Abstract
Vaccines are one of the most effective medical interventions to combat newly emerging and re-emerging diseases. Prophylactic vaccines against rabies, measles, etc., have excellent effectiveness in preventing viral infection and associated diseases. However, the host immune response is unable to inhibit virus replication or eradicate established diseases in most infected people. Therapeutic vaccines, expressing specific endogenous or exogenous antigens, mainly induce or boost cell-mediated immunity via provoking cytotoxic T cells or elicit humoral immunity via activating B cells to produce specific antibodies. The ultimate aim of a therapeutic vaccine is to reshape the host immunity for eradicating a disease and establishing lasting memory. Therefore, therapeutic vaccines have been developed for the treatment of some infectious diseases and chronic noncommunicable diseases. Various technological strategies have been implemented for the development of therapeutic vaccines, including molecular-based vaccines (peptide/protein, DNA and mRNA vaccines), vector-based vaccines (bacterial vector vaccines, viral vector vaccines and yeast-based vaccines) and cell-based vaccines (dendritic cell vaccines and genetically modified cell vaccines) as well as combinatorial approaches. This review mainly summarizes therapeutic vaccine-induced immunity and describes the development and status of multiple types of therapeutic vaccines against infectious diseases, such as those caused by HPV, HBV, HIV, HCV, and SARS-CoV-2, and chronic noncommunicable diseases, including cancer, hypertension, Alzheimer’s disease, amyotrophic lateral sclerosis, diabetes, and dyslipidemia, that have been evaluated in recent preclinical and clinical studies.
Introduction
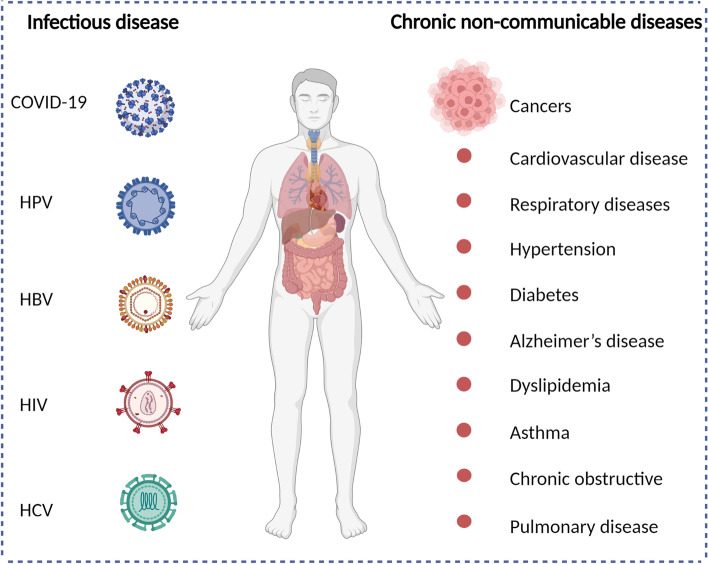
Infectious diseases are previously the leading cause of death [1].Vaccines have traditionally been used as the most effective medical interventions to reduce the death and morbidity caused by infectious diseases [2]. Vaccination has significantly reduced the burden of many dangerous infectious diseases, such as smallpox, poliomyelitis, diphtheria, tetanus and measles [3]. Faced with the epidemic of COVID-19, researchers have been racing to develop and test effective vaccines against COVID-19 that mimic the host immune response to the pathogen and elicit the activation of T cells and antibody production, leading to high efficacy in constraining this infectious disease. To date, several highly effective COVID-19 vaccines have been approved for use in humans or are still in clinical development worldwide [4]. The developed COVID-19 vaccines were shown to have > 90% efficacy and thus could protect most vaccinated individuals [5]. However, a considerable proportion of individuals still suffer from SARS-CoV-2 infection and the associated COVID-19 for several reasons, including limited global vaccine acceptance, inequitable global distribution of vaccines, limited cross-protection, the short duration of protection, virus variants, and individual immunosuppression [6]. Similarly, other infectious diseases continue to be main cause of mortality including HPV, HBV, HIV, HCV, influenza virus and so on (Fig. 1) [7]. Chronic non-communicable diseases represent a major source of morbidity and mortality in worldwide, resulting 71% of all deaths and serious global economic burden (Fig. 1) [8]. Chronic non-communicable diseases mainly include cardiovascular disease, cancer, respiratory diseases, diabetes, hypertension, Alzheimer’s disease, dyslipidemia, asthma, chronic obstructive and pulmonary disease [9]. The top four chronic non-communicable disease killers account for more than 80% of all deaths including cardiovascular diseases, cancers, respiratory diseases, and diabetes [8].
Fig. 1.
A summary of infectious disease and chronic non-communicable diseases
Therefore, there is a need for therapeutic vaccines that can break the body’s immune tolerance and enhance the body’s specific immune response for the purpose of eradicating an established disease. Therapeutic vaccines are used to stimulate antigen-specific immune responses to specifically target and kill infected cells [10]. In 1890, Koch developed a therapeutic vaccine for tuberculosis containing tuberculin and glycerol in suspension, setting a precedent for therapeutic vaccines, and proposed that vaccines can not only prevent diseases but also treat diseases [11]. After Almroth Wright reported the use of a corresponding antibacterial vaccine to treat long-term local bacterial infections in 1897, therapeutic vaccines entered a period of great development but also great controversy due to abuse [12]. Due to the advent of various chemicals and antibiotics, researchers in the development of therapeutic vaccines have been frustrated. Since the discovery of HIV in 1981, the basis for and application of antiviral immunology have developed rapidly. The increasing number of patients with chronic diseases and the emergence of antibiotic resistance have led to the redevelopment of therapeutic vaccines. Following the approval of Sipuleucel-T in 2010 [13], therapeutic vaccine development entered a booming stage. A number of studies have shown that therapeutic vaccines play a positive clinical role in the treatment of tumors and infectious diseases [14, 15], and an increasing number of therapeutic vaccines have been transferred from basic laboratory research to clinical trials. In fact, there are several therapeutic vaccines in clinical trials against infectious disease and chronic non-communicable diseases such as virus infection, cancer [16], hypertension, Alzheimer’s disease, diabetes and dyslipidemia [14]. Compared with current chemical drugs or other biological drugs, therapeutic vaccines have the advantages of high specificity, few side effects, long-lasting effects, and no drug resistance, making them a new hope for the treatment of infectious diseases and chronic noncommunicable diseases [17]. In this review, we have provided the development of therapeutic vaccines on some infectious diseases and chronic noncommunicable diseases.
Therapeutic vaccine-induced immunity
Researchers are racing to develop and test such effective prophylactic vaccines against infectious diseases that mimic the immune response against pathogens and elicit the activation of T cells and antibody production, leading to a highly efficacious constraint of infectious diseases. Prophylactic vaccines against hepatitis B virus (HBV) and human papillomavirus (HPV) have achieved great success [18, 19]. However, some viruses are able to cause persistent infection in some humans, for example, individuals at high risk for HPV infection. HPV has developed various approaches to escape immune surveillance, such as the low expression of viral proteins and the inhibition of antiviral immunity by suppressing APC function and the expression of MHC I molecules [20]. Similarly, cancer cells fail to be cleared by the immune response due to the low immunogenicity of the tumor antigen, the elimination of high-affinity T cells recognizing self-antigens and the immunosuppressive tumor microenvironment [21]. The ability of therapeutic vaccines to activate and amplify antigen-specific immune responses has been recognized as a potentially powerful tool for established diseases. The concept of therapeutic vaccines is based on the constant or unique expression of specific antigens, such as HPV viral E6 and E7, tumor neoantigens, and HBsAg, in established diseases [22–24]. Early, Saveria Campo et al. observed that an E7 protein vaccine against bovine papillomavirus type 4 induced a strong cellular immune response and resulted in the rejection of established tumors [25].
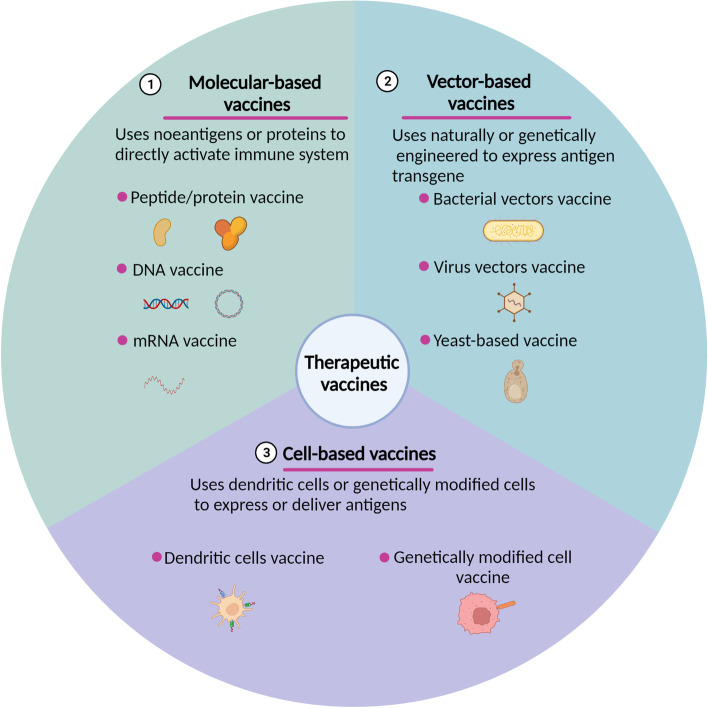
Therapeutic vaccines are mainly divided into three types used in preclinical and clinical phases: molecular-based vaccines, vector-based vaccines and cell-based vaccines (Fig. 2) [26]. Molecular-based vaccines include peptide/protein vaccines, DNA vaccines and mRNA vaccines, applying neoantigens, purified peptides/proteins or DNA/mRNA-encoded proteins with adjuvants to trigger immune responses [27]. Vector-based vaccines use naturally or genetically engineered bacteria, viruses, and yeast as effective carriers to express antigen transgenes [28, 29]. Cell-based vaccines consist of dendritic cell vaccines and genetically modified cell vaccines, which use dendritic cells or genetically modified cells to express or deliver antigens [30, 31]. Therapeutic vaccines typically involve endogenous or exogenous antigen delivery, in most cases, with an adjuvant to activate dendritic cells (DCs). The purpose of therapeutic vaccines is to target the existing antigens to maximize the induction of epitope-specific T cells that can reach the infection site and lesions to eliminate infection or B cells to produce specific antibodies that can neutralize the virus [32]. The vaccine immune response is complicated and occurs in multiple locations (Fig. 3).
Fig. 2.
An overview of therapeutic vaccine types in preclinical and clinical trials. Therapeutic vaccines have several types used in preclinical and clinical phase including molecular-based vaccines (peptide/protein vaccine, DNA and mRNA vaccine), vector-based vaccines (bacterial vectors vaccine, viral vectors vaccine and yeast-based vaccines) and cell-based vaccines (dendritic cells vaccines and genetically modified cell vaccines)
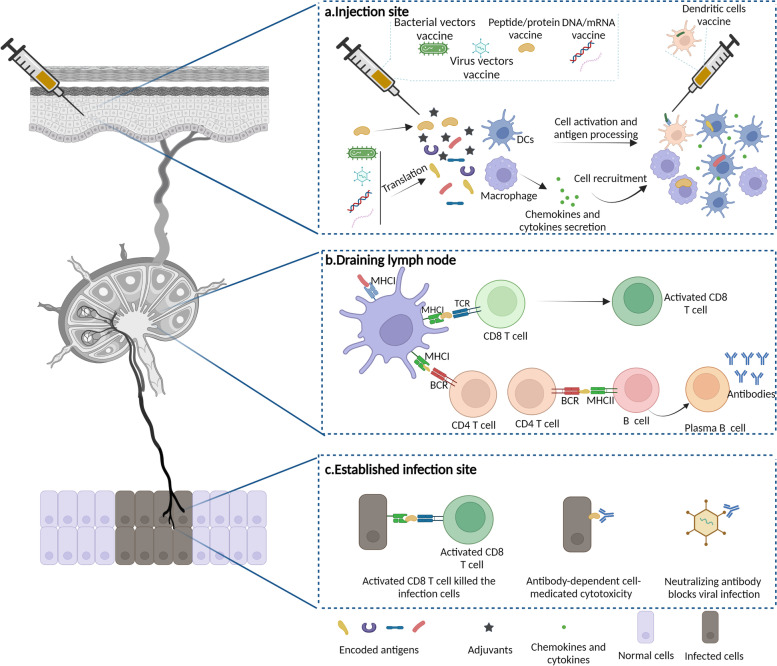
Fig. 3.
Therapeutic vaccine immunity. a Therapeutic vaccine, such as including bacterial vectors vaccine, viral vectors vaccine, peptide/protein vaccine, DNA and mRNA vaccine were injected locally. Adjuvants in the vaccine in local sites activate the resident innate immune cells and result in the release of chemokines (CCL2,CXCL1 et al) and cytokines, which subsequently recruits macrophages and DCs to the site of injection. The encoded antigen of the vaccine in the injection site is endocytosed by DCs; b Activated DCs in the vaccination sites travels to the draining lymph nodes. Upregulation of MHCII and co-stimulatory molecules such as CD40, CD80, and CD86 on the surface of DCs and cytokines are essential for DCs activation. Migratory and activated DCs present antigen in form of peptide-MHC complexes directly to T cells in the lymph nodes. Meanwhile receiving the co-stimulatory and cytokine signals, CD8+ T cells are activated. Antigen-activated B undergo maturation and expressed antigen-specific antibodies; c Antigen-specific CD8+ T cells and antigen-specific antibodies infiltrated into infection and lesions to conduct the function
Adjuvants are crucial components in vaccines to stimulate and enhance the magnitude and durability of the immune response against antigens [33]. Adjuvants in licensed vaccines include alum, MF59, CpG 1018, AS01, AS02, AS03 and AS04, and several high-potency adjuvants are being evaluated in preclinical and clinical studies. Adjuvants with immunostimulatory effects mimic damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) to activate innate immune cells through the stimulation of pattern recognition receptors (PRRs) [33]. Therapeutic vaccines are commonly injected via intramuscular, subcutaneous or intradermal routes in the clinic, with intramuscular administration being most frequently used [34]. Local inflammation plays a critical role in the magnitude and duration of the adaptive immune response [35]. At the vaccine delivery site, adjuvants in vaccines activate resident innate immune cells, resulting in the release of chemokines (CCL2, CXCL1, etc.) and cytokines, which subsequently recruit innate immune cells, including neutrophils, monocytes, macrophages and DCs, to the site of injection [36, 37]. DCs infiltrate the injection site and then serve as the main antigen-presenting cells (APCs), playing a vital role in eliciting a strong adaptive immune response [34]. The antigen component of the vaccine is endocytosed by DCs in the vaccination site.
DCs activated at the vaccination site and vaccine components travel to the draining lymph nodes [38]. The upregulation of MHCII and costimulatory molecules such as CD40, CD80, and CD86 on the surface of DCs and cytokines are essential for DC activation. Migratory and activated DCs present antigen in the form of peptide–MHC complexes directly to T cells in the lymph nodes. By receiving costimulatory and cytokine signals, CD8+ T cells are activated [39]. Antigen-activated B cells undergo maturation and rapid proliferation. Then, antigen-specific CD8+ T cells travel to infection sites and lesions to perform their function.
Therapeutic vaccines against infectious diseases
Multiple of severe infectious diseases, not least the COVID-19 pandemic, had a devastating impact on individuals [40]. For example, HPV-related malignancies account for 4.5% of all human cancers [41]. It is estimated that approximately 257 million people have chronic HBV infection and thus an increased risk of developing liver cirrhosis and hepatocellular carcinoma [42]. There were approximately 38 million HIV infections who need life-long antiretroviral treatment [43]. Therapeutic strategies need to be developed to solve persistent virus infection and associated lesions in clinic. Here, we review the development of therapeutic vaccines for HPV, HBV, HIV, HCV and SARS-CoV-2. Selected clinical trials of therapeutic vaccines against infectious disease were should in Table 1.
Table 1.
Selected clinical trials of therapeutic vaccines against infectious disease
| Therapeutic HPV vaccines | |||||
|---|---|---|---|---|---|
| Vaccine type | Vaccine Formulation | Combination agents | Condition | Clinical trial identifier | Phase/status |
| Bacterial vector vaccine | ADXS11–001(attenuated live Listeria Encoding HPV 16 E7 vector) | Cervical carcinoma | NCT02164461 |
Phase I-II Completed |
|
|
Cervical carcinoma Head and Neck Cance |
NCT02291055 |
Phase I-II Active, not recruiting |
|||
| Cervical cancer | NCT01266460 |
Phase II Completed |
|||
| Placebo | Cervical cancer | NCT02853604 |
Phase III Active, not recruiting |
||
|
Anal cancer Rectal cancer |
NCT02399813 |
Phase II Completed |
|||
|
Head and neck cancer, oropharyngeal squamous cell carcinoma |
NCT02002182 |
Phase II Active, not recruiting |
|||
| Viral vector vaccine | Vvax001(Semliki Forest virus expressing E6 and E7) |
CIN 2/3 Cervical cancer |
NCT03141463 |
Phase I Completed |
|
| HB-201 (lymphocytic choriomeningitis virus encoding HPV E6 and E7) | HPV-Related Squamous Cell Carcinoma | NCT04180215 |
Phase I/II Recruiting |
||
| Peptide/protein vaccine | ISA101(nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with Montanide ISA51) | Nivolumab | Solid Tumors | NCT02426892 |
Phase II Completed |
| TVGV-1(HPV16 E7 fused protein +adjuvant GPI-0100) | High-grade squamous intraepithelial lesions | NCT02576561 |
Phase IIa Active, not recruiting |
||
| DNA vaccine | VGX-3100(plasmid encoding E6 and E7 of HPV16/18) | Head and neck squamous cell cancer | NCT02163057 |
Phase I/IIa Completed |
|
| Cervical cancer | NCT02172911 |
Phase I/IIa Completed |
|||
| Placebo | High-grade squamous intraepithelial lesion of the cervix | NCT03185013 |
Phase III Completed |
||
| GX188E (HPV E6/E7 fused to Flt3L | Cervical intraepithelial neoplasia | NCT02139267 |
Phase II Completed |
||
| Placebo | Cervical Intraepithelial Neoplasia | NCT02596243 |
Phase II Active, not recruiting |
||
| Cervical Intraepithelial Neoplasia 3 | NCT03206138 |
Phase Ib/II Recruiting |
|||
| Therapeutic HBV vaccines | |||||
| Viral vector vaccines | TG1050(Adeno vector encoding core, polymerase, envelope fusion protein) | Placebo | Chronic HBV Infection | NCT02428400 | Phase I/Ib Completed |
| Protein vaccine | Theravax (DV-601, consisting of HBsAg, HBcAg and saponin-based ISCOMATRIX adjuvant) | Entecavir | Chronic HBV Infection | NCT01023230 | Phase IIb Completed |
| GS-4774(Heat-inactivated yeast containing S, core, X proteins) | Chronic HBV Infection | NCT01943799 | Phase II Completed | ||
| GS-4774(Heat-inactivated yeast containing S, core, X proteins) | Tenofovir | Chronic HBV Infection | NCT02174276 | Phase II Completed | |
| HeberNasvac (containing HBsAg and HBcAg) | Peg-IFN | Chronic HBV Infection | NCT01374308 | Phase II Completed | |
| HeberNasvac (containing HBsAg and HBcAg) | NUC | Chronic HBV Infection | NCT02249988 |
Phase IIB-III Completed |
|
| HepTcell vaccine (Synthetic peptide+ IC31 adjuvant) | Chronic HBV Infection | NCT02496897 | Phase I Completed | ||
| DNA vaccine | INO-1800 (DNA plasmids encoding S and core) | NUC | Chronic HBV Infection | NCT02431312 | Phase I Completed |
| HB-110(a mixed plasmid DNA) | Adefovir | Chronic HBV Infection | NCT00513968 | Phase I Completed | |
| Adefovir | Chronic HBV Infection | NCT01641536 | Phase I Completed | ||
| Therapeutic HIV vaccines | |||||
| DC-based vaccines | AGS-004(encoding the autologous HIV antigens Gag, Nef, Rev., and Vpr) | – | HIV Infection | NCT00672191 | Phase II Completed |
| HIV-1 ApB | – | HIV Infection | NCT00510497 | Phase I/II Completed | |
| mRNA vaccines | iHIVARNA (consisting of HIV immunogen sequence and a mixture of activation molecules (CD40L, CD70 and caTLR4)) | – | HIV-infection | NCT02413645 | Phase I Completed |
| – | HIV-infection | NCT02888756 | Phase IIa Terminated | ||
| Viral vector vaccines | MVA.HIVconsv(encoding 14 highly conserved regions of the viral proteome) | – | HIV-I infection | NCT01024842 | Phase I Terminated |
| HIVAX | – | HIV-infection | NCT01428596 | Phase I | |
| Therapeutic HCV vaccines | |||||
| Yeast vector vaccine | GI-5005(expressing an NS3-core fusion protein) | – | HCV infection | NCT00124215 | Phase I Completed |
| – | HCV infection | NCT00606086 | Phase II Completed | ||
| Viral vector vaccines | AdCh3NSmut/ Ad6NSmut (encoding HCV proteins) | – | HCV infection | NCT01094873 | Phase I |
| TG4040(expressing NS3/4/5B proteins) | – | HCV infection | NCT01055821 | Phase I Completed | |
Source: The clinical trials were from ClinicalTrials.gov
Therapeutic vaccines against HPV
Human papillomavirus (HPV) is recognized as the main cause of cervical cancer, the 4th most common cancer in women, accounting for 99.7% of cervical cancer cases and a subset of other diseases, such as vulvar, vaginal, penile, and anal cancers and head and neck cancers [44]. Persistent infection with HPVs precedes the development of high-grade squamous intraepithelial lesions, which can progress to malignant cancers [45]. It is estimated that HPVs cause 275,000 deaths from cervical cancer and result in 530,000 new cases every year, becoming a serious public health problem worldwide and causing a loss of life [46]. To decrease the prevalence of HPV-associated diseases, the development of prophylactic vaccines has been emphasized, and these vaccines have shown promising efficacy. Three commercially available HPV prophylactic vaccines, Cervarix, Gardasil and Gardasil 9, target several types of HPVs by producing neutralizing antibodies and preventing HPV infections and precancerous cervical lesions with almost 100% efficacy [47]. However, the incidence of HPV-related tumors remains high for several different reasons, including the low vaccination rate, lack of targeting all types of HPV, age-based recommended immunization practices and high costs of immunization [48]. More importantly, infected individuals cannot benefit from prophylactic vaccines [49]. Therefore, therapeutic vaccines against more types of oncogenic HPV are urgently needed for the HPV-infected population.
Therapeutic HPV vaccines mainly aim to expand or induce strong specific Th1-type and CTL responses to kill infected cells. An ideal antigen for a therapeutic vaccine against established HPV infections and HPV-associated lesions should have the qualities of being essential for the onset and maintenance of malignancy, constitutive expression at high levels and an absence of mutation. The HPV E6 and E7 oncoproteins represent near-ideal targets for the development of most HPV therapeutic vaccines, such as bacteria-, virus-, peptide-, DNA- and DC-based vaccines [50].
Bacterial vectors, including Listeria monocytogenes, Lactobacillus casei, Lactobacillus lactis and Salmonella vectors, have natural adjuvant properties and have the ability to modulate antigen presentation through MHC I and MHC II pathways, activating CD8+ T cells and CD4+ T cells [10]. ADXS11–001 is a live attenuated L. monocytogenes (Lm) which was modified to express HPV16 E7 joined to the protein listeriolysin-O (LLO) [51]. LLO contributes to the replication of Lm in APCs which allows antigens secreted by Lm and presented by APCs [52]. ADXS11–001 showed an acceptable safety profile and efficacy in activating HPV16 E7-specific T-cell responses in patients with invasive carcinoma of the cervix in a phase I clinical trial [51]. A phase II study showed increased survival in patients with advanced cervical cancer, with a 12-month combined survival rate of 34.9%, exceeding the historical overall survival [53]. Based on the encouraging data, a phase III trial is being conducted for advanced cervical cancer (NCT02653604). GLBL101c composited with an L. casei bacterial vector secreting full-length HPV16 E7 protein elicited E7-specific mucosal immunity and resulted in pathological downgrades in the cervix of CIN3 patients [54]. Another therapeutic vaccine based on L. casei, named NZ8123-HPV16-optiE6, was safe, increased the production of antibody and activated E6-specific IFN-γ-secreting CD8+ CTL responses in a phase I trial [55].
Virus vectors, including adenovirus, adeno-associated virus, alphavirus, and modified vaccinia Ankara (MVA) viral vectors, can be used to deliver antigens to induce an immune response [56]. In clinical trials, an MVA vector was used to express HPV16/18 E6 and E7 proteins, forming a TA-HPV vaccine. The TA-HPV vaccine was safe and immunogenic and generated HPV-specific CTL responses in phase I/II and II trials [57, 58]. More recently, Tipapkinogen Sovacivec (TS), an MVA-based vaccine that encodes human cytokine IL-2, HPV16 E6 and E7 proteins, significantly cleared viral DNA and achieved greater complete resolution rates of histological CIN3 disease [59]. Human adenovirus, another commonly used viral vector, was used to express HPV E6 and E7 to prepare the vaccine [60]. To avoid preexisting immunity against human adenovirus, a chimpanzee adenovirus vector was alternatively designed to deliver HPV antigens, and the efficacy alone or in combination with anti-PDL1/TGF-beta Trap is being tested in a clinical trial (NCT04432597). In addition to DNA viruses, RNA viral vectors have also been designed as antigen transporters. Vvax001, a Semliki Forest virus expressing E6 and E7, efficiently induces long-term CTL activity and a potent therapeutic antitumor effect in mice [61]. The phase I trial of Vvax001 was completed, but the results were not reported (NCT03141463). HB-201, encoding HPV E6 and E7, which is based on the arenavirus lymphocytic choriomeningitis virus, showed excellent therapeutic efficacy in a preclinical model [62]. Currently, a phase I/II study of Vvax001 in HPV16+ recurrent/metastatic head and neck squamous cell carcinoma is launching (NCT04180215).
Peptide-based vaccines are MHC-specific and easy to manufacture. However, adjuvants such as cytokines and TLR ligands are often mixed with peptide-based vaccines to enhance CD8+ T-cell responses due to the low antigenicity of peptides [63]. An early peptide vaccine consisting of HPV16 E7 12–20/86–93 epitopes and incomplete Freund’s adjuvant demonstrated decent biological and clinical effects [64]. ISA101 is an HPV16 vaccine consisting of nine HPV16 E6 and four HPV16 E7 synthetic peptides with the adjuvant Montanide ISA51 [65]. In the phase II study in women with high-grade vulvar intraepithelial neoplasia, clinical responses were observed in 15 of 19 patients (79%), and a complete response was observed in 9 of 19 patients (47%). Moreover, all patients had the capacity to develop vaccine-induced T-cell responses. The stronger CD4+ and CD8+ T-cell IFN-γ responses in patients contributed to the complete response [65]. Multiple studies have also demonstrated the excellent clinical effect of ISA101 [66–70]. To further augment the efficacy of ISA101, the combination of ISA101 and nivolumab showed promise in long-term follow-up (NCT02426892) [71]. The phase II trial of nivolumab and ISA101 vaccination showed a median overall survival of 15.2 months and 2-year overall survival rate of 33% among patients with incurable HPV16 + cancer. Moreover, the infiltration of cytotoxic T cells in tumors and the activation of the interferon signaling pathway strongly benefited the clinical response [71]. Additionally, a randomized phase II trial of ISA101 and cemiplimab (PD-1 blocking antibody) is ongoing (NCT03669718).
Compared to peptide-based vaccines, protein-based vaccines contain more epitopes and can induce memory CD8+ T-cell responses but show a preference for eliciting humoral immunity. Protein antigens are often designed to fuse with other proteins to expand antigen-specific immunity. GTL001 is formed by fusing the E7 proteins of HPV16 and HPV18 E7 proteins to catalytically inactive Bordetella pertussis CyaA, which specifically delivers E7 to CD11b+ antigen-presenting cells [72]. In a phase I trial (EudraCT No. 2010–018629-21), GTL001 in conjunction with imiquimod significantly reduced the viral load of HPV16/18 in patients infected by HPV16 or HPV18 [72]. A similar strategy was used to design SCN-00101, which fused HPV16 E7 protein to M. bovis BCG heat shock protein [73]. Of the 58 patients treated with SCN-00101 in the phase II trial (NCT00075569), 13 (22.5%) had a complete pathological response, 32 (55%) had a partial response and 11 (19%) had stable disease [73]. Recently, Da Silva et al. reported another fused protein vaccine, TVGV-1, which consisted of HPV16 E7 protein covalently linked to a bacterial exotoxin and an endoplasmic reticulum retention signal [74]. TVGV-1 significantly activated E7-specific CD8+ T-cell immunity in a mouse tumor model [74]. Based on the promising effect in vitro, the safety and efficacy of the TVGV-1 vaccine were assessed in a phase IIa clinical trial (NCT02576561).
DNA vaccinations consist of direct injection of plasmid DNA encoding antigens into host tissue, achieving sustained antigen expression. DNA vaccines do not induce neutralizing antibodies against the vector, allowing repeated vaccination [75]. Moreover, DNA vaccines are easily manufactured and can stimulate humoral and cell-mediated immunological responses, therefore serving as desirable therapeutic vaccine strategies. However, the poor immunogenicity and the risk of DNA integration into chromosomes remain the main drawbacks of DNA vaccines [76]. The immunogenicity of VGX-3100, a synthetic DNA vaccine in which the plasmid encodes the E6 and E7 proteins of HPV16/18, was assessed in women with CIN2/3 (NCT00685412) in a phase I clinical trial [77]. Overall, 78% of patients generated increased Th1-biased cellular immune responses. Based on the promising results, the efficacy, safety, and immunogenicity of VGX-3100 were further assessed in women with HPV16/18 and CIN2/3 (NCT01304524) in a phase II clinical trial [78]. In the per-protocol analysis, a total of 53/107 (49.5%) patients treated with VGX-3100 experienced histopathological regression, compared to 11/36 (30.6%) patients treated with placebo. Then, in the intention-to-treat analysis, increased histopathological regression among the VGX-3100 group (48.2%) compared with the placebo group (30.0%) was observed. A randomized, double-blind, placebo-controlled phase III study to determine the efficacy, safety, and tolerability of VGX-3100 in adult women with CIN2/3 associated with HPV16/18 was completed in 2021 (NCT03185013). However, the results have not been reported. Similarly, DNA vaccines have been rationally designed to link antigens with other proteins to enhance antigen immunogenicity. GX188E was engineered to coexpress HPV E6/E7 by fusing to Fms-like tyrosine kinase-3 ligand (Flt3L), which aimed to promote antigen presentation and trafficking [79]. In a clinical phase I trial (NCT01634503), nine patients with CIN3 received GX-188E by electroporation. A significant E6/E7-specific Th1-type cellular immune response was observed in all nine patients. Moreover, 7/9 (78%) patients showed a polyfunctional HPV16-specific CD8 T-cell response, which contributed to HPV clearance and a histological CR [79]. The efficacy of GX-188E for inducing the regression of CIN3 was further determined in a randomized, open-label, phase II trial (NCT02139267) [80]. After receiving the GX-188E vaccine, histopathological regression was observed in 52% (33/64) of patients in the per-protocol analysis and 67% (35/52) of patients in the extension analysis. Importantly, 73% of the patients with histological regression in the per-protocol analysis and 77% in the extension analysis showed HPV clearance, which was associated with enhanced IFN-γ production [80]. Other DNA vaccines, such as pNGVL4a-CRT-E7 [81] and ZYC101 [82], have also exhibited good efficacy and safety in phase I clinical trials.
Therapeutic vaccines for HBV
Chronic hepatitis B virus (HBV) infection has been considered a worldwide public health issue. HBV is a partially double-stranded DNA virus belonging to the Hepadnaviridae family that exclusively infects hepatocytes [83]. HBV prophylactic vaccination is safe and has been accepted worldwide as an effective method to avoid HBV infection. It was demonstrated that approximately 90% of followed individuals who received a usual three-dose HBV prophylactic vaccine remained protected for ≥30 years [84]. However, some people are still infected by HBV for several reasons, such as unresponsiveness to HBV vaccination, HBV mutants, and no opportunity for vaccination [85]. Once a chronic HBV infection is established, most people will remain infected for life, which largely increases the risk of liver-related death.
For the majority of affected individuals, the first-line therapies for the treatment of chronic HBV (CHB) infection are third-generation nucleot(s) ide analogs (NUCs) and interferon-alpha (IFN), which effectively improve the quality and duration of life by preventing progression to underlying liver disease [86]. Short-term treatment with IFN leads to a sustained virological response and subsequent HBsAg loss in only approximately 30% of patients and is frequently associated with poor tolerability, side effects and low effectiveness [87, 88]. Conversely, NUC therapy leads to the suppression of HBV replication in almost all treated patients; however, long-term administration is required to avoid virus reactivation after stopping treatment [89]. Therefore, there is an urgent need to develop new drugs to shorten the therapy administration time and achieve an HBV cure.
The main reason for HBV persistence after treatment with multiple drugs is dysfunctional HBV-specific T and B cells, with characteristics of low frequency, functional defects and exhaustion [90–92]. Therapeutic vaccination aims to reconstitute the systemic immune system and elicit or augment existing HBV-specific B and T-cell responses, representing a rational strategy to overcome immune tolerance and cure chronically HBV-infected patients. Therapeutic vaccination represents an attractive option for CHB infection therapy. At present, several different therapeutic vaccines have been assessed in clinical trials, including protein- or peptide-based, DNA- and viral vector-based vaccines.
Adenovirus induces several strong innate immune signaling pathways and subsequently effectively activates robust adaptive humoral and cellular immune responses; therefore, it is currently being applied in cancer vaccines [93]. TG1050, consisting of a nonreplicative adenoviral vector encoding HBV core, polymerase and envelope domains, is a promising vaccine candidate [94]. TG1050 effectively activated the cytolytic activity of T cells and exerted an antiviral effect in HBV-naïve and HBV-persistent mouse models [94].
The safety, immunogenicity and early efficacy of TG1050 in CHB patients were assessed in a phase I clinical trial (NCT02428400) [95]. IFN-γ-producing T cells and minor decreases in HBsAg were observed after TG1050 vaccination. Interestingly, the HBV-specific cellular immune response was enhanced by the combination of an NUC and TG1050 [95]. Additionally, some new strategies have been utilized to clear HBV with adenovirus vectors. For example, adenovirus was designed to deliver a CRISPR/Cas9 system and a single guide RNA (gRNA) system to degrade the HBV genome [96, 97].
Another effective virus vector was designed using a modified vaccinia Ankara (MVA) viral vector for the delivery of antigens [98]. To improve therapeutic vaccine efficacy, a novel HBV vaccine that consisted of chimpanzee adenovirus and MVA viral vectors encoding multiple HBV antigens administered through a prime-boost strategy was proposed [99]. Adenovirus prime followed by MVA boost vaccination coordinately enhanced polyfunctional HBV-specific CD8+ and CD4+ T-cell responses in mice [99].
Among the candidate vaccines, protein-based vaccines have attracted some attention. Theravax (DV-601) comprises recombinant HBV surface antigen (HBsAg) and HBV core antigen (HcAg), with a saponin-based ISCOMATRIX adjuvant. Theravax vaccination led to the development of an HBV-specific lymphoproliferative response, an HBc-specific interferon-gamma T-cell response and a reduction in HBV DNA [100]. Another protein-based vaccine, GS-4774, is a heat-inactivated and engineered yeast-based vaccine that recombinantly expresses a fusion protein consisting of HBsAg, HBcAg and HBx [101]. The yeast component exerts a strong adjuvant effect by enhancing DC presentation and eliciting a significant T-cell response [102]. GS-4774 was safe and well tolerated in healthy participants (NCT01779505). Then, the efficacy of GS-4774 as a therapeutic vaccine was tested in the phase II trial NCT01943799 in 178 virally suppressed patients with chronic hepatitis B infection. In the study, GS-4774 did not provide significant reductions in serum HBsAg, and only three patients had HBsAg declines ≥0.5 log10 IU/ml after receiving the highest vaccine dose. Although low HBV-specific T-cell responses were detected in all patients, GS-4774 did not result in a clinical benefit. To obtain a therapeutic benefit, GS-4774 was combined with tenofovir therapy in patients with chronic hepatitis (NCT02174276). Although HBsAg reduction or loss was not observed in participants, GS-4774 induced a strong CD8+ T-cell immune stimulatory effect, which paved the way for combination with other therapies, such as silencing RNA compounds and ICIs and modulating T-cell metabolism [103].
Among the new peptide-based vaccine candidates evaluated in clinical trials, HeberNasvac contained both HBsAg and HBcAg [104]. HeberNasvac showed safety, good tolerance and immunogenicity in a phase I study in healthy adults [105]. The safety and the ability to control the virus were further confirmed in subsequent clinical trials [105]. In a phase III trial of HeberNasvac versus Peg-IFN, HeberNasvac induced a superior reduction in HBV DNA load under the limit of detection (NCT01374308) [106]. Based on the clinical results, HeberNasvac was approved by the Cuban Regulatory Authority at the end of 2015. The phase IIB-III efficacy study was assessed as an adjunct therapy to NUCs to control HBV replication (NCT02249988). The HepTcell vaccine is formed by nine synthetic CD4+ and CD8+ T-cell peptides derived from the most conserved domains of HBV with an IC31 adjuvant (NCT02496897) [24].
DNA-based vaccines have also been studied and evaluated in clinical trials for CHB therapeutic vaccination. INO-1800 is a synthetic DNA vaccine that encodes HBsAg and the consensus sequence of HBcAg, and it induced a strong antigen-specific T-cell and B-cell response in immunized mice [107]. The addition of INO-9112, a plasmid expressing IL-12, to INO-1800 activated cytotoxic T lymphocytes, showing the efficacy of these synthetic plasmids as components of therapeutic HBV vaccines [108]. Another candidate vaccine, HB-110, is composed of plasmids encoding HBs, PreS1, HBc, Hbpol and IL-12 [109]. Higher T-cell and antibody responses were observed in mice [110].
Therapeutic vaccines for human immunodeficiency virus (HIV)
The first report of AIDS caused by HIV was published in 1981, and globally, there were approximately 38 million HIV infections and a total of 690,000 AIDS-related deaths in 2020 [43]. Most infected individuals need life-long antiretroviral treatment, which highlights the urgent need for a prophylactic HIV vaccine to prevent infection. Over the past 40 years, great efforts have been made in the development of HIV vaccines, but no effective vaccine has been approved. The main hurdles in developing an effective HIV vaccine include the high variability of HIV, genetic diversity and a lack of understanding about immune protection [111]. Among the many HIV vaccine candidates, RV-144 was the only vaccine that was tested in a clinical trial and achieved 31.2% efficacy in Thailand [112]. The failure of HIV prophylactic vaccine studies motivated the design idea of creating therapeutic vaccines to combat HIV. DNA, peptide, protein, viral, mRNA and DC vaccines have been tested in clinical trials. Protein-, peptide-, and DNA-based vaccines aim to induce cellular immunity and humoral immunity to viral proteins, but these vaccines have yet to provide effective treatment. Consequently, we mainly discuss DC-, mRNA- and viral-based vaccines.
DC-based vaccines are promising vaccine candidates that play a vital role in inducing an immune response against antigens [113]. Generally, autologous DCs are isolated from patients, loaded with antigens in vitro and returned to patients [114].AGS-004 is an autologous DC vaccine that was loaded in vitro with RNA encoding the autologous HIV antigens Gag, Nef, Rev., and Vpr [115]. The activity of AGS-004 was tested in a phase IIB study in which 54 HIV-1-infected patients were enrolled (NCT00672191) [116]. AGS-004 elicited an HIV-specific effector/memory CD8 T-cell response but showed no antiviral effect. Another study enrolled six male individuals to evaluate the immunogenicity of AGS-004 [115]. Multifunctional HIV-1-specific effector/memory CTLs were induced in all participants, which was strongly related to a longer time to viral rebound. The HIV-1 ApB DC vaccine also contained autologous DCs loaded with autologous HIV-1-infected apoptotic cells. The vaccine was safe and well tolerated in a phase I/II clinical trial but did not prevent viral rebound during treatment interruption (NCT00510497) [114].
The administration of naked mRNA represents a promising alternative to immunogens. Leal et al. performed a first-in-human phase I clinical trial with mRNA-based vaccines (NCT02413645) [117]. The naked mRNA in the vaccine consisted of a novel HIV immunogen sequence and a mixture of activation molecules (CD40L, CD70 and caTLR4). The safety and efficacy of the three intranodal doses of mRNA vaccine were evaluated in 21 patients with chronic HIV-1 infection. The results demonstrated that the mRNA vaccine was safe and activated HIV-specific T-cell responses. However, interim analysis did not show sufficient immunogenicity of IMP compared to placebo in a phase IIa study of the mRNA vaccine (NCT02888756) [118].
Recombinant viral vectors readily achieved intracellular antigen expression to induce a CTL response [119]. The MVA.HIVconsv vaccine consisted of a modified vaccinia Ankara (MVA) viral vector, which encodes a chimeric protein comprising 14 highly conserved regions of the viral proteome [120]. The immunogenicity and activity of the MVA.HIVconsv vaccine was tested in clinical trials (NCT01024842) [120]. Although the MVA.HIVconsv vaccine was safe in HIV-positive patients, and it displayed modest immunogenicity and weak antiviral activity. HIVAX is a replication-defective HIV-1 lentiviral vector vaccine that contains multiple mutations in its viral genome [121]. HIVAX enhanced the functionality of T cells and reduced the median viral load in HIV-1-infected participants (NCT01428596).
Therapeutic vaccines for HCV
Hepatitis C virus (HCV) is a major causative factor of chronic liver disease worldwide, and approximately 2 million people are newly infected every year [122]. Upon infection, HCV can initiate a strong, broad, and persistent antigen-specific T-cell response, leading to a state of chronic hepatic inflammation, even liver cirrhosis and primary liver cancer (hepatocellular carcinoma, HCC) [123]. The approval of novel HCV-specific direct-acting antiviral (DAA) drugs improved the treatment of HCV but was associated with a number of side effects [124]. Therefore, it is important to develop therapeutic vaccines to treat HCV to lower the chronicity rate and the disease burden. Studies have found that CTLs and CD4+ T cells recognize the majority of the viral epitopes in patients with HCV infection via the NS3 region and NS5A/B [125].
Peptide/protein vaccines can be generated relatively easily and are being developed for infectious diseases. Peptide vaccines are HLA-specific and present vaccine peptides to T-cell receptors via HLA molecules. The efficacy of peptide vaccines targeting E1, E2, NS3 and NS5A was evaluated in a phase I trial. Fifty percent of participants produced peptide-specific IFN-γ by CTLs, but only 25% of HCV RNA was reduced [126]. IC41, a peptide vaccine composed of five synthetic peptides derived from the core, NS3, and NS4 proteins of HCV genotypes 1 and 2, with a poly-L-arginine adjuvant, induced significant immunological responses in 128 HLA-A2+ healthy volunteers in a phase I trial. However, the T-cell responses were too weak to induce a decrease in HCV RNA in the serum of most of the volunteers [127]. The suboptimal immune response was most likely due to the low immunogenicity of synthetic peptides. In a randomized trial, intensified dosing and intradermal (i.d.) administration of IC41 could induce more robust peptide-specific immune responses than in a previous study [128]. Another peptide vaccine, consisting of HCV core region (C35–44) peptides with emulsified incomplete Freund’s adjuvant (ISA51), was shown to be safe and well tolerated in a phase I trial [129]. Furthermore, Pevion Biotech developed a virosome-based vaccine containing NS3 peptides, and a phase I study of this vaccine was recently completed, but data have not been released (NCT00445419).
GI-5005, a yeast vector vaccine expressing an NS3-core fusion protein for HCV [130], induced effective NS3- and core-specific cellular immune responses in both C57BL/6 and BALB/c mice [131]. A phase I clinical trial showed that GI-5005 was well tolerated and was able to induce a significant HCV-specific immune response in patients (NCT00124215). A phase II trial aiming to investigate the treatment effect of combining GI-5005 and standard-of-care treatment has been completed (NCT00606086).
Plasmid DNA encoding antigenic HCV protein(s) or epitope(s) can induce both humoral and cellular immune responses in vivo [132]. DNA vaccines include the nucleotides encoding structural proteins or nonstructural proteins, such as NS3, NS4, NS5, core, and the envelope proteins E1/E2 [133]. CIGB-230, containing a mixture of core/E1/E2-expressing plasmids, was the first therapeutic DNA vaccine for HCV evaluated in clinical trials [134, 135]. The second HCV DNA-based vaccine, now in phase II clinical trials, developed for HCV infection was ChronVac-C, which includes the most conserved regions (NS3 and NS4a) [136]. Initial results suggested the safety and immunogenicity of the vaccine, and it is now in phase II clinical trials for HCV infection (NCT01335711). Ratnoglik et al. constructed a series of DNA vaccines that express NS3 with mutations in the catalytic triad of the serine protease and the NTPase/RNA helicase domain [137], which overcame the effects of NS3 on normal cell function [138].
The use of viral vectors for the delivery of HCV RNA is an appealing vaccine choice. Replication-defective adenovirus (Ads) and the nonreplicative modified vaccinia Ankara (MVA) virus are commonly used vectors for HCV viral-based vaccines. An Ad6-based vaccine encoding NS3, NS4A, NS4B, NS5A, and inactivated NS5B can induce specific T-cell responses against the NS antigens of HCV in mice, rhesus macaques, and even chimpanzees [139, 140]. The vaccine was found to be safe and immunologically potent in a phase I clinical trial (NCT01094873). An MVA-based therapeutic vaccine (TG4040) that expresses NS3/4/5B proteins can induce potent, long-lasting and in vivo cross-reactive T-cell responses [141]. TG4040 in combination with standard PEG-IFN and ribavirin therapy is being evaluated in a phase II clinical trial (NCT01055821).
Recently, the results of a clinical trial of DC treatment among patients with chronic HCV infection were reported. DCs loaded and activated ex vivo with HCV-specific HLA-A2 restricted T-cell epitope were injected intradermally into patients. All six patients who received the vaccine exhibited weak HCV-specific CD8+ T-cell responses. The results showed the safety but weak efficacy of the DC vaccine. To improve the efficacy of treatment, alternative dosing regimens or vaccination routes should be considered [142].
Therapeutic effect of COVID-19 vaccines against SARS-CoV-2 infection
In addition to preventing the spread of SARS-CoV-2, multiple vaccines have been developed among countries worldwide, such as the CoronaVac, BNT162b2, Ad26.COV2.S, ChAdOx1 nCoV-19, mRNA-1273 and NVX-CoV2373 vaccines, against COVID-19 [143]. These vaccines activate the immune system and generate significantly high neutralizing antibodies against the virus, being highly efficacious in preventing SARS-CoV-2 infection [144]. Although millions of people have been infected and breakthrough infections (infections in fully vaccinated people [145]) do occur, COVID-19 vaccines are able to induce immunity to reduce persistent infection and severe disease, as well as hospitalizations and deaths [146].
Bradley et al. evaluated the efficacy of therapeutic mRNA vaccination in the context of persistent SARS-CoV-2 infection [147]. In this study, humoral and cellular responses were not detected in a 37-year-old Caucasian male with Wiskott-Aldrich syndrome after 120 days of PCR-confirmed SARS-CoV-2 infection. The immunodeficient man received two doses of the BNT162b2 mRNA COVID-19 vaccine one month apart. SARS-CoV-2-specific IFN-γ+ T cells and antibodies were increased at 14 days following the first vaccine dose. Interestingly, SARS-CoV-2 clearance was detected at 72 days following the first therapeutic vaccination. The researchers did not exclude viral clearance in an independent manner [147].
A study was conducted to evaluate the effectiveness of four COVID-19 vaccines (CoronaVac, ChAdOx1 nCoV-19, Ad26.COV2.S and BNT162b2) among individuals with previous SARS-CoV-2 infection in Brazil [148]. The vaccine effectiveness against symptomatic SARS-CoV-2 infection among the matched 22,566 individuals with previous infection was 39.4% for CoronaVac, 56.0% for ChAdOx1 nCoV-19, and 44.0% for Ad26.COV2.S, and 64.8% for BNT162b2. Furthermore, the effectiveness against hospitalization or death was 81.3% for CoronaVac, 89.9% for ChAdOx1 nCoV-19, 57.7% for Ad26.COV2.S, and 89.7% for BNT162b2 [148]. Similarly, individuals with previous infection plus vaccination had a lower risk of SARS-CoV-2 reinfection and COVID-19 hospitalization than previously infected people [146]. In another study of 1260 dialysis patients, patients who were not vaccinated had a mortality rate of 24.2%, compared with a mortality rate of 8.6% in patients who experienced breakthrough infections [149]. These data indicate that therapeutic COVID-19 vaccines may become an effective option in the treatment of SARS-CoV-2 infection and the associated disease.
Therapeutic vaccines against noncommunicable diseases
Cancers were defined as a chronic disease due to their turning into controllable conditions [150]. Therapeutic cancer vaccines have been envisioned as effective tool of cancer immunotherapy [151]. Despite the immense efforts, however, therapeutic cancer vaccines have shown modest benefit in mediating anti-tumor activity in humans. A deeper understanding of the tumor-associated antigens, neoantigens and checkpoint inhibitors has facilitated the improvement of therapeutic cancer vaccines. Meanwhile, vaccines have been developed as therapies against other diseases such as hypertension, dyslipidemia, Alzheimer’s disease, and amyotrophic lateral sclerosis. Based on the promising results in preclinical and clinical trials, vaccines may be an alternative strategy for lifestyle diseases. Here, we describe the representative knowledge and research progress of therapeutic vaccines for cancer, hypertension, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), diabetes, and dyslipidemia (Table 2).
Table 2.
Selected preclinical or clinical trials of therapeutic vaccines against chronic non-communicable diseases
| Therapeutic vaccines for cancers | |||||
|---|---|---|---|---|---|
| Vaccine type | Vaccine Formulation | Combination agents | Condition | Identifier/ reference | Phase/status |
| Personalized peptide vaccine | NeoVax (neoantigen + Poly-ICLC) | Ipilimumab | Kidney Cancer | NCT02950766 | Phase I Recruiting |
| NEO-PV-01 (neoantigen) + Poly-ICLC | Nivolumab | Melanoma, lung, or bladder cancer | NCT02897765 | Phase Ib Completed | |
| GRT-C903 (shared neoantigen prime)/GRT-R904(shared neoantigen boost) | Nivolumab/ipilimumab | NSCLC, colorectal cancer, pancreatic cancer, shared neoantigen-positive solid tumors | NCT03953235 | Phase I/II Recruiting | |
| GRT-C901(patient-specific neoantigen prime)/GRT-R902(patient-specific neoantigen boost) | Nivolumab/ ipilimumab | NSCLC, colorectal cancer, gastroesophageal, adenocarcinoma, urothelial carcinoma, | NCT03639714 | Phase I/II Active, not recruiting | |
| Multiple candidate tumor-derived neoantigens | Pembrolizumab | Advanced Cancer | NCT03568058 | Phase Ib Active, not recruiting | |
| GEN-009 (neoantigen) + Poly-ICLC | Nivolumab/ Pembrolizumab | Cutaneous melanoma,NSCLC, SCC ofhead and neck, urothelial carcinoma, RCC | NCT03633110 | Phase I/IIa Completed | |
| PGV001 (patient specific long peptides+ helper peptides) + Poly-ICLC | Atezolizumab | Urothelial/bladder cancer | NCT03359239 | Phase I Completed | |
| NeoVax(peptides) plus Montanide | Nivolumab/Ipilimumab | Melanoma | NCT03929029 | Phase Ib Recruiting | |
| personalized peptide | Pembrolizumab +imiquimod | Pancreatic and colorectal cancer (advanced) | NCT02600949 | Phase I Recruiting | |
| PGV001(patient specific long peptides+ helper peptides) + Poly-ICLC | Lenalidomide | Solid Tumors | NCT02721043 | Phase I Completed | |
| CDX-1401(DEC-205/NY-ESO-1 Fusion Protein) + Poly-ICLC | Recombinant Flt3 Ligand | Stage IIB-IV melanoma | NCT02129075 | Phase II Completed | |
| mRNA-based neoantigen vaccine | RO7198457(mRNA-based) | Atezolizumab | Locally advanced or metastatic tumor | NCT03289962 | Phase Ia/Ib Active, not recruiting |
| mRNA-4157(lipid encapsulated RNA) | Pembrolizumab | Solid Tumors | NCT03313778 | Phase I Recruiting | |
| mRNA-4157(lipid encapsulated RNA) | Pembrolizumab | Melanoma | NCT03897881 | Phase II Active, not recruiting | |
| RO7198457(mRNA-based) | Atezolizumab+ mFOLFIRINOX | Pancreatic Cancer | NCT04161755 | Phase I Active, not recruiting | |
| DNA-based neoantigen vaccine | Neoantigen DNA (TDS-IM system) | Durvalumab | TNBC | NCT03199040 | Phase I Active, not recruiting |
| GNOS PV02 (Personalized Neoantigen DNA Vaccine) | Pembrolizumab+INO9012(plasmid encoded IL-12) | HCC | NCT04251117 |
Phase I/IIa Recruiting |
|
| Therapeutic vaccines for hypertension | |||||
| Protein vaccine | PMD3117(Ang I analog with keyhole limpet haemocyanin(KLH)) | aluminum hydroxide | [152] | Phase II Completed | |
| Peptide vaccine | CYT006-AngQb (angiotensin II- conjugated to the VLP Qβ) | – | hypertension | [153] | Phase I Completed |
| – | Essential Hypertension | NCT00500786 | Phase I/II Completed | ||
| ATR12181 (peptide from rat AT1a receptor) | Complete Freund’s adjuvant | Spontaneously hypertension (in rat) | [154] | Preclinic trail | |
| ATRQβ-001(a peptide derived from human Ang II receptor type 1 conjugated with VLP Qβ) | Aluminum hydroxide | Hypertensive Animals | [155] | Preclinic trail | |
| DNA Vaccine | AGMG0201 (expressing angiotensin II) | – | Mild to moderate essential hypertension | ACTRN12617001192370 [156] | Phase I/IIa Completed |
| Therapeutic vaccines for Alzheimer’s disease | |||||
| Peptide vaccine | ABvac40(a conjugate of Aβx-40 with KLH) | Aluminum hydroxide | Mild to moderate Alzheimer’s disease | NCT03113812 | Phase I Completed |
| Aluminum hydroxide | Mild cognitive impairment Alzheimer Disease | NCT03461276 |
Phase II Active, not recruiting |
||
| AADvac1 (a synthetic peptide from a tau protein sequence coupled to KLH) | Aluminum hydroxide | Alzheimer’s Disease | NCT02031198 | Phase I Completed | |
| Aluminum hydroxide | Alzheimer’s Disease | NCT02579252 | Phase II Completed | ||
| UB311 | CpG ODN and alum | Alzheimer’s Disease | NCT00965588 | Phase I Completed | |
| CpG ODN and alum | Mild Alzheimer’s Disease | NCT02551809 | Phase II Completed | ||
| Therapeutic vaccines for other diseases | |||||
| Peptide vaccines | tgG-DSE2lim and tgG-DSE5b | Emulsigen-D | Amyotrophic lateral sclerosis (ALS) in mice | [157] | Preclinical trial |
| T-cell epitopes within DPP4 conjugated to KLH | Complete/incomplete Freund’s adjuvant | Type 2 diabetes mellitus in mice | [158] | Preclinical trial | |
| PCSK9Qβ-003(PCSK9 peptide conjugated with VLP Qβ) | Aluminum hydroxide | Dyslipidemia in mice | [159] | Preclinical trial | |
Source: The clinical trials were from ClinicalTrials.gov or Australian New Zealand Clinical Trials Registry (ANZCTR)
Neoantigen-based therapeutic cancer vaccines
Successful antitumor immunity requires multiple aspects of tumor immunity, including tumor antigen presentation, T-cell priming and activation, the recognition of tumor cells by T cells, and subsequent effector mechanisms to eliminate tumor cells [160]. Within the tumor microenvironment (TME), pattern recognition receptors of natural killer (NK) cells, neutrophils or macrophages, such as Toll-like receptors (TLRs), recognize pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), activate transcription factors, stimulate cytokine and chemokine production and recruit and activate lymphocytes, finally eliciting innate immunity [161]. Antigen-presenting cells (APCs), such as dendritic cells (DCs), are essential for initiating antitumor adaptive immunity, which captures and recognizes the immunogenic tumor antigens released from dead tumor cells by chemotherapy or immunogenic cell death. This promotes the expression of T-cell costimulatory signals and cytokines by APCs; APC maturation; and antigen uptake, processing and presentation on MHC molecules [162]. These APCs, especially DCs, migrate toward secondary lymphoid organs and interact with naïve CD4+ T cells and CD8+ T cells, which ultimately results in the priming and activation of T cells [163]. Activated T cells travel back to the TME to induce tumor killing or prevent tumor cell proliferation [16].
Therapeutic cancer vaccines commonly consist of adjuvants and tumor antigens selected with the aim of triggering an innate and adaptive antitumor response against tumor antigens to suppress tumor growth and induce tumor regression. In the early stages of tumor vaccine development, successful therapeutic vaccination against tumors was simply considered to activate tumor-specific T cells and then control tumor growth. Sipuleucel-T, a unique therapeutic cancer vaccine approved by the FDA in 2010, prolonged the overall survival of only 31.7% of male patients with metastatic castration-resistant prostate cancer (NCT00065442) [164]. Moreover, other therapeutic cancer vaccines, such as MAGE-A3 immunotherapeutic (NCT00480025) [165], Belagenpumatucel-L (NCT00676507) [166], tecemotide (L-BLP25) (NCT00409188) [167], and IMA901 (NCT01265901) [168], did not increase overall survival. The mechanisms of immune suppression, resistance and escape mediated by the highly complex heterogeneity of the TME were ignored when these cancer vaccines were designed, which consequently resulted in an inadequate number of T cells, insufficient durability of the T-cell response and failure of T cells to infiltrate the tumor core [169]. Immune checkpoint inhibitors (ICIs), such as anti-CTLA-4, anti-PD1 and anti-PD-L1 antibodies, interfere with the inhibitory pathways of T-cell reactivity, overcoming tumor escape and activating T-cell effector function [170]. The rapidly increasing understanding of immune resistance and escape and the promising efficacy of ICI treatment have encouraged investigators to combine therapeutic cancer vaccines with ICIs. Again, therapeutic cancer vaccines are considered strategies to increase response rates and survival [16].
Effective therapeutic vaccination against tumors depends on the design and screening of a high-quality tumor antigen, efficient antigen uptake by DCs, the sustained activation of CD4+ T cells and CD8+ T cells, T-cell infiltration into the TME and the strong persistence of the immune response. The success of antigen-specific therapeutic vaccines depends heavily on the choice of antigens in cancer vaccine design. An ideal antigen should be specifically present on the surface of all tumor cells and should be highly immunogenic. For many years, shared tumor-associated antigens (TAAs) have served as the focus of most cancer vaccines. These TAAs are self-molecules abnormally expressed by tumor cells or the “non-self” antigens of oncogenic viruses. They include cancer testis antigens (e.g., MAGE-A1, MAGE-A3, and NY-ESO-1), which are restricted to only immune privileged germline cells and have no or low expression in normal adult somatic cells [171, 172]; differentiation antigens (e.g., tyrosinase, gp100, MART-1, PSA and PAP), which are normally not expressed in adult tissue [173]; overexpressed antigens (e.g., RAGE-1, hTERT, HER2, mesothelin, and MUC-1), which are aberrantly overexpressed in tumor cells compared to normal cells [174]; and oncoviral products (e.g., E6/E7 proteins from HPV) [175]. However, several hurdles are associated with therapeutic vaccination focused on TAAs, resulting in unsuccessful and ineffective antitumor immune responses. This may be due to the low affinity between TAA-specific T cells and antigens due to central and/or peripheral tolerance, the loss of tumor antigen expression, a suppressive TME and collateral damage caused by the expression of some TAAs in nonmalignant tissues [21]. More recently, neoantigens have attracted attention as a subset of nonautologous antigens with individual specificity that are generated by nonsynonymous somatic mutations, frameshifting, insertion/deletion variants, alternative splicing, gene fusions and endogenous retroviruses [176, 177].
Neoantigen vaccines
Neoantigens differ from the traditionally used TAAs. Neoantigens are expressed exclusively by malignant cells but lack expression in normal tissues, which prevents collateral damage to nonmalignant tissues. Moreover, neoantigens possess strong immunogenicity toward and high affinity for T cells that are not subject to central tolerance in the thymus [178]. Neoantigen vaccines activate CD4+ and CD8+ T cells, which directly recognize autologous melanoma cells and discriminate antigens between mutant and wild-type cells [179].
Neoantigens are highly individual-specific and are derived from mutations occurring in the tumor cell genome [176]. Hence, the identification of neoantigens is critical for neoantigen vaccine development. While the tumor mutational burden (TMB) is heavily correlated with neoantigen formation, it is possible that a high level of mutation in the somatic exonic region will lead to increased neoantigen production and then recognition by CD8+ T cells [180]. Jaffee and colleagues evaluated the relationship between the TMB and the objective response rate for anti-PD-1/anti-PD-L1 therapy [181], which revealed TMB as a potential biomarker for the response to ICIs [182]. Most tumors can be categorized as having a high TMB, correlating with a correspondingly high number of neoantigens, and are more likely to respond to ICIs. However, a high TMB does not universally indicate the response to ICIs. For instance, a number of melanoma or NSCLC patients with a high TMB do not respond to ICIs, but some patients with renal cell carcinoma (RCC) with a low TMB respond to ICIs [182]. Hence, it is reasonable to assume that mechanisms other than the TMB may contribute to the quality of neoantigens. These mechanisms are as follows [183]: 1) The probability of being a T-cell-recognized neoantigen. A high level of mutation results in the accumulation of neoantigens, but only a small fraction is recognized by T cells. An opportunity for the generation of strong neoantigens exists in patients with a low TMB. 2) The clonality of neoantigens. Neoantigen-specific T-cell responses against clonal mutations have been observed, but not subclonal mutations with the possible loss of neoantigen expression. 3) TCR affinity for neoantigens; 4) Patients’ HLA class I genotype. CHOWELL et al. found that the maximal heterozygosity at HLA-I loci (HLA-A, HLA-B and HLA-C) was strongly associated with extended survival after ICI immunotherapy [184]. Therefore, the accurate prediction of neoantigens or the direct measurement of neoantigens on tumor cells is crucial for clinical success. The rapid development of high-throughput next-generation sequencing technology, including whole-genome sequencing and whole-exon sequencing, has provided opportunities to identify thousands of tumor-associated mutations in individual patients. Additionally, machine-learning-based algorithms for MHC-I (encoded by the HLA-A, HLA-B and HLA-C genes) or MHC II (encoded by the HLA-DR, HLA-DP and HLA-DQ genes) epitope prediction can expedite the identification of immunogenic neoantigens [185, 186]. Therefore, personalized therapeutic cancer vaccine-targeted neoantigens have become a promising method for tumor immunotherapy for individual patients.
Several clinical trials have shown that multiple types of personalized neoantigen vaccines can induce CD4+ T cell and CD8+ T cell antigen-specific responses and benefit patient survival. A notable study demonstrated that monocyte-derived DCs loaded with personalized neoantigens induced a T-cell-specific immune response in patients with melanoma [187]. Carreno et al. utilized exome sequencing to identify somatic mutations and in silico analysis to assess HLA-A*02:01 peptide-binding affinity, ultimately obtaining neoantigen candidates for each patient with stage III resected cutaneous melanoma. Subsequently, mature DCs loaded with neoantigen candidates were prepared in vitro and then transfused into the patients by intravenous infusion. Most vaccine-induced neoantigen-specific T cells exhibited a type I-skewed phenotype with high amounts of IFN-γ. DCs loaded with personalized neoantigens activated T-cell immunity and enhanced the breadth of antitumor immunity. Three patients benefited from the neoantigen vaccines and showed no autoimmune adverse events.
RNA vaccines are attractive because they carry genetic information for endogenous protein expression, and the neoepitopes can be translated without transcription. RNA vaccines are flexibly manufactured and induce both humoral and cellular immunity [188]. Sahin et al. [189]. first reported an RNA-based polyneoepitope against melanoma (NCT02035956). In this study, candidate neoantigens were identified by comparative exome and RNA sequencing and screened independent of high-affinity binding to HLA class I and II by computer simulation. The prepared RNA vaccine, injected into draining lymph nodes and capable of encoding candidate neoantigens, elicited CD4+ T cell and CD8+ T-cell responses. Eight of 13 patients who received the RNA vaccine showed no recurrence after 12 months of follow-up.
Similarly, Ott et al. [179]. identified tumor-specific mutations by whole-exome sequencing and RNA sequencing and predicted the binding activity of neoantigens to HLA molecules. The personalized peptide neoantigen vaccines consisting of neoantigens with poly-ICLC adjuvants induced T cell responses, among which 60% of neoantigens activated CD4+ T cells and 16% of neoantigens activated CD8+ T cells. Four of six vaccinated patients with untreated high-risk melanoma had no recurrence at 25 months after inoculation.
Several types of malignancies, including melanoma, pancreatic cancer, breast cancer and lung cancer, are being treated in clinical trials by neoantigen vaccines [190]. Two clinical trials have demonstrated that personalized neoantigen vaccination was feasible for glioblastoma with a low mutation load, an immunologically ‘cold’ TME and a low response to ICIs [191]. Hilf et al. [192]. prepared two synthesized vaccines: APVAC1 (targeting unmutated antigens) and APVAC2 (targeting neoantigens). Fifteen patients with glioblastomas were successively treated with APVAC1 and APVAC2. In 50 % of patients treated with APVAC1, mainly a CD8+ T-cell response was elicited. A CD4+ T-cell response was induced in 84.7% of patients treated with APVAC2. Vaccination improved the overall survival (OS) and progression-free survival (PFS) to 29.0 and 14.2 months, respectively. Patients in whom glioblastomas were surgically resected received another personalized peptide neoantigen vaccine that elicited neoantigen-specific CD4+ and CD8+ T-cell responses and showed an accumulation of neoantigen-specific T cells in the tumor site [193].
Neoantigen vaccines based on RNA, DCs or peptides aim to induce CD4+ and CD8+ T-cell responses and promote T-cell infiltration into the tumor core. The findings from these preliminary clinical trials indicated that neoantigen vaccines could benefit patients with malignant tumors.
Combination of neoantigen vaccines with other immunotherapies
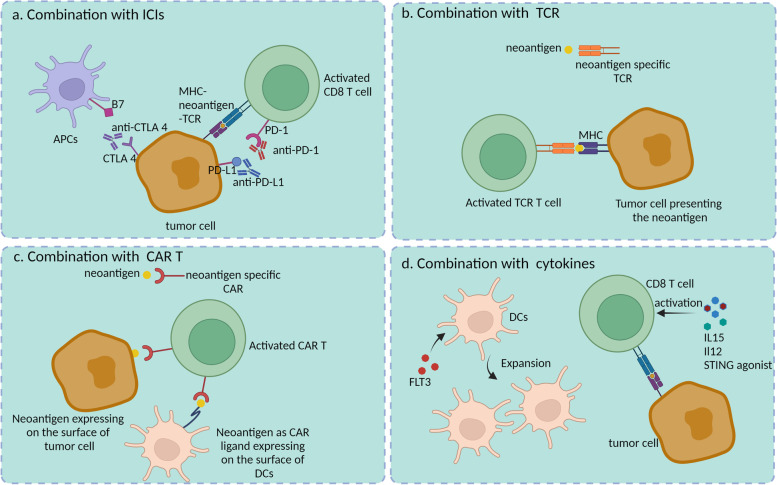
Neoantigen vaccines as monotherapy cannot completely eliminate malignant tumors. Several preclinical and clinical trials have investigated the combination of neoantigen vaccines with other immunotherapies, including ICIs, cytokines [194], immune-stimulatory molecules [195, 196], adaptive T-cell therapy, CAR-T therapy, and radiation therapy [197, 198], aiming to activate and expand the antitumor response by reversing the immunosuppressive TME with immune-suppressive cells and the expression of immune checkpoints (Fig. 4) [199].
Fig. 4.
Combination of neoantigen vaccines with other therapied for cancer. a Combination of neoantigen vaccines with ICI therapy such as anti-CTLA4, anti-PD-1 and anti-PD-L1 could relieve exhaustion of T cells and APCs; b Combination with TCR therapy, T cells were engineered with neoantigens-specific TCR to kill tumor cells; c T cells were equipped with neoantigens-specific CAR which could effectively recognize tumor cells which expressed neoantigens. Even neoantigen can be a CAR ligand to be transferred to the surface of DCs, activating the neoantigen-CAR T cells; d Cytokines induce the expansion of DCs or enhance the activity of T cells to improve the anti-tumor immunity
Tumor vaccines activate the CD4+ and CD8+ T-cell response, resulting in IFN-γ production [179, 189]. However, IFN-γ regulates the expression of PD-L1 in the TME, inhibiting the efficacy of tumor vaccines [200, 201]. Moreover, several studies have reported that neoantigen-specific T cells express high levels of PD-1 following treatment with monotherapy neoantigen. Yadav et al. [202]. immunized mice with mutated tumor neoantigens to evaluate immunogenicity. They found that the infiltration of neoantigen-reactive CD8+ T cells was increased. However, 76.9% of these tumor-specific CD8+ TILs coexpressed PD-1 and TIM-3, which may lead to T-cell exhaustion [203]. Sahin et al. detected the expression of PD-1 on neoepitope-specific T cells and the upregulation of PD-L1 in postvaccination lesions [189]. All evidence indicated that it is rational to combine the neoantigen vaccine with ICIs. Four of six patients with untreated high-risk melanoma benefited from peptide neoantigen vaccines [179]. However, two of six patients (stage IVM1b disease with lung metastases) had disease recurrence evident at follow-up. Both patients were subsequently treated with the anti-PD-1 antibody pembrolizumab for four doses. Complete elimination of tumors was observed in both patients, along with the expansion of neoantigen-specific T cells [179]. Another study also demonstrated that the choice of combination therapy can also significantly affect the therapy outcome [189]. One patient who received the RNA-based poly-neoepitope vaccine showed a strong immune response to neoantigens but experienced multiple relapses due to fast disease progression [189]. This patient was then given the anti-PD-1 antibody pembrolizumab after stopping vaccination. After pembrolizumab treatment, 80% of multiple melanoma lesions were reduced and eventually showed a complete response. Importantly, vaccine-mediated T cells persisted for up to 9 months [189]. Beyond these trials, several clinical trials are currently exploring the efficacy of neoantigen vaccines in combination with ICIs.
The efficacy of vaccines depends on the activated T-cell responses. However, the absence of active T cells in the system or low T-cell infiltration in the TME weakens the function of neoantigen vaccines. Therefore, combinations of neoantigen vaccines and T-cell therapy have the potential to be successfully used to achieve antitumor responses. T cells engineered to express a TCR targeting unique tumor-specific antigens were shown to eradicate large established cancers [204]. Liu et al. [205]. identified neoantigens from epithelial ovarian cancer that activated antigen-specific CD4+ and/or CD8+ T-cell responses by enhancing antigen processing and presentation. Peripheral T cells were engineered to express TCRs from neoepitope-specific T cells and demonstrated neoepitope-specific reactivity. The combination of neoantigens and adapted T cells is a promising strategy for expanding antitumor immunity.
CAR-T therapy has recently achieved inspiring clinical success in the treatment of hematological malignancies, but it has shown limited efficacy in the treatment of solid tumors owing to several problems, such as suboptimal trafficking of engineered T cells to tumors, antigen loss or heterogeneity, and poor fit with the TME. Amphiphile CAR-T ligands were designed by Ma and colleagues to combine therapeutic vaccines with CAR-T cells to alleviate the problem and enhance efficacy [206]. The amphiphile ligands consisted of an albumin binding/membrane-inserting domain as the tail where a CAR ligand was attached. When injected, amphiphile ligands (amph-ligands) associated with free albumin by binding with the albumin binding domain and were then rapidly trafficked to the draining LNs, where they were then transferred to the membrane of APCs with the exposure of the CAR ligand to activate CAR-T cells [206]. Ma and colleagues evaluated the efficacy of the combination of the amph-EGFRvIII vaccine with EGFRvIII-CAR-T cells in mice with gliomas and observed a great increase in CAR-T-cell infiltration, a significant delay in tumor growth and prolonged survival. Similarly, the enhancement of antitumor immunity was observed in B16F10 tumor-bearing mice.
In the TME, cytokines can suppress tumor cell growth by activating T cells and natural killer (NK) cells and have antiproliferative or proapoptotic activity, playing an important role in tumor treatment. The coadministration of FLT3, expanding DC and NK populations [207], with an RNA vaccine encoding antigen enhanced the priming and expansion of antigen-specific CD8+ T cells and T-cell infiltration into tumors [208]. Additionally, Lee et al. [194] utilized an IL15 superagonist, PD-L1 blockade and the tumor-targeted immunocytokine NHS-IL12 to improve the antitumor efficacies of neoantigen vaccines. They found that the combination therapy resulted in a significant increase in T-cell infiltration, enhanced expansion of T cells and efficient tumor clearance. Moreover, costimulatory molecules are necessary for APC function and the full activation of T cells. PancVAX, a neoantigen-targeted vaccine, was administered with the STING adjuvant ADU-V16 to activate neoepitope-specific T cells and promote tumor regression [195]. The addition of anti-PD-1 and agonist OX40 antibodies to the vaccine resulted in durable tumor regression and a survival benefit, which was associated with the reduced coexpression of the T-cell exhaustion markers Lag3 and PD-1 by OX40-targeted therapy [195].
Therapeutic vaccines for hypertension
Hypertension is the leading cause of cardiovascular disease and premature death worldwide [209]. In 2010, it was estimated that 31.1% of adults (1.39 billion) worldwide had hypertension, with a higher prevalence in low- and middle-income countries [209]. In China, the hypertension disease burden is increasing [210]. Based on the basic principle of vaccination, researchers began to develop therapeutic vaccines to control hypertension, such as ATR12181, pHAV-4Ang IIs, CYT006-AngQb, AngI-R, PMD3117 and ATRQβ-001 [211]. Downham et al. designed a vaccine, named “PMD-3117”, targeting angiotensin I, that is composed of an analog of angiotensin I fused to KLH and aluminum hydroxide adjuvant. PMD-3117 induced a significant immune response both in rats and in humans [212]. Then, the sustained immune response to angiotensin I induction and the efficacy of the antibodies to block the renin system by PMD-3117 were evaluated in a phase II clinical trial [152]. PMD-3117 induced anti-angiotensin I antibodies but did not sufficiently influence blood pressure. However, PMD-3117 significantly reduced the decrease in plasma renin [152]. AngQb is a conjugate angiotensin II vaccine composed of angiotensin II chemically conjugated to virus-like particles derived from the coat protein of the bacteriophage Qb [213]. In a phase IIa study in patients with mild to moderate hypertension, a 300 μg dose reduced blood pressure during the daytime, especially in the early morning (NCT00500786) [213]. All volunteers produced high IgG titers against angiotensin II. Similarly, the peptide vaccines ATR12181 and ATRQβ-001 targeting angiotensin II type 1 receptor showed promising immune responses in a mouse model [214]. Recently, the safety and tolerability AGMG0201(a modified angiotensin II DNA vaccine) were assessed in a phase I/IIa study which enrolled 12 patients with mild to moderate essential hypertension (ACTRN12617001192370) [156]. Patients who received the AGMG0201 immunization elicited anti-angiotensin II antibody, especially in the high-dose group.
Therapeutic vaccines for Alzheimer’s disease
Alzheimer’s disease (AD) is the most prevalent form of dementia, affecting 50 million people worldwide [215]. In the clinic, small-molecule drugs (such as rivastigmine, galantamine, and donepezil for inhibiting cholinesterase and memantine targeting the NMDA receptor) can relieve symptoms but cannot alter disease progression [216]. Therapeutic vaccines aim to clear the pathogenic peptide and protein aggregation associated with AD, and promise has been demonstrated in slowing cognitive decline in AD patients. Amyloid β-peptide (Aβ) aggregation pathologically results in β-amyloid plaques and cerebral amyloid angiopathy (CAA) deposits [217]. Hyperphosphorylated tau aggregates form neurofibrillary tangles [218]. Therefore, Aβ and tau are targets for candidate therapeutic vaccines against AD. The peptide vaccine ABvac40 is designed to target the C-terminal end of the Aβ40 peptide, which is an abnormal mutant of Aβ, and consists of Aβ33–40 conjugated with the carrier protein keyhole limpet hemocyanin (KLH) [219]. In a phase I clinical trial, 11 of 12 (92%) patients aged 50 to 85 years with mild to moderate AD received ABvac40 and produced specific anti-Aβ40 antibodies (NCT03113812). No ABvac40-immunized patients showed vasogenic edema, sulcal effusion or microhaemorrhages [219]. A phase II clinical trial is currently ongoing to determine the safety, tolerability and immune response of ABvac40 (NCT03461276). AADVvac1 is another peptide vaccine and contains tau294–395 coupled to the KLH carrier [220]. A significant protective humoral immune response and reduction in AD-type hyperphosphorylation of tau were detected in tau transgenic rats after AADVvac1 injection [220]. In a phase I clinical trial, 29 of 30 patients who received AADvac1 produced IgG titers and did not experience meningoencephalitis or vasogenic edema (NCT02031198) [221]. The safety and efficacy study of AADvac1 in patients with mild AD in phase II clinical trials was completed in 2019 (NCT02579252). However, the detailed results have not been reported. UB-311 is a novel synthetic peptide vaccine which mainly targets Th2-biased immunity by fusing two synthetic Aβ1–14–targeting B-cell peptides with different helper T-cell peptide epitopes [222]. UB-311 elicited high level of antibody targeting Aβ1–14 epitopes with 100% responder rate in patients with mild Alzheimer’s disease in the phase I trial (NCT00965588) [222]. UB-311 has completed the phase-II trial in early-to-mild Alzheimer’s disease patients (NCT02551809).
Therapeutic vaccines for other diseases
Overall, there are some other therapeutic vaccines against chronic diseases, such as amyotrophic lateral sclerosis (ALS), diabetes, and dyslipidemia. Superoxide dismutase 1 (SOD1) misfolding due to mutation is a risk factor for ALS pathogenesis and progression. Zhao et al. designed two peptide vaccines, tgG-DSE2lim and tgG-DSE5b, both targeting SOD1 mutations, to treat ALS [157]. Both vaccines elicited a rapid and sustained Th2-biased immune response and significantly prolonged the survival of hSOD1G37R transgenic mice. Importantly, tgG-DSE5b significantly delayed disease occurrence and progression [157]. The enzyme dipeptidyl peptidase 4 (DPP4) can rapidly degrade glucagon-like peptide 1 (GLP-1), which increases insulin secretion and improves insulin sensitivity, making it a promising therapeutic target for type 2 diabetes [223]. Pang et al. constructed a polypeptide vaccine targeting DPP4 containing the three epitopes within DPP4 conjugated to KLH [158]. DPP4 vaccination of C57BL/6 J mice successfully increased the DPP4-specific titer, inhibited plasma DPP4 activity, and increased the level of GLP-1, which was associated with an increase in both plasma insulin and pancreatic insulin content [158]. Recently, Zhang et al. prepared a proinflammatory cytokine IL-1β-targeted therapeutic vaccine composed of an IL-1β epitope peptide to treat type 2 diabetes [224]. The vaccine improved glucose tolerance and insulin sensitivity and enhanced B-cell function [224]. High levels of low-density lipoprotein cholesterol (LDL-C) lead to an increased risk of atherosclerosis and ischemic cardiovascular diseases. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a hepatic enzymatic protein that negatively regulates low-density lipoprotein receptor (LDLR), which results in the accumulation of LDL-C [159]. The PCSK9Qβ-003 vaccine, consisting of PCSK9 peptide conjugated with Qβ VLP, significantly elicited PCSK9-specific antibodies and obviously decreased plasma PCSK9 levels, which resulted in a reduction in plasma total cholesterol [159].
Discussion
Therapeutic vaccines offer a promising and attractive immunotherapy to combat established infectious diseases and chronic noncommunicable diseases by reason of their safety, specificity, effectiveness and even long-lasting response. Unfortunately, most of therapeutic vaccines were at early clinical stage. However, growing understanding of the spatial and temporal immune response elicited by vaccines and novel immunomodulatory approaches would accelerate the clinical feasibility and efficacy of therapeutic vaccines. Considerable efforts on the progress of therapeutic vaccines should be made on three areas: identification and selection of antigens; the choice of antigen delivery; the development of combination therapy. Better knowledge of the pathogenesis contributes to the selection of antigens owing the stable expression and high immunogenicity. For example, the better understanding of the breadth of TAAs, neo-antigens and the native immune response has facilitated and improved the rational vaccine design [16]. The quality of neoepitopes should be further enhanced for the best effect. The adjuvant used and the administration route acts as key determinants of antigen delivery [16]. Each type of therapeutic vaccines own advantages and defects respectively. Protein/peptide vaccines has the easy production, but low immunogenicity. Vector-based vaccines tend to deliver antigens with a high efficacy, but cause safety concerns. DNA vaccines lack high immune response [76]. The addition of adjuvants could effectively stimulate innate immunity and enhance the effect of Protein/peptide vaccines and DNA vaccines. Furthermore, novel carriers would improve the development of protein/peptide vaccines platforms, such as lipoplexes, liposomes and the self-assembling nanoparticles [16]. Additionally, combining with complementary therapies, such as ICIs, CAR-T and TCR-T therapies, will be important to the improvement of therapeutic vaccines. The better effectiveness was observed in the combination of therapeutic neoantigen vaccines with other these immunotherapies. In chronic viral infection, virus-specific T cells gradually become exhausted [225]. Blockade of PD-1/PD-L1 pathway in vivo provided a beneficial effect on therapeutic HBV vaccine in the LCMV mouse model [226].
Moving forward with those issues settled, therapeutic vaccines could be the alternative strategies and even provide a platform for combination therapy against established infectious diseases and chronic noncommunicable diseases, with minimal toxicity and a good safety profile contributing to improving human health.
Acknowledgments
The figures were drawn using BioRender (https://biorender.com).
Code availability
Not applicable.
Authors’ contributions
YMT and DH drafted initial manuscript. YHL and LY revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Sichuan University; and by the Talents project of Sichuan University of Science & Engineering undergrant number E10402637; 1.3.5 project for disciplines of excellence (No. ZYGD18007), West China Hospital; China Postdoctoral Science Foundation under grant number 2018 M643466.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have declared that no competing interest exist.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yaomei Tian, Email: yaomei226@163.com.
Die Hu, Email: hd13382070725@163.com.
Yuhua Li, Email: liyuhua@nifdc.org.cn.
Li Yang, Email: yl.tracy73@gmail.com.
References
- 1.Schlipköter U, Flahault A. Communicable diseases: achievements and challenges for public health. Public Health Rev. 2010;32:90–119. doi: 10.1007/bf03391594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Excler JL, Saville M, Berkley S, Kim JH. Vaccine development for emerging infectious diseases. Nat Med. 2021;27(4):591–600. doi: 10.1038/s41591-021-01301-0. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201(11):1607–1610. doi: 10.1086/652404. [DOI] [PubMed] [Google Scholar]
- 4.Binagwaho A, Mathewos K, Davis S. Equitable and effective distribution of the COVID-19 vaccines - a scientific and moral obligation. Int J Health Policy Manag. 2022;11(2):100–102. doi: 10.34172/ijhpm.2021.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodworth KR, Moulia D, Collins JP, Hadler SC, Jones JM, Reddy SC, et al. The advisory committee on immunization Practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years - United States, November 2021. MMWR Morb Mortal Wkly Rep. 2021;70(45):1579–1583. doi: 10.15585/mmwr.mm7045e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock G, Hellner K, Dorrell L. Therapeutic HPV vaccines. Best Pract Res Clin Obstet Gynaecol. 2018;47:59–72. doi: 10.1016/j.bpobgyn.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Masrour-Roudsari J, Ebrahimpour S. Causal role of infectious agents in cancer: an overview. Caspian J Intern Med. 2017;8(3):153–158. doi: 10.22088/cjim.8.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigna JJ, Noubiap JJ. The rising burden of non-communicable diseases in sub-Saharan Africa. Lancet Glob Health. 2019;7(10):e1295–e1296. doi: 10.1016/s2214-109x(19)30370-5. [DOI] [PubMed] [Google Scholar]
- 9.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006;100(3):191–199. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23(1):75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62(5):309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson JD, Gould KG, Brown K. Richard Pfeiffer's typhoid vaccine and Almroth Wright's claim to priority. Vaccine. 2021;39(15):2074–2079. doi: 10.1016/j.vaccine.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Handy CE, Antonarakis ES. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 2018;14(10):907–917. doi: 10.2217/fon-2017-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamaru R, Nakagami H, Rakugi H, Morishita R. Future directions of therapeutic vaccines for chronic diseases. Circ J. 2020;84(11):1895–1902. doi: 10.1253/circj.CJ-20-0703. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28. doi: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 17.Rethinking therapeutic cancer vaccines Nat Rev Drug Discov. 2009;8(9):685–686. doi: 10.1038/nrd2994. [DOI] [PubMed] [Google Scholar]
- 18.Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 2018;18(4):240–254. doi: 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim BK, Han KH, Ahn SH. Prevention of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Oncology. 2011;81(Suppl 1):41–49. doi: 10.1159/000333258. [DOI] [PubMed] [Google Scholar]
- 20.Gissmann L, Nieto K. The therapeutic vaccine: is it feasible? Arch Med Res. 2009;40(6):493–498. doi: 10.1016/j.arcmed.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Häfner N, Driesch C, Gajda M, Jansen L, Kirchmayr R, Runnebaum IB, et al. Integration of the HPV16 genome does not invariably result in high levels of viral oncogene transcripts. Oncogene. 2008;27(11):1610–1617. doi: 10.1038/sj.onc.1210791. [DOI] [PubMed] [Google Scholar]
- 23.Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18(1):128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobaina Y, Michel ML. Chronic hepatitis B: immunological profile and current therapeutic vaccines in clinical trials. Vaccine. 2017;35(18):2308–2314. doi: 10.1016/j.vaccine.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 25.Campo MS, Grindlay GJ, O'Neil BW, Chandrachud LM, McGarvie GM, Jarrett WF. Prophylactic and therapeutic vaccination against a mucosal papillomavirus. J Gen Virol. 1993;74(Pt 6):945–953. doi: 10.1099/0022-1317-74-6-945. [DOI] [PubMed] [Google Scholar]
- 26.Shahnazari M, Samadi P, Pourjafar M, Jalali A. Therapeutic vaccines for colorectal cancer: the progress and future prospect. Int Immunopharmacol. 2020;88:106944. doi: 10.1016/j.intimp.2020.106944. [DOI] [PubMed] [Google Scholar]
- 27.Lazoura E, Apostolopoulos V. Rational peptide-based vaccine design for cancer immunotherapeutic applications. Curr Med Chem. 2005;12(6):629–639. doi: 10.2174/0929867053202188. [DOI] [PubMed] [Google Scholar]
- 28.Ura T, Okuda K, Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2(3):624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar R, Kumar P. Yeast-based vaccines: new perspective in vaccine development and application. FEMS Yeast Res. 2019;19(2). 10.1093/femsyr/foz007. [DOI] [PubMed]
- 30.Santos PM, Butterfield LH. Dendritic cell-based cancer vaccines. J Immunol. 2018;200(2):443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue D, Liang Y, Duan S, He J, Su J, Zhu J, et al. Enhanced anti-tumor immunity against breast cancer induced by whole tumor cell vaccines genetically modified expressing α-gal epitopes. Oncol Rep. 2016;36(5):2843–2851. doi: 10.3892/or.2016.5128. [DOI] [PubMed] [Google Scholar]
- 32.Vermaelen K. Vaccine strategies to improve anti-cancer cellular immune responses. Front Immunol. 2019;10:8. doi: 10.3389/fimmu.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulendran B, Arunachalam PS, O'Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang F, Loré K. Local innate immune responses in the vaccine adjuvant-injected muscle. Clin Transl Immunology. 2016;5(4):e74. doi: 10.1038/cti.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang F, Lindgren G, Sandgren KJ, Thompson EA, Francica JR, Seubert A, et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci Transl Med. 2017;9(393). 10.1126/scitranslmed.aal2094. [DOI] [PubMed]
- 36.Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29(9):1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 37.Didierlaurent AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M, et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol. 2014;193(4):1920–1930. doi: 10.4049/jimmunol.1400948. [DOI] [PubMed] [Google Scholar]
- 38.Gornati L, Zanoni I, Granucci F. Dendritic cells in the cross hair for the generation of tailored vaccines. Front Immunol. 2018;9:1484. doi: 10.3389/fimmu.2018.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell. 2015;162(6):1322–1337. doi: 10.1016/j.cell.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022;20(4):193–205. doi: 10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smalley Rumfield C, Roller N, Pellom ST, Schlom J, Jochems C. Therapeutic vaccines for HPV-associated malignancies. Immunotargets Ther. 2020;9:167–200. doi: 10.2147/itt.S273327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cargill T, Barnes E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin Exp Immunol. 2021;205(2):106–118. doi: 10.1111/cei.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hokello J, Sharma AL, Tyagi M. An update on the HIV DNA vaccine strategy. Vaccines (Basel). 2021;9(6). 10.3390/vaccines9060605. [DOI] [PMC free article] [PubMed]
- 44.Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018;5:46–58. doi: 10.1016/j.pvr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Song Y, Wei X, Wang G, Sun R, Wang M, et al. Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol J. 2022;19(1):6. doi: 10.1186/s12985-021-01732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khairkhah N, Bolhassani A, Najafipour R. Current and future direction in treatment of HPV-related cervical disease. J Mol Med (Berl) 2022;100(6):829–845. doi: 10.1007/s00109-022-02199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garbuglia AR, Lapa D, Sias C, Capobianchi MR, Del Porto P. The use of both therapeutic and prophylactic vaccines in the therapy of papillomavirus disease. Front Immunol. 2020;11:188. doi: 10.3389/fimmu.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2016;215(2):212.e1–212.e15. doi: 10.1016/j.ajog.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrow MP, Yan J, Sardesai NY. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines. 2013;12(3):271–283. doi: 10.1586/erv.13.23. [DOI] [PubMed] [Google Scholar]
- 51.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated listeria monocytogenes vaccine: a phase I safety study of lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27(30):3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 52.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 2007;9(10):1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Basu P, Mehta A, Jain M, Gupta S, Nagarkar RV, John S, et al. A randomized phase 2 study of ADXS11-001 listeria monocytogenes-Listeriolysin O immunotherapy with or without Cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018;28(4):764–772. doi: 10.1097/igc.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine. 2014;32(47):6233–6239. doi: 10.1016/j.vaccine.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Taghinezhad SS, Mohseni AH, Keyvani H, Razavi MR. Phase 1 safety and immunogenicity trial of recombinant Lactococcus lactis expressing human papillomavirus type 16 E6 Oncoprotein vaccine. Mol Ther Methods Clin Dev. 2019;15:40–51. doi: 10.1016/j.omtm.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 2012;61(8):1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347(9014):1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 58.Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, Schneider A, et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res. 2002;8(12):3676–3685. [PubMed] [Google Scholar]
- 59.Harper DM, Nieminen P, Donders G, Einstein MH, Garcia F, Huh WK, et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: randomized controlled phase II trial with 2.5 years of follow-up. Gynecol Oncol. 2019;153(3):521–529. doi: 10.1016/j.ygyno.2019.03.250. [DOI] [PubMed] [Google Scholar]
- 60.Çuburu N, Khan S, Thompson CD, Kim R, Vellinga J, Zahn R, et al. Adenovirus vector-based prime-boost vaccination via heterologous routes induces cervicovaginal CD8(+) T cell responses against HPV16 oncoproteins. Int J Cancer. 2018;142(7):1467–1479. doi: 10.1002/ijc.31166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daemen T, Riezebos-Brilman A, Bungener L, Regts J, Dontje B, Wilschut J. Eradication of established HPV16-transformed tumours after immunisation with recombinant Semliki Forest virus expressing a fusion protein of E6 and E7. Vaccine. 2003;21(11–12):1082–1088. doi: 10.1016/s0264-410x(02)00558-3. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt S, Bonilla WV, Reiter A, Stemeseder F, Kleissner T, Oeler D, et al. Live-attenuated lymphocytic choriomeningitis virus-based vaccines for active immunotherapy of HPV16-positive cancer. Oncoimmunology. 2020;9(1):1809960. doi: 10.1080/2162402x.2020.1809960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang A, Farmer E, Lin J, Wu TC, Hung CF. The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus Res. 2017;231:148–165. doi: 10.1016/j.virusres.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, Small LA, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res. 2000;6(9):3406–3416. [PubMed] [Google Scholar]
- 65.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 66.de Vos van Steenwijk PJ, Ramwadhdoebe TH, Löwik MJ, van der Minne CE, Berends-van der Meer DM, Fathers LM, et al. A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol Immunother. 2012;61(9):1485–1492. doi: 10.1007/s00262-012-1292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Poelgeest MI, Welters MJ, van Esch EM, Stynenbosch LF, Kerpershoek G, Persijn van Meerten EL, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Vos van Steenwijk PJ, van Poelgeest MI, Ramwadhdoebe TH, Löwik MJ, Berends-van der Meer DM, van der Minne CE, et al. The long-term immune response after HPV16 peptide vaccination in women with low-grade pre-malignant disorders of the uterine cervix: a placebo-controlled phase II study. Cancer Immunol Immunother. 2014;63(2):147–160. doi: 10.1007/s00262-013-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med. 2016;8(334):334ra52. doi: 10.1126/scitranslmed.aad8307. [DOI] [PubMed] [Google Scholar]
- 70.Melief CJM, Welters MJP, Vergote I, Kroep JR, Kenter GG, Ottevanger PB, et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci Transl Med. 2020;12(535). 10.1126/scitranslmed.aaz8235. [DOI] [PubMed]
- 71.Sousa LG, Rajapakshe K, Rodriguez Canales J, Chin RL, Feng L, Wang Q, et al. ISA101 and nivolumab for HPV-16(+) cancer: updated clinical efficacy and immune correlates of response. J Immunother Cancer. 2022;10(2). 10.1136/jitc-2021-004232. [DOI] [PMC free article] [PubMed]
- 72.Van Damme P, Bouillette-Marussig M, Hens A, De Coster I, Depuydt C, Goubier A, et al. GTL001, a therapeutic vaccine for women infected with human papillomavirus 16 or 18 and Normal cervical cytology: results of a phase I clinical trial. Clin Cancer Res. 2016;22(13):3238–3248. doi: 10.1158/1078-0432.Ccr-16-0085. [DOI] [PubMed] [Google Scholar]
- 73.Einstein MH, Kadish AS, Burk RD, Kim MY, Wadler S, Streicher H, et al. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol Oncol. 2007;106(3):453–460. doi: 10.1016/j.ygyno.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Da Silva DM, Skeate JG, Chavez-Juan E, Lühen KP, Wu JM, Wu CM, et al. Therapeutic efficacy of a human papillomavirus type 16 E7 bacterial exotoxin fusion protein adjuvanted with CpG or GPI-0100 in a preclinical mouse model for HPV-associated disease. Vaccine. 2019;37(22):2915–2924. doi: 10.1016/j.vaccine.2019.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SJ, Yang A, Wu TC, Hung CF. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 2016;27(5):e51. doi: 10.3802/jgo.2016.27.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barra F, Della Corte L, Noberasco G, Foreste V, Riemma G, Di Filippo C, et al. Advances in therapeutic vaccines for treating human papillomavirus-related cervical intraepithelial neoplasia. J Obstet Gynaecol Res. 2020;46(7):989–1006. doi: 10.1111/jog.14276. [DOI] [PubMed] [Google Scholar]
- 77.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4(155):155ra138. 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed]
- 78.Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–2088. doi: 10.1016/s0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. doi: 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi YJ, Hur SY, Kim TJ, Hong SR, Lee JK, Cho CH, et al. A phase II, prospective, randomized, multicenter, open-label study of GX-188E, an HPV DNA vaccine, in patients with cervical intraepithelial Neoplasia 3. Clin Cancer Res. 2020;26(7):1616–1623. doi: 10.1158/1078-0432.Ccr-19-1513. [DOI] [PubMed] [Google Scholar]
- 81.Alvarez RD, Huh WK, Bae S, Lamb LS, Jr, Conner MG, Boyer J, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3) Gynecol Oncol. 2016;140(2):245–252. doi: 10.1016/j.ygyno.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia F, Petry KU, Muderspach L, Gold MA, Braly P, Crum CP, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2004;103(2):317–326. doi: 10.1097/01.Aog.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 83.Shih C, Yang CC, Choijilsuren G, Chang CH, Liou AT. Hepatitis B virus. Trends Microbiol. 2018;26(4):386–387. doi: 10.1016/j.tim.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214(1):16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 85.Das S, Ramakrishnan K, Behera SK, Ganesapandian M, Xavier AS, Selvarajan S. Hepatitis B vaccine and immunoglobulin: key concepts. J Clin Transl Hepatol. 2019;7(2):165–171. doi: 10.14218/jcth.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viganò M, Invernizzi F, Grossi G, Lampertico P. Review article: the potential of interferon and nucleos(t)ide analogue combination therapy in chronic hepatitis B infection. Aliment Pharmacol Ther. 2016;44(7):653–661. doi: 10.1111/apt.13751. [DOI] [PubMed] [Google Scholar]
- 87.Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136(7):2169–2179.e1–4. doi: 10.1053/j.gastro.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 88.van Zonneveld M, Honkoop P, Hansen BE, Niesters HG, Darwish Murad S, de Man RA, et al. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology. 2004;39(3):804–810. doi: 10.1002/hep.20128. [DOI] [PubMed] [Google Scholar]
- 89.Rapti I, Hadziyannis S. Risk for hepatocellular carcinoma in the course of chronic hepatitis B virus infection and the protective effect of therapy with nucleos(t)ide analogues. World J Hepatol. 2015;7(8):1064–1073. doi: 10.4254/wjh.v7.i8.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–4225. doi: 10.1128/jvi.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195(9):1089–1101. doi: 10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salimzadeh L, Le Bert N, Dutertre CA, Gill US, Newell EW, Frey C, et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J Clin Invest. 2018;128(10):4573–4587. doi: 10.1172/jci121957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang J. Adenovirus vectors: excellent tools for vaccine development. Immune Netw. 2021;21(1):e6. doi: 10.4110/in.2021.21.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin P, Dubois C, Jacquier E, Dion S, Mancini-Bourgine M, Godon O, et al. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice. Gut. 2015;64(12):1961–1971. doi: 10.1136/gutjnl-2014-308041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zoulim F, Fournier C, Habersetzer F, Sprinzl M, Pol S, Coffin CS, et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccin Immunother. 2020;16(2):388–399. doi: 10.1080/21645515.2019.1651141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schiwon M, Ehrke-Schulz E, Oswald A, Bergmann T, Michler T, Protzer U, et al. One-vector system for multiplexed CRISPR/Cas9 against hepatitis B virus cccDNA utilizing high-capacity adenoviral vectors. Mol Ther Nucleic Acids. 2018;12:242–253. doi: 10.1016/j.omtn.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kato Y, Tabata H, Sato K, Nakamura M, Saito I, Nakanishi T. Adenovirus vectors expressing eight multiplex guide RNAs of CRISPR/Cas9 efficiently disrupted diverse hepatitis B virus gene derived from heterogeneous patient. Int J Mol Sci. 2021;22(19). 10.3390/ijms221910570. [DOI] [PMC free article] [PubMed]
- 98.Moorthy VS, McConkey S, Roberts M, Gothard P, Arulanantham N, Degano P, et al. Safety of DNA and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2003;21(17–18):1995–2002. doi: 10.1016/s0264-410x(02)00771-5. [DOI] [PubMed] [Google Scholar]
- 99.Chinnakannan SK, Cargill TN, Donnison TA, Ansari MA, Sebastian S, Lee LN, et al. The design and development of a multi-HBV antigen encoded in chimpanzee adenoviral and modified Vaccinia Ankara viral vectors; a novel therapeutic vaccine strategy against HBV. Vaccines (Basel). 2020;8(2). 10.3390/vaccines8020184. [DOI] [PMC free article] [PubMed]
- 100.Boni C, Barili V, Acerbi G, Rossi M, Vecchi A, Laccabue D, et al. HBV immune-therapy: from molecular mechanisms to clinical applications. Int J Mol Sci. 2019;20(11). 10.3390/ijms20112754. [DOI] [PMC free article] [PubMed]
- 101.Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, et al. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32(39):4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 102.Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7(5):625–629. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 103.Boni C, Janssen HLA, Rossi M, Yoon SK, Vecchi A, Barili V, et al. Combined GS-4774 and Tenofovir therapy can improve HBV-specific T-cell responses in patients with chronic hepatitis. Gastroenterology. 2019;157(1):227–241.e7. doi: 10.1053/j.gastro.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 104.Aguilar JC, Lobaina Y, Muzio V, García D, Pentón E, Iglesias E, et al. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol Cell Biol. 2004;82(5):539–546. doi: 10.1111/j.0818-9641.2004.01278.x. [DOI] [PubMed] [Google Scholar]
- 105.Betancourt AA, Delgado CA, Estévez ZC, Martínez JC, Ríos GV, Aureoles-Roselló SR, et al. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int J Infect Dis. 2007;11(5):394–401. doi: 10.1016/j.ijid.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 106.Al Mahtab M, Akbar SMF, Aguilar JC, Guillen G, Penton E, Tuero A, et al. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial) PLoS One. 2018;13(8):e0201236. doi: 10.1371/journal.pone.0201236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Obeng-Adjei N, Choo DK, Saini J, Yan J, Pankhong P, Parikh A, et al. Synthetic DNA immunogen encoding hepatitis B core antigen drives immune response in liver. Cancer Gene Ther. 2012;19(11):779–787. doi: 10.1038/cgt.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Obeng-Adjei N, Hutnick NA, Yan J, Chu JS, Myles DJ, Morrow MP, et al. DNA vaccine cocktail expressing genotype a and C HBV surface and consensus core antigens generates robust cytotoxic and antibody responses in mice and rhesus macaques. Cancer Gene Ther. 2013;20(12):652–662. doi: 10.1038/cgt.2013.65. [DOI] [PubMed] [Google Scholar]
- 109.Yang SH, Lee CG, Park SH, Im SJ, Kim YM, Son JM, et al. Correlation of antiviral T-cell responses with suppression of viral rebound in chronic hepatitis B carriers: a proof-of-concept study. Gene Ther. 2006;13(14):1110–1117. doi: 10.1038/sj.gt.3302751. [DOI] [PubMed] [Google Scholar]
- 110.Yoon SK, Seo YB, Im SJ, Bae SH, Song MJ, You CR, et al. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015;35(3):805–815. doi: 10.1111/liv.12530. [DOI] [PubMed] [Google Scholar]
- 111.Mu Z, Haynes BF, Cain DW. HIV mRNA vaccines-Progress and future paths. Vaccines (Basel). 2021;9(2). 10.3390/vaccines9020134. [DOI] [PMC free article] [PubMed]
- 112.Kim JH, Excler JL, Michael NL. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu Rev Med. 2015;66:423–437. doi: 10.1146/annurev-med-052912-123749. [DOI] [PubMed] [Google Scholar]
- 113.Jonny J, Putranto TA, Sitepu EC, Irfon R. Dendritic cell vaccine as a potential strategy to end the COVID-19 pandemic. Why should it be ex vivo? Expert Rev Vaccines. 2022;21(8):1111–1120. doi: 10.1080/14760584.2022.2080658. [DOI] [PubMed] [Google Scholar]
- 114.Macatangay BJ, Riddler SA, Wheeler ND, Spindler J, Lawani M, Hong F, et al. Therapeutic vaccination with dendritic cells loaded with autologous HIV type 1-infected apoptotic cells. J Infect Dis. 2016;213(9):1400–1409. doi: 10.1093/infdis/jiv582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gay CL, DeBenedette MA, Tcherepanova IY, Gamble A, Lewis WE, Cope AB, et al. Immunogenicity of AGS-004 dendritic cell therapy in patients treated during acute HIV infection. AIDS Res Hum Retrovir. 2018;34(1):111–122. doi: 10.1089/aid.2017.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacobson JM, Routy JP, Welles S, DeBenedette M, Tcherepanova I, Angel JB, et al. Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA: a randomized, double-blind, placebo-controlled clinical trial. J Acquir Immune Defic Syndr. 2016;72(1):31–38. doi: 10.1097/qai.0000000000000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leal L, Guardo AC, Morón-López S, Salgado M, Mothe B, Heirman C, et al. Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection. Aids. 2018;32(17):2533–2545. doi: 10.1097/qad.0000000000002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Jong W, Aerts J, Allard S, Brander C, Buyze J, Florence E, et al. iHIVARNA phase IIa, a randomized, placebo-controlled, double-blinded trial to evaluate the safety and immunogenicity of iHIVARNA-01 in chronically HIV-infected patients under stable combined antiretroviral therapy. Trials. 2019;20(1):361. doi: 10.1186/s13063-019-3409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Travieso T, Li J, Mahesh S, Mello JDFRE, Blasi M. The use of viral vectors in vaccine development. npj Vaccines. 2022;7(1):75. doi: 10.1038/s41541-022-00503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hancock G, Morón-López S, Kopycinski J, Puertas MC, Giannoulatou E, Rose A, et al. Evaluation of the immunogenicity and impact on the latent HIV-1 reservoir of a conserved region vaccine, MVA.HIVconsv, in antiretroviral therapy-treated subjects. J Int AIDS Soc. 2017;20(1):21171. doi: 10.7448/ias.20.1.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tung FY, Tung JK, Pallikkuth S, Pahwa S, Fischl MA. A therapeutic HIV-1 vaccine enhances anti-HIV-1 immune responses in patients under highly active antiretroviral therapy. Vaccine. 2016;34(19):2225–2232. doi: 10.1016/j.vaccine.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 122.Tu T, Buhler S, Bartenschlager R. Chronic viral hepatitis and its association with liver cancer. Biol Chem. 2017;398(8):817–837. doi: 10.1515/hsz-2017-0118. [DOI] [PubMed] [Google Scholar]
- 123.Holz L, Rehermann B. T cell responses in hepatitis C virus infection: historical overview and goals for future research. Antivir Res. 2015;114:96–105. doi: 10.1016/j.antiviral.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spengler U. Direct antiviral agents (DAAs) - a new age in the treatment of hepatitis C virus infection. Pharmacol Ther. 2018;183:118–126. doi: 10.1016/j.pharmthera.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 125.Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, et al. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127(3):924–936. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 126.Yutani S, Yamada A, Yoshida K, Takao Y, Tamura M, Komatsu N, et al. Phase I clinical study of a personalized peptide vaccination for patients infected with hepatitis C virus (HCV) 1b who failed to respond to interferon-based therapy. Vaccine. 2007;25(42):7429–7435. doi: 10.1016/j.vaccine.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 127.Klade CS, Wedemeyer H, Berg T, Hinrichsen H, Cholewinska G, Zeuzem S, et al. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology. 2008;134(5):1385–1395. doi: 10.1053/j.gastro.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 128.Firbas C, Boehm T, Buerger V, Schuller E, Sabarth N, Jilma B, et al. Immunogenicity and safety of different injection routes and schedules of IC41, a hepatitis C virus (HCV) peptide vaccine. Vaccine. 2010;28(12):2397–2407. doi: 10.1016/j.vaccine.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 129.Yutani S, Komatsu N, Shichijo S, Yoshida K, Takedatsu H, Itou M, et al. Phase I clinical study of a peptide vaccination for hepatitis C virus-infected patients with different human leukocyte antigen-class I-A alleles. Cancer Sci. 2009;100(10):1935–1942. doi: 10.1111/j.1349-7006.2009.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Habersetzer F, Baumert TF, Stoll-Keller F. GI-5005, a yeast vector vaccine expressing an NS3-core fusion protein for chronic HCV infection. Curr Opin Mol Ther. 2009;11(4):456–462. [PubMed] [Google Scholar]
- 131.Haller AA, Lauer GM, King TH, Kemmler C, Fiolkoski V, Lu Y, et al. Whole recombinant yeast-based immunotherapy induces potent T cell responses targeting HCV NS3 and Core proteins. Vaccine. 2007;25(8):1452–1463. doi: 10.1016/j.vaccine.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 132.Ip PP, Nijman HW, Wilschut J, Daemen T. Therapeutic vaccination against chronic hepatitis C virus infection. Antivir Res. 2012;96(1):36–50. doi: 10.1016/j.antiviral.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 133.Shayeghpour A, Kianfar R, Hosseini P, Ajorloo M, Aghajanian S, Hedayat Yaghoobi M, et al. Hepatitis C virus DNA vaccines: a systematic review. Virol J. 2021;18(1):248. doi: 10.1186/s12985-021-01716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alvarez-Lajonchere L, Shoukry NH, Gra B, Amador-Canizares Y, Helle F, Bedard N, et al. Immunogenicity of CIGB-230, a therapeutic DNA vaccine preparation, in HCV-chronically infected individuals in a phase I clinical trial. J Viral Hepat. 2009;16(3):156–167. doi: 10.1111/j.1365-2893.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 135.Castellanos M, Cinza Z, Dorta Z, Veliz G, Vega H, Lorenzo I, et al. Immunization with a DNA vaccine candidate in chronic hepatitis C patients is safe, well tolerated and does not impair immune response induction after anti-hepatitis B vaccination. J Gene Med. 2010;12(1):107–116. doi: 10.1002/jgm.1407. [DOI] [PubMed] [Google Scholar]
- 136.Ahlen G, Nystrom J, Pult I, Frelin L, Hultgren C, Sallberg M. In vivo clearance of hepatitis C virus nonstructural 3/4A-expressing hepatocytes by DNA vaccine-primed cytotoxic T lymphocytes. J Infect Dis. 2005;192(12):2112–2116. doi: 10.1086/498218. [DOI] [PubMed] [Google Scholar]
- 137.Ratnoglik SL, Jiang DP, Aoki C, Sudarmono P, Shoji I, Deng L, et al. Induction of cell-mediated immune responses in mice by DNA vaccines that express hepatitis C virus NS3 mutants lacking serine protease and NTPase/RNA helicase activities. PLoS One. 2014;9(6):e98877. doi: 10.1371/journal.pone.0098877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morikawa K, Lange CM, Gouttenoire J, Meylan E, Brass V, Penin F, et al. Nonstructural protein 3-4A: the Swiss army knife of hepatitis C virus. J Viral Hepat. 2011;18(5):305–315. doi: 10.1111/j.1365-2893.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 139.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4(115):115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12(2):190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 141.Fournillier A, Gerossier E, Evlashev A, Schmitt D, Simon B, Chatel L, et al. An accelerated vaccine schedule with a poly-antigenic hepatitis C virus MVA-based candidate vaccine induces potent, long lasting and in vivo cross-reactive T cell responses. Vaccine. 2007;25(42):7339–7353. doi: 10.1016/j.vaccine.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 142.Gowans EJ, Roberts S, Jones K, Dinatale I, Latour PA, Chua B, et al. A phase I clinical trial of dendritic cell immunotherapy in HCV-infected individuals. J Hepatol. 2010;53(4):599–607. doi: 10.1016/j.jhep.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zinatizadeh MR, Zarandi PK, Zinatizadeh M, Yousefi MH, Amani J, Rezaei N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed Pharmacother. 2022;146:112527. doi: 10.1016/j.biopha.2021.112527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lipsitch M, Krammer F, Regev-Yochay G, Lustig Y, Balicer RD. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22(1):57–65. doi: 10.1038/s41577-021-00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22(6):781–790. doi: 10.1016/s1473-3099(22)00143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bradley RE, Ponsford MJ, Scurr MJ, Godkin A, Jolles S. Persistent COVID-19 infection in Wiskott-Aldrich syndrome cleared following therapeutic vaccination: a case report. J Clin Immunol. 2022;42(1):32–35. doi: 10.1007/s10875-021-01158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cerqueira-Silva T, Andrews JR, Boaventura VS, Ranzani OT, de Araújo OV, Paixão ES, et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case-control study. Lancet Infect Dis. 2022;22(6):791–801. doi: 10.1016/s1473-3099(22)00140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kikuchi K, Nangaku M, Ryuzaki M, Yamakawa T, Yoshihiro O, Hanafusa N, et al. Effectiveness of SARS-CoV-2 vaccines on hemodialysis patients in Japan: a nationwide cohort study. Ther Apher Dial. 2022. 10.1111/1744-9987.13887. [DOI] [PMC free article] [PubMed]
- 150.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62. doi: 10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18(3):168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brown MJ, Coltart J, Gunewardena K, Ritter JM, Auton TR, Glover JF. Randomized double-blind placebo-controlled study of an angiotensin immunotherapeutic vaccine (PMD3117) in hypertensive subjects. Clin Sci (Lond) 2004;107(2):167–173. doi: 10.1042/cs20030381. [DOI] [PubMed] [Google Scholar]
- 153.Ambühl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25(1):63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 154.Li LD, Tian M, Liao YH, Zhou ZH, Wei F, Zhu F, et al. Effect of active immunization against angiotensin II type 1 (AT1) receptor on hypertension & arterial remodelling in spontaneously hypertensive rats (SHR) Indian J Med Res. 2014;139(4):619–624. [PMC free article] [PubMed] [Google Scholar]
- 155.Chen X, Qiu Z, Yang S, Ding D, Chen F, Zhou Y, et al. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension. 2013;61(2):408–416. doi: 10.1161/hypertensionaha.112.201020. [DOI] [PubMed] [Google Scholar]
- 156.Nakagami H, Ishihama T, Daikyoji Y, Sasakura C, Yamada E, Morishita R. Brief report on a phase I/IIa study to assess the safety, tolerability, and immune response of AGMG0201 in patients with essential hypertension. Hypertens Res. 2022;45(1):61–65. doi: 10.1038/s41440-021-00755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao B, Marciniuk K, Gibbs E, Yousefi M, Napper S, Cashman NR. Therapeutic vaccines for amyotrophic lateral sclerosis directed against disease specific epitopes of superoxide dismutase 1. Vaccine. 2019;37(35):4920–4927. doi: 10.1016/j.vaccine.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 158.Pang Z, Nakagami H, Osako MK, Koriyama H, Nakagami F, Tomioka H, et al. Therapeutic vaccine against DPP4 improves glucose metabolism in mice. Proc Natl Acad Sci U S A. 2014;111(13):E1256–E1263. doi: 10.1073/pnas.1322009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pan Y, Zhou Y, Wu H, Chen X, Hu X, Zhang H, et al. A therapeutic peptide vaccine against PCSK9. Sci Rep. 2017;7(1):12534. doi: 10.1038/s41598-017-13069-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shemesh CS, Hsu JC, Hosseini I, Shen BQ, Rotte A, Twomey P, et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol Ther. 2021;29(2):555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol. 2019;10:379. doi: 10.3389/fimmu.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alloatti A, Kotsias F, Magalhaes JG, Amigorena S. Dendritic cell maturation and cross-presentation: timing matters! Immunol Rev. 2016;272(1):97–108. doi: 10.1111/imr.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Song Q, Zhang CD, Wu XH. Therapeutic cancer vaccines: from initial findings to prospects. Immunol Lett. 2018;196:11–21. doi: 10.1016/j.imlet.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 164.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 165.Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(6):822–835. doi: 10.1016/s1470-2045(16)00099-1. [DOI] [PubMed] [Google Scholar]
- 166.Giaccone G, Bazhenova LA, Nemunaitis J, Tan M, Juhász E, Ramlau R, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer. 2015;51(16):2321–2329. doi: 10.1016/j.ejca.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 167.Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(1):59–68. doi: 10.1016/s1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 168.Rini BI, Stenzl A, Zdrojowy R, Kogan M, Shkolnik M, Oudard S, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(11):1599–1611. doi: 10.1016/s1470-2045(16)30408-9. [DOI] [PubMed] [Google Scholar]
- 169.van der Burg SH. Correlates of immune and clinical activity of novel cancer vaccines. Semin Immunol. 2018;39:119–136. doi: 10.1016/j.smim.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 170.Yu Y. Multi-target combinatory strategy to overcome tumor immune escape. Front Med. 2022;16(2):208–215. doi: 10.1007/s11684-022-0922-5. [DOI] [PubMed] [Google Scholar]
- 171.Esfandiary A, Ghafouri-Fard S. MAGE-A3: an immunogenic target used in clinical practice. Immunotherapy. 2015;7(6):683–704. doi: 10.2217/imt.15.29. [DOI] [PubMed] [Google Scholar]
- 172.Thomas R, Al-Khadairi G, Roelands J, Hendrickx W, Dermime S, Bedognetti D, et al. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front Immunol. 2018;9:947. doi: 10.3389/fimmu.2018.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buonaguro L, Tagliamonte M. Selecting target antigens for cancer vaccine development. Vaccines (Basel). 2020;8(4). 10.3390/vaccines8040615. [DOI] [PMC free article] [PubMed]
- 174.Pao SC, Chu MT, Hung SI. Therapeutic vaccines targeting Neoantigens to induce T-cell immunity against cancers. Pharmaceutics. 2022;14(4). 10.3390/pharmaceutics14040867. [DOI] [PMC free article] [PubMed]
- 175.Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines (Basel). 2020;8(3). 10.3390/vaccines8030391. [DOI] [PMC free article] [PubMed]
- 176.Srivastava PK. Neoepitopes of cancers: looking Back, looking ahead. Cancer Immunol Res. 2015;3(9):969–977. doi: 10.1158/2326-6066.Cir-15-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smith CC, Selitsky SR, Chai S, Armistead PM, Vincent BG, Serody JS. Alternative tumour-specific antigens. Nat Rev Cancer. 2019;19(8):465–478. doi: 10.1038/s41568-019-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18(4):215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yarchoan M, Johnson BA, 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17(4):209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fumet JD, Truntzer C, Yarchoan M, Ghiringhelli F. Tumour mutational burden as a biomarker for immunotherapy: current data and emerging concepts. Eur J Cancer. 2020;131:40–50. doi: 10.1016/j.ejca.2020.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schumacher TN, Scheper W, Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 184.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359(6375):582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moore TV, Nishimura MI. Improved MHC II epitope prediction - a step towards personalized medicine. Nat Rev Clin Oncol. 2020;17(2):71–72. doi: 10.1038/s41571-019-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang X, Yu Z, Liu W, Tang H, Yi D, Wei M. Recent progress on MHC-I epitope prediction in tumor immunotherapy. Am J Cancer Res. 2021;11(6):2401–2416. [PMC free article] [PubMed] [Google Scholar]
- 187.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kramps T, Elbers K. Introduction to RNA vaccines. Methods Mol Biol. 2017;1499:1–11. doi: 10.1007/978-1-4939-6481-9_1. [DOI] [PubMed] [Google Scholar]
- 189.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 190.Dunn GP, Sherpa N, Manyanga J, Johanns TM. Considerations for personalized neoantigen vaccination in malignant glioma. Adv Drug Deliv Rev. 2022;186:114312. doi: 10.1016/j.addr.2022.114312. [DOI] [PubMed] [Google Scholar]
- 191.Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LA, Appin C, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19(18):4951–4960. doi: 10.1158/1078-0432.Ccr-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 193.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee KL, Benz SC, Hicks KC, Nguyen A, Gameiro SR, Palena C, et al. Efficient tumor clearance and diversified immunity through Neoepitope vaccines and combinatorial immunotherapy. Cancer Immunol Res. 2019;7(8):1359–1370. doi: 10.1158/2326-6066.Cir-18-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kinkead HL, Hopkins A, Lutz E, Wu AA, Yarchoan M, Cruz K, et al. Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer. JCI Insight. 2018;3(20). 10.1172/jci.insight.122857. [DOI] [PMC free article] [PubMed]
- 196.Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2020;71:47–58. doi: 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- 197.Lhuillier C, Rudqvist NP, Yamazaki T, Zhang T, Charpentier M, Galluzzi L, et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest. 2021;131(5). 10.1172/jci138740. [DOI] [PMC free article] [PubMed]
- 198.Lussier DM, Alspach E, Ward JP, Miceli AP, Runci D, White JM, et al. Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads. Proc Natl Acad Sci U S A. 2021;118(24). 10.1073/pnas.2102611118. [DOI] [PMC free article] [PubMed]
- 199.Lee KL, Schlom J, Hamilton DH. Combination therapies utilizing neoepitope-targeted vaccines. Cancer Immunol Immunother. 2021;70(4):875–885. doi: 10.1007/s00262-020-02729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol Res. 2015;3(8):946–955. doi: 10.1158/2326-6066.Cir-14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 203.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 204.Leisegang M, Engels B, Schreiber K, Yew PY, Kiyotani K, Idel C, et al. Eradication of large solid tumors by gene therapy with a T-cell receptor targeting a single cancer-specific point mutation. Clin Cancer Res. 2016;22(11):2734–2743. doi: 10.1158/1078-0432.Ccr-15-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu S, Matsuzaki J, Wei L, Tsuji T, Battaglia S, Hu Q, et al. Efficient identification of neoantigen-specific T-cell responses in advanced human ovarian cancer. J Immunother Cancer. 2019;7(1):156. doi: 10.1186/s40425-019-0629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ma L, Dichwalkar T, Chang JYH, Cossette B, Garafola D, Zhang AQ, et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365(6449):162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Maliszewski C. Dendritic cells in models of tumor immunity. Role of Flt3 ligand. Pathol Biol (Paris) 2001;49(6):481–483. doi: 10.1016/s0369-8114(01)00172-9. [DOI] [PubMed] [Google Scholar]
- 208.Kreiter S, Diken M, Selmi A, Diekmann J, Attig S, Hüsemann Y, et al. FLT3 ligand enhances the cancer therapeutic potency of naked RNA vaccines. Cancer Res. 2011;71(19):6132–6142. doi: 10.1158/0008-5472.Can-11-0291. [DOI] [PubMed] [Google Scholar]
- 209.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yin R, Yin L, Li L, Silva-Nash J, Tan J, Pan Z, et al. Hypertension in China: burdens, guidelines and policy responses: a state-of-the-art review. J Hum Hypertens. 2022;36(2):126–134. doi: 10.1038/s41371-021-00570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lu K, Su B, Meng X. Recent advances in the development of vaccines for diabetes, hypertension, and atherosclerosis. J Diabetes Res. 2018;2018:1638462. doi: 10.1155/2018/1638462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Downham MR, Auton TR, Rosul A, Sharp HL, Sjostrom L, Rushton A, et al. Evaluation of two carrier protein-angiotensin I conjugate vaccines to assess their future potential to control high blood pressure (hypertension) in man. Br J Clin Pharmacol. 2003;56(5):505–512. doi: 10.1046/j.1365-2125.2003.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371(9615):821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 214.Garay-Gutiérrez NF, Hernandez-Fuentes CP, García-Rivas G, Lavandero S, Guerrero-Beltrán CE. Vaccines against components of the renin-angiotensin system. Heart Fail Rev. 2021;26(3):711–726. doi: 10.1007/s10741-020-10033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–80. 10.1016/s1474-4422(18)30499-x. [DOI] [PMC free article] [PubMed]
- 216.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 217.Thal DR, Walter J, Saido TC, Fändrich M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer's disease. Acta Neuropathol. 2015;129(2):167–182. doi: 10.1007/s00401-014-1375-y. [DOI] [PubMed] [Google Scholar]
- 218.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14(7):399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lacosta AM, Pascual-Lucas M, Pesini P, Casabona D, Pérez-Grijalba V, Marcos-Campos I, et al. Safety, tolerability and immunogenicity of an active anti-Aβ(40) vaccine (ABvac40) in patients with Alzheimer's disease: a randomised, double-blind, placebo-controlled, phase I trial. Alzheimers Res Ther. 2018;10(1):12. doi: 10.1186/s13195-018-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimers Res Ther. 2014;6(4):44. doi: 10.1186/alzrt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer's disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017;16(2):123–134. doi: 10.1016/s1474-4422(16)30331-3. [DOI] [PubMed] [Google Scholar]
- 222.Wang CY, Wang PN, Chiu MJ, Finstad CL, Lin F, Lynn S, et al. UB-311, a novel UBITh(®) amyloid β peptide vaccine for mild Alzheimer's disease. Alzheimers Dement (N Y) 2017;3(2):262–272. doi: 10.1016/j.trci.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther. 2009;26(5):488–499. doi: 10.1007/s12325-009-0030-9. [DOI] [PubMed] [Google Scholar]
- 224.Zhang Y, Yu XL, Zha J, Mao LZ, Chai JQ, Liu RT. Therapeutic vaccine against IL-1β improved glucose control in a mouse model of type 2 diabetes. Life Sci. 2018;192:68–74. doi: 10.1016/j.lfs.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 225.Hoogeveen RC, Boonstra A. Checkpoint inhibitors and therapeutic vaccines for the treatment of chronic HBV infection. Front Immunol. 2020;11:401. doi: 10.3389/fimmu.2020.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10(1):e1003856. doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.