Abstract
Purpose
The aim of the study was to analyse patient and injury characteristics and the effects of weekend admissions on mortality rate and outcome after moderate and severe traumatic brain injuries.
Methods
This is an observational cohort study based on data from a prospectively maintained regional trauma registry in South Western Norway. Patients with moderate and severe traumatic brain injury admitted between January 1st, 2004 and December 31st, 2019 were included in this study.
Results
During the study period 688 patients were included in the study with similar distribution between moderate (n = 318) and severe (n = 370) traumatic brain injury. Mortality rate was 46% in severe and 13% in moderate traumatic brain injury. Two hundred and thirty-one (34%) patients were admitted during weekends. Patients admitted during weekends were significantly younger (median age (IQR) 32.0 (25.5–67.0) vs 47.0 (20.0–55.0), p < 0.001). Pre-injury ASA 1 was significantly more common in patients admitted during weekends (n = 146, 64%, p = 0.001) while ASA 3 showed significance during weekdays compared to weekends (n = 101, 22%, p = 0.013). On binominal logistic regression analysis mortality rate was significantly higher with older age (OR 1.03, 95% CI for OR 1.02–1.04, p < 0.001) and increasing TBI severity (OR 7.08, 95% CI for OR 4.67–10.73, p < 0.001).
Conclusions
Mortality rate and poor clinical outcome remain high in severe traumatic brain injury. While a higher number of patients are admitted during the weekend, mortality rate does not differ from weekday admissions.
Keywords: Epidemiology, Traumatic brain injury, Mortality, Weekend
Highlights
-
•
Moderate and severe traumatic brain injuries increase during weekends and patients are younger.
-
•
Mortality rate does not differ on weekends compared to weekdays.
-
•
Older age and more severe traumatic brain injury was related to higher mortality.
1. Introduction
Traumatic brain injuries (TBI) are a major global cause of mortality and morbidity and with increasing numbers these injuries are one of the major challenges in modern health care (James et al., 2019).
Trauma patients with coexisting TBI have an up to three times higher mortality rate than those without evidence of TBI (Baum et al., 2016). Many TBI patients are admitted outside general working hours, are of older age, and have associated comorbidities, thus making TBI a challenging condition to treat (Tverdal et al., 2020; Peeters et al., 2017; Pedersen et al., 2015; Heskestad et al., 2009).
Each year, approximately 2.5 million people will experience some form of TBI in Europe; of these, approximately 1 million patients will be admitted to hospital and around 75,000 of them will die (Maas et al., 2014).
In a systematic review on TBI from 23 European countries a mean hospitalization rate of 235 per 100,000 people was found, albeit there were extensive differences between countries (91–546 per 100,000) largely based on differences in inclusion criteria (Tagliaferri et al., 2006). Narrowing it down even further and only including CT verified TBI, Scandinavian countries show a rate of 26–42 per 100,000 inhabitants (Heskestad et al., 2009).
With an incrementally older population the incidence of TBI in older patients is increasing, and falls constitutes the main trauma mechanism (Tverdal et al., 2020; Pedersen et al., 2015; Heskestad et al., 2009; Roozenbeek et al., 2013). Traffic-related TBI, affecting primarily younger individuals are declining in high-income countries due to improved road safety regulations (Roozenbeek et al., 2013; Maas et al., 2008). However, there is a shift towards predominantly traffic related injuries with severe TBI, as highlighted in a recent review (Haller and Walder, 2015).
Moderate and severe TBI as well as general trauma admissions peak during the weekends (Bjarkø et al., 2019; Cantwell et al., 2015). Contrary to the observed trend in TBI with most patients being older and having associated comorbidities, weekend admissions are predominated by younger, healthier patients often admitted with coexisting alcohol intoxication (Bjarkø et al., 2019; Posti et al., 2021). The hypothesis that weekend admissions are associated with increased mortality rate is termed “weekend effect” and it has been demonstrated in various medical conditions for instance myocardial infarction and stroke (Kostis et al., 2007; Mekonnen et al., 2020). However, differential mortality was not seen in a study of a Level 1 trauma center comparing the days of the week, possibly due to trauma teams being adequately staffed throughout the week (Carr et al., 2010). There are few studies on TBI-related mortality during the weekend, but a recent Finnish study recognized increased mortality rate (Posti et al., 2021) while another study solely focusing on older patients (>65 years) with moderate and severe TBI reported increased mortality rate during the weekends (Schneider et al., 2012).
The aim of this study was to evaluate the epidemiology of moderate and severe TBI based on prospectively collected data from a regional trauma registry over a time period of 16 years and to evaluate if admission rate, clinical outcome or mortality rate varied during weekend compared to weekdays.
2. Methods
2.1. Study design and period
This study is an observational cohort study based on data on trauma patients with moderate or severe TBI retrieved from a prospectively maintained regional trauma registry. The study period was from January 1st, 2004 to December 31st, 2019. Ethical approval was granted by the Regional Committee for Medical and Health Research Ethics of Western Norway (#143902). The trauma registry has been approved by the hospitals’ data protection official. The study followed the STROBE guidelines for observational studies (von Elm et al., 2008).
2.2. Study population
Stavanger University Hospital (SUH) is one of six university hospitals with a neurosurgical department in Norway. It operates as a regional trauma centre in South-Western Norway covering about 370.000 inhabitants in the primary catchment area and receiving trauma patients from a wider population of about 550.000 (Wiik-Larsen et al., 2020).
2.3. Data collection (Stavanger Trauma Registry)
The SUH trauma registry has been operational since 2004, and approximately 500 patients are registered annually. An Association for the Advancement of Automotive Medicine (AAAM)-certified Abbreviated Injury Scale (AIS) coder (registered nurse) maintains the registry on a daily basis (Rogaland Trauma System Study Collaborating, 2012; Rating the Severity of Tissue, 1971). Patients assessed by a trauma team at SUH with an Injury Severity Score (ISS) > 10 were eligible for inclusion for this study (Baker et al., 1974). Brain injury was graded according to the Head Injury Severity Scale (HISS), which is based on Glasgow Coma Scale (GCS) and clinical characteristics and divide traumatic brain injuries into minimal (GCS 15), mild (GCS 14 or 15 plus amnesia or brief loss of consciousness), moderate (GCS 9–13) and severe (GCS 3–8) (Stein and Spettell, 1995; Teasdale and Jennett, 1974). Patients with moderate or severe TBI were included. All age groups were eligible for inclusion. Patients with minimal or mild TBI were not included as they are rarely admitted as trauma patients unless they have other serious concomitant injuries and therefore are not registered in the SUH trauma registry. Furthermore, patients primarily treated and transferred from other trauma centers were excluded from the study.
2.4. Variables
Patient demographics data and injury characteristics, as well as data regarding alcohol and narcotics intoxication were recorded from prehospital, in hospital and discharge records.
2.4.1. Injury mechanism
The mechanism of injury was registered as (i) traffic accidents (car, motorbike, bike and other), (ii) pedestrians hit by vehicle, (iii) falls (<1, 1–5 and >5 m), (iv) work accidents, (v) sports/recreation accidents. Injury mechanisms were recorded as blunt or penetrating.
2.4.2. Clinical outcome, mortality rate and discharge destination
Clinical outcome was measured with the Glasgow Outcome Scale (GOS) (Jennett and Bond, 1975). The GOS considers physical, social and cognitive sequela. It is organized into five outcome categories; death (GOS 1), persistent vegetative state (GOS 2), severe disability (GOS 3), moderate disability (GOS 4), good recovery (GOS 5). Good recovery and moderate disability are considered as favourable outcome and the latter being adverse or unfavourable outcomes (Oliveira et al., 2012; Collaborators et al., 2008). The same dichotomisation was applied in this study. Post-injury GOS was measured at time of hospital discharge, or death.
Other outcome variables were mortality rate (defined as death within 30 days), mortality rate due to TBI, location of death and discharge destination: ICU (specialized hospital), ICU (other hospital), other hospital normal ward, other hospital, psychiatry, rehab unit, nursing home, home or dead.
2.4.3. Injury dates
The injury dates were registered according to the day of the week the injury occurred. We dichotomized days into weekdays (Monday-Friday) and weekends (Saturday-Sunday).
2.5. Statistics
All statistical analyses were performed with SPSS version 26.0 (IBM, USA). The chi square test was used for categorical variables. The independent samples median test was used for continuous variables. Logistic regression analysis was performed to assess the impact of different variables on the 30-mortality rate and GOS (dependent variables) of severe TBI. The regression models contained 4 different variables (age, gender, TBI severity, weekend admission). Statistical significance was defined as p < 0.050 in both uni- and multivariate analyses.
3. Results
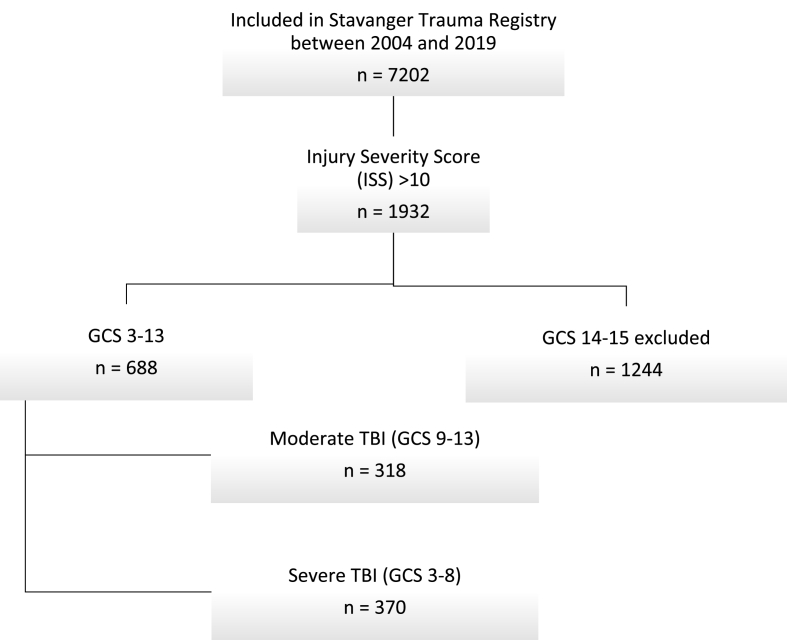
The registry included 7202 patients registered in the Stavanger Trauma Registry between 2004 and 2019 (Fig. 1). Among these, 688 (10%) patients with a GCS between 13 and 3 were identified. There was an equal distribution between moderate 318 (46%) and severe TBI 370 (54%). Data is presented for all patients as well as for both cohorts with moderate or severe TBI.
Fig. 1.
Flowchart showing patient selection.
3.1. Patient characteristics
Most patients were male (n = 513, 75%), mean age at time of injury was 44.5 years (range 0–99). Patients admitted during weekends were significantly younger than those admitted during weekends (median age (IQR) 32.0 (25.5–67.0) vs 47.0 (20.0–55.0), p < 0.001). Patients admitted during the weekend were more likely to have ASA grade 1 (n = 146, 64%, p = 0.001) while patients admitted during weekdays more commonly had an ASA score of 3 (n = 101, 22%, p = 0.013).
The patient characteristics are presented in Table 1, Table 3.
Table 1.
Patient and injury characteristics.
| Variable | All patients (GCS 3–13) | Moderate TBI (GCS 9–13) | Severe TBI (GCS 3–8) | p value |
|---|---|---|---|---|
| Number of patients | 688 (100%) | 318 | 370 | |
| Female sex | 175 (25%) | 84 (26%) | 91 (25%) | 0,585 |
| Age in years, median (IQR) | 42,0 (23,0–64,0) | 42,0 (23,8–64,0) | 42,0 (22,0–63,3) | 0,957 |
| Traffic accident | ||||
| Yes | 312 (45%) | 137 (43%) | 175 (47%) | 0,2542 |
| Car | 172 (25%) | 68 (21%) | 104 (28%) | 0,040 |
| Motorcycle | 56 (8%) | 23 (7%) | 33 (9%) | 0,413 |
| Bicycle | 63 (9%) | 36 (11%) | 27 (7%) | <0,001 |
| Other | 21 (2%) | 10 (4%) | 11 (3%) | 0,888 |
| Pedestrian hit by vehicle | 33 (5%) | 14 (4%) | 19 (5%) | 0,500 |
| Falls | ||||
| Yes | 273 (40%) | 136 (37%) | 138 (37%) | 0,130 |
| 0–1 m | 81 (12%) | 45 (14%) | 36 (10%) | 0,069 |
| 1–5 m | 139 (20%) | 73 (23%) | 66 (18%) | 0,089 |
| >5 m | 47 (7%) | 14 (4%) | 33 (9%) | 0,019 |
| Work accident | 72 (11%) | 33 (10%) | 39 (11%) | 0,944 |
| Sports/recreation accident | 365 (53%) | 178 (56%) | 187 (50%) | 0,154 |
| Injury mechanism | ||||
| Blunt | 664 (97%) | 312 (98%) | 352 (95%) | 0,031 |
| Penetrating | 24 (3%) | 6 (2%) | 18 (5%) | |
| Cardiac arrest | 52 (8%) | 2 (1%) | 50 (14%) | <0,001 |
| Respiratory arrest | 55 (8%) | 3 (1%) | 52 (15%) | <0,001 |
| Alcohol intoxication | 180 (26%) | 82 (26%) | 98 (27%) | 0,596 |
| Drug intoxication | 37 (5%) | 15 (5%) | 22 (6%) | 0,389 |
| ISS, median (IQR) | 26 (19–36) | 22 (17–29) | 30 (15–45) | <0,001 |
| NISS, median (IQR) | 38 (27–57) | 34 (22–41) | 50 (34–66) | <0,001 |
TBI – Traumatic Brain Injury; GCS – Glasgow Coma Score; ASA – American Society of Anesthesiologists; ISS – Injury Severity Score; NISS – New Injury Severity Score.
Table 3.
Patient and injury characteristics according to weekday/weekend admission.
| Variable | Weekday | Weekend | p value |
|---|---|---|---|
| Number of patients | 457 (66%) | 231 (34%) | |
| Female sex | 125 (27%) | 50 (22%) | 0,105 |
| Age in years, median (IQR) | 47,0 (25,25–67,0) | 32,0 (20,0–55,0) | <0,001 |
| ASA score pre-injury | |||
| 1 | 227 (50%) | 146 (64%) | 0,001 |
| 2 | 110 (24%) | 44 (19%) | 0,163 |
| 3 | 101 (22%) | 32 (14%) | 0,013 |
| 4 | 19 (4%) | 8 (4%) | 0,807 |
| GOS, pre-injury | |||
| 3 (severe disability) | 23 (5%) | 7 (3%) | 0,310 |
| 4 (moderate disability) | 83 (18%) | 25 (11%) | 0,017 |
| 5 (low/no disability) | 351 (77%) | 199 (86%) | 0,005 |
| Traffic accident | |||
| Yes | 216 (47%) | 96 (42%) | 0,145 |
| Car | 120 (26%) | 52 (23%) | 0,327 |
| Motorcycle | 36 (8%) | 20 (9%) | 0,841 |
| Bicycle | 46 (10%) | 17 (7%) | 0,306 |
| Other | 14 (3%) | 7 (3%) | 0,842 |
| Pedestrian hit by vehicle | 30 (14%) | 3 (3%) | 0,004 |
| Fall from height | |||
| Yes | 178 (39%) | 95 (41%) | 0,639 |
| 0–1 m | 58 (13%) | 23 (10%) | 0,354 |
| 1–5 m | 88 (19%) | 51 (22%) | 0,442 |
| >5 m | 29 (6%) | 18 (8%) | 0,584 |
| Work accident | 69 (15%) | 3 (1%) | <0,001 |
| Sports/recreation accident | 212 (46%) | 153 (66%) | <0,001 |
| Injury mechanism | |||
| Blunt | 441 (97%) | 223 (97%) | 0,980 |
| Penetrating | 18 (4%) | 8 (4%) | |
| Cardiac arrest | 37 (8%) | 15 (6%) | 0,452 |
| Respiratory arrest | 39 (9%) | 16 (7%) | 0.463 |
| Alcohol intoxication | 76 (17%) | 104 (46%) | <0,001 |
| Drug intoxication | 19 (4%) | 18 (8%) | 0,069 |
| ISS, median (IQR) | 26,0 (19,25–38,0) | 26,0 (19,0–35,0) | 0,444 |
| NISS, median (IQR) | 36,5 (27,0–57,0) | 38,0 (27,0–50,0) | 0,771 |
3.2. Injury characteristics
The primary cause of injury was traffic accidents (n = 312, 45%), with car accidents predominating (n = 172, 25%), followed by falls (40%, n = 273). The majority of accidents during weekends resulted from sports/recreational activities (66%, n = 153) and significantly more patients (n = 104, 46%, p < 0.001) were under the influence of alcohol.
3.3. Clinical outcome
There was a close to equal division between favourable (GOS 4–5) and poor outcome (GOS 1–3) at discharge from the primary hospital (Table 2). Namely, 354 (52%) patients had poor outcome, most with either GOS 1 or GOS 3, only 9 patients ended up in a persistent vegetative state (GOS 2). The majority of patients with severe TBI had poor outcome (71%, n = 261) in contrast to 29% (n = 93) of patients with moderate TBI. Age and TBI severity was associated with poorer outcome as demonstrated in Table 5.
Table 2.
Outcome.
| Variable | All patients (GCS 3–13) | Moderate TBI (GCS 9–13) | Severe TBI (GCS 3–8) | p value |
|---|---|---|---|---|
| Glasgow Outcome Scale | ||||
| 1 (dead) | 211 (31%) | 37 (11%) | 174 (47%) | <0,001 |
| 2 (persistent vegetative) | 9 (1%) | 0 (0%) | 9 (2%) | 0,004 |
| 3 (severe disability) | 134 (20%) | 56 (18%) | 78 (21%) | 0,245 |
| 4 (moderate disability) | 172 (25%) | 102 (32%) | 70 (19%) | <0,001 |
| 5 (low/no disability) | 161 (23%) | 123 (39%) | 38 (10%) | <0,001 |
| Mortality, 30 days | 211 (31%) | 40 (13%) | 171 (46%) | <0,001 |
| due to TBI | 128 (19%) | 19 (6%) | 109 (29%) | <0,001 |
| Location of death | ||||
| Emergency room | 54 (8%) | 4 (1%) | 50 (7%) | <0,001 |
| ICU | 107 (16%) | 14 (2%) | 93 (14%) | <0,001 |
| OR | 16 (2%) | 5 (1%) | 11 (2%) | 0,311 |
| Normal ward | 27 (4%) | 13 (2%) | 14 (2%) | 0,841 |
| Under transport | 3 (<1%) | 0 (0%) | 3 (<1%) | 0,253 |
| Discharge destination | ||||
| Specialized hospital, ICU | 26 (4%) | 15 (2%) | 11 (2%) | 0,222 |
| Other hospital, ICU | 7 (1%) | 1 (0%) | 6 (1%) | 0,132 |
| Other hospital, normal ward | 28 (4%) | 10 (2%) | 18 (3%) | 0,266 |
| Other hospital | 48 (7%) | 25 (4%) | 23 (3%) | 0,380 |
| Psychiatry | 9 (1%) | 6 (1%) | 3 (0%) | 0,314 |
| Rehab unit | 118 (17%) | 53 (8%) | 65 (8%) | 0,791 |
| Nursing home | 21 (3%) | 11 (2%) | 10 (2%) | 0,549 |
| Home | 227 (33%) | 162 (24%) | 65 (9%) | <0,001 |
| TBI – Traumatic Brain Injury; ICU – Intensive Care Unit; OR – Operating Room | ||||
Table 5.
Results from binominal logistic regression analysis for 30-day mortality and multinominal logistic regression analysis.
| Variables | p-value | OR | 95% CI for OR |
|---|---|---|---|
| 30-day mortality | |||
| Age | <0,001 | 1,03 | 1,02–1,04 |
| Gender | 0,162 | 1,33 | 0,89–1,97 |
| TBI severity | <0,001 | 7,08 | 4,67–10,73 |
| Weekend admission | 0,753 | 1,07 | 0,72–1,59 |
| GOS 1 (dead) | |||
| Age | <0,001 | 1,05 | 1,04–1,06 |
| Gender | 0,339 | 1,32 | 0,75–2,33 |
| TBI severity | <0,001 | 23,62 | 13,38–41,69 |
| Weekend admission | 0,214 | 1,39 | 0,83–2,34 |
| GOS 2 (persistent vegetative) | |||
| Age | 0,077 | 1,03 | 0,99–1,06 |
| Gender | 0,483 | 0,47 | 0,05–3,96 |
| TBI severity | – | – | – |
| Weekend admission | 0,973 | 1,03 | 0,25–4,22 |
| GOS 3 (severe disability) | |||
| Age | <0,001 | 1,03 | 1,02–1,04 |
| Gender | 0,864 | 0,95 | 0,53–1,67 |
| TBI severity | <0,001 | 5,84 | 3,44–9,89 |
| Weekend admission | 0,180 | 1,43 | 0,85–2,41 |
| GOS 4 (moderate disability) | |||
| Age | <0,001 | 1,02 | 1,01-1,03 |
| Gender | 0,966 | 0,99 | 0,59–1,66 |
| TBI severity | <0,001 | 2,59 | 1,59–4,23 |
| Weekend admission | 0,455 | 1,19 | 0,75–1,89 |
OR – Odds Ratio; CI – Confidence Interval; TBI – Traumatic brain injury.
3.4. Mortality rate
The overall 30-day mortality rate was 31% and comparing weekday vs weekend mortality rate was 32% and 27%, respectively (Table 4). Mortality rate in severe TBI was 46% compared to 13% in moderate TBI.
Table 4.
Outcome.
| Variable | Weekday | Weekend | p value |
|---|---|---|---|
| Glasgow Outcome Scale | |||
| 1 (dead) | 149 (33%) | 62 (27%) | 0,144 |
| 2 (persistent vegetative) | 5 (1%) | 4 (2%) | 0,729 |
| 3 (severe disability) | 92 (20%) | 42 (18%) | 0,610 |
| 4 (moderate disability) | 112 (25%) | 60 (26%) | 0,740 |
| 5 (low/no disability) | 98 (21%) | 63 (27%) | 0,107 |
| Mortality, 30 days | 148 (32%) | 63 (27%) | 0,199 |
| due to TBI | 90 (20%) | 38 (17%) | 0,354 |
| Location of death | |||
| Emergency room | 39 (9%) | 15 (7%) | 0,431 |
| ICU | 73 (16%) | 34 (15%) | 0,752 |
| OR | 14 (3%) | 2 (1%) | 0,124 |
| Normal ward | 20 (4%) | 7 (3%) | 0,517 |
| Under transport | 2 (1%) | 1 (1%) | 0,738 |
| Discharge destination | |||
| Specialized hospital, ICU | 10 (2%) | 16 (7%) | 0,004 |
| Other hospital, ICU | 7 (2%) | 0 | 0,056 |
| Other hospital, normal ward | 17 (4%) | 11 (5%) | 0,655 |
| Other hospital | 33 (7%) | 15 (7%) | 0,842 |
| Psychiatry | 7 (2%) | 2 (1%) | 0,370 |
| Rehab unit | 82 (18%) | 36 (16%) | 0,502 |
| Nursing home | 14 (3%) | 7 (3%) | 0,842 |
| Home | 141 (31%) | 86 37%) | 0,111 |
As depicted in Table 5, from binominal logistic regression analysis, 30-mortality rate was significantly higher in older age (OR 1.03, 95% CI for OR 1.02–1.04, p < 0.001) and with increasing TBI severity (OR 7.08, 95% CI for OR 4.67–10.73, p < 0.001).
Death due to TBI is significantly more common in severe TBI (29%) compared to moderate TBI (6%). Death most frequently occurred in the ICU (n = 107, 16%). However, 50 patients (7%) with severe TBI died in the emergency room. These patients were either dead on arrival or died in the emergency room before they received treatment.
3.5. Time of injury patterns
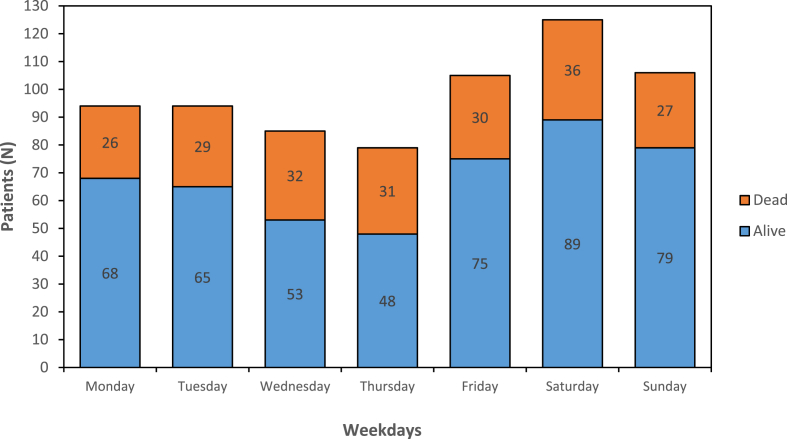
Two hundred and thirty-one (34%) patients were admitted during weekends and Saturday was the day with most admissions (18%). There was no significant increase in mortality rate during weekends compared to weekdays or when investigating individual days (Saturday vs rest of the week (p = 0.639), Sunday vs rest of the week (p = 0,196), Fig. 2). Other characteristics comparing weekday versus weekend admissions are shown in Table 3, Table 4, Table 5
Fig. 2.
Admissions and 30-day mortality according to day of the week.
4. Discussion
The results of this study on trauma patients with moderate and severe TBI over a 16-year period show that severe TBI is associated with high mortality rate and unfavourable short-term outcome. Young adult males still represent a large proportion of this patient group. Weekend admission did not significantly affect the mortality rate of TBI based on this data.
4.1. Patient characteristics
The overrepresentation of young adult males is demonstrated in several studies and is possibly explained by higher risk tolerance and is likely why traffic accidents were the leading cause of injury (Maegele et al., 2007; Farin et al., 2003). The median age of 44.5 and preponderance of male patients correlates well with the literature (Baum et al., 2016; Peeters et al., 2015; Andriessen et al., 2011; Jacobs et al., 2011). There was no second peak in elderly as demonstrated in other studies (Maegele et al., 2007; Andelic et al., 2012; Moppett, 2007), elderly are more prone to falls which consequently often lead to minor head trauma compared to road traffic accidents (Peeters et al., 2015).
4.2. Mechanism of injury
The main mechanism of injury was road traffic accidents and close to half of these were caused by other reasons than car accidents (bike, motorcycle, etc). Many studies, especially Scandinavian studies are reporting falls as the main etiology, but these are often studies including mild and minimal TBI (Tverdal et al., 2020; Pedersen et al., 2015; Andelic et al., 2012; Borg et al., 2011; Walder et al., 2013). Several studies including moderate-to-severe or solely severe traumatic brain injuries cite traffic accidents as the leading TBI cause (Haller and Walder, 2015; Maegele et al., 2007; Peeters et al., 2015; Jacobs et al., 2011; Murray et al., 1999a). Work accidents represent 15% of injuries during weekdays and thus need to be acknowledged as an important injury mechanism, that can be minimized with proper precautions and work-safety regulations.
4.3. Outcome
There was a slight predominance of patients with unfavourable outcome (354 patients, 52%). Separating the two cohorts, unfavourable outcome was observed in 70% of severe TBI and 29% of moderate TBI.
A large study of 650 patients with severe TBI from Germany measuring GOS at discharge found an equal division between unfavourable and favourable prognosis. However, other studies showed up to 78% unfavourable outcome after severe TBI (Oliveira et al., 2012; Agrawal et al., 2012).
Outcome after severe TBI is often evaluated at 6 months and unfavourable outcome range from 50 to 60% (Murray et al., 1999a, 1999b; Steyerberg et al., 2019; Lenartova et al., 2007; Myburgh et al., 2008; Rosenfeld et al., 2012). In severe TBI, the outcome is typically a U-shaped curve with patients either dying or recovering to a favourable outcome. Many patients suffer cognitive and physical problems and improvement is observed with physical rehabilitation and reintegration into society. Patients often meet their maximum level of recovery at 6 months (Maas et al., 2008; Oliveira et al., 2012; Jacobs et al., 2011; Heiden et al., 1983). With a follow-up at 6 months it's likely that more patient would improve from an unfavourable to a favourable outcome.
In moderate TBI the numbers are similar when compared to a study from the Netherlands that found unfavourable outcome in 31% of patients 6 months after moderate TBI (Jacobs et al., 2011). In moderate TBI the majority of patients are discharged with favourable outcome. It's important to bear in mind that these are multitrauma patients and not isolated head injuries.
4.4. Mortality rate
Mortality rate in severe TBI was significantly higher compared to moderate TBI and the majority of deaths were due to severe TBI (81%). Mortality rate increased with age which is in line with current literature (Tverdal et al., 2020; Andelic et al., 2012; Mushkudiani et al., 2007; Herou et al., 2015).
The mortality rate of trauma patients with severe TBI in this study was 46% which is in the higher range of comparable literature. However, studies show heterogenous results on early mortality rate in severe TBI spanning from 25 to 46% (Haller and Walder, 2015; Maegele et al., 2007; Andelic et al., 2012; Walder et al., 2013; Murray et al., 1999a). This heterogeneity can possibly be explained by differences in inclusion criteria as we included both pre-hospital and emergency department (ED) deaths and some studies exclude these deaths leading to a lower mortality rate (Walder et al., 2013; Masson et al., 2001). Among patients with severe TBI, we found a high number of fatalities before any neurosurgical intervention could be initiated, 3 patients died under transport and 50 died in the ED.
It is worth mentioning that several studies focus on mortality rate at 6 months and it ranges from 32 to 49%. However, the literature shows that the majority of patients die within 48 h after injury and only a minority of patients dies in the follow-up period (Haller and Walder, 2015; Maegele et al., 2007; Andriessen et al., 2011; Lenartova et al., 2007; Compagnone et al., 2009).
For the moderate TBI cohort we found a mortality rate of 13%, lower than that in the reported literature and only 19 patients (6%) died due to TBI (Andriessen et al., 2011; Jacobs et al., 2011; Compagnone et al., 2009).
4.5. Daily, weekly and seasonal variations
This study showed a peak in TBI admissions on Saturdays and patients admitted during weekends were younger, healthier and more often under the influence of alcohol. As discussed by Bjarkø et al., weekend-binge drinking rather than alcoholism is likely the culprit due to the younger population (Bjarkø et al., 2019).
Our results did not show any significant change in mortality rate during weekends. Several studies showed conflicting evidence regarding weekend-effect in trauma patients or patients with TBI (Carr et al., 2010, 2011; Schneider et al., 2012). It has been debated that reduced staffing during weekends might affect patient outcome, however level 1 trauma centers are equipped to deliver guaranteed all hour adequate service preventing a delay in patient care. The weekly trauma team simulation training and Advanced Trauma Life Support (Søreide, 2008) certification for all trauma leaders at SUH since 2012 also provides for a standard of care regardless of time of admission (Weber et al., 2022). Another aspect lies in patient characteristics and that those admitted during weekends are both younger and healthier patients leading to a lower overall mortality rate. There might also be a lower threshold to admit patients via trauma team with suspicion of alcohol intoxication and TBI giving them a standardized level of care. Altered staffing could possibly affect patients further down the chain of management (at the wards or OR), however it is worth mentioning that typical work hours can be subject to competition for personnel and availability of operating room theatre causing other problems and investigating this is beyond the scope of this study (Carr et al., 2011).
4.6. Strengths and limitations
A major strength of this cohort study is the long study period of 16 years, which allows for observations regarding time trends. Consecutive inclusion of patients in a prospective fashion minimized the selection bias and no age groups were excluded. The registry showed also a very high completeness of data.
One limitation of the study was that GOS was calculated at discharge and did not include a follow-up after 6 months. Longer follow-up data was also not available.
5. Conclusion
Weekend admissions were not associated with a higher mortality rate or worse outcome in moderate and severe TBI. This demonstrates that these patients receive proper care at all times when admitted via trauma teams. Severe TBI is overrepresented in younger patients, especially during weekends and overall early mortality rate is high. In a time where more focus is shifted towards older patients with comorbidities it's not to be overlooked that the most severely injured patients are often young adult males that are physically active.
Funding
Not applicable.
Ethics approval
Ethical approval was granted by the Regional Committee for Medical and Health Research Ethics of Western Norway (#143902). All patients and/or their legal guardians received written and oral information about the registry and its purpose.
Data availability
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agrawal D., Joshua S., Gupta D., Sinha S., Satyarthee G. Can glasgow score at discharge represent final outcome in severe head injury? J. Emergencies, Trauma, Shock. 2012;5(3):217–219. doi: 10.4103/0974-2700.99685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andelic N., Anke A., Skandsen T., Sigurdardottir S., Sandhaug M., Ader T., et al. Incidence of hospital-admitted severe traumatic brain injury and in-hospital fatality in Norway: a national cohort study. Neuroepidemiology. 2012;38(4):259–267. doi: 10.1159/000338032. [DOI] [PubMed] [Google Scholar]
- Andriessen T.M.J.C., Horn J., Franschman G., van der Naalt J., Haitsma I., Jacobs B., et al. Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J. Neurotrauma. 2011;28(10):2019–2031. doi: 10.1089/neu.2011.2034. [DOI] [PubMed] [Google Scholar]
- Baker S.P., O'Neill B., Haddon W., Jr., Long W.B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- Baum J., Entezami P., Shah K., Medhkour A. Predictors of outcomes in traumatic brain injury. World Neurosurg. 2016;90:525–529. doi: 10.1016/j.wneu.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Bjarkø V.V., Skandsen T., Moen K.G., Gulati S., Helseth E., Nilsen T.I.L., et al. Time of injury and relation to alcohol intoxication in moderate-to-severe traumatic brain injury: a decade-long prospective study. World Neurosurg. 2019;122:e684–e689. doi: 10.1016/j.wneu.2018.10.122. [DOI] [PubMed] [Google Scholar]
- Borg J., Röe C., Nordenbo A., Andelic N., de Boussard C., af Geijerstam J.-L. Trends and challenges in the early rehabilitation of patients with traumatic brain injury: a scandinavian perspective. Am. J. Phys. Med. Rehabil. 2011;90(1) doi: 10.1097/PHM.0b013e3181fc80e7. [DOI] [PubMed] [Google Scholar]
- Cantwell K., Morgans A., Smith K., Livingston M., Spelman T., Dietze P. Time of day and day of week trends in EMS demand. Prehosp. Emerg. Care. 2015;19(3):425–431. doi: 10.3109/10903127.2014.995843. [DOI] [PubMed] [Google Scholar]
- Carr B.G., Jenkins P., Branas C.C., Wiebe D.J., Kim P., Schwab C.W., et al. Does the trauma System protect against the weekend effect? J. Trauma. Acute Care Surg. 2010;69(5):1042–1048. doi: 10.1097/TA.0b013e3181f6f958. [DOI] [PubMed] [Google Scholar]
- Carr B.G., Reilly P.M., Schwab C.W., Branas C.C., Geiger J., Wiebe D.J. Weekend and night outcomes in a statewide trauma System. Arch. Surg. 2011;146(7):810–817. doi: 10.1001/archsurg.2011.60. [DOI] [PubMed] [Google Scholar]
- Collaborators M.C.T., Perel P., Arango M., Clayton T., Edwards P., Komolafe E., et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336(7641):425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone C., d'Avella D., Servadei F., Angileri F.F., Brambilla G., Conti C., et al. Patients with moderate head injury: a prospective multicenter study of 315 patients. Neurosurgery. 2009;64(4):690–697. doi: 10.1227/01.NEU.0000340796.18738.F7. [DOI] [PubMed] [Google Scholar]
- Farin A., Deutsch R., Biegon A., Marshall L.F. Sex-related differences in patients with severe head injury: greater susceptibility to brain swelling in female patients 50 years of age and younger. J. Neurosurg. 2003;98(1):32–36. doi: 10.3171/jns.2003.98.1.0032. [DOI] [PubMed] [Google Scholar]
- Haller C.S., Walder B. Severe neurotrauma in Switzerland: have short-term outcomes improved? Swiss Med. Wkly. 2015;145(3738) doi: 10.4414/smw.2015.14177. [DOI] [PubMed] [Google Scholar]
- Heiden J.S., Small R., Caton W., Weiss M., Kurze T. Severe head injury: clinical assessment and outcome. Phys. Ther. 1983;63(12):1946–1951. doi: 10.1093/ptj/63.12.1946. [DOI] [PubMed] [Google Scholar]
- Herou E., Romner B., Tomasevic G. Acute traumatic brain injury: mortality in the elderly. World Neurosurg. 2015;83(6):996–1001. doi: 10.1016/j.wneu.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Heskestad B., Baardsen R., Helseth E., Romner B., Waterloo K., Ingebrigtsen T. Incidence of hospital referred head injuries in Norway: a population based survey from the Stavanger region. Scand. J. Trauma Resuscitation Emerg. Med. 2009;17(1):6. doi: 10.1186/1757-7241-17-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B., Beems T., van der Vliet T.M., Diaz-Arrastia R.R., Borm G.F., Vos P.E. Computed tomography and outcome in moderate and severe traumatic brain injury: hematoma volume and midline shift revisited. J. Neurotrauma. 2011;28(2):203–215. doi: 10.1089/neu.2010.1558. [DOI] [PubMed] [Google Scholar]
- James S.L., Theadom A., Ellenbogen R.G., Bannick M.S., Montjoy-Venning W., Lucchesi L.R., et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennett B., Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;305(7905):480–484. doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Kostis W.J., Demissie K., Marcella S.W., Shao Y.-H., Wilson A.C., Moreyra A.E. Weekend versus weekday admission and mortality from myocardial infarction. N. Engl. J. Med. 2007;356(11):1099–1109. doi: 10.1056/NEJMoa063355. [DOI] [PubMed] [Google Scholar]
- Lenartova L., Janciak I., Wilbacher I., Rusnak M., Mauritz W. Severe traumatic brain injury in Austria III: prehospital status and treatment. Wien Klin. Wochenschr. 2007;119(1):35. doi: 10.1007/s00508-006-0762-3. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Menon D.K., Steyerberg E.W., Citerio G., Lecky F., Manley G.T., et al. Collaborative European NeuroTrauma effectiveness Research in traumatic brain injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2014;76(1):67–80. doi: 10.1227/neu.0000000000000575. [DOI] [PubMed] [Google Scholar]
- Maegele M., Engel D., Bouillon B., Lefering R., Fach H., Raum M., et al. Incidence and outcome of traumatic brain injury in an urban area in western Europe over 10 years. Eur. Surg. Res. 2007;39(6):372–379. doi: 10.1159/000107097. [DOI] [PubMed] [Google Scholar]
- Masson F., Thicoipe M., Aye P., Mokni T., Senjean P., Schmitt V., et al. Epidemiology of severe brain injuries: a prospective population-based study. J. Trauma. Acute Care Surg. 2001;51(3):481–489. doi: 10.1097/00005373-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Mekonnen B., Wang G., Rajbhandari-Thapa J., Shi L., Thapa K., Zhang Z., et al. Weekend effect on in-hospital mortality for ischemic and hemorrhagic stroke in US rural and urban hospitals. J. Stroke Cerebrovasc. Dis. 2020;29(10) doi: 10.1016/j.jstrokecerebrovasdis.2020.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moppett I.K. Traumatic brain injury: assessment, resuscitation and early management. Br. J. Anaesth. 2007;99(1):18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- Murray G.D., Teasdale G.M., Braakman R., Cohadon F., Dearden M., Iannotti F., et al. The European brain injury consortium survey of head injuries. Acta Neurochir. 1999;141(3):223–236. doi: 10.1007/s007010050292. [DOI] [PubMed] [Google Scholar]
- Murray L.S., Teasdale G.M., Murray G.D., Miller D.J., Pickard J.D., Shaw M.D.M. Head injuries in four British neurosurgical centres. Br. J. Neurosurg. 1999;13(6):564–569. doi: 10.1080/02688699943060. [DOI] [PubMed] [Google Scholar]
- Mushkudiani N.A., Engel D.C., Steyerberg E.W., Butcher I., Lu J., Marmarou A., et al. Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24(2):259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- Myburgh J.A., Cooper D.J., Finfer S.R., Venkatesh B., Jones D., Higgins A., et al. Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. Journal of Trauma and Acute Care Surgery. 2008;64(4) doi: 10.1097/TA.0b013e3180340e77. [DOI] [PubMed] [Google Scholar]
- Oliveira R.A.R.A., Ara˙jo S., Falc„o A.L.E., Soares SMdTP., Kosour C., Dragosavac D., et al. Glasgow outcome scale at hospital discharge as a prognostic index in patients with severe traumatic brain injury. Arquivos de neuro-psiquiatria. 2012;70 8:604–608. doi: 10.1590/s0004-282x2012000800009. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Fahlstedt M., Jacobsson A., Kleiven S., von Holst H. A national survey of traumatic brain injuries admitted to hospitals in Sweden from 1987 to 2010. Neuroepidemiology. 2015;45(1):20–27. doi: 10.1159/000381780. [DOI] [PubMed] [Google Scholar]
- Peeters W., van den Brande R., Polinder S., Brazinova A., Steyerberg E.W., Lingsma H.F., et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015;157(10):1683–1696. doi: 10.1007/s00701-015-2512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters W., Majdan M., Brazinova A., Nieboer D., Maas A.I.R. Changing epidemiological patterns in traumatic brain injury: a longitudinal hospital-based study in Belgium. Neuroepidemiology. 2017;48(1–2):63–70. doi: 10.1159/000471877. [DOI] [PubMed] [Google Scholar]
- Posti J.P., Kytö V., Sipilä J.O.T., Rautava P., Luoto T.M. High-risk periods for adult traumatic brain injuries: a nationwide population-based study. Neuroepidemiology. 2021;55(3):216–223. doi: 10.1159/000515395. [DOI] [PubMed] [Google Scholar]
- Rating the severity of Tissue damage: I. The abbreviated scale. JAMA. 1971;215(2):277–280. doi: 10.1001/jama.1971.03180150059012. [DOI] [PubMed] [Google Scholar]
- Rogaland Trauma System Study Collaborating G. Efficacy of a two-tiered trauma team activation protocol in a Norwegian trauma centre. Br. J. Surg. 2012;99(2):199–208. doi: 10.1002/bjs.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozenbeek B., Maas A.I.R., Menon D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013;9(4):231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J.V., Maas A.I., Bragge P., Morganti-Kossmann M.C., Manley G.T., Gruen R.L. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–1098. doi: 10.1016/S0140-6736(12)60864-2. [DOI] [PubMed] [Google Scholar]
- Schneider E.B., Hirani S.A., Hambridge H.L., Haut E.R., Carlini A.R., Castillo R.C., et al. Beating the weekend trend: increased mortality in older adult traumatic brain injury (TBI) patients admitted on weekends. J. Surg. Res. 2012;177(2):295–300. doi: 10.1016/j.jss.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Søreide K. Three decades (1978–2008) of Advanced Trauma Life Support (ATLS™) practice revised and evidence revisited. Scand. J. Trauma Resuscitation Emerg. Med. 2008;16(1):19. doi: 10.1186/1757-7241-16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S.C., Spettell C. The Head Injury Severity Scale (HISS): a practical classification of closed-head injury. Brain Inj. 1995;9(5):437–444. doi: 10.3109/02699059509008203. [DOI] [PubMed] [Google Scholar]
- Steyerberg E.W., Wiegers E., Sewalt C., Buki A., Citerio G., De Keyser V., et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–934. doi: 10.1016/S1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- Tagliaferri F., Compagnone C., Korsic M., Servadei F., Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006;148(3):255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tverdal C., Aarhus M., Andelic N., Skaansar O., Skogen K., Helseth E. Characteristics of traumatic brain injury patients with abnormal neuroimaging in Southeast Norway. Injury Epidemiol. 2020;7(1):45. doi: 10.1186/s40621-020-00269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Walder B., Haller G., Rebetez M.M.L., Delhumeau C., Bottequin E., Schoettker P., et al. Severe traumatic brain injury in a high-income country: an epidemiological study. J. Neurotrauma. 2013;30(23):1934–1942. doi: 10.1089/neu.2013.2955. [DOI] [PubMed] [Google Scholar]
- Weber C., Andreassen J.S., Behbahani M., Thorsen K., Søreide K. Characteristics, image findings and clinical outcome of moderate and severe traumatic brain injury among severely injured children: a population-based cohort study. Eur. J. Trauma Emerg. Surg. 2022 doi: 10.1007/s00068-021-01820-y. [DOI] [PubMed] [Google Scholar]
- Wiik-Larsen J., Thorsen K., Sandve K.O., Søreide K. Incidence and characteristics of pancreatic injuries among trauma patients admitted to a Norwegian trauma centre: a population-based cohort study. Scand. J. Gastroenterol. 2020;55(11):1347–1353. doi: 10.1080/00365521.2020.1829032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.