Abstract
This study examined the associations between childhood experiences and frailty trajectory among middle-aged and older Chinese adults. Data were derived from the China Health and Retirement Longitudinal Study. We used data from all four waves (i.e., 2011, 2013, 2015, 2018) and the life history survey in 2014. Data for 10,963 respondents were included. Latent growth curve models were conducted to examine the proposed model. The results show that adverse childhood experiences, self-rated childhood socioeconomic status, and the objective indicators of childhood health and health care were associated with both the baseline level and change rate of frailty. The educational attainment of fathers and perceived childhood health and healthcare conditions were associated with baseline frailty only. Our findings highlight the crucial role of childhood antecedents in the progression of frailty in later life. We further found strong evidence that childhood is an essential life stage for human development. Future social policies and interventions should use childhood experiences as a screening tool and promote child protection, health education, and life course interventions.
Keywords: Childhood experiences, Frailty trajectory, Latent growth curve model, CHARLS
Introduction
The world has witnessed a rapid growth of the older population in recent decades. As one of the most populous country, China has encountered even greater challenges. According to the seventh national census, more than 264 million people aged 60 or older live in China at present, accounting for about 18.70% of the total population (Zhang 2020b). Compared with the sixth census conducted in 2010, the proportion of older residents has increased by 5.44% (Zhang 2020b), and it is estimated that by 2050, about 487 million Chinese will be aged 60 or older (The Xinhua News Agency 2018). As people age, their health condition tends to decline over time. Among the health problems of older adults, a prevalent but still widely ignored health indicator is frailty.
Frailty is a unique result of gradual downstream changes in multiple physiologic functions (Fried et al. 2001; Rockwood et al. 2005). It refers to a condition that falls between healthy status and severe illness. Therefore, people might be considered frail even when they do not have a specific life-threatening disease (Rockwood and Mitnitski 2007). Although frailty is based on age-associated markers like activities of daily living, it is not just a simple summation of them. Frailty reflects a more comprehensive biologic syndrome, which often leads to vulnerability and decreased resistance to stressors (Fried et al. 2001; Rockwood and Mitnitski 2007; Rockwood et al. 2005). Moreover, it can predict adverse outcomes such as falls, hospitalization, and mortality (Chamberlain et al. 2016; Fried et al. 2001).
The frailty index (FI) is regarded as a valid measurement of frailty and has been widely used in studies in different countries. By averaging multidimensional age-related deficits, the FI is generated as a continuous indicator ranging from 0 to 1, with higher scores representing higher levels of frailty (Rockwood and Mitnitski 2007). Many studies have successfully drawn results using frailty measured at a single point in time (Li et al. 2020; Wu et al. 2017). However, frailty largely is not static. It is a dynamic, time-varying indicator. Therefore, it might be more precise and practical to study the trajectory of frailty (Jung et al. 2022; Raymond et al. 2020; Yu et al. 2018).
A range of factors might influence frailty trajectory. Studies have found that elements like weight, lifestyle, socioeconomic status (SES), and social resources can significantly affect the incidence and progression of frailty in later life (Jung et al. 2022; Liu et al. 2022; Raymond et al. 2020). However, most of these influencing factors only cover adulthood, whereas life course theory posits that an individual’s life features multiple stages and life events, each of which affects the next stages and eventually influences status during later life (Elder et al. 2003). Therefore, exploring childhood experiences can deepen our knowledge of how early life conditions might influence older adults’ health status throughout life.
In this study, childhood experiences refer to a comprehensive concept that contains all the physical and psychological, transient and sustaining experiences during childhood, which are deeply influenced by the surrounding family, community, and social environment. Among the limited studies examining the relationships between childhood experiences and frailty in later life, the indicators of childhood experiences generally fell into three categories: adverse childhood experiences (ACEs), childhood SES, and childhood health and health care. More specifically, ACEs refer to the exposure to negative and stressful events during childhood (Felitti et al. 1998), encompassing maltreatment and neglect of parents, household dysfunction (e.g., criminal behaviors, mental health illness, and substance abuse of parents), bullying, and unsafe living environment. More exposure to childhood adversities was found to be associated with higher levels of frailty (Linden et al. 2020; Mian et al. 2021). Another widely used indicator was childhood SES, which was often measured by family financial, educational, and occupational situation. Childhood SES acts as an important protective factor toward later-life frailty (Alvarado et al. 2008; Li et al. 2020; Linden et al. 2020). In addition, childhood health and health care also had a significant impact on frailty, in which poorer health and health care would lead to higher risks of being frail in later life (Alvarado et al. 2008; Li et al. 2020; Linden et al. 2020).
However, few studies examined how childhood experiences influenced the baseline level and change rate of frailty in the context of developing countries and regions such as China. Empirical studies in China have found partial evidence of the associations between the three childhood antecedents and trajectories of cognition, depression, and disease in later life (Pan et al. 2021; Yang and Wang 2020). Given that these symptoms are important components of frailty, we suspect that experiences in childhood might also have long-term influences on frailty trajectory among Chinese middle-aged and older adults.
Childhood experiences and frailty trajectory in later life
The mechanism linking childhood experiences to frailty in later life can be explained by both the latency model and the pathway model (Lyu and Burr 2016; Zhang et al. 2018). According to the latency model, childhood experiences are embedded in people’s development over their lifetime and significantly shape their health trajectories, leading to late-life frailty directly (Ben-Shlomo and Kuh 2002; Berens et al. 2017). Low income, poor health and health care, and ACEs would impose a permanent and irreversible impact on children’s brain development, endocrine function, immunity, and metabolic physiology (Berens et al. 2017; Black et al. 2017; Oh et al. 2018). As people age, these latent disadvantages, which have been determined during childhood, are unmasked and aggravated, giving rise to frailty-related impairment and disease (Ben-Shlomo and Kuh 2002; Zhang et al. 2018). Under this circumstance, childhood experiences can affect late-life frailty even after controlling for adulthood conditions (Hu 2021; Li et al. 2020).
Apart from this direct impact, the pathway model suggests that childhood experiences can also have an indirect effect on health status in later life by influencing SES and health during adulthood (Black et al. 2017; Kalmakis and Chandler 2015; Kendig et al. 2017). Negative childhood experiences, to some extent, will affect educational and occupational attainment, which are both strong protective factors of middle-aged and older adults’ health status (Zheng et al. 2021). In addition, adults who have been exposed to low SES, ACEs, illness, and low levels of health care have also been shown to have lower self-rated health and higher prevalence of risk behaviors, mental disorder, cognitive impairment, and chronic diseases such as cardiovascular disease (Kalmakis and Chandler 2015; Wang and Shen 2016; Zheng et al. 2021). Therefore, childhood experiences can influence frailty in later life directly, while also altering risk and protective factors in adulthood to cause an indirect impact.
Furthermore, based on the life course perspective, not only can the baseline level of frailty be affected by childhood experiences, but the change rate, or the trajectory of frailty, can also be influenced. According to cumulative advantage/disadvantage theory, interindividual divergence will be exacerbated with the passage of time. Therefore, regarding health status, the inequality will widen with the accumulation of advantages and disadvantages, which can be partially interpreted as “success breeds success” (Dannefer 2003). With successively unequal accumulation of disadvantages since childhood, the rate of decline of people’s health status will be strongly affected, and those with more negative childhood experiences might have increased risk of experiencing even more frailty in later life (Shi and Wu 2020).
In summary, these underlying mechanisms suggest that childhood experiences can influence late-life frailty directly and indirectly, simultaneously affecting both the baseline level and change rate of frailty.
Disparities in social backgrounds: China and Europe
Previous studies mainly focused on Western countries, especially in Europe (Linden et al. 2020). However, the function of the aging process and cumulative advantage or disadvantage can vary in different societies and cultural contexts (Dannefer 2003). Different geographic environments, philosophies, and histories have shaped the basic differences between China and Europe, and China is a unified country that pursues socialism, whereas Europe is a union of numerous divided countries with a capitalist ideology (Ko et al. 2018). On this basis, the disparities in political institutions and economic structures between the two regions might cause different linkages between childhood and later-life conditions.
More specifically, the development process of China, in which the current cohorts of middle-aged and older adults’ growth trajectories were embedded, differed greatly from that of Europe. In the middle twentieth century (e.g., 1950s–1960s), China has experienced a tremendously difficult time, both socially and economically. At the same time, though having met with some setbacks (e.g., oil crisis), the living standards of Europeans were still much higher than those of Chinese. However, the Chinese society has undergone significant transitions (e.g., from an agricultural society to industrial society) since the economic reform in 1978, whereas European countries have maintained steady development. Therefore, the living standards and available health resources of Chinese people have improved significantly, and it would be meaningful to investigate whether childhood experiences still affect late-life frailty trajectory after such a drastic social change.
In addition, from the mesoscopic and microscopic perspectives, the Chinese population’s dietary habits, healthy behavior patterns, and other lifestyle factors are different from those of Europeans. Furthermore, Chinese family structure has shifted from multigenerational households to a nuclear family structure in the past few decades (e.g., average family size has declined from 4.33 in 1953 to 2.62 in 2020; National Bureau of Statistics of China 1988; Zhang 2020a). Because individual habits like diet and healthy behaviors and family environment are two of the most important aspects of childhood health and development, these distinctions might also significantly shape the childhood experiences and developmental trajectories of Chinese adults.
Under such circumstances, local evidence is required to test the progression of frailty in later life and its antecedents from both childhood and adulthood in the Chinese context.
The present study
Using nationwide longitudinal data, the present study investigated the influence of childhood experiences on frailty trajectory among older Chinese adults. The hypotheses of this study were as follows:
H1
Severer ACEs are associated with higher frailty levels at baseline among older adults in China.
H2
Better family SES in childhood is associated with lower frailty levels at baseline among older adults in China.
H3
Better health and health care in childhood are associated with lower frailty levels at baseline among older adults in China.
H4
Severer ACEs are associated with higher change rates of frailty among older adults in China.
H5
Better family SES in childhood is associated with lower change rates of frailty among older adults in China.
H6
Better health and health care in childhood are associated with lower change rates of frailty among older adults in China.
Methods
Data
Data were drawn from the harmonized and life history data of the China Health and Retirement Longitudinal Study (CHARLS), a longitudinal cohort study featuring a nationally representative sample of individuals aged 45 years or older in China (Zhao et al. 2014). Using multistage stratified probability proportional to size sampling, the CHARLS team successfully conducted four waves of nationwide data collection in 150 counties and 28 provinces, ensuring the quality and representativeness of data. The baseline survey was conducted during 2011–2012, with follow-up surveys taking place in 2013, 2015, and 2018. The harmonized dataset is an integrated version of the four waves of data, which was created by the USC Gateway to Global Aging Data team (Phillips et al. 2021). A life history survey was conducted during 2014 to explore conditions during the respondents’ early life, including but not limited to their family information, education, and health.
In this study, we included respondents who completed all four longitudinal surveys and the life history survey. At baseline, 17,708 respondents participated, 11,988 of whom completed all follow-up surveys in 2013, 2015, and 2018. After matching and excluding those who did not answer the life history survey, had missing values on age, or were younger than 45 at baseline, 10,963 respondents were included in the final analytic sample.
Measurements
Dependent variable
The dependent variable in this study was frailty. According to Wang et al. (2021), 32 deficits in six domains were chosen to construct FI. First, we assessed self-rated health. Answers were measured by a 5-point Likert scale (1 = very good to 5 = very poor). For convenience, we reversed it and recoded the values (0 = very good, 0.25 = good, 0.50 = fair, 0.75 = poor, 1 = very poor). Second, we explored activities of daily living. Six indicators (i.e., difficulty with dressing, bathing, eating, getting in and out of bed, using the toilet, and controlling urination and defecation) were used, each of which was turned into a dichotomous variable (0 = no difficulty, 1 = difficulty). Third, we assessed instrumental activities of daily living. Similar to activities of daily living, five deficits (i.e., difficulty with managing money, taking medications, shopping for groceries, preparing a hot meal, and cleaning house) were used and recoded as dichotomous variables. Fourth, we examined cognitive function. We used three cognition tests that measured the respondents’ cognitive function: mathematical performance, orientation, and drawing an assigned picture. These tests were recoded into variables ranging from 0 to 1, depending on correct numbers. For example, in the mathematical test, respondents who answered all five questions correctly received a score of 0, and those who answered four questions correctly received 0.2 points. Fifth, we considered chronic diseases. The CHARLS assessed 13 chronic diseases, all as dichotomous variables (1 = disease diagnosed by doctor, 0 = no diagnosis). Finally, we explored psychological characteristics. Four items (i.e., sleep was restless, felt lonely, could not get going, and feel hopeful about the future) were used, and the last question was reverse recoded because it represents a positive domain. These 32 recoded indicators were then added and divided by 32. Thus, the FI was generated with a range from 0 to 1, with higher scores indicating higher frailty levels.
Regarding missing values, many respondents in all four surveys had no missing variable (i.e., 75.0%, 69.4%, 69.1%, and 59.2%, respectively), and more than 90% of the observations had no more than three missing values of the 32 deficits. Therefore, to make the maximum use of available data and ensure the quality of the indicator, we regarded missing 20% of the total number of deficits (i.e., six) as the threshold for exclusion, which has been commonly adopted in previous literature (Theou et al. 2013; Wang et al. 2021). If respondents had more than six missing values on these metrics, their FI would be considered as missing. For those with equal to or fewer than six missing values, the average score of the nonmissing values was calculated. For instance, if a respondent had 29 valid deficits with a summed score of 7, the frailty index was 7/29 = 0.24. In addition, we conducted sensitivity analysis using different thresholds such as 10%, and the results were robust.
Independent variable
The three types of independent variables selected in this study are ACEs, childhood SES, and childhood health and health care.
ACEs represent adversities experienced before age 17 in terms of family, friendship, community, and so on. Therefore, based on the life history data and previous studies, we selected 17 indicators as the criteria for ACEs (Lin et al. 2022). Overall, ACEs include deficits in abuse and neglect (i.e., emotional neglect and physical abuse, domestic violence), death (i.e., parents, sibling), illness and disability (parental disability and household mental illness), living environment outside the home (i.e., unsafe neighborhood and bullying), substance abuse, parental separation or divorce, and incarcerated household member. After converting these indicators into dichotomous variable (1 = yes, 0 = no), we calculated the average score, in line with how the FI score was generated. If the respondents had missing values on more than 20% of the indicators, their ACEs score was considered as missing. In this way, ACEs were transformed into a variable ranging from 0 to 1, with greater values indicating severer ACEs. Sensitivity analysis was conducted to test the separate influences of these 17 indicators, generating robust results (Appendix 1). Furthermore, different coding methods of ACEs (e.g., sum method) were also tested, and the results remained the same.
Childhood SES was measured by two indicators: self-rated SES and father’s education. In the life history survey of CHARLS, one question was used to measure self-rated SES during childhood: “Compared to the average family in the same community/village before age 17, how was your family’s financial situation?” This has been shown to be highly valid in previous literature (Zhang and Lu 2021). This was measured by a 5-point Likert scale, and we reversed and recoded it: 0 = a lot worse off than them, 2 = same as them, 4 = a lot better off than them. Furthermore, we measured the educational attainment of fathers because they were the main breadwinner of the family during the past century in China. Considering the high illiteracy rate, father’s education was dichotomized (0 = illiterate, 1 = literate; Yang and Wang 2020). We conducted additional sensitivity analysis by including father’s occupation, and the results indicated that it had no significant impact on either intercept or slope (Appendix 2).
As for childhood health and health care, self-rated health has been affirmed to have a strong correlation to retrospective childhood physical and psychological problems (Kendig et al. 2017). However, it was based on comparisons with other people in the same community, which can be affected by local health and medical standards. Therefore, we chose to use both subjective and objective measurements.
The subjective one was measured by self-reported health: “Before or when you were 15 years old, compared to other children of the same age, how was your health status?” Similar to self-reported childhood SES, we reversed and recoded it on a scale of 0–4, with higher scores indicating better health. As for objective health and health care, we calculated the total score of four dichotomous variables: confined to bed or home for a month or more (0 = yes, 1 = no), hospitalized for a month or more (0 = yes, 1 = no), received any vaccinations (0 = no, 1 = yes), had a usual source of care (0 = no, 1 = yes). The higher the value, the better the childhood health and health care (Pan 2020).
Covariates
Based on prior studies, 10 control variables were included in the model (Jung et al. 2022; Liu et al. 2022; Raymond et al. 2020). Sociodemographic characteristics of gender (1 = male, 0 = female), marital status (1 = married, 0 = other marital status), education (1 = received formal education, 0 = illiterate), place of residence (1 = rural, 0 = urban), and hukou status (1 = agricultural, 0 = nonagricultural) at baseline were recoded as dichotomous variables. Baseline age, body mass index, and household per capita consumption were also measured. Following the Chinese standard (National Health Commission of the People's Republic of China 2006), body mass index was further recoded as a dummy variable with normal weight as the reference group (e.g., normal weight vs. overweight, obesity, and underweight). Moreover, the log value of household per capita consumption was calculated. In addition, lifestyle (drinking and smoking) was also included by asking the respondents whether they consumed any alcohol in the last year (1 = yes, 0 = no) and whether they smoke cigarettes (1 = yes, 0 = no). Additional analysis was conducted to examine the impacts of other control variables such as daily activity (Appendix 3), and the conclusion remained same.
Data analysis
In this study, frequency analysis was conducted in Stata 16SE. Furthermore, we used Mplus 8.7 to conduct latent growth curve models to determine the relationship between childhood experiences and frailty trajectory. First, an unconditional model was estimated to identify frailty trajectory. Maximum likelihood estimation with robust standard errors was used, which can adjust to nonnormality and nonindependence of observations and handle missingness (Yuan and Bentler 2000; Yuan et al. 2012). To examine the model fit, we assessed several fit indexes: Chi-square value, comparative fit index (CFI), Tucker-Lewis index (TLI), root mean square error of approximation (RMSEA), and standardized root mean square residual (SRMR). Second, by constructing a conditional model, the effects of ACEs, childhood SES, and childhood health and health care on both the intercept (baseline level) and slope (rate of change) of frailty were tested, while controlling for baseline covariates.
Results
Descriptive statistics
As shown in Table 1, among the respondents, the mean age was 58.29 years, and 53% of them were female. The majority of the sample was married (83.6%). More than 80% of them held an agricultural hukou, although only 65.8% lived in a rural area. The percentage of respondents who had received formal education (elementary school or beyond) was 53.2%. Approximately half of the respondents had normal weight (44.1%), whereas others were underweight (5.4%), overweight (24.0%), or obese (9.8%). As for lifestyle at baseline, less than one-third of respondents had consumed alcohol in the last year (33.1%) and smoked cigarettes (29.0%).
Table 1.
Descriptive statistics (N = 10,963)
| N (%) | Mean (SD) | Missingness (%) | |
|---|---|---|---|
| Frailty index | |||
| 2011 | 0.16 (0.11) | 6.4 | |
| 2013 | 0.16 (0.10) | 6.8 | |
| 2015 | 0.18 (0.12) | 4.5 | |
| 2018 | 0.21 (0.13) | 7.1 | |
| Childhood experiences | |||
| ACEs | 0.13 (0.11) | 4.5 | |
| Self-rated childhood SES | 1.45 (0.97) | 0.7 | |
| Father’s education | 8.3 | ||
| Illiterate | 6066 (55.3) | ||
| Literate | 3988 (36.4) | ||
| Childhood self-rated health | 2.33 (1.01) | 0.7 | |
| Objective childhood health and health care | 3.66 (0.58) | 2.2 | |
| Control variables | |||
| Age | 58.29 (8.83) | 0.0 | |
| Gender | 0.0 | ||
| Male | 5154 (47.0) | ||
| Female | 5809 (53.0) | ||
| Hukou status | 0.7 | ||
| Agricultural | 9047 (82.5) | ||
| Nonagricultural | 1837 (16.8) | ||
| Place of residence | 0.0 | ||
| Urban | 3750 (34.2) | ||
| Rural | 7213 (65.8) | ||
| Marital status | 0.0 | ||
| Married | 9162 (83.6) | ||
| Other marital status | 1798 (16.4) | ||
| Education | 0.0 | ||
| Illiterate | 5135 (46.8) | ||
| Had received formal education or higher | 5828 (53.2) | ||
| Drank any alcohol last year | 3627 (33.1) | 0.4 | |
| Smoke at present | 3181 (29.0) | 2.8 | |
| Body mass index | 16.7 | ||
| Normal (18.5–23.9) | 4835 (44.1) | ||
| Underweight (lower than 18.5) | 587 (5.4) | ||
| Overweight (24–27.9) | 2631 (24.0) | ||
| Obesity (28 and above) | 1076 (9.8) | ||
| Household per capita consumption | 6865.64 (9077.86) | 15.1 | |
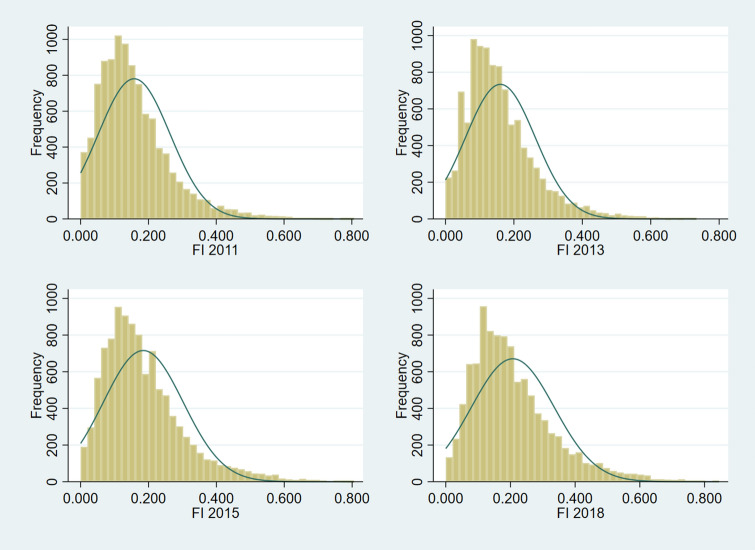
At baseline, the average FI score was nearly 0.16. It increased steadily over time, with average scores of 0.16, 0.18, 0.21 for the following three waves, respectively. Moreover, according to the commonly used cutoff point of FI (frail: ≥ 0.25; Rockwood et al. 2007), about 21% of the respondents were frail in 2011. That proportion increased to approximately 35% in the 2018 survey. The distribution of FI scores is shown in Appendix 4.
Furthermore, during childhood, the mean score of ACEs was 0.13. Self-rated childhood SES had a mean score of 1.45, indicating that a large proportion of respondents tended to regard themselves as poorer than others. Specifically, about 40.2% respondents thought they were somewhat or a lot worse off than others, whereas only 8.6% thought they were better off than their counterparts. As for their father’s educational attainment, around 55.3% of participants reported their father was illiterate. For childhood health and health care, the average score of the objective and subjective evaluation was 3.66 and 2.33, respectively, demonstrating that their actual health and health care level was better than what they reported.
Frailty trajectory and childhood experiences
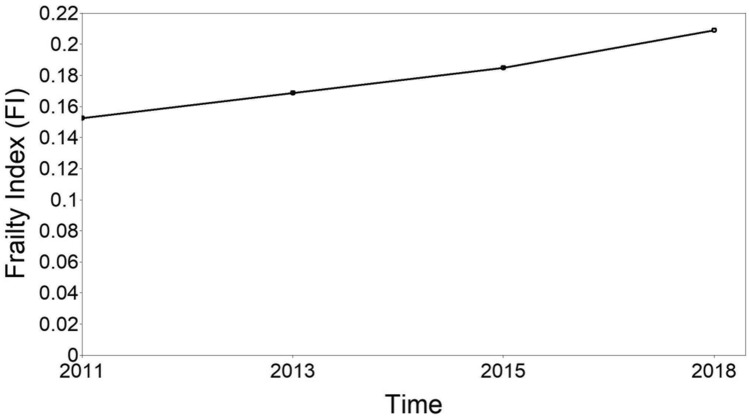
We built an unconditional linear latent growth curve model to identify the trajectory of FI scores (see Fig. 1). The model fit indexes showed a significant Chi-square test: χ2(5) = 337.135, p < 0.001. The significant Chi-square estimates might be because that test is sensitive to large sample sizes (Hair et al. 2010; Kline 2011). Furthermore, all other fit indexes suggested a good model fit (RMSEA = 0.078, CFI = 0.975, TLI = 0.969, SRMR = 0.036). Overall, there was an upward trend of the trajectory (intercept = 0.152, p < 0.001; slope = 0.016, p < 0.001). In other words, FI scores increased significantly during the survey period.
Fig. 1.
Unconditional latent growth curve model of frailty trajectory
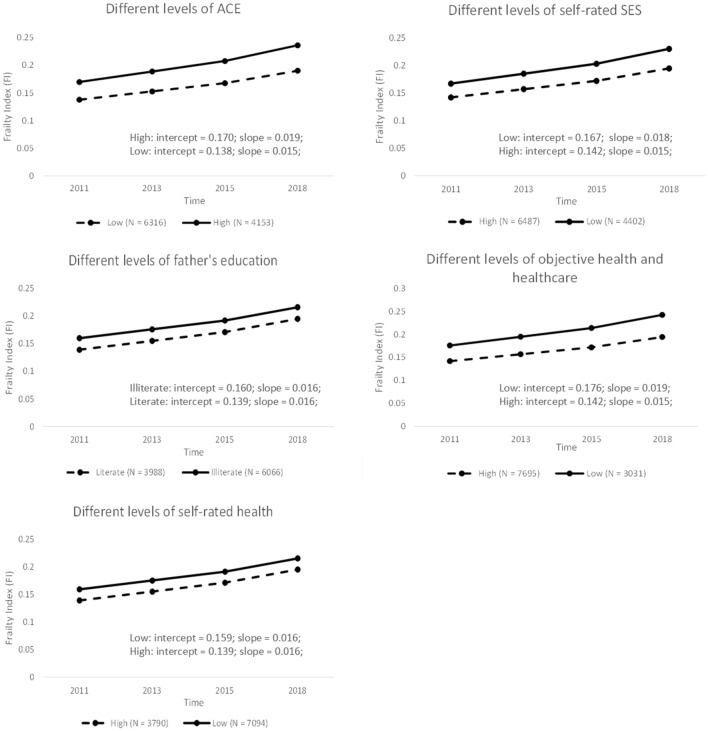
After considering all childhood variables and covariates, the model fit was also adequate: χ2(39) = 345.633, p < 0.001, RMSEA = 0.034, CFI = 0.981, TLI = 0.963, SRMR = 0.016. As shown in Table 2, all five childhood indicators were all found to be significantly associated with frailty at baseline (ACEs: b [SE] = 0.136 [0.011], β [SE] = 0.175 [0.014], p < 0.001; self-rated childhood SES: b [SE] = −0.006 [0.001], β [SE] = −0.069 [0.014], p < 0.001; father’s education: b [SE] = −0.006 [0.002], β [SE] = −0.034 [0.013], p < 0.01; childhood objective health and health care: b [SE] = −0.007 [0.002], β [SE] = −0.051 [0.014], p < 0.001; childhood self-rated health: b [SE] = −0.008 [0.001], β [SE] = −0.101 [0.014], p < 0.001). However, father’s education (b [SE] = 0.000 [0.001], β [SE] = −0.014 [0.021], p = 0.502) and childhood self-rated health (b [SE] = 0.000 [0.000], β [SE] =−0.029 [0.022], p = 0.177) were not associated with the rate of change of frailty, whereas ACEs (b [SE] = 0.018 [0.004], β [SE] = 0.117 [0.024], p < 0.001), self-rated childhood SES (b [SE] =−0.001 [0.000], β [SE] = −0.066 [0.022], p < 0.01), and objective health and health care (b [SE] = −0.002 [0.001], β [SE] = −0.064 [0.025], p < 0.05) were significantly associated with the slope. An additional graph was drawn to better visualize the influences of these five core variables on the intercept and slope of frailty, in which each indicator was further dichotomized according to the median. The graph and specific values of intercept and slope are shown in Appendix 5.
Table 2.
Latent growth curve model of frailty trajectory (N = 10,963)
| Variable | Intercept | Slope | ||
|---|---|---|---|---|
| b | SE | b | SE | |
| ACEs | .136*** | .011 | .018*** | .004 |
| Self-rated childhood SES | −.006*** | .001 | −.001** | .000 |
| Literate father | −.006** | .002 | .000 | .001 |
| Childhood self-rated health | −.008*** | .001 | .000 | .000 |
| Objective childhood health and health care | −.007*** | .002 | −.002* | .001 |
| Covariates | ||||
| Age | .002*** | .000 | .000*** | .000 |
| Male | −.024*** | .003 | −.002* | .001 |
| Agricultural hukou | .006 | .003 | −.001 | .001 |
| Living in rural area | .009** | .003 | .000 | .001 |
| Married | −.011** | .003 | .001 | .001 |
| Had received formal education or higher | −.026*** | .002 | .001 | .001 |
| Drank alcohol in the past year | −.012*** | .002 | .000 | .001 |
| Smoke at present | −.001 | .003 | .002* | .001 |
| Underweight | .018*** | .004 | .000 | .002 |
| Overweight | .007** | .002 | .003*** | .001 |
| Obesity | .023*** | .004 | .008*** | .001 |
| Household per capita consumption | .000 | .001 | .000 | .000 |
*p < 0.05. **p < 0.01. ***p < 0.001.; χ2(39) = 345.633, p < .001, RMSEA = .034, CFI = .981, TLI = .963, SRMR = .016
As for covariates (see Table 2), age, gender, marital status, place of residence, education, drinking, underweight, overweight, and obesity were associated with the baseline level of frailty, whereas only age, gender, overweight, and obesity were associated with its slope. Although smoking was not significantly associated with the intercept, it had a significant influence on the change rate of frailty. Finally, hukou status and household per capita consumption were not associated with either the intercept or slope of frailty trajectory.
Discussion
In this present study, we investigated the effects of ACEs, childhood SES, and childhood health and health care on frailty trajectory among Chinese middle-aged and older people from a life course perspective. Instead of examining the impact of childhood on later-life frailty at one point, our study revealed the impact of childhood experiences on both the intercept and slope of frailty. Results show that positive childhood experiences could not only reduce the initial level of frailty in later life but also slow its progression. Moreover, we made new contributions to the literature by employing a nationally representative sample in the Chinese context, which could further provide empirical evidence for practices in developing countries and regions.
In accordance with previous studies (Chamberlain et al. 2016; Liu et al. 2022), we have identified a pattern of increasing frailty over time. When considering childhood experiences, all three childhood antecedents affected frailty at baseline (Li et al. 2020; Mian et al. 2021). Among these childhood indicators, self-rated childhood SES also had a strong relationship with the trajectory of frailty. Nevertheless, father’s educational attainment, the other indicator of childhood SES, was not significantly related to frailty trajectory. As for ACEs, this study further expands our understanding on the significant and essential role of ACEs in the progression of frailty in Chinese older adults, verifying the importance of family, neighborhood, and school environments. Moreover, the findings of this study add new evidence that objective indicators of childhood health and health care, rather than subjective indicators, were significantly associated with the change rate of frailty in later life.
Furthermore, consistent with prior studies (Jung et al. 2022; Raymond et al. 2020; Yu et al. 2018), we found respondents who were older, female, overweight, and obese had a higher baseline level and steeper trajectory of frailty. Compared with their married counterparts, those who were not married at baseline (e.g., divorced or widowed) were more likely to be frail. Characteristics like living in a rural area, completing less education, and being underweight contributed to higher frailty scores at baseline, and smoking contributed to the risk of frailty development, findings that have been confirmed in previous studies (Jung et al. 2022; Li et al. 2020; Raymond et al. 2020; Yu et al. 2018).
Our findings confirm the application of life course theory and cumulative advantage/disadvantage theory in the Chinese context and highlight the importance of childhood in frailty development in later life. Additionally, this study has several implications for both policy and intervention. First, ACEs, childhood SES, and childhood health and health care can be effective screening tools for frailty in later life. Therefore, policy makers or social workers should make use of them to identify populations at risk of frailty. Second, social services programs of health education and self-management could be particularly important for middle-aged adults and older adults who are older, female, widowed or divorced, living in rural areas, overweight or obesity, drinking, smoking, and with relatively low educational attainment. Such programs could play an important role in mitigating the progression of frailty and further reduce morbidity and mortality in later life. Furthermore, networks of child protection and assistance should be better established to intervene in potential risk and protective factors. Specifically, for those who have already experienced childhood adversities, timely intervention is greatly needed to protect them from becoming frailer in later life. Finally, because childhood experiences are also integrated with family, community, school, and society, it is essential to develop social supportive sources for children in multiple social contexts. In a word, the integration of child and aged-care services should be built from a life course perspective, especially considering that the benefits and risks in childhood experiences could have a long-term impact on welfare at older ages.
Despite its contributions and strengths, the present study also has several limitations. First, we excluded respondents who did not participate in all five surveys, which might have caused selection bias. However, we conducted additional sensitivity analysis using the full sample, and it turned out to be robust (Appendix 6). Second, because childhood experiences were based on respondents’ retrospections, the measurements might suffer from information inaccuracy, especially among those who were older, even though multiple studies using 2014 CHARLS data have shown the adequate validity of these childhood indicators. Finally, apart from the direct impact of childhood experiences on frailty trajectories, indirect pathways might also exist. Therefore, future research should further study the mediation or moderation roles of adulthood conditions in these relationships.
Acknowledgements
This analysis uses data or information from the 2014 life history survey of the China Health and Retirement Longitudinal Study (CHARLS) and the Harmonized CHARLS dataset and Codebook, Version D as of June 2021 developed by the Gateway to Global Aging Data. The development of the Harmonized CHARLS was funded by the National Institute on Aging (R01 AG030153, RC2AG036619, R03 AG043052). For more information, please refer to https://g2aging.org/. We thank the CHARLS research team and field team for collecting the data and making the data publicly accessible.
Appendix 1
See Table 3.
Table 3.
Each ACEs indicator’s impact on intercept and slope of the model
| Variable | Intercept | Slope | ||
|---|---|---|---|---|
| b | SE | b | SE | |
| ACEs indicators | ||||
| Physical abuse | .007** | .003 | −.001 | .001 |
| Emotional neglect (love and affection) | .002 | .003 | .000 | .001 |
| Emotional neglect (effort) | .003 | .003 | .002* | .001 |
| Substance abuse | .006 | .004 | .000 | .001 |
| Mental illness (abnormality of mind) | .016* | .008 | .005 | .003 |
| Mental illness (continued signs of sadness or depression) | .013** | .004 | .005*** | .001 |
| Mental illness (duration of sadness or depression in the whole childhood) | .021*** | .006 | −.005** | .002 |
| Domestic violence | .010* | .005 | .005** | .002 |
| Incarcerated | −.035 | .021 | .009 | .008 |
| Separation/divorce | .003 | .022 | .000 | .007 |
| Unsafe neighborhood | .003 | .005 | −.001 | .001 |
| Bullying (in the neighborhood) | .007 | .004 | .002 | .001 |
| Bullying (in the school) | .004 | .005 | .001 | .002 |
| Parent death | .003 | .003 | −.002 | .001 |
| Sibling death | .004 | .003 | .000 | .001 |
| Parental disability (be sick on bed for a long time) | .019*** | .003 | .003** | .001 |
| Parental disability (a serious deformity) | .012* | .006 | .003 | .002 |
| Childhood SES | −.005** | .001 | −.001** | .000 |
| Father’s education | −.005* | .002 | −.001 | .001 |
| Childhood self-rated health | −.009*** | .001 | .000 | .000 |
| Objective childhood health and health care | −.009*** | .002 | −.002* | .001 |
| Covariates | ||||
| Age | .002*** | .000 | .000*** | .000 |
| Male | −.022*** | .003 | −.002 | .001 |
| Agricultural hukou | .007* | .004 | −.001 | .001 |
| Living in rural area | .005 | .003 | .001 | .001 |
| Married | −.010* | .004 | .003* | .001 |
| Had received formal education or higher | −.027*** | .003 | .001 | .001 |
| Drank alcohol in the past year | −.012*** | .003 | .000 | .001 |
| Smoke at present | −.002 | .003 | .002 | .001 |
| Underweight | .017** | .005 | .000 | .002 |
| Overweight | .008** | .003 | .003*** | .001 |
| Obesity | .023*** | .004 | .007*** | .001 |
| Household per capita consumption | −.001 | .001 | .001 | .000 |
(1) *p < 0.05. **p < 0.01. ***p < 0.001.; (2) χ2 (71) = 299.537, p < .001, RMSEA = 0.024, CFI = 0.983, TLI = 0.966, SRMR = 0.012
Appendix 2
See Table 4.
Table 4.
The impact of father’s education and occupation
| Variable | Intercept | Slope | ||
|---|---|---|---|---|
| b | SE | b | SE | |
| ACEs | .138*** | .011 | .018*** | .004 |
| Father’s education | −.006** | .002 | .000 | .001 |
| Father’s occupation | −.005 | .003 | .000 | .001 |
| Childhood SES | −.006*** | .001 | −.001** | .000 |
| Childhood self-rated health | −.008*** | .001 | −.001 | .000 |
| Objective childhood health and health care | −.008*** | .002 | −.002** | .001 |
| Covariates | ||||
| Age | .002*** | .000 | .000*** | .000 |
| Male | −.024*** | .003 | −.003* | .001 |
| Agricultural hukou | .005 | .003 | −.001 | .001 |
| Living in rural area | .008** | .003 | .000 | .001 |
| Married | −.011** | .003 | .001 | .001 |
| Had received formal education or higher | −.026*** | .002 | .001 | .001 |
| Drank alcohol in the past year | −.012*** | .002 | .000 | .001 |
| Smoke at present | −.002 | .003 | .002* | .001 |
| Underweight | .016*** | .004 | .000 | .002 |
| Overweight | .007** | .002 | .003*** | .001 |
| Obesity | .023*** | .004 | .008*** | .001 |
| Household per capita consumption | −.001 | .001 | .000 | .000 |
(1) *p < 0.05. **p < 0.01. ***p < 0.001.; (2) χ2 (41) = 309.461, p < .001, RMSEA = 0.032, CFI = 0.982, TLI = 0.967, SRMR = 0.015
Appendix 3
See Table 5.
Table 5.
The impact of daily activity
| Variable | Intercept | Slope | ||
|---|---|---|---|---|
| b | SE | b | SE | |
| ACEs | .137*** | .011 | .018*** | .004 |
| Childhood SES | −.006*** | .001 | −.001** | .000 |
| Father’s education | −.006** | .002 | .000 | .001 |
| Childhood self-rated health | −.008*** | .001 | .000 | .000 |
| Objective childhood health and health care | −.007*** | .002 | −.002* | .001 |
| Covariates | ||||
| Daily activity | −.010*** | .002 | −.001 | .001 |
| Age | .002*** | .000 | .000*** | .000 |
| Male | −.024*** | .003 | −.002* | .001 |
| Agricultural hukou | .006 | .003 | −.001 | .001 |
| Living in rural area | .009** | .003 | .000 | .001 |
| Married | −.012*** | .003 | .001 | .001 |
| Had received formal education or higher | −.026*** | .002 | .001 | .001 |
| Drank alcohol in the past year | −.011*** | .002 | .000 | .001 |
| Smoke at present | −.001 | .003 | .002* | .001 |
| Underweight | .017*** | .004 | .000 | .002 |
| Overweight | .007** | .002 | .003*** | .001 |
| Obesity | .024*** | .004 | .008*** | .001 |
| Household per capita consumption | .000 | .001 | .000 | .000 |
(1) *p < 0.05. **p < 0.01. ***p < 0.001.; (2) χ2 (41) = 357.210, p < .001, RMSEA = 0.034, CFI = 0.980, TLI = 0.962, SRMR = 0.016
Appendix 4
See Fig. 2.
Fig. 2.
Distribution of frailty index
Appendix 5
See Fig. 3.
Fig. 3.
The impact of different levels of ACEs, self-rated childhood SES, father’s education, self-rated childhood health, and objective health and health care on frailty trajectory
Appendix 6
See Table 6.
Table 6.
Latent growth curve model using the full sample (N=16,931)
| Variable | Intercept | Slope | ||
|---|---|---|---|---|
| b | SE | b | SE | |
| ACEs | .129*** | .010 | .017*** | .004 |
| Childhood SES | −.005*** | .001 | −.001** | .000 |
| Father’s education | −.007*** | .002 | .000 | .001 |
| Childhood self-rated health | −.009*** | .001 | .000 | .000 |
| Objective childhood health and health care | −.009*** | .002 | −.002* | .001 |
| Covariates | ||||
| Age | .002*** | .000 | .000*** | .000 |
| Male | −.023*** | .003 | −.001 | .001 |
| Agricultural hukou | .006* | .003 | −.001 | .001 |
| Living in rural area | .009*** | .002 | .000 | .001 |
| Married | −.013*** | .003 | .001 | .001 |
| Had received formal education or higher | −.026*** | .002 | .001 | .001 |
| Drank alcohol in the past year | −.013*** | .002 | .000 | .001 |
| Smoke at present | −.002 | .003 | .001 | .001 |
| Underweight | .017*** | .004 | .000 | .002 |
| Overweight | .007** | .002 | .003*** | .001 |
| Obesity | .023*** | .003 | .008*** | .001 |
| Household per capita consumption | −.001 | .001 | .000 | .000 |
Author’s contribution
YY contributed to statistical analysis, original draft preparation and writing, and revision. LC contributed to statistical analysis and revision. NL contributed to study design, supervision, data analysis, and paper writing and revision.
Funding
This work was supported by fund for building world-class universities (disciplines) of Renmin University of China.
Data availability
The datasets are available in the CHARLS repository, [http://charls.pku.edu.cn].
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Was obtained from the Ethical Review Committee of Peking University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuqi Yan, Email: yanyuqi@ruc.edu.cn.
Liqing Cai, Email: cailiqing@ruc.edu.cn.
Nan Lu, Email: nalv9728@ruc.edu.cn.
References
- Alvarado BE, Zunzunegui M-V, Béland F, Bamvita J-M. Life course social and health conditions linked to frailty in Latin American older men and women. The journals of gerontology. Ser Biol Sci Med Sci. 2008;63:1399–1406. doi: 10.1093/gerona/63.12.1399. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. doi: 10.1093/ije/31.2.285. [DOI] [PubMed] [Google Scholar]
- Berens AE, Jensen SKG, Nelson CA., 3rd Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, McCoy DC, Fink G, Shawar YR, Shiffman J, Devercelli AE, Wodon QT, Vargas-Barón E, Grantham-McGregor S, Committee LECDSS. Early childhood development coming of age: science through the life course. The Lancet. 2017;389:77–90. doi: 10.1016/s0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain AM, Rutten LJF, Manemann SM, Yawn BP, Jacobson DJ, Fan C, Grossardt BR, Roger VL, Sauver JLS. Frailty Trajectories in an Elderly Population-Based Cohort. J Am Geriatr Soc. 2016;64:285–292. doi: 10.1111/jgs.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannefer D. Cumulative advantage/disadvantage and the life course: cross-fertilizing age and social science theory. The journals of gerontology. Ser Psychol Sci Soc Sci. 2003;58:S327–S337. doi: 10.1093/geronb/58.6.s327. [DOI] [PubMed] [Google Scholar]
- Elder GH, Johnson MK, Crosnoe R. The emergence and development of life course theory. In: Mortimer JT, Shanahan MJ, editors. Handbook of the Life Course. US, Boston: Springer; 2003. pp. 3–19. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. the journals of gerontology. Ser Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Hair JF, Black WC, Babin BJ, Anderson RE. Multivariate Data Analysis. 7. Upper Saddle River: Prentice-Hall; 2010. [Google Scholar]
- Hu B. Childhood adversity and healthy ageing: a study of the Chinese older population. Eur J Ageing. 2021;18:523–535. doi: 10.1007/s10433-021-00608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Lyu J, Kim G. Multi-group frailty trajectories among older koreans: results from the korean longitudinal study of aging. Arch Gerontol Geriatr. 2022;98:104533. doi: 10.1016/j.archger.2021.104533. [DOI] [PubMed] [Google Scholar]
- Kalmakis KA, Chandler GE. Health consequences of adverse childhood experiences: a systematic review. J Am Assoc Nurse Pract. 2015;27:457–465. doi: 10.1002/2327-6924.12215. [DOI] [PubMed] [Google Scholar]
- Kendig H, Gong CH, Yiengprugsawan V, Silverstein M, Nazroo J. Life course influences on later life health in China: childhood health exposure and socioeconomic mediators during adulthood. SSM-Popul Health. 2017;3:795–802. doi: 10.1016/j.ssmph.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3. New York: The Guilford Press; 2011. [Google Scholar]
- Ko CY, Koyama M, Sng T-H. Unified China and Divided Europe. Int Econ Rev. 2018;59:285–327. doi: 10.1111/iere.12270. [DOI] [Google Scholar]
- Li Y, Xue Q-L, Odden MC, Chen X, Wu C. Linking early life risk factors to frailty in old age: evidence from the China Health and Retirement Longitudinal Study. Age Ageing. 2020;49:208–217. doi: 10.1093/ageing/afz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sun W, Lu C, Chen W, Guo VY. Adverse childhood experiences and handgrip strength among middle-aged and older adults: a cross-sectional study in China. BMC Geriatr. 2022;22:118. doi: 10.1186/s12877-022-02796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden BWAVD, Sieber S, Cheval B, Orsholits D, Guessous I, Gabriel R, Arx MV, Kelly-Irving M, Aartsen M, Blane D, Boisgontier MP, Courvoisier D, Oris M, Kliegel M, Cullati S. Life-course circumstances and frailty in old age within different European welfare regimes: a longitudinal study with share. the journals of gerontology. Ser Psychol Sci Soc Sci. 2020;75:1326–1335. doi: 10.1093/geronb/gbz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Chen B, Li Y, Morrow-Howell N. Neighborhood resources associated with frailty trajectories over time among community-dwelling older adults in China. Health Place. 2022;74:102738. doi: 10.1016/j.healthplace.2021.102738. [DOI] [PubMed] [Google Scholar]
- Lyu J, Burr JA. Socioeconomic status across the life course and cognitive function among older adults: an examination of the latency, pathways, and accumulation hypotheses. J Aging Health. 2016;28:40–67. doi: 10.1177/0898264315585504. [DOI] [PubMed] [Google Scholar]
- Mian O, Anderson LN, Belsky DW, Gonzalez A, Ma J, Sloboda DM, Bowdish DME, Verschoor CP. Associations of adverse childhood experiences with frailty in older adults: a cross-sectional analysis of data from the canadian longitudinal study on aging. Gerontology. 2021 doi: 10.1159/000520327. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics of China . Main data of the first National census —— Total number of households and total population. China Population Statistics Yearbook: China Prospect Publishing House, Beijing; 1988. p. 272. [Google Scholar]
- National Health Commission of the People's Republic of China (2006) Guidelines for the prevention and control of overweight and obesity in Chinese adults. People's Medical Publishing House, Beijing, pp 3
- Oh DL, Jerman P, Marques SS, Koita K, Boparai SKP, Harris NB, Bucci M. Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatr. 2018;18:83. doi: 10.1186/s12887-018-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. Late-life cognition: do childhood conditions play any role? China Econ Rev. 2020 doi: 10.1016/j.chieco.2020.101541. [DOI] [Google Scholar]
- Pan C, Wang C, Shrestha B, Wang P. 3-D health trajectories and related childhood predictors among older adults in China. Sci Rep. 2021;11:9874. doi: 10.1038/s41598-021-89354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D, Green H, Petrosyan S, Shao K, Wilkens J, Lee J (2021) Harmonized CHARLS Documentation, Version D (2011-2018).https://charls.charlsdata.com/pages/data/111/en.html. Accessed 1 March 2022
- Raymond E, Reynolds CA, Aslan AKD, Finkel D, Ericsson M, Hägg S, Pedersen NL, Jylhävä J. Drivers of Frailty from adulthood into old age: results from a 27-year longitudinal population-based study in Sweden. the journals of gerontology. Ser Biol Sci Med Sci. 2020;75:1943–1950. doi: 10.1093/gerona/glaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. the journals of gerontology. Ser Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. The journals of gerontology. Ser Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 173:489–495. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed]
- Shi Z, Wu C. Early life adversity and health inequality: a dual interaction model. J Chin Sociol. 2020 doi: 10.1186/s40711-020-00121-y. [DOI] [Google Scholar]
- The Xinhua News Agency (2018) The elderly will account for about one third of the total population in China by 2050. Xinhuanet. http://www.xinhuanet.com/politics/2018-07/19/c_1123151410.htm. Accessed 25 May 2022
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen JJ. childhood health status and adulthood cardiovascular disease morbidity in rural China: are they related? Int J Environ Res Public Health. 2016 doi: 10.3390/ijerph13060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen Z, Zhou C. Social engagement and physical frailty in later life: does marital status matter? BMC Geriatr. 2021;21:248. doi: 10.1186/s12877-021-02194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Smit E, Xue Q-L, Odden MC. Prevalence and correlates of frailty among community-dwelling chinese older adults: the China health and retirement longitudinal study. the journals of gerontology. Ser Biol Sci Med Sci. 2017;73:102–108. doi: 10.1093/gerona/glx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Z. Early-life conditions and cognitive function in middle-and old-aged Chinese adults: a longitudinal study. Int J Environ Res Public Health. 2020;17:3451. doi: 10.3390/ijerph17103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Wong M, Chong KC, Chang B, Lum CM, Auyeung TW, Lee J, Lee R, Woo J. Trajectories of frailty among Chinese older people in Hong Kong between 2001 and 2012: an age-period-cohort analysis. Age Ageing. 2018;47:254–261. doi: 10.1093/ageing/afx170. [DOI] [PubMed] [Google Scholar]
- Yuan K-H, Bentler PM. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol Methodol. 2000;30:165–200. doi: 10.1111/0081-1750.00078. [DOI] [Google Scholar]
- Yuan K-H, Yang-Wallentin F, Bentler PM. ML versus MI for missing data with violation of distribution conditions. Sociol Methods Res. 2012;41:598–629. doi: 10.1177/0049124112460373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Communiqué of the seventh national population census (No. 2)—Basic information of the national population. In: Zhang Y, editor. Key data from the Seventh National Population Census. Beijing: China Statistic Publishing House; 2020. pp. 50–54. [Google Scholar]
- Zhang Y. Communiqué of the seventh national population census (No. 5)—age composition. In: Zhang Y, editor. Key data from the Seventh National Population Census. Beijing: China Statistic Publishing House; 2020. pp. 66–71. [Google Scholar]
- Zhang J, Lu N. The association between childhood conditions and heart disease among middle-aged and older population in China: a life course perspective. BMC Geriatr. 2021;21:184. doi: 10.1186/s12877-021-02134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu J, Li L, Xu H. the long arm of childhood in china: early-life conditions and cognitive function among middle-aged and older adults. J Aging Health. 2018;30:1319–1344. doi: 10.1177/0898264317715975. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the china health and retirement longitudinal study (CHARLS) Int J Epidemiol. 2014;43:61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Fang Z, Shangguan S, Fang X. Associations between childhood maltreatment and educational, health and economic outcomes among middle-aged Chinese: The moderating role of relative poverty. Child Abuse Negl. 2021;130:105162. doi: 10.1016/j.chiabu.2021.105162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available in the CHARLS repository, [http://charls.pku.edu.cn].