Abstract
We are witnessing a revolution in our understanding of primary aldosteronism (PA). In the past 2 decades, we have learned that PA is a highly prevalent syndrome that is largely attributable to pathogenic somatic mutations, that contributes to cardiovascular, metabolic, and kidney disease, and that when recognized, can be adequately treated with widely available mineralocorticoid receptor antagonists and/or surgical adrenalectomy. Unfortunately, PA is rarely diagnosed, or adequately treated, mainly because of a lack of awareness and education. Most clinicians still possess an outdated understanding of PA; from primary care physicians to hypertension specialists, there is an urgent need to redefine and reintroduce PA to clinicians with a modern and practical approach. In this state-of-the-art review, we provide readers with the most updated knowledge on the pathogenesis, prevalence, diagnosis, and treatment of PA. In particular, we underscore the public health importance of promptly recognizing and treating PA and provide pragmatic solutions to modify clinical practices to achieve this.
Keywords: adrenal, aldosterone, blood pressure, hypertension, primary aldosteronism, renin
Graphical Abstract
Graphical Abstract.
INTRODUCTION AND CLINICAL PATHOPHYSIOLOGY
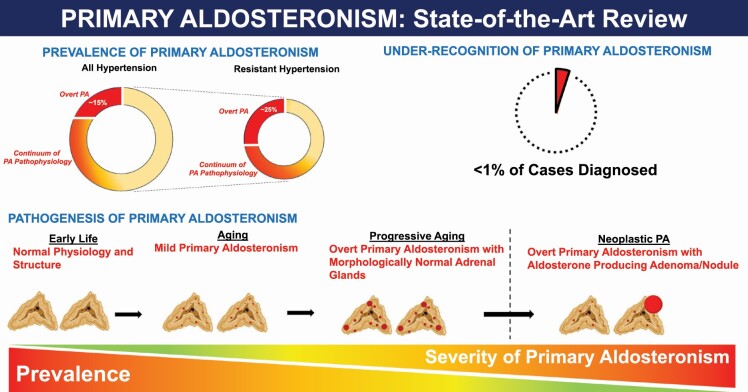
The past 20 years have witnessed a revolution in our understanding of primary aldosteronism (PA). PA is a pathologic form of aldosteronism, characterized by renin- and angiotensin II-independent aldosterone production that is relatively nonsuppressible. This PA pathophysiology can manifest across a broad continuum; thus, PA is best considered a syndrome rather than a binary disease. Once considered to be a rare hormonal cause of hypertension, we now recognize that the PA syndrome is common, and contributes to cardiovascular and kidney disease. Insights into the pathogenesis of PA have shown that it is largely driven by pathogenic somatic mutations that may progressively accrue across the lifespan, and occasionally merge with neoplasia to create the most severe forms of neoplastic PA. In parallel, prevalence studies have consistently shown that the prevalence of overt and categorically classified PA ranges from 10% to 25% in the hypertensive population. However, beyond this categorical definition of PA, we now understand that there is an expansive continuum of PA pathophysiology (relatively nonsuppressible and renin-independent aldosterone production) that exists beyond this categorical construct. Unfortunately, the reality is that fewer than 1% of patients with overt PA are ever diagnosed, largely due to a lack of awareness and testing, and virtually none of the patients with milder forms of PA pathophysiology are ever identified or treated. This constitutes an unrecognized public health crisis since PA pathophysiology can be treated with widely available targeted therapies that can mitigate the adverse health effects of PA.
In this state-of-the-art review, we aim to provide readers with a contemporary view of PA pathogenesis and epidemiology, while simultaneously sharing pragmatic approaches to diagnosis and treatment. It is our hope that after reading this review, readers will be informed and invigorated to improve clinical care for their patients by adapting to the new landscape of PA described herein.
THE PATHOGENESIS OF PA
Updates in the genetics and histopathology of PA
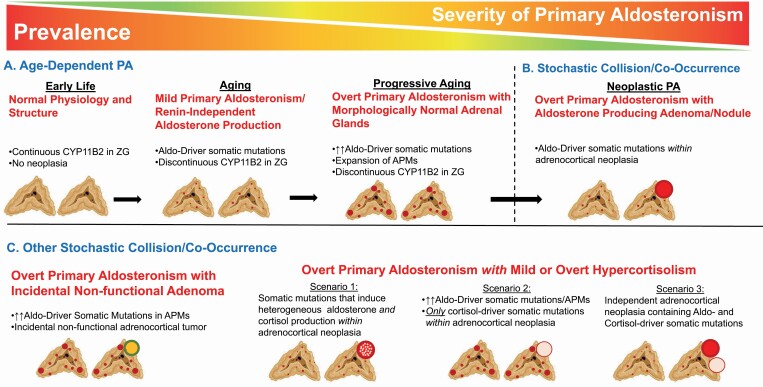
Significant progress has been made in determining the histopathologic and genetic characteristics of PA.1,2 The development of specific antibodies against human CYP11B2 (aldosterone synthase) and its application to immunohistochemistry have facilitated the identification of sources of aldosterone production in surgically resected adrenal tissue,3,4 revealing diverse histopathologic characteristics of adrenal glands from PA patients.5,6 Recently, an international consensus for the nomenclature and definition of adrenocortical lesions from patients with unilateral PA was developed and endorsed by the World Health Organization.7,8 Based on hematoxylin and eosin staining (for morphologic examination) and CYP11B2 immunohistochemistry (for functional characterization), the proposed classifications include: aldosterone-producing adenoma (APA), aldosterone-producing nodule (APN), aldosterone-producing micronodule (APM) (formerly known as “aldosterone-producing cell cluster”), aldosterone-producing diffuse hyperplasia, and rarely, aldosterone-producing adrenocortical carcinoma.7 It is important to note that multiple aldosterone-producing lesions can occur within a single adrenal gland, and that adrenal lesions identified by imaging studies are not necessarily the source of aldosterone excess9,10 (Figure 1). Incidental nonfunctioning adenomas can also commonly occur in patients with PA; in these cases, satellite APA or APN/APM in the adjacent or contralateral adrenal tissue may be the source of excess aldosterone production. The histopathology of PA when there is concomitant hypercortisolism can also be variable. The conceivable histologic subtypes include: an aldosterone and cortisol coproducing adenoma,11,12 a dominant cortisol-producing adenoma with multiple APMs in the adjacent adrenal tissue,13,14 and coexistence of separate APA and cortisol-producing adenoma.15–19 Multiple APMs appear to be a common histologic feature of idiopathic hyperaldosteronism (or bilateral PA).20
Figure 1.
Models for the pathogenesis of primary aldosteronism. (a) Young human adrenals display a continuous pattern of CYP11B2 expression in the zona glomerulosa. With aging, CYP11B2 expression becomes discontinuous, and there are greater numbers of aldosterone-producing micronodules (APMs), often harboring pathogenic aldosterone-driver somatic mutations; in parallel, there is greater renin-independent aldosterone production indicative of mild PA. With progressive aging, a proportion of individuals will have expansion of APMs, despite morphologically normal-appearing adrenal glands, contributing to overt PA. (b) Although the vast majority of PA is likely attributable to progressive acquisition of APMs and pathogenic somatic mutations, less commonly, these entities may co-occur or collide with adrenocortical neoplasia to form an APA. Although APAs manifest with the most severe PA clinical phenotype, they likely represent the minority of PA cases. (c) Other stochastic co-occurrences include the combination of APMs with incidental and nonfunctional adrenocortical neoplasia, and the combination of aldosterone production with cortisol production. Models of aldosterone and cortisol coproduction include (i) an aldosterone and cortisol coproducing adenoma; (ii) a cortisol-producing adenoma with a background of APMs; and (iii) separate cortisol- and aldosterone-producing adenomas. Abbreviations: Aldo, aldosterone; APA, aldosterone-producing adenoma; PA, primary aldosteronism; ZG, zona glomerulosa. (Figures created in BioRender.com.).
The application of next-generation sequencing has resulted in the detection of disease-causing somatic and germline mutations in APA and familial hyperaldosteronism (FH), respectively. The affected genes encode ion channels: Potassium Inwardly Rectifying Channel Subfamily J Member 5 (KCNJ5),21Calcium Voltage-Gated Channel Subunit Alpha1 D (CACNA1D),22Calcium Voltage-Gated Channel Subunit Alpha1 H (CACNA1H),23,24 and Chloride Voltage-Gated Channel 2 (CLCN2)25,26 and ATPases: ATPase Na+/K+Transporting Subunit Alpha 1 (ATP1A1) and ATPase Plasma Membrane Ca2+Transporting 3 (ATP2B3).27 Aldosterone-driver mutations in these genes either directly or indirectly (via cell membrane depolarization) increase intracellular calcium levels that stimulate aldosterone synthase (CYP11B2) expression and aldosterone production. Activating somatic mutations in exon 3 of the CTNNB1 gene that encodes β-catenin have also been identified in a small subset of APA.28,29CTNNB1-mutated tumor cells may require a second somatic mutation to obtain the capacity to produce aldosterone. For example, concomitant mutations in GNAQ/1130 or CACNA1D genes31 have been reported in CTNNB1-mutated APAs. Inheritable causes of PA are rare. FH-I is caused by the presence of a chimeric gene resulting from an unequal crossing over between CYP11B1 (11β-hydroxylase, the cortisol biosynthetic enzyme) and CYP11B2 genes, leading to abnormal aldosterone production that is regulated by adrenocorticotropic hormone (ACTH).32 Germline pathogenic variants in CLCN2,25,26KCNJ5,21 and CACNA1H24 have been identified as responsible for FH-II, FH-III, and FH-IV, respectively. De novo germline variants in the CACNA1D gene have also been identified as the cause of the rare PASNA syndrome (PA, seizures, and neurologic abnormalities).22
Although genetic causes of PA with hypercortisolism are not fully understood, some studies have shown somatic KCNJ5 mutations in adrenal tumors from such patients (presumably aldosterone and cortisol coproducing adenoma).12,33,34 The capacity to produce both aldosterone and cortisol may partly be explained by coexpression of steroidogenic enzymes CYP11B2 and CYP11B1 (11β-hydroxylase) in KCNJ5-mutated APA.35–39 Somatic mutations in PRKACA and GNAS, both known as genetic causes of cortisol-producing adenoma, have also been reported in adrenal tumors from patients with PA and autonomous cortisol secretion40,41; however, the pathologic role of these mutations needs further investigation.15,18,31
While heritable forms of PA are rare, somatic mutations causing PA are common. Using the combination of CYP11B2 immunohistochemistry (to identify lesions for DNA capture) and next-generation sequencing, pathogenic somatic mutations have been identified in approximately 90% of APAs.31,37,42 There appear to be sex and racial differences in the prevalence of somatic mutations in APA; for example, KCNJ5 is more common in East Asians and women regardless of race.43 Among these aldosterone-driver somatic mutations, CACNA1D mutations have frequently been documented in APMs in adrenals with bilateral or idiopathic aldosteronism20 as well as in normal adrenals.44,45 One important caveat on interpreting prevalence and demographic characteristics of somatic mutations is the multiple biases resulting in case detection and surgical adrenalectomy, which may skew the findings from available tissues in unpredictable ways.
Aging and adrenal histopathology
In 2010, Nishimoto et al.3 developed polyclonal antibodies that could independently detect human CYP11B1 and CYP11B2. Using these antibodies, the authors defined 2 distinct patterns of functional adrenocortical zonation, (i) conventional zonation with sporadic CYP11B2-positive cells in the zona glomerulosa (ZG) and (ii) variegated zonation with subcapsular CYP11B2-expressing cell clusters, termed aldosterone-producing cell clusters (recently redefined as APMs). Four years later, Gomez-Sanchez et al.4 successfully generated and characterized highly specific monoclonal CYP11B1 and CYP11B2 antibodies (RRID: AB_2650563 and AB_2650562, respectively). Since then, these monoclonal antibodies have been widely used for research and are currently commercially available.
To study CYP11B2 expression patterns in normal physiology, adrenal glands from deceased kidney donors and autopsy cases have been used. Using these specimens, 3 independent studies have demonstrated age-dependent accumulation of APMs,45–47 thereby implicating an age-dependent PA pathophysiology. In contrast, continuous zonal CYP11B2 expression in the ZG appears to be a feature of young adrenals that progressively dissipates with age.46,47 Aldosterone-driver somatic mutations, especially in the CACNA1D gene, are frequently found in APMs in normal adrenals, suggesting that an acquisition of pathogenic somatic mutations and APMs induces an age-dependent renin-independent aldosterone production.44,45,47,48 Dysregulated aldosterone production by APM has been further supported by mass spectrometry-based imaging analysis.49,50
Origins of PA
Considerable effort has been made to determine the cellular origins of PA. The number of APM per adrenal is significantly higher in patients with idiopathic aldosteronism or cross-sectional image-negative unilateral PA compared with normal adrenals.20,45 These histopathologic findings may explain the continuum of renin-independent aldosterone production that parallels the severity of clinical phenotypes.51
It is of great interest whether APM is a precursor of APA, as APM frequently harbor aldosterone-driver mutations. Some researchers have proposed an APM to APA progression model based on the observation of adrenocortical lesions termed, “possible APM-to-APA translational lesions,” that contains histologic features of both APM and APA.52,53 One in situ mass spectrometry imaging study revealed 2 distinct metabolic phenotypes of APM wherein 1 had a similar metabolic phenotype to APA, suggesting that a subset of APM may have the capacity to transition to APA.54 A possible 2-hit model of pathophysiologic convergence has also been proposed as an alternative hypothesis of APA development. In this model, abnormal cell proliferation due to genetic or environmental factors (neoplasia) occurs with somatic mutations in aldosterone-driver genes (aldosterone production).2,55,56 This latter model suggests that the high prevalence of PA is largely driven by a diffuse, bilateral, and nonneoplastic process of acquired pathogenic somatic mutations that likely result in mild-to-moderate PA pathophysiology. In a minority of cases, this process (and a parallel process inducing cortisol production) can collide with adrenocortical neoplasia to permit the emergence of more severe and overt cases of PA (Figure 1). Finally, it is important to note that beyond these pathogenic mutations and histopathologic morphologies, other circulating and paracrine factors, including ACTH, angiotensin II, LH/GnRH, GIP, vasopressin, leptin, serotonin, and mast cell activity, also play an important and variable role in determining the dysregulated production of aldosterone by activating their overexpressed, or ectopically expressed, receptors in PA tissues.57–70
EPIDEMIOLOGY AND PREVALENCE
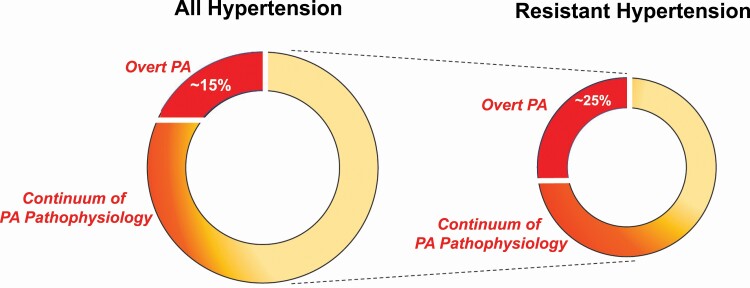
The prevalence of PA is considerably higher than previously realized (Figure 2).71 Several considerations that influence diagnostic interpretations account for the wide range of reported prevalence estimates. Although the diagnosis of PA has typically been made using relatively arbitrary categorical thresholds, growing evidence suggests that PA exists across a broad continuum.51,72–77 Thus, the reliance on specific categorical thresholds can yield dramatically different estimates of prevalence. For example, requiring an aldosterone-to-renin ratio (ARR) of >30 ng/dl per ng/ml/h as well as an aldosterone level >10 ng/dl to be considered a “positive” screen identifies 13.8% of hypertensive patients as potentially having PA; however, liberalizing these thresholds to an ARR >20 and omitting any requirement for a minimum aldosterone concentration can increase this yield to 33%.78,79 Similarly, using an aldosterone excretion rate of >10 µg/24 hours following an oral sodium loading test classifies up to 34% of patients with stage 2 hypertension as having PA, whereas using a threshold of >12 µg/24 hours only identifies 21.6%.51 These 2 examples highlight how the arbitrary reliance on categorical thresholds can distract clinicians from realizing the full spectrum of PA and its high prevalence.80 Herein, we will consider “overt” PA to describe PA defined by traditional categorical thresholds and to facilitate reporting of prevalence estimates; however, we also discuss in this review that overt PA represents the most severe extreme of the broad spectrum of PA that contributes to adverse health outcomes.
Figure 2.
The prevalence of primary aldosteronism. The prevalence of “overt PA” among untreated and/or new patients with hypertension has been reported to be as high as 14%–15% when using liberalized diagnostic interpretations; however, the prevalence of milder forms of PA pathophysiology is much higher. Among the subset of hypertensive individuals with resistant hypertension, the prevalence of “overt PA” is at least 25%, although when employing more liberalized criteria to define overt PA, it could be as high as 50%. Beyond this categorical definition of PA, there exists a broad continuum of “PA pathophysiology” in resistant hypertension, providing 1 reason why MR antagonists are especially effective in this situation. Abbreviations: MR, mineralocorticoid receptor; PA, primary aldosteronism.
In individuals without hypertension, the prevalence of overt PA is estimated to be 11%–14%.51,74,76 The clinical significance of this unrecognized burden of overt PA among normotensive individuals is that it identifies those at increased risk for developing hypertension in the future.72–75 Among patients with hypertension evaluated in the primary care or community setting, prevalence estimates for overt PA range from 0.7% to 14%,78,81–85 with higher prevalence paralleling greater hypertensive severity (3.9% in stage 1, 9.7% in stage 2, and 11.8% in stage 3).78 Notably, in a prospective study of untreated Australian patients with newly diagnosed hypertension, 25% had an elevated ARR and at least 14% (and up to 21%) were diagnosed with overt PA.82 These results are akin to the 15.7% prevalence reported in an untreated American cohort with stage I hypertension,51 thereby providing reproducible and consistent prevalence estimates.
In patients with hypertension and hypokalemia referred to specialty hypertension care, 28.1% had overt PA, though this increased to as much as 88.5% in the subgroup with severe hypokalemia.86 In referral centers for hypertension and among patients with resistant hypertension, estimates of prevalence range from 4.0% to 30%,51,87–94 and when patients with both resistant hypertension and renin suppression are considered, up to 50% may have overt PA.51
CLINICAL OUTCOMES AND PUBLIC HEALTH RELEVANCE
Without targeted treatment, PA results in disproportionately high rates of cardiovascular, kidney, and metabolic disease compared with essential hypertension (Table 1). While greater blood pressure elevation in PA may partially explain some of this excess risk, the increased rates of adverse outcomes are also independent of blood pressure. These blood pressure-independent effects are attributed to deleterious mineralocorticoid receptor (MR) activation in extrarenal tissues such as the myocardium and vascular endothelial and smooth muscle cells, resulting in fibrosis, necrosis, and endothelial dysfunction.95–112 Animal and human studies have shown that the combination of sodium/volume loading with excess aldosterone–MR interactions causes cardiovascular and kidney disease that can be mitigated by either restricting dietary sodium or by treatment with MR antagonists.113–116
Table 1.
Long-term health impact of primary aldosteronism prior to targeted treatment
| Long-term health impact of primary aldosteronism |
|---|
| Cardiovascular disease risk |
| Coronary artery disease |
| Congestive heart failure |
| Left ventricular hypertrophy |
| Atrial fibrillation |
| Stroke |
| Cardiovascular mortality |
| Kidney disease risk |
| Glomerular hyperfiltration |
| Accelerated decline in glomerular filtration rate |
| End-stage kidney disease |
| Proteinuria |
| Metabolic disease risk |
| Type 2 diabetes mellitus |
| Metabolic syndrome/obesity |
| Obstructive sleep apnea |
| Osteoporosis/fractures |
Numerous observational studies have demonstrated higher rates of adverse cardiovascular outcomes in patients with overt PA.78,117–131 A meta-analysis synthesizing the results from many of these observational studies reported that overt PA was associated with a higher risk of coronary artery disease (odds ratio [OR] 1.77 [95% confidence interval, CI 1.10–2.83]), heart failure (OR 2.05 [95% CI 1.11–3.78]), left ventricular hypertrophy (OR 2.29 [95% CI 1.65–3.17]), atrial fibrillation (OR 3.52 [95% CI 2.06–5.99]), and stroke (OR 2.58 [95% CI 1.93–3.45]).132 In addition, several observational studies have also reported a heightened cardiovascular mortality risk associated with overt PA compared with essential hypertension.123,125
Despite the historical focus on its cardiovascular impact, PA also contributes to a number of noncardiovascular adverse outcomes. PA causes glomerular hyperfiltration leading to increased glomerular filtration rate.133 As with other disease processes that result in glomerular hyperfiltration (e.g., diabetic nephropathy, obesity), this ultimately leads to a steeper longitudinal decline in glomerular filtration rate along with higher rates of incident chronic kidney disease and albuminuria.134–141 Moreover, PA is associated with an increased risk of type 2 diabetes mellitus and metabolic syndrome.125,142–145 This may be attributable to both decreased insulin secretion and increased insulin clearance in PA,146 as well as to concurrent cortisol cosecretion.142 Not surprisingly given its association with obesity and the pathophysiology of chronic volume retention, PA frequently co-occurs with obstructive sleep apnea.147–149 Prevalence estimates of obstructive sleep apnea in patients with PA range from 21% to 34%.150–152 Finally, PA increases the risk for both osteoporosis and fractures, possibly mediated by hypercalciuria-induced secondary hyperparathyroidism.153–156
Numerous population- and community-based cohort studies have also highlighted that even milder phenotypes of PA pathophysiology (that is renin-independent aldosterone production that lies below the categorical thresholds for overt PA), are independently associated with the development or progression of cardiovascular and kidney disease. Once hypertension has developed, the magnitude of PA pathophysiology is associated with risk for worsening hypertension, incident structural heart disease, adverse cardiovascular events, and death.77,157–159 PA pathophysiology is associated with lower renal plasma flow,134,160 and among individuals with established chronic kidney disease, the magnitude of aldosterone production is associated with lower serum potassium, greater kaliuresis, and accelerated chronic kidney disease progression to end-stage kidney disease.161
Thus, while the categorical construct of “overt PA” may be convenient for estimating prevalence in research studies, and in developing clinical practice guidelines, this categorization fails to recognize the large and clinically relevant spectrum of milder PA. This lack of recognition is particularly alarming given that even overt PA is rarely diagnosed.
UNDER-RECOGNITION AND APPROACH TO DIAGNOSIS
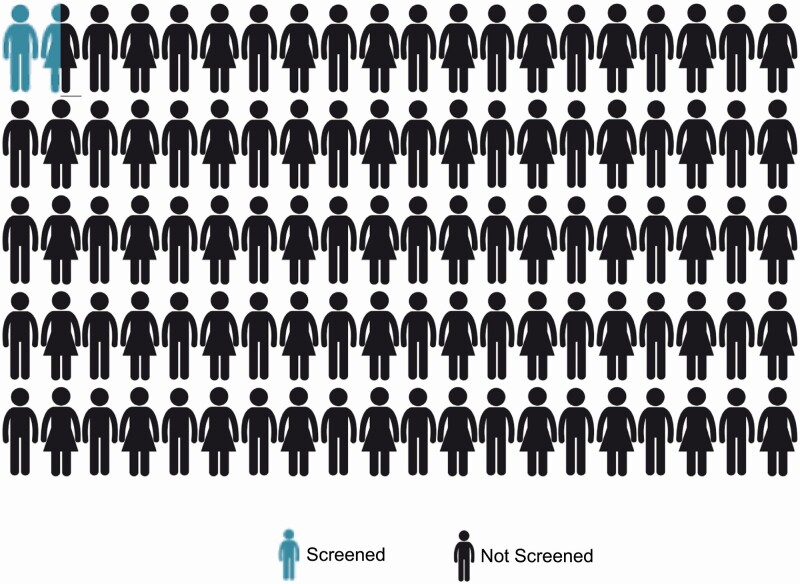
Despite its substantial clinical and public health implications, PA remains highly underdiagnosed. Uptake of guideline screening recommendations is abysmally low and approaches zero. Most recommendations suggest screening several high-risk populations in which PA prevalence is known to be high (Table 2).162–164 Yet numerous studies across the world consistently show that the overwhelming majority of patients meeting these criteria are never screened for PA (Figure 3).165–171 For instance, a mere 1.6% of patients with resistant hypertension who are followed in the United States Veterans Affairs Health System received guideline-recommended PA screening.169 Similarly, only 1.6% of patients in Ontario, Canada with hypertension and hypokalemia were screened for PA; even among those with hypertension and 5 or more episodes of hypokalemia, screening rates still remained below 5%.170 These alarmingly low rates of testing speak to the general lack of awareness throughout the medical community about the prevalence and health impact of PA, and imply that the vast majority of overt PA remains undiagnosed and untreated.
Table 2.
Populations considered to be “high-risk” for primary aldosteronism: high-risk populations are those in whom it has been demonstrated that the prevalence of primary aldosteronism is very high and mostly unrecognized
| High-risk populations |
|---|
| Severe or resistant hypertension |
| Unexplained or diuretic-induced hypokalemia |
| Hypertension with adrenal mass |
| Hypertension with sleep apnea |
| Hypertension with atrial fibrillation |
| Strong personal or family history |
| Debated expansion of eligible populations |
|---|
| New-onset hypertension |
| Stage 2 hypertension |
| All hypertension |
We suggest that all of these individuals be screened for primary aldosteronism. Beyond these high-risk individuals, there is growing consensus that screening for primary aldosteronism should be expanded to include more populations.
Figure 3.
Abysmal screening rates for PA: numerous studies from across the world have consistently shown that at best, even among high-risk populations, screening rates for PA are below 2%. This implies that much fewer than 1% of high-risk patients are ever diagnosed with PA. Abbreviation: PA, primary aldosteronism.
Who and how to screen
“High-risk” or “high prevalence” patient populations with the greatest risk for having PA should be screened (Table 2); however, there is growing momentum to consider testing every patient with hypertension at least once as a part of standard care.82,172
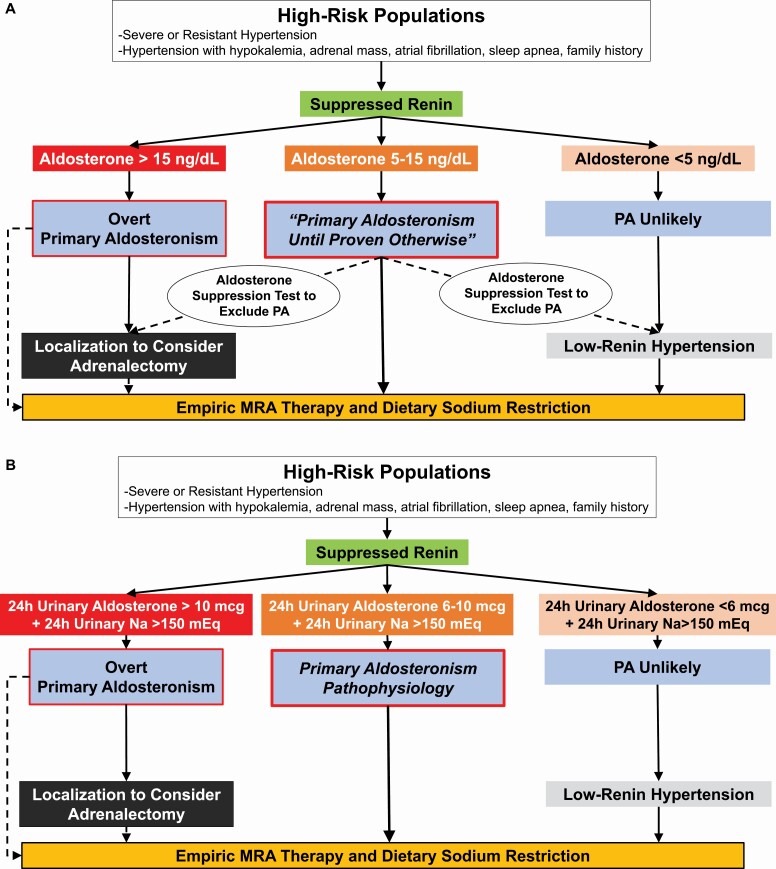
To maximize case detection and thus public health impact, we advocate a pragmatic approach that addresses the high prevalence of overt PA, the higher prevalence and unrecognized nature of a milder spectrum of PA,51,173 and the abysmally low detection rates.165,170 In a “high-risk” patient with a suppressed renin, the diagnostic mindset should be that the patient has “PA until proven otherwise.” This approach is distinct from the stepwise approach often prescribed by guidelines that places emphasis (or burden) on clinicians to “confirm” the diagnosis. By reforming the approach to assume PA until proven otherwise in high-risk phenotypes, we favor increasing the probability of making clinical diagnoses and/or initiating empiric treatment with MR antagonists for a wider array of PA phenotypes (Figure 4).
Figure 4.
(a) Pragmatic diagnostic approach for all clinicians. High-risk populations are those with a high pretest probability for having PA. In the context of a suppressed renin, a plasma aldosterone >15 ng/dl essentially confirms the diagnosis of overt PA and clinicians can proceed to localization or empiric MR antagonist therapy. In a high-risk patient with a suppressed renin and a very low aldosterone concentration, <5 ng/dl, PA is unlikely; however, MR antagonist therapy should still be considered as an effective treatment for low-renin hypertension. For all other high-risk patients (suppressed renin and aldosterone 5–15 ng/dl), the diagnostic mindset should be that this is “PA until proven otherwise.” Several options are available, including empiric initiation of MR antagonist therapy and dietary sodium restriction, or an aldosterone suppression test (including a 24-hour urine collection to measure aldosterone and sodium excretion) to exclude the possibility of PA or increase confidence in the diagnosis prior to pursuing localization and possible adrenalectomy. (b) Enhanced diagnostic approach for specialist clinicians. When available, specialists may opt to test high-risk populations with a suppressed renin with a 24-hour urine collection to gain a better integrated assessment of aldosterone production. This can be performed on an ad libitum diet since many patients may already be consuming sufficient dietary sodium, or after dietary sodium loading to ensure a high-sodium balance. Ideally, the desired 24-hour urinary sodium balance should be greater than 150 mEq/24 hours (reflective of a dietary intake of ~3.5 g of sodium per day). A 24-hour urinary aldosterone excretion rate of >10 mcg in this context likely confirms PA, whereas values between 6 and 10 mcg are suggestive of PA pathophysiology that is likely to still respond to targeted therapy. Twenty-four-hour urinary aldosterone excretion rates less than 6 mcg suggest PA is unlikely, but MR antagonist therapy may still be beneficial for low-renin hypertension. Abbreviations: MR, mineralocorticoid receptor; PA, primary aldosteronism.
Screening should occur irrespective of concurrent medications—including MR antagonists—or time of day to simplify the approach and maximize opportunities to test. When interpreting testing results, we suggest evaluating aldosterone and renin values independently, rather than relying on the integrated ARR alone.79,134,174 If renin is suppressed in a high-risk patient, irrespective of medications, the results can be interpreted.175,176 Renin suppression, representing volume expansion and downstream suppression of angiotensin II, can be conservatively defined as a plasma renin activity <1.0 ng/ml/h or corresponding plasma renin concentration <10 mU/l. Renin suppression despite the use of renin-angiotensin-aldosterone system inhibitors and diuretics, which are known to increase renin,177 should enhance clinician confidence in the presence of nonsuppressible aldosterone production.
If renin is not suppressed but clinical suspicion for PA is high, then repeat testing after withdrawal of MR antagonists and/or epithelial sodium channel inhibitors for up to 4 weeks is advisable. Many clinicians recommend withdrawal of other (or all) antihypertensive agents (such as converting enzyme inhibitors, angiotensin-receptor blockers, and diuretics); however, we find that this approach is impractical and infrequently necessary if clinicians adopt our proposed recalibrated diagnostic approach (Figure 4).177
Interpretations of screening
False-negative interpretations that erroneously exclude the diagnosis are far more common than false-positive interpretations,177,178 and several factors should be considered when evaluating results. First, there are systematic differences in the calibration of aldosterone assays. Several recent studies have demonstrated that aldosterone measurements by liquid chromatography–tandem mass spectrometry (LC–MS/MS), which is becoming more widespread, are 27%–87% lower when compared with immunoassay-based techniques.179–188 In 1 study of healthy volunteers who underwent simultaneous testing with both assay types under controlled physiologic conditions, a median aldosterone concentration of 19.6 ng/dl by immunoassay corresponded to only 10.5 ng/dl by LC–MS/MS (~50% lower); similarly, a median ARR of 23.3 ng/dl per ng/ml/h using immunoassay was only 6.2 ng/dl per ng/ml/h using LC–MS/MS (~75% lower).188 In a cohort of patients with resistant hypertension and overt PA, 1 quarter had a circulating aldosterone concentration <10 ng/dl by LC–MS/MS, a threshold below which most clinicians would have excluded the possibility of PA.51 Similarly, among overt PA patients undergoing adrenal venous sampling (AVS), 13%–26% are reported to have supine aldosterone concentration below 5 ng/dl by LC–MS/MS.189 Distinct, LC–MS/MS assay-specific cutoff values for the ARR and for aldosterone values during confirmatory tests have been proposed179–181,184,190 though are not yet incorporated into clinical practice guidelines.
Second, and related, aldosterone concentrations are not fixed but rather can vary substantially within an individual and across PA patients.191–195 Like many other hormones, the production of aldosterone can exhibit marked variability. In a study of patients with overt PA, aldosterone values on different days varied by 31% and the ARR by 45%, with a third of PA patients having at least 1 aldosterone value below the conventional threshold of 10 ng/dl and a quarter having at least 1 ARR below 20 ng/dl per ng/ml/h.195 This variability may be due to circulating factors and/or the variable expression of receptors for these factors in PA tissue.70 Studies have shown that aldosterone-producing foci in PA may express variable degrees of MC2R, thereby rendering some degree of aldosterone production under the control of ACTH57,62,64,66,68; dysregulated aldosterone production in PA, along with 18-hybrid steroids indicative of dysregulated CYP11B2 expression, can be enhanced by ACTH and depressed with dexamethasone.65,196,197 Further, variability in aldosterone production in PA may be induced by posture (angiotensin II modulation), variations in reproductive hormones (LH/GnRH), fasting or prandial status (GIP), vasopressin and serotonin activity, and procedural sedation or analgesia.57,62,68,69,189,194,198–200
Thus, clinicians should not be dissuaded from the possibility of PA because the aldosterone concentration appears “too low.” The categorization of aldosterone levels as “low” or “high” is overly simplistic and misleading; rather, clinicians should analyze whether aldosterone production is “physiologically appropriate vs. inappropriate.” In a high-risk patient with renin suppression, the clinician should assume “PA until proven otherwise” since virtually any aldosterone production in this context could represent the inappropriate and nonsuppressible pathophysiology that defines PA and enriches for response to MR antagonists.
Diagnosis and dynamic aldosterone suppression testing
Given the lack of an international, standardized, definition of PA, and the variable access to resources, time pressure on practicing clinicians, values of healthcare systems, and unique healthcare economics, we believe there is no single and uniform approach to diagnosing PA. Rather, there are multiple approaches that permit clinicians to use available resources, and their comfort level, to classify the patient appropriately. The most pragmatic approach relies on interpreting circulating measurements of renin and aldosterone. Although a single plasma aldosterone concentration may not reflect integrated daily aldosterone production, a liberalized approach to interpreting the results can provide a practical solution to this problem. Alternatively, specialists, and clinicians with more experience, can also consider using the results of 24-hour urinary aldosterone measurements to assess for PA (Figure 4). This approach involves more effort on the part of the clinician and patient, more sophisticated measurement techniques, and may require dietary and medication interventions; however, the results are generally more reliable and reflective of underlying physiology (Table 3).
Table 3.
The advantages and disadvantages of PA testing using plasma and 24-hour urinary aldosterone measurements
| Plasma aldosterone | 24-Hour urinary aldosterone | |
|---|---|---|
| Variability | • Aldosterone production varies throughout the day; single spot plasma value may not reflect daily production • Risk for erroneous (false-negative) classification can be overcome by using liberalized thresholds for positive interpretation (see Figure 4a) |
• Integrates aldosterone production throughout the day; less susceptible to daily variability • Only measures free and acid-labile aldosterone; does not adequately capture majority of aldosterone metabolites such as tetrahydroaldosterone that also reflect integrated production |
| Accessibility | • Venipuncture widely available • Assay for measurements generally widely available in locations where renin measurements also available |
• 24-Hour urine collection capabilities less widely available when compared with venipuncture |
| Patient perspective | • Venipuncture is safe and standardized • Minimal time and effort requirement |
• Requires patient education on how to collect a 24-hour urine collection adequately without over- or under-collecting • Requires patient effort and is usually inconvenient |
| Technical—protocol and interpretation | • Given aldosterone variability, requires a liberalized approach for interpretation (see Figure 4a) • Does not require any dietary preparation |
• Requires that patient be on a relatively high-sodium balance for interpretation (see Figure 4b) • Requires measurement of urinary sodium and creatinine to ensure adequate urine collection and appropriate sodium balance • May require dietary sodium loading protocol to ensure a high-sodium balance, which adds additional effort, inconvenience, and potential risk for worsening hypertension and/or hypokalemia |
| Technical—laboratory | • Plasma preparation is standard • Immunoassay and LC–MS/MS techniques protocolized at laboratories where available |
• Urine requires acidification prior to measurement • Following acidification, immunoassay and LC–MS/MS techniques protocolized at laboratories where available |
| Cost | • Variable depending on location and assay type • Cost of single plasma aldosterone measurement (in addition to renin) |
• Variable depending on location and assay type • Cost of urinary acidification, urine free aldosterone, urine sodium, and urine creatinine • Generally, more expensive than a spot plasma aldosterone |
Abbreviations: LC–MS, liquid chromatography–tandem mass spectrometry; PA, primary aldosteronism.
In patients with a high clinical pretest probability, the presence of suppressed renin and an aldosterone concentration greater than 15 ng/dl (via any assay) is sufficient to clinch the diagnosis, especially if in the presence of hypokalemia, and further dynamic testing is not necessary.177,201,202
In high-risk populations who have a suppressed renin but an aldosterone concentration <5 ng/dl, particularly using an immunoassay, the possibility of PA is unlikely. If these results were accompanied by hypokalemia, they should be repeated following potassium supplementation since hypokalemia can substantially lower aldosterone production.203 The exclusion of PA should not deter from the use of empiric MR antagonists as they have been shown to be effective at lowering blood pressure in low-renin hypertension, possibly via mechanisms independent of aldosterone.204,205 Alternatively, the lack of renin suppression in the absence of medications known to raise renin in PA makes the possibility of PA unlikely (though there are rare instances when PA does present with a nonsuppressed renin yet a very high ARR).
For all remaining instances, that is a high-risk patient with renin suppression and an aldosterone concentration between 5 and 15 ng/dl, clinicians should assume “PA until proven otherwise” (Figure 4a). Empiric MR antagonist therapy can be considered in those who prefer not to pursue surgery or in whom unilateral PA is unlikely. Dynamic aldosterone suppression testing can be considered if there are any doubts regarding the diagnosis or if increased confidence is desired prior to localization or surgery. These dynamic tests are often referred to as “confirmatory” tests; however, we prefer to regard their value as “exclusionary tests” since the diagnostic mindset should already be “PA until proven otherwise.” Further, there are multiple unvalidated aldosterone suppression testing protocols, each with their own relatively arbitrary thresholds; reliance on this type of testing can erroneously exclude participants who are likely to benefit from targeted therapies for PA.51,172,206,207 The complete suppression of aldosterone production using an aldosterone suppression test (such as oral or intravenous sodium loading, or fludrocortisone or captopril challenge) implies physiologic aldosterone production and excludes PA; however, the abject failure, or even marginal failure, to inhibit aldosterone production suggests some shade of mild, moderate, or overt PA that is likely to respond to targeted therapy.208
An alternative to relying on plasma aldosterone concentrations is to measure 24-hour urinary aldosterone excretion rates (Figure 4b).51,209,210 Since many patients in industrialized societies will already be in a relatively high dietary sodium state, urine collection without dietary modification may be sufficient; however, to ensure a high-sodium balance, it may be necessary to intervene with dietary sodium loading, which adds additional considerations to this methodology. When in a high-sodium balance, urine aldosterone values >10 mcg in high-risk individuals with suppressed renin likely confirms PA, whereas values between 6 and 10 mcg are strongly suggestive of PA pathophysiology,51,162,209,210 and values <6 mcg likely exclude the possibility of PA.
We recognize that there are many approaches to diagnosing PA164,208,209; however, given the high prevalence and low detection rates of PA, we emphasize to the reader that virtually any aldosterone production in the setting of renin suppression in a high-risk patient population should be regarded as a phenotype of PA pathophysiology that, at minimum, warrants MR antagonist therapy.
CORTISOL COSECRETION IN PA
It has become increasingly evident that cortisol cosecretion is a relatively common occurrence in PA.12,211–217 The prevalence of mild autonomous cortisol secretion (defined as a post-dexamethasone cortisol of ≥1.8 mcg/dl without overt clinical features of Cushing syndrome)218,219 in PA has been reported to range from 4% to 27%12,211–217,220–223; however, prevalence estimates have been even higher when using more sensitive methods at detecting glucocorticoid excess.142,212,216,224 The possibility of cortisol cosecretion in PA is generally restricted to patients who have a visible adrenal mass on cross-sectional imaging, as clinically relevant hypercortisolism usually requires a some degree of adrenocortical neoplasia. Therefore, all PA (and non-PA) patients with an adrenal mass should undergo dexamethasone suppression testing.
Cortisol cosecretion in PA has been associated with increased risk for adverse metabolic, vascular, kidney, and psychiatric outcomes.12,212–215,224,225 In addition, PA patients with cortisol cosecretion have a high risk of developing secondary adrenal insufficiency following a curative unilateral adrenalectomy. In some studies, 30%–50% of PA patients developed postoperative adrenal insufficiency.142,216,226 Although most patients were able to be weaned from glucocorticoid replacement over time,217 some did require long-term glucocorticoid replacement.216,217
IMAGING AND LOCALIZATION
Cross-sectional imaging is recommended once PA is diagnosed to evaluate the rare possibility of adrenocortical carcinoma, and to provide an anatomical map prior to AVS or surgery. Computed tomography and magnetic resonance imaging can detect morphological abnormalities in the adrenal glands; however, they cannot differentiate aldosterone-producing foci from common and incidental nonfunctional adrenal masses, nor can they detect APMs within otherwise morphologically normal-appearing adrenal glands. The discrepancy between cross-sectional imaging and functional histopathology has been well documented in many studies; more than half of morphologically normal-appearing adrenal glands may be a source of aldosteronism in PA when assessed by AVS.227 Despite the heterogeneity in AVS protocols,228–230 the discordance rates between imaging findings and AVS has been consistently reported to be at least 20%–55%.229–235
AVS remains the best method to differentiate unilateral from bilateral PA. Some consensus statements have suggested that young patients with florid PA and a unilateral adrenal mass may forego AVS and proceed directly to surgical adrenalectomy162,236,237; however, recent studies have suggested that this assumption may not be correct in a sizeable proportion of patients.227,238–240
AVS results are more reliable and reproducible when performed by experienced interventional radiologists at high-volume centers.241 However, AVS is a highly technical and operator-dependent procedure, with protocols that vary from institution to institution.228 The main differences in AVS protocols that may affect results and interpretations are summarized in Table 4.189,228,242–250 More stringent criteria to determine lateralization can increase the proportion of missed patients with unilateral PA who may benefit from adrenalectomy.227,234,251 There has also been some concern that cortisol cosecretion may interfere with AVS interpretations,217,252,253 namely misclassifying some patients who may have benefited from a curative unilateral adrenalectomy as having bilateral disease. Future studies to determine the precise impact of cortisol cosecretion on AVS interpretations are needed.
Table 4.
Variations in adrenal venous sampling protocols that could affect results and interpretations
| Advantages | Disadvantages | |
|---|---|---|
| Catheterization method | ||
| Sequential | Technically simpler | Could result in factitious lateralization, particularly when the time between 2 samplings is more than 15 minutes |
| Simultaneous | Helps minimize the difference due to sampling times between adrenal glands | Technically more involved |
| Sampling | ||
| Single adrenal venous sample | More convenient and less cost compared with multiple samples | Lacks precision and can result in misinterpretations owing to variability in aldosterone production |
| Multiple adrenal venous samples | Improves precision during AVS when calculating A/C ratio | Requires additional cost and time |
| Cosyntropin use | Enhances the selectivity index to increase confidence in successful adrenal vein cannulation Decreases the variability of aldosterone |
Can lower lateralization index and lateralization rates, leading to discordant interpretations, and may result in fewer patients diagnosed with lateralizing PA. Therefore, unstimulated data provide more reliable interpretations for lateralization |
| Confirmation of catheter placement | ||
| Cone-beam CT | Improves AVS success rate by confirming accurate catheterization, particularly to the right adrenal vein May reduce total radiation exposure during AVS |
Image degradation caused by several artifacts due to breath-hold or posture (patients are required to raise their arms above the head) Not available in all centers |
| Rapid intraprocedural cortisol assay | Improves AVS success rate by allowing biochemical confirmation of accurate catheterization during the procedure May reduce total radiation exposure during AVS |
May increase the procedural time (depending on the turnaround time for each laboratory) Not available at all centers and not validated |
| Standard cortisol assay | Available at nearly all AVS centers No additional cost |
Slow turnaround time |
Abbreviations: A/C, aldosterone-to-cortisol ratio; AVS, adrenal venous sampling; CT, computed tomography; PA, primary aldosteronism.
In the only randomized controlled trial to assess the value of AVS, when compared with cross-sectional imaging, the SPARTACUS trial found no short-term differences in blood pressure control between medical and surgical therapy.254 However, there were a number of critiques of this study, including its short duration, risk for potentially misclassifying PA patients who may have been eligible for curative adrenalectomy, and use of ACTH infusions during AVS (Table 4).255 Subsequently, several studies have shown that AVS-guided surgical treatment resulted in better clinical outcomes and more complete biochemical success than imaging-guided treatment alone, affirming the value of AVS, when available, as the most reliable method to differentiate between unilateral and bilateral PA.233,256
TREATMENT
The general approach to treating PA depends upon whether the aldosteronism is unilateral, and therefore amenable to a curative intervention, or bilateral, and therefore more amenable to chronic medical therapy. As discussed in the Pathogenesis of PA, the vast majority of PA is likely to be caused by diffuse and heterogeneous processes that occur bilaterally in the adrenal glands, whereas a minority is likely to represent entirely unilateral disease amenable to cure, particularly in younger individuals. Treatment of familial forms of PA is discussed elsewhere.257
Lateralizing PA
For patients with lateralizing PA who are healthy enough and willing to undergo surgery, adrenalectomy to cure (or substantially improve) PA is the treatment of choice.162 Adrenalectomy is now primarily performed via a laparoscopic or retroperitoneoscopic approach.258–261 Routinely, complete adrenalectomy should be performed as generally AVS is limited to determining the side of aldosterone secretion, but not whether a specific adrenal nodule is the source of aldosterone excess.162 Where available, segmental sampling during AVS may allow the surgeon to undertake partial adrenalectomy. Importantly, even when AVS indicates strong lateralization that benefits from adrenalectomy, there may still be residual or emergent PA from the contralateral gland (owing to APM/APN); while some forms of lateralizing PA represent truly unilateral disease, grossly asymmetric bilateral disease is common.262
Specific criteria for biochemical and clinical success following adrenalectomy have been set forth by the multinational Primary Aldosteronism Surgery Outcomes (PASO) study.263 Complete biochemical success is defined as normalization of the ARR, along with resolution of potential hypokalemia, and is achieved in the vast majority of cases including in 94% of patients in the PASO study.110,263–271 PASO characterized clinical success based upon blood pressure control, with complete clinical success defined by normalization of blood pressure without any antihypertensive medications, and partial clinical success defined by a reduction in the number of antihypertensive medications required or a reduction in blood pressure with the same number of antihypertensive medications.263 The PASO study demonstrated that 37% of patients achieved complete clinical success while an additional 47% of patients achieved partial clinical success263; similar results have been demonstrated in other studies.256,264,266,267,272–276
A common question that arises in the treatment of lateralizing PA is whether surgical adrenalectomy improves long-term outcomes beyond medical therapy alone. No randomized trials have answered this directly, largely due to a perceived lack of clinical equipoise. It should be noted that the observational studies that have compared surgical (almost exclusively lateralizing PA) vs. medical (primarily bilateral PA or PA with unconfirmed lateralization) therapy are confounded by inherent differences in the clinical presentation and underlying pathophysiology and severity between unilateral and bilateral PA. Nonetheless, observational studies that have attempted to control for these differences have demonstrated that adrenalectomy in unilateral PA significantly reduced the risk for cardiovascular events,124,125,131,277 kidney disease,135,278,279 type 2 diabetes mellitus,125,280 and mortality126,278 while improving quality of life271,281–283 compared with medical therapy. One large cohort study demonstrated that not only was adrenalectomy for patients with unilateral PA associated with lower all-cause mortality compared with essential hypertension patients, but also that adrenalectomy had a beneficial effect over MR antagonist therapy on mortality and cardiovascular events in lateralizing PA.284
Some centers have used ablative procedures in the treatment of unilateral APA. While concerns have been raised in regard to how ablation compares to surgical adrenalectomy given the lack of histopathology provided and the potential for leaving behind residual adrenal cortical tissue, a number of studies have suggested that ablation is safe and effective.285–289 One randomized controlled trial comparing ablation vs. medical therapy in the treatment of unilateral PA demonstrated that 81% of patients treated with ablation achieved complete or partial hypertension remission along with improvement in biochemical parameters.287 Future clinical trials of ablation vs. adrenalectomy are needed to compare their clinical and cost effectiveness and further define patient populations that are likely to benefit from each approach.
Bilateral PA
For patients with bilateral PA, or those with unilateral PA who do not undergo adrenalectomy, dietary sodium restriction and chronic MR antagonist therapy are the recommended treatments.162 Effective dietary sodium restriction can result in volume contraction, a rise in renin, and normalization of blood pressure and ARR, in patients with PA.79 However, sustaining this degree of sodium restriction (often <1,500 mg per day) can be challenging; therefore, combined therapy with MR antagonists is usually necessary.
The 2 most commonly prescribed MR antagonists are spironolactone and eplerenone. Spironolactone is more potent, longer acting, and generally less expensive than eplerenone. However, antiandrogenic effects (including gynecomastia, decreased libido, and menstrual irregularities) at higher and sustained doses can be limiting. In the future, newer generation nonsteroidal MR antagonists may expand the therapeutic options available for PA patients.290–292 When MR antagonists are not tolerated or sufficient, epithelial sodium channel inhibitors, such as amiloride, are also effective options to lower blood pressure and normalize potassium.80,293,294
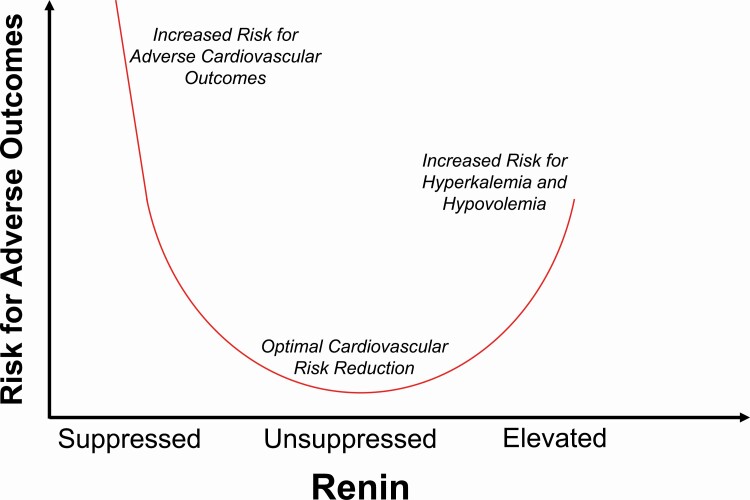
A common question that arises is “what is the goal of medical therapy in PA?” Historically, MR antagonists have been titrated to target normalization of blood pressure and serum potassium. However, recent data from at least 4 studies have shed more insights into pathophysiology-based approaches to treatment. Cohort studies have shown that PA patients treated with MR antagonists have a significantly higher risk for adverse cardiovascular events, atrial fibrillation, chronic kidney disease, and death, when compared with those with essential hypertension, despite achieving similar blood pressure control.124,125,135 In an American cohort, this excess risk was predominantly driven by PA patients whose renin remained suppressed despite MR antagonist therapy; in contrast, when MR antagonist therapy in PA resulted in substantial increases in renin, the excess risk was mitigated.124,125 Another large prospective study from Taiwan that evaluated patients with unilateral PA treated with MR antagonists, demonstrated similar findings, wherein a persistently suppressed renin was associated with a higher risk of major adverse cardiovascular events and death compared with those whose renin increased.284 Similarly, a German study reported a greater reduction in left ventricular hypertrophy among PA patients who achieved unsuppressed renin levels with MR antagonists compared with those whose renin levels remained suppressed.277 Collectively, these studies suggest that in addition to targeting normalization of blood pressure and serum potassium, clinicians should also consider targeting a rise in renin with MR antagonist therapy as a method to ensure optimized long-term risk reduction. A rise in renin will often require more aggressive MR antagonist dosing and reflects MR blockade sufficient enough to induce a level of volume contraction. With more aggressive MR antagonist dosing, other antihypertensive medications can be reduced or consolidated, and closer monitoring is required to avoid adverse effects such as antiandrogenic and progestational, as well as hyperkalemia and hypovolemia, particularly among patients with chronic kidney disease (Figure 5). Hyperkalemia in chronic kidney disease can now be managed with the use of novel potassium binders and with SGLT2 inhibitors.295,296
Figure 5.
Targeting renin levels during mineralocorticoid receptor antagonist therapy. The primary objectives of MR antagonist therapy include normalization of blood pressure and potassium. Once these objectives are achieved, a rise in renin is a biomarker that is associated with lower risk for adverse cardiovascular outcomes. An unsuppressed renin activity, 1.0–2.0 ng/ml/h, is an ideal range to target; however, it is likely that any increase in renin from its baseline suppressed state is indicative of beneficial MR blockade. Excessive uptitration of MR antagonists associated with much higher renin levels may herald increased risk for hyperkalemia and/or relative hypovolemia and renal hypoperfusion. Abbreviation: MR, mineralocorticoid receptor.
One remaining area of uncertainty in regard to the treatment of bilateral PA is whether unilateral adrenalectomy should be considered in certain cases where clinical optimization with MR antagonists cannot be achieved. In this scenario, unilateral adrenalectomy to attenuate disease severity, rather than biochemical cure, can be considered. Several case series and personal experiences have shown that noncurative unilateral adrenalectomy can be an effective and safe option to improve biochemical and clinical sequelae associated with bilateral PA.297–299
FUTURE DIRECTIONS
The next 5–10 years are likely to see emerging research focused on noninvasive methods to localize the source of PA as well as novel methods to treat PA. However, a key public health priority must include raising awareness for PA, increasing screening rates, and increasing the use of MR antagonists.
Newer imaging modalities and biomarkers have been investigated as a potential tool to aid subtype differentiation in PA. NP59 scans, although having low sensitivity in detecting lateralizing PA, can be used when AVS is unavailable or inconclusive.236,23711C-Metomidate positron emission tomography has shown some promise in subtype differentiation300,301; however, the concordance with AVS results is still not ideal.302 Adrenal steroid profiling is another promising approach to differentiate idiopathic hypertension from PA, and lateralizing from bilateral PA.303–305 Finally, many prediction models have been developed to predict subtype differentiation noninvasively, though they still lack reproducibility and external validation.306–308
Novel nonsteroidal MR antagonists have been shown to be safe and effective at lowering blood pressure in PA,309 and capable of lowering risk for cardiovascular and kidney disease progression in patients with chronic kidney disease290–292; whether they may provide long-term and durable risk reduction in PA remains to be seen. In addition, several phase II studies evaluating novel aldosterone synthase inhibitors are currently underway; the possibility of targeted CYP11B2 inhibition could be a game-changer for the treatment of PA and other aldosterone-driven conditions.
We hope new guidelines will bring together multiple medical societies to sound the alarm on the high prevalence and unrecognized nature of PA. Novel educational approaches to raise awareness and simplify the pathway to diagnosis or empiric treatment for PA (as suggested herein) are much needed.
Contributor Information
Anand Vaidya, Department of Medicine, Center for Adrenal Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Gregory L Hundemer, Department of Medicine (Division of Nephrology) and the Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario, Canada.
Kazutaka Nanba, Department of Endocrinology and Metabolism, National Hospital Organization Kyoto Medical Center, Kyoto, Japan; Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, Michigan, USA.
Wasita W Parksook, Department of Medicine, Division of Endocrinology and Metabolism, and Division of General Internal Medicine, Faculty of Medicine, Chulalongkorn University, and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand.
Jenifer M Brown, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
FUNDING
We thank our funding sources for supporting this work. A.V. was funded by National Institutes of Health awards: R01 DK115392, R01 HL153004, R01 DK16618, and R01 HL155834. K.N. was funded by a Japan Heart Foundation Research Grant. J.M.B. was funded by an American Heart Association Career Development Award 852429, a KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst UL1 TR002541, and by NIH/NHLBI grant K23HL159279. G.L.H. was funded by the Canadian Institutes of Health Research Institute of Nutrition, Metabolism and Diabetes (Reference # PJT-175027), the Kidney Foundation of Canada (Reference # 851937-21KHRG), the Kidney Research Scientist Core Education and National Training (KRESCENT) Program New Investigator Award (Reference # 2019KP-NIA626990), and the Lorna Jocelyn Wood Chair for Kidney Research.
DISCLOSURE
A.V. reports consulting fees from Mineralys, Corcept, HRA Pharma, all unrelated to the current work. J.M.B. reports consulting fees from Bayer, unrelated to the current work.
REFERENCES
- 1. Scholl UI. Genetics of primary aldosteronism. Hypertension 2022; 79:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zennaro MC, Boulkroun S, Fernandes-Rosa F. Genetic causes of functional adrenocortical adenomas. Endocr Rev 2017; 38:516–537. [DOI] [PubMed] [Google Scholar]
- 3. Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 2010; 95:2296–2305. [DOI] [PubMed] [Google Scholar]
- 4. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol 2014; 383:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A, Katabami T, Okumura A, Kawa G, Tanabe A, Naruse M. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab 2013; 98:1567–1574. [DOI] [PubMed] [Google Scholar]
- 6. Meyer LS, Wang X, Susnik E, Burrello J, Burrello A, Castellano I, Eisenhofer G, Fallo F, Kline GA, Knosel T, Kocjan T, Lenders JWM, Mulatero P, Naruse M, Nishikawa T, Peitzsch M, Rump LC, Beuschlein F, Hahner S, Gomez-Sanchez CE, Reincke M, Williams TA. Immunohistopathology and steroid profiles associated with biochemical outcomes after adrenalectomy for unilateral primary aldosteronism. Hypertension 2018; 72:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, Mete O, Yamazaki Y, Zerbini MCN, Beuschlein F, Satoh F, Burrello J, Schneider H, Lenders JWM, Mulatero P, Castellano I, Knosel T, Papotti M, Saeger W, Sasano H, Reincke M. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab 2021; 106:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mete O, Erickson LA, Juhlin CC, de Krijger RR, Sasano H, Volante M, Papotti MG. Overview of the 2022 WHO classification of adrenal cortical tumors. Endocr Pathol 2022; 33:155–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, Rainey WE, Auchus RJ, Turcu AF. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol 2017; 87:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Omata K, Yamazaki Y, Nakamura Y, Anand SK, Barletta JA, Sasano H, Rainey WE, Tomlins SA, Vaidya A. Genetic and histopathologic intertumor heterogeneity in primary aldosteronism. J Clin Endocrinol Metab 2017; 102:1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fallo F, Castellano I, Gomez-Sanchez CE, Rhayem Y, Pilon C, Vicennati V, Santini D, Maffeis V, Fassina A, Mulatero P, Beuschlein F, Reincke M. Histopathological and genetic characterization of aldosterone-producing adenomas with concurrent subclinical cortisol hypersecretion: a case series. Endocrine 2017; 58:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang L, Li X, Wang B, Ma X, Li H, Gao Y, Gu L, Nie W, Zhang X. Clinical characteristics of aldosterone- and cortisol-coproducing adrenal adenoma in primary aldosteronism. Int J Endocrinol 2018; 2018:4920841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teragawa H, Oshita C, Orita Y, Hashimoto K, Nakayama H, Yamazaki Y, Sasano H. Primary aldosteronism due to bilateral micronodular hyperplasia and concomitant subclinical Cushing’s syndrome: a case report. World J Clin Cases 2021; 9:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fushimi Y, Tatsumi F, Sanada J, Shimoda M, Kamei S, Nakanishi S, Kaku K, Mune T, Kaneto H. Concurrence of overt Cushing’s syndrome and primary aldosteronism accompanied by aldosterone-producing cell cluster in adjacent adrenal cortex: case report. BMC Endocr Disord 2021; 21:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nanba K, Omata K, Tomlins SA, Giordano TJ, Hammer GD, Rainey WE, Else T. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol 2016; 175:K1–K6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stenman A, Shabo I, Ramstrom A, Zedenius J, Juhlin CC. Synchronous aldosterone- and cortisol-producing adrenocortical adenomas diagnosed using CYP11B immunohistochemistry. SAGE Open Med Case Rep 2019; 7. doi: 10.1177/2050313X19883770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Y, Tan J, Yang Q, Du Z, Yang S, He W, Song Y, Hu J, Yang Y, Li Q, Zhang Y, He Y, Cheng Q; Chongqing Primary Aldosteronism Study (CONPASS) Group. Primary aldosteronism concurrent with subclinical Cushing’s syndrome: a case report and review of the literature. J Med Case Rep 2020; 14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen YJ, Peng KY, Chueh JS, Liao HW, Hsieh TY, Wu VC, Wang SM. Case report: primary aldosteronism due to bilateral aldosterone-producing micronodules with HISTALDO classical and contralateral non-classical pathology. Front Endocrinol 2022; 13:816754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ren K, Wei J, Liu Q, Zhu Y, Wu N, Tang Y, Li Q, Zhang Q, Yu Y, An Z, Chen J, Li J. Hypercortisolism and primary aldosteronism caused by bilateral adrenocortical adenomas: a case report. BMC Endocr Disord 2019; 19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, Anand SK, Guo Z, Stowasser M, Sasano H, Tomlins SA, Rainey WE. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension 2018; 72:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011; 331:768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013; 45:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nanba K, Blinder AR, Rege J, Hattangady NG, Else T, Liu CJ, Tomlins SA, Vats P, Kumar-Sinha C, Giordano TJ, Rainey WE. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension 2020; 75:645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife 2015; 4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scholl UI, Stolting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, Yoo T, Choi J, Xu S, Wu A, Kirchner M, Mertins P, Rump LC, Onder AM, Gamble C, McKenney D, Lash RW, Jones DP, Chune G, Gagliardi P, Choi M, Gordon R, Stowasser M, Fahlke C, Lifton RP. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet 2018; 50:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernandes-Rosa FL, Daniil G, Orozco IJ, Goppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, Schwarzmayr T, Strom TM, Jentsch TJ, Zennaro MC. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet 2018; 50:355–361. [DOI] [PubMed] [Google Scholar]
- 27. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 2013; 45:440–444, 444e1–2. [DOI] [PubMed] [Google Scholar]
- 28. Akerstrom T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stalberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Bjorklund P. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep 2016; 6:19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, Wang JJ, Connolly R, Hu YH, Gomez-Sanchez CE, Peng KY, Wu KD. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep 2017; 7:39121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou J, Azizan EAB, Cabrera CP, Fernandes-Rosa FL, Boulkroun S, Argentesi G, Cottrell E, Amar L, Wu X, O’Toole S, Goodchild E, Marker A, Senanayake R, Garg S, Akerstrom T, Backman S, Jordan S, Polubothu S, Berney DM, Gluck A, Lines KE, Thakker RV, Tuthill A, Joyce C, Kaski JP, Karet Frankl FE, Metherell LA, Teo AED, Gurnell M, Parvanta L, Drake WM, Wozniak E, Klinzing D, Kuan JL, Tiang Z, Gomez Sanchez CE, Hellman P, Foo RSY, Mein CA, Kinsler VA, Bjorklund P, Storr HL, Zennaro MC, Brown MJ. Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause. Nat Genet 2021; 53:1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, Vaidya A, Barletta JA, Else T, Giordano TJ, Tomlins SA, Rainey WE. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension 2019; 73:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992; 355:262–265. [DOI] [PubMed] [Google Scholar]
- 33. Yamada M, Nakajima Y, Taguchi R, Okamura T, Ishii S, Tomaru T, Ozawa A, Shibusawa N, Yoshino S, Toki A, Ishida E, Hashimoto K, Satoh T, Mori M. KCNJ5 mutations in aldosterone- and cortisol-co-secreting adrenal adenomas. Endocr J 2012; 59:735–741. [DOI] [PubMed] [Google Scholar]
- 34. Lerario AM, Nanba K, Blinder AR, Suematsu S, Omura M, Nishikawa T, Giordano TJ, Rainey WE, Else T. Genetics of aldosterone-producing adenomas with pathogenic KCNJ5 variants. Endocr Relat Cancer 2019; 26:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, Williams TA, Mulatero P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol 2015; 411:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inoue K, Yamazaki Y, Kitamoto T, Hirose R, Saito J, Omura M, Sasano H, Nishikawa T. Aldosterone suppression by dexamethasone in patients with KCNJ5-mutated aldosterone-producing adenoma. J Clin Endocrinol Metab 2018; 103:3477–3485. [DOI] [PubMed] [Google Scholar]
- 37. De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, Rocha A, Giscos-Douriez I, Meatchi T, Amar L, Travers S, Fernandes-Rosa FL, Zennaro MC. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension 2020; 75:1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology 2012; 153:1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hattangady NG, Karashima S, Yuan L, Ponce-Balbuena D, Jalife J, Gomez-Sanchez CE, Auchus RJ, Rainey WE, Else T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol 2016; 57:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rhayem Y, Perez-Rivas LG, Dietz A, Bathon K, Gebhard C, Riester A, Mauracher B, Gomez-Sanchez C, Eisenhofer G, Schwarzmayr T, Calebiro D, Strom TM, Reincke M, Beuschlein F. PRKACA somatic mutations are rare findings in aldosterone-producing adenomas. J Clin Endocrinol Metab 2016; 101:3010–3017. [DOI] [PubMed] [Google Scholar]
- 41. Nakajima Y, Okamura T, Horiguchi K, Gohko T, Miyamoto T, Satoh T, Ozawa A, Ishii S, Yamada E, Hashimoto K, Okada S, Takata D, Horiguchi J, Yamada M. GNAS mutations in adrenal aldosterone-producing adenomas. Endocr J 2016; 63:199–204. [DOI] [PubMed] [Google Scholar]
- 42. Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, Miller BS, Giordano TJ, Tomlins SA, Rainey WE. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J Clin Endocrinol Metab 2018; 103:3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nanba K, Rainey WE. Genetics in Endocrinology: impact of race and sex on genetic causes of aldosterone-producing adenomas. Eur J Endocrinol 2021; 185:R1–R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA 2015; 112:E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Omata K, Anand SK, Hovelson DH, Liu CJ, Yamazaki Y, Nakamura Y, Ito S, Satoh F, Sasano H, Rainey WE, Tomlins SA. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc 2017; 1:787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishimoto K, Seki T, Hayashi Y, Mikami S, Al-Eyd G, Nakagawa K, Morita S, Kosaka T, Oya M, Mitani F, Suematsu M, Kabe Y, Mukai K. Human adrenocortical remodeling leading to aldosterone-producing cell cluster generation. Int J Endocrinol 2016; 2016:7834356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation 2017; 136:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension 2018; 71:218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sugiura Y, Takeo E, Shimma S, Yokota M, Higashi T, Seki T, Mizuno Y, Oya M, Kosaka T, Omura M, Nishikawa T, Suematsu M, Nishimoto K. Aldosterone and 18-oxocortisol coaccumulation in aldosterone-producing lesions. Hypertension 2018; 72:1345–1354. [DOI] [PubMed] [Google Scholar]
- 50. Takeo E, Sugiura Y, Uemura T, Nishimoto K, Yasuda M, Sugiyama E, Ohtsuki S, Higashi T, Nishikawa T, Suematsu M, Fukusaki E, Shimma S. Tandem mass spectrometry imaging reveals distinct accumulation patterns of steroid structural isomers in human adrenal glands. Anal Chem 2019; 91:8918–8925. [DOI] [PubMed] [Google Scholar]
- 51. Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med 2020; 173:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nishimoto K, Seki T, Kurihara I, Yokota K, Omura M, Nishikawa T, Shibata H, Kosaka T, Oya M, Suematsu M, Mukai K. Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J Clin Endocrinol Metab 2016; 101:6–9. [DOI] [PubMed] [Google Scholar]
- 53. Nishimoto K, Koga M, Seki T, Oki K, Gomez-Sanchez EP, Gomez-Sanchez CE, Naruse M, Sakaguchi T, Morita S, Kosaka T, Oya M, Ogishima T, Yasuda M, Suematsu M, Kabe Y, Omura M, Nishikawa T, Mukai K. Immunohistochemistry of aldosterone synthase leads the way to the pathogenesis of primary aldosteronism. Mol Cell Endocrinol 2017; 441:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun N, Meyer LS, Feuchtinger A, Kunzke T, Knosel T, Reincke M, Walch A, Williams TA. Mass spectrometry imaging establishes 2 distinct metabolic phenotypes of aldosterone-producing cell clusters in primary aldosteronism. Hypertension 2020; 75:634–644. [DOI] [PubMed] [Google Scholar]
- 55. Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Genetic and genomic mechanisms of primary aldosteronism. Trends Mol Med 2020; 26:819–832. [DOI] [PubMed] [Google Scholar]
- 56. Vouillarmet J, Fernandes-Rosa F, Graeppi-Dulac J, Lantelme P, Decaussin-Petrucci M, Thivolet C, Peix JL, Boulkroun S, Clauser E, Zennaro MC. Aldosterone-producing adenoma with a somatic KCNJ5 mutation revealing APC-dependent familial adenomatous polyposis. J Clin Endocrinol Metab 2016; 101:3874–3878. [DOI] [PubMed] [Google Scholar]
- 57. St-Jean M, Bourdeau I, Martin M, Lacroix A. Aldosterone is aberrantly regulated by various stimuli in a high proportion of patients with primary aldosteronism. J Clin Endocrinol Metab 2021; 106:e45–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. St-Jean M, Ghorayeb NE, Bourdeau I, Lacroix A. Aberrant G-protein coupled hormone receptor in adrenal diseases. Best Pract Res Clin Endocrinol Metab 2018; 32:165–187. [DOI] [PubMed] [Google Scholar]
- 59. Lopez AG, Duparc C, Naccache A, Castanet M, Lefebvre H, Louiset E. Role of mast cells in the control of aldosterone secretion. Horm Metab Res 2020; 52:412–420. [DOI] [PubMed] [Google Scholar]
- 60. De Sousa K, Abdellatif AB, Giscos-Douriez I, Meatchi T, Amar L, Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Colocalization of Wnt/beta-catenin and ACTH signaling pathways and paracrine regulation in aldosterone producing adenoma. J Clin Endocrinol Metab 2021; 107:419–434. doi: 10.1210/clinem/dgab707 [DOI] [PubMed] [Google Scholar]
- 61. Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemele EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015; 132:2134–2145. [DOI] [PubMed] [Google Scholar]
- 62. El Ghorayeb N, Bourdeau I, Lacroix A. Role of ACTH and other hormones in the regulation of aldosterone production in primary aldosteronism. Front Endocrinol 2016; 7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gouli A, Kaltsas G, Tzonou A, Markou A, Androulakis II, Ragkou D, Vamvakidis K, Zografos G, Kontogeorgos G, Chrousos GP, Piaditis G. High prevalence of autonomous aldosterone secretion among patients with essential hypertension. Eur J Clin Invest 2011; 41:1227–1236. [DOI] [PubMed] [Google Scholar]
- 64. Lim JS, Plaska SW, Rege J, Rainey WE, Turcu AF. Aldosterone-regulating receptors and aldosterone-driver somatic mutations. Front Endocrinol 2021; 12:644382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Markou A, Sertedaki A, Kaltsas G, Androulakis II, Marakaki C, Pappa T, Gouli A, Papanastasiou L, Fountoulakis S, Zacharoulis A, Karavidas A, Ragkou D, Charmandari E, Chrousos GP, Piaditis GP. Stress-induced aldosterone hyper-secretion in a substantial subset of patients with essential hypertension. J Clin Endocrinol Metab 2015; 100:2857–2864. [DOI] [PubMed] [Google Scholar]
- 66. Funder JW. The potential of ACTH in the genesis of primary aldosteronism. Front Endocrinol 2016; 7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gagnon N, Caceres-Gorriti KY, Corbeil G, El Ghoyareb N, Ludwig N, Latour M, Lacroix A, Bourdeau I. Genetic characterization of GnRH/LH-responsive primary aldosteronism. J Clin Endocrinol Metab 2018; 103:2926–2935. [DOI] [PubMed] [Google Scholar]
- 68. De Sousa K, Abdellatif AB, Giscos-Douriez I, Meatchi T, Amar L, Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Colocalization of Wnt/beta-catenin and ACTH signaling pathways and paracrine regulation in aldosterone-producing adenoma. J Clin Endocrinol Metab 2022; 107:419–434. [DOI] [PubMed] [Google Scholar]
- 69. Lampron A, Bourdeau I, Oble S, Godbout A, Schurch W, Arjane P, Hamet P, Lacroix A. Regulation of aldosterone secretion by several aberrant receptors including for glucose-dependent insulinotropic peptide in a patient with an aldosteronoma. J Clin Endocrinol Metab 2009; 94:750–756. [DOI] [PubMed] [Google Scholar]
- 70. Zwermann O, Suttmann Y, Bidlingmaier M, Beuschlein F, Reincke M. Screening for membrane hormone receptor expression in primary aldosteronism. Eur J Endocrinol 2009; 160:443–451. [DOI] [PubMed] [Google Scholar]
- 71. Sinclair AM, Isles CG, Brown I, Cameron H, Murray GD, Robertson JWK.. Secondary hypertension in a blood pressure clinic. Arch Intern Med 1987; 147:1289–1289. [PubMed] [Google Scholar]
- 72. Vasan RS, Evans JC, Larson MG, Wilson PWF, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med 2004; 351:33–41. [DOI] [PubMed] [Google Scholar]
- 73. Newton-Cheh C, Guo C-YY, Gona P, Larson MG, Benjamin EJ, Wang TJ, Kathiresan S, O’Donnell CJ, Musone SL, Camargo AL, Drake JA, Levy D, Hirschhorn JN, Vasan RS. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension 2007; 49:846–856. [DOI] [PubMed] [Google Scholar]
- 74. Markou A, Pappa T, Kaltsas G, Gouli A, Mitsakis K, Tsounas P, Prevoli A, Tsiavos V, Papanastasiou L, Zografos G, Chrousos GP, Piaditis GP. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: a very high odds ratio for progression into arterial hypertension. J Clin Endocrinol Metab 2013; 98:1409–1416. [DOI] [PubMed] [Google Scholar]
- 75. Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH, Vaidya A. The spectrum of subclinical primary aldosteronism and incident hypertension. Ann Intern Med 2017; 167:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Baudrand R, Guarda FJ, Fardella CE, Hundemer G, Brown J, Williams GH, Vaidya A. Continuum of renin-independent aldosteronism in normotension. Hypertension 2017; 69:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi-ethnic study of atherosclerosis. Hypertension 2020; 76:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol 2017; 69:1811–1820. [DOI] [PubMed] [Google Scholar]
- 79. Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary sodium restriction increases the risk of misinterpreting mild cases of primary aldosteronism. J Clin Endocrinol Metab 2016; 101:3989–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev 2018; 39:1057–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Galati S-J, Cheesman KC, Springer-Miller R, Hopkins SM, Krakoff L, Bagiella E, Zhuk RA, Ying TK, Amer C, Boyajian MK, Inabnet WB, Levine AC. Prevalence of primary aldosteronism in an urban hypertensive population. Endocr Pract 2016; 22:1296–1302. [DOI] [PubMed] [Google Scholar]
- 82. Libianto R, Russell GM, Stowasser M, Gwini SM, Nuttall P, Shen J, Young MJ, Fuller PJ, Yang J. Detecting primary aldosteronism in Australian primary care: a prospective study. Med J Aust 2022; 216:408–412. [DOI] [PubMed] [Google Scholar]
- 83. Xu Z, Yang J, Hu J, Song Y, He W, Luo T, Cheng Q, Ma L, Luo R, Fuller PJ, Cai J, Li Q, Yang S, Mei M, Luo S, Liao K, Zhang Y, He Y, He Y, Xiao M, Peng B. Primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol 2020; 75:1913–1922. [DOI] [PubMed] [Google Scholar]
- 84. Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol 1994; 21:315–318. [DOI] [PubMed] [Google Scholar]
- 85. Schwartz GL, Chapman AB, Boerwinkle E, Kisabeth RM, Turner ST. Screening for primary aldosteronism: implications of an increased plasma aldosterone/renin ratio. Clin Chem 2002; 48:1919–1923. [PubMed] [Google Scholar]
- 86. Burrello J, Monticone S, Losano I, Cavaglia G, Buffolo F, Tetti M, Covella M, Rabbia F, Veglio F, Pasini B, Williams TA, Mulatero P. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension 2020; 75:1025–1033. doi: 10.1161/HYPERTENSIONAHA.119.14063 [DOI] [PubMed] [Google Scholar]
- 87. Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004; 89:1045–1050. [DOI] [PubMed] [Google Scholar]
- 88. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F, Investigators PS. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006; 48:2293–2300. [DOI] [PubMed] [Google Scholar]
- 89. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896. [DOI] [PubMed] [Google Scholar]
- 90. Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, Papadopoulos N, Vogiatzis K, Zamboulis C. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 2008; 371:1921–1926. [DOI] [PubMed] [Google Scholar]
- 91. Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, Rossato D, Mengozzi G, Ghigo E, Benso A, Maccario M. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertens 2020; 38:1841–1848. [DOI] [PubMed] [Google Scholar]
- 92. Memon SS, Lila A, Barnabas R, Goroshi M, Sarathi V, Shivane V, Patil V, Shah N, Bandgar T. Prevalence of primary aldosteronism in type 2 diabetes mellitus and hypertension: a prospective study from Western India. Clin Endocrinol 2022; 96:539–548. [DOI] [PubMed] [Google Scholar]
- 93. Voulgaris N, Tyfoxylou E, Vlachou S, Kyriazi E, Gravvanis C, Kapsali C, Markou A, Papanastasiou L, Gryparis A, Kassi E, Chrousos G, Kaltsas G, Piaditis G. Prevalence of primary aldosteronism across the stages of hypertension based on a new combined overnight test. Horm Metab Res 2021; 53:461–469. [DOI] [PubMed] [Google Scholar]
- 94. Hu Y, Zhang J, Liu W, Su X. Determining the prevalence of primary aldosteronism in patients with new-onset type 2 diabetes and hypertension. J Clin Endocrinol Metab 2020; 105:1079–1085. doi: 10.1210/clinem/dgz293 [DOI] [PubMed] [Google Scholar]
- 95. Hung CS, Chou CH, Liao CW, Lin YT, Wu XM, Chang YY, Chen YH, Wu VC, Su MJ, Ho YL, Chen MF, Wu KD, Lin YH; TAIPAI Study Group. Aldosterone induces tissue inhibitor of metalloproteinases-1 expression and further contributes to collagen accumulation: from clinical to bench studies. Hypertension 2016; 67:1309–1320. [DOI] [PubMed] [Google Scholar]
- 96. Hung CS, Sung SH, Liao CW, Pan CT, Chang CC, Chen ZW, Wu VC, Chen CH, Cheng HM, Lin YH; TAIPAI Study Group. Aldosterone induces vascular damage. Hypertension 2019; 74:623–629. [DOI] [PubMed] [Google Scholar]
- 97. Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, Ghiadoni L, Bernini M, Santoro G, Salvetti A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens 2008; 26:2399–2405. [DOI] [PubMed] [Google Scholar]
- 98. Demirkiran A, Everaars H, Elitok A, van de Ven PM, Smulders YM, Dreijerink KM, Tanakol R, Ozcan M. Hypertension with primary aldosteronism is associated with increased carotid intima-media thickness and endothelial dysfunction. J Clin Hypertens 2019; 21:932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen ZW, Tsai CH, Pan CT, Chou CH, Liao CW, Hung CS, Wu VC, Lin YH; TAIPAI Study Group. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci 2019; 20. doi: 10.3390/ijms20205214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van der Heijden C, Smeets EMM, Aarntzen E, Noz MP, Monajemi H, Kersten S, Kaffa C, Hoischen A, Deinum J, Joosten LAB, Netea MG, Riksen NP. Arterial wall inflammation and increased hematopoietic activity in patients with primary aldosteronism. J Clin Endocrinol Metab 2020; 105:e1967–e1980. doi: 10.1210/clinem/dgz306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tsai CH, Pan CT, Chang YY, Chen ZW, Wu VC, Hung CS, Lin YH. Left ventricular remodeling and dysfunction in primary aldosteronism. J Hum Hypertens 2021; 35:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rossi GP, Seccia TM, Maiolino G, Cesari M. The cardiovascular consequences of hyperaldosteronism. Ann Endocrinol 2021; 82:174–178. [DOI] [PubMed] [Google Scholar]
- 103. Briet M, Barhoumi T, Mian MOR, Coelho SC, Ouerd S, Rautureau Y, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced vascular remodeling and endothelial dysfunction require functional angiotensin type 1a receptors. Hypertension 2016; 67:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res 2013; 50:89–99. [DOI] [PubMed] [Google Scholar]
- 105. Diaz-Otero JM, Fisher C, Downs K, Moss ME, Jaffe IZ, Jackson WF, Dorrance AM. Endothelial mineralocorticoid receptor mediates parenchymal arteriole and posterior cerebral artery remodeling during angiotensin II-induced hypertension. Hypertension 2017; 70:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim SK, McCurley AT, DuPont JJ, Aronovitz M, Moss ME, Stillman IE, Karumanchi SA, Christou DD, Jaffe IZ. Smooth muscle cell-mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension 2018; 71:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012; 18:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension 2015; 66:988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tesch GH, Young MJ. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front Pharmacol 2017; 8:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rossi GP, Bolognesi M, Rizzoni D, Seccia TM, Piva A, Porteri E, Tiberio GA, Giulini SM, Agabiti-Rosei E, Pessina AC. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension 2008; 51:1366–1371. [DOI] [PubMed] [Google Scholar]
- 111. Rossi GP, Cesari M, Pessina AC. Left ventricular changes in primary aldosteronism. Am J Hypertens 2003; 16:96–98. [DOI] [PubMed] [Google Scholar]
- 112. Rossi GP, Di Bello V, Ganzaroli C, Sacchetto A, Cesari M, Bertini A, Giorgi D, Scognamiglio R, Mariani M, Pessina AC. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 2002; 40:23–27. [DOI] [PubMed] [Google Scholar]
- 113. Rocha R, Stier CT Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 2000; 141:3871–3878. [DOI] [PubMed] [Google Scholar]
- 114. Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension 2002; 39:614–618. [PubMed] [Google Scholar]
- 115. Gaddam K, Corros C, Pimenta E, Ahmed M, Denney T, Aban I, Inusah S, Gupta H, Lloyd SG, Oparil S, Husain A, Dell’Italia LJ, Calhoun DA. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical study. Hypertension 2010; 55:1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Redheuil A, Blanchard A, Pereira H, Raissouni Z, Lorthioir A, Soulat G, Vargas-Poussou R, Amar L, Paul JL, Helley D, Azizi M, Kachenoura N, Mousseaux E. Aldosterone-related myocardial extracellular matrix expansion in hypertension in humans: a proof-of-concept study by cardiac magnetic resonance. JACC Cardiovasc Imaging 2020; 13:2149–2159. [DOI] [PubMed] [Google Scholar]
- 117. Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med 2008; 168:80–85. [DOI] [PubMed] [Google Scholar]
- 118. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005; 45:1243–1248. [DOI] [PubMed] [Google Scholar]
- 119. Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab 2013; 98:4826–4833. [DOI] [PubMed] [Google Scholar]
- 120. Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 2013; 62:331–336. [DOI] [PubMed] [Google Scholar]
- 121. Murata M, Kitamura T, Tamada D, Mukai K, Kurebayashi S, Yamamoto T, Hashimoto K, Hayashi RD, Kouhara H, Takeiri S, Kajimoto Y, Nakao M, Hamasaki T, Otsuki M, Shimomura I. Plasma aldosterone level within the normal range is less associated with cardiovascular and cerebrovascular risk in primary aldosteronism. J Hypertens 2017; 35:1079–1085. [DOI] [PubMed] [Google Scholar]
- 122. Takeda R, Matsubara T, Miyamori I, Hatakeyama H, Morise T. Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. The Research Committee of Disorders of Adrenal Hormones in Japan. J Endocrinol Invest 1995; 18:370–373. [DOI] [PubMed] [Google Scholar]
- 123. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, Endres S; German Conn’s Registry-Else Kroner-Fresenius-Hyperaldosteronism Registry. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension 2012; 60:618–624. [DOI] [PubMed] [Google Scholar]
- 124. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol 2018; 3:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol 2018; 6:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu VC, Wang SM, Chang CH, Hu YH, Lin LY, Lin YH, Chueh SC, Chen L, Wu KD. Long term outcome of aldosteronism after target treatments. Sci Rep 2016; 6:32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rossi GP, Sacchetto A, Pavan E, Scognamiglio R, Pietra M, Pessina AC. Left ventricular systolic function in primary aldosteronism and hypertension. J Hypertens 1998; 16:2075–2077. [DOI] [PubMed] [Google Scholar]
- 128. Rossi GP, Sacchetto A, Visentin P, Canali C, Graniero GR, Palatini P, Pessina AC. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension 1996; 27:1039–1045. [DOI] [PubMed] [Google Scholar]
- 129. Rossi GP, Maiolino G, Flego A, Belfiore A, Bernini G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Muiesan ML, Mannelli M, Negro A, Palumbo G, Parenti G, Rossi E, Mantero F, Investigators PS. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension 2018; 71:585–591. [DOI] [PubMed] [Google Scholar]
- 130. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, Kurihara I, Itoh H, Umakoshi H, Tsuiki M, Ichijo T, Katabami T, Tanaka Y, Wada N, Shibayama Y, Yoshimoto T, Ogawa Y, Kawashima J, Takahashi K, Fujita M, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamoto K, Ogo A, Okamura S, Miyauchi S, Fukuoka T, Izawa S, Yoneda T, Hashimoto S, Yanase T, Suzuki T, Kawamura T, Tabara Y, Matsuda F, Naruse M; Nagahama Study; JPAS Study Group. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension 2018; 71:530–537. [DOI] [PubMed] [Google Scholar]
- 131. Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension 2013; 62:62–69. [DOI] [PubMed] [Google Scholar]
- 132. Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2018; 6:41–50. [DOI] [PubMed] [Google Scholar]
- 133. Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol 2005; 16:1320–1325. [DOI] [PubMed] [Google Scholar]
- 134. Hundemer GL, Baudrand R, Brown JM, Curhan G, Williams GH, Vaidya A. Renin phenotypes characterize vascular disease, autonomous aldosteronism, and mineralocorticoid receptor activity. J Clin Endocrinol Metab 2017; 102:1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension 2018; 72:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Reincke M, Rump LC, Quinkler M, Hahner S, Diederich S, Lorenz R, Seufert J, Schirpenbach C, Beuschlein F, Bidlingmaier M, Meisinger C, Holle R, Endres S; Participants of German Conn’s Registry. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab 2009; 94:869–875. [DOI] [PubMed] [Google Scholar]
- 137. Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palumbo G, Rizzoni D, Rossi E, Pessina AC, Mantero F; PAPY Study Participants. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension 2006; 48:232–238. [DOI] [PubMed] [Google Scholar]
- 138. Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA 2006; 295:2638–2645. [DOI] [PubMed] [Google Scholar]
- 139. Wu VC, Kuo CC, Wang SM, Liu KL, Huang KH, Lin YH, Chu TS, Chang HW, Lin CY, Tsai CT, Lin LY, Chueh SC, Kao TW, Chen YM, Chiang WC, Tsai TJ, Ho YL, Lin SL, Wang WJ, Wu KD; TAIPAI Study Group. Primary aldosteronism: changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J Hypertens 2011; 29:1778–1786. [DOI] [PubMed] [Google Scholar]
- 140. Kobayashi Y, Haze T, Yano Y, Tamura K, Kurihara I, Ichijo T, Yoneda T, Katabami T, Tsuiki M, Wada N, Ogawa Y, Kawashima J, Sone M, Inagaki N, Yamada T, Okamoto R, Fujita M, Kamemura K, Yamamoto K, Izawa S, Tanabe A, Naruse M; JPAS/JRAS Study Group. Associations between changes in plasma renin activity and aldosterone concentrations and changes in kidney function after treatment for primary aldosteronism. Kidney Int Rep 2020; 5:1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Monticone S, Sconfienza E, D’Ascenzo F, Buffolo F, Satoh F, Sechi LA, Veglio F, Mulatero P. Renal damage in primary aldosteronism: a systematic review and meta-analysis. J Hypertens 2020; 38:3–12. [DOI] [PubMed] [Google Scholar]
- 142. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, Riester A, Asbach E, Hughes BA, O’Neil DM, Bidlingmaier M, Tomlinson JW, Hassan-Smith ZK, Rees DA, Adolf C, Hahner S, Quinkler M, Dekkers T, Deinum J, Biehl M, Keevil BG, Shackleton CH, Deeks JJ, Walch AK, Beuschlein F, Reincke M. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2017; 2:e93136. doi: 10.1172/jci.insight.93136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chen W, Li F, He C, Zhu Y, Tan W. Elevated prevalence of abnormal glucose metabolism in patients with primary aldosteronism: a meta-analysis. Ir J Med Sci 2014; 183:283–291. [DOI] [PubMed] [Google Scholar]
- 144. Hanslik G, Wallaschofski H, Dietz A, Riester A, Reincke M, Allolio B, Lang K, Quack I, Rump LC, Willenberg HS, Beuschlein F, Quinkler M, Hannemann A; Participants of the German Conn’s Registry. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur J Endocrinol 2015; 173:665–675. [DOI] [PubMed] [Google Scholar]
- 145. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab 2006; 91:454–459. [DOI] [PubMed] [Google Scholar]
- 146. Adler GK, Murray GR, Turcu AF, Nian H, Yu C, Solorzano CC, Manning R, Peng D, Luther JM. Primary aldosteronism decreases insulin secretion and increases insulin clearance in humans. Hypertension 2020; 75:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, Oparil S, Cofield SS, Calhoun DA. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med 2010; 6:363–368. [PMC free article] [PubMed] [Google Scholar]
- 148. Pimenta E, Stowasser M, Gordon RD, Harding SM, Batlouni M, Zhang B, Oparil S, Calhoun DA. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest 2013; 143:978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007; 131:453–459. [DOI] [PubMed] [Google Scholar]
- 150. Di Murro A, Petramala L, Cotesta D, Zinnamosca L, Crescenzi E, Marinelli C, Saponara M, Letizia C. Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst 2010; 11:165–172. [DOI] [PubMed] [Google Scholar]
- 151. Chee MR, Hoo J, Libianto R, Gwini SM, Hamilton G, Narayan O, Young MJ, Fuller PJ, Yang J. Prospective screening for primary aldosteronism in patients with suspected obstructive sleep apnea. Hypertension 2021; 77:2094–2103. [DOI] [PubMed] [Google Scholar]
- 152. Dobrowolski P, Kolodziejczyk-Kruk S, Warchol-Celinska E, Kabat M, Ambroziak U, Wrobel A, Piekarczyk P, Ostrowska A, Januszewicz M, Sliwinski P, Lenders JWM, Januszewicz A, Prejbisz A. Primary aldosteronism is highly prevalent in patients with hypertension and moderate to severe obstructive sleep apnea. J Clin Sleep Med 2021; 17:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Salcuni AS, Carnevale V, Battista C, Palmieri S, Eller-Vainicher C, Guarnieri V, Pugliese F, Guglielmi G, Desina G, Minisola S, Chiodini I, Scillitani A. Primary aldosteronism as a cause of secondary osteoporosis. Eur J Endocrinol 2017; 177:431–437. [DOI] [PubMed] [Google Scholar]
- 154. Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, Guglielmi G, Desina G, Eller-Vainicher C, Beck-Peccoz P, Scillitani A, Chiodini I. Bone involvement in aldosteronism. J Bone Miner Res 2012; 27:2217–2222. [DOI] [PubMed] [Google Scholar]
- 155. Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, Chu TS, Chang YS, Chen L, Wu KD, Chueh SJ. Risk of fracture in primary aldosteronism: a population-based cohort study. J Bone Miner Res 2017; 32:743–752. [DOI] [PubMed] [Google Scholar]
- 156. Bayomy O, Zaheer S, Williams JS, Curhan G, Vaidya A. Disentangling the relationships between the renin-angiotensin-aldosterone system, calcium physiology, and risk for kidney stones. J Clin Endocrinol Metab 2020; 105:1937–1946. doi: 10.1210/clinem/dgaa123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Joseph JJ, Pohlman NK, Zhao S, Kline D, Brock G, Echouffo-Tcheugui JB, Sims M, Effoe VS, Wu WC, Kalyani RR, Wand GS, Kluwe B, Hsueh WA, Abdalla M, Shimbo D, Golden SH. Association of serum aldosterone and plasma renin activity with ambulatory blood pressure in African Americans: the Jackson Heart Study. Circulation 2021; 143:2355–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hu J, Shen H, Huo P, Yang J, Fuller PJ, Wang K, Yang Y, Ma L, Cheng Q, Gong L, He W, Luo T, Mei M, Wang Y, Du Z, Luo R, Cai J, Li Q, Song Y, Yang S. Heightened cardiovascular risk in hypertension associated with renin-independent aldosteronism versus renin-dependent aldosteronism: a collaborative study. J Am Heart Assoc 2021; 10:e023082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Brown JM, Wijkman M, Claggett B, Shah A, Ballantyne C, Coresh J, Grams M, Wang Z, Yu B, Boerwinkle E, Vaidya A, Solomon S. Cardiac structure and function across the spectrum of aldosteronism: the atherosclerosis risk in communities study. Hypertension 2022; e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Brown JM, Underwood PC, Ferri C, Hopkins PN, Williams GH, Adler GK, Vaidya A. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension 2014; 63:1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Verma A, Vaidya A, Subudhi S, Waikar S. Aldosterone in chronic kidney disease and renal outcomes. Eur Heart J 2022, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016; 101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 163. Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, Beuschlein F, Rossi GP, Nishikawa T, Morganti A, Seccia TM, Lin YH, Fallo F, Widimsky J. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens 2020; 38:1919–1928. [DOI] [PubMed] [Google Scholar]
- 164. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol 2021; 9:876–892. [DOI] [PubMed] [Google Scholar]
- 165. Jaffe G, Gray Z, Krishnan G, Stedman M, Zheng Y, Han J, Chertow GM, Leppert JT, Bhalla V. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension 2020; 75:650–659. [DOI] [PubMed] [Google Scholar]
- 166. Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery 2019; 165:221–227. [DOI] [PubMed] [Google Scholar]
- 167. Sivarajah M, Beninato T, Fahey TJ III. Adherence to consensus guidelines for screening of primary aldosteronism in an urban healthcare system. Surgery 2020; 167:211–215. [DOI] [PubMed] [Google Scholar]
- 168. Liu YY, King J, Kline GA, Padwal RS, Pasieka JL, Chen G, So B, Harvey A, Chin A, Leung AA. Outcomes of a specialized clinic on rates of investigation and treatment of primary aldosteronism. JAMA Surg 2021; 156:541–549. doi: 10.1001/jamasurg.2021.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Cohen JB, Cohen DL, Herman DS, Leppert JT, Byrd JB, Bhalla V. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among U.S. veterans: a retrospective cohort study. Ann Intern Med 2021; 174:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hundemer GL, Imsirovic H, Vaidya A, Yozamp N, Goupil R, Madore F, Agharazii M, Knoll G, Sood MM. Screening rates for primary aldosteronism among individuals with hypertension plus hypokalemia: a population-based retrospective cohort study. Hypertension 2022; 79:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Liu YY, King J, Kline GA, Padwal RS, Pasieka JL, Chen G, So B, Harvey A, Chin A, Leung AA. Outcomes of a specialized clinic on rates of investigation and treatment of primary aldosteronism. JAMA Surg 2021; 156:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rossi GP. Primary aldosteronism: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74:2799–2811. [DOI] [PubMed] [Google Scholar]
- 173. Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, Adler GK. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension 2013; 61:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hung A, Ahmed S, Gupta A, Davis A, Kline GA, Leung AA, Ruzicka M, Hiremath S, Hundemer GL. Performance of the aldosterone to renin ratio as a screening test for primary aldosteronism. J Clin Endocrinol Metab 2021; 106:2423–2435. [DOI] [PubMed] [Google Scholar]
- 175. Rossi GP, Ceolotto G, Rossitto G, Maiolino G, Cesari M, Seccia TM. Effects of mineralocorticoid and AT1 receptor antagonism on the aldosterone-renin ratio in primary aldosteronism-the EMIRA study. J Clin Endocrinol Metab 2020; 105:2060–2067. doi: 10.1210/clinem/dgaa080 [DOI] [PubMed] [Google Scholar]
- 176. Nanba AT, Wannachalee T, Shields JJ, Byrd JB, Rainey WE, Auchus RJ, Turcu AF. Adrenal vein sampling lateralization despite mineralocorticoid receptor antagonists exposure in primary aldosteronism. J Clin Endocrinol Metab 2019; 104:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016; 101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 178. Pecori A, Buffolo F, Burrello J, Mengozzi G, Rumbolo F, Avataneo V, D’Avolio A, Rabbia F, Bertello C, Veglio F, Mulatero P, Monticone S. Mineralocorticoid receptor antagonist effect on aldosterone to renin ratio in patients with primary aldosteronism. J Clin Endocrinol Metab 2021; 106:e3655–e3664. [DOI] [PubMed] [Google Scholar]
- 179. Guo Z, Poglitsch M, McWhinney BC, Ungerer JPJ, Ahmed AH, Gordon RD, Wolley M, Stowasser M. Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab 2018; 103:3965–3973. [DOI] [PubMed] [Google Scholar]
- 180. Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, Gordon RD, McWhinney BC, Ungerer JP, Stowasser M. Diagnosis of primary aldosteronism by seated saline suppression test - variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab 2019; 105:e477–e483. [DOI] [PubMed] [Google Scholar]
- 181. Eisenhofer G, Kurlbaum M, Peitzsch M, Constantinescu G, Remde H, Schulze M, Kaden D, Müller LM, Fuss CT, Kunz S, Kołodziejczyk-Kruk S, Gruber S, Prejbisz A, Beuschlein F, Williams TA, Reincke M, Lenders JWM, Bidlingmaier M. The saline infusion test for primary aldosteronism: implications of immunoassay inaccuracy. J Clin Endocrinol Metab 2022; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Baron S, Amar L, Faucon AL, Blanchard A, Baffalie L, Faucard C, Travers S, Pagny JY, Azizi M, Houillier P. Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography-tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens 2018; 36:1592–1601. [DOI] [PubMed] [Google Scholar]
- 183. Le Goff CM, Gonzalez-Antuña A, Peeters SD, Fabregat-Cabello N, Van Der Gugten JG, Vroonen L, Pottel H, Holmes DT, Cavalier E. Migration from RIA to LC-MS/MS for aldosterone determination: implications for clinical practice and determination of plasma and urine reference range intervals in a cohort of healthy Belgian subjects. Clin Mass Spectrom 2018; 9:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Fuss CT, Brohm K, Kurlbaum M, Hannemann A, Kendl S, Fassnacht M, Deutschbein T, Hahner S, Kroiss M. Confirmatory testing of primary aldosteronism with saline infusion test and LC-MS/MS. Eur J Endocrinol 2021; 184:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nishikawa T, Satoh F, Takashi Y, Yanase T, Itoh H, Kurihara I, Shibata H, Oki Y, Naruse M, Sasamoto H, Kuwa K. Comparison and commutability study between standardized liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) and chemiluminescent enzyme immunoassay for aldosterone measurement in blood. Endocr J 2022; 69:EJ21-0278. [DOI] [PubMed] [Google Scholar]
- 186. Ozeki Y, Tanimura Y, Nagai S, Nomura T, Kinoshita M, Shibuta K, Matsuda N, Miyamoto S, Yoshida Y, Okamoto M, Gotoh K, Masaki T, Kambara K, Shibata H. Development of a new chemiluminescent enzyme immunoassay using a two-step sandwich method for measuring aldosterone concentrations. Diagnostics 2021; 11. doi: 10.3390/diagnostics11030433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yin Y, Ma C, Yu S, Liu W, Wang D, You T, Cheng Q, Qiu L. Comparison of three different chemiluminescence assays and a rapid liquid chromatography tandem mass spectrometry method for measuring serum aldosterone. Clin Chem Lab Med 2020; 58:95–102. doi: 10.1515/cclm-2019-0706 [DOI] [PubMed] [Google Scholar]
- 188. Brown JM, Auchus RJ, Honzel B, Luther JM, Yozamp N, Vaidya A. Recalibrating interpretations of aldosterone assays across the physiologic range: immunoassay and liquid chromatography–tandem mass spectrometry measurements under multiple controlled conditions. J Endocr Soc 2022; 6. doi: 10.1210/jendso/bvac049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Variability of aldosterone measurements during adrenal venous sampling for primary aldosteronism. Am J Hypertens 2021; 34:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Fries CM, Bae YJ, Rayes N, Sandner B, Isermann B, Stumvoll M, Fagotto V, Reincke M, Bidlingmaier M, Mandy V, Kratzsch J, Fenske WK. Prospective evaluation of aldosterone LC-MS/ MS-specific cutoffs for the saline infusion test. Eur J Endocrinol 2020; 183:191–201. [DOI] [PubMed] [Google Scholar]
- 191. Vieweg WVR, Veldhuis JD, Carey RM. Temporal pattern of renin and aldosterone secretion in men: effects of sodium balance. Am J Physiol Renal Physiol 1992; 262:F871–F877. [DOI] [PubMed] [Google Scholar]
- 192. Siragy HM, Vieweg WV, Pincus S, Veldhuis JD. Increased disorderliness and amplified basal and pulsatile aldosterone secretion in patients with primary aldosteronism. J Clin Endocrinol Metab 1995; 80:28–33. [DOI] [PubMed] [Google Scholar]
- 193. Tanabe A, Naruse M, Takagi S, Tsuchiya K, Imaki T, Takano K. Variability in the renin/aldosterone profile under random and standardized sampling conditions in primary aldosteronism. J Clin Endocrinol Metab 2003; 88:2489–2494. [DOI] [PubMed] [Google Scholar]
- 194. Kline GA, Darras P, Leung AA, So B, Chin A, Holmes DT. Surprisingly low aldosterone levels in peripheral veins following intravenous sedation during adrenal vein sampling: implications for the concept of nonsuppressibility in primary aldosteronism. J Hypertens 2019; 37:596–602. [DOI] [PubMed] [Google Scholar]
- 195. Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Intraindividual variability of aldosterone concentrations in primary aldosteronism: implications for case detection. Hypertension 2021; 77:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Tezuka Y, Ishii K, Zhao L, Yamazaki Y, Morimoto R, Sasano H, Udager AM, Satoh F, Turcu AF. ACTH stimulation maximizes the accuracy of peripheral steroid profiling in primary aldosteronism subtyping. J Clin Endocrinol Metab 2021; 106:e3969–e3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Tsiavos V, Markou A, Papanastasiou L, Kounadi T, Androulakis II, Voulgaris N, Zachaki A, Kassi E, Kaltsas G, Chrousos GP, Piaditis GP. A new highly sensitive and specific overnight combined screening and diagnostic test for primary aldosteronism. Eur J Endocrinol 2016; 175:21–28. [DOI] [PubMed] [Google Scholar]
- 198. Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EA, Brown MJ. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med 2015; 373:1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yang J, Libianto R, Lau KK, Doery JCG, Wan KL, Chee NYN, Shen J, Fuller PJ, Chong W. Impact of intraprocedural sedation on adrenal vein sampling without corticotropin stimulation. Radiology 2022:212627. doi: 10.1148/radiol.212627 [DOI] [PubMed] [Google Scholar]
- 200. Stowasser M, Ahmed AH, Cowley D, Wolley M, Guo Z, McWhinney BC, Ungerer JP, Gordon RD. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab 2018; 103:4113–4124. [DOI] [PubMed] [Google Scholar]
- 201. Umakoshi H, Sakamoto R, Matsuda Y, Yokomoto-Umakoshi M, Nagata H, Fukumoto T, Ogata M, Ogawa Y. Role of Aldosterone and potassium levels in sparing confirmatory tests in primary aldosteronism. J Clin Endocrinol Metab 2020; 105:1284–1289. [DOI] [PubMed] [Google Scholar]
- 202. Wang K, Hu J, Yang J, Song Y, Fuller PJ, Hashimura H, He W, Feng Z, Cheng Q, Du Z, Wang Z, Ma L, Yang S, Li Q. Development and validation of criteria for sparing confirmatory tests in diagnosing primary aldosteronism. J Clin Endocrinol Metab 2020; 105:e2449–e2456. [DOI] [PubMed] [Google Scholar]
- 203. Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res 2012; 44:170–176. [DOI] [PubMed] [Google Scholar]
- 204. Williams B, Macdonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Maeoka Y, Su XT, Wang WH, Duan XP, Sharma A, Li N, Staub O, McCormick JA, Ellison DH. Mineralocorticoid receptor antagonists cause natriuresis in the absence of aldosterone. Hypertension 2022; 79:1423–1434. doi: 10.1161/HYPERTENSIONAHA.122.19159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Fukumoto T, Umakoshi H, Ogata M, Yokomoto-Umakoshi M, Matsuda Y, Motoya M, Nagata H, Nakano Y, Iwahashi N, Kaneko H, Wada N, Miyazawa T, Sakamoto R, Ogawa Y. Significance of discordant results between confirmatory tests in diagnosis of primary aldosteronism. J Clin Endocrinol Metab 2021; 106:e866–e874. [DOI] [PubMed] [Google Scholar]
- 207. Leung AA, Symonds CJ, Hundemer GL, Ronksley PE, Lorenzetti DL, Pasieka JL, Harvey A, Kline GA. Performance of confirmatory tests for diagnosing primary aldosteronism: a systematic review and meta-analysis. Hypertension 2022; 79:1835–1844. doi: 10.1161/HYPERTENSIONAHA.122.19377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Vaidya A, Carey RM. Evolution of the primary aldosteronism syndrome: updating the approach. J Clin Endocrinol Metab 2020; 105:3771–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Funder JW. Primary aldosteronism: three strikes and out. Hypertension 2021; 77:900–903. [DOI] [PubMed] [Google Scholar]
- 210. Helber A, Wambach G, Hummerich W, Bonner G, Meurer KA, Kaufmann W. Evidence for a subgroup of essential hypertensives with non-suppressible excretion of aldosterone during sodium loading. Klin Wochenschr 1980; 58:439–447. [DOI] [PubMed] [Google Scholar]
- 211. Peng KY, Liao HW, Chan CK, Lin WC, Yang SY, Tsai YC, Huang KH, Lin YH, Chueh JS, Wu VC. Presence of subclinical hypercortisolism in clinical aldosterone-producing adenomas predicts lower clinical success. Hypertension 2020; 76:1537–1544. [DOI] [PubMed] [Google Scholar]
- 212. Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, Nirschl N, Bidlingmaier M, Beuschlein F, Thorand B, Peters A, Reincke M, Roden M, Quinkler M. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J Clin Endocrinol Metab 2019; 104:3192–3202. [DOI] [PubMed] [Google Scholar]
- 213. Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, Yoneda T, Kurihara I, Itoh H, Katabami T, Ichijo T, Wada N, Shibayama Y, Yoshimoto T, Ashida K, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Fujita M, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamoto K, Ogo A, Okamura S, Miyauchi S, Fukuoka T, Izawa S, Hashimoto S, Yamada M, Yoshikawa Y, Kai T, Suzuki T, Kawamura T, Naruse M. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: a large, multicenter cohort study in Japan. Diabetes Care 2019; 42:938–945. [DOI] [PubMed] [Google Scholar]
- 214. Gendreitzig P, Künzel HE, Adolf C, Handgriff L, Müller L, Holler F, Sturm L, Heinrich DA, Reincke M, Quinkler M. Autonomous cortisol secretion influences psychopathological symptoms in patients with primary aldosteronism. J Clin Endocrinol Metab 2021; 106:e2423–e2433. [DOI] [PubMed] [Google Scholar]
- 215. Katabami T, Matsuba R, Kobayashi H, Nakagawa T, Kurihara I, Ichijo T, Tsuiki M, Wada N, Ogawa Y, Sone M, Inagaki N, Yoshimoto T, Takahashi K, Yamamoto K, Izawa S, Kakutani M, Tanabe A, Naruse M. Primary aldosteronism with mild autonomous cortisol secretion increases renal complication risk. Eur J Endocrinol 2022; 186:645–655. [DOI] [PubMed] [Google Scholar]
- 216. Heinrich DA, Adolf C, Holler F, Lechner B, Schneider H, Riester A, Nirschl N, Sturm L, Wang X, Ladurner R, Seidensticker M, Bidlingmaier M, Beuschlein F, Reincke M. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J Clin Endocrinol Metab 2019; 104:5658–5664. [DOI] [PubMed] [Google Scholar]
- 217. O’Toole SM, Sze WC, Chung TT, Akker SA, Druce MR, Waterhouse M, Pitkin S, Dawnay A, Sahdev A, Matson M, Parvanta L, Drake WM. Low-grade cortisol cosecretion has limited impact on ACTH-stimulated AVS parameters in primary aldosteronism. J Clin Endocrinol Metab 2020; 105:e3776–e3784. doi: 10.1210/clinem/dgaa519 [DOI] [PubMed] [Google Scholar]
- 218. Bancos I, Prete A. Approach to the patient with adrenal incidentaloma. J Clin Endocrinol Metab 2021; 106:3331–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Vaidya A, Hamrahian A, Bancos I, Fleseriu M, Ghayee HK. The evaluation of incidentally discovered adrenal masses. Endocr Pract 2019; 25:178–192. [DOI] [PubMed] [Google Scholar]
- 220. Fujimoto K, Honjo S, Tatsuoka H, Hamamoto Y, Kawasaki Y, Matsuoka A, Ikeda H, Wada Y, Sasano H, Koshiyama H. Primary aldosteronism associated with subclinical Cushing syndrome. J Endocrinol Invest 2013; 36:564–567. [DOI] [PubMed] [Google Scholar]
- 221. Fallo F, Bertello C, Tizzani D, Fassina A, Boulkroun S, Sonino N, Monticone S, Viola A, Veglio F, Mulatero P. Concurrent primary aldosteronism and subclinical cortisol hypersecretion: a prospective study. J Hypertens 2011; 29:1773–1777. [DOI] [PubMed] [Google Scholar]
- 222. Hiraishi K, Yoshimoto T, Tsuchiya K, Minami I, Doi M, Izumiyama H, Sasano H, Hirata Y. Clinicopathological features of primary aldosteronism associated with subclinical Cushing’s syndrome. Endocr J 2011; 58:543–551. [DOI] [PubMed] [Google Scholar]
- 223. Honda K, Sone M, Tamura N, Sonoyama T, Taura D, Kojima K, Fukuda Y, Tanaka S, Yasuno S, Fujii T, Kinoshita H, Ariyasu H, Kanamoto N, Miura M, Yasoda A, Arai H, Ueshima K, Nakao K. Adrenal reserve function after unilateral adrenalectomy in patients with primary aldosteronism. J Hypertens 2013; 31:2010–2017. [DOI] [PubMed] [Google Scholar]
- 224. Adolf C, Kohler A, Franke A, Lang K, Riester A, Low A, Heinrich DA, Bidlingmaier M, Treitl M, Ladurner R, Beuschlein F, Arlt W, Reincke M. Cortisol excess in patients with primary aldosteronism impacts left ventricular hypertrophy. J Clin Endocrinol Metab 2018; 103:4543–4552. [DOI] [PubMed] [Google Scholar]
- 225. Tsai CH, Liao CW, Wu XM, Chen ZW, Pan CT, Chang YY, Lee BC, Shun CT, Wen WF, Chou CH, Wu VC, Hung CS, Lin YH. Autonomous cortisol secretion is associated with worse arterial stiffness and vascular fibrosis in primary aldosteronism - a cross-sectional study with follow-up data. Eur J Endocrinol 2022; 187:187–208. doi: 10.1530/EJE-21-1157 [DOI] [PubMed] [Google Scholar]
- 226. DeLozier OM, Dream SY, Findling JW, Carroll TB, Evans DB, Wang TS. Selective glucocorticoid replacement following unilateral adrenalectomy for hypercortisolism and primary aldosteronism. J Clin Endocrinol Metab 2022; 107:e538–e547. [DOI] [PubMed] [Google Scholar]
- 227. Parksook WW, Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Morphologically normal-appearing adrenal glands as a prevalent source of aldosterone production in primary aldosteronism. Am J Hypertens 2022; 35:561–571. doi: 10.1093/ajh/hpab189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, Degenhart C, Deinum J, Fischer E, Gordon R, Kickuth R, Kline G, Lacroix A, Magill S, Miotto D, Naruse M, Nishikawa T, Omura M, Pimenta E, Plouin PF, Quinkler M, Reincke M, Rossi E, Rump LC, Satoh F, Schultze Kool L, Seccia TM, Stowasser M, Tanabe A, Trerotola S, Vonend O, Widimsky J Jr, Wu KD, Wu VC, Pessina AC. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab 2012; 97:1606–1614. [DOI] [PubMed] [Google Scholar]
- 229. Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, Deinum J. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151:329–337. [DOI] [PubMed] [Google Scholar]
- 230. Zhou Y, Wang D, Jiang L, Ran F, Chen S, Zhou P, Wang P. Diagnostic accuracy of adrenal imaging for subtype diagnosis in primary aldosteronism: systematic review and meta-analysis. BMJ Open 2020; 10:e038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Umakoshi H, Tsuiki M, Takeda Y, Kurihara I, Itoh H, Katabami T, Ichijo T, Wada N, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamto K, Ogo A, Yanase T, Suzuki T, Naruse M. Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J Clin Endocrinol Metab 2018; 103:900–908. [DOI] [PubMed] [Google Scholar]
- 232. Xu F, Gao Z, Wang G, Gao Y, Guo Y, Guo Y, Zhou Z. Prevalence, subtype classification, and outcomes of treatment of primary aldosteronism: a prospective study in China. Endocr Pract 2021; 27:478–483. [DOI] [PubMed] [Google Scholar]
- 233. Williams TA, Burrello J, Sechi LA, Fardella CE, Matrozova J, Adolf C, Baudrand R, Bernardi S, Beuschlein F, Catena C, Doumas M, Fallo F, Giacchetti G, Heinrich DA, Saint-Hilary G, Jansen PM, Januszewicz A, Kocjan T, Nishikawa T, Quinkler M, Satoh F, Umakoshi H, Widimský J Jr, Hahner S, Douma S, Stowasser M, Mulatero P, Reincke M. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension 2018; 72:641–649. [DOI] [PubMed] [Google Scholar]
- 234. Sam D, Kline GA, So B, Leung AA. Discordance between imaging and adrenal vein sampling in primary aldosteronism irrespective of interpretation criteria. J Clin Endocrinol Metab 2019; 104:1900–1906. [DOI] [PubMed] [Google Scholar]
- 235. Rossi GP, Crimì F, Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J, Naruse M, Deinum J, Schultze Kool L, Kocjan T, Negro A, Rossi E, Kline G, Tanabe A, Satoh F, Christian Rump L, Vonend O, Willenberg HS, Fuller PJ, Yang J, Chee NYN, Magill SB, Shafigullina Z, Quinkler M, Oliveras A, Cent Wu V, Kratka Z, Barbiero G, Seccia TM, Battistel M. Identification of surgically curable primary aldosteronism by imaging in a large, multiethnic International study. J Clin Endocrinol Metab 2021; 106:e4340–e4349. [DOI] [PubMed] [Google Scholar]
- 236. Naruse M, Katabami T, Shibata H, Sone M, Takahashi K, Tanabe A, Izawa S, Ichijo T, Otsuki M, Omura M, Ogawa Y, Oki Y, Kurihara I, Kobayashi H, Sakamoto R, Satoh F, Takeda Y, Tanaka T, Tamura K, Tsuiki M, Hashimoto S, Hasegawa T, Yoshimoto T, Yoneda T, Yamamoto K, Rakugi H, Wada N, Saiki A, Ohno Y, Haze T. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr J 2022; 69:327–359. doi: 10.1507/endocrj.EJ21-0508 [DOI] [PubMed] [Google Scholar]
- 237. Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, Lu CC, Chang CC, Lin JH, Lin YH, Wang TD, Wang CY, Tu ST, Jeff Chueh SC, Chang CC, Tseng FY, Wu KD. Case detection and diagnosis of primary aldosteronism—the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc 2017; 116:993–1005. [DOI] [PubMed] [Google Scholar]
- 238. Gkaniatsa E, Sakinis A, Palmér M, Muth A, Trimpou P, Ragnarsson O. Adrenal venous sampling in young patients with primary aldosteronism. extravagance or irreplaceable? J Clin Endocrinol Metab 2021; 106:e2087–e2095. [DOI] [PubMed] [Google Scholar]
- 239. Umakoshi H, Ogasawara T, Takeda Y, Kurihara I, Itoh H, Katabami T, Ichijo T, Wada N, Shibayama Y, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamto K, Ogo A, Yanase T, Okamura S, Miyauchi S, Suzuki T, Tsuiki M, Naruse M. Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin Endocrinol 2018; 88:645–651. [DOI] [PubMed] [Google Scholar]
- 240. Rossi GP, Crimì F, Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J, Naruse M, Deinum J, Kool LS, Kocjan T, Negro A, Rossi E, Kline G, Tanabe A, Satoh F, Rump LC, Vonend O, Willenberg HS, Fuller PJ, Yang J, Chee NYN, Magill SB, Shafigullina Z, Quinkler M, Oliveras A, Wu VC, Kratka Z, Barbiero G, Battistel M, Seccia TM. Feasibility of imaging-guided adrenalectomy in young patients with primary aldosteronism. Hypertension 2022; 79:187–195. [DOI] [PubMed] [Google Scholar]
- 241. Kline GA, Leung AA, Sam D, Chin A, So B. Repeat adrenal vein sampling in aldosteronism: reproducibility and interpretation of persistently discordant results. J Clin Endocrinol Metab 2021; 106:e1170–e1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Rossitto G, Battistel M, Barbiero G, Bisogni V, Maiolino G, Diego M, Seccia TM, Rossi GP. The subtyping of primary aldosteronism by adrenal vein sampling: sequential blood sampling causes factitious lateralization. J Hypertens 2018; 36:335–343. [DOI] [PubMed] [Google Scholar]
- 243. Laurent I, Astère M, Zheng F, Chen X, Yang J, Cheng Q, Li Q. Adrenal venous sampling with or without adrenocorticotropic hormone stimulation: a meta-analysis. J Clin Endocrinol Metab 2018; 104:1060–1068. doi: 10.1210/jc.2018-01324 [DOI] [PubMed] [Google Scholar]
- 244. Takeda Y, Umakoshi H, Takeda Y, Yoneda T, Kurihara I, Katabami T, Ichijo T, Wada N, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Takahashi K, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamto K, Ogo A, Yanase T, Suzuki T, Naruse M. Impact of adrenocorticotropic hormone stimulation during adrenal venous sampling on outcomes of primary aldosteronism. J Hypertens 2019; 37:1077–1082. [DOI] [PubMed] [Google Scholar]
- 245. Yozamp N, Hundemer GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Adrenocorticotropic hormone-stimulated adrenal venous sampling underestimates surgically curable primary aldosteronism: a retrospective cohort study and review of contemporary studies. Hypertension 2021; 78:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J, Naruse M, Deinum J, Schultzekool L, Kocjan T, Negro A, Rossi E, Kline G, Tanabe A, Satoh F, Rump LC, Vonend O, Willenberg HS, Fuller P, Yang J, Nian Chee NY, Magill SB, Shafigullina Z, Quinkler M, Oliveras A, Chang CC, Wu VC, Somloova Z, Maiolino G, Barbiero G, Battistel M, Lenzini L, Quaia E, Pessina AC, Rossi GP. Subtyping of primary aldosteronism in the AVIS-2 study: assessment of selectivity and lateralization. J Clin Endocrinol Metab 2020; 105:2042–2052. doi: 10.1210/clinem/dgz017 [DOI] [PubMed] [Google Scholar]
- 247. Hafezi-Nejad N, Gullotti DM, Bailey CR, Lessne ML, Holly BP. Does intraprocedural CT improve the success rate of adrenal venous sampling? A systematic review and meta-analysis of data from 809 patients. Cardiovasc Intervent Radiol 2022; 45:29–40. [DOI] [PubMed] [Google Scholar]
- 248. Busser WM, Arntz MJ, Jenniskens SF, Deinum J, Hoogeveen YL, de Lange F, Schultze Kool LJ. Image registration of cone-beam computer tomography and preprocedural computer tomography aids in localization of adrenal veins and decreasing radiation dose in adrenal vein sampling. Cardiovasc Intervent Radiol 2015; 38:993–997. [DOI] [PubMed] [Google Scholar]
- 249. Reardon MA, Angle JF, Abi-Jaoudeh N, Bruns DE, Haverstick DM, Matsumoto AH, Carey RM. Intraprocedural cortisol levels in the evaluation of proper catheter placement in adrenal venous sampling. J Vasc Interv Radiol 2011; 22:1575–1580. [DOI] [PubMed] [Google Scholar]
- 250. Augustin AM, Dalla Torre G, Fuss CT, Fassnacht M, Bley TA, Kickuth R. Reduction of radiation exposure in adrenal vein sampling: impact of the rapid cortisol assay [Reduktion der Strahlenbelastung bei der selektiven Nebennierenvenenblutentnahme: Einfluss des Kortison-Schnelltests]. Rofo 2021; 193:1392–1402. [DOI] [PubMed] [Google Scholar]
- 251. Kline G, Leung A, So B, Chin A, Harvey A, Pasieka JL. Application of strict criteria in adrenal venous sampling increases the proportion of missed patients with unilateral disease who benefit from surgery for primary aldosteronism. J Hypertens 2018; 36:1407–1413. [DOI] [PubMed] [Google Scholar]
- 252. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF Jr. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014; 63:151–160. [DOI] [PubMed] [Google Scholar]
- 253. Kline GA, So B, Campbell DJT, Chin A, Harvey A, Venos E, Pasieka J, Leung AA. Apparent failed and discordant adrenal vein sampling: a potential confounding role of cortisol cosecretion? Clin Endocrinol 2022; 96:123–131. [DOI] [PubMed] [Google Scholar]
- 254. Dekkers T, Prejbisz A, Kool LJS, Groenewoud H, Velema M, Spiering W, Kołodziejczyk-Kruk S, Arntz M, Kądziela J, Langenhuijsen JF, Kerstens MN, van den Meiracker AH, van den Born BJ, Sweep F, Hermus A, Januszewicz A, Ligthart-Naber AF, Makai P, van der Wilt GJ, Lenders JWM, Deinum J.. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol 2016; 4:739–746. [DOI] [PubMed] [Google Scholar]
- 255. Rossi GP, Funder JW. Adrenal venous sampling versus computed tomographic scan to determine treatment in primary aldosteronism (The SPARTACUS Trial): a critique. Hypertension 2017; 69:396–397. [DOI] [PubMed] [Google Scholar]
- 256. Rossi GP, Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J Jr, Naruse M, Deinum J, Schultze Kool L, Kocjan T, Negro A, Rossi E, Kline G, Tanabe A, Satoh F, Christian Rump L, Vonend O, Willenberg HS, Fuller PJ, Yang J, Chee NYN, Magill SB, Shafigullina Z, Quinkler M, Oliveras A, Dun Wu K, Wu VC, Kratka Z, Barbiero G, Battistel M, Chang CC, Vanderriele PE, Pessina AC. Clinical outcomes of 1625 patients with primary aldosteronism subtyped with adrenal vein sampling. Hypertension 2019; 74:800–808. [DOI] [PubMed] [Google Scholar]
- 257. Perez-Rivas LG, Williams TA, Reincke M. Inherited forms of primary hyperaldosteronism: new genes, new phenotypes and proposition of a new classification. Exp Clin Endocrinol Diabetes 2019; 127:93–99. [DOI] [PubMed] [Google Scholar]
- 258. Duncan JL III, Fuhrman GM, Bolton JS, Bowen JD, Richardson WS. Laparoscopic adrenalectomy is superior to an open approach to treat primary hyperaldosteronism. Am Surg 2000; 66:932–935; discussion 935–936. [PubMed] [Google Scholar]
- 259. Meria P, Kempf BF, Hermieu JF, Plouin PF, Duclos JM. Laparoscopic management of primary hyperaldosteronism: clinical experience with 212 cases. J Urol 2003; 169:32–35. [DOI] [PubMed] [Google Scholar]
- 260. Rossi H, Kim A, Prinz RA. Primary hyperaldosteronism in the era of laparoscopic adrenalectomy. Am Surg 2002; 68:253–256; discussion 256–357. [PubMed] [Google Scholar]
- 261. Jacobsen NE, Campbell JB, Hobart MG. Laparoscopic versus open adrenalectomy for surgical adrenal disease. Can J Urol 2003; 10:1995–1999. [PubMed] [Google Scholar]
- 262. Desrochers MJ, St-Jean M, El Ghorayeb N, Bourdeau I, So B, Therasse E, Kline G, Lacroix A. Basal contralateral aldosterone suppression is rare in lateralized primary aldosteronism. Eur J Endocrinol 2020; 183:399–409. [DOI] [PubMed] [Google Scholar]
- 263. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M; Primary Aldosteronism Surgery Outcome (PASO) investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol 2017; 5:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Katabami T, Fukuda H, Tsukiyama H, Tanaka Y, Takeda Y, Kurihara I, Ito H, Tsuiki M, Ichijo T, Wada N, Shibayama Y, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Fujita M, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamoto K, Ogo A, Yanase T, Suzuki T, Naruse M; JPAS/JRAS Study Group. Clinical and biochemical outcomes after adrenalectomy and medical treatment in patients with unilateral primary aldosteronism. J Hypertens 2019; 37:1513–1520. [DOI] [PubMed] [Google Scholar]
- 265. Swearingen AJ, Kahramangil B, Monteiro R, Krishnamurthy V, Jin J, Shin J, Siperstein A, Berber E. Analysis of postoperative biochemical values and clinical outcomes after adrenalectomy for primary aldosteronism. Surgery 2018; 163:807–810. [DOI] [PubMed] [Google Scholar]
- 266. Sellgren F, Koman A, Nordenstrom E, Hellman P, Hennings J, Muth A. Outcomes after surgery for unilateral dominant primary aldosteronism in Sweden. World J Surg 2020; 44:561–569. [DOI] [PubMed] [Google Scholar]
- 267. Picado O, Whitfield BW, Khan ZF, Jeraq M, Farra JC, Lew JI. Long-term outcome success after operative treatment for primary aldosteronism. Surgery 2021; 169:528–532. [DOI] [PubMed] [Google Scholar]
- 268. Takamatsu K, Takeda T, Hattori S, Tanaka N, Morita S, Matsumoto K, Kosaka T, Mizuno R, Shinojima T, Kikuchi E, Asanuma H, Kurihara I, Itoh H, Oya M. Appropriate timing for a biochemical evaluation after adrenalectomy for unilateral aldosterone-producing adenoma. Clin Endocrinol 2020; 92:503–508. [DOI] [PubMed] [Google Scholar]
- 269. Rutherford JC, Taylor WL, Stowasser M, Gordon RD. Success of surgery for primary aldosteronism judged by residual autonomous aldosterone production. World J Surg 1998; 22:1243–1245. [DOI] [PubMed] [Google Scholar]
- 270. Proye CA, Mulliez EA, Carnaille BM, Lecomte-Houcke M, Decoulx M, Wemeau JL, Lefebvre J, Racadot A, Ernst O, Huglo D, Carre A. Essential hypertension: first reason for persistent hypertension after unilateral adrenalectomy for primary aldosteronism? Surgery 1998; 124:1128–1133. [DOI] [PubMed] [Google Scholar]
- 271. Sukor N, Kogovsek C, Gordon RD, Robson D, Stowasser M. Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab 2010; 95:1360–1364. [DOI] [PubMed] [Google Scholar]
- 272. Vorselaars W, Nell S, Postma EL, Zarnegar R, Drake FT, Duh QY, Talutis SD, McAneny DB, McManus C, Lee JA, Grant SB, Grogan RH, Romero Arenas MA, Perrier ND, Peipert BJ, Mongelli MN, Castelino T, Mitmaker EJ, Parente DN, Pasternak JD, Engelsman AF, Sywak M, D’Amato G, Raffaelli M, Schuermans V, Bouvy ND, Eker HH, Bonjer HJ, Vaarzon Morel NM, Nieveen van Dijkum EJM, Vrielink OM, Kruijff S, Spiering W, Borel Rinkes IHM, Valk GD, Vriens MR; International CONNsortium study group. Clinical outcomes after unilateral adrenalectomy for primary aldosteronism. JAMA Surg 2019; 154:e185842. doi: 10.1001/jamasurg.2018.5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Hannon MJ, Sze WC, Carpenter R, Parvanta L, Matson M, Sahdev A, Druce MR, Berney DM, Waterhouse M, Akker SA, Drake WM. Clinical outcomes following unilateral adrenalectomy in patients with primary aldosteronism. QJM 2017; 110:277–281. [DOI] [PubMed] [Google Scholar]
- 274. Sawka AM, Young WF, Thompson GB, Grant CS, Farley DR, Leibson C, van Heerden JA. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001; 135:258–261. [DOI] [PubMed] [Google Scholar]
- 275. Celen O, O’Brien MJ, Melby JC, Beazley RM. Factors influencing outcome of surgery for primary aldosteronism. Arch Surg 1996; 131:646–650. [DOI] [PubMed] [Google Scholar]
- 276. Group TS, Wu VC, Chueh SC, Chang HW, Lin LY, Liu KL, Lin YH, Ho YL, Lin WC, Wang SM, Huang KH, Hung KY, Kao TW, Lin SL, Yen RF, Chen YM, Hsieh BS, Wu KD. Association of kidney function with residual hypertension after treatment of aldosterone-producing adenoma. Am J Kidney Dis 2009; 54:665–673. [DOI] [PubMed] [Google Scholar]
- 277. Kohler A, Sarkis AL, Heinrich DA, Muller L, Handgriff L, Deniz S, Schneider H, Kunzel H, Ladurner R, Reincke M, Adolf C. Renin, a marker for left ventricular hypertrophy, in primary aldosteronism: a cohort study. Eur J Endocrinol 2021; 185:663–672. [DOI] [PubMed] [Google Scholar]
- 278. Chen YY, Lin YH, Huang WC, Chueh E, Chen L, Yang SY, Lin PC, Lin LY, Lin YH, Wu VC, Chu TS, Wu KD. Adrenalectomy improves the long-term risk of end-stage renal disease and mortality of primary aldosteronism. J Endocr Soc 2019; 3:1110–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Kobayashi H, Abe M, Nakamura Y, Takahashi K, Fujita M, Takeda Y, Yoneda T, Kurihara I, Itoh H, Tsuiki M, Wada N, Ichijo T, Katabami T, Ogawa Y, Kawashima J, Yoshimoto T, Sone M, Inagaki N, Watanabe M, Kamemura K, Matsuda Y, Izawa S, Tanabe M, Tanabe A, Suzuki T, Naruse M; JPAS/JRAS Study Group. Association between acute fall in estimated glomerular filtration rate after treatment for primary aldosteronism and long-term decline in renal function. Hypertension 2019; 74:630–638. [DOI] [PubMed] [Google Scholar]
- 280. Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS; TAIPAI Study Group. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens 2017; 35:1698–1708. [DOI] [PubMed] [Google Scholar]
- 281. Ahmed AH, Gordon RD, Sukor N, Pimenta E, Stowasser M. Quality of life in patients with bilateral primary aldosteronism before and during treatment with spironolactone and/or amiloride, including a comparison with our previously published results in those with unilateral disease treated surgically. J Clin Endocrinol Metab 2011; 96:2904–2911. [DOI] [PubMed] [Google Scholar]
- 282. Velema M, Dekkers T, Hermus A, Timmers H, Lenders J, Groenewoud H, Schultze Kool L, Langenhuijsen J, Prejbisz A, van der Wilt GJ, Deinum J; SPARTACUS Investigators. Quality of life in primary aldosteronism: a comparative effectiveness study of adrenalectomy and medical treatment. J Clin Endocrinol Metab 2018; 103:16–24. [DOI] [PubMed] [Google Scholar]
- 283. Citton M, Viel G, Torresan F, Rossi GP, Iacobone M. Effect of unilateral adrenalectomy on the quality of life of patients with lateralized primary aldosteronism. BMC Surg. 2019; 18:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Wu VC, Wang SM, Huang KH, Tsai YC, Chan CK, Yang SY, Lin LY, Chang CC, Lu CC, Lin YH, Chen YM, Chueh JS. Long-term mortality and cardiovascular events in patients with unilateral primary aldosteronism after targeted treatments. Eur J Endocrinol 2021; 186:195–205. [DOI] [PubMed] [Google Scholar]
- 285. Liu SY, Chu CC, Tsui TK, Wong SK, Kong AP, Chiu PW, Chow FC, Ng EK. Aldosterone-producing adenoma in primary aldosteronism: CT-guided radiofrequency ablation-long-term results and recurrence rate. Radiology 2016; 281:625–634. [DOI] [PubMed] [Google Scholar]
- 286. Bouhanick B, Delchier MC, Lagarde S, Boulestreau R, Conil C, Gosse P, Rousseau H, Lepage B, Olivier P, Papadopoulos P, Trillaud H, Cremer A, group A. Radiofrequency ablation for adenoma in patients with primary aldosteronism and hypertension: ADERADHTA, a pilot study. J Hypertens 2021; 39:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhao Z, Liu X, Zhang H, Li Q, He H, Yan Z, Sun F, Li Y, Zhou X, Bu X, Wu H, Shen R, Zheng H, Yang G, Zhu Z; Chongqing Endocrine Hypertension Collaborative Team. Catheter-based adrenal ablation remits primary aldosteronism: a randomized medication-controlled trial. Circulation 2021; 144:580–582. [DOI] [PubMed] [Google Scholar]
- 288. Zhang H, Li Q, Liu X, Zhao Z, He H, Sun F, Hong Y, Zhou X, Li Y, Shen R, Bu X, Yan Z, Zheng H, Yang G, Zhu Z; Chongqing Endocrine Hypertension Collaborative Team. Adrenal artery ablation for primary aldosteronism without apparent aldosteronoma: an efficacy and safety, proof-of-principle trial. J Clin Hypertens 2020; 22:1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Sarwar A, Brook OR, Vaidya A, Sacks AC, Sacks BA, Goldberg SN, Ahmed M, Faintuch S. Clinical outcomes following percutaneous radiofrequency ablation of unilateral aldosterone-producing adenoma: comparison with adrenalectomy. J Vasc Interv Radiol 2016; 27:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 291. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385:2252–2263. [DOI] [PubMed] [Google Scholar]
- 292. Bakris G, Pergola PE, Delgado B, Genov D, Doliashvili T, Vo N, Yang YF, McCabe J, Benn V, Pitt B; BLOCK-CKD Study Group. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: results of the BLOCK-CKD study. Hypertension 2021; 78:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Mulatero P, Monticone S, Burrello J, Veglio F, Williams TA, Funder J. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens 2016; 34:2253–2257. [DOI] [PubMed] [Google Scholar]
- 294. Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, Ford I, Cruickshank JK, Caulfield MJ, Padmanabhan S, Mackenzie IS, Salsbury J, Brown MJ; British Hypertension Society Programme of Prevention and Treatment of Hypertension With Algorithm Based Therapy (PATHWAY) Study Group. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol 2018; 6:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB, Williams B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019; 394:1540–1550. [DOI] [PubMed] [Google Scholar]
- 296. Provenzano M, Puchades M, Garofalo C, Jongs N, D’Marco L, Andreucci M, De Nicola L, Gorriz J, Heerspink H. Albuminuria-lowering effect of dapagliflozin, eplerenone, and their combination in patients with chronic kidney disease: a randomized cross-over clinical trial. J Am Soc Nephrol 2022. doi: 10.1681/ASN.2022020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Groth H, Vetter W, Stimpel M, Greminger P, Tenschert W, Klaiber E, Vetter H. Adrenalectomy in primary aldosteronism: a long-term follow-up study. Cardiology 1985; 72:107–116. [DOI] [PubMed] [Google Scholar]
- 298. Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab 2009; 94:2437–2445. [DOI] [PubMed] [Google Scholar]
- 299. Hundemer GL, Vaidya A. Management of Endocrine Disease: the role of surgical adrenalectomy in primary aldosteronism. Eur J Endocrinol 2020; 183:R183–R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Burton TJ, Mackenzie IS, Balan K, Koo B, Bird N, Soloviev DV, Azizan EA, Aigbirhio F, Gurnell M, Brown MJ. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J Clin Endocrinol Metab 2012; 97:100–109. [DOI] [PubMed] [Google Scholar]
- 301. O’Shea PM, O’Donoghue D, Bashari W, Senanayake R, Joyce MB, Powlson AS, Browne D, O’Sullivan GJ, Cheow H, Mendichovszky I, Quill D, Lowery A, Lappin D, Gurnell M, Dennedy MC. (11)C-Metomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice. Clin Endocrinol 2019; 90:670–679. [DOI] [PubMed] [Google Scholar]
- 302. Soinio M, Luukkonen AK, Seppänen M, Kemppainen J, Seppänen J, Pienimäki JP, Leijon H, Vesterinen T, Arola J, Lantto E, Helin S, Tikkanen I, Metso S, Mirtti T, Heiskanen I, Norvio L, Tiikkainen M, Tikkanen T, Sane T, Välimäki M, Gomez-Sanchez CE, Pörsti I, Nuutila P, Nevalainen PI, Matikainen N. Functional imaging with 11C-metomidate PET for subtype diagnosis in primary aldosteronism. Eur J Endocrinol 2020; 183:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Eisenhofer G, Duran C, Cannistraci CV, Peitzsch M, Williams TA, Riester A, Burrello J, Buffolo F, Prejbisz A, Beuschlein F, Januszewicz A, Mulatero P, Lenders JWM, Reincke M. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw Open 2020; 3:e2016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Turcu AF, Wannachalee T, Tsodikov A, Nanba AT, Ren J, Shields JJ, O’Day PJ, Giacherio D, Rainey WE, Auchus RJ. Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension 2020; 75:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Wannachalee T, Zhao L, Nanba K, Nanba AT, Shields JJ, Rainey WE, Auchus RJ, Turcu AF. Three discrete patterns of primary aldosteronism lateralization in response to cosyntropin during adrenal vein sampling. J Clin Endocrinol Metab 2019; 104:5867–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Sam D, Kline GA, So B, Hundemer GL, Pasieka JL, Harvey A, Chin A, Przybojewski SJ, Caughlin CE, Leung AA. External validation of clinical prediction models in unilateral primary aldosteronism. Am J Hypertens 2022; 35:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Kobayashi H, Abe M, Soma M, Takeda Y, Kurihara I, Itoh H, Umakoshi H, Tsuiki M, Katabami T, Ichijo T, Wada N, Yoshimoto T, Ogawa Y, Kawashima J, Sone M, Inagaki N, Takahashi K, Watanabe M, Matsuda Y, Shibata H, Kamemura K, Yanase T, Otsuki M, Fujii Y, Yamamoto K, Ogo A, Nanba K, Tanabe A, Suzuki T, Naruse M. Development and validation of subtype prediction scores for the workup of primary aldosteronism. J Hypertens 2018; 36:2269–2276. [DOI] [PubMed] [Google Scholar]
- 308. Burrello J, Burrello A, Pieroni J, Sconfienza E, Forestiero V, Rabbia P, Adolf C, Reincke M, Veglio F, Williams TA, Monticone S, Mulatero P. Development and validation of prediction models for subtype diagnosis of patients with primary aldosteronism. J Clin Endocrinol Metab 2020; 105:e3706–e3717. doi: 10.1210/clinem/dgaa379 [DOI] [PubMed] [Google Scholar]
- 309. Satoh F, Ito S, Itoh H, Rakugi H, Shibata H, Ichihara A, Omura M, Takahashi K, Okuda Y, Iijima S. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res 2021; 44:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]