Abstract
The Kelch-like (KLHL) family members consist of three domains: bric-a-brac, tramtrack, broad complex/poxvirus and zinc finger domain, BACK domain and Kelch domain, which combine and interact with Cullin3 to form an E3 ubiquitin ligase. Research has indicated that KLHL family members ubiquitinate target substrates to regulate physiological and pathological processes, including tumorigenesis and progression. KLHL19, a member of the KLHL family, is associated with tumorigenesis and drug resistance. However, the regulation and cross talks of other KLHL family members, which also play roles in cancer, are still unclear. Our review mainly explores studies concerning the roles of other KLHL family members in tumor-related regulation to provide novel insights into KLHL family members.
Keywords: Kelch-like family member, E3 ubiquitin ligase, ubiquitination, degradation, human cancer
Introduction
Ubiquitin is a small protein composed of 76 amino acids with highly conserved sequences; it is widely present in all eukaryotic cells and is involved in ubiquitination modification [1]. Ubiquitination, an important post-translational modification type, links ubiquitin to target a protein and then triggers protein degradation by the 26S proteasome complex or regulates the biological functions of substrates [2,3]. This type of regulation is catalyzed by the sequential enzymatic reactions of ubiquitin-activating enzyme (E1), ubiquitin-binding enzyme (E2) and ubiquitin-ligase enzyme (E3). First, the ATP-dependent activation of ubiquitin occurs when the cysteine residues of E1 connect to the C-terminal lysine residues of ubiquitin. Second, activated ubiquitin is transferred and binds E2. Finally, through the action of E3, ubiquitin is transferred to a substrate’s lysine via a covalent bond; the transfer results in the ubiquitination of a target protein [4]. Notably, a ubiquitin molecule has seven lysine (K) residues (K6, K11, K27, K29, K33, K48, and K63) and one methionine residue (M1). And a variety of linkages can be formed in poly-ubiquitin chains; the linkage type determines the ultimate fate of target proteins [5,6] and affects various biological functions, including DNA damage repair [7,8], cell cycle regulation [9], autophagy [10], and immune response [11,12]. The main destination of ubiquitinated proteins is degradation by the ubiquitin-proteasome system (UPS), which is the main pathway of more than 80% of intracellular protein turnovers and plays significant roles in regulation of cellular proteins’ activities and functions [13,14]; thus, the aberrant regulation of the UPS is associated with various types of cancer [15].
A total of 2 species of E1, 40 species of E2, and over 600 species of E3 are found in mammals [16]. E3 ligases, which transfer ubiquitin to substrates, determine the fate of substrate proteins and regulate biological functions [17-19]. According to structural features and functional mechanisms, E3 ligases can be divided into three categories: the really interesting new gene (RING) family, homologous to the E6AP carboxyl terminus (HECT) family, and RING-between-RING (RBR) family [17]. At least one catalytically active cysteine residue is present in the HECT and RBR families and binds with the C-terminal amino acid residues of ubiquitin through thioester bonds, and E3 specifically recognizes its substrates and facilitates ubiquitin-substrate conjugation. However, the RING family has no active cysteine; E2 is necessarily involved in the combination of ubiquitin and then completes conjugation with E3 and substrates [20,21].
The Cullin-RING E3 ligases (CRLs) constitute the largest E3 ligase family, including CRL1, CRL2, CRL3, CRL4A, CRL4B, CRL5, CRL7 and CRL9, and are responsible for the ubiquitination of substrate proteins [22]. A CRL consists of four components: a Cullin protein functioning as a scaffold protein, a substrate-recognizing receptor that is vital to the identification of target substrates, an adaptor protein linking a Cullin protein to adaptor proteins, and one RING-Box (RBX) protein, which is crucial to recruitment to E2 [17]. CRL3 is a highly conserved member of the CRL family, consisting of a Cullin3 protein, an RBX1 protein, and a bric-a-brac tramtrack, broad complex (BTB) protein which serves as the roles of both the adaptor protein and the substrate-recognizing receptor [23,24].
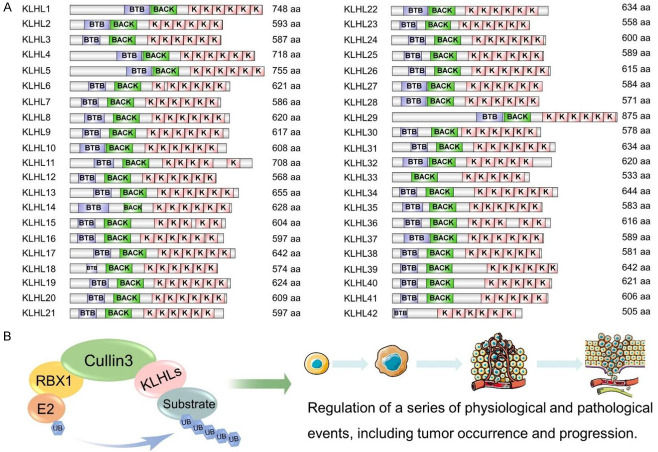
Kelch-like (KLHL) family members (KLHLs), regarded as the substrate-binding subunits of CRL3, commonly possess a BTB/poxvirus and zinc finger (POZ) domain, a BACK domain, and Kelch domain with 4-6 Kelch repeated motifs [25]. In a Cullin3-RBX1-KLHLs complex, the BTB/POZ domain is responsible for binding Cullin3, the Kelch domain determines substrate specificity, and the BACK domain forms a link between the BTB and Kelch domains. To date, 42 kinds of KLHLs in mammals have been identified [26] (Figure 1). Among these, KLHL19, also called Kelch-like-ECH-associated protein 1 (KEAP1), has been the most extensively studied, which is the substrate adaptor protein of the Cullin3 ubiquitin ligase complex, consisted of an N-terminal region, a BTB domain, an intermediate region (IVR), a double-glycine repeat (DGR) domain/Kelch domain with six repeated Kelch motifs, and a C-terminal region. The BTB domain is responsible for the dimerization of KLHL19 and promotes its binding to Cullin3 ligase. The Kelch repeated domain contains important binding sites interacting with specific substrates, such as nuclear factor E2-related factor 2 (NRF2) and P62. The IVR contains nuclear export-signals that regulate the cytoplasmic localization of KLHL19 [27]. A growing body of research has focused on other family members involved in regulating several biological functions, such as cell division, brain development, and blood pressure regulation [28-30]. A detailed classification of KLHLs and substrates are shown in Tables 1, 2 and Figures 2, 3.
Figure 1.
A: The Diagram of 42 KLHL family members with Amino acid numbers are listed on the right. B: The structural components of the Cullin3-RING-KLHLs complex and its ability to regulate tumor occurrence and development. A. Kelch-like protein family members (KLHLs) consist of the bric-a-brac, tramtrack, broad complex (BTB)/poxvirus and zinc finger (POZ) domain, the BACK domain and the Kelch repeated domain, the BTB/POZ domain is responsible for binding cullin3, the Kelch domain determines substrates recruitment, and the BACK domain forms a linker between BTB domain and Kelch domain. Data comes from the Uniprot and the Smart databases. B. The Cullin3-RBX1-KLHLs complex consists of three components, including the Cullin3 protein functioning as the scaffold protein, the KLHLs protein responsible for identifying the targeted substrates and binding the Cullin3 protein, and the RING-box protein crucial to recruitment to E2. The KLHLs have the abilities of triggering several substrates ubiquitination modification to influence its stability and localization, thereby taking part in the regulation of tumor occurrence and development.
Table 1.
The biological functions of interactions between KLHLs and different substrates
| KLHLs | Substrates | Degraded or not | Roles | References |
|---|---|---|---|---|
| KLHL2 | WNK4 | Yes | Anti-hypertension effect | [306] |
| WNK3 | Yes | Anti-hypertension effect | [331] | |
| NPCD | No | Regulation of neuronal development | [332] | |
| KLHL3 | WNK1 | Yes | Anti-hypertension effect | [333] |
| WNK4 | Yes | Anti-hypertension effect | [334-338] | |
| cMyBP-C | Yes | Regulation of heart development | [339] | |
| Claudin-8 | Yes | Anti-hypertension effect | [340] | |
| NCC | Yes | Anti-hypertension effect | [28] | |
| KLHL6 | HBXIP/Lamtor5 | No | Inhibition of B cell maturation | [341] |
| KLHL7 | TUT1 | Yes (K48) | Maintaining nucleolar integrity | [342] |
| KLHL8 | Rapsyn | Yes | Regulation of neuromuscular junction | [343] |
| KLHL9 | Aurora B | No | Regulation of Mitotic progression | [284] |
| IRS1 | Yes | Insulin resistance | [283] | |
| KLHL10 | dBruce | Yes | Regulation of spermiogenesis and male fertility | [344,345] |
| KLHL12 | DVLs | Yes | Inhibition of Wnt pathway | [140,141,147] |
| Lunapark | Yes | Regulation of neurodevelopment | [144] | |
| DRD4 | No | - | [346-348] | |
| SEC31 | No | Promotion of collagen secretion | [142,143] | |
| KHSRP | No | Inhibition of enterovirus ires-mediated translation | [349] | |
| KLHL13 | Aurora B | No | Regulation of mitotic progression | [284] |
| IRS1 | Yes | Insulin resistance | [283] | |
| KLHL15 | DCX, DCLK1 and DCLK2 | Yes | Regulation of neurogenesis | [350] |
| PP2A | Yes | Regulation of cellular dephosphorylation events | [351] | |
| CTIP | Yes | DNA-end resection and DSB repair | [352] | |
| KLHL16 | MAP1B-LC | Yes | Regulation of neuronal function | [161] |
| ATG16L1 | Yes (K48) | Regulation of autophagosomes production | [353] | |
| MAP8 | Yes | Regulation of neuronal function | [354] | |
| GFAF | Yes | Regulation of neuronal function | [162] | |
| TBCB | Yes | Regulation of neuronal function | [355] | |
| IFs | Yes | Regulation of neuronal function | [356] | |
| KLHL17 | GluR6 | Yes | Maintaining synaptic normality among neurons | [357] |
| KLHL18 | Aurora-A | Yes | Regulation of mitotic entry | [73] |
| UNC119 | Yes | Protection of cells from light damage | [358] | |
| KLHL19 | MCM3 | No | Maintaining the stability of cell cycle | [359,360] |
| PGAM5 | Yes | Regulation of oxidative stress | [361] | |
| PALB2 | No | Regulation of cell cycle | [362] | |
| P62 | No | Promotion of anti-oxidant response and autophagy | [363,364] | |
| MIRO-2 | Yes | Regulation of mitochondrial transport | [365] | |
| NRF1 | Yes | Cellular oxidative stress response | [237] | |
| NRF2 | Yes | Cellular oxidative stress response | [237] | |
| PGAM5, BCL2L1 | Yes | Promotion of cell apoptosis | [366] | |
| BCL2 | Yes | Promotion of cell apoptosis | [367] | |
| KLHL20 | Coronin 7 | No (K33) | Protein Trafficking | [368] |
| PDZ-RhoGEF | Yes | Neurite outgrowth | [369] | |
| DAPK, PML | Yes | Inhibition of apoptotic and autophagic death | [281] | |
| KLHL21 | EB | No | Promotion of cell migration | [370] |
| Aurora B | No | Regulation of mitosis | [371] | |
| KLHL22 | PLK1 | No | Regulation of mitosis | [98,372-374] |
| KLHL24 | KRT14 | Yes | Regulation of skin integrity | [375] |
| Keratin 14, 15 | Yes | Regulation of hair maintenance | [376] | |
| KLHL25 | 4E-BP1 | Yes | Promotion of translational activity | [377] |
| ACLY | Yes | Promotion of iTreg cell differentiation | [264] | |
| KLHL31 | FLNC | Yes | Maintenance of muscle function | [378] |
| KLHL38 | Myocardin | Yes | Promotion of heart failure | [67] |
| KLHL40 | DP | Yes | Regulation of skeletal muscle myogenesis | [379] |
| KLHL41 | Nebulin | No | Maintenance of muscle function | [380] |
| NRAP | Yes | Maintenance of muscle function | [381] | |
| KLHL42 | PPP2R5 | Yes | Promotion of connective tissue fibrosis | [382] |
| P60/Katanin | Yes | Regulation of mitosis | [383] |
Abbreviations: WNK1: WNK Lysine Deficient Protein Kinase 1; WNK3: WNK Lysine Deficient Protein Kinase 3; WNK4: WNK Lysine Deficient Protein Kinase 4; NPCD: Neuronal Pentraxin with Chromo Domain; cMyBP-C: Myosin Binding Protein C3; NCC: Na+-Cl- Cotransporter; HBXIP/Lamtor5: Late Endosomal/Lysosomal Adaptor; TUT1: Terminal Uridylyl Transferase 1; IRS1: Insulin Receptor Substrate 1; dBruce: One of the Inhibitor of Apoptosis Family of Proteins Family; DVLs: Disheveled Family Members; DRD4: Dopamine D4 Receptor; SEC31: Protein Transport Protein SEC31; KHSRP: KH-Type Splicing Regulatory Protein; DCX: Doublecortin; DCLK1: Doublecortin-Like Kinase 1; DCLK2: Doublecortin-Like Kinase 2; PP2A: Protein Phosphatase 2A; CTIP: Carboxy-Terminal Binding Protein 1 Interacting Protein; DSB: DNA Double Strand Breaks; MAP1B-LC: The Light Chain (LC) of Microtubule-Associated Protein 1B (MAP1B); ATG16L1: Autophagy Related 16 Like 1; MAP8: Microtubule-Associated Protein 8; GFAF: Glial Fibrillary Acidic Protein; TBCB: Tubulin Folding Cofactor B; IFs: Intermediate Filaments; GluR6: Glutamate Ionotropic Receptor Kainate Type Subunit 2; UNC119: Unc-119 Lipid Binding Chaperone; MCM3: Minichromosome Maintenance Complex Component 3; PGAM5: Phosphoglycerate Mutase Family Member 5; PALB2: Partner and Localizer of BRCA2; RHOT2/MIRO-2: Ras Homolog Family Member T2; NRF1: Nuclear Factor Erythroid-2-Related Factors 1; NRF2: Nuclear Factor Erythroid-2-Related Factors 2; BCL2L1: BCL2 Like 1; BCL2: BCL2 Apoptosis Regulator; PDZ-RhoGEF: Rho Guanine Nucleotide Exchange Factor 11 (also called ARHGEF11); DAPK: Death Associated Protein Kinase; PML: Promyelocytic Leukemia Protein; EB: End Binding Protein; PLK1: Polo Like Kinase 1; KRT14: Filamin-C (FlnC) Keratin 14; 4E-BP1: Eukaryotic Translation Initiation Factor 4E Binding Protein 1; ACLY: ATP-Citrate Lyase; iTreg: Inducible Regulatory T Cells; FLNC: Filamin C; DP: Dimerization Protein; NRAP: Nebulin-Related Anchoring Protein; PPP2R5: Phosphatase 2 Regulatory Subunit B’.
Table 2.
The interaction between KLHLs and specific substrates about cancers
| KLHLs | Substrates | Degraded or not | Roles | References |
|---|---|---|---|---|
| KLHL2 | ARHGEF7 | Yes | Inhibition of ccRCC progression | [322] |
| UCK1 | Yes | Promotion of AML progression and 5-AZA resistance | [313] | |
| KLHL6 | CDK2 | Yes | Inhibition of AML progression | [305] |
| Roquin2 | Yes | Inhibition of DLBCL progression | [191] | |
| KLHL7 | p53 | Yes | Promotion of BC progression | [42] |
| KLHL9 | C/EBPβ, C/EBPδ | Yes | Inhibition of GBM progression | [289] |
| KLHL12 | DVL1 | Yes | Inhibition of HCC progression | [147] |
| KLHL14 | BCR | Yes | Inhibition of DLBCL progression | [156] |
| KLHL16 | NF-κB | Yes | Maintaining chemotherapy sensitivity of HNC | [155] |
| KLHL18 | PI3K | Yes | Inhibition of LC progression | [72] |
| KLHL19 | IKKβ | Yes | Inhibition of tumorigenesis | [154] |
| Myosin 9b | Yes | Inhibition of NSCLC progression | [256] | |
| SOX9 | Yes | Inhibition of tumorigenesis | [263] | |
| KLHL20 | ULK1 | Yes (K48) | Maintaining chemotherapy sensitivity of CML | [236] |
| PML | Yes | Promotion of PCa progression | [44] | |
| DAPK, PML | Yes | Promotion of CRC progression | [282] | |
| KLHL22 | DEPDC5 | Yes (K48) | Promotion of BC progression | [103] |
| PD-1 | Yes | Inhibition of CRC progression | [99] | |
| KLHL25 | ACLY | Yes | Maintaining lipid metabolism balance to suppress LC progression | [273] |
| KLHL37 | LATS1/2 | Yes | Promotion of radio-resistance of BC | [34] |
| KLHL38 | BECN1 | Yes | Promotion of BC and OC progression | [68] |
| PTEN | Yes | Promoting of NSCLC progression | [71] |
Abbreviations: ARHGEF7: Rho Guanine Nucleotide Exchange Factor 7; ccRCC: Clear Cell Renal Cell Carcinoma; UCK1: Uridine-Cytidine Kinase 1; AML: Acute Myeloid Leukemia; 5-AZA: 5-Azacytidine; CDK2: Cyclin-Dependent Kinase 2; DLBCL: Diffused Large B-Cell Lymphoma; BC: Breast Cancer; C/EBP: CCAAT Enhancer Binding Protein; GBM: Glioblastoma; DVL1: Dishevelled Segment Polarity Protein 1; HCC: Hepatocellular Carcinoma; BCR: B Cell Receptor; NF-κB: Nuclear Factor Kappa-B; HNC: Head and Neck Cancer; PI3K: Phosphatidylinositol 3-Kinase; LC: Lung Cancer; IKKβ: IkB Kinase β; NSCLC: Non-Small Cell Lung Cancer; SOX9: Sex-Determining Region Y (SRY)-Box Transcription Factor 9; ULK1: UNC-51-Like Kinase 1; CML: Chronic Myelogenous Leukemia; PML: Promyelocytic Leukemia Protein; PCa: Prostate Cancer; DAPK: Death Associated Protein Kinase; CRC: Colorectal Cancer; DEPDC5: Dishevelled, Egl-10 and Pleckstrin Domain-Containing 5; PD-1: Programmed Death-1; ACLY: ATP-Citrate Lyase; LATS1/2: Large Tumor Suppressor Kinase 1/2; BECN1: Beclin1; OC: Ovarian Cancer; PTEN: Phosphatase and Tensin Homolog.
Figure 2.
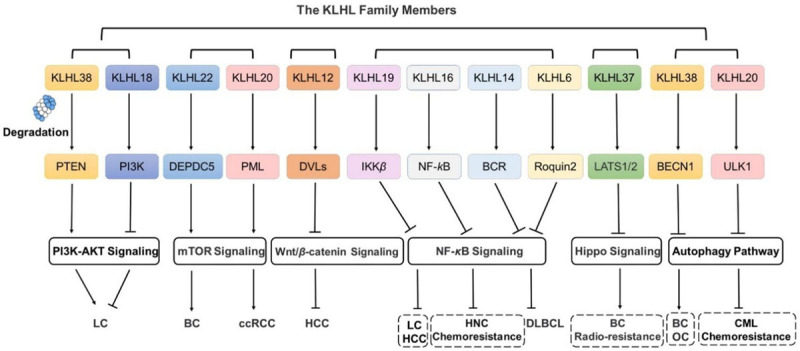
The Role of KLHLs in signaling pathways in cancer. The abnormity of signaling pathways is always closely related to tumor occurrence and development. KLHLs, as substrate adaptor proteins of Cullin3 E3 ligases, are involved in the occurrence and development of several types of cancers and regulates various signal pathways, including the PI3K/AKT pathway, mTOR signaling, Wnt/β-catenin signaling, NF-κB signaling, hippo signaling and autophagy-lysosome pathway.
Figure 3.
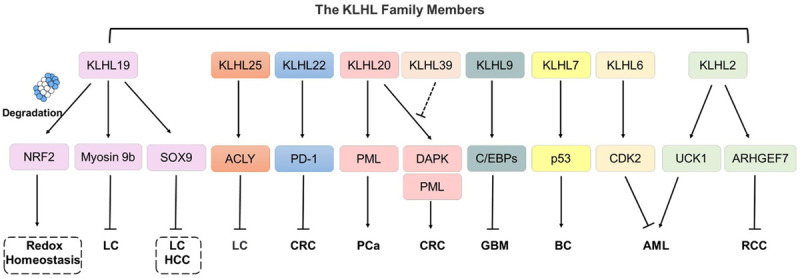
The Role of KLHLs in cancers. KLHLs are involved in the occurrence and progression of a variety of tumors via promoting ubiquitination of a series of substrate proteins, including NRF2, Myosin 9b, SOX9, ACLY, PD-1, PML, DAPK, C/EBPs, p53, CDK2, UCK1 and ARHGEF7.
Ubiquitination is closely involved in the physiological and pathological regulation of cancer, particularly through epithelial-to-mesenchymal transition (EMT) [31], stemness maintenance [32,33], resistance to radiotherapy [34,35], chemotherapy [36,37], endocrine therapy [38], and tumorigenesis, metastasis, and invasion [39-41]. Interestingly, the abnormal levels of KLHLs are tightly related to tumorigenesis and progression [42-44], and KLHLs are engaged in many signal pathways, including phosphatidylinositol 3-kinase (PI3K)/the protein kinase B (AKT), Wnt/β-catenin signaling, mammalian target of rapamycin (mTOR) signaling, hippo signaling, nuclear factor kappa-B (NF-κB) signaling and autophagy-lysosome pathway (Figure 2). Therefore, identifying the underlying mechanisms of KLHLs may be of great significance to tumor prevention, screening, precise treatment, prognosis assessment, and drug development.
PI3K/AKT signaling
The PI3K/AKT pathway is a signaling pathway activated by various growth factors, cytokines and insulin, and regulates normal physiological and pathological events, including glucose metabolism, lipid metabolism, biosynthesis, tumorigenesis, and tumor progression [45,46]. In addition, it is widely aberrantly upregulated in human cancer [47,48] and involved in the regulation of tumor metabolism, cell cycle, and angiogenesis [49].
PI3K is an intracellular phosphatidylinositol kinase activated by a series of upstream signals. Activated PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-triphosphate (PIP3). Then, PIP3 facilitates the membrane recruitment of AKT and promotes its phosphorylation at T308 [50,51]. Further, phosphorylated-AKT (p-AKT) activates downstream signaling [52,53], including mTOR [46], VEGF [54], MAPK [55] and NF-κB [56] signaling. PIK3CA, the gene encoding PI3K, is usually genetically dysregulated by amplification and somatic point mutations in various human cancer types [57]. At the protein modification level, the E3 ligase TRAF6 degrades P85α (the regulatory subunit of PI3K) through UPS, which promotes prostate cancer (PCa) migration [58].
In addition, the phosphatase and tensin homolog (PTEN) is a tumor suppressor that can antagonize the PI3K phosphorylation effect by dephosphorylating PIP3 into PIP2, thereby strictly regulating PIP3 levels and AKT signaling in normal cells [59,60]. PTEN (gene) loss or its decreased expression has been detected in a range of human cancer types [13], which is related to poor prognosis [61] and chemotherapy resistance of lung cancer (LC) [62].
Ubiquitination plays significant roles in various modes of signal transduction, including PI3K/AKT signaling, which participates in tumorigenesis, cell proliferation, invasion, apoptosis and chemotherapy resistance [55,63-66]. Currently, several studies have observed that KLHL38 and KLHL18 are involved in LC-related processes through ubiquitination degradation to associated substrates (PI3K and PTEN, respectively).
KLHL38-PTEN
As a member of the KLHL family, KLHL38 was found to be involved in heart failure formation due to its-mediated excessive degradation of myocardin, one protein essential in the differentiation and development of myocardium and smooth muscle [67]. In tumor-related research, KLHL38 is an oncogenic protein that participates in the regulation of the autophagy pathway and promotes tumor progression in breast cancer (BC) and ovarian cancer (OC) [68]. PTEN was recently found to be a substrate of KLHL38 in one LC-associated study.
One of the most common cancer types and the leading cause of death due to cancer [69], LC can be divided into non-small cell lung cancer (NSCLC) and small cell lung cancer, which account for approximately 85% and 15% of LC cases, respectively [70]. Xu et al. [71] showed a negative relationship existed between the survival times of patients with NSCLC and KLHL38 protein levels. They found that the mRNA and protein levels of KLHL38 in clinical LC tissues were dramatically higher than those in normal bronchi and alveoli, and also discovered that KLHL38 promoted the ubiquitination and proteasome degradation of PTEN to activate AKT signaling. Specifically, KLHL38 overexpression promoted LC cell proliferation by positively regulating CYCLIN D1, CYCLIN B, and C-MYC, simultaneously downregulating P21, and stimulated LC migration and invasion by upregulating RHOA and MMP9 and downregulating E-CADHERIN [71]. In general, KLHL38 acts as an oncoprotein facilitating LC occurrence and progression by degrading PTEN and activating the PI3K/AKT pathway. However, another research confirmed KLHL18 as a tumor suppressor inhibiting the PI3K/AKT pathway [72].
KLHL18-PI3K
KLHL18 was shown to interact with Aurora-A, a mitotic serine/threonine kinase essential to the regulation of mitosis and cell cycle progression, and promote its ubiquitination and proteasome degradation [73]. Moreover, it was suggested that KLHL18 inhibited LC progression by degrading PI3K [72]. Jiang et al. [72] have found that the expression of KLHL18 in LC tissues is much lower than that in normal bronchial epithelial tissues, and negatively correlated with tumor size, differentiation level, lymph node metastasis and TNM stage. A series of experiments in vivo and in vitro have indicated that KLHL18 inhibits NSCLC cells proliferation, migration and invasion by promoting the ubiquitination and proteasome degradation of P85α, an essential regulatory subunit of PI3K, thereby inhibiting the activity of PI3K/AKT pathway. In addition to this, KLHL18 is negatively correlated with the programmed death-1 ligand 1 (PD-L1) level.
PD-L1 is a transmembrane protein frequently expressed in human tumor cell membranes and interacts with programmed death-1 (PD-1), a co-inhibitory receptor expressed on immune cells, contributing to tumor immune escape [74]. The PI3K/AKT/mTOR pathway plays an essential role in carcinogenesis [75] and enhances the protein levels of PD-L1 by inhibiting the autophagy pathway, thus facilitating tumoral immune escape [76]. KLHL18 might suppress the PI3K/AKT pathway, which attenuates such pathway-induced inhibitory effects on PD-L1’s autophagy lysosomal degradation. Overall, KLHL18 is a tumor suppressor in LC and its low protein levels are closely relevant to high invasion, metastasis, immune tolerance and poor prognosis.
MTOR signaling
The mTOR is an atypical serine/threonine kinase in the PI3K-related kinase family [77]. It is the catalytic subunit existing in two complexes: mTOR complex 1 (mTORC1) and mTOR complex 2. mTORC1 is a trimer protein kinase composed of three core components: mTOR, Raptor, and mammalian lethal with Sec13 protein 8 [78], and plays a critical role in promoting anabolism processes (lipid synthesis, glycolysis, angiogenesis, and mitochondrial biogenesis) and inhibiting catabolism processes (autophagy and lysosome biogenesis). The mTOR is regulated by a series of upstream signals, which include growth factor, energy state, oxygen, and amino acid [79], and then signals to various downstream transcription factors, such as hypoxia-inducible factor-1 (HIF-1), peroxisome proliferators-activated receptor α/β, C-MYC, and transcription factor EB, which regulates physiological and pathological processes, including protein translation, cell proliferation, and normal cell cancerization [80-83].
Rag GTPases (Rags), heterodimers composed of RagA/RagB and RagC/RagD, can be activated and bind with the Raptor of mTORC1 to accelerate the activation of mTORC1 in response to amino acid stimulus [84,85]. GTPase-activating protein (GAP) activity toward Rags 1 complex (GATOR1) is composed of three proteins: dishevelled, Egl-10, and pleckstrin domain-containing 5 (DEPDC5); nitrogen permease regulator 2-like (NPRL2); and NPRL3 [86]. GATOR1, as one of the upstream-inhibiting regulators of Rags, has GAP activity and inactivates Rags [87,88], which further suppresses the mTORC1’s aggregation and activation as well as downstream pathways. Even so, the structure of GATOR1 and its specific forms that inhibit Rags are still unknown.
DEPDC5 is an integral component of GATOR1 involved in the inhibition of the mTOR pathway and plays an important role in maintaining embryonic and brain development [89]. DEPDC5 mutations are tightly associated with brain dysplasia and epilepsy occurrence [90]. Moreover, the aberrant activation of the mTOR pathway induced by DEPDC5 mutation is responsible for tumor occurrence and progression, including gastrointestinal stromal tumors [91] and hepatocellular carcinoma (HCC) [92]. Regarding the inhibition modes between DEPDC5 and mTORC1, scientists previously believed that DEPDC5 exerts its effect by promoting GTP hydrolysis to turn RagA/B into its inactive GDP state [93]. However, a recent study by Shen et al. revealed that at least two binding modes between Rag GTPases and a GATOR1 complex are required in the inhibition of mTORC1 in amino acid deficiency. A strong direct inhibiting interaction has been found between RagA and the SHEN domain of DEPDC5, and a weak interaction exists between the NPRL2-NPRL3 heterodimer and RagA, which stimulates GAP activity [94].
The mTORC1 signaling pathway is closely correlated with tumor occurrence and progression and is hyper-activated in many kinds of human cancer types, including BC [87,95,96] and renal carcinoma [97]. It has been shown that KLHL20 and KLHL22 promote the ubiquitination and degradation of promyelocytic leukemia protein (PML) and DEPDC5, respectively, thereby enhancing the mTOR signaling and accelerating cancer progression.
KLHL22-DEPDC5
As the substrate-specific adaptor of the Cullin3 E3 ligase, KLHL22 interacts with substrates and promotes its ubiquitination in mitosis [98] and tumor immunity [99]. It has been shown that KLHL22 can inhibit colorectal cancer (CRC) metastasis and invasion by suppressing the Wnt/β-catenin signaling [100] and promote malignant melanoma (MM) growth by activating the PI3K/AKT/mTOR signaling [101]. Moreover, KLHL22 promotes BC progression by activating the mTOR signaling.
BC is the most common malignant carcinoma in women worldwide, posing a considerable threat to women’s health and their lives; its incidence increases annually in aging populations [102]. At the protein level, Chen et al. observed that KLHL22 expression in human BC tissues was obviously higher than those in adjacent normal tissues. Their experiments at the cellular level showed that the depletion of KLHL22 negatively influenced BC cell proliferation [103]. Their research revealed that KLHL22 might be an oncogene of BC, promote its encoding protein aggregation on the lysosome surface, and initiate DEPDC5 K48-linked ubiquitination and proteasome degradation, thus activating the mTORC1 signaling pathway to promote BC growth [103].
KLHL20-PML
As an adaptor of CRL3, KLHL20 can promote Unc-51-like autophagy activating kinase 1 (ULK1) ubiquitination and proteasome degradation, a serine/threonine-protein kinase, which emerges as a regulator of autophagy termination [104,105]. In addition, KLHL20 is involved in regulation of tumor hypoxia environment [44] and tumor progression [106] through promoting specific substrates ubiquitination and proteasome degradation.
Clear cell renal cell carcinoma (ccRCC) is the most common kidney cancer [107]. The Von Hippel-Lindau (VHL) is a substrate-binding subunit of Cullin2 ubiquitin ligase and targets HIF1/2α for degradation. In clinical practice, VHL is lost or inactivated in the vast majority of ccRCCs, and this effect increases the expression level of HIF1/2, which in turn promotes angiogenesis and renal tumor growth [108,109]. PML inhibits neovascularization by inhibiting the activation of mTOR and downstream synthesis of HIF-1α [110].
First identified in patients with acute promyelocytic leukemia (APL) [111], PML is a tumor suppressor. Under normal conditions, it usually accumulates in the nucleus and forms PML nuclear bodies, which participates in the repair of DNA damage and regulation of protein synthesis, gene transcription and other biological functions [112]. In the regulation of the pathways, such as PTEN/AKT [113] and mTOR [110] signaling pathway, PML is essential to antitumor function, such as inhibition of tumor cell proliferation, invasion, migration and angiogenesis as well as activation of tumor cell apoptosis [110,114,115]. PML is downregulated in a range of tumors [116], and ubiquitination is key to PML’s dysregulation in tumors [117]. Intriguingly, with regard to PCa, Yuan et al. found that KLHL20 can promote PCa growth by degrading PML [44].
By investigating the relationship between PML and ccRCCs, Chen et al. observed that the small C-terminal domain phosphatases (SCPs) dephosphorylated PML at S518, blocking PML ubiquitination and proteasome degradation mediated by the prolyl isomerase 1 and KLHL20. They also found that the SCP1 expression was significantly downregulated compared with those in normal tissues, and the dephosphorylation effect of SCP1 can promote PML degradation, alleviate the inhibitory effect of PML on mTOR activity and increase the levels of downstream HIF1. Maintaining PML stability by suppressing KLHL20-mediated degradation can inhibit the mTOR-HIF1 pathway and ccRCC cell proliferation, migration, invasion and angiogenesis [106].
Wnt/β-catenin signaling
Wnt signaling is mainly divided into two types [118]: the canonical Wnt/β-catenin-dependent signaling cascade and noncanonical Wnt signaling cascades (Wnt/PCP and Wnt/Ca2+ pathways). The classic Wnt/β-catenin signaling pathway has been a focus of research in the areas of tumorigenesis, and the abnormal expression or activation of β-catenin is essential to tumorigenesis [119] and metastasis [120]. β-catenin in its inactivated state forms a degradation complex with Axin protein, casein kinase-1 (CK1), the kinases glycogen synthase kinase-3, and adenomatous polyposis coli protein, which induces the β-catenin phosphorylation [121-123] and β-TrCP-mediated ubiquitination and proteasome degradation [124,125]. Conversely, this signaling pathway is activated by the secretory glycoprotein, Wnt, which binds to the heterodimeric receptor complex consisting of frizzled (FZD) receptor and low-density-lipoprotein-related (LRP5/6) protein to stabilize β-catenin in the cytoplasm [126,127] and promote β-catenin nuclear translocation and the subsequent activation of T cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factors, thus regulating the expression of downstream target genes [128,129]. The Wnt/β-catenin signaling pathway is closely involved in the regulation of cell proliferation, differentiation and migration [130], and its dysregulation promotes carcinogenesis and progression in almost all human cancer types, including BC [131,132].
The three homologs of dishevelled (DVL) family members have been described: DVL1, DVL2 and DVL3, which all contain three domains: the N-terminal dishevelled, axin (DIX) domain responsible for DVL aggregation and interaction with Axin1 [133], the central postsynaptic density 95, disc large, zonula occuldens-1 domain responsible for interacting with the FZD receptor [134] and C-terminal Dvl, Egl-10, pleckstrin (DEP) domain responsible for DVL dimerization [122]. Upon stimulation by the Wnt protein, DVLs are rapidly phosphorylated and recruited to the FZD receptor, which inhibits the formation of degradation complex [126,127], thereby blocking the ubiquitination and proteasome degradation of β-catenin and promoting its nuclear translocation. DVLs are involved in tumorigenesis and are highly expressed in many human cancer types [135,136]. Several E3 ligases, such as SMURF2 [137], ITCH [138], and NEDD4L [139], perform DVLs ubiquitination and proteasome degradation. KLHL12 interacts with DVLs through the β-propeller regions of Kelch domains and triggers DVL1 ubiquitination and proteasome degradation, thereby suppressing the Wnt signaling [140,141].
KLHL12-DVL1
As an adaptor of CRL3 in KLHL family, KLHL12 regulates collagen secretion [142,143], neurodevelopment [144], and the Wnt signaling pathway [141] by ubiquitinating and degrading target substrates.
The sixth most common cancer, and the third leading cause of cancer death worldwide, primary liver cancers mainly consist of HCC (75%-85%) and intrahepatic cholangiocarcinoma (10%-15%) [145]. Abnormal spindle microtubule assembly (ASPM) is regarded as an oncoprotein highly expressed in HCC tissues. Its overexpression in HCC cells is associated with the activation of the Wnt signaling, which facilitates cell proliferation, invasion, migration, and induces EMT [146]. Kelvin et al. found that ASPM was upregulated in superpotent cancer stem cells (spCSCs) in HCC. They screened cancer stem cells (CSCs) from HCC cells with knockdown of ASPM and found that protein expression levels of β-catenin, DVL1 and CK1 were significantly downregulated compared with those in the control group. Furthermore, through co-immunoprecipitation and a series of experiments, they confirmed that ASPM regulated the Wnt pathway by modulating the stability of DVL1 rather than β-catenin and CK1. Finally, they demonstrated that ASPM and KLHL12 competitively bind with DVL1, which inhibited its degradation and activated the Wnt pathway in spCSCs to actively regulate its stemness and tumorigenic potential, thus leading to the progression of HCC [147].
NF-κB signaling
The NF-κB pathway is involved in regulating a variety of biological processes, including innate and adaptive immunity to infection, inflammation, stress responses, B cell development, lymphoid organogenesis, tumorigenesis and tumor progression [148-150]. The mammalian NF-κB family consists of five members: RelA/P65, C-REL, RelB, P50 (NF-κB1) and P52 (NF-κB2), which can form a variety of heterodimers or homologous dimers. The P50/P65 dimer (commonly referred to as NF-κB) is the most common dimer and exists in almost all cells. In mammals, the inhibitory proteins of κB family (IκB) consists of IκBα, IκBβ, IκBγ, IκBδ, IκBε, P100 and P105, which inhibits the NF-κB activity in the cytoplasm. Under resting conditions, IκB binds to the NF-κB dimer and forms a trimeric complex, which maintains its inactivated state [151]. The NF-κB signaling pathway can be divided into classical or nonclassical signaling cascades [152]. In mammalian cells, the classical pathways can be activated upon a variety of stimuli, such as tumor necrosis factor α (TNFα), lipopolysaccharide, and interleukin 1β (IL-1β). When the conformation of receptors, such as IL-1R, toll-like receptor (TLR) and TNFR, changes upon the binding of upstream signaling factors, it delivers the signals into IκB kinase (IKK), which induces the phosphorylation and ubiquitination of IκB, and dissociates IκB from trimers [153]. Subsequently, NF-κB rapidly enters nucleus from the cytoplasm and binds to specific sequences on the nuclear DNA, and further promotes the transcription of associated genes, including CYCLIN D1, C-MYC, and VEGF.
Under physiological conditions, NF-κB can promote cell apoptosis and inhibit tumorigenesis. Conversely, when the NF-κB is aberrantly activated, it can promote cell carcinogenesis by accelerating cell cycle evolution and inhibiting cell apoptosis. KLHL19 exerts adverse effects on LC and HCC progression by inhibiting the NF-κB signaling pathway [154]. Meanwhile, KLHL16 maintains chemotherapy sensitivity to head and neck cancer (HNC) by inhibiting the NF-κB signaling pathway [155]. Besides, several studies have demonstrated that KLHL14 [156] and KLHL6 [157] can suppress the NF-κB signaling pathway and then inhibit the progression of diffused large B-cell lymphoma (DLBCL).
KLHL19-IKKβ
IKKβ, as a serine/threonine kinase, contains four domains, including kinase domain (KD), scaffold/dimerization domain, ubiquitin-like domain and C-terminal NEMO binding domain [158]. It acts as a critical regulator in the NF-κB cascade. IKKβ undergoes phosphorylation and activation in response to multiple proinflammatory stimuli, which promotes the nuclear translocation of NF-κB and induces downstream gene transcription, playing an important role in the regulation of tumor invasion, metastasis and EMT. In addition, IKKβ is considered an oncogenic kinase promoting tumorigenesis and progression [159].
KLHL19 acts as a substrate-specific adaptor to IKKβ, inhibiting tumor progression by downregulating the NF-κB signaling [154]. During the physiologic motion, the Kelch domain of KLHL19 is primarily responsible for IKKβ KD domain binding, which promotes its K48-linked ubiquitination and proteasome degradation [160]. KLHL19 mutations occur frequently in tumors, and its abnormal mutation-induced IKKβ dysregulation has been also demonstrated in a variety of tumors. KLHL19 Kelch domain mutants (S404X and D479G) in liver cancer and Kelch domain mutants (G333C, G364C, G430C and R413L) in NSCLC can attenuate binding affinity and inhibit subsequent IKKβ ubiquitination and proteasome degradation, thereby activating NF-κB signaling to promote tumor progression [154]. In conclusion, KLHL19 may act as a tumor suppressor, inhibit IKKβ ubiquitination and proteolysis to suppress NF-κB signaling, and subsequently curb tumor progression.
KLHL16-NF-κB
Multiple studies have shown that KLHL16 (Gigaxonin) is critical for neuronal maintenance and survival [161,162]. KLHL16 mutations occur frequently in various tumors, including bone tumors, CRCs and hematopoietic/lymphocytic tumors [163]. Research has also suggested that KLHL16 mutations enhance NF-κB signaling in HNC cells and result in chemotherapy resistance [155].
HNC is the seventh most common type of cancer in the world and has many different histological types, the most common of which is squamous cell carcinoma (SCC) [164]. So far, platinum-based drugs remain the mainstays of chemotherapy regiments for HNC [165]. P16 is a tumor suppressor gene that plays a role in cell cycle regulation, and its mutations are associated with tumor progression and chemotherapy resistance [166,167]. In addition, cisplatin inhibits head and neck squamous cell carcinoma growth through P16-mediated cell cycle arrest, and reduction in P16 expression is associated with cisplatin resistance [168]. In HNC, Veena et al. have found that the inhibition of IKKβ leads to arrested tumor growth, and cisplatin treatment reduces NF-κB nuclear expression. Cisplatin treatment leads to the nuclear translocation of P16, which induces the recruitment of KLHL16 and promotes the ubiquitination and degradation of NF-κB.
KLHL14-BCR
KLHL14 is critical to cortical development by regulating the axon extension of corticospinal neurons [169]. In addition, KLHL14 has been found upregulated in OC and endometrial carcinoma (EC) tissues, and high KLHL14 expression in OC and EC patients is associated with poor prognosis [170,171]. Notably, KLHL14 is highly expressed in B cells and crucial to its differentiation and development [172]. Moreover, its encoded gene KLHL14 has been identified frequently mutated in mature B-cell malignancies [173].
DLBCL is the most common histological type of non-Hodgkin’s lymphoma, which is mainly divided into three subtypes: germinal center B-cell-like, activated B cell-like (ABC) and unclassified subgroups. Many patients still experience drug resistance or relapse during or after treatment [174] despite the rapid progress in DLBCL treatment. The B cell receptor (BCR) is a molecule on the surface of B cells responsible for specific recognition and conjugation to antigens. The BCR signaling pathway is closely associated with the pathogenesis and development of B cell malignancies and plays a significant role in DLBCL progression [175,176]. TLR, an important protein in nonspecific immune responses, is involved in the activation of NF-κB by binding myeloid differentiation factor 88 (MYD88), an important adaptor protein essential to innate immune responses and inflammatory response signal transduction [177]. In DLBCL-ABC cells, the BCR combines with MYD88 and TLR9 to form MyD88-TLR9-BCR (My-T-BCR) supramolecular complex, which facilitates the NF-κB signaling into the nucleus [178]. NF-κB activation is a principal feature of the DLBCL-ABC subgroup, which depends on constitutive NF-κB signaling to decrease tumor cell apoptosis and maintains its viability, called “chronic active” BCR signaling [179]. Blocking the BCR signaling pathway can inhibit NF-κB signaling and result in cell cycle arrest and apoptosis, thereby suppressing DLBCL progression [180,181].
George et al. have demonstrated that KLHL14, as a tumor suppressor, promotes the ubiquitination and degradation of BCR subunits, CD79A, CD79B and IgM, thus inhibiting My-T-BCR complex formation to block NF-κB signaling. In 574 DLBCL biopsy samples, KLHL14 mutations were most common in DLBCL-ABC (10.8%). They confirmed that the deletion of KLHL14 induced DLBCL resistance to brutinib, a bruton tyrosine kinase inhibitor that inhibits the degradation of BCR subunits and promotes the assembly of a My-T-BCR super complex, thereby promoting NF-κB activation and DLBCL progression [156].
KLHL6-Roquin2
KLHL6 is highly expressed in gastric cancer (GC) cells and tissues, promoting GC cell growth and lymphangiogenesis [182]. KLHL6 disrupts the formation of germinal centers in chronic lymphocytic leukemia (CLL) and its high level predicts poor clinical prognosis [183]. The recurrent mutations of KLHL6 have been found in CLL [184] and other mature B-cell malignancies, including DLBCL. The high frequency of KLHL6 mutations facilitated the proliferation of DLBCL-ABC cells [185].
Roquin2 is an RNA-binding protein in the Roquin family [186] and composed of a RING domain, conserved ROQ domain and zinc finger domain [187]. Roquin2 decays target mRNAs by binding with the conserved stem-loop motif in the 3’UTR of mRNA and its ROQ domain [187]. The tumor necrosis factor-α-inducible gene 3 (TNFAIP3) is a tumor suppressor gene in DLBCL [188,189], and its encoding protein, TNFα-induced protein 3 (TNFAIP3/A20), is a key NF-κB regulator and inhibits IKKγ activation [190]. Zhou et al. have shown that Roquin2 can induce the mRNA decay of TNFAIP3 and then suppress the accumulation of TNFAIP3. It has been shown that roquin2 is one of the substrates of KLHL6 which mediating roquin2 degradation through UPS [191]. Under physiological conditions, KLHL6 is upregulated during antigen-induced BCR/NF-κB activation and induces roquin2 degradation, which inhibits mRNA decay of TNFAIP3 and further leads to TNFAIP3 accumulation. Further, TNFAIP3 inhibits the IKK complex, which negatively regulates BCR signaling to maintain the homeostasis of the NF-κB signaling. By contrast, the high-frequency mutations of KLHL6 in DCLBC cells suppress the roquin2 degradation, accelerate TNFAIP3 decay and attenuate the inhibitory effect of TNFAIP3 on the IKK complex, which in turn induces the abnormal activation of NF-κB pathways. In conclusion, Zhou et al. suggested that the KLHL6-Roquin2 axis was of great importance to the regulation of B lymphoma cell proliferation, and regulated the mRNA decay and NF-κB activity [191,192].
Hippo pathway
The hippo pathway is composed of a series of conserved kinases and is involved in the regulation of several biological processes, including tumorigenesis [193-196]. The core components of the pathway in mammals include mammalian STE20-like protein kinase (MST1/2), cofactor human salvadorhomology 1, large tumor suppressor kinase 1/2 (LATS1/2) and its cofactor MOB kinase activator (MOB1) [197,198]. Under normal conditions, an extracellular growth signal induces a series of phosphorylation reactions of kinases: the phosphorylated MST1/2 (p-MST1/2) promotes LATS1/2 phosphorylation, and p-LATS1/2 induces the phosphorylation of downstream effector factors, Yes-associated protein (YAP) [199] and Tafazzin (TAZ) [200]. Then, p-YAP and p-TAZ interact to form a complex and stabilizes in the cytoplasm by binding 14-3-3 proteins and promote the β-TrCP-dependent proteasome degradation, which inhibits β-TrCP nuclear translocation and downstream transcription activation function [201-204]. A series of missense mutations in the hippo pathway appear in the early stage of tumor [205], which inhibits this pathway by affecting the phosphorylation cascade of key components and attenuating YAP/TAZ proteasome degradation. The accumulative unphosphorylated YAP/TAZ in the cytoplasm is transported to the nucleus and binds with TEAD transcription factors, thereby modulating the transcriptional activity of downstream oncogenes to facilitate cell proliferation and inhibit cell apoptosis [206].
Several E3 ligases have been suggested associated with tumor-related processes via regulating the hippo signaling pathway. For example, E3 ligase PARK2 can promote YAP K48-linked ubiquitination and proteasome degradation to inhibit esophageal squamous cell carcinoma procession [207]. E3 ligase ITCH can ubiquitinate and degrade LATS1 to promote BC progression [208]. Likewise, KLHL37 can induce radio-resistance in BC by ubiquitinating and degrading the key protein, LATS.
KLHL37-LATS1/2
KLHL37 is involved in various pathophysiologic processes, acting a vital role in nervous system development [209] and tumorigenesis and progression [210-212]. LATS [213], YAP [213,214] and TAZ [215-217] are closely associated with the regulation about BC progression. Li et al. have clarified that the overexpression of KLHL37 results in the hyperactivation of the hippo pathway in RaR (radio-resistant) BC cells. They found the expression of KLHL37 in BC samples from patients resistant to radiotherapy were significantly higher than those from patients sensitive to radiotherapy. In line with the histological outcome, the expression of KLHL37 are significantly higher in RaR BC cells compared with in non-RaR BC cells [34]. Specifically, they observed that the overexpression of KLHL37 degraded LATS1/2 through UPS and suppressed YAP/TAZ phosphorylation, promoting its nuclear translocation, further increasing the expression of the downstream anti-apoptotic genes GLI1, CTGF and FGF1, and enhancing radio-resistance in BC cells [34].
Autophagy-lysosome pathway
Autophagy is a tightly regulated pathway that plays an important role in basic metabolic functions and cellular homeostasis [218], and its dysregulation is closely related to tumor occurrence and progression [219,220]. The ULK1 complex and VPS34 complex are vital to autophagic processes and regulate autophagy initiation [221,222]. Under several types of autophagic stress, such as starvation, hypoxia and DNA damage, the ULK1 complex is activated and transmits signals that activate the VPS34 complex, which promotes autophagic protein localization to the phagophore [223]. The Beclin1 (BECN1), a protein of the VPS34 complex, plays a key role in autophagy regulation involved autophagosome formation, extension and maturation [224]. Several E3 ligases, such as HUWE1 [225] and WWP1 [226], promote the ubiquitination and proteasome degradation of crucial proteins in the autophagy pathway, affecting tumor progression. Among KLHL family, KLHL38 induces BECN1 degradation and promotes BC progression, and KLHL20 is vital to chemotherapy sensitivity by regulation of ULK1 degradation.
KLHL38-BECN1
As mentioned above, KLHL38 often acts as an oncoprotein and promotes tumor progression. It has been suggested that protein levels of BECN1 in tumor tissues are significantly lower than those in normal tissues, and its deficiency is closely related to tumorigenesis and tumor progression [227,228]. Xuan et al. analyzed 817 BC samples from the TCGA database and found the inconsistency between protein and mRNA expression levels of BECN1 in BC samples. They hypothesized that the reduced expression of BECN1 in tumor is due to post-translational modification, and discovered an obvious increase of BECN1 expression in BC cells after treatment with proteasome inhibitors. Through the TCGA database, they discovered elevated expression of KLHL12 and KLHL38 in triple-negative breast cancer (TNBC) tissues, and observed that BECN1 was significantly elevated only when KLHL38 was knocked down. Through a series of confirmatory experiments, they revealed that KLHL38 interacted with BECN1 and induced its K48-linked ubiquitination and proteasome degradation, thereby inhibiting autophagy and inducing BC progression [68].
KLHL20-ULK1
It has been established that KLHL20 can promote ULK1 ubiquitination and proteasome degradation to regulate autophagy termination [104]. ULK1 is a serine/threonine-protein kinase necessary for phagophore formation, which is directly regulated by mTOR and AMP-activated protein kinase and negatively correlates with the bone metastasis of BC [229].
Chronic myelogenous leukemia (CML) is an acquired clonal disease originating from pluripotent hematopoietic stem cells [230], and the inhibition of autophagy may increase therapeutic efficacy and improve patient prognosis [231]. Imatinib is a tyrosine kinase inhibitor widely used to treat CML and has a good therapeutic effect. However, a small number of patients still acquire therapeutic resistance to imatinib in the treatment process [232,233]. Autophagy dysregulation can result in imatinib resistance in CML therapy [234]. Grancalcin (GCA) is a cytoplasmic protein translocated to the cytoplasmic membrane when neutrophils are activated [235]. Seung et al. found that GCA was upregulated in peripheral blood mononuclear cells from patients with CML and imatinib resistance at the mRNA and protein levels [236] and showed that GCA can inhibit the imatinib-induced apoptosis of CML cells. They further confirmed that GCA facilitated the dimerization and activation of ULK1 by inducing the K63-linked ubiquitination of TRAF6 and inhibited the KLHL20-mediated proteasome degradation of ULK1, thus synergistically promoting the autophagy pathway to induce imatinib resistance.
Others
KLHL19-NRF2
NRF2, encoded by NFE2L2, belongs to the Cap’n’collar basic leucine zipper transcription factor family and can be divided into seven conserved functional domains (Neh1-Neh7). The Neh2 domain is located in the N-terminus and contains two important conserved regions (DLG and ETGE) responsible for the binding between NRF2 and KLHL19, which can induce NRF2 degradation and inhibit its transcriptional activity [237]. NRF2 is an important regulator of many antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and heme oxygenase-1, and interacts with the antioxidant response elements (AREs) of antioxidant and cell-protective genes, maintaining intracellular redox equilibrium [238].
Discovered in 1999, KLHL19 is an important negative regulator of NRF2 [239]. Under normal physiological conditions, NRF2 is strictly regulated by KLHL19. Through its N-terminal BTB domain, KLHL19 dimerizes and forms an E3 ligase complex with Cullin3 and RBX1, which promotes NRF2 ubiquitination and degradation to maintain NRF2 steady state. In the presence of multiple redox stimuli, the highly reactive cysteine residues of KLHL19 are immediately modified, and this effect inhibits the binding of KLHL19 to Cullin3 and facilitates NRF2 stabilization. And then, accumulative NRF2 is transported to the nucleus and binds to AREs located within the promoter region of a specific target gene, inducing the expression of a large number of cell-protective proteins with antioxidant and detoxification effects [27]. In recent years, the regulation of the KLHL19-NRF2 axis has attracted considerable interest. In addition to the crucial role in cell physiology and stress response, the abnormal regulation of the KLHL19-NRF2 axis has been confirmed involved in the progression of various cancer types, specifically promoting tumor progression, metastasis formation, drug resistance and radiotherapy resistance [240-242]. In the last few years, many scholars have extensively explored the KLHL19-NRF2 signaling pathway [240,242-248]. In this review, we mainly describe the regulation of KLHL proteins on specific substrates (other than NRF2) related to cancer progression.
KLHL19-myosin 9b
Myosin is a motor molecule based on actin with ATPase activity. The myosin family has fourteen distinct classes (1-14), which all contain a head (motor) domain containing ATP and actin binding sites, a neck domain containing one or more light chain binding sites, and a tail domain varying in size and structure [249]. Myosin 9 is an unconventional member of the myosin family because its tail domain contains a Rho-GTPase-activating protein and is closely associated with the regulation of cell motility [250]. Yang et al. found that the inhibition of myosin 9 can enhance LC cell migration [251]. Wang et al. discovered that tissue factor pathway inhibitor 2, a tumor suppressor, may inhibit the proliferation and invasion of BC cells partially by interacting with myosin 9 [252]. In neoplastic hematologic disorders, the expression levels of myosin 9 are positively correlated with the percentage of apoptosis in acute myeloid leukemia (AML) and CML cells [253].
RhoA, a member of the Rho-GTPase family, plays a significant role in the regulation of actin cytoskeleton dynamics and is crucial to the regulation of malignant transformation and cell migration [254]. The DGR domain of KLHL19 is responsible for interaction with the actin cytoskeleton [255]. KLHL19 promotes F-actin formation and inhibits the conversion of focal adhesion by enhancing RhoA activity. Zhou et al. have demonstrated that KLHL19 overexpression significantly inhibits the migration and invasion of LC cells, they have observed that its overexpression not only promotes the formation of stress fibers, but also inhibits the conversion of focal adhesion. Their study has indicated that KLHL19 triggers myosin 9b ubiquitination and proteasome degradation, which indirectly upregulates RhoA activity to stabilize the F-actin cytoskeleton, thereby inhibiting the migration and invasion of NSCLC [256].
KLHL19-SOX9
Sex-determining region Y-box transcription factor 9 (SOX9) is one of the transcription factors playing key roles in chondrocyte differentiation and bone development [257]. Ubiquitination modification is essential to the regulation of the activity, expression levels, and localization of SOX9 [258,259]. In recent years, the association among its abnormal protein levels, tumor development and progression have been investigated, particularly in HCC [260] and LC [261]. In addition, SOX9 is a potential therapeutic target based on human cancers [262].
Liu et al. found that KLHL19 negatively regulated the stability of SOX9 through UPS. In databases (https://cancer.sanger.ac.uk/cosmic and http://www.cbioportal.org/), at least fifty different mutations in KLHL19 have been recorded in a variety of cancer types and are largely concentrated in the BTB and Kelch domains. To explore the action mechanisms between KLHL19 and SOX9, they carried out a series of experiments and demonstrated that KLHL19 triggered SOX9 ubiquitination and degradation through the Kelch domain. In summary, they found that tumor-related KLHL19 mutations may impair the substrate recruitment function of the Kelch domain and thereby inhibit the oncoprotein SOX9 ubiquitination and degradation, which accelerates the progression of LC and HCC [263].
KLHL25-ACLY
KLHL25 is involved in the regulation of transition from fatty acid synthesis to fatty acid oxidation through the ubiquitination and degradation of ATP-citrate lyase (ACLY), which facilitates inducible regulatory T cell differentiation [264].
ACLY, a key enzyme in de novo lipid synthesis, links glucose metabolism to de novo lipid synthesis [265,266], is frequently overexpressed and activated in many types of cancer, including LC, and promotes lipid synthesis in cancer [267,268]. Abnormal lipid metabolism is a hallmark of cancer cells, which plays an important role in cancer progression [269-271], including LC [272]. To explore the relationship between KLHL25 and ACLY in LC progression, Zhang et al. conducted a series of experiments and revealed that KLHL25 can target ACLY and induce its ubiquitination and proteasome degradation to maintain lipid metabolism balance, thus suppressing LC cell proliferation and tumor progression [273].
KLHL22-PD-1
KLHL22 is involved in the regulation of the occurrence and progression of many tumors, including BC, MM and CRC. CRC is a high-incidence disease, ranking third among all diseases in terms of incidence and second in terms of mortality. PD-1 is an important immune-suppressive molecule expressed on the surfaces of activated T cells, suppresses T cell proliferation and function, and prevents the immune system from killing cancer cells [274]. The regulation of the PD-1/PD-L1 axis is significant for tumor occurrence, progression and therapy, and PD-1 inhibitors benefit a subset of patients with CRC [275].
PD-1 is regulated by post-translational modification, including a series of E3-mediated ubiquitination, which may influence immunosuppression effects [276]. Albert et al. have determined that KLHL22 is a major PD-1 interacting protein and maintains the homeostasis of PD-1 through UPS before PD-1 is transported to the cell surface [99]. In vitro cell experiments showed that KLHL22 knockdown increased the expression of PD-1 and leaded to excessive T cell suppression. And, the nude mouse tumorigenicity assays showed that nude mice injected subcutaneously with KLHL22 knockout CRC and MM cells have stronger tumor-forming abilities and shorter survival times compared to control group. In conclusion, their research showed that KLHL22 can regulate PD-1 expression through ubiquitination and maintain proper levels of PD-1 and T cell homeostasis, thereby inhibiting tumorigenesis and progression.
KLHL20-KLHL39
KLHL39 has been traditionally known as “influenza virus NS1A binding protein” in the past, which antagonizes the primary host anti-viral response by binding to the ubiquitin-like ISG15 protein and inhibiting its binding to a range of proteins [277]. In tumor-associated research, KLHL39 is considered a tumor suppressor inhibiting tumor invasion and migration [278]. It interacts with KLHL20 and inhibits the ubiquitination and degradation of the target substrates by KLHL20, which inhibits CRC progression.
The death-associated protein kinase (DAPK) is a calmodulin-regulated associated serine/threonine kinase that transmits apoptosis or autophagy death signals in presence of various cellular stress signals [279]. It acts as a suppressor protein and is downregulated in a variety of tumors [280]. PML, as mentioned above, is a pleiotropic tumor suppressor protein downregulated in many tumors [116]. KLHL20, as an adaptor of E3 ligase, promotes DAPK [281] and PML [44] ubiquitination and degradation, thus promoting tumor progression.
Hatano et al. showed that highly expressed KLHL39 inhibits the migration and invasion of colon cancer cells, and mice inoculated with colon cancer and melanoma cells with overexpressed KLHL39 tended to exhibit low rates of lung and liver metastasis and long survival periods [278]. To explore the underlying mechanisms of the anticancer effects of KLHL39, Chen et al. confirmed the interaction between KLHL20 and KLHL39 by yeast two-hybrid screening and co-immunoprecipitation. Interestingly, they have shown that KLHL39 interacts with the Kelch domain of KLHL20, is not a substrate for CRL3-KLHL20 ubiquitin ligase, and is not degraded through KLHL20-mediated ubiquitination modification. They hypothesized that KLHL39 might act as a pseudo-substrate preventing target substrate recruitment to the KLHL20 complex. Consistent with this hypothesis, a series of in vitro and in vivo experiments have demonstrated that KLHL39 overexpression can inhibit the binding of KLHL20 with its substrates, PML, and DAPK and mediated ubiquitination degradation, thereby suppressing CRC cell migration and invasion. Additionally, they observed that KLHL39 overexpression disrupted the formation of the Cullin3-RBX1-KLHL20 complex. In summary, their research indicated that KLHL39 not only blocked the binding of KLHL20 to its substrate but also disrupted the formation of E3 ligase complex, thereby inhibiting CRC progression [282].
KLHL9-C/EBPs
KLHL9 induces insulin receptor substrate 1 (IRS1) ubiquitination and proteasome degradation, therefore leading to insulin resistance [283], and regulates mitotic progression via inducing Aurora degradation [284]. In addition, the linkage between KLHL9 and brain tumor progression has been found, recently.
Glioblastoma (GBM) is the most common malignant tumor of the human brain and has a poor prognosis even after a series of aggressive treatments [285]. The mesenchymal subtype of GBM (MES-GBM) has the worst prognosis, and the regulation of the transition of GBM to a mesenchymal state is largely unknown. The CCAAT/enhancer binding protein (C/EBP) family consists of six transcription factors: C/EBPα, C/EBPβ, C/EBPγ, C/EBPδ, C/EBPε, and CHOP, which contain three structural regions: a C-terminal leucine-zipper, basic DNA-binding region, and N-terminal transactivating region [286]. C/EBPδ promotes the metastasis of pancreatic cancer [287], and C/EBPβ and C/EBPδ are necessary for the transition to mesenchymal-like states in GBM [288]. Michael et al. found that GBM cells with KLHL19 knockdown tended to have enhanced tumorigenicity ability. They confirmed C/EBPβ and C/EBPδ were substrates for KLHL9, and KLHL9 induced their degradation through UPS, which inhibited mesenchymal characteristics and reduced tumorigenesis in vitro and in vivo [289].
KLHL7-p53
KLHL7 plays crucial roles in maintaining nucleolar integrity by inducing terminal uridylyl transferase 1 ubiquitination and proteasome degradation [290]. Additionally, it has been observed that the tumor protein p53 is a substrate of KLHL7 and undergoes KLHL7-mediated ubiquitination and proteasome degradation, which promotes BC progression.
Tumor suppressor protein p53 participates in the coordination of multiple responses, including cell cycle arrest, DNA repair, antioxidant effects and anti-angiogenesis, thereby preventing tumor occurrence and progression [291]. The p53 mutations are in more than 50% tumors and provide selective advantages to tumor cells, enabling them to prevent apoptosis and senescence, and maintain proliferation when normal cells cannot proliferate [292]. Its role in the dysregulation of ubiquitination in tumor cells has been explored [293]. Two E3 ligases, MDM2 [294] and CRL4A [295], can promote BC progression by degrading p53, and E3 ligase TRIM47 can facilitate renal cell carcinoma (RCC) progression by degrading p53, [296]. Kurozumi et al. showed that patients with high KLHL7 levels tended to have shorter survival times despite that the mRNA levels of KLHL7 were not highly expressed in most BC specimens. They also found that KLHL7 protein expression increased in high histologic grade, ER (-) or HER-2(+) tumors and TNBC [42], and patients with higher expression of KLHL7 in BC tissues often have shorter survival times. Overall, their study indicated that KLHL7 might function as an adaptor protein and induce p53 ubiquitination and proteasome degradation, thereby boosting BC procession [42].
KLHL6-CDK2
KLHL6 is involved in the regulation of the formation of germinal centers about CLL, and the high expression of its encoded gene KLHL6 is considered a poor prognostic indicator [183]. Many studies have demonstrated the association between KLHL6 and hematological malignancies, including AML.
The disorder of hematopoietic stem cell differentiation is one of the principal hallmarks of AML, and altering differentiation stasis may be a potential treatment for AML. For example, all-trans retinoic acid (ATRA) functions as a differentiation-inducing drug and significantly improves the prognoses of patients with APL [297]. Cyclin-dependent kinase 2 (CDK2) is a serine/threonine-protein kinase and is essential to cell cycle regulation [298] and involved in DNA damage [299], intracellular material transport [300], protein degradation [301]. The overexpression of CDK2 is directly related to cancer progression and is considered a potential cancer therapeutic target [302,303]. It has been suggested that CDK2 in AML is specifically degraded during ATRA differentiation therapy [304]. Ying et al. found that CDK2 in AML cells was specifically degraded during intramedullary differentiation progression and this degradation can be blocked by proteasome inhibitors. Peroxiredoxin 2 (PRDX2) is one of the mercaptan-specific peroxidases, and its inhibition may promote the differentiation of AML cells [303]. They hypothesized the existence of E3 ligases interacting with CDK2, and identified that KLHL6 induced CDK2 ubiquitination and proteasome degradation to inhibit PRDX2 activation, promote AML cell differentiation [305] and suppress AML progression.
KLHL2-UCK1
As an adaptor of Cullin3-E3 ligase, KLHL2 promotes WNK kinase ubiquitination and proteasome degradation to regulate electrolyte balance in kidneys [306]. KLHL2 inhibits ccRCC progression by targeting a specific substrate and triggering its degradation.
AML is a malignant disease of the myeloid hematopoietic stem or progenitor cells. Currently, 5-azacytidine (5-AZA) is the first-line therapy and has a significant efficacy for AML, but the challenge of chemotherapy resistance remains persistent. [307-309]. Uridine-cytidine kinase 1 (UCK1) is an important member of the UCK family and phosphorylates uridine and cytidine into uridine monophosphate and cytidine monophosphate [310]. UCK1 is essential for the activation and metabolism of 5-AZA [311] with a low level in mononuclear cells from 5-AZA-resistant AML patients [312]. Huang et al. identified that KLHL2 and USP28 (one deubiquitinating enzyme) participated in the regulation of the ubiquitination of UCK1 in AML cells. In vitro, they observed that silencing KLHL2 not only inhibited the proliferation of AML cells but also enabled AML cells to be sensitive to 5-AZA. Overall, they revealed that KLHL2, as an oncoprotein, can facilitate AML cell proliferation and inhibit 5-AZA-induced cell apoptosis via inducing UCK1 ubiquitination and proteasome degradation [313].
KLHL2-ARHGEF7
Rho guanine nucleotide exchange factor 7 (ARHGEF7) is a guanine nucleotide exchange factor for Rho GTPases and plays a significant role in cell migration [314,315], cell spreading [316], cytoskeletal rearrangements [317], and protein polymerization in the trans-Golgi network [318]. In many types of cancer, such as BC and CRC, it acts as an oncoprotein and is highly expressed [319-321]. Zhang et al. found that ARHGEF7 levels in ccRCC tissues were significantly higher than that in normal tissues, in contrast to the low KLHL2 expression in ccRCCs. This result suggested that the protein expression levels of KLHL2 were negatively correlated with ARHGEF7 in ccRCCs. Further, they confirmed that ARHGEF7 was a substrate to KLHL2, promoted ubiquitination and proteasome degradation, and inhibited ccRCC cell growth, migration and invasion [322].
Discussion
KLHL family members, as the significant substrate-recognizing proteins of CRL3, play important biological functions, particularly in immune response, skeletal muscle maintenance and brain development [25,323]. Notably, emerging evidence has suggested the maladjustment of KLHLs in a series of human tumors [42-44,324]. Among the KLHL family, KLHL19 has been a subject of interest in research for several years, and many reviews have systematically introduced its association with tumor occurrence and progression [27,244,248,325]. Otherwise, the dysregulation of other KLHL family members may be also associated with tumorigenesis and progression by dysregulating the specific substrates, thus affecting their stability and localization. As mentioned earlier in this review, several signaling pathways are involved in tumor-associated regulation by KLHLs. Exploring the underlying mechanisms involved in tumor occurrence and progression may be of great significance to the precise treatment of malignancy. Our review mainly focuses on the role of KLHLs and their target substrates in tumorigenesis and progression, providing the potential insight for the discovery of diagnostic and prognostic markers as well as the development of KLHLs-targeted drugs.
Even though the members of the KLHL family have similar structural domains, their biological functions are varied due to the specificity of substrate selection. It has been found that the different or even same members recognize varied substrates, which exerts disparate effects on different tumor contexts. A better understanding of KLHLs molecular structures and their substrate selection mechanisms will be beneficial in finding more undetected substrates and further comprehending tumor pathogenesis and therapy. To date, only the crystal structure of KLHL19 has been elucidated. The Kelch domain of KLHL19 consists of six Kelch repeated motifs that form a highly conserved β-propeller structure. Specifically, each Kelch motif forms a β-fold structure consisting of four anti-parallel chains, and the loop structure between the four anti-parallel chains is complex and varies [326]. This variation may partly account for the specificity of the substrate. And, mountainous research has been devoted to KLHL19 not only due to its relatively lucid structure but also due to the discovery of a “star substrate”, NRF2. As noted above, NRF2, as a crucial substrate, is tightly associated with a series of physiological and pathological processes, including tumor occurrence, progression and metastasis [242]. This may provide us some hints and may be useful in determining whether other substrates with vital biological significance remain undiscovered.
Before this study, limited number of research explored the relationship between KLHL14 and tumor, one study first determined that KLHL14 induced BCR ubiquitination and proteasome degradation, which inhibited the NF-κB signaling to suppress DLBCL progression [156]. Moreover, KLHL37 was previously considered essential to nervous system development [209], and recent studies revealed that it not only inhibited the hippo signaling by ubiquitinating and degrading LATS1/2 to induce BC radiotherapy resistance [34], but also activated the Wnt/β-catenin signaling to facilitate BC growth, migration and invasion [327]. The activation mechanism of the Wnt/β-catenin signaling by KLHL37 is not yet fully understood, therefore, we raise a question: is there an unknown KLHL37 target-substrate which is closely involved in regulating the Wnt/β-catenin signaling in BC? No doubt, a large number of KLHLs target-substrates involved in tumor-associated regulations are waiting to be found, which may bring benefits to the tumor treatment.
With the rapid development of molecular biology in the past two decades, molecular targeted therapy has widely applied in tumor clinical therapy. Conventional one-target therapy has shown its advantages for tumor treatment indeed, however, there still exists many problems such as suboptimal therapy efficacy and drug resistance. Multi-target combination treatment, as a future direction, is one of the most promising approaches in tumor therapy, thus, discovering more promising therapeutic targets is of great urgency [328]. Several key proteins, such as ULK1, CDK2, PD-1 and p53, have been demonstrated to be the substrates targeted by a series of KLHLs and also the target molecules of various molecular-targeted drugs [302,329,330]. KLHLs function as their upstream regulators influencing their stability, structure, localization and function. Is it possible that KLHLs play roles as potential targets in development of molecular targeted drugs? There are still numerous unknown questions waiting to be answered. With further research and technological development, KLHLs may become markers or therapeutic targets in the near future.
Acknowledgements
This research was funded by The Medical and health science and Technology project of Zhejiang Province (2019337934), National Natural Science Foundation of China (Grant No. 32270821), Natural Science Foundation of Ningbo (Grant No. 2021J065), The Fundamental Research Funds for the Provincial Universities of Zhejiang (Grant No. SJLZ2022004), the Zhejiang Key Laboratory of Pathophysiology (No.202204), and The K. C. Wong Magna Fund in Ningbo University.
Disclosure of conflict of interest
None.
References
- 1.Zheng N, Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem. 2017;86:129–157. doi: 10.1146/annurev-biochem-060815-014922. [DOI] [PubMed] [Google Scholar]
- 2.Grumati P, Dikic I. Ubiquitin signaling and autophagy. J Biol Chem. 2018;293:5404–5413. doi: 10.1074/jbc.TM117.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai F, Li J, Ye M, Jin X. The functions and effects of CUL3-E3 ligases mediated non-degradative ubiquitination. Gene. 2022;832:146562. doi: 10.1016/j.gene.2022.146562. [DOI] [PubMed] [Google Scholar]
- 4.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. “Protein modifications: beyond the usual suspects” review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains-from structures to functions. Nat Rev Mol Cell Biol. 2009;10:659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JR, Solomon E. BRCA1: BARD1 induces the formation of conjugated ubiquitin structures, dependent on K6 of ubiquitin, in cells during DNA replication and repair. Hum Mol Genet. 2004;13:807–817. doi: 10.1093/hmg/ddh095. [DOI] [PubMed] [Google Scholar]
- 8.Baranes-Bachar K, Levy-Barda A, Oehler J, Reid DA, Soria-Bretones I, Voss TC, Chung D, Park Y, Liu C, Yoon JB, Li W, Dellaire G, Misteli T, Huertas P, Rothenberg E, Ramadan K, Ziv Y, Shiloh Y. The ubiquitin E3/E4 ligase UBE4A adjusts protein ubiquitylation and accumulation at sites of DNA damage, facilitating double-strand break repair. Mol Cell. 2018;69:866–878. e7. doi: 10.1016/j.molcel.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto ML, Wickliffe KE, Dong KC, Yu C, Bosanac I, Bustos D, Phu L, Kirkpatrick DS, Hymowitz SG, Rape M, Kelley RF, Dixit VM. K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol Cell. 2010;39:477–484. doi: 10.1016/j.molcel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Chen P, Gao H, Gu Y, Yang J, Peng H, Xu X, Wang H, Yang M, Liu X, Fan L, Chen S, Zhou J, Sun Y, Ruan K, Cheng S, Komatsu M, White E, Li L, Ji H, Finley D, Hu R. Ubiquitylation of autophagy receptor Optineurin by HACE1 activates selective autophagy for tumor suppression. Cancer Cell. 2014;26:106–120. doi: 10.1016/j.ccr.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang YK, Yang C, Chan W, Wang Z, Deibel KE, Pomerantz JL. Molecular determinants of scaffold-induced linear ubiquitinylation of B cell lymphoma/leukemia 10 (Bcl10) during T cell receptor and oncogenic caspase recruitment domain-containing protein 11 (CARD11) signaling. J Biol Chem. 2016;291:25921–25936. doi: 10.1074/jbc.M116.754028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Huang L, Hong Z, Lv Z, Mao Z, Tang Y, Kong X, Li S, Cui Y, Liu H, Zhang L, Zhang X, Jiang L, Wang C, Zhou Q. The E3 ubiquitin ligase RNF185 facilitates the cGAS-mediated innate immune response. PLoS Pathog. 2017;13:e1006264. doi: 10.1371/journal.ppat.1006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Dou QP. The ubiquitin-proteasome system as a prospective molecular target for cancer treatment and prevention. Curr Protein Pept Sci. 2010;11:459–470. doi: 10.2174/138920310791824057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, Chanda SK, Batalov S, Joazeiro CA. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS One. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buetow L, Huang DT. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat Rev Mol Cell Biol. 2016;17:626–642. doi: 10.1038/nrm.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Z, Li H, Zhu J, Wang H, Jin X. The roles of E3 ligases in Hepatocellular carcinoma. Am J Cancer Res. 2022;12:1179–1214. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Li J, Chen J, Ye M, Jin X. Functional roles of E3 ubiquitin ligases in prostate cancer. J Mol Med. 2022;100:1125–1144. doi: 10.1007/s00109-022-02229-9. [DOI] [PubMed] [Google Scholar]
- 20.Zheng N, Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem. 2017;86:129–157. doi: 10.1146/annurev-biochem-060815-014922. [DOI] [PubMed] [Google Scholar]
- 21.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 22.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 23.Genschik P, Sumara I, Lechner E. The emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): cellular functions and disease implications. EMBO J. 2013;32:2307–2320. doi: 10.1038/emboj.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Yasmin , Figg NL, Enchev R, Knebel A, O’Shaughnessy KM, Kurz T. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med. 2015;7:1285–1306. doi: 10.15252/emmm.201505444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koonin E, Senkevich T, Chernos V. A family of DNA virus genes that consists of fused portions of unrelated cellular genes. Trends Biochem Sci. 1992;17:213–214. doi: 10.1016/0968-0004(92)90379-n. [DOI] [PubMed] [Google Scholar]
- 26.Dhanoa BS, Cogliati T, Satish AG, Bruford EA, Friedman JS. Update on the Kelch-like (KLHL) gene family. Hum Genomics. 2013;7:13. doi: 10.1186/1479-7364-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey P, Singh AK, Singh M, Tewari M, Shukla HS, Gambhir IS. The see-saw of Keap1-Nrf2 pathway in cancer. Crit Rev Oncol Hematol. 2017;116:89–98. doi: 10.1016/j.critrevonc.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M International Consortium for Blood Pressure (ICBP) Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012;44:456–460. S1–3. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 29.Jiang S, Avraham HK, Park SY, Kim TA, Bu X, Seng S, Avraham S. Process elongation of oligodendrocytes is promoted by the Kelch-related actin-binding protein Mayven. J Neurochem. 2005;92:1191–1203. doi: 10.1111/j.1471-4159.2004.02946.x. [DOI] [PubMed] [Google Scholar]
- 30.Sumara I, Quadroni M, Frei C, Olma MH, Sumara G, Ricci R, Peter M. A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev Cell. 2007;12:887–900. doi: 10.1016/j.devcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Jin Y, Zhang Y, Li B, Zhang J, Dong Z, Hu X, Wan Y. TRIM21 mediates ubiquitination of Snail and modulates epithelial to mesenchymal transition in breast cancer cells. Int J Biol Macromol. 2019;124:846–853. doi: 10.1016/j.ijbiomac.2018.11.269. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Qiu T, Wu Y, Yang C, Li Y, Du G, He Y, Liu W, Liu R, Chen CH, Shi Y, Pan J, Zhou J, Jiang D, Chen C. Arginine methyltransferase PRMT5 methylates and stabilizes KLF5 via decreasing its phosphorylation and ubiquitination to promote basal-like breast cancer. Cell Death Differ. 2021;28:2931–2945. doi: 10.1038/s41418-021-00793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon HJ, Kim DH, Kim SJ, Jang JH, Surh YJ. Src-mediated phosphorylation, ubiquitination and degradation of Caveolin-1 promotes breast cancer cell stemness. Cancer Lett. 2019;449:8–19. doi: 10.1016/j.canlet.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Wang N, Zhu M, Xiong Y, Wang F, Guo G, Wang X, Gu Y. Aberrant super-enhancer-driven oncogene ENC1 promotes the radio-resistance of breast carcinoma. Cell Death Dis. 2021;12:777. doi: 10.1038/s41419-021-04060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Du L, Ren Y, Liu X, Jiao Q, Cui D, Wen M, Wang C, Wei G, Wang Y, Ji A, Wang Q. SKP2 promotes breast cancer tumorigenesis and radiation tolerance through PDCD4 ubiquitination. J Exp Clin Cancer Res. 2019;38:76. doi: 10.1186/s13046-019-1069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T, Tao Z, Zhu Y, Liu X, Wang L, Du Y, Cao J, Wang B, Zhang J, Hu X. Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer. Cell Death Dis. 2021;12:684. doi: 10.1038/s41419-021-03963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng KC, Lin RJ, Cheng JY, Wang SH, Yu JC, Wu JC, Liang YJ, Hsu HM, Yu J, Yu AL. FAM129B, an antioxidative protein, reduces chemosensitivity by competing with Nrf2 for Keap1 binding. EBioMedicine. 2019;45:25–38. doi: 10.1016/j.ebiom.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tecalco-Cruz AC, Ramirez-Jarquin JO, Cruz-Ramos E. Estrogen receptor alpha and its ubiquitination in breast cancer cells. Curr Drug Targets. 2019;20:690–704. doi: 10.2174/1389450119666181015114041. [DOI] [PubMed] [Google Scholar]
- 39.Jia Z, Wang M, Li S, Li X, Bai XY, Xu Z, Yang Y, Li B, Li Y, Wu H. U-box ubiquitin ligase PPIL2 suppresses breast cancer invasion and metastasis by altering cell morphology and promoting SNAI1 ubiquitination and degradation. Cell Death Dis. 2018;9:63. doi: 10.1038/s41419-017-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi YM, Kim KB, Lee JH, Chun YK, An IS, An S, Bae S. DBC2/RhoBTB2 functions as a tumor suppressor protein via Musashi-2 ubiquitination in breast cancer. Oncogene. 2017;36:2802–2812. doi: 10.1038/onc.2016.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsao SM, Hsu HY. Fucose-containing fraction of Ling-Zhi enhances lipid rafts-dependent ubiquitination of TGFβ receptor degradation and attenuates breast cancer tumorigenesis. Sci Rep. 2016;6:36563. doi: 10.1038/srep36563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurozumi S, Joseph C, Sonbul S, Gorringe KL, Pigera M, Aleskandarany MA, Diez-Rodriguez M, Nolan CC, Fujii T, Shirabe K, Kuwano H, Storr S, Martin SG, Ellis IO, Green AR, Rakha EA. Clinical and biological roles of Kelch-like family member 7 in breast cancer: a marker of poor prognosis. Breast Cancer Res Treat. 2018;170:525–533. doi: 10.1007/s10549-018-4777-z. [DOI] [PubMed] [Google Scholar]
- 43.Kang JJ, Liu IY, Wang MB, Srivatsan ES. A review of gigaxonin mutations in giant axonal neuropathy (GAN) and cancer. Hum Genet. 2016;135:675–684. doi: 10.1007/s00439-016-1659-5. [DOI] [PubMed] [Google Scholar]
- 44.Yuan WC, Lee YR, Huang SF, Lin YM, Chen TY, Chung HC, Tsai CH, Chen HY, Chiang CT, Lai CK, Lu LT, Chen CH, Gu DL, Pu YS, Jou YS, Lu KP, Hsiao PW, Shih HM, Chen RH. A Cullin3-KLHL20 Ubiquitin ligase-dependent pathway targets PML to potentiate HIF-1 signaling and prostate cancer progression. Cancer Cell. 2011;20:214–228. doi: 10.1016/j.ccr.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J. Clin. Oncol. 2016;34:3803–3815. doi: 10.1200/JCO.2014.59.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 48.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 51.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 53.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng J, He S, Wang M, Zhou L, Zhang Z, Feng X, Yu Y, Ma J, Dai C, Zhang S, Sun L, Gong Y, Wang Y, Zhao M, Luo Y, Liu X, Tian L, Li C, Huang Q. The caspase-3/PKCδ/Akt/VEGF-A signaling pathway mediates tumor repopulation during radiotherapy. Clin Cancer Res. 2019;25:3732–3743. doi: 10.1158/1078-0432.CCR-18-3001. [DOI] [PubMed] [Google Scholar]
- 55.Dogan T, Gnad F, Chan J, Phu L, Young A, Chen MJ, Doll S, Stokes MP, Belvin M, Friedman LS, Kirkpatrick DS, Hoeflich KP, Hatzivassiliou G. Role of the E3 ubiquitin ligase RNF157 as a novel downstream effector linking PI3K and MAPK signaling pathways to the cell cycle. J Biol Chem. 2017;292:14311–14324. doi: 10.1074/jbc.M117.792754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Wang S, Sun Q, Yang Z, Liu M, Tang H. DCLK1 promotes epithelial-mesenchymal transition via the PI3K/Akt/NF-κB pathway in colorectal cancer. Int J Cancer. 2018;142:2068–2079. doi: 10.1002/ijc.31232. [DOI] [PubMed] [Google Scholar]
- 57.Arafeh R, Samuels Y. PIK3CA in cancer: the past 30 years. Semin Cancer Biol. 2019;59:36–49. doi: 10.1016/j.semcancer.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Hamidi A, Song J, Thakur N, Itoh S, Marcusson A, Bergh A, Heldin CH, Landstrom M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci Signal. 2017;10:eaal4186. doi: 10.1126/scisignal.aal4186. [DOI] [PubMed] [Google Scholar]
- 59.Haddadi N, Lin Y, Travis G, Simpson AM, Nassif NT, McGowan EM. PTEN/PTENP1: “Regulating the regulator of RTK-dependent PI3K/Akt signalling”, new targets for cancer therapy. Mol Cancer. 2018;17:37. doi: 10.1186/s12943-018-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 61.Bepler G, Sharma S, Cantor A, Gautam A, Haura E, Simon G, Sharma A, Sommers E, Robinson L. RRM1 and PTEN as prognostic parameters for overall and disease-free survival in patients with non-small-cell lung cancer. J. Clin. Oncol. 2004;22:1878–1885. doi: 10.1200/JCO.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Kokubo Y, Gemma A, Noro R, Seike M, Kataoka K, Matsuda K, Okano T, Minegishi Y, Yoshimura A, Shibuya M, Kudoh S. Reduction of PTEN protein and loss of epidermal growth factor receptor gene mutation in lung cancer with natural resistance to gefitinib (IRESSA) Br J Cancer. 2005;92:1711–1719. doi: 10.1038/sj.bjc.6602559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang S, Xu Z, Yuan J, Chen H. Ubiquitin-specific peptidase 17 promotes cisplatin resistance via PI3K/AKT activation in non-small cell lung cancer. Oncol Lett. 2020;20:67–74. doi: 10.3892/ol.2020.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He L, Liu X, Yang J, Li W, Liu S, Liu X, Yang Z, Ren J, Wang Y, Shan L, Guan C, Pei F, Lei L, Zhang Y, Yi X, Yang X, Liang J, Liu R, Sun L, Shang Y. Imbalance of the reciprocally inhibitory loop between the ubiquitin-specific protease USP43 and EGFR/PI3K/AKT drives breast carcinogenesis. Cell Res. 2018;28:934–951. doi: 10.1038/s41422-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan M, Xu J, Siddiqui J, Feng F, Sun Y. Depletion of SAG/RBX2 E3 ubiquitin ligase suppresses prostate tumorigenesis via inactivation of the PI3K/AKT/mTOR axis. Mol Cancer. 2016;15:81. doi: 10.1186/s12943-016-0567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Goodfellow R, Li Y, Yang S, Winters CJ, Thiel KW, Leslie KK, Yang B. NEDD4 ubiquitin ligase is a putative oncogene in endometrial cancer that activates IGF-1R/PI3K/Akt signaling. Gynecol Oncol. 2015;139:127–133. doi: 10.1016/j.ygyno.2015.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo Y, Tian L, Liang C, Xu Y. KLHL38 facilitates STS-induced apoptosis in HL-1 cells via myocardin degradation. IUBMB Life. 2022;74:446–462. doi: 10.1002/iub.2602. [DOI] [PubMed] [Google Scholar]
- 68.Li X, Yang KB, Chen W, Mai J, Wu XQ, Sun T, Wu RY, Jiao L, Li DD, Ji J, Zhang HL, Yu Y, Chen YH, Feng GK, Deng R, Li JD, Zhu XF. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy. 2021;17:4323–4340. doi: 10.1080/15548627.2021.1912270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Mao C, Wang M, Liu N, Ouyang L, Liu S, Tang H, Cao Y, Liu S, Wang X, Xiao D, Chen C, Shi Y, Yan Q, Tao Y. Cancer progression is mediated by proline catabolism in non-small cell lung cancer. Oncogene. 2020;39:2358–2376. doi: 10.1038/s41388-019-1151-5. [DOI] [PubMed] [Google Scholar]
- 70.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y, Wang C, Jiang X, Zhang Y, Su H, Jiang J, Ren H, Qiu X. KLHL38 involvement in non-small cell lung cancer progression via activation of the Akt signaling pathway. Cell Death Dis. 2021;12:556. doi: 10.1038/s41419-021-03835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang X, Xu Y, Ren H, Jiang J, Wudu M, Wang Q, Guan J, Su H, Zhang Y, Zhang B, Guo Y, Hu Y, Jiang L, Liu Z, Wang H, Cheng Y, Sun L, Qiu X. KLHL18 inhibits the proliferation, migration, and invasion of non-small cell lung cancer by inhibiting PI3K/PD-L1 axis activity. Cell Biosci. 2020;10:139. doi: 10.1186/s13578-020-00499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moghe S, Jiang F, Miura Y, Cerny RL, Tsai MY, Furukawa M. The CUL3-KLHL18 ligase regulates mitotic entry and ubiquitylates Aurora-A. Biol Open. 2012;1:82–91. doi: 10.1242/bio.2011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Dang F, Ren J, Wei W. Biochemical aspects of PD-L1 regulation in cancer immunotherapy. Trends Biochem Sci. 2018;43:1014–1032. doi: 10.1016/j.tibs.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu LN, Gills JJ, Dennis PA. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 77.Chiang GG, Abraham RT. Determination of the catalytic activities of mTOR and other members of the phosphoinositide-3-kinase-related kinase family. Methods Mol Biol. 2004;281:125–141. doi: 10.1385/1-59259-811-0:125. [DOI] [PubMed] [Google Scholar]
- 78.Brown MC, Gromeier M. MNK inversely regulates TELO2 vs. DEPTOR to control mTORC1 signaling. Mol Cell Oncol. 2017;4:e1306010. doi: 10.1080/23723556.2017.1306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laplante M, Sabatini DM. MTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR. MTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015;34:2239–2250. doi: 10.1038/onc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pons B, Peg V, Vazquez-Sanchez MA, Lopez-Vicente L, Argelaguet E, Coch L, Martinez A, Hernandez-Losa J, Armengol G, Ramon Y Cajal S. The effect of p-4E-BP1 and p-eIF4E on cell proliferation in a breast cancer model. Int J Oncol. 2011;39:1337–1345. doi: 10.3892/ijo.2011.1118. [DOI] [PubMed] [Google Scholar]
- 82.Yuan L, Sheng X, Willson AK, Roque DR, Stine JE, Guo H, Jones HM, Zhou C, Bae-Jump VL. Glutamine promotes ovarian cancer cell proliferation through the mTOR/S6 pathway. Endocr Relat Cancer. 2015;22:577–591. doi: 10.1530/ERC-15-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mita MM, Mita A, Rowinsky EK. Mammalian target of rapamycin: a new molecular target for breast cancer. Clin Breast Cancer. 2003;4:126–137. doi: 10.3816/cbc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- 84.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shaw RJ. Cell biology. GATORs take a bite out of mTOR. Science. 2013;340:1056–1057. doi: 10.1126/science.1240315. [DOI] [PubMed] [Google Scholar]
- 87.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A tumor suppressor complex with GAP activity for the rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolfson RL, Chantranupong L, Wyant GA, Gu X, Orozco JM, Shen K, Condon KJ, Petri S, Kedir J, Scaria SM, Abu-Remaileh M, Frankel WN, Sabatini DM. KICSTOR recruits GATOR1 to the lysosome and is necessary for nutrients to regulate mTORC1. Nature. 2017;543:438–442. doi: 10.1038/nature21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iffland PH 2nd, Baybis M, Barnes AE, Leventer RJ, Lockhart PJ, Crino PB. DEPDC5 and NPRL3 modulate cell size, filopodial outgrowth, and localization of mTOR in neural progenitor cells and neurons. Neurobiol Dis. 2018;114:184–193. doi: 10.1016/j.nbd.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Myers KA, Scheffer IE. DEPDC5 as a potential therapeutic target for epilepsy. Expert Opin Ther Targets. 2017;21:591–600. doi: 10.1080/14728222.2017.1316715. [DOI] [PubMed] [Google Scholar]
- 91.Pang Y, Xie F, Cao H, Wang C, Zhu M, Liu X, Lu X, Huang T, Shen Y, Li K, Jia X, Li Z, Zheng X, Wang S, He Y, Wang L, Fletcher JA, Wang Y. Mutational inactivation of mTORC1 repressor gene DEPDC5 in human gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2019;116:22746–22753. doi: 10.1073/pnas.1914542116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu L, Yang C, Wang J, Li Z, Huang R, Ma H, Ma J, Wang Q, Xiong X. Persistent mTORC1 activation via Depdc5 deletion results in spontaneous hepatocellular carcinoma but does not exacerbate carcinogen- and high-fat diet-induced hepatic carcinogenesis in mice. Biochem Biophys Res Commun. 2021;578:142–149. doi: 10.1016/j.bbrc.2021.09.036. [DOI] [PubMed] [Google Scholar]
- 93.Baldassari S, Licchetta L, Tinuper P, Bisulli F, Pippucci T. GATOR1 complex: the common genetic actor in focal epilepsies. J Med Genet. 2016;53:503–510. doi: 10.1136/jmedgenet-2016-103883. [DOI] [PubMed] [Google Scholar]
- 94.Shen K, Huang RK, Brignole EJ, Condon KJ, Valenstein ML, Chantranupong L, Bomaliyamu A, Choe A, Hong C, Yu Z, Sabatini DM. Architecture of the human GATOR1 and GATOR1-rag GTPases complexes. Nature. 2018;556:64–69. doi: 10.1038/nature26158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaramillo MC, Zhang DD. Targeting the PI3K-AKT-mTOR signaling network in cancer. Genes Dev. 2013;32:253–265. doi: 10.5732/cjc.013.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, Wang W, Wu X, Ling S, Ma Y, Huang P. SLC7A5 serves as a prognostic factor of breast cancer and promotes cell proliferation through activating AKT/mTORC1 signaling pathway. Ann Transl Med. 2021;9:892. doi: 10.21037/atm-21-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Das F, Dey N, Bera A, Kasinath BS, Ghosh-Choudhury N, Choudhury GG. MicroRNA-214 reduces insulin-like growth factor-1 (IGF-1) receptor expression and downstream mTORC1 signaling in renal carcinoma cells. J Biol Chem. 2016;291:14662–14676. doi: 10.1074/jbc.M115.694331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beck J, Maerki S, Posch M, Metzger T, Persaud A, Scheel H, Hofmann K, Rotin D, Pedrioli P, Swedlow JR, Peter M, Sumara I. Ubiquitylation-dependent localization of PLK1 in mitosis. Nat Cell Biol. 2013;15:430–439. doi: 10.1038/ncb2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou XA, Zhou J, Zhao L, Yu G, Zhan J, Shi C, Yuan R, Wang Y, Chen C, Zhang W, Xu D, Ye Y, Wang W, Shen Z, Wang W, Wang J. KLHL22 maintains PD-1 homeostasis and prevents excessive T cell suppression. Proc Natl Acad Sci U S A. 2020;117:28239–28250. doi: 10.1073/pnas.2004570117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song Y, Yuan H, Wang J, Wu Y, Xiao Y, Mao S. KLHL22 regulates the EMT and proliferation in colorectal cancer cells in part via the Wnt/beta-catenin signaling pathway. Cancer Manag Res. 2020;12:3981–3993. doi: 10.2147/CMAR.S252232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu XR, Wang W, Li HM. KLHL22 promotes malignant melanoma growth in vitro and in vivo by activating the PI3K/Akt/mTOR signaling pathway. Neoplasma. 2020;67:1106–1113. doi: 10.4149/neo_2020_190923N954. [DOI] [PubMed] [Google Scholar]
- 102.Sopik V. International variation in breast cancer incidence and mortality in young women. Breast Cancer Res Treat. 2021;186:497–507. doi: 10.1007/s10549-020-06003-8. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Ou Y, Yang Y, Li W, Xu Y, Xie Y, Liu Y. KLHL22 activates amino-acid-dependent mTORC1 signalling to promote tumorigenesis and ageing. Nature. 2018;557:585–589. doi: 10.1038/s41586-018-0128-9. [DOI] [PubMed] [Google Scholar]
- 104.Liu CC, Lin YC, Chen YH, Chen CM, Pang LY, Chen HA, Wu PR, Lin MY, Jiang ST, Tsai TF, Chen RH. Cul3-KLHL20 ubiquitin ligase governs the turnover of ULK1 and VPS34 complexes to control autophagy termination. Mol Cell. 2016;61:84–97. doi: 10.1016/j.molcel.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Rong Z, Zheng K, Chen J, Jin X. Function and regulation of ULK1: from physiology to pathology. Gene. 2022;840:146772. doi: 10.1016/j.gene.2022.146772. [DOI] [PubMed] [Google Scholar]
- 106.Lin YC, Lu LT, Chen HY, Duan X, Lin X, Feng XH, Tang MJ, Chen RH. SCP phosphatases suppress renal cell carcinoma by stabilizing PML and inhibiting mTOR/HIF signaling. Cancer Res. 2014;74:6935–6946. doi: 10.1158/0008-5472.CAN-14-1330. [DOI] [PubMed] [Google Scholar]
- 107.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 108.Gossage L, Eisen T. Alterations in VHL as potential biomarkers in renal-cell carcinoma. Nat Rev Clin Oncol. 2010;7:277–288. doi: 10.1038/nrclinonc.2010.42. [DOI] [PubMed] [Google Scholar]
- 109.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 110.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 111.de Thé H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RAR alpha fusion mRNA generated by the t (15; 17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 112.Niwa-Kawakita M, Ferhi O, Soilihi H, Le Bras M, Lallemand-Breitenbach V, de The H. PML is a ROS sensor activating p53 upon oxidative stress. J Exp Med. 2017;214:3197–3206. doi: 10.1084/jem.20160301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455:813–817. doi: 10.1038/nature07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hsu KS, Kao HY. PML: regulation and multifaceted function beyond tumor suppression. Cell Biosci. 2018;8:5. doi: 10.1186/s13578-018-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reineke EL, Liu Y, Kao HY. Promyelocytic leukemia protein controls cell migration in response to hydrogen peroxide and insulin-like growth factor-1. J Biol Chem. 2010;285:9485–9492. doi: 10.1074/jbc.M109.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gurrieri C, Capodieci P, Bernardi R, Scaglioni PP, Nafa K, Rush LJ, Verbel DA, Cordon-Cardo C, Pandolfi PP. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J Natl Cancer Inst. 2004;96:269–279. doi: 10.1093/jnci/djh043. [DOI] [PubMed] [Google Scholar]
- 117.Chen RH, Lee YR, Yuan WC. The role of PML ubiquitination in human malignancies. J Biomed Sci. 2012;19:81. doi: 10.1186/1423-0127-19-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.James RG, Conrad WH, Moon RT. Beta-catenin-independent Wnt pathways: signals, core proteins, and effectors. Methods Mol Biol. 2008;468:131–144. doi: 10.1007/978-1-59745-249-6_10. [DOI] [PubMed] [Google Scholar]
- 119.Parsons MJ, Tammela T, Dow LE. WNT as a driver and dependency in cancer. Cancer Discov. 2021;11:2413–2429. doi: 10.1158/2159-8290.CD-21-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaplan Z, Zielske SP, Ibrahim KG, Cackowski FC. Wnt and β-catenin signaling in the bone metastasis of prostate cancer. Life Sci. 2021;11:1099. doi: 10.3390/life11101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 122.Gammons MV, Renko M, Johnson CM, Rutherford TJ, Bienz M. Wnt signalosome assembly by DEP domain swapping of dishevelled. Mol Cell. 2016;64:92–104. doi: 10.1016/j.molcel.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Zhang X, Garcia Abreu J, Peng L, He X. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ranes M, Zaleska M, Sakalas S, Knight R, Guettler S. Reconstitution of the destruction complex defines roles of AXIN polymers and APC in β-catenin capture, phosphorylation, and ubiquitylation. Mol Cell. 2021;81:3246–3261. doi: 10.1016/j.molcel.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 127.Malbon CC, Wang HY. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 2006;72:153–166. doi: 10.1016/S0070-2153(05)72002-0. [DOI] [PubMed] [Google Scholar]
- 128.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 129.Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4:a007906. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 131.Galluzzi L, Spranger S, Fuchs E, Lopez-Soto A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 2019;29:44–65. doi: 10.1016/j.tcb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Schie EH, van Amerongen R. Aberrant WNT/CTNNB1 signaling as a therapeutic target in human breast cancer: weighing the evidence. Front Cell Dev Biol. 2020;8:25. doi: 10.3389/fcell.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kan W, Enos MD, Korkmazhan E, Muennich S, Chen DH, Gammons MV, Vasishtha M, Bienz M, Dunn AR, Skiniotis G, Weis WI. Limited dishevelled/Axin oligomerization determines efficiency of Wnt/β-catenin signal transduction. Elife. 2020;9:e55015. doi: 10.7554/eLife.55015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu W, Li M, Wu J, Chen H, Zhao T, Zhang C, Wang Z. Inhibition of Dishevelled-2 suppresses the biological behavior of pancreatic cancer by downregulating Wnt/β-catenin signaling. Oncol Lett. 2021;22:769. doi: 10.3892/ol.2021.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang K, Song H, Yang P, Dai X, Li Y, Wang L, Du J, Pan K, Zhang T. Silencing dishevelled-1 sensitizes paclitaxel-resistant human ovarian cancer cells via AKT/GSK-3β/β-catenin signalling. Cell Prolif. 2015;48:249–258. doi: 10.1111/cpr.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bernatik O, Paclikova P, Sri Ganji R, Bryja V. Activity of smurf2 ubiquitin ligase is regulated by the Wnt pathway protein dishevelled. Cells. 2020;9:1147. doi: 10.3390/cells9051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wei W, Li M, Wang J, Nie F, Li L. The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol Cell Biol. 2012;32:3903–3912. doi: 10.1128/MCB.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ding Y, Zhang Y, Xu C, Tao QH, Chen YG. HECT domain-containing E3 ubiquitin ligase NEDD4L negatively regulates Wnt signaling by targeting dishevelled for proteasomal degradation. J Biol Chem. 2013;288:8289–8298. doi: 10.1074/jbc.M112.433185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Z, Wasney GA, Picaud S, Filippakopoulos P, Vedadi M, D’Angiolella V, Bullock AN. Identification of a PGXPP degron motif in dishevelled and structural basis for its binding to the E3 ligase KLHL12. Open Biol. 2020;10:200041. doi: 10.1098/rsob.200041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting dishevelled for degradation. Nat Cell Biol. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- 142.McGourty CA, Akopian D, Walsh C, Gorur A, Werner A, Schekman R, Bautista D, Rape M. Regulation of the CUL3 ubiquitin ligase by a calcium-dependent co-adaptor. Cell. 2016;167:525–538. doi: 10.1016/j.cell.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 143.Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, Rape M. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuniati L, Lauriola A, Gerritsen M, Abreu S, Ni E, Tesoriero C, Onireti JO, Low TY, Heck AJR, Vettori A, Cardozo T, Guardavaccaro D. Ubiquitylation of the ER-shaping protein Lunapark via the CRL3(KLHL12) ubiquitin ligase complex. Cell Rep. 2020;31:107664. doi: 10.1016/j.celrep.2020.107664. [DOI] [PubMed] [Google Scholar]
- 145.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 146.Zhang H, Yang X, Zhu L, Li Z, Zuo P, Wang P, Feng J, Mi Y, Zhang C, Xu Y, Jin G, Zhang J, Ye H. ASPM promotes hepatocellular carcinoma progression by activating Wnt/β-catenin signaling through antagonizing autophagy-mediated Dvl2 degradation. FEBS Open Bio. 2021;11:2784–2799. doi: 10.1002/2211-5463.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liao WY, Hsu CC, Chan TS, Yen CJ, Chen WY, Pan HW, Tsai KK. Dishevelled 1-regulated superpotent cancer stem cells mediate Wnt heterogeneity and tumor progression in hepatocellular carcinoma. Stem Cell Reports. 2020;14:462–477. doi: 10.1016/j.stemcr.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mei ZZ, Chen XY, Hu SW, Wang N, Ou XL, Wang J, Luo HH, Liu J, Jiang Y. Kelch-like protein 21 (KLHL21) targets IκB kinase-β to regulate nuclear factor κ-light chain enhancer of activated B cells (NF-κB) signaling negatively. J Biol Chem. 2016;291:18176–18189. doi: 10.1074/jbc.M116.715854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kulms D, Schwarz T. NF-kappaB and cytokines. Vitam Horm. 2006;74:283–300. doi: 10.1016/S0083-6729(06)74011-0. [DOI] [PubMed] [Google Scholar]
- 152.Shih VF, Tsui R, Caldwell A, Hoffmann A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011;21:86–102. doi: 10.1038/cr.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Christian F, Smith EL, Carmody RJ. The regulation of NF-κB subunits by phosphorylation. Cells. 2016;5:12. doi: 10.3390/cells5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, Li CW, Ding Q, Liao TL, Lai CC, Lin AC, Chang YH, Tsai SF, Li LY, Hung MC. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Veena MS, Wilken R, Zheng JY, Gholkar A, Venkatesan N, Vira D, Ahmed S, Basak SK, Dalgard CL, Ravichandran S, Batra RK, Kasahara N, Elashoff D, Fishbein MC, Whitelegge JP, Torres JZ, Wang MB, Srivatsan ES. P16 protein and Gigaxonin are associated with the ubiquitination of NFkappaB in cisplatin-induced senescence of cancer cells. J Biol Chem. 2014;289:34921–34937. doi: 10.1074/jbc.M114.568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Choi J, Phelan JD, Wright GW, Haupl B, Huang DW, Shaffer AL 3rd, Young RM, Wang Z, Zhao H, Yu X, Oellerich T, Staudt LM. Regulation of B cell receptor-dependent NF-kappaB signaling by the tumor suppressor KLHL14. Proc Natl Acad Sci U S A. 2020;117:6092–6102. doi: 10.1073/pnas.1921187117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choi J, Saraf A, Florens L, Washburn MP, Busino L. PTPN14 regulates Roquin2 stability by tyrosine dephosphorylation. Cell Cycle. 2018;17:2243–2255. doi: 10.1080/15384101.2018.1522912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu G, Lo YC, Li Q, Napolitano G, Wu X, Jiang X, Dreano M, Karin M, Wu H. Crystal structure of inhibitor of κB kinase β. Nature. 2011;472:325–330. doi: 10.1038/nature09853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 160.Jiang Z, Chu H, Xi M, Yang T, Jia J, Huang J, Guo X, Zhang X, You Q, Sun HP. Insight into the intermolecular recognition mechanism between Keap1 and IKKβ combining homology modelling, protein-protein docking, molecular dynamics simulations and virtual alanine mutation. Genes Dev. 2013;8:e75076. doi: 10.1371/journal.pone.0075076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Allen E, Ding J, Wang W, Pramanik S, Chou J, Yau V, Yang Y. Gigaxonin-controlled degradation of MAP1B light chain is critical to neuronal survival. Nature. 2005;438:224–228. doi: 10.1038/nature04256. [DOI] [PubMed] [Google Scholar]
- 162.Lin NH, Huang YS, Opal P, Goldman RD, Messing A, Perng MD. The role of Gigaxonin in the degradation of the glial-specific intermediate filament protein GFAP. Mol Biol Cell. 2016;27:3980–3990. doi: 10.1091/mbc.E16-06-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kang JJ, Liu IY, Wang MB, Srivatsan ES. A review of gigaxonin mutations in giant axonal neuropathy (GAN) and cancer. Hum Genet. 2016;135:675–684. doi: 10.1007/s00439-016-1659-5. [DOI] [PubMed] [Google Scholar]
- 164.Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398:2289–2299. doi: 10.1016/S0140-6736(21)01550-6. [DOI] [PubMed] [Google Scholar]
- 165.Thai AA, Rischin D. High-dose cisplatin for head and neck cancer lives on. J. Clin. Oncol. 2018;36:1055–1057. doi: 10.1200/JCO.2017.76.8614. [DOI] [PubMed] [Google Scholar]
- 166.Kudoh K, Ichikawa Y, Yoshida S, Hirai M, Kikuchi Y, Nagata I, Miwa M, Uchida K. Inactivation of p16/CDKN2 and p15/MTS2 is associated with prognosis and response to chemotherapy in ovarian cancer. Int J Cancer. 2002;99:579–582. doi: 10.1002/ijc.10331. [DOI] [PubMed] [Google Scholar]
- 167.Al-Mohanna MA, Manogaran PS, Al-Mukhalafi Z, A Al-Hussein K, Aboussekhra A. The tumor suppressor p16(INK4a) gene is a regulator of apoptosis induced by ultraviolet light and cisplatin. Oncogene. 2004;23:201–212. doi: 10.1038/sj.onc.1206927. [DOI] [PubMed] [Google Scholar]
- 168.Yip HT, Chopra R, Chakrabarti R, Veena MS, Ramamurthy B, Srivatsan ES, Wang MB. Cisplatin-induced growth arrest of head and neck cancer cells correlates with increased expression of p16 and p53. Arch Otolaryngol Head Neck Surg. 2006;132:317–326. doi: 10.1001/archotol.132.3.317. [DOI] [PubMed] [Google Scholar]
- 169.Sahni V, Itoh Y, Shnider SJ, Macklis JD. Crim1 and Kelch-like 14 exert complementary dual-directional developmental control over segmentally specific corticospinal axon projection targeting. Cell Rep. 2021;37:109842. doi: 10.1016/j.celrep.2021.109842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Han M, Yang HJ, Lin Q. KLHL14, an ovarian and endometrial-specific gene, is over-expressed in ovarian and endometrial cancer. Math Biosci Eng. 2019;17:1702–1717. doi: 10.3934/mbe.2020089. [DOI] [PubMed] [Google Scholar]
- 171.Wang X, Sun R, Hong X, Chen C, Ding Y. KLHL14: a novel prognostic biomarker and therapeutic target for ovarian cancer. J Oncol. 2022;2022:9799346. doi: 10.1155/2022/9799346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li S, Liu J, Min Q, Ikawa T, Yasuda S, Yang Y, Wang YQ, Tsubata T, Zhao Y, Wang JY. Kelch-like protein 14 promotes B-1a but suppresses B-1b cell development. Int Immunol. 2018;30:311–318. doi: 10.1093/intimm/dxy033. [DOI] [PubMed] [Google Scholar]
- 173.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, Hodson DJ, Xiao W, Yu X, Yang Y, Zhao H, Xu W, Liu X, Zhou B, Du W, Chan WC, Jaffe ES, Gascoyne RD, Connors JM, Campo E, Lopez-Guillermo A, Rosenwald A, Ott G, Delabie J, Rimsza LM, Tay Kuang Wei K, Zelenetz AD, Leonard JP, Bartlett NL, Tran B, Shetty J, Zhao Y, Soppet DR, Pittaluga S, Wilson WH, Staudt LM. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 175.Young RM, Shaffer AL 3rd, Phelan JD, Staudt LM. B-cell receptor signaling in diffuse large B-cell lymphoma. Semin Hematol. 2015;52:77–85. doi: 10.1053/j.seminhematol.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Profitos-Peleja N, Santos JC, Marin-Niebla A, Roue G, Ribeiro ML. Regulation of B-cell receptor signaling and its therapeutic relevance in aggressive B-cell lymphomas. Cancers. 2022;14:860. doi: 10.3390/cancers14040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Phelan JD, Young RM, Webster DE, Roulland S, Wright GW, Kasbekar M, Shaffer AL 3rd, Ceribelli M, Wang JQ, Schmitz R, Nakagawa M, Bachy E, Huang DW, Ji Y, Chen L, Yang Y, Zhao H, Yu X, Xu W, Palisoc MM, Valadez RR, Davies-Hill T, Wilson WH, Chan WC, Jaffe ES, Gascoyne RD, Campo E, Rosenwald A, Ott G, Delabie J, Rimsza LM, Rodriguez FJ, Estephan F, Holdhoff M, Kruhlak MJ, Hewitt SM, Thomas CJ, Pittaluga S, Oellerich T, Staudt LM. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560:387–391. doi: 10.1038/s41586-018-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen L, Monti S, Juszczynski P, Ouyang J, Chapuy B, Neuberg D, Doench JG, Bogusz AM, Habermann TM, Dogan A, Witzig TE, Kutok JL, Rodig SJ, Golub T, Shipp MA. SYK inhibition modulates distinct PI3K/AKT- dependent survival pathways and cholesterol biosynthesis in diffuse large B cell lymphomas. Cancer Cell. 2013;23:826–838. doi: 10.1016/j.ccr.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dunleavy K, Erdmann T, Lenz G. Targeting the B-cell receptor pathway in diffuse large B-cell lymphoma. Cancer Treat Rev. 2018;65:41–46. doi: 10.1016/j.ctrv.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 182.Deng J, Guo J, Ma G, Zhang H, Sun D, Hou Y, Xie X, Guo X, Nie Y, Liang H. Prognostic value of the cancer oncogene Kelch-like 6 in gastric cancer. Br J Surg. 2017;104:1847–1856. doi: 10.1002/bjs.10628. [DOI] [PubMed] [Google Scholar]
- 183.Kokubo Y, Gemma A, Noro R, Seike M, Kataoka K, Matsuda K, Okano T, Minegishi Y, Yoshimura A, Shibuya M, Kudoh S. The BTB-kelch protein KLHL6 is involved in B-lymphocyte antigen receptor signaling and germinal center formation. Br J Cancer. 2005;25:8531–8540. doi: 10.1128/MCB.25.19.8531-8540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim JA, Hwang B, Park SN, Huh S, Im K, Choi S, Chung HY, Huh J, Seo EJ, Lee JH, Bang D, Lee DS. Genomic profile of chronic lymphocytic leukemia in Korea identified by targeted sequencing. PLoS One. 2016;11:e0167641. doi: 10.1371/journal.pone.0167641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C, Cruz-Gordillo P, Knoechel B, Asmann YW, Slager SL, Novak AJ, Dogan A, Ansell SM, Link BK, Zou L, Gould J, Saksena G, Stransky N, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Hernandez-Lemus E, Schwarz-Cruz y Celis A, Imaz-Rosshandler I, Ojesina AI, Jung J, Pedamallu CS, Lander ES, Habermann TM, Cerhan JR, Shipp MA, Getz G, Golub TR. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pratama A, Ramiscal RR, Silva DG, Das SK, Athanasopoulos V, Fitch J, Botelho NK, Chang PP, Hu X, Hogan JJ, Mana P, Bernal D, Korner H, Yu D, Goodnow CC, Cook MC, Vinuesa CG. Roquin-2 shares functions with its paralog Roquin-1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity. 2013;38:669–680. doi: 10.1016/j.immuni.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 187.Leppek K, Schott J, Reitter S, Poetz F, Hammond MC, Stoecklin G. Roquin promotes constitutive mRNA decay via a conserved class of stem-loop recognition motifs. Cell. 2013;153:869–881. doi: 10.1016/j.cell.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 188.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano A, Bhagat G, Chadburn A, Dalla-Favera R, Pasqualucci L. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schmitz R, Hansmann ML, Bohle V, Martin-Subero JI, Hartmann S, Mechtersheimer G, Klapper W, Vater I, Giefing M, Gesk S, Stanelle J, Siebert R, Kuppers R. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J Exp Med. 2009;206:981–989. doi: 10.1084/jem.20090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell. 2011;44:559–571. doi: 10.1016/j.molcel.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Choi J, Lee K, Ingvarsdottir K, Bonasio R, Saraf A, Florens L, Washburn MP, Tadros S, Green MR, Busino L. Loss of KLHL6 promotes diffuse large B-cell lymphoma growth and survival by stabilizing the mRNA decay factor roquin2. Nat Cell Biol. 2018;20:586–596. doi: 10.1038/s41556-018-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Choi J, Zhou N, Busino L. KLHL6 is a tumor suppressor gene in diffuse large B-cell lymphoma. Cell Cycle. 2019;18:249–256. doi: 10.1080/15384101.2019.1568765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang M, Dai M, Wang D, Xiong W, Zeng Z, Guo C. The regulatory networks of the Hippo signaling pathway in cancer development. J Cancer. 2021;12:6216–6230. doi: 10.7150/jca.62402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 196.Juan WC, Hong W. Targeting the Hippo signaling pathway for tissue regeneration and cancer therapy. Genes. 2016;7:55. doi: 10.3390/genes7090055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Oka T, Mazack V, Sudol M. Mst2 and LATS kinases regulate apoptotic function of yes kinase-associated protein (YAP) J Biol Chem. 2008;283:27534–27546. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- 198.Ni L, Zheng Y, Hara M, Pan D, Luo X. Structural basis for Mob1-dependent activation of the core Mst-LATS kinase cascade in Hippo signaling. Genes Dev. 2015;29:1416–1431. doi: 10.1101/gad.264929.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 200.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the Hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19:480–494. doi: 10.1038/s41573-020-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–1098. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 204.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen Y, Han H, Seo G, Vargas RE, Yang B, Chuc K, Zhao H, Wang W. Systematic analysis of the Hippo pathway organization and oncogenic alteration in evolution. Sci Rep. 2020;10:3173. doi: 10.1038/s41598-020-60120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sarmasti Emami S, Zhang D, Yang X. Interaction of the Hippo pathway and phosphatases in tumorigenesis. Cancers. 2020;12:2438. doi: 10.3390/cancers12092438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhou X, Li Y, Wang W, Wang S, Hou J, Zhang A, Lv B, Gao C, Yan Z, Pang D, Lu K, Ahmad NH, Wang L, Zhu J, Zhang L, Zhuang T, Li X. Regulation of Hippo/YAP signaling and Esophageal Squamous Carcinoma progression by an E3 ubiquitin ligase PARK2. Theranostics. 2020;10:9443–9457. doi: 10.7150/thno.46078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Salah Z, Itzhaki E, Aqeilan RI. The ubiquitin E3 ligase ITCH enhances breast tumor progression by inhibiting the Hippo tumor suppressor pathway. Oncotarget. 2014;5:10886–10900. doi: 10.18632/oncotarget.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hernandez MC, Andres-Barquin PJ, Holt I, Israel MA. Cloning of human ENC-1 and evaluation of its expression and regulation in nervous system tumors. Exp Cell Res. 1998;242:470–477. doi: 10.1006/excr.1998.4109. [DOI] [PubMed] [Google Scholar]
- 210.Wu C, Wang X, Wu X, Chen X. Ectodermal-neural cortex 1 affects the biological function of lung cancer through the MAPK pathway. Int J Mol Med. 2021;47:79. doi: 10.3892/ijmm.2021.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cui Y, Yang J, Bai Y, Li Q, Yao Y, Liu C, Wu F, Zhang J, Zhang Y. ENC1 facilitates colorectal carcinoma tumorigenesis and metastasis via JAK2/STAT5/AKT axis-mediated epithelial mesenchymal transition and stemness. Front Cell Dev Biol. 2021;9:616887. doi: 10.3389/fcell.2021.616887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fan S, Wang Y, Sheng N, Xie Y, Lu J, Zhang Z, Shan Q, Wu D, Sun C, Li M, Hu B, Zheng Y. Low expression of ENC1 predicts a favorable prognosis in patients with ovarian cancer. J Cell Biochem. 2019;120:861–871. doi: 10.1002/jcb.27447. [DOI] [PubMed] [Google Scholar]
- 213.Britschgi A, Duss S, Kim S, Couto JP, Brinkhaus H, Koren S, De Silva D, Mertz KD, Kaup D, Varga Z, Voshol H, Vissieres A, Leroy C, Roloff T, Stadler MB, Scheel CH, Miraglia LJ, Orth AP, Bonamy GM, Reddy VA, Bentires-Alj M. The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERα. Nature. 2017;541:541–545. doi: 10.1038/nature20829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sorrentino G, Ruggeri N, Zannini A, Ingallina E, Bertolio R, Marotta C, Neri C, Cappuzzello E, Forcato M, Rosato A, Mano M, Bicciato S, Del Sal G. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. 2017;8:14073. doi: 10.1038/ncomms14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li YW, Shen H, Frangou C, Yang N, Guo J, Xu B, Bshara W, Shepherd L, Zhu Q, Wang J, Hu Q, Liu S, Morrison CD, Sun P, Zhang J. Characterization of TAZ domains important for the induction of breast cancer stem cell properties and tumorigenesis. Cell Cycle. 2015;14:146–156. doi: 10.4161/15384101.2014.967106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, Amabile MI, Pilozzi E, Patrizii M, Biffoni M, Maugeri-Sacca M, Piccolo S, De Maria R. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 217.Liu J, Li J, Li P, Jiang Y, Chen H, Wang R, Cao F, Liu P. DLG5 suppresses breast cancer stem cell-like characteristics to restore tamoxifen sensitivity by inhibiting TAZ expression. J Cell Mol Med. 2019;23:512–521. doi: 10.1111/jcmm.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rangel M, Kong J, Bhatt V, Khayati K, Guo JY. Autophagy and tumorigenesis. FEBS J. 2022;289:7177–7198. doi: 10.1111/febs.16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang Y, Du J, Wu X, Abdelrehem A, Ren Y, Liu C, Zhou X, Wang S. Crosstalk between autophagy and microbiota in cancer progression. Mol Cancer. 2021;20:163. doi: 10.1186/s12943-021-01461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 222.Wirth M, Joachim J, Tooze SA. Autophagosome formation-the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol. 2013;23:301–309. doi: 10.1016/j.semcancer.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 223.Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex-at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee J, Kim J, Shin J, Kang Y, Choi J, Cheong H. ATG101 degradation by HUWE1-mediated ubiquitination impairs autophagy and reduces survival in cancer cells. Int J Mol Sci. 2021;22:9182. doi: 10.3390/ijms22179182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jia X, Chen H, Ren Y, Dejizhuoga , Gesangyuzhen , Gao N, Feng H, Huang W, Liao Y, Yu H. BAP1 antagonizes WWP1-mediated transcription factor KLF5 ubiquitination and inhibits autophagy to promote melanoma progression. Exp Cell Res. 2021;402:112506. doi: 10.1016/j.yexcr.2021.112506. [DOI] [PubMed] [Google Scholar]
- 227.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the Beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Deng R, Zhang HL, Huang JH, Cai RZ, Wang Y, Chen YH, Hu BX, Ye ZP, Li ZL, Mai J, Huang Y, Li X, Peng XD, Feng GK, Li JD, Tang J, Zhu XF. MAPK1/3 kinase-dependent ULK1 degradation attenuates mitophagy and promotes breast cancer bone metastasis. Autophagy. 2021;17:3011–3029. doi: 10.1080/15548627.2020.1850609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fialkow P, Jacobson R, Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977;63:125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- 231.Tong Y, Liu YY, You LS, Qian WB. Perifosine induces protective autophagy and upregulation of ATG5 in human chronic myelogenous leukemia cells in vitro. Acta Pharmacol Sin. 2012;33:542–550. doi: 10.1038/aps.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jabbour EJ, Cortes JE, Kantarjian HM. Tyrosine kinase inhibition: a therapeutic target for the management of chronic-phase chronic myeloid leukemia. Expert Rev Anticancer Ther. 2013;13:1433–1452. doi: 10.1586/14737140.2013.859074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 234.Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi T, Yacobi R, Van Etten RA, Donato N, Hunter A, Dinsdale D, Tirro E, Vigneri P, Nicotera P, Dyer MJ, Holyoake T, Salomoni P, Calabretta B. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119:1109–1123. doi: 10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Teahan C, Totty N, Segal A. Isolation and characterization of grancalcin, a novel 28 kDa EF-hand calcium-binding protein from human neutrophils. Biochem J. 1992;286:549–554. doi: 10.1042/bj2860549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Han SH, Korm S, Han YG, Choi SY, Kim SH, Chung HJ, Park K, Kim JY, Myung K, Lee JY, Kim H, Kim DW. GCA links TRAF6-ULK1-dependent autophagy activation in resistant chronic myeloid leukemia. Autophagy. 2019;15:2076–2090. doi: 10.1080/15548627.2019.1596492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tian W, Rojo de la Vega M, Schmidlin CJ, Ooi A, Zhang DD. Kelch-like ECH-associated protein 1 (KEAP1) differentially regulates nuclear factor erythroid-2-related factors 1 and 2 (NRF1 and NRF2) J Biol Chem. 2018;293:2029–2040. doi: 10.1074/jbc.RA117.000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ma Q. Role of NRF2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by NRF2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yang H, Wang W, Zhang Y, Zhao J, Lin E, Gao J, He J. The role of NF-E2-related factor 2 in predicting chemoresistance and prognosis in advanced non-small-cell lung cancer. Clin Lung Cancer. 2011;12:166–171. doi: 10.1016/j.cllc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 242.Taguchi K, Yamamoto M. The KEAP1-NRF2 system in cancer. Front Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Schmidlin CJ, Shakya A, Dodson M, Chapman E, Zhang DD. The intricacies of NRF2 regulation in cancer. Semin Cancer Biol. 2021;76:110–119. doi: 10.1016/j.semcancer.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Song MY, Lee DY, Chun KS, Kim EH. The role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int J Mol Sci. 2021;22:4376. doi: 10.3390/ijms22094376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sanchez-Ortega M, Carrera AC, Garrido A. Role of NRF2 in lung cancer. Cells. 2021;10:1879. doi: 10.3390/cells10081879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Panda H, Wen H, Suzuki M, Yamamoto M. Multifaceted roles of the KEAP1-NRF2 system in cancer and inflammatory disease milieu. Antioxidants. 2022;11:538. doi: 10.3390/antiox11030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Taguchi K, Yamamoto M. The KEAP1-NRF2 system as a molecular target of cancer treatment. Cancers. 2020;13:46. doi: 10.3390/cancers13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Leinonen HM, Kansanen E, Polonen P, Heinaniemi M, Levonen AL. Dysregulation of the Keap1-Nrf2 pathway in cancer. Biochem Soc Trans. 2015;43:645–649. doi: 10.1042/BST20150048. [DOI] [PubMed] [Google Scholar]
- 249.Post PL, Bokoch GM, Mooseker MS. Human myosin-IXb is a mechanochemically active motor and a GAP for rho. J Cell Sci. 1998;111:941–950. doi: 10.1242/jcs.111.7.941. [DOI] [PubMed] [Google Scholar]
- 250.Hemkemeyer SA, Vollmer V, Schwarz V, Lohmann B, Honnert U, Taha M, Schnittler HJ, Bahler M. Local Myo9b RhoGAP activity regulates cell motility. J Biol Chem. 2021;296:100136. doi: 10.1074/jbc.RA120.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chen W, Wang W, Sun X, Xie S, Xu X, Liu M, Yang C, Li M, Zhang W, Liu W, Wang L, Zhou T, Yang Y. NudCL2 regulates cell migration by stabilizing both myosin-9 and LIS1 with Hsp90. Cell Death Dis. 2020;11:534. doi: 10.1038/s41419-020-02739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang G, Huang W, Li W, Chen S, Chen W, Zhou Y, Peng P, Gu W. TFPI-2 suppresses breast cancer cell proliferation and invasion through regulation of ERK signaling and interaction with actinin-4 and myosin-9. Sci Rep. 2018;8:14402. doi: 10.1038/s41598-018-32698-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 253.Zhang T, Shen S, Zhu Z, Lu S, Yin X, Zheng J, Jin J. Homoharringtonine binds to and increases myosin-9 in myeloid leukaemia. Br J Pharmacol. 2016;173:212–221. doi: 10.1111/bph.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M, Peng HB, Shao F. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell. 2009;35:841–855. doi: 10.1016/j.molcel.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 255.Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10:17–24. doi: 10.1016/s0962-8924(99)01673-6. [DOI] [PubMed] [Google Scholar]
- 256.Wu B, Yang S, Sun H, Sun T, Ji F, Wang Y, Xu L, Zhou D. Keap1 inhibits metastatic properties of NSCLC cells by stabilizing architectures of F-actin and focal adhesions. Mol Cancer Res. 2018;16:508–516. doi: 10.1158/1541-7786.MCR-17-0544. [DOI] [PubMed] [Google Scholar]
- 257.Lefebvre V, Angelozzi M, Haseeb A. SOX9 in cartilage development and disease. Curr Opin Cell Biol. 2019;61:39–47. doi: 10.1016/j.ceb.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Suryo Rahmanto A, Savov V, Brunner A, Bolin S, Weishaupt H, Malyukova A, Rosen G, Cancer M, Hutter S, Sundstrom A, Kawauchi D, Jones DT, Spruck C, Taylor MD, Cho YJ, Pfister SM, Kool M, Korshunov A, Swartling FJ, Sangfelt O. FBW7 suppression leads to SOX9 stabilization and increased malignancy in medulloblastoma. EMBO J. 2016;35:2192–2212. doi: 10.15252/embj.201693889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Akiyama H, Kamitani T, Yang X, Kandyil R, Bridgewater LC, Fellous M, Mori-Akiyama Y, de Crombrugghe B. The transcription factor Sox9 is degraded by the ubiquitin-proteasome system and stabilized by a mutation in a ubiquitin-target site. Matrix Biol. 2005;23:499–505. doi: 10.1016/j.matbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 260.Liu C, Liu L, Chen X, Cheng J, Zhang H, Shen J, Shan J, Xu Y, Yang Z, Lai M, Qian C. Sox9 regulates self-renewal and tumorigenicity by promoting symmetrical cell division of cancer stem cells in hepatocellular carcinoma. Hepatology. 2016;64:117–129. doi: 10.1002/hep.28509. [DOI] [PubMed] [Google Scholar]
- 261.Luanpitpong S, Li J, Manke A, Brundage K, Ellis E, McLaughlin SL, Angsutararux P, Chanthra N, Voronkova M, Chen YC, Wang L, Chanvorachote P, Pei M, Issaragrisil S, Rojanasakul Y. SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene. 2016;35:2824–2833. doi: 10.1038/onc.2015.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Behan FM, Iorio F, Picco G, Goncalves E, Beaver CM, Migliardi G, Santos R, Rao Y, Sassi F, Pinnelli M, Ansari R, Harper S, Jackson DA, McRae R, Pooley R, Wilkinson P, van der Meer D, Dow D, Buser-Doepner C, Bertotti A, Trusolino L, Stronach EA, Saez-Rodriguez J, Yusa K, Garnett MJ. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature. 2019;568:511–516. doi: 10.1038/s41586-019-1103-9. [DOI] [PubMed] [Google Scholar]
- 263.Shao N, Huang H, Idris M, Peng X, Xu F, Dong S, Liu C. KEAP1 mutations drive tumorigenesis by suppressing SOX9 ubiquitination and degradation. Adv Sci. 2020;7:2001018. doi: 10.1002/advs.202001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tian M, Hao F, Jin X, Sun X, Jiang Y, Wang Y, Li D, Chang T, Zou Y, Peng P, Xia C, Liu J, Li Y, Wang P, Feng Y, Wei M. ACLY ubiquitination by CUL3-KLHL25 induces the reprogramming of fatty acid metabolism to facilitate iTreg differentiation. Elife. 2021;10:e62394. doi: 10.7554/eLife.62394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72:3709–3714. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- 267.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–2120. [PubMed] [Google Scholar]
- 268.Lin R, Tao R, Gao X, Li T, Zhou X, Guan KL, Xiong Y, Lei QY. Acetylation stabilizes ATP-citrate lyase to promote lipid biosynthesis and tumor growth. Mol Cell. 2013;51:506–518. doi: 10.1016/j.molcel.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fritz V, Benfodda Z, Henriquet C, Hure S, Cristol JP, Michel F, Carbonneau MA, Casas F, Fajas L. Metabolic intervention on lipid synthesis converging pathways abrogates prostate cancer growth. Oncogene. 2013;32:5101–5110. doi: 10.1038/onc.2012.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Balaban S, Nassar ZD, Zhang AY, Hosseini-Beheshti E, Centenera MM, Schreuder M, Lin HM, Aishah A, Varney B, Liu-Fu F, Lee LS, Nagarajan SR, Shearer RF, Hardie RA, Raftopulos NL, Kakani MS, Saunders DN, Holst J, Horvath LG, Butler LM, Hoy AJ. Extracellular fatty acids are the major contributor to lipid synthesis in prostate cancer. Mol Cancer Res. 2019;17:949–962. doi: 10.1158/1541-7786.MCR-18-0347. [DOI] [PubMed] [Google Scholar]
- 271.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hess D, Igal RA. Genistein downregulates de novo lipid synthesis and impairs cell proliferation in human lung cancer cells. Exp Biol Med (Maywood) 2011;236:707–713. doi: 10.1258/ebm.2011.010265. [DOI] [PubMed] [Google Scholar]
- 273.Zhang C, Liu J, Huang G, Zhao Y, Yue X, Wu H, Li J, Zhu J, Shen Z, Haffty BG, Hu W, Feng Z. Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression. Genes Dev. 2016;30:1956–1970. doi: 10.1101/gad.283283.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chen Y, Liu C, Zhu S, Liang X, Zhang Q, Luo X, Yuan L, Song L. PD-1/PD-L1 immune checkpoint blockade-based combinational treatment: Immunotherapeutic amplification strategies against colorectal cancer. Int Immunopharmacol. 2021;96:107607. doi: 10.1016/j.intimp.2021.107607. [DOI] [PubMed] [Google Scholar]
- 276.Hu X, Wang J, Chu M, Liu Y, Wang ZW, Zhu X. Emerging role of ubiquitination in the regulation of PD-1/PD-L1 in cancer immunotherapy. Mol Ther. 2021;29:908–919. doi: 10.1016/j.ymthe.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sridharan H, Zhao C, Krug RM. Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins. J Biol Chem. 2010;285:7852–7856. doi: 10.1074/jbc.C109.095703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ohta Y, Fujimura L, Nishio S, Arima M, Sakamoto A, Shimada H, Ochiai T, Tokuhisa T, Hatano M. A kelch family protein Nd1-L functions as a metastasis suppressor in cancer cells via Rho family proteins mediated mechanism. Int J Oncol. 2010;36:427–434. [PubMed] [Google Scholar]
- 279.Van Eldik LJ. Structure and enzymology of a death-associated protein kinase. Trends Pharmacol Sci. 2002;23:302–304. doi: 10.1016/s0165-6147(02)02049-7. [DOI] [PubMed] [Google Scholar]
- 280.Gozuacik D, Kimchi A. DAPk protein family and cancer. Autophagy. 2006;2:74–79. doi: 10.4161/auto.2.2.2459. [DOI] [PubMed] [Google Scholar]
- 281.Lee YR, Yuan WC, Ho HC, Chen CH, Shih HM, Chen RH. The Cullin 3 substrate adaptor KLHL20 mediates DAPK ubiquitination to control interferon responses. EMBO J. 2010;29:1748–1761. doi: 10.1038/emboj.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chen HY, Hu JY, Chen TH, Lin YC, Liu X, Lin MY, Lang YD, Yen Y, Chen RH. KLHL39 suppresses colon cancer metastasis by blocking KLHL20-mediated PML and DAPK ubiquitination. Oncogene. 2015;34:5141–5151. doi: 10.1038/onc.2014.435. [DOI] [PubMed] [Google Scholar]
- 283.Frendo-Cumbo S, Jaldin-Fincati JR, Coyaud E, Laurent EMN, Townsend LK, Tan JMJ, Xavier RJ, Pillon NJ, Raught B, Wright DC, Brumell JH, Klip A. Deficiency of the autophagy gene ATG16L1 induces insulin resistance through KLHL9/KLHL13/CUL3-mediated IRS1 degradation. J Biol Chem. 2019;294:16172–16185. doi: 10.1074/jbc.RA119.009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sumara I, Peter M. A Cul3-based E3 ligase regulates mitosis and is required to maintain the spindle assembly checkpoint in human cells. Cell Cycle. 2007;6:3004–3010. doi: 10.4161/cc.6.24.5068. [DOI] [PubMed] [Google Scholar]
- 285.Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM. Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep. 2020;10:11622. doi: 10.1038/s41598-020-68011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wedel A, Ziegler-Heitbrock HW. The C/EBP family of transcription factors. Immunobiology. 1995;193:171–185. doi: 10.1016/s0171-2985(11)80541-3. [DOI] [PubMed] [Google Scholar]
- 287.Duitman J, Hartl L, Roelofs JJTH, Bijlsma MF, Spek CA. Non-tumor CCAAT/enhancer-binding protein delta potentiates tumor cell extravasation and pancreatic cancer metastasis formation. Biomolecules. 2021;11:1079. doi: 10.3390/biom11081079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, Califano A, Iavarone A. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chen JC, Alvarez MJ, Talos F, Dhruv H, Rieckhof GE, Iyer A, Diefes KL, Aldape K, Berens M, Shen MM, Califano A. Identification of causal genetic drivers of human disease through systems-level analysis of regulatory networks. Cell. 2014;159:402–414. doi: 10.1016/j.cell.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kim J, Tsuruta F, Okajima T, Yano S, Sato B, Chiba T. KLHL7 promotes TUT1 ubiquitination associated with nucleolar integrity: implications for retinitis pigmentosa. Biochem Biophys Res Commun. 2017;494:220–226. doi: 10.1016/j.bbrc.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 291.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Perri F, Pisconti S, Della Vittoria Scarpati G. P53 mutations and cancer: a tight linkage. Ann Transl Med. 2016;4:522. doi: 10.21037/atm.2016.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Dai C, Gu W. P53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Guo Y, Li Q, Zhao G, Zhang J, Yuan H, Feng T, Ou D, Gu R, Li S, Li K, Lin P. Loss of TRIM31 promotes breast cancer progression through regulating K48- and K63-linked ubiquitination of p53. Cell Death Dis. 2021;12:945. doi: 10.1038/s41419-021-04208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhou Z, Qiu R, Liu W, Yang T, Li G, Huang W, Teng X, Yang Y, Yu H, Yang Y, Wang Y. BCAS3 exhibits oncogenic properties by promoting CRL4A-mediated ubiquitination of p53 in breast cancer. Cell Prolif. 2021;54:e13088. doi: 10.1111/cpr.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chen JX, Xu D, Cao JW, Zuo L, Han ZT, Tian YJ, Chu CM, Zhou W, Pan XW, Cui XG. TRIM47 promotes malignant progression of renal cell carcinoma by degrading P53 through ubiquitination. Cancer Cell Int. 2021;21:129. doi: 10.1186/s12935-021-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Petrie K, Zelent A, Waxman S. Differentiation therapy of acute myeloid leukemia: past, present and future. Curr Opin Hematol. 2009;16:84–91. doi: 10.1097/MOH.0b013e3283257aee. [DOI] [PubMed] [Google Scholar]
- 298.Pagano M, Pepperkok R, Lukas J, Baldin V, Ansorge W, Bartek J, Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993;121:101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Muller-Tidow C, Ji P, Diederichs S, Potratz J, Baumer N, Kohler G, Cauvet T, Choudary C, van der Meer T, Chan WY, Nieduszynski C, Colledge WH, Carrington M, Koeffler HP, Restle A, Wiesmuller L, Sobczak-Thepot J, Berdel WE, Serve H. The cyclin A1-CDK2 complex regulates DNA double-strand break repair. Mol Cell Biol. 2004;24:8917–8928. doi: 10.1128/MCB.24.20.8917-8928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Horiuchi D, Huskey NE, Kusdra L, Wohlbold L, Merrick KA, Zhang C, Creasman KJ, Shokat KM, Fisher RP, Goga A. Chemical-genetic analysis of cyclin dependent kinase 2 function reveals an important role in cellular transformation by multiple oncogenic pathways. Proc Natl Acad Sci U S A. 2012;109:E1019–1027. doi: 10.1073/pnas.1111317109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Thacker G, Mishra M, Sharma A, Singh AK, Sanyal S, Trivedi AK. CDK2-instigates C/EBPα degradation through SKP2 in acute myeloid leukemia. Med Oncol. 2021;38:69. doi: 10.1007/s12032-021-01523-9. [DOI] [PubMed] [Google Scholar]
- 302.Tadesse S, Anshabo AT, Portman N, Lim E, Tilley W, Caldon CE, Wang S. Targeting CDK2 in cancer: challenges and opportunities for therapy. Drug Discov Today. 2020;25:406–413. doi: 10.1016/j.drudis.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 303.Chohan TA, Qian H, Pan Y, Chen JZ. Cyclin-dependent kinase-2 as a target for cancer therapy: progress in the development of CDK2 inhibitors as anti-cancer agents. Curr Med Chem. 2015;22:237–263. doi: 10.2174/0929867321666141106113633. [DOI] [PubMed] [Google Scholar]
- 304.Shao X, Xiang S, Fu H, Chen Y, Xu A, Liu Y, Qi X, Cao J, Zhu H, Yang B, He Q, Ying M. CDK2 suppression synergizes with all-trans-retinoic acid to overcome the myeloid differentiation blockade of AML cells. Pharmacol Res. 2020;151:104545. doi: 10.1016/j.phrs.2019.104545. [DOI] [PubMed] [Google Scholar]
- 305.Ying M, Shao X, Jing H, Liu Y, Qi X, Cao J, Chen Y, Xiang S, Song H, Hu R, Wei G, Yang B, He Q. Ubiquitin-dependent degradation of CDK2 drives the therapeutic differentiation of AML by targeting PRDX2. Blood. 2018;131:2698–2711. doi: 10.1182/blood-2017-10-813139. [DOI] [PubMed] [Google Scholar]
- 306.Zhang C, Meermeier NP, Terker AS, Blankenstein KI, Singer JD, Hadchouel J, Ellison DH, Yang CL. Degradation by Cullin 3 and effect on WNK kinases suggest a role of KLHL2 in the pathogenesis of familial hyperkalemic hypertension. Biochem Biophys Res Commun. 2016;469:44–48. doi: 10.1016/j.bbrc.2015.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Voso MT, Lo-Coco F, Fianchi L. Epigenetic therapy of myelodysplastic syndromes and acute myeloid leukemia. Curr Opin Oncol. 2015;27:532–539. doi: 10.1097/CCO.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 308.Ebrahem Q, Mahfouz RZ, Durkin L, Sekeres MA, Advani AS, Carraway HE, Mukherjee S, Hamilton BK, Gerds AT, Sobecks R, Reu FJ, Hsi ED, Maciejewski JP, Saunthararajah Y. Mechanisms of resistance to 5-azacytidine/decitabine in MDS-AML and pre-clinical in vivo proof of principle of rational solutions to extend response. Blood. 2015;126:678. [Google Scholar]
- 309.Simonicova K, Janotka L, Kavcova H, Sulova Z, Breier A, Messingerova L. Different mechanisms of drug resistance to hypomethylating agents in the treatment of myelodysplastic syndromes and acute myeloid leukemia. Drug Resist Updat. 2022;61:100805. doi: 10.1016/j.drup.2022.100805. [DOI] [PubMed] [Google Scholar]
- 310.Van Rompay AR, Norda A, Linden K, Johansson M, Karlsson A. Phosphorylation of uridine and cytidine nucleoside analogs by two human uridine-cytidine kinases. Mol Pharmacol. 2001;59:1181–1186. doi: 10.1124/mol.59.5.1181. [DOI] [PubMed] [Google Scholar]
- 311.Qian Y, Ding Q, Li Y, Zou Z, Yan B, Ou L. Phosphorylation of uridine and cytidine by uridine-cytidine kinase. J Biotechnol. 2014;188:81–87. doi: 10.1016/j.jbiotec.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 312.Valencia A, Masala E, Rossi A, Martino A, Sanna A, Buchi F, Canzian F, Cilloni D, Gaidano V, Voso MT, Kosmider O, Fontenay M, Gozzini A, Bosi A, Santini V. Expression of nucleoside-metabolizing enzymes in myelodysplastic syndromes and modulation of response to azacitidine. Leukemia. 2014;28:621–628. doi: 10.1038/leu.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhang H, Huang H, Feng X, Song H, Zhang Z, Shen A, Qiu X. Deubiquitinase USP28 inhibits ubiquitin ligase KLHL2-mediated uridine-cytidine kinase 1 degradation and confers sensitivity to 5’-azacytidine-resistant human leukemia cells. Theranostics. 2020;10:1046–1059. doi: 10.7150/thno.36503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Campa F, Machuy N, Klein A, Rudel T. A new interaction between Abi-1 and betaPIX involved in PDGF-activated actin cytoskeleton reorganisation. Cell Res. 2006;16:759–770. doi: 10.1038/sj.cr.7310091. [DOI] [PubMed] [Google Scholar]
- 315.Heidary Arash E, Song KM, Song S, Shiban A, Attisano L. Arhgef7 promotes activation of the Hippo pathway core kinase LATS. EMBO J. 2014;33:2997–3011. doi: 10.15252/embj.201490230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chang F, Lemmon CA, Park D, Romer LH. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX. Mol Biol Cell. 2007;18:253–264. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhou K, Wang Y, Gorski JL, Nomura N, Collard J, Bokoch GM. Guanine nucleotide exchange factors regulate specificity of downstream signaling from Rac and Cdc42. J Biol Chem. 1998;273:16782–16786. doi: 10.1074/jbc.273.27.16782. [DOI] [PubMed] [Google Scholar]
- 318.Anitei M, Stange C, Parshina I, Baust T, Schenck A, Raposo G, Kirchhausen T, Hoflack B. Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN. Nat Cell Biol. 2010;12:330–340. doi: 10.1038/ncb2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bae JY, Ahn SJ, Lee JE, Kim JE, Han MR, Han W, Kim SW, Shin HJ, Lee SJ, Park D, Noh DY. BetaPix-a enhances the activity of phospholipase Cgamma1 by binding SH3 domain in breast cancer. J Cell Biochem. 2005;94:1010–1016. doi: 10.1002/jcb.20357. [DOI] [PubMed] [Google Scholar]
- 320.Zhang Y, Davis C, Shah S, Hughes D, Ryan JC, Altomare D, Pena MM. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol Carcinog. 2017;56:272–287. doi: 10.1002/mc.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lei X, Deng L, Liu D, Liao S, Dai H, Li J, Rong J, Wang Z, Huang G, Tang C, Xu C, Xiao B, Li T. ARHGEF7 promotes metastasis of colorectal adenocarcinoma by regulating the motility of cancer cells. Int J Oncol. 2018;53:1980–1996. doi: 10.3892/ijo.2018.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhang E, Dong X, Chen S, Shao J, Zhang P, Wang Y, Wang X. Ubiquitin ligase KLHL2 promotes the degradation and ubiquitination of ARHGEF7 protein to suppress renal cell carcinoma progression. Am J Cancer Res. 2020;10:3345–3357. [PMC free article] [PubMed] [Google Scholar]
- 323.Shi X, Xiang S, Cao J, Zhu H, Yang B, He Q, Ying M. Kelch-like proteins: physiological functions and relationships with diseases. Pharmacol Res. 2019;148:104404. doi: 10.1016/j.phrs.2019.104404. [DOI] [PubMed] [Google Scholar]
- 324.Fu AB, Xiang SF, He QJ, Ying MD. Kelch-like proteins in the gastrointestinal tumors. Acta Pharmacol Sin. 2022 doi: 10.1038/s41401-022-01007-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Li X, Zhang D, Hannink M, Beamer LJ. Crystal structure of the Kelch domain of human Keap1. J Biol Chem. 2004;279:54750–54758. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- 327.Zhou Y, Tang X, Niu L, Liu Y, Wang B, He J. Ectodermal-neural cortex 1 as a novel biomarker predicts poor prognosis and induces metastasis in breast cancer by promoting Wnt/beta-catenin pathway. J Cell Mol Med. 2020;24:8826–8835. doi: 10.1111/jcmm.15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wang Z, Wu X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020;9:8086–8121. doi: 10.1002/cam4.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Deng J, Thennavan A, Dolgalev I, Chen T, Li J, Marzio A, Poirier JT, Peng DH, Bulatovic M, Mukhopadhyay S, Silver H, Papadopoulos E, Pyon V, Thakurdin C, Han H, Li F, Li S, Ding H, Hu H, Pan Y, Weerasekara V, Jiang B, Wang ES, Ahearn I, Philips M, Papagiannakopoulos T, Tsirigos A, Rothenberg E, Gainor J, Freeman GJ, Rudin CM, Gray NS, Hammerman PS, Pagano M, Heymach JV, Perou CM, Bardeesy N, Wong KK. ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1 mutant lung cancer. Nat Cancer. 2021;2:503–514. doi: 10.1038/s43018-021-00208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wang X, Yang X, Zhang C, Wang Y, Cheng T, Duan L, Tong Z, Tan S, Zhang H, Saw PE, Gu Y, Wang J, Zhang Y, Shang L, Liu Y, Jiang S, Yan B, Li R, Yang Y, Yu J, Chen Y, Gao GF, Ye Q, Gao S. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc Natl Acad Sci U S A. 2020;117:6640–6650. doi: 10.1073/pnas.1921445117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zeniya M, Morimoto N, Takahashi D, Mori Y, Mori T, Ando F, Araki Y, Yoshizaki Y, Inoue Y, Isobe K, Nomura N, Oi K, Nishida H, Sasaki S, Sohara E, Rai T, Uchida S. Kelch-like protein 2 mediates angiotensin II-with no lysine 3 signaling in the regulation of vascular tonus. J Am Soc Nephrol. 2015;26:2129–2138. doi: 10.1681/ASN.2014070639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Tseng LA, Bixby JL. Interaction of an intracellular pentraxin with a BTB-Kelch protein is associated with ubiquitylation, aggregation and neuronal apoptosis. Mol Cell Neurosci. 2011;47:254–264. doi: 10.1016/j.mcn.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Susa K, Sohara E, Rai T, Zeniya M, Mori Y, Mori T, Chiga M, Nomura N, Nishida H, Takahashi D, Isobe K, Inoue Y, Takeishi K, Takeda N, Sasaki S, Uchida S. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014;23:5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 334.Wu G, Peng JB. Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation. FEBS Lett. 2013;587:1717–1722. doi: 10.1016/j.febslet.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep. 2013;3:858–868. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 336.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A. 2013;110:7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451:111–122. doi: 10.1042/BJ20121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Guo Q, Zhang Y, Jiang GR, Zhang C. Decreased KLHL3 expression is involved in the activation of WNK-OSR1/SPAK-NCC cascade in type 1 diabetic mice. Pflugers Arch. 2021;473:185–196. doi: 10.1007/s00424-020-02509-8. [DOI] [PubMed] [Google Scholar]
- 339.Wang L, Lai G, Chu G, Liang X, Zhao Y. CMyBP-C was decreased via KLHL3-mediated proteasomal degradation in congenital heart diseases. Exp Cell Res. 2017;355:18–25. doi: 10.1016/j.yexcr.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 340.Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci U S A. 2015;112:4340–4345. doi: 10.1073/pnas.1421441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Bertocci B, Lecoeuche D, Sterlin D, Kuhn J, Gaillard B, De Smet A, Lembo F, Bole-Feysot C, Cagnard N, Fadeev T, Dahan A, Weill JC, Reynaud CA. Klhl6 deficiency impairs transitional B cell survival and differentiation. J Immunol. 2017;199:2408–2420. doi: 10.4049/jimmunol.1700708. [DOI] [PubMed] [Google Scholar]
- 342.Kim J, Tsuruta F, Okajima T, Yano S, Sato B, Chiba T. KLHL7 promotes TUT1 ubiquitination associated with nucleolar integrity: Implications for retinitis pigmentosa. Biochem Biophys Res Commun. 2017;494:220–226. doi: 10.1016/j.bbrc.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 343.Nam S, Min K, Hwang H, Lee HO, Lee JH, Yoon J, Lee H, Park S, Lee J. Control of rapsyn stability by the CUL-3-containing E3 ligase complex. J Biol Chem. 2009;284:8195–8206. doi: 10.1074/jbc.M808230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Arama E, Bader M, Rieckhof GE, Steller H. A ubiquitin ligase complex regulates caspase activation during sperm differentiation in Drosophila. PLoS Biol. 2007;5:e251. doi: 10.1371/journal.pbio.0050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kaplan Y, Gibbs-Bar L, Kalifa Y, Feinstein-Rotkopf Y, Arama E. Gradients of a ubiquitin E3 ligase inhibitor and a caspase inhibitor determine differentiation or death in spermatids. Dev Cell. 2010;19:160–173. doi: 10.1016/j.devcel.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 346.Skieterska K, Rondou P, Van Craenenbroeck K. Dopamine D4 receptor ubiquitination. Biochem Soc Trans. 2016;44:601–605. doi: 10.1042/BST20150281. [DOI] [PubMed] [Google Scholar]
- 347.Skieterska K, Rondou P, Lintermans B, Van Craenenbroeck K. KLHL12 promotes non-lysine ubiquitination of the dopamine receptors D4.2 and D4.4, but not of the ADHD-associated D4.7 variant. PLoS One. 2015;10:e0145654. doi: 10.1371/journal.pone.0145654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Rondou P, Haegeman G, Vanhoenacker P, Van Craenenbroeck K. BTB Protein KLHL12 targets the dopamine D4 receptor for ubiquitination by a Cul3-based E3 ligase. J Biol Chem. 2008;283:11083–11096. doi: 10.1074/jbc.M708473200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kung YA, Hung CT, Chien KY, Shih SR. Control of the negative IRES trans-acting factor KHSRP by ubiquitination. Nucleic Acids Res. 2017;45:271–287. doi: 10.1093/nar/gkw1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Song J, Merrill RA, Usachev AY, Strack S. The X-linked intellectual disability gene product and E3 ubiquitin ligase KLHL15 degrades doublecortin proteins to constrain neuronal dendritogenesis. J Biol Chem. 2021;296:100082. doi: 10.1074/jbc.RA120.016210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Oberg EA, Nifoussi SK, Gingras AC, Strack S. Selective proteasomal degradation of the B’beta subunit of protein phosphatase 2A by the E3 ubiquitin ligase adaptor Kelch-like 15. J Biol Chem. 2012;287:43378–43389. doi: 10.1074/jbc.M112.420281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ferretti LP, Himmels SF, Trenner A, Walker C, von Aesch C, Eggenschwiler A, Murina O, Enchev RI, Peter M, Freire R, Porro A, Sartori AA. Cullin3-KLHL15 ubiquitin ligase mediates CtIP protein turnover to fine-tune DNA-end resection. Nat Commun. 2016;7:12628. doi: 10.1038/ncomms12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Scrivo A, Codogno P, Bomont P. Gigaxonin E3 ligase governs ATG16L1 turnover to control autophagosome production. Nat Commun. 2019;10:780. doi: 10.1038/s41467-019-08331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ding J, Allen E, Wang W, Valle A, Wu C, Nardine T, Cui B, Yi J, Taylor A, Jeon NL, Chu S, So Y, Vogel H, Tolwani R, Mobley W, Yang Y. Gene targeting of GAN in mouse causes a toxic accumulation of microtubule-associated protein 8 and impaired retrograde axonal transport. Hum Mol Genet. 2006;15:1451–1463. doi: 10.1093/hmg/ddl069. [DOI] [PubMed] [Google Scholar]
- 355.Wang W, Ding J, Allen E, Zhu P, Zhang L, Vogel H, Yang Y. Gigaxonin interacts with tubulin folding cofactor B and controls its degradation through the ubiquitin-proteasome pathway. Curr Biol. 2005;15:2050–2055. doi: 10.1016/j.cub.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 356.Mahammad S, Murthy SN, Didonna A, Grin B, Israeli E, Perrot R, Bomont P, Julien JP, Kuczmarski E, Opal P, Goldman RD. Giant axonal neuropathy-associated gigaxonin mutations impair intermediate filament protein degradation. J Clin Invest. 2013;123:1964–1975. doi: 10.1172/JCI66387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Salinas GD, Blair LA, Needleman LA, Gonzales JD, Chen Y, Li M, Singer JD, Marshall J. Actinfilin is a Cul3 substrate adaptor, linking GluR6 kainate receptor subunits to the ubiquitin-proteasome pathway. J Biol Chem. 2006;281:40164–40173. doi: 10.1074/jbc.M608194200. [DOI] [PubMed] [Google Scholar]
- 358.Chaya T, Tsutsumi R, Varner LR, Maeda Y, Yoshida S, Furukawa T. Cul3-Klhl18 ubiquitin ligase modulates rod transducin translocation during light-dark adaptation. EMBO J. 2019;38:e101409. doi: 10.15252/embj.2018101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mulvaney KM, Matson JP, Siesser PF, Tamir TY, Goldfarb D, Jacobs TM, Cloer EW, Harrison JS, Vaziri C, Cook JG, Major MB. Identification and characterization of MCM3 as a Kelch-like ECH-associated protein 1 (KEAP1) substrate. J Biol Chem. 2016;291:23719–23733. doi: 10.1074/jbc.M116.729418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Tamberg N, Tahk S, Koit S, Kristjuhan K, Kasvandik S, Kristjuhan A, Ilves I. Keap1-MCM3 interaction is a potential coordinator of molecular machineries of antioxidant response and genomic DNA replication in metazoa. Sci Rep. 2018;8:12136. doi: 10.1038/s41598-018-30562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lo SC, Hannink M. PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J Biol Chem. 2006;281:37893–37903. doi: 10.1074/jbc.M606539200. [DOI] [PubMed] [Google Scholar]
- 362.Orthwein A, Noordermeer SM, Wilson MD, Landry S, Enchev RI, Sherker A, Munro M, Pinder J, Salsman J, Dellaire G, Xia B, Peter M, Durocher D. A mechanism for the suppression of homologous recombination in G1 cells. Nature. 2015;528:422–426. doi: 10.1038/nature16142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 363.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 364.Lee Y, Chou TF, Pittman SK, Keith AL, Razani B, Weihl CC. Keap1/Cullin3 modulates p62/SQSTM1 activity via UBA domain ubiquitination. Cell Rep. 2017;19:188–202. doi: 10.1016/j.celrep.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.O’Mealey GB, Plafker KS, Berry WL, Janknecht R, Chan JY, Plafker SM. A PGAM5-KEAP1-Nrf2 complex is required for stress-induced mitochondrial retrograde trafficking. J Cell Sci. 2017;130:3467–3480. doi: 10.1242/jcs.203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Niture SK, Jaiswal AK. Inhibitor of Nrf2 (INrf2 or Keap1) protein degrades Bcl-xL via phosphoglycerate mutase 5 and controls cellular apoptosis. J Biol Chem. 2011;286:44542–44556. doi: 10.1074/jbc.M111.275073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 367.Niture SK, Jaiswal AK. INrf2 (Keap1) targets Bcl-2 degradation and controls cellular apoptosis. Cell Death Differ. 2011;18:439–451. doi: 10.1038/cdd.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 368.Yuan WC, Lee YR, Lin SY, Chang LY, Tan YP, Hung CC, Kuo JC, Liu CH, Lin MY, Xu M, Chen ZJ, Chen RH. K33-linked polyubiquitination of coronin 7 by Cul3-KLHL20 ubiquitin E3 ligase regulates protein trafficking. Mol Cell. 2014;54:586–600. doi: 10.1016/j.molcel.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 369.Lin MY, Lin YM, Kao TC, Chuang HH, Chen RH. PDZ-RhoGEF ubiquitination by Cullin3-KLHL20 controls neurotrophin-induced neurite outgrowth. J Cell Biol. 2011;193:985–994. doi: 10.1083/jcb.201103015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Courtheoux T, Enchev RI, Lampert F, Gerez J, Beck J, Picotti P, Sumara I, Peter M. Cortical dynamics during cell motility are regulated by CRL3(KLHL21) E3 ubiquitin ligase. Nat Commun. 2016;7:12810. doi: 10.1038/ncomms12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Maerki S, Olma MH, Staubli T, Steigemann P, Gerlich DW, Quadroni M, Sumara I, Peter M. The Cul3-KLHL21 E3 ubiquitin ligase targets aurora B to midzone microtubules in anaphase and is required for cytokinesis. J Cell Biol. 2009;187:791–800. doi: 10.1083/jcb.200906117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Metzger T, Kleiss C, Sumara I. CUL3 and protein kinases: insights from PLK1/KLHL22 interaction. Cell Cycle. 2013;12:2291–2296. doi: 10.4161/cc.25369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.McGourty CA, Rape M. Cullin’ PLK1 from kinetochores. Nat Cell Biol. 2013;15:347–348. doi: 10.1038/ncb2722. [DOI] [PubMed] [Google Scholar]
- 374.Beck J, Peter M. Regulating PLK1 dynamics by Cullin3/KLHL22-mediated ubiquitylation. Cell Cycle. 2013;12:2528–2529. doi: 10.4161/cc.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Lin Z, Li S, Feng C, Yang S, Wang H, Ma D, Zhang J, Gou M, Bu D, Zhang T, Kong X, Wang X, Sarig O, Ren Y, Dai L, Liu H, Zhang J, Li F, Hu Y, Padalon-Brauch G, Vodo D, Zhou F, Chen T, Deng H, Sprecher E, Yang Y, Tan X. Stabilizing mutations of KLHL24 ubiquitin ligase cause loss of keratin 14 and human skin fragility. Nat Genet. 2016;48:1508–1516. doi: 10.1038/ng.3701. [DOI] [PubMed] [Google Scholar]
- 376.Cui J, Zhao Q, Song Z, Chen Z, Zeng X, Wang C, Lin Z, Wang F, Yang Y. KLHL24-mediated hair follicle stem cells structural disruption causes alopecia. J Invest Dermatol. 2022;142:2079–2087. doi: 10.1016/j.jid.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 377.Yanagiya A, Suyama E, Adachi H, Svitkin YV, Aza-Blanc P, Imataka H, Mikami S, Martineau Y, Ronai ZA, Sonenberg N. Translational homeostasis via the mRNA cap-binding protein, eIF4E. Mol Cell. 2012;46:847–858. doi: 10.1016/j.molcel.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Papizan JB, Garry GA, Brezprozvannaya S, McAnally JR, Bassel-Duby R, Liu N, Olson EN. Deficiency in Kelch protein Klhl31 causes congenital myopathy in mice. J Clin Invest. 2017;127:3730–3740. doi: 10.1172/JCI93445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Gong W, Gohla RM, Bowlin KM, Koyano-Nakagawa N, Garry DJ, Shi X. Kelch repeat and BTB domain containing protein 5 (Kbtbd5) regulates skeletal muscle myogenesis through the E2F1-DP1 complex. J Biol Chem. 2015;290:15350–15361. doi: 10.1074/jbc.M114.629956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Ramirez-Martinez A, Cenik BK, Bezprozvannaya S, Chen B, Bassel-Duby R, Liu N, Olson EN. KLHL41 stabilizes skeletal muscle sarcomeres by nonproteolytic ubiquitination. Elife. 2017;6:e26439. doi: 10.7554/eLife.26439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Jirka C, Pak JH, Grosgogeat CA, Marchetii MM, Gupta VA. Dysregulation of NRAP degradation by KLHL41 contributes to pathophysiology in nemaline myopathy. Hum Mol Genet. 2019;28:2549–2560. doi: 10.1093/hmg/ddz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Lear TB, Lockwood KC, Larsen M, Tuncer F, Kennerdell JR, Morse C, Valenzi E, Tabib T, Jurczak MJ, Kass DJ, Evankovich JW, Finkel T, Lafyatis R, Liu Y, Chen BB. Kelch-like protein 42 is a profibrotic ubiquitin E3 ligase involved in systemic sclerosis. J Biol Chem. 2020;295:4171–4180. doi: 10.1074/jbc.AC119.012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Cummings CM, Bentley CA, Perdue SA, Baas PW, Singer JD. The Cul3/Klhdc5 E3 ligase regulates p60/katanin and is required for normal mitosis in mammalian cells. J Biol Chem. 2009;284:11663–11675. doi: 10.1074/jbc.M809374200. [DOI] [PMC free article] [PubMed] [Google Scholar]