Abstract
Protection against a lethal challenge infection of Plasmodium falciparum was elicited in malaria-naive Aotus vociferans monkeys by vaccination with the C terminus 19-kDa protein of the major merozoite surface protein (MSP-119) fused to tetanus toxoid universal T-cell epitopes P30 and P2. Three of four monkeys were protected against a 104-parasite challenge. Four monkeys were challenged with 105 parasites; one self-cured the infection, two were protected against high parasitemia (<2%) but were treated for severe anemia (hematocrit of <25%), and the fourth was not protected. In this model system, anemia appears to be a manifestation of incomplete protection (prolonged low-level parasitemia). Enzyme-linked immunosorbent assay (ELISA) antibody titers correlated with protection. Antibodies from some protected monkeys inhibited secondary processing of MSP-142 to MSP-133 and MSP-119. To mimic the repeated reinfections seen in regions where malaria is endemic, a second malaria parasite challenge was administered 4 months later. All P30P2MSP-119-vaccinated monkeys were protected; thus, a single challenge infection may underestimate vaccine efficacy. ELISA antibody titers correlated with protection against a second infection but had decreased compared to the first challenge. As most target populations for asexual blood-stage malaria vaccines will have been exposed to malaria parasites, a malaria parasite-exposed monkey was vaccinated with P30P2MSP-119. This monkey was completely protected, while a malaria parasite-naive P30P2MSP-119-vaccinated monkey self-cured a low-grade parasitemia. Prior malaria parasite infection primed the production of anti-native MSP-119 antibodies, which were boosted by vaccination with recombinant P30P2MSP-119. Preliminary data suggest that immunogenicity studies of vaccines designed for malaria parasite-exposed populations should also be conducted in malaria parasite-exposed subjects.
In the search for effective malaria vaccines, numerous monkey studies have been conducted to evaluate the efficacy of the major merozoite surface protein 1 (MSP-1) (7, 14, 16, 19, 26). The MSP-1 precursor molecule is processed to form a four-polypeptide complex on the merozoite surface (21). The C-terminal 42-kDa fragment (MSP-142) undergoes further processing to form MSP-133, which is shed (3, 6), and MSP-119, which remains on the merozoite surface and is taken into the newly invaded red blood cells (RBC) (2, 4). This secondary processing of MSP-142 is thought to be a prerequisite for RBC invasion (5). MSP-119 has a highly conserved amino acid sequence (22) and is composed of two epidermal growth factor (EGF)-like motifs (4). MSP-1, particularly MSP-119, has been favored for vaccine development for several reasons: antibodies raised to MSP-119 inhibit parasite invasion in vitro (2, 8, 9, 23); antibodies from human hyperimmune sera that has been affinity purified to MSP-119 inhibit parasite invasion in vitro (12); vaccination of mice with the analogous region of Plasmodium yoelii MSP-1 elicits complete protection against a lethal challenge infection (10, 20); and seroepidemiologic studies in humans demonstrate significant associations between presence of antibodies to the C-terminal end of MSP-1 and resistance to clinical malaria (1, 13, 24).
We and others have used Aotus monkeys as a model for testing asexual blood-stage vaccines because Aotus monkeys, like nonimmune humans, are completely susceptible to P. falciparum infection, develop life-threatening parasitemia and/or anemia, and remain susceptible to repeated infections. Vaccination with MSP-142 (7) and MSP-119 (19) have been successful in protecting these monkeys against a lethal challenge with falciparum malaria parasites. The present work is a continuation of our earlier study in which protection from a lethal challenge infection of P. falciparum by vaccination with P30P2MSP-119 (a fusion of MSP-119 with the universal tetanus toxoid P30 and P2 helper T-cell epitopes) was induced in Aotus nancymai but not in A. vociferans monkeys. The latter appear to be more susceptible to the FVO strain of P. falciparum than are A. nancymai. In this study, we investigate whether vaccine-induced protective immunity can be elicited in A. vociferans by increasing the number of vaccinations, rechallenging with malaria parasites several months later, and/or exposing the animals to malaria parasites prior to vaccination. We also test whether these changes in vaccination protocol can protect against a 10-fold increase in parasite challenge inoculum.
MATERIALS AND METHODS
Immunogens.
The recombinant proteins used here have been described elsewhere (19). In brief, proteins were produced by the Malaria Vaccine Development Unit (MVDU) in recombinant yeast, Saccharomyces cerevisiae. The addition of a histidine tag to the C terminus of these fusion proteins enabled purification on nickel-nitrilotriacetic acid-agarose. The MSP-119 sequence is from the Wellcome allelic prototype with the Q/KNG amino acid version in the four major dimorphic positions. P30P2MSP-119 fusion protein contains the P30 and P2 universal T-cell epitopes from tetanus toxoid (19). Protein concentrations were determined by bicinchoninic acid protein assay reagent (Pierce) by using bovine serum albumin (BSA) as the standard. The amino acid sequence of the amino terminus of P30P2MSP-119 was determined by automated Edman degradation (Biological Resources Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health). MVDU lot 960427 was used for vaccinations in the first trial, MVDU lot 970108 used for the rechallenge trial, and MVDU lot 970301-970228 mix was used for enzyme-linked immunosorbent assay (ELISA).
Control monkeys were vaccinated with the malaria transmission-blocking vaccine (TBV25H), which acts as an irrelevant yeast-produced immunogen in this study. TBV25H is a vaccine candidate based on Pfs25, which is the predominant surface protein of P. falciparum zygotes (18).
ELISA.
Microtiter plates (96-well; Immulon 4; Dynatech) were coated overnight at 4°C with proteins diluted in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3; pH 9.5). The saturating concentration of protein was from 0.5 to 2 μg/ml, depending on the antigen. Plates were washed three times in phosphate-buffered saline (PBS; pH 7.2–0.05% (vol/vol) Tween 20 (PBS/T; washing buffer), blocked with a 1% (wt/vol) solution of nonfat powdered milk in PBS/T (blocking buffer) for 5 h at room temperature, and then washed again. Dilutions of Aotus sera were also preincubated for 5 h at room temperature in blocking buffer. Sera (100 μl) diluted in blocking buffer were added to wells in doubling dilutions and incubated overnight at 4°C. After a washing, alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG; 100 μl diluted 1:1,000 in PBS/T; Kirkegaard and Perry Laboratories) was added to the plates and incubated for 3 h at room temperature, and the plate was washed again. Plates were developed with a solution of alkaline phosphatase substrate (catalog no. 104-105; Sigma Chemical Co.) diluted in carbonate buffer. After 15 min at room temperature, the reaction was stopped by the addition of 30 μl of 10 M NaOH. Plates were read at 410 nm.
Competition ELISAs were performed to determine whether antibodies from P30P2MSP-119-vaccinated monkeys could inhibit binding of MSP-119-specific monoclonal antibodies (MAbs). Plates were coated with P30P2MSP-119 and blocked as described above. Plates were preincubated for 5 h at room temperature with doubling dilutions of Aotus sera starting at the saturating concentration. Washed plates were then incubated at 4°C overnight with MAbs diluted to saturating concentration. After a washing, plates were incubated with goat anti-mouse IgG (1:1,000 in PBS/T, 3 h, room temperature) and further processed as described above. The following MSP-119-specific murine MAbs were used: 2.2, 7.5, 12.8, and 12.10 (21); 111.2 and 111.4 (17); and 5.2 (25).
Disassociation ELISAs were performed to measure antibody avidity for the vaccinating immunogen, P30P2MSP-119. Monkey sera were diluted as described above and incubated with antigen-coated and blocked plates overnight at 4°C. Plates were washed and incubated with 0 to 6 M NH4SCN for 20 min at room temperature, washed, and then incubated with goat anti-human IgG and further processed as described above.
IgG isotype ELISA were not measured for Aotus antibodies due to a lack of validated cross-reactive human subclass reagents.
Immunofluorescence assays.
Immunofluorescence assays were performed with methanol-fixed P. falciparum parasites of the FVO strain. Air-dried parasites on toxoplasmosis slides (Bellco Glass, Inc.) were fixed with dry ice-cold methanol for 15 min, washed with PBS, and blocked with 3% BSA in PBS for 1 h. A 1:1,000 dilution of Aotus sera in 1% BSA-PBS was preabsorbed to washed human RBC for 1 h at room temperature. Wells were then incubated with 10 μl of dilutions of preabsorbed Aotus sera overnight at 4°C in a humid chamber. After a washing with PBS, 10 μl of fluorescein isothiocyanate-conjugated goat anti-human IgG (Cappel) at a 1:200 dilution in PBS was added to wells and incubated for 2 h at room temperature in the dark. After a washing with PBS, the slides were read by fluorescent light microscopy.
Western blot analysis.
For Western blot analysis, protein samples were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 4 to 20% gels (Novex Experimental Technology) electrophoretically transferred to nitrocellulose membranes. The membranes were blocked for 1 h at room temperature in blocking buffer (1% [wt/vol] solution of nonfat powdered milk in PBS/T). Aotus sera dilutions were also blocked for 1 h at room temperature in blocking buffer. Blots were then incubated with antibody diluted (1:500) in blocking buffer for 2 h at room temperature. After three washes in wash buffer, blots were incubated with alkaline phosphatase-conjugated goat anti-human IgG diluted 1:1,000 in blocking buffer for 1 h at room temperature and then washed three times. The protein bands were visualized by incubation with Western Blue (substrate for alkaline phosphatase; Promega).
Secondary processing assay.
This assay has been described in detail elsewhere (15). Parasites of the FCB-1 strain were used in this assay, as they have the same amino acid sequence as FVO (Wellcome allelic prototype) in the C-terminal end of MSP-1 and have been culture adapted (3). In brief, purified merozoites were washed in EGTA or EDTA in the presence of protease inhibitors to prevent secondary processing of MSP-1 while removing soluble MSP-133. Merozoites were then incubated on ice with a 1:10 dilution of test antibody to enable antibodies to bind while not allowing any MSP-1 proteolysis to occur. After 15 to 30 min, the samples were transferred to 37°C (a temperature at which MSP-1 processing is permissible). After 1 h, the samples were solubilized by Nonidet P-40 or Triton X-100 and subjected to SDS–12.5% PAGE. Gels were blotted onto nitrocellulose paper, and the membrane was probed with rabbit anti-MSP-133 and then labeled with radioiodinated anti-rabbit IgG. Bands were visualized by autoradiography. The autoradiograph was aligned with the blot, and the appropriate section was excised and counted in a gamma counter.
Immunization and parasite challenge of Aotus monkeys: first malarial infection.
Monkeys were housed at the Primate Research Facility, National Institutes of Health, in accordance with The Guide for the Care and Use of Laboratory Animals. Monkeys were stratified by weight and sex and randomly assigned to four groups by card draw. Grouping and group assignment were masked to investigators who cared for or vaccinated the animals, read smears, or determined when a monkey should be drug cured. Only when all control monkeys had been treated was the code revealed to these investigators.
Sixteen A. vociferans were used in the study. Four groups of four monkeys were selected. Two groups were vaccinated with P30P2MSP-119, and the other two groups were vaccinated with TBV25H, a control yeast-produced antigen.
Monkeys received 200 μg of the respective purified yeast-secreted recombinant protein per vaccination. Monkeys received four vaccinations, each 3 weeks apart. The first vaccination was an emulsion of 200 μl of antigen (in 200 μl of PBS) with 200 μl of complete Freund adjuvant (CFA) given subcutaneously at four sites on the back; the next two vaccinations were emulsions in incomplete Freund adjuvant (IFA) given as before, and the fourth was given intramuscularly in PBS in one site in a total volume of 200 μl. During the vaccination period, five monkeys in the control groups died of causes common to Aotus monkeys (congestive heart failure and nephritis). To compensate for these losses, four unvaccinated monkeys were added to the control groups prior to challenge infection. Monkey 2521 was inadvertently given P30P2MSP-119 at the third vaccination instead of TBV25H. Sera were obtained on vaccination days and on the day of challenge.
Ten days after the fourth vaccination, an A. vociferans donor monkey (2544) was infected intravenously with approximately 106 freshly thawed P. falciparum parasites of the FVO strain from a frozen sample from monkey 1588 (a frozen sample from monkey A1-936, kindly provided by W. E. Collins, Centers for Disease Control and Prevention, was passaged through monkey A11, which was used to infect monkey 1588). Four days later, a 3% parasitemia had been reached in the donor monkey; blood was collected, washed, and diluted in RPMI to 104 and 105 parasitized RBCs (pRBCs)/ml. The donor monkey then was drug cured with mefloquine. Monkeys from one P30P2MSP-119 group and one control group were each challenged by intravenous infusion of 1 ml of 104 pRBCs/ml, and monkeys in the remaining two groups were each challenged with 1 ml of 105 pRBCs/ml. The challenge infection was administered 14 days after the last vaccination.
Hematocrit and Giemsa-stained thin smears were made from blood collected by puncture of superficial veins in the dorsum of the calf. Hematocrits were taken biweekly; the plasma portions from hematocrits were retained for antibody analysis and the blood portion archived for later parasite analysis. Blood smears were taken from each monkey on challenge day 0 and then daily from day 3 until they were treated up to day 44. After chemotherapy, blood smears were taken daily until there was no detectable parasitemia for 3 consecutive days and then once weekly until the end of the trial. Monkeys were drug cured with 50 mg of mefloquine given orally at a parasitemia of ≥5% or a hematocrit of <25%. All untreated monkeys were given chemotherapy on day 44. Parasitemia was calculated based on examination of approximately 2,000 RBCs (equivalent to 10 high-power fields); if no parasites were seen, then 40 more high-power fields were examined.
Immunization and parasite challenge of Aotus monkeys: rechallenge infection.
Monkeys underwent a second malaria parasite challenge 4 months after the first infection. Three unvaccinated monkeys (2584, 2592, and 2594) that were control monkeys in the last challenge (i.e., parasite exposed) were vaccinated with P30P2MSP-119. Two new parasite-naive A. vociferans monkeys (2573 and 2583) were vaccinated with P30P2MSP-119, and two new parasite-naive A. vociferans monkeys (2575 and 2589) were vaccinated with TBV25H as positive and negative controls, respectively. All vaccinations were done as described above. Monkey 2584 died after the first vaccination, and monkey 2583 died after the second vaccination, both of congestive heart failure, which is a common cause of death in Aotus monkeys. A. vociferans donor monkey T619 was infected with approximately 106 thawed parasites from a frozen stock from the infection of the A. vociferans donor monkey 2544 in the first challenge experiment. Six days after the donor monkey was infected, a 7% parasitemia was reached; blood was collected, washed and diluted in RPMI to 105 pRBCs/ml and used to infect the rest. The donor monkey then received a drug cure. Blood smears, hematocrits, and chemotherapy were carried out as before. All untreated monkeys were given curative chemotherapy on challenge day 33. Monkey 2594 died 3 days after challenge infection. This was too early after challenge to be due to malaria, and in autopsy it was found that this monkey also died of congestive heart failure.
RESULTS
Malaria-naive vaccinated monkeys-antibody responses measured by ELISA.
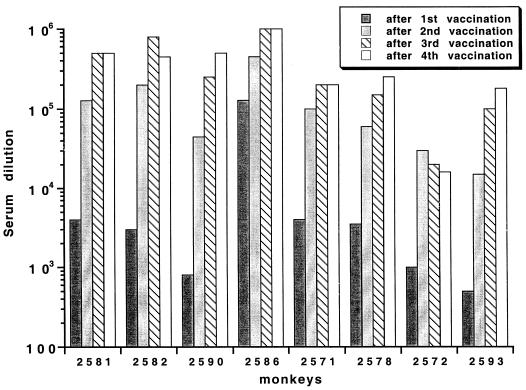
P30P2MSP-119-specific antibodies, with an optical density (OD) of approximately 1.0 at a 1:1,000 dilution of serum, are detectable in the majority of P30P2MSP-119-vaccinated monkeys 3 weeks after the first vaccination. Nearly identical results were obtained in all cases in which the titer of the serum was determined against yMSP119 rather than P30P2MSP-119 as the plate antigen (data not shown). Antibody responses are boosted by the second and third vaccinations (Fig. 1); however, there is very little boosting of the antibody response after a fourth vaccination.
FIG. 1.
P30P2MSP-119 antibody titers that give an OD of 1 (which is on the linear part of the titration curve for the majority of sera) using P30P2MSP-119 as the plate antigen. All bleeds before immunization are negative (data not shown). Similar results were obtained using recombinant yMSP-119 (19) as the plate antigen (data not shown).
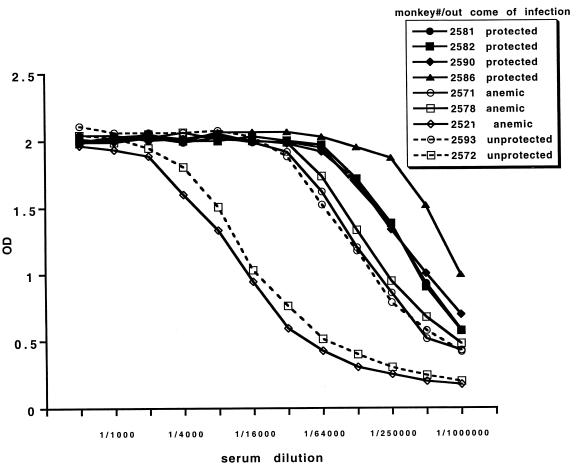
Unlike the previously published trial (19), antibody titers on the day of challenge in P30P2MSP-119-vaccinated A. vociferans monkeys do correlate with protection (Jonckheere-Terpstra nonparametric rank test of independence of two variables; P < 0.002) (Table 1; Fig. 2). Protected monkeys have the highest antibody titers (an OD of 1 at a dilution of 1/450,000 to 1/1,000,000), while anemic monkeys have intermediate titers (1/200,000 to 1/250,000) and unprotected monkeys have the lowest antibody titers (1/16,000 to 1/180,000). Control monkey 2521, which inadvertently received a single P30P2MSP-119 inoculation in IFA at the third vaccination, has a low antibody titer (1/16,000) to P30P2MSP-119.
TABLE 1.
The relationship between the course of infection and antibody assays in malaria-naive animals
| Vaccine | Monkey no. | Parasite challenge inoculation | Prepatent period (days) | % Peak parasitemiaa | Day of cure
|
Course of infection | Prechallenge sera
|
Secondary processing assaye
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dayb | % Parasitemia | % Hematocrit | ImFc | ELISAd | 1st | 2nd | ||||||
| P30P2MSP-119 | 2581 | 104 | 9 | 0.05 | Self-resolved | 105 | 1/500,000 | +++ | + | |||
| 2582 | 104 | 20 | 0.05 | Self-resolved | 105 | 1/450,000 | +++ | ++ | ||||
| 2590 | 104 | 8 | 0.05 | Self-resolved | 106 | 1/500,000 | +++ | +++ | ||||
| 2593 | 104 | 8 | 4.3 | 18 | 4.3 | 30 | Virulent | 105 | 1/180,000 | − | ++ | |
| 2586 | 105 | 12 | 0.05 | Self-resolved | 106 | 1/1,000,000 | +++ | +++ | ||||
| 2571 | 105 | 4 | 1.2 | 22 | 1.2 | 21 | Anemic | 106 | 1/200,000 | − | − | |
| 2578 | 105 | 11 | 0.1 | 17 | 22 | Anemic | 105 | 1/250,000 | +++ | +++ | ||
| 2572 | 105 | 5 | 6.0 | 10 | 6.0 | 48 | Virulent | 104 | 1/16,000 | − | − | |
| TBVg | 2521f | 104 | 10 | 0.2 | 11 | 0.2 | 19 | Anemic | 104 | 1/16,000 | − | − |
| TBV | T580 | 104 | 8 | 5.0 | 12 | 5.0 | 39 | Virulent | 103 | 1/700 | − | − |
| None | 2570 | 104 | 6 | 6.2 | 11 | 6.2 | 44 | Virulent | − | − | ||
| None | 2584 | 104 | 5 | 5.3 | 12 | 5.2 | 55 | Virulent | − | − | ||
| TBV | T562 | 105 | 6 | 6.9 | 15 | 6.9 | 30 | Virulent | 103 | 1/700 | − | − |
| None | 2592 | 105 | 4 | 5.9 | 9 | 5.9 | 44 | Virulent | − | − | ||
| None | 2594 | 105 | 6 | 5.0 | 9 | 5.0 | 46 | Virulent | − | − | ||
Parasitemia of 0.05% indicates that a total of one or two parasites were seen in the 50 high-power light microscopy fields of a blood smear.
Cured all remaining monkeys on day 44.
ImF, immunofluorescence for pRBCs.
ELISA titration on P30P2MSP-119; titers giving an OD of 1, which is on the linear part of the titration curve.
Secondary processing assay: −, no detectable processing inhibition; +/−, just-detectable inhibition; +, significant inhibition; ++, strong inhibition, +++, apparently complete inhibition.
Control monkey 2521 received one vaccination of P30P2MSP-119 instead of TBV25H at the third vaccination.
TBV, TBV25H.
FIG. 2.
Titration of serum on the day of challenge on P30P2MSP-119. Solid symbols, protected; open symbols, unprotected. Open symbol-solid line, drug cured for anemia; open symbol-dashed line, drug cured for parasitemia. Monkey 2521 is a control monkey that received one vaccination of P30P2MSP-119.
Antibody titers of postchallenge plasma are not boosted to P30P2MSP-119 in the protected monkeys during the infection (data not shown). In fact, after malaria parasite challenge antibody responses slightly decrease in all of the P30P2MSP-119-vaccinated monkeys; while control monkeys developed antibodies to MSP-119 (measured by ELISA to P30P2MSP-119), probably due to exposure to parasite-produced antigen during the course of the infection (data not shown).
Malaria-naive vaccinated monkeys—outcome of infection.
Malaria-naive, unvaccinated control monkeys experienced fulminant infections that would have been fatal if not treated (Table 1; Fig. 3). Upon challenge, the prepatent period was 5 to 10 days for control monkeys that received 104 parasites and 4 to 6 days for control monkeys that received 105 parasites. All control monkeys, except 2521, were treated for parasitemia between days 9 and 15. Control monkey 2521, which inadvertently received a single P30P2MSP-119 inoculation, was the only control monkey that was treated for anemia rather than for parasitemia. However, this monkey was anemic at the beginning of the study, and the monkey was cured as its initial hematocrit of 25% dropped to 19%. This monkey also had the longest prepatent period of the control monkeys, suggesting that even a single vaccination with P30P2MSP-119 in IFA has some protective effect. Control monkeys that were vaccinated with TBV25H had slightly longer prepatent periods and were drug cured slightly later than control monkeys that received no vaccine. This suggests that there is a slight, nonspecific protective effect of either the control antigen, a common yeast contaminant, and/or the adjuvant (CFA and/or IFA). There is antibody cross-reactivity to TBV25H in P30P2MSP-119-vaccinated monkeys (data not shown); likewise, TBV25H-vaccinated monkeys develop antibodies that recognize P30P2MSP-119 (Table 1). This cross-reactivity may reflect the similar structure of these two proteins (both contain EGF-like motifs). There is no antibody cross-reactivity for a control yeast-produced antigen glutathione S-transferase which does not contain EGF-like motifs (data not shown). Also, sera from TBV25H-vaccinated monkeys prior to malaria challenge have a weak immunofluorescence response to pRBCs, while sera from unvaccinated monkeys and from monkeys before vaccination do not recognize pRBCs (Table 1).
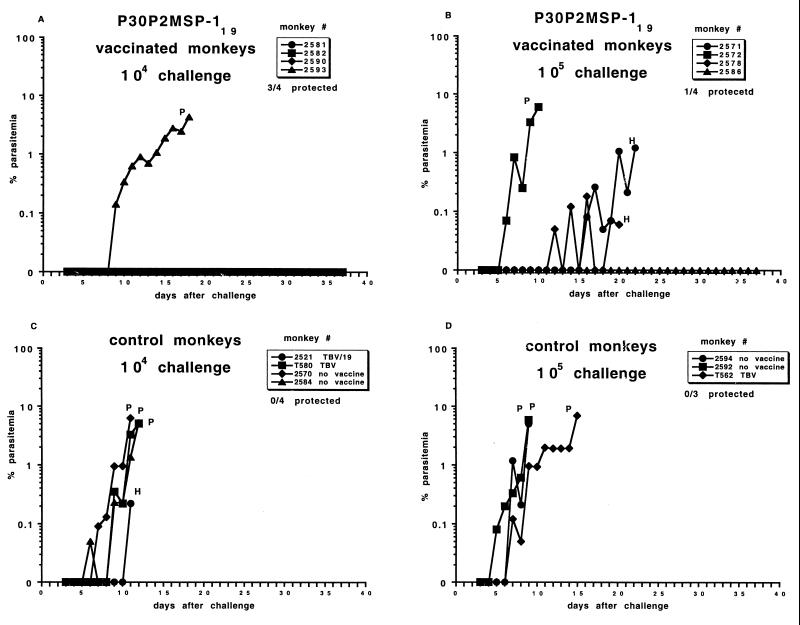
FIG. 3.
Course of infection of FVO P. falciparum in A. vociferans. (A) Vaccinated with P30P2MSP-119, challenged with 104 parasites. (B) Vaccinated with P30P2MSP-119, challenged with 105 parasites. (C) Control monkeys challenged with 104 parasites. (D) Control monkeys challenged with 105 parasites. P, drug cured for parasitemia above 5%; H, drug cured for hematocrit of <25%. Parasitemia of 0% can indicate that one or two parasites were seen in 50 high-power light microscopy fields of a blood smear.
Three of the four P30P2MSP-119-vaccinated monkeys challenged with 104 parasites were protected (only one or two parasites were seen in the blood smears in 30 days). The one monkey (animal 2593) that required treatment for high parasitemia was drug-cured on day 18, 6 days later than the equivalent controls.
One of the four P30P2MSP-119-vaccinated monkeys (animal 2586) challenged with 105 parasites was protected and had a prepatent period that was 6 days longer than for the control monkeys. Two monkeys (animals 2571 and 2578) had low parasitemia for several days and were drug cured for anemia on days 20 and 22, 5 to 7 days later than the equivalent controls. Monkey 2578 had a prepatent period 5 days greater than for the equivalent control monkeys, while monkey 2571 had a prepatent period similar to that for the control monkeys. Monkey 2572 was unprotected and had a prepatent period and day of drug cure similar to that for the control monkeys.
Rechallenge of vaccinated monkeys—antibody responses measured by ELISA.
Four months after the initial challenge, six P30P2MSP-119-vaccinated monkeys from the previous infection underwent a second challenge infection with 105 parasites (Table 2). Two control monkeys from the previous challenge infection, one vaccinated with TBV25H (animal T562) and the other unvaccinated (animal 2570), were also rechallenged.
TABLE 2.
Relationship between the course of infection and antibody assays in malaria-naive, -exposed, and/or -rechallenged animals
| Vaccine
|
Monkey no. | Prepatent period (days) | % Peak parasitemiaa | Day of cure
|
Course of previous infection | Course of this infection | Prechallenge sera
|
Secondary processing assaye
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | Dayb | % Parasitemia | % Hematocrit | ImFc | ELISAd | 1st | 2nd | |||||
| 19 | None | 2581 | 13 | 0.05 | Protected | Protected | >106 | 1/100,000 | ++ | ++ | |||
| 19 | None | 2582f | 18 | 0.05 | Protected | Protected | >106 | 1/200,000 | +++ | +++ | |||
| 19 | None | 2586 | 13 | 0.05 | Protected | Protected | >106 | 1/500,000 | ++(+) | +++ | |||
| 19 | None | 2578 | 15 | 0.05 | Anemic | Protected | 105 | 1/70,000 | ++(+) | +++ | |||
| 19 | None | 2593 | 14 | 0.1 | Virulent | Self-resolved | 106 | 1/32,000 | +/− | +/− | |||
| 19 | None | 2572 | 7 | 0.4 | Virulent | Self-resolved | 105 | 1/16,000 | +/− | +/− | |||
| None | 19 | 2592 | 19 | 0.05 | Virulent | Protected | 105 | 1/350,000 | ++ | ++ | |||
| TBVg | None | T562 | 9 | 0.8 | 21 | 0 | 24 | Virulent | Anemic | 104 | 1/1,000 | − | − |
| None | None | 2570 | 6 | 5.3 | 12 | 5.3 | 41 | Virulent | Virulent | 104 | − | − | |
| New | 19 | 2573 | 16 | 0.2 | Self-resolved | 105 | 1/350,000 | +/− | ++ | ||||
| New | TBV | 2575 | 6 | 10.9 | 12 | 10.9 | 37 | Virulent | 103 | 1/8,000 | ND | ND | |
| New | TBV | 2589 | 7 | 5.5 | 12 | 5.5 | 41 | Virulent | 104 | 1/1000 | ND | ND | |
Parasitemia of 0.05% indicates that a total of one or two parasites were seen in the 50 high-power light microscopy fields of a blood smear.
ImF, immunofluorescence for pRBCs.
ELISA titration on P30P2MSP-119; titers giving an OD of 1, which is on the linear part of the titration curve.
Secondary processing assay: −, no detectable processing inhibition; +/−, just-detectable inhibition; +, significant inhibition; ++, strong inhibition; +++, apparently complete inhibition.
Monkey 2582 died on day 20 due to heart seizure (not malaria related).
TBV, TBV25H (see Materials and Methods).
The control monkey that received no vaccine (animal 2570) had no antibodies to P30P2MSP-119 on the day of the first malaria parasite infection, while the control monkey that received TBV25H (animal T562) had a very low antibody titer response to P30P2MSP-119. On the day of the second challenge infection, antibody responses in these monkeys had slightly increased, possibly due to exposure to naive MSP-119 during the first malaria parasite infection; however, antibody responses of the P30P2MSP-119-vaccinated monkeys, with the exception of monkey 2572, decreased (by various amounts) on the day of the second challenge infection compared with 4 months earlier on the day of the first challenge infection (Fig. 4).
FIG. 4.
Comparison of P30P2MSP-119 antibody titers from serum on the day of challenge in the first challenge infection versus the second infection 4 months later. Dilution of serum to give an OD of 1 (which is on the linear part of the titration curve) is plotted for the two time periods. Solid symbol, previously protected; open symbol, previously unprotected; open symbol-solid line, previously drug cured for anemia; open symbol-dashed line, previously drug cured for parasitemia.
Although there was a decrease in P30P2MSP-119-specific antibody titers between the time of the first and second challenge, there was an increase in the percentage of monkeys protected. Nevertheless, ELISA antibody titers negatively correlate with the peak parasitemia observed during the second infection (Jonckheere-Terpstra nonparametric rank test of independence of two variables; P < 0.03) (i.e., monkeys 2593 and 2572, with the lowest antibody titers, developed a low-grade parasitemia). Of interest, titers had dropped at the time of the second challenge to a level that almost certainly would not have afforded the same level of protection in the first infection (Fig. 4).
Rechallenge of vaccinated monkeys—outcome of infection.
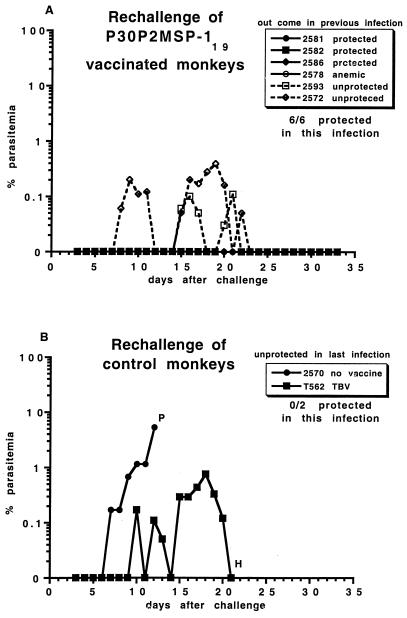
All six P30P2MSP-119-vaccinated monkeys were protected against a second infection (Table 2; Fig. 5). P30P2MSP-119-vaccinated-monkeys that self-resolved the infection or were anemic in the first challenge infection had extended prepatent periods and were protected in rechallenge (only one or two parasites were seen in their blood smears in 30 days). The two P30P2MSP-119-vaccinated-monkeys that were not protected during the first challenge infection (animals 2593 and 2572) developed a low-grade parasitemia in rechallenge that resolved without drug treatment.
FIG. 5.
Course of rechallenge infection with 105 FVO P. falciparum in A. vociferans monkeys (A) vaccinated with P30P2MSP-119. Solid symbol, previously protected; open symbol, previously unprotected; open symbol-solid line, previously drug cured for anemia; open symbol-dashed line, previously drug cured for parasitemia. (B) Control monkeys; 2570, unvaccinated; and T562, vaccinated with TBV25H. P, drug cured for parasitemia of >5%; H, drug cured for hematocrit of <25%. Parasitemia of 0% can indicate that one or two parasites were seen in 50 high-power light microscopy fields of a blood smear.
The unvaccinated monkey from the previous challenge infection (animal 2570) was unprotected against a second challenge infection. There was no reduction in the prepatent period or day of drug cure compared with malaria-naive, TBV25H-vaccinated control monkeys (for controls, see Fig. 6B). The TBV25H-vaccinated monkey from the previous challenge infection (T562) had a slightly prolonged prepatent period of 9 days and appeared to self-resolve its parasitemia but was drug cured on day 21 for anemia.
FIG. 6.
Course of infection of a 105-parasite challenge infection of FVO P. falciparum in A. vociferans monkeys. (A) Malaria parasite-exposed P30P2MSP-119, vaccinated (animal 2592); malaria parasite-naive P30P2MSP-119, vaccinated (animal 2573). (B) Malaria parasite-naive control monkeys vaccinated with TBV25H (animals 2575 and 2589). P, drug cured for parasitemia of >5%; H, drug cured for hematocrit of <25%. Parasitemia of 0% can indicate that one or two parasites were seen in 50 high-power light microscopy fields of a blood smear.
Malaria-exposed versus malaria-naive vaccinated monkeys—antibody responses measured by ELISA.
To address the question of the importance of prior malaria exposure on vaccination, three unvaccinated monkeys from the previous challenge infection were vaccinated with P30P2MSP-119; however, one monkey died after the first vaccination (animal 2584), and one monkey died 3 days after the challenge infection (animal 2594; too soon after challenge for the cause of death to be due to fulminant malaria). Three new malaria-naive monkeys, one vaccinated with P30P2MSP-119 (animal 2573) and two with TBV25H (animals 2575 and 2589), were challenged with 105 parasites (see Table 2).
After malaria parasite exposure, antibody titers in unvaccinated monkeys (animals 2592 and 2594) were similar to that in the malaria parasite-naive monkey 2573 after the single vaccination (Fig. 7; antibody titration not shown for monkey 2594, as it is very similar to that of 2592). After a single vaccination of the malaria parasite-exposed monkeys (animals 2592 and 2594), the antibody titers were equivalent to those in the malaria parasite-naive monkey (animal 2573) vaccinated two or more times. Subsequent vaccinations of the malaria parasite-exposed monkeys did not appreciably increase P30P2MSP-119 antibody titers boosted by the first vaccination.
FIG. 7.
Titration on P30P2MSP-119 comparing the antibody response of a malaria parasite-naive monkey vaccinated with P30P2MSP-119 (animal 2573) (A) versus a malaria parasite-exposed monkey vaccinated with P30P2MSP-119 (animal 2592) (B). Titration curves from the third and fourth vaccinations are not shown, as there is only slight boosting from the previous vaccination.
Malaria-exposed versus malaria-naive vaccinated monkeys—outcome of infection.
The malaria-exposed P30P2MSP-119-vaccinated monkey (animal 2592) had a long prepatent period (19 days) and was protected (only one or two parasites were seen in its blood smears in 30 days) (Fig. 6A; Table 2). The malaria-naive P30P2MSP-119-vaccinated monkey (2573) had a delayed prepatent period (16 days; 9 to 10 days greater than the controls) and a low-grade parasitemia that it was able to self-cure. The two malaria-naive TBV25H-vaccinated monkeys (animals 2575 and 2589) were not protected and had to be drug cured for parasitemia (Fig. 6B; Table 2).
In vitro correlates of protective immunity.
In an effort to identify an in vitro correlate of protection, antibody responses were measured in four assays. In none of the four assays could we detect a correlation with protection (data not shown): (i) competition ELISAs with sera from P30P2MSP-119-vaccinated monkeys against MSP-119-specific MAbs; (ii) avidity of antibody for the vaccinating antigen; (iii) Western blot of P30P2MSP-119—all bands are recognized by sera from all P30P2MSP-119-vaccinated monkeys; and (iv) immunofluorescence to pRBCs (Tables 1 and 2).
Sera from P30P2MSP-119-vaccinated monkeys that were protected against infection inhibit the secondary processing of MSP-142 to MSP-133 and MSP-119, while sera from unprotected monkeys (P30P2MSP-119-vaccinated monkeys and controls) do not inhibit (Table 1). The assay was performed twice, and there are some differences between the two independent runs of the assay. The correlation with protection is not as robust in the second assay, as serum from one unprotected monkey had processing-inhibitory activity in the second assay, while sera from two of the protected monkeys did not inhibit as strongly as in the first assay. These results suggest that there is good sensitivity but imperfect specificity in the association of secondary processing activity with protection. The assay does not distinguish between monkeys that were drug cured for anemia rather than for parasitemia: serum from one anemic monkey (animal 2578) inhibited processing, while serum from the other anemic monkey (animal 2571) did not. Secondary processing was inhibited by sera collected on the day of rechallenge from P30P2MSP-119-vaccinated monkeys that were protected against second infection, but sera collected from the partially protected monkeys that self-cured a low-grade parasitemia did not inhibit secondary processing (Table 2).
DISCUSSION
Previous studies in our laboratory have demonstrated protective immunity in A. nancymai monkeys vaccinated with P30P2MSP-119 but not in A. vociferans (19). In this study, P30P2MSP-119 vaccination protected A. vociferans against an otherwise lethal infection, indicating that protection is not Aotus species specific. Some protection was even detected when the parasite challenge was increased 10-fold. In previous trials with A. nancymai monkeys, some protected monkeys had low antibody titers, while some unprotected monkeys had high titers (19). Data from subsequent monkey studies indicate that antibody titers correlate with protection in A. vociferans monkeys but not in A. nancymai (unpublished observations), suggesting that the mechanisms of immunity differ between A. vociferans and A. nancymai monkeys.
The exact explanation for the protective immunity induced in A. vociferans in the present trial, compared with the previous trial, cannot be precisely identified, since there are multiple differences between the previous and present study. The two major variables that may explain the protection observed in the present study are (i) the immunogen used and (ii) the number and/or schedule of vaccinations. Recent studies by R. Shimp and D. C. Kaslow have demonstrated that fermentation conditions can change the ratio of conformers of P30P2MSP-119 produced (personal communication); therefore, variation in the immunogen used in these studies may account for the differences in protection obtained. Giving a fourth vaccination of soluble antigen in PBS intramuscularly did not boost the antibody titer between the third and fourth vaccinations but may have contributed to changes in antibody avidity, fine specificity, or in isotype switching. In addition, by giving a fourth vaccination, the day of challenge was delayed 3 weeks, which may have allowed time for the immune response to mature.
Vaccine efficacy can be strikingly different if determined by a single challenge or by reinfection. In rechallenge infection, all the P30P2MSP-119-vaccinated monkeys were protected, with only the monkeys unprotected in the first infection developing a low-level parasitemia. This is particularly encouraging for vaccine development with P30P2MSP-119, as repeated challenge will occur in areas where malaria is endemic. Boosting of total antibody to the vaccinating antigen may not be important for protection, since there was no boosting of the antibody response from the first to the second infection upon rechallenge; rather, a change in antibody isotype, specificity or avidity, cytokine profile, cell-mediated immunity, or an immune response to another antigen may come into effect. Such a phenomenon has been noted with other vaccines against blood-stage malaria parasites. For example, Deans et al. observed that monkeys vaccinated with apical merozoite antigen-1 were not protected against the first malaria parasite challenge infection but were completely protected against a second infection (11). The effectiveness of vaccines may be underestimated if the measure of efficacy is solely determined after a single challenge.
Prior exposure to malaria parasites primed the production of anti-native MSP-119 antibodies, which were further boosted by vaccination with recombinant P30P2MSP-119. Upon subsequent challenge, the malaria parasite-exposed vaccinated monkey appears to be better protected than the malaria parasite-naive vaccinated monkey. Unfortunately, limited access to healthy Aotus monkeys and non-malaria-related death of monkeys due to their fragility to handling during the trial resulted in a low number of animals per group, which compromised our ability to make firm conclusions from these studies. However, these preliminary data suggest that (i) vaccines should be evaluated in malaria parasite-exposed animals and (ii) vaccine efficacy determined in malaria parasite-naive volunteers may underestimate the effectiveness of vaccines in malaria parasite-exposed individuals. Whether prior malaria parasite exposure can overcome the requirement for CFA and reduce the number of vaccinations is currently being studied.
The mechanism of immunity in P30P2MSP-119-vaccinated monkeys is unknown. Antibodies from protected monkeys are able to inhibit the secondary processing of MSP-142 to MSP-133 and MSP-119, which is thought to be a prerequisite for RBC invasion (5); however, in rechallenge, antibodies from two monkeys (unprotected against the first infection) that self-cured a low-grade parasitemia exhibited little processing-inhibitory activity (Table 2). These data suggest that other mechanism(s) of immunity contribute to protection. Furthermore, the correlation observed between antibody titer and processing-inhibitory activity (Tables 1 and 2) may not reflect a causal relationship with protection.
In some areas where it is endemic, malaria tends to kill semi-immune children by anemia rather than by high parasite density (27). P30P2MSP-119 vaccination protects against a 104 parasite infection, but it only protects against parasitemia, not anemia, in a 105-parasite challenge infection, suggesting that anemia is a low-grade form of protection. The TBV25H-vaccinated monkey (animal T562) was protected against parasitemia in rechallenge infection but was drug cured for anemia, while an unvaccinated monkey (animal 2570) underwent a virulent infection. This suggests that anemia is a result of partial protection (i.e., with TBV25H vaccination in CFA) and may not necessarily be due to the P30P2MSP-119 vaccine. Antiparasite immunity is not equivalent to antidisease immunity; in fact, animals protected from high parasitemia become severely anemic and would die if not drug cured. Despite the very low parasitemia, when anemic monkeys receive antimalarial drug cure, the hematocrit increases and returns to normal within 2 to 3 weeks, indicating that their anemia is clearly associated with malaria parasite infection even though parasites are not detectable in the peripheral blood. Possible causes of this phenomenon include (i) sequestered parasites causing hemolytic anemia due to parasite antigens coating uninfected RBCs; (ii) sequestered parasites, in the bone marrow or elsewhere, invading or destroying erythroid precursor or downregulating erythropoesis, respectively; or (iii) failure to detect the peak of parasitemia of highly synchronized parasites due to time points chosen to monitor infection. It is possible that vaccination of nonimmune humans with P30P2MSP-119 will result in complete protection in some and partial protection in others, the latter resulting in anemia similar to that seen in semi-immune children living in areas where malaria is endemic. Whether vaccination may be beneficial in partially protected children by inducing complete protection against disease remains to be determined. In a subsequent study, monkeys with partial protection against infection (but drug cured for anemia) were completely protected against a second infection after vaccination with MSP-119 (manuscript in preparation).
Vaccination with P30P2MSP-119 can result in nearly complete protection against a 104-parasite challenge infection and can give partial protection against a 10-fold-higher parasite challenge inoculum. Monkeys that are not protected against the first infection are protected against a second. This demonstrates that the evaluation of blood-stage vaccines for protection of humans who are residents of areas where malaria is endemic should be measured by more than one challenge infection. Data presented here suggest that previous malaria parasite exposure elicits T-cell help to native MSP-119, which can be boosted by vaccination with recombinant P30P2MSP-119. In malaria-exposed individuals, antibody only needs to be boosted by, rather than primed by, vaccination. The protection afforded in this study was generated by using CFA, which is unacceptable for use in humans; however, it is possible that a more-effective delivery system and a less-potent adjuvant would be effective in malaria parasite-exposed animals that have already been primed to MSP-119 by native antigen.
ACKNOWLEDGMENTS
We thank William E. Collins for providing FVO parasites, David B. Keister for assistance in maintaining frozen stocks of parasites, Mark Garfield for N-terminal amino acid sequencing, Cherise Fenton for technical support in animal handling, Jose A. Guevara Patino for optimizing the processing assay, Mark VanRaden for statistical analysis, and Louis H. Miller for advice and support of this project.
REFERENCES
- 1.Al-Yuman F, Genton B, Anders R F, Falk M, Triglia T, Lewis D, Hii J, Beck H-P, Alpers M. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-1 and malaria morbidity in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg. 1994;51:593–602. doi: 10.4269/ajtmh.1994.51.593. [DOI] [PubMed] [Google Scholar]
- 2.Blackman M J, Heidrich H-G, Donachie S, McBride J S, Holder A A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman M J, Holder A A. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP-1) by a calcium-dependent membrane-bound serine protease: shedding of MSP-133 as a noncovalently associated complex with other fragments of the MSP-1. Mol Biochem Parasitol. 1992;50:307–316. doi: 10.1016/0166-6851(92)90228-c. [DOI] [PubMed] [Google Scholar]
- 4.Blackman M J, Ling I T, Nicholls S C, Holder A A. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–34. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 5.Blackman M J, Scott-Finnigan T J, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackman M J, Whittle H, Holder A A. Processing of the Plasmodium falciparum merozoite surface protein-1: identification of a 33 kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991;49:35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 7.Chang S P, Case S E, Gosnell W L, Hashimoto A, Kramer K J, Tam L Q, Hashiro C Q, Nikaido C M, Gibson H L, Lee-Ng C T, Barr P J, Yokota B T, Hui G S N. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang S P, Gibson H L, Lee-Ng C T, Barr P J, Hui G S N. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992;149:548–555. [PubMed] [Google Scholar]
- 9.Cooper J A, Cooper L T, Saul A J. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparum merozoite surface antigen MSA 1. Mol Biochem Parasitol. 1992;51:301–312. doi: 10.1016/0166-6851(92)90080-4. [DOI] [PubMed] [Google Scholar]
- 10.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deans J A, Knight A M, Jean W C, Waters A P, Cohen S, Mitchell G H. Vaccination trials in Rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66-kDa merozoite antigen. Parasite Immunol. 1988;10:535–552. doi: 10.1111/j.1365-3024.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 12.Egan A F, Chappel J A, Burghaus P A, Druilhe P, Holder A A, Riley E M. Human antibodies to the C-terminal fragment of Plasmodium falciparum merozoite surface protein-1 directly inhibit merozoite invasion in vitro and mediate antibody-dependent cell-mediated inhibition of parasite growth. Parasite Immunol. 1999;21:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 13.Egan A F, Morris J S, Barnish G, Allen S, Greenwood B, Kaslow D C, Holder A A, Riley E M. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19 kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 14.Etlinger H M, Caspers P, Matile H, Schenfield H-J, Stueber D, Tacaks B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991;59:3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guevara Patino J A, Holder A A, McBride J S, Blackman M J. Antibodies that inhibit malaria merozoite protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J Exp Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall R, Hyde J E, Goman M, Simmons D L, Hope I A, Mackay M, Scaife J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. Nature. 1984;311:379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- 17.Holder A A, Lockyer M J, Odink K G, Sandhu J S, Riveros-Moreno V, Nicholls S C, Hillman Y, Davey L S, Tizard M L V, Schwarz R T, Freeman R R. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985;317:270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- 18.Kaslow D C, Shiloach J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Bio/Technology. 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Yadava A, Keister D B, Tian J H, Ohl M, Perdue-Greenfield K, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 20.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 21.McBride J S, Heidrich H-G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol Biochem Parasitol. 1987;23:71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- 22.Miller L H, Roberts T, Shahabuddin M, McCutchan T F. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 23.Pirson P J, Perkins M E. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J Immunol. 1985;134:1946–1951. [PubMed] [Google Scholar]
- 24.Riley E M, Allen S J, Wheeler J G, Blackman M J, Bennett S, Takacs B, Schonfeld H-J, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui W A, Tam L Q, Kan S C, Kramer K J, Case S E, Palmer K L, Yamaga K M, Hui G S. Induction of protective immunity to monoclonal-antibody-defined Plasmodium falciparum antigens requires strong adjuvant in Aotus monkeys. Infect Immun. 1986;52:314–318. doi: 10.1128/iai.52.1.314-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddiqui W A, Tam L W, Kramer K J, Hui J S N, Case S E, Yamage K M, Chang S P, Chan E B T, Kan S. Merozoite surface coat surface protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snow R W, Omumbo J A, Lowe B, Molyneux C S, Obiero J O, Palmer A, Weber M W, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupter S, Marsh K. Relationship between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]