Abstract
Population fluctuations are widespread across the animal kingdom, especially in the order Rodentia, which includes many globally important reservoir species for zoonotic pathogens. The implications of these fluctuations for zoonotic spillover remain poorly understood. Here, we report a global empirical analysis of data describing the linkages between habitat use, population fluctuations and zoonotic reservoir status in rodents. Our quantitative synthesis is based on data collated from papers and databases. We show that the magnitude of population fluctuations combined with species’ synanthropy and degree of human exploitation together distinguish most rodent reservoirs at a global scale, a result that was consistent across all pathogen types and pathogen transmission modes. Our spatial analyses identified hotspots of high transmission risk, including regions where reservoir species dominate the rodent community. Beyond rodents, these generalities inform our understanding of how natural and anthropogenic factors interact to increase the risk of zoonotic spillover in a rapidly changing world.
Subject terms: Infectious diseases, Population dynamics, Ecological epidemiology, Macroecology
Many rodent species are known as hosts of zoonotic pathogens, but the ecological conditions that trigger spillover are not well-understood. Here, the authors show that population fluctuations and association with human-dominated habitats explain the zoonotic reservoir status of rodents globally.
Introduction
Rodents are globally abundant and famous for extreme population fluctuations that manifest as boom-and-bust events, eruptive outbreaks and/or cycles1–5. The contributors to these fluctuations are heterogeneous, and include density dependence6, weather conditions7 that affect food availability3, predation rates8, and land use change9,10, with the importance of these drivers varying across systems11,12. Given the near ubiquity of rodents and the diverse ecological roles they play as consumers, prey, and reservoirs for parasites and pathogens, these fluctuations are consequential for many ecological processes, including the transmission of zoonotic pathogens13–15.
Zoonotic diseases caused by these pathogens are an increasing threat to human health and welfare16, yet despite our increasing understanding of ecological factors that contribute to outbreaks, our ability to predict zoonotic spillover transmission remains limited. Rodents host a greater diversity of zoonotic pathogens than other mammal orders17 and, together with bats and primates, they harbour the majority of zoonotic viruses18. Within rodents, species with fast life history strategies (e.g., early and frequent reproduction) appear disproportionately likely to act as zoonotic reservoirs19, but the mechanisms underlying the effects of life history on reservoir status are poorly understood, as are the pathways leading to direct or indirect contact between rodents and humans18—a necessary condition for the transmission of many rodent-borne zoonoses.
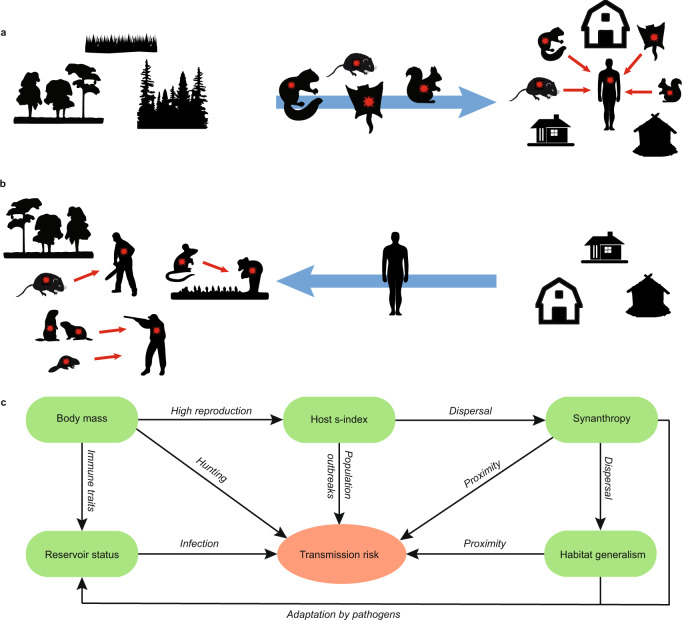
The propensity of some rodent species to live exclusively or occasionally in or near human dwellings (synanthropy) has long been acknowledged to increase transmission risk of important zoonoses threatening public health. Synanthropic behaviour and close human contact with globally distributed rodents like the house rat (Rattus rattus), brown rat (Rattus norvegicus) and house mouse (Mus musculus) have been linked to numerous zoonoses, including plague and typhus20. For the remaining majority of rodent reservoirs (282 species), synanthropy is not well documented21. In addition to synanthropy, which implies rodents moving into human habitats, contact between humans and rodents can arise from humans moving into rodent habitats (Fig. 1a, b). For example, compared to other professions, forest workers, agricultural workers, hunters and trappers are more frequently exposed to human orthohantavirus infections22, Lyme disease22–24, and tularemia25, with hunters and trappers being at risk either via environmental exposure or due to direct contact with infected animals26. As the incidence of zoonoses continues to increase globally, efforts to reduce spillover transmission will depend critically on understanding the mechanisms governing transmissible contact, which are mediated through reservoir traits and ecological context (Fig. 1c).
Fig. 1. Pathways increasing transmission risk from rodent-borne pathogens.
a, b Contact between rodents and humans increases transmission risk; a either with rodents moving into human dwellings and environments or with b, humans moving into rodent habitats or using rodents as a natural resource. c Factors (green boxes) and associated key traits of mechanisms (arrows) explaining increased transmission risk from rodent-borne pathogens. Description of mechanisms: Body mass as a key life history trait can dichotomously increase transmission risk. While rodents with high body mass are frequently hunted for fur or meat, many rodents with low body mass have less developed immune defence strategies and exhibit large population fluctuations (high s-index) resulting in population outbreaks. Periods of high rodent population density are frequently associated with abundant dispersal into human-dominated environments. As pathogens are frequently associated with synanthropic and habitat generalist rodents, these rodents increase transmission risk via proximity to humans. Being a reservoir poses an apparent transmission risk. In the light of many rodent-borne pathogens still being undetected16, factors like synanthropy, habitat generalism, high population fluctuations and/or high body mass are important predictors of transmission risk.
Here, we augment existing data on rodent reservoirs by compiling key information about rodent population dynamics and degree of synanthropy, which are hypothesized to underlie contact rates with humans across continents. Specifically, we collated or calculated s-index (Methods), which measures the degree of population fluctuation, across all rodent species for which data were available. This global dataset also includes life history traits, reservoir status for zoonotic pathogens, habitat associations, and spatial distributions of 436 rodent species (Supplementary Data 1). We use these data to test hypotheses about factors characterizing the probability that rodents would pose a strong risk for zoonotic spillover. Specifically, we hypothesised that synanthropic rodents would be overrepresented in the pool of rodent species identified as zoonotic reservoirs. We also hypothesised that zoonotic reservoir species, compared to non-reservoirs, would exhibit large population fluctuations and be habitat generalists. While we predicted that large population fluctuations are an important characteristic of rodent reservoirs, we also hypothesised that exploitation by humans (e.g., by hunting for meat and fur) poses increased transmission risk (Fig. 1). We show that the magnitude of population fluctuations combined with species’ synanthropy and degree of human exploitation together distinguish the vast majority of zoonotic rodents at a global scale. Beyond rodents, these generalities inform our understanding of how natural and anthropogenic factors interact to increase the risk of zoonotic spillover in a rapidly changing world.
Results
Predictors of reservoir status
Our analyses include all known rodent reservoirs for zoonotic pathogens (282 species). These reservoirs harbour a total of 95 known zoonotic pathogens (34 viruses, 26 bacteria, 17 helminths, 12 protozoa and six fungi) employing all known modes of transmission (43 vector-borne, 32 close-contact, 28 non-close contact, and 13 using multiple transmission modes) (Supplementary Data 2). Compared to presumed non-reservoirs (species currently not known to harbour any zoonotic pathogens), we observed that reservoir rodents are strikingly synanthropic (Figs. 2, 3a, Table 1). Despite potential geographic biases, and the general possibility that synanthropic species are better studied compared to non-synanthropic species (see Sampling bias and Supplementary Figs. 1, 2), synanthropy emerged as a defining characteristic of nearly all (95%) currently known rodent reservoirs. Of the 155 synanthropic species, only six are considered as truly synanthropic, i.e., predominately, if not exclusively, occurring in or near human dwellings, while the remaining species only occasionally show synanthropic behaviour (Supplementary Data 1).
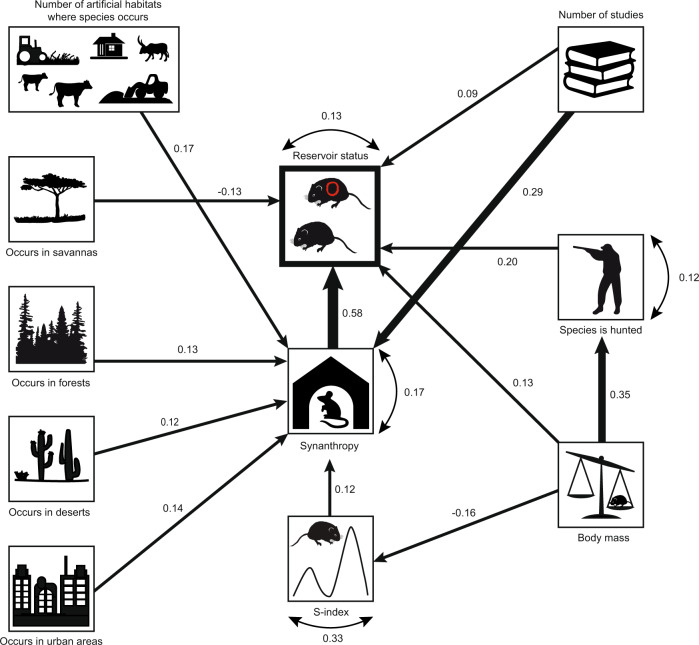
Fig. 2. Predictors of reservoir status.
Final structural equation model linking reservoir status of rodent species (n = 269) with their synanthropy and hunting status, population fluctuations (s-index, log-transformed), and adult body mass, controlling for their occurrence in a range of habitats and the number of studies available per species. One-sided (directional) arrows represent a causal influence originating from the variable at the base of the arrow, with the width of the arrow and associated value representing the standardised strength of the relationship. The small double-sided arrows and numbers next to each response (endogenous) variable represent the error variance.
Fig. 3. Characteristics of reservoir and synanthropic rodents.
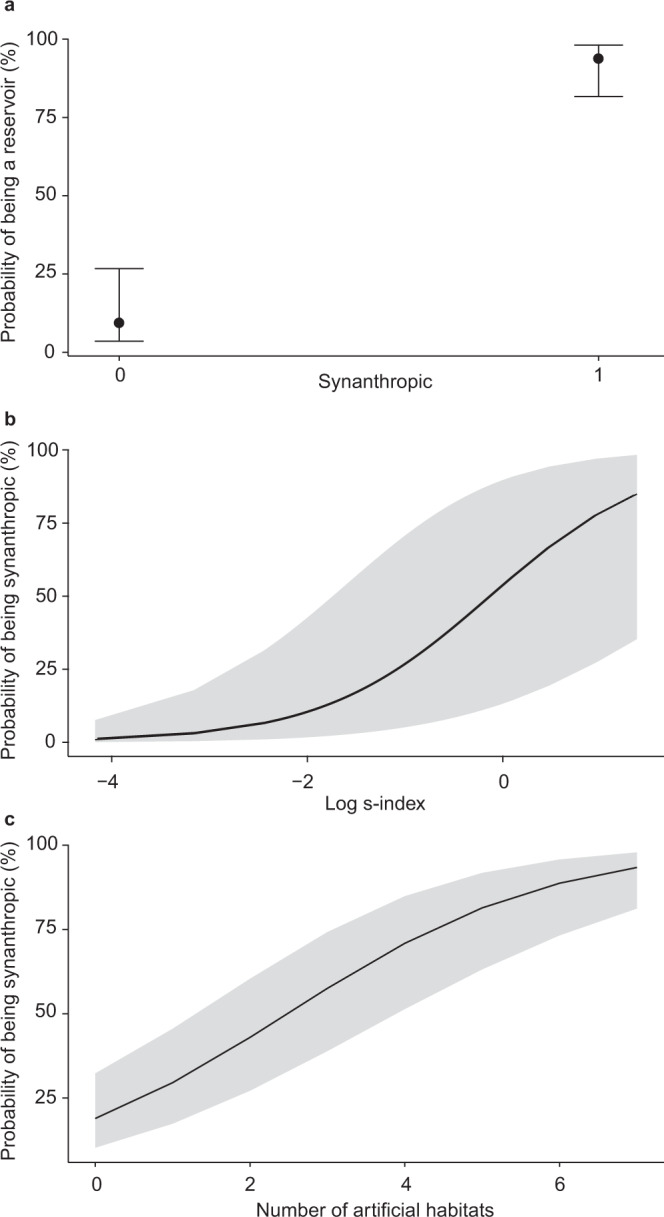
a Reservoir rodents are predominately synanthropic (n = 436 with n (non-reservoir) = 154, n (reservoir) = 282). b Synanthropic rodents display high population fluctuations (high s-index) (n = 269) and c, occur in multiple artificial habitats (n = 269) (Tables 1–3). In a, estimated probability and 95% confidence intervals are shown and in b–c, estimated probability is shown and shaded areas show 95 % confidence intervals.
Table 1.
Summary of best-fit generalized linear mixed effects model for reservoir status (n = 436)
| Predictors | Odds ratio | 95% CIa | P-value |
|---|---|---|---|
| Intercept | 0.12 | 0.03–0.51 | 0.004 |
| Log(adult mass, g) | 1.47 | 1.14–1.89 | 0.003 |
| Synanthropic | 132.49 | 58.16–301.83 | <0.001 |
| Occurring in grasslands | 0.37 | 0.22–0.62 | <0.001 |
| Occurring in savannas | 0.43 | 0.21–0.89 | 0.024 |
| Being hunted | 4.65 | 1.83–11.82 | 0.001 |
| Random effects | |||
| σ2 | 3.29 | ||
| τ00, Rodent family | 1.15 | ||
| ICCbRodent family | 0.26 | ||
| NRodent family | 24 | ||
aConfidence interval.
bIntra-class correlation coefficient.
Compared to non-reservoirs, we also found that rodent reservoirs are disproportionately exploited by humans (hunted for meat and fur). Seventy-two of the regularly hunted rodent species (n = 83) are reservoirs (87%), and hunted rodent species harbour on average five times the number of zoonotic pathogens than non-hunted species (Table 2).
Table 2.
Summary of rodent characteristics divided by rodent group with respect to hunting, reservoir status, and synanthropic behaviour
| Rodent characteristics | Mean | 95% CIa | Range | N |
|---|---|---|---|---|
| Number of zoonoses | ||||
| Hunted | 2.40 | 1.88–2.92 | 0–15 | 83 |
| Non-hunted | 0.44 | 0.35–0.53 | 0–3 | 216 |
| Number of habitats | ||||
| Reservoir | 3.26 | 3.00–3.51 | 1–11 | 282 |
| Non-reservoir | 2.87 | 2.58–3.16 | 1–10 | 154 |
| Number of artificial habitats | ||||
| Reservoir | 1.13 | 0.95–1.31 | 0–7 | 282 |
| Non-reservoir | 0.72 | 0.52–0.92 | 0–6 | 154 |
| Number of habitats | ||||
| Synanthropic | 3.96 | 3.56–4.36 | 1–11 | 155 |
| Non-synanthropic | 2.65 | 2.47–2.84 | 1–8 | 281 |
| Number of artificial habitats | ||||
| Synanthropic | 1.60 | 1.31–1.89 | 0–7 | 155 |
| Non-synanthropic | 0.64 | 0.52–0.77 | 0–5 | 281 |
| s-indexb | ||||
| Reservoir | 0.46 | 0.39–0.53 | 0.02–3.94 | 137 |
| Non-reservoir | 0.41 | 0.37–0.45 | 0.11–1.11 | 132 |
| Synanthropic | 0.52 | 0.42–0.62 | 0.09–3.94 | 94 |
| Non-synanthropic | 0.39 | 0.36–0.42 | 0.02–1.11 | 175 |
aConfidence interval.
bIndex of population fluctuations (Methods).
We explored causal pathways using a structural equation model (SEM) linking synanthropy, reservoir status, and their hypothesized predictors. The final model, which we established a priori, had 17 free parameters and 21 degrees of freedom (n = 269). The model fit, based on the SRMR (standardized root mean squared residual) and the RMSEA (root mean squared error of approximation) indicated a good fit (see Methods). From the initially formulated full model, the pathways linking reservoir status to population fluctuations (s-index, Methods), occurrence in grasslands, number of artificial habitats a species occurs in, and number of studies found per species were not significant and thus removed from the final model (Supplementary Fig. 3). Similarly, pathways linking synanthropy and occurrence in grasslands were not significant and also removed. All reported coefficients for pathways are standardized to facilitate comparisons among the different relationships. The relationships and coefficients below all refer to those in the final model.
The focal variable in the model was reservoir status, which was strongly and positively associated with synanthropy and had the highest estimated pathway coefficient (standardised estimate = 0.58, 95% CI 0.49–0.66, Fig. 2). Controlling for synanthropy, species were more likely to be a reservoir with increasing adult weight (0.13, 0.04–0.22). Species that occur in savanna were less likely to be reservoirs (−0.13, −0.22 to −0.04), while hunted species were more likely to be reservoirs (Fig. 2, 0.20, 0.11–0.30).
Synanthropy was influenced by four habitat variables: a species was more likely to be synanthropic if it occurs in a higher number of artificial habitats (0.17, 0.04–0.31), and occurs in urban areas (0.14, 0.01–0.27), deserts (0.12, 0.01–0.23), or forests (0.13, 0.02–0.24). Notably, species with higher s-index, and thus larger population fluctuations, were more likely to be synanthropic (0.12, 0.01–0.22), and the s-index itself decreased as adult weight increased (−0.16, −0.27 to −0.04). Finally, hunted species were characterized by higher adult bodyweight (0.35, 0.25–0.44) (Fig. 2).
The number of studies per species was positively associated with both a species’ synanthropic behaviour (0.29, 0.19–0.39) and its reservoir status (0.09, 0.00– 0.19), albeit with weaker evidence for the latter effect (p = 0.054) (Fig. 2),
The confirmatory generalized linear mixed effects models (GLMMs) (Tables 1, 3), which control for correlation among species within the same family, showed that our SEM results were robust. Indeed, synanthropy was a significant predictor of reservoir status. These models underscore synanthropy as the most important predictor of reservoir status in our analysis (Table 1, Figs. 2–3).
Table 3.
Summary of best-fit generalized linear mixed effects model for synanthropic status (n = 269)
| Predictors | Odds ratio | 95% CIa | P-value |
|---|---|---|---|
| (Intercept) | 0.26 | 0.03–2.10 | 0.205 |
| Number of artificial habitats | 1.96 | 1.58–2.42 | <0.001 |
| Occurring in urban areas | 3.63 | 1.34–9.82 | 0.011 |
| Occurring in deserts | 3.40 | 1.86–6.22 | <0.001 |
| Occurring in forests | 2.84 | 1.71–4.71 | <0.001 |
| Log(s-index)b | 3.18 | 2.01–5.01 | <0.001 |
| Occurring in grasslands | 0.44 | 0.27–0.72 | 0.001 |
| Random Effects | |||
| σ2 | 3.29 | ||
| τ00, Rodent family | 2.06 | ||
| ICCcRodent family | 0.39 | ||
| NRodent family | 19 | ||
aConfidence interval.
bIndex of population fluctuations (Methods).
cIntra-class correlation coefficient.
Population fluctuations affect transmission risk
Our newly compiled data on the magnitude of population fluctuations enabled comparative investigations beyond theoretically straightforward predictions that transmission risk increases with reservoir abundance for density-dependent systems. We show that while strong population fluctuations (measured as the s-index) are found frequently in both reservoir and non-reservoir rodents (Table 2), synanthropic rodents exhibit much larger population fluctuations compared to non-synanthropic rodents (Table 2, Figs. 2–3). This pattern was apparent despite broad confidence intervals in the relationship between the s-index and the probability of being synanthropic (Fig. 3b, Tables 2, 3). Taken together, our results suggest that larger population fluctuations in reservoir species increase zoonotic transmission risk via synanthropic behaviours of rodents, thereby increasing the likelihood of zoonotic spillover infection to humans.
Habitat generalism and habitat transformation increase transmission risk
We also find that reservoir species thrive in human-created (artificial) habitats (Fig. 3a, c, Tables 2–3), which reflects a general flexibility in their use of diverse habitat types compared to non-reservoir species (Fig. 4a, Table 2). In addition, the number of zoonotic pathogens harboured by a rodent species increased with habitat breadth (r436 = 0.34, p < 0.001). Despite the ability to persist in numerous habitat types, we found that reservoir rodents are underrepresented in some natural habitats, especially in savannas and grasslands (χ2 = 120.81, df = 8, p < 0.001), and they are overrepresented in artificial habitats (χ2 = 30.07, df = 7, p < 0.001; Fig. 4a). Of the 187 rodent species occurring in artificial habitats, 73% are reservoirs, while 59% of the 249 rodents occurring in natural habitats are reservoirs, making artificial habitats more reservoir-rich than natural habitats (χ2 = 9.28, df = 1, p < 0.01; Fig. 4a).
Fig. 4. Habitat types occupied by rodents.
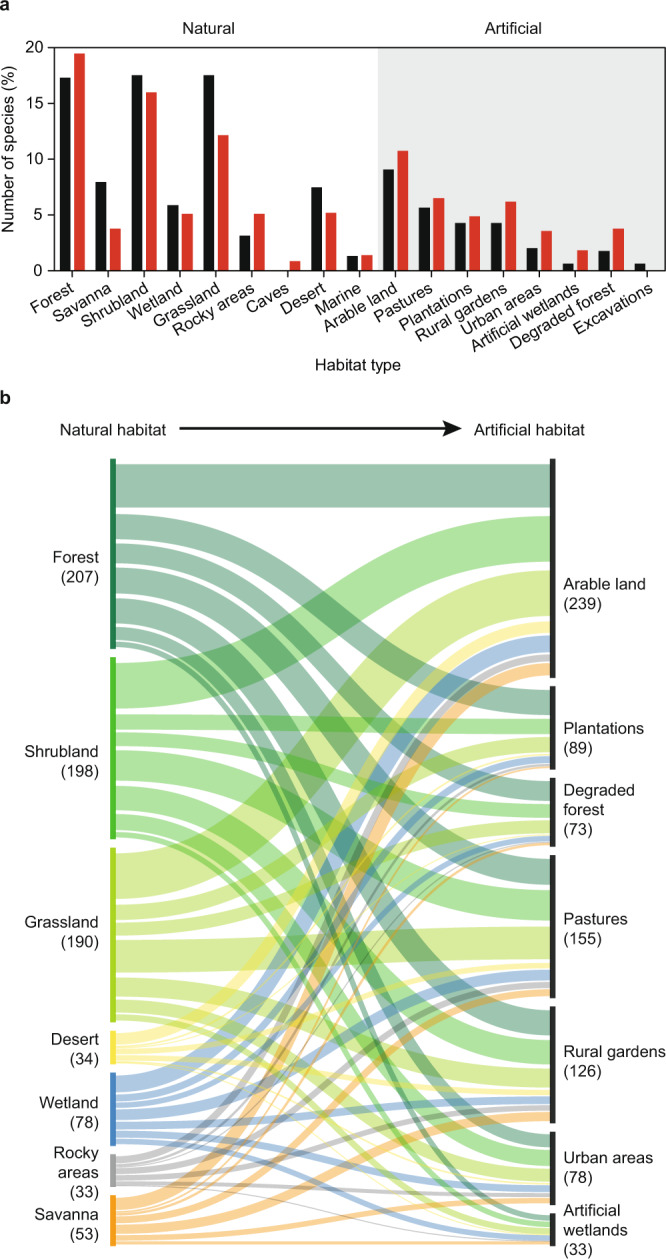
a Percentage of non-reservoir (black bars; n = 154) and reservoir rodents (red bars; n = 282), respectively, occurring in natural and artificial (shaded) habitats. b Habitat generalism of reservoir rodents. Reservoir rodents occurring in natural habitats potentially move into artificial habitats, e.g., during periods of high population density or when natural habitat is degraded. Thickness of curves represents number of rodents shared between natural and artificial habitat.
Our results also support an emerging consensus that changes in reservoir communities through the degradation of natural habitats increases transmission risk (Fig. 4b). We find evidence that the conversion of natural habitats to human-dominated uses may disproportionately support the persistence of generalist species (Table 2) and facilitate the influx of rodent reservoir species from nearby forest, shrubland and grassland into habitat types in which contact with humans is frequent and zoonotic transmission risk more likely (Fig. 4b).
Interplay between transmission mode and synanthropy
We examined whether the higher transmission risk imposed by synanthropic species varied with pathogen type or transmission mode. Compared to non-synanthropic species, synanthropic reservoirs harbour a higher number of zoonoses with “close” (transmission via grooming, biting, scratching, aerosols) and vector-borne transmission as the dominant modes (Table 4). The number of zoonoses caused by helminths, bacteria and viruses was also higher among synanthropic reservoirs (Table 4).
Table 4.
Summary of number of transmission modes, number of zoonoses with different transmission modes, and number of zoonoses caused by different pathogen types in synanthropic and non-synanthropic rodents
| Transmission type | Mean | 95% CIa | Range | N |
|---|---|---|---|---|
| Number of transmission modes | ||||
| Synanthropic reservoirs | 4.39 | 3.36–5.41 | 1–47 | 148 |
| Non-synanthropic reservoirs | 2.12 | 1.82–2.42 | 1–11 | 134 |
| Number of zoonoses with close transmission mode | ||||
| Synanthropic reservoirs | 1.28 | 1.03–1.54 | 0–10 | 148 |
| Non-synanthropic reservoirs | 0.65 | 0.53–0.77 | 0–3 | 134 |
| Number of zoonoses with non-close transmission mode | ||||
| Synanthropic reservoirs | 0.97 | 0.65–1.28 | 0–15 | 148 |
| Non-synanthropic reservoirs | 0.55 | 0.39–0.71 | 0–5 | 134 |
| Number of zoonoses transmitted by vectors | ||||
| Synanthropic reservoirs | 1.63 | 1.25–2.00 | 0–17 | 148 |
| Non-synanthropic reservoirs | 0.66 | 0.52–0.81 | 0–4 | 134 |
| Number of zoonoses with intermediate transmission mode | ||||
| Synanthropic reservoirs | 0.51 | 0.30–0.71 | 0–9 | 148 |
| Non-synanthropic reservoirs | 0.25 | 0.17–0.34 | 0–2 | 134 |
| Number of zoonoses caused by helminths | ||||
| Synanthropic reservoirs | 0.51 | 0.30–0.73 | 0–10 | 148 |
| Non-synanthropic reservoirs | 0.20 | 0.11–0.29 | 0–3 | 134 |
| Number of zoonoses caused by bacteria | ||||
| Synanthropic reservoirs | 1.18 | 0.88–1.49 | 0–12 | 148 |
| Non-synanthropic reservoirs | 0.36 | 0.25–0.46 | 0–3 | 134 |
| Number of zoonoses caused by viruses | ||||
| Synanthropic reservoirs | 0.99 | 0.78–1.20 | 0–8 | 148 |
| Non-synanthropic reservoirs | 0.38 | 0.29–0.47 | 0–3 | 134 |
| Number of zoonoses caused by fungi | ||||
| Synanthropic reservoirs | 0.03 | −0.01–0.06 | 0–2 | 148 |
| Non-synanthropic reservoirs | 0.05 | 0.01–0.10 | 0–2 | 134 |
| Number of zoonoses caused by Protozoa | ||||
| Synanthropic reservoirs | 0.72 | 0.55–0.90 | 0–7 | 148 |
| Non-synanthropic reservoirs | 0.57 | 0.46–0.69 | 0–3 | 134 |
aConfidence interval.
Hotspots of transmission risk
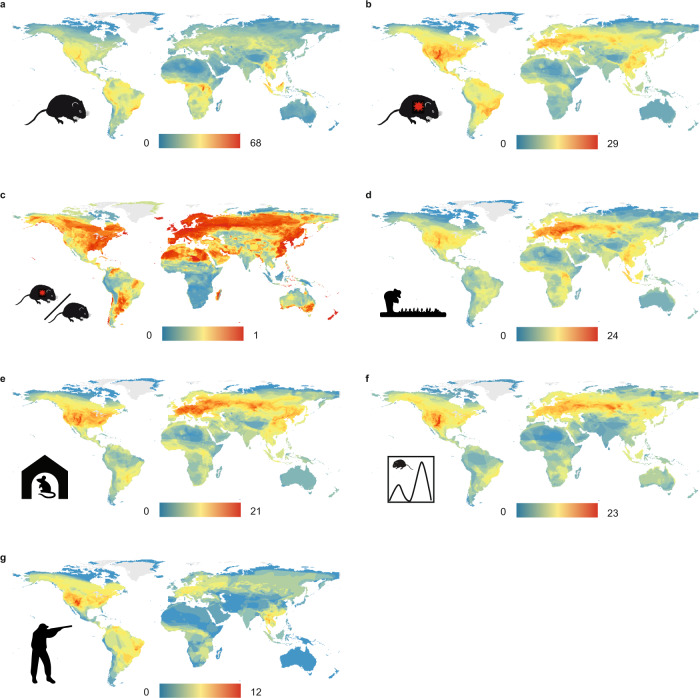
Global analyses of the richness of rodent reservoirs have previously identified hotspots in medium latitude North America and Europe, north-eastern parts of South America, south-eastern coastal Brazil and South-East Asia18,19. Our analyses identify additional regions where transmission risk is likely to be high owing to the occurrence of hunted, synanthropic rodents that occupy artificial habitats and show large population fluctuations. We also report regions where reservoir species dominate the rodent community (Fig. 5). These regions include Fennoscandia, South America west of the Andes, southern Australia and New Zealand, where our data suggest that zoonotic risk deriving from rodents is likely to be high because encountering a rodent species largely implies encountering a zoonotic reservoir species (Fig. 5c).
Fig. 5. Global distribution of the number of rodent species by category.
a All rodent species (n = 2308), b reservoir rodents (n = 282), c ratio between the number of reservoir and total number of rodents, d rodents occurring in artificial habitats (n = 186), e synanthropic rodents (n = 155), f rodents exhibiting pronounced population fluctuations (s-index >0.3, Methods; n = 159), g hunted rodents (n = 83). Warmer colours highlight areas of high species richness. See Methods for image licensing.
We observed particular regions in which overall rodent richness is low, but the richness of rodent species occupying artificial habitats is comparatively high (Fig. 5d). These regions include the north temperate zones of both hemispheres (Fig. 5d). In these areas, we postulate that artificial habitats, irrespective of the local species pool, are disproportionately occupied by multiple rodent reservoir species.
Sampling bias
Generally, the more a rodent species is studied for population fluctuations or zoonoses, the more zoonotic pathogens have been detected in it (Supplementary Fig. 1). The relationship between study effort and pathogen detection, however, is highly variable. For example, two of the five most studied rodent species (Rattus norvegicus and R. rattus) host 35 and 34 zoonotic pathogens, respectively, while the other three most studied reservoirs (Mus musculus, Myodes glareolus, and Peromyscus maniculatus) host comparatively few zoonotic pathogens (11, 6, and 10) (Supplementary Fig. 1). In addition, the greater Bandicoot rat (Bandicota indica) is a reservoir for the fifth highest number of zoonoses (15), despite comparatively low study effort.
The overall number of studies per rodent species on population dynamics and zoonoses varies among continents (H8 = 43.494, p < 0.001, Supplementary Fig. 2) with fewer studies on species occurring exclusively in Africa or Asia compared to those occurring exclusively in North America. However, variation is high and there is a similar number of studies on species occurring in both Africa and Asia compared to species on other continents (Supplementary Fig. 2).
Discussion
We addressed whether the tendency of some rodents to undergo dramatic fluctuations in population size interacts strongly with habitat generalism, synanthropy, and the tendency to be exploited by humans. These three critical factors combine to predict reservoir status for nearly all known rodent reservoirs of zoonotic disease. It has been long assumed that the nature of the contact interface between humans and reservoir species is important for driving spillover risk. However, our results provide comparative quantitative evidence of the nature of rodent-human contact interfaces for transmission risk, including the encroachment of rodent reservoirs into artificial habitats in addition to human encroachment into natural habitats.
Compared to other mammals, rodents host the highest viral richness of Bunya- and Arenaviruses and, together with bats, the highest number of Flaviviruses18. A majority of zoonotic viruses from these families (for revised virus taxonomy see ref. 27) are spread by vectors and/or by close contact (Supplementary Data 2). For such horizontally transmitted pathogens, infection prevalence generally increases concomitantly with reservoir abundance28. Periods of rapid population growth to a peak (illustrative of populations with high s-index), can result in rodents dispersing from natural sources to artificial sink habitats including urban areas4,5,29. Thus, increases in human transmission risk arise from the interaction between transmission modes, reservoir population fluctuations and habitat generalism in rodent reservoirs.
Our results contradict the perception that natural habitats, due to their high species diversity, pose greater risks of zoonotic transmission compared to artificial habitats30. On the contrary, species richness of reservoirs is actually higher in artificial habitats31, a finding that underscores the high zoonotic risks associated with the conversion of natural into artificial habitats32. Since habitat generalists favoured by such land conversions are disproportionally zoonotic reservoir species, our results suggest that the accelerating transformation of natural habitats into artificial habitats33,34 will continue to increase the transmission risk of rodent-borne zoonoses globally (Fig. 4). Our prediction that artificial habitats may be disproportionally occupied by multiple species of rodent reservoirs is supported by multiple previous studies that have shown, at local scales, that habitat loss and fragmentation result in reduced animal diversity35 with the remaining species dominated by generalists36 that can reach high density37. In addition, complex community responses arise if artificial habitat increases and the population dynamics of native rodents are affected by those of non-native invasive rodents38; processes that will likely be more common in the future due to accelerated global change. Our knowledge on the occurrence and distribution of wildlife pathogens, reservoirs and non-reservoirs is constantly increasing, implying that current pathogen-reservoir associations need to be evaluated with caution39, which also applies to our study (cf. Figs. 4–5). As more rodents will be studied, more pathogens are likely to be detected (Fig. 2)39.
Complexities of zoonotic transmission, including seasonality of rodent abundance, changes in land use creating artificial habitats, and diversity of transmission modes, cause many zoonotic disease systems to be considered as unique, with a need for control efforts tailored to the ecological nuances of each system. Our analyses demonstrate that across the majority of rodent-borne zoonotic diseases, population fluctuations and associated synanthropy are robust indicators of reservoir status and underscore particular pathways to potential spillover transmission. Transmission risk from rodents can however vary within a species’ geographic range due to, for example, spatial differences in immunogenetic properties of the reservoir and associated pathogen(s)40. The inclusion of reservoir lineages and pathogen strains in future models can therefore provide deeper insight into regional transmission risk.
Given that many rodent-borne pathogens are still undescribed18 the generalities identified here may serve as useful rules of thumb across rodent-borne zoonoses and also provide useful starting points from which to improve control of zoonotic transmission and more efficient discovery of novel pathogens and reservoir species. To better mitigate disease outbreaks, surveillance focusing on reservoir rodents exhibiting large population fluctuations appears to be a promising approach13. Surveillance should in addition intensify screening for new zoonotic pathogens and/or new reservoirs in rodents exhibiting large population fluctuations and those being hunted. These results also suggest the possibility that similar interactions between ecological and anthropogenic factors may exist for other reservoir groups, and may be leveraged to mitigate increasing risks of zoonotic disease emergence in humans.
Methods
Datasets
In our study, we included all rodent species classified as reservoirs of zoonotic diseases by the Global Infectious Disease and Epidemiology Network (GIDEON)41 by 1 October 2020. Due to the rather recent taxonomic split of Arvicola amphibius into A. amphibius and A. scherman42, we treated these species as one species complex (here called Arvicola amphibius) in the statistical analyses. In total, we studied 282 rodent reservoir species and the nomenclature of all rodents followed the International Union for Conservation of Nature (IUCN) Red List of Threatened Species43.
We complemented the dataset of reservoir rodents with data for 154 non-reservoir rodent species. These species were selected in a stratified approach. In a first step, we searched in the Clarivate Web of Science © (Copyright Clarivate 2020. All rights reserved) for the most studied rodent species per continent. For this search, we combined search strings for topics on countries, species (including their synonyms according to IUCN) and information on population dynamics. For information on population fluctuations, we searched for abundance, density, population dynamics, amplitude, cyclic or cyclicity as topics (search finalized 20 May 2020). The search string for each continent is represented as follows: “TS = (country) and TS = (species) and TS = (abundance or density or population dynamics or amplitude or cycl*)”, with “TS” representing “Topic”, “country” being a list of all countries per continent and “species” being a list of all known rodent species (incl. their synonyms) occurring in the respective continent. We imported the search results into a database and sorted the dataset by the number of references per species. In a second step, we then searched for information on population dynamics and their seasonality of the 20 most studied species per continent (search finalized 19 October 2020). When screening identified articles, we occasionally also found suitable data on population dynamics and/or seasonality for species that were not among the 20 most studied species and included the data in our dataset. For all reservoir rodents, we searched for and extracted literature data on population fluctuations in the same way as described for the non-reservoir species but included also studies listed in Google Scholar. If not available as raw data, we extracted seasonal and/or yearly abundance/density from figures with WebPlotDigitizer44 or received raw data from the authors. The extracted information per species included minimum and maximum abundance/density, if the respective species exhibits cyclicity, seasonality and/or outbreaks (Supplementary Data 1). For species with data from at least four consecutive years, we also calculated the s-index45. This index, calculated as the standard deviation of species abundance or density over time, has frequently been used to quantify the degree of fluctuation in rodent populations45 and we classified species with s-index >0.3 as those exhibiting pronounced population fluctuations. For species with multiple records for abundance/density, we used the data showing the largest amplitude and the highest value for the s-index, respectively.
As an index of publication bias, we also extracted for each species the number of studies that were available until 1 October 2020 on (a) population fluctuations, (b) zoonoses and (c) population fluctuations or zoonoses from Clarivate Web of Science © (Copyright Clarivate 2020. All rights reserved). For the search on population dynamics, we used the search string “TS = (species) and (TS = (abundance or density or population dynamics or amplitude or cycl*) not TS = (ovulatory cycl*) not TS = (lunar cycl* or lunar phase))”, for the search on zoonoses, the search string “TS = (species) and (TS = (zoonoses))” with “zoonoses” representing the list of zoonoses given in Supplementary Data 2, separated by “or”, and for the number of studies on population dynamics or zoonoses, we combined the search strings.
Information on habitat preferences of rodent species was extracted from the IUCN Red List of Threatened Species43. Forest habitats included woodlands and reforestations, while agricultural fields comprised crop fields, pastures, rural gardens and orchards, and wetlands comprised different types of aquatic habitats that were not used for crop fields. For details on habitat types, see ref. 43. We assigned each habitat type to either natural or artificial habitat (Supplementary Data 1; cf. Fig. 4). Information on synanthropy, i.e., if species live exclusively or occasionally in or near human dwellings, was limited in the IUCN database. We therefore systematically searched for synanthropy in Clarivate Web of Science © (Copyright Clarivate 2020. All rights reserved) (search finalized 19 October 2020) and used the following search terms combined by “or”: “synanthrop*”, “peridomestic”, “village”, “domestic”, “house”, “commensal”, “residen*”, “human dwelling*”, “urban”, “infest*”, “household*”, “anthropogenic” and “outbuilding*” in combination with the respective species names and their synonyms. We also contacted rodent experts to get an expert opinion on synanthropy for rodent species for which we did not find literature data. All species for which neither literature data nor experts indicated synanthropy, we assigned as non-synanthropic. For all rodent species, we also extracted information on whether they are hunted for fur or meat.
To detect global patterns in the distribution of reservoir rodents, synanthropy, habitat preferences, population fluctuations and hunted rodents, we extracted the distribution range of rodents as vector files from the IUCN43 database for further use in a geographic information system (GIS). As the IUCN data for introduced species mainly included native distribution ranges, we complemented the distribution ranges of these species (Cavia porcellus, Mus musculus, Myocastor coypus, Rattus norvegicus, R. rattus, Sciurus carolinensis, and Tamias sibiricus) with areas they have been introduced to. These data, we extracted from the Global Biodiversity Information Facility (GBIF)46,47. To map species richness per hexagon (865 km2), we used the IUCN Species Mapping Tools48 in ArcGIS Desktop49.
Statistical analyses
We used structural equation modelling (SEM) to evaluate the relationship between synanthropy and reservoir status of rodent species, the factors that influence them, and the pathways through which these factors are connected. SEM is a multivariate analysis technique that combines confirmatory factor analyses and multiple regression to test whether the data supports a pre-defined hypothetical model. SEM hypothesizes causal relationships among variables and tests them through linear equations and can include continuous or binomial variables. In SEM, the values assigned by the model to the pathways between variables are standardized estimates of the strength of that relationship after taking into account other relationships specified in the model50.
Here, we proposed a SEM model (full model) based on our a priori knowledge from previous studies and through preliminary data analysis (cf. Fig. 1). The candidate predictor variables for each model included habitat-related variables, log-transformed s-index (index of population fluctuations), and life history traits (see Supplementary Data 1 for the list of variables). Presented here in Fig. 2, the final model included the s-index, for which data was available for only a subset of the rodent species (n = 269). The full model, including all hypothesized pathways, is presented as Supplementary Fig. 3.
After fitting the full model, we removed non-significant pathways (p > 0.1). We used RMSEA (root mean squared error of approximation) and SRMSR (standardized root mean squared residual) to test the overall fit of the model. RMSEA is based on overall model χ2 but is standardized by degrees of freedom and is more appropriate for models with n > 200. An RMSEA value of <0.05 indicates a good fit. SRMSR is an absolute measure of fit, defined as the standardized difference between the observed correlation and the predicted correlation, for which a value of <0.08 is considered a good fit51. Our final model had a good fit to the data (SRMSR = 0.042). The RMSEA value of 0.071 (90% CI 0.046–0.097) was only slightly higher than the 0.05 suggested cut-off for a good fit. Thus, we proceeded with the interpretation of the final model and the relationships and coefficients therein without further adjustment in the model pathways.
We also fitted two generalized linear mixed effects models (GLMMs) to (a) confirm the results of the SEM and (b) account for the effect of species family on the SEM results and thus correlation among species within the same family, since it is not feasible to include family as a grouping factor in SEM models. The response variables for the two GLMMs were synanthropic and reservoir status, and the candidate predictor variables in the GLMMs were the variables maintained in the final SEM, and we included species family as a random effect.
Each predictor was first tested for its association with the response variable (either synanthropic or reservoirs status) using a generalized linear model with a binomial error distribution, and predictors with a p-value < 0.15 were then included in the full model. The full model was a generalized linear mixed effects model with a binomial error distribution. The response variables were reservoir status (Table 1) and synanthropic status (Table 3). Candidate predictors were included as fixed effects, rodent family as a random effect to control for phylogenetic correlations, and for each species, we weighted the observations by the number of studies with quantitative data included in our dataset (Supplementary Data 1).
Thus, the two models were as follows:
| 1 |
| 2 |
| 3 |
| 4 |
Y = 1 if species j in rodent family i was synanthropic (first model) or known reservoir (second model), and 0 if it was not. α is the intercept, X is a vector of fixed effects, bi is the random intercept for rodent family i, and εij is the error component.
We analysed differences in the percentage number of reservoir and non-reservoir rodents, respectively, occurring in natural and artificial habitats by two-tailed χ2-tests. Correlations between two variables were analysed with Spearman’s rank correlation coefficient (rs) and differences among groups with Kruskal-Wallis test (H) with Dunn test for posthoc comparisons. Data analyses were conducted in R 4.2.052.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Janet Foley, Virginia Hayssen, Jens Jacob, Charles Krebs, Herwig Leirs, Yonas Maheretu and Grant Singleton for their expert knowledge on the synanthropy of some rodent species. This study was funded by the Swedish Research Council Formas (Grant No. 2017-00578 and 2017-00867) and the Swedish Environmental Protection Agency (SEPA) through the Swedish Wildlife Management Fund (Grant No. 2020-00093).
Author contributions
F.E., R.S.O. and B.A.H. designed the study. F.E. and M.M. collected data. H.K. and F.E. performed statistical analyses. F.E., R.S.O., B.A.H., B.H., H.K., M.M., and N.J.S. were involved in writing the manuscript.
Peer review
Peer review information
Nature Communications thanks Rory Gibb, Serge Morand, and the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Data availability
Source data (including rodent species, reservoir status, synanthropy, habitat preferences, population fluctuations, list of reservoir–pathogen associations) needed to fully replicate and evaluate the analyses are provided as Supplementary Information. Global data used for the spatial mapping of rodent occurrences are available from the International Union for the Conservation of Nature and Natural Resources (IUCN, iucn.org) and the Global Biodiversity Information Facility (GBIF, gbif.org).
Code availability
The model details necessary to replicate the study are given in the Methods section.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-35273-7.
References
- 1.Krebs CJ, Myers JH. Population cycles in small mammals. Adv. Ecoligical Res. 1974;8:267–399. doi: 10.1016/S0065-2504(08)60280-9. [DOI] [Google Scholar]
- 2.Singleton GR, et al. One hundred years of eruptions of house mice in Australia–a natural biological curio. Biol. J. Linn. Soc. 2005;84:617–627. doi: 10.1111/j.1095-8312.2005.00458.x. [DOI] [Google Scholar]
- 3.Jaksic FM, Lima M. Myths and facts on ratadas: Bamboo blooms, rainfall peaks and rodent outbreaks in South America. Austral Ecol. 2003;28:237–251. doi: 10.1046/j.1442-9993.2003.01271.x. [DOI] [Google Scholar]
- 4.Krebs, C. J. Population Fluctuations In Rodents. (The University of Chicago Press, 2013).
- 5.Oli MK. Population cycles in voles and lemmings: state of the science and future directions. Mammal. Rev. 2019;49:226–239. doi: 10.1111/mam.12156. [DOI] [Google Scholar]
- 6.Hörnfeldt B. Delayed density dependence as a determinant of vole cycles. Ecology. 1994;75:791–806. doi: 10.2307/1941735. [DOI] [Google Scholar]
- 7.Meserve PL, Kelt DA, Previtali MA, Milstead WB, Gutiérrez JR. Global climate change and small mammal populations in north-central Chile. J. Mammal. 2011;92:1223–1235. doi: 10.1644/10-MAMM-S-267.1. [DOI] [Google Scholar]
- 8.Korpimäki E, Norrdahl K, Huitu O, Klemola T. Predator-induced synchrony in population oscillations of co-existing small mammal species. Proc. R. Soc. B. 2005;272:193–202. doi: 10.1098/rspb.2004.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duplantier JM, Duchemin JB, Chanteau S, Carniel E. From the recent lessons of the Malagasy foci towards a global understanding of the factors involved in plague reemergence. Vet. Res. 2005;36:437–453. doi: 10.1051/vetres:2005007. [DOI] [PubMed] [Google Scholar]
- 10.Van Wijngaarden, A. The rise and disappearance of continental vole plague zones in the Netherlands. Versl van Landbouwkd Onderz 63:1–21 (Plant Protection Service, Wageningen, 1957).
- 11.Andreassen HP, et al. Population cycles and outbreaks of small rodents: ten essential questions we still need to solve. Oecologia. 2021;195:601–622. doi: 10.1007/s00442-020-04810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers, J. H. Population cycles: generalities, exceptions and remaining mysteries. Proc. R. Soc. B: Biol. Sci.285 (2018). [DOI] [PMC free article] [PubMed]
- 13.Khalil H, Ecke F, Evander M, Bucht G, Hörnfeldt B. Population dynamics of bank voles predict human Puumala hantavirus risk. EcoHealth. 2019;16:545–555. doi: 10.1007/s10393-019-01424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis S, Calvet E, Leirs H. Fluctuating rodent populations and risk to humans from rodent-borne zoonoses. Vector-Borne Zoonotic Dis. 2005;5:305–314. doi: 10.1089/vbz.2005.5.305. [DOI] [PubMed] [Google Scholar]
- 15.Tian H, et al. Interannual cycles of Hantaan virus outbreaks at the human–animal interface in Central China are controlled by temperature and rainfall. Proc. Natl Acad. Sci. USA. 2017;114:8041–8046. doi: 10.1073/pnas.1701777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han BA, Kramer AM, Drake JM. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016;32:565–577. doi: 10.1016/j.pt.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olival KJ, et al. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reservoirs of future zoonotic diseases. Proc. Natl Acad. Sci. USA. 2015;112:7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kugeler KJ, Staples JE, Hinckley AF, Gage KL, Mead PS. Epidemiology of human plague in the United States, 1900-2012. Emerg. Infect. Dis. 2015;21:16–22. doi: 10.3201/eid2101.140564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornok S, et al. Synanthropic rodents and their ectoparasites as carriers of a novel haemoplasma and vector-borne, zoonotic pathogens indoors. Parasites Vectors. 2015;8:27. doi: 10.1186/s13071-014-0630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokarska-Rodak M, et al. Serological surveillance of vector-borne and zoonotic diseases among hunters in eastern Poland. J. Vector Borne Dis. 2016;53:355–361. [PubMed] [Google Scholar]
- 23.Prist PR, et al. Landscape, environmental and social predictors of Hantavirus risk in Sao Paulo, Brazil. PLoS ONE. 2016;11:e0163459. doi: 10.1371/journal.pone.0163459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy EC, Martyn CN, Bateman DE, Heckels JE, Lawton NF. Lyme disease: Prevalence and clinical importance of Borrelia burgdorferi specific IgG in forestry workers. Lancet. 1989;333:484–486. doi: 10.1016/S0140-6736(89)91377-9. [DOI] [PubMed] [Google Scholar]
- 25.Esmaeili S, et al. Epidemiological survey of tularemia in Ilam Province, west of Iran. Bmc Infect. Dis. 2019;19:502. doi: 10.1186/s12879-019-4121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friant S, Paige SB, Goldberg TL. Drivers of bushmeat hunting and perceptions of zoonoses in Nigerian hunting communities. PLoS Neglected Tropical Dis. 2015;9:e0003792. doi: 10.1371/journal.pntd.0003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams MJ, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017) Arch. Virol. 2017;162:2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 28.Calisher CH, Sweeney W, Mills JN, Beaty BJ. Natural history of Sin Nombre virus in western Colorado. Emerg. Infect. Dis. 1999;5:126–134. doi: 10.3201/eid0501.990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eskelinen, O. Studies on the Ecology of the Wood Lemming, Myopus Schisticolor (University of Joensuu, Finland, 2004).
- 30.Keesing F, Ostfeld RS. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl Acad. Sci. USA. 2021;118:e2023540118. doi: 10.1073/pnas.2023540118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibb R, et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;584:398–402. doi: 10.1038/s41586-020-2562-8. [DOI] [PubMed] [Google Scholar]
- 32.Gottdenker NL, Streicker DG, Faust CL, Carroll CR. Anthropogenic land use change and infectious diseases: a review of the evidence. EcoHealth. 2014;11:619–632. doi: 10.1007/s10393-014-0941-z. [DOI] [PubMed] [Google Scholar]
- 33.Popp A, et al. Land-use futures in the shared socio-economic pathways. Glob. Environ. Change. 2017;42:331–345. doi: 10.1016/j.gloenvcha.2016.10.002. [DOI] [Google Scholar]
- 34.Hoekstra JM, Boucher TM, Ricketts TH, Roberts C. Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 2005;8:23–29. doi: 10.1111/j.1461-0248.2004.00686.x. [DOI] [Google Scholar]
- 35.Hanski I. Habitat fragmentation and species richness. J. Biogeogr. 2015;42:989–993. doi: 10.1111/jbi.12478. [DOI] [Google Scholar]
- 36.Ecke F, Nematollahi Mahani SA, Evander M, Hörnfeldt B, Khalil H. Wildfire-induced short-term changes in a small mammal community increase prevalence of a zoonotic pathogen? Ecol. Evolution. 2019;9:12459–12470. doi: 10.1002/ece3.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriques R, Bizerril M, Palma A. Changes in small mammal populations after fire in a patch of unburned cerrado in Central Brazil. Mammalia. 2000;64:173–186. doi: 10.1515/mamm.2000.64.2.173. [DOI] [Google Scholar]
- 38.Fukasawa K, Miyashita T, Hashimoto T, Tatara M, Abe S. Differential population responses of native and alien rodents to an invasive predator, habitat alteration and plant masting. Proc. R. Soc. B: Biol. Sci. 2013;280:20132075. doi: 10.1098/rspb.2013.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibb R, et al. Mammal virus diversity estimates are unstable due to accelerating discovery effort. Biol. Lett. 2022;18:20210427. doi: 10.1098/rsbl.2021.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois A, et al. Microevolution of bank voles (Myodes glareolus) at neutral and immune-related genes during multiannual dynamic cycles: Consequences for Puumala hantavirus epidemiology. Infect., Genet. Evol. 2017;49:318–329. doi: 10.1016/j.meegid.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Berger SA. GIDEON: a comprehensive Web-based resource for geographic medicine. Int. J. Health Geographics. 2005;4:10. doi: 10.1186/1476-072X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson, D. E. & Reeder, D. M. Mammal Species of the World: a Taxonomic and Geographical Reference (John Hopkins University Press, Baltimore, 2005).
- 43.IUCN. IUCN Red List Categories. (International Union for the Conservation of Nature and Natural Resources, 2020).
- 44.WebPlotDigitizer v. 3.8 (Austin, Texas, USA, 2016).
- 45.Henttonen H, McGuire AD, Hansson L. Comparisons of amplitudes and frequencies (spectral analysis) of density variations in long-term data sets of Clethrionomys species. Annales Zoologici Fennici. 1985;22:221–227. [Google Scholar]
- 46.GBIF.org. Rattus norvegicus (30 October 2019) GBIF Occurrence Download10.15468/dl.f94bbc (2019).
- 47.GBIF.org. Rattus rattus (30 October 2019) GBIF Occurrence Download10.15468/dl.7tzpcv (2019).
- 48.IUCN. IUCN Species Mapping Tools. Toolbox Instructions for IUCN RedListToolbox 2019 08 (2019).
- 49.ArcGIS Desktop 10.6 (Redlands, CA, USA, 2017).
- 50.Hancock, G. R., Stapleton, L. M. & Mueller, R. O. The Reviewer’s Guide to Quantitative Methods in the Social Sciences. Second Edition. (Routledge, 2019).
- 51.Kenny DA, Kaniskan B, McCoach DB. The performance of RMSEA in models with small degrees of freedom. Sociological Methods Res. 2015;44:486–507. doi: 10.1177/0049124114543236. [DOI] [Google Scholar]
- 52.R: a language and environment for statistical computing. v. 4.2.0 (Foundation for Statistical Computing, Vienna, Austria, Vienna, Austria, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Source data (including rodent species, reservoir status, synanthropy, habitat preferences, population fluctuations, list of reservoir–pathogen associations) needed to fully replicate and evaluate the analyses are provided as Supplementary Information. Global data used for the spatial mapping of rodent occurrences are available from the International Union for the Conservation of Nature and Natural Resources (IUCN, iucn.org) and the Global Biodiversity Information Facility (GBIF, gbif.org).
The model details necessary to replicate the study are given in the Methods section.