Abstract
Introduction
Pneumocephalus after chronic subdural hematoma (CSDH) evacuation is a potential predictor of hematoma recurrence.
Research question
To study the feasibility and safety of a novel CSDH evacuation technique using a valve-controlled method to avoid pneumocephalus.
Material and methods
In a retrospective case series, we evacuated CSDH using very low-pressure valve-controlled drains and recorded the neurological, radiological, and functional outcomes. Patients with primary CSDH, without previous neurosurgical intervention, and who did not receive antiplatelet or anticoagulant therapy the week prior to the index surgery, were included in the study. Exclusion criteria were the evacuation with other treatment techniques and incomplete data files. Patients were assessed according to the Bender grading system to record the neurological status. The hematoma volume was estimated using the formula for ellipsoid volumes.
Results
Thirty-six patients with a mean age of 73 years (±9 years) fulfilled our eligibility criteria. Our technique was effective since it decreased the CSDH volume from 141 ml (IQR 97 ml) to 20.6 ml (IQR 26.59 ml; p < 0.001) and improved the neurological status according to the Bender grading system from two (IQR 0.25) to 1 (IQR 0). However, pneumocephalus and hematoma recurrence occurred in one case each (2.8%). At six months, all patients returned to their previous status, except for two patients (5.6%) who died due to irrelevant pathologies.
Conclusions
Valve-controlled CSDH evacuation aiming to decrease the postoperative pneumocephalus and hematoma recurrence constitutes an effective and safe alternative. However, larger randomized controlled studies are required to establish its role in CSDH management.
Keywords: Chronic subdural hematoma, Evacuation, Pneumocephalus, Recurrence, Outcome, Volume
Highlights
-
•
Pneumocephalus after CSDH evacuation is a potential predictor of hematoma recurrence.
-
•
Α very low-pressure valve-controlled drain was used in our study.
-
•
The neurological, radiological, and functional outcomes were recorded.
-
•
CSDH volume decreased, and neurological status improved.
-
•
Valve-controlled CSDH: effective and safe alternative.
1. Introduction
The chronic subdural hematoma (CSDH) constitutes a collection of fluid at the subdural space, presenting progressive neurologic manifestations weeks to months after moderate head trauma (Feghali et al., 2020; Yang and Huang, 2017). CSDH frequently present with headaches, motor and sensory disturbances, and disorders of consciousness but can also lead to death due to the hematoma mass effect (Feghali et al., 2020). The incidence of CSDH ranges from 1.72 to 20.6 per 100,000 persons, annually. Its severity depends on several risk factors, including advanced age, male gender, and use of anticoagulants (Feghali et al., 2020; Yang and Huang, 2017). The indolent and progressive nature of CSDH is attributed to osmotic hematoma volume expansion, associated with heme degradation byproducts and micro-bleeding of newly formed vessels and neomembranes (Feghali et al., 2020).
Hematoma evacuation, either using a closed drainage system or an open surgical technique, represents the primary treatment modality with satisfactory results (Feghali et al., 2020; Fomchenko et al., 2018). However, hematoma recurrence is the most frequent postoperative complication, with a reported incidence of 10%–20% (Feghali et al., 2020). Middle meningeal artery embolization is currently assessed to minimize the recurrence rate with ambiguous results (Feghali et al., 2020; Fomchenko et al., 2018).
Postoperative intracranial air accumulation, also known as pneumocephalus, is a frequent finding after hematoma evacuation (Chavakula et al., 2020; Ihab, 2012; Ishiwata et al., 1988; Ridwan et al., 2019). A recent study documented the benign nature of pneumocephalus, as it was not associated with poor prognosis, considering the patient's age and hematoma's volume (Huang et al., 2020). However, its clinical significance is challenged, as it may not necessitate urgent evacuation but has been associated with higher hematoma recurrence rates (Chavakula et al., 2020; Ihab, 2012; Ishiwata et al., 1988; Ridwan et al., 2019). Several tips and tricks have been evolved to minimize the impact of postoperative pneumocephalus on the clinical outcome and overall recurrence, including air aspiration to reduce air tension, reduce the subdural space, and promote cerebral expansion (Chavakula et al., 2020).
In the current study, we hypothesize that a valve-controlled CSDH evacuation through a closed drainage system could lower the incidence of pneumocephalus, and as a consequence, improve the hematoma recurrence figures. Meanwhile, the controlled hematoma evacuation would promote gradual cerebral expansion, aiming to prevent neomebrane re-bleeding and intraparenchymal hemorrhage. To the best of our knowledge, this is the first study reporting on the use of a valve-controlled CSDH evacuation, and our findings could be of interest to clinical neurosurgeons, CSDH patients, and medical device manufacturers.
2. Methods
2.1. Study design
We conducted a retrospective single-center case series during a period of two years. Two neurosurgeons with ten and eleven years of experience performed all operations. Approval from the Hospital's Ethical Committee of the Institutional Review Board for research on human subjects was not required for our study since it was based on retrospective anonymized hospital data. Every patient provided informed consent before surgery and study inclusion.
2.2. Eligibility criteria
We considered for eligibility in our study all consecutive adult patients with primary CSDH who were surgically treated using the closed valve-controlled drainage system. A non-enhanced computed tomography (CT) of the head set the diagnosis of CSDH. CSDH were classified as homogeneous, inhomogeneous, and septate. We also subclassified the former group into hypo-, iso-, and hyperdense according to the radiographic density. Surgery was indicated for patients with symptomatic CSDH with a measured thickness or midline shift of more than 1 cm. We excluded patients with septate CSDH, previous cranial interventions, undergoing other evacuation techniques, antiplatelet or anticoagulant therapy during the last week, and incomplete data files. Additionally, we avoided using the valve-controlled hematoma evacuation in acute and subacute subdural hematomas and mixed density hematomas based on the preoperative CT.
2.3. Surgical technique
Under patient sedation, we performed a twist burr hole at the site corresponding to the largest hematoma diameter. Then, we carried out a small cruciform incision on the dura under continuous saline irrigation and inserted a proximal valve silicone catheter ventricular catheter (inner diameter 1.4 mm, outer diameter 2.7 mm) in the subdural hematoma. After catheter tunneling, we connected a closed drainage system containing a Codman Hakim Precision Fixed® very low-pressure valve and a collection barrel. We paid every effort to connect the draining catheter to the collection barrel as soon as possible to permit a valve-controlled hematoma evacuation and prevent from pneumocephalus. In other words, we did not drain the hematoma freely before connecting to the collection system.
2.4. Postoperative management
The patient was mobilized at the same or the following day, with intensive pulmonary physiotherapy and repeated Valsalva maneuvers. Based on the hematoma volume at the postoperative CT, we kept the catheter for two to three days and discontinued the perioperative antibiotics as soon as we removed the catheter. In cases where the hematoma viscosity impeded the evacuation of the hematoma remnants, we aspirated the remaining hematoma by serial pumpings of the valve, and at the last catheter day, we removed the valve for a gravity drainage system. The patient was hospitalized until neurological status stabilization and returned for a follow-up visit at 15 days, three, and six months after surgery.
2.5. Data collection
Every consecutive eligible patient received a unique number. We recorded the patient's age, gender, clinical presentation, co-morbidity, and neurological status for each patient. We used the formula for ellipsoid volumes to measure the hematoma volume before and after surgery (Sachs and Sachs, 1977). We compared the preoperative and postoperative hematoma volumes according to the respective CT. We did not measure the hematoma volume on a daily basis since we performed serial peripheral flashing frequently. Accordingly, we defined pneumocephalus as the presence of more than 5 ml of intracranial air in the postoperative CT. Regarding the efficacy, we were particularly interested in hematoma recurrence. The patient's neurological status was co-registered according to the Bender grading scale, before and after surgery at the three- and six-month follow-up (Bender and Christoff, 1974; Wilson et al., 1998). We assessed the procedure's safety by recording all significant complications after surgery, including re-bleeding. Additional study parameters included the number of days with subdural catheters and the length of hospitalization. All records were gathered in an Excel spreadsheet by the principal investigator.
2.6. Statistical analysis
Mean and median values and standard deviations and interquartile ranges to summarize continuous parameters were used. For discrete parameters, we used counts and percentages. Additionally, we compared before and after values for continuous measures with paired t-tests and repeated measures analysis of variance (r-ANOVA). In contrast, for discrete data, we used the chi-square test. All statistical analyses was conducted with the JASP statistical software (JASP Team, 2021).
3. Results
3.1. Study sample
The study included 36 patients (7 females, 19.4%) with a mean age of 73 years (±9.5 years). The most common symptoms were hemi- or monoparesis (21, 58.3%), headache (15, 41.7%), and speech disorders or dizziness (12, 33.3%). Five patients discontinued their oral anticoagulants for at least five to seven days before surgery. The median GCS was 14 (IQR 1). Finally, one patient reported multiple co-morbidities from the respiratory and cardiovascular systems and the kidneys. On the head CT, the CSDH were unilateral and bilateral in 28 (77.8%) and eight (22.2%) cases, respectively (Table 1). The majority (31, 86%) were operated on the same day after hospital presentation. The catheters remained for a median of four days (IQR 1 day), and the patients were discharged from the hospital after eight days (IQR 2.5 days).
Table 1.
Summary table of our study sample.
| Parameter | Mean | Standard deviation |
|---|---|---|
| Age | 72.94 | 9.49 |
| INR | 1.130 | 0.25 |
| Median | IQR | |
| Catheter days | 4.0 | 1.0 |
| Hospitalization | 8.0 | 2.5 |
| CSDH volume (ml) | ||
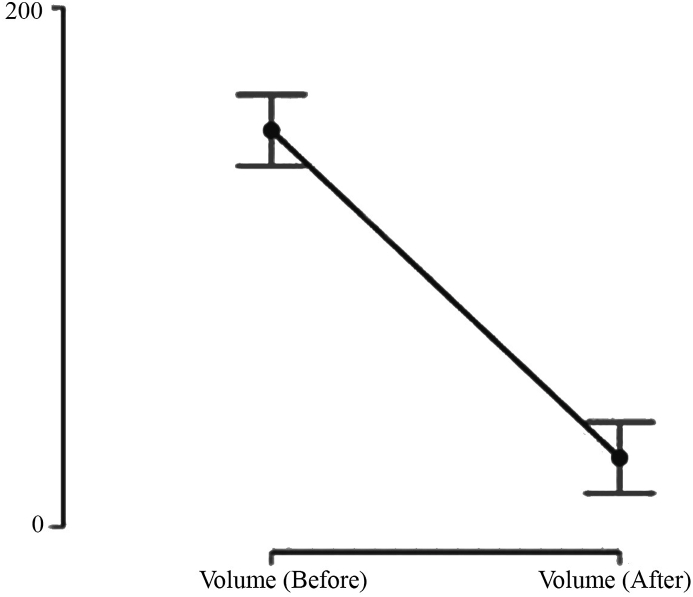
| Before surgery | 141 | 97 |
| After surgery | 20.6 | 25.59 |
| GCS | ||
| Before surgery | 13.50 | 1.261 |
| After surgery | 14.85 | 0.359 |
| 6 months follow-up | 15 | 0.000 |
| Bender score | ||
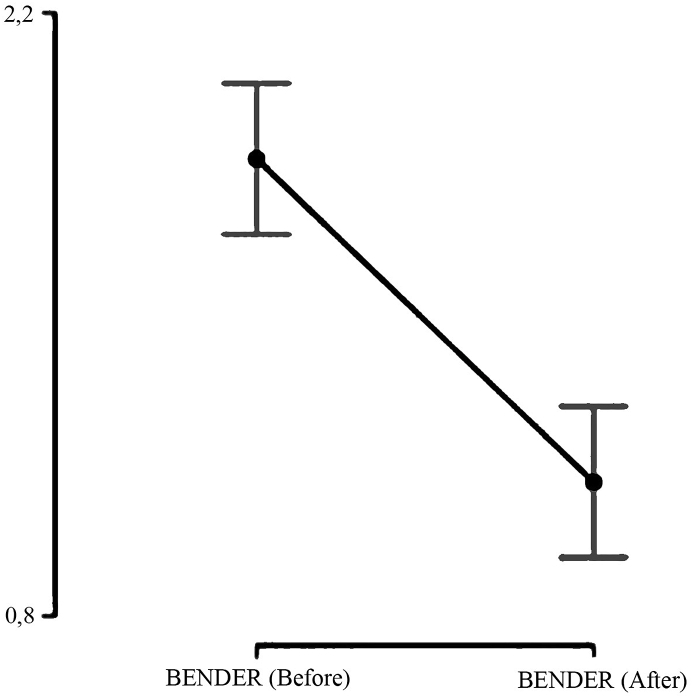
| Before surgery | 2 | 0.25 |
| After surgery | 1 | 0.00 |
| N | Percentage (%) | |
| Gender | ||
| Female | 7 | 19.4 |
| Male | 29 | 80.6 |
| Location | ||
| Left | 17 | 47.7 |
| Right | 11 | 30.6 |
| Bilateral | 8 | 22.2 |
| Presentation | ||
| Motor Weakness | 21 | 58.3 |
| Speech disturbances | 12 | 33.3 |
| Disturbances of consciousness | 5 | 13.9 |
| Headache | 15 | 41.7 |
| Dizziness | 12 | 33.3 |
| Epilepsy | 1 | 2.8 |
| Imbalance | 5 | 13.9 |
| GOS | ||
| 1 | 34 | 94.4 |
| 5 | 2 | 5.6 |
| Complications | ||
| Pneumocephalus | 1 | 2.8 |
| Recurrence | 1 | 2.8 |
3.2. Efficacy
Except for two cases (5.6%), the valve controlled CSDH drainage improved the neurological status, with an estimated efficacy as high as 94.4%. In one case (2.8%) the hematoma flow was obstructed at the level of the valve. In this case we removed the valve early in the first postoperative day. The technique was effective since it decreased the CSDH volume from 141 ml (IQR 97 ml) to 20.6 ml (IQR 26.59 ml; p < 0.001) and improved the neurological status according to the Bender grading system from two (IQR 0.25) to (1, IQR 0). (Fig. 1, Fig. 2). At the six-month follow-up, all remaining patients returned to their previous functional status.
Fig. 1.
Decrease of CSDH volume.
Fig. 2.
Improvement of the neurological status according to the Bender grading system.
3.3. Complications
Two patients died. One patient with multiple co-morbidities died from pulmonary embolism during hospitalization. Another patient died three months after surgery for reasons unrelated to the intervention. One patient developed pneumocephalus (2,8%) but did not require any further surgical intervention. Another patient presented with radiological CSDH recurrence twenty days after the index surgery. As he was still asymptomatic, a watchful follow-up was recommended until its resorption.
4. Discussion
4.1. Overview of our findings
The current study showed that valve-controlled drainage systems constitute an effective and safe alternative in CSDH treatment. Practically, we succeeded in evacuating all the hematomas with minimal air entrapment. The incidence of hematoma recurrence remained less than 5%. The morbidity and mortality rates were equally low. Despite our gratifying results, the nature of our study cannot establish a cause-and-effect relationship between our technique and the low hematoma recurrence rate.
4.2. Pathophysiology of CSDH
A minor trauma causes cleavage of the dural border cells, after which CSF with blood is interposed between the broken cell layer and the rest of the dura. Cytokines, attracting inflammatory cells are released by injured dural cells. Progressively more eosinophils are recruited, and the cavity is filled by plasminogen and thrombin. Fibrin clots are disintegrated, and platelets cannot aggregate. This process produces ongoing cell injury and causes a significant increase of inflammatory cells and VEGF production. The inflammation cycle perpetuates and the hygroma becomes a CSDH. The latter grows until it reaches a size that affects blood flow and metabolism of adjacent brain structures, leading to manifestation of symptoms (Holl et al., 2018).
4.3. Hypothesis
Our concept aimed to limit air accumulation in the subdural space when incising the dura and prevent backflow. Therefore, the incision was kept as small as the diameter of the silicone catheter. At the same time, the catheter was advanced under continuous saline irrigation without pressure to prevent pneumocephalus and reduce the hematoma viscosity, securing thus the valve mechanism to allow a low-pressure gradient hematoma evacuation, without backflow of air or hematoma contents. In addition, the valve mechanism permitted hematoma aspiration through serial valve pumping. Nevertheless, we were concerned about valve dysfunction from the high protein content of the hematoma. Thus, we planned to remove the valve mechanism if the postoperative CT of the head showed inadequate hematoma evacuation. However, we enhanced the hematoma flow after valve milking, particularly in viscous hematomas. Indeed, “milking” of the valve's pump was sufficient to maintain the flow, with the exception of one case, even in high-viscosity hematomas.
4.4. Comparison with other techniques
There are multiple treatment alternatives in the management of CSDH, aiming to reduce the recurrence rate. A large meta-analysis of 34,829 patients verified that the use of drain insertion following burr hole drainage reduced the risk of recurrence (RR, 0.46; 95% CI 0.27–0.76) (Almenawer et al., 2014). Nevertheless, another meta-analysis failed to prove the superiority of irrigation in the development of pneumocephalus (OR, 5.91; 95% CI, 0.65–56.86) and in preventing hematoma recurrence (OR, 1.14, 95% CI, 0.16–8.24) (Yuan et al., 2018). Likewise, the subperiosteal drain did not seemed to alter the improvement of the recurrence rate (OR, 0.73; 95% CI, 0.58–0.92; P = 0.07OR, 1.29; 95% CI, 1–1.68) compared to subdural drain in a recent meta-analysis (Ding et al., 2020). However, these results did not persist after sensitivity analysis because of heterogeneity among the analyzed studies. The superiority of subperiosteal drain regarding recurrence was also presented in other studies (Greuter et al., 2020; Pranata et al., 2020). Endoscopic treatment constitutes another alternative modality whose effect remains controversial. Nevertheless, the use of endoscope seems to reduce the postoperative bleeding and the need for reoperation (Amano et al., 2021). Furthermore, a second burr hole does not improve the risk of recurrence (OR, 1.28; 0.92–1.78) (Ding et al., 2020; Wan et al., 2019). On the contrary, the newly emerged middle meningeal artery embolization could be of value in reducing hematoma recurrence compared to conventional hematoma evacuation (Srivatsan et al., 2019; Dian et al., 2021). This minimally invasive technique showed lower rates of recurrence (OR = 0.087; 0.026–0.292; P < 0.001) with comparable rates of complications (OR = 0.563; 0.107–2.96; P = 0.497) (Srivatsan et al., 2019). Additionally, a most recent meta-analysis by Ironside et al. (2021) showed similar results regarding recurrence (OR = 0.15 (95% CI 0.03 to 0.75), p = 0.02) and complication rates (OR = 0.78 (0.34–1.76), p = 0.55). Finally, certain authors used a pump system for controlled irrigation in single cases (Tran et al., 2019; Himstead et al., 2021). These studies pointed out the need for gradient hematoma evacuation like we did.
4.5. Medical treatment and recurrence
The effect of drug treatment on reoperation rates has been also studied. The use of Dexamethasone, atorvastatin and tranexamic acid is examined in a number of trials. Recently, Yu et al. (2022) published his results of a Bayesian Network Meta-Analysis pointing out the improved recurrence rates following the use of the previous drug agents. As for the usage of dexamethasone, many authors observed that its use decreases the rate of recurrence, a recent multicenter, randomized trial study by Hutchinson et al. (2020) issued the subject of increased complications. However, in this study, the incidence of reoperation because of hematoma regrowth was as low as 1.7% in patients under dexamethasone.
4.6. Applicability of the results
Our results are particularly relevant to older patients. The elderly constitute a constantly enlarging population, particularly in industrialized societies due to the higher life expectancy. Thus, the altered brain turgor and the reduced cerebral volume with advanced age frequently result in large “dead” spaces, ready to harbor significant CSDH. Meanwhile, older people often suffer from repeated falls and commonly use antiplatelets or anticoagulants, all predisposing to CSDH. The valve-controlled hematoma evacuation could offer a safe and effective technique characterized by less risk for hematoma recurrence. However, comparative studies are required to establish its role compared to other techniques.
4.7. Limitations
Our study has some significant limitations. Firstly, our study sample was relatively small. Nevertheless, we were successful in reaching statistical significance, yet more extensive studies are needed to verify the reproducibility of our results. Secondly, we did not include a control group as the present study was a feasibility study, aiming to see if this drainage system works and if there is no unexpected morbidity. Thirdly we focused on a selected CSDH subgroup population after excluding septate hematomas. Membranous or septate CSDHs are compartmentalized. Therefore, they cannot be managed simply by drainage. Equally important, we need to establish the optimal candidate for the valve-controlled drainage system. Fourthly, we borrowed a valve-controlled drainage system that is contraindicated for high-viscosity fluids, such as CSDH. Ideally, a valve-controlled system could be redesigned, considering all CSDH peculiarities. Finally, additional research is needed to assess the cost-effectiveness of our new technique. Indeed, using a valve system raises the hospital costs for the treatment of CSDH and the length of hospitalization in some cases. All extra costs should be weight against the cost reduction from re-operation, re-hospitalization, and lengthy follow-ups using serial CT scans.
5. Conclusions
Valve-controlled CSDH drainage seems to be an effective and safe treatment alternative. We showed that this technique might permit hematoma evacuation without the risk of pneumocephalus. It remains to establish the reproducibility of our results and their role in CSDH management. We appreciate that future studies are required to address the incremental costs of using a closed valve drainage system in the management of chronic subdural hematomas, taking into consideration the cost reduction from re-operation, re-hospitalization, and lengthy follow-ups using serial CT scans.
Declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- CSDH
chronic subdural hematoma
- GCS
Glasgow coma scale
- GOS
Glasgow outcome scale
- IQR
interquartile range
References
- Almenawer S.A., Farrokhyar F., Hong C., Alhazzani W., Manoranjan B., Yarascavitch B., et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann. Surg. 2014;259:449–457. doi: 10.1097/SLA.0000000000000255. [DOI] [PubMed] [Google Scholar]
- Amano T., Miyamatsu Y., Otsuji R., Nakamizo A. Efficacy of endoscopic treatment for chronic subdural hematoma surgery. J. Clin. Neurosci. 2021;92:78–84. doi: 10.1016/j.jocn.2021.07.058. [DOI] [PubMed] [Google Scholar]
- Bender M.B., Christoff N. Nonsurgical treatment of subdural hematomas. Arch. Neurol. 1974;31:73–79. doi: 10.1001/archneur.1974.00490380021001. [DOI] [PubMed] [Google Scholar]
- Chavakula V., Yan S.C., Huang K.T., Liu J., Bi W.L., Rozman P., et al. Subdural pneumocephalus aspiration reduces recurrence of chronic subdural hematoma. Oper Neurosurg (Hagerstown) 2020;18:391–397. doi: 10.1093/ons/opz193. [DOI] [PubMed] [Google Scholar]
- Dian J., Linton J., Shankar J.J. Risk of recurrence of subdural hematoma after EMMA vs surgical drainage - systematic review and meta-analysis. Intervent Neuroradiol. 2021;27:577–583. doi: 10.1177/1591019921990962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Liu S., Quan X., Liao S., Liu L. Subperiosteal versus subdural drain after burr hole drainage for chronic subdural hematomas: a systematic review and meta-analysis. World Neurosurg. 2020;136:90–100. doi: 10.1016/j.wneu.2019.12.180. [DOI] [PubMed] [Google Scholar]
- Feghali J., Yang W., Huang J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020;141:339–345. doi: 10.1016/j.wneu.2020.06.140. [DOI] [PubMed] [Google Scholar]
- Fomchenko E.I., Gilmore E.J., Matouk C.C., Gerrard J.L., Sheth K.N. Management of subdural hematomas: Part II. Surgical management of subdural hematomas. Curr. Treat. Options Neurol. 2018;20:34. doi: 10.1007/s11940-018-0518-1. [DOI] [PubMed] [Google Scholar]
- Greuter L., Hejrati N., Soleman J. Type of drain in chronic subdural hematoma—a systematic review and meta-analysis. Front. Neurol. 2020:11. doi: 10.3389/fneur.2020.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himstead A.S., Davies J., Tran D.K., Vadera S. Net drainage as a novel metric for irrigating drainage systems in chronic subdural hematoma management: a case report. Oper Neurosurg (Hagerstown) 2021;20:E449–E453. doi: 10.1093/ons/opab070. [DOI] [PubMed] [Google Scholar]
- Holl D.C., Volovici V., Dirven C.M.F., Peul W.C., van Kooten F., Jellema K., et al. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World Neurosurg. 2018;116:402–411. doi: 10.1016/j.wneu.2018.05.037. e2. [DOI] [PubMed] [Google Scholar]
- Huang G.-H., Li X.-C., Ren L., Dai R.-X., Sun Z.-L., Jiang X.-F., et al. Take it seriously or not: postoperative pneumocephalus in CSDH patients? Br. J. Neurosurg. 2020;34:284–289. doi: 10.1080/02688697.2020.1729343. [DOI] [PubMed] [Google Scholar]
- Hutchinson P.J., Edlmann E., Bulters D., Zolnourian A., Holton P., Suttner N., et al. Trial of dexamethasone for chronic subdural hematoma. N. Engl. J. Med. 2020;383:2616–2627. doi: 10.1056/NEJMoa2020473. [DOI] [PubMed] [Google Scholar]
- Ihab Z. Pneumocephalus after surgical evacuation of chronic subdural hematoma: is it a serious complication? Asian J. Neurosurg. 2012;7:66–74. doi: 10.4103/1793-5482.98647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside N., Nguyen C., Do Q., Ugiliweneza B., Chen C.-J., Sieg E.P., et al. Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J. Neurointerventional Surg. 2021;13:951–957. doi: 10.1136/neurintsurg-2021-017352. [DOI] [PubMed] [Google Scholar]
- Ishiwata Y., Fujitsu K., Sekino T., Fujino H., Kubokura T., Tsubone K., et al. Subdural tension pneumocephalus following surgery for chronic subdural hematoma. J. Neurosurg. 1988;68:58–61. doi: 10.3171/jns.1988.68.1.0058. [DOI] [PubMed] [Google Scholar]
- JASP Team JASP. 2021 [Google Scholar]
- Pranata R., Deka H., July J. Subperiosteal versus subdural drainage after burr hole evacuation of chronic subdural hematoma: systematic review and meta-analysis. Acta Neurochir. 2020;162:489–498. doi: 10.1007/s00701-019-04208-5. [DOI] [PubMed] [Google Scholar]
- Ridwan S., Bohrer A.-M., Grote A., Simon M. Surgical treatment of chronic subdural hematoma: predicting recurrence and cure. World Neurosurg. 2019;128:e1010–e1023. doi: 10.1016/j.wneu.2019.05.063. [DOI] [PubMed] [Google Scholar]
- Sachs J., Sachs E. A simple formula for calculating the volume of subdural hematomas. Neurosurgery. 1977;1:60–61. doi: 10.1227/00006123-197707000-00012. [DOI] [PubMed] [Google Scholar]
- Srivatsan A., Mohanty A., Nascimento F.A., Hafeez M.U., Srinivasan V.M., Thomas A., et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg. 2019;122:613–619. doi: 10.1016/j.wneu.2018.11.167. [DOI] [PubMed] [Google Scholar]
- Tran D.K., Tretiakov P., Brock J., Chen J., Vadera S. Novel use of dual-lumen catheter for irrigation and drainage after evacuation of chronic subdural hematoma. World Neurosurg. 2019;132:343–346. doi: 10.1016/j.wneu.2019.08.225. [DOI] [PubMed] [Google Scholar]
- Wan Y., Xie D., Xue Z., Xie J., Song Z., Wang Y., et al. Single versus double burr hole craniostomy in surgical treatment of chronic subdural hematoma: a meta-analysis. World Neurosurg. 2019;131:e149–e154. doi: 10.1016/j.wneu.2019.07.097. [DOI] [PubMed] [Google Scholar]
- Wilson J.T., Pettigrew L.E., Teasdale G.M. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J. Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Yang W., Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg. Clin. 2017;28:205–210. doi: 10.1016/j.nec.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Yu W., Chen W., Jiang Y., Ma M., Zhang W., Zhang X., et al. Effectiveness comparisons of drug therapy on chronic subdural hematoma recurrence: a bayesian Network meta-analysis and systematic review. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.845386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Wang Q.-P., Cao Y.-L., Zhang H., Burkutally M.S.N., Budryte K., et al. Burr hole drainage and burr hole drainage with irrigation to treat chronic subdural hematoma: a systematic review and meta-analysis. Medicine (Baltim.) 2018;97 doi: 10.1097/MD.0000000000011827. [DOI] [PMC free article] [PubMed] [Google Scholar]