Postmortem microstructural studies together with in vivo magnetic resonance imaging show that human arachnoid granulations are porous channels that serve as transient filtration conduits for cerebrospinal fluid to flow directly into dural interstitial tissue, but not into venous sinuses.
Abstract
Postmortem microstructural studies together with in vivo magnetic resonance imaging show that human arachnoid granulations are porous channels that serve as transient filtration conduits for cerebrospinal fluid to flow directly into dural interstitial tissue, but not into venous sinuses (Shah et al. 2022. J. Exp. Med. https://doi.org/10.1084/jem.20220618).
In the present issue of JEM, detailed postmortem microstructural analyses performed on multiple specimens were combined with in vivo magnetic resonance imaging to fill an essential gap in our knowledge of human arachnoid granulations (AG; Shah et al., 2022).

Insights from Jean-Leon Thomas and Helene Benveniste.
In humans, AG are verrucous protrusions that stem from the intermediate layer of the meninges, that is, the arachnoid, and they are physically prominent close to the venous sinuses of the dura mater, which is the outer layer of the meninges. AG are observed in the meninges of humans, non-human primates, and large mammals but only form rudimentary budding structures in mice and rats (Proulx, 2021). In humans, AG are absent at birth and emerge robustly at the time of closure of the fontanelles, then they increase in number with aging (Mack et al., 2009). Human AG have early been proposed as the principal site of absorption of intracranial cerebrospinal fluid (CSF) into the blood stream of dural veins (Key and Retzius, 1875; Weller et al., 2018), and this view remains influential. Our general understanding of AG contribution to CSF reabsorption and central nervous system (CNS) fluid homeostasis, however, remains unclear.
Considering the lack of large-scale studies of AG structure among individuals and during aging, Shah et al. (2022) have carefully examined 400 AG resected from individuals spanning 24–104 yr of age. They used state-of-the-art high-resolution microscopic imaging techniques and immunohistological tools on tissue sections and whole-mount preparations to provide a quantitative analysis on the number, size, tissular and cellular composition and morphology, as well as on the immune cell content and cytokine profiles of AG. The AG architecture is described as a vimentin-expressing meningothelial capsule surrounded by a stromal core of collagen fibers. Importantly, in most AG, the perforated capsule abuts the perisinus space of the dural venous sinuses, but is not communicating, or in contact with, the venous endothelium or blood compartment. In the AG core, a fine and porous stromal framework showed complex cavities continuous with the subdural space. The AG core harbored leukocytes, mainly CD68+ macrophages, and expressed cytokine enrichment. These microstructural features of AG varied across individuals, while alterations in their shape and molecular composition, such as decreased collagen and reduced vimentin expressions, dramatically increased with aging. The authors also included anatomical magnetic resonance imaging studies on live patients, including T2-fluid attenuated inversion recovery sequences. MRI and histological data were shown to converge and confirm the existence of four AG types with a significant subset showing domes abutting the perisinus stroma, and other subsets imbedded within the calvarium bone marrow space. Based on the location of AG at the brain–meningeal lymphatic interface, the authors hypothesized that AG have alternate biological purposes including CSF filtration and neuroimmune surveillance, and further, that these functions may be altered with aging and represent targets for the diagnostic and monitoring of aged-related neurological diseases.
The careful collection of a high number of postmortem patient samples and the high quality of histological and MRI analyses are unique strengths of the study by Shah et al. (2022). Their work adds significant new information to our previous knowledge of the AG structure, composition, and relationship with the subarachnoid and dural tissues. This knowledge, and the associated state-of-the-art technical approaches, will be useful for future studies aiming to characterize AG microstructural features in patients with neurological pathologies.
Based on the authors’ anatomical findings, AG are expected to mediate a direct outflow of CSF into the dural interstitial compartment adjacent to the venous sinuses. This assumption is in line with previous reports of parasagittal CSF efflux observed in humans by MRI with gadobutrol contrast (Ringstad and Eide, 2020) as well as in rodents that, despite their lack of AG, showed small macromolecules passage across the arachnoid mater and infiltration of the dural stromal tissue (Rustenhoven et al., 2021). Altogether, these data indicate that the dura mater is not a strict barrier that isolates the brain and locally is in direct communication with subarachnoid CS. However, the model of Shah et al. (2022) appears to be in contradiction with the prevalent view that human AG drain CSF into the blood of dural venous sinuses. Functional studies will be needed for further validation of the AG as a local, one-way CSF conduit into the dural stroma and meningeal lymphatics. In humans, such functional evidence may be provided by dynamic MRI studies acquired with sufficient spatial resolution to correlate the position of AG with local CSF outflow into the meninges. Dynamic imaging after injection of a contrast agent into the CSF could also directly demonstrate CSF flow dynamics and drainage through AG. In this respect, MRI sequences for contrast-enhanced imaging of meningeal lymphatic drainage have been reported (Absinta et al., 2017; Jacob et al., 2022). However, considering the small size of AG, these approaches may be technically challenging and will require high spatial and temporal resolution, and possibly high-field (7Tesla) MRI. MRI studies on large number of patients with neurological diseases affecting CSF outflow, such as hydrocephaly, venous stenosis, or idiopathic intracranial hypertension, may offer an easier, although indirect, way of assessing whether AG are CSF drainage regulators, by determining if the type of pathology correlates with alterations of the number, size, and structure of AG and if these features can change with therapy. Considering that AG features vary highly between individuals, such MRI studies will require large cohorts of patients. Alternative approaches using AG-bearing animal models, especially large-size species, should also be considered, including in vivo imaging using MRI, ultrasound imaging or infrared imaging, which could be combined with manipulations to ablate AG using laser ablation, or to alter CSF drainage routes, for example by intraventricular administration of LPS.
Lymphatic vessels of the dura mater contribute to the drainage of CSF outflow in the dorsal, basal, and anterior regions of the skull. The lymphatic nature of these meningeal vessels has been assessed by their expression of a panel of markers, including podoplanin and, more commonly, Prospero homeobox protein 1 and lymphatic vessel endothelial hyaluronan receptor-1 (Louveau et al., 2018; Aspelund et al., 2015). In perisinus tissue adjacent to dorsal granulations, Shah et al. (2022) have identified vessel-like structures expressing podoplanin in some AG specimens. Considering podoplanin+ structures as lymphatic endothelium elements, the meningeal lymphatics neighboring AG may further drain CSF from the dura mater. AG may thus mediate CSF-to-dural stroma and likely CSF-to-lymph transfer, but the consistent presence of meningeal lymphatics associated with AG remains to be confirmed in additional human specimens and large-mammal animal models, using a full panel of lymphatic markers.
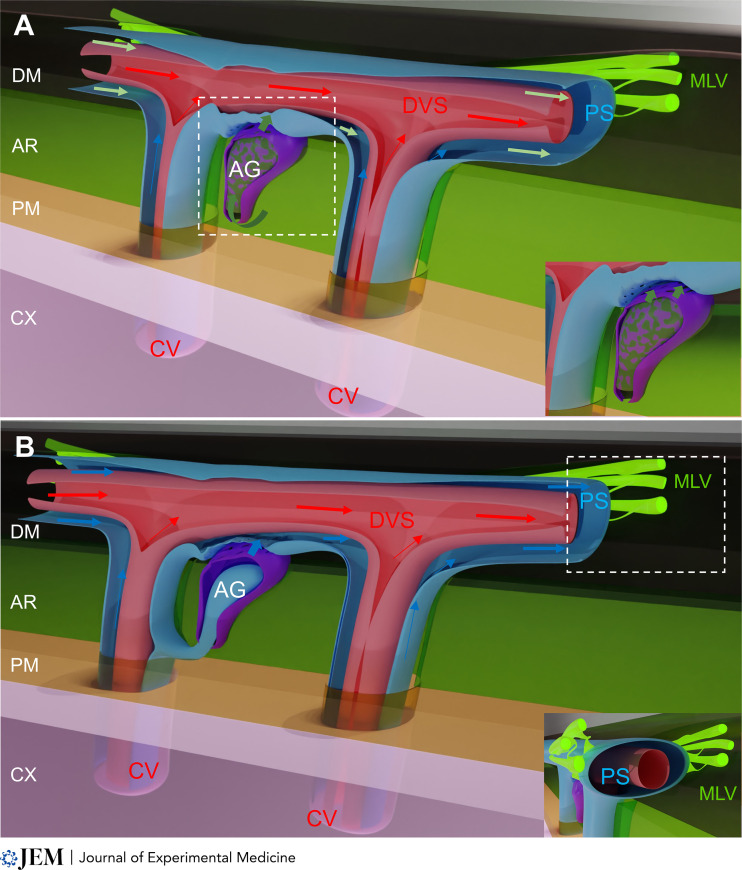
In the case that future studies confirm AG as a new piece of the brain fluid drainage machinery, how would AG integrate with the glymphatic system? Briefly, the glymphatic system is a network of perivascular channels that functions as a transit passageway for CSF to exchange with interstitial fluid to facilitate removal of soluble waste from the brain in a manner dependent of sleep state (Iliff et al., 2012; Xie et al., 2013). The glymphatic model postulates that waste solutes are transported out of the brain via perivenous channels that connect with meningeal lymphatics (Zhao et al., 2022). In humans, cranial nerves exiting on the skull base, the parasagittal dura, and AG are considered the major CSF reabsorption sites (Ringstad and Eide, 2020). According to Shah et al. (2022), AG would serve as an alternate outlet for CSF to communicate with the dural interstitial space and possibly also meningeal lymphatics. AG may thus directly channel CSF into the perisinus stroma or be in continuity with the perivenous space of large cerebral veins and thereby contribute to the glymphatic drainage (see figure). It is as yet unknown if CSF exiting via the AG carries waste solutes (e.g., amyloid β or τ protein), and this information would be necessary to draw further conclusions. Indeed, if CSF passing through AG was carrying waste, this would support a direct downstream connection to the glymphatic system.
Two models for the AG-mediated transfer of CNS-derived fluids into the dura. Both models postulate that perivenous space of cerebral veins (CV) in the brain and leptomeninges are continuous, and perivenous fluids and macromolecules (blue arrows) collect into the perisinus space (PS) in the dura. (A) CSF drainage: AG (purple) located in the arachnoid (AR, light green) contact the dura mater (DM, black). AG channel the CSF (dark green arrows) into the perisinus stroma of the dural venous sinus (DVS, red). In this first model, the perisinus collects CSF and glymphatic fluids (green arrows). Inset shows a closer view of the contact between AG and the perisinus. (B) Glymphatic drainage: AG (purple) are connected with the perivenous space (blue) of large cerebral veins (red) and contribute to cerebral waste drainage. In this second model, the perisinus only collects glymphatic fluids (blue arrows). Inset shows dural lymphatics (MLV, fluorescent green) contacting the perisinus. In both models, dural lymphatics allow the uptake and drainage of perisinus fluids and macromolecules into CNS-draining lymph nodes. Cortex (CX, pink); Pia mater (PM, yellow). Arrows: fluid flow directions.
The meninges, especially the dura mater, are enriched in circulating immune cells compared to the brain. Immune cells are more concentrated along the dural venous sinuses and in the perisinus space that provides a neuroimmune interface, where brain antigens are surveyed under steady-state conditions (Rustenhoven et al., 2021). As expected, considering the location of AG along the perisinus space of dural venous sinuses, Shah et al. (2022) found macrophages, dendritic cells, and few lymphocytes within the AG stroma. AG may thus offer an additional interface for immune sampling of CSF components and immune cell interactions, thereby contributing to the immune surveillance of the meninges and brain tissues. This observation correlates with the detection of a broad spectrum of secreted cytokines in adult AG, and pro-inflammatory signatures of AG in aged individuals. Altogether, these premises justify future in-depth investigation of the AG immune environment, whether it displays specific responses to inflammation, injury, and infection of the meninges, as well as to neurological diseases. In humans, recent progress in imaging by positron emission tomography allows the detection of different immune cell populations in live individuals, and this approach may be relevant for monitoring immune signatures in AG and their changes during pathological states in patients. Alternatively, AG-bearing animal models will facilitate experimental approaches and ease tissue sampling for further single cell multi-omics and flow cytometry analysis of AG. These studies could allow to identify AG-specific gene signatures and their variation with pathological conditions and aging, profile the immune environment, and follow immune cell trafficking to finally predict immune interactions according to disease state. Similar analyses could be conducted during meningeal development to identify signals and pathways regulating AG development and aging.
Hopefully, the timely study of Shah et al. (2022) will raise new experimental approaches by imaging and single cell analysis to integrate AG function into the brain clearance and neuroimmune communication machineries.
Acknowledgments
We thank Stephanie Lenck, Philip Coish, and Luiz Geraldo for critical reading, and Jean-Mickael Thomas for graphic art.
References
- Absinta, M., et al. 2017. Elife. 10.75554/eLife.29738 [DOI] [Google Scholar]
- Aspelund, A., et al. 2015. J. Exp. Med. 10.1084/jem.20142290 [DOI] [Google Scholar]
- Iliff, J.J., et al. 2012. Sci. Transl. Med. 10.1126/scitranslmed.3003748 [DOI] [Google Scholar]
- Jacob, L., et al. 2022. J. Exp. Med. 10.1084/jem.20220035 [DOI] [Google Scholar]
- Key, A., and Retzius G.. 1875. resource.nlm.nih.gov/66211410RX2.
- Louveau, A., et al. 2018. Nat. Neurosci. 10.1038/s41593-018-0227-9 [DOI] [Google Scholar]
- Mack, J., et al. 2009. Pediatr. Radiol. 10.1007/s00247-008-1084-6 [DOI] [Google Scholar]
- Proulx, S.T. 2021. Cell. Mol. Life Sci. 10.1007/s00018-020-03706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad, G., and Eide P.K.. 2020. Nat. Commun. 10.1038/s41467-019-14195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven, J., et al. 2021. Cell. 10.1016/j.cell.2020.12.040 [DOI] [Google Scholar]
- Shah, T., et al. 2022. J. Exp. Med. 10.1084/jem.20220618 [DOI] [Google Scholar]
- Weller, R.O., et al. 2018. Acta Neuropathol. 10.1007/s00401-018-1809-z [DOI] [Google Scholar]
- Xie, L., et al. 2013. Science. 10.1126/science.1241224 [DOI] [Google Scholar]
- Zhao, L., et al. 2022. Physiology. 10.1152/physiol.00015.2022 [DOI] [Google Scholar]