilewicz M. Overexpression of ADAM10 in oral squamous cell carcinoma with metastases. Polish J Pathol. 2018;69(1):67–72. doi: 10.5114/pjp.2018.75339. [DOI] [PubMed] [Google Scholar]
- 9.Jones AV, Lambert DW, Speight PM, Whawell SA. ADAM 10 is over expressed in oral squamous cell carcinoma and contributes to invasive behaviour through a functional association with αvβ6 integrin. FEBS Lett. 2013;587(21):3529–3534. doi: 10.1016/j.febslet.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 10.ADAM10 mediates the cell invasion and metastasis of human esophageal squamous cell carcinoma via regulation of E-cadherin activity [Internet]. [cited 2022 Jun 4]. Available from: https://www.spandidos-publications.com/10.3892/or.2016.4667?text=fulltext [DOI] [PubMed]
- 11.Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 2009;38(8):639–643. doi: 10.1111/j.1600-0714.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 12.Ganvir SM, Bamane SA, Katkade SP, Khobragade PG, Hazarey VP, Gosavi SR. Depth of invasion and GLUT-1 as risk predictors in oral squamous cell carcinoma: an immunohistochemical study. Transl Res Oral Oncol. 2017;2:2057178X16689690. [Google Scholar]
- 13.Correlation between invasion mode and the histologic risk assessment model in oral squamous cell carcinoma | SpringerLink [Internet]. [cited 2022 Jun 4]. Available from: https://link.springer.com/article/10.1007/s10006-016-0572-3 [DOI] [PubMed]
- 14.Etemad-Moghadam S, Alaeddini M. Invasion phenotypes of oral squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2015;23(8):e12–e16. doi: 10.1097/PAI.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 15.Etemad-Moghadam S, Alaeddini M. Pattern of invasion in squamous cell carcinomas of the lower lip and oral cavity. J Oral Biol Craniofacial Res. 2017;7(3):167–170. doi: 10.1016/j.jobcr.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—a review. PMC [Internet]. [cited 2022 Jun 4]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4260254/ [DOI] [PMC free article] [PubMed]
- 17.Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8(9):11884. [PMC free article] [PubMed] [Google Scholar]
- 18.Goto M, Hasegawa Y, Terada A, Hyodo I, Hanai N, Ijichi K, et al. Prognostic significance of late cervical metastasis and distant failure in patients with stage I and II oral tongue cancers. Oral Oncol. 2005;41(1):62–69. doi: 10.1016/j.oraloncology.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Yu B, Cao W, Zhang C, Xia R, Liu J, Yan M, et al. Prediction of lymph node metastasis in oral squamous cell carcinoma based on protein profile. Expert Rev Proteomics. 2019;16(4):363–373. doi: 10.1080/14789450.2019.1584039. [DOI] [PubMed] [Google Scholar]
- 20.Seals DF. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17(1):7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 21.Bao X, Shi J, Xie F, Liu Z, Yu J, Chen W, et al. Proteolytic release of the p75NTR intracellular domain by ADAM10 promotes metastasis and resistance to Anoikis. Cancer Res. 2018;78(9):2262–76. doi: 10.1158/0008-5472.CAN-17-2789. [DOI] [PubMed] [Google Scholar]
- 22.Ko S, Lin S, Wong Y, Liu C, Chang K, Liu T. Increase of disintergin metalloprotease 10 (ADAM10) expression in oral squamous cell carcinoma. Cancer Lett. 2007;245(1–2):33–43. doi: 10.1016/j.canlet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Mullooly M, McGowan PM, Crown J, Duffy MJ. The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol Ther. 2016;17(8):870–880. doi: 10.1080/15384047.2016.1177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.


