Abstract
Pancreatic cancer (PC) is the fourth-most-deadly cancer in the United States with a 5-year survival rate of only 8%. The majority of patients with locally advanced pancreatic cancer undergo chemotherapy and/or radiation therapy (RT). However, current treatments are inadequate and novel strategies are desperately required. 3-Bromopyruvate (3-BP) is a promising anticancer drug against pancreatic cancer. It exerts potent anticancer effects by inhibiting hexokinase II enzyme (HK2) of the glycolytic pathway in cancer cells while not affecting the normal cells. 3-BP killed 95% of Panc-2 cells at 15 μM concentration and severely inhibited ATP production by disrupting the interaction between HK2 and mitochondrial Voltage Dependent Anion Channel-1 (VDAC1) protein. Electron microscopy data revealed that 3-BP severely damaged mitochondrial membrane in cancer cells. We further examined therapeutic effect of 3-BP in syngeneic mouse pancreatic cancer model by treating animals with 10, 15 and 20 mg/kg dose. 3-BP at 15 & 20 mg/kg dose level significantly reduced tumor growth by approximately 75-80% in C57BL/6 female mice. Immunohistochemistry data showed complete inhibition of hexokinase II (HK2) and TGFβ, in animals treated with 3-BP drug. We also observed enhanced expression of active caspase-3 in tumor tissues exhibited apoptotic death. Flow Cytometry analysis showed significant inhibition in MDSC (CD11b) population in treated tumor which may have allowed infiltration of CD8+ T cells and inhibited tumor growth. Notably, metabolomic data also revealed severe inhibition in glycolysis, NADP, ATP and lactic acid production in cancer cells treated with 40 μM 3-BP. Importantly, we also observed inhibition in lactic acid production responsible for tumor aggression. These results provide new evidence that 3-BP severely inhibit glucose metabolism in cancer cells by blocking hexokinase II, and disrupting mitochondria by suppressing BCL2L1 in pancreatic cancer.
Keywords: Pancreatic cancer, 3-Bromopyruvate, hexokinase II, glycolysis, mitochondria, voltage dependent anion channel 1 (VDAC1), apoptosis, ATP production
Introduction
Pancreatic cancer (PC) is the fourth most lethal cancer in the United States. In 2022, there will be an estimated diagnosis of 62,210 new cases, and 49,830 deaths [1]. PC has the highest mortality rate amongst all other cancers in both men and women, with a one-year relative survival rate of 20%, and a five-year relative survival rate of 8%. The pancreaticoduodenectomy (Whipple procedure), can increase survival for patients with resectable PC; however, less than 20% of patients are candidates for surgery at the time of presentation. Most patients have advanced PC, often with regional and distant metastasis at the time of their diagnosis. In these advanced cases, chemotherapy and radiation have shown limited tumor control. Consequently, PC continues to be refractory to treatment and new treatment options are desperately needed.
3-bromopyruvate (3-BP) is an analog of pyruvic acid that exhibits strong anticancer activity and is a promising anti-cancer agent [2-4]. In human cancer cells, 3-BP is transported across the membrane by the lactate/pyruvate H+ symporter. Once, it enters into the cell it becomes highly reactive, showing strong alkylating properties towards proteins having SH groups [5-7]. Cancer cells rely on glycolysis as a source of energy, and they overexpress lactate transporters to import additional glucose and efflux excess lactic acid. This overexpression of lactate transporters, which transport 3-BP into the cell, provide a novel mechanism by which 3-BP is strongly toxic for cancer cells without affecting normal cells. 3-BP has demonstrated therapeutic potential by targeting energy metabolism in cancer cells by inhibiting glycolysis. Specifically, 3-BP irreversibly alkylates the glycolytic enzyme, Hexokinase II (HK2), resulting in the disruption of glucose metabolism leading to cancer cell death [8]. 3-BP also exert potent anticancer effects through an oxidative stress mechanism which causes cancer cell death [2,5,9,10]. Recent investigations have also shown that 3-BP treatment produces increased levels of reactive oxygen species (ROS), which causes DNA damage with reduction of free glutathione levels [11]. Considerably, cancer cells heavily rely on glycolysis as a source of energy and produce lactic acid in presence of oxygen by overexpressing lactate transporters in cancer cells and making tumor microenvironment more acidic. The novelty 3-BP is its selectivity and specificity, i.e., it doesn’t affect the normal cells but strongly toxic to cancer cells making 3-BP a promising candidate as an anticancer drug. In the present study, we have investigated the anti-tumor effects of 3-BP on highly aggressive Panc-2 pancreatic cancer cells and found that 3-BP can be effectively combined with low doses of radiation to treat locally advanced pancreatic cancer. Furthermore, we have also tested anti-tumor activity of 3-BP in syngeneic mouse model of locally advanced pancreatic cancer.
Material and methods
Panc-2 cell culture
Murine pancreatic adenocarcinoma Panc-2 cells were obtained from Dr. Jeffrey Schlom, National Cancer Institute, National Institutes of Health, Bethesda, MD. The cells were grown and maintained in culture with RPMI 1640 (ATCC 30-2002, Manassas, VA, USA) supplemented with 10% FBS at 37°C and in a 5% CO2 atmosphere.
Clonogenic survival assay
The desired number of cells were seeded in six well plate 24 hours prior to 3BP and radiation treatment. After 48 hours of treatment, media was removed, and cells were rinsed with PBS. Before treatment cells were trypsinized and resuspended in fresh media in a centrifuge tube. The cells were then centrifuged at 1000 × g at 4°C supernatant was removed and then cells were resuspended in fresh media. Clonogenic survival assay was performed as described previously [12].
Co-immunoprecipitation
Immunoprecipitation was carried out according to the protocol described by the antibody provider Santa Cruz Biotechnology, USA. Briefly, 250 µg protein was incubated with 2 µg VDAC1 antibodies (Santa Cruz Biotechnology) for 1 hour at 4°C in a cold room with a rotator. For control samples, instead of primary antibody, 2 µg mouse IgG was incubated with the protein lysates. Protein G Agarose (Santa Cruz Biotechnology) was equilibrated with protein lysis buffer and 10 µl was added in each sample. Subsequently, samples were incubated in a cold room at 4°C with rotation overnight. Following overnight incubation, beads were pelleted by centrifugation at 1000 × g and washed with 500 µl lysis buffer with rotation for 5 min; the washing was repeated four times. After final wash, the bound protein was eluted with 30 μl 1 × SDS-PAGE buffer and boiled for 10 mins. The eluted proteins were separated on 4-20% Tris Glycine SDS-PAGE and immunoblot was performed using hexokinase-2 antibodies (Protein Tech, USA) as described in the western blot protocol.
Western blot analysis
After drug treatment for 48 hours Panc-2 cells were harvested with a cell scraper, transferred to a 15 ml tube, and incubated on ice for 15 min. The cells were pelleted by centrifugation at 1000 rpm for 5 min, and then washed with cold 1 × PBS and suspended in 1 × RIPA buffer. Cells were sonicated for 30 second, pulse 01, amplitude 50% using a Sonicator (Fisher scientific, USA). The sonicated cells were kept on ice for 15 mins, transferred to 1.5 ml Eppendorf tubes and centrifuged at 12000 rpm for 15 mins at 4°C. The supernatant was collected, and protein concentration was measured using Pierce BCA protein assay kit (Thermofisher Scientific, USA). Immunoblot analysis was performed as described previously [12].
Metabolomics analysis of Panc-2 cells treated with 40 µM of 3-BP
Panc2 cells (5 × 105 cells) were grown in 100 mm dish with RPMI 1640 media and treated with 40 µM of 3-BP for 48 hours, along with untreated control. After treatment media was aspirated, and the cells were washed with cold 1 × PBS twice and metabolites were extracted as described previously [13].
Cellular ATP assay
Cellular ATP content was measured using Cell Titer-Gl0 2.0 reagent (Promega, USA) according to the manufacturer’s instructions. Briefly, 1500 Panc-2 cancer cell were seeded in each well of a 96 well luminescence cell culture plate (clear bottom with white solid sides; ThermoFisher Scientific, USA) in 100 µl RPMI 1640 cell culture media. After 24 hours, cells were treated with 10-50 µM of 3BP for 48 hours, using four wells for each replicate. After treatment ATP assay reagent was added to the cells and placed on a shaker for 2 min, and then without shaking for 10 min at room temperature, luminescence was measured using a BIO-TEK ELISA reader (USA).
TUNEL assay
Panc2 cells were trypsinized and seeded in a chamber slide with 3 × 103 cells/well. The following day, cells were treated with 0, 20, 40 µM 3-BP for 48 hours and media was removed. Floating cells were collected and fixed with 10% formalin for 30 mins at 4°C. After washing twice with 1 × PBS cells were treated with 0.2% triton X-100 in 1 × PBS for 5 mins and chamber wells were removed and slides were washed with 1 × PBS and processed for staining for TUNEL according to the manufacturer’s instructions (Promega, USA).
Electron microscopy
Panc2 cells (3 × 105) were seeded in 100 mm cell culture plate with RPMI 1640. The following day cells were treated with 40 µM 3-BP for 48 hours. After treatment media was removed, and cells were fixed with 5 ml fixing buffer for EM imaging (University of Maryland EM CORE).
Development of Panc-2 based syngeneic mouse model of pancreatic cancer
After approval of animal protocol from the Office of Animal Welfare Assurance (OAWA) University of Maryland School of Medicine (IACUC Protocol No. #1019006), we developed a syngeneic mouse model of pancreatic cancer. We subcutaneously injected Panc-2 cells in the right flank of 8 weeks old C57BL/6 female mice. The animals were anesthetized using 3.5-4% isoflurane in 100% oxygen. For tumor implantation, the sub confluent Panc-2 cells were trypsinized, washed with ice cold 1 × PBS, and then 1 × 106 cells were mixed with Matrigel in 1:1 ratio (50 µl + 50 µl) and drawn into a cold 0.5 ml syringe (BD Bioscience, USA) fitted with a 27-1/2-gauge needle. The cell mixture (100 µl) was carefully injected subcutaneously in the right flank of the animal. After one week, tumor volume was measured (approximately 100 mm3 in size), animals were randomized into 4 groups (n = 8 animals/group). Animals were treated with vehicle control (saline and buffer only), 10, 15 and 20 mg/kg 3-BP drug. Each animal was injected with 100 µl drug or vehicle via intra-peritoneal (IP) injection three days per week (i.e., every alternate day) for 30 days. After the treatment period, animals were euthanized, and tumor was imaged and processed for further analysis. Tumor volume was measured every alternate day with digital vernier caliper using the following formula: Volume = (L*W*W)/2, where L = large length, W = smaller length. Body weights were measured once a week. Pictures of each animal were taken to show the tumor site after euthanizing with CO2 and tumors were excised and preserved for further analysis.
Immunohistochemistry (IHC) of mouse tumor tissues
IHC staining of pancreatic tumor tissues was performed using the following antibodies: hexokinase II (Santa Cruz Biotechnology, 1:100 dilution), Cleaved caspase-3 (Cell Signaling Technology, USA, 1:300 dilution), TGFβ-1 (Santa Cruz Biotechnology, 1:50 dilution), as described previously [12-14]. Biotin labelled secondary antibodies were used using ABC Vectastain universal quick kit, and DAB substrate (vector laboratories, USA), for signal amplification. Counter stain was performed with hematoxylin QS from Vector Lab (USA). The slides were washed with xylene and mount with cytoseal 60 (Thermofisher USA). Images were captured with Evos microscope color xl (Thermofisher USA).
Flow cytometry
Tumors were collected after euthanasia at 30 days post-treatment. Tissues were then collected, and single cell suspensions were obtained for flow cytometry analysis [15]. Briefly, tumors were processed through a cell strainer and washed in PBS 2% FBS (Gemini) 1% PenStrep 1% NEAA (Gibco, Life Technologies, Gaithersburg, MD, USA). Cells were stained at the supplier recommended concentrations of the antibody for 30 min at 4°C in 100 ul FACS buffer. All antibodies were from BioLegend (San Diego, CA, USA). Two panels of mouse monoclonal antibodies were used; Panel 1: CD4 (clone GK 1.5, product # 100421), CD8a (clone 53-6.7, product # 100705) and Panel 2: CD11b (clone M1/70, product # 101205). All flow cytometry was performed at the University of Maryland Greenebaum Cancer Center Flow Cytometry Shared Services. Flow cytometry acquisition [16] was performed using a LSRII instrument (BD Biosciences) and data were analyzed using FlowJo software (Version 10.6, Tree Star Inc., Ashland, OR, USA).
Statistical analysis
Prior to the analysis, each survival fraction datum was log transformed to ensure a good model fit at the higher dose levels. The signed rank test in excel was performed to analyze and compare the distribution of differences in means for different doses of RT and 3-BP drug. A statistically significant difference was considered when p value was less than < 0.05 and highly significant when p value was less than < 0.01. For metabolomic sample analysis all results are shown as the mean ± SEM fold change. Statistical significance was designated as *P < 0.05, **P < 0.01 using Student’s t-test.
Results
3-BP exhibit promising anti-tumor response against Panc-2 cells
To evaluate anti-tumor effect of 3-BP against pancreatic cancer, we treated Panc-2 cells in graded concentration of 3-BP and performed a clonogenic survival assay. The clonogenic survival data demonstrated dose-dependent cell killing of cancer cells treated with 3-BP. Treatment with 10 µM 3-BP resulted in 55% survival, treatment with 12.5 µM 3-BP resulted in 25% survival, and about 10% survival was observed at 15 µM of 3-BP (Figure 1A, 1B). We observed significant cancer cell death beyond 15 µM concentration of 3-BP and we noticed that surviving colony size was very small (Figure 1A). The brightfield microscopy also showed 3-BP induced morphological changes in cancer cells treated with 40 µM (Figure 1C).
Figure 1.
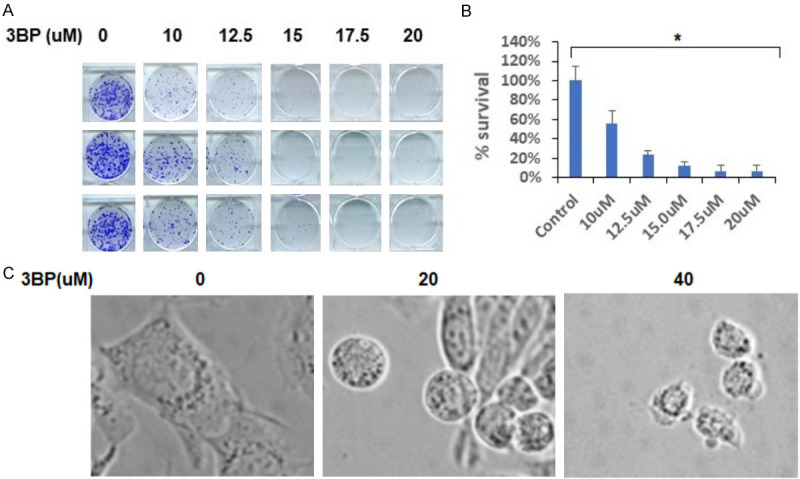
A, B. Clonogenic survival assay of Panc-2 cell treated with different doses of 3-BP. C. Morphological changes in Panc-2 cells after 3-BP treatment.
3-BP inhibited glycolysis, ATP production and dismantled mitochondrial structure in cancer cell
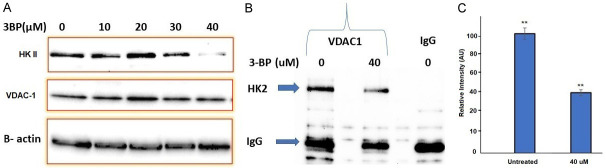
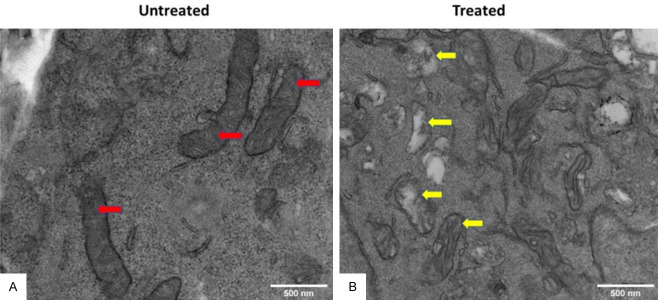
Pancreatic cancer cells overexpress hexokinase II enzyme (HK2), and enhance glucose consumption and glycolysis in Panc-2 cells. In the present investigation we treated Panc-2 cells with 10, 20, 30 and 40 µM of 3-BP up to 48 hours and measured the expression of hexokinase II and Voltage-Dependent Anion Channel (VDAC-1) by immunoblot analysis. The data showed that expression of hexokinase II was significantly inhibited at 40 µM, and expression level of VDAC-1 was not affected at 40 µM of 3-BP (Figure 2A). Moreover, Co-IP data showed that 3-BP (40 µM) treatment disrupted interaction between HK2, and VDAC-1, and the level of hexokinase expression was inhibited by almost 50% (Figure 2C). VDAC-1 is an important HK2 interacting partner helping cancer cells in ATP transport, and maintaining anti-apoptotic characteristics by interacting with BCL2L1 (Figure 3A). The Data presented in Figure 3 showed interaction between HKII, VDAC-1 and BCL2L1 responsible for suppressing mitochondrial apoptosis in Panc-2 cells. Further, treatment with 40 µM of 3-BP suppressed BCL2L1 expression and causing activation of mitochondrial caspases (Figure 3B, lane 3). Notably, we also observed that 40 µM of 3-BP treatment dismantled mitochondrial structure in Panc-2 cancer cells (Figure 4A, 4B), severely affecting ATP production (Figure 5A). In addition, Panc-2 cells treated with 3-BP showed TUNEL-positive Panc-2 cells, suggesting cancer cell death might be due to apoptosis and interaction breakdown between HKII and VDAC-1 (Figure 5B).
Figure 2.
A. Effect of different doses of 3-BP in HKII inhibition in Panc-2 cells. B. 3-BP induced disruption of interaction between HKll and mitochondrial VDAC-1 in Panc-2 cells. C. Quantification of HKII in immunoblot in panel B after Co-IP (*P < 0.05).
Figure 3.
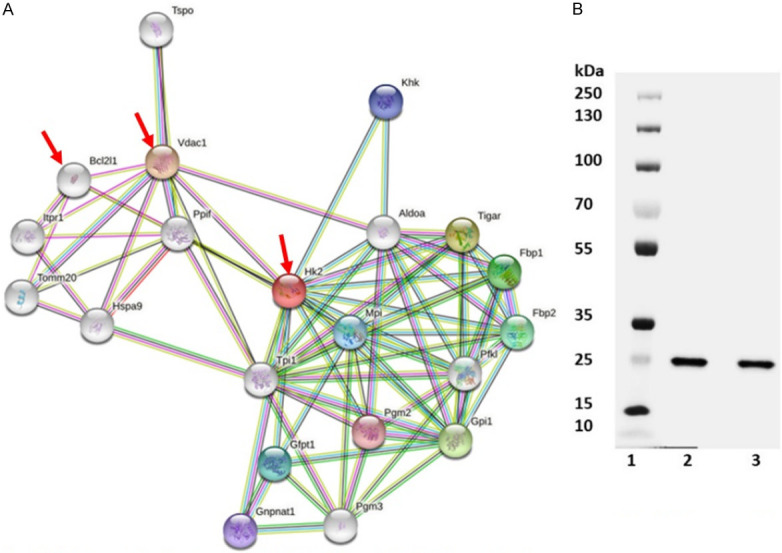
A. Interaction between HKII, VDAC-1 and BCL2L1 proteins in cancer cells. B. Suppression of BCL21 in cancer cells treated with 40 μM of 3-BP.
Figure 4.
Transmission electron micrograph (TEM) of Panc-2 cells treated with 3-BP. A. Untreated. B. Treated.
Figure 5.
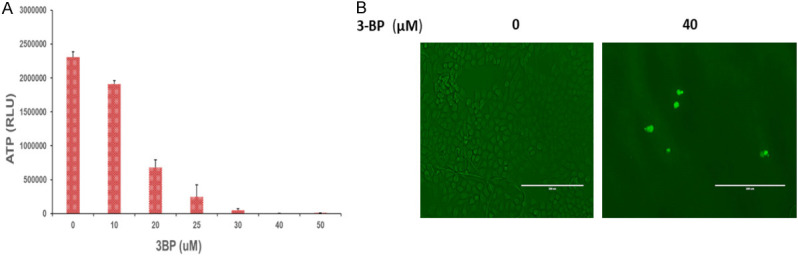
A. ATP production in Panc-2 cells treated with (0-50 μM) of 3-BP drug. B. 3-BP untreated (0 μM), and treated (40 μM), Panc-2 cells showing TUNEL positive cells.
Metabolic inhibition of glucose consumption and lactic acid production in cancer cells treated with 3-BP
The metabolomics data show that 3-BP treatment at 40 µM inhibited creatinine level by almost 70%, and lactic acid production by approximately 62% (Figure 6A, 6D). We also observed almost 40% inhibition in aspartate levels in Panc-2 cells treated with 40 µM of 3-BP (Figure 6C). Interestingly, in recent reports creatinine has also been identified as prognostic biomarker in lung and vulvar cancer. We also observed 3-BP induced inhibition in lactic acid production in cancer cells which might be responsible for causing acidic tumor microenvironment and tumor aggression. Metabolomics data also showed inhibition in aspartate, serine, threonine metabolism and formylglycinamide ribonucleotide (FGAR) in purine metabolism pathway (Supplementary Figure 1).
Figure 6.
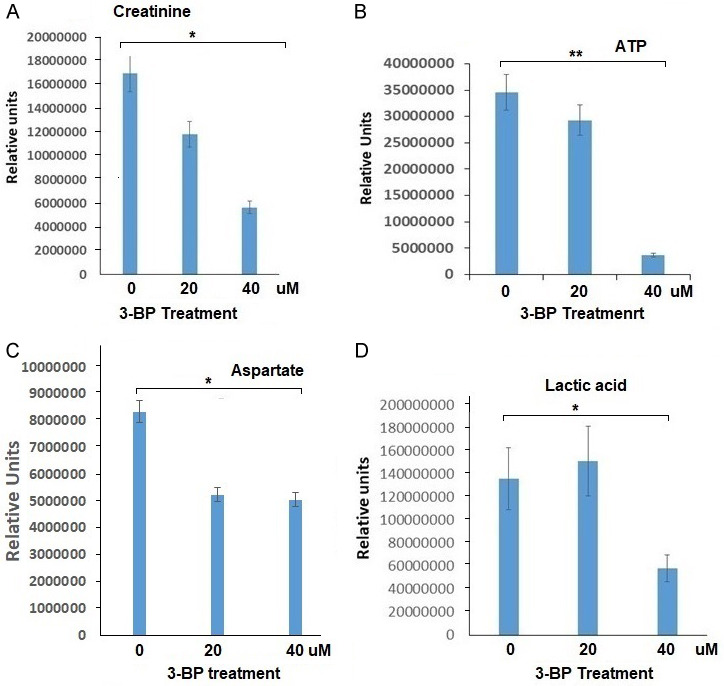
Metabolomic analysis of Panc-2 cells treated with 20 and 40 μM of 3-BP (*P < 0.05, **P < 0.01).
3-BP inhibit in vivo pancreatic tumor growth in C57BL/6 mouse model
Syngeneic pancreatic cancer was successfully developed by subcutaneously injecting 1 × 106 Panc-2 cells in the right flank of 8-week-old C57BL/6 female mice (Figure 7A). When the tumor was grown to 100 mm3 in size, animals were randomized into four groups and treated twice per week with intraperitoneal injections of vehicle (Saline), and 10, 15 and 20 mg/kg of 3-BP for 30 days. Tumor volumes and body weights were recorded every alternate day. We observed that the 20 mg/kg dose of 3-BP suppressed 80% of tumor growth and prolonged animal survival as compared to non-treated control group (Figure 7C). After 30 days animals were euthanized, and the tumor tissue were processed for H&E and IHC staining. We observed apoptotic cell death and necrosis in H & E-stained tumor tissues treated with 10-20 mg/kg 3-BP as compared to mock group (Figure 7D). The data showed that there was complete inhibition of HK II in tumor treated with high dose of 3-BP. Immunohistochemistry staining showed complete inhibition of HK2 and TGF-β1 in tumor tissues treated with 3-BP (Figure 7D). The pronounced staining of cleaved caspase 3 in treated tumor tissues was indicative of 3-BP-induced apoptosis. Thus, in vivo data have shown that 3-BP treatment at higher dose (10-20 mg/kg) significantly reduced pancreatic tumor growth and prolonged animal survival (Figure 8).
Figure 7.
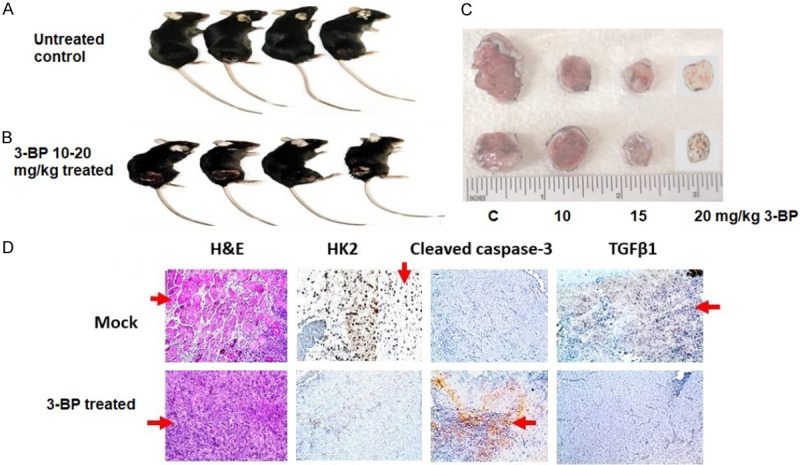
Treatment of Panc-2 induced subcutaneous pancreatic tumor with 3-BP and its IHC characterization.
Figure 8.
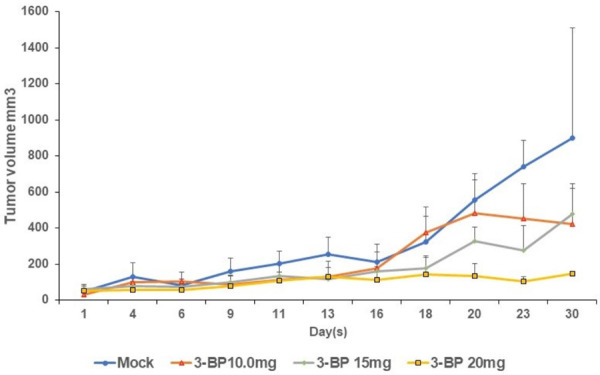
Longitudinal tumor growth in pancreatic tumor bearing mice treated with different doses of 3-BP.
3-BP treatment suppressed MDSC (CD11b) in pancreatic tumor microenvironment
The myeloid derived suppressor cells (MDSC) are responsible for immunosuppressive tumor microenvironment in pancreatic cancer which protects the tumor from the patient’s immune system. Flow cytometry data have precisely shown that animals treated with higher doses of 3-BP (10-20 mg/kg) CD11b (myeloid derived suppressor cells) population was reduced to 7% as compared to 17% in untreated control (Figure 9). Notably, we also observed only 3.2% CD8+ T cells in untreated control, and this might be due elevated MDSC population which may have suppressed CD8+ T cell and CD4+ T helper cells infiltration. However, after 3-BP treatment there was significant reduction in MDSC population and increase in infiltration of CD8+ T cells population in tumor tissues (Figure 9). Thus, the data have shown that after 3-BP treatment the tumor reduction could be due to strong immune response due to infiltration of CD8 T cells in tumor tissues.
Figure 9.
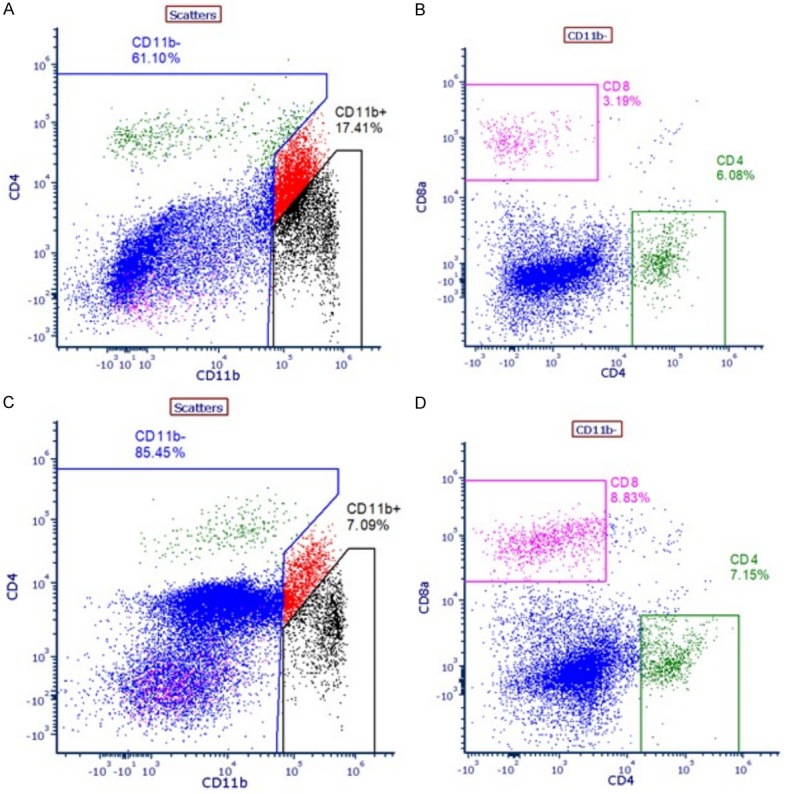
A. Level of CD11b in untreated tumor tissues. B. Level of CD8+ T cells in untreated tumor tissues. C. Level of CD11b in 3-BP treated tumor tissues. D. Level of CD8+ T cells in 3-BP treated tumor tissues.
Discussion
Pancreatic tumor cells reprogram cellular glucose metabolism to meet their increased energy requirements. Pancreatic tumor cells consume higher levels of glucose and have enhanced glycolysis that provides increased ATP production and lactic acid generation for tumor proliferation and aggression [17,18]. Hexokinase II is highly expressed in Panc-2 cells and is a key enzyme involved in metabolism of glucose through aerobic glycolysis. In the present investigation, we have demonstrated that 3-BP blocks expression of hexokinase II in pancreatic cancer cells and inhibits tumor growth in an in vivo model of pancreatic cancer [2,19-21]. The clonogenic survival data have shown that 3-BP drug was quite effective in killing over 90% of cancer cells (Figure 1A, 1B). We observed that 15-40 µM of drug caused cell death and significantly affected Panc-2 cell viability and altered cell morphology (Figure 1C), which could be due to alteration in glucose consumption.
Pancreatic cancer cells are highly adaptable to the changed glucose metabolism [22,23]. This is achieved by elevated expression of hexokinase II which efficiently convert glucose and feeds into aerobic glycolysis and produces ATP for tumor growth [24,25]. Based on our experimental evidence it is delineated that Panc-2 cells over express hexokinase II and its N-terminal domain interacts and binds with VDAC-1, an outer mitochondrial membrane protein and regulates glycolysis and ATP transport across mitochondrial membrane. Their interaction also suppresses apoptosis by interacting with BCL2L1 (Figure 3) [26]. We observed that HK-II expression was almost completely abolished after treatment with 40 µM of 3-BP (Figure 2A), and there was no change in expression level of VDAC-1 protein (Figure 2A). Further, the Co-IP data suggest that 3-BP treatment disrupted the interaction between hexokinase II and VDAC-1, and blocked the transport of ATP across mitochondrial membrane and suppresses BCL2L1 an anti-apoptotic protein and causes cancer cell death (Figures 2B, 3B) [11,22,27,28]. 3-BP severely inhibited ATP generation in cancer cells treated with 25 µM and higher concentrations, which could be due to inhibition in glycolysis and ATP production (Figure 5A). The corollary findings have also suggested 3-BP induced cellular ATP inhibition in prostate cancer [29]. We also investigated mitochondrial integrity in 3-BP treated Panc-2 cells by performing transmission electron microscopy (TEM). Interestingly, there was collapse of mitochondrial structure in 3-BP treated Panc-2 cells. This is the first report that we have shown collapse of mitochondria in 3-BP treated Panc-2 cells (Figure 4A, 4B). Thus, it seems relevant to conclude that 3-BP treatment dismantled mitochondrial structure and ATP generation and activated mitochondrial caspases which led to cancer cell death [11,21]. Further, the metabolomics data also corroborated that 3-BP treatment of Panc-2 cells inhibited ATP and lactic acid production, which is responsible for energy supply in cancer cells, and creating acidic tumor microenvironment [21,30]. 3-BP also severely inhibited aspartate, serine, threonine and formylglycinamide (FGAR) metabolites production in treated pancreatic cancer cells. Specifically, upregulation of aspartate has been linked to tumor aggression and its inhibition has been proposed to be a potential therapeutic target in solid tumors [31].
We also tested the efficacy of 3-BP in a syngeneic mouse model of pancreatic cancer. The in vivo data showed significant inhibition of tumor growth, with a 75-80% tumor reduction in animals treated with 20 mg/kg of 3-BP (Figure 7A-C). The H&E and IHC staining demonstrated apoptosis and necrosis in drug treated tumor tissues, and inhibition in HKII and TGFβ expression (Figure 7D), and activation of cleaved caspase 3. Taken together, the in vivo data have demonstrated that 3-BP inhibits HKII and TGFβ and induces activation of caspase-3 and causes cancer cell death [21,32]. The flow cytometry data presented in Figure 9 strongly suggest that 3-BP treatment suppress MDSCs population in pancreatic cancer tumor microenvironment and allows infiltration of T effector cells. The corollary reports also suggest that eliminating MDSCs improves the ability of the host’s immune system to attack the cancer and improves the efficacy of immunotherapy treatment [33,34].
Acknowledgements
Authors thanks Dr. William Regine, Chair Department of Radiation Oncology, University of Maryland School of Medicine for valuable suggestions. We also thank Electron Microscopy, and Flow Cytometry Core services, University of Maryland School of Medicine. We also acknowledge financial support from New G Lab Pharma Inc.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Ko YH, Pedersena PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Letters. 2001;173:83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Liu Z, Zou X, Lan Y, Sun X, Wang X, Zhao S, Jiang C, Liu H. Mechanisms underlying 3-bromopyruvate-induced cell death in colon cancer. J Bioenerg Biomembr. 2015;47:319–329. doi: 10.1007/s10863-015-9612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Sun G, Huang Y, Hao Y, Tang X, Zhang N, Zhao L, Zhong R, Peng Y. 3-Bromopyruvate regulates the status of glycolysis and BCNU sensitivity in human hepatocellular carcinoma cells. Biochem Pharmacol. 2020;177:113988. doi: 10.1016/j.bcp.2020.113988. [DOI] [PubMed] [Google Scholar]
- 5.Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunjithapatham R, Geschwind JF, Rao PP, Boronina TN, Cole RN, Ganapathy-Kanniappan S. Systemic administration of 3-bromopyruvate reveals its interaction with serum proteins in a rat model. BMC Res Notes. 2013;6:277. doi: 10.1186/1756-0500-6-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TA, Xian SL, Guo XY, Zhang XD, Lu YF. Combined 18F-FDG PET/CT imaging and a gastric orthotopic xenograft model in nude mice are used to evaluate the efficacy of glycolysis-targeted therapy. Oncol Rep. 2018;39:271–279. doi: 10.3892/or.2017.6060. [DOI] [PubMed] [Google Scholar]
- 8.Dell’Antone P. Targets of 3-bromopyruvate, a new, energy depleting, anticancer agent. Med Chem. 2009;5:491–496. doi: 10.2174/157340609790170551. [DOI] [PubMed] [Google Scholar]
- 9.Zou X, Zhang M, Sun Y, Zhao S, Wei Y, Zhang X, Jiang C, Liu H. Inhibitory effects of 3-bromopyruvate in human nasopharyngeal carcinoma cells. Oncol Rep. 2015;34:1895–1904. doi: 10.3892/or.2015.4147. [DOI] [PubMed] [Google Scholar]
- 10.Cal M, Matyjaszczyk I, Litwin I, Augustyniak D, Ogórek R, Ko Y, Ułaszewski S. The anticancer drug 3 bromopyruvate induces DNA damage potentially through reactive oxygen species in yeast and in human cancer cells. Cells. 2020;9:1161. doi: 10.3390/cells9051161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petricciuolo M, Davidescu M, Fettucciari K, Gatticchi L, Brancorsini S, Roberti R, Corazzi L, Macchioni L. The efficacy of the anticancer 3-bromopyruvate is potentiated by antimycin and menadione by unbalancing mitochondrial ROS production and disposal in U118 glioblastoma cells. Heliyon. 2020;6:e05741. doi: 10.1016/j.heliyon.2020.e05741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leiser D, Samanta S, Eley J, Strauss J, Creed M, Kingsbury T, Staats PN, Bhandary B, Chen M, Dukic T, Roy S, Mahmood J, Vujaskovic Z, Shukla HD. Role of caveolin-1 as a biomarker for radiation resistance and tumor aggression in lung cancer. PLoS One. 2021;16:e0258951. doi: 10.1371/journal.pone.0258951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elgogary A, Xu Q, Poore B, Alt J, Zimmermann SC, Zhao L, Fu J, Chen B, Xia S, Liu Y, Neisser M, Nguyen C, Lee R, Park JK, Reyes J, Hartung T, Rojas C, Rais R, Tsukamoto T, Semenza GL, Hanes J, Slusher BS, Le A. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc Natl Acad Sci U S A. 2016;113:E5328–36. doi: 10.1073/pnas.1611406113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Ming L, Xinyou Y, Feng G, Wei L. Repression of hexokinases II-mediated glycolysis contributes to Piperlongumine-induced tumor suppression in non-small cell lung cancer cells. Int J Biol Sci. 2019;15:826–837. doi: 10.7150/ijbs.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Horst A, Versteijne E, Besselink MGH, Daams JG, Bulle EB, Bijlsma MF, Wilmink JW, van Delden OM, van Hooft JE, Franken NAP, van Laarhoven HWM, Crezee J, van Tienhoven G. The clinical benefit of hyperthermia in pancreatic cancer: a systematic review. Int J Hyperth. 2018;34:969–979. doi: 10.1080/02656736.2017.1401126. [DOI] [PubMed] [Google Scholar]
- 16.Chuong M, Chang ET, Choi EY, Mahmood J, Lapidus RG, Davila E, Carrier F. Exploring the concept of radiation “booster shot” in combination with an anti-PD-L1 mAb to enhance anti-tumor immune effects in mouse pancreas tumors. JSM Clin Oncol Res. 2017;5:1058. [PMC free article] [PubMed] [Google Scholar]
- 17.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeWaal D, Nogueira V, Terry AR, Patra KC, Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR, Hay N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nature. 2018;9:446. doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson M, Marayati R, Moffitt R, Yeh JJ. Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget. 2017;8:56081–56094. doi: 10.18632/oncotarget.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra KC, Hay N. Hexokinase 2 as oncotarget. Oncotarget. 2013;4:1862–1863. doi: 10.18632/oncotarget.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, Jha AK, Smolen GA, Clasquin MF, Robey B, Hay N. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell. 2017;31:5–19. doi: 10.1016/j.ccell.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Cao X, Bloomston M, Zhang T, Frankel WL, Jia G, Wang B, Hall NC, Koch RM, Cheng H, Knopp MV, Sun D. Synergistic antipancreatic tumor effect by simultaneously targeting hypoxic cancer cells with HSP90 inhibitor and glycolysis inhibitor. Clin Cancer Res. 2008;14:1831–9. doi: 10.1158/1078-0432.CCR-07-1607. [DOI] [PubMed] [Google Scholar]
- 24.Qin C, Yang G, Yang Y, Ren B, Wang H, Chen G, Zhao F, You L, Wang W, Zhao Y. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50. doi: 10.1186/s12943-020-01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, Yip YM, Li L. In silico construction of HK2-VDAC1 complex and investigating the HK2 binding-induced molecular gating mechanism of VDAC1. Mitochondrion. 2016;30:222–8. doi: 10.1016/j.mito.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Ko YH, Niedźwiecka K, Casal MJ, Pedersen PL, Ułaszewski S. 3-Bromopyruvate as a potent anticancer therapy in honor and memory of the late Professor André Goffeau. Yeast. 2019;36:211–221. doi: 10.1002/yea.3367. [DOI] [PubMed] [Google Scholar]
- 28.Wang TA, Zhang XD, Guo XY, Xian SL, Lu YF. 3‑Bromopyruvate and sodium citrate target glycolysis, suppress survivin, and induce mitochondrial-mediated apoptosis in gastric cancer cells and inhibit gastric orthotopic transplantation tumor growth. Oncol Rep. 2016;35:1287–1296. doi: 10.3892/or.2015.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valenti D, Vacca RA, de Bari L. 3-Bromopyruvate induces rapid human prostate cancer cell death by affecting cell energy metabolism, GSH pool and the glyoxalase system. J Bioenerg Biomembr. 2015;47:493–506. doi: 10.1007/s10863-015-9631-y. [DOI] [PubMed] [Google Scholar]
- 30.Parks S, Mueller-Klieser W, Pouysségur J. Lactate and acidity in the cancer microenvironment. Annu Rev Cancer Biol. 2020;4:141–58. [Google Scholar]
- 31.Garcia-Bermudez J, Baudrier L, La K, Zhu XG, Fidelin J, Sviderskiy VO, Papagiannakopoulos T, Molina H, Snuderl M, Lewis CA, Possemato RL, Birsoy K. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumors. Nat Cell Biol. 2018;20:775–781. doi: 10.1038/s41556-018-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Z, Kang D, Joo Y, Lee J, Oh GH, Choi S, Ko S, Je S, Choi HJ, Song JJ. TGF-β downregulation-induced cancer cell death is finely regulated by the SAPK signaling cascade. Exp Mol Med. 2018;50:1–19. doi: 10.1038/s12276-018-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Zhang L, Yang Y, Xia Z, Liu T, Wu G. Polymorphonuclear-MDSCs facilitate tumor regrowth after radiation by suppressing CD8+ T cells. Int J Radiat Oncol Biol Phys. 2021;109:1533–1546. doi: 10.1016/j.ijrobp.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 34.Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, Breden MA, Li X, Krisnawan VE, Khan SQ, Schwarz JK, Rogers BE, Fields RC, Hawkins WG, Gupta V, DeNardo DG. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med. 2019;11:eaau9240. doi: 10.1126/scitranslmed.aau9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.