Abstract
Anaplastic thyroid cancer (ATC) is a rare but lethal thyroid cancer. Dabrafenib and trametinib has been the standard treatment for the patients with BRAF mutation based on phase II study. This study aimed to exam the impact of dabrafenib and trametinib in ATC patients. ATC patients treated in three institutes in Taiwan were retrospectively reviewed. The clinical features, BRAF status, and survivals were collected. Multivariate analysis was performed to determine the independent prognostic factors. A total of 44 ATC patients were enrolled in current study. Twelve (50%) out of 24 detected patients had BRAF V600E mutation and eleven received dabrafenib and trametinib treatment. Patients treated with dabrafenib and trametinib had longer overall survival (OS) than the patients without treatment with dabrafenib and trametinib (median OS: 10.4 months vs. 3.3 months, P=0.05). The objective response rate was 81.8% and progress-free survival was 7.4 months. Multivariate analysis identified prior surgery, treatment with dabrafenib and trametinib and metastasis to lung, brain, and bone were significant prognostic factors for OS. The benefit of prior surgery was significant in patients receiving dabrafenib and trametinib (P=0.017) rather than those without dabrafenib and trametinib (P=0.067). The current study provides the real-world evidence that targeted therapy with dabrafenib and trametinib was effective and significantly improved the OS for ATC patients. The role of prior surgery became important in the era of targeted therapy. Future studies should focus on resistance mechanisms and combination with immunotherapy for ATC patients.
Keywords: Anaplastic thyroid cancer, BRAF mutation, dabrafenib, trametinib, surgery
Introduction
Thyroid cancer accounts for about 3% of all malignant cancers [1], and anaplastic thyroid cancer (ATC) is even rare. ATC only accounted from 1.3% to 9.8% of all thyroid cancers based on various studies in different areas [2], but it is the most lethal thyroid-derived tumor with a disease-specific mortality of approaching 100 percent. The median overall survival of all ATC is only 4-6 months, and one-year survival rate is only 44% even in resectable disease [3]. Previous studies worked hard on improving the survival, by improving surgical techniques, radiotherapy design, and development of medications [4-6], however, the treatment outcomes of ATC remained unsatisfied. In addition, several prognostic factors were also identified including age, leukocytosis, acute symptoms, disease stage, tumor size, prior surgery, prior radiotherapy, and chemotherapy in previous studies [4,7,8].
BRAF mutation was commonly found in thyroid carcinoma, and the prevalence of BRAF mutation was reported to be 35-40% in ATC [9]. BRAF mutation activates the mitogen-activated protein (MAP) kinase pathway including downstream proteins called MEK and ERK. The activation of ERK finally leads to cell proliferation and oncogenesis of thyroid tumor [10]. Dabrafenib and trametinib are tyrosine-kinase inhibitors (TKIs) against BRAF V600E and MEK1/2 respectively. Combined use of both drugs has demonstrated promising effects on several cancers with BRAF mutation, including melanoma [11], lung cancer [12], and ATC [13]. The use of dabrafenib and trametinib in ATC was approved based on a phase 2 study with single arm [13], however, the impact of this combination on clinical practice had not been well evaluated. Our study aimed to retrospectively analyze the impact of dabrafenib and trametinib for BRAF mutated ATC in real world practice and the prognosis factors of ATC with or without this combination of dabrafenib and trametinib.
Materials and methods
Patients diagnosed of ATC from 2000 to 2020 were enrolled in three hospitals in Taiwan (Linkou Chang Gung Memorial Hospital, National Taiwan University Hospital, and National Taiwan University Hospital Hsin-Chu Branch). The diagnosis was made by either surgical resected tissue or core-needle biopsy. Patients’ characteristics including age, gender, disease stage, metastatic sites, treatments (such as prior surgery, prior radiotherapy, etc.), and survival status were retrospectively collected for analysis of clinical prognostic factors. Prior surgery and radiotherapy indicated local treatment performed before starting targeted therapy of dabrafenib and tremetinib.
The mutations of BRAF V600E were detected by either immunohistochemical stain (IHC) using VE1 antibody, polymerase chain reaction (PCR), and/or next-general sequencing (NGS). The patients may undertake more than one test.
Progression free survival (PFS) was defined from the date of receiving dabrafenib and tremetinib to the date of death or disease progression evaluated by image study. Overall survival (OS) was defined from the date of diagnosis to the date of death or last following-up. The association between variables and OS was calculated using univariate Cox proportional hazards model. If the variables were statistically significant (P<0.10), they were included in the following multivariate analysis. The survival curve based on variables were analyzed by Kaplan-Meier method, and the log-rank p value was presented. Hazard ratio (HR) was calculated using Cox proportional hazards model and presented along with 95% confidence interval (C.I.) and p value.
IBM SPSS Statistics for Windows (Version 22.0, Armonk, NY, USA) was used to perform all statistical analyses, and P<0.05 was considered significant. Survival curves were plotted by SPSS.
This study was approved by Institutional Review Boards of Chang Gung Medical Foundation (202100148B0) and National Taiwan University Hospital (202105037RINA).
Results
The baseline characteristics of 44 ATC patents based on dabrafenib and trametinib treatment
A total of forty-four patients with pathologically confirmed ATC were enrolled. Until May of 2021, the median follow-up time was 3.6 months ranged 0.3-22.1 months. The median age was 75.2 years old. Female were more than male patients (56.8% vs. 43.2%). Most patients had good or intermediate performance status (PS0-2 of 77.3%), no previous history of differentiated thyroid cancer (de novo ATC: 79.5%), and distant metastasis disease when ATC was firstly diagnosed (stage IVc: 65.9%). There was no statistical difference of baseline characteristics between patients who received dabrafenib plus trametinib and patients who did not (Table 1).
Table 1.
Baseline characteristics of all ATC patients
| All (n=44) | Dabrafenib + Trametinib (n=11) | No Dabrafenib + Trametinib (n=33) | p | ||||
|---|---|---|---|---|---|---|---|
| Age (years, median, IQR) | 75.2 (66.2-80.0) | 70.0 (66.6-77.1) | 75.4 (66.2-80.6) | 0.623 | |||
| Age | N | % | N | % | N | % | |
| ≤75 | 21 | 47.7% | 6 | 54.5% | 15 | 45.5% | 0.601 |
| >75 | 23 | 52.3% | 5 | 45.5% | 18 | 54.5% | |
| Gender | |||||||
| Male | 19 | 43.2% | 5 | 45.5% | 14 | 42.4% | 0.861 |
| Female | 25 | 56.8% | 6 | 54.5% | 19 | 57.6% | |
| Performance status | |||||||
| 0-2 | 34 | 77.3% | 7 | 63.6% | 27 | 81.8% | 0.145 |
| 3-4 | 9 | 20.5% | 4 | 36.4% | 5 | 15.2% | |
| Missing | 1 | 2.3% | 0 | 0.0% | 1 | 3.0% | |
| De novo ATC | 35 | 79.5% | 6 | 54.5% | 29 | 87.9% | 0.018 |
| Prior Surgery | 23 | 52.3% | 5 | 45.5% | 17 | 51.5% | 0.728 |
| Prior Radiotherapy | 24 | 54.5% | 4 | 36.4% | 20 | 60.6% | 0.162 |
| Stage | |||||||
| IVa | 7 | 15.9% | 2 | 18.2% | 5 | 15.2% | 0.606 |
| IVb | 8 | 18.2% | 3 | 27.3% | 5 | 15.2% | |
| IVc | 29 | 65.9% | 6 | 54.5% | 23 | 69.7% | |
| Metastasis | |||||||
| Lung | 24 | 54.5% | 5 | 45.5% | 19 | 57.6% | 0.484 |
| Bone | 6 | 13.6% | 1 | 9.1% | 5 | 15.2% | 0.612 |
| Brain | 2 | 4.5% | 0 | 0.0% | 2 | 6.1% | 0.403 |
| Others | 5 | 11.4% | 2 | 18.2% | 3 | 9.1% | 0.411 |
Twenty-four out of 44 (54.5%) patients received examinations for BRAF status. Twenty patients did not check BRAF mutation because of financial concerns (BRAF study was not reimbursed by Taiwan National Health Insurance). Regarding the methods to detect BRAF V600E mutation, 4 were done by IHC, 15 were done by PCR, and 4 were done by both IHC and PCR. Only one was done by both PCR and NGS. Positive BRAF V600E mutations were found in 2, 8, 2, and 0 by IHC, PCR, IHC plus PCR, and PCR plus NGS respectively and only 2, 6, 2, and 0 patients received dabrafenib plus trametinib respectively. The mutation rate was 50% among the patients undergoing genetic tests. All 5 patients undergoing more than one detection method had concordant results (Figure 1; Table 2).
Figure 1.
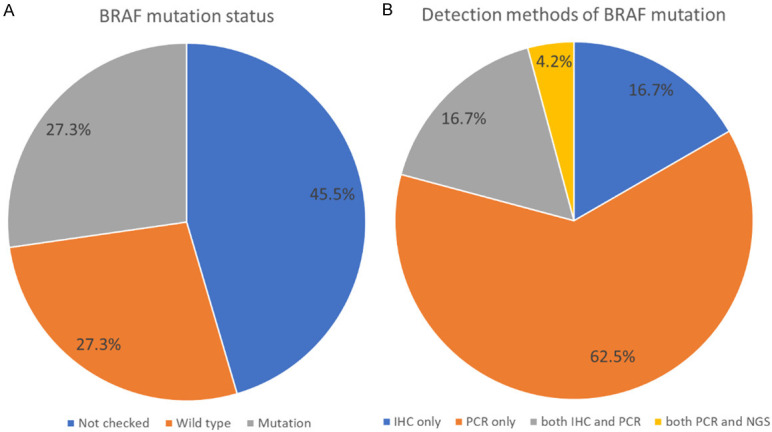
The detection of BRAF mutation. A. Of 44 ATC patients, 24 (55.4%) patients had BRAF detection and half of patients detected had BRAF mutation. B. Of 24 patients with BRAF detection, most patients (62.5%) were detected by PCR followed by immunohistochemistry (IHC) (16.7%) and IHC/PCR (16.7%).
Table 2.
BRAF mutation status in tested group (n=24)
| Detection method | Total | ||||
|---|---|---|---|---|---|
|
| |||||
| IHC only | PCR only | both IHC and PCR* | both PCR and NGS* | ||
| Positive | 2 | 8# | 2 | 0 | 12 (50%) |
| Negative | 2 | 7 | 2 | 1 | 12 (50%) |
| Total | 4 | 15 | 4 | 1 | 24 (100%) |
All 5 patients undergoing more than one detection methods had concordant results.
One patient was positive for BRAF mutation but did not receive dabrafenib plus trametinib.
Eleven out of 12 patients with BRAF mutation received dabrafenib and trametinib treatment. One did not receive dabrafenib and trametinib treatment because of financial consideration. In terms of tumor response, one patient had complete response, eight patients had partial response and two patients had progressive disease resulting in objective response rate of 81.8%. The median PFS and OS were 7.4 and 10.4 months respectively.
The overall OS was 3.9 months (95% confident interval, C.I., 0.7-7.2 months) among 44 ATC patients. In univariate analysis, prior surgery (vs. no prior surgery, hazard ratio, HR: 0.44, 95% C.I.: 0.21-0.91, P=0.027), lung metastases (vs. no lung metastases, HR: 1.98, 95% C.I.: 0.95-4.14, P=0.068), bone metastases (vs. no bone metastases, HR: 2.31, 95% C.I.: 0.87-6.15, P=0.093), brain metastases (vs. no brain metastases, HR: 4.95, 95% C.I.: 1.11-21.99, P=0.036), and BRAF mutation with dabrafenib plus trametinib (vs. no dabrafenib and trametinib, HR: 0.40, 95% C.I.: 0.16-1.00, P=0.050) were prognostic factors associated with OS. All the prognostic factors with P<0.10 were included in multivariate analysis. Prior surgery (vs. no prior surgery, hazard ratio, HR: 0.21, 95% C.I.: 0.09-0.50, P<0.001), lung metastases (vs. no lung metastases, HR: 3.29, 95% C.I.: 1.41-7.69, P=0.006), bone metastases (vs. no bone metastases, HR: 4.28, 95% C.I.: 1.35-13.54, P=0.014), brain metastases (vs. no brain metastases, HR: 13.55, 95% C.I.: 2.11-86.86, P=0.006), and BRAF mutation with dabrafenib plus trametinib (vs. no dabrafenib and trametinib, HR: 0.25, 95% C.I.: 0.09-0.67, P=0.006) were still significantly associated with OS in multivariate analysis (Table 3).
Table 3.
Univariate and multivariate analysis of ATC patients (n=44)
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| HR | 95% C.I. | p | HR | 95% C.I. | p | |||
|
|
|
|||||||
| lower | upper | lower | upper | |||||
| Age (>75 vs. ≤75) | 1.45 | 0.70 | 3.02 | 0.321 | ||||
| Gender (Female vs. Male) | 1.22 | 0.59 | 2.53 | 0.586 | ||||
| PS (3-4 vs. 0-2) | 1.52 | 0.63 | 3.68 | 0.349 | ||||
| De novo ATC | 1.28 | 0.52 | 3.14 | 0.594 | ||||
| Prior Surgery | 0.44 | 0.21 | 0.91 | 0.027 | 0.21 | 0.09 | 0.50 | <0.001 |
| Prior Radiotherapy | 0.56 | 0.27 | 1.15 | 0.112 | ||||
| Stage | ||||||||
| IVa | 1 | |||||||
| IVb | 0.86 | 0.25 | 3.02 | 0.816 | ||||
| IVc | 1.39 | 0.51 | 3.77 | 0.518 | ||||
| Metastatic sites | ||||||||
| Lung | 1.98 | 0.95 | 4.14 | 0.068 | 3.29 | 1.41 | 7.69 | 0.006 |
| Bone | 2.31 | 0.87 | 6.15 | 0.093 | 4.28 | 1.35 | 13.54 | 0.014 |
| Brain | 4.95 | 1.11 | 21.99 | 0.036 | 13.55 | 2.11 | 86.86 | 0.006 |
| Others | 0.70 | 0.23 | 2.11 | 0.530 | ||||
| Dabrafenib/Trametinib | 0.40 | 0.16 | 1.00 | 0.050 | 0.25 | 0.09 | 0.67 | 0.006 |
The patients with BRAF mutation who received dabrafenib plus trametinib had better OS than the patients who did not (median OS: 10.4 months, 95% C.I., 1.6-19.3 months vs. 3.3 months, 95% C.I. 0.0-6.6 months, P=0.05) (Figure 2A). No difference in baseline characteristics existed between patients treated with or without dabrafenib and trametinib except the rate of de novo ATC, as patients who received dabrafenib and trametinib treatment had a lower rate of de novo ATC (Table 1). The patients receiving prior surgery had better OS (median: 6.5 months, 95% C.I. 2.1-10.8 months) than the patients without prior surgery (3.2 months, 95% C.I. 0.1-6.3 months, P=0.027) (Figure 2B).
Figure 2.
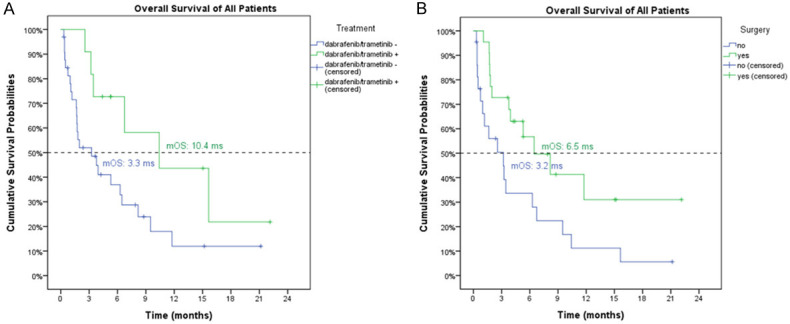
The overall survivals for ATC patients based on the use of dabrafenib and trametinib (A) and surgery (B). (A) The patients with BRAF mutation who received dabrafenib plus trametinib had better OS than the patients who did not (median OS: 10.4 months, 95% C.I., 1.6-19.3 months vs. 3.3 months, 95% C.I. 0.0-6.6 months, P=0.05). (B) The patients receiving prior surgery had better OS (median: 6.5 months, 95% C.I. 2.1-10.8 months) than the patients without prior surgery (3.2 months, 95% C.I. 0.1-6.3 months, P=0.027).
Among the patients treated with dabrafenib plus trametinib, the patients received prior surgery had better OS (median OS, not reached, 95% C.I. 0.0-7.7 months) than those who did not (median OS, 3.5 months, 95% C.I. cannot be assessed, P=0.017, Figure 3A). Less significantly, all patients treated without dabrafenib plus trametinib, the patients who received prior surgery (median OS, 5.3 months, 95% C.I., 2.6-8.0 months) had longer OS than those who did not (median OS, 1.2 months, 95% C.I., 0.4-1.9 months, P=0.067, Figure 3B). Among four subgroups based on use of dabrafenib/trametinib and prior surgery, the patients undergoing both experienced the best overall survival (Figure S1).
Figure 3.
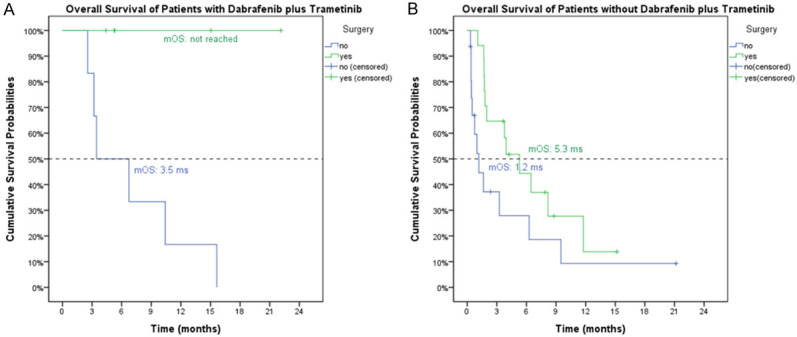
The impact of surgery for overall survivals for the ATC patients treated with (A) and without (B) dabrafenib and trametinib. (A) In patients treated with dabrafenib plus trametinib, the patients who received prior surgery had better OS (median OS, not reached, 95% C.I. 0.0-7.7 months) than those who did not (median OS, 3.5 months, 95% C.I. cannot be assessed, P=0.017). (B) In patients treated without dabrafenib plus trametinib, the patients who received prior surgery (median OS, 5.3 months, 95% C.I., 2.6-8.0 months) had longer OS than those who did not (median OS, 1.2 months, 95% C.I., 0.4-1.9 months, P=0.067, (B)).
The efficacy of dabrafenib and trametinib was further evaluated. One patient had complete response, and eight patients had partial response. The objective response rate (ORR) was 81.8%. The median PFS was 7.3 months (95% C.I.: 1.6-13.1 months) (Figure 4), median OS was 10.3 months 95% C.I., 1.6-19.3 months) (Figure 2A).
Figure 4.
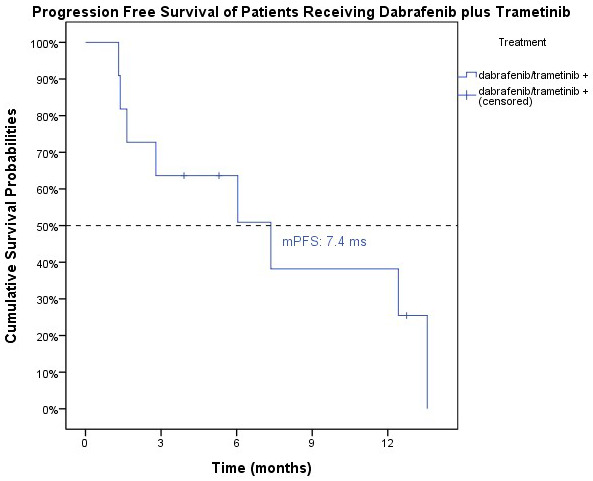
The progression-free survival for the ATC patients treated with dabrafenib and trametinib. The median PFS was 7.3 months (95% C.I.: 1.6-13.1 months) for the patients treated with dabrafenib and trametinib.
Discussion
To our knowledge, this study is the first and largest real-world experience of ATC including BRAF mutation status and targeted therapy in Asia. Most patients were old, female, de novo ATC, and in advanced stage. The prevalence of BRAF mutation was 50% in our cohort. The patients receiving dabrafenib and trametinib experienced ORR of 81.8%, median PFS of 7.3 months, and median OS of 10.3 months. The most significant prognostic factors among all ATC patients were prior surgery, metastases to bone, brain, and lung, and BRAF mutation receiving dabrafenib and trametinib. The patients treated with prior surgery and dabrafenib/trametinib experienced excellent OS.
In current study, the positive rate of BRAF V600E was 50% among the ATC patients undergoing BRAF mutation detection by either IHC, PCR or NGS studies. IHC staining using VE1 antibody has been widely used to detect BRAF mutation with high sensitivity and specificity in melanoma [14,15], lung cancer [16] and also thyroid cancer [17]. The concordance rate was 100% among the samples examined by both IHC and PCR/NGS although the case number was limited. Therefore, IHC staining could be a rapid and convenient method to detect BRAF V600E and screen the suitable candidate for targeted therapy. Of note, IHC only detected BRAF V600E rather than BRAF non-V600E mutation from experience of melanoma [14,15]. Therefore, BRAF V600 mutation other than V600E (most commonly V600K) may be missed by IHC staining and those patients with such mutation are suitable for targeted therapy with dabrafenib and trametinib. Therefore, the patents with negative IHC may need additional sequencing either by PCR or NGS.
In current study, 11 patients with BRAF mutation were treated with targeted therapy of dabrafenib and trametinib. The ORR was 81.8%. The median PFS and OS were 7.4 and 10.4 months respectively. The pivot phase II study demonstrated that ORR was 69% and all patients undergoing prior radiotherapy in 16 ATC patients [13]. The updated data showed the ORR was 67%. The PFS and OS were 1.2 and 1.7 years respectively in 27 ATC evaluable patients [18]. Although the ORR was higher, the PFS and OS in our study were worse than the pivot study. The major reason was possibly the clinical trial enrolling the patients with good PS of 0-1 but in contrast 36.4% patients in our cohort had PS >2. In addition, only 36.4% patients undergoing prior radiotherapy but 100% and 82% undergoing prior radiotherapy in preliminary and updated results. The only published real world data of targeted therapy which was conducted at The University of Texas MD Anderson Cancer Center [19], median OS for patients treated with targeted therapy was 15.7 months, compared with 7.6 months in patients not having received any targeted therapy, with an adjusted HR of 0.49. The OS reported was much longer than other real-world studies [3] possibly due to the selection bias from referral hospital. In terms of HR, the crude and adjusted HR were 0.40 and 0.25 respectively in our cohort indicating the critical impact of targeted therapy for ATC patients.
Prior surgery, dabrafenib and trametinib, and metastases to lung, bone, and brain were independently significant factors for overall survival among ATC patients. These findings were compatible with a retrospective study of 50 ATC patients in China which reported that distant metastases, prior surgery, prior radiotherapy, and tumor residue were the most important factors affecting the prognosis [20]. However, above study did not report any genetic study and targeted therapy.
In a recently published data, the impact of BRAF mutation in treatment strategy was also demonstrated. Anastasios Maniakas and his colleges had retrospectively analyzed 479 patients of ATC at The University of Texas MD Anderson Cancer Center [19]. Similar to our findings, they found that targeted therapy and prior surgery played important role for improvement of overall survival. In addition, addition of immunotherapy on targeted therapy provided more benefit for overall survival. Only one case in current study received concurrent immunotherapy and targeted therapy so the impact of adding immunotherapy was unknown in current cohort. The study concluded that the traditional approaches to ATC should be gradually replaced by molecular-based personalized therapies [19].
In previous research, patients with ATC gained little benefits from prior surgery, even with more extensive surgeries [5,21]. Most of the patients died of asphyxiation of rapid local recurrence or distant metastases after primary prior surgery. However, in our study, we found that those people with BRAF mutation received dabrafenib plus trametinib would gain greater benefits from prior surgery than the without dabrafenib plus trametinib (Figures 3 and S1). Therefore, the role of prior surgery may become important in the era with active treatment such as targeted therapy with dabrafenib and trametinib.
Although targeted therapy largely improved the survivals in ATC patients with BRAF mutation, unfortunately, most patients experienced recurrence and died of ATC after variable duration of response. To understand and overcome resistance mechanisms, molecular profiling and preclinical studies are warranted to continue the progress of treatment for ATC patients. Patient-derived xenograft (PDX) and cell lines provide more mechanistic studies in terms of resistance and should be the future of ATC treatment [22].
Some limitations exist in our study. First, selection bias should be considered owning to the retrospective design. Patients with better performance and less aggressive disease presentation tended to receive more genetic test and multimodality treatments, though no statistical significance was found. Second, two patients with BRAF mutation cannot afford dabrafenib plus trametinib because of financial issue. And half of our patients even did not receive mutation study as no targeted therapy in early era. BRAF mutation tests including IHC, PCR, and NGS become the standard of care in current daily practice once ATC is diagnosed. Third, due to the low prevalence of disease, our sample size is relatively small. Following investigation should be done to confirm our findings. In the future, further preclinical and prospective investigation with comprehensive genetic profiling is needed.
In conclusion, current study provides the real-world evidence that targeted therapy with dabrafenib and trametinib was effective and significantly improved the survivals for ATC patients. The role of prior surgery became important in the era of targeted therapy. Future studies should focus on resistance mechanisms and combination with immunotherapy for ATC patients.
Acknowledgements
This work was supported by grants from Linkou Chang-Gung Memorial Hospital and National Taiwan University Hospital in Taiwan. Please refer to funding section. This work was funded by Linkou Chang Gung Memorial Hospital, grant numbers CMRPG3J0971-3, NMRPG3K6201-3, CMRPG3K2171, CMRPG3L0911, and CMRPG3M0521-2 to CEW.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. Vol. 3. Lyon, France: International Agency for Research on Cancer; 2018. p. 2019. [Google Scholar]
- 2.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 2010;22:486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DY, Won JK, Choi HS, Park do J, Jung KC, Sung MW, Kim KH, Hah JH, Park YJ. Recurrence and survival after gross total removal of resectable undifferentiated or poorly differentiated thyroid carcinoma. Thyroid. 2016;26:1259–1268. doi: 10.1089/thy.2016.0147. [DOI] [PubMed] [Google Scholar]
- 4.Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 5.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 6.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill JP, Shaha AR. Anaplastic thyroid cancer. Oral Oncol. 2013;49:702–706. doi: 10.1016/j.oraloncology.2013.03.440. [DOI] [PubMed] [Google Scholar]
- 8.Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S. Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg. 2012;36:1247–1254. doi: 10.1007/s00268-012-1437-z. [DOI] [PubMed] [Google Scholar]
- 9.Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. 2019;40:1573–1604. doi: 10.1210/er.2019-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begum S, Rosenbaum E, Henrique R, Cohen Y, Sidransky D, Westra WH. BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment. Mod Pathol. 2004;17:1359–1363. doi: 10.1038/modpathol.3800198. [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, Chiarion Sileni V, Schachter J, Garbe C, Bondarenko I, Gogas H, Mandalá M, Haanen JBAG, Lebbé C, Mackiewicz A, Rutkowski P, Nathan PD, Ribas A, Davies MA, Flaherty KT, Burgess P, Tan M, Gasal E, Voi M, Schadendorf D, Long GV. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 12.Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, Rigas JR, Upalawanna A, D’Amelio AM Jr, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018;36:7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang WK, Kuo TT, Wu CE, Cheng HY, Hsieh CH, Hsieh JJ, Shen YC, Hou MM, Hsu T, Chang JW. A comparison of immunohistochemical and molecular methods used for analyzing the BRAF V600E gene mutation in malignant melanoma in Taiwan. Asia Pac J Clin Oncol. 2016;12:403–408. doi: 10.1111/ajco.12574. [DOI] [PubMed] [Google Scholar]
- 15.Lo MC, Paterson A, Maraka J, Clark R, Goodwill J, Nobes J, Garioch J, Moncrieff M, Rytina E, Igali L. A UK feasibility and validation study of the VE1 monoclonal antibody immunohistochemistry stain for BRAF-V600E mutations in metastatic melanoma. Br J Cancer. 2016;115:223–227. doi: 10.1038/bjc.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gow CH, Hsieh MS, Lin YT, Liu YN, Shih JY. Validation of immunohistochemistry for the detection of BRAF V600E-mutated lung adenocarcinomas. Cancers (Basel) 2019;11:866. doi: 10.3390/cancers11060866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghossein RA, Katabi N, Fagin JA. Immunohistochemical detection of mutated BRAF V600E supports the clonal origin of BRAF-induced thyroid cancers along the spectrum of disease progression. J Clin Endocrinol Metab. 2013;98:E1414–1421. doi: 10.1210/jc.2013-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keam B, Kreitman R, Wainberg Z, Cabanillas M, Cho D, Italiano A, Stein A, Cho J, Schellens J, Wen P. Updated efficacy and safety data of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E-mutated anaplastic thyroid cancer (ATC) Annals of Oncology. 2018;29:viii645–viii646. [Google Scholar]
- 19.Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, Williams MD, Gunn GB, Hofmann MC, Cote G, Sperling J, Gross ND, Sturgis EM, Goepfert RP, Lai SY, Cabanillas ME, Zafereo M. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol. 2020;6:1397–1404. doi: 10.1001/jamaoncol.2020.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu TR, Xiao ZW, Xu HN, Long Z, Wei FQ, Zhuang SM, Sun XM, Xie LE, Mu JS, Yang AK, Zhang GP, Fan Y. Treatment and prognosis of anaplastic thyroid carcinoma: a clinical study of 50 cases. PLoS One. 2016;11:e0164840. doi: 10.1371/journal.pone.0164840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66:321–330. doi: 10.1002/1097-0142(19900715)66:2<321::aid-cncr2820660221>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Maniakas A, Henderson YC, Hei H, Peng S, Chen Y, Jiang Y, Ji S, Cardenas M, Chiu Y, Bell D, Williams MD, Hofmann MC, Scherer SE, Wheeler DA, Busaidy NL, Dadu R, Wang JR, Cabanillas ME, Zafereo M, Johnson FM, Lai SY. Novel anaplastic thyroid cancer PDXs and cell lines: expanding preclinical models of genetic diversity. J Clin Endocrinol Metab. 2021;106:e4652–e4665. doi: 10.1210/clinem/dgab453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


