Abstract
Human health is linked to climatic factors in complex ways, and climate change can have profound direct and indirect impacts on the health status of any given region. Susceptibility to climate change is modulated by biological, ecological and socio-political factors such as age, gender, geographic location, socio-economic status, occupation, health status and housing conditions, among other.
In the Eastern Mediterranean and Middle East (EMME), climatic factors known to affect human health include extreme heat, water shortages and air pollution. Furthermore, the epidemiology of vector-borne diseases (VBDs) and the health consequences of population displacement are also influenced by climate change in this region.
To inform future policies for adaptation and mitigation measures, and based on an extensive review of the available knowledge, we recommend several research priorities for the region. These include the generation of more empirical evidence on exposure-response functions involving climate change and specific health outcomes, the development of appropriate methodologies to evaluate the physical and psychological effects of climate change on vulnerable populations, determining how climate change alters the ecological determinants of human health, improving our understanding of the effects of long-term exposure to heat stress and air pollution, and evaluating the interactions between adaptation and mitigation strategies.
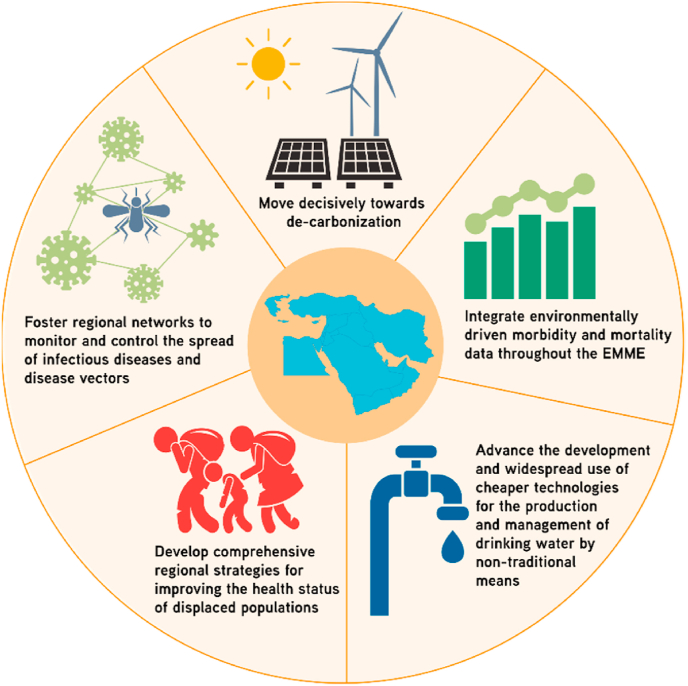
Because national boundaries do not limit most climate-related factors expected to impact human health, we propose that adaptation/mitigation policies must have a regional scope, and therefore require collaborative efforts among EMME nations. Policy suggestions include a decisive region-wide decarbonisation, the integration of environmentally driven morbidity and mortality data throughout the region, advancing the development and widespread use of affordable technologies for the production and management of drinking water by non-traditional means, the development of comprehensive strategies to improve the health status of displaced populations, and fostering regional networks for monitoring and controlling the spread of infectious diseases and disease vectors.
Keywords: Middle East, Eastern Mediterranean, Climate change, Health, Extreme heat, Water shortage, Air pollution, Vector-borne diseases, Population displacement
Highlights
-
•
Extreme heat, water shortages and air pollution affect human health in the Eastern Mediterranean and Middle East.
-
•
Population displacement and infectious disease biology are also affected by climate change in the region.
-
•
In this manuscript, we provide a comprehensive review of the relevant literature and identify research gaps.
-
•
We suggest mitigation and adaptation policies which can improve regional resilience to environmental change.
1. Introduction
The Eastern Mediterranean and Middle East (EMME) is a broad and diverse geographic area encompassing the countries of Bahrain, Cyprus, Egypt, Greece, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Oman, Palestine, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates (UAE) and Yemen (Fig. 1). Climate is very variable within this region, with temperate conditions (i.e. hot, dry summers and relatively mild winters) prevailing in the north, while in the south climatic conditions are those normally associated with desert areas: arid, hot, and mostly devoid of precipitation and vegetation (Lelieveld et al., 2012).
Fig. 1.
Map of the EMME region. The countries included in the EMME region are Bahrain, Cyprus, Egypt, Greece, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Oman, Palestine, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates (UAE) and Yemen.
It is estimated that the EMME is home to approximately 460 million inhabitants, with three countries (Egypt, Iran and Turkey) accounting for over 58% of the region's total human population (United Nations Department of Economic and Social Affairs, 2019). Significant inequalities in economic development are prevalent among the countries of this region; for example, per capita gross domestic product (PC-GDP) in 2018 ranged from $758 in Yemen to $65,908 in Qatar (The World Bank, 2020). In fact, economic data reported for the same year show that the combined per-capita wealth (as measured by PC-GDP) of the region's five wealthiest countries (Qatar, UAE, Israel, Kuwait and Cyprus) is roughly 18 times higher than that of the five poorest countries (Yemen, Syria, Egypt, Palestine and Iran) combined (The World Bank, 2020).
Climate change affects every aspect of human life in complex and inter-connected ways. The impacts of climate change on health can be either direct (e.g., exposure to extreme and unusual temperatures, drought and flooding) or indirect (e.g., through changes in infectious disease epidemiology, vector ecology and variations in the availability and/or quality of food, water and air). These effects are further compounded by a variety of biological, ecological and socio-political factors. For countries in the EMME region, the vulnerability to climate change is heightened by factors such as high rates of population growth and urbanization, aging population, political and military conflicts, mass population displacement and, in some cases, low economic performance (Al-Delaimy et al., 2020; Linares et al., 2020a; Watts et al., 2021).
In this review, we synthetize the current knowledge regarding the effects that climate change exerts on the health of people living on the EMME region, with particular focus on exposure to extreme heat, water shortage, air pollution, vector-borne diseases and the health of displaced populations. Furthermore, we identify gaps in knowledge and research infrastructures that, when addressed, will help us better understand and monitor the impacts of climate change on human health. Finally, we offer policy suggestions aimed at ameliorating some of these effects especially among vulnerable individuals including pregnant women, infants, older adults, people with chronic or pre-existing medical conditions, and socially marginalised groups such as forcefully displaced populations and the poor.
For these purposes, we performed a comprehensive revision of relevant literature using on-line scholarly databases including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/) and Google Scholar (https://scholar.google.com/). Our selection criteria did not explicitly follow PRISMA guidelines. We used different combinations of the following terms as keywords for our database searches: “climate change”, “middle east”, “eastern Mediterranean”, “human health”, “public health”, “heat wave”, “water scarcity”, “air quality”, “dust”, “wildfire”, “vector borne disease” and “population displacement”. We selected articles published in peer-review journals, as well as relevant books, book chapters, technical reports and on-line resources published by authoritative sources (e.g. World Bank, United Nations, World Health Organization, national statistical services). We excluded papers published before the year 2000, and papers published in languages other than English. We performed an in-depth qualitative reading of the selected literature and, when deemed necessary, we obtained and included in our analysis specific references cited in it.
2. Extreme heat
The ability to regulate body temperature is part of the basic physiological characteristics that have allowed humans to survive and function in a diverse range of environments across the globe (Lim, 2020). Normally, the human body regulates its core temperature to remain between ∼37 °C and 40 °C1 (Lim, 2020; Lim et al., 2008). The body's ability to stay within these functional limits is intricately linked to the temperature of the direct surroundings, with higher environmental temperatures making it more difficult to dissipate the heat produced by metabolic processes, especially when it is combined with high humidity, which in turn can lead to various acute and chronic health issues.
When the body's core temperature rises above 40.5 °C, there is an elevated risk of heat stroke (Gauer and Meyers, 2019). It has been postulated that the thermal regulation capacity of humans reaches its sustainable limit when environmental “wet bulb” temperature (a measure of combined temperature and humidity) exceeds 35 °C (Pal and Eltahir, 2016); this temperature is considered to represent the limit for human survival. However, health problems related to increased environmental temperatures can also arise from exposure to temperatures well below this limit, especially if the exposure is maintained for extended periods of time.
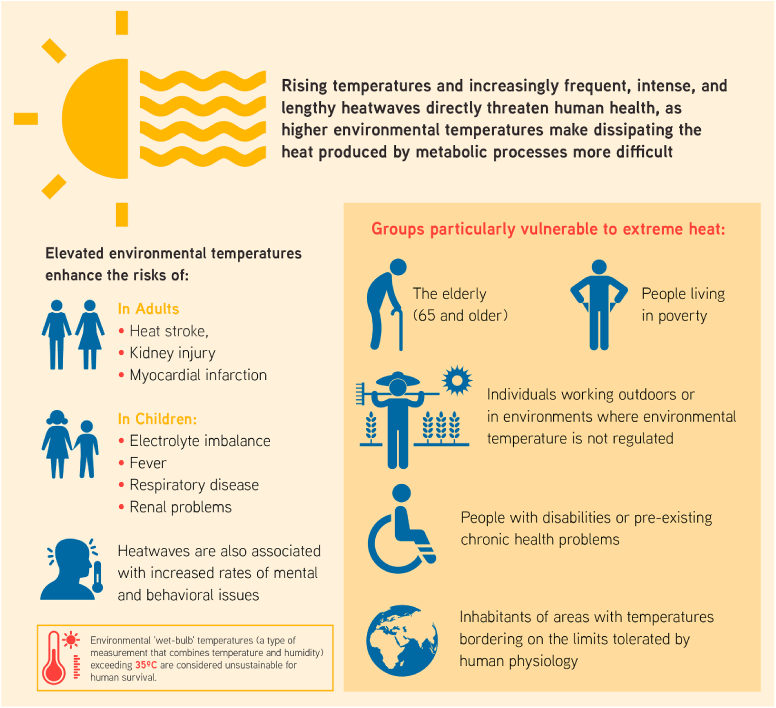
Rising average environmental temperatures, as well as the increased frequency, intensity and duration of heatwaves,2 are among the most evident and most direct threats to human health caused by climate change. The main health conditions associated with heat exposure include heat stress, heat stroke, acute and chronic kidney injury and myocardial infarction in adults (Parker et al., 2019; Watts et al., 2019), as well as electrolyte imbalance, fever, respiratory disease and renal problems in children (Watts et al., 2019). Additionally, heatwaves have been associated with increased rates of mental and behavioural issues such as sleep disturbances, cognitive deficits, aggressive and criminal behavior, collective violence, assault, homicide and suicide (Watts et al., 2019; Palinkas and Wong, 2020; Levy et al., 2017) (Fig. 2).
Fig. 2.
The effects of increasing environmental temperatures on human health.
On a global scale, one in three heat-related deaths recorded between 1990 and 2018 are attributable to man-made global warming (Vicedo-Cabrera et al., 2021). This estimate is believed to be much higher for Middle Eastern countries, where up to two in three deaths from heat are attributable to human-made global warming (e.g. 63% in Iran and 68% in Kuwait) (Vicedo-Cabrera et al., 2021).
Older adults (i.e., persons aged 65 years and older) are among the groups most vulnerable to extreme heat exposure (Watts et al., 2021; Meade et al., 2020). Other susceptible populations include people living in poverty, people with disabilities or pre-existing chronic health problems, individuals working outdoors or in environments where the ambient temperature is not regulated and the inhabitants of certain regions of the planet where temperatures already border the limits tolerated by human physiology (Watts et al., 2021) (Fig. 2). Furthermore, the effects of heat on human health are also modulated by urban characteristics (such as population density and air pollution levels) and socio-economic factors (i.e., gross domestic product and income inequality). It has been reported that both an abundance of green spaces and a high prevalence of air-conditioning units represent effective adaptation strategies to mitigate the negative health effects of heat (Sera et al., 2019, 2020).
Because of the interaction between age and susceptibility to rising environmental temperatures, ongoing global demographic shifts are particularly relevant: in 2018, the global proportion of people over 65 years became larger than the proportion of those under 5 years (Chen et al., 2020) for the first time in history, and is expected to reach 16% of the world's population by 2050 (United Nations, 2019). Older individuals are particularly susceptible to environmental threats due to factors such as the reduction of their social support networks, increased prevalence of chronic health problems, reduced cognitive capabilities and the physiological decline normally associated with aging (which importantly includes a significant reduction in mobility and thermoregulatory capacity) (Watts et al., 2021; Levy et al., 2017; Meade et al., 2020; Sera et al., 2019, 2020; Millyard et al., 2020). In this context, several studies have also identified gender as a risk factor for heat-related mortality in older adults, with women showing greater susceptibility than men (van Steen et al., 2019). Furthermore, extreme heat exposure has been reported to cause severe negative effects in pregnant women and unborn children (Zhang et al., 2019). Therefore, child-bearing women can also be considered a group particularly susceptible to extreme heat exposure.
Chronic conditions that increase susceptibility to environmental heat include diabetes and other endocrine disorders, cardiovascular disease, mental and neurological disorders (such as Alzheimer's, dementia, schizophrenia and Parkinson's), obesity, cystic fibrosis, renal disease and respiratory disease (World Health Organization (WHO), 2011; Smith, 2019). In addition to the physiological, cognitive and/or behavioural problems normally associated with these conditions (which directly impair the affected individuals' ability to successfully cope with increased temperatures), the medicines required to manage them can also interfere with the body's thermoregulatory processes, further increasing vulnerability in those afflicted by chronic health issues (World Health Organization (WHO), 2011; Li et al., 2015).
In a study looking at the relationship between increasing environmental temperature and daily mortality, Vicedo-Cabrera et al. (2018) have shown that the mortality caused by increased heat has declined over the past decades in many countries. However, this trend is not uniform across the globe, or even across regions relatively homogeneous in their social and economic structures, such as continental Europe. De’Donato et al. (de’ Donato et al., 2015) found that the reduction of mortality due to heat in the past 20 years has been mostly observed in Europe's warmer regions close to the Mediterranean, but not in Northern Europe, where in fact mortality associated to heat exposure has risen (de’ Donato et al., 2015; Linares et al., 2020b). Therefore, it seems that the reduction in heat-related mortality can be attributed, at least in part, to the fact that people living in warmer regions have developed an enhanced awareness of the health risks posed by increasingly warmer weather, leading them to adopt protective practices and behaviours such as the implementation of ‘heat health action plans’, early warning and response systems, air conditioning, building designs that maintain cool temperatures in indoor spaces and medical services that efficiently prevent and/or treat heat-related health issues (Linares et al., 2020b; Díaz et al., 2018). These protective factors might not be as prevalent in more temperate regions, increasing their inhabitants' vulnerability to extreme heatwaves (de’ Donato et al., 2015). In this context, Ramis and Amengual (2017) conclude that vulnerability is temporally and spatially variable, with steeper increases commonly observed in regions where extreme temperatures are rare.
2.1. Heat-related morbidity and mortality in the Eastern Mediterranean and Middle East
In Europe and the EMME, a combination of an ageing population, high prevalence of chronic diseases and intense urbanization renders these regions highly vulnerable to heat exposure (Watts et al., 2019; Kendrovski et al., 2017). This enhanced vulnerability has become evident in recent years, as heatwaves have broken temperature records and caused tens of thousands of deaths across Western and Central Europe (as was the case during the heatwave of 2003) (Robine et al., 2008), as well as Eastern Europe (heatwave of 2010) (Barriopedro et al., 2011). More recently, excess mortality attributable to increased environmental temperatures has been recorded in several cities in the region, including Athens, Crete and Istanbul (Can et al., 2019; Tsekeri et al., 2020; Zafeiratou et al., 2019; Paravantis et al., 2017). Within the first seven months of 2022, over 1700 heat-related deaths have been reported in Spain and Portugal alone, with many additional fatalities expected in the region before the summer is over (WHO, 2022).
Countries in the EMME region have also reported increased health concerns related to increasing temperatures. Studies in both Cyprus and Kuwait have found an increase in relative mortality risk due to cardiovascular disease associated with increased average daily temperatures (Lubczyńska et al., 2015; Alahmad et al., 2020). In Iran, Mohammadi et al. (2018) reported increased rates of acute myocardial infarctions due to elevated environmental temperatures, particularly in males and older adults. In a report about the interaction between temperature and mortality in Cyprus, Heaviside et al. (2016) projected that heat-related mortality would double with a 1 °C increase in the daily maximum temperature, and a 5 °C increment would cause an almost 8-fold increase in mortality. The risk of heat-related mortality in Nicosia (Cyprus's capital) is reported to be higher due to the urban heat island effect3 (Pyrgou and Santamouris, 2018; Oke, 1982; Li and Bou-Zeid, 2013). In Egypt, it has been estimated that under a business-as-usual scenario (Representative Concentration Pathway 8.5) approximately 80% of the days in the last decade of the 21st century (2090–2100) will be hotter than 90% of the days of the 2006–2015 period (Mostafa et al., 2019).
The epidemiology of some infectious diseases has been linked to variations in temperature and humidity due to climate change. Saad-Hussein et al. (Saad Hussein et al., 2011) found that the incidence of fungal keratitis in the greater Cairo area increased significantly with rises in minimum temperature and maximum atmospheric humidity during 1997–2007. Predicted increases in the incidence of this disease up to the year 2030 correspond to expected increases in surface temperature and greenhouse gas emissions in the region (Saad Hussein et al., 2011).
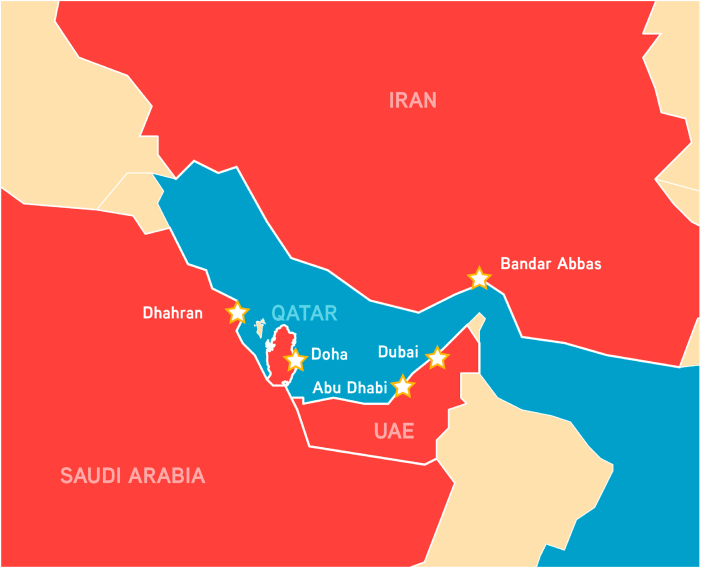
In the future, under a business-as-usual greenhouse gas concentration growth scenario, the death toll caused by heatwaves in Europe is predicted to account for 99% of all weather-related disaster fatalities by the end of the century (Forzieri et al., 2017). Parts of the EMME region are expected to reach maximum daytime temperatures of around 50 °C (Lelieveld et al., 2016), and could in exceptional cases exceed 60 °C (Pal and Eltahir, 2016). The effect of this marked increase in environmental temperature could be further amplified in densely populated areas due to the urban heat island effect (Li and Bou-Zeid, 2013). As a result, by the end of the century cities around the Persian (Arabian) Gulf, including Abu Dhabi and Dubai (United Arab Emirates), Doha (Qatar), Dhahran (Saudi Arabia) and Bandar Abbas (Iran) could experience periods of such intense heat that they might surpass the thermal limits suitable for human survival (Pal and Eltahir, 2016) (Fig. 3).
Fig. 3.
Cities where future environmental temperatures could surpass the limits for human survival. By the year 2100, five cities (represented with stars) located in four EMME countries (Iran, Qatar, Saudi Arabia and the United Arab Emirates) could reach environmental “wet bulb” temperatures above 35 °C, surpassing the threshold for the human body's physiological adaptability. UAE: United Arab Emirates. Source: Pal et al. 2016 (Pal and Eltahir, 2016).
3. Water scarcity
The Middle East and North Africa (MENA) is among regions with the lowest water availability in the world, with only approximately 1100 cubic metres (m3) of natural renewable water resources (NRWR) per capita per year (North Atlantic Treaty Organization (NATO), 2019; Wold Bank, 2007). In comparison, water-rich regions such as Australia and Latin America count over 34 000 m3 NRWR per capita per year, and Western Europe has over 5000 m3 NRWR (Wold Bank, 2007). The most widely accepted measure of water availability, known as the “Falkenmark indicator” or “water stress index”, establishes 1700 m3 NRWR per capita per year as the minimum threshold to sustain a population's basic needs. Countries that fall below this figure are considered to experience water stress. Countries with less than 1000 m3 suffer from water scarcity, and countries with less than 500 m3 suffer from absolute water scarcity (Falkenmark et al., 1989; Rijsberman, 2006).
While in several of the EMME countries water supply is based on desalination projects, 9 out of 15 countries in the Middle East experience absolute water scarcity, with one country (Kuwait) possessing practically no internal NRWR (North Atlantic Treaty Organization (NATO), 2019; World Bank, 2018). Overall, more than 60% of the region's population is estimated to live under either “high” or “very high” water stress conditions (North Atlantic Treaty Organization (NATO), 2019). Projections suggest that under a business-as-usual scenario, all countries in the region could deplete their groundwater reserves by the year 2050 (North Atlantic Treaty Organization (NATO), 2019). Chenoweth et al. (2011) have projected that “If the internal water footprint of the region declines in line with precipitation but the total water footprint of the region increases in line with population, then by mid-century, as much as half the total water needs of the region may need to be provided through desalination and imported in the form of virtual water”.
In the Southeast Mediterranean region water availability is similarly constrained, with an estimated 180 million inhabitants experiencing water scarcity conditions, and about 80 million people living under absolute water scarcity (Ferragina et al., 2010). This situation is further complicated by the region's quickly growing population, increasing rural-to-urban migration and heavy dependence on water-intensive farming, which is the main source of income for up to 79% of its rural population (Dassonville and Fé D'ostiani, 2006). Additionally, the renowned appeal of the Mediterranean coasts as tourist destinations often creates a large seasonal increase in the demand for potable water. This effect can be clearly observed in Cyprus, a country with less than 900 000 inhabitants that attracted between 3.1 and 3.9 million tourists per year between 2016 and 2019 (The Statistical Service of Cyprus (CYSTAT), 2021). It has been estimated that water demands associated with tourism account for 4.7% of the total water consumption in Cyprus (Sofroniou and Bishop, 2014).
3.1. Direct impacts of safe water scarcity on human health
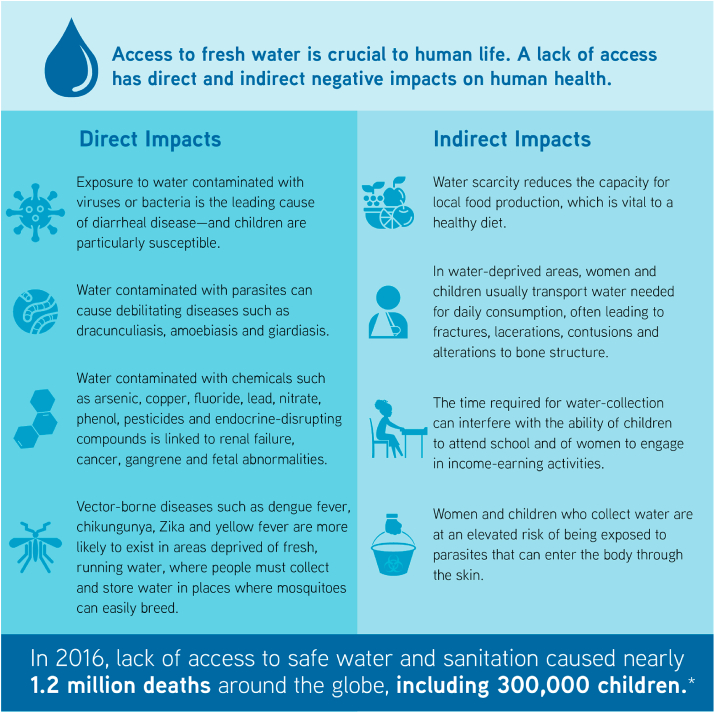
Freshwater is vital to life, and the health of human populations is heavily dependent on the availability of a “safe, reliable, affordable and easily accessible” water supply (Hunter et al., 2010). The lack of access to potable water affects human health in a variety of ways. Perhaps most important, exposure to water contaminated with viruses or bacteria is the leading cause of diarrhoeal diseases, one of the top five contributors to the global disease burden (Hunter et al., 2010; Abbafati et al., 2020). Children are particularly susceptible to the effect of water-borne infections, with diarrhoeal diseases ranking as the third-most important cause of diseases among children aged nine or younger, surpassed only by neonatal disorders and lower respiratory infections (Abbafati et al., 2020). In 2016 alone, the lack of access to safe water and sanitation caused nearly 1.2 million deaths worldwide, including 300 000 children aged five years or less who died of diarrhoea (World Health Organization (WHO), 2020a).
Although several countries in the MENA have substantial death rates caused by diarrhoea in young children, Syria is currently by far the country with the worst outlook, with approximately 15% of its infant mortality attributable to this cause (a value well above the world's average, which is 9%) (World Bank, 2018). When comparing countries of similar gross domestic product, those with better access to improved water services display significantly lower infant mortality rates (Hunter et al., 2010). It is also worth mentioning that outbreaks of water-borne diseases are not exclusive to developing nations, as disease outbreaks linked to either drinking or recreational water are reported every year in developed countries (Younes and Bartram, 2001). Furthermore, it has been suggested that about one-third of all diarrhoea cases are linked to the consumption of poor-quality water from systems supposedly compliant with robust safety standards (Younes and Bartram, 2001).
Besides diarrhoeal diseases, sub-optimal water access can affect health in several other ways. Ingestion of water contaminated with parasites can cause debilitating diseases such as dracunculiasis, amoebiasis and giardiasis, among others (Tarrass and Benjelloun, 2012; Ngowi, 2020). Acute or chronic exposure to water contaminated with chemicals (including arsenic, copper, fluoride, lead, nitrate, phenol, pesticides and endocrine-disrupting compounds) has been linked to disorders ranging from nausea and skin rashes to serious and life-threatening conditions such as renal failure, cancer, gangrene and foetal abnormalities (Hunter et al., 2010; Younes and Bartram, 2001; Rahman et al., 2009; Saha, 2003). Furthermore, an inconsistent water supply forces people to collect and store water where important disease vectors, such as mosquitoes responsible for the transmission of dengue fever, chikungunya, Zika and yellow fever, can easily breed (Tarrass and Benjelloun, 2012), enhancing the likelihood of VBD transmission in water-deprived areas (Fig. 4).
Fig. 4.
Direct and indirect impacts of freshwater scarcity on human health. *Source: World Health Organization (WHO), (2020a).
3.2. Indirect impacts of safe water scarcity on human health
A deficient water supply can indirectly affect the health of a region's population by reducing local food production, thereby interfering with people's ability to obtain a healthy and nutritious diet from local sources. In this context, several studies have shown that improving irrigation of local farming lands resulted in significantly better nutrition, particularly among children (Hunter et al., 2010; Veronicah et al., 2007; Mathew, 2005). Ferald et al. (Fernald and Grantham-McGregor, 1998) have proposed that undernourishment in children can cause elevated cortisol levels, which in turn can reduce their cognitive capabilities, decrease their functional immunity and increase their risk of cardiovascular disease later in life (Fig. 4).
In water-scarce settings, water sources are often located away from homes, and people need to transport water for daily consumption from the source to their dwellings. This task, often assigned to women and children, can impose heavy workloads on those transporting the water, leading to injuries (such as bone fractures, lacerations and contusions) and alterations to normal bone structure due to the repeated transport of heavy weights (Tarrass and Benjelloun, 2012; United Nations Human Settlements Programme (UN-HABITAT), 2003; Venkataramanan et al., 2020). The time required for these journeys can also interfere with other important daily activities, such as children's school attendance (impeding their educational process) and adult engagement in business endeavours, thereby contributing to the financial instability of already impoverished households (Tarrass and Benjelloun, 2012; United Nations Human Settlements Programme (UN-HABITAT), 2003). Furthermore, water collection practices increase the likelihood of exposure to water contaminated with certain parasites, like those belonging to the genus Schistosoma, which can enter the body through the skin, putting those responsible for these activities at an elevated risk of acquiring parasitic diseases (Colley et al., 2014; Elmorshedy et al., 2020).
3.3. The nexus between water stress, economy and food security
Water availability is necessary for virtually all economic activities, and therefore its scarcity represents a threat not only to a region's public health, but also to its economy. Food production requires large quantities of water and, often, expensive investments are required in order to obtain, purify and transport the required water resources from their source to the end users (North Atlantic Treaty Organization (NATO), 2019).
Agricultural activity is responsible for approximately 70% of global water consumption. However, the very arid climatic conditions and dry soils of the Middle East increase the water requirements of its agricultural sector to approximately 80% of all water withdrawals, with certain countries (such as Syria, Oman, Saudi Arabia, Iran and Yemen) using as much as 90% of water withdrawals for agricultural practices (World Bank, 2018). Additionally, countries in this part of the world have some of the highest rates of freshwater resource losses per capita within their food supply chain, with certain countries wasting as much as 177 m3 of freshwater per capita per year during production, processing and distribution of food (Kummu et al., 2012).
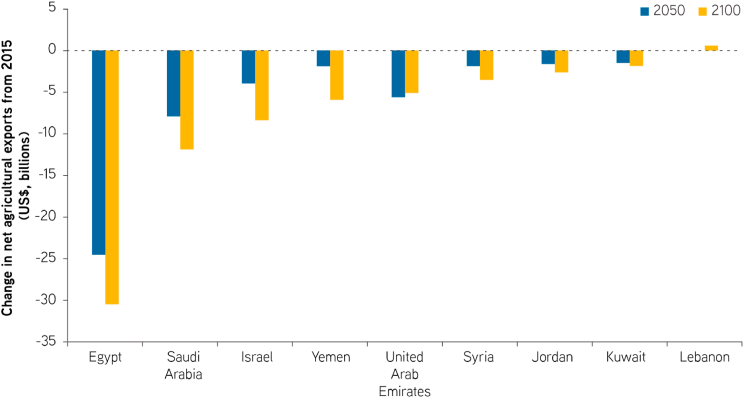
The expected scarcity of water resources in the Middle East may cause certain countries in the area to lose up to 60% of their agricultural productivity by the year 2050 (Borgomeo et al., 2018). In addition to directly affecting the availability of food for the local population, this reduction in agricultural output would also cause a massive drop in the gross domestic product of these countries (estimated between 6% and 14%) and negatively affect the livelihoods of almost one-third of the region's population who work in agriculture-related businesses (North Atlantic Treaty Organization (NATO), 2019; Borgomeo et al., 2018). Furthermore, diminished agricultural productivity is expected to reduce revenues from agricultural exports in the range of billions of dollars, with countries such as Egypt, Saudi Arabia, Israel, Yemen and the United Arab Emirates being the most affected by this phenomenon (Borgomeo et al., 2018) (Fig. 5).
Fig. 5.
Expected changes in revenue from agricultural exports in selected EMME countries. Source: Figure modified from Borgomeo et al. (2018).
On a positive note, several countries in the region have successfully implemented strategies that allow them to increase the productivity of their water resources, as well as to obtain useable water through non-conventional means such as desalination and wastewater re-use (World Bank, 2018). In Israel, for example, water generated by non-conventional processes accounts for approximately 25% of the total water supply, and provides about half of the water used for agricultural purposes (Fridman et al., 2021). However, the high financial and energy costs associated with some of these processes (particularly desalination) currently limit their widespread use (Martinez-Alvarez et al., 2020).
In summary, the projected reduction in water availability in the EMME region will greatly affect crucial aspects of life in the region, including the health, education and economic status of the population. To quote a North Atlantic Treaty Organization (NATO, 2019) report on the topic, in this region “water scarcity has the potential to deeply undermine the structure of society” (North Atlantic Treaty Organization (NATO), 2019).
4. Air quality: dust events, wildfires and pollution
4.1. Dust events
The Sahara, the Arabian Peninsula, the Arabian deserts (i.e. Iraq, Syria and Jordan's arid lands) and the Sistan region are the four major sources of airborne dust in the EMME region, strongly affecting the concentration and deposition of dust particles across and beyond the region (Schweitzer et al., 2018; Rezazadeh et al., 2013) (Fig. 6). Saharan dust plumes have been detected in several countries, including the United States, Spain, Italy, Greece, Cyprus, Israel and Lebanon (Polymenakou et al., 2008; Salvador et al., 2014; Kumar et al., 2018), and dust storms from the western Middle East (i.e. the Arabian Peninsula and Arabian deserts) have been shown to reach Iran (Givehchi et al., 2013; Ahmady-Birgani et al., 2015) and India (Badarinath et al., 2010). Ahmady-Birgani et al. concluded that the Persian Gulf States, Syria and Iraq are the main sources of dust affecting western Iran (Ahmady-Birgani et al., 2018). Additionally, the Sistan region between Iran and Afghanistan is now a main source of dust events in the eastern Middle East, affecting Iran, Afghanistan, Pakistan and India (Rashki et al., 2015; Karami et al., 2021; Aswini et al., 2020).
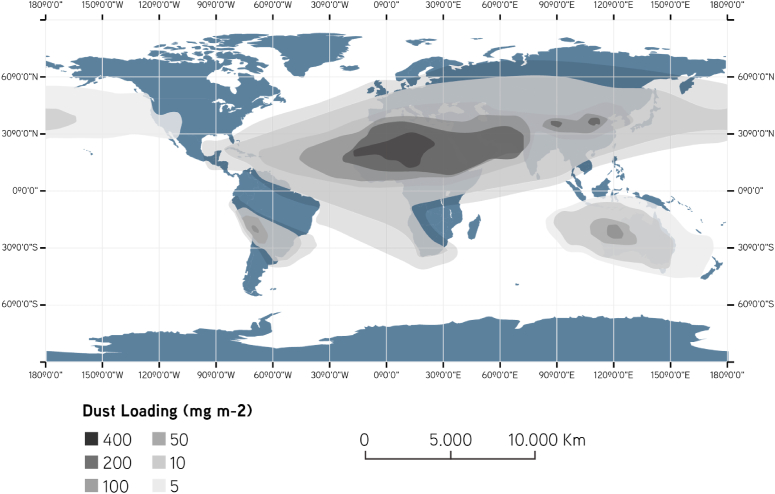
Fig. 6.
Distribution of atmospheric dust loads around the world. Source: Figure modified from De Longueville et al. (2010)).
Any review of dust events across the EMME region should consider changes in air pressure, temperature, wind speed, soil moisture and geomorphological settings. It is projected that the temperature in this region will rise by 3–9 °C by the year 2100 (Ozturk et al., 2018), which would lead to changes in air pressure across large sections of the EMME region. For example, the Sistan region is affected by higher pressures coming from the Caspian Sea and lower pressures coming from India and Pakistan, resulting in an intensification of the phenomenon known as the “120-day winds” (also known as the Levar winds) (Hamidianpour et al., 2021).
Regional drying and warming trends are strong drivers of the increase in the frequency and intensity of dust storms (Zittis et al., 2016; Gat et al., 2017), while water shortages, poor water management, land surface erosion, high evaporation, high wind erosion, desertification and deforestation may create new drylands and sources of dust (Keyantash and Dracup, 2002; Keramat et al., 2011; Mousavi et al., 2020; Ahmady-Birgani et al., 2017). Although the exact nature of the interaction between climate change and dust events remains elusive, some studies have emphasised the fact that these phenomena are interconnected. For example, Klingmüller et al. have postulated that increases in temperature and reductions in relative humidity associated with climate change during the past decade have increased dust emissions in the Middle East (Klingmüller et al., 2016), and Naderi-Beni et al. have reported that dust events throughout the Persian Gulf region are directly related to drought and water scarcity attributable to climate change (Naderi Beni et al., 2021).
In Iran, Baghbanan et al. reported that different phenomena associated with climate change (including rising temperatures, low surface moisture, vegetation loss, wetland drying, high-speed winds and successive droughts) have increased the frequency of dust storms (Baghbanan et al., 2019). Furthermore, it has been shown that wind erosion is prevalent in many Iranian provinces, and susceptibility to drought is expected to increase considerably in the near future (Keramat et al., 2011) (Mousavi et al., 2020).
Meanwhile, studies in Israel reported an increase in concentrations of particulate matter (PM) with a diameter of less than 10 μm (PM10) due to dust storms between 2001 and 2015, and detected more extreme dust events, with higher daily and hourly levels since 2009, which was attributed to climate change (Krasnov et al., 2016) (Gat et al., 2017) (Linares et al., 2020b).
The impacts of dust exposure on chronic health conditions and mortality have not been sufficiently characterised in contrast to the impacts of anthropogenic air pollution (Lelieveld et al., 2015). However, available data point towards increased risks of hospitalization and mortality due to circulatory and respiratory diseases resulting from dust events in the EMME region (Linares et al., 2020b) (Middleton et al., 2008) (Neophytou et al., 2013) (Tobias et al., 2019) (Giannadaki et al., 2014). Research performed in Greece has revealed associations between exposure to airborne PM (including desert dust) and increased hospital admissions due to asthma in Athens (Samoli et al., 2011), as well as increased admissions due to chronic obstructive pulmonary disease in Crete (Lorentzou et al., 2019). And in Iran, it has been reported that the presence of PM in the environment is associated with an increase in daily mortality in the city of Ahvaz (Shahsavani et al., 2020; Goudarzi et al., 2021). Also in Iran, Bonyadi et al. concluded that atmospheric PM and sulphur dioxide have negatively affected the health of the people in the city of Shiraz by increasing the incidence of cardiovascular disorders (including acute myocardial infarction) as well as respiratory diseases (Bonyadi et al., 2020).
Many microorganisms, including human pathogens, have been detected in dust plumes transported over long distances (Polymenakou, 2012; Mazar et al., 2016; Lang-Yona et al., 2020). The nature of transported microorganisms may depend on their origin, and their abundance and diversity could be enhanced due to climate change causing the appearance of new sources, as well as the spread of microbial species previously not subjected to transportation and deposition (Gat et al., 2017).
Although the viability and pathogenicity of these bio-agents is unclear, chronic and episodic exposure to high dust levels has been associated with allergies, silicosis, lung disease, chronic respiratory diseases and infections such as granulomatous, sarcoidosis and meningococcal meningitis (Schweitzer et al., 2018; Thomson et al., 2009). Pardo et al. have shown that injuries observed in lung cells exposed to dust extracts can be attributed to dust-containing bacterial material (such as endotoxins) that induces lung injury upon inhalation (Pardo et al., 2017). As with exposure to extreme heat, groups that are particularly vulnerable to the exposure of high dust concentrations include very young children and older adults (due to underdeveloped or deteriorating immunity, respectively), as well as people with chronic cardiopulmonary diseases. Studies in Iran have reported a link between temperature, humidity, atmospheric dust and peaks of shigellosis cases in summer months (Aminharati et al., 2018). Furthermore, research performed in the city of Ilam (western Iran) revealed that the concentration of bio-aerosols in the environment is elevated during dust storms (Amarloei et al., 2020).
Airborne dust originating from agricultural areas could potentially facilitate the spread of antibiotic resistance genes, a growing global health problem (Gat et al., 2017). Traces of pesticides and herbicides, as well as microbial and heavy metal contaminants of anthropogenic origin (including those generated by military conflict), could coincide with dust storms and increase their risk to public health in the region (Gat et al., 2017; Keramat et al., 2011; Mousavi et al., 2020; Mazar et al., 2016; Lang-Yona et al., 2020; Sanayei et al., 2009; Falkovich and Rudich, 2001; Falkovich et al., 2004). Atmospheric dust has also been associated with the incidence of food-borne disease in the region (Mousavi et al., 2020).
4.2. Wildfires
Reduced precipitation and relative humidity and higher temperatures are expected to increase the risk of forest fires and associated air pollution (Şeker et al., 2020) (Lelieveld et al., 2012). In the latest report of the Lancet Countdown, the largest increase in the risk of wildfires between 2001 and 2019 was observed in Lebanon along with Kenya and South Africa (Watts et al., 2021). In Turkey, storms, floods, droughts and forest fires are among the most frequent natural disasters, and an increasing trend has been reported especially in the past two decades (Şeker et al., 2020).
In addition to their direct health impacts, wildfires increase the number of patients checking into emergency departments, and are implicated with an increase in acute and chronic respiratory diseases (Şeker et al., 2020). Furthermore, a recent review and meta-analysis by Karanasiou et al. (2021) established that exposure to the emissions of biomass burning (including wildfires) is positively associated with mortality, including deaths caused by cardiovascular problems.
4.3. Air pollution
Outdoor air pollution in the form of fine PM with a diameter of 2.5 μm or less (PM2.5) is implicated in up to 9 million excess deaths per year globally (Cohen et al., 2017; Burnett et al., 2018) (Lelieveld et al., 2020). In 2018 alone, air pollution was responsible for approximately 8.7 million deaths around the world – approximately 20% of that year's total global mortality (Vohra et al., 2021).
The largest epidemiological study conducted to date on the short-term health effects of air pollution with PM10 and PM2.5 showed a concentration-response function with a consistent increase in daily mortality as PM concentrations increased, with no discernible thresholds (Liu et al., 2019). This finding is particularly worrisome for megacities in the EMME region. Similar concentration-response functions with no discernible thresholds have recently been reported for carbon monoxide and nitrogen dioxide, the latter also being a main cause of respiratory disease in children (Chen et al., 2021; Meng et al., 2021; Chowdhury et al., 2021).
In Europe and the Eastern Mediterranean, up to 1 million people die prematurely each year due to air pollution (Lelieveld et al., 2020; Paz et al., 2020). High concentrations of PM2.5 and ozone in outdoor air are associated with adverse health impacts, including chronic obstructive pulmonary disease, acute lower respiratory illness, cerebrovascular disease, ischaemic heart disease and lung cancer (Burnett et al., 2014) (Lelieveld et al., 2015).
Increased PM concentrations in ambient air from energy generation, industrial pollutants, agriculture, traffic, domestic energy use and increasingly frequent wildfires are implicated with respiratory and cardiovascular diseases, potentially aggravated by the growing abundance of desert dust in outdoor air.
The strong urbanization trend in the region, in combination with the increase in human-induced emissions, are both major contributors to air pollution, causing air quality degradation in several megacities, such as Cairo, Istanbul and Tehran (Lelieveld et al., 2012; Heger and Sarraf, 2018). Excess morbidity and mortality associated with ground-level ozone and air pollution (caused by fine PM) are predicted in the region (Lelieveld et al., 2012).
In the EMME region, especially in the Persian Gulf states, fossil fuels dominate the energy supply. The by-products of fossil fuel burning include greenhouse gases, fine PM and hazardous gases such as ground-level ozone, nitrogen dioxide and volatile organic compounds. Because heat and sunlight facilitate the formation of some of these noxious compounds, urban heat islands, heatwaves and the high intensity of sunlight contribute to the deterioration of air quality in the densely populated environments of the EMME region.
A modelling study performed by Lelieveld et al. in 2012 suggested that the expected warming and increased frequency and intensity of heatwaves will strongly increase the demand for air conditioning in the region (Lelieveld et al., 2012). The increasing energy demand for industry, traffic, air conditioning and desalination due to water shortage will exacerbate air pollution and greenhouse gases, which in turn will feed into environmental and climate change.
5. Vector-borne diseases
Arthropods capable of transmitting human pathogens, also known as “disease vectors”, are unable to self-regulate their body temperatures by physiological means, and must therefore rely on external sources of heat to maintain their temperature within functional limits. Consequently, environmental conditions are major determinants in the development, physiology, behavior and ecology of disease vectors, and can also influence important biological processes in the life cycle of pathogens (Reisen et al., 2006; Blanford et al., 2013; Paaijmans et al., 2013). Therefore, the epidemiological landscape of VBDs is heavily influenced by climate change (Reiter, 2001; Lafferty, 2009; Tabachnick, 2016).
Environmental factors that can influence the transmission rate of VBDs include temperature, precipitation, relative humidity, wind and duration of daylight (Reiter, 2001; Negev et al., 2015). Among these, temperature and precipitation play particularly determinant roles in the geographic distribution, seasonality and ecology of VBDs (Ogden, 2017; Yé et al., 2007; Ciota and Keyel, 2019).
The interaction between temperature and VBD transmission is complex, often non-linear, and variable among different vector/pathogen combinations (Mordecai et al., 2019; Mordecai et al., 2020). In some instances, it has been proposed that global warming will cause changes to the geographic distribution and seasonality of disease vector species, allowing them to survive in areas and/or seasons currently deemed unsuitable due to their low temperatures, therefore enhancing the potential for transmission of VBDs (António et al., 2018). However, it has also been proposed that in other regions, global warming could cause environmental temperatures to rise above the maximum tolerable levels for certain vector/pathogen systems, resulting in a reduction of current geographic ranges, alterations to seasonal transmission patters or shifts in the relevance of different VBDs for specific geographic settings (Mordecai et al., 2020; Murdock et al., 2016; Brady et al., 2014).
Besides climate, the epidemiology of VBDs is also influenced by socio-economic factors such as population density, access to running water; housing quality; and local knowledge, attitudes and practices, among others (Kenneson et al., 2017; Schmidt et al., 2011; Padmanabha et al., 2010; Heydari et al., 2017; Horstick and Runge-Ranzinger, 2019). Presently, a combination of ecological and socio-economic factors found in the EMME region creates appropriate conditions for the local transmission of several VBDs, including malaria, dengue, leishmaniasis and West Nile fever (Negev et al., 2015; Waha et al., 2017) (Table 1).
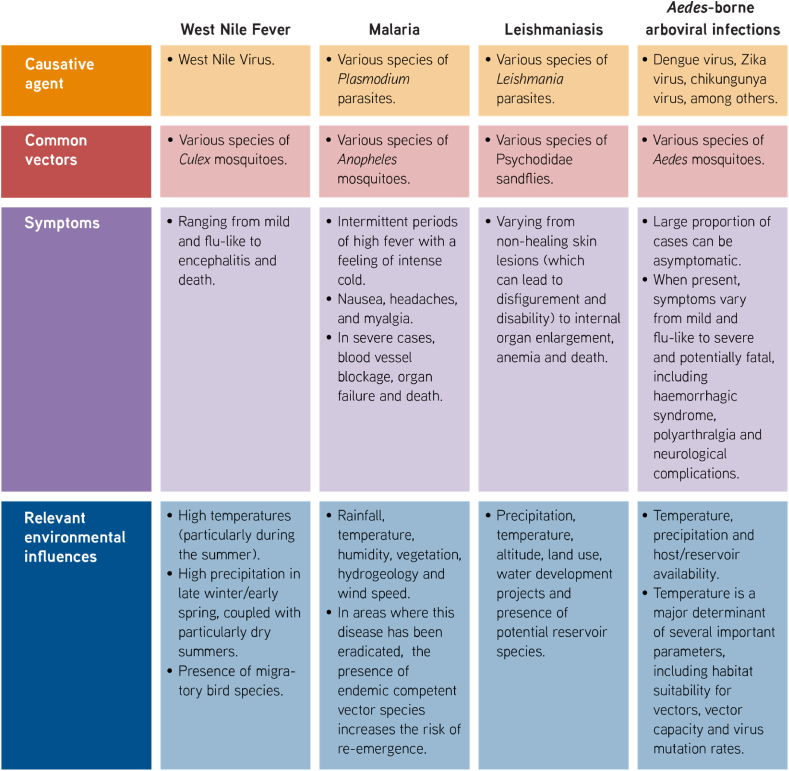
Table 1.
Principal vector-borne diseases affected by climate change in the EMME region.
It has been proposed that warming climatic trends observed for the region will cause the incidence of these infections to rise (Watts et al., 2021; Linares et al., 2020b; Lillepold et al., 2019; Cramer et al., 2018). Below, we address several VBDs of particular concern in the region, and describe the expected effects of climate change on their regional epidemiology.
5.1. West Nile Fever
West Nile Fever is caused by the West Nile Virus (WNV), a member of the Flaviviridae family of viruses. During its natural (i.e. enzootic) transmission cycle, WNV is normally transmitted between birds by mosquitoes (Diptera: Culicidae) belonging to the Culex genus. A few mosquito species that feed on both birds and mammals can act as “bridge vectors”, transmitting the virus to a broad diversity of mammalian hosts, including humans and horses. Mammalian hosts usually do not develop high enough viremias to infect blood-feeding mosquitoes, and are therefore considered to be dead-end hosts.
Infection with WNV in humans is generally sub-clinical. However, symptomatic cases might range from mild and flu like to paralysis, full-blown encephalitis and even death (Colpitts et al., 2012). Fatality rates vary depending on age and co-morbidities, reaching up to 29% in people aged 70 years and over. Moreover, among clinical cases that recover, up to 50% can still present consequences lasting for a year or longer post infection (Campbell et al., 2002).
Although WNV is originally endemic to Africa, it is currently considered the most widespread of all flaviviruses due to its presence in Africa, Eurasia and the Americas. In countries of the EMME region such as Greece, Israel and Turkey, a clear correlation has been established between WNV outbreaks in humans and the unusually warm summers between 2010 and 2014 (Negev et al., 2015; Paz et al., 2013; Tran et al., 2014; Stilianakis et al., 2016). In Greece, Stilianakis et al. found that increased soil and air temperatures were correlated with an incremental increase in the incidence of WNV cases in humans, and proposed that higher temperatures might contribute to the spread of the disease by favouring mosquito larval development and reducing generation times (Stilianakis et al., 2016).
Studies in the Mediterranean region have also demonstrated the importance of water bodies and precipitation for WNV epidemiology: One study showed that areas with positive anomalies of the Modified Normalized Difference Water Index during the month of June are at a higher risk of transmission (Tran et al., 2014). Another study proposed that high precipitation in late winter/early spring, coupled with particularly dry summers, are good predictors of WNV outbreaks (Marcantonio et al., 2015). On the other hand, it was found in other regions that drought conditions can be as much a risk factor for WNV outbreaks (Cotar et al., 2016). A recent study in Italy found that WNV outbreaks are associated with the local pool of mosquitoes infected in previous years (Marini et al., 2018). When combined with an increasing mosquito abundance and the number of WNV cases in recent years, the association between the risk of WNV outbreaks and climate change is strengthened (Marini et al., 2020). Furthermore, because of the role of birds as reservoirs of WNV, the epidemiology of this pathogen is also dependent on variables such as bird migration pathways over the area, which are in turn affected by climate change (Stilianakis et al., 2016; European Academies Science Advisory Council (EASAC), 2019).
5.2. Malaria
Malaria is a mosquito-borne disease caused by several species of protozoans from the Plasmodium genus. Human Plasmodium species are transmitted by mosquitoes belonging to the Anopheles genus. The co-evolution of the ancestors of humans and Plasmodium parasites is ancient, stretching millions of years before the appearance of modern human beings (Garnham, 1963).
Persons infected with Plasmodium sp. generally present periods of high fever during which they experience a feeling of intense cold and shivering. These symptoms are often accompanied by nausea, headaches and myalgia. Severe cases can present life-threatening symptomatology including blood vessel blockage, organ failure, brain swelling, seizures and coma. Pregnant women, children under five years of age and older adults present a higher risk of malaria-related complications and mortality (Centers for Disease Control and Prevention (CDC), 2021). According to the World Health Organization (WHO), in the last two decades the global average number of malaria cases per year is 236 million (ranging from 217 to 248 million). Over the same period, the number of yearly deaths due to malaria decreased from 738 000 (average for the 2000–04 period) to 425 000 (average for the 2015–19 period) (World Health Organization, 2020).
There have been several successful efforts to control the spread of malaria at the national and regional levels across the globe. The current geographic spread of this disease responds in part to these control efforts, as well as climatic factors (Reiter, 2001; Ogden, 2017). For example, although malaria was historically endemic to Europe, intense and well-coordinated control efforts led to its eradication from the entire continent by the late 1970s (Reiter, 2001; Piperaki and Daikos, 2016). However, local transmission of malaria resumed in Europe during the late 1990s, and has occurred sporadically in several countries of the region during the past decade, including in Greece and Cyprus (Piperaki and Daikos, 2016; European Centre for Disease Prevention and Control (ECDC), 2017). On the other hand, the WHO's most recent World Malaria Report highlights several countries in the EMME region for their achievements in reducing malaria incidence. For instance, Iran reported progressively decreasing malaria cases throughout the past decade, and zero cases in 2018 and 2019. Saudi Arabia reported only 38 indigenous cases in 2019, and Iraq, Oman and Syria have not reported indigenous cases since 2009, 2011 and 2004, respectively (World Health Organization, 2020).
Environmental determinants of malaria transmission in Eastern Mediterranean countries include rainfall, temperature, humidity, vegetation index, hydrogeology and wind speed (Khader et al., 2015). Furthermore, it has been proposed that due to the existence of competent vector species in the region, future climate change could create conditions that facilitate the reappearance of malaria in countries where the disease is currently considered to be eradicated (Negev et al., 2015).
5.3. Leishmaniasis
Leishmaniasis is a parasitic disease caused by several species of parasites belonging to the genus Leishmania. In addition to humans, these parasites can infect a diversity of vertebrate hosts, which act as reservoirs. Leishmania parasites are transmitted by over 90 species of sandflies (Diptera: Psychodidae) belonging to the phlebotominae sub-family (Maroli et al., 2013). In the EMME region, leishmaniasis vectors belong to the Phlebotomus genus (Aspöck et al., 2008).
According to the WHO (World Health Organization (WHO), 2020b), this disease has three main forms:
-
1)
Visceral leishmaniasis, also known as kala-azar, is characterised by the enlargement of the spleen and liver, fever, anaemia and weight loss. If not treated, its fatality rate is >95%. Most cases of this kind of leishmaniasis occur in Brazil, East Africa and India. However, Iraq (part of the EMME region) has reported a high incidence of this disease in the recent past.
-
2)
Cutaneous leishmaniasis is characterised by non-healing skin lesions that can lead to severe scarring and disability. This is the most common form of leishmaniasis, with up to 1 million new cases occurring each year, and is particularly prevalent in the Americas, the Mediterranean region, the Middle East and Central Asia. Approximately 70% of all cutaneous leishmaniasis cases reported worldwide come from countries within the Eastern Mediterranean region. EMME countries reporting high incidence of cutaneous leishmaniasis in the recent past include Iran, Iraq and Syria.
-
3)
Mucocutaneous leishmaniasis is characterised by the destruction of mucous membranes in the upper respiratory tract (nose, mouth and throat), often leading to dramatic scarring and disfigurement. Most cases of this form of the disease occur in Bolivia, Brazil, Ethiopia and Peru.
In the Eastern Mediterranean region, significant correlations between leishmaniasis incidence and climatic parameters have been shown, with a positive correlation between incidence and precipitation, and a negative correlation between incidence and temperature (Khader et al., 2015). Furthermore, a study in Israel showed that much of the variance in sandfly spatio-temporal activity and population growth is determined by early night temperatures, and that the effect of rising temperatures on sandfly populations is more marked at lower elevations (Waitz et al., 2018). Other factors associated with the incidence of leishmaniasis in the region include elevation, land use, presence of potential reservoirs and water development projects (Khader et al., 2015; Iliopoulou et al., 2018).
Ecological niche modelling predicts that future climate change will alter the geographic distribution of the existing Phlebotomus species within the EMME region. On the one hand, it is expected that these changes will allow sandflies to spread towards areas currently not suitable for their survival (eventually reaching Western and Central Europe), while on the other hand some sandfly populations are expected to disappear from currently suitable areas (Chalghaf et al., 2018).
5.4. Arboviral diseases transmitted by Aedes mosquitoes
Mosquitoes (Diptera: Culicidae) belonging to the Aedes (Ae.) genus, particularly Ae. aegypti and Ae. albopictus, are the main vectors for several arboviruses of major medical relevance, including the dengue, chikungunya and Zika viruses. All three viruses contain ribonucleic acid as their genetic material, and cause infections that present a wide range of symptoms, from mild and flu-like, to severe and potentially life threatening. Furthermore, a large proportion of infections with these viruses are asymptomatic (Paixão et al., 2018).
Originally endemic to Africa, Ae. aegypti is a highly synanthropic mosquito species which has successfully adapted to urban environments thanks to traits such as the capability of breeding in artificial containers (such as discarded plastic, tires, etc.) and a preference for feeding almost exclusively on humans (Ryan et al., 2019). Ae. albopictus, on the other hand, is a species of Asian origin and has a more flexible ecology, being able to adapt to urban, rural and agricultural habitats, and breed in either natural or man-made containers as well as feed on a wider range of vertebrate hosts, including birds and mammals. Because of its diverse host preferences, Ae. albopictus has the potential to act as an important vector of zoonotic viruses (Waldock et al., 2013). An additional important difference between Ae. aegypti and Ae. albopictus is their tolerance to low temperatures: while Ae. aegypti is generally unable to thrive in temperatures below 10 °C, Ae. albopictus can survive below-freezing temperatures by undergoing diapause. Therefore, Ae. albopictus has the capacity to colonise a broader latitudinal gradient than Ae. aegypti (Ryan et al., 2019; Lounibos et al., 2003; Paupy et al., 2009). On the other hand, Ae. albopictus is less tolerant to very high temperatures than Ae. aegypti, limiting the capability of Ae. albopictus to survive in extremely hot zones (Brady et al., 2014).
Temperature is not only a major determinant of habitat suitability for vectors but can also affect important biological parameters related to disease transmission. For example:
•Because female mosquitoes feed on blood to provide the nutrients required for egg development, warmer temperatures increase the biting rate of female mosquitoes by reducing the time period required for egg maturation (gonotrophic cycle) (Brady et al., 2014). Since the transmission of mosquito-borne viruses happens during blood feeding, higher biting rates mean higher disease incidence. Therefore, increases in environmental temperature will intensify epidemics by decreasing the time between successive infections in humans (Siraj et al., 2017).
-
•
In the case of dengue, it has been proposed that the number of secondary infections will increase with environmental temperature until reaching an optimal temperature of approximately 33 °C, after which it will start decreasing (Siraj et al., 2017).
-
•
Due to the high mutation rates and short generation times characteristic of arboviruses, the increased numbers of infections caused by rising temperatures might promote faster evolution rates in these pathogens. The increased genetic variability generated by this process could result in the emergence of novel virus strains and/or serotypes, with different properties regarding virulence and/or transmissibility (Tozan et al., 2020).
It is often the case that an increase in environmental temperature leads to an increase in arbovirus transmission rates, although this trend does not apply to all arboviruses (Ciota and Keyel, 2019). The interactions between temperature and disease transmission are complex and not always linear, with factors such as spatiotemporal temperature variations and the effects of rearing temperature on adult vector biology playing important roles (Ciota and Keyel, 2019; Parham et al., 2015).
Besides temperature, other environmental factors such as precipitation and host availability have been shown to be major determinants of an area's suitability for Aedes vectors (Ducheyne et al., 2018; Kraemer et al., 2019). Furthermore, in currently endemic areas, factors such as population growth can deeply influence the epidemiology of Aedes-borne diseases; as an example, it has been estimated that between 2015 and 2080, the number of people at risk of contracting dengue will increase by 2.25 billion, reaching a global total of over 6.1 billion (equivalent to 60% of the planet's population). This increase will be largely due to growing population sizes in endemic areas (Messina et al., 2019).
The global geographic distribution of both Ae. aegypti and Ae. albopictus is expected to shift as a result of climate change, potentially resulting in enhanced disease transmission (Reinhold et al., 2018). Both Ae. aegypti and Ae. albopictus are currently present in several countries of the EMME region (Fig. 7), and it has been shown that all countries in the Eastern Mediterranean region have at least some areas with suitable conditions for the establishment of both species, making it possible for the expansion of their geographic ranges to regions which they have either never before colonised, or where they have been successfully eradicated in the past (Brady et al., 2014; Ducheyne et al., 2018). Furthermore, future climate change is expected to render additional areas suitable for the survival of these mosquito species, including regions within the MENA region and continental Europe (Ryan et al., 2019; Iwamura et al., 2020).
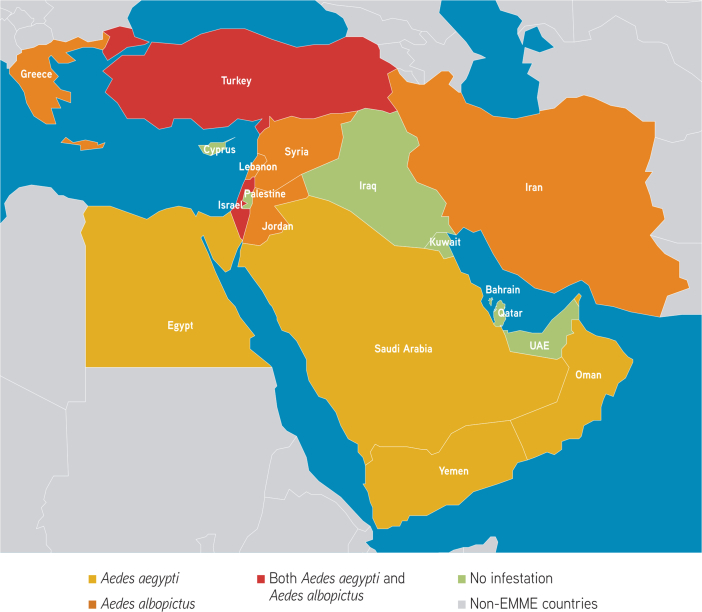
Fig. 7.
Current distribution of Aedes aegypti and Aedes albopictus in the EMME region. Data sources: Ducheyne et al. (2018) and European Centre for Disease Prevention and Control (ECDC), 2021a, European Centre for Disease Prevention and Control (ECDC), 2021b.
6. Population displacement
Climate change has been intimately linked to the geographic displacement of human populations throughout the history of our species, and might have played an important role in shaping early societies (Finlayson, 2005). Climate change has been associated with such notable events as the mass migrations resulting from the Little Ice Age in 17th century Europe, and the population displacement resulting from the 19th century Irish Potato Famine, among many others (McMichael et al., 2012; Fraser, 2003).
During the past 50 years, the displacement of human groups has surged significantly due to complex environmental, economic and socio-political issues. Currently, it is estimated that approximately 13% of the world's population (∼1 billion people) do not live in their place of birth, and approximately 3.5% of the world's population (∼270 million people) have moved between countries (Balsari et al., 2020). The United Nations Refugee Agency reported that by 2019 the global number of forcibly displaced persons had reached 79.5 million, 40% of which were children (United Nations High Commissioner for Refugees (UNHCR), 2020).
Climate change can enhance population displacement in several ways, including increases in the intensity and frequency of extreme weather events, loss of land to sea-level rise and the deterioration of life-sustaining ecosystems, among others (McMichael, 2015; Shultz et al., 2019). Furthermore, it has been proposed that the negative effects of environmental events might influence people's reaction to pre-existing socio-political problems, sometimes leading to turmoil and violence, which in turn force people to move out of their lands. Bowles et al. (2015) state that:
“Decreased availability of essential resources could directly increase the probability of violence. Diminished arable land or available water could lead to conflict between ethnic groups or between nations. Somewhat less directly, countries may respond to water shortages by building dams or contravening water treaties, which may provoke conflict, including through attacks on water infrastructure by downstream countries”.
The recent armed conflict in Syria can be viewed as a key example of the interaction between environmental change, socioeconomic factors and population displacement: Kelley et al. (2015) propose that the severe drought that affected the greater Fertile Crescent region between 2007 and 2010 caused a devastating loss of crops and livestock, forcing as many as 1.5 million Syrians to migrate from rural areas towards urban centres in search of better livelihoods. The authors contend that the intense pressure this migration exerted on urban areas, together with pre-existing problems such as overcrowding, unemployment, corruption and poverty, triggered the civil unrest that started in 2011 (Kelley et al., 2015). However, other authors argue that the uprising responded to decades of socio-political problems, and would have happened even in the absence of extreme drought conditions (Al-Delaimy et al., 2020). Although it is often impossible to know with certainty whether environmental factors are direct triggers of conflict, a widely accepted notion is to understand climatic events as “risk multipliers” – that is, elements that exacerbate pre-existing tensions and increase the likelihood of violent confrontations being triggered by economic, social or political factors (Al-Delaimy et al., 2020; Bowles et al., 2015). Furthermore, it has been proposed that in regions experiencing active turmoil, climatic events can also act as “peace inhibitors” by creating conditions that undermine conflict resolution efforts (Bowles et al., 2015; Zittis et al., 2021).
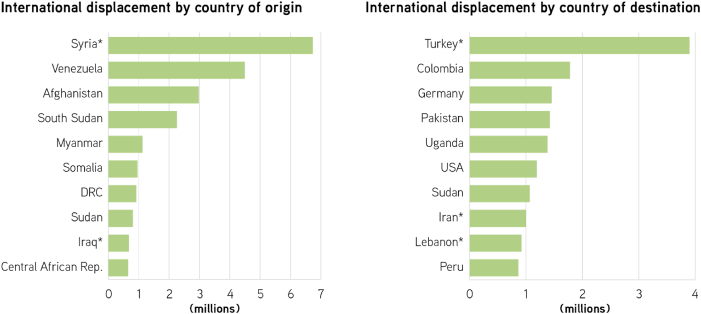
Countries in the MENA region have some of the largest shares of migrants anywhere in the world (Baldwin-Edwards, 2005) (Fig. 8). The reasons behind this massive movement of people can be traced to failing economies, climate change, natural disasters or violent conflicts (Al-Delaimy et al., 2020; Mowafi, 2011). Protracted conflicts in many of the region's countries have caused the displacement of millions of people – by 2019, Syria was the country with the highest number of displaced people in the world (13.2 million, 6.7 million of which are internationally displaced). Iraq, another country of the EMME region, ranks ninth in the global list of countries with the highest number of displaced persons – 0.6 million (United Nations High Commissioner for Refugees (UNHCR), 2020). Furthermore, several EMME countries serve as hosts to very large displaced populations: Turkey alone has received approximately 3.9 million people, becoming the world's largest host of internationally displaced populations. Iran and Lebanon are also home to large international refugee populations, receiving approximately 1 million displaced people each (United Nations High Commissioner for Refugees (UNHCR), 2020).
Fig. 8.
International population displacement by country of origin and destination, 2019. Figure shows the 10 countries either generating (left panel) or receiving (right panel) the largest internationally displaced populations in the world. *EMME countries. DRC: Democratic Republic of the Congo. USA: United States of America. Source: Figure modified from UNHCR (2020).
6.1. Direct effects of human displacement on health
The displacement of human populations can have detrimental health effects at various levels. Direct effects can include bodily injuries and psychological trauma (in cases of displacement caused by violence), as well as the problems caused by rough conditions prevalent either during the displaced person's journey, or at the temporary dwellings where displaced populations are usually housed (Al-Delaimy et al., 2020; Bowles et al., 2015; Daynes, 2016). The main health problems associated with refugees' poor living conditions include respiratory, gastrointestinal and skin conditions, as well as dehydration, hypothermia, burns and malnutrition (Bowles et al., 2015; Daynes, 2016; Shortall et al., 2017). Additionally, the sub-optimal nature of the housing usually available to refugees tends to render them particularly vulnerable to climatic phenomena such as extreme temperatures, flooding or air pollution (Fig. 9).
Fig. 9.
Direct and indirect effects of population displacement on human health.
6.2. Indirect effects of human displacement on health
6.2.1. Infectious diseases
The displacement of people across borders has important epidemiological consequences for infectious disease transmission: on the one hand, migrating individuals coming into new geographic areas can be exposed to infections which they have not faced before, and against which they lack both immunity and protective practices. On the other hand, migrants themselves can act as carriers of pathogens to the areas through which they journey, or where they settle (McMichael, 2015). Displaced populations are often sheltered under sub-optimal conditions where overcrowding, poor ventilation and lack of access to basic sanitation are common, creating an environment where diarrhoeal diseases (including cholera), measles, meningitis, acute respiratory infections, scabies and tuberculosis are easily transmitted (McMichael, 2015; De Bruijn, 2009)– (Kouadio et al., 2010). Importantly, in areas where vector-borne pathogens are endemic, these sub-optimal living conditions create ideal environments for the transmission of diseases such as malaria, dengue, leishmaniasis, epidemic typhus, relapsing fever and trench fever, making them particularly serious threats to displaced populations (McMichael et al., 2012; Kwak et al., 2021; Roll Back Malaria Partnership, 2020; Abdul-Ghani et al., 2019). A clear example of this are the outbreaks of cutaneous leishmaniasis observed among Syrian refugees sheltered in Lebanon and Jordan (Alawieh et al., 2014; Kanani et al., 2019). Furthermore, the epidemiology of parasitic diseases can also be affected by climate change: Ahmed et al. (2014) have suggested that increases in the incidence of bladder cancer due to schistosomiasis in the Red Sea area of Egypt are linked to the internal migration of farmers from Delta governorates, who had lost their lands to sea-level rise.
Reporting on refugees (originating mostly from the Middle East) seeking shelter in Greece during 2016, Shortall et al. (2017) found that more than half of the cases reported by volunteer physicians could be attributed to infectious diseases, highlighting the threat that pathogens pose to displaced populations in the EMME region. This issue is further compounded by the fact that vaccination rates tend to be much lower among displaced children than in the general population, increasing the risk of infection even more in this vulnerable group. As an example, Mowafi et al. (Mowafi, 2011) report that while overall vaccination rates for children in Iraq are up to 78%, only 48% of displaced Iraqi children have completed their basic vaccination courses against diphtheria, pertussis, tetanus, polio and measles.
Infection with the human immunodeficiency virus (HIV) is a concern for displaced populations, as turmoil and displacement increase the probability of its transmission (Mowafi, 2011; Brennan and Nandy, 2001). Although the overall prevalence of HIV in the MENA region is still one of the lowest in the world, with an estimated 0.1% (Gökengin et al., 2016), it is possible that official numbers underreport the actual prevalence of this disease, particularly among certain high-risk groups (Mowafi, 2011). Adding to this complexity is the fact that new HIV infections in this part of the world have increased at alarming rates during the past two decades (Gökengin et al., 2016). For refugees hosted in certain countries of the region, being diagnosed as HIV + implies not only a devastating social stigma, but also the possibility of being forcibly deported to their country of origin (a practice known as “refoulement”) (Mowafi, 2011; DeJong et al., 2005). Such dramatic consequences create important barriers for testing and reporting HIV in displaced populations.
6.2.2. Non-communicable diseases
Globally, non-communicable diseases (NCDs) such as diabetes, cardiovascular disease, cancer and chronic respiratory disease are the leading cause of death, causing 63% of all reported fatalities (World Health Organization (WHO), 2013; United Nations Inter-Agency Task Force on the Prevention and Control of Non-communicable Diseases, 2019). EMME countries are no exception to this trend: in 2018, NCDs were reportedly responsible for 84% of deaths in Lebanon, 76% in Jordan and 78% in Saudi Arabia. In Syria, NCDs were responsible for 77% of deaths prior to the recent armed conflict (McNatt, 2020). The high prevalence of these diseases represents an additional risk to displaced individuals, as the harsh conditions of their journeys and temporary settlements usually curtail the access to medicines and health-care facilities required to manage NCDs (Daynes, 2016). For internationally displaced individuals, language, cultural differences and legal status might represent additional barriers to obtaining access to health care. The WHO reports that “being undocumented can be considered a risk factor for poor health among migrants in Europe” (World Health Organization (WHO), 2019). Furthermore, the high prevalence of NCDs, together with the high burden these diseases place on public health systems, can represent significant economic and logistic problems for countries hosting large displaced populations (Mowafi, 2011).
Unhealthy behaviours, such as tobacco and alcohol use, physical inactivity and poor diet greatly increase the severity of NCDs. Unfortunately, the stressors associated with forced displacement can significantly enhance the likelihood of individuals engaging in such behaviours, further complicating their health status (United Nations Inter-Agency Task Force on the Prevention and Control of Non-communicable Diseases, 2019; World Health Organization (WHO), 2019).
6.2.3. Sexual and reproductive health
Population displacement can place the most vulnerable individuals (i.e. women and children) at an elevated risk of being the target of sexual violence and exploitation. In some instances, the lack of resources and uncertain legal status associated with displacement can push individuals to engage in sex work in order to support themselves and their families. In other instances, displaced persons can be kidnapped, raped or otherwise sexually exploited (Brennan and Nandy, 2001; Chynoweth and McKenna, 2007). In all these scenarios, the victims of sexual violence and exploitation are at an elevated risk of sexually transmitted infections and unwanted pregnancies. Unfortunately, social customs in several countries of the region can make it very difficult for victims to access reproductive health services, or even basic family planning options (Mowafi, 2011; DeJong et al., 2005).
Displacement can be particularly risky for pregnant women due to the lack of appropriate hygiene, nutrition and antenatal/postnatal care. In this group, problems such as reproductive tract infections and malnourishment, as well as the lack of access to medical services, enhance the risk for maternal morbidity and mortality, pre-term birth, miscarriage, stillbirth and other forms of perinatal and neonatal morbidity (World Health Organization (WHO), 2018).
6.2.4. Mental health
Mental health problems are reportedly among the most important and yet most neglected issues faced by displaced populations (Al-Delaimy et al., 2020). People fleeing areas of armed conflict are at an elevated risk of experiencing issues such as post-traumatic stress disorder, depression, anxiety and suicide (Al-Delaimy et al., 2020; Arnetz et al., 2020). The study of mental health issues in displaced populations is greatly complicated by the lack of appropriate psychological assessment tools to evaluate the effects of extreme forms of trauma often endured during armed conflict, such as kidnappings, assassinations, torture, genocide and mass rape (Shultz et al., 2019; Brennan and Nandy, 2001).
Even in cases when displacement is not caused by armed conflict, the displaced individuals invariably experience profound psychological distress triggered by the separation from their usual material and social environments. Not only do they leave behind their home, land, crops, vehicles and animals, but also their community affiliations, social network, social status and civic roles. Importantly, their occupational identity can be drastically altered, as any skills that were used to provide livelihood might not be useful following resettlement, creating enormous obstacles in the pursuit of financial stability (Shultz et al., 2019).
Mental health issues are highly prevalent among displaced populations. The WHO estimates that following a humanitarian emergency, 15–20% of adults will suffer mild to moderate mental disorders, while 3–4% will suffer severe mental problems (World Health Organization & United Nations High Commissioner for Refugees, 2012). Other studies suggest that at least 50% of refugees experience some form of psychological disorder, and often multiple disorders simultaneously. Children are particularly susceptible to psychological trauma during armed conflict, when it is likely they will witness acts of violence being committed, often against their loved ones (Daynes, 2016; Brennan and Nandy, 2001). Accordingly, physicians working with refugees from the EMME region have reported high rates of psychosocial distress in displaced populations, including increased suicide attempts (Hermans et al., 2017) (Fig. 9).
An important complicating factor for refugees in the EMME region is the scarcity of psychological support. In many countries of the region, access to psychological counselling can be scarce even for the local population. As an example, the estimated number of psychiatrists working in the Middle East is in the order of 1 per 100 000 to 200 000 inhabitants (Al-Delaimy et al., 2020; Mowafi, 2011). Various authors have highlighted this fact as a major obstacle for the well-being of displaced populations in the area (Al-Delaimy et al., 2020; Mowafi, 2011; Daynes, 2016; Hermans et al., 2017).
It is important to highlight that psychological trauma differs from physical trauma in that it often has very long-lasting effects, which can even be passed on from one generation to the next (East et al., 2018). For this reason, author Wakel Al-Delaimy states that “… poor mental health is going to be a major health impact of climate change in the MENA region among displaced populations, whether resulting from climate impacts on the environment and livelihood of populations in the region or from ongoing civil unrest and violence” (Al-Delaimy et al., 2020).
7. Research recommendations
Based on our analysis of the current state of the knowledge regarding the effects of climate change on human health in the EMME, we have identified several topics where more research is urgently needed in order to address important knowledge gaps and to inform future policies for adaptation to climate change and mitigation of its effects on human health. These are detailed below:
7.1. Empirical evidence on exposure-response functions involving climate change and health outcomes
Our capacity to predict the effects of climate change on human health is largely based on our understanding of the exposure-response functions4 of human beings. However, the empirical evidence available to evaluate these functions does not cover all health aspects potentially influenced by climatic factors (Madrigano et al., 2021). More research is required, for example, on the effects of dust exposure on chronic health conditions (Lelieveld et al., 2015), the effects of air pollution caused by wildfires on respiratory and neurologic health, and the effects of climate change on children's overall health (Madrigano et al., 2021; Sheffield et al., 2018). Furthermore, we need to develop better tools to evaluate the psychological effects of human displacement, particularly for children and women (Brennan and Nandy, 2001).
7.2. Assessment of the effects of climate change on ecological determinants of human health
In addition to the aforementioned scarcity of data linking climate change and certain health outcomes, there is also a dearth of empirical data about the influence of changing climate on ecological factors that ultimately affect human health. This situation is particularly relevant in the field of vector-borne diseases due to the complex nature of their natural cycles, which involve pathogens, vectors and reservoirs in addition to human beings.
Several recent studies have sought to estimate the effects of altered environmental temperatures on the vectorial capacity of some of the main species of disease vectors (Mordecai et al., 2019; Liu-Helmersson et al., 2014; Mordecai et al., 2017). However, these studies are based on predictive models, which often rely on generalizations about biological and physiological parameters, and rarely incorporate variables such as the genetic variability of pathogens and vectors. Furthermore, it is difficult (and often impossible) for modelling studies to account for complex aspects such as progressive adaptive changes in the biology, physiology and/or behavior of the many species involved in the transmission cycles of infectious diseases.
More and better empirical data could in turn be used to produce and refine predictive models. Priorities for study include (a) the effects of environmental variables on vector physiology, longevity, fertility, behavior, morphology and competence; (b) effects of climate change on the biology, abundance and distribution of reservoirs, and (c) the effects of climate change on the genetic variability and pathogenicity of vector-borne microbes.
7.3. Effects of long-term exposure to climate change
Most of our current understanding of the influence of climate change on human health is based on the effects of severe weather events (such as heat waves), which are typically short-lived. Little information is available, however, on the effects of long-term exposure to persistent climatic change, such as the gradual increase in average temperatures or low-level increases in the concentration of air pollutants (Madrigano et al., 2021; Wolf et al., 2021; Dirgawati et al., 2019). In addition, data on long-term exposure to climatic change is often focused on mortality instead of disease incidence (Wolf et al., 2021).
One important consequence of the current focus on the human health effects of severe, short-term weather events is that without data on long-term exposure to low-level environmental change, it is not possible to properly assess a population's adaptation responses, whether autonomous (e.g. changes in local attitudes and practices) or planned (e.g. development of early warning systems and response strategies) (Madrigano et al., 2021).
7.4. Evaluation of the interactions between adaptation and mitigation strategies
Although it is generally accepted that strategies aimed at either mitigating climate change or adapting to it are beneficial for human health (Milner et al., 2020; Haines et al., 2009), literature reporting on the complex, and sometimes conflicting, interactions between mitigation and adaptation strategies is rather scarce. An example is outlined by Madrigano et al. (2021):
“the most effective adaptation strategy for preventing heat-related morbidity and mortality is to increase access to air conditioning [ …]. However, without sufficient renewable electricity generation, such a strategy will result in an increase in greenhouse gas emissions, directly conflicting with mitigation goals.”
The complexity of these interactions is rooted in the inherent differences of the two types of strategies. While both adaptation and mitigation seek to address climate change, they usually work at different temporal and geographic scales, with adaptation strategies aiming to achieve local changes in the short and medium terms, while mitigation strategies generally seek global changes over the long term. Furthermore, there are also important differences in the relative relevance of these two types of strategies to different sectors, with mitigation strategies being mainly relevant to the energy and finance sectors, while adaptation strategies are generally more applicable to the health, water management, farming and tourism sectors, among others (Tol, 2005).
Therefore, research in this area should focus on establishing the cross-linking and trade-offs between mitigation and adaptation efforts (i.e. how global mitigation efforts impact options for adaptation in specific regions, and how adaptation practices can alter greenhouse gas emissions). Ultimately, researchers and policy makers should strive to devise strategic plans that commingle adaptation and mitigation tools, ensuring their acceptability across social, economic and environmental perspectives (Klein et al. et al., 2007).
8. Policy suggestions
8.1. Move decisively towards de-carbonization
Achieving meaningful reductions in greenhouse gas emissions is a key component of any plan aimed at protecting human health from future climate change. Lelieveld et al. (2019) have estimated that the burning of fossil fuels causes an excess mortality of approximately 3.61 million people per year, globally. Although phasing out fossil fuels is one of the most important steps to protect human health from climate change, our current rate of fossil fuel consumption is deeply rooted in complex and often outdated political and societal practices. Thus, achieving meaningful and sustainable changes will require profound commitments on the side of consumers, institutions and policymakers alike.
Several technologies with the potential to reduce greenhouse gas emissions (including improvements in energy efficiency, carbon sequestration and renewable energies, among other advances) have existed for decades, so the current barriers to de-carbonization are not necessarily technical or even economic (Watts et al., 2015). Therefore, only strong political determination at the national and regional levels can drive a concerted and efficient de-carbonization movement. As clearly stated in a recent editorial by Atwoli et al., endorsed by over 200 scientific journals:
“The current strategy of encouraging markets to swap dirty for cleaner technologies is not enough. Governments must intervene to support the redesign of transport systems, cities, production and distribution of food, markets for financial investments, health systems, and much more.” (Atwoli et al., 2021).
8.2. Integration of environmentally driven morbidity and mortality data throughout the EMME
After a careful revision of scientific and medical data available to date, it has become apparent that the EMME region would greatly benefit from the development of a system that facilitates the tracking of morbidity and mortality data attributable to climatic factors.
Such a system would allow us not only to establish clearer correlations between regional variations in climatic parameters and the incidence of specific health issues, but also to measure the efficiency of policies designed to prevent or mitigate the effects of climate change at the national or regional levels.
To develop a system of this nature, the EMME nations need to take several important steps. Namely:
-
a)
Reach a scientific consensus regarding which health conditions are directly and indirectly influenced by climatic factors
-
b)
Homogenize reporting criteria for the aforementioned health conditions throughout EMME countries
-
c)
Create a regional data repository where researchers and policy makers can openly access up-to-date clinical and epidemiological information.
-
d)
Archive validated data –both historical and current-on climate and pollution in the air, fresh water, oceans, etc.
-
e)
Establish environmental health funds to finance region-wide research on climate change and its effects on health.
8.3. Advancing the development and widespread use of cheaper technologies for the production and management of drinking water by non-traditional means
Water scarcity is a common threat for the future of most EMME countries. It is therefore vitally important to develop region-wide policies aimed at either managing or generating water for human consumption.
Efforts should be made to optimize the efficiency of water distribution systems as well as to stimulate rational consumption. To this end, the implementation of smart-metering systems might improve cost recovery and reduce waste on the users’ side by increasing awareness of consumption (World Bank, 2018).
Although recent advances in desalination and membrane technology have decreased the costs associated with the production of drinking water by alternative means, the price tag of these technologies remains high enough as to become prohibitive for many countries in the region. Therefore, EMME countries should prioritize investments in research aimed at optimizing (and therefore reducing the cost of) alternative technologies for the generation of drinking water. Furthermore, it is also necessary to develop technologies that mitigate the ecological costs of these technologies (for example, the safe disposal of the highly concentrated brine generated by seawater desalination processes).
Potential mechanisms to achieve the required improvements include the creation of funding schemes for academic groups working in this research field, as well as policies that foster startup companies focused on developing relevant novel technologies.
8.4. Comprehensive regional strategies for improving the health status of displaced populations
The particular circumstances surrounding displaced populations require special healthcare considerations. Mazhin and collaborators (2020) have stated that “Public health responses and policies related to health risks associated with migration from climate change must be consistent with the nature of the migration and the demographic characteristics of those who migrate” (Mazhin et al., 2020). In this context, policies related to the health of migrant populations need to consider aspects such as the inherent inequalities in healthcare access of the displaced population vs. the local populations, as well as the cultural complexities of refugee settlements, which often intermix populations with an array of cultural, ethnic and religious backgrounds.
We recommend that EMME countries hosting settlements of displaced individuals implement comprehensive health policies that offer access to:
-
a)
Healthy food and water
-
b)
Mental health services and crisis help lines
-
c)
Maternal and reproductive health resources
-
d)
Medicines to control chronic health conditions
-
e)
Infant vaccination
-
f)
Diagnosis and treatment of infectious diseases
-
g)
Control of disease vectors
In order to plan and implement such comprehensive health policies, national governments should coordinate efforts with nongovernmental organizations (e.g. the International Organization for Migration, the United Nations High Commission for Refugees, The Red Cross, Médecins Sans Frontières, among other), private institutions, local authorities and the displaced communities themselves.
8.5. Fostering regional networks to monitor and control the spread of infectious diseases and disease vectors
Due to the high mobility of people and goods characteristic of modern society, national boundaries no longer represent meaningful barriers to the spread of infectious diseases – a fact made painfully clear during the current COVID-19 pandemic. As a consequence, any efforts towards the monitoring and control of pathogens and disease vectors at the national level need to be complemented with carefully coordinated regional strategies. Indeed, there is evidence that region-wide initiatives aimed at producing epidemiological data, identifying dissemination routes and facilitating control strategies have been key in successfully reducing the disease burden of malaria in certain parts of Africa, as well as Chagas disease in South America (Negev et al., 2015).
In the EMME region, several initiatives have attempted to create regional networks for the monitoring and control of infectious diseases. These include the EpiSouth project, which in its initial stages sought to connect infectious disease experts from southern Europe, the Mediterranean and the Balkans (Negev et al., 2015), and the Middle East Consortium on Infectious Disease Surveillance (MECIDS), an initiative that facilitates communication and coordination among the ministries of health in Jordan, Palestine and Israel (Linares et al., 2020a). Ideally, these early steps toward regional integration should inspire infectious disease experts and policy makers from EMME nations to develop novel, more comprehensive collaborative networks that are able to transcend the language, cultural and political barriers characteristic of this highly complex region.
Fig. 10 presents a visual summary of the five policy proposals presented as part of this report.
Fig. 10.
Visual summary of policy suggestions. If implemented on a regional scale, these policies would enhance the resilience of the EMME against climate change.
Author contributions
H. Ahmady-Birgani: Investigation, writing -original draft, writing – review & editing.
G. Christophides: conceptualization, supervision, funding acquisition, writing -original draft, writing – review & editing.
N. DaifAllah AL-Hmoud: writing – review & editing.
K. Erguler: Investigation, writing -original draft, writing – review & editing.
R. Fears: writing – review & editing.
C. Gogos: writing – review & editing.
N. Hobbhahn: writing – review & editing.
M. Koliou: writing – review & editing.
L. Kostrikis: writing – review & editing.
J. Lelieveld: writing – review & editing.
A. Majeed: writing – review & editing.
M. Neira: conceptualization, supervision, investigation, visualization, methodology, writing -original draft, writing – review & editing.
S. Paz: writing – review & editing.
Y. Rudich: writing – review & editing.
A. Saad-Hussein: investigation, writing -original draft, writing – review & editing.
M. Shaheen: writing – review & editing.
A. Tobias: investigation, writing -original draft, writing – review & editing.
Funding
This project received funding from the Cyprus Institute's Core funds and from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 856612 and the Cyprus Government. All authors are members of the Task Force on Health of the Eastern Mediterranean and Middle East Climate Change Initiative (EMME-CCI).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Dr. Maria Christou, Dr. Pantelis Georgiades and Ms. Aayushi Sharma for their generous help in proof-reading this document.
Footnotes
Most people regulate body temperature to stay around 37 °C. However, under particular circumstances (such as during high-performance athletic training), the body temperature of normal individuals can temporarily rise above this limit without permanent negative consequences (Lim, 2020).
Although there is no standardized definition for the concept of heatwaves, they are generally described as periods of “consecutive days where conditions are excessively hotter than normal” (Perkins and Alexander, 2013).
It has been documented that environmental temperature in cities is generally higher than in the surrounding rural areas (Oke, 1982). This is known as the “urban heat island” effect.
Exposure-response functions are defined as the potential associations between climate-sensitive environmental hazards and health outcomes (Kintziger et al., 2017).
Contributor Information
Marco Neira, Email: m.neira@cyi.ac.cy.
George Christophides, Email: g.christophides@cyi.ac.cy.
Data availability
No data was used for the research described in the article.
References
- Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Ghani R., et al. Impact of population displacement and forced movements on the transmission and outbreaks of Aedes-borne viral diseases: dengue as a model. Acta Trop. 2019;197 doi: 10.1016/j.actatropica.2019.105066. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Ahmady-Birgani H., Mirnejad H., Feiznia S., McQueen K.G. Mineralogy and geochemistry of atmospheric particulates in western Iran. Atmos. Environ. 2015;119:262–272. doi: 10.1016/j.atmosenv.2015.08.021. [DOI] [Google Scholar]
- Ahmady-Birgani H., McQueen K.G., Moeinaddini M., Naseri H. Sand dune encroachment and desertification processes of the Rigboland Sand Sea, Central Iran. Sci. Rep. 2017. 2017;7(1):1–10. doi: 10.1038/s41598-017-01796-z. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmady-Birgani H., McQueen K.G., Mirnejad H. Characteristics of mineral dust impacting the Persian Gulf. Aeolian Res. 2018;30:11–19. doi: 10.1016/J.AEOLIA.2017.11.001. [DOI] [Google Scholar]
- Ahmed S.A., Saad-Hussein A., El Feel A., Hamed M.A. Time series trend of Bilharzial bladder cancer in Egypt and its relation to climate change: a study from 1995-2005. Int. J. Pharm. Clin. Res. 2014;6(1):46–53. [Google Scholar]
- Al-Delaimy W. In: Health of People, Health of Planet and Our Responsibility: Climate Change, Air Pollution and Health. Al-Delaimy W., Ramanathan V., Sanchez Sorondo M., editors. Springer Open; Cham, Switzerland: 2020. Vulnerable populations and regions: Middle East as a case study; pp. 121–133. [Google Scholar]
- Alahmad B., et al. Cardiovascular mortality and exposure to heat in an inherently hot region: implications for climate change. Circulation. 2020;141(15):1271–1273. doi: 10.1161/CIRCULATIONAHA.119.044860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawieh A., Musharrafieh U., Jaber A., Berry A., Ghosn N., Bizri A.R. Revisiting leishmaniasis in the time of war: the Syrian conflict and the Lebanese outbreak. Int. J. Infect. Dis. 2014;29:115–119. doi: 10.1016/j.ijid.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Amarloei A., Fazlzadeh M., Jafari A.J., Zarei A., Mazloomi S. Particulate matters and bioaerosols during Middle East dust storms events in Ilam, Iran. Microchem. J. 2020;152 doi: 10.1016/J.MICROC.2019.104280. [DOI] [Google Scholar]
- Aminharati F., et al. The effect of environmental parameters on the incidence of Shigella outbreaks in Yazd province, Iran. Water Supply. 2018;18(4):1388–1395. doi: 10.2166/ws.2017.205. [DOI] [Google Scholar]
- António D.C., Sanseverino I., Pozzoli L., Lettieri T. JRC Technical Report; 2018. Toward Climate Change Impact : Vectors Carrying Viral Infection What We Should Know. [DOI] [Google Scholar]
- Arnetz B.B., et al. Dysfunctional neuroplasticity in newly arrived Middle Eastern refugees in the U.S.: association with environmental exposures and mental health symptoms. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspöck H., Gerersdorfer T., Formayer H., Walochnik J. Sandflies and sandfly-borne infections of humans in Central Europe in the light of climate change. Wien Klin. Wochenschr. 2008;120(Suppl. 4):24–29. doi: 10.1007/s00508-008-1072-8. Wien Klin Wochenschr. [DOI] [PubMed] [Google Scholar]
- Aswini M.A., Kumar A., Das S.K. Quantification of long-range transported aeolian dust towards the Indian peninsular region using satellite and ground-based data - a case study during a dust storm over the Arabian Sea. Atmos. Res. 2020;239:104910. doi: 10.1016/J.ATMOSRES.2020.104910. [DOI] [Google Scholar]
- Atwoli L., et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health. BMJ. 2021;374:1734. doi: 10.1136/BMJ.N1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badarinath K.V.S., Kharol S.K., Kaskaoutis D.G., Sharma A.R., Ramaswamy V., Kambezidis H.D. Long-range transport of dust aerosols over the Arabian Sea and Indian region — a case study using satellite data and ground-based measurements. Global Planet. Change. 2010;72(3):164–181. doi: 10.1016/j.gloplacha.2010.02.003. [DOI] [Google Scholar]
- Baghbanan P., Ghavidel Y., Farajzadeh M. Spatial analysis of spring dust storms hazard in Iran. Theor. Appl. Climatol. 2019;139(3):1447–1457. doi: 10.1007/S00704-019-03060-Y. 2019 1393. [DOI] [Google Scholar]
- Baldwin-Edwards M. Global Commission on International Migration; Athens, Greece: 2005. Migration in the Middle East and Mediterranean.” Policy Analysis and Research Programme. [Google Scholar]
- Balsari S., Dresser C., Leaning J. vol. 7. Springer Science and Business Media Deutschland GmbH; 2020. Climate change, migration, and civil strife; pp. 404–414. (Current Environmental Health Reports). 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriopedro D., Fischer E.M., Luterbacher J., Trigo R.M., García-Herrera R. The hot summer of 2010: redrawing the temperature record map of Europe. Science (80-.) 2011;332(6026):220–224. doi: 10.1126/science.1201224. [DOI] [PubMed] [Google Scholar]
- Blanford J.I., et al. Implications of temperature variation for malaria parasite development across Africa. Sci. Rep. 2013;3 doi: 10.1038/srep01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonyadi Z., Arfaeinia H., Ramavandi B., Omidvar M., Asadi R. Quantification of mortality and morbidity attributed to the ambient air criteria pollutants in Shiraz city, Iran. Chemosphere. 2020;257:127233. doi: 10.1016/J.CHEMOSPHERE.2020.127233. [DOI] [PubMed] [Google Scholar]
- Borgomeo E., Jägerskog A., Talbi A., Wijnen M., Hejazi M., Miralles-Wilhelm F. The World Bank; Washington, D.C.: 2018. The Water-Energy-Food Nexus in the Middle East and North Africa Scenarios for a Sustainable Future. [Google Scholar]
- Bowles D.C., Butler C.D., Morisetti N. Climate change, conflict and health. J. R. Soc. Med. 2015;108(10):390–395. doi: 10.1177/0141076815603234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady O.J., et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites Vectors. 2014;7(1):338. doi: 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan R.J., Nandy R. Complex humanitarian emergencies: a major global health challenge. Emerg. Med. 2001;13(2):147–156. doi: 10.1046/j.1442-2026.2001.00203.x. [DOI] [PubMed] [Google Scholar]
- De Bruijn B. United Nations Development Programme; 2009. Human Development Research Paper 2009/25: the Living Conditions and Well-Being of Refugees. [Google Scholar]
- Burnett R.T., et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014;122(4):397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett R., et al. Global estimates of mortality associated with longterm exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. U.S.A. 2018;115(38):9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G.L., Marfin A.A., Lanciotti R.S., Gubler D.J. West Nile virus. Lancet Infect. Dis. 2002;2(9):519–529. doi: 10.1016/S1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- Can G., et al. Excess mortality in Istanbul during extreme heat waves between 2013 and 2017. Int. J. Environ. Res. Public Heal. Artic. 2019 doi: 10.3390/ijerph16224348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), “Malaria.” https://www.cdc.gov/malaria/about/disease.html (accessed Jan. 15, 2021).
- Chalghaf B., et al. Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: impact of climate change. Parasites Vectors. 2018;11(1):461. doi: 10.1186/s13071-018-3019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Vicedo-Cabrera A.M., Dubrow R. Projections of ambient temperature- and air pollution-related mortality burden under combined climate change and population aging scenarios: a review. Curr. Environ. Health Rep. 2020;7(3):243–255. doi: 10.1007/s40572-020-00281-6. Springer. [DOI] [PubMed] [Google Scholar]
- Chen K., et al. Ambient carbon monoxide and daily mortality: a global time-series study in 337 cities. Lancet Planet. Health. 2021;5(4):e191–e199. doi: 10.1016/S2542-5196(21)00026-7. [DOI] [PubMed] [Google Scholar]
- Chenoweth J., et al. Impact of climate change on the water resources of the eastern Mediterranean and Middle East region: modeled 21st century changes and implications. Water Resour. Res. 2011;47(6) doi: 10.1029/2010WR010269. [DOI] [Google Scholar]
- Chowdhury S., et al. Global and national assessment of the incidence of asthma in children and adolescents from major sources of ambient NO2. Environ. Res. Lett. 2021;16(3):35020. doi: 10.1088/1748-9326/ABE909. [DOI] [Google Scholar]
- Chynoweth S., McKenna M. Women’s Commission for Refugee Women and Children; 2007. Iraqi Refugee Women and Youth in Jordan: Reproductive Health Findings (A Snapshot from the Field) [Google Scholar]
- Ciota A.T., Keyel A.C. The role of temperature in transmission of zoonotic arboviruses. Viruses. 2019;11:11. doi: 10.3390/v11111013. MDPI AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.J., et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet (London, England) 2014;383(9936):2253. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpitts T.M., Conway M.J., Montgomery R.R., Fikrig E. West Nile virus: biology, transmission, and human infection. Clin. Microbiol. Rev. 2012;25(4):635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotar A.I., Falcuta E., Prioteasa L.F., Dinu S., Ceianu C.S., Paz S. Transmission dynamics of the West Nile virus in mosquito vector populations under the influence of weather factors in the Danube Delta, Romania. EcoHealth. 2016;13(4):796–807. doi: 10.1007/s10393-016-1176-y. [DOI] [PubMed] [Google Scholar]
- Cramer W., et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change. 2018;8(11):972–980. doi: 10.1038/s41558-018-0299-2. [DOI] [Google Scholar]
- Dassonville L., Fé D’ostiani L. Proceedings of the International Watershed Management Conference on Water Resources for the Future. The Food and Agriculture Organization of the United Nations (FAO); Sardinia, Italy: 2006. Mediterranean watershed management: overcoming water crisis in the Mediterranean; pp. 61–70. [Google Scholar]
- Daynes L. The health impacts of the refugee crisis: a medical charity perspective. Clin. Med. J. R. Coll. Phys. Lond. 2016;16(5):437–440. doi: 10.7861/clinmedicine.16-5-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong J., Jawad R., Mortagy I., Shepard B. The sexual and reproductive health of young people in the Arab countries and Iran. Reprod. Health Matters. 2005;13(25):49–59. doi: 10.1016/S0968-8080(05)25181-9. [DOI] [PubMed] [Google Scholar]
- Díaz J., Carmona R., Mirón I.J., Luna M.Y., Linares C. Time trend in the impact of heat waves on daily mortality in Spain for a period of over thirty years (1983–2013) Environ. Int. 2018;116:10–17. doi: 10.1016/j.envint.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Dirgawati M., et al. Long-term exposure to low air pollutant concentrations and the relationship with all-cause mortality and stroke in older men. Epidemiology. 2019;30:S82–S89. doi: 10.1097/EDE.0000000000001034. [DOI] [PubMed] [Google Scholar]
- de’ Donato F., et al. Changes in the effect of heat on mortality in the last 20 Years in nine European cities. Results from the PHASE project. Int. J. Environ. Res. Publ. Health. 2015;12(12):15567–15583. doi: 10.3390/ijerph121215006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducheyne E., et al. Current and future distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in WHO Eastern Mediterranean region. Int. J. Health Geogr. 2018;17(1):4. doi: 10.1186/s12942-018-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East P.L., Gahagan S., Al-Delaimy W.K. The impact of refugee mothers' trauma, posttraumatic stress, and depression on their children's adjustment. J. Immigr. Minority Health. 2018;20(2):271–282. doi: 10.1007/s10903-017-0624-2. [DOI] [PubMed] [Google Scholar]
- Elmorshedy H., et al. Elimination of schistosomiasis requires multifactorial diagnostics: evidence from high- and low-prevalence areas in the Nile Delta, Egypt. Infect. Dis. Poverty. 2020;9(1) doi: 10.1186/S40249-020-00648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Academies Science Advisory Council (EASAC) vol. 38. EASAC policy report; Halle (Saale), Germany: 2019. p. 76. (The Imperative of Climate Action to Protect Human Health in Europe). [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) ECDC; Stockholm: 2017. Multiple Reports of Locally-acquired Malaria Infections in the EU - 20 September 2017. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) 2021. Aedes aegypti - Current Known Distribution: March 2021.https://www.ecdc.europa.eu/en/publications-data/aedes-aegypti-current-known-distribution-march-2021 accessed Sep. 13, 2021) [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) 2021. Aedes albopictus - Current Known Distribution: March 2021.https://www.ecdc.europa.eu/en/publications-data/aedes-albopictus-current-known-distribution-march-2021 accessed Sep. 13, 2021) [Google Scholar]
- Falkenmark M., Lundqvist J., Widstrand C. Macro‐scale water scarcity requires micro‐scale approaches: aspects of vulnerability in semi‐arid development. Nat. Resour. Forum. 1989;13(4):258–267. doi: 10.1111/j.1477-8947.1989.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Falkovich A.H., Rudich Y. Analysis of semivolatile organic compounds in atmospheric aerosols by direct sample introduction thermal desorption GC/MS. Environ. Sci. Technol. 2001;35(11):2326–2333. doi: 10.1021/es000280i. [DOI] [PubMed] [Google Scholar]
- Falkovich A.H., Schkolnik G., Ganor E., Rudich Y. Adsorption of organic compounds pertinent to urban environments onto mineral dust particles. J. Geophys. Res. Atmos. 2004;109(2):2208. doi: 10.1029/2003jd003919. [DOI] [Google Scholar]
- Fernald L.C., Grantham-McGregor S.M. Stress response in school-age children who have been growth retarded since early childhood. Am. J. Clin. Nutr. 1998;68(3):691–698. doi: 10.1093/ajcn/68.3.691. [DOI] [PubMed] [Google Scholar]
- Ferragina E. In: Environmental and Sustainable Development in the Mediterranean. Scoullos M., Ferragina E., editors. 2010. The water issue in the Mediterranean; pp. 53–78. [Google Scholar]
- Finlayson C. Biogeography and evolution of the genus Homo. Trends Ecol. Evol. 2005;20(8):457–463. doi: 10.1016/j.tree.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Forzieri G., Cescatti A., e Silva F.B., Feyen L. Increasing risk over time of weather-related hazards to the European population: a data-driven prognostic study. Lancet Planet. Health. 2017;1(5):e200–e208. doi: 10.1016/S2542-5196(17)30082-7. [DOI] [PubMed] [Google Scholar]
- Fraser E.D.G. Social vulnerability and ecological fragility: building bridges between social and natural sciences using the Irish Potato famine as a case study. Ecol. Soc. 2003;7(2) doi: 10.5751/es-00534-070209. [DOI] [Google Scholar]
- Fridman D., Biran N., Kissinger M. Beyond blue: an extended framework of blue water footprint accounting. Sci. Total Environ. 2021;777 doi: 10.1016/j.scitotenv.2021.146010. [DOI] [PubMed] [Google Scholar]
- Garnham P.C. Distribution of simian malaria parasites in various hosts. J. Parasitol. 1963;49:905–911. doi: 10.2307/3275716. [DOI] [PubMed] [Google Scholar]
- Gat D., Mazar Y., Cytryn E., Rudich Y. Origin-dependent variations in the atmospheric microbiome community in Eastern Mediterranean dust storms. Environ. Sci. Technol. 2017;51(12):6709–6718. doi: 10.1021/acs.est.7b00362. [DOI] [PubMed] [Google Scholar]
- Gauer R., Meyers B.K. Heat-related illnesses. Am. Fam. Physician. 2019;99(8):482–489. [PubMed] [Google Scholar]
- Giannadaki D., Pozzer A., Lelieveld J. Modeled global effects of airborne desert dust on air quality and premature mortality. Atmos. Chem. Phys. 2014;14(2):957–968. doi: 10.5194/ACP-14-957-2014. [DOI] [Google Scholar]
- Givehchi R., Arhami M., Tajrishy M. Contribution of the Middle Eastern dust source areas to PM10 levels in urban receptors: case study of Tehran, Iran. Atmos. Environ. 2013;75:287–295. doi: 10.1016/j.atmosenv.2013.04.039. [DOI] [Google Scholar]
- Gökengin D., Doroudi F., Tohme J., Collins B., Madani N. vol. 44. Elsevier B.V.; 2016. HIV/AIDS: trends in the Middle East and North Africa region; pp. 66–73. (International Journal of Infectious Diseases). [DOI] [PubMed] [Google Scholar]
- Goudarzi G., Hopke P.K., Yazdani M. Forecasting PM2.5 concentration using artificial neural network and its health effects in Ahvaz, Iran. Chemosphere. 2021;283:131285. doi: 10.1016/J.CHEMOSPHERE.2021.131285. [DOI] [PubMed] [Google Scholar]
- Haines A., et al. Public health benefits of strategies to reduce greenhouse-gas emissions: overview and implications for policy makers. Lancet. 2009;374(9707):2104–2114. doi: 10.1016/S0140-6736(09)61759-1. [DOI] [PubMed] [Google Scholar]
- Hamidianpour M., Jahanshahi S.M.A., Kaskaoutis D.G., Rashki A., Nastos P.G. Climatology of the Sistan Levar wind: atmospheric dynamics driving its onset, duration and withdrawal. Atmos. Res. 2021;260:105711. doi: 10.1016/J.ATMOSRES.2021.105711. [DOI] [Google Scholar]
- Heaviside C., et al. Heat-related mortality in Cyprus for current and future climate scenarios. Sci. Total Environ. 2016;569(570):627–633. doi: 10.1016/j.scitotenv.2016.06.138. [DOI] [PubMed] [Google Scholar]
- Heger M., Sarraf M. World Bank; Washington, DC: 2018. Air Pollution in Tehran : Health Costs, Sources, and Policies,” Environment and Natural Resources Global Practice Discussion Paper No. 6. [DOI] [Google Scholar]
- Hermans M.P.J., Kooistra J., Cannegieter S.C., Rosendaal F.R., Mook-Kanamori D.O., Nemeth B. Healthcare and disease burden among refugees in long-stay refugee camps at Lesbos, Greece. Eur. J. Epidemiol. 2017;32(9):851–854. doi: 10.1007/s10654-017-0269-4. [DOI] [PubMed] [Google Scholar]
- Heydari N., et al. Household dengue prevention interventions, expenditures, and barriers to Aedes aegypti control in Machala, Ecuador. Int. J. Environ. Res. Publ. Health. 2017;14(2) doi: 10.3390/ijerph14020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstick O., Runge-Ranzinger S. Multisectoral approaches for the control of vector-borne diseases, with particular emphasis on dengue and housing. Trans. R. Soc. Trop. Med. Hyg. 2019;113(12):822–827. doi: 10.1093/trstmh/trz020. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- Hunter P.R., Macdonald A.M., Carter R.C. Water supply and health. PLoS Med. 2010;7(11) doi: 10.1371/journal.pmed.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulou P., et al. Risk mapping of visceral leishmaniasis: a spatial regression model for Attica region, Greece. Trav. Med. Infect. Dis. 2018;3(3) doi: 10.3390/tropicalmed3030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura T., Guzman-Holst A., Murray K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 2020;11(1):1–10. doi: 10.1038/s41467-020-16010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanani K., et al. Cutaneous leishmaniasis among Syrian refugees in Jordan. Acta Trop. 2019;194:169–171. doi: 10.1016/j.actatropica.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Karami S., Hamzeh N.H., Alam K., Noori F., Saadat Abadi A.R. Spatio-temporal and synoptic changes in dust at the three islands in the Persian Gulf region. J. Atmos. Sol. Terr. Phys. 2021;214:105539. doi: 10.1016/J.JASTP.2021.105539. [DOI] [Google Scholar]
- Karanasiou A., et al. Short-term health effects from outdoor exposure to biomass burning emissions: a review. Sci. Total Environ. 2021;781 doi: 10.1016/j.scitotenv.2021.146739. [DOI] [PubMed] [Google Scholar]
- Kelley C.P., Mohtadi S., Cane M.A., Seager R., Kushnir Y. Climate change in the Fertile Crescent and implications of the recent Syrian drought. Proc. Natl. Acad. Sci. U.S.A. 2015;112(11):3241–3246. doi: 10.1073/pnas.1421533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrovski V., Baccini M., Martinez G.S., Wolf T., Paunovic E., Menne B. Quantifying projected heat mortality impacts under 21st-Century warming conditions for selected European countries. Int. J. Environ. Res. Publ. Health. 2017;14(7) doi: 10.3390/ijerph14070729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneson A., et al. Social-ecological factors and preventive actions decrease the risk of dengue infection at the household-level: results from a prospective dengue surveillance study in Machala, Ecuador. PLoS Neglected Trop. Dis. 2017;11(12) doi: 10.1371/journal.pntd.0006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramat A., Marivani B., Samsami M. Climatic change, drought and dust crisis in Iran. World Acad. Sci. Eng. Technol. 2011;81(9):10–13. [Google Scholar]
- Keyantash J., Dracup J.A. The quantification of drought: an evaluation of drought indices. Bull. Am. Meteorol. Soc. 2002;83(8):1167–1180. doi: 10.1175/1520-0477-83.8.1167. [DOI] [Google Scholar]
- Khader Y.S., et al. Climate change and health in the Eastern Mediterranean countries: a systematic review. Rev. Environ. Health. 2015;30(3):163–181. doi: 10.1515/reveh-2015-0013. [DOI] [PubMed] [Google Scholar]
- Kintziger K.W., et al. Climate and Health Technical Report Series. Climate and Health Program. Centers for Disease Control and Prevention.; 2017. Technical documentation on exposure-response functions for climate-sensitive health outcomes; pp. 1–54. [Google Scholar]
- Klein R.J., et al. In: Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. van der L P.J., Parry C.E.H.M.L., Canziani O.F., Palutikof J.P., editors. Cambridge University Press; Cambridge, UK: 2007. Inter-relationships between adaptation and mitigation; pp. 745–777. [Google Scholar]
- Klingmüller K., Pozzer A., Metzger S., Stenchikov G.L., Lelieveld J. Aerosol optical depth trend over the Middle East. Atmos. Chem. Phys. 2016;16(8):5063–5073. doi: 10.5194/acp-16-5063-2016. [DOI] [Google Scholar]
- Kouadio I.K., Kamigaki T., Oshitani H. Measles outbreaks in displaced populations: a review of transmission, morbidity and mortality associated factors. BMC Int. Health Hum. Right. 2010;10(1) doi: 10.1186/1472-698X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer M.U.G., et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019;4(5):854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnov H., Katra I., Friger M. Increase in dust storm related PM10 concentrations: a time series analysis of 2001–2015. Environ. Pollut. 2016;213:36–42. doi: 10.1016/j.envpol.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Kumar A., et al. Seasonal radiogenic isotopic variability of the African dust outflow to the tropical Atlantic Ocean and across to the Caribbean. Earth Planet Sci. Lett. 2018;487:94–105. doi: 10.1016/J.EPSL.2018.01.025. [DOI] [Google Scholar]
- Kummu M., de Moel H., Porkka M., Siebert S., Varis O., Ward P.J. Lost food, wasted resources: global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci. Total Environ. 2012;438:477–489. doi: 10.1016/j.scitotenv.2012.08.092. [DOI] [PubMed] [Google Scholar]
- Kwak R., Kamal K., Charrow A., Khalifian S. Mass migration and climate change: dermatologic manifestations. Int. J. Women’s Dermatol. 2021;7(1):98–106. doi: 10.1016/j.ijwd.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D. The ecology of climate change and infectious diseases. Ecology. 2009;90(4):888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Lang-Yona N., et al. Links between airborne microbiome, meteorology, and chemical composition in northwestern Turkey. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138227. 138227. [DOI] [PubMed] [Google Scholar]
- Lelieveld J., Proestos Y., Hadjinicolaou P., Tanarhte M., Tyrlis E., Zittis G. Strongly increasing heat extremes in the Middle East and North Africa (MENA) in the 21st century. Clim. Change. 2016 doi: 10.1007/s10584-016-1665-6. [DOI] [Google Scholar]
- Lelieveld J., et al. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Change. 2012;114(3–4):667–687. doi: 10.1007/s10584-012-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J., Evans J.S., Fnais M., Giannadaki D., Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Lelieveld J., Klingmüller K., Pozzer A., Burnett R.T., Haines A., Ramanathan V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. U.S.A. 2019;116(15):7192–7197. doi: 10.1073/pnas.1819989116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J., Pozzer A., Pöschl U., Fnais M., Haines A., Münzel T. Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc. Res. 2020;116(11):1910–1917. doi: 10.1093/CVR/CVAA025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B.S., Sidel V.W., Patz J.A. Climate change and collective violence. Annu. Rev. Publ. Health. 2017;38(1):241–257. doi: 10.1146/annurev-publhealth-031816-044232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Bou-Zeid E. Synergistic interactions between urban heat islands and heat waves: the impact in cities is larger than the sum of its parts. J. Appl. Meteorol. Climatol. 2013;52(9):2051–2064. doi: 10.1175/JAMC-D-13-02.1. [DOI] [Google Scholar]
- Li M., Gu S., Bi P., Yang J., Liu Q. Heat waves and morbidity: current knowledge and further direction-a comprehensive literature review. Int. J. Environ. Res. Publ. Health. 2015;12(5):5256–5283. doi: 10.3390/ijerph120505256. MDPI AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillepold K., Rocklöv J., Liu-Helmersson J., Sewe M., Semenza J.C. More arboviral disease outbreaks in continental Europe due to the warming climate? J. Trav. Med. 2019;26(5) doi: 10.1093/jtm/taz017. [DOI] [PubMed] [Google Scholar]
- Lim C.L. Fundamental concepts of human thermoregulation and adaptation to heat: a review in the context of global warming. Int. J. Environ. Res. Publ. Health. 2020;17(21):1–33. doi: 10.3390/ijerph17217795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.L., Byrne C., Lee J.K. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann. Acad. Med. Singapore. 2008;37(4):347–353. [PubMed] [Google Scholar]
- Linares C., Díaz J., Negev M., Martínez G.S., Debono R., Paz S. Impacts of climate change on the public health of the Mediterranean Basin population - current situation, projections, preparedness and adaptation. Environ. Res. 2020;182:109107. doi: 10.1016/j.envres.2019.109107. Academic Press Inc. [DOI] [PubMed] [Google Scholar]
- Linares C., Díaz J., Negev M., Martínez G.S., Debono R., Paz S. Impacts of climate change on the public health of the Mediterranean Basin population - current situation, projections, preparedness and adaptation. Environ. Res. 2020;182 doi: 10.1016/j.envres.2019.109107. Academic Press Inc. [DOI] [PubMed] [Google Scholar]
- Liu C., et al. Ambient particulate air pollution and daily mortality in 652 cities. N. Engl. J. Med. 2019;381(8):705–715. doi: 10.1056/nejmoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Helmersson J., Stenlund H., Wilder-Smith A., Rocklöv J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS One. 2014;9(3) doi: 10.1371/JOURNAL.PONE.0089783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Longueville F., Hountondji Y.C., Henry S., Ozer P. What do we know about effects of desert dust on air quality and human health in West Africa compared to other regions? Sci. Total Environ. 2010;409(1):1–8. doi: 10.1016/J.SCITOTENV.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Lorentzou C., et al. Extreme desert dust storms and COPD morbidity on the island of crete. Int. J. COPD. 2019;14:1763–1768. doi: 10.2147/COPD.S208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L.P., Escher R.L., Lourenço-De-Oliveira R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2003;96(4):512–518. doi: 10.1603/0013-8746(2003)096[0512:AEOPDI]2.0.CO;2. [DOI] [Google Scholar]
- Lubczyńska M.J., Christophi C.A., Lelieveld J. Heat-related cardiovascular mortality risk in Cyprus: a case-crossover study using a distributed lag non-linear model. Environ. Heal. A Glob. Access Sci. Source. 2015;14(1) doi: 10.1186/s12940-015-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigano J., Shih R.A., Izenberg M., Fischbach J.R., Preston B.L. Science policy to advance a climate change and health research agenda in the United States. Int. J. Environ. Res. Publ. Health. 2021;18(15) doi: 10.3390/ijerph18157868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio M., et al. Identifying the environmental conditions favouring West Nile Virus outbreaks in Europe. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini G., et al. West Nile virus transmission and human infection risk in Veneto (Italy): a modelling analysis. Sci. Rep. 2018;8(1):14005. doi: 10.1038/s41598-018-32401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini G., et al. A quantitative comparison of West Nile virus incidence from 2013 to 2018 in Emilia-Romagna, Italy. PLoS Neglected Trop. Dis. 2020;14(1) doi: 10.1371/journal.pntd.0007953. e0007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroli M., Feliciangeli M.D., Bichaud L., Charrel R.N., Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013;27(2):123–147. doi: 10.1111/j.1365-2915.2012.01034.x. Med Vet Entomol. [DOI] [PubMed] [Google Scholar]
- Martinez-Alvarez V., Bar-Tal A., Javier Diaz Peña F., Maestre Valero J.F. Desalination of seawater for agricultural irrigation. Water (Switzerland) 2020;12(6) doi: 10.3390/W12061712. MDPI AG. [DOI] [Google Scholar]
- Mathew B. IRC International Water and Sanitation Centre - Occasional Paper Series 40. 2005. Ensuring sustained beneficial outcomes for water and sanitation programmes in the developing world; pp. 1–223. Delft, the Netherlands. [Google Scholar]
- Mazar Y., Cytryn E., Erel Y., Rudich Y. Effect of dust storms on the atmospheric microbiome in the Eastern Mediterranean. Environ. Sci. Technol. 2016;50(8):4194–4202. doi: 10.1021/acs.est.5b06348. [DOI] [PubMed] [Google Scholar]
- Mazhin S., Khankeh H., Farrokhi M., Aminizadeh M., Poursadeqiyan M. Migration health crisis associated with climate change: a systematic review. J. Educ. Health Promot. 2020;9(1) doi: 10.4103/jehp.jehp_4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael C. Climate change-related migration and infectious disease. Virulence. 2015;6(6):548–553. doi: 10.1080/21505594.2015.1021539. Taylor and Francis Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael C., Barnett J., McMichael A.J. An III wind? Climate change, migration, and health. Environ. Health Perspect. 2012;120(5):646–654. doi: 10.1289/ehp.1104375. Public Health Services, US Dept of Health and Human Services. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatt Z.Z. Addressing noncommunicable diseases among urban refugees in the Middle East and North Africa - a scoping review. Conflict Health. 2020;14(1) doi: 10.1186/s13031-020-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade R.D., et al. Physiological factors characterizing heat-vulnerable older adults: a narrative review. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.105909. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Meng X., et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: multilocation analysis in 398 cities. BMJ. 2021;372 doi: 10.1136/bmj.n534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina J.P., et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019;4(9):1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton N., et al. A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: the effect of short-term changes in air pollution and dust storms. Environ. Health. 2008;7(1):39. doi: 10.1186/1476-069X-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millyard A., Layden J.D., Pyne D.B., Edwards A.M., Bloxham S.R. Impairments to thermoregulation in the elderly during heat exposure events. Gerontol. Geriatr. Med. 2020;6 doi: 10.1177/2333721420932432. 233372142093243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., et al. Health benefits of policies to reduce carbon emissions. BMJ. 2020;368 doi: 10.1136/bmj.l6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi R., Soori H., Alipour A., Bitaraf E., Khodakarim S. The impact of ambient temperature on acute myocardial infarction admissions in Tehran, Iran. J. Therm. Biol. 2018;73:24–31. doi: 10.1016/j.jtherbio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Mordecai E.A., et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Neglected Trop. Dis. 2017;11(4) doi: 10.1371/journal.pntd.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai E.A., et al. Thermal biology of mosquito‐borne disease. Ecol. Lett. 2019;22(10):1690–1708. doi: 10.1111/ele.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai E.A., Ryan S.J., Caldwell J.M., Shah M.M., LaBeaud A.D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health. 2020;4(9):e416–e423. doi: 10.1016/S2542-5196(20)30178-9. Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa A.N., et al. Past (1950–2017) and future (−2100) temperature and precipitation trends in Egypt. Weather Clim. Extrem. 2019;26 doi: 10.1016/j.wace.2019.100225. [DOI] [Google Scholar]
- Mousavi A., Ardalan A., Takian A., Ostadtaghizadeh A., Naddafi K., Bavani A.M. Climate change and health in Iran: a narrative review. J. Environ. Heal. Sci. Eng. 2020;18(1):367–378. doi: 10.1007/s40201-020-00462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowafi H. Conflict, displacement and health in the Middle East. Global Publ. Health. 2011;6(5):472–487. doi: 10.1080/17441692.2011.570358. Glob Public Health. [DOI] [PubMed] [Google Scholar]
- Murdock C.C., Sternberg E.D., Thomas M.B. Malaria transmission potential could be reduced with current and future climate change. Sci. Rep. 2016;6(1):1–7. doi: 10.1038/srep27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi Beni A., et al. Climate change: a driver of future conflicts in the Persian Gulf Region? Heliyon. 2021;7(2) doi: 10.1016/J.HELIYON.2021.E06288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negev M., et al. Impacts of climate change on vector borne diseases in the mediterranean basin — implications for preparedness and adaptation policy. Int. J. Environ. Res. Publ. Health. 2015;12(6):6745–6770. doi: 10.3390/ijerph120606745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou A.M., et al. Particulate matter concentrations during desert dust outbreaks and daily mortality in Nicosia, Cyprus. J. Expo. Sci. Environ. Epidemiol. 2013;23(3):275–280. doi: 10.1038/jes.2013.10. [DOI] [PubMed] [Google Scholar]
- Ngowi H.A. Prevalence and pattern of waterborne parasitic infections in eastern Africa: a systematic scoping review. Food Waterborne Parasitol. 2020;20 doi: 10.1016/j.fawpar.2020.e00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North Atlantic Treaty Organization (NATO) NATO Strategic Direction South Hub; Naples, Italy: 2019. Water Scarcity in the Middle East. [Google Scholar]
- Ogden N.H. Climate change and vector-borne diseases of public health significance. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2017;364(19) doi: 10.1093/femsle/fnx186. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- Oke T.R. The energetic basis of the urban heat island. Q. J. R. Meteorol. Soc. 1982;108(455):1–24. doi: 10.1002/qj.49710845502. [DOI] [Google Scholar]
- Ozturk T., Turp M.T., Türkeş M., Kurnaz M.L. Future projections of temperature and precipitation climatology for CORDEX-MENA domain using RegCM4.4. Atmos. Res. 2018;206:87–107. doi: 10.1016/J.ATMOSRES.2018.02.009. [DOI] [Google Scholar]
- Paaijmans K.P., et al. Temperature variation makes ectotherms more sensitive to climate change. Global Change Biol. 2013;19(8):2373–2380. doi: 10.1111/gcb.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabha H., Soto E., Mosquera M., Lord C.C., Lounibos L.P. Ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. EcoHealth. 2010;7(1):78–90. doi: 10.1007/s10393-010-0301-6. [DOI] [PubMed] [Google Scholar]
- Paixão E.S., Teixeira M.G., Rodrigues L.C. Zika, chikungunya and dengue: the causes and threats of new and reemerging arboviral diseases. BMJ Glob. Heal. 2018;3(Suppl. 1) doi: 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal J.S., Eltahir E.A.B. Future temperature in southwest Asia projected to exceed a threshold for human adaptability. Nat. Clim. Change. 2016;6(2):197–200. doi: 10.1038/nclimate2833. [DOI] [Google Scholar]
- Palinkas L.A., Wong M. Global climate change and mental health. Curr. Opin. Psychol. 2020;32:12–16. doi: 10.1016/j.copsyc.2019.06.023. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Paravantis J., Santamouris M., Cartalis C., Efthymiou C., Kontoulis N. Mortality associated with high ambient temperatures, heatwaves, and the urban heat Island in Athens, Greece. Sustainability. 2017;9(4):606. doi: 10.3390/su9040606. [DOI] [Google Scholar]
- Pardo M., Katra I., Schaeur J.J., Rudich Y. Mitochondria-mediated oxidative stress induced by desert dust in rat alveolar macrophages. GeoHealth. 2017;1(1):4–16. doi: 10.1002/2016GH000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P.E., et al. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philos. Trans. R. Soc. B Biol. Sci. 2015;370(1665):1–17. doi: 10.1098/rstb.2013.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C.L., Wellbery C.E., Mueller M. The changing climate: managing health impacts. Am. Fam. Physician. 2019;100(10):618–626. [PubMed] [Google Scholar]
- Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microb. Infect. 2009;11(14–15):1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Paz S., et al. Permissive summer temperatures of the 2010 European West Nile fever upsurge. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz S., Díaz J., Linares C., Negev M., Sánchez Martínez G. In: Marsewille, France: MedECC, Union for the Mediterranean, Plan Bleu. Cramer W., Guiot J., Marini K., editors. UNEP/MAP; 2020. Society (health),” in climate and environmental change in the Mediterranean Basin – current situation and risks for the future; pp. 493–514. [Google Scholar]
- Perkins S.E., Alexander L.V. On the measurement of heat waves. J. Clim. 2013;26(13):4500–4517. doi: 10.1175/JCLI-D-12-00383.1. [DOI] [Google Scholar]
- Piperaki E.T., Daikos G.L. Malaria in Europe: emerging threat or minor nuisance? Clin. Microbiol. Infect. 2016;22(6):487–493. doi: 10.1016/j.cmi.2016.04.023. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Polymenakou P.N. Atmosphere: a source of pathogenic or beneficial microbes? Atmosphere (Basel). 2012;3(1):87–102. doi: 10.3390/atmos3010087. [DOI] [Google Scholar]
- Polymenakou P.N., Mandalakis M., Stephanou E.G., Tselepides A. Particle size distribution of airborne microorganisms and pathogens during an Intense African dust event in the eastern Mediterranean. Environ. Health Perspect. 2008;116(3):292–296. doi: 10.1289/ehp.10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrgou A., Santamouris M. Increasing probability of heat-related mortality in a mediterranean city Due to urban warming. Int. J. Environ. Res. Publ. Health. 2018;15(8) doi: 10.3390/ijerph15081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.F., Yanful E.K., Jasim S.Y. Endocrine disrupting compounds (EDCs) and pharmaceuticals and personal care products (PPCPs) in the aquatic environment: implications for the drinking water industry and global environmental health. J. Water Health. 2009;7(2):224–243. doi: 10.2166/wh.2009.021. [DOI] [PubMed] [Google Scholar]
- Ramis C., Amengual A. vol. 1–5. Elsevier; 2017. Climate change effects on European heat waves and human health; pp. 209–216. (Encyclopedia of the Anthropocene). [Google Scholar]
- Rashki A., Kaskaoutis D.G., Francois P., Kosmopoulos P.G., Legrand M. Dust-storm dynamics over Sistan region, Iran: seasonality, transport characteristics and affected areas. Aeolian Res. 2015;16:35–48. doi: 10.1016/J.AEOLIA.2014.10.003. [DOI] [Google Scholar]
- Reinhold J.M., Lazzari C.R., Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: a review. Insects. 2018;9(4) doi: 10.3390/insects9040158. MDPI AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W.K., Fang Y., Martinez V.M. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae) J. Med. Entomol. 2006;43(2):309–317. doi: 10.1603/0022-2585(2006)043[0309:eotott]2.0.co. [DOI] [PubMed] [Google Scholar]
- Reiter P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001;109(Suppl. 1):141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezazadeh M., Irannejad P., Shao Y. Climatology of the Middle East dust events. Aeolian Res. 2013;10:103–109. doi: 10.1016/J.AEOLIA.2013.04.001. [DOI] [Google Scholar]
- Rijsberman F.R. Water scarcity: fact or fiction? Agric. Water Manag. 2006;80(1–3):5–22. doi: 10.1016/j.agwat.2005.07.001. SPEC. ISS. [DOI] [Google Scholar]
- Robine J.M., et al. Death toll exceeded 70,000 in Europe during the summer of 2003. Comptes Rendus Biol. 2008;331(2):171–178. doi: 10.1016/j.crvi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Roll Back Malaria Partnership . 2020. Vector Control in Humanitarian Emergencies.https://endmalaria.org/vector-control-humanitarian-emergencies [Google Scholar]
- Ryan S.J., Carlson C.J., Mordecai E.A., Johnson L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Neglected Trop. Dis. 2019;13(3) doi: 10.1371/journal.pntd.0007213. e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Hussein A., El Mofty H.M., Hassanien M.A. Climate change and predicted trend of fungal keratitis in Egypt. EMHJ - East. Mediterr. Heal. J. 2011;17(6):468–473. 2011. [PubMed] [Google Scholar]
- Saha K.C. Review of arsenicosis in West Bengal, India - a clinical perspective. Crit. Rev. Environ. Sci. Technol. 2003;33(2):127–163. doi: 10.1080/10643380390814514. [DOI] [Google Scholar]
- Salvador P., et al. African dust outbreaks over the western Mediterranean Basin: 11-year characterization of atmospheric circulation patterns and dust source areas. Atmos. Chem. Phys. 2014;14(13):6759–6775. doi: 10.5194/acp-14-6759-2014. [DOI] [Google Scholar]
- Samoli E., Nastos P.T., Paliatsos A.G., Katsouyanni K., Priftis K.N. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ. Res. 2011;111(3):418–424. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Sanayei Y., Ismail N., Talebi S.M. Determination of heavy metals in Zayandeh Rood River, Isfahan-Iran. World Appl. Sci. J. 2009;6(9):1209–1214. [Google Scholar]
- Schmidt W.P., et al. Population density, water supply, and the risk of dengue fever in vietnam: cohort study and spatial analysis. PLoS Med. 2011;8(8) doi: 10.1371/journal.pmed.1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer M.D., et al. Lung health in era of climate change and dust storms. Environ. Res. 2018;163:36–42. doi: 10.1016/j.envres.2018.02.001. February. [DOI] [PubMed] [Google Scholar]
- Şeker M., Koyuncu İ., Öztürk İ. The Turkish Academy of Sciences; Ankara, Turkey: 2020. The Report on Climate Change and Public Health in Turkey. [DOI] [Google Scholar]
- Sera F., et al. How urban characteristics affect vulnerability to heat and cold: a multi-country analysis. Int. J. Epidemiol. 2019;48(4):1101–1112. doi: 10.1093/ije/dyz008. [DOI] [PubMed] [Google Scholar]
- Sera F., et al. Air conditioning and heat-related mortality: a multi-country longitudinal study. Epidemiology. 2020;31(6):779–787. doi: 10.1097/EDE.0000000000001241. [DOI] [PubMed] [Google Scholar]
- Shahsavani A., et al. Short-term effects of particulate matter during desert and non-desert dust days on mortality in Iran. Environ. Int. 2020;134:105299. doi: 10.1016/J.ENVINT.2019.105299. [DOI] [PubMed] [Google Scholar]
- Sheffield P.E., Herrera M.T., Kinnee E.J., Clougherty J.E. Not so little differences: variation in hot weather risk to young children in New York City. Publ. Health. 2018;161:119–126. doi: 10.1016/j.puhe.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortall C.K., Glazik R., Sornum A., Pritchard C. On the ferries: the unmet health care needs of transiting refugees in Greece. Int. Health. 2017;9(5):272–280. doi: 10.1093/inthealth/ihx032. [DOI] [PubMed] [Google Scholar]
- Shultz J.M., Rechkemmer A., Rai A., McManus K.T. Public health and mental health implications of environmentally induced forced migration. Disaster Med. Public Health Prep. 2019;13(2):116–122. doi: 10.1017/dmp.2018.27. [DOI] [PubMed] [Google Scholar]
- Siraj A.S., et al. Temperature modulates dengue virus epidemic growth rates through its effects on reproduction numbers and generation intervals. PLoS Neglected Trop. Dis. 2017;11(7) doi: 10.1371/journal.pntd.0005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.J. Pediatric thermoregulation: considerations in the face of global climate change. Nutrients. 2019;11(9) doi: 10.3390/nu11092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniou A., Bishop S. Water scarcity in Cyprus: a review and call for integrated policy. Water. 2014;6(10):2898–2928. doi: 10.3390/w6102898. [DOI] [Google Scholar]
- van Steen Y., Ntarladima A.M., Grobbee R., Karssenberg D., Vaartjes I. Sex differences in mortality after heat waves: are elderly women at higher risk? Int. Arch. Occup. Environ. Health. 2019;92(1):37–48. doi: 10.1007/s00420-018-1360-1. Springer Verlag. [DOI] [PubMed] [Google Scholar]
- Stilianakis N.I., et al. Identification of climatic factors affecting the epidemiology of human West Nile Virus infections in Northern Greece. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161510. e0161510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick W.J. Climate change and the Arboviruses: lessons from the evolution of the dengue and yellow fever Viruses. Annu. Rev. Virol. 2016;3:125–145. doi: 10.1146/annurev-virology-110615-035630. [DOI] [PubMed] [Google Scholar]
- Tarrass F., Benjelloun M. The effects of water shortages on health and human development. Perspect. Public Health. 2012;132(5):240–244. doi: 10.1177/1757913910391040. Perspect Public Health. [DOI] [PubMed] [Google Scholar]
- The Statistical Service of Cyprus (CYSTAT) 2021. Arrivals of Tourists by Country of Usual Residence, 1980-2019.https://www.mof.gov.cy/mof/cystat/statistics.nsf/services_71main_en/services_71main_en?OpenForm&sub=1&sel=2 [Google Scholar]
- The World Bank . 2020. GDP Per Capita (Current US$),” World Bank National Accounts Data, and OECD National Accounts Data Files.https://data.worldbank.org/indicator/NY.GDP.PCAP.CD [Google Scholar]
- Thomson M.C., Jeanne I., Djingarey M. Dust and epidemic meningitis in the Sahel: a public health and operational research perspective. IOP Conf. Ser. Earth Environ. Sci. 2009;7(1):12017. doi: 10.1088/1755-1307/7/1/012017. [DOI] [Google Scholar]
- Tobias A., Karanasiou A., Amato F., Querol X. Health effects of desert dust and sand storms. Environ. Epidemiol. 2019;3:396. doi: 10.1097/01.ee9.0000610424.75648.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol R.S.J. Adaptation and mitigation: trade-offs in substance and methods. Environ. Sci. Pol. 2005;8(6):572–578. doi: 10.1016/J.ENVSCI.2005.06.011. [DOI] [Google Scholar]
- Tozan Y., Sjödin H., Muñoz Á.G., Rocklöv J. Transmission dynamics of dengue and chikungunya in a changing climate: do we understand the eco-evolutionary response? Expert Rev. Anti Infect. Ther. 2020;18(12):1187–1193. doi: 10.1080/14787210.2020.1794814. [DOI] [PubMed] [Google Scholar]
- Tran A., et al. Environmental predictors of West Nile fever risk in Europe. Int. J. Health Geogr. 2014;13 doi: 10.1186/1476-072X-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsekeri E., Kolokotsa D., Santamouris M. On the association of ambient temperature and elderly mortality in a Mediterranean island - Crete. Sci. Total Environ. 2020;738:139843. doi: 10.1016/j.scitotenv.2020.139843. [DOI] [PubMed] [Google Scholar]
- United Nations . 2019. World Population Prospects - Population Division - United Nations.https://population.un.org/wpp/ Mar. 04, 2021. [Google Scholar]
- United Nations Department of Economic and Social Affairs . 2019. World Population Prospects 2019.https://population.un.org/wpp/Download/Standard/Population/ May 05, 2022) [Google Scholar]
- United Nations High Commissioner for Refugees (UNHCR) UNHCR Global Data Service; Copenhagen, Denmark: 2020. Global Trends: Forced Displacement in 2019. [Google Scholar]
- United Nations Human Settlements Programme (UN-HABITAT) Earthscan Publications Ltd.; London, UK: 2003. Water and Sanitation in the World's Cities: Local Action for Global Goals. [Google Scholar]
- United Nations Inter-Agency Task Force on the Prevention and Control of Non-communicable Diseases . United Nations Development Programme; 2019. Responding to the Challenge of Non-communicable Diseases. [Google Scholar]
- Venkataramanan V., Geere J.A.L., Thomae B., Stoler J., Hunter P.R., Young S.L. In pursuit of ‘safe’ water: the burden of personal injury from water fetching in 21 low-income and middle-income countries. BMJ Glob. Heal. 2020;5(10) doi: 10.1136/bmjgh-2020-003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronicah K., Kogi-Makau W., Muroki N.M., Kirogo V. The role of irrigation on improvement of nutritional status of young children in central Kenya. Afr. J. Food Nutr. Sci. 2007;7(2):1–16. [Google Scholar]
- Vicedo-Cabrera A.M., et al. A multi-country analysis on potential adaptive mechanisms to cold and heat in a changing climate. Environ. Int. 2018;111:239–246. doi: 10.1016/j.envint.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Vicedo-Cabrera A.M., et al. The burden of heat-related mortality attributable to recent human-induced climate change. Nat. Clim. Change. 2021;11(6):492–500. doi: 10.1038/s41558-021-01058-x. 2021 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra K., Vodonos A., Schwartz J., Marais E.A., Sulprizio M.P., Mickley L.J. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: results from GEOS-Chem. Environ. Res. 2021;195:110754. doi: 10.1016/j.envres.2021.110754. [DOI] [PubMed] [Google Scholar]
- Waha K., et al. Climate change impacts in the Middle East and Northern Africa (MENA) region and their implications for vulnerable population groups. Reg. Environ. Change. 2017;17(6):1623–1638. doi: 10.1007/s10113-017-1144-2. [DOI] [Google Scholar]
- Waitz Y., Paz S., Meir D., Malkinson D. Temperature effects on the activity of vectors for Leishmania tropica along rocky habitat gradients in the Eastern Mediterranean. J. Vector Ecol. 2018;43(2):205–214. doi: 10.1111/jvec.12304. [DOI] [PubMed] [Google Scholar]
- Waldock J., et al. The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathog. Glob. Health. 2013;107(5):224–241. doi: 10.1179/2047773213Y.0000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts N., et al. Health and climate change: policy responses to protect public health. Lancet. 2015;386(10006):1861–1914. doi: 10.1016/S0140-6736(15)60854-6. [DOI] [PubMed] [Google Scholar]
- Watts N., et al. The 2019 report of the Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019;394(10211):1836–1878. doi: 10.1016/S0140-6736(19)32596-6. Lancet Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts N., et al. The 2020 report of the Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397(10269):129–170. doi: 10.1016/S0140-6736(20)32290-X. Lancet Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2022. Heatwave in Europe: Local Resilience Saves Lives – Global Collaboration Will Save Humanity.https://www.who.int/europe/news/item/22-07-2022-heatwave-in-europe--local-resilience-saves-lives---global-collaboration-will-save-humanity [Google Scholar]
- Wold Bank . 2007. Making the Most of Scarcity Accountability for Better Water Management in the Middle East and North Africa. Washington, D.C. [DOI] [Google Scholar]
- Wolf K., et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet. Health. 2021;5(9):e620–e632. doi: 10.1016/S2542-5196(21)00195-9. [DOI] [PubMed] [Google Scholar]
- World Bank . World Bank; Washington, DC: 2018. Beyond Scarcity: Water Security in the Middle East and North Africa,” MENA Development Series. [DOI] [Google Scholar]
- World Health Organization & United Nations High Commissioner for Refugees . 2012. Assessing Mental Health and Psychosocial Needs and Resources: Toolkit for Humanitarian Settings. Geneva, Switzerland. [Google Scholar]
- World Health Organization . World Health Organization; Geneva, Switzerland: 2020. World Malaria Report 2020: 20 Years of Global Progress and Challenges. [Google Scholar]
- World Health Organization (WHO) WHO Regional Office for Europe; Copenhagen, Denmark: 2011. Public Health Advice on Preventing Health Effects of Heat. [Google Scholar]
- World Health Organization (WHO) 2013. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013-2020. Geneva, Switzerland. [Google Scholar]
- World Health Organization (WHO) WHO Regional Office for Europe; Copenhagen, Denmark: 2018. Improving the Health Care of Pregnant Refugee and Migrant Women and Newborn Children. [Google Scholar]
- World Health Organization (WHO) WHO Regional Office for Europe; Copenhagen, Denmark: 2019. Prevention and Control of Noncommunicable Diseases in Refugees and Migrants. [Google Scholar]
- World Health Organization (WHO) The World Health Organization (WHO); Geneva, Switzerland: 2020. World Health Statistics 2020: Monitoring Health for the Sustainable Development Goals. [Google Scholar]
- World Health Organization (WHO) 2020. Leishmaniasis.https://www.who.int/news-room/fact-sheets/detail/leishmaniasis [Google Scholar]
- Yé Y., Louis V.R., Simboro S., Sauerborn R. Effect of meteorological factors on clinical malaria risk among children: an assessment using village-based meteorological stations and community-based parasitological survey. BMC Publ. Health. 2007;7:101. doi: 10.1186/1471-2458-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M., Bartram J. Waterborne health risks and the WHO perspective. Int. J. Hyg Environ. Health. 2001;204(4):255–263. doi: 10.1078/1438-4639-00081. [DOI] [PubMed] [Google Scholar]
- Zafeiratou S., et al. Spatial variability in the effect of high ambient temperature on mortality: an analysis at municipality level within the greater Athens area. Int. J. Environ. Res. Public Heal. Artic. 2019 doi: 10.3390/ijerph16193689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., et al. Projected changes in maternal heat exposure during early pregnancy and the associated congenital heart defect burden in the United States. J. Am. Heart Assoc. 2019;8(3) doi: 10.1161/JAHA.118.010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittis G., Hadjinicolaou P., Fnais M., Lelieveld J. Projected changes in heat wave characteristics in the eastern Mediterranean and the Middle East. Reg. Environ. Change. 2016;16(7):1863–1876. doi: 10.1007/s10113-014-0753-2. [DOI] [Google Scholar]
- Zittis G., et al. Business-as-usual will lead to super and ultra-extreme heatwaves in the Middle East and North Africa. npj Clim. Atmos. Sci. 2021;4(1):1–9. doi: 10.1038/s41612-021-00178-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.