Abstract
During COVID-19 pandemic, lung ultrasound (LUS) proved to be of great value in the diagnosis and monitoring of patients with pneumonia. However, limited data exist regarding its use to assess aeration changes during follow-up (FU).
Our study aims to prospectively evaluate 232 subjects who underwent a 3-month-FU program after hospitalization for COVID-19 at the University Hospital of Pisa. The goals were to assess the usefulness of standardized LUS compared with the gold standard chest computed tomography (CT) to evaluate aeration changes and to verify LUS and CT agreement at FU.
Patients underwent in the same day a standardized 16-areas LUS and high-resolution chest CT reported by expert radiologists, assigning interpretative codes.
Based on observations distribution, LUS score cut-offs of 3 and 7 were selected, corresponding to the 50th and 75th percentile, respectively. Patients with LUS scores above both these thresholds were older and with longer hospital stay. Patients with a LUS score ≥3 had more comorbidities. LUS and chest CT showed a high agreement in identifying residual pathological findings, using both cut-off scores of 3 (OR 14,7; CL 3,6–64,5, Sensitivity 91%, Specificity 49%) and 7 (OR 5,8; CL 2,3–14,3, Sensitivity 65%, Specificity 79%).
Our data suggest that LUS is very sensitive in identifying pathological findings at FU after a hospitalization for COVID-19 pneumonia, compared to CT. Given its low cost and safety, LUS could replace CT in selected cases, such as in contexts with limited resources or it could be used as a gate-keeper examination before more advanced techniques.
Keywords: Lung ultrasound, COVID-19: Follow-up, Pneumonia
1. Introduction
1.1. Background
From December 2019, the medical scenario has been characterized by the SARS-CoV-2 pandemic that put a strain on the healthcare systems all around the world. Human-to-human aerosol transmission as source of contagion [1] explains the rapid spread of the Coronavirus disease 2019 (COVID-19) [2].
The respiratory tract is the most affected by the disease and the clinical manifestations of SARS-CoV-2 infected patients ranged from mild non-specific symptoms to severe pneumonia with organ function damage [2,3].
Diagnosis of COVID-19 is carried out on nasopharyngeal swab by polymerase chain reaction (PCR) [4]. The lung involvement in COVID-19 has been investigated by chest computed tomography (CT), as the radiological gold standard for its very high sensitivity [5].
Since the beginning of this pandemic, clinical and radiological findings of COVID-19 pneumonia have pushed physicians to use more frequently low-impact diagnostic tools. Thus, lung ultrasound (LUS) has been used for the diagnosis and management of this disease [6,7] since the peripheral distribution of pulmonary lesions can be easily visualized by ultrasounds [8,9]. LUS is a very useful technique, with many advantages in terms of logistics, costs, applicability and safety for both for patients and healthcare providers [10,11]. Although LUS is widely employed as diagnostic tool in several lung pathologies, such as pleural effusion, pneumothorax, lung consolidation and interstitial syndrome [12,13], the need to set up a standardized scanning scheme was essential to assess the overall lung aeration due to the typical uneven distribution of COVID-19 pneumonia. Thus, relying on a scheme already validated for acute respiratory distress syndrome (ARDS) [14], a 16-areas scanning scheme LUS was proposed in COVID-19 pneumonia [15]. However, very few studies have used LUS in patients follow-up (FU) and the usefulness of this method in long-term evaluations has yet to be demonstrated [7,16,17].
1.2. Aim of the study
The main objective of our study was to assess the usefulness of standardized LUS compared with the gold standard CT to assess aeration changes during 3-months follow-up of COVID-19 patients. The secondary outcome was to verify the agreement between standardized LUS and chest CT at 3-months follow-up.
2. Methods
2.1. Setting
This study was carried out at University Hospital of Pisa (Italy) on 232 hospitalized patients for COVID-19 undergoing imaging follow-up program after 3 months from discharge. Patients of first Italian pandemic wave were included in the study. In this phase, hospitalization was required in all cases of manifest respiratory symptoms requiring oxygen support.
2.2. Study design
We conducted a prospective single-center study. The research followed the Declaration of Helsinki ethical principles and the international standards of Good Clinical Practice. Local Ethics Committee approved this protocol on 7th April 2020 (protocol number 17828). The written informed consent was obtained from all the patients.
2.3. Study population
In our study, 232 patients hospitalized for COVID-19 were enrolled from 20 June 2020 to 6 February 2021 and underwent a 3-month follow-up after Pisa University Hospital hospitalization. Patients discharged were identified by PCR swab test result and Hospital Discharge Form data, without exclusion criteria. They were contacted 4 weeks after discharge by telephone and asked to perform a questionnaire for their availability to be included in the study. Approximately 3-months (within a 12-to-15-week range) after discharge, patients performed complete imaging tests and clinical evaluation.
Imaging follow-up evaluation was conducted by performing high-resolution chest CT scan (HRCT) and LUS on the same day, with blinded assessment, by two different operators not involved in the clinical management of the patient. Among the study population, 12 were not evaluated by follow-up HRCT but only by LUS, to avoid excessive radiation exposure (e.g., young age, pregnancy). Imaging tests at follow-up were compared with those performed during hospitalization for Sars-Cov2 infection. At baseline, all chest CTs were performed at the Emergency Department. All LUS evaluations within 48 h of hospital's admission.
As regards the clinical aspect, expert pulmonologists assessed the qualitative evolution of three respiratory symptoms (dyspnea, cough, sputum) at 3-months clinical evaluation.
2.4. LUS topographic scheme and scoring
Due to the peculiar distribution of lung lesions in COVID-19 pneumonia, we decided to adopt a 16-areas scanning scheme (8 scans for each hemi-thorax) to emphasize posterior chest analysis [15] . We used convex probes (frequency 2.5–5 MHz) along the intercostal spaces with the transverse approach, to cover the largest possible surface with a single scan. The focus was set on the pleural line and the progressive TGC (time gain compensation) was adjusted to optimize the image. For each of the 16 areas we acquired a video, including at least one complete respiratory cycle (4–6 s). The standardized scanning scheme allowed to evaluate each area and to assign a numerical score based on lung aeration. The score is similar to the one used in ARDS, with score 0 in case of normal aeration (only A-lines or less than 3 separated B-lines); score 1 in case of 3 or more B-lines or coalescent B-lines occupying ≤ 50% of the screen; score 2 for coalescent B-lines occupying > 50% of the screen; and score 3 for consolidation. A final LUS score, achieved from the sum of all values obtained within the 16 areas can range from 0 to 48 and indicates a decrease in aeration as the score increases. Each exam was recorded and reviewed by expert sonographers (L.G., G.B.) to verify methodology and scoring assignment. All sonographers had undergone and successfully passed a LUS training on B-lines [18] and a dedicated LUS training on COVID-19 findings. The B-lines inter-observer variability was examined by intraclass correlation coefficient (ICCs) on 50 previously acquired LUS videos evaluated by an expert reader (L.G.).
2.5. Chest CT methodology
Radiologists with a specific expertise in chest diagnostics and interstitial disease, reported CT images, while a code was assigned to each report obtaining 8 different groups of patients as shown in Table 1 . Each group showed a comparison between baseline chest CT and 3 months follow-up HRCT. Chest CTs were not analysed with quantitative or semi-quantitative scores but with comparative methods between baseline and 3 months follow-up CT scans.
Table 1.
Radiological codes used for interpretation of follow-up chest CT and number of patients in each category
COVID-19: CO-ronaVI-rus d-isease 2019, CT: computed tomography, FU: follow-up.
| GROUP | CODE | DESCRIPTION | N (%) Tot.220 |
|---|---|---|---|
| 0 | [0–0] | Without COVID-19 pneumonia (chest CT signs of pneumonia absent at baseline and absent at FU) | 11 (5%) |
| 1 | [1–0] | COVID-19 pneumonia resolution (chest CT signs of pneumonia present at baseline and absent at FU) | 91 (41,4%) |
| 2 | [1–01] | Resolution and new findings (CT chest CT signs of COVID-19 pneumonia present at baseline and resolved but present elsewhere at FU | 11 (5%) |
| 3 | [1–11] | Stable (chest CT signs of COVID-19 pneumonia present at baseline and present unchanged at FU) | 4 (1,8%) |
| 4 | [1–10] | Resolving pneumonia (CT chest signs of COVID-19 pneumonia present at baseline and reduced at FU) | 85 (38,6%) |
| 5 | [1–12] | Worsened (CT chest CT signs of COVID-19 pneumonia present at baseline and increased at FU) | 0 |
| 6 | [0–1] | Onset (chest CT signs of COVID-19 pneumonia absent at baseline and present at FU) | 0 |
| 7 | [x-0] | Absence (chest CT not performed at baseline and no signs of COVID-19 pneumonia at FU) | 10 (4,5%) |
| 8 | [x-11] | Missing / finding (chest CT not performed at baseline and signs of COVID-19 pneumonia present at FU) | 5 (3,7%) |
Chest CT was considered worsened in case of new or more extensive lesions compared to the previous exam. Pathological findings considered were those typical of COVID-19 pneumonia: ground-glass, crazy paving, and consolidations. During hospitalization, 2 types of CT scan were used: 64-row General Electric Light Speed (collimation width 0.625, reconstruction thickness 1.25 mm, standard kernel, soft and boneplus) and 40-row Siemens Somatom Sensation (collimation width 0.6, reconstruction thickness 1.5 mm, kernel B31, B35 and B60). At 3 month follow-up, CT scans were acquired with a 64-slice Siemens Somatom Sensation scanner, Siemens Healthineers (collimation width 0.6, reconstruction thickness 1.5 mm, kernel B60 or B31).
2.6. Statistical analysis
Data are expressed as mean ± standard deviation, median and interquartile range (IQR) for continuous numeric variables and as percentage for categorical variables. Differences between groups were analysed with a parametric test (Student's T test) for normally distributed variables and a non-parametric test (Mann-Whitney U test) for non-normally distributed variables. χ2 test was used for comparisons between variables expressed in the form of frequencies. Receiving Operator Curves (ROC) were used to identify the best cut-off values of the LUS aeration score and their diagnostic accuracy in identifying pathological findings Logistic regression was used to verify the ability of LUS cut-offs to predict pathological changes on chest CT. Regression coefficient (β) and odds ratio (OR) with the corresponding 95% confidence interval (CI) were assumed as outputs of the logistic regression models. P value 0.05 was considered statistically significant.
3. Results
3.1. Descriptive analysis of population subjected to follow-up
The mean age of patients was 62.2 ± 14.5 years (minimum 18, maximum 96). Out of the 232 patients examined, 144 (62.1%) were males and 88 (37.9%) females. The mean hospital stay was 17.6 ± 11.3 days. Regarding the setting, 131 (81.9%) patients were hospitalized in the medical area and 29 (18.1%) in intensive care units (ICU), while among the latter, 17 (58,6%) underwent endotracheal intubation (ETI). The most frequent comorbidities of the 232 hospitalized patients were arterial hypertension (n.89, 38.5%),; cardiovascular disease (n = 57, 24%); diabetes mellitus (n = 42, 18.2%); and respiratory diseases (n = 28, 12,1%).
As regards the 3-month follow-up clinical evaluation, the majority of the patients showed resolution of respiratory symptoms (60.9%), while improvement, stability or worsening were observed in 20.5%, 15.5% and 3.1% of the patients, respectively.
3.2. LUS score in the follow-up population
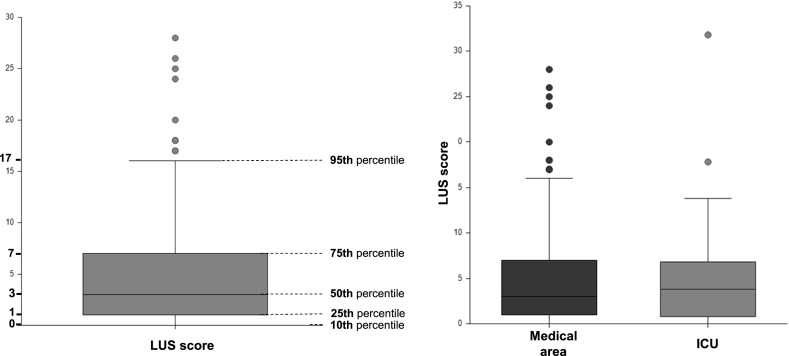
At follow-up, the average LUS score of the whole population of 232 patients was 4.9 ± 5.7. Fig. 1 shows LUS score distribution. The 50th percentile was a LUS score of 3, and the 75th percentile a LUS score of 7.
Fig. 1.
LUS score values distribution in total of patients undergoing follow-up and in population divided by hospital setting
LUS: lung ultrasound, ICU: intensive care unit.
LUS score of patients admitted to the medical area was not different from that of patients admitted to intensive care (respectively 4.9 ± 5.4 and 5.4 ± 6.0, p 0.3) as shown in Fig. 1.
Patients with LUS greater than 3 were older, more frequently males, with longer hospitalisations and higher incidence of comorbidities as compared to those with LUS < 3 (Table 2 ). Patients with LUS greater than 7 were older, with longer hospitalization as compared to those with LUS < 7 (Table 2).
Table 2.
Clinical characteristics of population divided according to LUS cut-offs of 3 and 7.
LUS: lung ultrasound, CV: cardiovascular, ICU: intensive care unit.
|
LUS score ≤3 (50th perc) Tot. 122 |
LUS score >3 (50th perc) Tot. 110 |
P value |
LUS score ≤7 (75th perc) Tot. 181 |
LUS score >7 (75th perc) Tot. 51 |
P value | |
| Male sex | 67 (46.5%) | 77 (53.5%) | 0.02 | 111 (77.1%) | 33 (22.9%) | 0.66 |
| Female sex | 55 (62.5%) | 33 (37%) | 70 (79.5%) | 18 (20.5%) | ||
| Age | 55.8 ± 13.1 | 69.5 ± 12.4 | < 0.001 | 58.6 ± 13.08 | 75.2 ± 11.5 | < 0.001 |
| Arterial hypertension | 34 (38.2%) | 55 (61.8%) | 0.006 | 60 (67.4%) | 29 (32.6%) | 0.002 |
| CV disease | 21 (36.8%) | 36 (63.2%) | 0.006 | 36 (63.2%) | 21 (36.8%) | 0.002 |
| Diabetes mellitus | 14 (33.3%) | 28 (66.7%) | 0.006 | 29 (69%) | 13 (31%) | 0.12 |
| Respiratory disease | 13 (46.4%) | 15 (53.6%) | 0.5 | 18 (64.3%) | 10 (35.7%) | 0.06 |
| ICU | 19 (44.2%) | 24 (55.8%) | 0.3 | 33 (76.7) | 10 (23.3%) | 0.9 |
| Days of hospitalization | 13.41 ± 7.8 | 19.3 ± 12.3 | < 0.001 | 14.9 ± 9.9 | 20.9 ± 11.5 | < 0.001 |
| P/F | 315.3 ± 84.9 | 306.5 ± 96.2 | 0.5 | 313.6 ± 91.4 | 302.4 ± 84.7 | 0.5 |
| Persistence respiratory symptoms at 3-month FU | 5 (41.8%) | 40 (96.4%) | 0.34 | 68 (37.6) | 23 (45.1) | 0.37 |
No statistically significant differences emerged regarding the arterial partial pressure of oxygen / fractional inspired oxygen ratio (P/F) at admission and follow-up ultrasound findings. The persistence of subjective respiratory symptoms at 3-month follow-up also showed no significant association with LUS (Table 2).
In those patients (n = 80) where LUS was available both during hospitalization and at follow-up, LUS score changed from 14.4 ± 8.5 to 5.1 ± 5.6 (p<0.001).
3.4. Correlation of LUS and chest CT at 3-month follow-up
The population was divided into 2 groups based on chest CT results (Table 1): those with a resolved or resolving pneumonia (CT codes 0–0, 1–0, x-0, 1–10) and those who still presented some pathological findings (CT codes 1- 01, 1–11, 1–12, 0–1, x-10, x-11).
To verify the correlation between ultrasound and CT findings, we compared the group of patients with LUS score ≤ 3, corresponding to the 50th percentile, and resolution on CT examination, as shown in Table 3 .
Table 3.
Concordance between chest CT and LUS using cut-off of 3 and 7
LUS: lung ultrasound, CT: computed tomography.
| LUS score ≤3 | LUS score >3 | p-value |
LUS score ≤7 |
LUS score >7 |
p-value | |
|---|---|---|---|---|---|---|
|
CT Resolved/resolving pneumonia Tot. 197 |
115 (58.4%) | 82 (41.6%) | < 0.001 | 161 (94.2%) | 10 (5.8%) | < 0.001 |
|
CT pathological findings Tot. 23 |
2 (1.7%) | 21 (91.3%) | 10 (43.5%) | 13 (56.5%) |
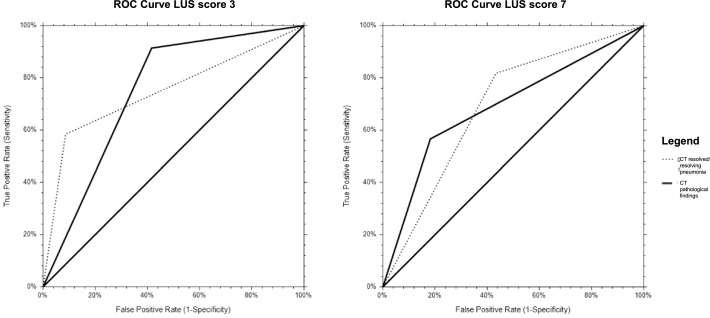
Then, we verified the validity and predictivity of LUS score cut-offs by univariate logistic regression analysis (with reference to chest CT findings), obtaining an Odds Ratio of 14.7 for score 3 and 5.8 for score 7. Finally, we established sensitivity and specificity of the 2 cut-offs using the ROC curves (Table 4 and Fig. 2 ).
Table 4.
Univariate logistic regression analysis, sensitivity, and specificity values for 3 and 7 cut-offs
LUS: lung ultrasound.
| Odds ratio | Beta | Confidence limits | p-value | Sensibility | Specificity | |
|---|---|---|---|---|---|---|
| LUS 3 | 14.7 | 2.7 | 3.6–64.5 | 0.005 | 91% | 49% |
| LUS 7 | 5.8 | 1.7 | 2.3–14.3 | 0.008 | 65% | 79% |
Fig. 2.
ROC curves corresponding to the cut-offs of 3 and 7
CT: computed tomography.
3.5. B-lines inter-observer variability
All readers had a mean ICC on B-lines number assessment >0.90 for single measurements (p<0.0001) and >0.90 for average measurements (p<0.0001). Intra-observer variability was assessed on 20 consecutive videos, with an overall concordance rate on LUS score of 95%.
4. Discussion
A very large number of publications on COVID-19 were issued in 2020–2021 regarding pathogenesis, pathophysiology, epidemiology, clinical course, diagnosis, and complications. However, systematic studies on medium to long-term imaging follow-up from hospital discharge of surviving patients are scarce [16,17,19].
LUS is considered a valid diagnostic technique in the context of several lung diseases (including as heart failure), with higher sensitivity compared to X-ray [10,12,13]. Furthermore, LUS has many logistical advantages, particularly significant in the context of SARS-CoV-2 pandemic, in terms of costs, safety and applicability [7,15].
The present study demonstrates that LUS can assess aeration changes at follow-up of COVID-19 patients, and that a good correlation exists between LUS and HCRT, 3 months after hospitalization for COVID-19 disease.
The correlation between different imaging methods has been already studied [20,21]. Zieleskiewicz et al. investigated the relationship between ultrasound and chest CT in patients with SARS-CoV-2 pneumonia in acute phase. In patients with proven SARS-CoV-2 pneumonia, their calculated LUS score was significantly associated with the severity of pneumonia as assessed by chest CT and clinical characteristics of the patients [20]. A semi-quantitative analysis by Deng et al. showed a high consistency between LUS and CT results in critically ill patients with COVID-19, promoting LUS as a potential tool for dynamic monitoring of ICU patients in the absence of CT [21]. Conversely, we evaluated the conformity between LUS and chest CT at 3-months follow-up, according to the comparative approach based on the evolution of HRCT pathological findings.
We divided the population based on LUS cut-offs that identified the 50th and 75th percentiles of our observations (LUS score 3 and 7, respectively). Our analysis showed that both cut-offs of 3 and 7 are effective in identifying HRCT abnormality (OR 14.7, CL 3.6–64.5; OR 5.8 with CL 2.3–14.3, respectively). Nevertheless, ROC curves demonstrated a high sensitivity (91%) of the cut-off of 3 compared to 7 (65%). Thus, the use of a lower cut-off could allow a more "prudential" approach but burdened by false positives due to other pathological conditions, particularly in the elderly (pulmonary congestion due to heart failure or renal failure). Conversely, the cut-off of 7 showed a higher specificity (70%) compared to 3 (49%). These data agree with the closer relationship of the cut-off value of 3 with preexisting comorbidities.
A comparable ultrasound cut-off proposal was formulated by Clofent and collaborators, who suggested LUS score 3 demonstrating a strong correlation with HRCT alterations in 352 patients after 2–5 months after hospitalization [19]. Similarly to our study, ROC curve analysis revealed an excellent ability of LUS score ≥ 3 to discriminate patients with HCRT abnormalities with of sensitivity 94.2% [19].
Baseline characteristics of our study population, dating back to the very first pandemic wave, showed a high prevalence of males, cardiovascular diseases and hospital management influenced not only by clinical conditions but also by organizational difficulties [22], [23], [24], [25].
Our analyses showed that the groups with LUS score above both cut-offs at follow-up had a higher age and a longer hospitalization. The group with a LUS score ≥ 3 showed greater comorbidities. Statistical significance is achieved in all categories when the cut-off of 3 was used. This result may suggest that older and comorbid patients require a longer recovery time. On the other hand, these data could be related to a more severe disease in the acute phase, or concomitants pathologies (for example heart failure).
There was no difference in LUS score between patients managed in different care settings or with worse respiratory conditions on admission, probably due, to the high mortality of ICU, which resulted in a hyper-selected population at post-hospitalization follow-up.
As regards the 3-month follow-up clinical evaluation, approximately 40% patients reported the persistence of respiratory symptoms. The LUS aeration score was not dissimilar in patients with or without persistence of symptoms at follow-up. This finding could be due to the non-specificity and subjectivity of the symptoms reported, while a correlation with functional tests was already demonstrated [19]. Indeed, Clofent and al. showed an inverse association for LUS score ≥ 3 with lung diffusing capacity for carbon monoxide (DLCO) [19].
Results of our and previous studies [11,19,26] suggest that LUS could be used in selected cases as an alternative to CT, with a significant reduction in timing, costs, and exposure to X-rays [27]. The LUS examination could be considered as a "gate-keeper" before CT, particularly in younger patients or in limited-resource setting. Accordingly, a subgroup of patients with LUS assessment both at baseline and at follow-up, showed a significant lung improvement at follow-up compared to the values of the acute phase (p<0.001). Considering that LUS score expresses the degree of lung aeration, our data show that standardized LUS technique allows the monitoring of this phenomenon over time.
5. Limitations
This research has some limitations. The first is the single-center design of the study and the limited number of cases. The study was designed according to the scarce data regarding early evaluation of imaging at follow-up of patients hospitalized for COVID during the first pandemic wave in Italy. Patients' pre-existing comorbidities could be responsible for pathological findings on imaging examinations, independently of COVID-19 disease, introducing confounding to interpretation. The two imaging techniques compared, despite being standardized and performed at the same time, use methods that are not completely overlapping [28]. Furthermore, CT and LUS findings identified at 3 months FU could be interpreted as normal evolution after COVID-19 pneumonia, as part of the slow resolution process. Therefore, our considerations could be re-evaluated considering the subsequent follow-up checks and correlation with clinical data, details that would be valued in future studies.
6. Conclusion
The evaluation of LUS at follow-up showed a substantial resolution of COVID-19 pneumonia in a large percentage of patients hospitalized 3 months earlier. Patients with higher LUS score were older with associated co-morbidities and longer hospital stay.
The comparison between LUS and CT findings (through qualitative categories identified by expert radiologists) shows an excellent correlation between the two methods. Considering the high sensitivity demonstrated by the proposed cut-offs, we believe that a standardized LUS approach could be applied effectively in COVID-19 patients follow-up, limiting the use of expensive, unsafe, and not always available methods, such as chest CT.
7. Perspectives
LUS data could be analysed with clinical conditions and respiratory functional tests, aiming at the optimization of COVID-19 patient's assessment after hospitalization. Indeed, the association of LUS findings associated with the clinical evaluation, could overcome the limit of the low specificity of the technique.
A further perspective is represented by the development of automated interpretation for LUS, involving online-computerized measurements of the percentage of pleural line presenting B-lines [29]. This could extend the application in all pandemic contexts overcoming the limits derived from the operator-dependency [26]
CRediT authorship contribution statement
Greta Barbieri: Conceptualization, Formal analysis, Investigation, Writing – review & editing. Luna Gargani: Conceptualization, Methodology, Writing – review & editing. Vittoria Lepri: Data curation. Stefano Spinelli: Investigation, Data curation, Writing – review & editing. Chiara Romei: Investigation, Data curation, Writing – review & editing. Annalisa De Liperi: Writing – review & editing. Davide Chimera: Investigation, Data curation, Writing – review & editing. Francesco Pistelli: Writing – review & editing. Laura Carrozzi: Writing – review & editing. Francesco Corradi: Methodology, Writing – review & editing. Lorenzo Ghiadoni: Conceptualization, Formal analysis, Writing – review & editing.
Conflict of Interest
The authors declare no potential conflict of interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2022.12.002.
Contributor Information
Pisa COVID-19 Study Group:
Joanne Spataro, Margherita Malacarne, Elisabetta Addante, Sabrina Agostini o Degl'Innocenti, Paolo De Carlo, Alessio Gregori, Sara Manieri, Chiara Deri, Sara Perelli, Arianna Sabattini, Simonetta Salemi, Federica Volpi, Leonardo Colligiani, Salvatore Claudio Fanni, Laura Tavanti, Roberta Pancani, Massimiliano Desideri, Nicoletta Carpenè, Luciano Gabbrielli, Alessandro Celi, Antonio Fideli, Chiara Cappiello, Claudia Meschi, Luca Visconti, Giovanna Manfredini, and Ferruccio Aquilini
Appendix
Pisa COVID-19 Study Group: Joanne Spataro, Margherita Malacarne, Elisabetta Addante, Sabrina Agostini o Degl'Innocenti, Paolo De Carlo, Alessio Gregori, Sara Manieri, Chiara Deri, Sara Perelli, Arianna Sabattini, Simonetta Salemi, Federica Volpi, Leonardo Colligiani, Salvatore Claudio Fanni, Laura Tavanti, Roberta Pancani, Massimiliano Desideri, Nicoletta Carpenè, Luciano Gabbrielli, Alessandro Celi, Antonio Fideli, Chiara Cappiello, Claudia Meschi, Luca Visconti, Giovanna Manfredini, Ferruccio Aquilini.
Appendix. Supplementary materials
References
- 1.Pascarella G., Strumia A., Piliego C., et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge H., Wang X., Yuan X., et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39(6):1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GdSaHdCdBd SIAARTI. SIAARTI guidelines for admission to and discharge from Intensive Care Units and for limitation of treatment in intensive care. Minerva Anestesiol. 2003;69(3):111–118. 101-11. [PubMed] [Google Scholar]
- 4.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 5.Jalaber C., Lapotre T., Morcet-Delattre T., Ribet F., Jouneau S., Lederlin M. Chest CT in COVID-19 pneumonia: a review of current knowledge. Diagn Interv Imaging. 2020;101(7–8):431–437. doi: 10.1016/j.diii.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpicelli G., Gargani L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020;12(1):22. doi: 10.1186/s13089-020-00171-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpicelli G., Gargani L., Perlini S., et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med. 2021;47(4):444–454. doi: 10.1007/s00134-021-06373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetrugno L., Baciarello M., Bignami E., et al. The "pandemic" increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. doi: 10.1186/s13089-020-00185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetrugno L., Mojoli F., Boero E., et al. Level of diffusion and training of lung ultrasound during the COVID-19 pandemic - A National Online Italian Survey (ITALUS) from the Lung Ultrasound Working Group of the Italian Society of Anesthesia, Analgesia, Resuscitation, and Intensive Care (SIAARTI) Ultraschall Med. 2021 doi: 10.1055/a-1634-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargani L., Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25. doi: 10.1186/1476-7120-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vetrugno L., Sala A., Orso D., et al. Lung ultrasound signs and their correlation with clinical symptoms in COVID-19 pregnant women: the "PINK-CO" observational study. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.768261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hew M., Tay T.R. The efficacy of bedside chest ultrasound: from accuracy to outcomes. Eur Respir Rev. 2016;25(141):230–246. doi: 10.1183/16000617.0047-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpicelli G., Elbarbary M., Blaivas M., et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 14.Bouhemad B., Mongodi S., Via G., Rouquette I. Ultrasound for "lung monitoring" of ventilated patients. Anesthesiology. 2015;122(2):437–447. doi: 10.1097/ALN.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 15.Gargani L., Soliman-Aboumarie H., Volpicelli G., Corradi F., Pastore M.C., Cameli M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging. 2020;21(9):941–948. doi: 10.1093/ehjci/jeaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen M., Gaujard G., Guinot P.G., Bouhemad B., Group L.U.S.S. Using the lung ultrasound score to monitor disease progression for COVID-19-associated ARDS. Intensive Care Med. 2021;47(11):1329–1331. doi: 10.1007/s00134-021-06515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Píriz A., Tung-Chen Y., Jiménez-Virumbrales D., et al. Importance of lung ultrasound follow-up in patients who had recovered from coronavirus disease 2019: results from a prospective study. J Clin Med. 2021;10(14) doi: 10.3390/jcm10143196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gargani L., Sicari R., Raciti M., et al. Efficacy of a remote web-based lung ultrasound training for nephrologists and cardiologists: a LUST trial sub-project. Nephrol Dial Transplant. 2016;31(12):1982–1988. doi: 10.1093/ndt/gfw329. [DOI] [PubMed] [Google Scholar]
- 19.Clofent D., Polverino E., Felipe A., et al. Lung ultrasound as a first-line test in the evaluation of post-COVID-19 pulmonary sequelae. Front Med. 2021;8 doi: 10.3389/fmed.2021.815732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zieleskiewicz L., Markarian T., Lopez A., et al. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020;46(9):1707–1713. doi: 10.1007/s00134-020-06186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Q., Zhang Y., Wang H., et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363–1372. doi: 10.1016/j.acra.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbieri G., Cipriano A., Carrara S., et al. SARS-CoV-2 management in Emergency Department: risk stratification and care setting identification proposal based on first pandemic wave in Pisa University Hospital. Emerg Care J. 2021;17(4) [Google Scholar]
- 23.Falcone M., Tiseo G., Barbieri G., et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome Coronavirus 2 Pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7(12):ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coppelli A., Giannarelli R., Aragona M., et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 study. Diabetes Care. 2020 doi: 10.2337/dc20-1380. [DOI] [PubMed] [Google Scholar]
- 25.Barbieri G., Spinelli S., Filippi M., et al. COVID-19 pandemic management at the Emergency Department: the changing scenario at the University Hospital of Pisa. Emerg Care J. 2020 [Google Scholar]
- 26.Corradi F., Via G., Forfori F., Brusasco C., Tavazzi G. Lung ultrasound and B-lines quantification inaccuracy: B sure to have the right solution. Intensive Care Med. 2020;46(5):1081–1083. doi: 10.1007/s00134-020-06005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meroi F., Orso D., Vetrugno L., Bove T. Lung ultrasound score in critically ill COVID-19 patients: a waste of time or a time-saving tool? Acad Radiol. 2021;28(9):1323–1324. doi: 10.1016/j.acra.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corradi F., Ball L., Brusasco C., et al. Assessment of extravascular lung water by quantitative ultrasound and CT in isolated bovine lung. Respir Physiol Neurobiol. Jul 01 2013;187(3):244–249. doi: 10.1016/j.resp.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Corradi F., Vetrugno L., Isirdi A., Bignami E., Boccacci P., Forfori F. Ten conditions where lung ultrasonography may fail: limits, pitfalls and lessons learned from a computer-aided algorithmic approach. Minerva Anestesiol. 2022;88(4):308–313. doi: 10.23736/S0375-9393.22.16195-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.