Abstract
The ability to activate macrophages in vitro for nitric oxide production and killing of Leishmania major parasites is dependent on tumor necrosis factor, although L. major-infected mice lacking the TNF receptor p55 (TNFRp55−/− mice) or both the TNFRp55 and TNFRp75 (TNFRp55p75−/− mice) are able to produce NO in vivo and eliminate the parasites. Here we report that activated T cells cocultured with macrophages results in TNFR-independent activation sufficient to control parasites and that both CD40/CD40L and LFA-1 contribute to T-cell-mediated macrophage activation. Thus, anti-CD3-stimulated T cells activated TNFR-deficient macrophages, while T cells from CD40L−/− mice were partially defective in triggering NO production by TNFRp55p75−/− macrophages. Moreover, in the presence of gamma interferon, anti-CD40 monoclonal antibody (MAb) activated TNFR-deficient macrophages. Finally, MAb blockade of LFA-1 completely inhibited macrophage NO production. Our data indicate that T cells can activate macrophages in the absence of TNF, thus providing a mechanism for how TNFR-deficient mice can control intracellular pathogens.
The protozoan parasite Leishmania major infects mononuclear phagocytes, and control of infection depends on adequate activation of the infected macrophages to kill parasites and inhibit their replication (15). In vitro studies with murine macrophages revealed that soluble factors secreted by activated T cells mediate activation of macrophages to produce nitric oxide (NO), resulting in killing or control of L. major parasites (2). Macrophage activation by soluble factors (cytokines) depends on gamma interferon (IFN-γ) as well as tumor necrosis factor (TNF) (11, 12, 19, 41). Optimal NO production occurs in macrophages via upregulation of inducible nitric oxide synthase (iNOS) mRNA, which is itself optimally induced when IFN regulatory factor 1 is upregulated by IFN-γ, and NF-κB is activated by a second signal (22, 32). TNF has been shown to be a major NF-κB-activating signal for macrophage activation. Thus, IFN-γ and autocrine secretion of TNF by macrophages are sufficient to mediate production of NO and killing of L. major parasites (11, 18).
We previously demonstrated that macrophages derived from TNFR (TNF receptor)p55−/− or TNFRp55p75−/− mice failed to produce NO and control parasites upon stimulation with IFN-γ in vitro, whereas TNFRp75−/− mice lacked this defect (25). This suggested that the TNF dependence of in vitro macrophage activation to produce NO and kill parasites was mediated by the TNFRp55. However, work with receptor knockouts, soluble TNFR-Ig (immunoglobulin) overexpression transgenics, and neutralizing antibodies show that TNF is not required for in vivo control of parasites, for the development of the type 1 IFN-γ response to antigen restimulation, or for upregulation of iNOS at the site of infection in vivo (9, 20, 25, 42, 44). These data suggest that an in vivo mechanism exists that permits macrophages to produce NO and control parasites independent of TNF.
Since T cells are present in the lesions of infected mice, and activated T cells can mediate macrophage NO production and control of parasites in vitro, we hypothesized that activated T cells could compensate for the lack of the TNFRp55 on macrophages (25, 34, 37, 40). Activated T cells can mediate macrophage activation via secretion of soluble macrophage-activating factors or via a IFN-γ-dependent cognate interaction between the T cell and the macrophage. Several costimulatory molecules on activated T cells have been implicated in macrophage activation, including CD40L and LFA-1 (35, 36, 40). It is not known, however, whether the contributions of CD40L and LFA-1 depend on TNF.
Therefore, to define a mechanism of TNFRp55-independent macrophage activation, we asked whether activated T cells could mediate macrophage activation in the absence of the TNFRp55 or both receptors, and if this activation resulted in control of parasites in vitro. We report here that T cells can activate TNFRp55−/− macrophages to produce NO and kill L. major parasites and that CD40/CD40L and LFA-1 contribute to T-cell-mediated macrophage activation in a TNF-independent system.
MATERIALS AND METHODS
Mice.
Receptor-deficient and control mice were bred and housed at the University of Pennsylvania. Mice were used at 6 and 8 weeks of age. TNFRp55 mice were backcrossed onto the C57BL/6 background for seven generations (26). The TNFRp55p75−/− mice were maintained on a random C57BL/6 × 129 hybrid background and were initially provided by Mark Moore (Genentech, South San Francisco, Calif.) (8). Wild-type (+/+) littermates from the seventh backcross to C57BL/6 (wild-type mice) and C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) were maintained for use as controls. SCID (Jackson) mice were purchased for use as a source of amastigotes. No significant differences between wild-type and C57BL/6 (Jackson) mice were detected, and only data from wild-type mice are shown. CD40L−/− mice were kindly provided by Christopher Hunter (University of Pennsylvania, Philadelphia) after generation on a 129/B6 background by Immunex (Seattle, Wash.). We found no differences in macrophage activation by cells from 129/Sv or 129/B6 mice compared to C57BL/6 mice.
Parasites.
L. major (WHO MHOM/IL-1/80 Friedlin clone) amastigotes were isolated from SCID mice infected 6 weeks previously with metacyclic promastigotes. Amastigotes were frozen in liquid nitrogen for storage. Upon thawing, viable amastigotes were counted by FDA fluorescence as described elsewhere (14).
Cell culture medium.
Endotoxin-free (<1 endotoxin unit/ml by the Limulus amebocyte lysate assay performed by the Cell Center, University of Pennsylvania) reagents were used. Wash medium (4.5 mg of glucose Dulbecco modified Eagle medium [DMEM] per ml, 2% fetal calf serum [FCS], 25 mM HEPES, 5 × 10−5 β-2-mercaptoethanol [2ME], 100 U of penicillin-6-potassium per ml, 100 μg of streptomycin sulfate, 2 mM l-glutamine, 10 μg of polymyxin B sulfate [Sigma] per ml) and Complete tissue culture medium (CTCM; 4.5 mg of glucose DMEM per ml, 10% FCS, 25 mM HEPES, 5 × 10−5 2ME, 100 U of penicillin-6-potassium per ml, 100 μg of streptomycin sulfate, 2 mM l-glutamine, 10 μg of polymyxin B sulfate per ml) were used for harvesting and culturing of cells, respectively. Red blood cells (RBCs) were lysed with lysing buffer (0.017 M Tris, 0.16 M NH4Cl [pH 7.2]) at room temperature as needed. Nylon wool columns were primed, loaded, and eluted with RPMI-10 (RPMI 1640, 10% FCS, 25 mM HEPES, 5 × 10−5 2ME, 100 U of penicillin-6-potassium per ml, 100 μg of streptomycin sulfate, 2 mM l-glutamine, 10 μg of polymyxin B sulfate per ml). Cultures using lipopolysaccharide (LPS) were prepared with CTCM without polymyxin B sulfate. L-cell conditioned medium (30% L-cell supernatants [43], 20% FCS, 4.5 mg of glucose DMEM per ml, 10% FCS, 25 mM HEPES, 100 U of penicillin-6-potassium per ml, 100 μg of streptomycin sulfate, 2 mM l-glutamine) was used for bone marrow macrophages.
Preparation of cells. (i) Macrophages.
Peritoneal exudate cells (PECs) from naive mice were used as a source of resident macrophages. PECs were harvested by lavage of the peritoneal cavity with wash medium; the percentage of macrophages was determined by differential stain (Hema 3 stain set; Fisher Scientific, Swedesboro, N.J.), and the population was typically 50 to 70% macrophages. PECs were adjusted to contain 106 macrophages/ml (final concentration). Bone marrow macrophages were derived as a source of T-cell-free naive macrophages. Briefly, marrow was eluted from the femurs of naive mice. RBCs were lysed with lysing buffer, and cells were plated at 107/50 ml of L-cell conditioned medium. Cultures were fed with 30% volume on day 3 of culture. Macrophages were harvested at day 6 of culture by incubating on ice with endotoxin-free phosphate-buffered saline, washed, and cultured at 106/ml in CTCM (43).
(ii) T cells.
T cells were obtained from two sources: those resident to the peritoneal cavity (10 to 15% CD4+, 5 to 8% CD8+) and enriched splenic T cells from naive mice. Splenic T cells were prepared by harvesting spleens from naive mice and disruption in glass tissue grinders. After lysis of RBCs with lysing buffer, splenocytes were washed and filtered through a 70-μm-pore-size cell strainer (Falcon). Cells were loaded onto RPMI-10-primed sterile nylon wool columns at 108 per column and incubated at 37°C–6% CO2 for 45 min. Nonadherent cells (>90% CD3+, <2% Mac-1+, <5% B220+) were eluted with 25 ml of warm RPMI-10, washed, and resuspended in CTCM for coculture with macrophages.
Cell activation and in vitro infection.
Enriched T cells were incubated with macrophages and soluble anti-CD3 (145-2C11; Pharmingen, San Diego, Calif.) at 5 μg/ml for 72 h. Macrophages were incubated with T cells and anti-CD3 monoclonal antibody (MAb) or with recombinant IFN-γ (10 to 100 U/ml, as indicated; Genzyme, Cambridge, Mass.), with or without parasites. Other cultures were incubated with recombinant IFN-γ and the agonistic MAb to CD40 (3/23; 0.1 to 10 μg/ml; Pharmingen) or LPS (Sigma L5014). Activated cultures were incubated with or without anti-IFN-γ MAb XMG-6 (50 μg/ml, final concentration), the l-arginine analogue l-NMMA or its control d-NMMA (10 μM) for the duration of the cultures to block activation or NO production (11, 12, 23). In addition, clones specific for LFA-1 (FD441.8) (28) and ICAM-1 (YN1/1.7.4) (38) were generously provided by Ellen Pure (The Wistar Institute, Philadelphia, Pa.). Antibody specific for ICAM-2 (3C4 [mIC2/4]) was purchased from Pharmingen. Macrophage cultures were infected in suspension cultures (polypropylene tubes) based on efficient in vitro infection as previously described (24), using viable amastigotes at a 2:1 amastigote ratio. Aliquots were removed at 2 and 72 h and stained for visual quantitation of the infection.
IFN-γ ELISA.
IFN-γ was measured in supernatants of T cell-macrophage cocultures after 72 h of stimulation with anti-CD3 using a previously described enzyme-linked immunosorbent assay (ELISA) (29).
Indicators of macrophage activation.
NO production was assessed by measuring NO2 in supernatants harvested at 72 h using the Greiss reagent (10). Control of parasite replication was determined by quantitation of the number of intracellular parasites per 100 macrophages 72 h postinfection in vitro.
Statistics.
Significance was determined by Student's paired t test, with a P value of <0.05 considered significant.
RESULTS
Production of NO by TNFR-deficient macrophages stimulated by activated T cells.
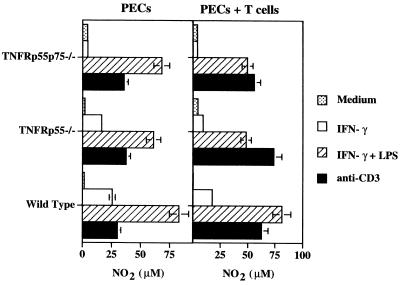
We previously demonstrated that recombinant IFN-γ fails to activate resident peritoneal macrophages lacking the p55 TNFR (TNFRp55−/− and TNFRp55p75−/−) to produce NO and control L. major in vitro (25, 44). However, during L. major infection in TNFR-deficient mice, iNOS is upregulated and parasites are reduced in number to levels equivalent to that seen in wild-type mice (25). Moreover, antigen-elicited peritoneal macrophages from infected mice produced NO and were leishmanicidal (25). We hypothesized that the difference between our in vitro and in vivo findings might be due to the presence of T cells in vivo and their absence in vitro. Therefore, we tested whether T cells might mediate TNFRp55-independent macrophage activation. PECs, which contained 10% CD4+, 3 to 6% CD8+, and 50 to 70% Mac-1+ cells, from naive wild-type, TNFRp55−/−, and TNFRp55p75−/− mice were incubated with anti-CD3, and NO production was measured. As a control, we stimulated macrophages with LPS plus IFN-γ, which is known to be a TNF-independent pathway of NO production (25, 44, 45). Anti-CD3 treatment of PECs induced NO production in both wild-type and TNFR-deficient cells, although the levels of NO were always less than those seen when cells were stimulated with IFN-γ plus LPS (Fig. 1).
FIG. 1.
Triggering NO production by treatment of TNFR−/− PECs with anti-CD3. Resident PECs pooled from three to five wild-type, TNFRp55−/−, or TNFRp55p75−/− mice were cultured with medium, IFN-γ (100 U/ml), IFN-γ plus LPS (100 ng/ml), or soluble anti-CD3 MAb 145-2C11 (5 μg/ml) for 72 h, at which time NO2 was measured in supernatants by using the Greiss reagent. Cultures were normalized to contain 106 macrophages/ml. Left, PECs (10 to 20% CD4+ T cells; 50 to 60% Mac-1+ F4/80+ B220− CD19− T cells). Right, PECs plus nylon wool-enriched splenic T cells from naive mice (ratio, 1 T cell/1 macrophage). T cells incubated without macrophages in the presence of anti-CD3 or IFN-γ and LPS produced less than 10 μM NO2 (data not shown). Limit of detection for all Greiss reactions was 4 μM. The data represent one experiment showing the mean NO2 production of duplicate cultures (±SE). Similar results were obtained from four additional experiments.
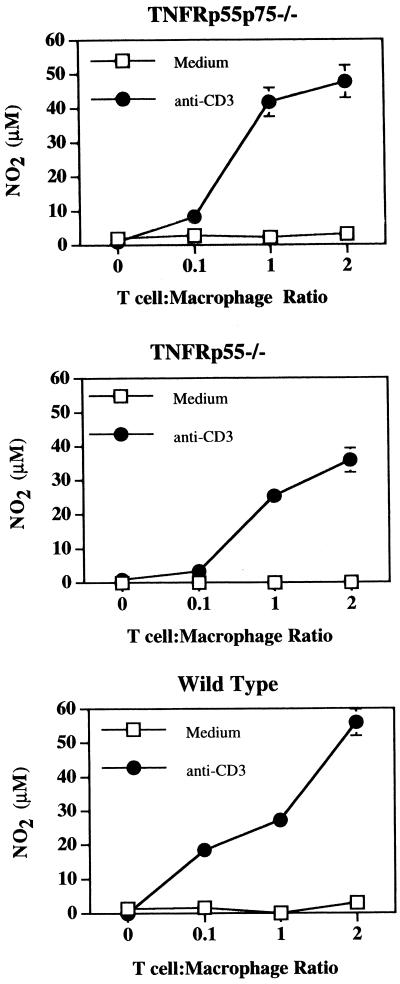
Since T cells represent only a small percentage of cells in the peritoneal cavity, we asked if addition of T cells to the PECs would increase NO production. As seen in Fig. 1, NO production could be augmented by addition of splenic T cells to levels similar to that induced by IFN-γ and LPS. On the other hand, T cells incubated without macrophages failed to produce NO (data not shown), confirming previous reports that T cells do not produce NO (39). To define the optimal T cell/macrophage ratio for activation, we cocultured bone marrow-derived macrophages with increasing numbers of naive anti-CD3-treated T cells. Higher T cell/macrophage ratios yielded the most efficient macrophage activation in all groups (Fig. 2). While the highest level of NO produced by normal macrophages varied between experiments, the ability of macrophages from TNFR-deficient mice to produce NO in the presence of anti-CD3-activated T cells at levels close to that observed by wild-type macrophages was consistent. Taken together, these results demonstrate that T cells can activate macrophages in the absence of TNFRs.
FIG. 2.
T-cell dose dependence of NO production by TNFR−/− macrophages. Bone marrow-derived macrophages were incubated with increasing numbers of T cells in the presence or absence of anti-CD3 (5 μg/ml), and NO was measured as in Fig. 1. The data show either TNFRp55p75−/− bone marrow macrophages plus TNFRp55p75−/− T cells, TNFRp55−/− bone marrow macrophages plus TNFRp55−/− T cells, or wild-type bone marrow macrophages plus wild-type T cells. The data represent one experiment showing the mean NO2 production of duplicate cultures (±SE). Similar results were obtained from six additional experiments.
Leishmanicidal activity of TNFR-deficient macrophages stimulated by activated T cells.
Next, we investigated whether T-cell-mediated macrophage activation was sufficient to induce control of L. major parasites. PEC cultures infected with L. major amastigotes were stimulated with anti-CD3, and the number of parasites per 100 macrophages (mean ± standard error [SE]) was determined 72 h after infection by differential staining. Macrophages from PEC cultures stimulated with anti-CD3 controlled parasite growth over 72 h and produced NO (Table 1). Macrophage activation was TNFR independent, suggesting that if two signals are required for T-cell-mediated macrophage activation in vitro, TNF is not one of the required signals.
TABLE 1.
NO production and control of parasites during T-cell-mediated activation of TNFR-deficient PECsa
| Cells | Treatment | NO2 (μM) | Parasites/100 macrophages |
|---|---|---|---|
| TNFRp55p75−/− | Medium | <4 | 348 ± 29 |
| Anti-CD3 | 47 ± 1 | 42 ± 19 | |
| Anti-CD3 + XMG | 5 ± 1 | 331 ± 14 | |
| Anti-CD3 + l-NMMA | 5 ± 1 | 376 ± 2 | |
| Anti-CD3 + d-NMMA | 41 ± 1 | 16 ± 4 | |
| TNFRp55−/− | Medium | <4 | 215 ± 110 |
| Anti-CD3 | 47 ± 1 | 16 ± 1 | |
| Anti-CD3 + XMG | <4 | 465 ± 6 | |
| Anti-CD3 + l-NMMA | 6 ± 1 | 436 ± 19 | |
| Anti-CD3 + d-NMMA | 42 ± 1 | 15 ± 11 | |
| Wild type | Medium | <4 | 264 ± 10 |
| Anti-CD3 | 57 ± 1 | 26 ± 7 | |
| Anti-CD3 + XMG | <4 | 301 ± 19 | |
| Anti-CD3 + l-NMMA | 8 ± 1 | 292 ± 29 | |
| Anti-CD3 + d-NMMA | 53 ± 1 | 16 ± 4 |
Resident PECs were harvested, suspended at 106 macrophages/ml, and infected for 2 h with two amastigotes per macrophage in the presence of the anti-CD3 (5 μg/ml) and indicated blocking reagents. NO2 was quantitated from the supernatants at 72 h of culture by the Greiss reagent, and parasites per 100 macrophages was determined by counting differential stains of duplicate cultures (minimum of 400 macrophages/experimental group) 72 h postinfection. The data shown are the mean NO2 levels ±SE or the mean number of parasites per 100 macrophages ± SE from one experiment. The experiment was repeated, with similar results.
To confirm that the leishmanicidal activity we observed was dependent on NO, we blocked the l-arginine–NO synthetic pathway by culturing cells with the l-arginine analogue l-NMMA. As seen in Table 1, l-NMMA but not its enantiomer, d-NMMA, blocked the effect of anti-CD3 treatment on the parasite numbers and NO production. As expected, neutralization of IFN-γ with MAb XMG-6 demonstrated that IFN-γ is required for T-cell-mediated macrophage activation (4, 11, 12, 19).
Activation of TNFR-deficient macrophages by IFN-γ and agonistic anti-CD40 MAb.
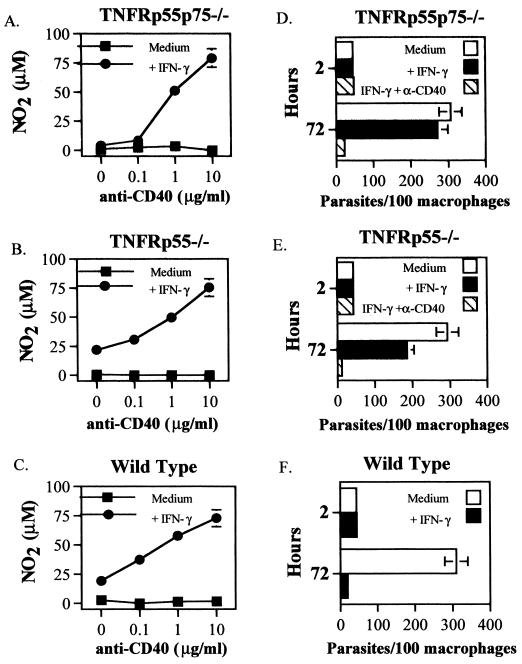
One T-cell costimulatory molecule which could synergize with IFN-γ to induce NO in TNFR-deficient macrophages is CD40. It has been shown that IFN-γ and CD40 cross-linking results in NO production by macrophages, but it is not known whether this depends on autocrine production of TNF (35, 40). We observed constitutive, low-level expression of CD40 on macrophages from wild-type, TNFRp55−/−, and TNFRp55p75−/− mice by flow cytometry (data not shown). To determine whether NO induced by IFN-γ and CD40 cross-linking required TNFRs, we incubated resident PECs from uninfected mice with IFN-γ and an agonistic MAb to CD40. In the presence of IFN-γ, anti-CD40 MAb induced a dose-dependent induction of NO by macrophages from TNFRp55p75−/−, TNFRp55−/−, and wild-type mice (Fig. 3A to C). Further, the activation induced by IFN-γ and anti-CD40 was sufficient to permit killing of parasites in TNFR-deficient or wild-type mice (Fig. 3D to F).
FIG. 3.
Activation of TNFR−/− PECs by cross-linking CD40 in the presence of IFN-γ. Resident PECs pooled from three to five mice were incubated with IFN-γ (100 U/ml) and increasing concentrations of anti-CD40 MAb. PECs were also infected with amastigotes as described in Materials and Methods and exposed to either IFN-γ (100 U/ml) or IFN-γ and anti-CD40 MAb (10 μg/ml). Nitrite production (72 h) or parasites per 100 macrophages (2 and 72 h) in TNFRp55p75−/− macrophages (A and D), TNFRp55−/− macrophages (B and E), or wild-type macrophages (C and F) was determined. The data shown represent one experiment showing the mean NO2 production of duplicate cultures (±SE). The parasite data shown represent the mean number of parasites in two separate culture tubes (±SE). (F) Anti-CD40 was not used. Similar results were obtained in two additional experiments.
Contributions of CD40L and LFA-1 to T-cell-mediated macrophage activation.
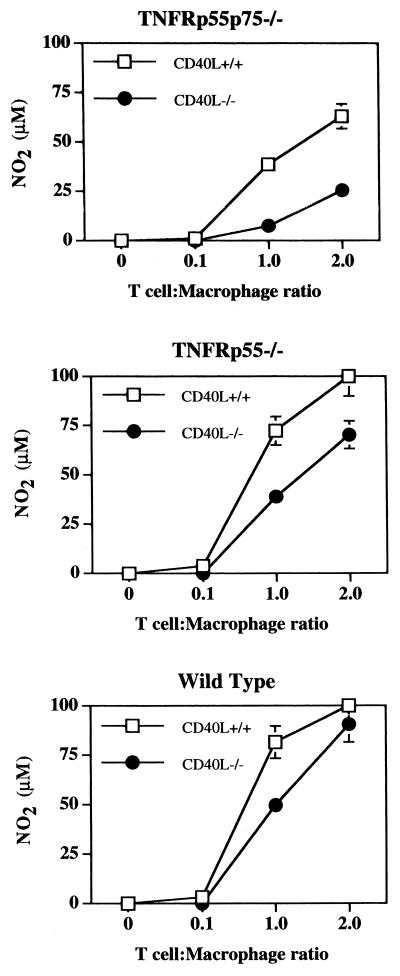
Since cross-linking CD40 in the presence of IFN-γ is sufficient to activate macrophages lacking the TNFRp55, we asked whether CD40L was required for optimal T-cell-mediated macrophage activation. CD40L is upregulated rapidly on CD4+ T cells upon activation with immobilized anti-CD3 or soluble anti-CD3 and antigen-presenting cells (5, 35). Flow cytometric analysis of T cells activated on immobilized anti-CD3 revealed a rapid upregulation of CD40L independent of the TNFRs (data not shown). To address the role of CD40L in T-cell-mediated macrophage activation, we incubated bone marrow-derived macrophages from TNFRp55−/− or TNFRp55p75−/− mice with T cells from the spleens of naive TNFR-deficient, wild-type, or CD40L−/− mice. While a lack of the TNFRs on T cells did not impair macrophage activation (Fig. 2), we observed a decrease in NO production when CD40L−/− T cells were used to activate TNFRp55p75−/− or TNFRp55−/− macrophages (Fig. 4A, B, and D). However, as is clear from these data, there was not a complete inhibition of NO when CD40L−/− T cells were used to activate macrophages, indicating that other factors must be involved. Thus, it appears that CD40-CD40L interactions contribute to T-cell-mediated activation in the absence of both of the TNFRs, but that other factors may play a role as well.
FIG. 4.
Efficiency of CD40L−/− T cells in triggering macrophage NO production by TNFR−/− macrophages. Bone marrow-derived macrophages were incubated with increasing numbers of T cells from CD40L−/− or CD40L+/+ (wild-type) mice, and NO was measured as in Fig. 1. The data show the mean NO2 levels (±SE) in one experiment with TNFRp55p75−/− macrophages, TNFRp55−/− macrophages, or wild-type macrophages after 72 h of incubation. The experiment was repeated seven times, and Student's paired t test indicated that NO production was significantly less (P < 0.05) for TNFRp55p75−/− or TNFRp55−/− macrophages incubated with CD40L−/− T cells than for those incubated with CD40+/+ T cells. CD40L+/+ and CD40L−/− T cells induced similar levels of NO in wild-type macrophages.
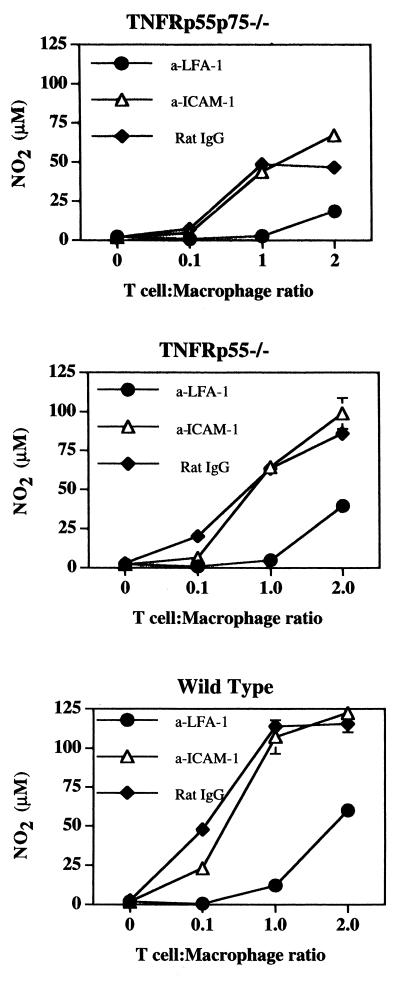
Next, we investigated the role of adhesion molecules LFA-1 and ICAM-1 in T-cell-mediated macrophage activation by blockade with MAb. NO production was blocked by anti-LFA-1 MAb (Fig. 5). At present, we have not been able to identify the ligand that binds LFA-1 and contributes to T-cell-mediated macrophage activation. As seen in Fig. 5, anti-ICAM-1 MAb failed to block NO production. These results are similar to those of a previous study where anti-LFA-1, but not anti-ICAM-1, blocked T-cell-induced NO production by macrophages (40). There are two other known ligands for LFA-1, termed ICAM-2 and ICAM-3 (6, 7). We found that blockade of ICAM-2 also failed to influence T-cell-mediated macrophage activation but were unable to block ICAM-3 due to lack of reagents (Table 2). Thus, neither ICAM-1 nor ICAM-2 is required for T-cell-mediated macrophage activation, and ICAM-3 remains a candidate as a critical ligand in this system.
FIG. 5.
Blockade of T-cell-mediated macrophage activation and IFN-γ production in vitro by anti-LFA-1 MAb. Bone marrow macrophages from TNFR-deficient or wild-type mice were cultured with T cells and anti-CD3 (5 μg/ml) in the presence of anti-LFA-1 (10 μg/ml), anti-ICAM-1 (10 μg/ml), or control rat IgG (10 μg/ml) for 72 h. Nitrites were measured as in Fig. 1. The data shows the mean NO2 levels (±SE) of one experiment with TNFRp55p75−/− macrophages, TNFRp55−/− macrophages, and wild-type macrophages after 72 h of incubation. The experiment was repeated three times, with similar results. Student's paired t test indicated that NO production was significantly less (P < 0.05) for all macrophages incubated with anti-LFA-1 but not for those incubated with anti-ICAM-1.
TABLE 2.
Blockade of T-cell-mediated macrophage NO production by anti-LFA-1 MAba
| Treatment | NO2 (μM)
|
|
|---|---|---|
| Wild type | TNFRp55−/− | |
| Anti-CD3 | 49 ± 2 | 33 ± 3 |
| Anti-CD3 plus: | ||
| Anti-LFA-1 | <4 | <4 |
| Anti-ICAM-1 | 53 ± 1 | 35 ± 3 |
| Anti-ICAM-2 | 51 ± 2 | 32 ± 3 |
| Anti-ICAM-1 + anti-ICAM-2 | 47 ± 2 | 27 ± 1 |
| Rat IgG | 50 ± 2 | 29 ± 2 |
Bone marrow-derived macrophages were incubated with T cells at a T cell/macrophage ratio of 2:1 and treated with anti-CD3 (5 μg/ml) for 72 h. Some cultures were also treated with MAbs to LFA-1 or with ICAM-1 and/or ICAM-2 (10 μg/ml, final concentration). The data represent the mean NO levels of duplicate cultures ± SE. Similar results were obtained in three additional experiments.
DISCUSSION
Macrophages produce NO when treated with IFN-γ and infected with L. major parasites (12). This activation is dependent on TNF, which is produced by macrophages at the time of parasite infection (11). However, we previously reported that mice lacking TNFRs were able to eliminate L. major parasites. The aim of this study was to identify a mechanism of macrophage activation which compensates for the absence of TNFR signaling. We found that T cells cocultured with TNFR-deficient macrophages induced NO production and leishmanicidal activity. This activation depended on IFN-γ and l-arginine for NO production and control of Leishmania parasites in vitro. T cells produce a variety of soluble factors upon activation, but supernatants taken from activated T cells failed to mediate macrophage NO production in TNFR-deficient macrophages (data not shown). Hence, we investigated whether molecules expressed by activated T cells might mediate activation in a TNFR-independent manner. Two logical candidates were CD40L and LFA-1, which have been shown to contribute to macrophage activation (40). We report here that both CD40L and LFA-1 can participate in T-cell-mediated macrophage activation in the absence of the TNFRs.
Previous reports show that fixed activated T cells from CD40L−/− mice are defective in triggering IFN-γ-dependent NO production by macrophages (35). Furthermore, draining lymph node cells from Leishmania amazonensis-infected CD40L−/− mice were defective in stimulating NO production by infected macrophages (31). We found that stimulation of TNFRp55−/− and TNFRp55p75−/− macrophages with anti-CD40 MAb in the presence of IFN-γ induced TNFR-independent NO production. This observation suggested that CD40 could be responsible for macrophage activation and parasite elimination in TNFR-deficient mice. However, activated T cells from CD40L−/− mice were not completely defective in triggering TNFR-independent NO by macrophages. These data are consistent with previous studies where it was found that the requirement for CD40L when T cells were used to activate macrophages was apparent only early after T-cell activation, since after 24 h of activation, CD40L−/− T cells were capable of activating macrophages in vitro (35). Therefore, while CD40L on T cells maximizes NO production by TNFR−/− macrophages, it is evident that factors other than CD40L contribute to T-cell-mediated macrophage activation.
To examine other pathways of T-cell-mediated, TNFR-independent NO production by macrophages, we explored the role of LFA-1, another surface protein found on activated T cells (40). In assessing the ability of an anti-LFA-1 MAb to suppress NO production in macrophages cocultured with wild-type or CD40L−/− T cells, we found dramatic suppression of macrophage NO production when LFA-1 was blocked. Interestingly, blocking with an anti-ICAM-1 and/or anti-ICAM-2 MAb failed to inhibit macrophage activation. The inability of ICAM-1 blockade to inhibit NO production was surprising, since ICAM-1 has been implicated in NO production by another macrophage type, Kupffer cells (13, 16, 17). There are several possible reasons that blockade of LFA-1, but not ICAM-1, might abrogate T-cell-mediated macrophage activation, the simplest of which is that LFA-1 mediates macrophage activation via another ligand such as ICAM-3, which in human monocytes is expressed constitutively. Blockade of ICAM-3, but not ICAM-1 or ICAM-2, inhibited with high efficiency peripheral blood dendritic cell-stimulated mixed lymphocyte responses (33), suggesting that ICAM-3 can have unique roles in T cell–antigen-presenting cell interactions. However, without antibodies to block murine ICAM-3, we are unable to directly test whether ICAM-3 is important in our system.
LFA-1 is an important adhesion molecule in the immune system, and blockade of cell-cell adhesion can prevent the Th1-associated mixed lymphocyte reactions, antigen-specific stimulation, and anti-CD3 MAb stimulation (27, 30, 38). Therefore, we investigated if blockade of LFA-1 might result in suppression of IFN-γ production by T cells in these cultures and thus lead to the absence of macrophage activation. Indeed, we found that anti-LFA-1 partly blocked IFN-γ production by the T cell-macrophage cocultures (data not shown). Interestingly, anti-ICAM-1 also blocked IFN-γ production, but the cultures nevertheless retained the ability to activate macrophages to produce NO. These results suggest that while anti-ICAM-1 MAb reduces IFN-γ production by T cells, there is nevertheless sufficient IFN-γ available to stimulate macrophage NO production. To confirm that the reduced IFN-γ production observed when LFA-1 was blocked was not responsible for the reduced macrophage activation, we added IFN-γ to PEC cultures treated with anti-LFA-1 MAb. Addition of IFN-γ failed to bypass the blocking effect of anti-LFA-1 (data not shown). Therefore, the effects of blockade by anti-LFA-1 MAb were probably not due to suboptimal IFN-γ production, and these data argue for a positive signal either on the T cells or on the macrophages.
One mechanism to account for the ability of LFA-1 to trigger macrophage NO production, whereas ICAM-1 or ICAM-2 blockade will not, is that LFA-1 can mediate signals to the T cell, enhancing the production of additional soluble factors or the expression of other costimulatory molecules (21). LFA-1 can also stabilize the T cell-macrophage cognate interaction for efficient contact of other costimulatory molecules with their ligands (40). Work with transfectants suggests that activated LFA-1 may have different binding sites for the three ICAMs (3). These studies suggest that there may be multiple effects of LFA-1 on the T cell-macrophage cognate interaction and signaling by LFA-1 and its ligand.
Recent studies of another TNFR family member, TRANCE, shows that TRANCE is critical to the development of CD40-CD40L-independent CD4+ T-cell response to lymphocytic choriomeningitis virus via induction of interleukin-12 (1). Thus, blockade of TRANCE-TRANCE receptor interactions completely abrogated IFN-γ production in virus-infected CD40−/− mice. In contrast to the TRANCE dependence of interleukin-12 production by dendritic cells and the subsequent IFN-γ production by CD4+ T cells, pilot studies in this laboratory suggest that CD40L-independent T-cell-mediated macrophage activation does not require TRANCE (data not shown). Thus, some factor besides TRANCE mediates TNFR-independent NO production by CD40L−/− T cells.
The presence of two distinct pathways of macrophage activation—one mediated by IFN-γ and TNF requiring the TNFRp55; the other mediated by T cell-macrophage cognate interactions—implies that the immune response can mediate either a generalized or a local macrophage-activating response in vivo. Theoretically, IFN-γ and TNF can stimulate macrophage activation away from the site of production, whereas T-cell-mediated macrophage activation requires that the activated T cell be in contact with the infected macrophage. Advantages to both mechanisms of macrophage activation are apparent. It is clear from these studies that when the TNFRp55 is present, there is highly efficient macrophage activation in the presence of L. major and IFN-γ. When this pathway is functional, there is early upregulation of iNOS and control of parasites. However, there are potential advantages to the T-cell-mediated mechanism of macrophage activation. One advantage is that the macrophage activation in vivo is mediated by a specific T cell activating an infected macrophage, instead of generalized systemic macrophage activation mediated by the presence of large amounts of IFN-γ, which could potentially lead to excessive NO production. Another advantage to T-cell-mediated macrophage activation is that less IFN-γ may be required when the T cells activate the macrophage at the site of infection, preventing nonspecific macrophage activation and extensive pathology. The presence of multiple pathways, however, ensures that L. major, as well as other pathogens, can be controlled by the immune response.
ACKNOWLEDGMENTS
We thank Mark Moore for providing the TNFRp75−/− and TNFRp55p75−/− mice, C. Hunter for providing the CD40L−/− mice, and Hunter and Farrell for helpful discussions and review of the manuscript. Finally, we thank Nadine Blanchard for technical assistance.
This work was supported by NIH grant AI 41880.
REFERENCES
- 1.Bachmann M F, Wong B R, Josien R, Steinman R M, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belosevic M, Davis C E, Meltzer M S, Nacy C A. Regulation of activated macrophage antimicrobial activities: identification of lymphokines that cooperate with IFN-γ induction of resistance to infection. J Immunol. 1988;141:890–896. [PubMed] [Google Scholar]
- 3.Binnerts M E, van Kooyk Y, Simmons D L, Figdor C G. Distinct binding of T lymphocytes to ICAM-1, -2, or -3 upon activation of LFA-1. Eur J Immunol. 1994;24:2155–2160. doi: 10.1002/eji.1830240933. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan C, Moll H, Solbach W, Rollinghoff M. Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol. 1990;20:1131–1135. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- 5.Castle B E, Kishimoto K, Stearns C, Brown M L, Kehry M R. Regulation of expression of the ligand for CD40 on T helper lymphocytes. J Immunol. 1993;151:1777–1788. [PubMed] [Google Scholar]
- 6.de Fougerolles A R, Springer T A. Intracellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Fougerolles A R, Stacker S A, Schwarting R, Springer T A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991;174:253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson S L, De Sauvage F J, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan K C F, Schreiber R D, Goeddel D V, Moore M W. Decreased sensitivity to tumour-necrosis factor but normal T cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 9.Garcia I, Miyazaki Y, Araki K, Araki M, Lucas R, Grau G E, Milon G, Belkaid Y, Montixi C, Lesslauer W, Vassalli P. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol. 1995;25:2401–2407. doi: 10.1002/eji.1830250841. [DOI] [PubMed] [Google Scholar]
- 10.Green L, Wagner D, Glogowski J, Skipper P, Wishnok J, Tannebaum S. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 11.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 12.Green S J, Meltzer M S, Hibbs J B, Jr, Nacy C A. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–283. [PubMed] [Google Scholar]
- 13.Igietseme J U, Uriri I M, Hawkins R, Rank R G. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J Leukoc Biol. 1996;59:656–662. doi: 10.1002/jlb.59.5.656. [DOI] [PubMed] [Google Scholar]
- 14.Jackson P R, Pappas M G, Hansen B D. Fluorogenic substrate detection of viable intracellular and extracellular pathogenic protozoa. Science. 1985;227:435–438. doi: 10.1126/science.2578226. [DOI] [PubMed] [Google Scholar]
- 15.James S L. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurose I, Ebinuma H, Higuchi H, Yonei Y, Saito H, Miura S, Ishii H. Nitric oxide mediates mitrochondrial dysfunction in hepatoma cells induced by non-activated Kupffer cells: an evidence implicating ICAM-1-dependent process. J Gastroenterol Hepatol. 1995;10:S68–S71. doi: 10.1111/j.1440-1746.1995.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurose I, Saito H, Miura S, Ebinuma H, Hajime H, Watanabe N, Zeki S, Nakamura T, Takaishi M, Ishii H. CD18/ICAM-1-dependent oxidative NF-κB activation leading to nitric oxide production in rat Kupffer cells cocultured with syngenic hepatoma cells. J Clin Investig. 1997;99:867–878. doi: 10.1172/JCI119251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liew F Y, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 19.Liew F Y, Millott S, Parkinson C, Palmer R M, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- 20.Liew F Y, Parkinson C, Millott S, Severn A, Carrier M. Tumor necrosis factor (TNFα) in leishmaniasis. I. TNFα mediates host protection against cutaneous leishmaniasis. Immunology. 1990;69:570–573. [PMC free article] [PubMed] [Google Scholar]
- 21.Lub M, van Kooyk Y, Figdor C G. Ins and outs of LFA-1. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 22.MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 23.Nacy C A. Macrophage activation to kill Leishmania tropica: characterization of a T cell-derived factor that suppresses lymphokine-induced intracellular destruction of amastigotes. J Immunol. 1984;133:448–453. [PubMed] [Google Scholar]
- 24.Nacy C A, Diggs C L. Intracellular replication of Leishmania tropica in suspension cultures of mouse peritoneal macrophages. Infect Immun. 1981;34:310–313. doi: 10.1128/iai.34.1.310-313.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major in mice lacking TNF receptors. J Immunol. 1998;160:5506–5513. [PubMed] [Google Scholar]
- 26.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Weignmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 27.Salomon B, Bluestone J A. Cutting edge: LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–5142. [PubMed] [Google Scholar]
- 28.Sanchez-Madrid F, Simon P, Thompson S, Springer T A. Mapping of antigenic and functional epitopes on the α and β subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semnani R T, Nutman T B, Hochman P, Shaw S, van Seventer G A. Costimulation by purified intercellular adhesion molecule 1 and lymphocyte function-associated antigen 3 induces distinct proliferation, cytokine and cell surface antigen profiles in human “naive” and “memory” CD4+ T cells. J Exp Med. 1994;180:2125–213. doi: 10.1084/jem.180.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soong L, Xu J C, Grewal I S, Kima P, Sun J, Longley B J, Jr, Ruddle N H, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 32.Spink J, Evans J. Binding of the transcription factor interferon regulatory factor-1 to the inducible nitric-oxide synthase promoter. J Biol Chem. 1997;272:24417–24425. doi: 10.1074/jbc.272.39.24417. [DOI] [PubMed] [Google Scholar]
- 33.Starling G C, McLellan A D, Egner W, Sorg R V, Fawcett J, Simmons D L, Hart D N. Intracellular adhesion molecule-3 is the predominant co-stimulatory ligand for leukocyte function antigen-1 on human blood dendritic cells. Eur J Immunol. 1995;28:2528–2532. doi: 10.1002/eji.1830250918. [DOI] [PubMed] [Google Scholar]
- 34.Stout R D, Bottomly K. Antigen specific activation of effector macrophages by interferon-IFN-γ producing (Th1) T cell clones. Failure of IL-4 producing (Th2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–765. [PubMed] [Google Scholar]
- 35.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 36.Suttles J, Miller R W, Tao X, Stout R D. T cells which do not express membrane tumor necrosis factor-α activate macrophage effector function by cell contact-dependent signaling of macrophage tumor necrosis factor-α production. Eur J Immunol. 1994;24:1736–1742. doi: 10.1002/eji.1830240803. [DOI] [PubMed] [Google Scholar]
- 37.Sypek J P, Panosian C B, Wyler D J. Cell contact-mediated macrophage activation for antileishmanial defense. II. Identification of effector cell phenotype and genetic restriction. J Immunol. 1984;133:3351–3357. [PubMed] [Google Scholar]
- 38.Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2) J Immunol. 1985;134:1403–1407. [PubMed] [Google Scholar]
- 39.Thuring H, Stenger S, Gmehling D, Rollinghoff M, Bogdan C. Lack of inducible nitric oxide synthase activity in T cell clones and T lymphocytes from naive and Leishmania major infected mice. Eur J Immunol. 1995;25:3229–3234. doi: 10.1002/eji.1830251205. [DOI] [PubMed] [Google Scholar]
- 40.Tian L, Noelle R J, Lawrence D A. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995;25:306–309. doi: 10.1002/eji.1830250152. [DOI] [PubMed] [Google Scholar]
- 41.Titus R G, Kelso A, Louis J A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984;55:157–165. [PMC free article] [PubMed] [Google Scholar]
- 42.Titus R G, Sherry B, Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989;170:2097–2103. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tushinski R J, Oliver I T, Guilbert L J, Tynan P W, Warner J R, Stanley E R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- 44.Vieira L Q, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 45.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55 and p75 deficient mice to chronic toxoplasmosis despite normal activation of iNOS in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]