Abstract
Background
Despite the increasing prevalence of type 2 diabetes mellitus (T2DM), limited pharmacologic options are available for prevention. Cholesteryl ester transfer protein inhibitors (CETPis) have been studied primarily as a therapy to reduce cardiovascular disease, but have also been shown to reduce new-onset diabetes. As new trial data have become available, this meta-analysis examines the effect of CETP inhibitors on new-onset diabetes and related glycaemic measures.
Methods and results
We searched MEDLINE, EMBASE, and Cochrane databases (all articles until 4 March, 2021) for randomised controlled trials (RCT) ≥1-year duration, with at least 500 participants, comparing CETPi to placebo, and that reported data on new-onset diabetes or related glycaemic measures [haemoglobin A1C (HbA1C), fasting plasma glucose, insulin, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR)]. A fixed effects meta-analysis model was applied to all eligible studies to quantify the effect of CETPi therapy on new-onset diabetes. Four RCTs (n = 75 102) were eligible for quantitative analysis of the effect of CETPi on new-onset diabetes. CETPis were found to significantly decrease the risk of new-onset diabetes by 16% (RR: 0.84; 95% CI: 0.78, 0.91; P < 0.001), with low between-trial heterogeneity (I2 = 4.1%). Glycaemic measures were also significantly improved or trended towards improvement in those with and without diabetes across most trials.
Conclusion
Although RCTs have shown mixed results regarding the impact of CETPi on cardiovascular disease, they have shown a consistent reduction in the risk of new-onset diabetes with CETPi therapy. Future trials of CETPis and potentially other HDL-raising agents should therefore specify new-onset diabetes and reversal of existing T2DM as secondary endpoints.
Introduction
Type 2 Diabetes Mellitus (T2DM)1 is growing exponentially. T2DM nearly doubles the risk of coronary heart disease (CHD) and stroke, and correlates with worse prognosis and increased cardiovascular disease (CVD) mortality.1 The impact of T2DM on CVD is partly mediated through diabetic dyslipidaemia, characterised by low high-density lipoprotein (HDL), hypertriglyceridaemia, and increased Apo-B levels.
Unfortunately, beyond lifestyle, pharmacologic therapies to prevent the onset of diabetes are limited. Metformin can lower the risk of diabetes in those with prediabetes.2 Emerging T2DM therapies, sodium-glucose co-transporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) agonists, may improve glycaemic control,3,4 but have not yet been evaluated for T2DM prevention. Statins are a cornerstone of cardiovascular risk reduction in persons with and without diabetes, but have been shown to increase the risk of new-onset T2DM.5,6
Cholesteryl ester transfer protein inhibitors (CETPis) are a class of lipid lowering medications with potential to lower the risk of new onset diabetes. Cholesteryl ester transfer protein (CETP) promotes exchange of triglycerides (TGs) and cholesterol ester (CE) from HDL to atherogenic ApoB100-containing lipoproteins. CETP inhibition also increases cholesterol-efflux from peripheral tissues, raising HDL-cholesterol (HDL-C) by shifting the partitioning of cholesterol towards HDL particles7 (Figure 1, Appendix 8). Unfortunately, randomized controlled trial (RCT) data have largely failed to show a benefit for CETPis as a class, with only one large RCT showing cardiovascular benefit from CETP inhibition,8 and several other studies showing no effect.9–12 A recent Mendelian randomization analysis points to compound specific failures, but not to a lack of class effect on cardiovascular outcomes.13 In fact, genetic work suggests that the impact on major cardiac events may depend mainly on absolute LDL-C and time of trial, as with other LDL-C lowering therapies.14
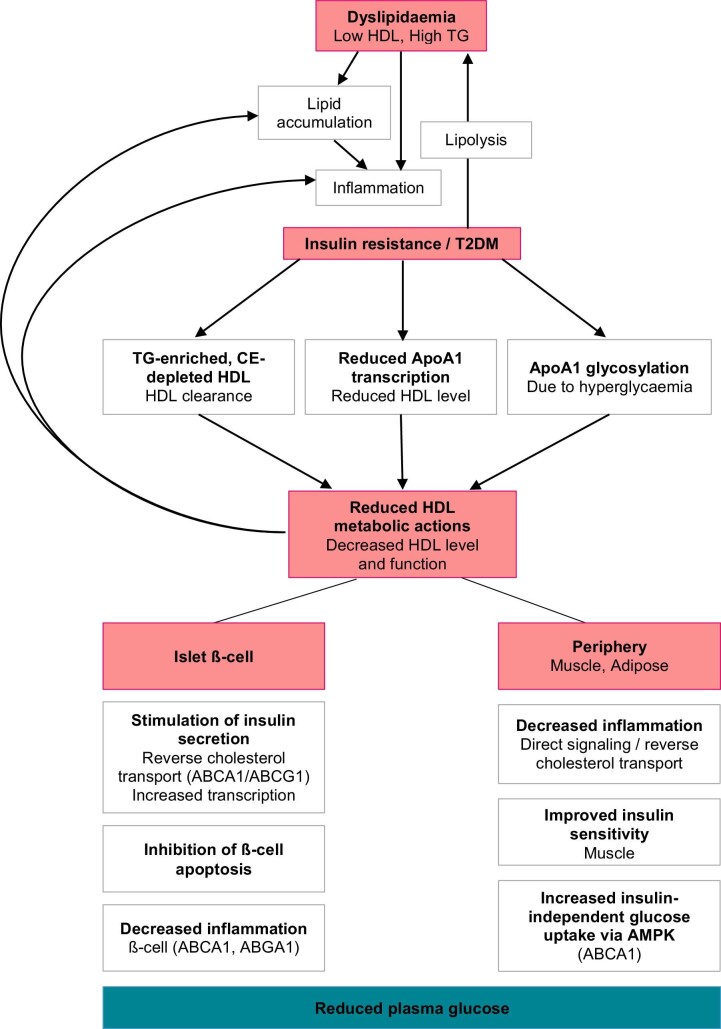
Figure 1.
Mechanisms linking HDL-C and diabetes. Dyslipidaemia (low HDL) causes lipid accumulation and inflammation propagating insulin resistance and T2DM. T2DM increases ApoA1 glycation, reduces ApoA1 transcription and changes HDL composition to increase clearance, reducing HDL level and function. HDL normally has reverse cholesterol transport and anti-inflammatory actions at ß-cells and in periphery that decrease plasma glucose.7 HDL promotes insulin secretion via ApoA130 and ABCA1/ABCG (1) or through stimulating insulin transcription. HDL-C may inhibit ER-stress-induced ß-cell apoptosis31 and islet cell inflammation via ABCA1 and ABCG.32 Loss of HDL particles and function exacerbates lipid accumulation and inflammation and increases plasma glucose, contributing to a vicious cycle.
Interestingly, some CETPi trials have trended towards reduction in new-onset diabetes. Biologic plausibility exists for the ability to prevent diabetes through CETP inhibition: Individuals with CETP polymorphisms that increase HDL-C have been shown to have lowered risk of T2DM and improved glycaemic status.15 Cellular and mechanistic studies also support a potential mechanistic link between CETP, HDL-C, and T2DM.16,17
Most initial RCTs of CETPi did not pre-specify or report on new-onset diabetes as an outcome, with the exception of randomized evaluation of the effects of anacetrapib through lipid modification (REVEAL) (anacetrapib vs. placebo, n = 30 449), which did find a reduction in new-onset diabetes with anacetrapib therapy.8 One prior meta-analysis of 4 RCTs evaluating the link between CETPi and new-onset diabetes18 found that CETPis reduced incidence of new-onset diabetes by 12%. Since its publication, novel post-hoc analyses of major trials, assessment of clinical effects of cholesteryl ester transfer protein inhibition with evacetrapib in patients with a high risk for vascular outcomes (ACCELERATE) (evacetrapib vs. placebo) and Dal-Outcomes (dalcetrapib vs. placebo) have been published19,20 with additional data now released on new-onset diabetes and glycaemic measures.
Hence, this meta-analysis sought to use all available large randomized clinical trial data available to evaluate the effect of CETPi on new-onset diabetes. Further, we sought to determine whether changes in related measures [haemoglobin A1C (HbA1C), fasting plasma glucose, insulin, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR)] were concordant with any observed effect.
Methods
MEDLINE, Embase, and Cochrane databases were systematically searched (all articles prior to 4 March, 2021) to identify results from randomised controlled trials (RCTs) that assess efficacy or safety of CETPi against placebo. Search strategies (Appendix 2) were constructed with drug names (obicetrapib, evacetrapib, dalcetrapib, anacetrapib, torcetrapib). McMaster RCT-hedge was adapted to restrict MEDLINE and Embase searches to RCTs.21 Reference lists of identified trials and ClinicalTrials.gov were also searched for additional relevant RCTs.
Title and abstract were screened independently. Full text was extracted from chosen publications and subsequently evaluated. Inclusion criteria were (a) RCTs comparing CETPi to placebo (b) participants were adults (c) not reviews or editorials (d) planned treatment period >1-year (e) >500 participants and (f) reported on one of new-onset diabetes, HbA1C, plasma glucose, insulin, or HOMA-IR. Conference abstracts were excluded if published manuscript was included. Quantitative analysis was limited to studies reporting new-onset diabetes.
Study characteristics, patient characteristics (age, sex, diabetes, statin use), and HDL-C and low-density lipoprotein cholesterol (LDL-C) at baseline and at >1-year were extracted (Tables 1, 3). The definition of new-onset diabetes and all data on new-onset diabetes, HbA1C, fasting plasma glucose, and insulin were also extracted. Quality of included studies was assessed independently by two reviewers using Cochrane Collaboration's tool for assessing risk of bias.22 Manuscript screening and quality assessment were done by K.D. and a senior consultant at the University of Oxford. Data extraction and analysis was done by K.D.
Table 1.
Characteristics of randomized controlled trials comparing effect of CETP inhibitor (CETPi) to placebo
| Trial | Enrollment | Size | Arm (n) | Inclusion | Diabetes at baseline (%) | New-onset diabetes (n) | New-onset diabetes criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Drug | Type | sites | Duration | (n) | Treatment | Criteria | ||||||||
| CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | |||||||||
| ILLUMINATE9 | 2007 | Torcetrapib | R, DB, PC, P | 221 sites in NA, Eu, Aus | 4.5y† | 15067 | 7533 | 7534 | 60 mg CETPi with Atorvastatin | Placebo with Atorvastatin | 45–75 yo with history CVD 1–5 mo prior or T2DM who met ADA criteria or on hypoglycemic therapy | 43.5 | 45.2 | 76 | 96 | Not reported. |
| Dal-OUTCOMES12 | 2012 | Dalcetrapib | R, DB, PC, P | 27 countries in NA, Eu, Asia, Aus | 31 mos* | 15871 | 7938 | 7933 | 600 mg CETPi with Standard of Care‡ | Placebo with Standard of Care | >45 yo with recent ACS, has completed planned coronary revascularization procedures | 24 | 25 | 403 | 516 | Post-randomization diabetes-related adverse event, new use of antihyperglycaemic medication, haemoglobin A1c ≥6.5%, or a combination of at least two measurements of serum glucose ≥7.0 mmol/L (fasting) or ≥11.1 mmol/L (random) for a person without evidence of diabetes mellitus at baseline. |
| ACCELERATE10 | 2017 | Evacetrapib | R, DB, PC, P | 36 countries in NA, SA, Eu, Asia, Aus | 26 mos* | 12092 | 6038 | 6054 | 130 mg CETPi with Standard of Care‡ | Placebo with Standard of Care | >18 yo with high risk vascular disease, treated with statin 1 month pre-screening | 68.4 | 67.9 | 175 | 200 | Fasting plasma glucose ≥7 mmol/L or 2-hour plasma glucose ≥11.1 mmol/L during an oral glucose tolerance test or HbA1c levels ≥6.5% (≥48 mmol/mol). Confirm by repeat testing on a second day. |
| REVEAL8 | 2017 | Anacetrapib | R, DB, PC, P | 41 sites in NA, Eu, China | 4.1 y | 30449 | 15225 | 15224 | 100 mg CETPi with Atorvastatin | Placebo with Atorvastatin | >50 yo with high CV risk | 37.1 | 37.2 | 510 | 571 | Post-randomization diabetes-related adverse event or the use of antihyperglycaemic medication (insulin or oral treatment) recorded on at least one follow-up visit form for a person without evidence of diabetes mellitus at baseline. |
| DEFINE11 | 2010 | Anacetrapib | R, DB, PC, P | 20 countries in NA, Eu, Asia, Aus | 18 mos | 1623 | 811 | 812 | 100 mg CETPi with Atorvastatin | Placebo with Atorvastatin | 18–80 yo with CHD or high risk of CHD | 53 | 53.2 | NA | NA | NA |
R, randomised; DB, double-blind; PC, placebo-controlled; P, parallel; CO, crossover; NA, North America; Eu, Europe; Aus, Australia; SOC, Standard of Care; ADA, American Diabetes Association; ACS, acute coronary syndrome; CV, cardiovascular; CHD, coronary heart disease; CVD, cardiovascular disease; T2DM, Type 2 Diabetes Mellitus.
*Median.
†Study was terminated at 1 year.
‡Standard of Care is statin therapy.
Table 3.
Effect of CETPi on lipids
| LDL-C (mg/dL) | HDL-C (mg/dL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Drug | Arm (n) | Baseline (SD) | >1 year (SD) | Change from baseline (%) | Baseline (SD) | >1 year (SD) | Change from baseline (%) | |||||||||
| CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | CETPi-Placebo (%) | CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | CETPi-Placebo (%) | ||
| ILLUMINATE9 | Torcetrapib | 7533 | 7534 | 79.7 (20.4) | 79.9 (20.4) | NR | NR | –24.9 | 3.0 | –27.9%† | 48.6 (12.0) | 48.5 (12.2) | NR | NR | 72.1 | 1.8 | 70.3%† |
| Dal-OUTCOMES12 | Dalcetrapib | 7938 | 7933 | 76.4 (26.4) | 75.8 (25.9) | NR | NR | NR | NR | 0.0% | 42.5 (11.7) | 42.2 (11.5) | 58.6 (NR) | 46.1 (NR) | 40.0 | 11.0 | 29%† |
| ACCELERATE10 | Evacetrapib | 6038 | 6054 | 81.6 (28.4) | 81.1 (27.8) | 54.7 (26.4) | 83.7 (30.8) | –31.1 | 6.0 | –37.1% | 45.3 (11.7) | 45.3 (11.7) | 104.1 (31.4) | 45.6 (12.3) | 133.2 | 1.6 | 131.6% |
| REVEAL8 | Anacetrapib | 15225 | 15224 | 61 (15) | 61 (15) | 38 (NR) | 64 (NR) | NR | NR | –41.0% | 40 (NR) | 40 (NR) | 85 (NR) | 42 (NR) | NR | NR | 104.0% |
| DEFINE11 | Anacetrapib | 811 | 812 | 81.2 (21.3) | 82.2 (20.7) | 48.9 (NR) | 76.7 (NR) | –40.5 | –4.3 | –36.2% | 40.5 (9.3) | 40.4 (9.1) | 102.3 (NR) | 44.9 (NR) | 151.1 | 12.3 | 138.8% |
*ACCELERATE only reported lipid levels at 3 months.
†Calculated based on available data.
To evaluate potential association of CETPi and new-onset diabetes, risk ratios (RR) and 95% confidence intervals (CI) were calculated from available data for patients without T2DM at baseline and those who developed T2DM in follow-up. RRs were pooled using a fixed-effects meta-analysis model. All analyses were conducted using STATA (College Station, TX, USA). All analyses were planned and conducted in accordance with preferred reporting items for systematic reviews and meta-analyses checklist (Appendix 7).
Results
Search results and characteristics of included trials
Electronic search identified 1716 relevant publications (Appendix 3), of which 57 underwent full-text review. Five RCTs met inclusion criteria8–12 and were included, along with all relevant post-hoc analyses,19,20,23 though one of the trials determining the efficacy and tolerability of CETP inhibition with anacetrapib (DEFINE) did not report new-onset diabetes, and was thus excluded from quantitative meta-analysis.8–10,12 All included trials had low risk of bias (Appendix 4).
Table 1 shows characteristics of CETPi trials in this meta-analysis, which included 75 102 persons with CVD or at high risk of CVD randomized to CETPi vs. placebo. The average duration of follow-up ranged from 1.5 to 4.5y (Table 1). CETP inhibitors evaluated were torcetrapib, dalcetrapib, evacetrapib, and anacetrapib; no studies of obicetrapib (TA-8995) met our inclusion criteria. Table 2 shows patient characteristics in those included in the trials. The mean age of participants was between 60–67 years, over 75% were male, and nearly all (96.4% or higher) were on a statin at baseline.
Table 2.
Baseline characteristics of patients enrolled in each arm of trials comparing effect of CETPi to placebo
| Arm (n) | Mean Age (SD) | Male sex (%) | Diabetes (%) | Mean BMI (SD) | Statin use (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Drug | CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | CETPi | Placebo | CETPi | Placebo |
| ILLUMINATE9 | Torcetrapib | 7533 | 7534 | 61.3±7.6 | 61.3±7.6 | 77.7 | 77.8 | 43.5 | 45.2 | 30.1±5.7 | 30.2±5.6 | 100 | 100 |
| Dal-OUTCOMES12 | Dalcetrapib | 7938 | 7933 | 60.3±9.1 | 60.1±9.1 | 80 | 81 | 24 | 25 | 28.6±5.0 | 28.6±5.1 | 97 | 98 |
| ACCELERATE10 | Evacetrapib | 6038 | 6054 | 64.8±9.4 | 65.0+9.5 | 77 | 77 | 68.4 | 67.9 | NR | NR | 96.4 | 96.6 |
| REVEAL8 | Anacetrapib | 15225 | 15224 | 67±8 | 67±8 | 83.9 | 83.8 | 37.1 | 37.2 | 28.6±5.0 | 28.6±5.1 | 97.2 | 96.9 |
| DEFINE11 | Anacetrapib | 811 | 812 | 62.5±8.7 | 62.9±9.0 | 77.6 | 76.1 | 53 | 53.2 | 30.4±5.5 | 30.1±5.2 | 99.5 | 99.1 |
BMI, body mass index; CETPi, CETP inhibitor.
Lipid measures at baseline and on-treatment are summarized in Table 3. The baseline LDL-C ranged from 61 to 82 mg/dL, and baseline HDL-C ranged from 40–49 mg/dL. The effects of CETPi on HDL-C varied by agent, with the greatest increases in HDL-C seen with anacetrapib and evacetrapib. All reduced LDL-C by 20–30% relative to placebo except dalcetrapib which had minimal effect on LDL-C (Table 3).
Impact of cholesteryl ester transfer protein inhibitors on new onset diabetes
New-onset diabetes was defined as the development of diabetes after initiation of the pharmacological intervention in patients who were not diabetic at baseline. The precise criteria varied between trials (Table 1). While REVEAL based diagnosis on physician reporting,8 ACCELERATE diagnosed with biomarkers,10 and dal-OUTCOMES with both.12 The rates of diabetes in the included populations at baseline varied from 24% in Dal-OUTCOMES to 68.4% in ACCELERATE. A total of 41 739 persons free of diabetes were included in the four trials available for quantitative meta-analysis.
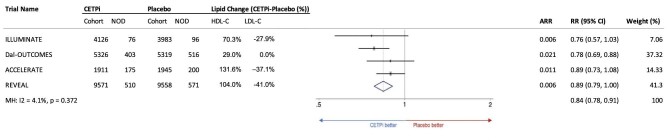
Figure 2 shows results from individual trials and pooled results from the meta-analysis on the risk of new onset diabetes among those free of diabetes at baseline. All four trials reported lower rates of new onset diabetes among those given CETPi vs. placebo, though this was only statistically significant in Dal-OUTCOMES. In pooled analysis, CETPi therapy was associated with a reduced risk of new onset diabetes relative to placebo (RR: 0.84; 95% CI: 0.78–0.91; P <0.001) (Figure 2), with low between-study heterogeneity (I2 = 4.1%). Absolute risk reduction ranged from 0.6–2.1% with CETPi therapy (Figure 2). No indication of publication bias was observed for new-onset diabetes according to the funnel plot (Appendix 5).
Figure 2.
Forest plot indicating reduction in risk of new-onset diabetes with CETP inhibitor therapy in using a fixed-effects model (weights are from Mantel–Haenszel model). Included ILLUMINATE,23 dal-OUTCOMES,20 ACCELERATE19 and REVEAL.8 RR, risk ratio; NOD, new-onset diabetes.
Impact of cholesteryl ester transfer protein inhibitors on glycaemic measures in those with diabetes
All but one trial (dal-OUTCOMES) reported showed differences in haemoglobin A1c between placebo and treatment arms among those with diabetes (Table 4). Investigation of lipid level management to understand its impact in atherosclerotic events (ILLUMINATE) and ACCELERATE each demonstrated a statistically significant reduction in HbA1c for Torcetrapib and Evacetrapib, respectively, while no difference was seen in HbA1c in REVEAL or DEFINE for anacetrapib. In the ILLUMINATE trial, within the cohort of persons with diabetes, patients in placebo group were more frequently given add-on insulin [200 patients (5.9%) in placebo and 165 patients (5.04%) in torcetrapib; P = 0.13] and oral antidiabetic therapy [456 patients (13.45%) in placebo and 394 (12.05%); P = 0.09] than in torcetrapib group, potentially suggesting that the true effect of torcetrapib on glucose homeostasis may have been even stronger.23
Table 4.
Glycemic measures
| Persons with diabetes | Persons without diabetes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Drug | HbA1C | Plasma Glucose | Insulin | HOMA-IR | HbA1C | Plasma glucose | Insulin | HOMA-IR |
| ILLUMINATE23 | Torcetrapib | At 12 mo: placebo arm (n = 2999) 7.36%; CETPi arm (n = 2796) 7.16% (P < 0.001) | At 3 mo: 0.34 mmol/L lower in CETPi arm (n = 3137) than placebo arm (n = 3288) (P < 0.001) | At 3 mo: 11.7 μU/mL lower in CETPi arm (n = 3092) than placebo arm (n = 3230) (P < 0.0001) | At 3 mo: Decreased from 49.1 to 47.3 in CETPi arm (n = 3053) (P < 0.0001), increase in placebo arm (n = 3200) | At 12 mo: placebo arm (n = 240) 6.52%; CETPi (n = 219) 6.27% (p<0.001) | At 12 mo: –0.06 Placebo (n = 3857)-CETPi (n = 3972) (P = 0.03) | At 3 mo: 6.6 lower in CETPi arm (n = 3092) than placebo arm (n = 3947) (P < 0.001) | At 3 mo: Decreased from 22.35 to 21.89 in CETPi arm (n = 4026) while increase in placebo arm (n = 3906) |
| Dal-OUTCOMES20 | Dalcetrapib | Not reported | Not reported | Not reported | Not reported | At 12 mo: placebo arm (n = 6923) 5.9%; CETPi (n = 6847) 5.8%, (P < 0.01)* | At 12 mo: 5.6 mmol/L in placebo arm (n = 5397); CETPi arm (n = 5297) (P > 0.05)* | At 3 mo: –0.61 μU/mL CETPi (n = 1071)-Placebo (n = 1097) (P > 0.05) | At 3 mo: Placebo (n = 1097) 2.09; CETPi (n = 1071) 1.95 (P > 0.05) |
| ACCELERATE19 | Evacetrapib | At 6 mo: placebo arm 7.15%; CETPi arm 7.08% (P = 0.023) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| REVEAL8 | Anacetrapib | Placebo arm 6.9%; CETPi arm 6.9% (P = 0.92) (n = NR)† | Not reported | Not reported | Not reported | –0.03 CETPi-placebo (P < 0.001) (n = NR)† | Not reported | Not reported | Not reported |
| DEFINE11 | Anacetrapib | At 76 w: CETPi-placebo: –0.11 (P = 0.083) (n = NR) | Not reported | Not reported | Not reported | Not reported | At 76 w: –2.5 CETPi-Placebo (P = 0.11) (n = NR) | Not reported | Not reported |
DEFINE showed changes in HbA1C between CETPi and placebo groups, but the results of the two groups were not individually reported. Green = significant improvement, Yellow = trending towards improvement, Red = no effect. HbA1C, haemoglobin A1C; HOMA-IR, Homeostatic model assessment of insulin resistance; DM, baseline cohort of persons with diabetes; NDM, baseline persons without diabetes; NR, not reported.
*This data reflects the whole cohort (DM + NDM), as majority of dal-OUTCOMES cohort is NDM, it is most representative of this group.
†Data collected at final study visit, precise timing not reported.
Only ILLUMINATE reported other diabetes-related biomarkers in persons with diabetes. Plasma glucose (fasting), insulin, and HOMA-IR were all lowered at 3 months in the torcetrapib arm compared with the placebo arm (Table 4).
Among those free of diabetes at baseline, three trials reported changes in HbA1c, with similar findings as in those with diabetes; torcetrapib, and dalcetrapib, but not anacetrapib, was associated with a reduction in HbA1C. Diabetes-related biomarkers were reported in participants without diabetes for torcetrapib and dalcetrapib. Torcetrapib showed a reduction in plasma fasting glucose, insulin, and HOMA-IR, while dalcetrapib found no statistically significant differences between the treatment and placebo arms in any of these three markers, possibly explained by the modest HDL-C increase conferred by dalcetrapib.
Additional data reported from cholesteryl ester transfer protein inhibitors trials
Dal-OUTCOMES20 further reported on bidirectional transitions among three glycaemic states: normoglycaemia, prediabetes, and diabetes. Prediabetes to diabetes transition was reduced [dalcetrapib: 364/3394 (10.7); placebo: 473/3301 (14.3); P < 0.001]. However, normoglycaemia to diabetes transitions were not significantly reduced [dalcetrapib: 39/1932 (2.0); placebo: 43/2018 (2.1); P = 0.80]. Normoglycaemia to prediabetes transition was also decreased by dalcetrapib relative to placebo [dalcetrapib: 711/1846 (38.5%); placebo: 826/1915 (43.1%); P = 0.004]. Interestingly, dalcetrapib also significantly increased reversal of diabetes to no diabetes [dalcetrapib: 325/2354 (13.8%); placebo: 271/2393 (11.3), P = 0.01].
Finally, torcetrapib was shown to increase systolic blood pressure by a mean of 5.4 mmHg vs. 0.9 mmHg in the atorvastatin-only group (P < 0.0001) and it was thought that this increase in blood pressure was associated with adverse outcomes.9 Other trials showed very modest changes in systolic blood pressure of <1 mmHg, which is unlikely to be clinically significant.8,10,12
Discussion
This meta-analysis indicates that CETPi, when added to statin therapy, resulted in a statistically significant 16% reduction in new-onset diabetes risk in patients with CVD or at high risk of CVD, with a consistent effect across trials of different CETPis. Glycaemic measures were rarely reported, but trended towards supporting this effect, with decreases in haemoglobin A1c among those with pre-existing diabetes.
While, CETPis are associated with decreased risk of diabetes despite reducing LDL-C, other LDL-C lowering agents are mostly associated with increased or no change in T2DM risk.6 Niacin has been associated with a moderately increased risk of incident diabetes,24 ezetimibe trials have not reported on new-onset diabetes, bempedoic acid trials indicate at most a very mild (<3%) decrease.25 Statins have been shown to increase the risk of incident diabetes, with the highest risk among those who had risk factors for diabetes.5
Genetic studies on cholesteryl ester transfer protein inhibitors and diabetes risk
Genetic studies support a role for CETPi in prevention of diabetes, but with some mixed results. CETP loci are most strongly associated with HDL-C, but also LDL-C and TG, resulting in complex associations with gene-sets related to cholesterol metabolism, lipid transport, and foam-cell differentiation, amongst others.26 Polymorphisms that increase CETP and decrease HDL-C have been hypothesized to worsen glycaemic status, while polymorphisms that decrease CETP activity and increase HDL-C may improve glycaemic status.15 Indeed, in healthy adults the B2 allele at CETP-related Taq1B locus, which affects CETP promotor activity, is associated with increased-HDL-C and reduced insulin resistance,27 while the B1B1-genotype is associated with lower HDL-C28 and higher insulin resistance and T2DM risk. These results are consistent with improved diabetes-related measures with CETP. However, the largest candidate-gene study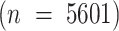 found no association between presence of CETP SNP rs3764261 (which increases HDL-C) and T2DM in CVD patients.29
found no association between presence of CETP SNP rs3764261 (which increases HDL-C) and T2DM in CVD patients.29
The most important evidence suggesting that new-onset diabetes reduction is an on-target effect of CETP inhibition comes from a recent large mendelian randomization study of CETP loci on >190 pharmacologically relevant outcomes with 480 698–21 770 samples and over 74 million events. Low CETP haplotypes were found to be causally related to T2DM, with HDL as the mediator.13 Importantly, CETP polymorphisms tend to alter HDL-C levels by ≤5% whereas CETPi change HDL-C by up to 130%, which may explain some of the discordance between candidate-gene studies and clinical trials, though it must also be considered that CETPis are acting acutely whereas genetic polymorphisms act chronically.
Potential mechanism for cholesteryl ester transfer protein inhibitors and diabetes
If the association between CETPi and reduced risk of new onset diabetes is mediated by changes in lipids, it is unlikely to be due to changes in LDL-C. CETPis induce variable magnitudes of lipid changes but all increased HDL-C and all but dalcetrapib decreased LDL-C. Lack of LDL-C reduction with dalcetrapib is attributed to relatively low CETP inhibition (∼30%), as LDL-C reduction is proportional to the degree of CETP inhibition. Despite this difference, dalcetrapib exerted at least as large a reduction in new-onset diabetes as other CETPi (Figure 2) and significantly increased T2DM reversal [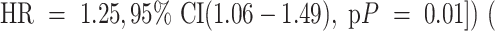 20), indicating an overall positive effect on glycaemic transitions.
20), indicating an overall positive effect on glycaemic transitions.
All CETPis increase HDL-C which may play a role in their association with diabetes. ACCELERATE10 and REVEAL,8 studying evacetrapib and anacetrapib respectively, did not exhibit the largest reduction in new-onset diabetes despite largest on-treatment increase in HDL-C beyond placebo (>100% baseline) (Figure 2). This implies either a non-linear relationship between HDL-C changes and diabetes risk, or a pathway between CETP inhibition and diabetes that is independent of HDL-C. However, these trial-level evaluations of overall HDL-C changes and diabetes risk are likely insufficient to determine if changes in HDL-C mediate or correlate with diabetes risk reduction, which would be best answered with individual-level data. Future research is needed to elucidate the specific mechanisms of the link between CETP inhibition and incident diabetes, including the impact of CETP inhibition on cholesterol efflux from beta-cells, induction of insulin synthesis by beta cells, and increased glucose uptake by muscle.
There is biological plausibility for a role in HDL in T2DM, including significant mechanistic evidence for a bi-directional relationship between dyslipidaemia and T2DM.16 Lipid accumulation and inflammation leads to insulin resistance, while T2DM changes HDL composition and reduces ApoA116 exacerbating dyslipidaemia.7 (Figure 1)
Cellular and rodent studies have characterised multiple HDL glucose-lowering actions insofar as HDL acts centrally at ß-cells to inhibit apoptosis, reduce inflammation and promote insulin secretion, reviewed elsewhere.7,16 In the periphery, HDL-mediated cholesterol-efflux increases insulin sensitivity and glucose uptake, especially by muscle.7 Together, these mechanisms reduce plasma glucose, suggesting a role for HDL-C in diabetes pathogenesis. Accordingly, in T2DM patients, recombinant-HDL stimulated insulin secretion and reduced plasma glucose.17 Interestingly, plasma glucose reduced at 30 min whereas insulin secretion rose after 1.5h, suggesting an insulin-independent glucose-lowering mechanism, potentially AMPK-dependent glucose uptake, which may be enhanced by ApoA1.16 This study acutely altered HDL-C, so suggested mechanisms may reflect the impact of chronic changes in HDL-C.
Overall, HDL appears to play a role in glucose metabolism, but the exact chronology and clinical relevance of these mechanisms, and the specific role for CETP in this pathway, remain unclear. On a cellular level, dalcetrapib7 may stimulate cholesterol efflux at ß-cells encouraging insulin secretion, and torcetrapib has been shown to do so in vivo in rodents,7 but evidence remains preliminary.
Of note, our study found that the association between CETPi and new-onset diabetes (RR: 0.84; 95% CI: 0.78–0.91; P <0.001) appeared stronger than the association between CETPi and glycaemic control among those who already had diabetes (Table 4). Thus, the evidence does not support potential role for CETPi to improve glycaemic control in those who have already developed diabetes. Given this small change in glycaemic indices, it is possible that the mechanism of CETPi on T2DM risk is due to direct effects on beta cell survival. Future work should evaluate the degree to which any reduction in risk of T2DM is mediated by HDL-C, as well as other potential measures of HDL including HDL function.
Limitations
There are several key limitations to the present analysis. First, analysis of new-onset diabetes was post-hoc (except REVEAL8), increasing potential for false positive results. However, the consistency across trials increases our pooled result's credibility, and warrants further study through prospective randomised trials with pre-specification of T2DM as an endpoint. Second, methods for new-onset diabetes diagnosis varied between trials. While REVEAL based diagnosis on physician reporting,8 ACCELERATE diagnosed on basis of biochemical lab testing10 and dal-OUTCOMES accepted both12 (Table 1). Importantly, however a sensitivity analysis in dal-OUTCOMES suggested both approaches yielded a similar result,12,20 suggesting minimal impact on our overall findings.
Furthermore, there was a lack of individual patient-level data as well as a lack of systematic collection of insulin/glucose measurements and diabetes medication or insulin use, which limited the extent of analysis. Specifically, ILLUMINATE did not report criteria for new onset diabetes. However, the hazard ratio for treatment on new onset diabetes for ILLUMINATE was similar to other trials, and exclusion of this trial from the fixed effects model did not change the overall result.
Finally, all of the trials evaluated the effect of CETP inhibitors when added to a background of high-intensity statin therapy. It is not known, therefore, what the consequences might be of CETP inhibitor monotherapy for the new-onset of diabetes. The impact of the last CETP-inhibitor in clinical development, obicetrapib, is as of yet unknown but is prospectively investigated in a large phase III programme currently underway.
Conclusions
This meta-analysis indicates that CETPis provide a significant 16% reduction in new-onset diabetes risk. Together with increasing mechanistic and genetic evidence, these results support a role for HDL in T2DM pathogenesis and prevention, though further characterization is needed. Prospective randomized trials are needed to evaluate the role of CETPi as a possible adjunct to statin therapy to improve lipid profile in vulnerable patient populations. Future trials of CETPi and potentially other HDL-raising agents should therefore specify new-onset diabetes and reversal of existing T2DM as secondary endpoints.
Supplementary Material
Contributor Information
Katerina Dangas, Magdalen College University of Oxford, Oxford, OX1 4AU UK.
Ann-Marie Navar, Department of Internal Medicine, UT Southwestern Medical Center, Dallas, TX 75390, , USA.
John J P Kastelein, Department of Vascular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam 1081, Netherlands.
Funding
None disclosed.
Conflict of interest: A.M.N. has received consulting fees from New Amsterdam Pharma for work on obicetrapib. K.D. has no relevant disclosures. J.J.P.K. has received consulting fees from New Amsterdam Pharma and stock and stock options from New Amsterdam Pharma. J.J.P.K. is founder and CSO of NewAmsterdam Pharma.
Data availability
The data was extracted using publically available data from previously published randomized clinina trials. No patient identified data were used in these analyses (8-12, 19, 20, 23).
Contributors
K.D. conducted the literature search, design, data collection, formal analysis, and writing. A.M.N. contributed to supervision and writing. J.J.P.K. contributed to supervision and writing.
Registration information
The review was registered at Research Registry with unique identifier: reviewregistry1280.
References
- 1. Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CDA, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J, Tipping RW, Ford CE, Pressel SL, Folsom AR, Chambless LE, Wagenkneckt DB, Panagiotakos C, Pitsavos C, Chysoohou C, Stefanidis C, Knuiman M, Whincup PH, Wannamethee SG, Morris RW, Kiechl S, Willeit J, Oberhollenzer F, Mayr A, Wald N, Ebrahim S, Yarnell JW, Gallacher J, Casiglia E, Tikhonoff V, Nietert PJ, Sutherland SE, Bachman DL, Keil JE, de Boer IH, Kizer JR, Mukamal KJ, Grandits G. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knowler WC, Barret-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde A-M, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 5. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 6. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 7. Siebel AL, Heywood SE, Kingwell BA. HDL and glucose metabolism: current evidence and therapeutic potential. Front Pharmacol 2015;6:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ;HPS3/TIMI55—REVEAL Collaborative Group . Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 9. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 10. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE; ACCELERATE Investigators . Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 11. Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Stepanavage M, Liu SX, Gibbons P, Ashraf TB, Zafarino J, Mitchel Y, Barter P; Determining the Efficacy and Tolerability Investigators . Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010;363:2406–2415. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS; dal-OUTCOMES Investigators . Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt AF, Hunt NB, Gordillo-Marañón M, Charoen P, Drenos F, Kivimaki M, Lawlor DA, Giambartolomei C, Papacosta O, Chaturvedi N, Bis JC, O'Donnell CJ, Wannamethee G, Wong A, Price JF, Hughes AD, Gaunt TR, Franceschini N, Mook-Kanamori DO, Zwierzyna M, Sofat R, Hingorani AD, Finan C. Cholesteryl ester transfer protein (CETP) as a drug target for cardiovascular disease. Nat Commun 2021;12:5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, Packard CJ, Laufs U, Brook RD, Oliver-Williams C, Butterworth AS, Danesh J, Smith GD, Catapano AL, Sabatine MS. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA 2017;318:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ridker PM, Paré G, Parker AN, Zee RY, Miletich JP, Chasman DI. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: genomewide analysis among 18 245 initially healthy women from the women's genome health study. Circ Cardiovasc Genet 2009;2:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol 2012;8:237–245. [DOI] [PubMed] [Google Scholar]
- 17. Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009;119:2103–2111. [DOI] [PubMed] [Google Scholar]
- 18. Masson W, Lobo M, Siniawski D, Huerín M, Molinero G, Valéro R, Nogueira JP. Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Diabetes Metab 2018;44:508–513. [DOI] [PubMed] [Google Scholar]
- 19. Menon V, Kumar A, Patel DR, St John J, Riesmeyer J, Weerakkody G, Ruotolo G, Wolski KE, McErlean E, Cremer PC, Nicholls SJ, Lincoff AM, Nissen SE. Effect of CETP inhibition with evacetrapib in patients with diabetes mellitus enrolled in the ACCELERATE trial. BMJ Open Diabetes Res Care 2020;8:e000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz GG, Leiter LA, Ballantyne CM, Barter PJ, Black DM, Kallend D, Laghrissi-Thode F, Leitersdorf E, McMurray JJV, Nicholls SJ, Olsson AG, Preiss D, Shah PK, Tardif JC, Kittelson J. Dalcetrapib reduces risk of new-onset diabetes in patients with coronary heart disease. Diabetes Care 2020;43:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilczynski NL, McKibbon KA, Haynes RB. Enhancing retrieval of best evidence for health care from bibliographic databases: calibration of the hand search of the literature. Stud Health Technol Inform 2001;84:390–393. [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barter PJ, Rye KA, Tardif JC, Waters DD, Boekholdt SM, Breazna A, Kastelein JJ. Effect of torcetrapib on glucose, insulin, and haemoglobin A1c in subjects in the investigation of lipid level management to understand its impact in atherosclerotic events (ILLUMINATE) trial. Circulation 2011;124:555–562. [DOI] [PubMed] [Google Scholar]
- 24. Goldie C, Taylor AJ, Nguyen P, McCoy C, Zhao XQ, Preiss D. Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials. Heart 2016;102:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM; CLEAR Harmony Trial . Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 26. Willer CJ, Mohike KL. Finding genes and variants for lipid levels after genome-wide association analysis. Curr Opin Lipidol 2012;23:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. López-Ríos L, Pérez-Jiménez P, Martínez-Quintana E, Rodriguez González G, Díaz-Chico BN, Nóvoa FJ, Serra-Majem L, Chirino R. Association of taq 1B CETP polymorphism with insulin and HOMA levels in the population of the canary islands. Nutr Metab Cardiovasc Dis 2011;21:18–24. [DOI] [PubMed] [Google Scholar]
- 28. Kuivenhoven JA, Jukema JW, Zwinderman AH, de Knijff P, McPherson R, Bruschke AV, Lie KI, Kastelein JJ. The role of a common variant of the cholesteryl ester transfer protein gene in the progression of coronary atherosclerosis. The regression growth evaluation statin study group. N Engl J Med 1998;338:86–93. [DOI] [PubMed] [Google Scholar]
- 29. Koopal C, van der Graaf Y, Asselbergs FW, Westerink J, Visseren FL; SMART study group . Association between CETP gene polymorphism, insulin resistance and risk of diabetes mellitus in patients with vascular disease. Atherosclerosis 2015;242:605–610. [DOI] [PubMed] [Google Scholar]
- 30. Fryirs MA, Barter PJ, Appavoo M, Tuch BE, Tabet F, Heather AK, Rye KA. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler Thromb Vasc Biol 2010;30:1642–1648. [DOI] [PubMed] [Google Scholar]
- 31. Yalcinkaya M, Kerksiek A, Gebert K, Annema W, Sibler R, Radosavljevic S, Lütjohann D, Rohrer L, von Eckardstein A. HDL inhibits endoplasmic reticulum stress-induced apoptosis of pancreatic β-cells in vitro by activation of smoothened. J Lipid Res 2020;61:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kruit JK, Brunham LR, Verchere CB, Hayden MR. HDL and LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr Opin Lipidol 2010;21:178–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data was extracted using publically available data from previously published randomized clinina trials. No patient identified data were used in these analyses (8-12, 19, 20, 23).