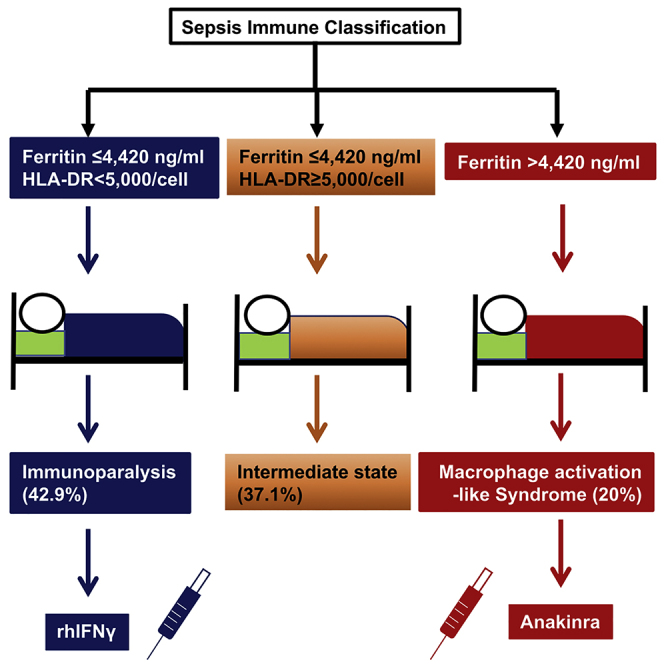
Summary
The state of immune activation may guide targeted immunotherapy in sepsis. In a double-blind, double-dummy randomized clinical study, 240 patients with sepsis due to lung infection, bacteremia, or acute cholangitis were subjected to measurements of serum ferritin and HLA-DR/CD14. Patients with macrophage activation-like syndrome (MALS) or immunoparalysis were randomized to treatment with anakinra or recombinant interferon-gamma or placebo. Twenty-eight-day mortality was the primary endpoint; sepsis immune classification was the secondary endpoint. Using ferritin >4,420 ng/mL and <5,000 HLA-DR receptors/monocytes as biomarkers, patients were classified into MALS (20.0%), immunoparalysis (42.9%), and intermediate (37.1%). Mortality was 79.1%, 66.9%, and 41.6%, respectively. Survival after 7 days with SOFA score decrease was achieved in 42.9% of patients of the immunotherapy arm and 10.0% of the placebo arm (p = 0.042). Three independent immune classification strata are recognized in sepsis. MALS and immunoparalysis are proposed as stratification for personalized adjuvant immunotherapy. Clinicaltrials.gov registration NCT03332225.
Keywords: macrophage activation, immunoparalysis, sepsis, ferritin, mortality, monocytes
Graphical abstract
Highlights
-
•
Immune classification of sepsis is done by ferritin and HLA-DR on monocytes
-
•
Macrophage activation-like syndrome is classified by ferritin levels >4,420 ng/mL
-
•
Immunoparalysis is classified by <5,000 HLA-DR receptors per CD14-monocyte
-
•
Anakinra treatment in macrophage activation-like syndrome improves 7-day outcomes
Leventogiannis et al. introduce immune classification of sepsis into macrophage activation-like syndrome, immunoparalysis, and intermediate state. Ferritin more than 4,420 ng/mL and HLA-DR less than 5,000 receptors on CD14-monocytes without hyperferritinemia are diagnostic tools of macrophage activation-like syndrome and immunoparalysis, respectively. Anakinra treatment provides short-term improvement of macrophage activation-like syndrome.
Introduction
Sepsis is defined as a life-threatening organ dysfunction due to the dysregulated response of the host to an infection.1 This definition, based on the results of extensive research conducted over the past three decades, aimed to pinpoint the importance of the dysregulation of the host immune response. Over the past two decades, large randomized clinical trials with immunomodulators in sepsis failed to show benefit.2 A major explanation for these failures is the significant heterogeneity in the clinical and immunological phenotypes of sepsis. The extremes of the immune dysregulation phenotypes in sepsis are a state of hyperinflammation and a state of immune paralysis, both deleterious to short- and long-term outcomes. If we can understand in the early phase of sepsis which patient is suffering from which type of immune dysregulation, we might be able to design personalized adjunctive immunotherapeutic approaches that might be more effective than standard immunotherapy.
The patients in a hyperinflammatory state have a high risk for death during the first 10 days. These patients have excess production of interleukin (IL)-1β by tissue macrophages, leading to pancytopenia, bone marrow hemophagocytosis, liver dysfunction, and disseminated intravascular coagulation (DIC), a complex of features named macrophage activation syndrome (MAS).3 Using the HS score4 and the criteria proposed by Shakoory et al.,5 we suggested that features of MAS may present in 3.7% to 4.3% of the total of patients with sepsis, an entity that we called macrophage activation-like syndrome (MALS). Concentrations of ferritin exceeding 4,420 ng/mL have specificity of 98.0% and negative predictive value of 97.2% for the diagnosis of MALS.6 The post hoc analysis of a large randomized clinical trial showed that the subgroup of patients with MALS experienced survival benefit when treated with the recombinant antagonist of the IL-1 receptor, anakinra).6
The other extreme are patients who present with immunoparalysis.7 The cells of the immune system are in an exhausted state, and this results in susceptibility for secondary infections, prolonged hospitalization, and increased mortality. The decrease of the expression of the human leukocyte antigen (HLA)-DR expression on the membrane of circulating monocytes has been put forward as the hallmark of immunoparalysis.8 Pilot studies have shown that this state can be reversed by recombinant human interferon-γ (rhIFNγ).9 Since a minority of patients present with immunoparalysis (probably not more than 25%–30%), it is useless and perhaps even dangerous to treat all sepsis patients with rhIFNγ.
PROVIDE (a Personalized Randomized trial Of Validation and restoration of Immune Dysfunction in severE infections and Sepsis) is a randomized phase II, investigator-initiated and sponsored, pilot clinical trial conducted with two main objectives: (1) to provide tools to classify the immune state of sepsis patients as MALS, intermediate, or immunoparalysis, and to investigate whether this classification reflects final outcome (as defined by survival); and (2) to assess whether anakinra or rhIFNγ in patients with MALS and immunoparalysis, respectively, beneficially influence the outcome of the patients.
Results
Patient population
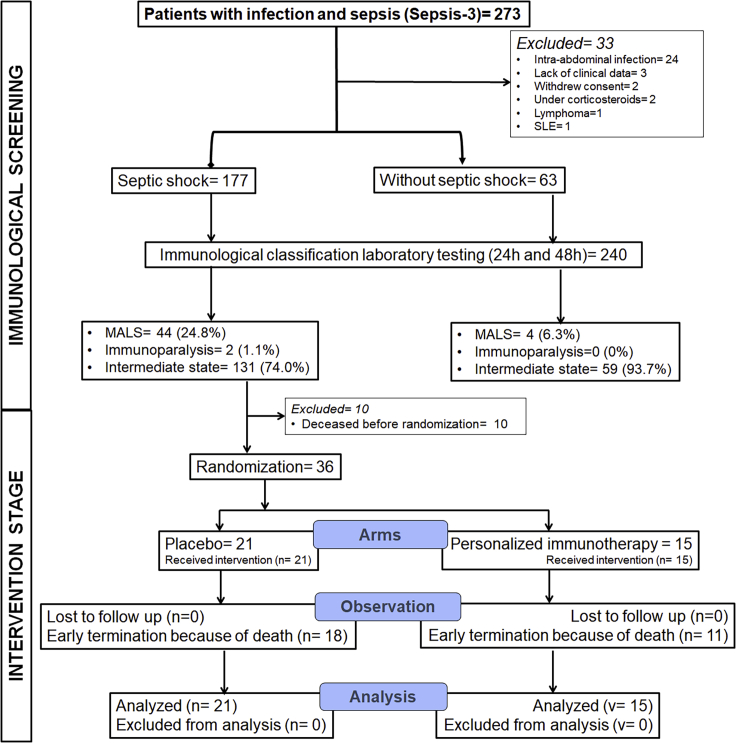
The flow chart of both stages of PROVIDE is presented in Figure 1. During the first stage of immunological screening, 240 patients were studied; 177 with septic shock and 63 patients without septic shock. Most of the patients were males. The most common infection leading to sepsis was community-acquired pneumonia; the most common comorbidities were chronic heart failure and chronic obstructive pulmonary disease (Table 1). Using ferritin and the expression of HLA-DR on CD14-monocytes, patients were classified into three states of immune function, namely MALS, sepsis-induced immunoparalysis, and intermediate state (Table 2). Among the 177 patients with septic shock, 46 met the inclusion criteria for the second interventional stage of PROVIDE. Eventually, 36 patients were randomized to the blind intervention since 10 patients died before randomization.
Figure 1.
Flow of patients among the two stages of the PROVIDE trial
The first stage of immunological screening was aiming to classify patients with septic shock and without septic shock into MALS (macrophage activation-like syndrome), immunoparalysis, and intermediate state. The second intervention stage was a double-blind, double-dummy randomized clinical trial where patients with septic shock and MALS or immunoparalysis received blind adjunctive treatment with placebo or personalized immunotherapy. SLE, systemic lupus erythematosus.
Table 1.
Patient characteristics according to immune classification
| Intermediate state (n = 89) | Immunoparalysis (n = 103) | MALS (n = 48) | p | |
|---|---|---|---|---|
| Age, years, mean ± SD | 71.7 ± 16.5 | 74.2 ± 12.5 | 70.8 ± 14.2 | 0.308∗ |
| Male gender, n (%) | 50 (56.2) | 63 (61.2) | 29 (60.4) | 0.767∗∗ |
| CCI, mean ± SD | 4.8 ± 2.3 | 5.2 ± 2.4 | 4.9 ± 2.3 | 0.398∗ |
| Septic shock, n, % | 55 (61.8) | 78 (75.7) | 44 (91.7) | 0.001∗∗ |
| ΑPACHE II score, mean ± SD | 21.1 ± 8.7 | 24.9 ± 8.5 | 29.5 ± 9.2 | <0.0001∗ |
| SOFA score, mean ± SD | 9.4 ± 4.0 | 11.0 ± 3.8 | 13.8 ± 3.5 | <0.0001∗ |
| CRP, mg/L, mean ± SD | 108.4 ± 105.7 | 108.6 ± 102.1 | 96.8 ± 104.8 | 0.843∗ |
| Type of infection, n (%) | ||||
| Community-acquired pneumonia | 40 (44.9) | 45 (43.7) | 19 (39.6) | 0.740∗∗ |
| Hospital-acquired pneumonia | 21 (23.5) | 21 (20.4) | 12 (25.0) | 0.407∗∗ |
| Ventilator-associated pneumonia | 8 (9.0) | 15 (14.6) | 5 (10.4) | 0.465∗∗ |
| Primary bacteremia | 13 (14.6) | 16 (15.5) | 10 (20.8) | 0.620∗∗ |
| Acute cholangitis | 7 (7.9) | 6 (5.8) | 2 (4.2) | 0.676∗∗ |
| Gram-negative pathogens, n (%) | 19 (21.3) | 23 (19.4) | 11 (22.9) | 0.825∗∗ |
| Pathogens, n (%) | ||||
| Escherichia coli | 1 (1.1) | 1 (0.9) | 1 (2.1) | 0.840∗∗ |
| Acinetobacter baumannii | 3 (3.3) | 7 (6.7) | 1 (2.1) | 0.338∗∗ |
| Klebsiella pneumoniae | 5 (5.6) | 2 (1.9) | 4 (8.4) | 0.182∗∗ |
| Staphylococcus aureus | 2 (2.2) | 6 (5.8) | 2 (4.2) | 0.465∗∗ |
| Candida albicans | 4 (4.5) | 2 (1.9) | 2 (4.2) | 0.578∗∗ |
| Main comorbidities, n (%) | ||||
| Type 2 diabetes mellitus | 27 (30.7) | 27 (26.2) | 16 (33.3) | 0.639∗∗ |
| Chronic heart failure | 17 (19.1) | 28 (27.2) | 17 (35.4) | 0.105∗∗ |
| Chronic renal disease | 9 (10.1) | 12 (11.7) | 5 (10.4) | 0.938∗∗ |
| Chronic obstructive pulmonary disease | 21 (23.6) | 25 (24.3) | 12 (25.0) | 0.983∗∗ |
| Administered antimicrobials, n (%) | ||||
| Piperacillin/tazobactam | 35 (42.7) | 37 (37.8) | 13 (27.7) | 0.236∗∗ |
| Carbapenems | 25 (30.5) | 34 (34.3) | 21 (44.7) | 0.261∗∗ |
| Tigecycline | 11 (13.4) | 19 (19.4) | 12 (25.5) | 0.223∗∗ |
| Colistin | 25 (30.5) | 34 (34.3) | 25 (53.2) | 0.029∗∗ |
| Vancomycin | 17 (20.7) | 24 (24.2) | 9 (19.1) | 0.690∗∗ |
∗Comparison using ANOVA test. ∗∗Comparison using Pearson chi-squared test.
APACHE, acute physiology and chronic health evaluation; CCI, Charlson’s comorbidity index; CRP, C-reactive protein; SD, standard deviation; SOFA, sequential organ failure assessment.
Table 2.
Classification of the immune states of sepsis used in the PROVIDE study
| Macrophage activation-like syndrome | Sepsis-induced immunoparalysis | Intermediate | |
|---|---|---|---|
| Ferritin | >4,420 ng/mL | ≤4,420 ng/mL | ≤4,420 ng/mL |
| Percentage of CD45/CD14- monocytes that express HLA-DR | All values | <30% | ≥30% |
First stage: Ferritin as classifier of MALS
During the first stage, it was found that the frequency of MALS was significantly higher in patients with septic shock (44 of 177 patients, 24.9%) than in patients without septic shock (4 of 63 patients, 6.3%, p = 0.001). When the entire cohort of 240 studied patients was divided into those with MALS (as diagnosed with ferritin >4,420 ng/mL) and into those without MALS (as diagnosed with ferritin ≤4,420 ng/mL), we found that liver dysfunction and disseminated intravascular coagulation were more common in MALS. More precisely, patients with MALS had lower absolute counts of platelets, higher international normalized ratio (INR), prolonged activated partial thromboplastin time (aPTT), and higher levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (Figure S1). These findings corroborate that ferritin levels above 4,420 ng/mL are consistent with the characteristics of MAS.
First stage: The number of HLA-DR receptors on CD14-monocytes classifies sepsis-induced immunoparalysis
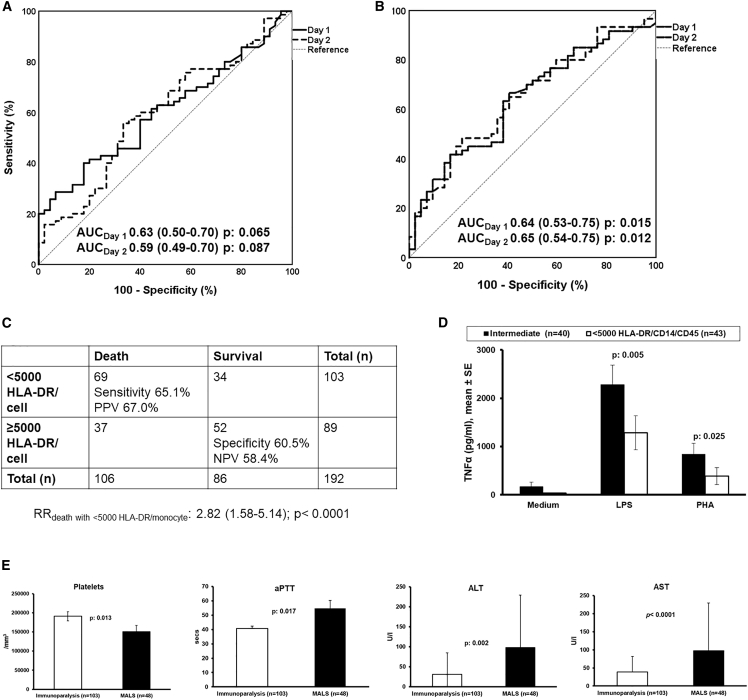
In this study, we used HLA-DR expression on CD14-monocytes less than 30% to classify patients with immunoparalysis as described in an earlier study.8 This cutoff of HLA-DR expression was associated with greater likelihood for death after 28 days. Surprisingly, only two patients had HLA-DR expression on CD14-monocytes less than 30% to become classified with immunoparalysis. This led us to conclude that the percentage of HLA-DR/CD14/CD45 is not an appropriate tool for the diagnosis of immunoparalysis. Since the expression of HLA-DR on CD14-monocytes is an indirect measure of the risk for 28-day mortality, we investigated for the cutoff of the percentage of HLA-DR/CD14/CD45 that is prognostic for death. We performed receiver operating characteristics (ROC) curve analysis of the screened population without considering patients with MALS. It was found that the prognostic performance of the percentage of HLA-DR/CD14/CD45 for 28-day mortality was poor (Figure 2A). In order to develop the number of HLA-DR receptors on CD14/CD45 monocytes as classifier for sepsis-induced immunoparalysis, we applied four criteria: (1) the association with mortality; (2) the compatibility with immune exhaustion of sepsis-induced immunoparalysis; (3) the association with patient deterioration10; and (4) the lack of signs of macrophage activation. For criterion (1) we repeated ROC curve analysis for the number of HLA-DR receptors on CD14/CD45 monocytes and the prognostic performance was substantially improved (Figure 2B). Using Youden index analysis, a cutoff of HLA-DR receptors less than 5,000/monocyte was found to be a better predictor of death after 28 days and it could be considered as diagnostic tool of immunoparalysis (Figure 2C). For criterion (2), we found that patients with less than 5,000 HLA-DR/monocyte had lower production capacity for tumor necrosis factor alpha (TNFα) by their peripheral blood mononuclear cells (PBMCs) (Figure 2D). For the criterion (3), we analyzed the incidence of multiple-organ dysfunction, which is an indirect measure of patient deterioration. This was 21.3% (19 patients) among patients with an intermediate immune state and 35.9% (37 patients) among patients with less than 5,000 HLA-DR receptors per CD14-monocyte (p = 0.038). For the criterion (4), we found that the laboratory results of impaired coagulation and liver dysfunction were less evident among patients with less than 5,000 HLA-DR/monocyte than among patients with MALS (Figure 2E). However, as this analysis was not available until the end of the trial, it was too late to be used for the real-time diagnosis of immunoparalysis and subsequent randomization for treatment with placebo/rhIFNγ during the trial. Using the cutoff of less than 5,000 HLA-DR/monocyte, allowed us to retrospectively identify 103 patients as suffering from immunoparalysis.
Figure 2.
Development of the number of HLA-DR receptors/monocyte as diagnostic tool for sepsis-induced immunoparalysis
(A and B) ROC curve (A) of percentage and (B) of number of HLA-DR receptors/monocyte of each day (day 1 and day 2) as a mortality predictor.
(C) Prognostic performance of <5,000 HLA-DR molecules/monocyte for 28-day mortality.
(D) Comparison the capacity ability of peripheral blood mononuclear cells for tumor necrosis factor alpha (TNF-α) production, following stimulation by endotoxin (LPS) and phytohemagglutinin (PHA), between patients with <5,000 HLA-DR molecules/monocyte and with an intermediate state (ferritin ≤4,420 ng/mL and ≥5,000 HLA-DR molecules/monocyte).
(E) Comparison of coagulation parameters and liver function enzyme levels between the immunoparalysis group and macrophage activation-like syndrome (MALS) group. The p values of the indicated comparisons are shown. Values are provided as means and standard error. ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartic aminotransferase; AUC, area under curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; RR, relative risk.
First stage: Intermediate immune state classification
Among patients diagnosed with neither MALS nor immunoparalysis, 21 (23.6%) had ferritin concentrations between 1,000 and 4,420 ng/mL, which is consistent with intense inflammatory reaction.11 Also 23 of these patients (25.9%) had HLA-DR receptors between 5,000 and 8,000/monocyte, which is consistent with partial dysfunction of the immune system.11 Therefore, these patients were classified to have an intermediate immune status.
First stage: Immune classification and 28-day outcome
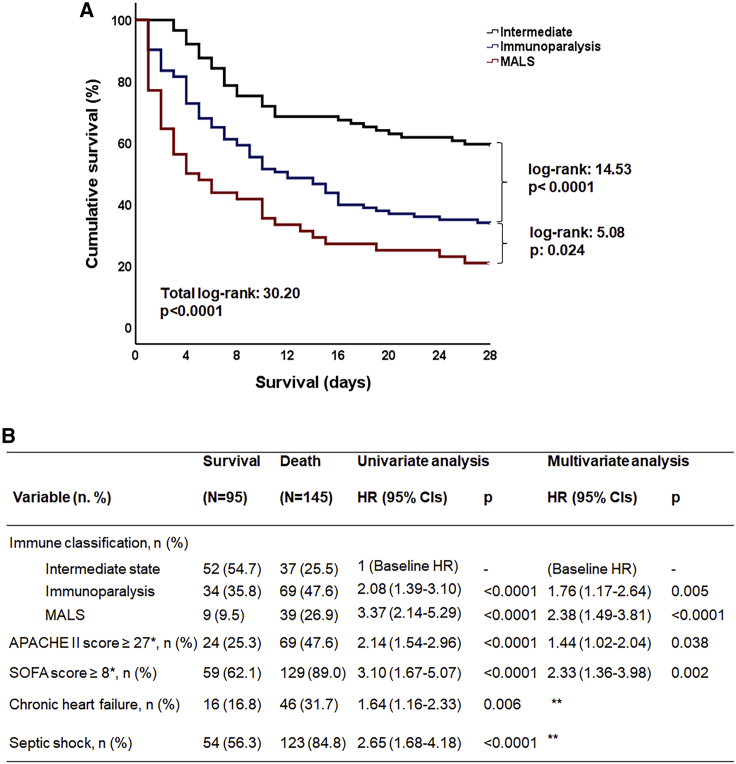
As a consequence, the enrolled 240 patients were classified into three groups of immune activation: MALS group (n = 48) (20.0%), immunoparalysis (n = 103) (42.9%), and intermediate immune state (n = 89) (37.1%). The repeatability of this classification of the first and second day of sampling was 88.5%. Comparison of the clinical characteristics of these three groups is presented in Table 1. Apart from larger values on severity scores and greater frequency of septic shock in MALS, no other differences were found. The classification groups differed significantly on 28-day mortality: mortality of the MALS group was 79.1% (95% confidence interval [CI] 65.7%–88.3%); of immunoparalysis group 66.9% (95% CI 56.4%–74.4%); and of the intermediate-state group 41.6% (95% CI 31.9%–51.9%) (Figure 3A). Multivariate analysis showed that this immune classification was an independent predictor of 28-day mortality (hazard ratio [HR]immunoparalysis versus intermediate 1.76; 95% CI 1.17–2.64; and HRMALS versus intermediate 2.38; 95% CI 1.49–3.81) regardless of severity scores and presence of septic shock (Figure 3B). It is important to state that most of the administered antimicrobials were similar in all classification groups, therefore difference on survival is not attributable to differences in medical treatment. However, the percentage of patients with MALS treated with colistin was somewhat greater than the other groups. Multivariate Cox regression analysis of survival was repeated using colistin among covariates. The inclusion of colistin did not change the significance of the independent impact of MALS and sepsis-induced immunoparalysis on 28-day outcome (Table S1).
Figure 3.
Association of immune classification of sepsis and 28-day mortality
(A) Survival curves of each classification group: macrophage activation-like syndrome (MALS), immunoparalysis, and intermediate state. Log rank values and p values are presented.
(Β) Twenty-eight-day mortality-related risk factors after univariate and multivariate Cox regression analysis. Hazard ratios (HRs), 95% confidence intervals (CIs), and p values are presented. APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment. ∗Values represent Youden Index of ROC curves for mortality. ∗∗Not entering the equation after four steps of forward analysis.
Second stage: Interventional trial
As presented in Figure 1, 36 patients were enrolled to the double-blind, double-dummy randomized clinical stage of PROVIDE and were randomized to administration of personalized immunotherapy (n = 15) or of placebo (n = 21). The first patient was randomized on 2 December 2017 and the last on 1 December 2019. By December 2019, it was decided to prematurely stop the study because of the slow enrollment of patients. The slow rate of enrollment was because the 30% cutoff of HLA-DR/CD14/CD45 used as a tool of enrollment in the study for patients with immunoparalysis was inappropriate, as discussed above. Of the two patients classified as immunoparalysis, one was randomized to receive rhIFNγ and one to receive placebo (Table 3).
Table 3.
Clinical characteristics of treatment groups
| Placebo (n = 21) | Personalized immunotherapy (n = 15) | p | |
|---|---|---|---|
| Age, years, mean ± SD | 69.7 ± 10.8 | 70.6 ± 15.1 | 0.838∗ |
| Male gender, n (%) | 12 (57.1) | 10 (66.7) | 0.732∗∗ |
| CCI, mean ± SD | 4.3 ± 2.2 | 5.7 ± 2.2 | 0.062∗ |
| ΑPACHE II score, mean ± SD | 18.2 ± 8.7 | 30.5 ± 9.4 | 0.376∗ |
| SOFA score, mean ± SD | 14.5 ± 2.8 | 14.3 ± 3.1 | 0.843∗ |
| Type of infection, n (%) | |||
| Community-acquired pneumonia | 10 (47.6) | 2 (13.3) | 0.040∗∗ |
| Hospital-acquired pneumonia | 6 (28.6) | 8 (58.3) | 0.175∗∗ |
| Ventilator-associated pneumonia | 3 (14.3) | 3 (20.0) | 0.677 |
| Other | 2 (13.3) | 2 (13.3) | 1.00 |
| Main comorbidities, n (%) | |||
| Type 2 diabetes mellitus | 6 (28.6) | 6 (40.0) | 0.499∗∗ |
| Chronic heart failure | 4 (19.0) | 8 (53.3) | 0.071∗∗ |
| Chronic renal disease | 0 (0) | 4 (26.6) | 0.023∗∗ |
| Chronic obstructive pulmonary disease | 7 (33.3) | 3 (20.0) | 0.468∗∗ |
| Administered antimicrobials, n (%) | |||
| Piperacillin/tazobactam | 3 (14.3) | 2 (13.3) | 1.00∗∗ |
| Carbapenems | 10 (47.6) | 12 (80.0) | 0.083∗ |
| Tigecycline | 6 (28.6) | 3 (20.0) | 0.705∗∗ |
| Colistin | 13 (61.9) | 10 (66.7) | 1.00∗ |
| Vancomycin | 3 (14.3) | 4 (26.7) | 0.418∗∗ |
| Antimicrobial treatment according to ESCMID guidelines | 18 (85.7) | 12 (80.0) | 0.677∗∗ |
| Hydrocortisone replacement | 19 (90.5) | 10 (80.0) | 0.639∗∗ |
∗Comparison by the Student’s t test. ∗∗Comparison by Fisher’s exact test.
APACHE, acute physiology and chronic health evaluation; CCI, Charlson’s comorbidity index; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; SD, standard deviation; SOFA, sequential organ failure assessment.
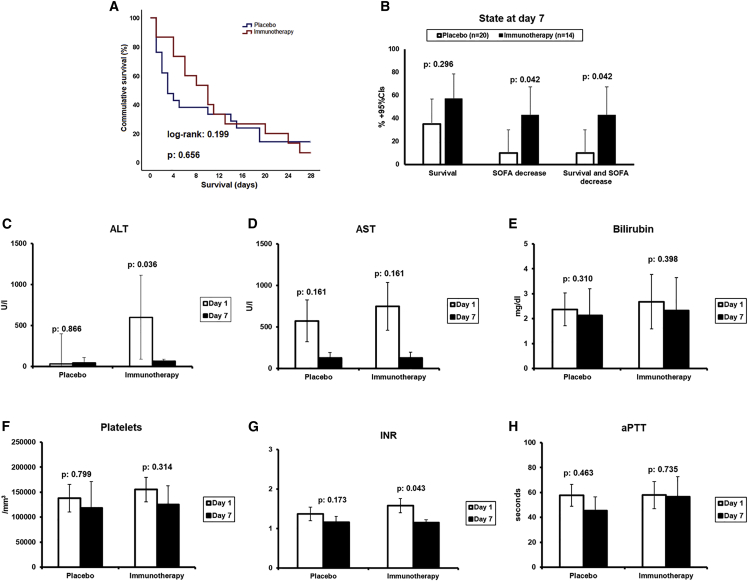
The 28-day survival did not differ between groups (Figure 4A): 18 deaths occurred in the placebo arm (28-day mortality 85.7%; 95% CI 65.4%–95.1%); and 14 deaths in the personalized immunotherapy arm (28-day mortality 93.3%; 95% CI 70.2%–98.8%). Mortality after 90 days was the same. No difference was found between the two groups on the incidence of secondary infections.
Figure 4.
Effect of personalized immunotherapy
(A) Survival curves of both treatment arms. Log rank value and p value are presented.
(B) State of patients at day 7; patients are presented according to the survival status, decrease of SOFA score from baseline, or both.
(C–H) Comparison of alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, absolute platelet count, international normalized ratio (INR), and activated partial thromboplastin time (aPTT) between days 1 and 7 for each treatment arms; p values of indicated statistical comparison are presented. CI, confidence interval; SOFA, sequential organ failure assessment.
The exploratory endpoint of the study was survival associated with SOFA decrease at the end of treatment. Since only one patient was treated with rhIFNγ, we limited the analysis to the patients treated with anakinra. By day 7 (the end of anakinra treatment), two patients allocated to placebo were alive with decrease of baseline SOFA (10%) compared with six (42.9%) of anakinra-treated patients (Figure 4B). On the same day 7, there was a significant reduction of ALT and of INR, the hallmark of MALS but not of AST, bilirubin, absolute platelet count, and aPTT (Figures 4C and 4H). This signifies a positive treatment effect of anakinra in these patients with MALS. However, this was not translated in improved mortality by day 28, most likely because anakinra treatment was stopped prematurely on day 7, when patients still had important hyperinflammatory features. Follow-up measurements of ferritin and of the expression of HLA-DR/CD14/CD45 did not differ between the placebo arm and the personalized immunotherapy arm (Figure S2).
There was no difference in the incidence of adverse events between the two arms of the study (Table S2).
Discussion
In this investigator-initiated pilot study, we were able to demonstrate that sepsis patients could be distinguished into three groups based on their immunological phenotype: a group with MALS (identified by serum ferritin >4,420 ng/mL), an intermediate group without extreme immune dysregulation, and an immunoparalysis group (identified by <5,000 HLA-DR/monocyte). This distinction allowed us to prospectively study personalized immunotherapy in a randomized double-blind trial.
In those patients with MALS who were randomized to receive anakinra, we found that their SOFA score, ALT, and INR improved during the first week of treatment. However, the decrease in SOFA was not translated into survival benefit, probably because of the need for more prolonged treatment. The reason for the lack of long-term benefit of anakinra is most likely the premature discontinuation of treatment, at a moment that the hyperinflammatory state was still present. In this respect, it is important to note that ferritin concentrations were still significantly increased when anakinra was stopped. These data strongly argue for a future approach in which anakinra in given for longer periods of time, preferably until normalization of inflammatory markers.
The concept that anakinra might be of benefit in patients with MALS came from a retrospective analysis of a phase III clinical trial of the efficacy of intravenous anakinra in severe sepsis.12 This trial had failed to show a survival benefit, but post hoc analysis showed 30% reduction of 28-day mortality in the small subgroup of 43 patients with liver dysfunction and DIC.5 Although serum ferritin concentrations were not known, this group of patients most likely suffered from MALS. In that study, anakinra was administered for 3 days only but at a considerably higher dose, i.e., 2 mg/kg per hour in a continuous infusion, which would mean for a 70-kg person, a daily dose of 3,360 mg per day; in the PROVIDE study we used a fixed dose of 600 mg per day. As mentioned above, based on our findings that the hyperinflammatory response has not subsided after 7 days, we argue for a prolonged anakinra treatment and perhaps a higher dose in future trials.
At the other end of the spectrum of immune dysregulation of sepsis, we identify immunoparalysis, responsible for the later infectious complications and deterioration in the sepsis patients. Our measurements of absolute number of HLA-DR molecules on monocytes showed that immunoparalysis is present in 42.9% of patients from sepsis onset and that it occurs in sepsis patients with or without septic shock, being associated with a high mortality rate. As immunostimulation is an attractive treatment strategy for immunoparalysis,13 we included an rhIFNγ arm in the PROVIDE trial. This was based on the results of a small open-label non-randomized clinical trial, in which rhIFNγ treatment was administered in nine patients with septic shock. Reversal of immunoparalysis was achieved in all nine patients, with increase of expression of HLA-DR molecules on monocytes alongside ex vivo increase of TNFα production following stimulation by LPS. In that study, clinical resolution occurred in eight patients.14 Additional support for this treatment came from a study in six human healthy volunteers in whom immunoparalysis was induced with bacterial endotoxin; these subjects received rhIFNγ subcutaneously or placebo. In the volunteers receiving rhIFNγ, significant increase of HLA-DR expression on monocytes was observed.9
The original marker chosen to diagnose immunoparalysis, i.e., less than 30% expression of HLA-DR on CD14-monocytes, failed to identify patients with immune paralysis and poor outcome. As a result, very few patients were classified as suffering from immunoparalysis and hence eligible to be randomized to rhIFNγ treatment or matched placebo. In contrast, an explorative biomarker for immunoparalysis investigated in the study, the total number of HLA-DR molecules on monocytes (as assessed by Quantibrite), offers a better alternative. Patients with less than 5,000 HAL-DR receptors on CD14-monocytes are meeting all four applied criteria for classification of immunoparalysis, i.e., greater risk for 28-day mortality, association with immune exhaustion of PBMCs, greater risk for progression into multiple-organ dysfunction, and absence of signs of MAS. In a recent large prospective study of 592 patients in the intensive care unit, it was found that the trajectory toward decrease of the absolute count of HLA-DR receptors on CD14-monocytes is associated with greater likelihood for secondary infections, with the authors using a decrease of less than 8,000 HLA-DR receptors per CD14-cell.15 While this cutoff is higher than the one coming from the analysis of the PROVIDE trial, treatment with rhIFNγ in 13 patients with secondary infections and immunoparalysis led to the HLA-DR increase above 8,000/cell in nine of 13 patients and SOFA score decrease in 10 of 13 patients.16
A pertinent question is how the immunological stratification used in this study relates to other immune endotypes described in the literature. Endotypes have been defined as a compilation of disease mechanisms that explain disease expression in groups of patients.17 Two recent studies have proposed endotypes of sepsis that are associated with unfavorable outcome. The first study is GAiNS (UK Genomic Advances in Sepsis), which analyzed 384 patients with sepsis due to community-acquired pneumonia divided into one discovery cohort (n = 270) and one validation cohort (n = 114). Two transcriptomic sepsis-response signatures (SRS) were identified; SRS1 and SRS2. Gene expression of DYRK2, CCNB1IP1, TDRD9, ZAP70, ARL14EP, MDC1, and ADGRE3 is driving the classification into SRS1, which is characterized by downregulation of HLA-DR and T cell activation. Patients with the SRS1 have worse outcome than SRS2 in both the discovery and validation cohorts and this is independent of age, gender, and the type of pathogen.18 Recently Scicluna et al. reported four endotypes in sepsis based on transcriptomic profiles.19 These authors studied two cohorts; one discovery cohort with 306 patients and a first validation cohort with 216 patients both recruited in the context of the MARS (Molecular Diagnosis and Risk Stratification of Sepsis) study. A second validation cohort with 265 patients from the GAiNS study was also studied. Patients were classified into four endotypes, namely Mars1 to Mars 4, using the immune pathways they characterized. Mars1 endotype was characterized by pronounced decrease of immune function, especially antigen presentation and T cell function; these patients had the worse outcome in all cohorts. The authors of the study elaborate the need to identify single gene expressions as easier-to-use biomarkers for the rapid diagnosis of the endotype of each patient.19 In the PROVIDE study, we do not have transcriptome data in all the patients in order to compare our classification to the transcriptional endotypes, but one could hypothesize that our immunoparalysis immunotype would at least partially overlap with the Mars1 endotype. Future studies are needed to explore this.
PROVIDE defines a rapid classification of sepsis from an immunological standpoint so that MALS and immunoparalysis may be the basis of an algorithm of personalized immunotherapy. In our study, anakinra treatment was promising, as it showed a therapeutic effect during treatment; this, however, needs optimization. Based on the present study, a new randomized multicenter trial of personalized immunotherapy is currently on-going (EudraCT number 2020-005768-74; Clinicaltrials.gov NCT04990232). The lessons learned from PROVIDE (longer anakinra treatment, novel biomarker of immunoparalysis based on the number of HLA-DR molecules per monocyte) are crucial for an improved chance of success in future immunotherapy trials in sepsis.
Limitations of the study
This pilot study, which was investigator-initiated, has several shortcomings. First, because of the unreliable assessment of immunoparalysis, we were unable to enroll more than a few patients in the rhIFNγ arm. Second, the treatment with anakinra has been most likely stopped prematurely, which led to the loss of therapeutic benefit after the end of the treatment. Third, treatment with anakinra was started only on day 2 after diagnosis, due to the necessity to confirm on two consecutive days the high ferritin concentrations. This led to a late administration of the drug, with a high mortality in the first day after inclusion. As the correlation between ferritin concentrations in the patients was very good, we argue for a swift initiation of therapy in future trials.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD14-FITC (RMO52 clone) | Beckman Coulter | Cat# IM0645U; RRID: AB_130992 |
| CD45-PC5 (J33 clone) | Beckman Coulter | Cat# IM2653U; RRID: AB_10641226 |
| HLA-DR-RD1 (9-49 clone) | Beckman Coulter | Cat# 6604366; RRID: AB_2832962 |
| IgG1-PE isotype control (679.1Mc7 clone) | Beckman Coulter | Cat# A07796; RRID: AB_2832963 |
| IgG1-FITC isotype control (679.1Mc7 clone) | Beckman Coulter | Cat# A07795; RRID: AB_2832964 |
| Quantibrite Anti-HLA-DR/Anti-Monocyte (L243/MφP9) | BD Biosciences | Cat# 340827; RRID: AB_400137 |
| Mouse Anti-Human IL-6-PE (AS12 clone) | BD Biosciences | Cat# 340527; RRID: AB_400442 |
| Chemicals, peptides, and recombinant proteins | ||
| VersaLyse Lysing Solution | Beckman Coulter | A09777 |
| Fixative Solution | Beckman Coulter | A07800 |
| RPMI 1640 W/Stable Glutamine W/25 MM HEPES | SigmaAldrich | R0833 |
| Lymphosep, Lymphocyte Separation Media | Biowest | L0560 |
| PBS Dulbecco’s Phosphate Buffered Saline w/o Magnesium, w/o Calcium | Biowest | L0615 |
| FBS Superior; standardized Fetal Bovine Serum, EU-approved | Biochrom | S0615 |
| Gentamycin Sulfate BioChemica | PanReac AppliChem | A1492 |
| Penicillin G Potassium Salt BioChemica | PanReac AppliChem | A1837 |
| Lipopolysaccharides from Escherichia coli O 55:B5 | Sigma-Aldrich | L2880 |
| Phytohaemagglutinin from Phaseolus vulgaris | Roche | SKU 11249738001 |
| Critical commercial assays | ||
| Human TNFa uncoated ELISA | Invitrogen | 88–7346 |
| Human Ferritin ELISA | ORGENTEC Diagnostika GmbH | ORG 5FE |
| Software and algorithms | ||
| SPSS | IBM | https://www.ibm.com/analytics/spss-statistics-software |
| Other | ||
| Kineret | Swedish Orphan Biovitrum | https://www.ema.europa.eu/en/documents/product-information/kineret-epar-product-information_en.pdf |
| Immukine | Boehringer Ingelheim | https://www.ema.europa.eu/en/documents/psusa/interferon-gamma-list-nationally-authorised-medicinal-products-psusa/00001760/201601_en.pdf |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Evangelos J. Giamarellos-Bourboulis (egiamarel@med.uoa.gr).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
PROVIDE is a double-blind, double-dummy randomized clinical trial that took place between November 2017 and December 2019 in 14 study sites in Greece (nine Intensive Care Units and five departments of Internal Medicine) (EudraCT 2017-002171-26; approval by the National Ethics Committee of Greece 78/17; approval IS 75–17 by the National Organization for Medicines of Greece; Clinicaltrials.gov registration NCT03332225). Participants were adults (age ≥18 years) of either gender with lower respiratory tract infection (community-acquired pneumonia or hospital-acquired pneumonia or ventilator-associated pneumonia) or primary bacteremia or acute cholangitis, and sepsis. Infections were defined according to international criteria (Table S3).20 Sepsis was defined as total Sequential Organ Failure Assessment (SOFA) score 2 or more for new admissions or as 2 or more point-increase of the total SOFA score for hospitalized patients. Septic shock was defined as persisting mean arterial pressure below 65mmHg despite adequate intravenous administration fluids with plasma lactate greater than 2 mmol/L necessitating treatment with vasopressors. Exclusion criteria were: other type of infections, stage IV malignancies, HIV infection, primary immunodeficiency, chronic intake of corticosteroids defined as total daily dose equal or greater than 0.4 mg/kg of equivalent prednisone for more than the last 15 days, intake of anti-cytokine biological agents, history of systemic lupus erythematosus, history of demyelinating disorders, pregnancy, lactation and decision of no resuscitation. For all patients written Informed Consent Form (ICF) was provided by themselves or by their legal representatives.
Method details
Study design
PROVIDE consisted of one screening/observation stage and one intervention stage. During the screening/observation stage there was a daily recording of the following variables: type of infection, co-morbidities, total blood cell count, coagulation tests, renal and hepatic biochemistry, arterial blood gases, culture results of blood, urine and tracheo-bronchial secretions, imaging results and overall survival. Each patient had a blood draw of 10mL via a peripheral vein within the first 24 h from study inclusion (day 1) which was repeated after 24 h (day 2). Samples were collected in EDTA-containing and clean vials and transferred via courier to the central laboratory. Ferritin was measured in serum by an enzyme immunosorbent assay (ORGENTEC Diagnostika GmbH, Mainz, Germany; lower detection limit 75 ng/mL). Using whole blood from the EDTA vial, white blood cells were incubated for 15 min in the dark with the following monoclonal antibodies: anti-CD14 FITC, anti-HLA-DR-PE, anti-CD45 PC5 (Immunotech, Marseille, France) and Quantibrite HLA-DR/anti-monocyte PerCP-Cy5 (Becton Dickinson, Cockeysville Md). After incubation, cells were analyzed by flow cytometer FC500 versus cells stained with anti-CD45 PC5 and anti-idiotypic IgG1. HLA-DR molecules on CD14/CD45 monocytes were expressed as a percentage of positive stained cells and as number of molecules per cell.
MALS was defined as ferritin more than 4420 ng/mL on both samples or on day 2 sample, regardless of flow cytometry results.6 Immunoparalysis was diagnosed as expression of HLA-DR/CD14/CD45 less than 30% of both days or on the sample of day 2 provided that ferritin concentration was ≤4420 ng/ml8. Patients who could not be classified as MALS or immunoparalysis were considered having an intermediate immune state.
Additionally, peripheral blood mononuclear cells (PBMCs) were isolated from each EDTA blood sample using gradient centrifugation over Ficoll-Hypaque density gradient. After three washings in ice-cold PBS (phosphate buffered saline, pH: 7,2), PBMCs were counted using a Neubauer plate with trypan blue exclusion of dead cells and distributed on 96-wells plate at final volume 200 μL and density 2.5 × 106/mL. PBMCs were incubated in RPMI 1640 (SigmaAldrich, Berlin, Germany) enriched with 2 mM of L-glutamine, 100 U/mL penicillin G, 0.1 mg/mL streptomycin without/with 10 ng/mL lipopolysaccharide (LPS) of Escherichia coli O55:B5 or 5 μg/mL phytohaemagglutinin (PHA). After incubation for 48 h at 37°C and 5% CO2 the supernatants were collected and tumor necrosis factor alpha (TNF-α) concentration was measured in duplicate using an enzyme immunosorbent assay (Invitrogen Waltham, Massachusetts; lower detection limit 20 pg/mL).
Intervention stage
Patients diagnosed with septic shock and MALS or immunoparalysis were allowed to participate in the intervention stage of the randomized clinical trial. They were randomized into adjunctive treatment either with personalized immunotherapy or placebo. If classified as MALS, patients were randomized to anakinra (Kineret, Swedish Orphan Biovitrum, Athens) 200 mg intravenously (iv) in 20mL saline every 8 h (q8h) for 7 days and placebo consisting of 0.5mL of NaCl 0.9% subcutaneously (sc) every 48 h (q48h) for 15 days; if classified with immunoparalysis they were administered 20mL of NaCl 0.9% iv q8h for 7 days or 100μg rhIFNγ (Immukine, Boehringer Ingelheim, Athens) at 0.5 mL sc q48h for 15 days. Patients randomized to placebo arm were administered NaCl 0.9% iv and sc as described above. The above dose of anakinra was adjusted to 100 mg q8h when creatinine clearance was less than 30 mL/min. Adverse events were daily captured. A separate computer-generated allocation sequence was prepared for each study site and preparation of the study drug was done by an unblinded pharmacist. Blood draw for measurements of ferritin and of the expression of HLA-DR/CD14/CD45 were repeated before start of the study drug and on days 4 and 7.
The endpoint of the screening/observation stage was the association of the immunological endotype with the 28-day survival outcome. The primary study endpoint of the intervention stage was the effect of personalized immunotherapy on 28-day mortality. Secondary endpoints were 90-day mortality, secondary infections and the appropriateness of immune classification using the total of screened patients from the original screening stage. Exploratory endpoint was survival with decrease of SOFA score at the end of treatment. Using the hypothesis that immunotherapy would decrease mortality from MALS from 60% to 35% and mortality from immunoparalysis from 50% to 35%, it was calculated that 278 patients should be enrolled to demonstrate these differences with 80% power at the 10% level of significance.
Quantification and statistical analysis
Qualitative variables were expressed as percentages and 95% confidence intervals (CIs) and compared by the Fisher exact test. Quantitative variables were expressed as mean ± SD (standard deviation) or SE (standard error) and compared using the Student’s t-test or the Mann Whitney U test. Paired comparisons were done by the Wilcoxon test. The prognostic output of the percentage and of absolute counts of HLA-DR molecules on monocytes for 28-day mortality was studied by ROC curve analysis. The co-ordinates showing the highest sensitivity and specificity (Youden index) were selected. Multivariate stepwise Cox regression analysis was used to validate if immunological classification was an independent factor of unfavorable outcome; hazard ratio (HR) and 95% confidence intervals (CIs) were calculated. Any p value lower than 0.05 was considered statistically significant.
Additional resources
The PROVIDE trial is registered at EudraCT 2017-002171-26 and at Clinicaltrials.gov registration NCT03332225.
Acknowledgments
The study was supported in part by the Hellenic Institute for the Study of Sepsis, in part by bioMérieux, Lyon, France, and in part by a Spinoza grant of the Netherlands Organisation for Scientific Research (to M.G.N.).
Author contributions
K.L., E.K., N.A., and A.K. collected the data and generated the study database, drafted the manuscript for content, and approved the version to be submitted. I.T., D.M., I.G., N.R., V.T., E.A., I.K., G.D., G.V., K.A., V.K., A.K., T.K., A.P., A.K., and G.D. participated in patient enrollment, drafted the manuscript for content, and approved the version to be submitted. I.G. participated in lab analysis, drafted the manuscript for content, and approved the version to be submitted. J.W.M.V.D.M. participated in study design, drafted the manuscript for content, and approved the version to be submitted. M.K. analyzed the data, drafted the manuscript for content, and approved the version to be submitted. M.G.N. and E.J.G.-B. designed the study, analyzed the data, wrote the manuscript, and approved the version to be submitted.
Declaration of interests
G.D. has acted as Advisor/Lecturer for Abbvie, Bristol-Myers Squibb, Gilead, Novartis, Roche, Amgen, MSD, Janssen, Ipsen, Genkyotex, Sobi, and Pfizer; has received grant support from Bristol-Myers Squib, Gilead, Roche, Janssen, Abbvie, and Bayer; and was or is currently principal investigator in national and international protocols sponsored by Abbvie, Bristol-Myers Squibb, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics Inc, Tiziana Life Sciences, Bayer, Astellas, Ipsen, Pfizer, Amyndas Pharamaceuticals, CymaBay Therapeutics Inc., and Roche.
M.G.N. is supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. He is a scientific founder of TTxD and has received independent educational grants from TTxD, GSK, Ono Pharma, and ViiV HealthCare.
E.J.G.-B. has received honoraria from Abbott CH, bioMérieux, ThermoFisherBrahms GmbH, GSK, InflaRx GmbH, Sobi, and XBiotech Inc; independent educational grants from Abbott CH, AxisShield, bioMérieux Inc, InflaRx GmbH, Johnson & Johnson, MSD, Sobi, and XBiotech Inc.; and funding from the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), the Horizon 2020 European Grants ImmunoSep and RISCinCOVID (granted to the Hellenic Institute for the Study of Sepsis), and the Horizon Health European Grant EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis).
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: November 15, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100817.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., Kumar A., Sevransky J.E., Sprung C.L., Nunnally M.E., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 3.Minoia F., Davì S., Horne A., Demirkaya E., Bovis F., Li C., Lehmberg K., Weitzman S., Insalaco A., Wouters C., et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66:3160–3169. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 4.Fardet L., Galicier L., Lambotte O., Marzac C., Aumont C., Chahwan D., Coppo P., Hejblum G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 5.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A., Cron R.Q., Opal S.M. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyriazopoulou E., Leventogiannis K., Norrby-Teglund A., Dimopoulos G., Pantazi A., Orfanos S.E., Rovina N., Tsangaris I., Gkavogianni T., Botsa E., et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017;15:172. doi: 10.1186/s12916-017-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss R.S., Monneret G., Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monneret G., Lepape A., Voirin N., Bohé J., Venet F., Debard A.L., Thizy H., Bienvenu J., Gueyffier F., Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 9.Leentjens J., Kox M., Koch R.M., Preijers F., Joosten L.A.B., van der Hoeven J.G., Netea M.G., Pickkers P. Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. Am. J. Respir. Crit. Care Med. 2012;186:838–845. doi: 10.1164/rccm.201204-0645OC. [DOI] [PubMed] [Google Scholar]
- 10.Venet F., Tissot S., Debard A.L., Faudot C., Crampé C., Pachot A., Ayala A., Monneret G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: correlation with severity and secondary septic shock. Crit. Care Med. 2007;35:1910–1917. doi: 10.1097/01.CCM.0000275271.77350.B6. [DOI] [PubMed] [Google Scholar]
- 11.Rosário C., Zandman-Goddard G., Meyron-Holtz E.G., D'Cruz D.P., Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opal S.M., Fisher C.J., Jr., Dhainaut J.F., Vincent J.L., Brase R., Lowry S.F., Sadoff J.C., Slotman G.J., Levy H., Balk R.A., et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit. Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Allantaz-Frager F., Turrel-Davin F., Venet F., Monnin C., de Saint Jean A., Barbalat V., Cerrato E., Pachot A., Lepape A., Monneret G. Identification of biomarkers of response to IFNg during endotoxin tolerance: application to septic shock. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Döcke W.D., Randow F., Syrbe U., Krausch D., Asadullah K., Reinke P., Volk H.D., Kox W. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nat. Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 15.De Roquetaillade C., Dupuis C., Faivre V., Lukaszewicz A.C., Brumpt C., Payen D. Monitoring of circulating monocyte HLA-DR expression in a large cohort of intensive care patients: relation with secondary infections. Ann. Intensive Care. 2022;12:39. doi: 10.1186/s13613-022-01010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payen D., Faivre V., Miatello J., Leentjens J., Brumpt C., Tissières P., Dupuis C., Pickkers P., Lukaszewicz A.C. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect. Dis. 2019;19:931. doi: 10.1186/s12879-019-4526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agache I., Akdis C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Invest. 2019;129:1493–1503. doi: 10.1172/JCI124611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport E.E., Burnham K.L., Radhakrishnan J., Humburg P., Hutton P., Mills T.C., Rautanen A., Gordon A.C., Garrard C., Hill A.V.S., et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir. Med. 2016;4:259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scicluna B.P., van Vught L.A., Zwinderman A.H., Wiewel M.A., Davenport E.E., Burnham K.L., Nürnberg P., Schultz M.J., Horn J., Cremer O.L., et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir. Med. 2017;5:816–826. doi: 10.1016/S2213-2600(17)30294-1. [DOI] [PubMed] [Google Scholar]
- 20.Calandra T., Cohen J., International Sepsis Forum Definition of Infection in the ICU Consensus Conference The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit. Care Med. 2005;33:1538–1548. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request