Abstract
Background
Endolymphatic sac tumors are rare neoplasia characterized by slow growth. However, their clinical impact should not be underestimated, considering their potential for local aggressive behavior and strong association with von Hippel–Lindau syndrome. Therefore, early detection with emerging theragnostic examinations such as 68Ga-DOTATATE-PET/CT might improve patient management and reduce morbidity.
Methods
We report the clinicopathological features of seven endolymphatic sac tumors. In this cohort, we performed immunohistochemical analysis of somatostatin receptor 2A (SSTR2A) and prostate specific membrane antigen (PSMA) protein expression patterns; two targets providing rationale for novel imaging modalities such as PSMA- or SSTR-targeted PET.
Results
The tumor cells of all cases were negative for prostate specific membrane antigen and somatostatin receptor 2A, however immunolabeling was consistently detected in intratumoral endothelial cells of endolymphatic sac tumors for PSMA (7/7 cases, 100%), and for SSTR2A (5/7 cases, 71%).
Conclusions
Our results show a high rate of PSMA and SSTR2A expression in the tumor vasculature of endolymphatic sac tumors. PSMA and SSTR2A can be targeted with appropriate radioligands for diagnostic and therapeutic purposes. This finding provides a rationale for prospective clinical studies to test this approach as a sensitive screening tool for patients with suspected endolymphatic sac tumors including an improved management of von Hippel–Lindau syndrome.
Keywords: Endolymphatic sac, von Hippel-Lindau syndrome, Early detection of cancer, Ear neoplasms, Endothelial cells, Positron emission tomography computed tomography
Introduction
Endolymphatic sac tumors (ELSTs) are low-grade malignant epithelial tumors of neuroectodermal origin arising in the endolymphatic sac of the inner ear, located in the temporal bone and the dura of the posterior cranial fossa. The endolymphatic sac has been associated with several functions in the inner ear, such as regulation of the endolymph ionic content and volume, as well as immunologic functions [1–3].
ELSTs are extremely rare and growing slowly, presenting with unspecific symptoms, including tinnitus, hearing loss, vertigo and facial palsy [4, 5]. ELSTs occasionally show invasive and locally destructive growth, infiltrating the middle ear, middle and posterior cranial fossae and the cerebellopontine angle, with exceptional cases giving rise to metastatic disease [6]. Histologically, they are typically small, well-vascularized neoplasms with variable papillary, glandular or cystic morphology with a single layer of neoplastic epithelial cells [7]. Age at diagnosis is highly variable and surgical excision represents the treatment of choice, with the possibility of radiotherapy in advanced cases. At least a third of ELSTs are detected in patients with VHL syndrome, with up to 30% occurring bilaterally. In this setting, ELSTs are usually detected at a younger age and often as the first disease manifestation [8].
Von Hippel–Lindau disease is an autosomal dominantly inherited tumor predisposition syndrome with germline mutations in a tumor suppressor gene on chromosome 3 (VHL gene 3p25.3) [9]. In addition to ELSTs, VHL patients typically develop multiple hemangioblastomas of the central nervous system, clear cell renal cell carcinomas (ccRCC), pheochromocytomas, extra-adrenal paragangliomas, as well as pancreatic adenomas and neuroendocrine tumors [10]. Inactivation of the VHL tumor suppressor gene due to genetic alterations of wild type alleles has been identified in VHL patients as well as in sporadic ELSTs [11, 12]. This finding was recently confirmed in a larger cohort. Of note, in a minority of cases without VHL-alterations, Schweizer et al. found TERT promotor mutations [13]. Interestingly, these findings are similar to clear cell renal cell carcinoma, which may also harbor inactivating VHL mutations in sporadic cases [14].
Bi-allelic inactivation of the VHL gene leads to constitutive activation of the hypoxia-inducible factors (HIF-1 and HIF-2), promoting neovascularization through downstream targets such as vascular endothelial growth factor (VEGF), which is strongly expressed in the tumor cells of ELSTs [15, 16].
Immunohistochemical studies have consistently reported that ELSTs are immunoreactive for specific cytokeratins (CK7, CK8, CK18), as well as epithelial membrane antigen (EMA), vimentin, glial fibrillary acidic protein (GFAP), S100, paired-box-proteins 8 and 2 (PAX-8/ PAX-2) and carbonic anhydrase IX (CAIX). In contrast, thyroid transcription factor 1 (TTF-1), anti-renal cell carcinoma (RCC), CD10, CK20, as well as synaptophysin, chromogranin A, thyroglobulin and transthyretin are negative [15, 17, 18]. Reported proliferation index is very low, ranging between 1 and 3%; however, cases showing progression and invasive behavior suggest the existence of possible clones with higher proliferative potential [15].
The early detection of limited disease remains crucial, with complete surgical excision representing the most successful therapy to limit morbidity and mortality [5]. Next to conventional computed tomography (CT) and magnetic resonance imaging (MRI), gallium-68 (68Ga)-DOTATATE positron emission tomography (PET)/CT technique has emerged as a potential screening tool for ELSTs, with the original aim of detecting VHL-associated tumors such as hemangioblastomas, pancreatic neuroendocrine tumors, as well as paragangliomas [19]. Initially, somatostatin receptor (SSTR) expression was suspected to reside on the cell-surface of tumor cells; however, localization of somatostatin receptor type 2A (SSTR2A) expression has only recently been reported in the tumor vasculature with corresponding low-level radiotracer uptake in the tumor area in a single case [20].
Furthermore, SSTR2A expression has gained importance as a possible prognostic biomarker as well as potential diagnostic and therapeutic (theragnostic) target in tumor entities such as neuroendocrine tumors or nasopharyngeal carcinoma [21–24]. In this so-called theragnostic approach, structures on tumor cells and/or in tumor-associated neovasculature are targeted for diagnostic and therapeutic purposes. This concept was first applied to thyroid diseases, where it is part of standard therapeutic practice [25]. Novel radioligands are currently becoming an integral part of the diagnosis and therapy of prostate adenocarcinoma and neuroendocrine tumors. Considering the emerging applications of this technique, the aim of our study is to further characterize the immunohistochemical expression patterns of SSTR2A in ELSTs. Due to the established use of PSMA-PET in the staging and follow up of prostate cancer patients, and recognition of PSMA expression in a variety of other tumor entities, PSMA staining was also evaluated as a potential theragnostic target.
Materials and Methods
The study cohort consists of seven cases of ELSTs, all diagnosed between 1993 and 2018 at the Institute of Pathology and Molecular Pathology, in collaboration with the Institute of Neuropathology, University Hospital Zurich. All cases have been comprehensively reviewed by experienced head and neck pathologists (M.D.B., N.J.R.) and a neuropathologist (E.J.R.). Histopathological evaluation of hematoxylin and eosin (HE)-stained slides confirmed the initial diagnosis, with a representative tumor tissue block chosen for further investigations. Immunohistochemical staining of tumor was performed using commercially available antibodies on an automated staining system (Ventana Benchmark) with the following antibodies: pancytokeratin AE1/AE3 (monoclonal, 1:50, DAKO A/S); PAX8 (monoclonal, 1:100, Abcam Limited), somatostatin receptor 2A (SSTR2A) (polyclonal, 1:75, Zytomed Systems), Ki-67 (monoclonal, 30–9, prediluted) and prostate-specific membrane antigen (PSMA) (monoclonal, 1:25, DAKO A/S). Immunostaining for cytokeratin and PAX8 was assessed as either present or absent, while for SSTR2A and PSMA, staining intensity was scored as either negative (0), weak (1+), moderate (2+) or strong (3+). External controls for SSTR2A and PSMA included pancreatic and prostatic tissue.
Results
Clinical Data and Demographics
Table 1 contains clinical presentation, tumor size and location, as well as treatment approach of our cohort. The gender distribution (male:female ratio = 1.3:1) for the seven patients diagnosed with endolymphatic sac tumor was almost equal. Median age at diagnosis was 26 years and clinical information identified six patients with underlying von Hippel–Lindau (VHL) syndrome (6/7 cases, 86%). Available follow-up data of 6 patients with ELST, ranging from 12 to 130 months, showed disease recurrence in 2 patients (2/6, 33.3%), while one patient was lost to follow up.
Table 1.
Cohort clinicopathological data: case nr. 1–7 endolymphatic sac tumor
| Case number | Age at diagnosis | Gender | Tumor size (mm) | Tumor location | Morphology | Clinical presentation | Therapy |
|---|---|---|---|---|---|---|---|
| 1 | 44 | Male | 10 | Right cerebellopontine angle/posterior cranial fossa | Glandular-cystic | Hearing loss, tinnitus | Tumor resection by subtotal petrosectomy with resection of otic capsule |
| 2 | 18 | Female | 12 | Left vestibular aqueduct | Papillary/cystic | Vertigo, hearing loss | Tumor resection by subtotal petrosectomy without resection of otic capsule |
| 3 | 23 | Male | 15 | Left vestibular aqueduct | Papillary/cystic | Hearing loss, tinnitus | Translabyrinthine tumor resection |
| 4 | 21 | Female | 23 | Right posterior cranial fossa with labyrinthic infiltration | Papillary/cystic | No audiovestibular symptoms | Tumor resection by infratemporal fossa approach (Fisch type A) and subtotal petrosectomy |
| 5 | 43 | Male | 13 | Right posterior cranial fossa | Papillary/cystic | Deafness, vertigo | Tumor resection by subtotal petrosectomy with resection of otic capsule |
| 6 | 14 | Female | 5 | Right vestibular aqueduct | Papillary/cystic | Tinnitus | Transmastoid retrolabyrinthine tumor resection |
| 7 | 20 | Male | 10 | Left vestibular aqueduct and posterior semicircular canal | Papillary/cystic | No audiovestibular symptoms | Transmastoid tumor resection with partial resection of posterior semicircular canal |
Morphological Description
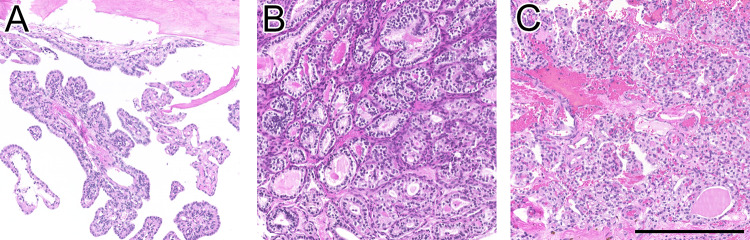
The histomorphological spectrum of ELSTs included both papillary and glandular-cystic growth patterns, with admixed patterns in most cases (5/7 cases, 71.3%). Representative growth patterns are illustrated in Fig. 1. Interestingly, in one case, the tumor initially showed papillary features, while one year later at relapse, cystic growth pattern dominated.
Fig. 1.
H&E-stained sections depicting the growth patterns of three representative ELSTs: A papillary; B glandular-cystic and C mixed papillary and glandular-cystic growth. Scale bar: 250 μm
Immunohistochemical Expression Characteristics
All cases showed diffuse, robust staining for cytokeratin (AE1/AE3) with positivity for PAX8 in all ELSTs.
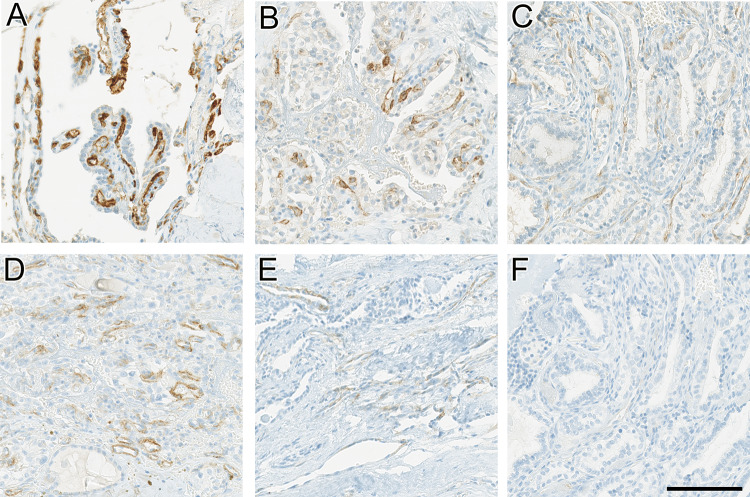
Immunostainings for PSMA and SSTR2A were negative in the constituent tumor cells in all cases of ELST. However, tumor-associated vessels immunolabeled with PSMA in all cases, including four cases (4/7, 66.7%) with moderate (2+) to strong (3+) intensity, and three cases (3/7, 33.3%) with weak (1+) immunostaining. In five cases (5/7, 71.4%), SSTR2A immunostaining was weaker than PSMA staining, but clearly detectable: Staining intensity was moderate (2+) in a single case (1/7, 14.3%), and weak (1+) in four cases (4/7, 57.1%). Figure 2 illustrates representative immunohistochemical expression patterns for PSMA and SSTR2A. No expression of SSTR2A or PSMA was noted in adjacent normal tissue. Similar to previous reports, the Ki-67 proliferation rate was very low, not exceeding 1%. Table 2 summarizes the staining results of the seven ELSTs.
Fig. 2.
Immunohistochemical expression patterns of PSMA and SSTR2 in our cohort of ELSTs, displaying the various staining intensities in the tumor vasculature, while epithelial tumor cells lining papillae and glandular structures remain negative. For each staining intensity, one representative case is shown: A strong (3+) PSMA expression in endothelial cells. B and C moderate (2+) and weak (1+) PSMA expression, respectively. D moderate (2+) SSTR2A expression in endothelial cells of the tumor vasculature. E and F weak (1+) and negative (0) SSTR2A expression. Scale bar: 100 μm
Table 2.
Cohort immunohistochemical results (staining intensity scores)
| Case number | PAX8 | SSTR2A/PSMA tumor cells | SSTR2A vessels | PSMA vessels | MIB-1 (Ki-67) |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 1 | < 1% |
| 2 | 1 | 0 | 1 | 3 | < 1% |
| 3 | 1 | 0 | 1 | 1 | < 1% |
| 4 | 1 | 0 | 1 | 3 | < 1% |
| 5 | 1 | 0 | 2 | 2 | < 1% |
| 6 | 1 | 0 | 0 | 1 | < 1% |
| 7 | 1 | 0 | 1 | 3 | < 1% |
PAX8 (0 = negative, 1 = positive), SSTR2A/PSMA (0 = negative, 1 = weak, 2 = moderate, 3 = strong)
Discussion
Endolymphatic sac tumors are part of the spectrum of mass lesions involving the inner ear. Their rarity as well as their characteristic slow growth accompanied by non-specific symptoms render the diagnosis challenging [5]. Conventional imaging techniques are limited in differentiating ELST from jugulotympanic paraganglioma, middle ear adenoma, meningioma, and choroid plexus papilloma [26]. Therefore, finding alternate screening modalities is of clinical interest and relevance.
To our knowledge, this represents the first study evaluating the expression of two commonly used radioligand targets, SSTR2A and PSMA, in a series of ELSTs. In more than 70% of our cases, we detected weak to moderate immunohistochemical expression of SSTR2A in the tumor vasculature of ELSTs, while the tumor cells remained negative. This staining pattern is in concordance with a recent case report of an ELST, in which the staining pattern was proposed as a histopathological correlate to weak but distinct tracer uptake in 68Ga-DOTATATE-PET/CT [20]. To date, two case reports have documented mild but distinct radiotracer uptake on 68Ga-DOTATATE-PET/CT of ELST (SUVmax 10.9 and 6.29) [19, 20]. The level of uptake was considerably lower than that reported for head and neck paragangliomas, an important differential diagnosis, indicating applicability as a possible clinical screening tool [27]. However, to the best of our knowledge, no prospective data have yet been reported on the correlation of endothelial SSTR2 expression in ELSTs and corresponding SSTR-targeting PET examinations. Paragangliomas and meningiomas strongly express SSTR2 on tumor cells, providing a rationale for the binding and accumulation of SSTR targeting agents such as DOTATATE [28, 29]. Middle ear adenoma, currently renamed as middle ear neuroendocrine tumor (MeNET) [30], usually show neuroendocrine differentiation and SSTR expression is indicated by positive octreotide scan [31]. Furthermore, high uptake on 68Ga-DOTATOC PET/CT followed by effective peptide receptor radionuclide therapy (PRRT) was reported [32]. Histologically, metastasis from renal, thyroid and prostate cancer might mimic ELST and need to be excluded.
Prostate-specific membrane antigen (PSMA)-PET has evolved as an important adjunct to conventional imaging techniques in the management of patients with prostate cancer [33]. Notably, immunohistochemical tumor expression patterns correlate with 68Ga-PSMA-11 accumulation in the staging and restaging setting of prostate cancer [34, 35].
Next to prostate cancer cells, PSMA targeted tracer uptake can be observed in normal tissue, with the strongest uptake in the kidneys and salivary glands [36]. However, in salivary glands evidence exists for a distinct PSMA-unrelated uptake mechanism [37, 38]. Additionally, PSMA targeted tracer uptake has been described in multiple malignancies from different organ systems, most likely attributable to PSMA expression in the endothelial cells of the tumor neovasculature. This observation has been consistently documented in clear cell renal cell carcinoma, and somewhat less consistently in breast cancer and non-small cell lung cancer [39]. Among the histologic mimics of ELSTs, thyroid and renal cancer showed variable PSMA expression in tumoral microvessels [39]. Here, we present first evidence for recurrent PSMA expression in the tumor neovasculature of ELSTs, comparable to the tumor entities described above. Even though staining intensity is variable, almost 70% of our cases show strong or clearly visible PSMA expression in tumor endothelial cells.
In summary, SSTR2, as well as PSMA are nonspecific markers found in many tumor entities with variable expression patterns. In the literature concordant data exist with our own experience of variable vascular PSMA and SSTR2 expression in clear cell renal cell carcinoma (unpublished data), as the morphologically closest differential diagnosis [39–41]. With our results, we added ELST to the list, although with a recurrent vascular expression pattern in a high percentage of cases for both markers. PSMA and SSTR2 do not aid in histopathologic diagnosis of this entity, which leans on morphology and other more specific immunohistochemical markers. However, our findings highlight the potential utility of SSTR- and/or PSMA-targeting PET examinations in the detection of ELSTs.
Limitations to our retrospective study include the lack of a preoperative PET/CT or PET/MR scan to demonstrate a relationship with possible radiotracer uptake. Further, our cohort is relatively small, yet representative, considering the rarity of this tumor.
Conclusion
Our data provide further insight into the protein expression patterns of endolymphatic sac tumors. PSMA and SSTR2A, two well-established radiotracer targets, were not detected in the epithelial tumor cells in our cohort. However, PSMA expression was noted in the tumor neovasculature of all ELSTs, and SSTR2A expression in the neovasculature of the majority of ELSTs. These results corroborate noninvasive techniques such as 68Ga-DOTATATE-PET as a potentially useful screening tool in patients with suspected ELST. This observation awaits validation as a more sensitive screening and follow-up modality for ELSTs in prospective and multidisciplinary studies with larger cohorts.
Author Contributions
All authors contributed significantly to this work, and approved the final manuscript.
Funding
Open access funding provided by University of Zurich. This work has been supported by a grant from the Iten-Kohaut Foundation/University Hospital Zurich Foundation to NJR. DB is supported by a national MD-PhD scholarship from the Swiss National Science Foundation (SNSF). AHE is supported by a career development grant (“Filling the Gap”) from the University of Zurich, Switzerland.
Data Availability
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to ethical/privacy restrictions.
Code Availability
Not applicable.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This study was performed after approval of the local ethics committee (Kantonale Ethikkommission Zürich, BASEC No 2020-01663). Written consent was obtained from all patients treated from 01/2016 for the use of their tissue and data. For cases treated earlier approval was obtained through a waiver from the ethics committee.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J Assoc Res Otolaryngol. 2003;4(2):139–147. doi: 10.1007/s10162-002-3025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckhard AH, Zhu M, O'Malley JT, Williams GH, Loffing J, Rauch SD, et al. Inner ear pathologies impair sodium-regulated ion transport in Meniere’s disease. Acta Neuropathol. 2019;137(2):343–357. doi: 10.1007/s00401-018-1927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bächinger D, Egli H, Goosmann MM, Monge Naldi A, Eckhard AH. Immunolocalization of calcium sensing and transport proteins in the murine endolymphatic sac indicates calciostatic functions within the inner ear. Cell Tissue Res. 2019;378(2):163–173. doi: 10.1007/s00441-019-03062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae SH, Kim SS, Kwak SH, Jung JS, Choi JY, Moon IS. Clinical features and treatment of endolymphatic sac tumor. Acta Otolaryngol. 2020;140(6):433–437. doi: 10.1080/00016489.2020.1722855. [DOI] [PubMed] [Google Scholar]
- 5.Wick CC, Manzoor NF, Semaan MT, Megerian CA. Endolymphatic sac tumors. Otolaryngol Clin N Am. 2015;48(2):317–330. doi: 10.1016/j.otc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Tay KY, Yu E, Kassel E. Spinal metastasis from endolymphatic sac tumor. AJNR Am J Neuroradiol. 2007;28(4):613–614. [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson LD. Endolymphatic sac tumor. Ear Nose Throat J. 2013;92(4–5):184–188. doi: 10.1177/014556131309200408. [DOI] [PubMed] [Google Scholar]
- 8.Bausch B, Wellner U, Peyre M, Boedeker CC, Hes FJ, Anglani M, et al. Characterization of endolymphatic sac tumors and von Hippel-Lindau disease in the international endolymphatic sac tumor registry. Head Neck. 2016;38(Suppl 1):E673–E679. doi: 10.1002/hed.24067. [DOI] [PubMed] [Google Scholar]
- 9.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd RV, Osamura RY, Klöppel G, Rosai J, editors. WHO Classification of Tumours of Endocrine Organs. 4. Lyon: IARC; 2017. [Google Scholar]
- 11.Hamazaki S, Yoshida M, Yao M, Nagashima Y, Taguchi K, Nakashima H, et al. Mutation of von Hippel-Lindau tumor suppressor gene in a sporadic endolymphatic sac tumor. Hum Pathol. 2001;32(11):1272–1276. doi: 10.1053/hupa.2001.28961. [DOI] [PubMed] [Google Scholar]
- 12.Vortmeyer AO, Huang SC, Koch CA, Governale L, Dickerman RD, McKeever PE, et al. Somatic von Hippel-Lindau gene mutations detected in sporadic endolymphatic sac tumors. Cancer Res. 2000;60(21):5963–5965. [PubMed] [Google Scholar]
- 13.Schweizer L, Thierfelder F, Thomas C, Soschinski P, Kim HY, Jodicke R, et al. Molecular characterisation of sporadic endolymphatic sac tumours and comparison to von Hippel-Lindau disease-related tumours. Neuropathol Appl Neurobiol. 2021;47(6):756–767. doi: 10.1111/nan.12741. [DOI] [PubMed] [Google Scholar]
- 14.Razafinjatovo C, Bihr S, Mischo A, Vogl U, Schmidinger M, Moch H, et al. Characterization of VHL missense mutations in sporadic clear cell renal cell carcinoma: hotspots, affected binding domains, functional impact on pVHL and therapeutic relevance. BMC Cancer. 2016;16:638. doi: 10.1186/s12885-016-2688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horiguchi H, Sano T, Toi H, Kageji T, Hirokawa M, Nagahiro S. Endolymphatic sac tumor associated with a von Hippel-Lindau disease patient: an immunohistochemical study. Mod Pathol. 2001;14(7):727–732. doi: 10.1038/modpathol.3880380. [DOI] [PubMed] [Google Scholar]
- 16.Mao ML, Zhao YH, Ma DL, Liu HG. Expression of VHL, VEGF and HIF-1α in endolymphatic sac tumors. Zhonghua Bing Li Xue Za Zhi = Chin J Pathol. 2021;50(11):1228–1233. doi: 10.3760/cma.j.cn112151-20210324-00231. [DOI] [PubMed] [Google Scholar]
- 17.Jester R, Znoyko I, Garnovskaya M, Rozier JN, Kegl R, Patel S, et al. Expression of renal cell markers and detection of 3p loss links endolymphatic sac tumor to renal cell carcinoma and warrants careful evaluation to avoid diagnostic pitfalls. Acta Neuropathol Commun. 2018;6(1):107. doi: 10.1186/s40478-018-0607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson LDR, Magliocca KR, Andreasen S, Kiss K, Rooper L, Stelow E, et al. CAIX and pax-8 commonly immunoreactive in endolymphatic sac tumors: a clinicopathologic study of 26 cases with differential considerations for metastatic renal cell carcinoma in von Hippel-Lindau patients. Head Neck Pathol. 2019;13(3):355–363. doi: 10.1007/s12105-018-0973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadakis GZ, Millo C, Sadowski SM, Bagci U, Patronas NJ. Endolymphatic sac tumor showing increased activity on 68Ga DOTATATE PET/CT. Clin Nucl Med. 2016;41(10):783–784. doi: 10.1097/RLU.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou R, Lazor JW, Baraban E, Ware JB, Cooper K, Pantel AR. 68Ga-dotatate uptake in an endolymphatic sac tumor: radiologic-pathologic correlation. Clin Nucl Med. 2020;45(7):563–565. doi: 10.1097/RLU.0000000000003092. [DOI] [PubMed] [Google Scholar]
- 21.Emanuel O, Liu J, Schartinger VH, Nei WL, Chan YY, Tsang CM, et al. SSTR2 in Nasopharyngeal carcinoma: relationship with latent EBV infection and potential as a therapeutic target. Cancers. 2021;13(19):4944. doi: 10.3390/cancers13194944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner M, Schartinger VH, Steele CD, Nei WL, Ooft ML, Schreiber LM, et al. Somatostatin receptor 2 expression in nasopharyngeal cancer is induced by epstein barr virus infection: impact on prognosis, imaging and therapy. Nat Commun. 2021;12(1):117. doi: 10.1038/s41467-020-20308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Pang Y, Wang Y, Chen J, Zhuang Y, Zhang J, et al. Somatostatin receptor imaging with [68Ga] Ga-DOTATATE positron emission tomography/computed tomography (PET/CT) in patients with nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging. 2021;49(4):1360–1373. doi: 10.1007/s00259-021-05587-7. [DOI] [PubMed] [Google Scholar]
- 24.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhury PS, Gupta M. Differentiated thyroid cancer theranostics: radioiodine and beyond. Br J Radiol. 2018;91(1091):20180136. doi: 10.1259/bjr.20180136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel NP, Wiggins RH, Shelton C. The radiologic diagnosis of endolymphatic sac tumors. Laryngoscope. 2006;116(1):40–46. doi: 10.1097/01.mlg.0000185600.18456.36. [DOI] [PubMed] [Google Scholar]
- 27.Chang CA, Pattison DA, Tothill RW, Kong G, Akhurst TJ, Hicks RJ, et al. 68Ga-DOTATATE and (18)F-FDG PET/CT in paraganglioma and pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging. 2016;16(1):22. doi: 10.1186/s40644-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leijon H, Remes S, Hagström J, Louhimo J, Mäenpää H, Schalin-Jäntti C, et al. Variable somatostatin receptor subtype expression in 151 primary pheochromocytomas and paragangliomas. Hum Pathol. 2019;86:66–75. doi: 10.1016/j.humpath.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W, Zhou Y, Wang Y, Liu L, Lou J, Deng Y, et al. Clinical significance of somatostatin receptor (SSTR) 2 in meningioma. Front Oncol. 2020;10:1633. doi: 10.3389/fonc.2020.01633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandison A. Update from the 5th edition of the World Health Organization classification of head and neck tumours: tumours of the ear. Head Neck Pathol. 2022;16(1):76. doi: 10.1007/s12105-022-01450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinelli JP, Cass SP, Mann SE, Haynes DS, Hunter JB, Isaacson B, et al. Adenomatous neuroendocrine tumors of the middle ear: a multi-institutional investigation of 32 cases and development of a staging system. Otol Neurotol. 2018;39(8):e712–e721. doi: 10.1097/MAO.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 32.Lima Ferreira J, Marques B, der Menke-van H, van Oordt CW, de Herder WW, Brabander T, Hofland J. Treatment with somatostatin analogues and PRRT in metastatic middle ear adenoma with neuroendocrine features. Endocrinol Diabetes Metab Case Rep. 2021 doi: 10.1530/EDM-20-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 34.Ferraro DA, Rüschoff JH, Muehlematter UJ, Kranzbühler B, Müller J, Messerli M, et al. Immunohistochemical PSMA expression patterns of primary prostate cancer tissue are associated with the detection rate of biochemical recurrence with 68Ga-PSMA-11-PET. Theranostics. 2020;10(14):6082–6094. doi: 10.7150/thno.44584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rüschoff JH, Ferraro DA, Muehlematter UJ, Laudicella R, Hermanns T, Rodewald AK, et al. What's behind. Eur J Nucl Med Mol Imaging. 2021;48(12):4042–4053. doi: 10.1007/s00259-021-05501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40(4):486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 37.Rupp NJ, Umbricht CA, Pizzuto DA, Lenggenhager D, Töpfer A, Müller J, et al. First clinicopathologic evidence of a non-PSMA-related uptake mechanism for 68Ga-PSMA-11 in Salivary Glands. J Nucl Med. 2019;60(9):1270–1276. doi: 10.2967/jnumed.118.222307. [DOI] [PubMed] [Google Scholar]
- 38.Tönnesmann R, Meyer PT, Eder M, Baranski AC. [177Lu]Lu-PSMA-617 salivary gland uptake characterized by quantitative in vitro autoradiography. Pharmaceuticals. 2019;12(1):18. doi: 10.3390/ph12010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backhaus P, Noto B, Avramovic N, Grubert LS, Huss S, Bögemann M, et al. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur J Nucl Med Mol Imaging. 2018;45(5):860–877. doi: 10.1007/s00259-017-3922-y. [DOI] [PubMed] [Google Scholar]
- 40.Peter L, Sänger J, Hommann M, Baum RP, Kaemmerer D. Molecular imaging of late somatostatin receptor-positive metastases of renal cell carcinoma in the pancreas by 68Ga DOTATOC PET/CT: a rare differential diagnosis to multiple primary pancreatic neuroendocrine tumors. Clin Nucl Med. 2014;39(8):713–716. doi: 10.1097/RLU.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 41.Höög A, Kjellman M, Mattsson P, Juhlin CC, Shabo I. Somatostatin receptor expression in renal cell carcinoma–a new front in the diagnostics and treatment of renal cell carcinoma. Clin Genitourin Cancer. 2018;16(3):e517–e520. doi: 10.1016/j.clgc.2018.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to ethical/privacy restrictions.
Not applicable.