Abstract
Background
Oral Squamous Cell Carcinoma (OSCC), a major debilitating illness demands focus in recent times due to a constant upsurge in cases and poor prognostic implications. An urgent mandate upon finding evidence of relevant prognostic markers is the need of the hour. This systematic review and meta-analysis, therefore, elect an objective assessment of Lymphatic Vessel Density (LVD) as a pertinent parameter governing OSCC prognosis.
Methods
The study protocol was registered at the International Prospective Register Of Systematic Reviews (PROSPERO). Databases were searched using the MeSH keywords for all study types following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The exposure under consideration was the evaluation of LVD in patients of OSCC. The outcome was measured as pooled Hazard/Odd’s/Risk ratios in survived versus non-survived OSCC population. The risk of bias assessment was performed using the QUIPS tool. Heterogeneity was assessed by Chi-square and I2 statistics whereas publication bias was investigated using Egger’s test of significance. All the statistical analysis was conducted using STATA version 13.0.
Results
The initial search of 226 records were screened and filtered through the inclusion and exclusion criteria to achieve an outcome of 15 studies for qualitative synthesis out of which seven studies were eligible for meta-analysis. Pooled Hazard of enhanced Lymphatic Vessel Density was not found to be statistically significant (HR = 1.98, p = 0.553); contrary to the pooled Odd’s/Risk for patient survival which was statistically significant (RR = 1.33, p = 0.046). The I2 test of heterogeneity was also significant (58.8%, p = 0.046).
Conclusions
This meta-analysis helps to generate pathfinding evidence for a noteworthy role of Lymphatic Vessel Density evaluation in suggesting OSCC prognosis.
Keywords: Lymphatic vessel density, Oral squamous cell carcinoma, Podoplanin, Lymphatic vessel endothelial Receptor-1
Introduction
Oral Squamous Cell Carcinoma (OSCC) accounts for nearly 3.01% of malignancies and ranks at the sixth position globally [1–3]. It can potentially affect any segment of the oral mucosal lining [4], characterizing a high degree of local invasiveness and cervical lymph node (LN) metastasis [5]. It has been hypothesized that since OSCC is a heterogeneous malignancy, specific histologic and molecular features must be identified for better recognition of the behavior and progression of the disease [6].
In OSCC (an epithelial malignancy), metastatic tumor cells choose to polarize towards cervical lymph nodes via lymphatics rather than the vasculature [7]. Research over recent years has highlighted [8] lymphangiogenesis (formation of new lymphatic vessels), as an important process in tumor metastasis [9]. An interplay between a multitude of growth factors such as Vascular Endothelial Growth Factor, Platelet-Derived Growth Factor, basic Fibroblast Growth Factor, Insulin-like Growth Factors, etc. moderates the tumor microenvironment to exhibit an increased lymphangiogenic activity [9]. They are believed to act as a driving force for the survival, proliferation, and persistence of lymphatic endothelial cells [7]. There is a striking correlation between the aforementioned growth factors with tumor lymphangiogenesis and lymph node metastasis in several tumor types as reported in the literature [9]. This loco-regional metastatic dissemination of tumor cells is also a common feature of many epithelial malignancies [10, 11], and is considered a chief prognostic indicator for disease progression in OSCC [12, 13]. However, there exists an ongoing debate regarding the utilization of pre-existing lymphatic vessels or neo-lymphangiogenesis for metastatic dissemination [10, 11]. Also, the metastatic potential of OSCC is independent of its clinical staging. Therefore, it is absolutely imperative to identify the prognostic factors histopathologically that may have a bearing on the occurrence and likelihood of locoregional and distant metastasis in OSCC [14, 15].
This mandates an objective understanding of lymphangiogenesis through the assessment of Lymphatic Vessel Density (LVD) for predicting prognosis in patients with OSCC [7, 16], which can now be possibly achieved by the advent of markers such as Podoplanin/D2-40/Lymphatic Vessel Endothelial Receptor-1 (LYVE-1)/Prox l specific to lymphatic endothelium [15, 17].
This systematic review and meta-analysis have been therefore taken up to understand the current evidence on the exact role of LVDs in governing Oral Squamous Cell Carcinoma prognosis which may ultimately determine the exact pathways of disease progression, dissemination, and metastasis in OSCC and help to re-evaluate the patient-specific therapeutic regimens.
Methodology
Study registration: The protocol was registered at the International Prospective Register Of Systematic Reviews (PROSPERO) (Registration number CRD42021292079) available at https://www.crd.york.ac.uk/prospero. The systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Search Strategy
Database: The following bibliographic databases were searched: PubMed, Medical Literature Analysis and Retrieval System Online, or MEDLARS Online (MEDLINE), Cumulative Index of Nursing and Allied Health Literature (CINAHL), Web of Science, Global Index Medicus, Cochrane Central Register of Controlled Trials (CENTRAL), Allied and Complementary Medicine Database (AMED) and Embase. We had also manually searched the National Institute of Health and Clinical Trials Database (http://www.ClinicalTrials.gov/), WHO’s International Clinical Trials Registry Platform (https://www.who.int/ictrp/en/), Clinical Trials Registry - India (http://www.ctri.nic.in), Science Direct, and Google Scholar for any current trials/research. All published/accepted articles from inception till 30 November 2021 were included.
Language
Only English language literature was searched.
Searching terms utilized
(“lymphatic vessels“[MeSH Terms] OR (“lymphatic“[All Fields] AND “vessels“[All Fields]) OR “lymphatic vessels“[All Fields] OR (“lymphatic“[All Fields] AND “vessel“[All Fields]) OR “lymphatic vessel“[All Fields]) AND (“densities“[All Fields] OR “density“[All Fields]) AND (“oral squamous cell carcinoma“[MeSH Terms] OR (“squamous“[All Fields] AND “cell“[All Fields] AND “carcinoma“[All Fields] OR “oral squamous cell carcinoma “[All Fields] OR (“oral“[All Fields] AND “squamous“[All Fields] AND “cell“[All Fields] AND “carcinoma“[All Fields]) OR “oral squamous cell carcinoma“[All Fields]).
Eligibility Criteria
Inclusion criteria
Randomized controlled studies (RCTs), analytical, case-control, and cohort studies describing the assessment of Lymphatic Vessel Density in Oral Squamous Cell Carcinoma were included.
Exclusion criteria
Animal studies and studies on head and neck squamous cell carcinomas were excluded.
Participants/Population
Patients with Oral Squamous Cell Carcinoma (all stages and grades).
Exposure(s)
The exposure studied was Lymphatic Vessel Density (Average/Intra-tumoral and/or Peri-tumoral) at any time since diagnosis.
Comparator(s)
Survived versus non-survived based on quantification of Lymphatic Vessel Density.
Outcome
Prognostic significance of assessing Lymphatic Vessel Densities in Oral Squamous Cell Carcinoma.
Measures of Effect
Pooled Hazard Ratio (HR)/ Odd’s Ratio (OR) and Relative Risk (RR) at 95% Confidence Interval were computed to determine the prognostic significance of Lymphatic Vessel Density in cases of Oral Squamous Cell Carcinoma.
Strategy for Data Extraction
The aforementioned strategy was utilized for an exhaustive database search. A simultaneous manual search of English literature was also performed for other suitable evidence. The data was manually cleaned by removing all the duplicate and irrelevant articles.
Data extraction: All the eligible articles were thoroughly evaluated and the relevant data was extracted autonomously by two authors (AT, NNS) in a Microsoft Excel sheet which was later revised by the two authors (AT, KS). The following information was extracted: (1) basic study information including first author, publication year, place of study, duration of the study; (2) patient and tumor information, including sample size, mean/median age, gender, name, and source of the molecular marker used (and its dilution), sample type (paraffin-embedded or frozen), tumor size, and clinical stage of the disease; (3) assessment of average Lymphatic Vessel Density; (4) outcome measures including survival data, Hazard Ratio, Odd’s Ratio, Risk ratio; (5) evidence of lymph node metastasis and recurrence of the primary tumor. Inconsistencies were resolved through discussions with another author (AK).
Assessment of Risk of Bias: The risk of bias was assessed by two authors (AT, KS). All of the eligible studies were appraised for the Risk of Bias (RoB) through the Quality in Prognosis Studies (QUIPS) tool as recommended by the Cochrane Prognosis Methods Group [10]. The risk of bias was summarized as low, moderate, and high based on six domains: (1) Bias due to participation, (2) Bias due to attrition, (3) Bias due to prognostic factor measurement, (4) Bias due to outcome measurement, (5) Bias due to confounding and (6) Bias in statistical analysis and reporting. Disagreements, if any, were solved by the discussion with a third author (AK).
Strategy for Data Synthesis and Analysis
Considering the variability between the included studies a random-effect model was employed for determining the pooled effect size. Pooled Hazard Ratio, pooled Odd’s/Risk Ratio at 95% Confidence Interval were computed to determine the prognostic significance of Lymphatic Vessel Density. Publication bias, if any, was assessed using Egger’s significance test [18]. Heterogeneity levels of the studies included in the review were performed using the Chi-Square test of heterogeneity with the significance level at p < 0.05 and the I2 statistic at significance level ˃50%. The findings were depicted in forest and funnel plots. All the statistical analysis was conducted using STATA version 13.0 (Stata Corp 2013. Stata Statistical Software: Release 13. College Station, TX: Stata Corp LP).
Results
Literature Exploration:
A total of 210 records for assessment of Lymphatic Vessel Density in Oral Squamous Cell carcinoma were recovered after the databases search, following the aforementioned keywords. Additional 16 records were identified through other sources and cross-references. 172 records were removed after duplicate removal, and the remaining 54 records were selected for screening. Of these articles, 21 were excluded as these were based on head and neck squamous cell carcinoma and involved descriptions of laryngeal and oropharyngeal squamous cell carcinomas. Out of a total of 33 full articles eligible for the systematic review 18 were excluded, as the result data format was not suitable for inclusion. 15 records were included for qualitative synthesis and 07 records qualified for Meta-analysis (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart
Description of Studies Included
A total of 15 studies were eligible for this systematic review. The studies were conducted between 2003 and 2021. All included studies have performed Lymphatic Vessel Density (LVD) analysis through immunohistochemistry on Formalin Fixed Paraffin Embedded tissues using either D2-40/Podoplanin (n = 11) or LYVE-1 (n = 3) or PA2.26 (n = 1). The LVD in stained slides was identified through 3–5 hotspots and an average LVD value was calculated per field of the tumor tissue with immunoreactivity ranging between 74 and 100% of the stained slides. The sample sizes extended between 28 and 160, with an average age of participants ranging between 50 and 62 years. Seven studies were eligible for meta-analysis {LVD with a Hazard ratio(n = 2) [19, 20]; LVD with Odd’s Ratio (n = 4) [7, 21–23]; LVD with Risk ratio (n = 1) [14]} including 600 cases and incorporating a total of 354 (59%) males (One study did not mention gender characteristics of the sample [14]). The basic features of the studies included in the meta-analysis have been summarized in Table 1.
Table 1.
Basic characteristics of included studies
| Author | Place of study | Sample Size | Gender (M/F) |
Age (years) |
Lymph node metastasis | Recurrence | IHC Molecule used | Hazard Ratio (CI) | Odd’s Ratio (CI) |
Relative Risk (CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen J et al. (2021) | China | 128 | 90/38 | Median 60 (31–87) | 51 | 92 | LYVE-1 | - |
1.291 (1.191–1.401) |
- |
| Jardim JF et al. (2021) | Brazil | 88 | 64/24 |
Mean 57 (27–82) |
60 | 56 | D2-40 |
2.19 (1.29–3.73) |
- | - |
| Al-Shareef H et al. (2016) | Japan | 80 | 55/25 | Median 62 (22–92) | 40 | - | D2-40 | - |
2.527 (1.412–4.522) |
- |
| Seppälä M et al. (2016) | Finland | 61 | 33/28 |
Mean 61 (17–91) |
31 | 29 | D2-40 | - |
2.27 (1.00-5.11) |
- |
| Ding L et al. (2014) | China | 50 | 25/25 | Mean 53.5 (26–74) | 19 | 50 | LYVE-1 |
1.524 (0.551–4.21) |
- | - |
| Sugiura T et al. (2009) | Japan | 160 | 87/73 | Median 64 (24–94) | 65 | - | D2-40 | - |
1.967 (1.512–2.557) |
- |
| Muñoz-Guerra MF et al. (2004) | Spain | 33 | - | - | 0 | 10 | PA 2.26 | - | - |
4.45 (0.97–20.36) |
LYVE-1: Lymphatic Vessel Endothelial Receptor-1, D2-40: Podoplanin, PA 2.26: Small mucin-like transmembrane glycoprotein; CI: Confidence Interval
Risk of Bias Assessment
Quality in Prognosis Studies (QUIPS) tool suggested that out of seven studies, four had an overall low risk of bias [7, 20, 22, 23], two studies had an overall moderate risk of bias [19, 21], and one had a high risk of bias [14]. The summary for risk of bias assessment has been illustrated in Fig. 2a and b.
Fig. 2.
Risk of Bias assessment (a, b)
Outcomes
A total of seven studies involving 600 subjects fulfilled the inclusion criteria of this meta-analysis. Pooled Hazard Ratio of 1.98 (95% CI, 0.97-3.00) derived from two studies correlating survival of cases with LVD (Fig. 3a) was observed in our analysis. However, pooled Odd’s and Risk ratio revealed an effect score of 1.33 (95% CI, 1.22–1.43) derived from five studies correlating LVDs with Odd’s/Risk ratio as measures of survival (Fig. 3b). In-consistency measured by I2 statistics were significant (58.8% with p = 0.046 for Odd’s/Risk ratio analysis) (Fig. 3b).
Fig. 3.
Forest plots; (a) Hazard Ratio, (b) Odd’s/Risk Ratio
Effect on clinical outcome of disease:
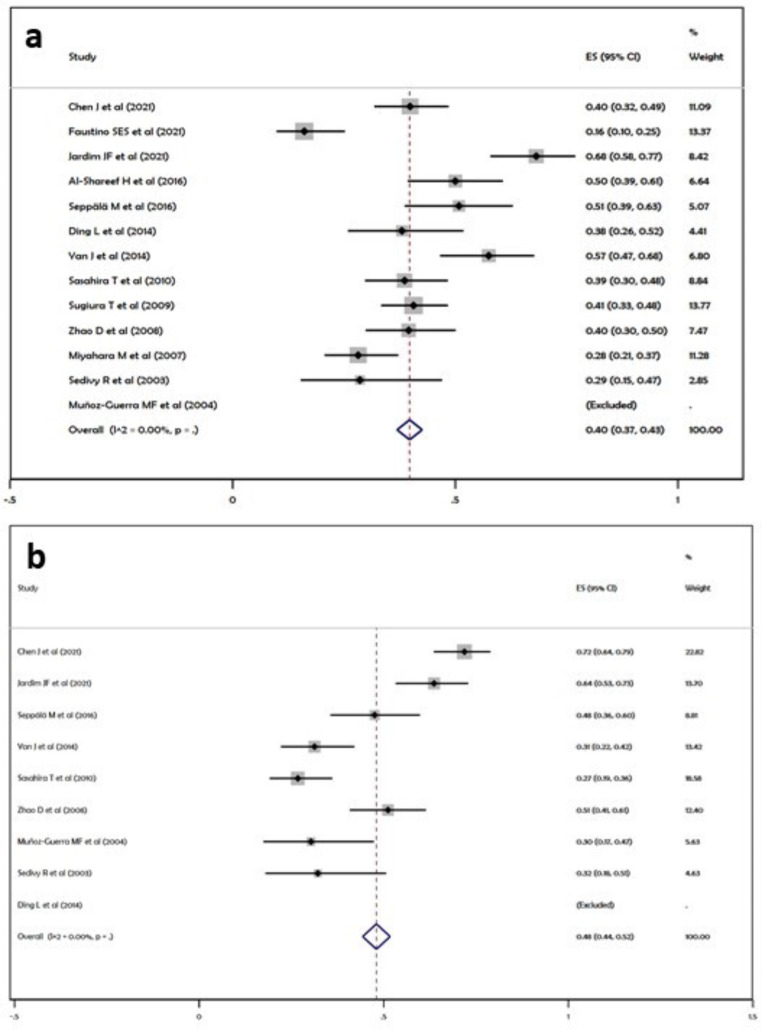
Lymph node metastasis: A total of 13 studies included for qualitative synthesis revealed lymph node involvement in 438 (40.1%) cases out of a total of 1092 study participants enrolled for assessment of LVD. The pooled proportion of Lymph node metastasis was 40% as observed by the forest plot i.e., an overall effect score of 0.40 (95%CI, 0.37–0.43) (Fig. 4a).
Fig. 4.
Clinicopathologic co-relations; (a) Lymph node metastasis, (b) Recurrence of the primary tumor
-
2)
Recurrence: The pooled proportion of recurrences in OSCC as suggested by the forest plot was 48% i.e., an overall effect score of 0.48 (95% CI, 0.44–0.52) (Fig. 4b).
Risk of Publication Bias
In this meta-analysis, the funnel plot did not reveal any significant publication bias (p = 0.069) suggesting that the study findings were consistent (Fig. 5).
Fig. 5.
Funnel Plot for assessment of Publication Bias
Discussion
This meta-analysis is pathfinding research on the assessment of lymphangiogenesis in terms of objective assessment of LVD correlating the clinicopathologic outcomes of Oral Squamous Cell carcinoma and overall patient survival.
Despite aggressive and often mutilating therapeutic regimens [20] in the past few years of vigorous research, the overall 5-year survival rate of OSCC has not shown significant improvement [24] and the long-term survival remains < 50%, motivating the researchers to seek reliable prognostic factors for improvement in therapeutic strategies of OSCC cases [20].
It is a general hypothesis that during the progression of OSCC, lymphatic vessels are repeatedly destroyed and regenerated with the invasion of cancer cells [12, 13] providing a major track for their spread [25]. The thinner walls of the lymphatic vessels, with one layer of endothelial cells, and the lack or discontinuity of the basal membrane makes the lymphatic pathway more favorable for metastatic diffusion than the hematogenous pathway, thereby increasing the metastatic efficiency of tumor cells [26]. The high LVD, therefore, enables direct interactions between the lymphatic vessels and potentially metastatic tumor cells to enhance the likelihood of invasion [14, 27]. Furthermore, the characterization of new molecular markers specific to lymphatic endothelial cells generates strong evidence that lymphangiogenesis can contribute to metastasis as they can clearly differentiate lymphatic vessels from blood capillaries [28, 29].
In our meta-analysis, we observed the Hazard of enhanced Lymphatic Vessel Density for Patient survival, however, the observation was not statistically significant (HR = 1.98, p = 0.553) (Fig. 3a). The findings could only be attributed to insufficient data available in the literature. We also observed Odd’s/Risk of enhanced Lymphatic Vessel Density for Patient survival, and the association was found to be statistically significant (RR = 1.33, p = 0.046) (Fig. 3b). The descriptive evidence suggests that the assessment of lymphangiogenesis in terms of LVDs in epithelial malignancies such as OSCC is of prime importance and is often considered a significant prognostic indicator of disease outcome.
The assessment of LVD is also extremely important in clinical decision-making because a subgroup of cases with histologically lymph node-negative disease may develop persistent metastatic disease and vice-versa. Hence, it is very clear that the potentiality of OSCC to metastasize to lymph nodes does not always correlate with the clinical staging [14]. This makes the findings of this meta-analysis extremely relevant for clinicians as the objective assessment of LVD can prove as a gold standard in clinical judgment regarding the treatment options instituted for OSCC patients.
The literature also emphasizes that lymphangiogenesis acts as a major drainage channel for cancer cells and hence is closely associated with an increased risk of lymph node metastasis [12, 13]. Similar findings have been observed in our analysis where 40% of cases with high LVDs revealed lymph node metastasis which is crucial in the judgment of patient prognosis. Also, 48% of cases enrolled in the meta-analysis revealed recurrences and a significant correlation with increased LVDs in tissues of primary pathology.
Limitations
Assessment of tumor micro-environment along with LVDs
The inconsistencies observed in literature regarding the study of lymphangiogenesis could largely be attributed to the role of tumor micro-environment (cancer-associated fibroblasts, inflammatory cells) [7]. The growth of lymphatic vessels is not an isolated phenomenon and requires an interplay of multiple growth factors (VEGF, Prostaglandin E2, IGF-2, etc.) and chemokines [24, 30] to facilitate the process. This meta-analysis could not venture into this arena because of little existing evidence in this regard.
Assessment of the Individual Significance of Intra-tumoral (ILVD) and Peri-Tumoral LVD (PLVD)
Invasion of tumor cells into the existing/new lymphatic vessels in and around the tumor facilitates metastasis in epithelial malignancies [31]. Therefore, both ILVD and PLVD could be used as predictors for lymphatic metastasis in OSCC [7]. This meta-analysis could not assess the individual roles of ILVD and PLVD in disease progression and patient prognosis because of the paucity of data in the existing literature.
Conclusions
Through years of intensive research, it is now extremely crucial to recognize the exact prognostic features that encourage the loco-regional and distant metastasis in OSCC. The evidence gained through this meta-analysis may pave pathways for future research in a similar direction to empower the clinicians for an appropriate choice of therapeutic modality and alter the treatment strategies as the case demands.
Funding (information that explains whether and by whom the research was supported)
Self.
Data Availability (data transparency)
Data is available with the corresponding author. The data will be submitted if the journal requires it.
Code Availability (software application or custom code)
Not applicable.
Declarations/Compliance with ethical Standards
Conflicts of Interest/Competing Interests (include appropriate disclosures)
None.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Ethics approval (include appropriate approvals or waivers)
Not applicable.
Consent to participate (include appropriate statements)
Not applicable.
Consent for publication (include appropriate statements)
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ankita Tandon, Email: drankitatandon7@gmail.com.
Kumari Sandhya, Email: drksandhya@gmail.com.
Narendra Nath Singh, Email: naren_cancer@hotmail.com.
Amit Kumar, Email: amits52003@gmail.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, Soerjomataram I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67:51–64. doi: 10.3322/caac.21384. [DOI] [PubMed] [Google Scholar]
- 3.Subash A, Bylapudi B, Thakur S, Rao VUS. Oral cancer in India, a growing problem: Is limiting the exposure to avoidable risk factors the only way to reduce the disease burden? Oral Oncol. 2022;125:105677. doi: 10.1016/j.oraloncology.2021.105677. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Wada K, Taniguchi Y, Al-Shareef H, Masuda T, Usami Y, Aikawa T, Okura M, Kamisaki Y, Kogo M. Expression of fatty acid binding protein 4 is involved in the cell growth of oral squamous cell carcinoma. Oncol Rep. 2014;31:1116–20. doi: 10.3892/or.2014.2975. [DOI] [PubMed] [Google Scholar]
- 5.Arimoto S, Hasegawa T, Takeda D, Saito I, Amano R, Akashi M, Komori T. Lymphangiogenesis and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Anticancer Res. 2018;38:6157–62. doi: 10.21873/anticanres.12968. [DOI] [PubMed] [Google Scholar]
- 6.Faustino SES, Tjioe KC, Assao A, Pereira MC, Carvalho AL, Kowalski LP, Oliveira DT. Association of lymph vessel density with occult lymph node metastasis and prognosis in oral squamous cell carcinoma. BMC Oral Health. 2021;21:114. doi: 10.1186/s12903-021-01459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Zhang F, Hua M, Song X, Liu S, Dong Z. Prognostic value of lymphatic vessel density in oral squamous cell carcinoma. Life Sci. 2021;265:118746. doi: 10.1016/j.lfs.2020.118746. [DOI] [PubMed] [Google Scholar]
- 8.Achen MG, Stacker SA. Tumor lymphangiogenesis and metastatic spread-new players begin to emerge. Int J Cancer. 2006;119:1755–60. doi: 10.1002/ijc.21899. [DOI] [PubMed] [Google Scholar]
- 9.Ali MA. Lymphatic microvessel density and the expression of lymphangiogenic factors in oral squamous cell carcinoma. Med Princ Pract. 2008;17:486–92. doi: 10.1159/000151572. [DOI] [PubMed] [Google Scholar]
- 10.Saaristo A, Karpanen T, Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene. 2000;19:6122–9. doi: 10.1038/sj.onc.1203969. [DOI] [PubMed] [Google Scholar]
- 11.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Helman JI, Li LJ. Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer–a review of mechanisms. Int J Oral Sci. 2010;2:5–14. doi: 10.4248/IJOS10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal D, Pardhe N, Bajpai M, Gupta S, Mathur N, Vanaki SS, Puranik RS, Mittal M. Characterization, Localization and Patterning of Lymphatics and Blood Vessels in Oral Squamous Cell Carcinoma: A Comparative Study Using D2-40 and CD-34 IHC Marker. J Clin Diagn Res. 2014;8:ZC86–9. doi: 10.7860/JCDR/2014/10311.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muñoz-Guerra MF, Marazuela EG, Martín-Villar E, Quintanilla M, Gamallo C. Prognostic significance of intratumoral lymphangiogenesis in squamous cell carcinoma of the oral cavity. Cancer. 2004;100:553–60. doi: 10.1002/cncr.11933. [DOI] [PubMed] [Google Scholar]
- 15.Zhao D, Pan J, Li XQ, Wang XY, Tang C, Xuan M. Intratumoral lymphangiogenesis in oral squamous cell carcinoma and its clinicopathological significance. J Oral Pathol Med. 2008;37:616–25. doi: 10.1111/j.1600-0714.2008.00707.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Zhang D, Gong M, Wen L, Liao C, Zou L. High lymphatic vessel density and presence of lymphovascular invasion both predict poor prognosis in breast cancer. BMC Cancer. 2017;17:335. doi: 10.1186/s12885-017-3338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mashhadiabbas F, Mahjour F, Mahjour SB, Fereidooni F, Hosseini FS. The immunohistochemical characterization of MMP-2, MMP-10, TIMP-1, TIMP-2, and podoplanin in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:240–50. doi: 10.1016/j.oooo.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–94. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding L, Zhang Z, Shang D, Cheng J, Yuan H, Wu Y, Song X, Jiang H. α-Smooth muscle actin-positive myofibroblasts, in association with epithelial-mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43:335–43. doi: 10.1111/jop.12143. [DOI] [PubMed] [Google Scholar]
- 20.Jardim JF, Galvis MM, Fabelo IR, Soares FA, Pinto CAL, Kowalski LP. Intratumoral lymphatic vascular density is an independent factor for disease-free and overall survival in advanced stage oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132:580–8. doi: 10.1016/j.oooo.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Al-Shareef H, Hiraoka SI, Tanaka N, Shogen Y, Lee AD, Bakhshishayan S, Kogo M. Use of NRP1, a novel biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to predict lymph node metastasis in squamous cell carcinoma of the tongue. Oncol Rep. 2016;36:2444–54. doi: 10.3892/or.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seppälä M, Pohjola K, Laranne J, Rautiainen M, Huhtala H, Renkonen R, Lemström K, Paavonen T, Toppila-Salmi S. High relative density of lymphatic vessels predicts poor survival in tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2016;273:4515–24. doi: 10.1007/s00405-016-4150-y. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura T, Inoue Y, Matsuki R, Ishii K, Takahashi M, Abe M, Shirasuna K. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: Implications for use as a prognostic marker. Int J Oncol. 2009;34:673–80. doi: 10.3892/ijo_00000193. [DOI] [PubMed] [Google Scholar]
- 24.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers, and perspectives (I) Oral Oncol. 2010;46:630–5. doi: 10.1016/j.oraloncology.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Xiao F, Dai Y, Hu Y, Lu M, Dai Q. Expression profile analysis identifies IER3 to predict overall survival and promote lymph node metastasis in tongue cancer. Cancer Cell Int. 2019;19:307. doi: 10.1186/s12935-019-1028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maturana-Ramírez A, Espinoza I, Reyes M, Aitken JP, Aguayo F, Hartel S, Rojas-Alcayaga G. Higher blood vessel density in comparison to the lymphatic vessels in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:13677–86. [PMC free article] [PubMed] [Google Scholar]
- 27.Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr) 2016;39:397–410. doi: 10.1007/s13402-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–95. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2(8):573–83. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–21. doi: 10.1016/S1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 31.Sales CB, Buim ME, de Souza RO, de Faro Valverde L, Mathias Machado MC, Reis MG, Soares FA, Ramos EA, Gurgel Rocha CA. Elevated VEGFA mRNA levels in oral squamous cell carcinomas and tumor margins: a preliminary study. J Oral Pathol Med. 2016;45:481–5. doi: 10.1111/jop.12398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available with the corresponding author. The data will be submitted if the journal requires it.
Not applicable.