Abstract
Uterine endometrial cancer (EC) incidence and deaths are on the rise. Hormone therapy, a traditional treatment regimen for this disease, uses progesterone and its synthetic analogue, progestin, to induce cell differentiation, apoptosis, and inhibition of invasion. This therapy is highly effective for progesterone receptor (PR) positive tumors in the short term. However, responsiveness decreases over time due to loss of PR expression; acquired resistance leads to treatment failure and poor prognosis. Primary resistance occurs in advanced, PR-negative tumors. Regardless, progestin therapy can be effective if the PR downregulation mechanism is reversed and if functional PR expression is restored. Using histone deacetylase inhibitors (HDACi), we inhibited cell proliferation in three EC cell lines and restored functional PR expression at the mRNA and protein levels. Two HDACi were tested using an endometrial xenograft tumor model: entinostat, an oral drug, and romidepsin, an IV drug. In vitro and in vivo studies support that entinostat decreased EC tumor growth, induced differentiation, and increased expression of the PR-targeted gene, PAEP. These findings supported the approval of a new NIH NCTN clinical trial, NRG-GY011, which concluded that dual treatment of MPA and entinostat, decreased expression of the proliferation marker, Ki67, but did not increase PR expression relative to single treatment with MPA in this short-term study. Therefore, a more potent HDACi, romidepsin, was investigated. Romidepsin treatment inhibited tumor growth and enhanced progestin treatment efficacy. More importantly, PR, PAEP, and KIAA1324 expressions were upregulated. Using a chromatin immunoprecipitation assay, we verified that HDACi can reverse PR downregulation mechanisms in mice models. Other potential drug efficacy markers, such as CD52, DLK1, GALNT9, and GNG2, were identified by transcriptome analysis and verified by q-PCR. Many of the upregulated drug efficacy markers predict favorable patient outcomes, while downregulated genes predict worse survival. Here, our current data suggests that romidepsin is a more potent HDACi that has the potential to achieve more robust upregulation of PR expression and may be a more promising candidate for future clinical trials.
Keywords: Progesterone receptor, progestin, epigenetic regulation, hormone therapy, endometrial cancer
Introduction
Uterine endometrial cancer is the most common gynecologic cancer and the fourth most common cancer in women [1]. It is estimated that 65,950 new cases and 12,550 deaths will occur in the United States alone in 2022 [1]. The incidences of this disease have increased by 50% from 1987 to 2008, with associated deaths increasing by 300% [2]. Survival rates for endometrial cancer are worse today than in 1975 (81% vs. 87%, P < 0.05), highlighting the need for better treatment strategies. The currently available treatment options for this disease include surgery, radiation, hormone therapy, chemotherapy, and immunotherapy. Compared to the other treatments, hormone therapy provides a safer treatment strategy.
Progestin-based hormone therapy has been used to treat endometrial cancer for over 70 years, due to the role of progesterone as the ultimate tumor suppressor in the endometrium [3]. Progestin therapy has been found to be primarily successful in tumors where hormone receptors are expressed. PR expression has been reported to be positively correlated to both a heightened response to progestin treatment and a favorable prognosis, whereas loss of PR expression underlies treatment failure and poor patient survival. The impact of progestin therapy in advanced disease where receptors are inherently lost in the tumor (primary resistance) is thereby limited. In addition, resistance to PR can be acquired over the course of treatment (acquired resistance), reducing the effects of treatment. However, progestin therapy can be effective if the PR downregulation mechanism is identified and if functional PR expression is restored to resensitize endometrial cancer patients to progestin therapy.
Multiple mechanisms of PR downregulation have been reported by our group and others. In our previous report, we systematically summarized that PR expression can be downregulated at four levels: 1) ligand-dependent proteasomal degradation, 2) miRNA fine tuning, 3) polycomb repressor complex 2-induced transcriptional repression, and 4) permanent DNA methylation. Our group has found that histone deacetylase inhibitors (HDACi) have shown to be promising solutions to reverse transcriptional repression and restore functional PR expression. Previously, HDACi have not been used to enhance PR expression in in vivo EC models. The objectives of this study are to 1) confirm that HDACi can reverse PR downregulation in multiple endometrial cancer cell lines, and 2) validate the use of HDACi in in vivo models and provide a rationale for future clinical trials. We hypothesize that progesterone therapy in endometrial cancer can be sensitized by combining progestin with epigenetic modulators in in vivo models. In this study, we demonstrated that the combination of progesterone with HDACi can sensitize endometrial cancer to progesterone therapy which we termed “molecularly enhanced progestin therapy”.
Our positive data led to the approval of a national clinical trial NRG GY011 to test the molecularly-enhanced progestin therapy in endometrial cancer patients. Entinostat was chosen in this trial due to its safety and oral route of administration. 50 patients in total were recruited over a span of 4 months. This rapid recruitment of patients across the nation displays the enthusiasm for such clinical trials expressed by patients and clinicians alike. Overall, the proliferation marker, Ki67, was downregulated in 90% of patient specimens treated with MPA + entinostat, compared to 68% of specimens treated with progestin treatment alone. However, PR expression was not enhanced by the addition of entinostat to progestin. Indeed, the initial down-regulation of PR is now considered to be a sign of PR transcriptional activity [4,5]. Nevertheless, the sustained loss of PR over the long term likely signals the initiation of hormone resistance, the development of which may be blocked by a potent HDACi. In this study, we find that romidepsin is a more potent HDACi when compared to entinostat, and when combined with MPA, reverses MPA-induced PR loss efficiently in all tested mice models and may be even more effective at reaching the primary endpoint of maintaining PR expression.
Material and methods
Antibodies and reagents
HDAC inhibitors were generously provided by Novartis (panobinostat), Celgene (romidepsin), Syndax (entinostat), and TopoTarget & Spectrum Pharmaceuticals (belinostat). MPA (USP 150 mg/ml) was acquired from Pfizer. Western blotting antibodies against PRA/B (#3153), PRB (#3157), FOXO1 (#2880), Myc (#13987), p21 (#2947), p27 (#3686), H3Ace (#9671) and cyclin D1 (#2926) were from Cell Signaling. ERα (#sc8002) and HR23B/RAD23B antibody (#sc137088) were obtained from Santa Cruz. β-actin antibody (#A1978) was obtained from Sigma-Aldrich. Chromatin immunoprecipitation antibodies against Histone H3 tri-methylated at lysine 4 (H3K4Me3, #9751), Histone H3 tri-methylated at lysine 9 (H3K9Me3, #39161), Histone H3 tri-methylated at lysine 27 (H3K27Me3, #9733) and RNA polymerase II (#39097) were obtained from Active Motif. The SUZ12 antibody (#3737) was obtained from Cell Signaling.
Cell lines and cell culture
Human endometrial cancer cell lines: ECC1 and KLE were purchased from the ATCC. Ishikawa cells were kindly gifted by Dr. Erlio Gurpide (New York University). The ECC1 and KLE cells were cultured in RPMI 1640 and the Ishikawa cells were cultured in DMEM. All cell lines were supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco). All cell lines have been authenticated using STR analysis by BioSynthesis.
Cell proliferation assay
Cell proliferation was tested under different HDACi treatments and concentrations. 10,000 cells were seeded into each well of 24 well-plates (Corning, #3527). Cells were allowed to grow for 1 day before being treated with panobinostat, romidepsin, entinostat and belinostat. Ishikawa and ECC1 cells were treated with 0-50 nM of panobinostat and 0-10 nM of romidepsin. KLE cells were treated with 0-100 nM of panobinostat and 0-100 nM of romidepsin. All three cell lines were treated with 0-2 µM of entinostat. Ishikawa and ECC1 cells were treated with 0-0.5 nM of belinostat and KLE cells were treated with 0-2 µM of belinostat. Crystal violet was added to visualize cell proliferation.
Western blotting
Expression of PR, FOXO1, p21, p27, cyclin D1, Myc, H3Ace, ERα, HR23B and β-actin were assessed by Western blotting as previously described [3,5].
Real-time PCR
Quantitative real-time PCR (qPCR) was performed as previously described [3]. Comparisons of normalized expression values (ΔCt) applied to the conventional ΔΔCt fold change method [4]. Specific primer sequences are listed in Table 1.
Table 1.
Specific primer sequences
| A. Primers for real-time PCR | |||
|
| |||
| Primer name | Forward primer (F) | Reverse primer (R) | |
|
| |||
| 1 | ESR1 | CCCACTCAACAGCGTGTCTC | CGTCGATTATCTGAATTTGGCCT |
| 2 | FOXO1 | TCGTCATAATCTGTCCCTACACA | CGGCTTCGGCTCTTAGCAAA |
| 3 | PGR | ATGTGGCAGATCCCACAGGAGTTT | ACTGGGTTTGACTTCGTAGCCCTT |
| 4 | Myc | GGCTCCTGGCAAAAGGTCA | CTGCGTAGTTGTGCTGATGT |
| 5 | p21 | TGTCCGTCAGAACCCATGC | AAAGTCGAAGTTCCATCGCTC |
| 6 | AREG | GTGGTGCTGTCGCTCTTGATA | CCCCAGAAAATGGTTCACGCT |
| 7 | PAEP | GAGATCGTTCTGCACAGATGG | CGTTCGCCACCGTATAGTTGAT |
| 8 | 18S | AACTTTCGATGGTAGTCGCCG | CCTTGGATGTGGTAGCCGTTT |
| 9 | KIAA1324 | GGAGCTTCATGCCTGCAAAGA | CATCAAACCGAATGCCTGTGC |
| 10 | CD52 | TCTTCCTCCTACTCACCATCAG | CCTCCGCTTATGTTGCTGGA |
| 11 | DLK1 | AGGGTCCCCTTTGTGACCA | GCAGGCCCGAACATCTCTATC |
| 12 | FOXA3 | GAGATGCCGAAGGGGTATCG | TGATTCTCCCGGTAGTAAGGG |
| 13 | GALNT9 | GGAAGCCCTACAACAACGACA | GGGTTCGACATGGGGATGTTC |
| 14 | GDNF | GGCAGTGCTTCCTAGAAGAGA | AAGACACAACCCCGGTTTTTG |
| 15 | GNG2 | CTCAGGCTTTAGGAACTGAAGAG | GCTGCCTTGGACACCTTTAT |
| 16 | IL1A | TGGTAGTAGCAACCAACGGGA | ACTTTGATTGAGGGCGTCATTC |
| 17 | SCUBE1 | TGGACGAGTGTCAGGACAATA | GCAGTTCATACCCTCATTGGAG |
| 18 | SPIB | CCAGCTCTGCTACGAACCC | TCCAACGGTAAGTCTTCCTCC |
| 19 | TEMEM35A | CCCAGAACCGTAACTATTGTGG | GTTTCATCTCACTGTAGGCATCC |
| 20 | EFHB | ATGAACATGGAGATTGGACATCC | GCCATCCTTCCCTCACACTTA |
| 21 | CTSS | TGACAACGGCTTTCCAGTACA | GGCAGCACGATATTTTGAGTCAT |
| 22 | S100B | TGGCCCTCATCGACGTTTTC | ATGTTCAAAGAACTCGTGGCA |
| 23 | DHRS3 | ACTGAGTGCCATTACTTCATCTG | CATCACTGTCCATTAGGCTCTTC |
| 24 | SPOCK1 | ACCCCTGCCTGAAGGTAAAAT | GGCTTGCACTTGACCAAATTC |
| 25 | UNC5C | ACCTGTACTGTAAAGCAAGCC | GGACAATGAGACCGGAAGTTT |
| 26 | SFRP2 | CTGGCCCGACATGCTTGAG | GCTTCACATACCTTTGGAGCTT |
|
| |||
| B. Primers for ChIP assay | |||
|
| |||
| Primer name | Forward primer (F) | Reverse primer (R) | |
|
| |||
| 1 | chPR | GAGGAGGAGGCGTTGTTAGA | GCCTCGGGTTGTAGATTTCA |
| 2 | chPAEP | TCTGCCCTCCTCCTACATAA | CAGGCCTCCCACAGTAAAG |
| 3 | chMyc | TACCCTTCTTTCCTCCACTCT | GCATCCTTGTCCTGTGAGTATAA |
Mouse model for endometrial cancer
The mouse studies were authorized by the Institutional Animal Care and Use Committee at the University of Iowa. Immunodeficient mice were obtained from Jackson Laboratory (NOD.Cg-Prkdc<scid>, 005557). All mice were maintained at the University of Iowa MERF Animal Facility under barrier conditions. 106 Ishikawa cells in 200 μL of medium were subcutaneously injected into the right flank regions of the mice. After one week, mice were ovariectomized. The mice were randomized into four treatment groups: control (n = 6), MPA (n = 7), HDACi (n = 7), and MPA + HDACi (n = 7). MPA was given once per week, i.m., 1 mg/ml. Romidepsin was given twice per week via i.p. injection, 2.4 mg/kg in saline. Tumor sizes were routinely measured and recorded. Mice were euthanized if the tumor length exceeded 2 cm, displayed an ulcer on their tumor, or appeared to be unhealthy.
H&E staining
A slice of tumor tissue was taken from each tumor of the mice during sacrificing and was fixed in formalin, embedded in paraffin, sliced, and followed by H&E staining. The slides were applied with xylene, 100% alcohol, 70% alcohol, and tap water. Haematoxylin was applied for 4 minutes followed by a 20-second differentiation in ammonia after which eosin was applied for 20 seconds.
Transcriptome analysis
Mice treatment was identical to the aforementioned mouse model section with the following modification: control (n = 3), MPA (n = 3), HDACi (n = 3), and MPA + HDACi (n = 3). MPA was given once for 6 hours, i.m., 1 mg/ml. Romidepsin was given once via i.p. injection, 4.4 mg/kg in saline for 24 hours. Total RNA was extracted using Qiagen RNeasy Kit, followed by DNase I treatment to remove DNA. RNA quality was assessed using Agilent and DropSense. The RNA sequencing library was prepared by the University of Iowa Genomic DNA Facility. RNA-seq was conducted using Illumina TruSeq stranded mRNA (oligo-dT) and the Illumina HiSeq4000 sequencing system with 75 bp paired end read. Data was analyzed by the University of Iowa Bioinformatics CORE Facility.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was conducted using the SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling, #9005), followed by q-PCR using the primers listed in Table 1. Results are representative of at least three independent replicates.
MRNA correlation analysis and prediction of survival
The mRNA expression data was obtained from the Cancer Genome Atlas (TCGA) which includes three sets of data: (1) normal versus tumor samples, (2) correlation to PR expression, (3) and Kaplan Meier curve prediction of patient survival [6,7].
Statistical analysis
Student’s t-test was used for comparisons of two groups. All pairwise multiple comparisons were performed by one-way ANOVA and comparisons between two groups were performed by one-tailed two-sample student t-test with an overall significance level at 0.05 (P ≤ 0.05).
Results
Multiple HDAC inhibitors inhibit cell proliferation and restore functional PR expression in endometrial cancer cells
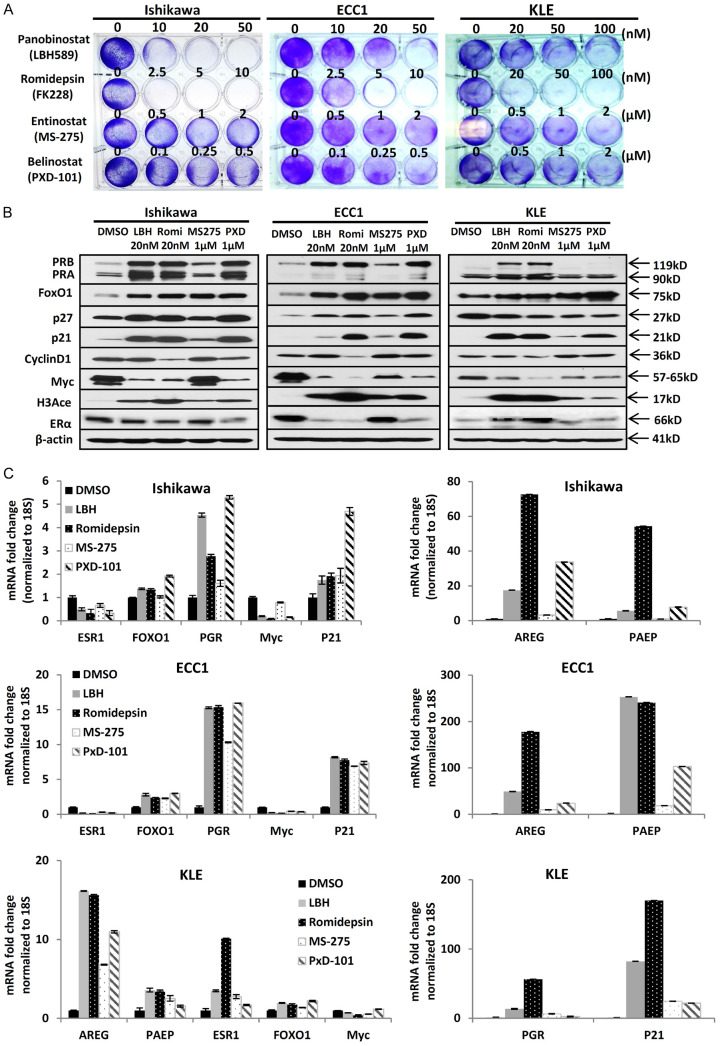
To investigate the ability of HDACi to inhibit endometrial cancer cell growth, panobinostat, romidepsin, entinostat, and belinostat were tested in three endometrial cancer cell lines: Ishikawa, ECC1, and KLE. A cell proliferation assay was conducted to determine optimal dosage; in all three endometrial cancer cell lines, HDACi decreased cell proliferation, albeit with different efficacy (Figure 1A). Panobinostat and romidepsin more effectively inhibited cell proliferation at low nanomolar concentrations, however, entinostat and belinostat inhibited cell proliferation at micromolar concentrations. Next, immunoblotting was used to assess the effects of treatment with HDACi on PR and its downstream genes. As shown in Figure 1B, various HDACi increased the expression of PR and downstream targets FOXO1, p27, and p21. Furthermore, expression of oncogenes, Myc and CCND1, were decreased after treatment with HDACi. QPCR studies were used to validate the restoration of functional PR expression at the mRNA level which indicates transcriptional regulation by HDACi (Figure 1C). At the same time, multiple PR downstream genes, including FOXO1, p21, AREG, and PAEP were also increased after the treatment. The responses to HDACi were cell-specific. The responsiveness of drugs were cell-specific. Notably, AREG and PAEP were especially upregulated and exhibited significantly higher fold changes with romidepsin in Ishikawa and ECC1 cell lines. However, in the KLE cell line, PGR and p21 exhibited the greatest fold changes. Conversely, oncogene Myc was decreased which is consistent with alterations on the protein level. Although HDACi can increase ERα expression in breast cancer [8], we demonstrated that HDACi decreased ERα expression in two ER-positive endometrial cancer cell lines, Ishikawa and ECC1. In the ER-negative KLE cells which have low ESR1 expression, ESR1 was upregulated 2 to 10 fold, depending on the treatment.
Figure 1.
Multiple HDAC inhibitors inhibit cell proliferation and restore functional PR expression in endometrial cancer cells. A. Cell proliferation assay: cell proliferation in Ishikawa, ECC1, and KLE endometrial cancer cell lines was evaluated after different HDACi treatments. B. Western blot analysis of PRB, PRA, FOXO1, p27, p21, Cyclin D1, Myc, histone H3 acetylation (H3Ace) and ERα in Ishikawa, ECC1 and KLE cells. β-actin serving as loading control. C. QPCR: ESR1, PGR, FOXO1, p21, AREG and PAEP mRNA expression normalized to 18S displayed as mean ± SD.
Entinostat sensitizes progestin treatment in endometrial tumors
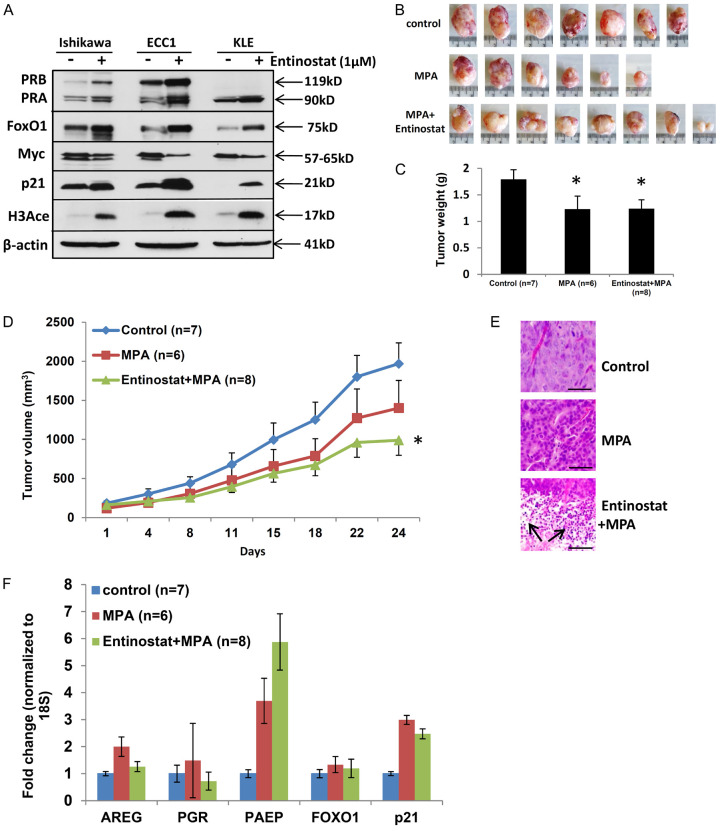
Entinostat is not the most active HDACi evaluated in our preclinical studies, however, it was chosen for NRG-GY011 based upon its safety profile and oral route of administration. The efficacy of this drug was very carefully evaluated in vitro and in vivo. First, entinostat was found to increase PR and its target gene expression at protein levels (Figure 2A) in three endometrial cancer cell lines. This is consistent with the observation that entinostat increased functional PR expression at the mRNA level (Figure 1C). Next, an endometrial xenograft model was used to assess if entinostat could enhance progestin therapy. The combination of entinostat + MPA reduced tumor volume and weight compared to MPA and control treatments (Figure 2B-D). In vivo, entinostat + MPA induced expression of the PR target gene, PAEP, however, did not restore PR expression (Figure 2F). Furthermore, dual treatment induced cellular differentiation and tumor necrosis (Figure 2E). Unlike tumors from control or MPA groups, tumors from the entinostat + MPA group were 80% necrotic tissue (as shown with arrows), which may explain the low PR mRNA levels on day 24 (Figure 2E). Together, these findings resulted in the approval of clinical trial NRG-GY011, the first in-human trial to evaluate molecularly-enhanced progestin therapy. In this clinical trial, tumor proliferation marker, Ki67, was downregulated in 90% of the dual treatment group compared to 68% of the MPA group. However, because PR was not shown to be upregulated in the timeframe of this study, this trial was deemed a negative trial. We next studied whether another, potentially more potent HDACi, romidepsin, could be useful for future studies.
Figure 2.
Entinostat sensitizes progestin treatment in endometrial tumors. A. Western blot analysis of PRB, PRA, FOXO1, Myc, p21 and H3Ace in Ishikawa, ECC1 and KLE cells with entinostat treatment. β-actin serving as loading control. B-D. Mice were injected subcutaneously with 1 × 106 Ishikawa cells and treated with DMSO, MPA, and MPA + entinostat 2 times a week. Tumor growth was monitored by measuring tumor size 2 times a week for 24 days. Ishikawa tumors were harvested after 24 days of drug treatment. B. Tumor picture. C. Tumor weight displayed as mean ± SEM. D. Tumor growth displayed as mean + SEM or mean - SEM. *P < 0.05 vs. control. E. H&E staining of tumor specimens (scale bar = 50 µm, and magnification = 20x). F. QPCR: AREG, PGR, PAEP, FOXO1, p21 mRNA expression in tumor samples were normalized to 18S displayed as mean ± SEM.
Romidepsin sensitizes progestin therapy and restores PR expression in endometrial tumors
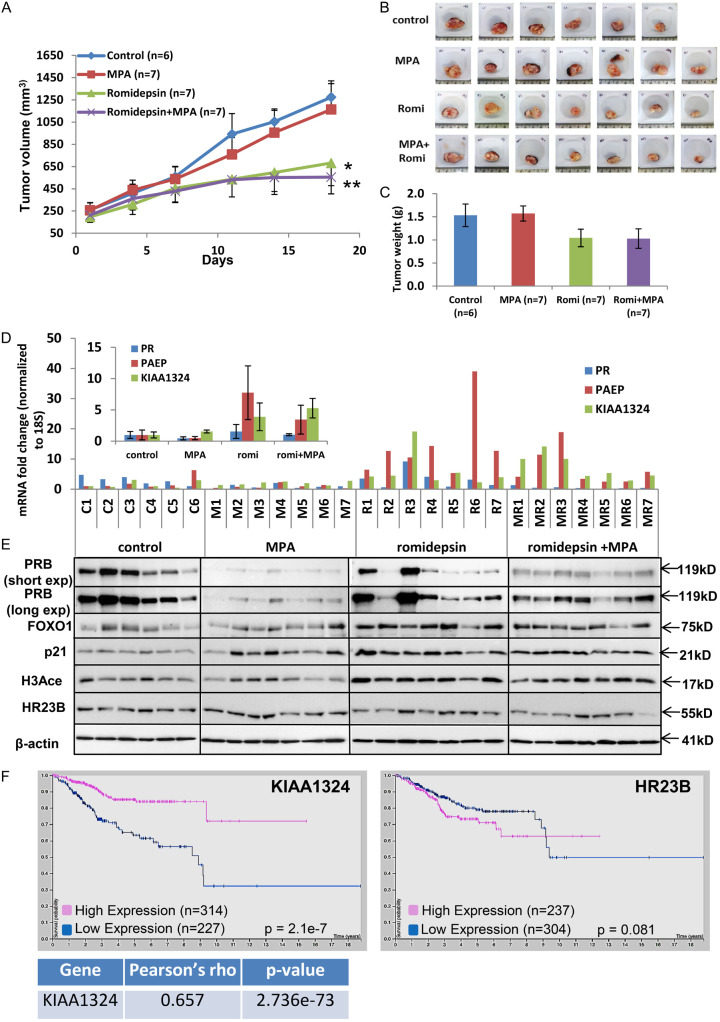
Romidepsin, a much more potent HDACi which targets only one class of HDACs, provided the next logical step to study the effects of HDACi on PR expression. As shown in Figure 3A, 3B, dual treatment dramatically decreased tumor volume over an 18-day course. Furthermore, tumor weight of the romidepsin + MPA group was significantly lower than the control group on day 18 (Figure 3C). Following these findings, immunoblotting and qRT-PCR analyses were completed to assess expression levels of PR, PAEP, and KIAA1324 in all tumors. As shown in Figure 3D, the addition of romidepsin to traditional progestin treatment led to a significant expression increase in both the PR downstream gene, PAEP, and the tumor suppressor KIAA1324. Furthermore, PRB, FOXO1, p21, and H3Ace were shown to be upregulated in the romidepsin + MPA treatment group compared to MPA treatment alone. HR23B/RAD23B, a DNA repair protein which also plays a role in ubiquitin-mediated proteolysis, was found to be downregulated with the treatment of HDACi in most tumor samples (Figure 3E).
Figure 3.
Romidepsin sensitizes progestin therapy and restores PR expression in endometrial tumors. A-C. Mice were injected subcutaneously with 1 × 106 Ishikawa cells, ovariectomized and treated with DMSO, MPA, romidepsin, and Romidepsin + MPA. Tumor growth was monitored by measuring tumor size 2 times a week for 20 days. Ishikawa tumors were harvested after 20 days of drug treatment. A. Tumor size displayed as mean ± SEM. *P < 0.05 vs. control. **P < 0.01 vs. control. B. Tumor picture. C. Tumor weight displayed as mean ± SEM. D. QPCR: PR, PAEP, KIAA1324 mRNA expression in tumor samples were normalized to 18S. Insert, Average ± SEM mRNA expression of genes in each group. E. Western blot analysis of PRB, FOXO1, p21, H3Ace and HR23B in harvested mice tumor cells. β-actin served as loading control. F. Kaplan-Meier plots of survival data of KIAA1324 and HR23B obtained from The Human Protein Atlas in the TCGA endometrial cancer data set (n = 541).
Consistent with the upregulated KIAA1324 by the romidepsin + MPA group (Figure 3D), KIAA1324 was found to be a favorable prognostic marker in endometrial cancer as well as having a strong correlation with PR (r = 0.657), as shown in Figure 3F. Conversely, high HR23B expression was found to be associated with worse survival rates among endometrial cancer patients, suggesting that downregulation will be beneficial for patient survival (Figure 3F). Indeed, romidepsin + MPA treatment decreases HR23B expression in mice tumors.
HDACi treatment reverses PR and PAEP transcriptional repression
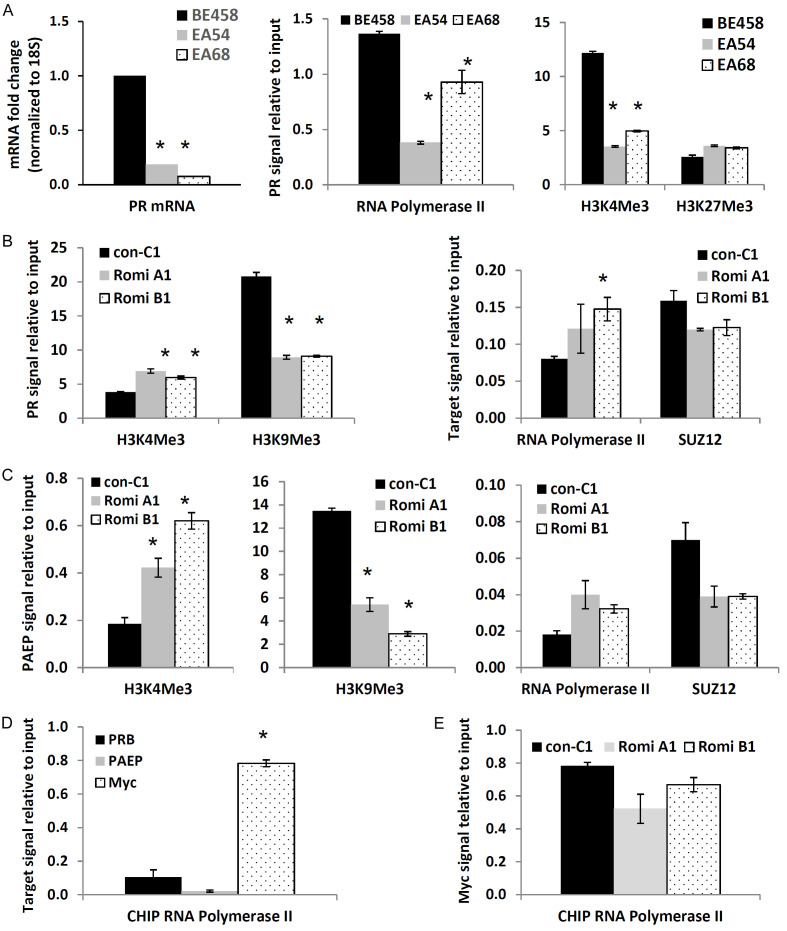
Epigenetic repression of PR is a key mechanism of PR silencing in endometrial cancer reported by our group and others [9-12]. First, we confirmed this mechanism using endometrial cancer patient tumors. First, we confirmed this mechanism using endometrial cancer patient tumors. As shown in Figure 4A, PR expression was shown to be greatly decreased in representative endometrial cancer patient tumors (EA54 & EA68) compared to the benign endometrial sample (BE458). Similarly, RNA Polymerase II and active transcription marker, H3K4Me3, were also found to have diminished binding to the PR promoter regions when compared to benign samples, while H3K27Me3 exhibited enhanced binding. Next, we investigated the effect of romidepsin on these markers. After treatment of romidepsin in mice, favorable H3K4Me3 binding was elevated while H3K9Me3 binding was decreased in the PR promoter region when compared to control groups. Binding by RNA Polymerase II was also enhanced while the binding by SUZ12, a component of the polycomb repressor complex 2, was downregulated, as shown in Figure 4B. Furthermore, binding to the PAEP promoter region by H3K4Me3 and RNA Polymerase II increased, while binding by H3K9Me3 and SUZ12 decreased (Figure 4C). Additionally, greater RNA polymerase II binding to the promoter of oncogene Myc was observed compared to PRB and PAEP in mice tumor samples (Figure 4D). Romidepsin was found to reduce the binding of RNA polymerase II to the promoter region of Myc (Figure 4E).
Figure 4.
HDACi treatment reverses PR and PAEP transcriptional repression. A. PR expression and binding of RNA polymerase II (RNA PII), H3K4Me3 and H3K27Me3 to the PR promoter region in the benign tissue (BE458) sample and two endometrial tumor samples (EA54 & EA68). *P < 0.05 vs. control. B, C. ChIP followed by q-PCR determined binding of H3K4Me3, H3K9Me3, RNA PII and SUZ12 to the different promoter regions in mice tumor samples in control (con C1) and romidepsin treated samples (Romi A1 & Romi B1). *P < 0.05 vs. control. B. In the PR promoter region. C. In the PAEP promoter region. D. ChIP followed by q-PCR determined the binding of RNA PII to the promoter regions of PR, PAEP, and Myc in Ishikawa mice tumor samples. *P < 0.05 vs. control. E. ChIP followed by q-PCR determined the binding of RNA PII to the Myc promoter region in control (con C1) and romidepsin treated mice tumors (Romi A1 & Romi B1).
Novel romidepsin + MPA drug response genes
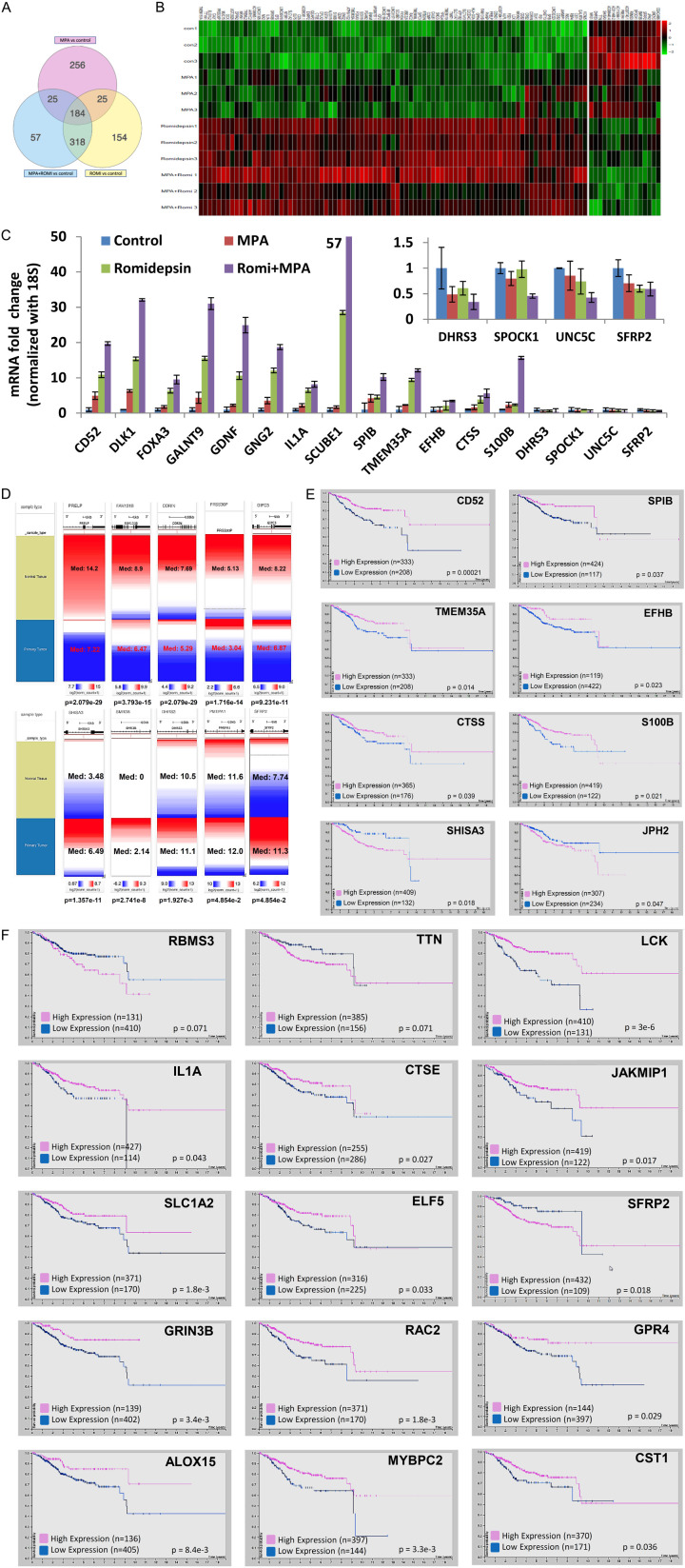
In order to discover the transcriptome alterations associated with romidepsin + MPA treatment, RNA-sequencing was done on 24-hour treated samples of four groups: control, MPA, romidepsin, and romidepsin + MPA. To determine genes that the dual treatment may have acted through to allow for superior tumor suppression, iPathwayGuide was used to analyze RNA-seq data to identify differentially expressed genes. As shown in Figure 5A, iPathwayGuide found 184 genes that were differentially expressed from all three treatment groups compared to the control group and 57 genes were differentially expressed only in the dual treatment group. In Figure 5B and Table 2, 103 transcripts were identified with the expression significantly upregulated or downregulated (fold > 2 and false discovery rate (FDR) < 0.02). From these potential candidates, several genes were chosen. Real-time PCR was used to validate the expression of these genes in each of the 4 treatment groups. CD52, DLK1, FOXA3, GALNT9, GDNF, GNG2, IL1A, SCUBE1, SPIB, TMEM35A, EFHB, CTSS, and S100B were significantly upregulated in the dual treatment group compared to other treatment groups. Meanwhile, DHRS3, SPOCK1, UNC5C and SFRP2 were downregulated in the romidepsin + MPA group (Figure 5C). Using UCSC Xena to analyze the TCGA TARGET GTEx study, strong differential expression of several genes identified from Figure 5B were identified in normal and tumor tissue. PRELP, FAM131B, CORIN, PRSS30P and GIPC3 were upregulated in the dual treatment group and were found to have low expression in tumor tissue, while SHISA3, SMR3B, DHRS3, PMEPA1 and SFRP2 were downregulated in our treatment and found to be overexpressed in tumor tissue (Figure 5D). Furthermore, upregulated genes CD52, SPIB, TMEM35A, EFHB, CTSS, and S100B were correlated with favorable survival in endometrial cancer patients while downregulated genes SHISA3 and JPH2 were correlated with worse survival (Figure 5E). More genes were identified and listed in Figure 5F and Table 3. Altogether, these altered genes provide clinical evidence supporting direct or indirect regulation of several transcripts by romidepsin + MPA; these genes may also serve as drug efficacy markers for future clinical trials.
Figure 5.
Novel Romidepsin + MPA drug response genes. A. IPathwayGuide Meta Analysis was used to identify 1,019 differentially expressed genes among the three treatment groups relative to the control group. B. Heatmap of differentially expressed genes, color-coded to show change in expression levels based on treatment. C. qPCR: CD52, DLK1, FOXA3, GALNT9, GDNF, GNG2, IL1A, SCUBE1, SPIB, TMEM35A, EFHB, CTSS, S100B, DHRS3, SPOCK1, UNC5C and SFRP2 mRNA expression in mice tumor samples were normalized to 18S. Insert, qPCR: downregulated genes DHRS3, SPOCK1, UNC5C and SFRP2 mRNA expression in mice tumor samples were normalized to 18S. D. UCSC Xena was used to compare expression of differentially expressed genes in normal tissue from the TCGA TARGET GTEx dataset for normal tissue (n = 78) and endometrial tumor samples (n = 57). Red represents higher gene expression while blue represents lower gene expression. Ranges, median log expressions, and p-values are labeled. E and F. Kaplan-Meier plots of survival data obtained from The Human Protein Atlas of differentially expressed genes in the TCGA endometrial cancer data set (n = 541).
Table 2.
Fold change of 103 genes in heatmap compare with control
| A. Fold change of upregulated genes compare with control | ||||
|
| ||||
| Gene Name | MPA | Romidepsin | MPA + Romi | |
|
| ||||
| 1 | TMEM151A | 1.57 | 8.72 | 5.44 |
| 2 | TTYH1 | 2.63 | 8.27 | 6.63 |
| 3 | ELOVL2 | 1.67 | 7.52 | 6.07 |
| 4 | CALY | 1.99 | 8.08 | 8.04 |
| 5 | PRSS30P | 0.77 | 6.96 | 8.29 |
| 6 | MUC15 | 1.00 | 4.09 | 5.64 |
| 7 | DCX | 1.14 | 7.33 | 13.50 |
| 8 | UGT2B28 | 0.82 | 3.99 | 5.38 |
| 9 | DPPA2 | 2.21 | 4.16 | 6.78 |
| 10 | NRXN1 | 2.46 | 10.98 | 18.21 |
| 11 | TREML2 | 1.40 | 4.25 | 6.65 |
| 12 | AKR1C2 | 1.44 | 2.41 | 5.62 |
| 13 | AC108865.1 | 2.43 | 7.03 | 7.22 |
| 14 | LINC01305 | 2.19 | 5.75 | 6.68 |
| 15 | MX2 | 1.92 | 6.08 | 7.73 |
| 16 | IL1A | 2.36 | 6.45 | 11.76 |
| 17 | GRIN3B | 2.83 | 7.19 | 7.84 |
| 18 | EFS | 4.10 | 11.06 | 10.88 |
| 19 | LINC00616 | 1.70 | 5.98 | 5.59 |
| 20 | NTSR1 | 2.46 | 5.11 | 5.26 |
| 21 | SLC1A2 | 1.47 | 5.63 | 6.31 |
| 22 | ELF5 | 1.44 | 5.85 | 5.29 |
| 23 | CD5 | 2.70 | 24.42 | 22.43 |
| 24 | AC005381.1 | 1.90 | 8.65 | 8.25 |
| 25 | ENPP2 | 1.36 | 6.56 | 5.63 |
| 26 | IQGAP2 | 1.87 | 7.06 | 6.43 |
| 27 | CTSE | 1.51 | 9.03 | 6.82 |
| 28 | CPLX2 | 2.07 | 3.18 | 5.13 |
| 29 | GSTA1 | 2.29 | 3.00 | 6.23 |
| 30 | ALOX15 | 1.17 | 4.77 | 5.07 |
| 31 | S100B | 2.85 | 12.49 | 20.72 |
| 32 | GDNF | 1.65 | 7.77 | 24.56 |
| 33 | GNG2 | 2.16 | 4.24 | 6.90 |
| 34 | ATP6V1G2 | 2.84 | 3.62 | 5.42 |
| 35 | TMEM35A | 3.60 | 9.53 | 12.68 |
| 36 | FOXA3 | 4.83 | 18.72 | 18.92 |
| 37 | SOX18 | 2.27 | 7.71 | 14.76 |
| 38 | PGAM2 | 1.70 | 5.80 | 6.61 |
| 39 | ENAM | 8.46 | 20.35 | 21.04 |
| 40 | AP000757.1 | 2.41 | 8.42 | 7.67 |
| 41 | IGF2 | 2.55 | 8.21 | 5.88 |
| 42 | LINC02158 | 1.42 | 3.40 | 5.94 |
| 43 | MYBPC2 | 6.13 | 22.99 | 20.60 |
| 44 | LINC01571 | 1.20 | 2.79 | 5.15 |
| 45 | SCUBE1 | 1.33 | 6.99 | 16.18 |
| 46 | FXYD4 | 1.29 | 8.25 | 8.10 |
| 47 | UBE2L6 | 1.66 | 8.40 | 6.51 |
| 48 | RAC2 | 1.55 | 5.96 | 5.72 |
| 49 | TMEM59L | 0.92 | 12.84 | 7.17 |
| 50 | GIPC3 | 0.85 | 20.35 | 11.74 |
| 51 | RTBDN | 0.91 | 17.97 | 13.38 |
| 52 | COMP | 1.03 | 7.85 | 5.33 |
| 53 | CALB1 | 0.90 | 6.02 | 5.33 |
| 54 | SNCG | 1.00 | 4.97 | 5.28 |
| 55 | YPEL4 | 1.73 | 22.35 | 23.38 |
| 56 | TMEM119 | 0.71 | 18.46 | 17.09 |
| 57 | TNNT1 | 1.37 | 16.45 | 11.11 |
| 58 | SLC17A7 | 1.35 | 14.19 | 9.33 |
| 59 | ATP1B2 | 1.17 | 8.25 | 6.24 |
| 60 | SLC7A10 | 1.00 | 104.16 | 83.16 |
| 61 | C2CD4C | 0.57 | 24.12 | 9.55 |
| 62 | HLA-DOA | 1.08 | 14.05 | 6.39 |
| 63 | GPR4 | 1.70 | 31.70 | 24.22 |
| 64 | FAM131B | 1.24 | 23.52 | 14.87 |
| 65 | PIANP | 1.89 | 25.33 | 15.53 |
| 66 | PRELP | 1.38 | 34.72 | 17.70 |
| 67 | CEND1 | 1.08 | 31.09 | 16.49 |
| 68 | AC007384.1 | 1.26 | 13.42 | 6.17 |
| 69 | ALPL | 1.29 | 10.50 | 6.91 |
| 70 | FSD1 | 1.07 | 21.10 | 10.63 |
| 71 | LCK | 0.82 | 7.32 | 5.87 |
| 72 | NRXN2 | 3.63 | 13.97 | 9.82 |
| 73 | CGB2 | 0.54 | 9.48 | 5.17 |
| 74 | CRB2 | 6.61 | 5.50 | 5.05 |
| 75 | CORIN | 5.18 | 4.15 | 5.51 |
| 76 | DLK1 | 16.63 | 13.01 | 28.47 |
| 77 | RS1 | 5.07 | 7.40 | 7.25 |
| 78 | CHST13 | 3.72 | 13.41 | 12.33 |
| 79 | SPIB | 7.29 | 11.54 | 13.30 |
| 80 | LINC02223 | 4.65 | 14.83 | 13.39 |
| 81 | CD52 | 6.64 | 6.77 | 15.59 |
| 82 | JAKMIP1 | 2.89 | 5.58 | 6.12 |
| 83 | INSC | 3.88 | 4.32 | 6.04 |
| 84 | RBP4 | 2.71 | 3.94 | 5.22 |
| 85 | CST1 | 4.91 | 6.81 | 12.80 |
| 86 | GALNT9 | 9.68 | 9.79 | 23.79 |
| 87 | KCNJ5 | 3.77 | 2.73 | 5.43 |
|
| ||||
| B. Fold change of downregulated genes compare with control | ||||
|
| ||||
| Gene Name | MPA | Romidepsin | MPA + Romi | |
|
| ||||
| 1 | NRG1 | 0.99 | 0.27 | 0.18 |
| 2 | DHRS3 | 0.36 | 0.26 | 0.15 |
| 3 | TTN | 0.80 | 0.47 | 0.19 |
| 4 | SHISA3 | 0.67 | 0.18 | 0.20 |
| 5 | AC008443.4 | 0.56 | 0.19 | 0.12 |
| 6 | AC011498.2 | 0.68 | 0.29 | 0.19 |
| 7 | AC115099.1 | 1.13 | 0.18 | 0.15 |
| 8 | PMEPA1 | 0.59 | 0.19 | 0.09 |
| 9 | AC007743.1 | 0.45 | 0.17 | 0.16 |
| 10 | RBMS3 | 0.44 | 0.25 | 0.12 |
| 11 | SPOCK1 | 0.29 | 0.26 | 0.11 |
| 12 | SMR3B | 0.59 | 0.25 | 0.19 |
| 13 | SFRP2 | 0.42 | 0.16 | 0.21 |
| 14 | JPH2 | 0.25 | 0.18 | 0.16 |
| 15 | UNC5C | 0.24 | 0.18 | 0.09 |
| 16 | CEACAM6 | 0.26 | 0.47 | 0.19 |
Table 3.
Correlation of MPA + HDACi drug response genes
| Gene | Pearson’s rho | p-value | |
| 1 | GDNF | 0.2508 | 8.244e-10 |
| 2 | SCUBE1 | 0.1952 | 2.055e-6 |
| 3 | EFHB | 0.3170 | 4.490e-15 |
| 4 | CTSS | 0.1591 | 1.142e-4 |
| 5 | CD52 | 0.2131 | 2.064e-7 |
| 6 | ENPP2 | 0.2348 | 9.631e-9 |
| 7 | CTSE | 0.09096 | 2.808e-2 |
| 8 | SLC1A2 | 0.4768 | 2.028e-34 |
| 9 | ELF5 | 0.2049 | 6.011e-7 |
| 10 | GRIN3B | 0.2140 | 1.824e-7 |
| 11 | ALOX15 | 0.2612 | 1.505e-10 |
| 12 | GSTA1 | 0.1046 | 1.147e-2 |
| 13 | MYBPC2 | 0.1110 | 7.278e-3 |
| 14 | TMEM119 | 0.1932 | 2.620e-6 |
| 15 | ATP1B2 | 0.2154 | 1.514e-7 |
| 16 | GIPC3 | 0.1297 | 1.699e-3 |
| 17 | ALPL | 0.1782 | 1.509e-5 |
| 18 | DCX | 0.1751 | 2.126e-5 |
| 19 | FAM131B | 0.1495 | 2.919e-4 |
| 20 | C2CD4C | 0.08856 | 3.252e-2 |
| 21 | PRELP | 0.1184 | 4.211e-3 |
| 22 | NRXN2 | 0.1182 | 4.256e-3 |
| 23 | CORIN | 0.1474 | 3.550e-4 |
| 24 | SFRP2 | -0.2010 | 9.993e-7 |
Discussion
Progestin therapy has long been used to treat endometrial cancer due to its tolerability and ease of administration; it has been shown to be very effective for patients with PR-positive tumors in the short term [3,13,14]. It is often prescribed to preserve patient fertility or to help patients who cannot undergo surgery [4]. However, as treatment progresses, PR is found to be significantly downregulated, eventually leading to endocrine resistance [13,15]. In patients with PR negative tumors, primary resistance prevents promising outcomes from progestin therapy. Thus, in tumors displaying either acquired or primary resistance, it is necessary to find treatment options that can ameliorate this event and resensitize tumor cells to progestin. In this study, we found that supplementing traditional progestin therapy, MPA, with HDACi, entinostat and romidepsin, enhanced tumor suppression through epigenetically regulating the transcription of PR and other key genes. Our data suggests that romidepsin would be a promising candidate for future clinical trials to noticeably restore functional PR expression and sensitize progestin therapy.
It has been known that HDACi-induced histone hyperacetylation allows transcriptional activation of a distinct set of genes resulting in inhibition of cell proliferation, induction of terminal differentiation and apoptosis, and enhancement of antitumor immune responses [16,17]. Further, HDACi also have non-histone targets and regulate protein expression at the post-translational level. HDACi have been widely applied to treat various cancers; four HDACi have already been FDA-approved to treat lymphoma and leukemia (SAHA, panobinostat, romidepsin, and belinostat) with tolerable side effects [17]. Specifically, romidepsin is a potent HDACi that targets class 1 HDACs [18], has been applied in the clinic for over a decade, and has been shown to be safely utilized in the clinic.
Restoration of PR expression through HDACi utilization in endometrial cancer cells has been reported by our group and others [9-11]. Following our studies, Ando et al. reported that panobinostat can recover PR expression on the mRNA level and further enhance progestin’s effect on inhibiting endometrial cancer cell viability in vitro, however, no tumor regression was investigated in this study [19]. Romidepsin has also been shown to suppress endometrial cancer cell proliferation and induce apoptosis by upregulating p53 and p21 in vitro which is consistent with our findings [20]. A newer preclinical HDACi, MHY2256, has been tested in mice models and was found to induce cell death through acetylation of p53 in endometrial cancer [21]. Therefore, this is the first time PR expression has been evaluated through HDACi treatment in in vivo models to enhance existing hormone therapies.
In our studies, well-studied PR downstream genes FoxO1, p27, p21, Cyclin D1 and Myc were used as markers for PR function [3,9,22,23]. Tumor suppressors FoxO1, p27, and p21 were upregulated at both the protein (Figure 1B) and mRNA levels (Figure 1C) by HDACi treatment, with the exception of p27 in KLE cells. In contrast, expression of oncogenes, cyclin D1 and Myc, was decreased upon HDACi treatment. Myc was especially downregulated in Ishikawa and ECC1 cells. More importantly, upregulation of FoxO1 and p21 by romidepsin was also observed in in vivo EC tumor models (Figure 3D). Of note, MPA treatment also increased p21 expression which is consistent with its reported role in cell cycle arrest [3]. These proteins may serve as potential drug effect markers.
As a principal inducer, ERα has a strong correlation with PR in breast and endometrial cancer [23,24]. Previously, our group has shown that ERα upregulation is not necessary to induce PR upregulation through treatment with HDACi [23]. This effect is replicated in Figure 1B, 1C as PR expression is shown to be upregulated despite ERα loss. Romidepsin downregulated ERα in two ER-positive endometrioid endometrial cancer cell lines Ishikawa and ECC1. ERα repression by all HDACi, except entinostat, is more dramatic in ECC1 cells which harbor high basal ERα levels. In ER-negative KLE cells, romidepsin treatment increased ERα expression to similar levels observed in ECC1 and Ishikawa. HDACi-induced ERα repression was also reported by Fiskus et al. [25].
Next, we aimed to understand the molecular mechanism of HDACi-induced PR upregulation. Compared to endometrial tumors, PR level is significantly lower in 2D cell culture as reported in uterine leiomyomas [26,27]. Consequently, HDACi have a significantly higher impact on PR expression in cell lines. As shown in Figure 1B, 1C, HDACi induce PR expression at the transcriptional level by increasing PR mRNA expression. This mechanism was further validated using tissue ChIP as shown in Figure 4. However, when Ishikawa cells were grown in vivo, PR expression was much higher when compared to 2D cell cultures. In the presence of MPA, ligand-induced PR activation followed by proteasomal degradation is the dominant mechanism to downregulate PR expression [28,29]. In this respect, romidepsin restores PR expression by preventing PR degradation rather than epigenetic regulation. As shown in Figure 3D, 3E, MPA activates PR, evidenced by an upper-shifted band. This activation is followed by proteasomal degradation. Combination of MPA with romidepsin greatly rescues PR expression at the protein level with both activated and inactivated forms, but not at the mRNA level, suggesting that romidepsin might prevent MPA-induced proteasome degradation. HR23B exists as a possible key factor that links HDAC inhibition and proteasome degradation.
HR23B has been found to be closely tied to HDACi and is involved in DNA excision repair and the transportation of ubiquitinated proteins for proteasomal degradation [30]. HDACi treatment has been reported to both increase and decrease HR23B expression [31-33]. As shown in Figure 3E, the addition of romidepsin led to the downregulation of HR23B which may be a potential mechanism for the prevention of functional PR degradation in the cell. In endometrial cancer patients, lower HR23B expression has been associated with better survival (Figure 3F). Therefore, downregulation of HR23B would be beneficial for patient outcomes. Further research may be needed to validate HR23B’s role in PR regulation.
KIAA1324 (EIG121/ELAPOR1), an estrogen-induced gene, is a reported tumor suppressor due to its anti-proliferative and pro-apoptotic effects by blocking oncogene GRP78 and has been shown to be suppressed by HDACi in gastric cancer [34]. It is reported that high expression of KIAA1324 correlates with good survival for prostate, breast, and endometrioid ovarian cancer [35-37]. As shown in Figure 3F, it has been linked to significantly better survival in endometrial cancer patients and has a very strong association to PR expression. KIAA1324 was found to be upregulated by romidepsin treatment in vivo (Figure 3D), suggesting it may be a promising drug effect marker. Indeed, the expression of KIAA1324 is high in estrogen-related type I endometrioid endometrial cancer, a low-grade endometrial cancer with generally good prognosis. Contrastingly, KIAA1324 expression is extremely low in non-estrogen related type II endometrial cancer, a higher grade endometrial cancer associated with worse prognosis [38].
As shown in Figure 3, we have demonstrated that romidepsin is effective at preventing acquired hormone resistance by rescuing PR expression through blocking ligand-triggered proteasomal degradation. Similar to our previous reports, we found that romidepsin is also effective at reversing PR repression at the transcriptional level (Figures 1C and 4). As shown in Figure 4A, low PR expression in endometrial tumors can be attributed to less active transcription, as demonstrated by decreased RNA polymerase II binding on the PR promoter region. Romidepsin treatment indeed reverses this transcriptional repression by recruiting active transcription factor, H3K4Me3, and RNA polymerase II, while diminishing repressive factor, H3K9Me3, and SUZ12 binding on the PR promoter region as previously reported [9]. In summary, we propose two potential mechanisms underlying romidepsin-induced PR expression: (1) prevention of proteasome degradation and (2) derepression at the transcriptional level.
Compared to entinostat, romidepsin is more efficient in restoring PR expression in endometrial tumors in in vivo models. Three reasons can explain the lesser effectiveness of entinostat on restoring PR expression (Figure 2F): (1) entinostat is a less potent HDACi with IC50 = 3.9-7.8 µM to inhibit HDAC1-3 [39], (2) long treatment window; to achieve a better tumor volume reduction, the mice were given MPA and/or entinostat for over three weeks, generating significant tumor necrosis and decreased PR expression, and (3) alteration of mice estrous cycle; estrogen and progesterone secreted from the mouse ovary can change PR expression by 10-fold. Based on the aforementioned reasons, we chose romidepsin as the next candidate drug and modified our protocol. Three reasons might account for the success of romidepsin restoring functional PR expression: (1) romidepsin is a much more potent HDACi with IC50 = 8-17 nM to inhibit HDAC1-3, (2) the mice were ovariectomized to ablate the rapidly cycling hormone production, and (3) the mice were treated over a shorter time course to prevent massive tumor necrosis but long enough to reach the PR upregulation window.
To search for novel drug effect markers, our transcriptome analysis identified 103 transcripts whose expression was significantly upregulated (n = 87) or downregulated (n = 16) (fold > 2 and false discovery rate (FDR) < 0.02). These altered genes provide in vivo evidence supporting direct or indirect regulation of many new transcripts by romidepsin + MPA. We selected 17 altered genes and validated them with real-time PCR (Figure 5C). These genes are related to endometrium function or tumor progression: CD52, a glycosylphosphatidylinositol (GPI)-anchored protein, was upregulated 15.59-fold after treatment of romidepsin + MPA in mice tumor samples (Figure 5C). CD52 has been found to be dramatically increased by progestin treatment in pregnant mice and rats and is decreased in women refractory to embryo implantation [40]. Higher expression of CD52 also led to increased anti-tumor immune cell infiltration in breast cancer [41]. According to the Cancer Genome Atlas Database, CD52 is a strong predictor of favorable survival in endometrial cancer (Figure 5E). DLK1, Delta-Like Non-Canonical Notch Ligand 1, is an inhibitor of the PI3K/Akt pathway and leads to inhibitory effects on chondrogenesis and was increased 28.47-fold after dual treatment (Figure 5C). A study found that increasing expression of DLK1 and SCUBE1 resulted in significantly decreased prostate cancer tumor size and the inhibition of the tumorigenic activities of human prostate cancer-associated fibroblasts [42]. GALNT9 (an initiator of O-glycosylation) is also a favorable marker and was upregulated 23.79-fold (Figure 5C). Literature suggests GALNT9 is a putative tumor suppressor due to its downregulation by DNA methylation from breast to brain metastasis [43]. Low expression of GALNT9 predicts worse progression-free survival for breast cancer and neuroblastoma patients [43,44]. G-protein γ2 subunit (GNG2), observed 18.72-fold increase in expression through dual treatment. It has been shown to be a tumor suppressor in malignant melanomas, and Mazur et al. have reported that PR directly binds to GNG2 in decidualized human endometrial stromal cells, modulating its expression [45]. A 13.3-fold increase was observed in SPIB (Spi-B transcription factor) after romidepsin + MPA treatment. SPIB functions as a tumor suppressor in colorectal cancer by triggering NFkB and JNK signaling. SPOCK1 is a Ca2+-binding matricellular glycoprotein belonging to the SPARC family and was downregulated 0.11-fold by romidepsin + MPA treatment. SPOCK1 is upregulated in colorectal cancer and promotes cell proliferation through the PI3K/AKT signaling pathway [46,47]. High expression of SPOCK1 correlates with a poor prognosis and promotes tumor growth in multiple tumor types, including breast cancer and lung cancer [47-49]. Knockdown of SPOCK1 inhibits tumor cell proliferation and invasion in non-small cell lung cancer, ovarian cancer and colon cancer [50-53]. Secreted Frizzled Related Protein 2 (SFRP2), is an oncogenic factor that was suppressed 0.60-fold in the romidepsin + MPA treatment group. Progesterone has been shown to abrogate SFRP2 expression in the endometrium [54], suggesting increased PR activity is the underlying mechanism for this gene suppression in our study. SFRP2 modulates the Wnt signaling pathway, leading to cell proliferation, differentiation, and tumorigenesis [54,55]. These genes may serve as potential drug effect markers for romidepsin + MPA treatment, however, more studies are needed to validate these genes.
The strength of this study is that we confirmed that PR expression can be restored in three endometrial cancer cell lines with four clinical-grade HDACi. Two HDACi were used to test drug efficacy in in vivo endometrial tumor models. To support that the restored PR expression is functional, multiple well-studied PR target genes were tested. Dozens of novel romidepsin + MPA drug efficacy markers were also proposed in this study. The limitation of this study is that only one endometrial tumor model was used and a small number of patient tumor samples were tested. In the future, various endometrial tumor models and a larger endometrial tumor sample size may further support our data. Taken together, our study supports that romidepsin is a promising HDACi that can sensitize progestin therapy in future endometrial cancer clinical trials. It is noteworthy that with an enhanced understanding of PR biology, it is now more clear that PR upregulation as an endpoint in response to targeted agents in combination with progestins must be studied in the long-term, not just in the short-term. This is because the initial drop in PR in response to progestins is expected during ligand-dependent downregulation and signals hormone/PR transcriptional activity [5]. The ability of an HDACi to stave off the eventual silencing of PR expression and activity in the long-term is the goal of our current studies, where we propose that romidepsin has such an impact.
Acknowledgements
This project was supported by NIH R01CA99908 (KKL), NIH R37CA238274 (SY), the Department of Obstetrics and Gynecology Research Fund (KKL), and the Department of Pathology StartUp Fund (SY).
Disclosure of conflict of interest
None.
Abbreviations
- PR
Progesterone Receptor
- HDACi
Histone Deacetylase Inhibitor
- MPA
Medroxyprogesterone Acetate
- H3K4Me3
Histone H3 Trimethylation at Lysine 4
- H3K9Me3
Histone H3 Trimethylation at Lysine 9
- H3K27Me3
Histone H3 Trimethylation at Lysine 27
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Makker V, Green AK, Wenham RM, Mutch D, Davidson B, Miller DS. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol Oncol Res Pract. 2017;4:19. doi: 10.1186/s40661-017-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22:145–152. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duska LR, Filiaci VL, Walker JL, Holman LL, Hill EK, Moore RG, Ring KL, Pearl ML, Muller CY, Kushnir CL, Lankes HA, Samuelson MI, Carrick KS, Rajan A, Rodgers WH, Kohn EC, Piekarz R, Leslie KK. A surgical window trial evaluating medroxyprogesterone acetate with or without entinostat in patients with endometrial cancer and validation of biomarkers of cellular response. Clin Cancer Res. 2021;27:2734–2741. doi: 10.1158/1078-0432.CCR-20-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol. 2001;21:6122–6131. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman MJ, Craft B, Hastie M, Repecka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38:675–678. doi: 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, Sanli K, von Feilitzen K, Oksvold P, Lundberg E, Hober S, Nilsson P, Mattsson J, Schwenk JM, Brunnstrom H, Glimelius B, Sjoblom T, Edqvist PH, Djureinovic D, Micke P, Lindskog C, Mardinoglu A, Ponten F. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 8.Sabnis GJ, Goloubeva O, Chumsri S, Nguyen N, Sukumar S, Brodie AM. Functional activation of the estrogen receptor-alpha and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res. 2011;71:1893–1903. doi: 10.1158/0008-5472.CAN-10-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S, Jia Y, Liu X, Winters C, Wang X, Zhang Y, Devor EJ, Hovey AM, Reyes HD, Xiao X, Xu Y, Dai D, Meng X, Thiel KW, Domann FE, Leslie KK. Systematic dissection of the mechanisms underlying progesterone receptor downregulation in endometrial cancer. Oncotarget. 2014;5:9783–9797. doi: 10.18632/oncotarget.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S, Xiao X, Jia Y, Liu X, Zhang Y, Wang X, Winters CJ, Devor EJ, Meng X, Thiel KW, Leslie KK. Epigenetic modification restores functional PR expression in endometrial cancer cells. Curr Pharm Des. 2014;20:1874–1880. doi: 10.2174/13816128113199990532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong Y, Dowdy SC, Gonzalez Bosquet J, Zhao Y, Eberhardt NL, Podratz KC, Jiang SW. Epigenetic-mediated upregulation of progesterone receptor B gene in endometrial cancer cell lines. Gynecol Oncol. 2005;99:135–141. doi: 10.1016/j.ygyno.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MR, Reske JJ, Koeman J, Adams M, Joshi NR, Fazleabas AT, Chandler RL. SWI/SNF antagonism of PRC2 mediates estrogen-induced progesterone receptor expression. Cells. 2022;11:1000. doi: 10.3390/cells11061000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Thiel KW, De Geest K, Leslie KK. Endometrial cancer: reviving progesterone therapy in the molecular age. Discov Med. 2011;12:205–212. [PubMed] [Google Scholar]
- 14.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes HD, Carlson MJ, Devor EJ, Zhang Y, Thiel KW, Samuelson MI, McDonald M, Yang S, Stephan JM, Savage EC, Dai D, Goodheart MJ, Leslie KK. Downregulation of FOXO1 mRNA levels predicts treatment failure in patients with endometrial pathology conservatively managed with progestin-containing intrauterine devices. Gynecol Oncol. 2016;140:152–160. doi: 10.1016/j.ygyno.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly RM, Rudek MA, Piekarz R. Entinostat: a promising treatment option for patients with advanced breast cancer. Future Oncol. 2017;13:1137–1148. doi: 10.2217/fon-2016-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18:1414. doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolewski P, Robak T. The discovery and development of romidepsin for the treatment of T-cell lymphoma. Expert Opin Drug Discov. 2017;12:859–873. doi: 10.1080/17460441.2017.1341487. [DOI] [PubMed] [Google Scholar]
- 19.Ando H, Miyamoto T, Kashima H, Higuchi S, Ida K, Mvunta DH, Shiozawa T. Panobinostat enhances growth suppressive effects of progestin on endometrial carcinoma by increasing progesterone receptor and mitogen-inducible gene-6. Horm Cancer. 2017;8:257–267. doi: 10.1007/s12672-017-0295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LH, Zhang PR, Cai PY, Li ZC. Histone deacetylase inhibitor, romidepsin (FK228) inhibits endometrial cancer cell growth through augmentation of p53-p21 pathway. Biomed Pharmacother. 2016;82:161–166. doi: 10.1016/j.biopha.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 21.De U, Son JY, Sachan R, Park YJ, Kang D, Yoon K, Lee BM, Kim IS, Moon HR, Kim HS. A new synthetic histone deacetylase inhibitor, MHY2256, induces apoptosis and autophagy cell death in endometrial cancer cells via p53 acetylation. Int J Mol Sci. 2018;19:2743. doi: 10.3390/ijms19092743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Huang C, Kavlashvili T, Fronk A, Zhang Y, Wei Y, Dai D, Devor EJ, Meng X, Thiel KW, Leslie KK, Yang S. Loss of progesterone receptor through epigenetic regulation is associated with poor prognosis in solid tumors. Am J Cancer Res. 2020;10:1827–1843. [PMC free article] [PubMed] [Google Scholar]
- 23.Kavlashvili T, Jia Y, Dai D, Meng X, Thiel KW, Leslie KK, Yang S. Inverse relationship between progesterone receptor and myc in endometrial cancer. PLoS One. 2016;11:e0148912. doi: 10.1371/journal.pone.0148912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siadati S, Sharbatdaran M, Nikbakhsh N, Ghaemian N. Correlation of ER, PR and HER-2/Neu with other prognostic factors in infiltrating ductal carcinoma of breast. Iran J Pathol. 2015;10:221–226. [PMC free article] [PubMed] [Google Scholar]
- 25.Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, Herger B, Yang Y, Atadja P, Wu J, Bhalla K. Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res. 2007;13:4882–4890. doi: 10.1158/1078-0432.CCR-06-3093. [DOI] [PubMed] [Google Scholar]
- 26.Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod. 2006;12:187–207. doi: 10.1093/molehr/gal018. [DOI] [PubMed] [Google Scholar]
- 27.Severino MF, Murray MJ, Brandon DD, Clinton GM, Burry KA, Novy MJ. Rapid loss of oestrogen and progesterone receptors in human leiomyoma and myometrial explant cultures. Mol Hum Reprod. 1996;2:823–828. doi: 10.1093/molehr/2.11.823. [DOI] [PubMed] [Google Scholar]
- 28.Khan JA, Amazit L, Bellance C, Guiochon-Mantel A, Lombes M, Loosfelt H. P38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization. Mol Endocrinol. 2011;25:1710–1724. doi: 10.1210/me.2011-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olzscha H. Posttranslational modifications and proteinopathies: how guardians of the proteome are defeated. Biol Chem. 2019;400:895–915. doi: 10.1515/hsz-2018-0458. [DOI] [PubMed] [Google Scholar]
- 31.Fotheringham S, Epping MT, Stimson L, Khan O, Wood V, Pezzella F, Bernards R, La Thangue NB. Genome-wide loss-of-function screen reveals an important role for the proteasome in HDAC inhibitor-induced apoptosis. Cancer Cell. 2009;15:57–66. doi: 10.1016/j.ccr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Khan O, Fotheringham S, Wood V, Stimson L, Zhang C, Pezzella F, Duvic M, Kerr DJ, La Thangue NB. HR23B is a biomarker for tumor sensitivity to HDAC inhibitor-based therapy. Proc Natl Acad Sci U S A. 2010;107:6532–6537. doi: 10.1073/pnas.0913912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelika Ihle M, Merkelbach-Bruse S, Hartmann W, Bauer S, Ratner N, Sonobe H, Nishio J, Larsson O, Aman P, Pedeutour F, Taguchi T, Wardelmann E, Buettner R, Schildhaus HU. HR23b expression is a potential predictive biomarker for HDAC inhibitor treatment in mesenchymal tumours and is associated with response to vorinostat. J Pathol Clin Res. 2016;2:59–71. doi: 10.1002/cjp2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JM, Park S, Kim SJ, Kim H, Lee B, Kim J, Park J, Kim ST, Yang HK, Kim WH, Kim SJ. KIAA1324 suppresses gastric cancer progression by inhibiting the oncoprotein GRP78. Cancer Res. 2015;75:3087–3097. doi: 10.1158/0008-5472.CAN-14-3751. [DOI] [PubMed] [Google Scholar]
- 35.Meseure D, Drak Alsibai K, Vacher S, Hatem R, Nicolas A, Callens C, Lerebours F, Bieche I. Altered expression of three egfr posttranslational regulators MDGI, MIG6, and EIG121 in invasive breast carcinomas. Anal Cell Pathol (Amst) 2020;2020:9268236. doi: 10.1155/2020/9268236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stinnesbeck M, Kristiansen A, Ellinger J, Hauser S, Egevad L, Tolkach Y, Kristiansen G. Prognostic role of TSPAN1, KIAA1324 and ESRP1 in prostate cancer. APMIS. 2021;129:204–212. doi: 10.1111/apm.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dieters-Castator DZ, Rambau PF, Kelemen LE, Siegers GM, Lajoie GA, Postovit LM, Kobel M. Proteomics-derived biomarker panel improves diagnostic precision to classify endometrioid and high-grade serous ovarian carcinoma. Clin Cancer Res. 2019;25:4309–4319. doi: 10.1158/1078-0432.CCR-18-3818. [DOI] [PubMed] [Google Scholar]
- 38.Deng L, Broaddus RR, McCampbell A, Shipley GL, Loose DS, Stancel GM, Pickar JH, Davies PJ. Identification of a novel estrogen-regulated gene, EIG121, induced by hormone replacement therapy and differentially expressed in type I and type II endometrial cancer. Clin Cancer Res. 2005;11:8258–8264. doi: 10.1158/1078-0432.CCR-05-1189. [DOI] [PubMed] [Google Scholar]
- 39.Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon AM, Schlegl J, Abraham Y, Becher I, Bergamini G, Boesche M, Delling M, Dumpelfeld B, Eberhard D, Huthmacher C, Mathieson T, Poeckel D, Reader V, Strunk K, Sweetman G, Kruse U, Neubauer G, Ramsden NG, Drewes G. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat Biotechnol. 2011;29:255–265. doi: 10.1038/nbt.1759. [DOI] [PubMed] [Google Scholar]
- 40.Dhakal P, Rumi MA, Kubota K, Chakraborty D, Chien J, Roby KF, Soares MJ. Neonatal progesterone programs adult uterine responses to progesterone and susceptibility to uterine dysfunction. Endocrinology. 2015;156:3791–3803. doi: 10.1210/en.2015-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma YF, Chen Y, Fang D, Huang Q, Luo Z, Qin Q, Lin J, Zou C, Huang M, Meng D, Huang Q, Lu GM. The immune-related gene CD52 is a favorable biomarker for breast cancer prognosis. Gland Surg. 2021;10:780–798. doi: 10.21037/gs-20-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orr B, Grace OC, Brown P, Riddick AC, Stewart GD, Franco OE, Hayward SW, Thomson AA. Reduction of pro-tumorigenic activity of human prostate cancer-associated fibroblasts using Dlk1 or SCUBE1. Dis Model Mech. 2013;6:530–536. doi: 10.1242/dmm.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pangeni RP, Channathodiyil P, Huen DS, Eagles LW, Johal BK, Pasha D, Hadjistephanou N, Nevell O, Davies CL, Adewumi AI, Khanom H, Samra IS, Buzatto VC, Chandrasekaran P, Shinawi T, Dawson TP, Ashton KM, Davis C, Brodbelt AR, Jenkinson MD, Bieche I, Latif F, Darling JL, Warr TJ, Morris MR. The GALNT9, BNC1 and CCDC8 genes are frequently epigenetically dysregulated in breast tumours that metastasise to the brain. Clin Epigenetics. 2015;7:57. doi: 10.1186/s13148-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berois N, Gattolliat CH, Barrios E, Capandeguy L, Douc-Rasy S, Valteau-Couanet D, Benard J, Osinaga E. GALNT9 gene expression is a prognostic marker in neuroblastoma patients. Clin Chem. 2013;59:225–233. doi: 10.1373/clinchem.2012.192328. [DOI] [PubMed] [Google Scholar]
- 45.Mazur EC, Vasquez YM, Li X, Kommagani R, Jiang L, Chen R, Lanz RB, Kovanci E, Gibbons WE, DeMayo FJ. Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology. 2015;156:2239–2253. doi: 10.1210/en.2014-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Zhi X, Shi S, Tao R, Chen P, Sun S, Bian L, Xu Z, Ma L. SPOCK1 is up-regulated and promotes tumor growth via the PI3K/AKT signaling pathway in colorectal cancer. Biochem Biophys Res Commun. 2017;482:870–876. doi: 10.1016/j.bbrc.2016.11.126. [DOI] [PubMed] [Google Scholar]
- 47.Xu M, Zhang X, Zhang S, Piao J, Yang Y, Wang X, Lin Z. SPOCK1/SIX1axis promotes breast cancer progression by activating AKT/mTOR signaling. Aging (Albany NY) 2020;13:1032–1050. doi: 10.18632/aging.202231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan LC, Jeng YM, Lu YT, Lien HC. SPOCK1 is a novel transforming growth factor-beta-induced myoepithelial marker that enhances invasion and correlates with poor prognosis in breast cancer. PLoS One. 2016;11:e0162933. doi: 10.1371/journal.pone.0162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miao L, Wang Y, Xia H, Yao C, Cai H, Song Y. SPOCK1 is a novel transforming growth factor-beta target gene that regulates lung cancer cell epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2013;440:792–797. doi: 10.1016/j.bbrc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Zhang LQ, Wang Y, Zhang L. Effects of shRNA-mediated knockdown of SPOCK1 on ovarian cancer growth and metastasis. Cell Mol Biol (Noisy-le-grand) 2015;61:102–109. [PubMed] [Google Scholar]
- 51.Zhao P, Guan HT, Dai ZJ, Ma YG, Liu XX, Wang XJ. Knockdown of SPOCK1 inhibits the proliferation and invasion in colorectal cancer cells by suppressing the PI3K/Akt pathway. Oncol Res. 2016;24:437–445. doi: 10.3727/096504016X14685034103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Liu X, Tian Q, Liang T, Chang P. Reduced SPOCK1 expression inhibits non-small cell lung cancer cell proliferation and migration through Wnt/beta-catenin signaling. Eur Rev Med Pharmacol Sci. 2018;22:637–644. doi: 10.26355/eurrev_201802_14288. [DOI] [PubMed] [Google Scholar]
- 53.Liu HX, Cao YY, Qu JY. SPOCK1 promotes the proliferation and migration of colon cancer cells by regulating the NF-kappaB pathway and inducing EMT. Neoplasma. 2021;68:702–710. doi: 10.4149/neo_2021_201031N1158. [DOI] [PubMed] [Google Scholar]
- 54.Heinosalo T, Gabriel M, Kallio L, Adhikari P, Huhtinen K, Laajala TD, Kaikkonen E, Mehmood A, Suvitie P, Kujari H, Aittokallio T, Perheentupa A, Poutanen M. Secreted frizzled-related protein 2 (SFRP2) expression promotes lesion proliferation via canonical WNT signaling and indicates lesion borders in extraovarian endometriosis. Hum Reprod. 2018;33:817–831. doi: 10.1093/humrep/dey026. [DOI] [PubMed] [Google Scholar]
- 55.van Loon K, Huijbers EJM, Griffioen AW. Secreted frizzled-related protein 2: a key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metastasis Rev. 2021;40:191–203. doi: 10.1007/s10555-020-09941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]