Abstract
Porphyromonas gingivalis is considered among the etiological agents of human adult periodontitis. Although in vitro studies have shown that P. gingivalis has the ability to invade epithelial cell lines, its effect on the epithelial barrier junctions is not known. Immunofluorescence analysis of human gingival epithelial cells confirmed the presence of tight-junction (occludin), adherens junction (E-cadherin), and cell-extracellular matrix junction (β1-integrin) transmembrane proteins. These transmembrane proteins are expressed in Madin-Darby canine kidney (MDCK) cells. In addition, MDCK cells polarize and therefore serve as a useful in vitro model for studies on the epithelial cell barrier. Using the MDCK cell system, we examined the effect of P. gingivalis on epithelial barrier function. Exposure of the basolateral surfaces of MDCK cells to P. gingivalis (>109 bacteria/ml) resulted in a decrease in transepithelial resistance. Immunofluorescence microscopy demonstrated decreases in the amounts of immunoreactive occludin, E-cadherin, and β1-integrin at specific times which were related to a disruption of cell-cell junctions in MDCK cells exposed to basolateral P. gingivalis. Disruption of cell-cell junctions was also observed upon apical exposure to bacteria; however, the effects took longer than those seen upon basolateral exposure. Cell viability was not affected by either basolateral or apical exposure to P. gingivalis. Western blot analysis demonstrated hydrolysis of occludin, E-cadherin, and β1-integrin in lysates derived from MDCK cells exposed to P. gingivalis. Immunoprecipitated occludin and E-cadherin molecules from MDCK cell lysates were also degraded by P. gingivalis, suggesting a bacterial protease(s) capable of cleaving these epithelial junction transmembrane proteins. Collectively, these data suggest that P. gingivalis is able to invade the deeper structures of connective tissues via a paracellular pathway by degrading epithelial cell-cell junction complexes, thus allowing the spread of the bacterium. These results also indicate the importance of a critical threshold concentration of P. gingivalis to initiate epithelial barrier destruction.
The gram-negative, black-pigmented bacterium Porphyromonas gingivalis is recognized as one of the primary etiologic agents of adult periodontal disease. P. gingivalis has been commonly isolated from periodontal disease sites, and patients with periodontitis have serum antibodies specific to this pathogen. Thus, P. gingivalis has the ability to invade host tissues. Recent studies have also provided evidence that the effects of periodontal pathogens on the host are not limited to periodontal tissues; they can be more widespread and systemic. Associations between periodontal pathogens and cardiovascular disease (5) and low birth weight (37) have been suggested. Perhaps this represents the tip of the iceberg as to the consequences of harboring pathogens such as P. gingivalis for the systemic manifestation of diseases.
Bacterial colonization of soft-tissue structures is central to periodontal disease pathogenesis since microbes and their products gain access to the subepithelial connective tissue from the sulcular region of gingiva, where the pathophysiological process of periodontal disease is initiated (60). P. gingivalis can invade gingival epithelial cells (48), and its replication in oral epithelial cell lines and primary cultures of gingival epithelial cells has been shown in vitro (10, 28, 49, 50). However, it is not clear how this pathogen gains access to the underlying connective tissue. Studies with the periodontal pathogen Actinobacillus actinomycetemcomitans have shown that this bacterium invades the oral epithelium and that invasion can be an actin-dependent process (6, 54). These results support the concept that this bacterium gains access to the deeper tissues by cell-cell spread.
Epithelial cells throughout the body are polarized, which plays a significant role in resistance to infection (14). Polarity is established through cell surface signals and requires both cell-cell and cell-extracellular matrix adhesion to create the specialized apical and basolateral domains. The apical domain is separated from the lateral domain by the zonula occludens, which forms the tight junction. The transmembrane protein occludin is among the tight-junction components (16). Adjacent to the tight junction is the zonula adherens (also known as the adherens junction), whose major structural protein, E-cadherin, is responsible for homotypic cell-cell adhesion and the development of a polarized phenotype in the epithelium (47).
Basolateral membranes have been shown to express specific receptors by which certain enteropathogens such as Listeria monocytogenes and Yersinia species invade host cells. For Yersinia pseudotuberculosis and Yersinia enterocolitica, the most efficient pathway for invasion is promoted by invasin, which binds directly to β1-integrin receptors on the mammalian cell membrane (22, 23, 46). Unlike Yersinia species, the gram-positive bacterium L. monocytogenes binds E-cadherin as the ligand for internalin, a protein essential for entry of L. monocytogenes into epithelial cells (32). In addition to intracellular pathways for tissue invasion, paracellular proteolysis of the epithelial barrier could render the subepithelial connective-tissue structures liable to bacterial invasion. Breakdown of the interconnecting epithelial cell adhesions has been shown through a by-product of Bacteroides fragilis. This enteropathogen secretes a proteinase which specifically cleaves tight and adherens junctions (36, 65). The elimination of E-cadherin molecules from the basolateral surfaces of the epithelial cells renders them and underlying tissues susceptible to infection.
P. gingivalis possesses a number of factors of potential importance in the periodontal disease process. Among these factors are fimbriae (19, 24, 38, 63, 66), lipopolysaccharide (20), hemagglutinins (11, 34, 40, 44, 45), capsule (55, 61), and proteases (1, 9, 42, 43, 59). The proteolytic capability of P. gingivalis strains is known; however, only recently have protease genes been cloned. Although studies have reported that P. gingivalis expresses arginine- and lysine-specific proteases (4, 39, 41, 43), the direct involvement of the proteases in the pathogenesis of periodontal disease is not yet known.
The aim of the present study was to determine the effects of P. gingivalis on epithelial cell barrier function and junctional complexes. Specifically, we set out to determine the involvement of E-cadherin (adherens junction), occludin (zonula occludens junction), and β1-integrin (cell-extracellular matrix junction) in the pathogenesis of periodontal disease. In the present study, we have utilized as an in vitro model the Madin-Darby canine kidney (MDCK) cells. MDCK cells have been widely used for studies in epithelial cell biology, since they form a well-polarized epithelial monolayer, essentially reconstituting a simple epithelial tissue. In addition, polarized MDCK cells express adherens, zonula occludens, and cell-extracellular matrix junctions.
MATERIALS AND METHODS
Bacteria.
P. gingivalis ATCC 33277 was used in these studies. The bacteria were cultured and maintained on enriched Trypticase soy agar (ETSA) plates consisting of Trypticase soy agar supplemented with yeast extract (1%), 5% defribinated sheep blood, hemin (5 mg/liter), and menadione (1 mg/liter) at 37°C in an anaerobic atmosphere of 10% H2, 5% CO2, and 85% N2 (56, 57). For the preparation of P. gingivalis for in vitro studies, cultures were grown in basal anaerobic broth (57) at 37°C under anaerobic conditions (26, 27, 57). The bacteria were harvested, washed in sterile Dulbecco's phosphate-buffered saline containing Mg2+ (0.4919 mM MgCl2 · 6H2O) and Ca2+ (0.9009 mM CaCl2) (PBS+), and centrifuged (6,000 × g for 20 min). The number of bacteria in the suspension was determined by reading the optical density at 580 nm and extrapolating from a standard curve. The bacteria were then centrifuged and resuspended in antibiotic-free minimal essential medium (MEM) containing Earl's balanced salt solution (Cellgro; Mediatech, Inc., Washington, D.C.) supplemented with 5% fetal calf serum (Hyclone, Logan, Utah).
Cell culture.
Type II MDCK cells were used between passages 5 and 15. Cells were cultured in MEM containing Earl's balanced salt solution supplemented with 5% fetal calf serum and 100 U of penicillin, 100 mg of streptomycin, and 0.25 μg of amphotericin B/ml. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air. For all our experiments, MDCK cells were seeded at confluency on Transwell filter units (Costar, Cambridge, Mass.) with either 3- or 0.4-μm-diameter pores. Cell monolayers were used for experiments after 3 days of culture with daily changes in media.
Primary cultures of gingival epithelial cells.
Biopsies of human gingiva were obtained from patients undergoing periodontal surgery or preprosthetic surgical extractions in the Department of Periodontics, University of Alabama School of Dentistry. Specimens were obtained from clinically healthy sites and diseased sites from which tissue was removed due to functional or aesthetic considerations or as part of periodontal treatment. Gingival tissue samples were placed in culture medium (described above) on ice immediately after surgical excision. Within 1 h, tissues were prepared to obtain epithelial cells by a modified explanation technique (64). Briefly, under sterile conditions, gingival specimens were cut into small pieces and washed by gentle shaking in 70% ethanol to remove bacteria and reduce the risk of contamination. The tissue pieces were rinsed in medium (described above) and placed in culture flasks for 30 to 60 min to allow their attachment to the plastic surface. The tissue explants were incubated in serum-free keratinocyte (SFK) medium to which supplements were added according to the manufacturer's instructions (GIBCO, Grand Island, N.Y.). The SFK medium was supplemented with 5% fetal calf serum for the first 4 to 6 weeks, and then SFK medium alone was used. For immunofluorescence studies (see below), cells were used at passage one or two and plated at confluency on Transwell filter units with 0.4-μm-diameter pores.
Bacterial translocation and measurement of electrical resistance.
Bacterial translocations from apical to basolateral surfaces and from basolateral to apical surfaces were assessed using monolayers of MDCK cells cultured on 3-μm-pore-size filters. The total volumes of antibiotic-free medium in the apical and basolateral chambers were 600 and 1,200 μl, respectively. Freshly harvested P. gingivalis cells (10-fold dilutions, ranging from 1011 to 106 bacteria/ml) were added to the apical (200-μl) or basolateral (400-μl) compartment. Aliquots were removed from the apical (10 μl) and basolateral (20 μl) compartments at 0, 2, 4, 6, and 8 h and cultured on ETSA plates at 37°C in an anaerobic environment. The CFU were counted after 7 days of incubation. Controls included wells with MDCK cells alone or with bacteria and no cells. Measurements of the transepithelial resistance (TER) in monolayers cultured on 0.4-μm-pore-size filters at 0, 2, 4, 6, 8, and 24 h were done using an EVOM electrical resistance system (World Precision Instruments, New Haven, Conn.) (17). Filter units with no MDCK cells or with bacteria only were used to obtain baseline levels. All conditions were established in triplicate for each experiment. The TER results are expressed as the measured resistance in ohms multiplied by the area of the filter (1 cm2).
Invasion assay.
An antibiotic protection assay as modified for P. gingivalis (10, 14, 49, 58) was used to quantitate bacterial invasion of the epithelial cells. For this assay, MDCK cells were cultured on 0.4-μm-pore-size filters. The medium was removed from the apical compartment, and 200 μl of antibiotic-free medium containing 1010 P. gingivalis cells/ml (total volume of 600 μl) was added. Fresh antibiotic-free medium (1,200 μl) was also added to the basolateral compartment. The cultures were incubated for 15, 45, or 90 min at 37°C. External nonadherent bacteria were removed by washing the MDCK cells three times with PBS+. MDCK cells were then incubated for 60 min at 37°C with gentamicin (300 μg/ml) and metronidazole (200 μg/ml) to kill extracellular bacteria. The MDCK cells were then washed three times with PBS+, and internalized bacteria were released by incubating cells in sterile distilled water for approximately 45 min at 37°C for cell lysis. The lysates were plated on ETSA plates and incubated at 37°C under anaerobic conditions. Black-pigmented colonies were counted after 7 days, and the numbers of CFU in the lysates (invasive bacteria) were determined. All conditions were established in triplicate.
Assessment of epithelial cell injury.
MDCK cell monolayers, cultured as described above, were incubated with 1010 P. gingivalis cells/ml for 4 or 24 h. The culture medium in the apical compartment was collected, and nonadherent cells were recovered by centrifugation. The adherent cells were washed one time with PBS− (PBS+ without Ca2+ or Mg2+) and detached from the filter by treatment with EDTA-trypsin. A portion of the cells was resuspended in 0.4% trypan blue (Sigma), and viability was assessed by trypan blue exclusion using light microscopy. Cytotoxicity was also determined using the LIVE/DEAD EukoLight viability/cytotoxicity assay (Molecular Probes, Inc., Eugene, Oreg.), which differentiates between viable and dead cells by using green and red fluorescent dyes, respectively. The percentages of viable and dead cells were determined using a fluorescence microscope (see below).
Immunofluorescence analysis of epithelial cells.
MDCK cells were grown on 0.4-μm-pore-size Transwell filters. P. gingivalis cells (1010/ml) were added to the apical or basolateral compartments of sets of wells prepared in triplicate. A third set of wells had no bacteria added. Wells with bacteria in the apical compartment were incubated for 1, 6, 12, 24, or 48 h, whereas wells with bacteria in the basolateral domain were incubated for 0.5, 1, 2, 4, or 8 h. Cultures of MDCK cells or primary epithelial cells were fixed and labeled as previously described (3) with minor modifications. Briefly, at the indicated times, the filters were washed once in cold PBS+ and the cells were fixed with ice-cold 4% paraformaldehyde in PBS+ for 20 min. After the filters were washed three times with PBS+, the cells were quenched with 75 mM NH4Cl–20 mM glycine, pH 8.0, plus KOH (quench solution) for 10 min at room temperature (RT). Filters were washed one time with PBS+ and permeabilized with PBS+–0.7% fish skin gelatin–0.025% saponin (PFS) for 15 min at 37°C. Cells were labeled with mouse anti-human β1-integrin monoclonal antibody (MAb; 1:100 dilution in PFS) (Life Technologies, Inc., Gaithersburg, Md.), mouse anti-human E-cadherin MAb (1:500) (Transduction Laboratories, Lexington, Ky.), or rabbit anti-human occludin polyclonal antibody (PAb) (1:500) (Zymed Laboratories Inc., San Francisco, Calif.). Cells were incubated for 60 min at 37°C with the primary antibody, washed four times for 5 min each with PFS at RT, and then incubated with the appropriate fluorescein-labeled secondary antibody (rabbit anti-mouse or goat anti-rabbit immunoglobulin G [IgG]; Jackson ImmunoResearch Laboratories, West Grove, Pa.) in PFS for 60 min at 37°C. Filters were rinsed four times for 5 min each with PFS, one time with PBS+, two times with PBS+ containing 0.1% TX-100, and one time with PBS+. Cells were postfixed with 4% paraformaldehyde for 15 min at RT. Filters were cut from the support with a scalpel and mounted in Vectashield mounting medium (Vector Laboratory, Burlingame, Calif.). Immunofluorescent images were obtained with a Leica fluorescence microscope equipped with a Hamamatsu C5810 digital camera as previously described (2). The generated photomicrographs were captured and labeled using Adobe Photoshop on a Power Macintosh G3.
Cell lysate preparation and immunoprecipitation.
Three sets of cultures, similar to those described in the immunofluorescence studies, were used for the preparation of cell lysates. Adherent cells on filters were then exposed to 0.2 ml of 20 mM Tris-HCl (pH 7.4)–150 mM NaCl–0.1% sodium dodecyl sulfate–1% TX-100–1% deoxycholic acid–5 mM EDTA (radioimmunoprecipitation assay [RIPA] buffer) containing inhibitors of proteases (2 mM phenylmethylsulfonyl fluoride; 5 μg of pepstatin, 10 μg of chymostatin, 5 μg of leupeptin, and 10 μg of antipain/ml; 500 μM benzamidine; 5 μU of aprotinin/ml) for 15 min on ice. Nonadherent cells in the apical compartment were collected by centrifugation and added to the RIPA buffer on the respective filters. Adherent cells were scraped from the filter with a rubber policeman. Total cell lysates were sedimented in a 4°C microcentrifuge. The protein concentration of each cell lysate was determined by using the bicinchoninic acid protein determination assay (Pierce Chemical Co., Rockford, Ill.). For the immunoprecipitation of E-cadherin and occludin, MDCK cells were cultured in 10-cm-diameter tissue culture petri dishes until confluent. Confluent monolayers were rinsed once in ice-cold PBS+ and lysed in RIPA buffer. Total cell lysates were sedimented in a 4°C microcentrifuge. Soluble lysates were rotated with either an E-cadherin MAb or an occludin PAb. Immunocomplexes were collected with affinity-purified, rabbit anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) coupled to protein A-Sepharose beads or protein A-Sepharose beads alone. Immunoprecipitated beads were washed three times with PBS+ and resuspended in 200 μl of the same. Twenty microliters of immunoprecipitated E-cadherin or occludin was exposed to 5 μl of 0.25% trypsin, bacterial suspension (1010 cells/ml), bacterial culture supernatant, or PBS+ (control) for specific incubation periods. The reactions were terminated by the addition of 8 μl of 4× Laemmli buffer containing 100 mM dithiothreitol and boiling for 5 min.
Electrophoresis and Western blotting.
Cell lysates or immunoprecipitates of E-cadherin or occludin incubated with bacteria or trypsin were electrophoresed in sodium dodecyl sulfate–8% polyacrylamide gel electrophoresis minigels and transferred to Immobilon P filters (Millipore Corp., Bedford, Mass.). Filters were blocked for 60 min at RT with PBS−–5% milk–0.1% Tween 20 (block solution) and probed with an occludin PAb (1:500 dilution), an E-cadherin MAb (1:500), and a β1-integrin MAb (1:500) (Transduction Laboratories). The filters were then washed five times for 5 min each with PBS−–0.1% Tween 20 (wash solution) and probed with horseradish peroxidase-labeled goat anti-mouse (1:25,000 for E-cadherin; 1:10,000 for β1-integrin) or goat anti-rabbit (1:25,000) IgG (Jackson ImmunoResearch Laboratories, Inc.) diluted in block solution for 60 min. Filters were washed five times for 5 min each with wash solution. All filters were visualized on Kodak X-OMAT AR film with an enhanced chemiluminescence kit (ECL; Amersham Corp.). Autoradiographs were scanned and saved as Adobe Photoshop files with a UMAX PowerLook II scanner.
RESULTS
Characterization of gingival epithelial cells for transmembrane proteins.
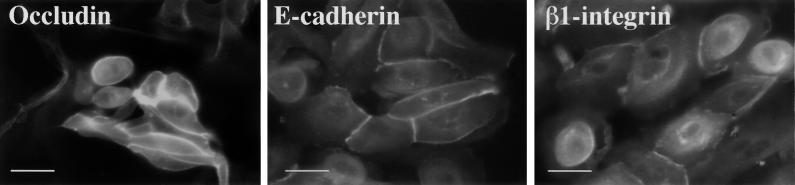
MDCK cells have been used as a model to study interactions between bacterial pathogens and epithelial cells (13, 33). When grown on permeable supports, MDCK cells are well polarized with tight and adherens junctions characterized by the presence of occludin (18), E-cadherin (3), and integrins (51). Since P. gingivalis is a periodontal pathogen, we wanted to determine if the junctional proteins occludin, E-cadherin, and β1-integrin were present in the gingival epithelium in a manner analogous to that previously observed in MDCK cells. The transmembrane proteins occludin, E-cadherin, and β1-integrin were present in primary cultures of gingival epithelial cells (Fig. 1). Immunofluorescence staining of gingival epithelial cells showed junctional and cytoplasmic localization of E-cadherin, occludin, and β1-integrin. No staining was seen with cells incubated with secondary antibody only (data not shown). Furthermore, junctional complexes in the epithelial layers of gingival tissue sections were demonstrated (data not shown). These data demonstrated the presence of junctional proteins in gingival tissue similar to those detected in MDCK cells and support the use of the MDCK cell system as an in vitro model to study the interaction of P. gingivalis with the gingival epithelium.
FIG. 1.
Immunofluorescence analysis of primary cultures of gingival epithelial cells for cell-cell junction complexes. Primary cultures of gingival epithelial cells were incubated with antibodies to occludin, E-cadherin, or β1-integrin followed by incubation with the appropriate fluorescein-labeled secondary antibody. Bars, 50 μm.
Changes in transepithelial resistance.
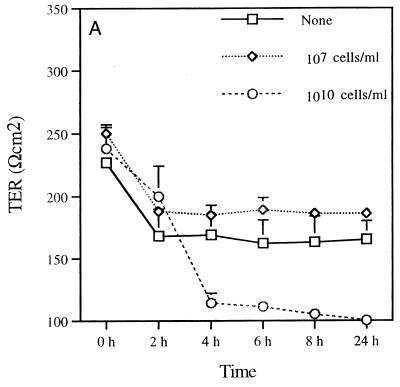
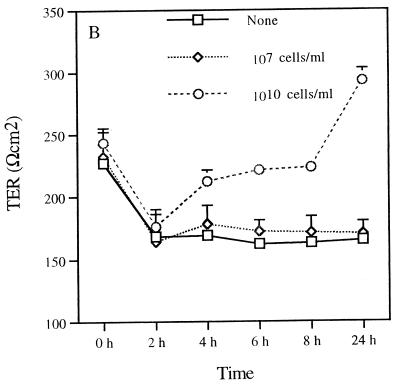
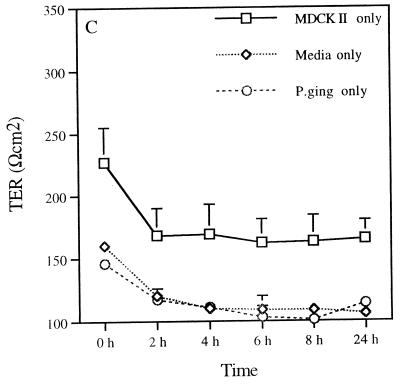
The permeability of the epithelial monolayer tight-junction barrier is commonly assessed by measuring TER. This is a measure of the barrier to small ions (predominantly Na+ and Cl−) in an experimentally applied electrical field in the bathing medium (12). Therefore, the measurement of the TER of MDCK cells reflects the integrity of tight junctions across the monolayer (17). Assessment of the TER after incubating P. gingivalis with MDCK cells provided information on the ability of this pathogen to alter the epithelial cell barrier function. Changes in the TER of the MDCK cell monolayer upon exposure to P. gingivalis were seen (Fig. 2). Basolateral exposure of MDCK cell monolayers to P. gingivalis (1010 cells/ml) decreased the TER of MDCK cells in a time-dependent manner (Fig. 2A). The most acute drop in resistance was observed between 2 and 4 h. In some experiments, the pronounced drop in resistance occurred slightly later (between 5 and 7 h; data not shown). After 4 h of basolateral exposure, the resistance was not significantly different from the resistance found in filters without cells (Fig. 2C). A more rapid decrease in the TER of MDCK cells was seen when 1011 bacteria/ml were added. In contrast, exposure of the apical surfaces of MDCK cells to P. gingivalis (1010 cells/ml) resulted in an increase in the TER up to 24 h (Fig. 2B). The resistance then dropped between 24 and 48 h (not shown). The initial drop in resistance between 0 and 2 h (Fig. 2A and B) was due to the change of media at the beginning of the experimental period. It can be seen that after 2 h the system is equilibrated. These data indicate that P. gingivalis disrupts epithelial barrier function and that the basolateral surface is more susceptible to this effect than the apical surface.
FIG. 2.
Effect of exposing MDCK cells to P. gingivalis on TER. The TER of monolayers of MDCK cells cultured in Transwell filter units with 0.4-μm-diameter pores was assessed at various times following incubation with P. gingivalis (107 or 1010 bacteria/ml) on the basolateral (A) or apical (B) surface. Transwell units incubated with MDCK cells, media, or apical P. gingivalis (P. ging) only served as controls (C). The results are expressed as means ± standard deviations.
Because these experiments were carried out using MDCK cell monolayers grown on filters with a 0.4-μm pore size, the effects of basolaterally applied P. gingivalis on TER suggest that direct contact of the bacterium with the epithelial cells is not necessary for the effects observed. These results strongly suggest the involvement of a bacterial soluble factor which is able to diffuse through the membrane pores to elicit the effect on TER. In addition, the finding that a lower concentration of P. gingivalis (<109 bacteria/ml) did not alter the TER (Fig. 2A and B and data not shown) suggests that a critical threshold concentration of P. gingivalis is required to disrupt the barrier function. In control wells containing MDCK cells without bacteria, the TER was almost twofold higher than that seen in medium-only wells or in wells with apically applied P. gingivalis but no MDCK cells (Fig. 2C). The TER measurements in wells with media only or with P. gingivalis alone were similar.
Bacterial translocation and invasion of MDCK cells.
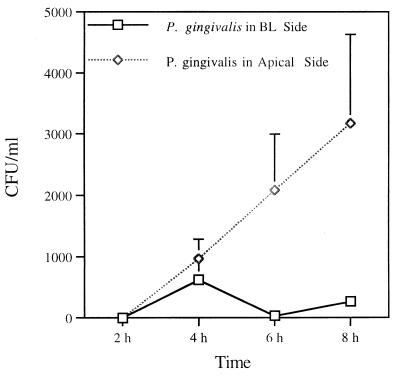
When P. gingivalis was added to the apical compartment of MDCK cells cultured on 3-μm-pore-size filters, the number of P. gingivalis cells recovered in the basolateral medium increased over time (Fig. 3). However, an assessment of translocation following addition of bacteria in the basolateral compartment revealed variability and only a few P. gingivalis cells in the apical medium. This finding indicates that this bacterium does not translocate efficiently from the basolateral to the apical compartment.
FIG. 3.
Quantitation of translocation of P. gingivalis across the MDCK cell monolayer. Freshly harvested P. gingivalis was added to either the basolateral (BL) or apical surfaces of MDCK cells in Transwell filter units. Aliquots were removed from the basolateral (20 μl) or apical (10 μl) compartments and plated on ETSA plates under anaerobic conditions for 7 days. Results for triplicate cultures are shown.
We next assessed the invasion of MDCK epithelial cells by P. gingivalis using a modified invasion assay (see Materials and Methods). Monolayers of MDCK cells were harvested at 15, 45, and 90 min following incubation with P. gingivalis, and aliquots of cell lysates were cultured on ETSA plates. The mean numbers of black-pigmented colonies recovered from the cell lysates at 15, 45, and 90 min were 2.9 × 105, 1.7 × 104, and 2.5 × 104 CFU/ml, respectively.
Immunofluorescence studies.
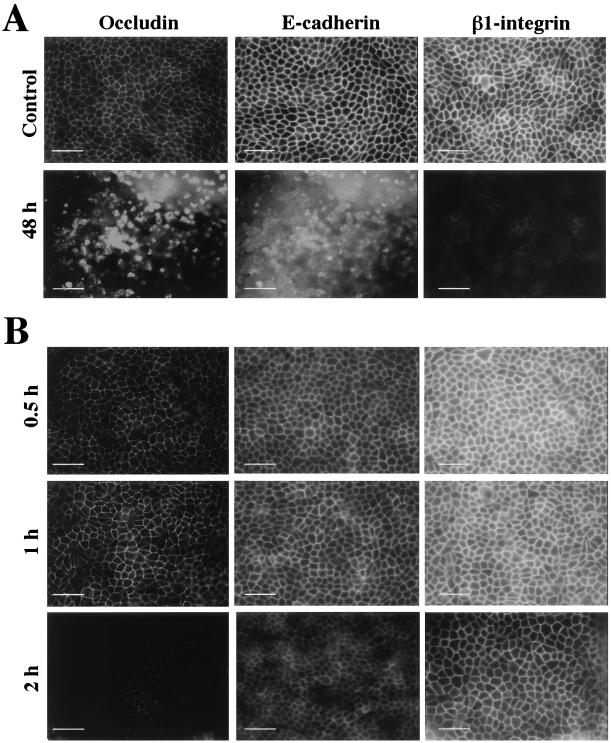
The effect of P. gingivalis on the epithelial cell barrier proteins of MDCK cells was evaluated by immunofluorescence using antibodies to occludin, E-cadherin (intracellular domain), and β1-integrin. MDCK cells were incubated with P. gingivalis (1010 bacteria/ml) added to either the apical or basolateral surface for specific time periods. Control MDCK cells (no bacteria) showed uniform staining for occludin, E-cadherin, and β1-integrin (Fig. 4A; control). The effects of apically applied P. gingivalis after 1, 6, 12, 24, and 48 h were assessed. No difference in the levels of immunolabeling of occludin, E-cadherin, and β1-integrin was detected for up to 24 h following exposure of the apical surfaces of cells to P. gingivalis (data not shown). However, after 48 h, immunofluorescence labeling of the cells showed disruption of junctional occludin, E-cadherin, and β1-integrin (Fig. 4A; 48 h). These observations suggested either a loss of junctional proteins or perhaps cell death following 48 h of apical exposure to P. gingivalis. The effect of incubating P. gingivalis on the basolateral surfaces of MDCK cells for 0.5, 1, 2, 4, and 8 h was also assessed. After 2 h of exposure, there was a qualitative decrease in the labeling intensities of occludin, E-cadherin, and β1-integrin (Fig. 4B). However, after 4 h, immunofluorescence labeling of the cells became technically difficult because the cells detached from the filter during the fixation and labeling procedure. This observation suggested a loss of cell-cell junctional proteins or perhaps that the cells were dead. These data correlate with the TER data in that they demonstrate a differential sensitivity of polarized epithelial cells to the effects of P. gingivalis. That is, the apical surface of the cell monolayer seems to be relatively more resistant than the basolateral surface to the effects of P. gingivalis. Exposure of cell monolayers to lower numbers of P. gingivalis cells (108 to 102 bacteria/ml) did not cause detachment of MDCK cells even after 72 h of bacterial exposure (not shown).
FIG. 4.
Immunofluorescence analysis of MDCK cells following exposure of their apical or basolateral surfaces to P. gingivalis (1010 bacteria/ml). (A) Effects of apical exposure to bacteria at 48 h. Control, MDCK cells without bacteria. (B) Basolateral exposure to P. gingivalis at 0.5, 1, and 2 h. Bars, 50 μm.
Cell viability.
It was important to ascertain if the lack of visualization of junctional proteins by immunofluorescence was due to degradation of epithelial cell junctions, not merely to the cytotoxicity of P. gingivalis. To address this issue, cell viability was assessed following exposure of MDCK cells to P. gingivalis for up to 24 h. Two methods were utilized to assess viability, namely, trypan blue dye exclusion and the LIVE/DEAD viability/cytotoxicity assay. No difference in the viability of MDCK cells, as determined by trypan blue exclusion, was seen following incubation for 24 h with P. gingivalis cells in either the apical (75.4%) or basolateral (81.3%) surface, compared to that of control cells (75.2%). Similar results were observed with the LIVE/DEAD fluorescence assay.
Western blotting.
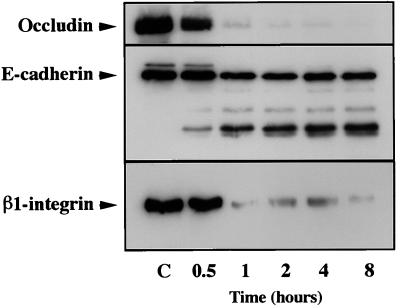
To assess whether the mechanism for the loss of occludin, E-cadherin, and β1-integrin, as identified by immunofluorescence, was indeed a degradative process, Western blot analysis with antibodies specific for occludin, E-cadherin, and β1-integrin was used to analyze lysates prepared from MDCK cells exposed to basolateral P. gingivalis. The time course of the effect of basolateral exposure to P. gingivalis (1010 cells/ml) on MDCK cells was evaluated (Fig. 5). After 30 min of bacterial exposure, amounts of mature occludin, E-cadherin, and β1-integrin were decreased. For occludin and β1-integrin, over 90% of the protein was degraded by 1 h. E-cadherin was also degraded during basolateral exposure to P. gingivalis with the generation of several fragments. The most prominent of these fragments has an apparent molecular mass of 81 kDa. Because we used an antibody which recognizes an intracellular epitope of E-cadherin and because 80% of the mature 124-kDa E-cadherin molecule is predicted to be extracellular (35), this suggests that cleavage of E-cadherin by P. gingivalis occurred close to the extracellular surface of the plasma membrane. A similarly sized fragment of E-cadherin is generated by exposure of MDCK cells to trypsin in the absence of Ca2+ (17). Similar results were obtained following exposure of the apical surfaces of MDCK cells to P. gingivalis for periods extended to 24 h. These results provide evidence that epithelial exposure to P. gingivalis results in the degradation of epithelial junction proteins. Exposure of MDCK cells to P. gingivalis at a concentration of 107 cells/ml for periods extended to 24 h did not result in any apparent degradation of these junctional proteins, as determined by Western blot analysis (data not shown). Taken together, these data agree with previous observations that a certain concentration of bacteria is necessary for the degradation of epithelial junction complexes at the time points evaluated in this study.
FIG. 5.
Immunoblot of MDCK cell lysates basolaterally exposed to P. gingivalis (1010 bacteria/ml) for 0.5, 1, 2, 4, and 8 h and reacted with occludin, E-cadherin, and β1-integrin. Controls (C), blots of lysates prepared from MDCK cells not exposed to P. gingivalis.
Effect of P. gingivalis and culture supernatant on immunoprecipitated E-cadherin and occludin molecules.
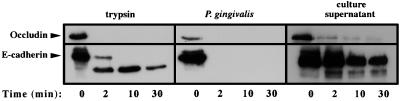
In order to test whether the observed degradation of junctional proteins was due to a direct protease produced by P. gingivalis or by a mechanism in which an epithelial cell protease(s) is activated by exposure to P. gingivalis, we next examined the proteolytic effect of P. gingivalis and P. gingivalis culture supernatant on immunoprecipitated E-cadherin and occludin molecules (β1-integrin antibodies used in this study did not work well for immunoprecipitation). Immunoprecipitated E-cadherin or occludin molecules were incubated with trypsin, P. gingivalis, or P. gingivalis culture supernatant for 2, 10, and 30 min. After a 2-min incubation with 1010 bacteria/ml, occludin and E-cadherin were completely degraded (Fig. 6, middle). After 2 min of incubation with trypsin, occludin was completely degraded. E-cadherin was also sensitive to trypsin digestion. However, after 30 min of incubation with trypsin, the E-cadherin 81-kDa proteolytic fragment was still detectable (Fig. 6, right, 30-min time point). The lag in the degradation of E-cadherin by trypsin may be due to the presence of Ca2+, which is known to stabilize E-cadherin digestion by trypsin (17). While not as potent as P. gingivalis or trypsin, P. gingivalis culture supernatant alone degraded immunoprecipitated occludin and E-cadherin molecules (Fig. 6, left). These studies indicate a very high proteolytic activity of the periodontal pathogen P. gingivalis for junctional proteins of epithelial cells.
FIG. 6.
Effect of trypsin, P. gingivalis, and P. gingivalis culture supernatant on immunoprecipitated occludin and E-cadherin after 2, 10, and 30 min. The immunoblot analysis was performed as described in Materials and Methods.
DISCUSSION
The integrity of the epithelium plays an important role in host defense against pathogens. In the present study, we investigated the effects of the periodontal pathogen P. gingivalis on occludin, E-cadherin, and β1-integrin, which are important molecules involved in cell-cell and cell-extracellular matrix adhesion and in maintaining the integrity and polarity of the epithelium. These transmembrane proteins are expressed by gingival epithelial cells as established by immunofluorescence using antibodies specific to these proteins. Previous studies have reported the presence of β1-integrin in gingival tissue (21, 31); however, to our knowledge the present study is the first report of the presence of occludin and E-cadherin in primary cultures of gingival epithelial cells. Our findings showed that E-cadherin and β1-integrin were colocalized adjacent to occludin, thus indicating that β1-integrin was present in areas of cell-cell adhesion. Studies by Schoenenberger et al. (51) and Larjava et al. (30) have shown the presence of β1-integrin at similar sites in MDCK and epidermal keratinocytes, respectively. Although the studies by Larjava et al. (30) indicated a role of β1-integrins in cell-cell adhesion, there is currently no consensus as to the function of these molecules.
MDCK cells were used in the present study as an in vitro model to investigate P. gingivalis effects on the epithelial cell barrier. MDCK cells form a well-polarized epithelial monolayer and express occludin, E-cadherin, and β1-integrin. It has been previously reported that a decrease in the TER of MDCK cells is associated with an alteration of the epithelial cell barrier (17). In the present study, we have shown a decrease in the TER of MDCK cells within 4 h after exposure of their basolateral surfaces to P. gingivalis. Exposure of the apical surfaces of MDCK cells to P. gingivalis caused an increase in resistance during the first 24 h prior to a decrease. These results suggest that basolateral surfaces are more susceptible than apical surfaces to the effects of P. gingivalis. Fleiszig et al. (14) demonstrated that cell polarization characterized by apical and basolateral domains is involved in defense against Pseudomonas aeruginosa infection and that increased susceptibility to bacterial invasion occurred at exposed basolateral surfaces. Furthermore, Wu et al. (65) demonstrated that B. fragilis toxin affects epithelial cell morphology and function in a more potent and rapid manner when exposed to the basolateral membranes.
The drop in the resistance of MDCK cells after the addition of P. gingivalis to the basolateral (after 2 h) or apical (after 24 h) surface was consistent with detachment of MDCK cells from the filters, as established by immunofluorescence analysis, implying a disruption of the epithelial barrier proteins. Studies by Kallman and Kihlstrom (25) showed that group B streptococcus U5 caused a decrease in resistance and some widening of intercellular spaces after 24 h of apical bacterial inoculation. However, Finlay et al. (13), who examined the interactions between Salmonella enterica serovar Choleraesuis and MDCK cells showed a drop in resistance without an alteration of intercellular morphology. The various observations can perhaps be reconciled on the basis of the specific bacterial pathogens used in the studies.
The increase in the TER of MDCK cells following apical bacterial inoculation could represent a defense mechanism by the epithelial cell barrier before succumbing to the bacterial load (24 to 48 h). Immunofluorescence and Western blot analysis did not reveal a more pronounced staining of occludin, E-cadherin, or β1-integrin. Furthermore, the accumulation of P. gingivalis in the pores of the filters could not account for the increased resistance, since there was no change in the TER of filters incubated with bacteria only. Therefore, at this time we cannot provide a sound explanation for this observation.
The change in TER of MDCK cells was dependent on the number of P. gingivalis cells added since the addition of a lower number of bacteria to the cultures had no effect on the TER. Others have shown a dependency on the number of S. enterica serovar Choleraesuis cells and the loss of resistance of MDCK cells (13). These findings indicate that the threshold of bacteria is important for the resulting effects. Specifically, for P. gingivalis, this point is of paramount importance in relation to prevention, treatment, and periodontal health maintenance.
P. gingivalis was shown to cross the MDCK epithelial cell monolayer from the apical to the basolateral compartment but not to cross in the opposite direction. Studies with group B streptococci (25), but not with Salmonella (13), have shown similar results. Whether cell- and/or bacterium-specific receptors are involved in this process, as in the binding of L. monocytogenes to E-cadherin (32) or that of Yersinia species to β1-integrin (23), is at this time not known. The movement of P. gingivalis through the epithelium could involve the intra- or paracellular pathway or both. Since black-pigmented colonies were recovered from the lysates of MDCK cells following incubation with P. gingivalis, it is likely that the intracellular pathway is involved in the translocation/transcytosis of P. gingivalis from the apical to the basolateral surface. Studies by Lamont et al. (28) showed that P. gingivalis invasion of healthy derived gingival epithelial cells occurred at about 13% of the initial inoculum. Furthermore, invasion efficiency increased over time for up to 90 min. Duncan et al. (10) assessed P. gingivalis invasion of the human oral epidermoid KB cell line, and, although invasion took place, it was less than 0.1%. In the present study, about 0.01% of the initial inoculum was detected after 15 min in lysates of MDCK cells. This low level of bacterial invasion may be due to the observed increased resistance of the MDCK cells. Meyer et al. (33) have shown that invasion of MDCK cells by A. actinomycetemcomitans is substantially lower than that seen with KB cells. It is known that MDCK cells form a well-polarized epithelial monolayer and thus are excellent for studies of microbial effects on the epithelial cell barrier (13, 14). It is known that KB cells do not polarize well. This is no surprise, since KB cells do not express occludin or E-cadherin, which are important in cell-cell interactions, although they do express β1-integrin (personal observations).
Passage of P. gingivalis across the MDCK monolayer was at least in part through a paracellular route based on the evidence that P. gingivalis did not appear to have an effect on MDCK cell viability but did have an effect on the integrity of the epithelial cell barrier. Results of Western blot analysis indicated that following basolateral incubation of P. gingivalis with MDCK cells, degradation of occludin, E-cadherin, and β1-integrin was apparent. This was confirmed by immunoprecipitation studies with purified E-cadherin and occludin and demonstrated that the paracellular proteolysis observed was most likely due to a proteinase(s) derived from P. gingivalis and not the result of a eukaryotic enzymatic activation. Paracellular proteolysis of HT29/C1 cloned epithelial cells (36) and of type II alveolar and MDCK cells (65) has been demonstrated by a zinc metalloproteinase produced by B. fragilis, the most commonly isolated anaerobe of the human colonic microflora implicated in diarrheal disease (36, 65). Furthermore, a cysteine proteinase derived from Dermatophagoides pteronyssinus (62) causes disruption of occludin, thus suggesting that openings of the paracellular barrier may be involved in the development of asthma. It is known that P. gingivalis produces a series of proteases in cell-associated (8, 29) and secretory forms (7, 15, 42). At this time we do not know the protease(s) of P. gingivalis that might be responsible for the observed results. Studies by Scragg et al. (52, 53) have shown the loss of β1-integrin on human gingival fibroblasts due to arginine-specific protease activities derived from P. gingivalis. Future work in our laboratories will investigate the possible P. gingivalis enzyme(s) involved in the effect on the integrity of the epithelial cell barrier that we observed as well as the epithelial cell mechanisms involved.
In conclusion, this study demonstrates for the first time the presence of E-cadherin and occludin in primary cultures of gingival epithelial cells. Furthermore, our results suggest that P. gingivalis is able to invade the deeper structures of connective tissues via a paracellular pathway by degrading epithelial cell-cell junction complexes, thus allowing the spread of the bacterium. Our results also indicated the importance of a critical threshold concentration of P. gingivalis to initiate epithelial barrier destruction. This is a very relevant concept not only for the prevention and treatment of periodontal disease but also for the potential systemic consequences of periodontal infection.
ACKNOWLEDGMENTS
These studies were supported by U.S. Public Health Service grants DE 10607, DE 13269, and DE 08228, a grant from the University of Alabama at Birmingham Research Center in Oral Biology, and the Medical Research Service of the Department of Veterans Affairs. D.F.B. is a recipient of a Veterans Affairs Career Development Award.
REFERENCES
- 1.Aduse-Opoku J, Muir J, Slaney J M, Rangarajan M, Curtis M A. Characterization, genetic analysis, and expression of a protease antigen (PrpR1) of Porphyromonas gingivalis W50. Infect Immun. 1995;63:4744–4754. doi: 10.1128/iai.63.12.4744-4754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkovetz D F. Evidence that hepatocyte growth factor abrogates contact inhibition of mitosis in Madin Darby canine kidney cell monolayers. Life Sci. 1999;64:1393–1401. doi: 10.1016/s0024-3205(99)00073-9. [DOI] [PubMed] [Google Scholar]
- 3.Balkovetz D F, Pollack A L, Mostov K E. Hepatocyte growth factor alters the polarity of Madin Darby canine kidney cell monolayers. J Biol Chem. 1997;272:3471–3477. doi: 10.1074/jbc.272.6.3471. [DOI] [PubMed] [Google Scholar]
- 4.Barkocy-Gallagher G A, Han N, Patti J M, Whitlock J, Progulske-Fox A, Lantz M S. Analysis of the prtP gene encoding porphypain, a cysteine protease of Porphyromonas gingivalis. J Bacteriol. 1996;178:2734–2741. doi: 10.1128/jb.178.10.2734-2741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(Suppl.):1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 6.Brissette C A, Fives-Taylor P M. Actinobacillus actinomycetemcomitans may utilize either actin-dependent or actin-independent mechanisms of invasion. Oral Microbiol Immunol. 1999;14:137–142. doi: 10.1034/j.1399-302x.1999.140301.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Potempa J, Polanowski A, Wikstrom M, Travis J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J Biol Chem. 1992;267:18896–18901. [PubMed] [Google Scholar]
- 8.Ciborowski P, Nishikata M, Allen R D, Lantz M S. Purification and characterization of two forms of high-molecular-weight cysteine proteinase (porphypain) from Porphyromonas gingivalis. J Bacteriol. 1994;176:4549–4557. doi: 10.1128/jb.176.15.4549-4557.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis M A, Ramakrishnan M, Slaney J M. Characterization of the trypsin-like enzymes of Porphyromonas gingivalis W83 using a radiolabelled active-site-directed inhibitor. J Gen Microbiol. 1993;139:949–955. doi: 10.1099/00221287-139-5-949. [DOI] [PubMed] [Google Scholar]
- 10.Duncan M J, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dusek D M, Progulske-Fox A, Whitlock J, Brown T A. Isolation and characterization of a cloned Porphyromonas gingivalis hemagglutinin from an avirulent strain of Salmonella typhimurium. Infect Immun. 1993;61:940–946. doi: 10.1128/iai.61.3.940-946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanning A S, Mitic L L, Anderson J M. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- 13.Finlay B B, Gumbiner B, Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988;107:221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiszig S M, Evans D J, Do N, Vallas V, Shin S, Mostov K. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimura S, Nakamura T. Purification and characterization of a 43-kDa protease of Bacteroides gingivalis. Oral Microbiol Immunol. 1990;5:360–362. doi: 10.1111/j.1399-302x.1990.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 16.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haskins J, Gu L, Wittchen E S, Hibbard J, Stevenson B R. ZO 3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO 1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirose K, Isogai E, Mizugai H, Ueda I. Adhesion of Porphyromonas gingivalis fimbriae to human gingival cell line Ca9-22. Oral Microbiol Immunol. 1996;11:402–406. doi: 10.1111/j.1399-302x.1996.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 20.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 21.Hormia M, Ylanne J, Virtanen I. Expression of integrins in human gingiva. J Dent Res. 1990;69:1817–1823. doi: 10.1177/00220345900690120601. [DOI] [PubMed] [Google Scholar]
- 22.Isberg R R. Pathways for the penetration of enteroinvasive Yersinia into mammalian cells. Mol Biol Med. 1990;7:73–82. [PubMed] [Google Scholar]
- 23.Isberg R R, Leong J. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 24.Isogai H, Isogai E, Yoshimura F, Suzuki T, Kagota W, Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the fimbriae. Arch Oral Biol. 1988;33:479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- 25.Kallman J, Kihlstrom E. Penetration of group B streptococci through polarized Madin-Darby canine kidney cells. Pediatr Res. 1997;42:799–804. doi: 10.1203/00006450-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Katz J, Leary R M, Ward D C, Harmon C C, Michalek S M. Humoral response to Porphyromonas (Bacteroides) gingivalis in rats: time course and T-cell dependence. Infect Immun. 1992;60:3579–3585. doi: 10.1128/iai.60.9.3579-3585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz J, Ward D C, Michalek S M. Effect of host responses on the pathogenicity of strains of Porphyromonas gingivalis. Oral Microbiol Immunol. 1996;5:309–318. doi: 10.1111/j.1399-302x.1996.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 28.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lantz M S, Allen R D, Ciborowski P, Holt S C. Purification and immunolocalization of a cysteine protease from Porphyromonas gingivalis. J Periodontal Res. 1993;28:467–469. doi: 10.1111/j.1600-0765.1993.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 30.Larjava H, Peltonen J, Akiyama S K, Yamada S S, Gralnick H R, Uitto J, Yamada K M. Novel function for β1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990;110:803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larjava H, Zhou C, Larjava I, Rahemtulla F. Immunolocalization of β1 integrins in human gingival epithelium and cultured keratinocytes. Scand J Dent Res. 1992;100:266–273. doi: 10.1111/j.1600-0722.1992.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 32.Mengaud J, Ohayon H, Gounon P, Mege R, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 33.Meyer D H, Lippmann J E, Fives-Taylor P M. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouton C, Bouchard D, Deslauriers M, Lamonde L. Immunochemical identification and preliminary characterization of a non-fimbrial hemagglutinating adhesin of Bacteroides gingivalis. Infect Immun. 1989;57:566–573. doi: 10.1128/iai.57.2.566-573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- 36.Obiso R J, Azghani A O, Wilkins T D. The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells. Infect Immun. 1997;65:1431–1439. doi: 10.1128/iai.65.4.1431-1439.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a risk factor for preterm low birth weight. J Periodontol. 1996;67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa T, Shimauchi H, Hamada S. Mucosal and systemic immune responses in BALB/c mice to Bacteroides gingivalis fimbriae administered orally. Infect Immun. 1989;57:3466–3471. doi: 10.1128/iai.57.11.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine proteinase (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with the arginine-specific cysteine proteinase (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 40.Okuda K, Yamamoto A, Naito Y, Takazoe I, Slots J, Genco R. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986;54:659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavloff N, Pemberton P A, Potempa J, Chen W-C A, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 42.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 43.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Progulske-Fox A, Tumwasorn S, Holt S C. The expression and function of a Bacteroides gingivalis hemagglutinin gene in Escherichia coli. Oral Microbiol Immunol. 1989;4:121–131. doi: 10.1111/j.1399-302x.1989.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 45.Progulske-Fox A, Tumwasorn S, Lepine G, Whitlock J, Savett D, Ferretti J, Banas J. The cloning, expression and sequence analysis of a second Porphyromonas gingivalis gene that codes for a protein involved in hemagglutination. Oral Microbiol Immunol. 1995;10:311–318. doi: 10.1111/j.1399-302x.1995.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 46.Rankin S, Isberg R R, Leong J M. The integrin-binding domain of invasin is sufficient to allow bacterial entry into mammalian cells. Infect Immun. 1992;60:3909–3912. doi: 10.1128/iai.60.9.3909-3912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Boulan E, Nelson W J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 48.Saglie F R, Marfany A, Camargo P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J Periodontol. 1988;59:259–265. doi: 10.1902/jop.1988.59.4.259. [DOI] [PubMed] [Google Scholar]
- 49.Sandros J, Papapanou P N, Dahlen G. Porphyromonas gingivalis invades oral epithelial cells in vitro. J Periodontol Res. 1993;28:219–226. doi: 10.1111/j.1600-0765.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 50.Sandros J, Papapanou P N, Nannmark U, Dahlen G. Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontol Res. 1994;29:62–69. doi: 10.1111/j.1600-0765.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 51.Schoenenberger C A, Zuk A, Zinkl G M, Kendall D, Matlin K S. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J Cell Sci. 1994;107:527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- 52.Scragg M A, Cannon S J, Rangarajan M, Williams D M, Curtis M A. Targeted disruption of fibronectin-integrin interactions in human gingival fibroblasts by the RI protease of Porphyromonas gingivalis W50. Infect Immun. 1999;67:1837–1843. doi: 10.1128/iai.67.4.1837-1843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scragg M A, Cannon S J, Williams D M. The secreted products of Porphyromonas gingivalis alter human gingival fibroblast morphology by selective damage to integrin-substrate interactions. Microb Ecol Health Dis. 1996;9:167–179. [Google Scholar]
- 54.Sreeivasan P K, Meyer D H, Fives-Taylor P M. Requirements for invasion of epithelial cells by Actinobacillus actinomycetemcomitans. Infect Immun. 1993;61:1239–1245. doi: 10.1128/iai.61.4.1239-1245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundqvist G, Bloom G D, Enberg K, Johansson E. Phagocytosis of Bacteroides melaninogenicus and Bacteroides gingivalis in vitro by human neutrophils. J Periodontol Res. 1982;17:113–121. doi: 10.1111/j.1600-0765.1982.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 56.Syed S A. Characteristics of Bacteroides asaccharolyticus from dental plaques of beagle dogs. J Clin Microbiol. 1980;11:522–526. doi: 10.1128/jcm.11.5.522-526.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syed S A, Loesche W J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978;21:821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang P, Foubister V, Pucciarelli M G, Finlay B B. Methods to study bacterial invasion. J Microbiol Methods. 1993;18:227–240. [Google Scholar]
- 59.Tokuda M, Karunakaran T, Duncan M, Hamada N, Kuramitsu H. Role of Arg-gingipain A in virulence of Porphyromonas gingivalis. Infect Immun. 1998;66:1159–1166. doi: 10.1128/iai.66.3.1159-1166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uitto V, Larjava H. Extracellular matrix molecules and their receptors: an overview with special emphasis on periodontal tissues. Crit Rev Oral Biol Med. 1991;2:323–354. doi: 10.1177/10454411910020030301. [DOI] [PubMed] [Google Scholar]
- 61.van Steenbergen T J, Kastelein P, Touw J J, de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontol Res. 1982;17:41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 62.Wan H, Winton H L, Soeller C, Tovey E R, Gruenert D C, Thompson P J, Stewart G A, Taylor G W, Garrod D R, Cannell M B, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Investig. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinberg A, Belton C M, Park Y, Lamont R J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wille J J, Mansson-Rahemtulla B, Rahemtulla F. Characterization of human gingival keratinocytes cultured in a serum-free medium. Arch Oral Biol. 1990;35:967–976. doi: 10.1016/0003-9969(90)90016-4. [DOI] [PubMed] [Google Scholar]
- 65.Wu S, Lim K-C, Huang J, Saidi R F, Sears C L. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA. 1998;95:14979–14984. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshimura F, Sugano T, Kawanami M, Kato H, Suzuki T. Detection of specific antibodies against fimbriae and membrane proteins from the oral anaerobe Bacteroides gingivalis in patients with periodontal disease. Microbiol Immunol. 1987;31:935–941. doi: 10.1111/j.1348-0421.1987.tb03154.x. [DOI] [PubMed] [Google Scholar]