Abstract
Malignant pleural effusion (MPE) is associated with advanced stages of various malignant diseases, especially lung cancer, and is a poor prognostic indicator in these patients. However, the management of MPE remains palliative. A better understanding of the pathogenesis of MPE may lead to the development of new and more effective therapeutic options. Here, we shed light on recent advances in the mechanisms of MPE formation and provide an overview of current targeted therapies for the vascular endothelial growth factor pathway. We also retrospectively enrolled 19 patients with lung adenocarcinoma from the West China Hospital to analyze the efficacy of bevacizumab for MPE using different routes of administration.
Keywords: Malignant pleural effusion (MPE), vascular endothelial growth factor, angiogenesis, treatment, cancer, non-small cell lung cancer
Introduction
Patients with advanced tumors frequently develop malignant pleural effusion (MPE). MPE is defined as the presence of tumor cells in the pleural effusion [1]. MPE, almost all of which are exudative, frequently occurs in patients with advanced tumors and is often predominantly infiltrated by lymphocytes, especially CD4+ T cells [2-4]. Lung cancer is the most common malignancy associated with MPE; approximately one-third of all MPEs occur in patients with lung cancer [5]. Metastatic breast cancer and lymphoma are the second and third most commonly associated cancers, respectively. Malignant pleural mesothelioma is the most common primary pleural tumor associated with MPE, with approximately 90% of patients with malignant pleural mesothelioma also having MPE [6]. Approximately 15% of cancer patients die from MPE [7].
MPE treatment mainly aims to shrink tumors and absorb pleural fluid to relieve dyspnea, cough, and other symptoms. Dyspnea is the most common symptom in patients with MPE and requires palliative intervention [8]. Current options for treatment include repeated thoracentesis, pleurodesis, tube thoracostomy, pleurectomy, and internal or external drainage catheters [9-11]. However, these therapeutic options are not without limitations. Pleurodesis is accompanied by fever, chest pain, and coughing. Indwelling pleural catheters are costly and can only be used in patients who have failed pleurodesis or are unsuitable for pleurodesis [12]. Therefore, these palliative methods are unsatisfactory.
Pleural fluid accumulates when production is in excess of clearance, and drainage is impaired when tumors metastasize. Previous studies have identified that MPE occurs when tumor cells initially invade the visceral pleura mainly through the hyperpermeable pleural vasculature networks [7,13,14], after which inflammatory, mesothelial, and endothelial cells interact with invading tumor cells and promote MPE formation. MPE is also associated with immune dysfunction; however, there is very little information about the underlying mechanism [15-18]. This review provides new insights into MPE pathophysiology and examines the current state of MPE treatment.
Pathophysiology of MPE
Fluid drainage obstruction: blockade of the drainage system
Pleural effusion accumulates when production outweighs removal. Necropsy studies have reported that pleural fluid clearance via the lymphatic system originates from the parietal pleura stomata and drains through the mediastinal nodes. Mediastinal lymph node invasion has been defined as a predictor of effusion. Therefore, impaired pleural fluid drainage caused by tumor invasion of the drainage system is believed to be one of the mechanisms of MPE formation [19]. However, patients without parietal pleural invasion have also been reported to develop MPE [20]. In addition, the protein level in MPE is higher than that in normal pleural fluid, indicating that there may be plasma leakage in MPE [21,22].
Increased fluid filtration: abnormal permeability of vasculature networks
In the past few decades, related studies have discovered that redundant plasma leakage through hyperpermeable vasculature networks is involved in MPE formation [23]. Vasoactive mediators, such as vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), and angiopoietin (ANG)-1, are vital to this process [13,14,24]. VEGF is a family of proteins, including VEGF-A (hereafter referred to as VEGF), VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor, that are essential for angiogenesis during homeostasis and disease states by regulating vascular permeability and neoangiogenesis [25]. Clinical work has discovered that VEGF levels are much higher in MPE than in parapneumonic effusion, indicating that VEGF plays a central role in MPE formation and is a potential therapeutic target [13,23,26-30]. In addition, antiangiogenic treatment can temporarily “normalize” tumor vasculature [31]. Bevacizumab, a humanized anti-VEGF monoclonal neutralizing antibody that blocks VEGF from binding to its receptor, has been shown to suppress pleural effusion formation in preclinical and clinical studies [32,33]. VEGF has two tyrosine kinase receptors, VEGFR-1 (FLT-1) and VEGFR-2 (FLK-1/KDR), which are predominantly expressed in endothelial cells [25]. Some studies have also examined the role of VEGFR-2 in MPE formation. ZD6474, a novel anti-VEGFR-2 inhibitor with additional activity against epidermal growth factor receptor (EGFR), can control established lung metastases and pleural effusions in a mouse model of lung cancer produced by human lung cancer cells. These results indicate that ZD6474 could be used to control MPE by inhibiting the activation of VEGFR-2 and reducing tumor vascularization and tumor cell proliferation [34]. Another VEGFR/platelet-derived growth factor receptor tyrosine kinase phosphorylation inhibitor, PTK787, inhibits MPE formation in a xenograft model using human lung adenocarcinoma (PC14PE6) cells [35]. Because the VEGF-VEGFR signaling pathway regulates vascular permeability, these results suggest that neoangiogenesis is the primary mechanism of MPE formation.
Factors indirectly involved in MPE formation by regulating VEGF accumulation in the pleural space have also been studied. The transforming growth factor-beta (TGF-β) signaling pathway is crucial during normal development and carcinogenesis [36]. Recently, TGF-β was found to participate in MPE formation by stimulating mesothelial cells to produce more VEGF both in vivo and in vitro [37]. Interleukin-6 (IL-6), a cytokine with pro- and anti-inflammatory properties, can be produced by almost all stromal and immune cells. It regulates both innate and adaptive immunities [38]. Yeh et al. found that IL-6-induced activation of STAT3 in lung cancer may be involved in MPE formation by upregulating VEGF [39]. Additionally, osteopontin (OPN), a multifunctional cytokine that participates in lung cancer development, metastasis, and angiogenesis, is involved in the formation of MPE by promoting VEGF secretion [40]. However, Psallidas et al. proposed that OPNs of different origins can promote MPE formation in different ways. Host-originated OPN recruits macrophages to cancer cells to participate in pleural fluid accumulation and promote tumor angiogenesis. By contrast, tumor-derived OPN induces MPE formation by blocking apoptosis in cancer cells. Additionally, OPN can directly cause vascular hyperpermeability in a VEGF-independent manner [41]. TNF-α is also involved in MPE formation. TNF-α functions via nuclear factor-kappa B and neutral sphingomyelinase-dependent pathways to induce TNF-α and VEGF, respectively [42]. These factors may be effective target molecules for reducing MPE in patients with cancer.
Ang-1 and Ang-2 may also contribute to MPE formation. Ang-1 and Ang-2 are essential regulators of angiogenesis and bind to a tyrosine kinase receptor (Tie-2) that is mainly expressed on endothelial cells. Ang-2 acts as a natural antagonist of Ang1/Tie-2 signaling [43]. Kalomenidis et al. discovered that Ang-2 levels are elevated in exudative pleural fluid and correlate with VEGF levels in the fluid [44]. Furthermore, Economidou et al. found that VEGF regulates exudative pleural fluid formation in an Ang-1/Tie-2 pathway-independent manner [24]. These results indicate that, in addition to VEGF, the Ang-2/Tie-2 pathway might participate in MPE formation; however, this needs to be investigated further.
VEGF-independent mediators of vascular hyperpermeability have also recently been identified. Myo9b is a multidomain motor protein expressed in immune tissues, such as the spleen, and various immune cells, such as macrophages and dendritic cells [45,46]. Myo9b expression is positively correlated with MPE development, and Myo9b deficiency inhibits MPE formation in a mouse model, in addition to prolonging survival by decreasing vascular permeability and inhibiting tumor angiogenesis and tumor cell proliferation [47]. Furthermore, high levels of monocyte chemoattractant protein (MCP-1), also known as chemokine ligand 2, have been detected in mouse and human MPE samples [48,49]. MCP-1 regulates MPE formation by affecting vascular permeability and recruiting macrophages into the pleural fluid. Marazioti et al. determined that C-C motif chemokine ligand 2 (CCL2) promotes MPE formation: in a mouse model, CCL2 blockade reduced MPE induced by murine and human adenocarcinoma cells [50]. In summary, during MPE production, tumor cells promote vascular permeability by directly affecting the VEGF/VEGFR pathway or increasing VEGF production. Immune cells and tumor cells can also directly increase vascular permeability by producing Ang-1/2, Myo9b, and MCP-1 (Figure 1).
Figure 1.

Mechanisms of MPE pathogenesis. Fluid drainage obstruction and increased filtration are the main causes of pleural effusion. During MPE production, tumor cells promote vascular permeability by directly affecting the VEGF/VEGFR pathway or increasing VEGF production. Immune cells and tumor cells can also directly increase vascular permeability by producing Ang-1/2, Myo9b, and MCP-1. The immune component of malignant pleural effusion mainly includes T helper cells, macrophages, Tregs, mast cells, and γδT17 cells. Abbreviations: MPE, malignant pleural effusion; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; IL-6, interleukin 6; TGF-β, transforming growth factor-β; OPN, osteopontin; TNF-α, tumor necrosis factor α; PlGF, placental growth factor; Ang, Angiopoietin; Myo9b, myosin IXB; MCP-1, monocyte chemoattractant protein-1; Treg, regulatory T cell.
The MPE microenvironment as a contributor to tumorigenesis
Studies on the MPE microenvironment have shown that a variety of immune and non-immune cells accumulate in the pleural fluid. The MPE microenvironment contains cancer cells and various immune cells (>108/L), such as diverse lymphoid cells and myeloid subsets [51,52].
Lymphocytes, especially CD4+ helper T cells, are frequently present in MPE. CD4+ T cells can be divided into Th1 and Th2 subsets according to the type of cytokine production, and these subsets have distinct functions. Th1 cells predominantly produce IL-2, TNF-α, and IFN-γ, which stimulate the development of CD8+ effector T cells. Th2 helper cells primarily produce IL-4, IL-5, IL-10, and IL-13. The balance of Th1 and Th2 cells in pleural effusion is controversial; some believe that Th2 cells are dominant in MPE and secrete soluble ST2 protein [4,53], whereas others have demonstrated that T cells in MPE are mainly naïve or not definitely polarized to Th1 cells [54]. However, the Th1/Th2 cell balance in MPE inevitably influences the pathophysiological processes of pleural diseases.
Th17 cells are also involved in MPE formation. Th17 cells differentiate from naïve CD4+ T cells with the addition of IL-1β, IL-6, IL-23, and TGF-β and are characterized by the production of IL-17 [55,56]. Patients with lung cancer have more Th17 cells in the pleural fluid than in the blood, and Th17 cells in the pleural fluid predict better survival. Th17 cells in MPE can be chemoattracted from the blood by the CCR4-CCL22 and CCR6-CCL20 pathways and can differentiate from naïve CD4+ T cells [56]. However, the role of IL-17 in MPE remains controversial. Lin et al. demonstrated that IL-17 inhibits MPE formation and improves survival xenograft mouse models of Lewis lung cancer and colon adenocarcinoma [16]. However, Nieto et al. suggested that the concentrations of IL-17 in MPE in lung cancer patients are higher than those in patients with heart failure-related effusion, and IL-17 levels are negatively correlated with survival [57]. Heart failure-related effusion is generally transudative pleural effusion with low cell and protein content [58]. Other immune cells, such as NK cells, γδT cells, and myeloid cells, also secrete IL-17 [59]. Therefore, further efforts toward understanding the function of IL-17 and Th17 cells in MPE formation are needed.
In addition to Th17 cells, γδT17 cells also suppress MPE development. Increased γδT17 cells in the pleura predict improved survival in murine and human MPE. However, Wei et al. found that IL-10 suppresses secretion of IL-17A from γδT cells in MPE via RORγt [60]. Moreover, IL-10 was also reported to participate in MPE formation by suppressing the differentiation of Th1 cells from T cells and inhibiting the CXCR3-CXCL10 signaling pathway, which recruits Th1 and Th17 cells into MPE [61]. Considering the critical roles of γδT17 cells and IL-10 in MPE, they may be promising targets for controlling MPE.
Regulatory T cells (Tregs) have attracted much attention for their role in MPE formation. MPE has more Tregs than blood and benign pleural effusions. Tregs are considered immunosuppressive cells [62,63] and can not only differentiate from naïve CD4+ T cells in the presence of TGF-β but also chemoattract into the MPE via the CXCL1-CXCR2 pathway, CCL17, and CCL22 [18,63,64]. In addition, natural CD4+CD25- T cells in MPE can transform into CD4+CD25+FOXP3+ T cells, which have suppressive functions [65]. Tregs suppress immunity in MPE in several ways. They upregulate immune checkpoints, such as CTLA-4 and PD-L1. They also highly express TNFR2, which binds to TNF-α to promote its immunosuppressive function; blockade of TNFR2 increases IFN-γ-expressing CD8+ T cells in MPE [66]. A higher Treg/Th17 cell ratio is partially related to poor survival in lung cancer patients with MPE [17,63]. Ye et al. demonstrated that CD39+ Tregs in MPE inhibit the differentiation of Th17 cells in a latency-associated peptide-dependent manner [56]. These findings lay the foundation for developing novel immunotherapy strategies based on Treg clearance in patients with MPE.
Macrophages and mast cells have significant effects on MPE formation. Macrophages are significantly increased in MPE, and CD206+CD14+ macrophages can be used as biomarkers of MPE [67]. Further, stimulated macrophages in the pleura can chemoattract lymphocytes by producing IL-8 [68]. They also impair T cell cytotoxicity by inducing TGF-β [69]. Tumor-associated macrophage (TAM)-derived TGF-β upregulates the expression of CCL22 in TAMs via c-Fos. Subsequently, CCL22 chemoattracts Tregs to the pleural space to further stimulate TGF-β production by TAMs in an IL-8-dependent manner [70]. These findings indicate that macrophages participate in building an immunosuppressive tumor microenvironment in MPE. Anastasios et al. found that CCL2 and OPN in the pleural space attract mast cells, which induce pleural vasculature leakiness and trigger NF-κB activation by producing tryptase AB1 and IL-1β [71]. These data suggest that immune treatments based on TAMs or mast cells may be effective strategies for controlling MPE.
With the development of molecular techniques, activating mutations in EGFR and KRAS have also been found to affect MPE pathogenesis [72,73]. The EGFR L858R mutant promotes cancer cell invasion and MPE formation by activating the CXCL12-CXCR4 pathway. Mutant KRAS upregulates CCL2 in the blood to mobilize myeloid cells from the bone marrow to the pleural space [73]. These studies indicate that patients with MPE may benefit from targeted therapies based on EGFR or KRAS mutations.
Novel therapies for MPE based on the VEGF/VEGFR pathway
Currently, the management of MPE is palliative, including repeated thoracentesis, pleurodesis, tube thoracostomy, pleurectomy, and internal or external drainage catheters. The advantages and disadvantages of these treatments have been thoroughly reviewed by Neragi-Miandoab [8]. Several preclinical studies have assessed novel therapeutic interventions against MPE, including monoclonal neutralizing antibodies, soluble receptors, and small-molecule inhibitors. Blockade of VEGF, VEGFR, TNFR2, IL-5, IL-10, CCL2, and Ang signaling improves MPE in preclinical models [34,35,50,61,66,74]. Among these, the VEGF/VEGFR pathway is the most well-studied in MPE formation because of its prominent role in blood vessel formation. In this section, we shed new light on the strategies of targeting the VEGF/VEGFR pathway in MPE.
Many studies have explored the efficacy of VEGF/VEGFR blockade in patients with MPE and mouse models of MPE. In an early study, treatment of mice implanted with human lung adenocarcinoma with PTK 787, a VEGF/VPE receptor tyrosine kinase phosphorylation inhibitor, significantly reduced MPE formation (Table S1) [35]. ZD6474, a novel orally active inhibitor of VEGFR-2, can also control MPE in a mouse model of non-small cell lung cancer (NSCLC). ZD6474 inhibits the production of pleural fluid by inhibiting the activation of VEGFR-2 and reducing tumor vascularization (Table S1) [34]. Related clinical trials and retrospective studies have also been recently conducted (Tables 1 and S2). The earliest clinical analysis was performed by Kitamura et al. [75]. They treated 13 patients with MPE secondary to NSCLC with conventional chemotherapy plus bevacizumab (15 mg/kg) and examined the pleural effusion control rate (PECR), defined as the proportion of patients without reaccumulation of MPE for 8 weeks from the initiation of treatment. Twelve patients (92.3%) achieved pleural effusion control. In addition, prospective studies have examined the efficacy of bevacizumab in controlling MPE. Tamiya et al. enrolled 23 patients with lung adenocarcinoma accompanied by MPE. The patients were treated with platinum-paclitaxel plus bevacizumab, and PECR was defined as the percentage of patients without re-accumulation of MPE on chest radiography or computed tomography (CT) during treatment. In that study, the PECR was 91.3% (21/23) [76]. Usui et al. also performed a single-arm, open-label phase II trial of bevacizumab plus chemotherapy [77], in which PECR was defined as the proportion of patients without pleurodesis at 9 weeks. They enrolled 28 patients with NSCLC, and the PECR was 92.9% (26/28). A more recent study performed by Rintaro et al. also demonstrated that bevacizumab (15 mg/kg) was effective in controlling MPE, with a PECR of 80% [78].
Table 1.
Clinical Studies of VEGF/VEGFR blockade in MPE
| Type of clinical research | Primary disease | Drug | Intervention | Patients number | *Control rate of MPE (PECR) or OR | Ref. |
|---|---|---|---|---|---|---|
| Retrospective study | NSCLC | Conventional chemotherapy plus bevacizumab (15 mg/kg) | Intravenous | 13 | PECR: 92.3% | [75] |
| Prospective study | Lung adenocarcinoma | Platinum-paclitaxel plus bevacizumab (15 mg/kg) | Intravenous | 23 | PECR: 91.3% | [76] |
| Prospective study | NSCLC | Carboplatin-pemetrexed and bevacizumab (15 mg/kg) | Intravenous | 28 | PECR: 92.9% | [77] |
| Prospective study | NSCLC | Chemotherapy plus bevacizumab (15 mg/kg) | Intravenous | 20 | PECR: 80% | [78] |
| Prospective study | NSCLC | Cisplatin (30 mg) plus bevacizumab (300 mg) | Intrapleural injection | 72 | OR: 83.33% | [80] |
| Retrospective study | NSCLC | Pemetrexed (100-600 mg) plus bevacizumab (200 mg) | Intrapleural injection | 45 | OR: 86.36% | [81] |
The pleural effusion control rate (PECR) was described as the proportion of patients without reaccumulation of MPE for eight weeks from the initiation of treatment. *Complete remission (CR) was defined as when the pleural fluid had disappeared and lasted for at least four weeks; partial remission (PR) was defined as when >50% of the pleural fluid had disappeared, symptoms had improved, and the fluid did not increase for at least four weeks; OR, overall response was the sum of CR and PR.
Intrapleural therapies have been adopted to treat a vast array of pleural diseases, and intrapleural injection has become an effective route for the administration of traditional chemotherapeutics and targeted agents [79]. Intracavitary injection of bevacizumab has been shown to control MPE. In 2013, Du et al. prospectively enrolled 72 patients with NSCLC accompanied by MPE to receive thoracentesis followed by either intrapleural cisplatin (30 mg) plus bevacizumab (300 mg) or cisplatin (30 mg) alone [80]. The curative efficacy of the combination therapy was significantly superior to that of cisplatin alone (overall response 83.33% vs. 50.00%). Additionally, Song et al. found that intrapleural injection of bevacizumab (200 mg) plus pemetrexed could effectively control MPE in patients with NSCLC [81]. These studies indicate that intrapleural injection of bevacizumab can effectively control MPE caused by NSCLC. However, the optimal dose of bevacizumab has not yet been established. Chen et al. recently attempted to optimize intrapleural bevacizumab dosing in MPE secondary to NSCLC [82]. They retrospectively enrolled 71 patients with MPE secondary to NSCLC who received a low dose of bevacizumab (100 mg/week, 200 mg/2 weeks, or 200 mg/3 weeks) or a high dose of bevacizumab (200 mg/week, 400 mg/2 weeks, or 400 mg/3 weeks). Complete response was defined as complete disappearance of pleural effusion within 4 weeks. In that study, patients who received a low dose of bevacizumab had better overall survival (OS) and less toxicity than those who received a high dose, whereas those who received a high dose of bevacizumab had better progression-free survival (PFS).
We also retrospectively enrolled 19 patients with lung adenocarcinoma accompanied by MPE from the West China Hospital, Sichuan University, between October 2017 and May 2018 (the study was approved by the Medical Ethics Committee of West China Hospital, Sichuan University). After draining the pleural fluid by thoracentesis, patients were administered either a combination of 100 mg bevacizumab plus 30-50 mg cisplatin, 30 mg cisplatin alone, or 100 mg bevacizumab alone by intrapleural injection every 2 weeks. One patient was administered intravenous bevacizumab at 7.5 mg/kg. CT was performed at the end of the first treatment cycle. Complete remission (CR) was defined as disappearance of the pleural fluid for at least 4 weeks; partial remission (PR) was defined as disappearance of >50% of the pleural fluid with improved symptoms and no increase in the remaining fluid for at least 4 weeks; remission not obvious was defined as disappearance of <50% of the accumulated fluid; and progressive disease was defined as an increase in fluid accumulation. The overall response rate (ORR) was calculated as the proportion of patients achieving CR and PR. The median PFS and OS were assessed. Adverse reactions were evaluated using the Common Toxicity Evaluation Criteria according to the National Cancer Institute. Quality of life was assessed using the Karnofsky performance score (KPS). Data analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Kaplan-Meier plots were used to evaluate PPFS, PFS, and OS. Median PPFS and PFS were compared by log-rank test. The median values and 95% confidence intervals (CIs) are reported. Differences with a two-sided P value of <0.05 were considered statistically significant.
The patient characteristics are summarized in Table S3. In combination therapy with bevacizumab, cisplatin chemotherapy was implemented in 11 patients (57.89%). In monotherapy, 4 patients (21.05%) received intrapleural bevacizumab therapy, 3 patients (15.79%) received intrapleural cisplatin therapy, and 1 patient (5.26%) received intravenous bevacizumab therapy. Pleural effusion was discovered in fifteen patients before treatment commenced, while 4 patients developed new fluid effusion during the treatment process. After 4 weeks of treatment, 42.10% (8/19) of patients exhibited a noticeable effusion decrease, 26.32% (5/19) of patients experienced an effusion increase, and 31.58% of patients (6/19) experienced no apparent changes in the pleural effusion volume. Radiological changes after 4 weeks of combination therapy are shown in Figure 2. The MPE responses are listed in Table 2. The ORRs of MPE treated with intrapleural perfusion of bevacizumab plus cisplatin, bevacizumab monotherapy, and cisplatin monotherapy were 81.82%, 50.00%, and 66.67%, respectively. The ORR of MPE treated with intravenous bevacizumab injection was 100%. No significant differences (P>0.05) were observed between the groups.
Figure 2.
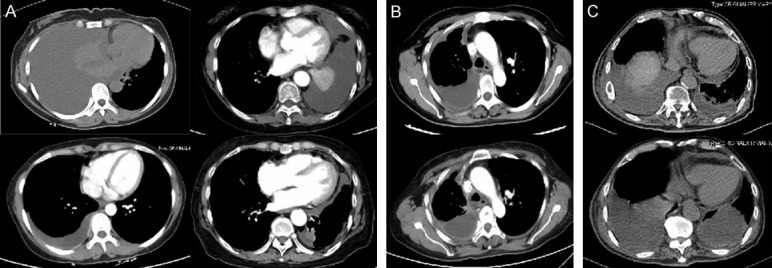
Chest computed tomography scans showed the outcomes of four weeks of treatment. A. Two patients with MPE achieved partial remission (PR) of pleural effusion. B. One MPE patient with stable disease (SD). C. One MPE patient obtained progressive disease (PD) of pleural effusion.
Table 2.
Short-term efficacy of bevacizumab-containing treatment on MPE
| Clinical outcomes | Bev + Cis via IP | Bev via IP | Cis via IP | Bev via I.V |
|---|---|---|---|---|
| n = 11 | n = 4 | n = 3 | n = 1 | |
| CR | 0 | 0 | 0 | 0 |
| PR | 3 | 2 | 2 | 1 |
| SD | 6 | 0 | 0 | 0 |
| PD | 2 | 2 | 1 | 0 |
| ORR (%) | 81.82 | 50.00 | 66.67 | 100.00 |
MPE, malignant pleural effusion; Bev, bevacizumab; Cis, cisplatin; IP, intrapleural perfusion; I.V, intravenous injection; complete remission (CR) was considered when the pleural fluid had disappeared and was stable for at least four weeks; partial remission (PR) was considered when >50% of the pleural fluid had disappeared, symptoms had improved, and the remaining fluid had failed to increase for at least four weeks; remission not obvious (NC) was considered when <50% of the accumulated fluid had disappeared; progression disease (PD) was considered when the accumulated fluid had increased. The overall response rate (ORR) was calculated by taking the sum of CR and PR.
The median OS were 22 months among patients who received intrapleural perfusion of bevacizumab plus cisplatin (n = 9), 15 months for those who received intrapleural perfusion of bevacizumab alone (n = 3), and 22 months for those who received intrapleural perfusion of cisplatin alone (n = 2) (Figure 3A). However, there was no significant difference in the median OS among the three groups (P>0.05). The median OS of patients diagnosed with hydrothorax before or after treatment were also not significantly different (P = 0.2664) (Figure 3B) in all patients who received treatment. However, Kaplan-Meier curves showed a significant difference in PPFS and PFS (17.5 months vs. 11 months; P = 0.0336) (Figure 3C).
Figure 3.

Overall survival and progression-free survival: A. Overall survival of patients with intrapleural administration. B. Overall survival in patients diagnosed with hydrothorax before or after treatment. C. Kaplan-Meier curves for pleural progression-free survival (PPFS) and progression-free survival (PFS) of patients.
To test the levels of bevacizumab and VEGF in the pleural fluid and serum, the pleural fluid and serum were centrifuged at 4,000 rpm for 10 min at 4°C, after which the supernatant was collected and assessed by ELISA using the BEVACIZUMAB ELISA kit and VEGF-A ELISA kit (USCN, Wuhan, China) according to the manufacturer’s instructions. The assay plates were read on a microplate reader (Bio-Rad, model 550, USA). Bevacizumab in the pleural fluid and serum were measured on days 1, 2, and 3 after intrathoracic perfusion (Figure 4A) and intravenous injection (Figure 4B). In patients with intrathoracic perfusion of bevacizumab, the mean ± standard error levels of bevacizumab in the pleural fluid on days 1, 2, and 3 were 78,697.83 ± 35,248.67 ng/mL, 49,388.34 ± 23,610.83 ng/mL, and 43,859.71 ± 26,241.54 ng/mL, respectively. The mean ± standard error levels of bevacizumab in the serum on days 1, 2, and 3 were 1844.84 ± 500.11 ng/mL, 3070.95 ± 872.65 ng/mL, and 3688.27 ± 924.71 ng/mL, respectively. The concentration of bevacizumab in the pleural effusion decreased in a time-dependent manner, whereas that in the serum increased in a time-dependent manner. In patients receiving bevacizumab intravenously, the bevacizumab levels in the pleural fluid on days 1, 2, and 3 were 13,171.87 ng/mL, 23,623.04 ng/mL, and 37,662.23 ng/mL, respectively. The serum levels on days 1, 2, and 3 were 70,138.23 ng/mL, 123,681.70 ng/mL, and 67,565.68 ng/mL, respectively. At the individual level, the concentration of bevacizumab in the serum among patients who received intravenous bevacizumab reached a maximum on day 2. Nevertheless, the concentration of bevacizumab in the pleural effusion was lower than that in serum. However, intrathoracic perfusion administration significantly improved the overall level of bevacizumab in pleural effusion. Furthermore, the VEGF concentration in the pleural fluid quickly reduced 1 day after intrapleural bevacizumab administration (Figure 5).
Figure 4.

Bevacizumab concentrations in pleural fluid and serum of patients with intrapleural administration (A) and one patient with intravenous administration (B). The serum bevacizumab level was significantly lower than that in the pleural fluid of patients with intrapleural administration (P<0.05).
Figure 5.

VEGF concentrations in the pleural fluid of patients with intrapleural administration.
However, these studies were small-sample studies, and more effort is needed to explore optimized bevacizumab dosing in controlling MPE.
Discussion and future perspectives
The pathogenesis of MPE is complex. In addition to tumor cells, various somatic and immune cells and related signaling pathways, such as mast cells, T cells, myeloid cells, and the NF-κB pathway, are involved in MPE formation. A detailed understanding of the pathogenesis of MPE will allow us to develop more effective prevention and treatment strategies and prolong the survival of cancer patients. The VEGF/VEGFR signaling pathway plays a vital role in the development of MPE, and therapeutic strategies based on the VEGF pathway provide hope for the treatment of MPE. MPE secondary to NSCLC or lung adenocarcinoma can be effectively controlled by either intravenous chemotherapy plus bevacizumab or intrapleural chemotherapy plus bevacizumab. However, the studies investigating the efficacy of bevacizumab in controlling MPE are all small-sample studies, and there is a lack of uniform standards for intrapleural doses of bevacizumab. High-quality randomized controlled trials are necessary to compare the efficacy and safety of intravenous and intrapleural administration of bevacizumab in MPE.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81872489, 82073369).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Heffner JE. Management of the patient with a malignant pleural effusion. Semin Respir Crit Care Med. 2010;31:723–733. doi: 10.1055/s-0030-1269831. [DOI] [PubMed] [Google Scholar]
- 2.Dalbeth N, Lee YC. Lymphocytes in pleural disease. Curr Opin Pulm Med. 2005;11:334–339. doi: 10.1097/01.mcp.0000166490.92659.17. [DOI] [PubMed] [Google Scholar]
- 3.Lucivero G, Pierucci G, Bonomo L. Lymphocyte subsets in peripheral blood and pleural fluid. Eur Respir J. 1988;1:337–340. [PubMed] [Google Scholar]
- 4.Atanackovic D, Block A, de Weerth A, Faltz C, Hossfeld DK, Hegewisch-Becker S. Characterization of effusion-infiltrating T cells: benign versus malignant effusions. Clin Cancer Res. 2004;10:2600–2608. doi: 10.1158/1078-0432.ccr-03-0239. [DOI] [PubMed] [Google Scholar]
- 5.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83:235–250. doi: 10.4065/83.2.235. [DOI] [PubMed] [Google Scholar]
- 6.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: british thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii32–40. doi: 10.1136/thx.2010.136994. [DOI] [PubMed] [Google Scholar]
- 7.Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J. 1989;2:366–369. [PubMed] [Google Scholar]
- 8.Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer. 2006;54:1–9. doi: 10.1016/j.lungcan.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Thomas R, Roy B, Maldonado F, Lee YCG. Management of malignant pleural effusions-what is new. Semin Respir Crit Care Med. 2019;40:323–339. doi: 10.1055/s-0039-1698285. [DOI] [PubMed] [Google Scholar]
- 10.Doelken P. Management of pleural effusion in the cancer patient. Semin Respir Crit Care Med. 2010;31:734–742. doi: 10.1055/s-0030-1269833. [DOI] [PubMed] [Google Scholar]
- 11.Koegelenberg CFN, Shaw JA, Irusen EM, Lee YCG. Contemporary best practice in the management of malignant pleural effusion. Ther Adv Respir Dis. 2018;12:1753466618785098. doi: 10.1177/1753466618785098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Brahmer J. Management of malignant pleural effusion. Curr Oncol Rep. 2008;10:287–293. doi: 10.1007/s11912-008-0045-4. [DOI] [PubMed] [Google Scholar]
- 13.Economidou F, Margaritopoulos G, Antoniou KM, Siafakas NM. The angiogenetic pathway in malignant pleural effusions: pathogenetic and therapeutic implications. Exp Ther Med. 2010;1:3–7. doi: 10.3892/etm_00000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep. 2013;15:207–216. doi: 10.1007/s11912-013-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye ZJ, Zhou Q, Yin W, Yuan ML, Yang WB, Xiang F, Zhang JC, Xin JB, Xiong XZ, Shi HZ. Interleukin 22-producing CD4+ T cells in malignant pleural effusion. Cancer Lett. 2012;326:23–32. doi: 10.1016/j.canlet.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Tong ZH, Xu QQ, Wu XZ, Wang XJ, Jin XG, Ma WL, Cheng X, Zhou Q, Shi HZ. Interplay of Th1 and Th17 cells in murine models of malignant pleural effusion. Am J Respir Crit Care Med. 2014;189:697–706. doi: 10.1164/rccm.201310-1776OC. [DOI] [PubMed] [Google Scholar]
- 17.Ye ZJ, Zhou Q, Zhang JC, Li X, Wu C, Qin SM, Xin JB, Shi HZ. CD39+ regulatory T cells suppress generation and differentiation of Th17 cells in human malignant pleural effusion via a LAP-dependent mechanism. Respir Res. 2011;12:77. doi: 10.1186/1465-9921-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y, Liu B, Hou Y, Wang T. miR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther. 2014;13:3152–3162. doi: 10.1158/1535-7163.MCT-14-0448. [DOI] [PubMed] [Google Scholar]
- 19.Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437–443. doi: 10.1136/thx.21.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701–1702. doi: 10.1183/09031936.97.10081701. [DOI] [PubMed] [Google Scholar]
- 21.Maker AV, Nguyen DM. Images in cardiothoracic surgery. Active malignant pleural effusion captured through the thoracoscope. Ann Thorac Surg. 2005;80:1941. doi: 10.1016/j.athoracsur.2003.11.065. [DOI] [PubMed] [Google Scholar]
- 22.Sakr L, Maldonado F, Greillier L, Dutau H, Loundou A, Astoul P. Thoracoscopic assessment of pleural tumor burden in patients with malignant pleural effusion: prognostic and therapeutic implications. J Thorac Oncol. 2011;6:592–597. doi: 10.1097/JTO.0b013e318208c7c1. [DOI] [PubMed] [Google Scholar]
- 23.Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Fidler IJ. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol. 2000;157:1893–1903. doi: 10.1016/S0002-9440(10)64828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Economidou F, Antoniou KM, Tzanakis N, Sfiridaki K, Siafakas NM, Schiza SE. Angiogenic molecule Tie-2 and VEGF in the pathogenesis of pleural effusions. Respir Med. 2008;102:774–779. doi: 10.1016/j.rmed.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shubayev VI, Strongin AY. Tissue inhibitors of metalloproteases strike a nerve. Neural Regen Res. 2018;13:1890–1892. doi: 10.4103/1673-5374.239437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax. 1999;54:707–710. doi: 10.1136/thx.54.8.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zebrowski BK, Yano S, Liu W, Shaheen RM, Hicklin DJ, Putnam JB, Ellis LM. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res. 1999;5:3364–3368. [PubMed] [Google Scholar]
- 29.Mulet M, Zamora C, Porcel JM, Nieto JC, Pajares V, Muñoz-Fernandez AM, Calvo N, Esquerda A, Vidal S. Platelet factor 4 regulates T cell effector functions in malignant pleural effusions. Cancer Lett. 2020;491:78–86. doi: 10.1016/j.canlet.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Hsu IL, Su WC, Yan JJ, Chang JM, Lai WW. Angiogenetic biomarkers in non-small cell lung cancer with malignant pleural effusion: correlations with patient survival and pleural effusion control. Lung Cancer. 2009;65:371–376. doi: 10.1016/j.lungcan.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Yano S, Ogino H, Wang W, Uehara H, Nishioka Y, Sone S. The therapeutic efficacy of anti vascular endothelial growth factor antibody, bevacizumab, and pemetrexed against orthotopically implanted human pleural mesothelioma cells in severe combined immunodeficient mice. Clin Cancer Res. 2007;13:5918–5925. doi: 10.1158/1078-0432.CCR-07-0501. [DOI] [PubMed] [Google Scholar]
- 33.Sabang RL, Gandhiraj D, Fanucchi M, Epelbaum O. Role of bevacizumab in the management of the patient with malignant pleural effusion: more questions than answers. Expert Rev Respir Med. 2018;12:87–94. doi: 10.1080/17476348.2018.1417042. [DOI] [PubMed] [Google Scholar]
- 34.Matsumori Y, Yano S, Goto H, Nakataki E, Wedge SR, Ryan AJ, Sone S. ZD6474, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, inhibits growth of experimental lung metastasis and production of malignant pleural effusions in a non-small cell lung cancer model. Oncol Res. 2006;16:15–26. doi: 10.3727/000000006783981260. [DOI] [PubMed] [Google Scholar]
- 35.Yano S, Herbst RS, Shinohara H, Knighton B, Bucana CD, Killion JJ, Wood J, Fidler IJ. Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation. Clin Cancer Res. 2000;6:957–965. [PubMed] [Google Scholar]
- 36.Massagué J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gary Lee YC, Melkerneker D, Thompson PJ, Light RW, Lane KB. Transforming growth factor beta induces vascular endothelial growth factor elaboration from pleural mesothelial cells in vivo and in vitro. Am J Respir Crit Care Med. 2002;165:88–94. doi: 10.1164/ajrccm.165.1.2104006. [DOI] [PubMed] [Google Scholar]
- 38.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 39.Yeh HH, Lai WW, Chen HH, Liu HS, Su WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene. 2006;25:4300–4309. doi: 10.1038/sj.onc.1209464. [DOI] [PubMed] [Google Scholar]
- 40.Cui R, Takahashi F, Ohashi R, Yoshioka M, Gu T, Tajima K, Unnoura T, Iwakami S, Hirama M, Ishiwata T, Iwase A, Takahashi K. Osteopontin is involved in the formation of malignant pleural effusion in lung cancer. Lung Cancer. 2009;63:368–374. doi: 10.1016/j.lungcan.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Psallidas I, Stathopoulos GT, Maniatis NA, Magkouta S, Moschos C, Karabela SP, Kollintza A, Simoes DC, Kardara M, Vassiliou S, Papiris SA, Roussos C, Kalomenidis I. Secreted phosphoprotein-1 directly provokes vascular leakage to foster malignant pleural effusion. Oncogene. 2013;32:528–535. doi: 10.1038/onc.2012.57. [DOI] [PubMed] [Google Scholar]
- 42.Stathopoulos GT, Kollintza A, Moschos C, Psallidas I, Sherrill TP, Pitsinos EN, Vassiliou S, Karatza M, Papiris SA, Graf D, Orphanidou D, Light RW, Roussos C, Blackwell TS, Kalomenidis I. Tumor necrosis factor-alpha promotes malignant pleural effusion. Cancer Res. 2007;67:9825–9834. doi: 10.1158/0008-5472.CAN-07-1064. [DOI] [PubMed] [Google Scholar]
- 43.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 44.Kalomenidis I, Kollintza A, Sigala I, Papapetropoulos A, Papiris S, Light RW, Roussos C. Angiopoietin-2 levels are elevated in exudative pleural effusions. Chest. 2006;129:1259–1266. doi: 10.1378/chest.129.5.1259. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Pektor S, Balkow S, Hemkemeyer SA, Liu Z, Grobe K, Hanley PJ, Shen L, Bros M, Schmidt T, Bähler M, Grabbe S. Dendritic cell motility and T cell activation requires regulation of Rho-cofilin signaling by the Rho-GTPase activating protein myosin IXb. J Immunol. 2014;192:3559–3568. doi: 10.4049/jimmunol.1300695. [DOI] [PubMed] [Google Scholar]
- 46.Moalli F, Ficht X, Germann P, Vladymyrov M, Stolp B, de Vries I, Lyck R, Balmer J, Fiocchi A, Kreutzfeldt M, Merkler D, Iannacone M, Ariga A, Stoffel MH, Sharpe J, Bähler M, Sixt M, Diz-Muñoz A, Stein JV. The Rho regulator Myosin IXb enables nonlymphoid tissue seeding of protective CD8+ T cells. J Exp Med. 2018;215:1869–1890. doi: 10.1084/jem.20170896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi FS, Zhang X, Zhai K, Huang ZY, Wu XZ, Wu MT, Shi XY, Pei XB, Dong SF, Wang W, Yang Y, Du J, Luo ZT, Shi HZ. TSAd plays a major role in Myo9b-mediated suppression of malignant pleural effusion by regulating TH1/TH17 cell response. J Immunol. 2020;205:2926–2935. doi: 10.4049/jimmunol.2000307. [DOI] [PubMed] [Google Scholar]
- 48.Stathopoulos GT, Psallidas I, Moustaki A, Moschos C, Kollintza A, Karabela S, Porfyridis I, Vassiliou S, Karatza M, Zhou Z, Joo M, Blackwell TS, Roussos C, Graf D, Kalomenidis I. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst. 2008;100:1464–1476. doi: 10.1093/jnci/djn325. [DOI] [PubMed] [Google Scholar]
- 49.Antony VB, Godbey SW, Kunkel SL, Hott JW, Hartman DL, Burdick MD, Strieter RM. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol. 1993;151:7216–7223. [PubMed] [Google Scholar]
- 50.Marazioti A, Kairi CA, Spella M, Giannou AD, Magkouta S, Giopanou I, Papaleonidopoulos V, Kalomenidis I, Snyder LA, Kardamakis D, Stathopoulos GT. Beneficial impact of CCL2 and CCL12 neutralization on experimental malignant pleural effusion. PLoS One. 2013;8:e71207. doi: 10.1371/journal.pone.0071207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhupar R, Okusanya OT, Eisenberg SH, Monaco SE, Ruffin AT, Liu D, Luketich JD, Kammula US, Bruno TC, Lotze MT, Soloff AC. Characteristics of malignant pleural effusion resident CD8+ T cells from a heterogeneous collection of tumors. Int J Mol Sci. 2020;21:E6178. doi: 10.3390/ijms21176178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salaroglio IC, Kopecka J, Napoli F, Pradotto M, Maletta F, Costardi L, Gagliasso M, Milosevic V, Ananthanarayanan P, Bironzo P, Tabbò F, Cartia CF, Passone E, Comunanza V, Ardissone F, Ruffini E, Bussolino F, Righi L, Novello S, Di Maio M, Papotti M, Scagliotti GV, Riganti C. Potential diagnostic and prognostic role of microenvironment in malignant pleural mesothelioma. J Thorac Oncol. 2019;14:1458–1471. doi: 10.1016/j.jtho.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 53.Oshikawa K, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Expression of ST2 in helper T lymphocytes of malignant pleural effusions. Am J Respir Crit Care Med. 2002;165:1005–1009. doi: 10.1164/ajrccm.165.7.2105109. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto M, Hasegawa Y, Hara T, Hashimoto N, Imaizumi K, Shimokata K, Kawabe T. T-helper type 1/T-helper type 2 balance in malignant pleural effusions compared to tuberculous pleural effusions. Chest. 2005;128:4030–4035. doi: 10.1378/chest.128.6.4030. [DOI] [PubMed] [Google Scholar]
- 55.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 57.Nieto JC, Zamora C, Porcel JM, Mulet M, Pajares V, Muñoz-Fernandez AM, Calvo N, Espinosa I, Pascual-García M, Bielsa S, Vidal S. Migrated T lymphocytes into malignant pleural effusions: an indicator of good prognosis in lung adenocarcinoma patients. Sci Rep. 2019;9:2996. doi: 10.1038/s41598-018-35840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karkhanis VS, Joshi JM. Pleural effusion: diagnosis, treatment, and management. Open Access Emerg Med. 2012;4:31–52. doi: 10.2147/OAEM.S29942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 60.Wei XS, Pei XB, Liu YL, Wu XZ, Shi HZ, Zhou Q. IL-17A-producing γδT cells inhibit the formation of malignant pleural effusions. Am J Respir Cell Mol Biol. 2019;61:174–184. doi: 10.1165/rcmb.2018-0201OC. [DOI] [PubMed] [Google Scholar]
- 61.Wu XZ, Zhai K, Yi FS, Wang Z, Wang W, Wang Y, Pei XB, Shi XY, Xu LL, Shi HZ. IL-10 promotes malignant pleural effusion in mice by regulating TH 1- and TH 17-cell differentiation and migration. Eur J Immunol. 2019;49:653–665. doi: 10.1002/eji.201847685. [DOI] [PubMed] [Google Scholar]
- 62.Chen YQ, Shi HZ, Qin XJ, Mo WN, Liang XD, Huang ZX, Yang HB, Wu C. CD4+CD25+ regulatory T lymphocytes in malignant pleural effusion. Am J Respir Crit Care Med. 2005;172:1434–1439. doi: 10.1164/rccm.200504-588OC. [DOI] [PubMed] [Google Scholar]
- 63.Yang G, Li H, Yao Y, Xu F, Bao Z, Zhou J. Treg/Th17 imbalance in malignant pleural effusion partially predicts poor prognosis. Oncol Rep. 2015;33:478–484. doi: 10.3892/or.2014.3576. [DOI] [PubMed] [Google Scholar]
- 64.Qin XJ, Shi HZ, Deng JM, Liang QL, Jiang J, Ye ZJ. CCL22 recruits CD4-positive CD25-positive regulatory T cells into malignant pleural effusion. Clin Cancer Res. 2009;15:2231–2237. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 65.Mao C, Wang S, Jiang Q, Tong J, Ma J, Yang M, Xu X, Qiu G, Shao Q, Li L, Xu H. Increased CD4CD25+FOXP3+ regulatory T Cells in cancer patients from conversion of CD4+CD25- T cells through tumor-derived factors. Onkologie. 2008;31:243–248. doi: 10.1159/000121360. [DOI] [PubMed] [Google Scholar]
- 66.Ye LL, Peng WB, Niu YR, Xiang X, Wei XS, Wang ZH, Wang X, Zhang SY, Chen X, Zhou Q. Accumulation of TNFR2-expressing regulatory T cells in malignant pleural effusion of lung cancer patients is associated with poor prognosis. Ann Transl Med. 2020;8:1647. doi: 10.21037/atm-20-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pei XB, Wu XZ, Yi FS, Zhai K, Shi HZ. Diagnostic value of CD206+CD14+ macrophages in diagnosis of lung cancer originated malignant pleural effusion. J Thorac Dis. 2019;11:2730–2736. doi: 10.21037/jtd.2019.06.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pace E, Gjomarkaj M, Melis M, Profita M, Spatafora M, Vignola AM, Bonsignore G, Mody CH. Interleukin-8 induces lymphocyte chemotaxis into the pleural space. Role of pleural macrophages. Am J Respir Crit Care Med. 1999;159:1592–1599. doi: 10.1164/ajrccm.159.5.9806001. [DOI] [PubMed] [Google Scholar]
- 69.Li L, Yang L, Wang L, Wang F, Zhang Z, Li J, Yue D, Chen X, Ping Y, Huang L, Zhang B, Zhang Y. Impaired T cell function in malignant pleural effusion is caused by TGF-β derived predominantly from macrophages. Int J Cancer. 2016;139:2261–2269. doi: 10.1002/ijc.30289. [DOI] [PubMed] [Google Scholar]
- 70.Wang D, Yang L, Yue D, Cao L, Li L, Wang D, Ping Y, Shen Z, Zheng Y, Wang L, Zhang Y. Macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment via IL-8 in malignant pleural effusion. Cancer Lett. 2019;452:244–253. doi: 10.1016/j.canlet.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 71.Giannou AD, Marazioti A, Spella M, Kanellakis NI, Apostolopoulou H, Psallidas I, Prijovich ZM, Vreka M, Zazara DE, Lilis I, Papaleonidopoulos V, Kairi CA, Patmanidi AL, Giopanou I, Spiropoulou N, Harokopos V, Aidinis V, Spyratos D, Teliousi S, Papadaki H, Taraviras S, Snyder LA, Eickelberg O, Kardamakis D, Iwakura Y, Feyerabend TB, Rodewald HR, Kalomenidis I, Blackwell TS, Agalioti T, Stathopoulos GT. Mast cells mediate malignant pleural effusion formation. J Clin Invest. 2015;125:2317–2334. doi: 10.1172/JCI79840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai MF, Chang TH, Wu SG, Yang HY, Hsu YC, Yang PC, Shih JY. EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci Rep. 2015;5:13574. doi: 10.1038/srep13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agalioti T, Giannou AD, Krontira AC, Kanellakis NI, Kati D, Vreka M, Pepe M, Spella M, Lilis I, Zazara DE, Nikolouli E, Spiropoulou N, Papadakis A, Papadia K, Voulgaridis A, Harokopos V, Stamou P, Meiners S, Eickelberg O, Snyder LA, Antimisiaris SG, Kardamakis D, Psallidas I, Marazioti A, Stathopoulos GT. Mutant KRAS promotes malignant pleural effusion formation. Nat Commun. 2017;8:15205. doi: 10.1038/ncomms15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stathopoulos GT, Sherrill TP, Karabela SP, Goleniewska K, Kalomenidis I, Roussos C, Fingleton B, Yull FE, Peebles RS, Blackwell TS. Host-derived interleukin-5 promotes adenocarcinoma-induced malignant pleural effusion. Am J Respir Crit Care Med. 2010;182:1273–1281. doi: 10.1164/rccm.201001-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitamura K, Kubota K, Ando M, Takahashi S, Nishijima N, Sugano T, Toyokawa M, Miwa K, Kosaihira S, Noro R, Minegishi Y, Seike M, Yoshimura A, Gemma A. Bevacizumab plus chemotherapy for advanced non-squamous non-small-cell lung cancer with malignant pleural effusion. Cancer Chemother Pharmacol. 2013;71:457–461. doi: 10.1007/s00280-012-2026-4. [DOI] [PubMed] [Google Scholar]
- 76.Tamiya M, Tamiya A, Yamadori T, Nakao K, Asami K, Yasue T, Otsuka T, Shiroyama T, Morishita N, Suzuki H, Okamoto N, Okishio K, Kawaguchi T, Atagi S, Kawase I, Hirashima T. Phase2 study of bevacizumab with carboplatin-paclitaxel for non-small cell lung cancer with malignant pleural effusion. Med Oncol. 2013;30:676. doi: 10.1007/s12032-013-0676-7. [DOI] [PubMed] [Google Scholar]
- 77.Usui K, Sugawara S, Nishitsuji M, Fujita Y, Inoue A, Mouri A, Watanabe H, Sakai H, Kinoshita I, Ohhara Y, Maemondo M, Kagamu H, Hagiwara K, Kobayashi K North East Japan Study Group. A phase II study of bevacizumab with carboplatin-pemetrexed in non-squamous non-small cell lung carcinoma patients with malignant pleural effusions: North East Japan Study Group Trial NEJ013A. Lung Cancer. 2016;99:131–136. doi: 10.1016/j.lungcan.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Noro R, Kobayashi K, Usuki J, Yomota M, Nishitsuji M, Shimokawa T, Ando M, Hino M, Hagiwara K, Miyanaga A, Seike M, Kubota K, Gemma A North East Japan Study group. Bevacizumab plus chemotherapy in nonsquamous non-small cell lung cancer patients with malignant pleural effusion uncontrolled by tube drainage or pleurodesis: a phase II study North East Japan Study group trial NEJ013B. Thorac Cancer. 2020;11:1876–1884. doi: 10.1111/1759-7714.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huggins JT, Doelken P, Sahn SA. Intrapleural therapy. Respirology. 2011;16:891–899. doi: 10.1111/j.1440-1843.2011.02011.x. [DOI] [PubMed] [Google Scholar]
- 80.Du N, Li X, Li F, Zhao H, Fan Z, Ma J, Fu Y, Kang H. Intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancer-mediated malignant pleural effusion. Oncol Rep. 2013;29:2332–2340. doi: 10.3892/or.2013.2349. [DOI] [PubMed] [Google Scholar]
- 81.Song X, Chen D, Guo J, Kong L, Wang H, Wang Z. Better efficacy of intrapleural infusion of bevacizumab with pemetrexed for malignant pleural effusion mediated from nonsquamous non-small cell lung cancer. Onco Targets Ther. 2018;11:8421–8426. doi: 10.2147/OTT.S184030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen D, Song X, Zhang Y, Kong L, Wang H, Yu J. Optimizing intrapleural bevacizumab dosing in non-small-cell lung cancer-mediated malignant pleural effusion: less is more. Future Oncol. 2018;14:2131–2138. doi: 10.2217/fon-2018-0089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


